Translate this page into:
Genus Tabebuia: A comprehensive review journey from past achievements to future perspectives
⁎Corresponding author. Marwa.taher@nub.edu.eg (Marwa A. Taher)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Tabebuia is the largest genus of Bignoniaceae. It is commonly recognized as a therapeutic alternative by rural or remote populations. The results of ethnopharmacological studies indicate the potential use of these plants to treat a large variety of diseases. Tabebuia species have been used empirically as anti-inflammatory, anticancer and antimicrobial agents in rural areas of Colombia, Bolivia, Brazil and other Latin-American countries. Due to its great importance in traditional and modern medicine, several Tabebuia species have been phytochemically investigated and the potential toxicity of these plants has also been discussed. Variable phytoconstituents are isolated from genus Tabebuia, among which; naphthoquinones and phenolic compounds are the most prevalent. The present review aims to provide a critical and comprehensive details about the traditional uses, phytochemical, pharmacological and toxicological properties of twenty Tabebuia species. In addition, the reported pharmaceutical documents that support the importance of Tabebuia species in traditional systems, are provided. On the other hand, the review also clarify the remaining gaps and thus supply a basis for further investigations. Although recent experimental evidence confirms the pharmacological interest of this genus, further bioguided isolation studies are required to understand the role of a particular compound in the observed biological activities.
Keywords
Bignoniaceae
Tabebuia
Traditional uses
Phytochemical content
Naphthoquinones
Biological activities
1 Introduction
Traditional medicine represents the knowledge, skills and also the practices that depend on beliefs or even experiences belong to specific cultures, for maintenance of health and for prevention, diagnosis or treatment of different illness (Benzie and Wachtel-Galor, 2011). According to World Health Organization, medicinal plants still represent the best source of different drugs (Krishnan, 2018). Plants belonging to family Bignoniaceae are commonly employed in traditional medicinal systems (Raju et al., 2011).
Bignoniaceae comprises around 116–120 genera and 650–750 species, among them 12 genera and 35 species exist in China, where 21 species are endemic (Zhang and Santisuk, 1998). Mabberley, divided this family into seven tribes mainly distributed in the tropical and sub-tropical parts of the world (Mabberley, 2008; Madhukar et al., 2012). Bignoniaceae gets its name from genus Bignonia and is also commonly known as trumpet vine or trumpet creeper family (Choudhury et al., 2011), and (Deka et al., 2013). Tecoma, Catalpa, Tabebuia and Jacaranda are some of the well-known members of the family. This family is extensively used in traditional medicine in a number of countries, including Bangladesh (Rahmatullah et al., 2010).
Tabebuia is the largest and most important genus of Bignoniaceae (Grose and Olmstead, 2007; Ferraz-Filha et al., 2017). Antonio Gomes was the first taxonomist used the word “Tabebuia” in the literature in 1803 and then the word used as a generic name by de Candolle in 1838 (Gentry, 1969). The word “Tabebuia” comes from the contraction of “tacyba bebuya” meaning “ant wood” referring to ants living in the hollow twigs of some Tabebuia species (Gentry, 1970). Tabebuia, is a large flowering trees genus that include about 100 species in tropical and subtropical areas (Jimenez-Gonzalez et al., 2013; Gentry, 1970; Bussmann, 2018). Tabebuia species are widely used in traditional medicine in treatment of syphilis, malaria, cutaneous infections, stomach disorders, cancer, inflammation, pain, bacterial and fungal infections, anxiety, poor memory, irritability, depression, and for treating diabetes, prostatitis, constipation and allergies (Corrêa and de Azeredo, 1984; Park et al., 2006; Sichaem et al., 2012; Cragg et al., 2014; Ferreira-Júnior et al., 2015; Regalado et al., 2017; Ferraz-Filha et al., 2017). Several studies stated the biological efficacy of secondary metabolites isolated from some members of this genus, e.g. lapachol, used in clinical studies as adjuvant in cancer therapy (Rao et al., 1968; Santana and Silva, 1980; Barbosa-Filho et al., 2004).
Bark extract of Tabebuia species is known as “taheebo”, “lapacho”, “pau d’arco” or “ipê” and their active components include napthoquinones, quinines, furanonapthoquinones, benzoic acid, cyclopentenes dialdehydes and flavonoids (Sharma et al., 1988; Koyama et al., 2000a).
Figures are the simplest way to translate the huge recorded data into informative points. In addition, the aim of the present study is not only to represent the recorded data, but also to explore all the defects and gaps that needed further future investigation. So, we used these statistical figures and information to explore what could the researchers work about in future investigation regarding this genus.
2 Traditional uses of some Tabebuia species
Portuguese and Spanish population used the names of Pau d’arco and lapacho to identify about 26 species of shrubs and trees belong to Tabebuia. These species are indigenous to the American tropics from Mexico to southern South America, the majority of species are found in Brazil and neighboring countries. For curative purposes, native people preferred the inner bark, although the heartwood is more potent. Leaves and flowers are less commonly used (Lewis et al., 2005). In the early 1980s, d’arco became known in North America and Europe. The infusion and decoction of the bark or wood was ingested regularly by at least one million people (Jones, 1995; Lewis et al., 2005). In 1995, d’arco is listed among the top 25 selling herbs in the United States, representing 1.7% of herb sales in United States in 1996 (Arenas, 1977; Lewis et al., 2005). Old native populations used Tabebuia extracts as an antidote for snake bites (Rizzini et al., 1988; Ruppelt et al., 1991; Martz, 1992). Table 1 lists the reported traditional uses of different Tabebuia species and the region where they are employed.
Species name
Common name
Region
Traditional uses
Ref.
T. avellanedae Lorentz ex Griseb
‘‘divine tree’’
Tropical rain forests of northeastern Brazil, Central and Latin American
Folk treatment of cancer
(Rao and Kingston, 1982; Lubeck, 1998; Alonso, 2004; Zhang et al., 2015)(Hashimoto, 1996; Lee et al., 2012)
For treating eczema, psoriasis, fungal infections, and even skin cancers.
(Suo and Yan, 2016).
For treatment of ulcers, bacterial and fungal infections
(Goel et al., 1987; Guiraud et al., 1994; Schultes and Raffauf, 1990; de Miranda et al., 2001; Twardowschy et al., 2008)
For treating malaria, leishmaniasis, fevers, fungal, bacterial infections and syphilis
(Schultes and Raffauf, 1990; Duke, 1985; Duke and Vasquez, 1994)
For gastrointestinal disturbances, inflammation and tropical diseases
(Rodrigues, 2006)
To treat colds, coughs and flu
(Grenand et al., 2004)
To treat uterine cancer and liver cirrhosis
(Schunke, 1993)
Anticancer
(Plowman, 1967)
Astringent and as a treatment of cutaneous ulcers
(Jones, 1995; Bussmann, 2018)
T. impetiginosa (Mart. ex DC) (T. avellanedae Lorentz ex Griseb, synome)
Pau d’arco, ipê roxo, taheebo, red (or purple) lapacho (Luebeck, 1999; Mowrey, 2001; Taylor, 2005).
Amazon rain forest, Argentina, Bolivia, Brazil, Colombia, Ecuador, French Guinea,Paraguay, Perú, Surinam, Trinidad, Tobago, and Venezuela.
To treat diabetes, malignant tumors, leukemia, other cancers, anemia, and Parkinson’s disease
(Lewis et al., 2005)
Anti-inflammatory and for treatment of fungal infections
(Taylor, 2005; Castellanos et al., 2009)
T. aurea (Manso) S. Moore
“craibeira”, “paratudo” and “ipê-amarelo”
South America (from Venezuela to Argentina)
Anti-inflammatory
(Nunes et al., 2003; Reis et al., 2014; Malange et al., 2019)
Anti-inflammatory and for treatment of influenza
(Agra, 1996)
Anticancer
(Bandoni et al., 1972; Barbosa-Filho et al., 2004)
For treating snake bites
(Pott and Pott, 1994; Agra et al, 2007; Hajdu and Hohmann, 2012)
T. argentea Britt (T. aurea (Manso) S. Moore synome)
Silver-trumpet tree, ‘‘craibeira’’, ‘paratudo’’, and ‘‘ipê-amarelo’’
South America (from Venezuela to Argentina) and India
Anti-inflammatory and for treating influenza
(Daulatabad and Hosamani, 1991; Agra, 1996; De Abreu et al., 2014)
T. chrysotricha (Mart. ex DC.) Standley
‘ipe”-amarelo’ or ‘ipe”’
Brazil
Analgesic, antitumor agent, Antidiabetic and for treatment of peptic ulcer
(Oga and Sekino, 1969; Grazziotin et al., 1992).
T. incana A.H. Gentry
Amazonian tree, “ipê amarelo” and “pau d’arco”
Amazon
Anti-inflammatory, antimalarial, anticancer and for the treatment of kidney and liver disorders
(da Silva et al., 1977; de Oliveira et al., 1993).
T. heptaphylla (Vell. Conc.)
“tayï pytá” or “lapacho”
Eastern Paraguay
Anti-inflammatory, anticancer and for treating wounds
(Gupta, 1995; Bernal and Correa, 1989; Ortega Torres et al., 1989; Schmeda-Hirschmann and Papastergiou., 2003).
T. ochracea ssp. neochrysantha (A. Gentry)
“To hua ri”, “Vero”, and “Cañahuate”
Tropical America, from El Salvador to northwest Venezuela and Colombia
Antimalarial and for healing ulcers
(Gentry, 1982; Bernal and Correa, 1989; Pérez et al., 1997)
T. rosea (Bertol.) DC.,
“Pink Trumpet Tree”
Guatemala, Costa Rica, Colombia
Antipyretic and for treating eyes infections
(Gentry, 1992).
Antimalaria and for treatment of rabies, fever, colds, headache, and snake bites
(Morton, 1981; lewis et al., 2005)
For treating throat ailments, fever, and as an astringent
(Garcı́a Barriga, 1975; lewis et al., 2005)
Antimicrobial activity
(Binutu and Lajubutu, 1994)
Astringent, anti-inflammatory, antimicrobial, diuretic, and laxative
(de Almeida et al., 1990; Arenas, 1987; Ramalakshmi and Muthuchelian, 2011; Sichaem et al., 2012)
Antimalaria and anticancer (uterine cancer) and for treatment of anaemia, constipation, fever, pain and tonsillitis
(Madhumitha et al., 2015)
T. billbergii
guayacán
Amazon
Antimicrobial, for treatment of fever, syphilis, malaria, trypanosomiasis, stomach and bladder disorders, and for tumors
(Gómez-Estrada et al., 2012)
3 Phytochemical studies
To date, about 292 chemical constituents have been isolated from Tabebuia, among which, naphthoquinones are considered the main constituents. Other reported classes of secondary metabolites are tannins, flavonoids, alkaloids, and iridoids (Ferreira-Júnior et al., 2015). Several studies provide the preliminary phytochemical screening as a first step for chemical classes’ identification (Jimenez-Gonzalez et al., 2018; Hemamalini et al., 2012a; Sathiya and Muthuchelian, 2008; Madhumitha et al., 2015; da Silva et al., 2017; Mota and Duarte, 2015).
For best knowledge it’s valuable to know that some reported studies consider T. avellanedae Lorentz ex Griseb and T. impetiginosa Mart. ex DC are synonymous to each other (Fujimoto et al., 1991; Castellanos et al., 2009; Bussmann, 2018).
Table 2 summarizes up all reported data about the phytochemical composition of Tabebuia species. The reported phytoconstituents copmrise 66 naphthoquinones, 73 flavonoids and phenolic compounds, 26 lignans, 8 coumarins, 31 aldehydes, acids and esters, 30 hydrocarbons, triterpenoids and sterols, 54 irridoids and 4 carotenoids. Each phytochemical is numbered from (1–292) and cited in the text. The structures of chemical constituents are illustrated in Figs. 1–8 according to the chemical classes.
Metabolite classes
Compound name
Cpd. no.
Species name
Part used
References
1. Naphthoquinones
(Naphthofurandione derivatives)
1) 2-ethyl-naphtho[2,3- b]furan-4,9-dione.
2) 2-isopropyl-naphtho[2,3- b]furan-4,9-dione.
3) 2-ethyl-5-hydroxynaphtho[2,3-b]furan-4,9-dione.1
T. serratifolia
Trunk wood
(Vidal-Tessier et al., 1988)
1, 2
T. avallandae
Inner bark
(Steinert et al., 1996)
3
T. incana
Trunk wood
(de Oliveira et al., 1993)
(Acetyl derivatives of naphthofurandione)
4) 2-acetyl-naphtho[2,3-b]furan-4,9-dione.
5) 5-hydroxy-2-acetyl-naphtho[2,3-b]furan-4,9-dione.
6) 8-hydroxy-2-acetyl-naphtho[2,3-b]furan-4,9-dione.
7) 6- methoxy-2-acetyl-naphtho[2,3-b]furan-4,9-dione.
8) 7-methoxy-2-acetyl-naphtho[2,3-b]furan-4,9-dione.
9) 8-methoxy-2-acetyl-naphtho[2,3-b]furan-4,9-dione.
10) 7-hydroxy-8-methoxy-2-acetyl-naphtho[2,3-b]furan-4,9-dione.
11) 7-methoxy-8- hydroxy-2-acetyl-naphtho[2,3-b]furan-4,9-dione.
12) 7,8-dimethoxy-2-acetyl-naphtho[2,3-b]furan-4,9-dione.4
T. avellanedae
T. impetiginosa (T. avellanedae synome)Inner bark
Heart wood
Bark(Zhang et al., 2015)
(Steinert et al., 1996)
(Koyama et al., 2000a; Girard et al., 1988)
T. chrysantha
Bark
(Girard et al., 1988)
T. cassinuides
Stem bark
(Rao and Kingston, 1982)
T. palmeri
Stem
(Sakhuja et al., 2014)
T. rosea
Bark
(Girard et al., 1988)
4, 5, 6
T. avellanedue
Stem bark
(Wagner et al., 1989)
T. barbata
Bark
(de Saizarbitoria Colman et al., 1997)
4, 6
T. avellanedae
Inner bark
(Steinert et al., 1996)
4, 7, 9, 12
T. ochracea
Trunk wood
(Zani et al., 1991)
4, 8, 11
T. ochracea ssp. neochrysanta
Inner stem bark
(Díaz and Medina, 1996)
4, 9, 10, 12
T. Billbergii
Inner bark
Inner bark and trunk wood(Gómez-Estrada et al., 2012)
11
T. ochracea ssp. neochrysanta
Inner stem bark
(Pérez et al., 1997)
(1ꞌ-hydroxyethyl derivatives of naphthofuran- dione)
13) 2-(1ꞌ-hydroxyethyl naphtho[2,3-b]furan-4,9-dione.
14) 5-hydroxy-2-(1ꞌ-hydroxyethyl-naphtho[2,3-b]furan-4,9-dione.
15) 8-hydroxy-2-(1ꞌ-hydroxyethyl-naphtho[2,3-b]furan-4,9-dione.
16) 5,8-dihydroxy-2-(1ꞌ-hydroxyethyl-naphtho[2,3-b]furan-4,9-dione.
17) 6-methoxy-2-(1ꞌ-hydroxyethyl-naphtho[2,3-b]furan-4,9-dione.
18) 7-methoxy-2-(1ꞌ-hydroxyethyl-naphtho[2,3-b]furan-4,9-dione.
19) 8-methoxy-2-(1ꞌ-hydroxyethyl-naphtho[2,3-b]furan-4,9-dione.
20) 7-methoxy-8-hydroxy-2-(1ꞌ-hydroxyethyl-naphtho[2,3-b]furan-4,9-dione.
21) 7,8-dimethoxynaphtho-2-(1ꞌ-hydroxyethyl-naphtho[2,3-b]furan-4,9-dione.13
T. chrysantha
Bark
(Girard et al., 1988)
13, 14
T. rosea
Bark
(Girard et al., 1988)
13, 14, 15
T. avellanedue
Inner bark
(Wagner et al., 1989)
T. impetiginosa
(T. avellanedae, synome)Bark
(Fujimoto et al., 1991; Girard et al., 1988; Koyama et al., 2000a)
T. cassinoides
Stem bark
(Rao and Kingston,1982)
13, 15
T. avallandae
Inner bark
(Steinert et al., 1995; Steinert et al., 1996)
13, 18, 19, 21
T. ochracea
Trunk wood
(Zani et al., 1991)
14
T. rosea
Roots
(Sichaem et al., 2012)
T. chrysotricha
Wood
(Grazziotin et al., 1992)
14, 15
T. avellanedae
Inner bark
(Yamashita et al., 2009)
14, 15, 16
T. ochracea ssp. neochrysanta
Stem bark
(Pérez et al., 1997)
14, 20
T. avellanedae
Inner bark
(Zhang et al., 2015)
T. incana
Trunk wood
(de Oliveira et al., 1993)
15
T. barbata
Bark
(de Saizarbitoria Colman et al., 1997)
16, 18, 20
T. ochracea ssp. neochrysanta
Bark
(Díaz and Medina, 1996)
22) 2-(1,2-dihydroxy-1-methyl-ethyl)-5-hydroxy-naphtho[2,3- b]furan-4,9-dione.
22
T. avellanedae
Inner bark
(Zhang et al., 2015)
23) 2-(1′-methylethenyl)-5-hydroxynaphtho [2,3-b]furan-4,9-dione
23
T. rosea
Root
(Sichaem et al., 2012)
24) Lapachol
24
T. avellanedae
Inner bark and Heart wood
(Yamashita et al., 2009; Steinert et al., 1995; wagner et al., 1989; Steinert et al., 1996; Jeon et al., 2011)
T. impetiginosa
Inner bark
(Park et al., 2006)
T. aurea
Stem bark
(Barbosa-Filho et al., 2004)
T. barbata
Bark
(de Saizarbitoria Colman et al., 1997)
T. billbergii
Trunk wood
(Gomez Estrada et al., 2012)
T. chrysantha
Heart wood
(Burnett and Thomson, 1968)
T. chrysotricha
Wood
(Grazziotin et al., 1992)
T. guayacan
Bark
(Manner et al., 1974)
T. heptaphylla
Trunk wood
(Schmeda-Hirschmann and Papastergiou, 2003)
T. incana
Trunk wood
(Oliveira et al., 1990)
T. ochracea
Trunk wood
(Zani et al., 1991)
T. rosea
Roots
Heart wood(Joshi et al., 1977; Sichaem et al., 2012)
(Joshi et al., 1973; Girard et al., 1988)
T. palmeri
Stem
Wood(Sakhuja et al., 2014)
(Villegas et al., 1995)
T. pentaphylla
Stem bark
Heart wood
Leaves and heart wood(Prakash and Singh, 1980)
(Rohatgi et al., 1983)
(Prakash and Singh, 1981)
T. serratifolia
Trunk wood
(Oliveira et al., 1999 andVidal-Tessier et al., 1988)
25) Lapachol methylether.
