Translate this page into:
Forced degradation of mometasone furoate and development of two RP-HPLC methods for its determination with formoterol fumarate or salicylic acid
⁎Corresponding author. Tel.: +20 1002768761. nosso2@yahoo.com (Enas H. Tolba)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Two simple, selective and precise stability-indicating reversed-phase liquid chromatographic methods were developed and validated for the determination of mometasone furoate in two binary mixtures, with formoterol fumarate (Mixture 1) and salicylic acid (Mixture 2). Also, a forced degradation study of mometasone furoate was carried out including acid and alkali hydrolysis, oxidation, thermal and photo-degradation. For mixture 1, the method was based on isocratic elution using a mobile phase consisting of (Acetonitrile: 3 mM Sodium lauryl sulfate) (60:40, v/v) at a flow rate of 1 ml min−1. Quantitation was achieved applying dual wavelength detection where mometasone furoate and its degradation products were detected at 247 nm and formoterol fumarate and its degradation product were detected at 214 nm at 30 °C. For mixture 2 and for the forced degradation study, separation was based on isocratic elution of mometasone furoate, its degradation products and salicylic acid on a reversed phase C8 column using a mobile phase consisting of acetonitrile:water:methanol:glacial acetic acid (60:30:10:0.1, v/v) at a flow rate of 2 mL min−1. Quantitation was achieved with UV detection at 240 nm. In addition, products from alkaline forced degradation of mometasone furoate were verified by LC–MS. Linearity, accuracy and precision were found to be acceptable over the concentration range of 10–800 μg mL−1 and 5–60 μg mL−1 for mometasone furoate and formoterol fumarate, respectively and over the concentration range of 5–320 μg mL−1 and 20–1280 μg mL−1 for mometasone furoate and salicylic acid, respectively. The two proposed methods could be successfully applied for the routine analysis of the studied drugs in their pharmaceutical preparations without any preliminary separation step.
Keywords
Mometasone furoate
Formoterol fumarate
Salicylic acid
Stability-indicating assay
Forced degradation
1 Introduction
Mometasone furoate (MF), 9α,21-dichloro-11β,17-dihydroxy-16α- methylpregna-1,4-diene-3,20-dione 17-(2-furoate), (Fig. 1a), is a topical corticosteroid. It has anti-inflammatory, anti-pruritic, and vasoconstrictive properties. It is indicated for a number of conditions such as allergic reactions, eczema and psoriasis. Corticosteroids act by the induction of phospholipase A2 inhibitory proteins, collectively called lipocortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting the release of their common precursor arachidonic acid (Shaikh et al., 2009).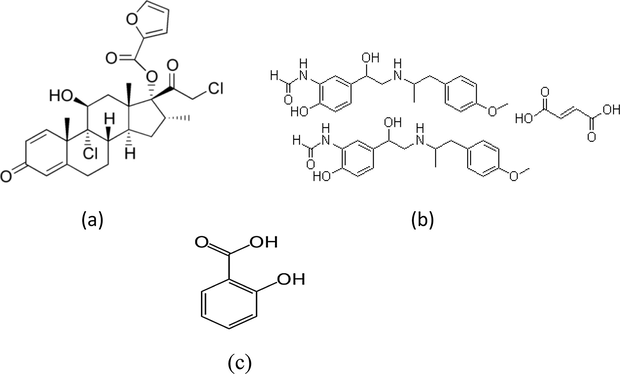
Chemical structure of (a) mometasone furoate, (b) formoterol fumarate and (c) salicylic acid.
Formoterol, ((RR)-(±)-N-[2-hydroxy-5-[1-hydroxy-2-[[2-(4- methoxyphenyl)-1 methylethyl]amino]ethyl]phenyl]formamide), (Fig. 1b) is an adrenergic agent with high selectivity for the β2 receptor (β2-agonist) and has proven to be a very effective bronchodilating agent (Sardela et al., 2012). Hence, it is frequently applied therapeutically by means of metered dose inhalers for the treatment of asthma, bronchospasm and prophylaxis of exercise-induced bronchospasm.
Salicylic acid (SA), 2-hydroxybenzoic acid, (Fig. 1c) is widely used as a peeling or keratolytic agent to treat callus, keratosis or warts. Salicylic acid is further applied in the treatment of acne, psoriasis and photo ageing in various concentrations depending on the desired amount of keratolysis (Bashir et al., 2005). Salicylic compounds have also been synthesized for analgesics (acetylsalicylic acid or aspirin) and antipyretics.
Literature survey revealed the determination of mometasone furoate (MF) in plasma by LC-tandem mass spectrometry (Sahasranaman et al., 2005), and by HPLC with stability studies (Teng et al., 2001). Also, MF was determined in its dosage form by HPLC (Ourique et al., 2012). Kinetic study of MF in aqueous system (Teng et al., 2003) and supercritical fluid chromatographic impurity analysis (Wang et al., 2011) were also carried out. Moreover, determination of MF with fucidic acid, tazarotene using HPLC (Shaikh et al., 2009; Ankam et al., 2009) and with nadifloxacin using HPTLC (Kulkarni et al., 2010) was reported.
Besides, Several methods have been reported for the determination of formoterol fumarate (FF) by HPLC in mixtures (Assi et al., 2006; Sule et al., 2012; TRIVEDI et al., 2012; malik et al., 2011), by Chiral analysis (Samuel et al., 2009), and with its related substances (Samuel and Muhammad, 2003; Hassib et al., 2011). Several qualitative mass spectrometry based methods for the detection of formoterol in the field of doping analysis have been also described (Kang et al., 2007; Mazzarino and Botre, 2006; Thevis et al., 2003; Ventura et al., 2000; Peters et al., 2010; Kolmonen et al., 2009). For its quantitation, several non-mass spectrometric methods have been described in the frame of pharmacokinetic experiments (Nadarassan et al., 2007; Butter et al., 1996; Rosenborg et al., 1999). Quantitative detection methods applying mass spectrometric detection are limited to a GC–MS method (Kamimura et al., 1982), a UPLC-MS (Sardela et al., 2012), and two LC–MS/MS methods (Deventer et al., 2012; Marzo et al., 2000).
Also, several analytical methods have been reported for the determination of salicylic acid (SA) in mixtures using spectrophotometry (Salinas et al., 1990), colorimetry (Saha and Baksi, 1985), liquid chromatography (Aguiar et al., 2009; Akay et al., 2008), flow-through UV spectrophotometric sensor (Ruiz-Medina et al., 2001) and titrimetric method (Ruiz-Medina et al., 2001).
Mometasone furoate is incorporated in many pharmaceutical preparations, as inhaled corticosteroids for the treatment of bronchial asthma and as anti-inflammatory corticosteroids for the treatment of many skin disorders. The two pharmaceutical preparations analysed in this manuscript represent these binary usages of mometasone furoate.
