Translate this page into:
Fabrication of zirconium(IV) cross-linked alginate/kaolin hybrid beads for nitrate and phosphate retention
⁎Corresponding author. drnviswanathan@gmail.com (Natrayasamy Viswanathan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The extreme nitrate (NO3−) species in drinking water leads to methemoglobinemia (blue baby syndrome) disease in new born toddlers whereas the excess phosphate (PO43−) and NO3− contents lead to the eutrophication (algae growth) problem of water sources. Upto date, the environmental researchers have developing the suitable adsorbent materials for providing NO3− and PO43− free water system. In present study, a low-cost alginate (Alg) assisted kaolin (KN) (AlgKN) composite beads were prepared and utilized for the removal of NO3− and PO43−. To improve the sorption capacity (SC) and stability, Zr4+ ions were coated onto AlgKN to get Zr@AlgKN composite beads which were prepared via., hydrothermal (Hydro) and in situ precipitation (In situ) methods. The hydro assisted Zr@AlgKN composite beads possess an enhanced SC than the in situ assisted adsorbents. In batch scale, the parameters responsible for the adsorption process such as contact time, co-ions, adsorbent dosage, pH, initial ions concentration and temperature were optimized. The adsorbents were characterized by XRD, FTIR, BET, EDAX and SEM analysis. The adsorption experimental data was fitted with isotherms, kinetics and thermodynamic parameters. The regeneration and field applicability study of the Zr@AlgKN composite beads were also investigated.
Keywords
Alginate
Kaolin
Zr4+ ions
Zr@AlgKN beads
NO3− and PO43− adsorption
Reuse
1 Introduction
Nitrogen (N) and phosphorus (P) are the essential macronutrients for all the existing organisms (Aswin Kumar and Viswanathan, 2018a). But, the extreme nitrate (NO3−) species in drinking water leads to methemoglobinemia (blue baby syndrome) in new born toddlers whereas the excess phosphate (PO43−) and NO3− contents lead to the eutrophication (algae growth) problem of water sources (Fewtrell, 2004). The World Health Organization (WHO) has fixed the NO3− and PO43− contents with 40 and <0.5 mg/L respectively are to be the tolerance limit in drinking water (Aswin Kumar and Viswanathan, 2018b). The rapid utilization of NO3− and PO43− made vibration on the environmental scientists to expand the modern techniques for removing them from water. The numerous NO3− and PO43− removal technologies such as biological treatment (Meinhold et al., 1999), chemical precipitation (Guo et al., 2010), adsorption (Aswin Kumar and Viswanathan, 2018c), ion-exchange (Xing et al., 2010) and membrane process (Kyu-Hong et al., 2003) were investigated in which adsorption method seems to be cost-effective and suitable at industrial level.
Kaolin (KN) is a silicate type natural clay which containing the elemental constituents of aluminum oxide (Al2O3) (39.50%), silicon dioxide (SiO2) (46.54%) and water (H2O) molecule (13.96%) in its layered structure (Wang et al., 2009). The isomorphous replacement of Si4+ by Al3+ ions in the silicate double layer of KN clay makes it as the good adsorbent material for the contaminant adsorption from water (Adebowale et al., 2006). However, the bottle-necks of KN clay such as pressure drop during filtration and low-cation exchange capacity makes it as the unsuitable material for the practical use. To resolve this shortcut, biopolymeric composite beads have been investigated in recent years (Aswin Kumar and Viswanathan, 2017a).
Alginate (Alg) is a natural polysaccharide derived from the brown seaweeds. It has the monomers of (1/4) a-L-guluronate and (1/4) b-D-mannuronate in a unit (Pandi and Viswanathan, 2014). Alg has advantages like biodegradable, biocompatible and eco-friendly which make it as the prominent adsorbent material. In addition, the chemical modification of Alg increases its stability and reactivity toward the toxic ions adsorption. Hence, alginate supported kaolin (AlgKN) composite beads were prepared by dispersing KN clay in Alg matrix. In addition, Alg has the tendency to interact with higher valence metal ions to form metal-alginate complex beads (Pandi and Viswanathan, 2015).
Zirconium (IV) (Zr4+) is an inorganic metal ion belonging to d-block family. Zr4+ ion fit in to Lewis acid category which easily binds the Lewis bases like NO3− and PO43−. Hence, Zr4+ ions were uniformly cross-linked with AlgKN composite beads to form Zr4+ loaded AlgKN (Zr@AlgKN) composite beads. The adsorbent preparation by hydrothermal method using an autoclave has enriches the material properties via, smaller the particle size with larger the specific surface area which leads to the higher sorption capacity (SC) of the adsorbent (Ahmed et al., 2017). Sorption capacity is defined as the amount of adsorbate (mg) adsorbed per unit gram of the adsorbent.
This present investigation was focused to synthesize in situ and hydro assisted Zr@AlgKN composite beads for the NO3− and PO43− adsorption. The characterization studies such as XRD, FTIR, BET, EDAX and SEM analysis of the adsorbents were performed. The parameters responsible for adsorption process such as contact time, pH, dosage, initial ions concentration, temperature and co-ions were optimized in batch scale. The isotherms and study of thermodynamic parameters were carried out. The kinetic study of Zr@AlgKN composite beads (Hydro) were performed to find the order of NO3− and PO43− adsorption. The field study and regeneration of the Zr@AlgKN composite beads were also investigated.
2 Experimental section
2.1 Materials
Sodium alginate (70,000–80,000 of molecular weight) was acquired from Himedia, India. ZrOCl2·8H2O (98.0%), Kaolin clay, NaOH (≥98.0%), HCl (35–38%), NH4VO3 (98.0%) and (NH4)6Mo7O24 (≥99.0%) was acquired from Merck, India. The typical PO43− and NO3− stock solutions were prepared by dissolving of 1.4329 g of anhydrous KH2PO4 (≥98.0%) and 1.6305 g of anhydrous KNO3 (≥98.0%) in 1000 mL of double distilled (DD) water separately. AR grade of all other reagents were utilized.
2.2 Synthesis of the composite beads
2.2.1 Preparation of alginate/kaolin (AlgKN) composite beads
About 2% of alginate solution was prepared by pouring 2 g of sodium alginate in 100 mL of DD water. About 10 g of Kaolin clay was dispersed in DD water which is slowly poured into alginate medium and continuously stirred using magnetic stirrer for 3 h to get homogeneous AlgKN composite solution. Then, AlgKN composite solution was taken in the burette and slowly dropped into 2% CaCl2 to obtain AlgKN composite beads. Further, it was kept undisturbed in the same solution upto 24 h for ageing as well as strengthening of the beads. For hydro synthesis, the wet AlgKN composite beads were transmitted into Teflon shielded autoclave and heated to 130 °C for 6 h. Finally, AlgKN composite beads were filtered by centrifuge and dried in hot air oven at 80 °C upto 4 h for NO3− and PO43− adsorption studies.
2.2.2 Preparation of Zr4+ ions coated AlgKN (Zr@AlgKN) composite beads
To prepare Zr@AlgKN composite beads, about 3% of Zr4+ solution was prepared which is taken in the glass beaker (100 mL). Further, the prepared homogeneous AlgKN composite solution was taken in the burette and slowly dropped into Zr4+ medium. Immediately, the usable Zr@AlgKN composite beads were formed. The obtained in situ assisted Zr@AlgKN composite beads were kept undisturbed in the mother liquid upto 24 h for ageing and as well as strengthening purpose. For hydro synthesis, the wet Zr@AlgKN composite beads were transmitted into Teflon shielded autoclave and heated to 130 °C for 7 h. Finally, the Zr@AlgKN composite beads were filtered by centrifuge and dried in hot air oven at 80 °C upto 4 h for NO3− and PO43− adsorption studies.
2.3 NO3− and PO43− adsorption by batch method
The batch adsorption tests were executed for NO3− and PO43− adsorption. About 0.1 g of the adsorbent was added with 50 mL of 100 mg/L of the NO3− and PO43− solutions which were taken in the iodine flask. The reaction contents were shaken under mechanical shaker at assorted time interval of 10 to 60 min and then the adsorbents were filtered by centrifuge followed by the final concentration of NO3− and PO43− ions was analyzed by UV–Visible spectrophotometer. For hydrothermal preparation of the adsorbent, Teflon shielded autoclave (100 mL) was utilized at 130 °C. The parameters responsible for the adsorption process such as contact time, adsorbent dosage, pH, co-ions and initial ions concentration were carried out. About 0.1 N HCl/ NaOH were used to adjust the pH of the NO3− and PO43− solution. The adsorption isotherms and kinetics study were executed for 80, 100, 120 and 140 mg/L of NO3− and PO43− solution at 303, 313 and 323 K. The regeneration of the prepared Zr@AlgKN composite beads was studied using the suitable eluent NaOH in batch mode. The SC of the adsorbent and the removal percentage toward NO3−/PO43− can be calculated by the Eqs. (1) and (2) as follows
2.4 Analysis and characterization details
The NO3− and PO43− concentration was analyzed using UV–Visible spectrophotometer kit (model: Spectroquant Pharo 300, Merck) at 202 and 400 nm respectively. To check the PO43− concentration, the reagents such as NH4VO3 and (NH4)6Mo7O24 were utilized. To identify the pH of NO3− and PO43− solution, pH electrode with Thermo Orion Benchtop multiparameter kit (model: VERSA STAR92) was used. The pH drift method was applied to determine the pH at zero point charge (pHzpc) of the adsorbent (Lopez-Ramon et al., 1999). The quality parameters of drinking water such as chloride, total dissolved solids and total hardness was also studied using the standard methods (APHA, 2005).