26) Desoxy-lapachol
27) Menaquinone-125, 26, 27
T. avellanedae
Heart wood
(Steinert et al., 1995; Steinert et al., 1996)
28) α-Lapachone
29) Rhinacantin A28
T. avellanedae
Heart wood
(Steinert et al., 1996)
T. chrysantha
Heartwood
(Burnett and Thomson, 1968)
T. guayacan
Bark
(Manner et al., 1974)
T. pentaphylla
Heart wood
(Rohatgi et al., 1983)
T. serratifolia
Trunk wood
(Vidal-Tessier et al., 1988)
28, 29
T. heptaphylla
Trunk wood
(Schmeda-Hirschmann and Papastergiou, 2003)
30) Dehydro-α-Lapachone
30
T. avellanedae
Stem bark
Inner bark
Inner and heart wood(Wagner et al., 1989)
(Steinert et al., 1995)
(Steinert et al., 1996)
T. chrysantha
Heartwood
(Burnett and Thomson, 1968)
T. chrysotricha
Wood
(Grazziotin et al., 1992)
T. guayacan
Bark
(Manner et al., 1974)
T. heptaphylla
Trunk wood
(Schmeda-Hirschmann and Papastergiou, 2003)
T. palmeri
Wood
(Villegas et al., 1995)
T. pentaphylla
Heart wood
(Rohatgi et al., 1983)
T. rosea
Heartwood
Roots(Joshi et al.,1973; Girard et al., 1988)
(Joshi et al.,1977)
T. serratifolia
Trunk wood
(Oliveira et al., 1999; Vidal-Tessier et al., 1988)
31) Dehydro iso-α-lapachone
32) 5-hydroxydehydro- iso-α-lapachone.31
T. avellanedae
Inner bark
(Steinert et al., 1995)
T. heptaphylla
Trunk wood
(Schmeda-Hirschmann and Papastergiou, 2003)
T. incana
Trunk wood
(de Oliveira et al., 1993)
T. pentaphylla
Heart wood
(Rohatgi et al., 1983)
T. rosea
Heart wood
Root(Joshi et al., 1973)
(Joshi et al., 1977)
32
T. rosea
Root
(Sichaem et al., 2012)
31, 32
T. avellanedae
Stem bark
(Wagner et al., 1989)
33) 2,3-dihydro-2-(2‘-methylethenyl) naphtho[2,3-b]furan-4,9-dione).
33
T. avallandae
Inner bark
(Steinert et al., 1996)
34) Stenocarpone B
35) Avicequinone A34, 35
T. heptaphylla
Trunk wood
(Schmeda-Hirschmann and Papastergiou, 2003)
36) β-Lapachone
37) Stenocarpoquinone A
36
T. avellanedae
Heartwood
Inner bark(Steinert et al., 1995; Steinert et al., 1996)
(Yamashita et al., 2009)
T. chrysantha
Stem
(Panda et al., 2019)
T. guayacan
Bark
(Manner et al., 1974)
T. pentaphylla
Heart wood
(Rohatgi et al., 1983)
37
T. heptaphylla
Trunk wood
(Schmeda-Hirschmann and Papastergiou, 2003)
36, 37
T. chrysanrha
Heart and sapwood
(Burnett and Thomson,1968)
38) Lapachenol
38
T. avallandae
Heart wood
(Steinert et al., 1996)
T. chrysantha
Heart wood
and sap wood(Burnett and Thomson,1968)
T. heptaphylla
Trunk wood
(Schmeda-Hirschmann and Papastergiou, 2003)
T. incana
Trunk wood
(de Oliveira et al., 1993)
T. palmeri
Wood
(Villegas et al., 1995)
(lapachenole derivatives)
39) Dihydro-lapachenole
40) Nordihydro-lapachenole
41) 2,2-dimethyl-3-hydroxy-3,4-dihydro-6-methoxy-4H-naphtho[1,2-b]pyran.
42) 2,2-dimethyl-3α,4β-dihydroxy-3,4-dihydro-6-methoxy-4H-naphtho [1,2-b]pyran.
43) 2,2-dimethyl-3-hydroxy-3α,4β-dihydro-4-oxo-6-methoxy-4H-naphtho[1,2-b]pyran.39, 40
T. chrysantha
Heart woodand sap wood
(Burnett and Thomson, 1968)
41, 42, 43
T. heptaphylla
Trunk wood
(Schmeda-Hirschmann and Papastergiou, 2003)
(Anthraquinone derivatives)
44) 1-hydroxyanthraquinone.
45) 1-methoxyanthraquinone.
46) 2-methylanthraquinone.
47) 2-hydroxymethylanthraquinone.
48) 2-acetoxymethylanthraquinone.
49) 2-hydroxy-3-methyl-anthraquinone.
50) l-hydroxy-2-methyl- anthraquinone.
51) Anthraquinone-2-carboxylic acid44, 45, 46, 47, 48, 51
T. avallandae
Heart wood
(Steinert et al., 1996)
47, 51
T. impetiginosa
Inner bark
(Park et al., 2006)
49, 50
T. chrysantha
Heart wood
(Burnett and Thomson, 1968)
(Naphthalene derivatives)
52) 2,4-dihydroxy-3-(2,3-dihydroxy-3-methyl-1-oxobutyl)-1-methoxynaphthalene.
53) (1–methoxy-naphthalene)
54) 3,5-dihydroxy-3-methyl-N-(1-(naphthalen-1-yl) ethyl)pentanamide
55) 2-hydroxynaphthalene-1,4-dione.
56) 2-((dimethylamino)methyl)-3 methoxy-naphthalene-1,4-dione.52, 53
T. heptaphylla
Trunk wood
(Schmeda-Hirschmann and Papastergiou, 2003)
53
T. chrysantha
Sap wood
(Burnett and Thomson, 1968)
54
T. avallandae
Bark
(Zhang et al., 2014)
55, 56
T. chrysantha
Stem
(Panda et al., 2019)
(Naphthofuran derivatives)
57) 2,3-dihydro-2-(1-hydroxy-1-methylethyl)-3,4,9-trihydroxynaphtho [2,3-b] furan.
58) 4,9-dihydroxynaphtho[2,3-b] furan.57, 58
T. heptaphylla
Trunk wood
(Schmeda-Hirschmann, and Papastergiou, 2003)
59) 9-hydroxy-3-methylnaphto[2,3-b]pyran-2,5,10-trione
59
T. impetiginosa
Stem bark
(Koyama et al., 2000a)
60) Dehydrotectol
61) Tetrahydrotectol
62) Dimethyl ether tetrahydrotectol60
T. pentaphylla
Root bark
Stem bark
Leaves and heart wood(Prakash and Garg, 1980)
(Prakash and Singh, 1980)
(Prakash and Singh, 1981)
T. rosea
Heart wood
Root(Joshi et al., 1973)
(Joshi et al., 1977)
60, 61, 62
T. chrysantha
Heart wood
(Burnett and Thomson, 1968)
63) Tecomaquinone I
64) Tecomaquinone II
65) Tecomaquinone III
66) Tabebuin63
T. incana
Trunk wood
(de Oliveira et al., 1993)
63, 64, 65
T. pentaphylla
Heart wood
(Sharma et al., 1988)
65, 66
T. rosea
Heartwood
(Khandelwal and Singh, 2008)
2. Flavonoid and phenolics
67) Kaempferol
68) Quercetin
69) Luteolin67, 68
T. pentaphylla
Leaves
(Bishay et al., 1987)
67, 68, 69
T. argentea
Flowers
(Dixit and Srivastava, 1992)
70) Kaempferol 3-O-b-D-glucopyranoside
71) Kaempferol 3-O-rutinoside
72) kaempferol 3-O-(2′'-α-methyl p-coumaryl)-β-D-glucoside70
T. ochracea
Leaves
(Blatt et al., 1998)
70, 71
T. argentea Britt.
Leaves
(De Abreu et al., 2014)
72
T. rosea
Flowers
(Senthamilselvi et al., 2016)
73) Quercetin 3-O-b-D-glucopyranoside
74) Quercetin 3-O-sambubioside
75) Quercetin 3-O-robinobioside
76) Quercetin-3-O-galactoside
77) 3-O-diglycoside of quercetin based on galactose and rhamnose.73 or 76
T. ochracea
Leaves
(Blatt et al., 1998)
73, 74, 75
T. argentea Britt.
Leaves
(De Abreu et al., 2014)
73, 76, 77
T. caraiba
Leaves
(Blatt et al., 1996) and (Blatt et al., 1998)
78) kaempferol-3-O-diglucoside
79) Quercetin-3-O-diglucoside78, 79
T. pentaphylla
Leaves
(Bishay et al., 1987)
80) Luteolin-7-O-glucoside
81) 6-Hydroxyluteolin
82) 6-OH-luteolin-7-O-glucoside80, 81
T. caraiba
Leaves
(Blatt et al., 1996) and (Blatt et al., 1998)
80, 82
T. ochracea
Leaves
(Blatt et al., 1998)
82
T. caraiba
Leaves
(Blatt et al., 1998)
83) Cyanidin-3-rutinoside
84) Cyanidin-3-rhamnogluco-5-glucoside83
T. argentea
Flowers
(Dixit and Srivastava, 1992)
84
T. argentea
Pods
(Swarnalakshmi et al., 1982)
85) Naringenin
86) Naringenin-7-glucorhamnoside85, 86
T. argentea
Pods
(Swarnalakshmi et al., 1982)
87) 5,7,4′-Trihydroxyflavone
87
T. palmeri
Flowers
(Sakhuja et al., 2014)
88) 3,4́,5-Trihydroxy-7-methoxyflavone
88
T. aurea
Stem bark
(Barbosa-Filho et al., 2004)
89) Rutin
89
T. argentea
Leaves
Flowers
Flowers(De Abreu et al., 2014)
(Vinod et al., 2011)
(Swarnalakshmi et al., 1982)
T. caraiba
Leaves
(Blatt et al., 1996; Blatt et al., 1998)
T. ochracea
Leaves
(Blatt et al., 1998)
T. roseo-alba
Leaves
(Ferraz-Filha et al., 2016)
90) Epigallocatechin gallate
90
T. argentea
Flower
(Vinod et al., 2011)
91) 4a,5,8,8α-tetrahydro-5-hydroxy-3,7,8-trimethoxy-2-(3,4-dimethoxyphenyl) chromen-4-one (TMF)
91
T. chrysantha
Stem
(Panda et al., 2020)
92) Benzyl-b-D-glucopyranoside.
92
T. argentea Britt.
Leaves
(De Abreu et al., 2014)
93) 1′-O-β-(3,4-dihydroxyphenyl)-ethyl-4′-O-caffeoyl-α-L-rhamnopyranosyl-(l-3′)-D-glucopyranoside. (Acteoside)
94) 2-(3,4-dihydroxyphenyl)ethy1 O-α-L-rhamnopyranosy1-(1–3)-(6-O-cafeoy1)-β-D-glucopyranoside (Isoacteoside)
95) 2-(3,4-dihydroxypheny1)ethy1 O-α-Lrhamnopyranosy1-(1–3)-(4-O-caffeoy1)-2-O-acety1-β-D-glucopyranoside (2′-acety1acteoside)
96) 1′-O-β-(3,4-dihydroxyphenyl)-ethyl-4′-O-caffeoyl-α-L-fucopyranosyl-(l-3′)-D-glucopyranoside.
97) 1′-O-β-(3,4-dihydroxyphenyl)-ethyl-[4′-O-caffeoyl-(α-L-rhamnopyranosyl)]-(l-3′)-D-galactopyranoside.
98) 1′-O-β-(3,4-dihydroxyphenyl)-ethyl-[4′'-O-caffeoyl-(α-L-rhamnopyranosyl)]-(l-3′)-D-galactopyranoside.
99) 1′-O-β-(3,4-dihydroxyphenyl)-ethyl-[4′'-O-caffeoyl-(α-L-fucopyranosyl)]-(l-3′)-D-galactopyranoside.93
T. heptaphylla
Trunk bark
(Garcez et al., 2007)
93, 94, 95
T. chrysotricha
Immature legumes
(Ogihara et al., 2015)
93, 94, 96, 97, 98, 99
T. avellanedae
Bark
(Suo et al., 2013)
100) 4-hydroxymethyl-2-methoxyphenyl 1-O-b-D-[5-O-(3,4-dimethoxybenzoyl)]-apiofuranosyl-(1 → 6)-b-D-glucopyranoside.
101) 4-hydroxymethyl-2-methoxyphenyl 1-O-b-D-[5-O-(4-hydroxybenzoyl)]-apiofuranosyl-(1 → 6)-b-D-glucopyranoside.
102) 4-hydroxymethyl-2-methoxyphenyl 1-O-b-D-[5-O-(4-methoxybenzoyl)]-apiofuranosyl-(1 → 6)-b-D-glucopyranoside.
103) 4-(1,2-dihydroxyethyl)-2-methoxyphenyl 1-O-b-D-[5-O-(3,4-dimethoxybenzoyl)]- apiofuranosyl-(1 → 6)-b-D-glucopyranoside.
104) 4-(1,2-dihydroxyethyl)-2-methoxyphenyl 1-O-b-D-[5-O-(4 hydroxy,5-methoxybenzoyl)]-apiofuranosyl-(1 → 6)-b -D-glucopyranoside.
105) 4-(1,2-dihydroxyethyl)-2-methoxyphenyl 1-O-b-D-[5-O-(4,5-dimethoxybenzoyl)]-apiofuranosyl-(1 → 6)-b -D-glucopyranoside.100, 101, 102, 103
T. impetiginosa
Bark
(Warashina et al., 2005)
103, 104, 105
T. impetiginosa
Bark
(Warashina et al., 2006)
106) 3,4-dimethoxyphenyl 1-O-b -D-[5-O-(4-hydroxybenzoyl)]-apiofuranosyl-(1 → 6)-b -D-glucopyranoside.
107) 3,4-dimethoxyphenyl 1-O-b -D-[5-O-(3,4-dimethoxybenzoyl)]-apiofuranosyl-(1 → 6)-b -D-glucopyranoside.
108) 3,4,5-trimethoxyphenyl 1-O-b -D-[5-O-(4-methoxybenzoyl)]-apiofuranosyl-(1 → 6)-b -D-glucopyranoside.
109) 3,4-dimethoxyphenyl 1-O-b -D-[5-O-(4-
methoxybenzoyl)]-apiofuranosyl-(1 → 6)-b -D-glucopyranoside.
110) 3,4,5-trimethoxyphenyl 1-O-b -D-[5-O-(3,4-methoxybenzoyl)]-apiofuranosyl-(1 → 6)-b -D-glucopyranoside.
111) 4-methoxyphenyl 1-O-b -D-[5-O-(3,4-dimethoxybenzoyl)]-apiofuranosyl-(1 → 6)-b -D-glucopyranoside.
112) 2,4-dimethoxyphenyl 1-O-b -D-[5-O-(3,4-dimethoxybenzoyl)]-apiofuranosyl-(1 → 6)-b -D-glucopyranoside106
T. chrysotricha
Branches
(Takahashi et al., 2015)
106, 107, 108, 109
T. avellanedae
Bark
(Awale et al., 2005)
106, 107, 108, 109, 110, 111, 112
T. impetiginosa
Bark
(Warashina et al., 2004)
113) 2-(4-hydroxyphenyl)ethyl-1-O-b-D-[5-O-(4-hydroxybenzoyl)]-apiofuranosyl-(1 → 6)-b–Dglucopyranoside.
114) 2-(4 hydroxyphenyl)ethyl-1-O-b-D-[5-O-(3,4-dimethoxybenzoyl)]-apiofuranosyl-(1 → 6)-b -D-glucopyranoside.
115) 2-(4-hydroxyphenyl)ethyl-1-O-b -D-[5-O-(4-methoxybenzoyl)]-apiofuranosyl-(1 → 6)-b -D-glucopyranoside.
116) 2-(4-hydroxyphenyl)ethyl-1-O-b-D-[5-O-(3,4,5-trimethoxybenzoyl)]-apiofuranosyl-(1 → 6)-b -D-glucopyranoside.113, 114, 115
T. avellanedae
Bark
(Awale et al., 2005)
113, 114, 116
T. chrysotricha
Branches
(Takahashi et al., 2015)
114, 115, 116
T. impetiginosa
Bark
(Warashina et al., 2004)
117) 2-methoxy-4-[(1S,2S)-1,2,3-trihydroxypropyl]phenyl 1-O-b-D-[6-O-(4-methoxybenzoyl)]-glucopyranoside.
118) 2-methoxy-4-[(1S,2S)-1,2,3trihydroxypropyl]phenyl 1-O-b -D-[6-O-(4-hydroxybenzoyl)]-glucopyranoside.117
T. impetiginosa
Bark
(Warashina et al., 2005)
117, 118
T. impetiginosa
Bark
(Warashina et al., 2006)
119) Osmanthuside H
120) 2-(4-hydroxyphenyl)ethyl 5-O- 3″',4″'-dimethoxycinnamate-b-D-apiosyl-(l → 6)-β-D-glucopyranoside.
121) 2-(4-hydroxyphenyl)ethyl 5-O-trans-feruloyl-β-D-apiosyl-(l-→6)-β-D-glucopyranoside (osmanthuside J)119
T. impetiginosa
Bark
Bark
(Warashina et al., 2004)
(Warashina et al., 2006)
120, 121
T. chrysotricha
Branches
(Takahashi et al., 2015)
122) 3,4 dimethoxyphenyl 1-O-b-D-apiofuranosyl-(1 → 6)-b –D glucopyranoside.