The combination of MF with FF or SA is not officially reported in any pharmacopoeia. So far, no RP-HPLC stability-indicating method has been reported for the rapid simultaneous determination of MF with any of them. Besides, only degradation kinetics in different pH solutions (Teng et al., 2003) and thermostability studies in plasma (Teng et al., 2001) were found in the literature but no full forced degradation study of mometasone furoate was published. Thus, the aim of the present work was to develop simple, sensitive and selective LC methods for the simultaneous determination of mometasone furoate and formoterol fumarate (Mixture 1), and for the determination of mometasone furoate and salicylic acid (Mixture 2). Selectivity was demonstrated by enhancing alkaline and acidic degradation of mometasone furoate and formoterol fumarate in mixture 1 and by alkaline degradation of mometasone furoate in mixture 2. Mometasone furoate was found to be stable in acidic conditions while formoterol fumarate was stable in alkaline conditions. The two methods were successfully applied to laboratory prepared mixtures and their pharmaceutical preparations. Moreover, the present manuscript describes the degradation behaviour of mometasone furoate under acidic, basic, oxidative, thermal and photolytic conditions. The method validation is generally performed as per the guidelines published by the International Conference on Harmonization (ICH).
2 Experimental
2.1 Instrumentation
A chromatographic system consists of Agilent 1200 series (CA, USA): interface equipped with an Agilent quaternary pump G1311A, Agilent UV–visible detector G1314B, an Agilent manual injector G1328B equipped with (20 μL) injector loop, and an Agilent degasser G1322A.
Separation and quantitation were made on C18 column (250 × 4.6 mm, 5 μm) –XTerra® for mixture 1 and C8 column (250 mm × 4.6 mm, 7 μm) – Phenomenex® for mixture 2 and for forced degradation study.
2.2 Reagents and reference samples
Mometasone furoate was kindly supplied by Sigma–Aldrich and was certified to contain 99.6%. Formoterol fumarate was supplied by Novartis and was certified to contain 99.76% and salicylic acid was supplied by Pharco Pharmaceutical and was certified to contain 99.7%. HPLC grade acetonitrile and methanol were supplied by Scharlau, Spain. Sodium lauryl sulfate was supplied by Parchem. Bi-distilled water was produced in-house (Aquatron Water Still, A4000D, UK). Membrane filters of 0.45 μm were purchased from Teknokroma (Barcelona, Spain). All the chemicals were of analytical grade.
Dulera® inhaler labelled to contain 200 μg/5 μg of Mometasone furoate and Formoterol fumarate, respectively, per actuation (Batch No. H015615), was supplied by MERCK & CO. Elicasal® ointment, nominally containing 0.1%w/w mometasone furoate and 5%w/w Salicylic acid (Batch No. M0062), was supplied by Jamjoom Pharmaceuticals Company, KSA.
2.3 Chromatographic conditions
2.3.1 Mixture 1
Chromatographic separation was achieved on a C18 column (250 × 4.6 mm, 5 μm) –XTerra® using the mobile phase consisting of acetonitrile: 3 mM sodium lauryl sulfate) (60:40, v/v) at a flow rate of 1 ml min−1. The column temperature was adjusted at 30 °C. UV detector was operated at 247 nm for MF and its alkaline degradation products and at 214 nm for FF and its acidic degradation product.
2.3.2 Mixture 2 and forced degradation study
Chromatographic separation was achieved on a C8 column (250 mm × 4.6 mm, 7 μm) Phenomenex® applying isocratic elution based on a mobile phase consisting of acetonitrile:water:methanol:glacial acetic acid (60:30:10:0.1, v/v). The mobile phase was pumped through the column at a flow rate of 2 mL min−1. Analyses were performed at ambient temperature and detection was carried out at 240 nm. The injection volume was 20 μL.
2.4 Solutions
2.4.1 Stock standard solutions
2.4.1.1 Mixture 1
Stock standard solutions of MF (2 mg mL−1) and FF (0.5 mg mL−1) were prepared in methanol. Appropriate dilutions of theses stock solutions were prepared using the same solvent used for linearity studies and assay purposes.
2.4.1.2 Mixture 2
Stock standard solutions of MF (0.5 mg mL−1) and SA (2 mg mL−1) were prepared in acetonitrile. Appropriate dilutions of theses stock solutions were prepared using the same solvent used for linearity studies and assay purposes.
2.4.2 Laboratory-prepared mixtures
2.4.2.1 Mixture 1
Solutions containing different ratios of MF (200–680 μg mL−1) and FF (5–17 μg mL−1) were prepared by transferring aliquots from their stock solutions into a series of 10-mL volumetric flasks and the volume of each was completed to the mark with methanol.
2.4.2.2 Mixture 2
Solutions containing different ratios of MF (6–24 μg mL−1) and SA (300–1200 μg mL−1) were prepared by transferring aliquots from their stock solutions into a series of 10-mL volumetric flasks and the volume of each was completed to the mark with acetonitrile.
2.4.3 Sample preparation
2.4.3.1 Mixture 1
The pressurized canister was removed from the actuator and placed in a plastic bag in upright position and chilled to −20 °C for 30 min then carefully a small hole was pierced on the shoulder of the canister, allowing the propellants to evaporate and the top was removed. The top and valve of the opened canister were washed with methanol. 5 mL of diluent was added in the canister and sonicated to dissolve at ambient temperature. The content of canister was transferred to 25 mL volumetric flask. The above procedure was repeated 2 times with 5 mL methanol and the solution was sonicated until dissolved. Then the volumetric flask was filled up to the mark with methanol and mixed.
2.4.3.2 Mixture 2
Two grams of Elicasal® ointment (for mixture 2) was transferred to a conical flask, taking care to avoid sticking ointment to the walls of the flask. Twenty-five mL of acetonitrile was added and the mixture was heated in a water bath at 80 °C until the ointment melted completely; in between, the flask was occasionally swirled. The conical flask content was quantitatively transferred to a 50 mL volumetric flask. The flask was washed with 2 × 10 mL acetonitrile. Volume was made up to the mark with acetonitrile and mixed. Solution was gently cooled then supernatant solution was filtered through a 0.45-μm membrane filter.
2.5 Procedures
2.5.1 Construction of the calibration curves
For mixture 1, aliquots equivalent to 10–800 μg mL−1 of MF and 5–60 μg mL−1 of FF were accurately transferred into two series of 10-mL volumetric flasks and the volumes were completed to the mark with methanol.
For mixture 2, aliquots equivalent to 5–320 μg mL−1 of MF and 20–1280 μg mL−1 of SA were accurately transferred into two series of 10 mL volumetric flasks and the volumes were completed to the mark with acetonitrile.
A volume of 20 μL of each solution was injected in triplicate into the chromatograph under the specified chromatographic conditions described in Section 2.3.1. for mixture 1 and 2.3.2. for mixture 2. A calibration curve for each compound was obtained by plotting area under the peak (AUP) against concentration (C).
2.5.2 Assay of laboratory-prepared mixtures
The solutions prepared in Section 2.4.2.1. (For mixture 1) and in Section 2.4.2.2. (For mixture 2) were injected in triplicate into the chromatograph under the specified chromatographic conditions described in section 2.3.1. and 2.3.2., respectively. The concentration of each drug was calculated using the specified regression equation.
2.5.3 Assay of Dulera inhaler
The sample solution, prepared in Section 2.4.3.1., was serially diluted to get concentrations equivalent to 240–480 μg mL−1 and 6–12 μg mL−1 of MF and FF, respectively. Samples then were injected in triplicate and concentrations of MF and FF were calculated using calibration equations.