The crystalline nature of the adsorbent was studied using X-ray diffraction (XRD) analysis by X’pert 173 PRO model PAN-alytical instrument. The surface textural properties of the adsorbents were observed by BET surface analyzer (model: NOVA 1000) at N2 atmosphere. The functional groups present in the adsorbents were examined by Fourier transform infrared (FTIR) spectrometer (model: JASCO-460 plus). The particle size and surface topography of the beads were observed by scanning electron microscope (SEM) (model: Vega3 Tescan). The elemental accumulation of the hydro supported Zr@AlgKN composite beads and NO3−/PO43− sorbed Zr@AlgKN composite beads were studied using energy dispersive X-ray analyzer (EDAX) (model: Bruker Nano GMBH).
2.5 Statistical tools
All the experimental data was computed by Microcal Origin (version 15) software. In addition, the chi-square analysis (χ2), standard deviation (sd) and regression correlation coefficient (r) was utilized to fit the appropriate isotherm model.
3 Results and discussion
3.1 Characterization studies
3.1.1 FTIR investigation
FTIR study was used to identify the functional groups of the sodium alginate, kaolin (KN) clay, hydro assisted ZrO(OH)2, AlgKN composite beads, Zr@AlgKN composite beads, NO3− and PO43− adsorbed Zr@AlgKN composite beads which are shown in Fig. 1a–c. In FTIR spectra of sodium alginate, the -OH stretching vibration was observed at 3443 cm−1 whereas the asymmetric and symmetric vibrations of —COO group were attained at 1614 and 1423 cm−1 respectively (Aswin Kumar and Viswanathan, 2017b) (cf. Fig. 1a). In FTIR spectra of KN clay, the —OH stretching bands were attained at 3677 and 3429 cm−1 which due to the grafting of —OH with Al site of KN clay (Njoya et al., 2006). Further, Si—O and Si—O—Si vibrations in KN clay were observed at 1608 and 1049 cm−1 respectively. In addition, the -OH bending vibration of Al—OH in KN clay was pertained at 963 cm−1 (Georges-Ivo, 2005) (cf. Fig. 1a).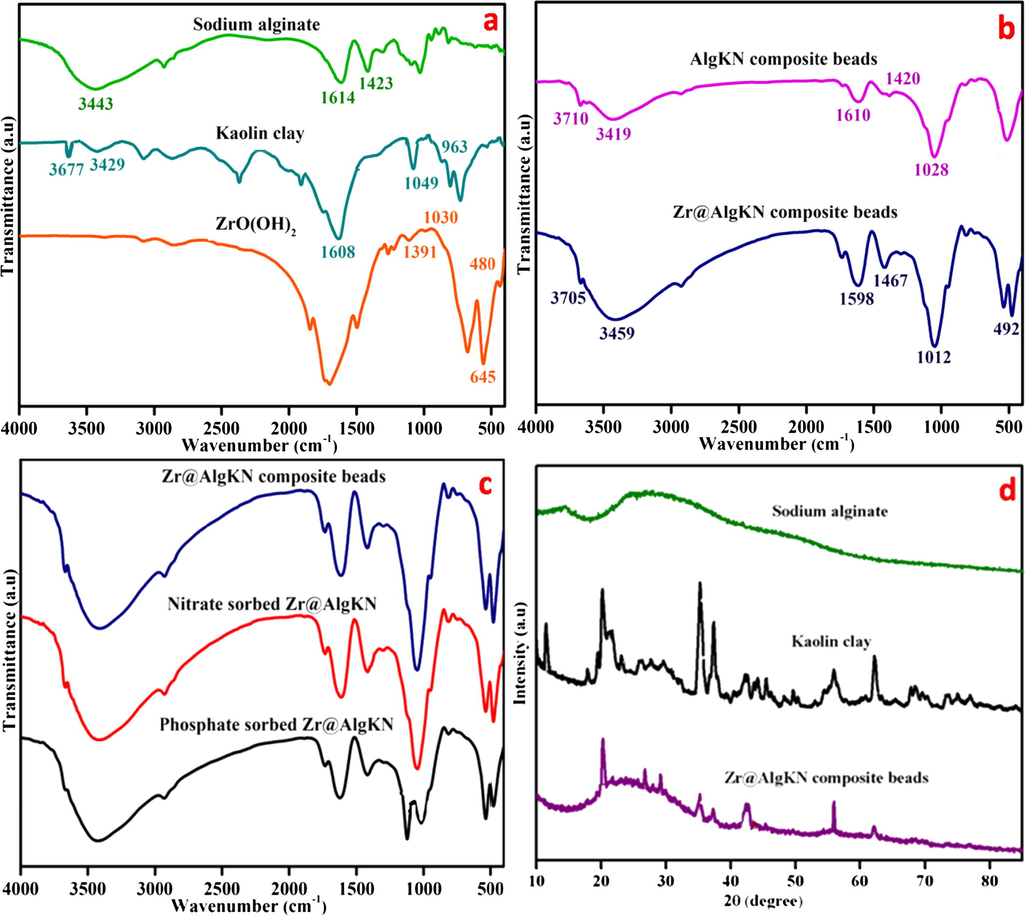
FTIR spectra of (a) sodium alginate, kaolin clay and hydro assisted ZrO(OH)2, (b) AlgKN and Zr@AlgKN composite beads, (c) Zr@AlgKN composite beads, nitrate sorbed Zr@AlgKN and phosphate sorbed Zr@AlgKN composite beads, and (d) XRD images of sodium alginate, kaolin clay and hydro assisted Zr@AlgKN composite beads.
In FTIR spectra of ZrO(OH)2, the symmetric and asymmetric frequencies of Zr—O—H was observed at 1391 and 1278 cm−1, while Zr—O and O—H bending modes was attained at 1030 cm−1. Mainly, the vibration bands of Zr—OH and Zr—O—Zr of ZrO(OH)2 were appeared at 645 and 480 cm−1 respectively (Mekhemer, 1998) (cf. Fig. 1a). The individual FTIR bands of sodium alginate, KN clay and ZrO(OH)2 were retained in the FTIR spectra of Zr@AlgKN composite beads which shows its good formation (cf. Fig. 1b). In FTIR spectra of NO3− and PO43− adsorbed Zr@AlgKN composite beads, most of the significant FTIR bands of Zr@AlgKN composite beads were retained and shifted in wave-numbers which may confirms the NO3− and PO43− adsorption. In addition, the asymmetric stretching and bending modes of PO43− at 1032 and 560 cm−1 in PO43− sorbed Zr@AlgKN composite beads may confirms PO43− adsorption in Zr@AlgKN composite beads (cf. Fig. 1c) (Niwas et al., 2000).
3.1.2 XRD study
XRD spectra of sodium alginate, KN clay, hydro assisted Zr@AlgKN composite beads are shown in Fig. 1d. The two typical crystalline peaks of sodium alginate was appeared at 14.30° and 21.30° on the crystal planes (4 2 2) and (5 1 1) respectively (Zhao et al., 2015). In KN clay, the XRD signals at 12.30°, 21.92°, 23.98°, 34.02°, 36.80°, 42.98° and 61.95° was observed on the planes (0 0 1), (1 1 1), (0 2 1), (1 0 2), (2 0 0), (0 4 1) and (0 0 2) respectively [JCPDS File No. 78-2110] (Fardjaoui et al., 2017). Moreover, the XRD peaks of ZrO(OH)2 are preserved at 30.01°, 35.55°, 50.09° and 61.02° in the crystalline planes (1 0 0), (1 0 2), (1 3 0) and (1 2 0) (Cui et al., 2012). It was concluded that the strong and individual XRD peaks of sodium alginate, KN clay and ZrO(OH)2 were retained in the XRD spectra of Zr@AlgKN composite beads (Hydro) with crystalline nature which enhances the structural stability of the Zr@AlgKN composite beads (Hydro).