123) 3,4,5-trimethoxyphenyl 1-O-b -D-apiofuranosyl-(1 → 6)-b -D-glucopyranoside.122, 123
T. impetiginosa
Bark
(Warashina et al., 2006)
124) Erythro1,2-bis(4-hydroxy-3-methoxyphenyl)-1,3-propanediol-4′-O-b –Dglucopyranoside.
125) Threo-1,2-bis(4-hydroxy-3-methoxyphenyl)-1,3-propanediol-4′-O-b –glucopyranoside.124, 125
T. impetiginosa
Bark
(Warashina et al., 2006)
126) 2,4-dimethoxyphenyl 1-O-b -D-apiofuranosyl-(1 → 6)-b -D-glucopyranoside.
126
T. impetiginosa
Bark
(Warashina et al., 2006)
127) 4-[[(3,4-dimethoxybenzoyl)oxy]-methyl]-2 methoxyphenyl 1-O-b -D-[5-O-(3,4-dimethoxybenzoyl)]-apiofuranosyl-(1 → 6)-b -D-glucopyranoside.
128) 4-[[(3,4-dimethoxybenzoyl)oxy]methyl]-2-methoxyphenyl 1-O-b -DJanuary[5-O-(4-hydroxybenzoyl)]-apiofuranosyl-(1 → 6)-b -D-glucopyranoside.
129) 4-[[(4-methoxybenzoyl)oxy]methyl]-2-methoxyphenyl 1-O-b-D-[5-O-(4-hydroxybenzoyl)]-apiofuranosyl-(1 → 6)-b -Dglucopyranoside.
130) 4-[[(3methoxy-4hydroxybenzoyl)oxy]-
methyl]-2-methoxyphenyl 1-O-b -D-[5-O-(3,4-dimethoxybenzoyl)]-apiofuranosyl-(1 → 6)-b -D-glucopyranoside.127, 128, 129, 130
T. impetiginosa
Bark
(Warashina et al., 2006)
130
T. impetiginosa
Bark
(Warashina et al., 2004)
131) 5′-O-3,4-dimethoxybenzoyl-β-D-apiofuranoside.
132) 5′-O-4-methoxybenzoyl-β-D-apiofuranoside.
133) 5′-O-4-hydroxybenzoyl-β-D-apiofuranoside.
134) 5′-O-3, 4-dihydroxybenzoyl-β-D-apiofuranoside.131, 132, 133, 134
T. avellanedae
Bark
(Suo et al., 2012)
135) Guayin
136) Guayacanin135, 136
T. guayacan
Bark
(Manners et al., 1975)
137) ((4S)-3,4-dihydroxy-5-(((2R,3S,4S,5S,6S)-3,4,5-trihydroxy-6-(3,4,5-trimethoxyphenoxy)tetrahydro-2H-pyran-2-yl)methoxy)tetrahydrofuran-3-yl)methyl 4-hydroxybenzoate.
138) ((5R)-5-(((2R,3S,4S,5S,6S)-4,5-dihydroxy-6-(hydroxymethyl)-2-(4-hydroxyphenethoxy)tetrahydro-2H-pyran-3-yl)oxy)-3,4-dihydroxytetrahydrofuran-3-yl)methyl 3,4-dimethoxybenzoate.137, 138
T. chrysotricha
Branches
(Takahashi et al., 2015)
139) Tyrosol
139
T. caraiba
Flowers
(Soares et al., 2020)
3. Lignans
140) 5-hydroxysesamin 5-O-β-D-glucopyranosyl-(1–2)-[β-D-gluco- pyranosyl-(1–6)]-β-D-glucopyranoside.
140
T. argentea Britt.
Leaves
(De Abreu et al., 2014)
(Dihydrobenzofuran lignan)
141) Trans-Dihydro-dehydrodiconiferylalcohol 4-O-a-Lrhamnopyranoside (icariside E4)141
T. roseo-alba
Bark
(Ferreira-Júnior et al., 2015)
142) Avellanedae A
142
T. avellanedae
Bark
(Suo et al., 2012)
143) Secoisolariciresinol
143
T. heptaphylla
Trunk wood
(Schmeda-Hirschmann, and Papastergiou, 2003)
T. palmeri
Flowers
(Sakhuja et al., 2014)
144) Secoisolariciresinol-4-O-b-D-[6-O-(4-methoxybenzoyl)]-glucopyranoside.
145) Secoisolariciresinol-4-O-b-D-[6-O-(3,4-methoxybenzoyl)]-glucopyranoside.144, 145
T. impetiginosa
Bark
(Warashina et al., 2004)
146) (−)-isolariciresinol 3α-O-β-D-glucopyranoside
146
T. chrysotricha
Branches
(Takahashi et al., 2015)
147) Cycloolivil
147
T. heptaphylla
Trunk wood
(Schmeda-Hirschmann, and Papastergiou, 2003)
T. incana
Trunk wood
(de Oliveira et al., 1993)
T. ochracea
Trunk wood
(Zani et al., 1991)
T. palmeri
Flowers
(Sakhuja et al., 2014)
T. serratifolia
Trunk wood
(Oliveira et al., 2001)
148) Cycloolivil acetonide
148
T. incana
Trunk wood
(de Oliveira et al., 1993)
149) Olivil
149
T. serratifolia
Trunk wood
(Oliveira et al., 2001)
150) (+)-lyoniresinol-3α-O-b-D-gluco-pyranoside.
151) (+)-lyoniresinol-3α-O-(2″-O-β-D-apiofuranosyl)-β-D-glucopyranoside.150
T. impetiginosa
Bark
(Warashina et al., 2005)
150, 151
T. chrysotricha
Branches
(Takahashi et al., 2015)
152) [(1S,2R,3R)-7-Hydroxy-1-(4-hydroxy-5-methoxyphenyl)-3-(hydroxymethyl)-8-dimethoxy-1,2,3,4-tetrahydro-2-naphthalenyl]methyl β-D-apiofuranosyl)-β-D-glucopyranoside.
153) [(1S,2R,3R)-7-Hydroxy-1-(4-hydroxy-3,5-dimethoxyphenyl)-3-(hydroxymethyl)-8-dimethoxy-1,2,3,4-tetrahydro-2-naphthalenyl]methyl β-D-apiofuranosyl)-β-D-glucopyranoside.152, 153
T. chrysotricha
Branches
(Takahashi et al., 2015)
154) Dihydrodehydro-diconiferyl alcohol 9-O-b-D-glucopyranoside.
155) Dihydrodehydrodiconiferyl alcohol 9′-O-b-D glucopyranoside.
156) Dihydrodehydro-diconiferyl alcohol
4-O-b-D-glucopyranoside.154, 155, 156
T. impetiginosa
Bark
(Warashina et al., 2005)
157) Balanophonin,
158) Balanophonin 4-O-b-D-glucopyranoside.157
T. avellanedae
Inner bark
(Zhang et al., 2014)
158
T. impetiginosa
Bark
(Warashina et al., 2005)
159) Isopaulownin
159
T. rosea
Roots
(Sichaem et al., 2012)
160) Pawlownin
160
T. incana
Trunk wood
(de Oliveira et al., 1993)
161) Pinoresinol
162) Epipinoresinol
163) 1-(benzo[d][1,3]dioxol-6-yl)-4-(4-hydroxy-3-methoxyphenyl)hexahydrofuro[3,4-c]furan-3a-ol.
164) Salicifoliol161, 162, 163, 164
T. avellanedae
Bark
(Zhang et al., 2014)
165) 4-Aryltetralin
165
T. palmeri
Wood
(Villegas et al., 1995)
4. Coumarins
166) 3,4-Dihydro-6,8-dihydroxy-3-methylisocoumarin (6-hydroxymellein)
166
T. avellanedue
Inner bark
(Wagner et al., 1989)
T. impetiginosa
Bark
(Koyama et al., 2000)
167) 6 Hydroxymellein-6-O-b-D-apiofuranosyl-(1 → 6)-b-D-glucopyranosyl.
167
T. impetiginosa
Bark
(Warashina et al., 2006)
168) 6 Hydroxymellein-6-O-b-D-xylopyranosyl-(1 → 6)-b-D-glucopyranosyl.
168
T. impetiginosa
Bark
(Warashina et al., 2006)
169) 6-Hydroxymellein 6-O-b-D-[5-O-(4-methoxybenzoyl)]-apiofuranosyl-(1 → 6)-b-D-glucopyranoside.
170) 6-Hydroxymellein 6-O-b-D-[5-O-(3,4 dimethoxybenzoyl)]-apiofuranosyl-(1 → 6)-b-D-glucopyranoside.
171) 6-hydroxymellein 6-O-b-D-[5-O-(3,4,5 trimethoxybenzoyl)]apiofuranosyl-(1 → 6)-b-D-glucopyranoside.
169
T. impetiginosa
Bark
(Warashina et al., 2006)
170, 171
T. impetiginosa
Bark
(Warashina et al., 2004)
172) 6-Hydroxymellein-6-O-b-D-[6-O-(4-methoxybenzoyl)]-glucopyranoside.
172
T. impetiginosa
Bark
(Warashina et al., 2006)
173) 1-(5-(hydroxymethyl)furan-2-yl)isochroman-6,7-diol.
173
T. avellanedae
Bark
(Zhang et al., 2014)
5. Aldehydes, acids and esters
174) 4-methoxybenzaldehyde (anisaldehyde).
175) 4-hydroxy-3methoxy benzaldehyde.
176) 3,4 dimethoxy benzaldehyde.174, 175, 176
T. avellanedae
Inner stem bark
(Wagner et al., 1989)
177) Benzo[b]furan-6-carboxaldehyde.
177
T. avellanedae
Inner bark
(Wagner et al., 1989)
178) 3,4-dimethoxybenzoic acid (veratric acid).
179) 4-methoxybenzoic acid (p-anisic acid).
180) 4-hydroxybenzoic acid.
181) 3,4-dihydroxybenzoic acid.
182) 4-hydroxy-3-methoxybenzoic acid (vanillic acid).
183) 3,4,5-trimethoxybenzoic acid.
184) 2-methyl Benzoic acid.
185) 4-O-β-glucosylbenzoic acid.178
T. rosea
Bark
(Oliveira et al., 1999)
178, 179
T. aurea
Stem bark
(Barbosa-Filho et al., 2004)
T. rosea
Roots
(Sichaem et al., 2012)
178, 179, 180
T. heptaphylla
Trunk bark
(Garcez et al., 2007)
T. avellanedae
Inner bark
(Awale et al., 2005)
178, 179, 180, 182, 183
T. avellanedae
Inner bark
(Wagner et al., 1989)
178, 180, 181
T. palmeri
Stem
(Sakhuja et al., 2014)
180, 181, 185
T. palmeri
Flowers
(Sakhuja et al., 2014)
182
T. serratifolia
Bark
(Oliveira et al., 1999)
186) 4-hydroxycinnamic acid (E-p-coumaric acid)
186
T. caraiba
Flowers
(Soares et al., 2020)
T. rosea
Roots
(Sichaem et al., 2012; Oliveira et al., 1999)
187) Caffeic acid
187
T. roseo-alba
Leaves
(Ferraz-Filha et al., 2016)
(Cyclopentenyl esters)
188) Avellaneine A
189) Avellaneine B
190) Avellaneine C
191) Avellaneine D
192) 2-formyl-5-(4′-methoxybenzoyl-oxy)-3-methyl-2-cyclopentene-1-acetaldehyde.
193) 2-formyl-5-(3′,4′-dimethoxybenzoyloxy)-3-methyl-2-cyclopentene-1-acetaldehyde.
194) Tabebuialdehyde A
195) Avellaneine E
196) Avellaneine F188, 189, 190, 191, 192, 193, 194, 196
T. avellanedae
Inner bark
(Zhang et al., 2016)
192, 193
T. impetiginosa
Bark
(Koyama et al., 2000b)
192, 193, 194
T. rosea
Roots
(Sichaem et al., 2012)
193
T. heptaphylla
Trunk bark
(Garcez et al., 2007)
(Cyclopentyl esters)
197) Avellaneine G
198) Avellaneine H197, 198
T. avellanedae
Inner bark
(Zhang et al., 2016)
199) Tabebuialdehyde B
200) Tabebuialdehyde C199, 200
T. rosea
Roots
(Sichaem et al., 2012)
201) Methyl 3,4-dimethoxybenzoate
201
T. palmeri
Stem
(Sakhuja et al., 2014)
202) 4ꞌ-methoxybenzyl-4-methoxybenzoate
202
T. impetiginosa
Stem bark
(Koyama et al., 2000b)
203) Methyl cinnamate.
204) Ethyl p-hydroxycinnamate.203, 204
T. aurea
Stem bark
(Barbosa-Filho et al., 2004)
6. Hydrocarbons, triterpenoids and sterols
205) 1-hexadecanol
206) 1-triacontanol
207) 1-hentriacontanol205
T. palmeri
Stem and leaves
(Sakhuja et al., 2014)
206
T. palmeri
Stem
(Sakhuja et al., 2014)
207
T. pentaphylla
Leaves
(Prakash and Singh, 1981)
208) Linoleic acid
209) Palmitic acid208, 209
T. palmeri
Leaves
(Sakhuja et al., 2014)
210) Hexacosane
211) Nonacosane
212) Hentriacontane
213) Hepacosane210, 213
T. pentaphylla
Root bark
(Prakash and Garg, 1980)
211
T. pentaphylla
Stem bark
Heart wood(Prakash and Singh, 1980)
(Prakash and Singh, 1981)
212
T. pentaphylla
Leaves
(Prakash and Singh, 1981)
T. rosea
Flowers
(Madhumitha et al., 2015)
214) Squalene
214
T. heptaphylla
Trunk bark
(Garcez et al., 2007)
215) 6-(1-hydroxyundec-3-enyl)-tetrahydropyran-2-one.
215
T. palmeri
Flowers
(Sakhuja et al., 2014)
216) Stigmast-5-en-3β-ol.
216
T. palmeri
Stem and flowers
(Sakhuja et al., 2014)
217) β sitosteryl-β-D-galactoside
217
T. palmeri
Flowers
(Sakhuja et al., 2014)
218) 3β-hydroxy-12-ursen-28-oic acid (ursolic acid
218
T. palmeri
T. caraiba
Flowers
Flowers(Sakhuja et al., 2014)
(Soares et al., 2020)
219) 3-O-E-p-coumaroylursolic acid
220) 2α-hydroxyursolic acid (corosolic acid)
221) 3β-6β-19α-trihydroxy-urs-12-en-28-oic acid119
T. caraiba
Bark
(Soares et al., 2006)
119, 120, 121
T. caraiba
Flowers
(Soares et al., 2020)
221
T. rosea
Bark
(Oliveira et al., 1999)
222) Stigmasterol
T. Billbergii
Inner bark
(Gómez-Estrada et al., 2012)
T. Impetiginosa
Bark
(Koyama et al., 2000b)
T. roseo-alba
Leaves
(Ferraz-Filha et al., 2016)
223) β-Sitosterol
223
T. aurea
Stem bark
(Barbosa-Filho et al., 2004)
T. Billbergii
nner bark
(Gómez-Estrada et al., 2012)
T. caraiba
Flowers
(Soares et al., 2020)
T. heptaphylla
Trunk bark
(Garcez et al., 2007)
T. ochracea
Trunk wood
(Zani et al., 1991)
T. impetiginosa
Bark
(Koyama et al., 2000b)
T. pentaphylla
Root bark
Stem bark
Leaves
Heart wood(Prakash and Garg, 1980)
(Prakash and Singh, 1980)
(Bishay et al., 1987) (Prakash and Singh, 1981)
T. rosea
T. rosea
Roots
Heart wood
(Joshi et al.,1977)
(Joshi et al.,1973; Oliveira et al., 1999)
T. roseo-alba
Leaves
(Ferraz-Filha et al., 2016)
224) β-sitosterol-3-O-β-D-glucopyranoside
225) β-sitosterol-3-O-β-D-(6́-O-acyl)-glucopyranoside224
T. rosea
Bark
(Oliveira et al., 1999)
224, 225
T. caraiba
Flowers
(Soares et al., 2020)
226) Sitostenone
226
T. heptaphylla
Trunk bark
(Garcez et al., 2007)
T. rosea
Heart wood
(Joshi et al.,1973)
227) α-amyrin
228) β-amyrin227
T. pentaphylla
Leaves
(Bishay et al., 1987)
228
T. caraiba
Bark
Flowers(Soares et al., 2006)
(Soares et al., 2020)
227, 228
T. roseo-alba
Leaves
(Ferraz-Filha et al., 2016)
229) Olean-12-en-3-one (beta-Amyrone)
229
T. caraiba
Bark
Flowers(Soares et al., 2006)
(Soares et al., 2020)
230) Betulinic acid
231) Betulin230
T. aurea
Stem bark
(Barbosa-Filho et al., 2004)
T. caraiba
Bark
Flowers(Soares et al., 2006)
(Soares et al., 2020)
230, 231
T. pentaphylla
Leaves
(Bishay et al., 1987)
232) Oleanolic acid
232
T. caraiba
Bark
Flowers(Soares et al., 2006)
(Soares et al., 2020)
T. pentaphylla
Leaves and bark
Root(Bishay et al., 1987)
(Prakash and Garg, 1980)
233) 3-β-O-E-p-cumaroyl-ol-12-en-28-oic
233
T. caraiba
Bark
(Soares et al., 2006)
234) 3 β, 6 β, 21 β-trihydroxyolean-12ene.