2.5.4 Assay of Elicasal ointment
The sample solution, prepared in Section 2.4.3.2., was serially diluted to get concentrations equivalent to 10–20 μg mL−1 and 500–1000 μg mL−1 of MF and SA, respectively. Samples then were injected in triplicate and concentrations of MF and SA were calculated using calibration equations.
2.6 Forced degradation of mometasone furoate
Forced degradation studies of bulk drug included appropriate solid state and solution state stress conditions in accordance with the ICH regulatory guidance (Q1A (R2), 2003; Elkady and Fouad, 2011). The stock solution was used for the forced degradation study to provide an indication of the stability indicating property and specificity of proposed method.
2.6.1 Acid- and base-induced degradation
To 5 mL of MF methanolic stock solution, an appropriate volume of 2 M HCl was added and the mixture was diluted with water to 10 mL to reach molarity of 0.1 M, 0.5 M and 1 M HCl, separately. The mixtures were kept at room temperature for 8 h. Alkaline degradation studies were carried out in a similar manner with molarities of 0.1 M, 0.5 M and 1 M NaOH for 8 h. These experiments were repeated at higher temperature of 75 °C for 0.5 h while keeping all other conditions constant. For study in neutral condition, drug was held in methanol at room temperature for 24 h. The forced degradation in acidic and basic media was performed in the dark in order to exclude the possible degradative effect of light. Prior to injection, samples were withdrawn at appropriate time and were neutralized (in case of acid and alkali hydrolysis) and the solutions were diluted with methanol. The total chromatographic run time was about two times the retention of the drug peak.
2.6.2 Hydrogen peroxide-induced degradation
To 1 mL of MF methanolic stock solution, 5 mL of 6% (v/v) H2O2 and 30% (v/v) H2O2 were separately added to reach final concentrations of 3% and 15% (v/v) H2O2, respectively. The prepared mixtures were kept at room temperature for 12 and 5 h, respectively. Also, oxidative degradation reactions were performed with the aid of heating at 80 °C for 1 h.
2.6.3 Thermal and photolytic degradation
The dry powder of the drug was placed in oven at 55 °C for 72 h to study dry heat degradation. Powder was dissolved and diluted with methanol to get final concentrations of (125 μg ml−1). The photochemical stability of the drug was also studied by exposing the drug solution (125 μg ml−1) to sunlight for 72 h.
Twenty microlitres of the resultant solutions were injected onto column and the chromatograms were run as described in Section 2.3.2.
2.7 Preparation of mometasone furoate alkaline degradation products
Mometasone furoate methanolic solution with a concentration of 50 μg mL−1 was incubated in 0.5 N NaOH at room temperature for 1 h. Solution was neutralized before incorporation in the analysis. This procedure resulted in four degradation products.
2.8 Preparation of desformyl derivative of formoterol fumarate
Formoterol fumarate (5 mg) was heated at 80 °C for 1 hour in 0.5 M hydrochloric acid (10 ml). The solution was cooled and diluted to 50 ml with water to produce a solution of desformyl derivative (equivalent to 0.1 mg mL−1 FF) in 0.1 M hydrochloric acid (Hassib et al., 2011).
3 Results and discussion
3.1 Method development
3.1.1 Mixture 1
Several chromatographic conditions were attempted using C18 and C8 column. Various mobile phase compositions such as (methanol: H2O), (Acetonitrile: H2O) and (Acetonitrile: methanol: H2O) in different ratios were tried in an isocratic mode but optimized separation of all contents was not obtained. Finally, by using a mixture of 3 mM sodium lauryl sulfate and acetonitrile, an improvement has been noticed. The chromatographic parameters were optimized and the ratio was kept as acetonitrile:sodium Lauryl sulfate) (60:40 v/v) at a flow rate of 1 mL min−1 and a column temperature of 30 °C. UV detector was operated at 247 nm for determination of MF and at 214 nm for determination of FF. These conditions resulted in optimal resolution and peaks sharpness of MF and FF (Fig. 2a) and their degradation products (Fig. 2b and c) with a chromatographic analysis time less than 10 min. The apparent advantages of using surfactant solutions as the mobile phase in liquid chromatography include the following: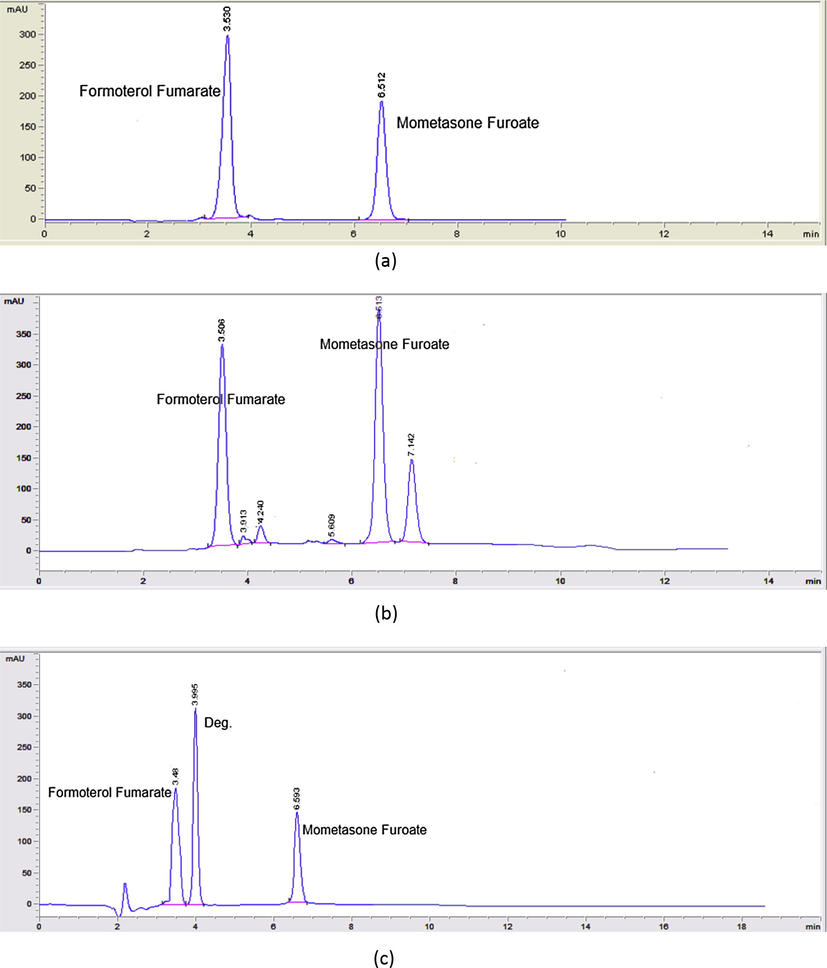
(a) HPLC chromatogram of mometasone furoate (40 μg mL−1) and formoterol fumarate (35 μg mL−1). (b) HPLC chromatogram of mometasone furoate (70 μg mL−1), mometasone furoate degradation products and formoterol fumarate (30 μg mL−1). (c) HPLC chromatogram of mometasone furoate (25 μg mL−1), formoterol fumarate desformyl derivative and formoterol fumarate (20 μg mL−1).
