Translate this page into:
Exploring high pressure structural transformations, electronic properties and superconducting properties of MH2 (M = Nb, Ta)
⁎Corresponding authors. scujyy@163.com (Yuanyuan Jin), zcz19870517@163.com (Chuanzhao Zhang) jyy@163.com (Chuanzhao Zhang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The high-pressure structures and properties of MH2 (M = Nb, Ta) are explored through an ab initio evolutionary algorithm for crystal structure prediction and first-principles calculations. It is found that NbH2 undergoes a phase transition from a cubic Fm m structure with regular NbH8 cubes to an orthorhombic Pnma structure with fascinating distorted NbH9 tetrakaidecahedrons at 48.8 GPa, while the phase transition pressure of TaH2 from a hexagonal P63mc phase with slightly distorted TaH7 decahedron to an orthorhombic Pnma phase with attractive distorted TaH9 tetrakaidecahedrons is about 90.0 GPa. Besides, the calculated electronic band structure and density of states demonstrate that all of these structures are metallic. The Poisson’s ratio, electron localization function, and Bader charge analysis suggest that these phases possess dominant ionic bonding character with the effective charges transferring from the metal atom to H. From our electron–phonon calculations, the calculated superconducting critical temperature Tc of the Pnma-NbH2 is 6.903 K at 50 GPa. Finally, via the quasi-harmonic approximation method, the phase diagrams at pressure up to 300 GPa and temperature up to 1000 K of MH2 (M = Nb, Ta) are established, where the transition pressure of Fm m-NbH2 → Pnma-NbH2 and P63mc-TaH2 → Pnma-TaH2 were found to decrease with increasing temperature.
Keywords
Metal hydrides
First-principles calculation
High pressure
Electron localization function
Superconductivity
Pressure–temperature phase diagram
1 Introduction
Metal hydrides, especially under high pressure, have attracted wide attention because of their unique physical properties and attractive applications, such as potential high-temperature superconductivity and clean energy with the ability to replace fossil fuels as hydrogen storage materials. For example, some hydrogen-rich compounds, including YH6 (Troyan et al., 2021; Kong et al., 2021), YH9 (Snider et al., 2021), CeH9 (Salke et al., 2019; Li et al., 2019), CeH10 (Chen et al., 2021), ThH9 (Semenok et al., 2020), ThH10 (Semenok et al., 2020), LaH10 (Drozdov et al., 2019), SnH12 (Hong et al., 2021; Talantsev, 2021); UH7 (Kruglov et al., 2018); and CaH6 (Ma et al., 2022; Li et al., 2022), have been proved to have good superconductivity with high superconducting temperatures ranging from 44 to 262 K at high pressures and a hydrogen-rich compound rhodium trihydride (RhH3) with a high volumetric hydrogen density of 212.5 g H2/L could be used as a hydrogen storage material (Shao et al., 2021).
In recent years, transition metal hydrides have also been extensively studied under high pressure, which can be summarized as the following two aspects. On the one hand, the late transition metals with higher and comparable electronegativity values (e.g., 2.2 for Ru, Pd, Os, and Ir; 2.28 for Pt; 2.36 for W; 2.54 for Au) than that of hydrogen (2.2) are unfavorable react with hydrogen at ambient pressure. However, fortunately, high pressure can make the chemical potential of hydrogen rise sharply, thus effectively stimulating the chemical reactivity of elements, which makes the reaction between hydrogen and post-transition metals become a reality (Miao et al., 2020). Previous high-pressure experiments have shown that high pressure makes it possible to synthesize Rh, Ir, and Pt hydrides. For example, a potential hydrogen source material rhodium dihydride RhH2 (Li et al., 2011) with a volume hydrogen density of 163.7 g H2/L was discovered at room temperature at 8 GPa, and iridium trihydride IrH3 (Scheler et al., 2013) was successfully synthesized above 65 GPa at room temperature by in-situ X-ray diffraction measurement. Besides, PtH is a superconductor with Tc = 10–25 K at high pressure (Kim et al., 2011). On the other hand, as for the early transition metals, although it is found that they are easy to form hydrides under environmental conditions, such as ScH2/ScH3, YH2/YH3, TiH2, ZrH2, HfH2, VH2, and NbH2, after compression, some transition metal hydrides show attractive characteristics and undergo complex phase transitions that cannot be found under normal conditions (Miwa and Fukumoto, 2002; Quijano et al., 2009; Kanagaprabha et al., 2013; Smithson et al., 2002; Purans et al., 2021). For example, theoretical studies show that TiH2 undergoes a structural transition of I4/mmm → P4/nmm → P21/m under high pressure at the corresponding transition pressures of 63 GPa and 294 GPa, respectively, and the phase transition from I4/mmm to P4/nmm at 63 GPa is more suitable for the experimental XRD pattern and the resulting unit cell parameters (Gao et al., 2013; Kalita et al., 2010). A subsequent experiment on the structures and properties of ZrH2 were also investigated under high pressure, which provided a new method for finding hydrogen structures with higher bulk density in transition metal hydrides without static pressure or shear stress (Huang et al., 2014). Subsequently, the theoretical study of HfH2 revealed that it undergoes pressure-induced structural phase transition I4/mmm → Cmma → P21/m at 180 GPa and 250 GPa, respectively, and the Tc of P21/m-HfH2 at 260 GPa is 10.62–12.8 K (Liu et al., 2015). Moreover, Ye et al. reported that the structural sequence of ScH2 should be Fm m → C2/m, with transformation pressures of 65 GPa (Ye et al., 2015). Recent ab initio calculations predicted that Fm m-ScH2 (Wei et al., 2016) with Tc up to 38.11 K at 30 GPa is close to that of MgB2 (39 K) (Choi et al., 2002; Bohnen et al., 2001). This motivated us to find its new structures and explore the high-temperature superconductive property by compressing compounds of early transition metals with hydrogen.
With regard to group 5 element dihydrides, the high-pressure structures and related structural and electronic properties of VH2 and NbH2 have also been deeply explored under high pressure. The two groups predicted that VH2 transforms from cubic Fm m structure to orthogonal Pnma phase at about 50 GPa and the Tc estimate of two stable VH2 structures is only several Kelvins (Chen et al., 2014; Wang et al., 2020). As for NbH2, Gao et al. report a phase transition sequence of Fm m → Pnma at 50 GPa (Gao et al., 2013; Liu et al., 2017). However, Chen et al. used an evolutionary algorithm to detect their stability in the pressure range of 0–100 GPa and found that the ground state Fm m-NbH2 transformed to P63mc-NbH2 at about 43 GPa (Chen et al., 2014). However, the obtained phase transition sequences of NbH2 were not in agreement with each other. In recent experiments, the tantalum dihydride phase was synthesized at about 5 GPa. Despite large amounts of experimental research on TaH2 (Saitoh et al., 2021; Kuzovnikov et al., 2017; Ying et al., 2019; Kuzovnikov et al., 2020), there are few theoretical studies on the high-pressure phase transition of TaH2 and its mechanical properties, chemical bonding nature, dynamical properties, and superconductivity under high pressures. Therefore, this stimulates us to explore of high-pressure structures of MH2 (M = Nb, Ta) and completely understand the structural characters, mechanical properties, electronic properties, and superconductivity of both dihydrides.