234
T. heptaphylla
Trunk bark
(Garcez et al., 2007)
7. Irridoids
235) 6-epi-aucubin
235
T. chrysantha
bark
(Bianco et al., 1982a)
236) 6-O-p-OH-benzoyl-6-epi-aucubin
(derwentioside B)236
T. alba
Bark
(Von poser et al., 2000)
T. argentea
leaves
(Piaz et al., 2013)
T. chrysantha
Bark
(Bianco et al.,1982c)
T. chrysotricha
Bark
Branches(Von poser et al., 2000)
(Takahashi et al., 2015)
T. heptaphylla
Leaves
(Von Poser et al., 2000; Bianco et al., 1982c)
T. impetiginosa
Bark
(Warashina et al., 2005)
T. palmeri
flowers
(Sakhuja et al., 2014)
237) 6-epi-monomelittoside
237
T. heptaphylla
Leaves
(Bianco et al., 1982b)
238) 6-O-p-OH-benzoyl-epi-monomelittoside.
239) 6-O-p-methoxy-benzoyl-epi-monomelittoside238, 239
T. heptaphylla
Leaves
(Bianco et al., 1982c)
240) 6-O-p-OH-benzoyl-ajugol (6-O-4-OH-benzoyl-ajugol) (6-O-4″-hydroxy benzoyl-leonuride)
241) 6-O-p-methoxybenzoyl-ajugol or 6-O-4-methoxybenzoyl-ajugol.
242) 6-O-3,4-dimethoxybenzoyl-ajugol.
243) 6-O-(3,4,5 trimethoxy-benzoyl)-ajugol.
244) 6-O-2,4-dimethoxybenzoyl-ajugol.
245) 6-O-(4-hydroxy-3-methoxybenzoyl)ajugol.(6-O-vanilloyl-ajugol or 6-O-vanilloylleonuride)240, 241, 242
T. avellanedae
Inner bark and trunk wood
(Nakano et al., 1993; Awale et al., 2005)
T. heptaphylla
Trunk bark
(Garcez et al., 2007)
240, 241, 242, 243, 244, 245
T. impetiginosa
Bark
(Warashina et al., 2005)
240, 245
T. chrysotricha
Branches
(Takahashi et al., 2015)
241, 242, 243, 244
T. impetiginosa
Bark
(Warashina et al., 2004)
245
T. serratifolia
Trunk wood
(Oliveira et al., 2001)
246) 6- O -(p-coumaroyl)-catalpol (specioside)
246
T. argentea
Leaves
(Piaz et al., 2013)
T. aurea
Stem bark
(Nocchi et al., 2020)
T. pentaphylla
Bark
(Bishay et al., 1987)
T. rosea
Bark
(Compadre et al., 1982)
247) Catalposide
248) Amphicoside
249) 6-O-veratrylcatalposide247
T. argentea
Leaves
(Piaz et al.,2013)
247, 248, 249
T. chrysotricha
Branches
(Takahashi et al., 2015)
250) Catalpol
250
T. serratifolia
Seeds
(Hegnauer and Kooiman, 1978)
251) Avellanedaesides A
252) Avellanedaesides B
253) Avellanedaesides C
254) Avellanedaesides D
255) Avellanedaesides E251, 252, 253, 254, 255
T. avellanedae
Inner bark
(Suo and Yan, 2016)
256) Avelladoids A
257) Avelladoids B
258) Avelladoids C
259) Avelladoids D
260) Avelladoids E
261) Avelladoids F
262) Avelladoids G
263) Avelladoids H256, 257, 258, 259, 260, 261, 262, 263
T. avellanedae
Inner bark
(Zhang et al., 2017)
264) 7-hydroxy-1,3-dimethoxy-7-methyl-octa hydro-cyclopenta [c]pyran-5-yl 4-hydroxybenzoate.
265) 7-hydroxy-1,3-dimethoxy-7-methyl-octa hydro-cyclopenta[c]pyran-5-yl 4-hydroxybenzoate.264, 265
T. avellanedae
Bark
(Awale et al., 2005)
266) 6-O-(4-methoxybenzoyl)-5,7-bisdeoxy-cynanchoside.
267) 10-O-(4-methoxybenzoyl)-impetiginoside A.
268) 6-O-(3,4-dimethoxybenzoyl)-
crescentin IV 3-O-b-D-glucopyranoside.
269) 6-O-(4-methoxybenzoyl)-crescentin IV 3-O-b-Dglucopyranoside.
270) 3-O-(4-hydroxybenzoyl)-10-deoxyeucommiol 6-O-b-Dglucopyranoside.266, 267, 268, 269, 270
T. impetiginosa
Bark
(warashina et al., 2005; warashina et al., 2006)
271) 4-O-methylcedrusin
272) 1-dehydroxy-3,4-dihydroaucubigenin271, 272
T. avellanedae
Inner bark
(Iwamoto et al., 2016)
273) 3-deoxy-artselaenin
273
T. avellanedae
Bark
(Zhang et al., 2014)
274) 8 α-methyl-8 β-hydroxy-6 β-(3′,4′-dimethoxy)benzoyloxy-1 α,3 α-dimethoxy-octahydro-cyclopenta[c]pyran.
275) 8 α-methyl-8 β-hydroxy-6 β-(4′-hydroxy)benzoyloxy-1 α,3 α-dimethoxy-octahydro-cyclopenta[c]pyran.274, 275
T. heptaphylla
Trunk bark
(Garcez et al., 2007)
276) 6-O-E-p-cumaroylcatoalpol
277) 6-O-E-p-cumaroyljuglutin-A
278) Rehmaglutin-D
279) Juglutin-D276, 277, 278, 279
T. caraiba
Bark
(Soares et al., 2006)
276, 278, 279
T. caraiba
Trunk bark
(Soares et al., 2020)
280) 6-O-E-p-coumaroyljuglutin D
281) 6-O-E-p-coumaroyl-3-demethyl-3-O-ethyljuglutin D
282) 6-O-E-p-coumaroyl-1-demethyl-1-O-ethyljuglutin D.
283) 7-O-E-p-coumaroyljiofuranaldehyde.280, 281, 282, 283
T. caraiba
Trunk bark
(Soares et al., 2020)
284) Argenteoside A
285) Argenteoside B
286) Rehmaglutin A
287) Stereospermoside
288) Picroside II284, 285, 296, 297, 298
T. argentea
Leaves
(Piaz et al., 2013)
8. Carotenoids
289) Lycopene
290) Capsanthin
291) B-carotene
292) Zeaxanthin299, 290, 291, 292
T. argentea
Flowers
(Dixit and Srivastava, 1992)
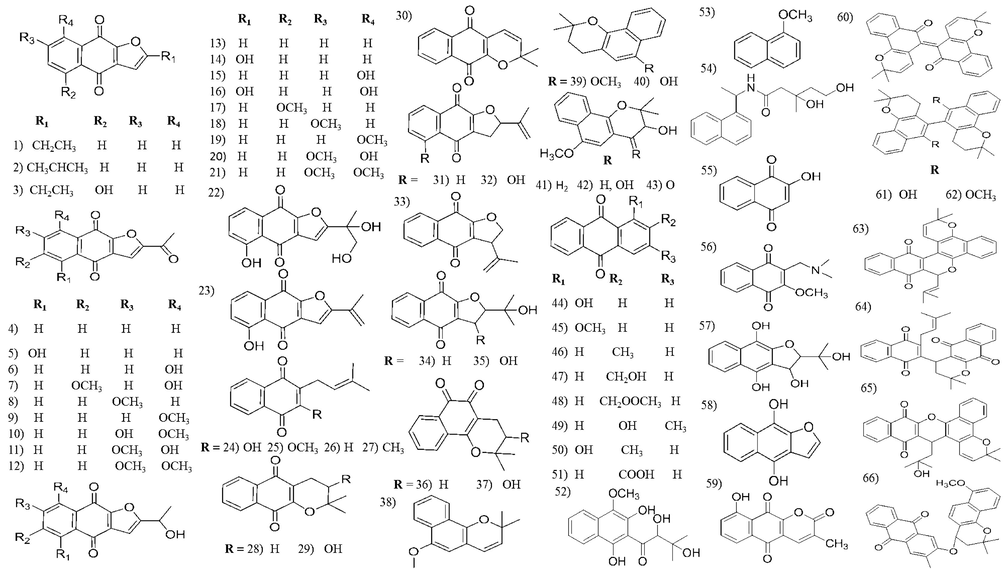
Chemical structures of naphthoquinones isolated from Tabebuia species.
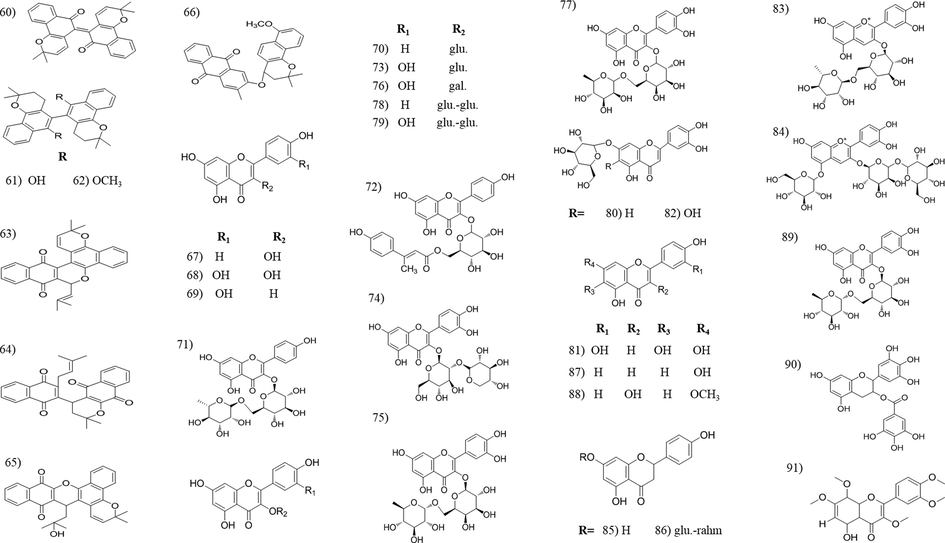
Chemical structures of naphthoquinones and flavonoids isolated from Tabebuia species.
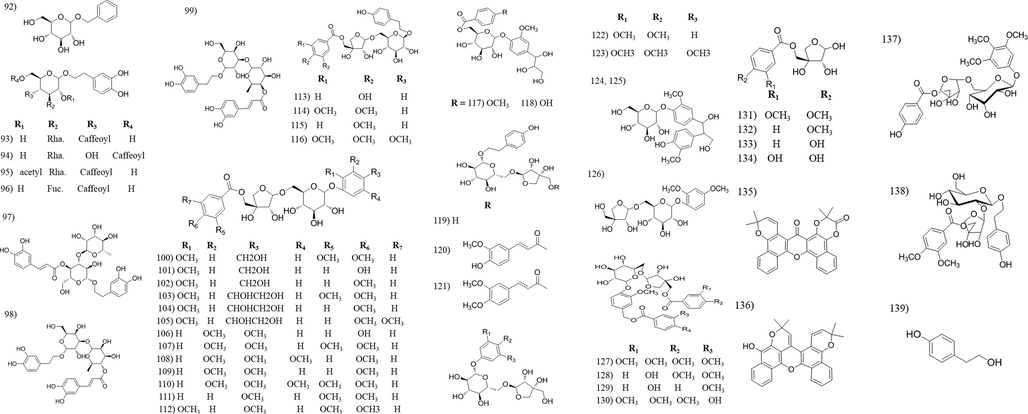
Chemical structures of phenolic compounds isolated from Tabebuia species.
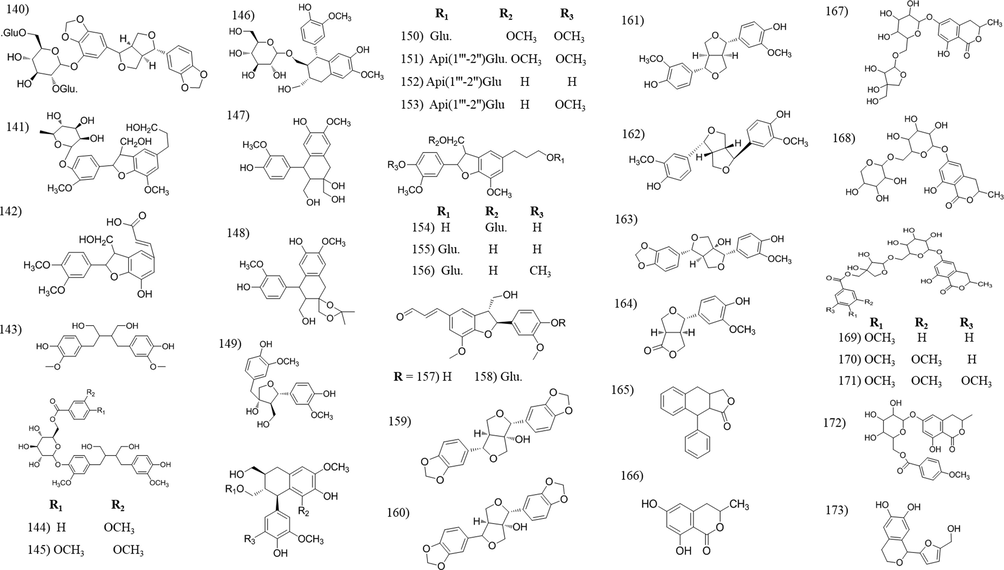
Chemical structures of lignans and coumarin compounds isolated from Tabebuia species.
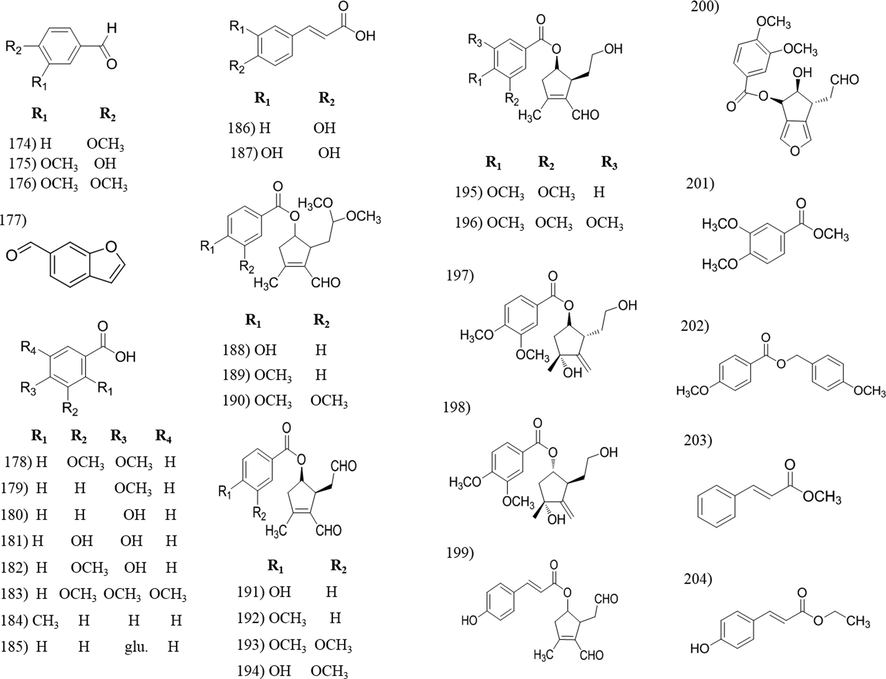
Chemical structures of aldehyde, acid and ester compounds isolated from Tabebuia species.
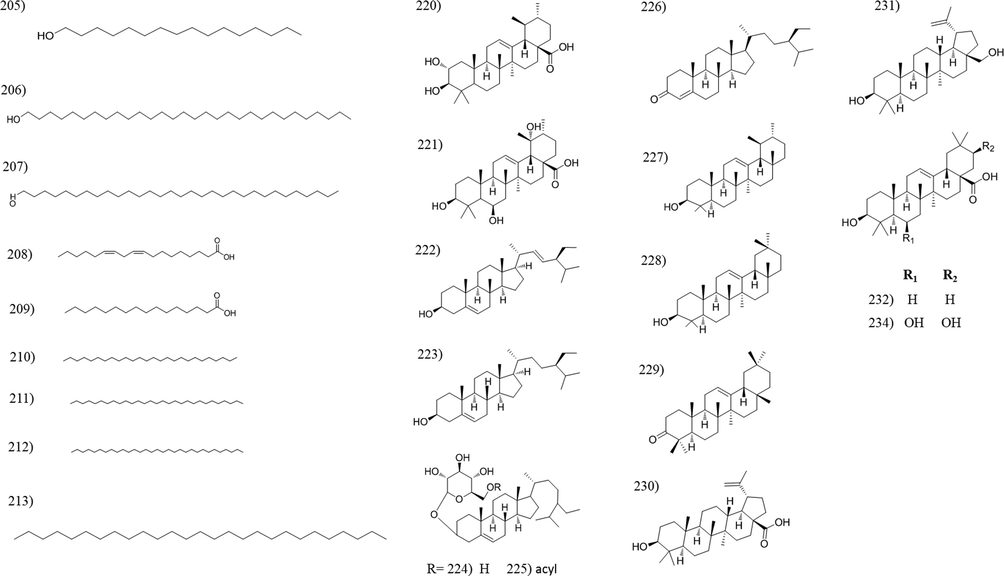
Chemical structures of hydrocarbons, triterpenes and sterols isolated from Tabebuia species.
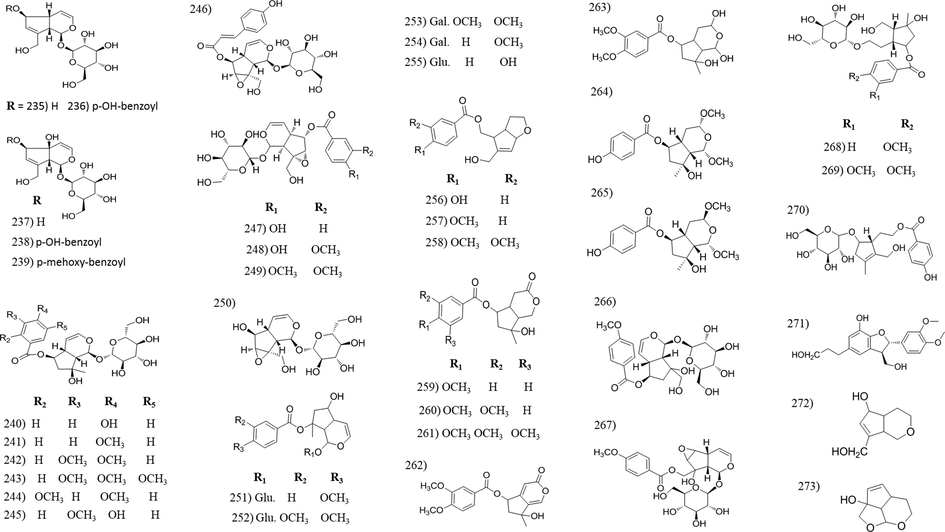
Chemical structures of irridoids isolated from Tabebuia species.