(i) their ability to solubilize hydrophobic compounds; (ii) highly selective partitioning of many solutes into micelles; (iii) low cost; and (iv) the ability to change the polarity of the micellar mobile phase simply by changing the concentration of surfactant in solution. The unique possibilities of separating structurally similar solutes with a micellar mobile phase arise because the micelles solubilize and bind a variety of solute molecules via hydrophobic, electrostatic, and hydrogen-bonding interactions (Mohammed and Jabeen, 2003).
3.1.2 Mixture 2
During the optimization cycle, several chromatographic conditions were attempted using C18 column. Various mobile phase compositions such as methanol with water, acetonitrile with water, methanol with water and glacial acetic acid, in different proportions, were tried in an isocratic mode. Using the third composition in different ratio was applicable for separation of MF and SA but with excessive peak tailing of both. Thus, introduction of acetonitrile was necessary to optimize shape and symmetry of peaks. Also, sharpness and symmetry of SA peak were optimized through switching from C18 to C8 column.
After all these attempts, it was found that a mobile phase composition of acetonitrile:methanol:water:glacial acetic acid, (60:10:30:0.1, v/v) at a flow rate of 2 mL min−1 and UV detection at 240 nm using C8 column was the optimum for the separation of MF and SA (Fig. 3a) along with the degradation products of MF in minimum runtime (Fig. 3b).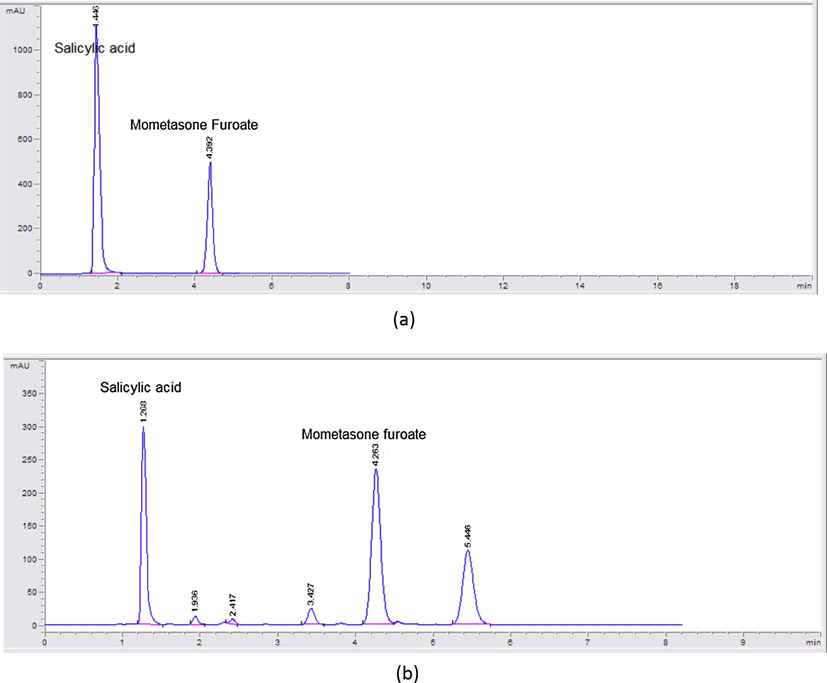
(a) HPLC chromatogram of mometasone furoate (160 μg mL−1) and salicylic acid (640 μg mL−1). (b) HPLC chromatogram of mometasone furoate (75 μg mL−1), mometasone furoate degradation products and salicylic acid (85 μg mL−1).
3.2 System suitability tests
System suitability tests are an integral part of liquid chromatographic methods in the course of optimizing the conditions of the proposed method. They are used to verify that the resolution and reproducibility were adequate for the analysis performed. The parameters of these tests are column efficiency (number of theoretical plates), tailing of chromatographic peak, repeatability as %R.S.D of peak area for six injections and reproducibility of retention as %R.S.D of retention time. Solutions of 360 μg mL−1 and 25 μg mL−1 of MF and FF, respectively, (For mixture 1) and 160 μg mL−1 and 640 μg mL−1 of MF and SA, respectively, (For mixture 2) were used for these tests. The results of system suitability tests for the proposed method are listed in Table 1.
Parameter
Mixture 1
Mixture 2
MF
FF
MF
SA
Na
5936
1289
5394
1230
Tb
1
1.05
1
0.85
K′
2.72
1.00
2.44
1.00
Resolution
9.06
12.08
%R.S.Dc of 6 injections of
Peak area
0.383
0.641
0.39
0.32
tRd (min)
0.148
1.02
0.07
0.13
3.3 Method Validation
3.3.1 Range and linearity
The linearity of the chromatographic methods for the determination of MF, FF and SA was evaluated by analysing a series of different concentrations of each drug. In this study, for mixture 1, nine and seven concentrations ranging between 10–800 μg mL−1 and 5–60 μg mL−1 for MF and FF, respectively, were chosen. For mixture 2, seven and nine concentrations ranging between 5–320 μg mL−1 and 20–1280 μg mL−1 for MF and SA, respectively, were chosen. Each concentration was analysed three times. Good linearity of the calibration curve was verified by the high correlation coefficient. The analytical data of the calibration curve including standard deviations for the slope and intercept (Sb, Sa) are summarized in Table 2.
Parameter
Mixture 1
Mixture 2
MF
FF
MF
SA
Retention time (min)
6.51
3.53
4.39
1.4
Wavelength of detection (nm)
247
214
240
240
Range of linearity (μg mL−1)
10–800
5–60
5–320
20–1280
Regression equation
Area = 59.834
Area = 106.55
Area = 26.006
Area = 15.135
Cμg mL−1 − 150.46
Cμg mL−1 + 33.166
Cμg mL−1 − 0.0746
Cμg mL−1 + 52.038
Regression coefficient (r2)
0.9998
0.9998
0.9999
0.9995
LOD (μg mL−1)
1.571
0.611
0.335
0.705
LOQ (μg mL−1)
4.762
1.852
1.016
2.136
Sb
0.301
0.676
0.0899
0.1342
Sa
131.037
24.425
12.56
101.62
Confidence limit of the slope
59.834 ± 141.208
106.55 ± 1.739
26.006 ± 0.2311
15.135 ± 0.3166
Confidence limit of the intercept
−150.46 ± 309.249
33.166 ± 62.772
0.0746 ± 32.267
52.038 ± 239.8023
Standard error of the estimation
265.293
33.969
25.256
196.016
Precision the regression
1.1
1.06
0.67
2.02
Inter-day (%R.S.D.)
0.096–0.478
0.142–0.646
0.34–0.52
0.17–0.36
Intra-day (%R.S.D.)