In this paper, we examine in detail the crystal structures of MH2 (M = Nb, Ta) within the pressure range of 0–300 GPa by employing an ab initio evolutionary algorithm in combination with first-principles calculations. Interestingly, our calculated results show that NbH2 and TaH2 adopt the Fm m and P63mc structure, respectively, at low pressures. However, at high pressures, NbH2 and TaH2 possess the same Pnma phases. Band structures and density of states indicate that these structures are metallic, while the estimated Tc is 0.945–1.836 K for Fm m-NbH2 at 1 atm, 0.775–1.528 K for P63mc-TaH2 at 1 atm, 4.485–6.795 K for Pnma-NbH2 at 100 GPa, and 3.586–5.542 K for Pnma-TaH2 at 100 GPa, respectively. In addition, the analyses of the Poisson’s ratio, electron localization function (ELF), and Bader charge show that these phases are ionic crystals with the charges transferring from the metal atom to H. Furthermore, the calculation results of the pressure–temperature phase diagram of MH2 (M = Nb, Ta) based on the quasi-harmonic approximation method indicate that high temperature and low pressure are more favorable to synthesizing the high-pressure phase of MH2 (M = Nb, Ta). Our present research attempts to better understand the behavior and properties of MH2 (M = Nb, Ta) under pressure and temperature, and promote further experimental and theoretical research on transition metal hydrides.
2 Computational method
We use the evolutionary algorithm USPEX code (Universal Structure Predictor: Evolutionary Xtallograph) to predict crystal structure, so as to extensively explore stable low-energy structures under high pressure with a different number of formula units (f.u.) at zero temperature (Oganov and Glass, 2006; Oganov et al., 2011; Lyakhov et al., 2013). A similar method has been successfully applied to discover transition metal dichalcogenides under high pressure with a similar composition to MH2 (M = Nb, Ta) and provides good guidance for the synthesis of experiments (Saqib et al., 2021; Rahman et al., 2022). Here, we have predicted the crystal structure of MH2 (M = Nb, Ta) at 1 atm, 50, 100, 150, 200, 250, and 300 GPa, in which the unit cell is as high as 8 formula units. The 50 structures of the first generation are randomly generated by the evolutionary algorithm, 60 % of the structures of each subsequent generation come from the parent generation, and the new structures of each subsequent generation are generated by mutation operator inheritance (60 %), substitution (10 %) and lattice mutation (30 %). The energy calculations, electronic structure calculations, and elastic properties introduced in this paper are carried out in density functional theory within the Perdew-Burke-Ernzerhof (PBE) parameterization of the generalized gradient approximation (GGA) as implemented in the Vienna ab initio simulation software package (VASP) (Kresse and Furthmüller, 1996; Perdew et al., 1996). The Projector-Augmented Wave (PAW) potentials with 13 valence electrons (4s24p65s14d4) for Nb, 11 valence electrons (5p66s25d3) for Ta, and 1 valence electron (1 s1) for H were applied with a plane wave basis set up to cutoff energy of 600 eV and the k-point grid with 0.2 Å-1 spacing was used to sample the Brillouin zone to ensure that all enthalpy calculations are well converged to<1 meV/atom (Kresse and Joubert, 1999; Monkhorst and Pack, 1976). We use Bader charge analysis (Tang et al., 2009) and electronic localization function (Becke and Edgecombe, 1990; Savin et al., 1992) to describe electronic properties. In order to determine the dynamic stability and thermodynamic properties of the studied structure, the phonon calculation is carried out by using the supercell method with PHONOPY program (Togo et al., 2008; Togo and Tanaka, 2015). The independent single crystal elastic constants could be determined by applying a stress tensor generated by a small strain to an optimized unit cell. The bulk modulus (B), shear modulus (G), Young’s modulus (E), and Poisson’s ratio (ν) of the material were determined by Voigt-Reuss-Hill (VRH) approximation (Hill, 1952). Electron–phonon coupling (EPC) parameter λ of NbH2 and TaH2 is performed using the density functional perturbation theory as implemented in the QUANTUM-ESPRESSO package (Giannozzi et al., 2009). The Projector-Augmented Wave (PAW) potentials were applied with a plane wave basis set up to cutoff energy of 60 Ry and charge density cutoff of 600 Ry. The q-point mesh of EPC calculations adopted 6 × 6 × 6 for Fm m-NbH2, 2 × 4 × 2 for Pnma-NbH2, 4 × 4 × 2 for P63mc-TaH2, and 2 × 4 × 2 for Pnma-TaH2, respectively.
3 Results and discussion
3.1 Crystal structure searching and phase transition under pressure
Firstly, to extensively explore the stable MH2 (M = Nb, Ta) under different pressures, we carry out the evolutionary structural search for MH2 (M = Nb, Ta) using simulation sizes ranging from one to eight MH2 (M = Nb, Ta) formula units (f.u.) in the unit cell at 0 K with selected pressures of 1 atm, 50, 100, 150, 200, 250, and 300 GPa. Subsequently, the analysis of the predicted structures provides some candidate structures with space groups Pm, C2/m, Pnma, Imma, P4/nmm, P
m1, P63mc, and Fm
m for NbH2, and Pm, Pnma, P63/mmc, P4/nmm, Fm
m, P
m1, P63mc, and P
2m for TaH2. The enthalpy-pressure (H—P) relations of the candidate structures relative to the cubic Fm
m phase under the pressure range of 0–300 GPa for NbH2 and TaH2 are presented in Fig. 1a and Fig. 1c. According to our simulation, NbH2 and TaH2 at 1 atm adopt the structures of Fm
m and P63mc, respectively, which is consistent with the experimental observations (Reilly and Wiswall, 1970; Saitoh et al., 2021; Kuzovnikov et al., 2017; Ying et al., 2019; Kuzovnikov et al., 2020). The fact that all the experimental and theoretical structures are successfully reconstructed in a specific pressure range verifies the rationality of our method. Under compression, we observed the orthorhombic Pnma structure is favored over the cubic Fm
m phase above 48.8 GPa for NbH2 and the hexagonal P63mc phase above 90.0 GPa for TaH2, which does not include zero point energy (ZPE). Because of the extremely light mass of hydrogen atoms, it is expected that quantum effects will play an important role, and the (ZPE) of hydrogen may be large enough to affect the structural stability of the calculated phase (Pickard and Needs, 2007). We will discuss the influence of zero point energy on phase stability in Section 3.5. The calculated equations of state (EOS) for ambient-pressure structures and the orthorhombic Pnma structures show the volumetric changes are discontinuous at the transition point as plotted in Fig. 1b and Fig. 1d. We observed a volume collapse of 3.7 % occur at 48.8 GPa for NbH2 and 1.2 % occur at 90.0 GPa for TaH2, suggesting that the two-phase transitions are the first-order transition.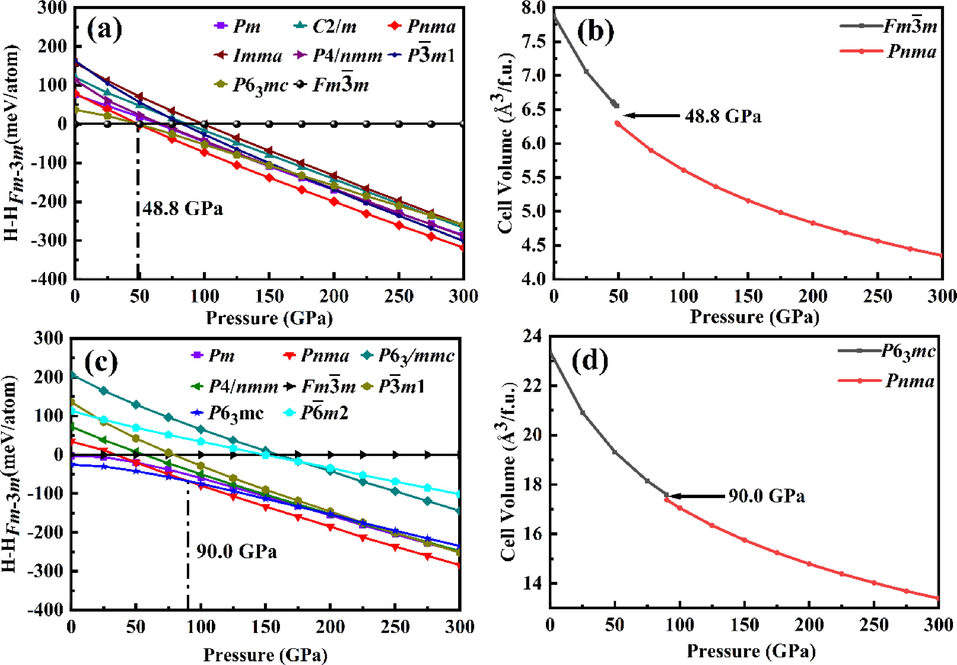
(a) Enthalpy-pressure relation concerning the Fm
m phase and (b) volume-pressure relations for NbH2. (c) Enthalpy-pressure relation concerning the Fm
m phase and (d) volume-pressure relations for TaH2.