3.1.3 BET study
The textural properties of the in situ and hydro assisted Zr@AlgKN composite beads were studied using BET analysis. The N2 adsorption/desorption isotherm graph of Zr@AlgKN composite beads (Hydro) were studied at 77 K and the pore size distribution of Zr@AlgKN composite beads (Hydro) were demonstrated in Fig. 2a and b respectively. The non-local density functional theory (NLDFT) method was applied to find the BET property of the beads. The specific surface area, total pore width and as well as pore volume of the hydro assisted Zr@AlgKN composite beads were found to be 78.93 m2/g, 3.61 nm and 0.024 cm3/g whereas for in situ assisted Zr@AlgKN composite beads it was found to be 67.15 m2/g, 3.02 nm and 0.018 cm3/g respectively. From BET results, it was observed that the hydro supported Zr@AlgKN composite beads possess the higher specific surface area, larger pore width and as well as pore volume compared to the in situ supported Zr@AlgKN composite beads. Hence, the NO3− and PO43− ions can easily occupies the active sites of the hydro supported Zr@AlgKN composite beads than the in situ assisted Zr@AlgKN composite beads.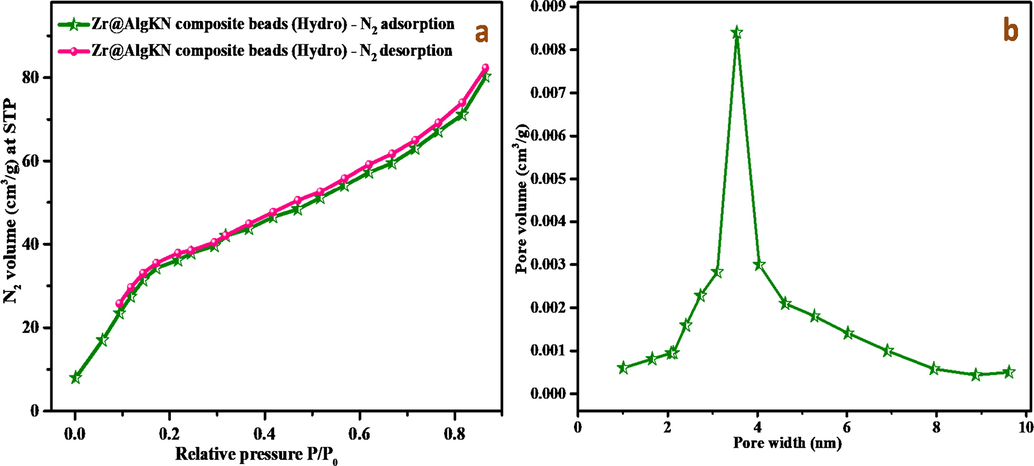
(a) N2 adsorption/desorption isotherm of Zr@AlgKN composite beads (Hydro) at 77 K, and (b) pore size distribution of Zr@AlgKN composite beads (Hydro).
3.1.4 SEM analysis
The surface morphology of in situ and hydro supported Zr@AlgKN composite beads with their NO3−/PO43− sorption were studied by SEM analysis which are illustrated in Fig. 3b to i. The digital image of Zr@AlgKN composite beads were shown in Fig. 3a. The particle size of in situ and hydro assisted Zr@AlgKN composite beads were measured using SEM which found to be 1.597 and 1.362 mm respectively (cf. Fig. 3b and f). The close view of the in situ assisted Zr@AlgKN composite beads surface were taken at 10 µm which shows the irregular surface (cf. Fig. 3c) whereas hydro assisted Zr@AlgKN composite beads possess the uneven and needle like surface with some pores which is presented in Fig. 3g. The uneven surface of in situ assisted Zr@AlgKN composite beads was changed into smoother after NO3− and PO43− adsorption (cf. Fig. 3d and e). Likewise, the active surface of hydro assisted Zr@AlgKN composite beads were almost blocked by NO3− and PO43− ions result in the smoother surface which confirms the NO3− and PO43− adsorption (cf. Fig. 3h and i).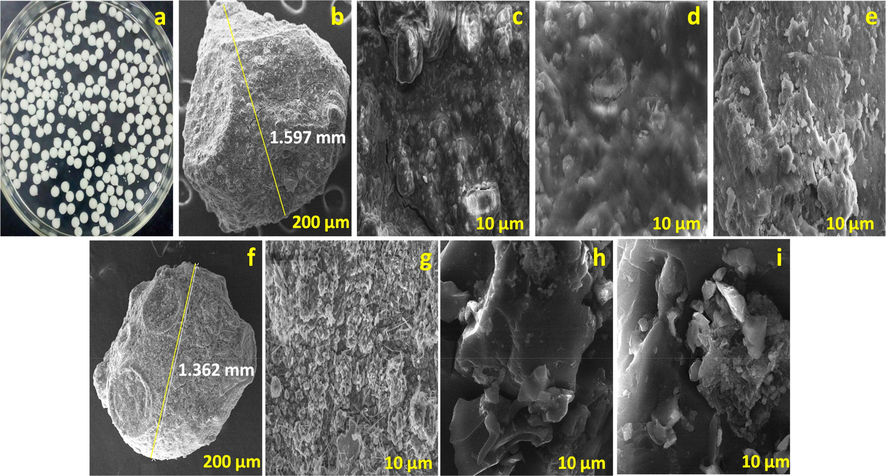
(a) Digital image of Zr@AlgKN composite beads, SEM images of (b) particle size measured Zr@AlgKN composite beads (In situ) at 200 µm, (c) Zr@AlgKN composite beads (In situ) at 10 µm, (d and e) NO3− and PO43− sorbed Zr@AlgKN composite beads (In situ), (f) particle size measured Zr@AlgKN composite beads (Hydro) at 200 µm, (g) Zr@AlgKN composite beads (Hydro) at 10 µm, and (h and i) NO3− and PO43− sorbed Zr@AlgKN composite beads (Hydro).
3.1.5 EDAX analysis
The elemental components of hydro assisted Zr@AlgKN composite beads with their NO3− and PO43− sorption were studied by EDAX analysis. In Fig. 4a, the significant elements of Zr@AlgKN composite beads such as O and C peaks from alginate, Si and Al peaks from kaolin clay and Zr peak were observed. Fig. 4b of NO3− sorbed Zr@AlgKN composite beads, in addition to C, O, Si, Al and Zr, the N (2.96%) peak were appeared with good percentage which may confirms NO3− sorption on Zr@AlgKN composite beads. In the case of PO43− sorbed Zr@AlgKN, the new peak of P (3.37%) was appeared due to PO43− adsorption (cf. Fig. 4c). It was also observed that from Fig. 4b and c, the atomic percentages of O, Si, Al and Zr was slightly low compared to the same in Fig. 4a may due to their interaction with NO3− and PO43− during the adsorption process.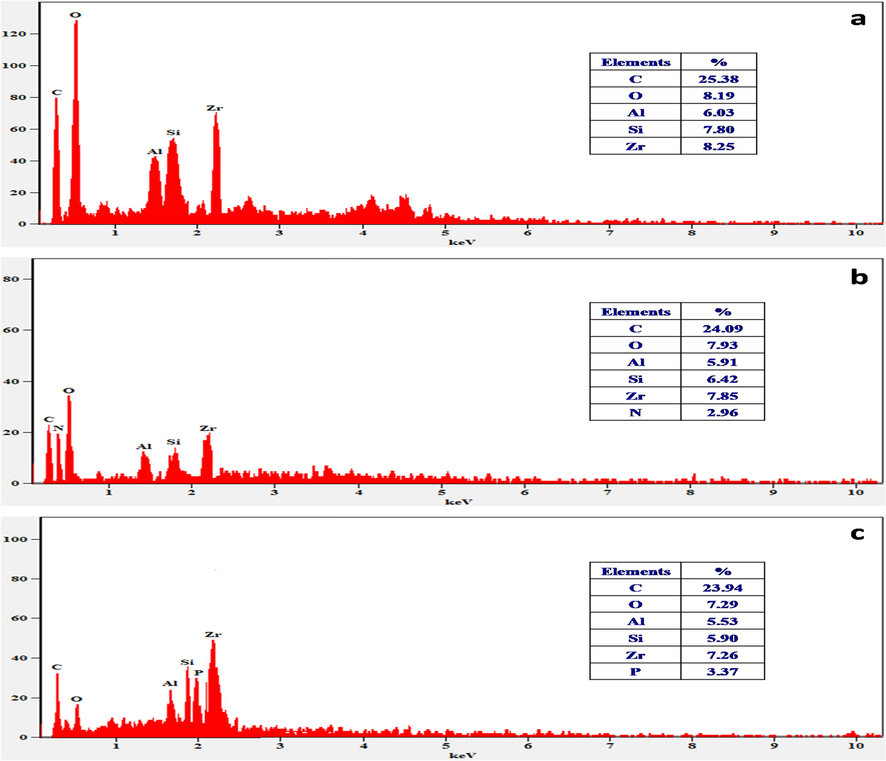
EDAX spectra of hydro supported (a) Zr@AlgKN composite beads; (b) nitrate sorbed Zr@AlgKN composite beads, and (c) phosphate sorbed Zr@AlgKN composite beads.