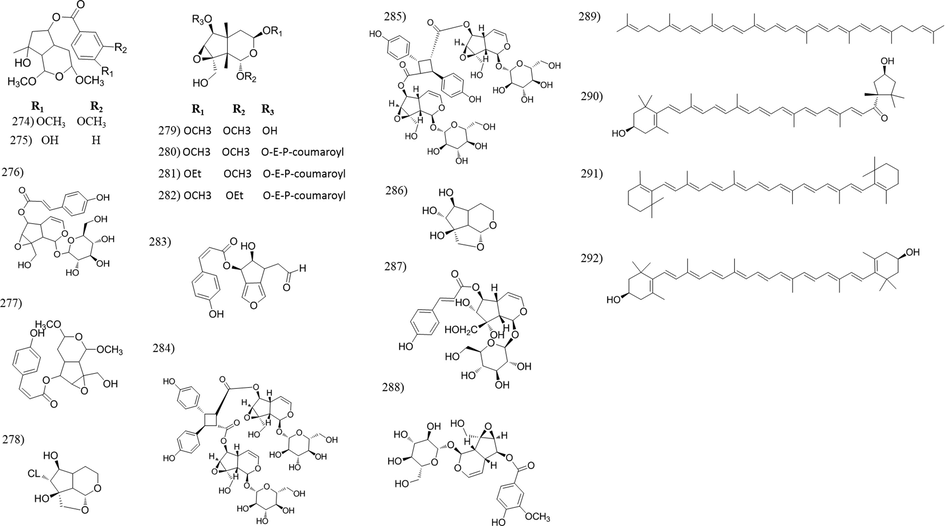
Chemical structures of irridoids and carotenoid compounds isolated from Tabebuia species.
3.1 Naphthoquinones
Naphthoquinones are natural aromatic compounds, structurally related to naphthalene, found in several plant families and commercially used for dyeing properties. They are highly reactive organic compounds where their biological activities are attributed to naphthoquinones redox and acid-base properties (Ramos-Peralta et al., 2015). Naphthoquinones are the major constituents of Tabebuia. About 66 quinones have been isolated and identified in Table 2. Lapachol is a naturally occurring 1,4- naphthoquinone widely distributed in this genus (Epifano et al., 2014), as well as β-lapachone, the most common naphthoquinone isolated from the genus and is now in clinical trial phase as plant derived anticancer agents (Nirmala et al., 2011). Additionally, β-lapachone, is a potential depigmentation agent for various hyperpigmentation disorders in skin care preparations (Kim et al., 2015b). Naphthoquinones received a special consideration in Tabebuia species due to their pharmacological activities (Moura et al., 2001), as anti-inflammatory and wound healing activity (Grazziotin et al., 1992; Kung et al., 2008), antimicrobial activity (Machado et al., 2003; Velasquez et al., 2004; Park et al., 2005; Pereira et al., 2006; Park et al., 2006; Yamashita et al., 2009), antimalarial activity (Pérez et al., 1997), antileishmanial activity (Ali et al., 2010; Gonzalez-Coloma et al., 2012), insecticidal activity (Jeon and Lee, 2011; Jeon et al., 2011; Kim et al., 2013; Borges et al., 2019) and cytotoxic activity (Ueda, et al., 1994; de Saizarbitoria Colman et al., 1997; Yamashita et al., 2009; Morais et al., 2007; Zhang et al., 2015; Sichaem et al., 2012; Woo and Choi, 2005; Woo et al., 2006; Queiroz et al., 2008).
3.2 Flavonoids and phenolic compounds
Flavonoids are common plant constituents with a wide range of biological activities, e.g., anti-oxidant, hepatoprotective, antitumour, etc. Most of the Tabebuia flavonoids have flavonol structure, whereas the presence of other flavonoid seems to be limited. The majority of the reported flavonoids were isolated from the leaves and flowers of T. argentea, T. pentaphylla, T. ochracea and T. caraiba. Phenylethanoids and phenylpropanoid are known for its anti-oxidant, anti-inflammatory and neuroprotective activity (Pan et al., 2003). The majority of these compounds were isolated from T. avellanedae and T. chrysotricha. To our observation, the anti-oxidant activity of Tabebuia extracts is credited to its content of flavonoids and phenolic compounds (Pires et al., 2015; Rahman et al., 2015; Rahman et al., 2019; Suo et al., 2013).
3.3 Lignans
Lignans are a large class of secondary metabolites with numerous biological effects, including anticancer, anti-oxidant, antihypertensive, antiviral, estrogenic, and insecticidal properties (Simpson and Amos, 2017). Plant lignans, such as sesamin, can converted by intestinal microbiota to mammalian lignans, which have protective effects against hormone-related diseases such as breast cancer (Sato and Matsui, 2012) and fortunately, 5-hydroxysesamin 5-O-β-D-glucopyranosyl-(1–2)-[β-D-gluco- pyranosyl-(1–6)]-β-D-glucopyranoside (140) was isolated and identified from the leaves of T. argentea. Twenty-six lignans were isolated and identified among which, avallandae A (142) exhibit anti-inflammatory activity (Suo et al., 2012), icariside E4 (141), had antinociceptive activity (Ferreira-Júnior et al., 2015) and lyoniresinol-3a-O-b-D-gluco-pyranoside (150), showed a potent anti-oxidant activity (Takahashi et al., 2015).
3.4 Coumarins
Coumarins are phenolic substances composed of fused benzene and α-pyrone rings. They exhibit antithrombotic, anti-inflammatory, vasodilatory and can also antibacterial activities (Bor et al., 2016). Eight coumarin compounds were isolated and identified, from which six of them were isolated and identified by Warashina et al., from the year of 2004–2006 (Warashina et al., 2004; Warashina et al., 2006). The last two, 6-hydroxymellein (166) and the new coumarin 1-(5-(hydroxymethyl) furan-2-yl) isochroman-6,7-diol (173) both isolated from the bark of T. avellanedae (Wagner et al., 1989; Zhang et al., 2014, respectively).
3.5 Aldehyde, acids and esters
All reported aldehydic compounds, (174–177), were isolated from T. avellanedae. The isolated acidic compounds, including eight derivatives of benzoic acid (178–185), 4-hydroxycinnamic acid (186) and caffeic acid (187); were distributed in different Tabebuia species including, T. rosea, T. heptaphylla, T. aurea, T. avellanedae, T. palmeri and T. roseo-alba. [Table 2]. Seventeen ester compounds were isolated from Tabebuia species, nine of them identified as cyclopentenyl esters (188–196) isolated from T. avellanedae, T. rosea and T. heptaphylla. Two are cyclopentyl esters (197–198) and isolated only from T. avellanedae. Tabebui-aldehyde B and C, were isolated from the roots of T. rosea while the two benzoate derivatives were isolated from the stem bark of T. palmeri and T. impetiginosa. The last two are cinnamate derivatives and isolated from the stem bark of T. aurea.
3.6 Hydrocarbons, triterpenoids and sterols
Three fatty alcohols (205–207) and two fatty acids (208 and 209) were reported upon investigation of T. palmeri and T. pentaphylla extracts. Additionally, four hydrocarbons were isolated from T. pentaphylla and T. rosea. Squalene, a linear triterpene, was isolated from the trunk bark of T. heptaphylla (Garcez et al., 2007). Several studies reported squalene to inhibit the tumor growth in the colon, skin, lung, and breast, and stimulate the immune system against HIV, H1N1, leukemia and herpes (Lozano-Grande et al., 2018). Plant sterols are famous for its ability to reduce cholesterol levels, help in preventing heart disease and heart attacks. Nineteen sterol and triterpene compounds were isolated from different Tabebuia species (Table 2, Fig. 6).
3.7 Irridoid compounds
Iridoids are reported for its health benefits including anti-inflammatory, anticancer, antimicrobial, antispasmodic, cardioprotective, hepatoprotective, hypoglycemic etc. (Leisner et al., 2017). They are widely distributed in Tabebuia. Fifty four irridoid compounds were identified from fifteen Tabebuia species [Table 2]. The majority of irridoids were isolated from the bark and wood organs, however tewelve irridoids were isolated from other plant organs such as; (296) isolated from leaves of both T. argentea and T. heptaphylla and flowers of T. palmeri, (297–299) from leaves of T. heptaphylla, (246, 247 and 284–288) from leaves of T. argentea and (250) isolated from the seeds of T. serratifolia.
3.8 Carotenoids
Tabebuia species are rich with carotenoids, this may be the reason for decorative flower colours. Four carotenoid compounds (288–292) were isolated and identified from the yellow flowers of T. argentea.
3.9 Other constituents identified by GC/MS and other assays from Tabebuia species
The GC/MS analysis of T. impetiginosa inner bark lead to identification 4-methoxybenzaldehyde, and 4-methoxyphenol as a major volatile constituents (Park et al., 2003). Oleic and linoleic were the most abundant unsaturated fatty acids. In addition, Oxalic, citric, and succinic acids were also identified with α-tocopherol as the most predominant tocopherols present (Pires et al., 2015). While, the unsaturated fatty acids of T. argentea seed oil were expressed in form of linoleic, oleic, vernolic and linolenic acids (Daulatabad and Hosamani, 1991). Moreover, the essential oils analysis of T. rosea identified methyl cyclohexane and methyl benzene which representing 65.88% of the total leaf essential oil while the stem bark enclosed n-amyl ketone, methyl cyclohexane and methyl benzene that representing 84.67% (Oloyede et al., 2010).
On the other hand, GC–MS analysis of T. rosea leaf extract lead to identification of different classes with aromatic aldehydes (21.81%), representing the main class, in which, 2-furancarboxaldehyde-(5-hydroxy methyl) was the main constituents (Ramalakshmi and Muthuchelian, 2011). In addition, the flower extract showed four major peaks in which Dispiro[1,3-dioxolane 2,2′bicyclo[2.2.1]heptane- 3′,2′'(1′',3′'dioxolane)] the main component (Madhumitha et al., 2015).
T. heptaphylla wood extract revealed ten compounds from hexane extract in which 2,6-di-tert-butylnaphthalene (53.32%) the main and only two compounds were identified in chloroform extract (Borges et al., 2019). For T. aurea bark, lapachol with five more compounds were identified (Brito et al., 2020). While. HPLC/DAD/HRESIM of T. caraiba trunk bark, identified nine compounds differ from isolated compounds (Soares et al., 2020).
4 Pharmacological and toxicological activity:
4.1 Anti-inflammatory activity
Nitric oxide (NO) is an important molecule that regulates a lot of physiological processes. NO is excessively produced when the cell is activated by pro-inflammatory agents such as; tumor necrosis factor (TNF), interferon-gamma (IFN-g) and interleukin-1 (IL-1)), leading to tissue damage or even septic shock (Vincent et al., 2000). The inhibitory activity of NO production, of T. avellanedae further supports the traditional utility of this plant as an anti-inflammatory agent. Compounds (113, 240–242 and 264), isolated from T. avellanedae water extract, displayed a significant dose-dependent inhibition of NO production in LPS-activated macrophage-like cells with compound 241 being the most potent. The results proved that iridoids are active as inhibitors of NO production, while simple phenolic compounds are inactive (Awale et al., 2005). This was confirmed by Zhang et al., where the new iridoid esters (256, 257 and 258) were shown to exhibit anti-inflammatory activity through inhibition NO and PGE2 production in a dose-dependent manner, without alteration in cell viability (Zhang et al., 2017). Additionally, the aldehydic compounds, (189–191, 193 and 195) reduced the NO production and 193 and 195 decreased the PGE2 production in a dose-dependent manner, without alteration in cell viability. Make the NO production inhibition represented the most pharmacologically target of most Tabebuia species (Zhang et al., 2016). The neolignan (142) and benzoyl apiosides (131–134), from the water extract of T. avellanedae, inhibited the production of (TNF and (IL-1) in cultured human myeloma THP-1 cells stimulated with LPS without any cytotoxicity, the inhibitory activity of both (131 and 132) were more than (133 and 134), suggesting that methyoxy groups may play a vital role in activity (Suo et al., 2012). The iridoid glycosides (251–255) also inhibit IL-1β and TNF-α cytokine production and cytochrome CYP3A4 enzyme (Suo and Yan, 2016). In addition, β- lapachone (36) inhibited the neutrophil migration and reduced the concentrations of TNF-α, IL-6 and NO in animals with peritonitis (Sitônio et al., 2013). Not only the active constituents but also water extract of T. avellanedae (100 mg/kg for one week, oral administration) completely reduced the mouse ear edema induced by arachidonic acid through inhibition the production of prostaglandin (PG) E2 and NO in LPS stimulated RAW264.7 cells. This suggests a new strategy for using T. avellanedae extract for inflammatory diseases such arthritis and atherosclerosis (Byeon et al., 2008). As discussed by Park et al., upon using taheebo water extract (TWE) with colitis induced by dextran sulfate sodium treatment, TWE reduced body weight loss and colonic tissue inflammation, via up regulating type II T helper immune responses (Park et al., 2017a). In another investigation T. avellanedae ethanolic extract (Ta-EE) improved the symptoms associated with osteoarthritis and reduced the serum levels of inflammatory mediators without any toxicity (Park et al., 2017b). These results support park et al., to test TaEE on atopic dermatitis (AD) disease. Ta-EE inhibited the mRNA expression of T helper 2 and other proinflammatory cytokines (Park et al., 2018).
Tabebuia is traditionally used for its neutralization activity against venom effect. Otero et al investigated the in vitro antihaemorrhagic effect of seventy five plant extracts against Bothrops atrox venom where T. rosea displayed 100% effectiveness (Otero et al., 2000). Similarly, the hydro-ethanolic extract of T. aurea reduced the hemorrhagic and myotoxic activities induced by B. neuwiedi venom (Reis et al., 2014), in addition to reducing the hyperalgesia and neuronal injury induced by B. mattogrossensis venom (VBm)). The study related the activity to the iridoid glycosides content of the plant (Malange et al., 2019).
For uric acid and carrageenan induced inflammatory oedema, the ethanolic extract with (222 and 228) from the leaves of T. roseo alba, reduced the serum uric acid levels and decreased the paw edema induced by monosodium urate crystals (Ferraz-Filha et al., 2016). Moreover caffeic and chlorogenic acids, the constituents of the aqueous extract, reduced the serum uric acid and decreased the paw edema (Ferraz-Filha et al., 2017). Both leaves and flowers extracts of T. aurea had anti-edematogenic action (Santos et al., 2015). Alcohol and aqueous extracts of the leaves showed dose dependent anti-inflammatory activity in carrageenan induced paw oedema. While 500 mg/kg of alcohol extract showed the highest inhibition (76.92%) after only 24 hrs. (Chandrika et al., 2014). Specioside (246), isolated from T. aurea, inhibited leucocyte recruitment into the peritoneal cavity in mice injected with carrageenan (Nocchi et al., 2020).
500 mg/kg of T. hypoleuca stem extract showed a significant anti-inflammatory activity against carrageenan-induced paw edema and anti-inflammatory activity at all doses against croton oil induced auricular edema. The activity may be attributed to the presence of tannins, phenols and alkaloids. (Regalado et al., 2015).
The ethanolic extract with lapachol (24), from T. crhysotricha wood, showed a significant difference in the response times to heat stimulus in mice relative to control group (Grazziotin et al., 1992). In contrast, β-lapachone did not showed any protective effect against the lesions induced by azoxymethane in the colon of mice (Higa et al., 2011).
4.2 Anti-ulcer activity
The bark extract of T. avellanedae, had a protective effect against gastric lesions in acute and chronic ulceration models, by maintenance the protective factors, such as mucus, prostaglandin and reduction the gastric acidity (Twardowschy et al., 2008). The chronic treatment with T. avellanedae ethanolic extract twice a day for 7 days revealed a contraction in the gastric ulcer size and an increase in the mucus layer and cell proliferation (Pereira et al., 2013). Also, the methanolic extract of T. rosea (Bertol.) DC exhibited significant anti-ulcerogenic effects using ranitidine as standard drug, these effect might be due the presence of flavonoids (Kiranmai et al., 2013).
4.3 Wound healing activity
The macroscopic analysis showed a complete epithelization after 14 days treatment with T. avellanedae extract on the cutaneous wounds, while the control group still show fibroblasts and lower collagen than treated group (Coelho et al., 2010a). Likewise, bark extract of T. rosea reduced the wound diameter as well as epithelialization time and 100% healing was achieved at the 14th day post excision (Nwonu et al., 2010). On the other hand, ethanolic extract of T. aurea leaves showed no scar development better than control groups, and absence of the total re-epithelialization, at the end of fourteen days of treatment (Povoas et al., 2016). Interestingly, β-lapachone (36) was found to increase the cell proliferation, including keratinocytes, and endothelial cells, and thus accelerate wound healing (Kung et al., 2008).
4.4 Antinociceptive activity
Oral administration of T. avellanedae aqueous extract (100, 200 and 400 mg/kg), reduced the acetic acid induced nociception by 49.9%, 63.7% and 43.8%, respectively. Also, 200 mg/kg dose reduced the formalin effects at the second phase of experiment by 49.3% and inhibited the edema by 12.9% in rat paw edema model (De Miranda et al., 2001). Moreover, the same dose of the ethanolic extract, induced a significant antinociceptive activity and increased the pain threshold around 30% compared with the control. The extract also inhibited the inflammation by 30–50% (Lee et al., 2012).