To further verify the stability of four structures in comparison with other M−H (M = Nb, Ta) compositions, the typical convex hull diagrams of the M−H system are plotted in Fig. S1 at 1 atm, 100 GPa, 200 GPa, and 300 GPa. (Fig. S1 in the Supplementary Material) It is found that MH2 (M = Nb, Ta) always lie on the convex hull curve, suggesting that they are thermodynamically stable under both high pressure and low pressure. Besides, we approach the pressure dependence of the MH2 (M = Nb, Ta) decomposition reaction (see Fig. S2). Fig. S2a shows that NbH2 can’t decompose to
or
. For TaH2, we consider three substantially different decomposition paths displayed in Fig. S2b:
,
, or
. (Fig. S2 in the Supplementary Material) Apparently, TaH2 can be stabilized in 0–300 GPa. Through the above discussion, we can conclude that MH2 (M = Nb, Ta) are ground states at 0–300 GPa without phase separation.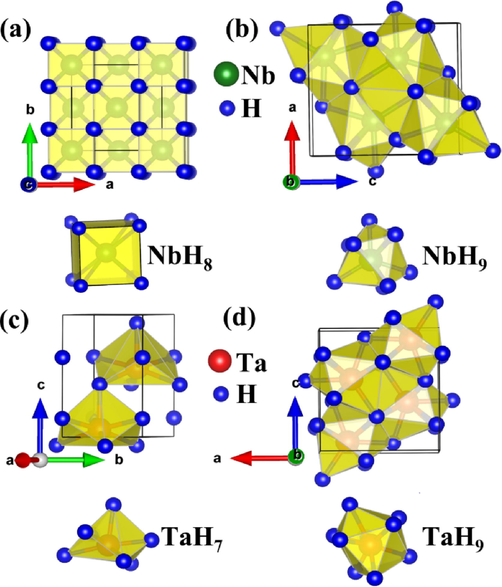
Crystal Structures of MH2 (M = Nb, Ta) phases, together with metal coordination polyhedral: (a) Fm
m-NbH2 at 1 atm, (b) Pnma-NbH2 at 100 GPa, (c) P63mc-TaH2 at 1 atm, and (d) Pnma-TaH2 at 100 GPa. Green atoms depict Nb and red atoms depict Ta, while blue atoms present H.
3.2 Structural features
The crystal structures of the thermodynamically stable phases at the corresponding pressures are illustrated in Fig. 2 and their structural parameters, together with previous experimental data, are presented in Table 1 and Table S1. (Table S1 in the Supplementary Material) The Fm
m-NbH2 phase at 1 atm, shown in Table 1, has lattice parameters of a = b = c = 4.556 Å, which is in good agreement with previous experimental results (a = b = c = 4.53 Å) with a lattice parameter difference of 0.57 % and other theoretical results (Zhang et al., 2017; Gao et al., 2013; Liu et al., 2017; Saitoh et al., 2021). For Fm
m-NbH2 at atmospheric pressure, each Nb atom is surrounded by 8H atoms to form a regular NbH8 cube with an Nb-H separation of 1.982 Å. For Pnma phase at 100 GPa, each Nb atom is surrounded by 9H atoms. The Nb-H distances range from 1.791 Å to 2.077 Å, and the H-Nb-H angle ranges from 57° to 121°, which indicates that the NbH9 arrangement in Pnma phase is a highly distorted tetrakaidecahedron. Obviously, with the increase of pressure, the Nb-H environment in NbH2 changes from regular NbH8 cube to highly distorted NbH9 tetrakaidecahedron.
Space group
Pressure
Refs
a (Å)
b (Å)
c (Å)
V (Å3)
B0
B0′
NbH2
Fm
m
1 atm
This work
4.556
178
3.92
Experiment (Reilly and Wiswall, 1970)
4.530
Calculation (Chen et al., 2014)
4.558
Calculation (Zhang et al., 2017)
4.577
NbH2
Pnma
100 GPa
This work
4.682
2.838
5.067
Calculation (Gao et al., 2013)
4.704
2.860
5.085
Calculation (Zhang et al., 2017)
4.696
2.862
5.092
TaH2
P63mc
1 atm
This work
3.231
3.231
5.162
23.34
180
4.08
Calculation (Zhuang et al., 2017)
3.222
3.222
5.153
Experiment (Kuzovnikov et al., 2020)
3.223
3.223
5.143
23.14
210(20)
4
TaH2
Pnma
100 GPa
This work
4.709
2.842
5.095
17.04
For the P63mc-TaH2 at ambient pressure, the lattice parameter (a = b = 3.231 Å, c = 5.162 Å) is in excellent agreement with the previous results (a = b = 3.222 Å, c = 5.153 Å) calculated by Zhang et al. (Zhuang et al., 2017) and experimental data (Kuzovnikov et al., 2020) (a = b = 3.223 Å, c = 5.143 Å), with a lattice constant deviation of 0.4 %. The third-order Birch-Murnaghan equation of state (EOS (Birch, 1947) is used to determine the bulk modulus B0, its pressure derivative B0′ and the unit cell volume V0 at ambient conditions of P63mc-TaH2 (see Table 1).