0.119–0.869
0.011–0.503
0.08–0.12
0.05–0.34
Drug in bulk
99.476% ± .445
100.624% ± 0.587
99.664% ± 0.941
100.08% ± 0.988
Drug in dosage form
97.81% ± 0.702
99.18% ± 0.871
100.57% ± 0.677
99.59% ± 0.345
Standard added
99.015% ± 0.728
98.552 ± 0.335
99.95% ± 0.683
100.87 ± 0.235
3.3.2 Accuracy
Accuracy of the results was calculated by % recovery of laboratory prepared mixtures of 6 different concentrations of MF and FF and 5 different concentrations of MF and SA and also by standard addition technique for Dulera® inhaler and Elicasal® ointment. The results obtained including the mean of the recovery and standard deviation are displayed in Table 2.
3.3.3 Precision
The repeatability of the method was assessed by six determinations for each of the three concentrations representing 80%, 100%, 120% for each drug, and for mixture 1, these percentages represent (280, 360 and 420 μg mL−1) for MF and (20, 25 and 30 μg mL−1) for FF. For mixture 2, these percentages represent (128, 160 and 192 μg mL−1) for MF and (512, 640 and 768 μg mL−1) for SA. The repeatability of sample application and peak area measurement for active compounds were expressed in terms of percentage relative standard deviation (%R.S.D.) and found to be less than 1% in the three concentrations. All experiments described in repeatability were repeated in three consecutive days by the same analyst to evaluate day to day ruggedness.
3.3.4 Specificity
Specificity is the ability of the analytical method to measure the analyte response in the presence of interferences. The chromatograms of MF and FF or SA in the sample solutions were found to be identical to the chromatograms obtained by the standard solution. Also, no chromatographic interference from any of the degradation products was found by mixing alkaline degraded MF solution with FF or SA and by mixing acidic degraded FF solution with MF (Figs. 2 and 3). In addition, no chromatographic interference from any of the excipients was found at the retention time of the examined drug after extraction of the active ingredient (Figs. 4 and 5). Besides, the chromatograms of the pharmaceutical formulation samples were checked for the appearance of any extra peaks. Good resolution and absence of interference from any of the degradation products resulted under all stress conditions are shown in (Figs. 6–8). So the proposed methods could be successfully applied for the routine analysis of the studied drugs in their dosage forms without any preliminary separation step. Results for determination of these drugs by the proposed method in their dosage forms along with standard addition technique are displayed in Table 2.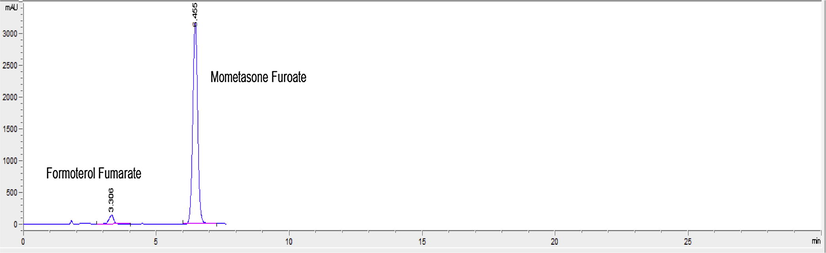
HPLC chromatogram of Dulera inhaler containing 680 μg mL−1 mometasone furoate and 17 μg mL−1 formoterol fumarate.
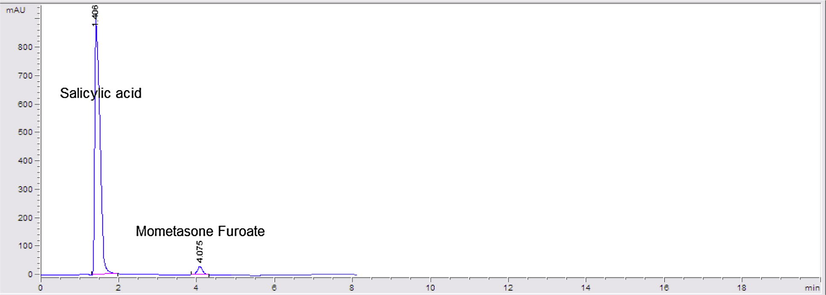
HPLC chromatogram of Elicasal ointment containing 10 μg mL−1 of mometasone furoate and 500 μg mL−1 of salicylic acid.
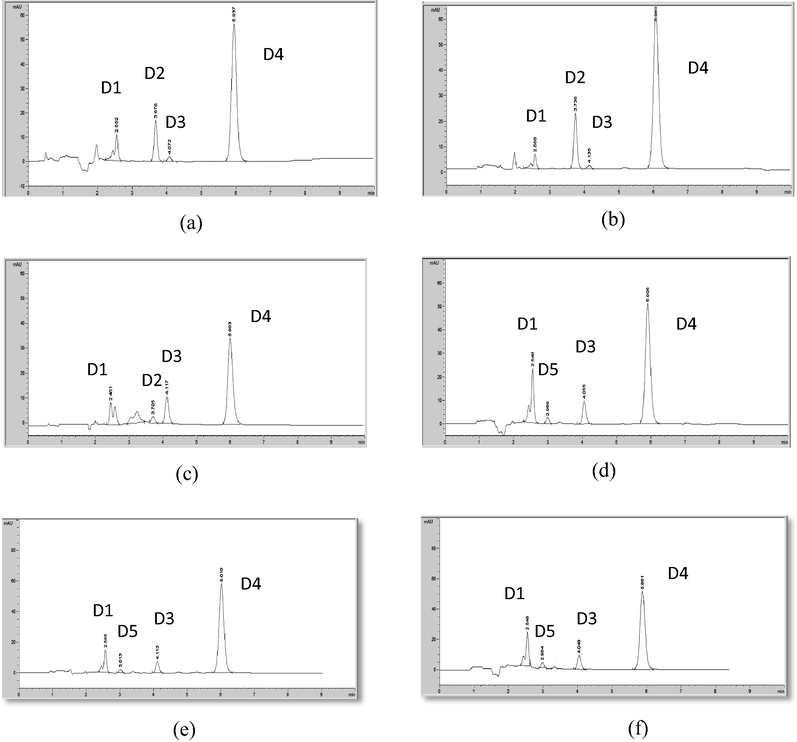
HPLC chromatogram of degraded mometasone furoate in (a) 0.1 M NaOH at RT; (b) 0.5 M NaOH at RT; (c) 1 M NaOH at RT; (d) 0.1 M NaOH at 75 °C; (e) 0.5 M NaOH at 75 °C; (f) 1 M NaOH at 75 °C.