3.2 Effect of contact time
The contact time experiment was performed by adding 0.1 g of the adsorbent in 50 mL of 100 mg/L of the respective NO3− and PO43− solution followed by stirred under mechanical shaker by assorted time interval of 10–60 min. The direct use of alginate (Alg) doesn’t suits for the adsorption process due to its water soluble nature. Hence, the SCs of calcium alginate, KN clay, ZrO(OH)2 (In situ), ZrO(OH)2 (Hydro), AlgKN composite beads (In situ), AlgKN composite beads (Hydro), Zr@AlgKN beads (In situ) and Zr@AlgKN composite beads (Hydro) toward NO3− and PO43− adsorption was shown in Fig. 5a and b respectively. It was found that Zr@AlgKN composite beads (Hydro) exhibit an enhanced SC of 31.24 and 37.18 mg/g toward NO3− and PO43− with the equilibrium time of 30 min and other adsorbents were saturated at 40 min. In addition, Zr@AlgKN composite beads (In situ) possess the considerable SC. Hence, the further NO3− and PO43− adsorption studies were investigated for both in situ and hydro assisted Zr@AlgKN composite beads with fixed contact time of 40 and 30 min respectively.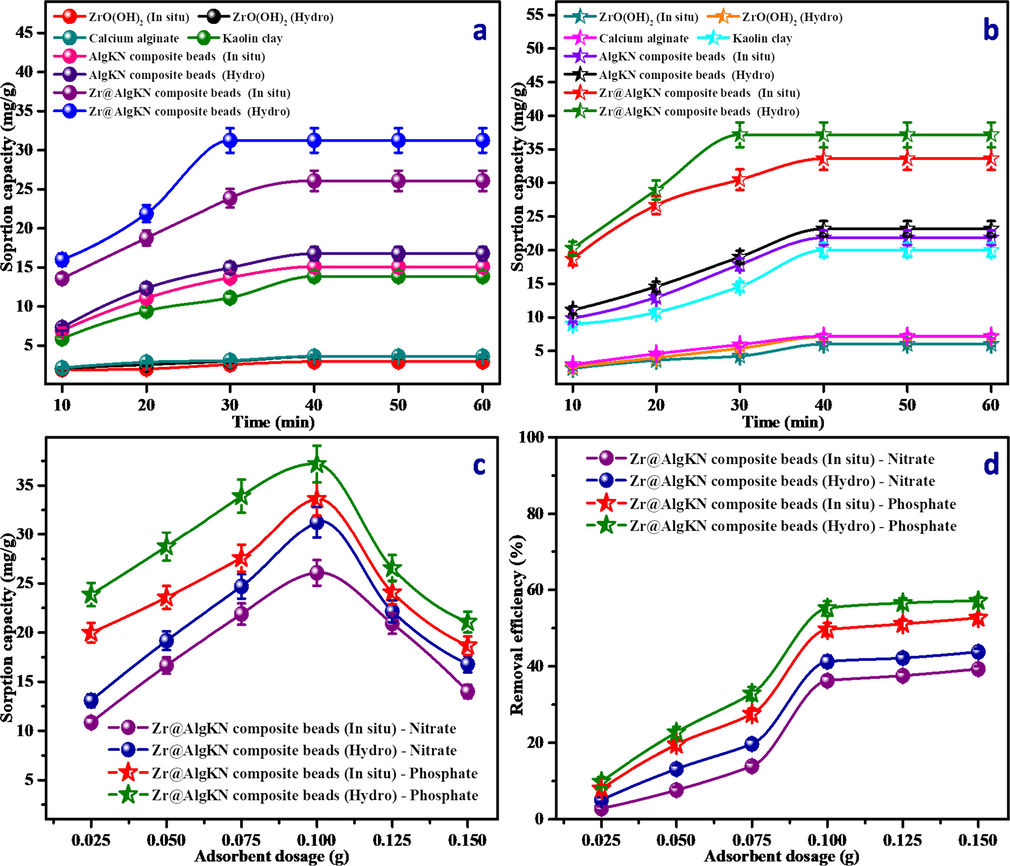
Effect of (a and b) contact time of ZrO(OH)2 (In situ), ZrO(OH)2 (Hydro), calcium alginate, KN clay, AlgKN composite beads (In situ), AlgKN composite beads (Hydro), Zr@AlgKN composite beads (In situ) and Zr@AlgKN composite beads (Hydro) on the nitrate and phosphate SC respectively with 0.1 g of dosage at 10 to 60 min. Effect of (c and d) adsorbent dosage of in situ and hydro supported Zr@AlgKN composite beads on the nitrate and phosphate SC and removal efficiency respectively.
3.3 Effect of adsorbent dosage
The dosage effect of in situ and hydro assisted Zr@AlgKN composite beads toward NO3− and PO43− adsorption were studied by taking the different dosages of Zr@AlgKN composite beads from 0.025 to 0.150 g. The correlation between SC and removal efficiency of the Zr@AlgKN composite beads toward NO3−/PO43− adsorption with respect to varying the adsorbent dosage are shown in Fig. 5c and d respectively. The result portrays that raise of Zr@AlgKN composite beads dosage leads to increases the SC at initial and decreases after 0.1 g, whereas the removal percentage (%) was gradually increases because it does not depend on the amount of dosage. The active sites of the adsorbent would be more when the adsorbent dosage is high which results in the gradual increase in the removal efficiency toward the NO3−/PO43− and lower in the SC after 0.1 g of the adsorbent dosage added. This is due to when the dosage of Zr@AlgKN composite beads increased, the availability in active sites is greater than the initial concentration of the NO3−/PO43− thereby SC decreases after 0.1 g. (Farzana and Meenakshi, 2015). Hence, 0.1 g of the Zr@AlgKN composite beads was chosen as the optimum dosage.
3.4 Influence of initial ions concentration
The effect of initial concentrations of NO3− and PO43− solution on its removal was studied. The varied initial concentrations such as 20, 40, 60, 80, 100, 120 and 140 mg/L of the NO3− and PO43− solution was taken and added with 0.1 g of Zr@AlgKN composite beads. The results in Fig. 6a portrays that SC toward NO3− and PO43− was gradually increased with the raise of initial ions concentration followed by saturation was attained. The higher concentration of NO3−/PO43− ions offer the mobile force which surmounts the mass transfer resistant at adsorbent/adsorbate surface thereby SC was increased (Karimi et al., 2012). Moreover, it was observed that the active sites of Zr@AlgKN composite beads were almost filled by NO3− and PO43− ions at 100 mg/L. Hence, 100 mg/L of initial NO3−/PO43− concentration was chosen as optimal concentration.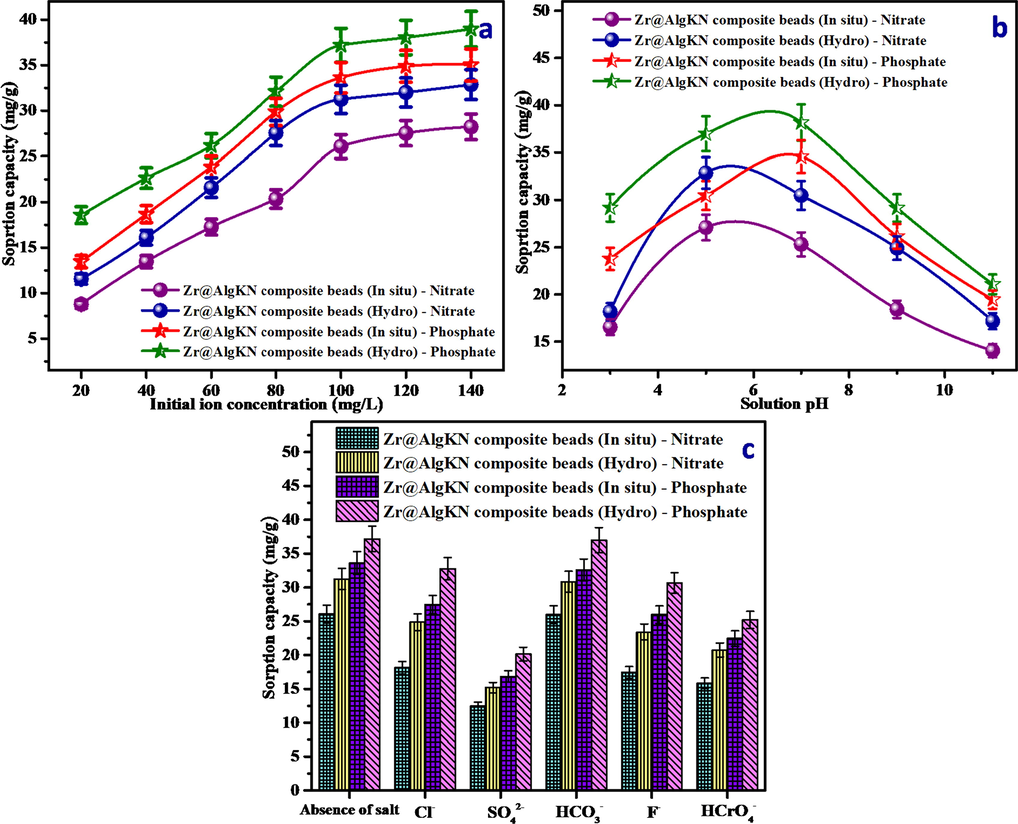
Effect of (a) initial ions concentration, (b) pH of the solution, and (c) co-ions of the in situ and hydro assisted Zr@AlgKN composite beads on the nitrate and phosphate SC.