By fitting for P63mc-TaH2, the following parameters are obtained: B0 = 180 GPa, B0′ = 4.08, and V0 = 23.34 Å3. These values are in agreement with previous Energy-dispersive X-ray diffraction studies (B0 = 210(20) GPa, B0′ = 4, and V0 = 23.14 Å3) (Kuzovnikov et al., 2020). As displayed in Fig. 2a, each Ta atom is surrounded by one H atom at a separation of 1.889 Å, three H atoms at a separation of 1.921 Å, and a further three H atoms at a separation of 1.990 Å. In addition, the H-Ta-H angles vary from 68° to 115°. Therefore, the Ta environment in the P63mc configuration can be described as distorted TaH7 decahedrons. In the Pnma phase at 100 GPa (see Fig. 2b), the Ta-H distances range from 1.787 to 2.06 Å and the H-Ta-H angles vary from 58° to 164°. These results describe that the Ta environment in the Pnma phase is strongly distorted TaH9 tetrakaidecahedron. In short, the structural features of P63mc and Pnma TaH2 can be summarized as slightly distorted TaH7 decahedrons and strongly distorted TaH9 tetrakaidecahedron, respectively.
3.3 Mechanical stability, mechanical properties, and electronic properties
To inspect the mechanical stabilities of MH2 (M = Nb, Ta) structures, we calculated the elastic constants of MH2 (M = Nb, Ta) at different pressures by using the strain–stress method, as summarized in Table 2. As given in Table 2, four phases satisfy their respectively mechanical stability standards, which state that four structures are mechanically stable at ambient pressure. Besides, Fig. S3 shows that the Pnma-NbH2 and Pnma-TaH2 are dynamically stable at 1 atm, which means that the high-pressure phases Pnma-NbH2 and Pnma-TaH2 can be quenched to normal pressure. (Fig. S3 in the Supplementary Material) At the same time, the bulk modulus (B), shear modulus (G), B/G, Young’s modulus (E), and Poisson’s ratio (ν) can be derived from the calculated elastic constants on the basis of the Voigt-Reuss-Hill method. Firstly, the bulk modulus B of Fm
m-NbH2 and P63mc-TaH2 is consistent with the zero-pressure bulk modulus B0 fitted by EOS (see Table 1), which underlines the accuracies of our calculations. The calculated bulk modulus (B), shear modulus (G), and Young’s modulus (E) of MH2 (M = Nb, Ta) at 1 atm are all larger than those of MgH2 and Pnma-TiH2 hydrogen storage materials, implying that these MH2 (M = Nb, Ta) dihydrides present good mechanical properties (Junkaew et al., 2014; Pan and Chen, 2020). As we all know, the ductility or brittleness of materials can be estimated by the value of B/G with 1.75 as the critical value (Pugh, 1954). Our results show that the calculated identical ratio values of B/G for Fm
m-NbH2, Pnma-NbH2, P63mc-TaH2, and Pnma-TaH2, are 2.31, 2.07, 2.20, and 2.27, respectively, indicating their toughness behavior. Poisson’s ratio is an important parameter to describe the type of bonding in a material (Haines et al., 2001). The threshold value of Poisson’s ratio ν for covalent, and ionic materials is 0.25: large ν values (>0.25) correspond to ionic or metallic materials, while smaller ν values (<0.25) correspond to covalent materials. The Poisson’s ratios of Fm
m-NbH2, Pnma-NbH2, P63mc-TaH2, and Pnma-TaH2, are all larger than 0.25, suggesting that they can be classified into ionic materials and that the ionic bond component is dominant. Vickers hardness was estimated according to the empirical model proposed by Chen et al (Chen et al., 2011). This model is applied as follows: Hv = 2(K2G)0.585-3, where K = G/B. The estimated hardness values are 6.6 GPa for Fm
m-NbH2, 7.6 GPa for Pnma-NbH2, 7.5 GPa for P63/mc-TaH2, and 6.6 GPa for Pnma-TaH2 at 1 atm, which are lower than that of Pd2Mo3N with the strong three-dimensional covalent network (17.1 GPa) (Errandonea et al., 2010). Due to strong ionic bonds, the hardness values of all MH2 (M = Nb, Ta) phases are lower than 10 GPa.
Phase
NbH2
TaH2
MgH2 (Junkaew et al., 2014)
TiH2 (Pan and Chen, 2020)
Fm
m
Pnma
P63mc
Pnma
P42/mnm
Pnma
Pressure
1 atm
1 atm
1 atm
1 atm
1 atm
1 atm
C11
273
273
312
288
74.4
262.6
C22
242
284
273.3
C33
248
313
263
136.0
248.0
C44
84
77
78
74
37.6
45.1
C55
68
70
83.7
C66
81
73
85
53.0
13.4
C12
133
102
166
140
38.8
55.8
C13
89
95
105
31.4
104.8
C23
121
122
75.6
B
180
154
183
174
54.2
139.5
G
78
75
83
77
37.8
51.5
E
204
193
216
200
92.1
51.5
B/G
2.31
2.07
2.20
2.27
2.71
ν
0.31
0.29
0.3
0.31
Hv
6.6
7.6
7.5
6.6
To further gain insight into the electronic properties of four stable MH2 (M = Nb, Ta) compounds, we calculated the electronic band structures and densities of states (DOS), as displayed in Fig. 3. We can see that they all exhibit metallic characteristics, confirmed by the overlap between the conduction bands and the valence bands, and the finite electronic DOS at the Fermi level (Ef). The metallic behavior of these phases indicates that these materials may be magnetic. We have carried out calculations taking into account the spin polarization. Results suggest that MH2 (M = Nb, Ta) are all nonmagnetic because the magnetic moment of each mental atom is zero. For these phases, it is found that there is a deep valley below the Fermi level, and the localized hybridization between M-d and H-s is observed in the energy region below the pseudogap, while the contributions of M-d (M = Nb, Ta) states mainly dominate in this energy range above pseudogap, so the metallic properties are mainly due to partially filled Nb-4d or Ta-5d shell. Note that the contribution from H-s states at the Fermi level is almost zero, which is of great significance to superconductivity according to previous studies as will be discussed later.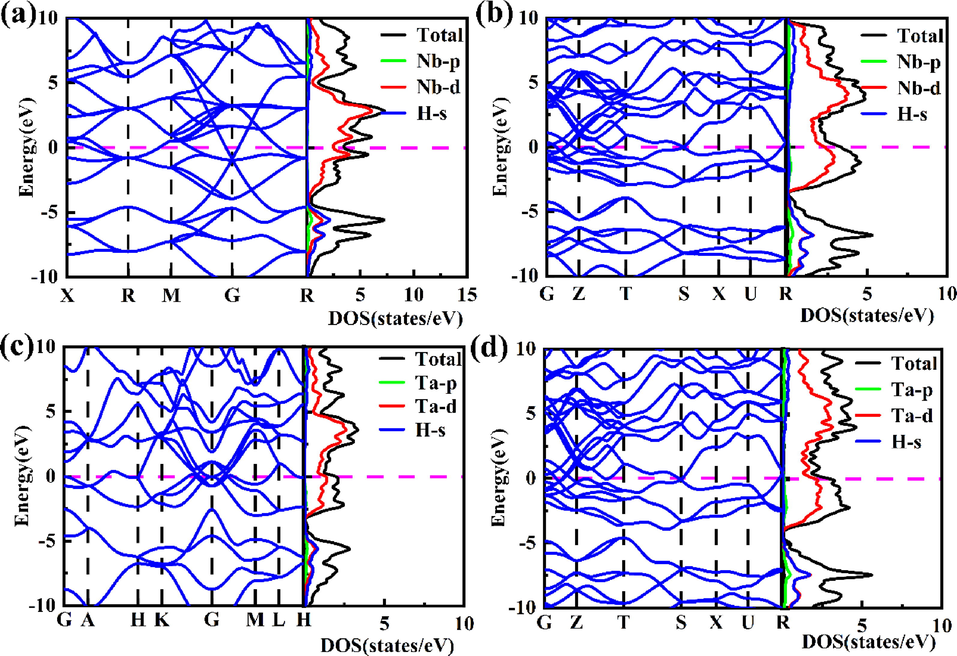
The electronic band structure and densities of states (DOS) of (a) Fm
m-NbH2 at 1 atm, (b) Pnma-NbH2 at 100 GPa, (c) P63mc-TaH2 at 1 atm, and (d) Pnma-TaH2 at 100 GPa.