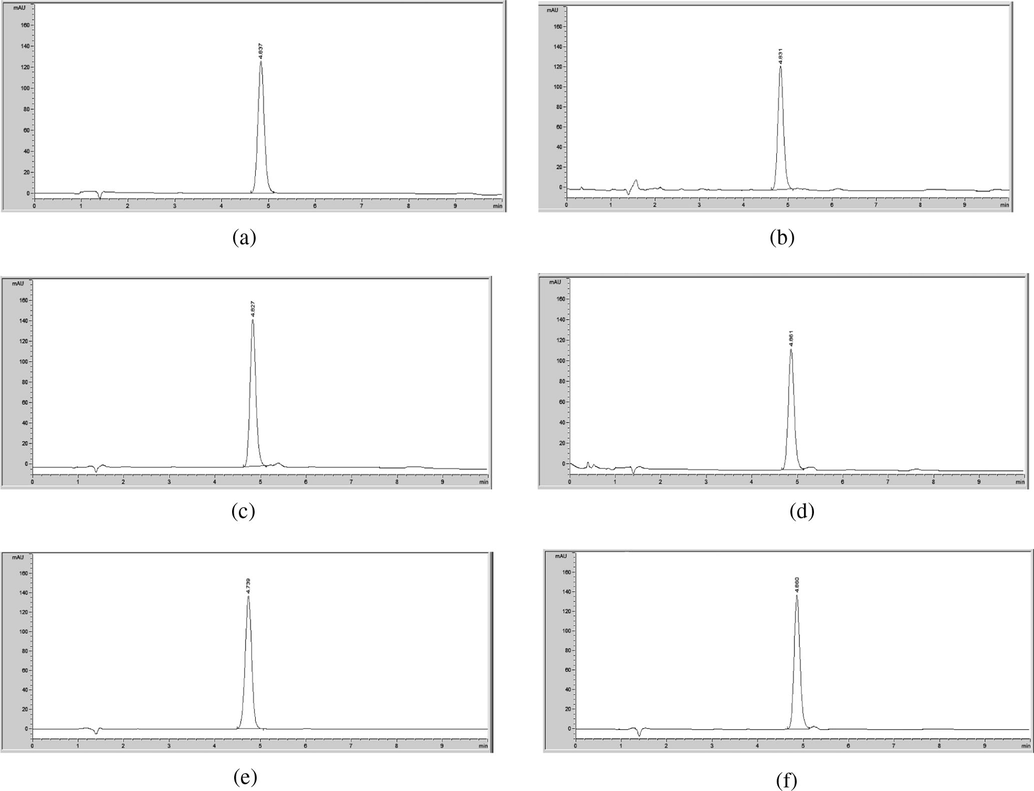
HPLC chromatogram of degraded mometasone furoate in (a) 0.1 M HCl at RT; (b) 0.5 M HCl at RT; (c) 1 M HCl at RT; (d) 0.1 M HCl at 75 °C; (e) 0.5 M HCl at 75 °C; (f) 1 M HCl at 75 °C.
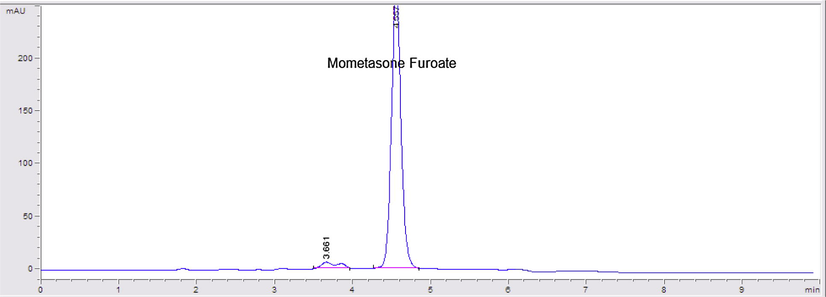
HPLC chromatogram of sunlight degraded mometasone furoate.
3.3.5 Limit of detection and limit of quantitation
Limit of detection (LOD) which represents the concentration of analyte at S/N ratio of 3 and limit of quantification (LOQ) at which S/N ratio is 10 were determined experimentally for the proposed methods and results are given in Table 2.
3.3.6 Robustness
Robustness was performed by deliberately changing the chromatographic conditions. For both mixtures, the flow rate of the mobile phases was changed by ±0.2 units. The organic strengths were varied by ±2%. These variations did not have significant effect on chromatographic resolution by the proposed LC methods.
4 Degradation behaviour of mometasone furoate
4.1 Acid- and base-induced degradation
The chromatograms of the alkaline degraded samples for mometasone furoate using different molarities of NaOH on cold, showed complete disappearance of the intact drug and appearance of additional four peaks (D1, D2, D3 & D4) at retention times of ≈2.5, 3.7, 4 and 5.9 min. (Fig. 6a–c), while using different molarities of NaOH on hot showed complete disappearance of degradation product which appear at 3.7 min (D2) and introduction of a new one at 2.9 min (D5) (Fig. 6d–f).
Acidic degradation studies showed no additional peaks, which indicates that mometasone furoate is acid stable (Fig. 7).
Verification of the products was carried out using LC–MS, and all mass spectra are illustrated in Fig. 9. The major peak [D4 (Mwt: 466.453), Retention time 5.9 min] has a molecular ion peak 466.92 (M+), which may correspond to structure (a) in Fig. 10. The mechanism of the reaction has been proposed by Foe et al. (1998). It involves a stereospecific nucleophilic attack of the 11β-hydroxyl on C-9 on departure of the 9-halogen, leading to the formation of 9β, 11β-epoxide derivative in addition to a rearrangement of the furoate moiety and dehydration on the C-17 side-chains of MF. The two other peaks [D1 (Mwt: 390.453) and D3 (Mwt: 484.453), Retention time 2.5 and 4.0 min] have the molecular ion peaks of 388.3 (M-2) and 484.12 (M+) which may correspond to structures (b) & (c), respectively. Epoxide formation was accompanied by ester hydrolysis resulted in the formation of structure (b) while structure (c) was found as a result from epoxide formation without the rearrangement of the furoate moiety and the dehydration. In addition, a fourth degradation product D2 (Mwt: 426.906), (Retention time 3.7 min with a molecular ion peak 426.82 (M+) which may correspond to structures (d) in Fig. 10 was found as a result from furoate ester hydrolysis. Structure (a) was the major degradation product.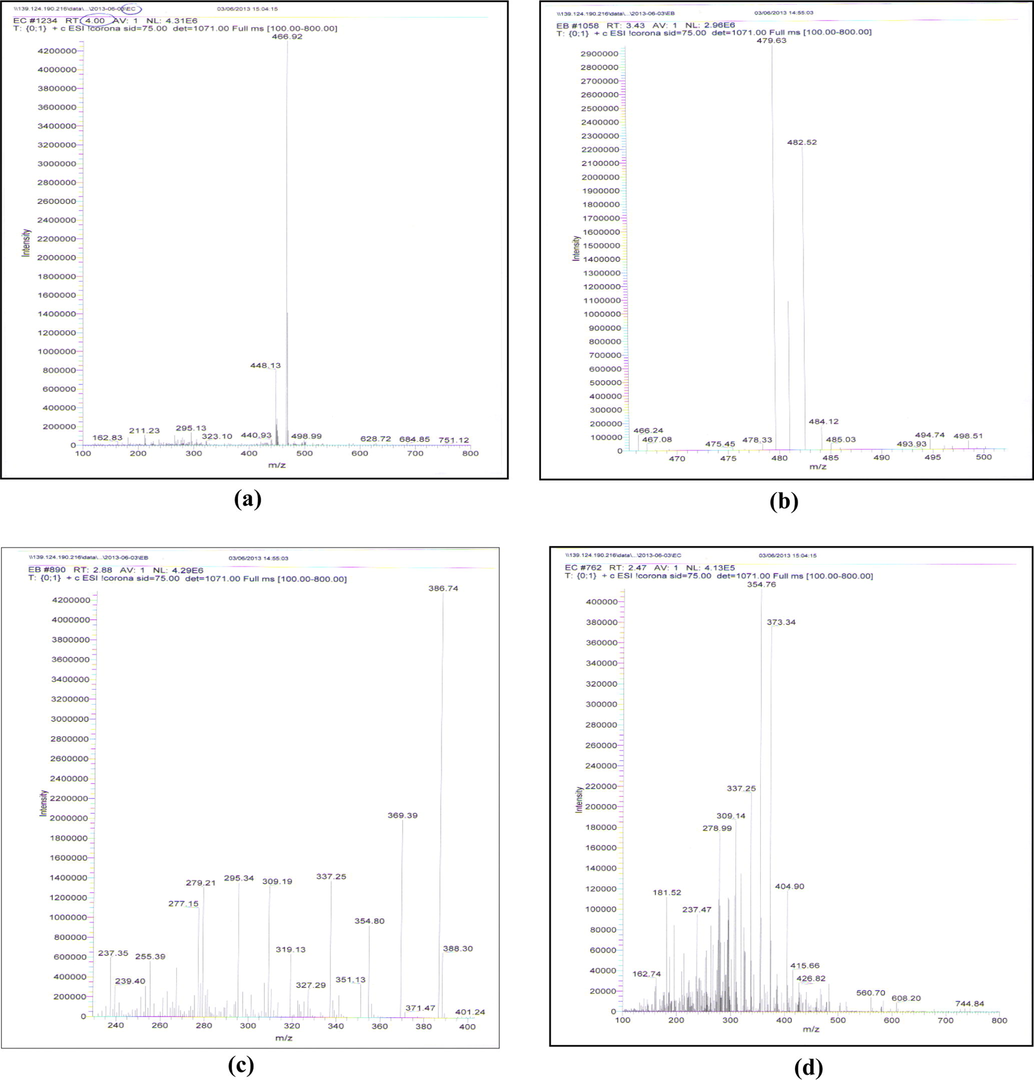
Mass spectrum of mometasone furoate alkaline degradation products.