3.5 Influence of adsorbate pH
The solution pH is an important parameter which often affects the surface charge of the adsorbent. The effect of pH of NO3− and PO43− solution on its adsorption was shown in Fig. 6b. In the case of NO3−, increasing pH from 3 to 5, SC was gradually increased and after pH 7 it was decreased. In the case of PO43−, there was a gradual increase in SC which attained from pH 3 to 7 followed by decreased after pH 7. The several form of phosphate in water are H3PO4 (pH < 2), H2PO4− (pH 2 ∼ 7), HPO42− (pH 7 ∼ 11) and PO43− (pH > 11) respectively (Aswin Kumar and Viswanathan, 2018d). Amongst, H2PO4− (pH 2 ∼ 7) is stable at acidic pH condition which leads to the increased phosphate SC upto pH 7. The pHzpc values of the in situ and hydro supported Zr@AlgKN composite beads were found to be 5.31 and 5.64 respectively. From this values, it was concluded that the surfaces chare of Zr@AlgKN composite beads were positive and negative when the pH < pHzpc and pH > pHzpc respectively. Hence, during pH < pHzpc, NO3− and PO43− ions were surrounded with the protonated Zr@AlgKN composite beads by electrostatic attraction. However, in the basic pH condition, the predominant OH− ions competes the NO3− and PO43− adsorption by occupying the active sites of Zr@AlgKN composite beads during pH > pHzpc. It was also observed that the pH of NO3− and PO43− solution attaining neutral after the adsorption process which denotes the suitability of Zr@AlgKN composite beads at assorted pH conditions.
3.6 Influence of co-anions
In addition to NO3− and PO43−, the other anions such as Cl−, HCO3−, SO42−, F− and HCrO4− are also present in the natural water which may affects the NO3− and PO43− adsorption. About 0.1 g of Zr@AlgKN composite beads were added into 50 mL mixture containing 200 mg/L of the individual co-ion solution and 100 mg/L of NO3−/PO43− solution. From Fig. 6c, it was observed that HCO3− ion does not compete significantly whereas the electronegative F− and Cl− ions slightly give the competition on the NO3−/PO43− adsorption. The maximum SC toward NO3−/PO43− adsorption was observed at acidic pH condition which was explained in the Section 3.5. However, HCrO4− ions are also stable at acidic pH condition and hence it contends the NO3−/PO43− adsorption. Moreover, SO42− ion exhibit the predominant competing effect on the both NO3−/PO43− adsorption due to its higher electronic charge and reactivity which make it as a competitor for NO3− and PO43− by filling the active sites of the Zr@AlgKN composite beads instead of NO3−/PO43− (Saad et al., 2007). The competing order of co-anions on NO3− and PO43− adsorption was found to be SO42− > HCrO4− > F− > Cl− > HCO3−.
3.7 Adsorption isotherms study
The isotherm study was performed to find the mechanism involved during NO3− and PO43− adsorption by Zr@AlgKN composite beads. The experimental data was fitted with Freundlich (1906), Langmuir (1916) and Dubinin-Radushkevich (D-R) (Dubinin et al., 1947) isotherm models. About 0.1 g of Zr@AlgKN composite beads (Hydro) were added into 50 mL of individual NO3− and PO43− solution having initial concentrations of 80, 100, 120 and 140 mg/L at 303, 313 and 323 K. The isotherm equations and their linear plot details are shown in Table 1a. The single layer type of adsorption was governed by Langmuir which was plotted by Ce vs Ce/qe. The multilayer type adsorption was governed by Freundlich which was plotted by log qe vs log Ce and the best fit in these parameters are listed in Table 1b (Pahlavanzadeh et al., 2012). The 1/n and n values should be around 0 to 1 and 1 to 10 for the adsorption to be feasible. In Freundlich isotherm, it was detected that 1/n values dropping between 0 and 1 whereas n values falling between 1 and 10 denotes the favorable nature of NO3− and PO43− adsorption onto Zr@AlgKN composite beads (Hydro).
Isotherms
Linear form
Linear plot
Parameters
Freundlich
log qe vs log Ce
qe - Amount of NO3− and PO43− adsorbed per unit weight of the sorbent (mg/g)
Ce - Equilibrium concentration of NO3− and PO43− solution (mg/L)
kF - Measure of sorption capacity
1/n - Adsorption intensity
Langmuir
Ce/qe vs Ce
Qo - Amount of NO3− and PO43− at complete monolayer coverage (mg/g)
b - Langmuir isotherm constant (L/mg)
D-R
ln qe vs ε2
Xm - NO3− and PO43− adsorption capacity (mg/g)
k - D-R isotherm constant
ε2 - Polanyi potential
Isotherms
Parameters
Nitrate
Phosphate
303 K
313 K
323 K
303 K
313 K
323 K
Freundlich
1/n
0.824
0.825
0.827
0.597
0.601
0.604
n
6.315
6.317
6.319
7.051
7.055
7.058
kF (mg/g) (L/mg)1/n
28.135
28.129
28.120
32.816
32.827
32.831
r
0.908
0.910
0.911
0.997
0.998
0.999
sd
1.985
1.984
1.987
0.754
0.761
0.763
χ2
0.197
0.205
0.211
0.038
0.041
0.045
Langmuir
Qo(mg/g)
31.241
31.218
31.189
37.182
37.201
37.230
b (L/g)
0.931
0.938
0.944
1.065
1.068
1.067
RL
3.731
3.734
3.735
6.261
6.270
6.273
r
0.992
0.990
0.993
0.986
0.987
0.989
sd
0.567
0.582
0.634
2.764
2.767
2.769
χ2
0.017
0.019
0.021
0.437
0.442
0.446
Dubinin - Radushkevich
kDR (mol2/J2)
4.86E-01
4.91E-01
4.95E-01
7.63E-01
7.68E-01
7.72E-01
Xm (mg/g)
25.086
25.082
25.079
30.015
30.020
30.024
E (kJ/mol)
8.097
8.122
8.123
9.164
9.169
9.701
r
0.834
0.835
0.837
0.794
0.796
0.797
sd
1.376
1.379
1.402
1.989
1.993
1.996
χ2
0.687
0.691
0.693
2.324
2.329
2.331
The significant parameters such as KDR, Xm, E, χ2 and r of D-R isotherm was acquired by the plot of ln qe vs ε2 which are shown in Table 1b. The mean adsorption energy (E) value of Zr@AlgKN composite beads were found to be in the range of 7 to 10 kJ/mol which denotes the physisorption nature of NO3− and PO43− adsorption (Wu, 2007). Mainly, the values of significant parameters such as χ2, r and sd were used to determine the suitable isotherm model for NO3− and PO43− adsorption. Langmuir isotherm exhibited the highest r value and lowest sd as well as χ2 values toward NO3− adsorption whereas the same condition was applied for PO43− adsorption by Freundlich isotherm which designates their respective suitability. The experimentally measured raw isotherm (c vs q) data was given in the supplementary file as Figs. S1 and S2, where the experimental data was compared with various isotherms.
3.8 Study of adsorption thermodynamics
The NO3− and PO43− adsorption onto Zr@AlgKN composite beads (Hydro) were investigated through various thermodynamic parameters such as standard entropy change (ΔS°), Gibbs free energy change (ΔG°) and standard enthalpy change (ΔH°). The equations which were used to find out the thermodynamic parameters and their plot details are shown in Table 2a. From Table 2b, it was noticed that the decrease of Gibbs free energy change (ΔG°) with an increase of the temperature suggested the feasible adsorption process (Aswin Kumar and Viswanathan, 2018a). The negative enthalpy change (ΔH°) (−0.59 kJ/mol) points the exothermic nature of NO3− adsorption while the positive ΔH° (4.67 kJ/mol) spots the endothermic nature of PO43− adsorption (Bhatnagar and Sillanpaa, 2011; Thagira Banu et al., 2018). The positive entropy change (ΔS°) value exposes the improved randomness in the liquid-solid interface in the surface of Zr@AlgKN composite beads during NO3− and PO43− adsorption (Bhatnagar et al., 2008; Aswin Kumar et al., 2019).