To clarify the bonding futures for these phases MH2 (M = Nb, Ta), we calculated the electronic localization function (ELF), as depicted in Fig. 4. It is worthy pointing out that these four crystals display a common feature of the ELF that there is no electron localization toward the neighboring M−H and H—H connections, thus suggesting that no covalent interaction exists for neighboring M−H and H—H. Moreover, the ELF values between M and the nearest H atom in four crystals are all smaller than 0.5, implying that ionic bonds are present between M and H atoms. This conclusion also demonstrates the validity of the analyses of Poisson’s ratio. In addition, in order to evaluate the strength of ionic bonds, we explicitly calculated the actual charge transfer between MH2 (M = Nb, Ta) and H atoms, which is summarized in Table 3. Each H atom respectively gains approximately 0.65 e, 0.51 e, 0.59 e, and 0.52 e for Fm
m-NbH2, Pnma-NbH2, P63mc-TaH2, and Pnma-TaH2, while the M (M = Nb, Ta) atoms lose approximately 1.30 e, 1.02 e, 1.18 e, and 1.04 e. Therefore, there is a large amount of charge transfer from M (M = Nb, Ta) to the H atoms, demonstrating the ionic character of the M−H bonding in MH2 (M = Nb, Ta) crystals. Such ionic bonding properties are similar in RhH2 (Shao et al., 2021), ThH2 (Zhang et al., 2015), TcH2 (Li et al., 2016), TiH2 (Zhuang et al., 2018), HfH2 (Quijano et al., 2009), and VH2 (Zhuang et al., 2017; Li and Peng, 2017), but differ from many hydrogen-rich polycrystalline forms (i.e. SiH4 (Zhang et al., 2015), SnH4 (Zhang et al., 2016), MgH12 (Lonie et al., 2013), MgH16 (Lonie et al., 2013), AsH8 (Fu et al., 2016), RuH6 (Liu et al., 2016); and MoH11 (Du et al., 2021) where H atoms are bonded to the nearest H atoms to form H2 or H3 units and/or they are covalently bonded to X (X = Si, Sn) to form X-H bonds. Combination analysis of ELF and Bader charges reveals that MH2 (M = Nb, Ta) forms an ionic bond between M and H, and MH2 (M = Nb, Ta) belongs to ionic crystal with the charge transfers from M atom to H atom, which is consistent with Poisson’s ratio analysis mentioned above.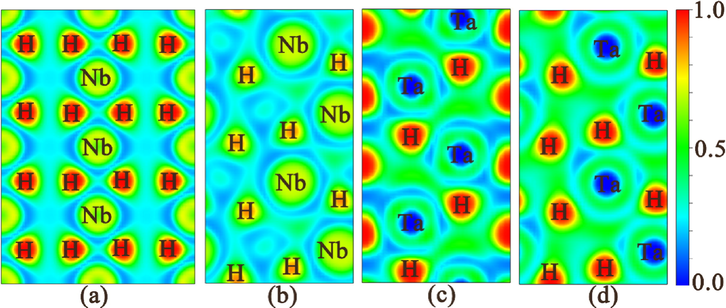
Electron localization function (ELF) of for stable MH2 (M = Nb, Ta) phases: (a) ( −1 1 0) plane for the Fm
m-NbH2 phase at 1 atm, (b) (0 1 0) plane for the Pnma-NbH2 phase at 100 GPa, (c) (1 1 0) plane for the P63mc-TaH2 phase at 1 atm, and (d) (0 1 0) plane for the Pnma-TaH2 phase at 100 GPa.
Phase
Space group
Pressure
Atom
Charge value (e)
δ (e)
NbH2
Fm
m
1 atm
Nb
11.70
1.30
H
1.65
−0.65
Pnma
100 GPa
Nb
11.98
1.02
H
1.51
−0.51
TaH2
P63mc
1 atm
Ta
9.82
1.18
H
1.59
−0.59
Pnma
100 GPa
Ta
9.96
1.04
H
1.52
−0.52
3.4 Electron–phonon coupling
For exploring the superconductivity, we calculate the logarithmic average phonon frequency ωlog, the electronic DOS at the Fermi level N(Ef), and the EPC parameter λ as shown in Table 4. It is found that for materials with λ < 1.5, it is more accurate to estimate the superconducting temperature of the obtained structure by using the modified Allen-Dynes McMillan equation
(Allen and Dynes, 1975). The estimated Tc of NbH2 and TaH2 with a typical choice of Coulomb pseudopotential μ* = 0.1–0.13 is presented in Table 4, which shows that Pnma-NbH2 has the highest Tc, and can reach 6.9 K at 50 GPa, higher than the maximal 4 K predicted for Pnma-VH2 (Chen et al., 2014) at 60 GPa. The estimated Tc of P63mc-TaH2 is 0.775–1.528 K at 1 atm, which is consistent with the experimental measurement of no superconducting transition in the temperature range of 4.4–300 K for TaH2 (Kuzovnikov et al., 2017). In addition, the calculated Tc of the Pnma-TaH2 is 3.586–5.542 K at 100 GPa, which is smaller than that of the Pnma-NbH2 at 100 GPa. The main reason is that the electron DOS of Pnma-TaH2 at Fermi level N (Ef) is much smaller than that of Pnma-NbH2.