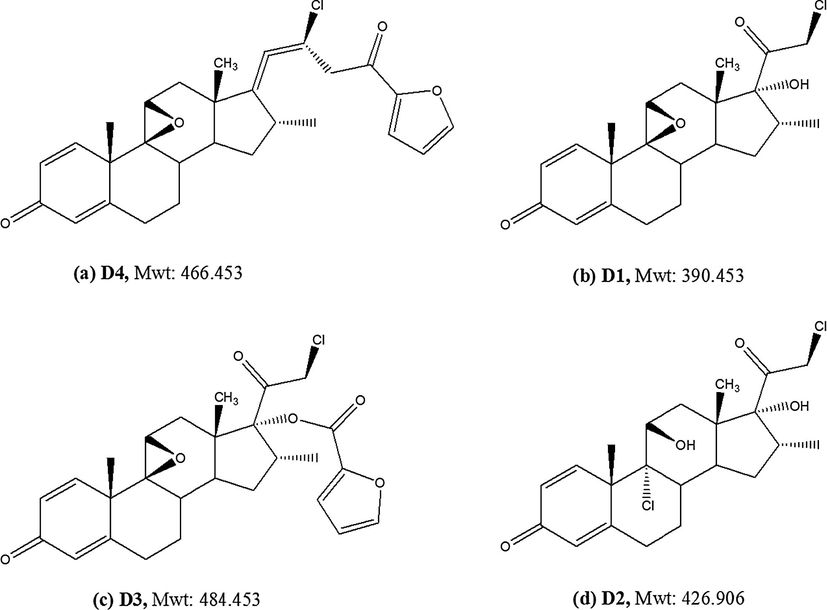
Structures of mometasone furoate alkaline degradation products.
4.2 Hydrogen peroxide-induced degradation
The sample degraded with 3% and 15% v/v hydrogen peroxide either on cold or after heating at 80 °C for 1 h showed no additional peaks. This was confirmed by good percentage recovery of the intact drug.
4.3 Thermal and photolytic degradation
The samples degraded under dry heat conditions showed no additional peaks. On the other hand, the photodegraded sample showed an additional peak D2 at retention time ≈3.7 min when drug solution was exposed to sunlight for 72 h (Fig. 8).
From the aforementioned data, the drug was found to be susceptible to base hydrolysis and photodegradation but resistant to acid hydrolysis, oxidation and dry heat degradation.
5 Statistical studies
Statistical comparison between the results of the proposed methods and those obtained by the reference methods for MF according to U.S.P 37 (USP, 2014) and for FF and SA according to BP (BP, 2014), respectively showed no significant difference. Results were compared using the Student’s t-test and F-ratio. It can be concluded that the proposed analytical methods are sufficiently accurate and precise and results are given in Tables 3 and 4.
Statistical Term
MF
FF
Reference method
HPLC method
Reference method
HPLC method
Mean
100.02
99.48
99.76
100.62
±S.D.
0.542
0.444
0.939
0.587
±S.E.
0.221
0.181
0.383
0.239
%R.S.D
0.542
0.446
0.941
0.583
n
6
6
6
6
Variance
0.294
0.197
0.882
0.345
t-value
1.890
1.905
(2.228a)
(2.228a)
F-value
1.492
2.557
(5.050a)
(5.050a)
Statistical term
MF
SA
Reference method
HPLC method
Reference method
HPLC method
Mean
100.02
99.65
99.77
100.08
±S.D.
0.542
0.950
0.845
0.988
±S.E.
0.221
0.425
0.345
0.442
%R.S.D
0.542
0.953
0.847
0.987
n
6
5
6
5
Variance
0.294
0.903
0.714
0.976
t-value
0.772
0.553
(2.260⁎)
(2.260⁎)
F-value
3.071
1.367
(5.190⁎)
(5.190⁎)
6 Conclusion
Two validated LC methods were developed for the simultaneous determination of (mometasone furoate, formoterol fumarate) and (mometasone furoate, salicylic acid) mixtures along with mometasone furoate alkaline degradation products for both mixtures and with Formoterol fumarate acidic degradation product for mixture 1. Moreover, a full study of the degradation behaviour of mometasone furoate under acid, alkali, oxidation, thermal and photolysis conditions was carried out. The drug was found to be degraded in alkaline and photolytic conditions and found to be stable to acidic, oxidation and dry heat conditions. The proposed methods could be successfully applied for the routine analysis of the studied drugs either in their pure bulk powders or in their dosage forms without any preliminary separation step.
References
- Development of a new analytical method for determination of acetylsalicylic and salicylic acids in tablets by reversed phase liquid chromatography. Brazil. J. Pharm. Sci.. 2009;45:723-727.
- [Google Scholar]
- Rapid and simultaneous determination of acetylsalicylic acid, paracetamol, and their degradation and toxic impurity products by HPLC in pharmaceutical dosage forms. Turkish J. Med. Sci.. 2008;38:167-173.
- [Google Scholar]
- Simultaneous assay determination of mometasone and tazarotene in ointment formulation. Acta Ciencia Indica. 2009;35:523-526.
- [Google Scholar]
- High performance liquid chromatography assay method for simultaneous quantitation of formoterol and budesonide in Symbicort Turbuhaler. J. Pharm. Biomed. Anal.. 2006;41:325-328.