Thermodynamic parameters
Thermodynamic equation
Thermodynamic linear plot
Parameters
Standard free energy change ΔG° (kJ/mol)
ln (qe/Ce) vs Ce
T – Temperature
R - Universal gas constant
(8.314 J/mol K)
Ko – Adsorption distribution
coefficient
Standard enthalpy change ΔH° (kJ/mol)
ln Ko vs 1/T
Standard entropy change ΔS° (J/K mol)
ln (1 − θ) vs 1/T
Thermodynamic parameters
Nitrate
Phosphate
ΔGo (kJ/mol)
303 K
−3.16
−7.28
313 K
−3.21
−7.35
323 K
−3.29
−7.41
ΔHo (kJ/mol)
−0.59
4.67
ΔSo (J/K mol)
18.07
29.61
3.9 Kinetics study
To find the reaction rate of NO3− and PO43− adsorption onto the hydro assisted Zr@AlgKN composite beads, the kinetic models such as reaction-based and diffusion-based models were investigated at 303, 313 and 323 K. The pseudo-first-order and pseudo-second-order kinetic models were categorized under the reaction based kinetic models. The kinetic equation and the linear plot details of these kinetic models are shown in Table 3a. The linear plot of pseudo-first-order was governed by log (qe − qt) vs t which shows its applicability whereas the linear plot of t/qt vs t shows the applicability of the pseudo-second-order kinetic model (Periyasamy et al., 2018).
Kinetic models
Kinetic equation
Linear plot
(i) Reaction-based
Pseudo-first-order
log (qe − qt) vs t
Pseudo-second-order
t/qt vs t
(ii) Diffusion-based
Particle diffusion
ln (1 − Ct /Ce) vs t
Intraparticle diffusion
qt vs t1/2
The rate constant (kad) and r values of the pseudo-first-order and qe, k, h and r values of the pseudo-second-order kinetic model of Zr@AlgKN composite beads toward NO3− and PO43− adsorption are listed in Tables 3b and 3c respectively. The qe value in Table 3b was slightly decreased with increase in temperature during NO3− sorption whereas for PO43− it was slightly increased with increase in temperature which is shown in Table 3c. Moreover, the higher r value and lower sd value for the pseudo-second-order model than the pseudo-first-order indicates the suitability of the pseudo-second-order kinetic model towards NO3− and PO43− adsorption.
Kinetic models
Parameters
303 K
313 K
323 K
80 mg/L
100 mg/L
120 mg/L
140 mg/L
80 mg/L
100 mg/L
120 mg/L
140 mg/L
80 mg/L
100 mg/L
120 mg/L
140 mg/L
Pseudo-first-order
kad (min−1)
0.020
0.023
0.021
0.019
0.023
0.035
0.039
0.027
0.031
0.038
0.033
0.037
r
0.946
0.945
0.948
0.949
0.950
0.955
0.957
0.959
0.955
0.960
0.962
0963
sd
0.516
0.524
0.525
0.527
0.511
0.526
0.529
0.561
0.509
0.524
0.558
0.563
Pseudo-second-order
qe (mg/g)
21.462
31.264
31.269
31.271
21.405
31.259
31.263
31.266
21.389
31.220
31.255
31.261
k (g/mg min)
0.009
0.017
0.014
0.025
0.008
0.021
0.020
0.012
0.010
0.029
0.030
0.034
h (mg/g min)
16.234
23.625
23.627
23.630
16.230
23.623
23.625
23.629
16.227
23.621
23.622
23.625
r
0.995
0.997
0.996
0.999
0.992
0.995
0.996
0.997
0.999
0.997
0.995
0.998
sd
0.241
0.233
0.230
0.229
0.239
0.235
0.232
0.231
0.237
0.236
0.238
0.240
Particle diffusion
kp (min−1)
0.043
0.087
0.093
0.097
0.067
0.099
0.105
0.107
0.109
0.113
0.115
0.0119
r
0.942
0.940
0.939
0.941
0.943
0.942
0.940
0.944
0.948
0.949
0.951
0.950
sd
0.396
0.398
0.399
0.397
0.402
0.415
0.414
0.416
0.416
0.419
0.423
0.417
Intra particle diffusion
ki (mg/g min0.5)
1.023
1.085
1.095
1.099
1.046
1.134
1.128
1.130
1.077
1.154
1.149
1.152
r
0.985
0.986
0.987
0.990
0.991
0.992
0.988
0.986
0.990
0.994
0.993
0.997
sd
0.104
0.103
0.106
0.108
0.111
0.113
0.116
0.117
0.120
0.115
0.118
0.121
Kinetic models
Parameters
303 K
313 K
323 K
80 mg/L
100 mg/L
120 mg/L
140 mg/L
80 mg/L
100 mg/L
120 mg/L
140 mg/L
80 mg/L
100 mg/L
120 mg/L
140 mg/L
Pseudo-first-order
kad (min−1)
0.034
0.037
0.035
0.027
0.031
0.049
0.053
0.057
0.044
0.043
0.046
0.041
r
0.966
0.965
0.963
0.969
0.973
0.970
0.972
0.977
0.978
0.986
0.983
0.984
sd
0.346
0.382
0.386
0.389
0.345
0.376
0.380
0.384
0.343
0.378
0.387
0.392
Pseudo-second-order
qe (mg/g)
26.032
37.246
37.249
37.252
26.037
37.278
37.284
37.291
26.041
37.290
37.295
37.299
k (g/mg min)
0.011
0.020
0.028
0.033
0.006
0.025
0.031
0.033
0.015
0.038
0.036
0.031
h (mg/g min)
19.235
27.135
27.137
27.139
19.236
27.140
27.142
27.144
19.239
27.143
27.146
27.150
r
0.996
0.998
0.997
0.999
0.993
0.997
0.998
0.999
1.000
0.999
0.997
0.996
sd
0.154
0.179
0.181
0.177
0.159
0.180
0.178
0.182
0.160
0.175
0.183
0.186
Particle diffusion
kp (min−1)
0.104
0.125
0.128
0.122
0.149
0.167
0.173
0.177
0.159
0.183
0.195
0.199
r
0.953
0.955
0.952
0.957
0.960
0.954
0.959
0.963
0.961
0.960
0.962
0.965
sd
0.456
0.469
0.471
0.470
0.459
0.473
0.476
0.478
0.457
0.482
0.485
0.488
Intra particle diffusion
ki (mg/g min0.5)
1.246
1.574
1.678
1.735
1.439
1.628
1.682
1.699
1.538
1.707
1.714
1.735
r
0.994
0.996
0.995
0.997
0.995
0.994
0.996
0.999
0.998
0.996
0.999
0.995
sd
0.276
0.293
0.295
0.294
0.277
0.299
0.301
0.302
0.280
0.306
0.309
0.311
The particle diffusion and intraparticle diffusion kinetic models were used to investigate the solute transfer during solid-liquid sorption process. The linear plots of ln (1 − Ct/Ce) vs t and qt vs. t0.5 denote the suitability of the particle diffusion and intraparticle diffusion kinetic models respectively (Viswanathan et al., 2019). In addition, the values of kp, ki and r at 303, 313 and 323 K of both models toward NO3− and PO43− adsorption are presented in Tables 3b and 3c respectively. It was concluded that the higher r value and lower sd values of the intraparticle diffusion kinetic model declares it suitability for NO3− and PO43− adsorption compared to particle diffusion kinetic model.