Phase
Structure
Pressure
ωlog(K)
N(εf)
λ
Tc(K)μ* = 0.1
Tc(K)μ* = 0.13
NbH2
Fm
m
1 atm
262.565
7.208
0.441
1.836
0.945
Pnma
50 GPa
376.899
26.477
0.560
6.903
4.668
100 GPa
394.013
20.208
0.550
6.795
4.485
300 GPa
547.141
16.527
0.554
9.694
6.439
TaH2
P63mc
1 atm
229.106
10.402
0.436
1.528
0.775
50 GPa
297.577
7.146
0.325
0.299
0.071
Pnma
100 GPa
348.496
16.927
0.537
5.542
3.586
300 GPa
371.099
14.736
0.532
5.719
3.672
According to Fig. 5, we computed phonon dispersions, phonon density of states (PHDOS), Eliashberg spectral function (α2F(ω)/ω), and EPC integrated (λ(ω)) for the Fm
m-NbH2 and P63mc-TaH2 at 0 GPa, and the Pnma-NbH2 and Pnma-TaH2 at 100 GPa. As a result, we found that all structure is dynamically stable because it exhibits the absence of any imaginary frequencies (Zhang et al., 2022; Sun et al., 2020; Zheng et al., 2021; Li et al., 2022; Jin et al., 2022). A clear gap between acoustic and optical phonon modes is observed in the theoretical phonon density of states (Fig. 5), which is due to the huge difference in mass between the M (M = Nb, Ta) atom and H atom, thus dividing the phonon density of states into low-frequency vibrations of M (M = Nb, Ta) atom and high-frequency vibrations of H atom. Note that the low-frequency vibrational modes below 10 THz come from M (M = Nb, Ta) atoms, accounting for 90.8 %, 85.5 %, 84.5 %, and 78 % of the total λ, respectively, while the high-frequency vibrational modes above 10 THz come from H atoms, accounting for 9.2 %, 14.5 %, 15.5 %, and 22 % of the total λ, respectively. We, therefore, conclude that the coupling between electron and phonon vibration of M (M = Nb, Ta) is the reason why MH2 (M = Nb, Ta) shows superconductivity, which is consistent with the dominant DOS of M-d (M = Nb, Ta) shell at Fermi energy. For MH2 (M = Nb, Ta), contrary to the case of superconducting H3S (Drozdov et al., 2015; Duan et al., 2014), YH3 (Kim et al., 2009), and PdH (Gupta, 1982), the electron-optical phonon contribution and consequently to Tc is small, due essentially to the low value of the DOS of H-s states at the Fermi energy, which leads to the low-Tc features of all MH2 (M = Nb, Ta) phases.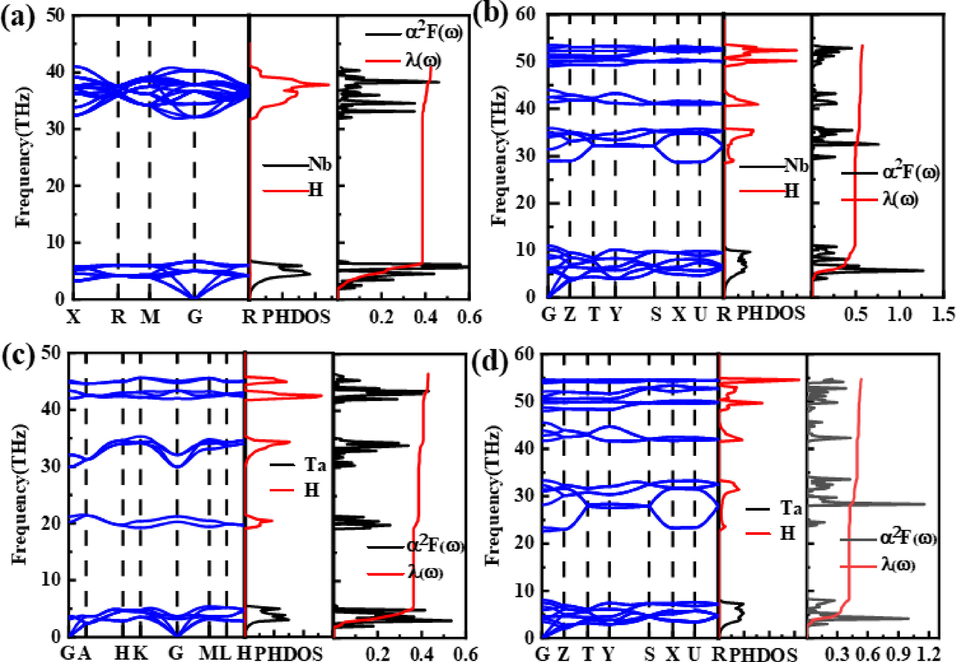
Phonon dispersion relations, projected phonon densities of states (PHDOS), and Eliashberg spectral function for (a) Fm
m-NbH2 at 1 atm, (b) Pnma-NbH2 at 100 GPa, (c) P63mc-TaH2 at 1 atm, and (d) Pnma-TaH2 at 100 GPa.
3.5 Pressure–temperature phase diagram
As discussed earlier, we established a phase transition sequence of MH2 (M = Nb, Ta) under pressure at 0 K. High pressure and high-temperature technology are very important for the synthesis of new materials. Here, the main function of pressure and temperature is to change the free energy diagram of materials or the reaction barrier between materials, so as to promote phase transition or chemical reaction, which leads to the formation and synthesis of the target phase, which can be stored in environmental conditions. To further discover the high-pressure synthesis conditions and the stable pressure–temperature region of each phase, the phase diagram of MH2 (M = Nb, Ta) was constructed by using the quasi-harmonic approximation (QHA) method (Togo and Tanaka, 2015). At a given pressure and temperature, the Helmholtz free energy is expressed as:
Therefore, based on the calculated Gibbs free energies of these phases at different temperatures and pressures, we obtain a pressure–temperature phase diagram (Fig. 6), which can determine the possible existence region of MH2 (M = Nb, Ta) phases. According to Fig. 6, by comparing the transition pressures with and without the influence of temperature, we find that the transition pressure of NbH2 from cubic Fm
m phase to orthorhombic Pnma phase at 0 K is 27.9 GPa, while the transition pressure of TaH2 from the hexagonal P63mc phase to orthorhombic Pnma phase at 0 K is 54.0 GPa. Therefore, ZPE does not change the phase transition series, but only reduces the phase transition pressure, especially for high-pressure transition, which is also observed in other hydrides, such as MgH6 (Feng et al., 2015), AcH2 (Jiang et al., 2014); and PtH (Gao et al., 2012). It is obvious that the transition pressures of Fm
m-NbH2 → Pnma-NbH2 and P63mc-TaH2 → Pnma-TaH2 decrease obviously with the increase of temperature, which originates mainly from the contributions of the thermal vibration at the atomic position to the high-pressure structures more significantly than the low-pressure structures, thus significantly expanding their high-pressure structural stability fields.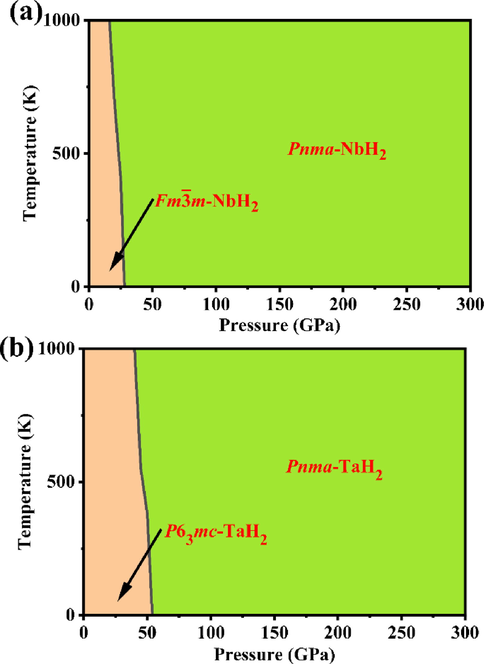
Calculated temperature–pressure phase diagram of (a) NbH2 and (b) TaH2 based on quasi-harmonic approximation.