- [Google Scholar]
- Cutaneous bioassay of salicylic acid as a keratolytic. Int. J. Pharm.. 2005;292:187-194.
- [Google Scholar]
- British Pharmacopoeia (BP). The Stationary Office on Behalf of The Medicines and Healthcare Products Regulatory Agency, London, UK, 2014.
- Deter-mination by HPLC with electrochemical detection of formoterol RR and SS enantiomers in urine. J. Liq. Chromatogr. Related Technol.. 1996;19:993-1005.
- [Google Scholar]
- Quantitative detection of inhaled formoterol in human urine and relevance to doping control analysis. J. Drug Testing Anal.. 2012;4:449-454.
- [Google Scholar]
- Forced degradation study to develop and validate stability-indicating RP-LC method for the determination of ciclesonide in bulk drug and metered dose inhalers. Talanta. 2011;87:222-229.
- [Google Scholar]
- Degradation products of beclomethasone dipropionate in human plasma. Drug Metabol. Dispos.. 1998;26:132-137.
- [Google Scholar]
- Validated stability-indicating derivative and derivative ratio methods for the determination of some drugs used to alleviate respiratory tract disorders and their degradation products. J. Drug Testing Anal.. 2011;3:306-318.
- [Google Scholar]
- Quantitative-determination of the beta-adrenoceptor stimulant formoterol in urine by gas-chromatography mass-spectrometry. J. Chromatogr. B. 1982;229:337-345.
- [Google Scholar]
- Validation and application of a screening method for beta 2-agonists, anti-estrogenic substances and mesocarb in human urine using liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom.. 2007;21:252-264.
- [Google Scholar]
- Generic sample preparation and dual polarity liquid chromatography-time-of-flight mass spectrometry for high-throughput screening in doping analysis. J. Drug Testing Anal.. 2009;1:250-266.
- [Google Scholar]
- Simultaneous estimation of Nadifloxacin and Mometasone Furoate in topical cream by HPTLC method. Der Pharma Chemica. 2010;2:25-30.
- [Google Scholar]
- Simultaneous Quantitative Determination of Formoterol Fumarate and Fluticasone Propionate by Validated Reversed-Phase HPLC Method in Metered dose inhaler. Der Pharmacia Sinica.. 2011;2(6):77-84.
- [Google Scholar]
- Bioequivalence of inhaled formoterol fumarate assessed from pharmacodynamic, safety and urinary pharmacokinetic data. Arzneimittelforschung. 2000;50:559-563.
- [Google Scholar]
- A fast liquid chromatographic/mass spectrometric screening method for the simultaneous detection of synthetic glucocorticoids, some stimulants, anti-estrogen drugs and synthetic anabolic steroids. Rapid Commun. Mass Spectrom.. 2006;20:3465-3476.
- [Google Scholar]
- Reversed-phase Chromatography of amines, phenols, and metal cations on silica layers imprignated with tributyl phosphate, using surfactant-mediated mobile phases. ACTA CHROMATOGRAPHICA. 2003;13
- [Google Scholar]
- Validation of high-performance liquid chromatography assay for quantification of formoterol in urine samples after inhalation using UV detection technique. J. Chromatogr. B. 2007;850:31-37.
- [Google Scholar]
- Set-up of a method using LC-UV to assay mometasone furoate in pharmaceutical dosage forms. Quimica Nova. 2012;35:818-821.
- [Google Scholar]
- Generic sample preparation combined with high-resolution liquid chromatography-time-of-flight mass spectrometry for unification of urine screening in doping-control laboratories. Anal. Bioanal. Chem.. 2010;396:2583-2598.
- [Google Scholar]
- Q1A (R2), 2003. Stability testing of new drug substances and products, International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use, Geneva. Commission of the European Communities.
- Mass balance and metabolism of [H-3] formoterol in healthy men after combined IV and oral administration mimicking inhalation. Drug Metabol. Dispos.. 1999;27:1104-1116.
- [Google Scholar]
- Flow-through UV spectrophotometric sensor for determination of (acetyl) salicylic acid in pharmaceutical preparations. Int. J. Pharm.. 2001;216:95-104.
- [Google Scholar]
- Spectrophotometric determination of salicylic acid in pharmaceutical formulations using copper (II) acetate as a colour developer. Analyst. 1985;110:739-741.
- [Google Scholar]
- A sensitive liquid chromatography–tandem mass spectrometry method for the quantification of mometasone furoate in human plasma. J. Chromatogr. B. 2005;819:175-179.
- [Google Scholar]
- A new spectrophotometric method for quantitative multicomponent analysis resolution of mixtures of salicylic and salicyluric acids. Talanta. 1990;37:347-351.
- [Google Scholar]
- Validation of a RP-HPLC method for the assay of formoterol and its related substances in formoterol fumarate dehydrate drug substance. J. Pharm. Biomed. Anal.. 2003;33:935-945.
- [Google Scholar]
- Chiral HPLC analysis of formoterol stereoisomers and thermodynamic study of their interconversion in aqueous pharmaceutical formulations. J. Pharm. Biomed. Anal.. 2009;49:632-637.
- [Google Scholar]
- Development and validation of a ultra high performance liquid chromatography–tandem mass spectrometric method for the direct detection of formoterol in human urine. J. Pharm. Biomed. Anal.. 2012;70:471-475.
- [Google Scholar]
- Simple RP-HPLC method for the simultaneous quantitation of chlorocresol, mometasone furoate, and fusidic acid in creams. J. Chromatogr. Sci.. 2009;47:178-183.
- [Google Scholar]
- Simultaneous determination of impurities from a combination metered dose inhaler consisting of a long acting beta 2 agonist and an inhaled corticosteroid by RP-HPLC. Int. J. Pharma World Res.. 2012;3
- [Google Scholar]
- High-performance liquid chromatographic analysis of mometasone furoate and its degradation products application to in vitro degradation studies. J. Pharm. Biomed. Anal.. 2001;26:313-319.
- [Google Scholar]
- Degradation kinetics of mometasone furoate in aqueous systems. Int. J. Pharm.. 2003;259:129-141.
- [Google Scholar]
- Liquid chromatography/electrospray ionization tandem mass spectrometric screening and confirmation methods for beta (2)-agonists in human or equine urine. J. Mass Spectrom.. 2003;38:1197-1206.
- [Google Scholar]
- A Rapid, Stability-Indicating RP-HPLC Method for the Simultaneous Determination of Formoterol Fumarate, Tiotropium Bromide, and Ciclesonide in a Pulmonary Drug Product. Scientia Pharmaceutica. 2012;80:591-603.
- [Google Scholar]
- The United States Pharmacopoeia (USP 37), 2014. National Formulary (NF 32), Asian edition, The United States Pharmacopoeal Convention, vol. 2 and 3.
- Analytical methodology for the detection of beta(2)-agonists in urine by gas chromatography–mass spectrometry for application in doping control. Anal. Chim. Acta. 2000;418:79-92.
- [Google Scholar]
- Development of an orthogonal method for mometasone furoate impurity analysis using supercritical fluid chromatography. J. Chromatogr. A. 2011;1218:2311-2319.
- [Google Scholar]