3.10 Exploration of adsorption mechanism
The electrostatic interaction, ion exchange and surface complexation were formed during the adsorption of NO3− and PO43− by in situ and hydro supported Zr@AlgKN composite beads which are illustrated in Fig. 7. According to Pearson’s Hard Soft Acid Base (HSAB) concept, the metal ions with higher positive charge are act as Lewis acid which has an affinity to strongly bind the hard bases such as NO3− and PO43−. The mobility of NO3− and PO43− in solution would be the fast towards the protonated Zr—O—OH2+, Al—OH4+ and Si—OH5+ in order to form the electrostatic bond (Lu et al., 2014; Sowmya and Meenakshi, 2014). This electrostatic adsorption was further explained by pH study (cf. Section 3.4). PO43− could exist in a multivalent form in water. The dihydrogen phosphate (H2PO4−) forms complexation with the protonated Zr—O—OH2+, Al—OH4+ and Si—OH5+ (Fang et al., 2015). Further, the interstitial OH− ions of kaolin clay may get exchanged for both NO3− and PO43− by ion-exchange mechanism (Li et al., 2016).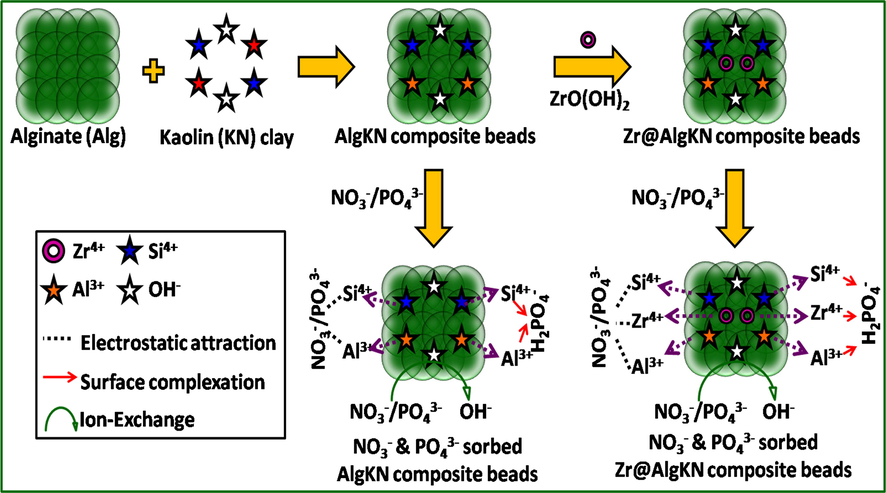
The possible adsorption mechanism of NO3− and PO43− using in situ and hydro assisted Zr@AlgKN composite beads.
3.11 Field study of Zr@AlgKN composite beads
The quality parameters of the collected field water samples were investigated using in situ and hydro assisted Zr@AlgKN composite beads and the results are presented in Table 4a. The initial NO3− and PO43− concentration of the field water sample was found to be 28.16 and 37.42 mg/L respectively. However, the final concentration of NO3− and PO43− after the treatment was found to be nil. In addition, Cl− content, total dissolved solids and total hardness of the collected field water was also controlled by the prepared Zr@AlgKN composite beads which ensure its applicability at field conditions.
Water quality parameters
Before treatment
After treatment
Zr@AlgKN (Hydro)
Zr@AlgKN (In situ)
Initial NO3− concentration (mg/L)
28.16
Nil
Nil
Initial PO43− concentration (mg/L)
37.42
Nil
Nil
pH
5.97
6.63
6.51
Cl− (mg/L)
328
197
204
Total hardness (mg/L)
539
367
391
Total dissolved solids (mg/L)
454
186
193
3.12 Adsorption capacity comparison
The adsorption capacity of the prepared Zr@AlgKN composite beads (Hydro) was compared with the other adsorbents in the market and the comparison is shown in Table 4b. The prepared Zr@AlgKN composite beads possess the appreciable adsorption capacity toward NO3− and PO43− removal. Although, most of the other adsorbents possess the good adsorption capacity, the powder nature of them leads to attain the pressure drops during filtration which may limit their field applications. However, the prepared Zr@AlgKN composite beads overcome such technological bottle-neck. In addition, Zr@AlgKN composite beads can be easily separated after its usage which designates its applicability at industry level.
S.No
Name of the adsorbent
Nitrate
Phosphate
Refs.
Sorption capacity (mg/g)
Best fit isotherm
Temp. (K)
Sorption capacity (mg/g)
Best fit isotherm
Temp. (K)
1
Zr@AlgKN composite beads (Hydro)
31.24
Langmuir
303
37.18
Freundlich
303
Present study
2
Modified cellulose from corn stalks
13.60
Langmuir
298
22.88
Langmuir
298
(Fan and Zhang, 2018)
3
Activated carbon prepared from prosopis juliflora
10.99
Langmuir
308
17.33
Langmuir
308
(Manjunath and Kumar, 2018)
4
Fe(0) supported activated carbon
4.60
Langmuir
298
1.75
Langmuir
298
(Khalil et al., 2017)
5
Poly(styrene divinylbenzene)
–
–
–
12.20
Langmuir
303
(Pan et al., 2009)
6
Carbon silica nano composite
11.34
–
–
–
–
–
(Muthu et al., 2017)
7
Zr/quaternary ammonium powder with polyvinylidene fluoride
9.66
–
298
15.58
–
298
(Gao et al., 2019)
8
Bentonite modified with La(III)
–
–
–
14.00
Langmuir
298
(Kuroki et al., 2014)
9
Cu and Mg impregnated alumina
8.00
Freundlich
–
–
–
–
(Jain et al., 2015)
10
Granular chitosan Fe3+ complex
8.35
Langmuir & Freundlich
298
–
–
–
(Hu et al., 2015)
11
Biomass
11.20
Langmuir
298
30.20
Langmuir
298
(Kilpimaa et al., 2015)
12
Chitosan saturated with copper (II)
–
–
–
28.86
Langmuir
298
(Dai et al., 2011)
3.13 Regeneration study of Zr@AlgKN composite beads
To regenerate the metal ions surrounded adsorbent, a NaOH eluent was used (Wang et al., 2017). Once, the sorption process over, the NO3− and PO43− sorbed Zr@AlgKN composite beads were completely soaked in 50 mL of 0.1 N NaOH for 1 h followed by filtered, dried at 80 °C and reused for the adsorption again. The same procedure was repeated for six times and their removal efficiency toward NO3− and PO43− was illustrated in Fig. S3. The increasing cycles of NaOH added decreases the removal percentage. This may governed by the competing effect of basic OH− ions which occupies the active sites of Zr@AlgKN composite beads surface instead of both NO3− and PO43−. It was also concluded that there is a significant loss in the NO3− and PO43− removal percentage was observed after 3 and 4 cycles for in situ and hydro assisted Zr@AlgKN composite beads. Hence, Zr@AlgKN composite beads can be reused as the efficient recyclable adsorbent up to 3 and 3 cycles for water treatment which reveals its cost effective nature.
4 Conclusions
The hydro assisted Zr@AlgKN composite beads exhibited an enhanced SC toward NO3− and PO43− compared to other adsorbents prepared by in situ precipitation method. The solution pH was predominantly affects NO3− and PO43− sorption and during the protonation of Zr@AlgKN composite beads surface the electrostatic attraction was formed with both NO3− and PO43−. The serious competence of SO42− for NO3− and PO43− was governed in natural water. FTIR, XRD, SEM, EDAX and BET studies of the adsorbents were studied in detail. The experimental data was fitted with Langmuir and Freundlich isotherms for NO3− and PO43− adsorption respectively. The order of the NO3− and PO43− adsorption follows pseudo-second order kinetics and as well as intraparticle diffusion model. The negative ΔH° values of Zr@AlgKN composite beads (Hydro) denote the exothermic nature of NO3− adsorption while the positive ΔH° value indicates the endothermic nature of the PO43− adsorption. The electrostatic adsorption, surface complexation, and ion-exchange mechanism were involved during NO3− and PO43− adsorption. The reuse recovery was achieved upto three and four extraction cycles for in situ and hydro assisted Zr@AlgKN composite beads. Moreover, the prepared Zr@AlgKN composite beads were controls the other water quality parameters in addition to NO3− and PO43− in the collected field water sample which facilitates its applicability at field conditions.
Acknowledgements
The authors were gratefully acknowledging University Grants Commission (F. No. 43-179/2014(SR)), New Delhi, India, for providing financial support to carry out this research work. The first author (I. Aswin Kumar) is sincerely thanks the Council of Scientific and Industrial Research (CSIR), New Delhi, India for awarding Senior Research Fellowship.
References
- The effect of some operating variables on the adsorption of lead and cadmium ions on kaolinite clay. J. Hazard. Mater.. 2006;134:130-139.
- [Google Scholar]
- Standard methods for the examination of water and wastewater. Washington, DC: American Public Health Association; 2005.
- Hexamethylene tetramine-assisted hydrothermal synthesis of porous magnesium oxide for high-efficiency removal of phosphate in aqueous solution. J. Environ. Chem. Eng.. 2017;5:4649-4655.
- [Google Scholar]
- Development of multivalent metal ions imprinted chitosan biocomposites for phosphate sorption. Int. J. Biol. Macromol.. 2017;104:1539-1547.
- [Google Scholar]
- Fabrication of metal ions cross-linked alginate assisted biocomposite beads for selective phosphate removal. J. Environ. Chem. Eng.. 2017;5:1438-1446.