4 Conclusions
In a word, we have extensively investigated the systematic structure evolution of MH2 (M = Nb, Ta) compounds in the pressure range of 0 to 300 GPa by ab initio evolutionary simulation. Based on the structural prediction, the phase transition sequence of NbH2 is Fm m → Pnma at 48.8 GPa, while the TaH2 is P63mc → Pnma at 90.0 GPa, respectively. The calculations of elastic constants and phonon dispersions confirmed that these phases are stable. The analyses of DOS, Poisson’s ratio, ELF, and Bader charge indicate that four MH2 (M = Nb, Ta) phases are metallic and an ionic crystal with large amounts of charge transferring from M (M = Nb, Ta) atoms to H. The estimated superconducting transition temperature Tc values of Pnma-NbH2 are 6.903 K at 50 GPa. In addition, it is found that the high-pressure phase transition of MH2 (M = Nb, Ta) is more favorable upon heating, and this originates mainly from the contributions of the thermal vibration at the atomic position. The current research will stimulate further high-temperature and high-pressure experiments to synthesize these transition metal dihydrides and make structural, mechanical, and superconducting measurements.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 11804031, 11904297 and 11747139), the Scientific Research Project of Education Department of Hubei Province (No. Q20191301) and Talent and High Level Thesis Development Fund of Department of Physics and Optoelectronic Engineering of Yangtze University (C. Z. Z.).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Transition temperature of strong-coupled superconductors reanalyzed. Phys. Rev. B. 1975;12:905-922.
- [Google Scholar]
- A simple measure of electron localization in atomic and molecular systems. J. Chem. Phys.. 1990;92:5397-5403.
- [Google Scholar]
- Phonon dispersion and electron-phonon coupling in MgB2 and AlB2. Phys. Rev. Lett.. 2001;86:5771.
- [Google Scholar]
- Modeling hardness of polycrystalline materials and bulk metallic glasses. Intermetallics. 2011;19:1275-1281.
- [Google Scholar]
- High-temperature superconducting phases in cerium superhydride with a Tc up to 115 K below a pressure of 1 megabar. Phys. Rev. Lett.. 2021;127:117001.
- [Google Scholar]
- Pressure induced phase transition in MH2 (M = V, Nb) J. Chem. Phys.. 2014;140:114703.
- [Google Scholar]
- The origin of the anomalous superconducting properties of MgB2. Nature. 2002;418:758-760.
- [Google Scholar]
- Conventional superconductivity at 203 kelvin at high pressures in the sulfur hydride system. Nature. 2015;525:73-76.
- [Google Scholar]
- Superconductivity at 250 K in lanthanum hydride under high pressures. Nature. 2019;569:528-531.
- [Google Scholar]
- High-temperature superconductivity in transition metallic hydrides MH11 (M = Mo, W, Nb, and Ta) under high pressure. PCCP. 2021;23:6717-6724.
- [Google Scholar]
- Pressure-induced metallization of dense (H2S)2H2 with high-Tc superconductivity. Sci. Rep.. 2014;4:1-6.
- [Google Scholar]
- High-pressure x-ray diffraction and ab initio study of Ni2Mo3N, Pd2Mo3N, Pt2Mo3N, Co3Mo3N, and Fe3Mo3N: Two families of ultra-incompressible bimetallic interstitial nitrides. Phys. Rev. B. 2010;82:174105.
- [Google Scholar]
- Compressed sodalite-like MgH6 as a potential high-temperature superconductor. RSC Adv.. 2015;5:59292-59296.
- [Google Scholar]
- High-pressure phase stability and superconductivity of pnictogen hydrides and chemical trends for compressed hydrides. Chem. Mater.. 2016;28:1746-1755.
- [Google Scholar]
- Pressure-induced formation of noble metal hydrides. J. Phys. Chem. C. 2012;116:1995-2000.
- [Google Scholar]
- Theoretical study of the ground-state structures and properties of niobium hydrides under pressure. Phys. Rev. B. 2013;88:184104.
- [Google Scholar]
- QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J. Phys.: Condens. Matter. 2009;21:395502.
- [Google Scholar]
- Electronic properties and electron-phonon coupling in zirconium and niobium hydrides. Phys. Rev. B. 1982;25:1027.
- [Google Scholar]
- The elastic behaviour of a crystalline aggregate. Proc. Phys. Soc. London, Sect. A. 1952;65:349.
- [Google Scholar]
- Hong, F., Shan, P.F., Yang, L.X., Yue, B.B., Yang, P.T., Liu, Z.Y., Sun, J.P., Dai, J.H., Yu, H., Yin, Y.Y., Yu, X.H., Cheng, J.G., Zhao, Z.X., 2021. Superconductivity at 70 K in tin hydride SnHx under high pressure. arXiv preprint arXiv:2101.02846.
- Structural stability and compressive behavior of ZrH2 under hydrostatic pressure and nonhydrostatic pressure. RSC Adv.. 2014;4:46780-46786.
- [Google Scholar]
- Phase transitions of actinium dihydride: Pressure-induced charge transfer driving effect. Int. J. Hydrog. Energy. 2014;39:15827-15835.
- [Google Scholar]
- Pressure-induced novel structure with graphene-like boron-layer in titanium monoboride. Chin. Phys. B 2022
- [CrossRef] [Google Scholar]
- Ab-initio calculations of the elastic and finite-temperature thermodynamic properties of niobium-and magnesium hydrides. Int. J. Hydrog. Energy. 2014;39:15530-15539.
- [Google Scholar]
- Equation of state of TiH2 up to 90 GPa: A synchrotron x-ray diffraction study and ab initio calculations. J. Appl. Phys.. 2010;108:043511.
- [Google Scholar]
- First principles study of stability and electronic structure of TMH and TMH2 (TM = Y, Zr, Nb) Acta Phys. Pol.. 2013;123:126-131.
- [Google Scholar]
- Predicted high-temperature superconducting state in the hydrogen-dense transition-metal hydride YH3 at 40 K and 17.7 GPa. Phys. Rev. Lett.. 2009;103:077002.
- [Google Scholar]
- Predicted formation of superconducting platinum-hydride crystals under pressure in the presence of molecular hydrogen. Phys. Rev. Lett.. 2011;107:117002.
- [Google Scholar]
- Superconductivity up to 243 K in the yttrium-hydrogen system under high pressure. Nat. Commun.. 2021;12:1-9.
- [Google Scholar]
- Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B. 1996;54:11169.
- [Google Scholar]
- From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B. 1999;59:1758.
- [Google Scholar]
- Uranium polyhydrides at moderate pressures: Prediction, synthesis, and expected superconductivity. Sci. Adv.. 2018;4:eaat9776.
- [Google Scholar]
- New tungsten borides, their stability and outstanding mechanical properties. J. Phys. Chem. Lett.. 2018;9:3470-3477.
- [Google Scholar]
- Rhodium dihydride (RhH2) with high volumetric hydrogen density. PNAS. 2011;108:18618-18621.
- [Google Scholar]
- Superconductivity above 200 K discovered in superhydrides of calcium. Nat. Commun.. 2022;13:1-5.