The alcoholic and aqueous extracts of T. aurea leaves produced an increase in latency time compared to vehicle and a significant inhibition of writhing activity in hot plate and acetic acid induced writhing, where alcohol extract showed the highest activity after 150 min in hot plate method (4.63 ± 0.08 sec) (Chandrika et al., 2014). Moreover, 100 and 200 mg/kg of the ethanolic extract reduced the nociceptive response in acetic acid and glutamate models (Silva et al., 2018). The methanolic extract of T. hypoleuca stems showed significant antinociceptive activity using several nociception models at a doses of 300 and 500 mg/kg. Except, the second phase of formalin test, only the dose of 500 mg/kg give the antinociceptive activity (Regalado et al., 2017a). In another way, the dihydrobenzofuran lignin (141), from T. roseo-alba bark, reduced the number of writhes evoked by acetic acid injection and reduced the nociceptive behavior in the second phase of formalin test by reduction the licking time (Ferreira-Júnior et al., 2015).
4.5 Hepatoprotective and nephroprotective activity
The methanolic extract of T. rosea displayed a hepatoprotective effect against the injury induced by paracetamol in rats. The activity was confirmed by the significant reduction in the serum liver enzymes (Hemamalini et al., 2012b). The ethyl acetate and aqueous fractions of T. aurea leaves showed remarkable anti-oxidant and nephroprotective activities against carbon tetrachloride (CCl4)-induced nephrotoxicity in rats, proved by the improvements of renal serum biomarkers and histopathological features (Mahmoud et al., 2019).
4.6 Anti-obesity activity
Pancreatic lipase inhibitors are used for obesity treatment. Among 24 extracts that showed a lipase inhibitory activity more than 45%, only T. impetiginosa ethanolic extract exhibited a significant decrease in the postprandial accumulation of triglyceride levels in rats (Roos et al., 2008). Moreover, this extract can regulate the gene expression related to lipid metabolism in high fat diet-induced obesity in mice (Choi et al., 2014). Feeding with 0.5% n-BuOH fraction of T. avellanedae for sixteen weeks showed significant decrease in the body weight of mice compared to control, and significant decrease in the fat mass and triglyceride (TG) levels in ovariectomized (OVX) induced obesity (Iwamoto et al., 2016). β-lapachone decreased the body weight gain by stimulating the browning of white adipose tissue, in addition to increasing the expression of brown adipocyte–specific genes in a high-fat diet mice (Choi et al., 2016).
4.7 Antidepressant activity
The ethanolic extract of T. avellanedae (EET) produced antidepressant effect in forced swimming test and tail suspension test (TST) models in mice. The effect depends on the serotonergic, noradrenergic and dopaminergic systems. Furthermore, the extract produced a synergistic effect when combined with conventional antidepressants (Freitas et al., 2010). The Chronic administration of the EET reversed the hyperactivity like behavior and increased the immobility time happened in the TST model, in addition to, reversed biochemical changes (Freitas et al., 2013).
4.8 Antimicrobial activity
Among fourteen plant species used in Paraguay, T. avellanedae showed a broad antifungal activity. The dichloromethane (DCM) extract of T. avellanedae, displayed a growth inhibition zones against Aspergillus fumigatus, Cryptococcus neoformans, Microsporum gypseum, Penicillium purpurogenum, Saccharomyces cerevisiae and Trichophyton mentagrophytes. Methanol (MeOH) and aqueous (Aq.) extracts exhibited activity against only C. neoformans, M. gypseum, P. purpurogenum and T. mentagrophytes (Portillo et al. 2001). Another study reported that, MeOH extract, of the same species, inhibited the growth of ten Candida species, while DCM extract had inhibitory activity only against Candida krusei (Hofling et al., 2010). Additionally, the ethanolic extract had moderate inhibitory activity against Staphylococcus aureus and no activity against both, Escherichia coli and Pseudomonas aeruginosa (Lipinski et al., 2013). Hexane extract of the heartwood of T. avellanedae displayed antibacterial activity against both methicillin-resistant S. aureus and methicillin-sensitive S. aureus. The activity was attributed to α-lapachone and α-xiloidone, their MIC values were 62.5 mg/L and 125 mg/L, respectively (Machado et al., 2003).
The hydro alcoholic extract of T. avellanedae was tested for antimycobacterial activity using a time-to-kill assay. The extract reduced the bacterial growth by 2 orders of magnitude in CFU/mL within half to one hour contact, and no bacterial growth was observed after three hours contact (Oliveira et al. 2009). Another species, T. rosea, was tested for antimycobacterial activity where 500 mg/mL methanolic extract exhibited a significant activity against H37RV strain of Mycobacterium tuberculosis. Moreover, the antibacterial activity was tested against 5 human pathogens. It was found that E. coli was the highly susceptible pathogen (Anupriya et al., 2016).
Binutu and Lajubutu, reported that T. rosea (Bertol) D.C. stem bark extract showed better antimicrobial activity than that of the leaf extract (Binutu and Lajubutu, 1994). However, another study stated that leaf extract showed good inhibitory activity against tested strains with a dose dependent manner. Klebsiella pneumonia was more susceptible with inhibition zone ranging from 9.9 to 16.0 mm, while, S. epidermis was the least susceptible with inhibition zone ranging from 8.4 to 13.8 mm (Sathiya and Muthuchelian, 2008). Furthermore, leaf extract was more effective against gram positive bacterial strain and fungal strain with inhibition zone of 19 mm. The gram negative strain E. coli was least susceptible with the inhibition zone of 16 mm (Saravanan et al., 2011).
Similarly the antimicrobial activity of T. roseo-alba (Ridl.) stem bark extracts was tested. Results indicated activity of the bark methanol extract against E. coli and both ethanol and methanol extracts against S. epidermidis from (da Silva et al., 2017).
The flower extract of T. aurea showed bactericidal action, against S. epidermidis (MIC 0.06 mg/ml) and moderate action against recto S. epidermidis (MIC: 0.25 mg/mL) while against S. aureus (MIC: 0.50 mg/mL) bacteriostatic action was observed. T. aurea did not show antiradical activity but the flower extract was cytotoxic in concentrations above >0.5 mg/mL (Santos et al., 2015). The bark extract showed MIC values of 12.5 and 25 mg/mL for both S. aureus and E. coli, respectively. For C. albicans, a MIC of 25 mg/mL was obtained (Brito et al., 2020). Furthermore, all the constituents of T. aurea stem bark except 230 showed inhibition activity against S. aureus and Enterococcus faecalis. Although, 204 showed weak activity against E. coli, it showed a marked activity against yeast and filamentous fungi (Barbosa-Filho et al., 2004).
T. chrysantha leaves methanolic extract, showed mild antibacterial activity against S. aureus, while chloroform and ether extracts did not show any-bacterial activity (Pérez et al., 2007).
The ethanolic extract of T. caraiba is one of four extracts, traditionally used in Cerrado region to inhibit the growth of C. albicans. Moreover, hexane and DCM extracts inhibited the growth of Trichophyton rubrum (e Silva et al. 2009).
Vinay et al investigated the antimicrobial activity of nano formulation, where silver nanoparticles of T. argentea flower extract showed significant effect against gram-positive and gram-negative bacteria (Vinay et al., 2017).
Interrestingly, the synergistic effect of T. impetiginosa ethanolic extract with ciprofloxacin against P. aeruginosa was confirmed (Mehmood et al., 2018). Also, additive potentiation was noted for combinations containing the water extract with erythromycin, chloramphenicol or penicillin-G against E. coli and S. aureus (Fernandez and Cock, 2020).
The hydro-alcoholic extract of T. impetiginosa inhibited 36% of Helicobacter pylori growth but had no effect on Campylobacter jejuni (Cwikla et al. 2010). Several studies discussed antimicrobial activity of the active constituents from T. impetiginosa, where, Lapachol (24), displayed fungicidal activity against Gloeophyllum trabeum and Tinea versicolor at 60 µg/mL and fungistatic activity between 30 and 50 µg/mL (Velasquez et al., 2004). Also, 24 and 51, were tested against ten human intestinal bacteria, where, 51 showed a very strong inhibition against Clostridium paraputrificum, and 24 showed a moderate activity. Both compounds exhibited weak activity against both, C. perfringens and E. coli, and no activity against Bifidobacterium strains and Lactobacillus stains. It was concluded that the methyl group in the C-2 position of 1, 4-naphthoquinone derivatives might play an important role in the antibacterial activity (Park et al., 2005). Compounds 36, 37 and 38, were tested against MRSA, where, all showed antibacterial, but not bactericidal activity. Moreover, 36 and 37, displayed a considerable inhibitory activity against S. aureus (Pereira et al., 2006) and 47 exhibited strong activity against H. pylori. In the MIC bioassay, 24, 47 and 51 were more active than metronidazole but less effective than amoxicillin and tetracycline (Park et al., 2006). 14 and its enantiomer 15 showed the same activity against both fungal and Gram-positive bacteria and were inactive against Gram-negative bacteria (Yamashita et al., 2009).
4.9 Antimalarial activity
The mixture of the naphthoquinones (14 and 15), scored the highest antimalarial activity with significant (IC50) 1.67 × 10–7 against Plasmodium berghei and 6.77 × 10–7 against P. falciparum (Pérez et al., 1997). All constituents of T. billbergii inner bark and trunk wood proved to have anti-malarial activity, with very encouraging LC50′s ranging from (28–163 µg/ml). The strongest inhibitory activity against P. berghei was observed for 2-acetyl-naphtho-[2,3b]-furan-4,9-dione (4) with (LC50 28 µg/ml) (Gómez-Estrada et al., 2012).
4.10 Antileishmanial activity
The n-hexane and DCM fractions of T. avellanedae displayed the highest antileishmanial activity with IC50 of 64 µg/ml and 41 µg/ml, respectively. Compound 24, isolated from n-hexane fraction, exhibited antileishmanial activity with IC50 values of 33 µM and 115 µM, respectively. A mixture of 14 and 15, from DCM fraction, showed activity with IC50 of 4 µM that is more active than 24. These results suggested that presence of a furan ring may increase the antileishmanial activity of naphthoquinones (Ali et al., 2010).
The chloroform extract of T. serratifolia showed activity against T. cruzi and L. infantum parasites, with inhibition percentages greater than 96%. Compound (13) was the most active constituent against L. infantum and T. cruzi, with a growth-inhibition concentration of 0.01 μg/mL and this value was lower than Nifurtimox and similar to Amphotericin B (Gonzalez-Coloma et al., 2012).
4.11 Antiviral activity
The ethanolic exytracts of both, T. aurea stem and T. cassinoides leaf and stem had no activity against encephalomyocarditis virus, human herpes virus 1 and vaccinia virus Western Reserve strain. The lack of activity may be due to the high cytotoxicity of naphthoquinones present in the extracts (Brandão et al. 2010a). However, another study estimated the antiviral activity of the ethanolic extracts of both, T. impetiginosa and T. serratifolia against the same viruses and concluded that T. impetiginosa extract exerted activity against HHV-1, with a one-half maximal effective concentration (EC50) of 166.6 μg/mL (Brandão et al. 2010b).
4.12 Insecticidal activity
6-(1-hydroxyundec-3-enyl)-tetrahydropyran-2-one (215), isolated from T. palmeri, was previously tested with insecticidal activity against Bruchus chinensis using oviposition inhibition assay. Compound 215 showed oviposition inhibition, so helped for the disruption of egg laying in the field and reduced the pest population (Upadhyay et al., 2006). Depending on LC50 values lapachol (24) was about 20.8 times more toxic than abamectin against Tetranychus urticae. While, benzyl benzoate exhibited higher acaricidal activity than 24 against T. putrescentiae (Jeon and Lee, 2011). Both, 24 and its analogues gave similar results against Laodelphax striatellus, except for 2, 3-Dichloro-1,4-naphtoquinone and 5,8- Dihydroxy-1,4-naphtoquinone, but naphtho[2,3- b]furan-4,9-dione was the most active compound against Nilaparvata lugens (0.042 µg/female), followed by its analogue 5,8-Dihydroxy-1,4-naphtoquinone (0.080 µg/female) (Jeon et al., 2011). 24 also scored similar results against Aedes aegypti and Ochlerotatus togoi larvae with its derivatives (Kim et al., 2013). However, 24 did not exhibit repellent activity against Reticulitermes termites, but showed activity to other termites as, Microcerotermes crassus and Kalotermes flavicollis. On the other hand, 38 and 46, showed repellence activity against various Reticulitermes, as well as Termitidae and Kalotermitidae species. These study concluded that extracts worked better than isolated compound and small changes in the molecules significantly change the activity (Becker et al., 1972; Castillo and Rossini, 2010). In a recent research, docking analysis was performed to predict the interactions between the major constituents of T. heptaphylla wood extracts and the odorant binding receptor of A. aegypti. The analysis predicted significant binding of 24 with the internal active pocket of the mosquito odorant binding receptor, that explain why the gel and cream formulations containing T. heptaphylla extracts protect up to 3 hr. against the bites of A. aegypti (Borges et al., 2019).
A year before, Borges et al., proved that the acetone and ethyl acetate extracts of T. avellanedae were more toxic against 3rd instar A. aegypti larvae, with CL50 of 100.1 and 151.0 μg/mL, respectively. The mortality values (LT50 and LT95) were 38.66 and 66.74 min for ethyl acetate extract, respectively, and 53.47 and 119.96 min for acetone extract, respectively. All extracts presented 100% mortality after 12 hr. The ethanol extract at 333.3 μg/mL strongly deterred oviposition by 89.89% while the ethyl acetate and acetone extracts presented 89.04 and 68.10% deterrence, respectively (Borges et al., 2018).
4.13 Anti-oxidant activity
T. impetiginosa volatiles extract was able to inhibit the oxidation of hexanal for 40 days at a level of 5 µg/mL (Park et al., 2003). Moreover, the syrup and methanolic extract of T. impetiginosa exhibited the highest anti-oxidant activity, related to their highest amount of phenolics and flavonoids (Pires et al., 2015).
Young and old leaf extracts of T. heptaphylla showed a lipid peroxidation inhibition induced by H2O2 and FeSO4 in concentrations of 20 and 200 μg/mL and 2 and 20 mg/mL, respectively (Budni et al., 2007).
In the interested comparative studies, Franco Ospina et al., concluded that the ethanolic extracts of T. rosea was more active as anti-inflammatory, while, T. ochracea was more potent as antioxidant. But both species revealed significant antibacterial activity against S. aureus (Franco Ospina et al., 2013). In another way, the ethanolic extracts of T. rosea and T. argentea flower represent a promising natural sources of anti-oxidants suitable for application in nutritional and pharmaceutical fields (Sobiyana et al., 2019). Also, the ethyl acetate fraction of T. rosea leaves, scored the highest DPPH radical scavenging activity. Moreover, n-hexane, chloroform, and aqueous extracts, in addition to inner bark aqueous extract inhibit the nitric oxide production by over 90%. Furthermore, the inner bark extracts significantly inhibited prostaglandins E2 and tumor necrosis factor alpha (>90%) (Jimenez-Gonzalez et al., 2018).
T. pallida leaves (TPL) extract displayed the highest total anti-oxidant capacity in DPPH and hydroxyl radical scavenging activity, and the strongest radical scavenging activity when compared with standards (Rahman et al., 2015). The ethyl acetate fraction (EAF) exhibited the highest phenolic and flavonoids content, and scored the highest total anti-oxidant capacity than other extracts (Rahman et al., 2019). The phenylpropanoid glycosides (93, 94 and 96–99), from T. avellanedae water extract, displayed anti-oxidant activity in DPPH assay. Compound 98 exhibited the highest activity with IC50 of 0.12 µM, however all compounds showed moderate inhibitory activity on CYP3A4 enzyme except, 99 that was the most active with IC50 value of 15.1 µM. Compounds 97, 98 and 99 were more active than of 93, 94 and96 in both assays, suggesting that galactose group plays important role in the activity (Suo et al., 2013).
The lignan (150), from T. chrysotricha, exhibited the highest DPPH radical-scavenging activity (IC50; 17.7 ± 0.2 μM), giving an indication that increasing the number of methoxy groups positively affected the activity (Takahashi et al., 2015).
4.14 Cytotoxic activity
Naphthoquinones are commonly used for treating a number of diseases, including cancer. The antitumor activity of Tabebuia was evaluated in several studies. Both compounds 14 and 15 exhibited significant dose-dependent inhibitory effects against Epstein-Barr virus (EBV) expression assay (Ueda, et al., 1994). de Saizarbitoria Colman et al., proved that lapachol (24) is less antiproliferative than other naphthoquinone derivatives, where all the compounds isolated from T. barbata except lapachol had a significant cytotoxic activity against A-549 human lung adenocarcinoma, MCF-7 human breast carcinoma and HT-29 human colon carcinoma cells with IC50 values (15–82.5 µM) (de Saizarbitoria Colman et al., 1997). Also 14 exhibited more potent antiproliferative and higher cancer chemopreventive activity against several human tumor cell lines than its enantiomer 15 with lower effect against normal human cell lines. The study revealed that the presence of hydroxyl group at C-5 is increases antiproliferative activity (Yamashita et al., 2009). The ethanolic extract of T. incana and its chloroform fraction showed significant lethality (LC50 167 ± 39 and 12 ± 4 mg.ml−1, respectively), however, hexane and water-methanol fractions were inactive. The mixture of 14 and 15 was about as active as chloroform fraction (LC50 15 ± 10 mg.ml−1), from which they were isolated, with the existence of other cytotoxic components (Morais et al., 2007). Compounds; 15 and 22, from T. avellanedae inner bark, were evaluated against A549, SiHa and MCF-7 cell lines and they were able to induce a cell cycle arrest and apoptosis at G2/M phase in A549 cells by strongly decreasing the levels of cyclin protein (A and B) with time dependent manner (Zhang et al., 2015). 14, 23 and 32 showed significant cytotoxic activity against both KB, and HeLa cell lines where 23 was the most active suggesting that the presence of methylethenyl furan-moiety, causes better cytotoxicity against both cell lines (Sichaem et al., 2012). β-lapachone (36) inhibited the growth and induce apoptosis in a time- and dose-dependent manner in the human lung carcinoma cell line A549. The apoptosis was ascribed to down regulation of the levels of both, human telomerase RNA (hTR) and c-myc expression (Woo and Choi, 2005). The activity of 36 on the human hepatoma cell line HepG2 was related to the apoptosis by the formation of apoptotic bodies and DNA fragmentation (Woo et al., 2006). In addition, 36 had anti-proliferative and apoptotic effects on human malignant melanoma by regulation of Sp1-mediated gene products (Bang et al., 2016). Furthermore, 120 mg/kg of T. avellanedae extract and 1 mg/kg of 36 prolonged the life span of tumour-bearing mice, and produced the same level of survival. They act synergistically with specific cytokines to enhance the macrophage activation against tumour cells (Queiroz et al., 2008). The activity of T. avellanedae inner bark extract against estrogen receptor positive human breast cancer cells was related to the down-regulation of the cell cycle regulatory and estrogen responsive genes, in addition to, up-regulation of both apoptosis and biotic metabolism specific genes (Mukherjee et al., 2009). Furthermore, T. avellanedae inner bark under the name of (TNM) was used as an effective nutritional alternative for aromatase positive, post-menopausal breast cancer (Telang et al., 2019).