- [Google Scholar]
- Hydrothermal fabrication of zirconium oxyhydroxide capped chitosan/kaolin framework for highly selective nitrate and phosphate retention. Ind. Eng. Chem. Res.. 2018;57(43):14470-14481.
- [Google Scholar]
- Development and reuse of amine-grafted chitosan hybrid beads in the retention of nitrate and phosphate. J. Chem. Eng. Data. 2018;63:147-158.
- [Google Scholar]
- Preparation and testing of a tetra-amine copper(ii) chitosan bead system for enhanced phosphate remediation. Carbohydr. Polym.. 2018;183:173-182.
- [Google Scholar]
- A facile synthesis of magnetic particles sprayed gelatin embedded hydrotalcite composite for effective phosphate sorption. J. Environ. Chem. Eng.. 2018;6:208-217.
- [Google Scholar]
- Hydrothermal encapsulation of lanthanum oxide derived Aegle marmelos admixed chitosan bead system for nitrate and phosphate retention. Int. J. Biol. Macromol.. 2019;130:527-535.
- [Google Scholar]
- Removal of nitrate from water by adsorption onto zinc chloride treated activated carbon. Sep. Sci. Technol.. 2008;43:886-907.
- [Google Scholar]
- A review of emerging adsorbents for nitrate removal from water. Chem. Eng. J.. 2011;168:493-504.
- [Google Scholar]
- Strong adsorption of arsenic species by amorphous zirconium oxide nanoparticles. J. Ind. Eng. Chem.. 2012;18:1418-1427.
- [Google Scholar]
- Phosphate adsorption from aqueous solutions by disused adsorbents: chitosan hydrogel beads after the removal of copper(II) Chem. Eng. J.. 2011;166:970-977.
- [Google Scholar]
- Kaolin-issued zeolite A as efficient adsorbent for bezanyl yellow and nylomine green anionic dyes. Micropor. Mesopor. Mater.. 2017;243:91-101.
- [Google Scholar]
- Adsorption isotherms, kinetics and thermodynamics of nitrate and phosphate in binary systems on a novel adsorbent derived from corn stalks. J. Geochem. Explor.. 2018;188:95-100.
- [Google Scholar]
- Facile upscaled synthesis of layered iron oxide nanosheets and their application in phosphate removal. J. Mater. Chem. A. 2015;3:7505-7512.
- [Google Scholar]
- Photocatalytic aptitude of titanium dioxide impregnated chitosan beads for the reduction of Cr(VI) Int. J. Biol. Macromol.. 2015;72:1265-1271.
- [Google Scholar]
- Drinking-water nitrate, methemoglobinemia, and global burden of disease: a discussion. Environ. Health Perspect.. 2004;112:1371-1374.
- [Google Scholar]
- Ultrafiltration membrane micro reactor (MMR) for simultaneous removal of nitrate and phosphate from water. Chem. Eng. J.. 2019;355:238-246.
- [Google Scholar]
- Fourier transform infrared spectrophotometry and X-ray powder diffractometry as complementary techniques in characterizing clay size fraction of kaolin. J. Appl. Sci. Environ. Manage.. 2005;9:43-48.
- [Google Scholar]
- The removal of nitrogen and phosphorus from reject water of municipal wastewater treatment plant using ferric and nitrate bioreductions. Bioresour. Technol.. 2010;101:3992-3999.
- [Google Scholar]
- Nitrate adsorption from aqueous solution using granular chitosan-Fe3+ complex. Appl. Surf. Sci.. 2015;347:1-9.
- [Google Scholar]
- Enhancing adsorption of nitrate using metal impregnated alumina. J. Environ. Chem. Eng.. 2015;3:2342-2349.
- [Google Scholar]
- Column study of Cr (VI) adsorption onto modified silica–polyacrylamide microspheres composite. Chem. Eng. J.. 2012;210:280-288.
- [Google Scholar]
- Optimized nano-scale zero-valent iron supported on treated activated carbon for enhanced nitrate and phosphate removal from water. Chem. Eng. J.. 2017;309:349-365.
- [Google Scholar]
- Physical activation of carbon residue from biomass gasification: novel sorbent for the removal of phosphates and nitrates from aqueous solution. J. Ind. Eng. Chem.. 2015;21:1354-1364.
- [Google Scholar]
- Use of La(III)-modified bentonite for effective phosphate removal from aqueous media. J. Hazard. Mater.. 2014;274:124-131.
- [Google Scholar]
- Enhanced biological phosphorus and nitrogen removal using a sequencing anoxic/anaerobic membrane bioreactor (SAM) process. Desalination. 2003;15:345-352.
- [Google Scholar]
- The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc.. 1916;38:2221-2295.
- [Google Scholar]
- Enhancing phosphate adsorption by Mg/Al layered double hydroxide functionalized biochar with different Mg/Al ratios. Sci. Total Environ.. 2016;559:121-129.
- [Google Scholar]
- On the characterization of the acidic and basic surface sites on carbons by various techniques. Carbon. 1999;37:1215-1221.
- [Google Scholar]
- Phosphate removal from water using freshly formed Fe-Mn binary oxide: adsorption behaviors and mechanisms. Colloids Surf., A. 2014;455:11-18.
- [Google Scholar]
- Evaluation of single and multi-component adsorption of metronidazole, phosphate and nitrate on activated carbon from prosopis juliflora. Chem. Eng. J.. 2018;346:525-534.
- [Google Scholar]
- Effect of nitrite on anoxic phosphate uptake in biological phosphorus removal activated sludge. Water Res.. 1999;33:1871-1883.
- [Google Scholar]
- Characterization of phosphated zirconia by XRD, Raman and IR spectroscopy. Colloids Surf., A. 1998;141:227-235.
- [Google Scholar]
- Unprecedented nitrate adsorption efficiency of carbon-silicon nanocomposites prepared from bamboo leaves. Mater. Chem. Phys.. 2017;189:12-21.
- [Google Scholar]
- The adsorption of phosphamidon on the surface of styrene supported zirconium (IV) tungstophosphate: A thermodynamic study. Colloids Surf., A. 2000;164:115-119.
- [Google Scholar]
- Genesis of mayouom kaolin deposit (Western Cameroon) Appl. Clay Sci.. 2006;32:125-140.
- [Google Scholar]
- Synthesize of polypyrrole nanocomposite and its application for nitrate removal from aqueous solution. J. Ind. Eng. Chem.. 2012;18:948-956.
- [Google Scholar]
- Development of polymer-based nanosized hydrated ferric oxides (HFOs) for enhanced phosphate removal from waste effluents. Water Res.. 2009;43:4421-4429.
- [Google Scholar]
- Synthesis of alginate bioencapsulated nano-hydroxyapapatite composite for selective fluoride sorption. Carbohydr. Polym.. 2014;112:662-667.
- [Google Scholar]
- A novel metal coordination enabled in carboxylated alginic acid for effective fluoride removal. Carbohydr. Polym.. 2015;118:242-249.
- [Google Scholar]
- Hydrothermal assisted magnetic nanohydroxyapatite encapsulated alginate beads for efficient Cr (VI) uptake from water. J. Environ. Chem. Eng.. 2018;6:1443-1454.
- [Google Scholar]
- Adsorption of phosphate and nitrate anions on ammonium-functionalized MCM-48: effects of experimental conditions. J. Colloid Interface Sci.. 2007;311:375-381.
- [Google Scholar]
- Effective removal of nitrate and phosphate anions from aqueous solutions using functionalized chitosan beads. Desalin. Water Treat.. 2014;52:2583-2593.
- [Google Scholar]
- Lanthanum (III) encapsulated chitosan-montmorillonite composite for the adsorptive removal of phosphate ions from aqueous solution. Int. J. Biol. Macromol.. 2018;112:284-293.
- [Google Scholar]
- Development of chitosan encapsulated tricalcium phosphate biocomposite for fluoride retention. Int. J. Biol. Macromol.. 2019;133:811-816.
- [Google Scholar]
- The chemical bonding of copper ions on kaolin from suzhou, China. Desalination. 2009;249:991-995.
- [Google Scholar]
- Preparation of hollow Fe-Al binary metal oxyhydroxide for efficient aqueous fluoride removal. Colloids Surf. A. 2017;520:580-589.
- [Google Scholar]
- Adsorption of reactive dye onto carbon nanotubes: equilibrium, kinetics and thermodynamics. J. Hazard. Mater.. 2007;144:93-100.
- [Google Scholar]
- Preparation of agricultural by-product based anion exchanger and its utilization for nitrate and phosphate removal. Bioresour. Technol.. 2010;101:8558-8564.
- [Google Scholar]
- Alginate fibers embedded with silver nanoparticles as efficient catalysts for reduction of 4-nitrophenol. RSC Adv.. 2015;5:49534-49540.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2019.06.006.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