- [Google Scholar]
- Crystal structures and superconductivity of technetium hydrides under pressure. PCCP. 2016;18:28791-28796.
- [Google Scholar]
- Superconductivity of pressure-stabilized vanadium hydrides. Inorg. Chem.. 2017;56:13759-13765.
- [Google Scholar]
- Polyhydride CeH9 with an atomic-like hydrogen clathrate structure. Nat. Commun.. 2019;10:1-7.
- [Google Scholar]
- Superconductivity in compressed ternary alkaline boron hydrides. Phys. Rev. B. 2022;105:224107.
- [Google Scholar]
- First-principles study on the structural and electronic properties of metallic HfH2 under pressure. Sci. Rep.. 2015;5:11381.
- [Google Scholar]
- Stability and properties of the Ru−H system at high pressure. PCCP. 2016;18:1516-1520.
- [Google Scholar]
- Metallization of magnesium polyhydrides under pressure. Phys. Rev. B. 2013;87:054107.
- [Google Scholar]
- New developments in evolutionary structure prediction algorithm USPEX. Comput. Phys. Commun.. 2013;184:1172-1182.
- [Google Scholar]
- High-temperature superconducting phase in clathrate calcium hydride CaH6 up to 215 K at a pressure of 172 GPa. Phys. Rev. Lett.. 2022;128:167001.
- [Google Scholar]
- First-principles study on 3d transition-metal dihydrides. Phys. Rev. B. 2002;65:155114.
- [Google Scholar]
- Crystal structure prediction using ab initio evolutionary techniques: principles and applications. J. Chem. Phys.. 2006;124:244704.
- [Google Scholar]
- How evolutionary crystal structure prediction works−and why. Acc. Chem. Res.. 2011;44:227-237.
- [Google Scholar]
- Exploring the novel structure, transportable capacity and thermodynamic properties of TiH2 hydrogen storage material. Int. J. Energy Res.. 2020;44:4997-5007.
- [Google Scholar]
- Xcii. relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Phil. Mag.. 1954;45:823-843.
- [Google Scholar]
- Local electronic structure rearrangements and strong anharmonicity in YH3 under pressures up to 180 GPa. Nat. Commun.. 2021;12:1-10.
- [Google Scholar]
- Electronic structure and energetics of the tetragonal distortion for TiH2, ZrH2, and HfH2: A first-principles study. Phys. Rev. B. 2009;80:184103.
- [Google Scholar]
- Pressure-induced metallization and robust superconductivity in pristine 1T-HfSe2. Mater. Today Phys.. 2022;25:100698.
- [Google Scholar]
- Structure, stability, and mechanical properties of boron-rich Mo–B phases: a computational study. J. Phys. Chem. Lett.. 2020;11:2393-2401.
- [Google Scholar]
- Pressure temperature phase diagram of Ta-H system up to 9 GPa and 600 ℃. Appl. Sci.. 2021;11:6719.
- [Google Scholar]
- Synthesis of clathrate cerium superhydride CeH9 at 80–100 GPa with atomic hydrogen sublattice. Nat. Commun.. 2019;10:1-10.
- [Google Scholar]
- Evolution of structural and electronic properties of TiSe2 under high pressure. J. Phys. Chem. Lett.. 2021;12:9859-9867.
- [Google Scholar]
- Electron localization in solid-state structures of the elements: the diamond structure. Angew. Chem. Int. Ed.. 1992;31:187-188.
- [Google Scholar]
- High-pressure synthesis and characterization of iridium trihydride. Phys. Rev. Lett.. 2013;111:215503.
- [Google Scholar]
- Superconductivity at 161 K in thorium hydride ThH10: Synthesis and properties. Mater. Today. 2020;33:36-44.
- [Google Scholar]
- First-principles investigation of rhodium hydrides under high pressure. Phys. Rev. B. 2021;104:054110.
- [Google Scholar]
- First-principles study of the stability and electronic structure of metal hydrides. Phys. Rev. B. 2002;66:144107.
- [Google Scholar]
- Synthesis of yttrium superhydride superconductor with a transition temperature up to 262 K by catalytic hydrogenation at high pressures. Phys. Rev. Lett.. 2021;126:117003.
- [Google Scholar]
- Second group of high-pressure high-temperature lanthanide polyhydride superconductors. Phys. Rev. B. 2020;102:144524.
- [Google Scholar]
- Comparison of highly-compressed C2/m-SnH12 superhydride with conventional superconductors. J. Phys.: Condens. Matter. 2021;33:285601.
- [Google Scholar]
- A grid-based bader analysis algorithm without lattice bias. J. Phys.: Condens. Matter. 2009;21:084204.
- [Google Scholar]
- First-principles calculations of the ferroelastic transition between rutile-type and CaCl2-type SiO2 at high pressures. Phys. Rev. B. 2008;78:134106.
- [Google Scholar]
- First principles phonon calculations in materials science. Scr. Mater.. 2015;108:1-5.
- [Google Scholar]
- Structural, mechanical and electronic properties and hardness of ionic vanadium dihydrides under pressure from first-principles computations. Sci. Rep.. 2020;10:8868.
- [Google Scholar]
- Pressure induced superconductivity and electronic structure properties of scandium hydrides using first principles calculations. RSC Adv.. 2016;6:81534-81541.
- [Google Scholar]
- High-temperature-and high-pressure-induced formation of the Laves-phase compound XeS2. Phys. Rev. B. 2016;93:214112.
- [Google Scholar]
- Theoretical study of phase separation of scandium hydrides under high pressure. J. Phys. Chem. C. 2015;119:5614-5625.
- [Google Scholar]
- Synthesis and stability of tantalum hydride at high pressures. Phys. Rev. B. 2019;99:224504.
- [Google Scholar]
- Zero-point effects on phase transitions of thorium dihydride under high pressure. J. Phys. Chem. C. 2015;119:13465-13471.
- [Google Scholar]
- High-temperature superconductivity in compressed solid silane. Sci. Rep.. 2015;5:8845.
- [Google Scholar]
- Pressure-induced phase transition of SnH4: a new layered structure. RSC Adv.. 2016;6:10456-10461.
- [Google Scholar]
- The crystal structures, phase stabilities, electronic structures and bonding features of iridium borides from first-principles calculations. RSC Adv.. 2022;12:11722-11731.
- [Google Scholar]
- Prediction of novel high-pressure structures of magnesium niobium dihydride. ACS Appl. Mater. Interfaces. 2017;9:26169-26176.
- [Google Scholar]
- Pressure-driven structural phase transitions and superconductivity of ternary hydride MgVH6. J. Phys. Chem. C. 2021;125:3150-3156.
- [Google Scholar]
- Pressure-stabilized superconductive ionic tantalum hydrides. Inorg. Chem.. 2017;56:3901-3908.
- [Google Scholar]
- Investigation of superconductivity in compressed vanadium hydrides. PCCP. 2017;19:26280-26284.
- [Google Scholar]
- Effect of electrons scattered by optical phonons on superconductivity in MH3(M = S, Ti, V, Se) Phys. Rev. B. 2018;98:024514.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104347.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1
Supplementary data 2
Supplementary data 2