The n-Hexane, chloroform and ethyl acetate fractions of T. impetiginosa displayed a significant inhibition of platelet aggregation induced by collagen and arachidonic acid (AA) in a dose-dependent manner. The chloroform fraction, significantly suppressed AA liberation and inhibited the cell proliferation and DNA synthesis (Son et al., 2006). The methanolic extract was evaluated against human tumor and non-tumor cells lines. The extract showed cytotoxic activity, without any toxicity on PLP2 non tumer cell line (Pires et al., 2015).
Total alkaloid extract of T. rosea (Bertol.) DC. leaves showed higher toxicity towards human leukemic cells (MOLT-4) than the normal cells in a dose and time dependent manner (Sathiya and Muthuchelian, 2010). The chloroform extract of inner bark displayed the best antiproliferative activity against both HepG2 and B16F10 cell lines (Jimenez-Gonzalez et al., 2018). On the other hand, the cytotoxic activity of T. roseo-alba (Ridl.) was observed at the concentration of 500 μg/mL for all samples, while at 100 μg/mL only the proliferation of the macrophages was observed (da Silva et al., 2017).
The hydroethanolic extract of T. aurea bark was able to inhibit the growth of cervical carcinoma lineage (HELA) by about 50% at 24–72 h with no significant toxic effects against normal cells such as human fibroblasts (GM0749) (Brito et al., 2020).
For T. chrysantha stem, the methanolic extract, showed a direct cytotoxic effect against Ehrlich Ascites Carcinoma (EAC) in a dose-dependent manner with IC50 value 463.27 μg/mL in MTT assay and 443.58 μg/mL in trypan blue dilution assay (Panda et al., 2019). Panda et al., suggested that a low dose of T. chrsantha extract can be used as a novel product to suppress angiogenesis and cell proliferation associated with angiosarcoma and that the isolated flavonoid (91) functions as specific regulators of target protein-associated angiosarcoma (Panda et al., 2020).
4.15 Cosmotics and skin care activity
T. avellanedae extracts inhibited the biosynthesis of prostaglandin E2, thus relieves the skin irritation caused by lactic acid and the erythema caused by UV radiation (Woo et al., 2009). Moreover, the ethanolic extract inhibited both tyrosinase activity as well as melanin biosynthesis (Kim et al., 2015a). β-lapachone (36) was proved to be useful as a potential depigmentation agent for various hyperpigmentation disorders due to its ability to inhibit melanin synthesis and tyrosinase activity at 0.8 lM in melan-a cells, reducing melanogenesis in the human 3D skin tissue culture, as well as inhibition of body pigmentation of zebrafish (Kim et al., 2015b).
T. impetiginosa extracts were reported to have a degranulation inhibitory activity which improves skin pigmentation, dermatitis, wrinkles, pruritus, and pain caused by chemicals. Also, T. impetiginosa has skin whitening, anti-inflammatory, anti-allergic, and anti-oxidant effects (Osawa et al., 2006). Moreover, the bark extract could stimulate collagen synthesis by human follicle dermal papilla cell (Iwano et al., 2013). In Addition, the cosmetic combination of T. impetiginosa and Codium Tomentosum extracts can selectively proliferate the beneficial microorganism present in the skin, inhibit the pathogenic microorganism and help the skin-beneficial microorganism to maintain the barrier function against the external environment (Lee, 2017).
4.16 Miscellaneous bioactivities
The dimeric iridoid (284), from T. argentea efficiently inhibit the chaperone in biochemical and cellular assays. The results revealed C9-type iridoids as a novel class of heat shock protein 90 inhibitors as a therapeutic target for numerous diseases (Piaz et al., 2013).
T. hypoleuca stem methanolic extract (500 mg/kg), induced a significant decrease in the fever from the first hour to 4 h. After administration without exerting sedative or hypnotic effects at the tested doses (Regalado et al., 2017b).
T. impetiginosa extract could manage the hyper-triglyceridemia and other factors of cardiovascular disease that common in obesity and diabetes (Kiage-Mokua et al., 2018).
T. avellanedae is a great candidate for treatment of primary dysmenorrhea as it inhibits the production of PGE2 and reduces COX-2 activity. Quality of life, pain intensity and inflammatory markers were evaluated and the trial approved by the Institutional Review Board at Helfgott Research Institute and the National University of Natural Medicine (McClure et al., 2019).
4.17 Genotoxic activity
The genotoxicity, evaluated via wing somatic mutation and recombination test, revealed that the bark and stem extracts of T. impetiginosa were toxic, however not genotoxic by itself, but it possesses a significant potentiating effect on DXR genotoxicity, considering that T. impetiginosa possess anticarcinogenic potential (Sousa et al., 2009). The genotoxic activity of the flower extract was estimated on the blood and liver cells of Wistar rats. Except the dose of 100 mg /kg body weight, a significant increase in DNA damage compared to the control was noted. The genotoxic potential was higher in liver cells but the response in both tissues was related to dose-dependency. While, the DNA damage can be corrected before conversion into mutations (Lemos et al., 2012).
The genotoxic potential of the alkaloid extract of T. rosea was tested using micronucleus assay. The number of micronuclei formed even at the highest concentration was insignificant with that of the positive control mitomycin-C, supporting the absence of genotoxicity (Sathiya and Muthuchelian, 2010).
The LD50 of methanolic and aqueous extracts of T. aurea bark was estimated as 4608 μg/mL and 104,656 μg/mL, respectively. The results indicated that both extracts did not induce a significant changes in mitotic index of Allium cepa roots or induced the formation of micronuclei. Accordingly, they are cytotoxic, but not mutagenic (Lucas et al., 2019).
5 Discussion and future perspectives
Tabebuia has been used for a long time as therapeutic alternative by rural population. The present review summarizes the research progress regarding Tabebuia species, with particular consideration to the traditional uses, chemical constituents and biological activities. Pharmacological studies that carried out on crude extracts and pure metabolites provided pragmatic documents for its traditional uses, as Tabebuia has been effectively used traditionally for treating syphilis, malaria, skin and stomach disorders, cancer, inflammation, pain, irritability, depression, diabetes, prostatitis, constipation and allergies.
The presented data clearly states that all the reported phytochemical and pharmacological studies, focus extensive attention towards only some species, however, the majority of Tabebuia species still require more extensive future investigated as showed in Fig. 9.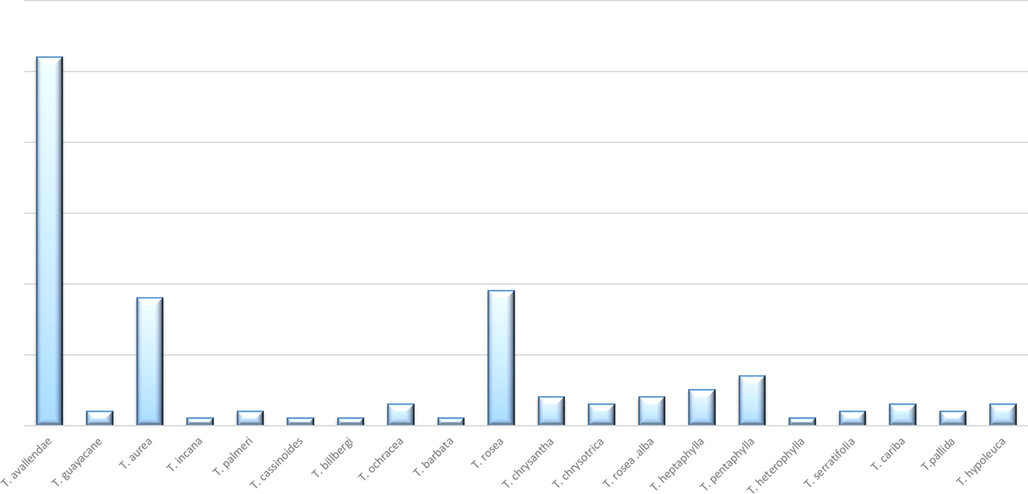
The relative percentage of all published chemical and biological reports regarding Tabebuia species.
Additionally, the state of the art on Tabebuia chemistry gives considerable opportunities for future discoveries. Approximately 292 chemical constituents have been isolated from different Tabebuia species (Fig. 10). These metabolites belong to different classes; naphthoquinones, flavonoids, lignans, coumarins, aldehydes, acids, esters, fatty acids, sterols, irridoids and carotenoids. Throughout the chemical achievements, there are still scientific gaps.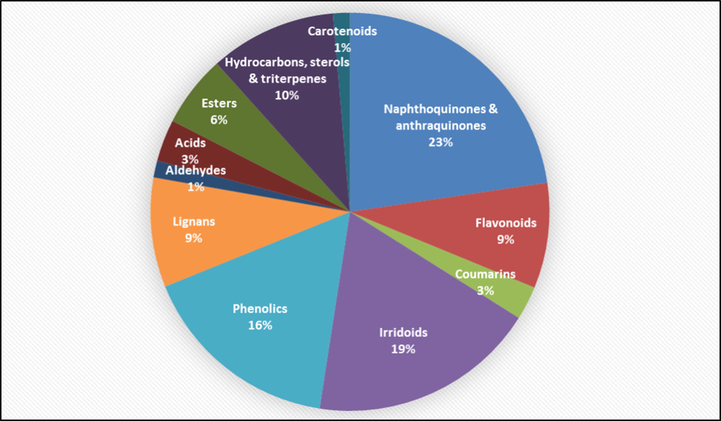
The distribution of the secondary metabolites among Tabebuia species.
First, the total alkaloids extract of T. rosea leaves showed cytotoxic activity against human T-cell leukemia (MOLT-4) cells. As this chemical class is unique to Tabebuia and the alkaloids are famous for its valuable pharmacological activities. Therefore, future studies are required for precise isolation and identification of each alkaloid structure by an in-depth exploration techniques.
Second, the research of flavonoids, lignans, aldehydes, acids and esters was relatively slow compared with the study of naphthoquinones, anthraquinones and irridoids, while, the study of coumarin compounds is still in its initial stage. Thus, it may be possible that more bio-active components could be identified by using bioactivity guided isolation strategies.
Third, napthoquinones and anthraquinones in addition to phenyl ethanoid and phenyl propanoid compounds were mainly isolated from the bark and wood organs of Tabebuia species. However, screening other organs may provide more chances for discovering a new bioactive principles, likewise flavonoids; that were mainly isolated from the leaves and flowers of Tabebuia organs, while the new derivative (88) and the new flavonoid (TMF) (91) were isolated from the stem of T.aurea and T. chrysantha, respectively.
Fourth, Fig. 11 illustrates the relative percentage of the secondary metabolites isolated from each Tabebuia species under investigation. These results indicated that T. pallida and T. hypoleuca are only biologically explored (Rahman et al., 2015; Rahman et al., 2019; Regalado et al., 2015), while other species such as; T. guayacan, T. cassinoids, T. barbata, T. heterophylla, T. serratifolia and T. rosea alba are insufficiently chemically studied. Taking these in consideration, more studies are needed for better understanding their chemical bases to explain the claimed biological activities.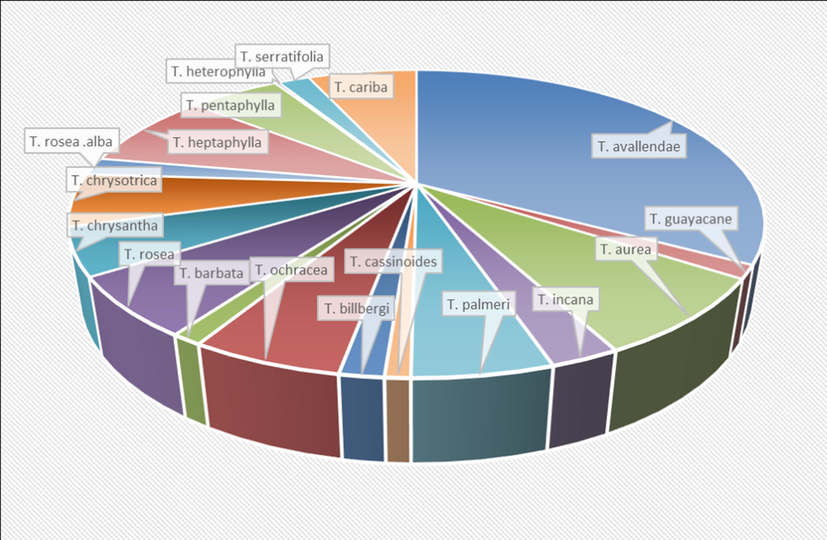
The relative percentage of secondary metabolites isolated from each Tabebuia species.
For further in-depth phytochemical scanning, Fig. 12 is performed to illustrate the type and the relative percentage of each chemical class isolated from Tabebuia species. Although there have been marked achievements in the phytochemical studies regarding Tabebuia species, there are still some notifications that have not been clarified. These notifications are as the following: (1) Flavonoids are not isolated from T. avallandae, although these species take extensive phytochemical attention. (2) Phenyl ethanoid and phenyl propanoid compounds from phenolic chemical class are mainly isolated from T. avallandae, although they are recently isolated from other species like; T. chrysotricha and T. caraiba (Takahashi et al., 2015; Soares et al., 2020), respectively, so further studies are required. (3) Finally, the species and chemical classes that require more phytochemical studies are also obvious. However, other chemical classes like; naphthoquinones, lignans, irridoids, hydrocarbons, fatty acids and sterols, are widely distributed among different Tabebuia species.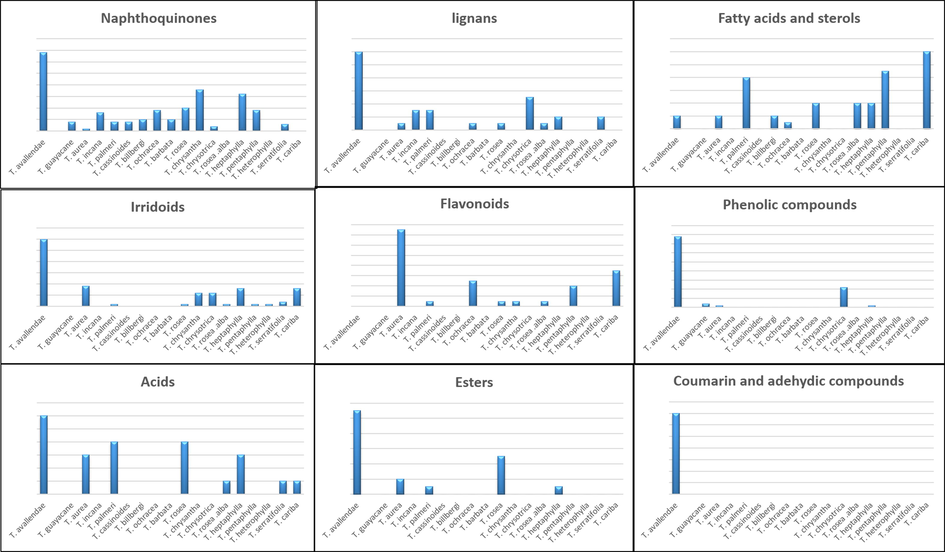
The relative percentage of each chemical classes among different Tabeuia species.
Furthermore, in spite of the large number of pharmacological studies regarding the medicinal importance of Tabebuia species, there are still several gaps in our understanding of the applications of these plants. First gap, is that some of the pharmacological activities in vitro and in vivo studies have been obtained with doses that can be high for clinical study. For example, doses of Tabebuia extracts that applied to evaluate anti-inflammatory, antinociceptive and sedative or hypnotic effects (administrated 500 mg/kg of extract in mice) are too high for application in clinical studies.
Second, pharmacokinetic data and the penetration capacity of the total extract or the plant's ingredients into the central nervous system is still unstudied. In addition, there are poor reported information focused on the main side effect or the safety of the plant extract or its components.
Third, the promising results confirmed by animal models should be further investigated by clinical studies, like, β-lapachone, the most common naphthoquinone isolated from T. avallandae and other Tabebuia species is now in clinical trial phase as plant derived anticancer agents (Nirmala et al., 2011).
Fourth, modern studies are now focused on nanosize materials. T. argentea silver nanoparticles were successes to possess significant antimicrobial activity against both gram positive and gram negative bacteria (Vinay et al., 2017). So, further studies are needed to illustrate the activity of Tabebuia extracts and isolated compound nanoparticles against different pharmacological aspects.
Fifth, analyses of the structure–activity relationships studies are still insufficient.
Sixth, despite, the numerous pharmacological activities of Tabebuia species, most of functional mechanisms remain unclear and need further exploration through in vivo and in vitro experiments.
The different pharmacological activities performed on Tabebuia species are illustrated in Fig. 13. The presented data indicated extensive pharmacological studies of some species e.g. T. avallandae, T. aurea and T. rosea, other species like T. billbergi, T. palmeri, T. ochracea, T. chrysotrica, T. rosea alba, T. serratifolia and T. cariba, remain insufficiently studied. Furthermore, some species as T. guayacan, T. cassinoids, T. barbata, T. heterophylla, T. incana T. pentaphylla are not pharmacologically reported till now.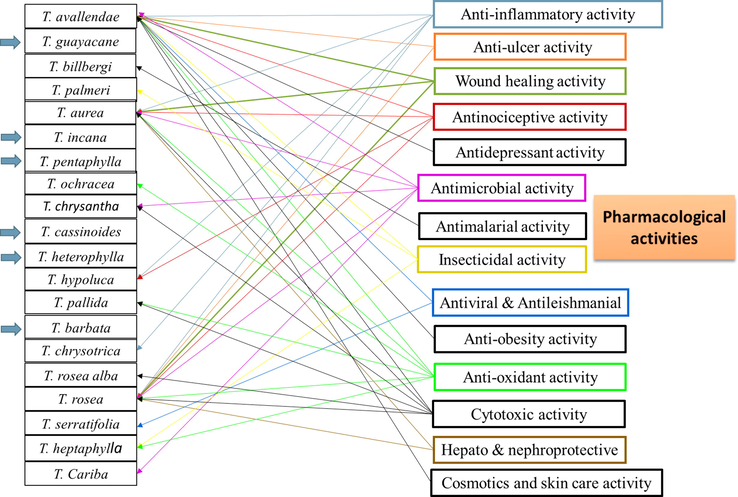
The pharmacological activities of different Tabebuia species.
Second, T. avallandae showed antidepressant, antimalarial and anti-obesity activities. Furthurmore, T. rosea and T. aurea showed significant hepato and nephroprotective activities, respectively. These results suggest similar biological testing for other Tabebuia species extracts as well as their isolated pure compounds.
6 Conclusion
The current review helps to develop a high resolution picture about genus Tabebuia, its most studied species, main active constituents and reported biological activities. It also helps to recognize the importance of different species in traditional systems of medicine. Additionally, it provides suggestion for some Tabebuia species that need further phytochemical and/or pharmacological investigations.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Plantas da medicina popular dos Cariris Velhos, Paraíba, Brasil. João Pessoa, Editora União; 1996.
- Medicinal and poisonous diversity of the flora of “Cariri Paraibano”, Brazil. J. Ethnopharmacol.. 2007;111:383-395.
- [Google Scholar]
- Lapachol and isomeric 5-and 8-hydroxy-2-(1′-hydroxyethyl) naphtho [2, 3-b] furan-4, 9-diones are effective antileishmanial constituents of Tabebuia avellanedae. Planta Med.. 2010;76:471.
- [Google Scholar]
- Screening of Tabubeia rosea dc: for antituberculosis, antibacterial and antioxidant studies; an in vitro approach. Indo Am. J. Pharm. Sci.. 2016;6:5297-5306.
- [Google Scholar]
- Medicine and magic among the Maka Indians of the Paraguayan Chaco. J. Ethnopharmacol.. 1987;21:279-295.
- [Google Scholar]
- Plants of common use in Paraguayan folk medicine for regulating fertility. Econ. Bot.. 1977;31:298-300.
- [Google Scholar]
- Nitric oxide (NO) production inhibitory constituents of Tabebuia avellanedae from Brazil. Chem. Pharm. Bull.. 2005;53:710-713.
- [Google Scholar]
- Bandoni, A.L., Mendiondo, M.E., Rondina, R.V.D., Coussio, J.D., 1972. Survey of Argentine medicinal plants. I. Folklore and phytochemical screening. Lloydia.
- β-lapachone suppresses the proliferation of human malignant melanoma cells by targeting specificity protein 1. Oncol. Rep.. 2016;35:1109-1116.
- [Google Scholar]
- Botanical study, phytochemistry and antimicrobial activity of Tabebuia aurea:(with 1 table and 1 figure) Phyton (Buenos Aires). 2004;73:221-228.
- [Google Scholar]
- Unterschiede im Verhalten und der Giftempfindlichkeit verschiedener Termiten-Arten gegenüber einigen Kernholzstoffen. Zeitschrift für. Angew Entomol.. 1972;71:201-214.
- [Google Scholar]
- Benzie I.F., Wachtel-Galor S., eds. Herbal Medicine: Biomolecular and Clinical Aspects. CRC Press; 2011.
- Bernal, H.Y., Correa, J.E., 1989. Especies vegetales promisorias de los paı́ses del convenio André s Bello, Tomo II, Bogotá, 226–259.
- Iridoids in Equatorial and Tropical flora - III. Isolation and partial synthesis of 6-epiaucubin, a new glucosidic iridoid. Tetrahedron. 1982;38:359-362.
- [Google Scholar]
- Iridoids in Equatorial and Tropical flora. Part 4. Isolation of amareloside. Planta Med.. 1982;46:33-37.
- [Google Scholar]
- Iridoids in Equatorial and Tropical flora. V. A new glucosidic iridoid from Tecoma chrysantha Jacq. Gazz. Chim. Ital.. 1982;112:227-229.
- [Google Scholar]
- Antimicrobial potentials of some plant species of the Bignoniaceae family. Afr. J. Med. Med. Sci.. 1994;23:269-273.
- [Google Scholar]
- Phytochemical Study of Tabebuia Pentaphylla Hemsl Cultivated In Egypt. Bull. Pharm. Sci, Assiut University. 1987;10:1-20.
- [Google Scholar]
- Flavonoids of Bignoniaceae from the “cerrado” and their possible taxonomic significance. Plant Syst. Evol.. 1998;210:289-292.
- [Google Scholar]
- Antimicrobials from herbs, spices, and plants. In: Fruits, Vegetables, and Herbs. Academic Press; 2016. p. :551-578.
- [Google Scholar]
- Chemical composition, oviposition deterrent and larvicidal activities of the wood extracts of Tabebuia avellanedae from the Cerrado of Brazil. J. Med. Plant Res.. 2018;12:404-414.
- [Google Scholar]
- Mosquiticidal and repellent potential of formulations containing wood residue extracts of a Neotropical plant, Tabebuia heptaphylla. Ind. Crops Prod.. 2019;129:424-433.
- [Google Scholar]
- Antiviral activity of Bignoniaceae species occurring in the State of Minas Gerais (Brazil): part 1. Lett. Appl. Microbiol.. 2010;51:469-476.
- [Google Scholar]
- b. Antiviral activities of plants occurring in the state of Minas Gerais, Brazil: Part 2. Screening Bignoniaceae species. Rev. Bras. Farmacogn.. 2010;20:742-750.
- [Google Scholar]
- Brito, M.C.A., Pereira, L.P.L.A., Guimarães, S.J.A., de Castro Júnior, J.R., Chagas, V.T., Arruda, M.O., Coutinho, D.F., 2020. Bioprospection of Tabebuia aurea (Silva Manso) Benth. and Hook. f. ex S. Moore: chemical, biological and toxicity studies.
- Preliminary studies of the antioxidant activity of adult and young leaf extract hydroetanclic of Tabebuia heptaphylla (Vell) Latin. Am. J. Pharm.. 2007;26:394-398.
- [Google Scholar]
- Naturally occurring quinones. Part XII. Extractives from Tabebuia chrysantha nichols and other bignoniaceae. J. Chem. Soc. C Org.. 1968;850–853
- [Google Scholar]
- Tabebuia avellanedae Lorentz ex Griseb. In: Medicinal and Aromatic Plants of South America. Dordrecht: Springer; 2018. p. :439-451.
- [Google Scholar]
- In vitro and in vivo anti-inflammatory effects of taheebo, a water extract from the inner bark of Tabebuia avellanedae. J. Ethnopharmacol.. 2008;119:145-152.
- [Google Scholar]
- Red Lapacho (Tabebuia impetiginosa)-a global ethnopharmacological commodity. J. Ethnopharmacol.. 2009;121:1-13.
- [Google Scholar]
- Antinociceptive and anti-inflammatory activity of Tabebeuia aurea leaf extracts. Int. J. Ayurvedic Herb Med.. 2014;4:1520-1526.
- [Google Scholar]
- Beta-lapachone prevents diet-induced obesity by increasing energy expenditure and stimulating the browning of white adipose tissue via down-regulation of miR-382 expression. Diabetes. 2016;65:2490-2501.
- [Google Scholar]
- Ethanolic extract of Taheebo attenuates increase in body weight and fatty liver in mice fed a high-fat diet. Molecules. 2014;19:16013-16023.
- [Google Scholar]
- Phytochemistry of the family bignoniaceae-A review. Assam Univ. J. Sci. Technol.. 2011;7:145-150.
- [Google Scholar]
- Effects of silver sulfadiazine, ipê roxo (Tabebuia avellanedae) extract and barbatimão (stryphnodendron adstringens) extract on cutaneous wound healing in rats. Rev. Col. Bras. Cir.. 2010;37:45-51.
- [Google Scholar]
- Isolation of 6-O-(p-Coumaroyl)-Catalpol from Tabebuia rosea. Planta Med.. 1982;46(1):42-44.
- [Google Scholar]
- Corrêa, M.P., de Azeredo Penna, L., 1984. Dicionário das plantas úteis do Brasil e das exóticas cultivadas: HL, Vol. 4. Ministério da Agricultura, Instituto Brasileiro de Desenvolvimento Florestal.
- Investigations into the antibacterial activities of phytotherapeutics against Helicobacter pylori and Campylobacter jejuni. Phytother. Res. 2010;24:649-656.
- [Google Scholar]
- Evaluation of the Cytotoxic, Antimicrobial and Antioxidant Activity of the Plant Especies Tabebuia roseo-alba (Ridl) Sand. J. Chem. Pharm. Res.. 2017;9:148-153.
- [Google Scholar]
- Nomes vulgares de plantas amazônicas. Manaus, Amazonas: INPA/CNPq; 1977. p. :222.
- Vernolic acid intabebuia argentia seed oil: A moderate source of oil. J. Am. Oil Chem. Soc.. 1991;68:520-521.
- [Google Scholar]
- Phenolic glycosides from Tabebuia argentea and Catalpa bignonioides. Phytochem. Lett.. 2014;7:85-88.
- [Google Scholar]
- Antinociceptive and antiedematogenic properties and acute toxicity of Tabebuia avellanedae Lor. ex Griseb. inner bark aqueous extract. BMC Pharmacol.. 2001;1:6.
- [Google Scholar]
- Lignans and naphthoquinones from Tabebuia incana. Phytochemistry. 1993;34:1409-1412.
- [Google Scholar]
- Bioactive furonaphtoquinones from Tabebuia barbata (Bignoniaceae) Acta Cient. Venez.. 1997;48:42-46.
- [Google Scholar]
- Oroxylum indicum–a medicinal plant of North East India: an overview of its nutritional, remedial, and prophylactic properties. J. Appl. Pharm. Sci.. 2013;3:104-112.
- [Google Scholar]
- Furanonaphthoquinones from Tabebuia ochracea ssp. neochrysanta. J. Nat. Prod.. 1996;59:423-424.
- [Google Scholar]
- Handbook of Medicinal Herbs. Boca Ratón: CRC Press; 1985.
- Amazonian Ethnobotanical Dictionary. Ann Arbor: CRC Press; 1994.
- Evaluation of the antifungal potential of Brazilian Cerrado medicinal plants. Mycoses. 2009;52:511-517.
- [Google Scholar]
- Lapachol and its congeners as anticancer agents: a review. Phytochem. Rev.. 2014;13:37-49.
- [Google Scholar]
- Tabebuia impetiginosa (Mart. Ex DC. Mattos) bark extracts inhibit the growth gastrointestinal bacterial pathogens and potentiate the activity of some conventional antibiotics. Phcog. Commn.. 2020;10:75-82.
- [Google Scholar]
- Tabebuia roseoalba: in vivo hypouricemic and anti-inflammatory effects of its ethanolic extract and constituents. Planta Med.. 2016;82:1395-1402.
- [Google Scholar]
- Effects of the aqueous extract from Tabebuia roseoalba and phenolic acids on hyperuricemia and inflammation. Evid. Based Complement. Altern. Med.. 2017;2017:1-10.
- [Google Scholar]
- Isolation of a dihydrobenzofuran lignan, icariside E 4, with an antinociceptive effect from Tabebuia roseo-alba (Ridley) Sandwith (Bignoniaceae) bark. Arch. Pharm. Res.. 2015;38:950-956.
- [Google Scholar]
- Actividad antiinflamatoria, antioxidante y antibacteriana de dos especies del género Tabebuia. Rev. cuba plantas Med.. 2013;18:34-46.
- [Google Scholar]
- Antidepressant-like action of the ethanolic extract from Tabebuia avellanedae in mice: evidence for the involvement of the monoaminergic system. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34:335-343.
- [Google Scholar]
- Antidepressant-like action of the bark ethanolic extract from Tabebuia avellanedae in the olfactory bulbectomized mice. J. ethnopharmacol.. 2013;145:737-745.
- [Google Scholar]
- Studies on the structure and stereochemistry of cytotoxic furanonaphthoquinones from Tabebuia impetiginosa: 5-and 8-hydroxy-2-(1-hydroxyethyl) naphtho [2, 3-b] furan-4, 9-diones. J. Chem. Soc. Perkin Trans. 1991;I:2323-2327.
- [Google Scholar]
- New constituents from the trunk bark of Tabebuia heptaphylla. Quim. Nova. 2007;30:1887-1891.
- [Google Scholar]
- Garcı́a Barriga, 1975. H. Flora Medicinal de Colombia.Instituto de Ciencias: Naturales, Bogota, Vol. 3.
- A revision of tabebuia (bignoniaceae) in Central America. Brittonia. 1970;22:246-264.
- [Google Scholar]
- A synopsis of Bignoniaceae ethnobotany and economic botany. Ann. Missouri Bot. Garden. 1992;79:53-64.
- [Google Scholar]
- Effect of lapachol, a naphthaquinone isolated from Tectona grandis, on experimental peptic ulcer and gastric secretion. J. Pharm. Pharmacol.. 1987;39:138-140.
- [Google Scholar]
- In vitro antimalarial activity of fractions and constituents isolated from Tabebuia billbergii. Rev. cuba plantas med.. 2012;17:172-180.
- [Google Scholar]
- Antileishmanial, antitrypanosomal, and cytotoxic screening of ethnopharmacologically selected Peruvian plants. Parasitol Res.. 2012;110:1381-1392.
- [Google Scholar]
- Phytochemical and analgesic investigation of Tabebuia chrysotricha. J. Ethnopharmacol.. 1992;36:249-251.
- [Google Scholar]
- Grenand, P., Moretti, C., Jacquemin, H., Prévost, M., 2004. Pharmacopées Traditionnelles en Guyane:Créoles, Wayãpi, Palikur. IRD Éditions, Paris.
- Taxonomic revisions in the polyphyletic genus Tabebuia s. I. (Bignoniaceae) Syst. Bot.. 2007;32:660-670.
- [Google Scholar]
- Comparison of antibacterial and antifungal activities of lapachol and β-lapachone. Planta Med.. 1994;60:373-374.
- [Google Scholar]
- Gupta M., (Ed.) 1995. 270 Plantas Medicinales Ibero-americanas. CYTED-SECAB, Santafé de Bogotá, Co-lombia, pp. 191–193.
- An ethnopharmacological survey of the traditional medicine utilized in the community of Porvenir, Bajo Paraguá Indian Reservation. Bolivia. J. Ethnopharmacol.. 2012;139:838-857.
- [Google Scholar]
- Illustrated Cyclopedia of Brazilian Medicinal Plants. Aboc-sha; 1996.
- Die systematische Bedeutung von iridoiden Inhaltsstoffen im Rahmen von Wettstein's Tubiflorae. Planta Med.. 1978;33:1-33.
- [Google Scholar]
- Hepatoprotective activity of Tabebuia rosea and Solanum pubescens against paracetamol induced hepatotoxicity in rats. Asian J. Pharm. Clin. Res.. 2012;5:153-156.
- [Google Scholar]
- Anti-ulcer activity of methanolic extracts of Wattakaka volubilis and Tabebuia rosea in rats. Asian J. Pharm. Clin. Res.. 2012;5:242-246.
- [Google Scholar]
- Study of the antineoplastic action of Tabebuia avellanedae in carcinogenesis induced by azoxymethane in mice. Acta Cir. Bras.. 2011;26:125-128.
- [Google Scholar]
- Antimicrobial potential of some plant extracts against Candida species. Braz. J. Biol.. 2010;70:1065-1068.
- [Google Scholar]
- The anti-obesity effect of Taheebo (Tabebuia avellanedae Lorentz ex Griseb) extract in ovariectomized mice and the identification of a potential anti-obesity compound. Biochem. Biophys. Res. Commun.. 2016;478:1136-1140.
- [Google Scholar]
- Iwano, H., Sawaki, S., Sawaki, S., 2013. Collagen synthesis stimulator for papilla and hair cosmetic containing it for prevention and improvement of hair color change. Jpn. Kokai Tokkyo Koho, JP 2013213002 A 20131017.
- Acaricidal activity of Tabebuia impetiginosa bark-derived constituent against domestic and spider mites (Arachnida: Acari) J. Korean Soc. Appl. Biol. Chem.. 2011;54:551-557.
- [Google Scholar]
- Insecticidal effects of Tabebuia avellanedae-derived Constituent and its analogues against Nilaparvata lugens and Laodelphax striatellus. J. Korean Soc. Appl. Biol. Chem.. 2011;54:822-826.
- [Google Scholar]
- Antioxidant, anti-inflammatory, and antiproliferative activity of extracts obtained from Tabebuia Rosea (Bertol.) DC. Pharmacogn. Mag.. 2018;14:25.
- [Google Scholar]
- Anti-infectious activity in plants of the genus Tabebuia/Actividad anti-infecciosa en plantas del genero Tabebuia/Atividade anti-infecciosa em plantas do genero Tabebuia. Revista Universitas Scientarum 2013:257-268.
- [Google Scholar]
- Pau d’Arco: Immune Power from the Rain Forest. Rochester, VT: Healing Arts Press; 1995.
- Chemical examination of the roots of Tabebuia rosea and heartwood of Oroxylum indicum. Planta Med.. 1977;31:257-258.
- [Google Scholar]
- Tabebuin and tecomaquinone-III-dimeric quinones from Tabebuia rosea. J. Indian Chem. Soc.. 2008;85:310-312.
- [Google Scholar]
- Kiage-Mokua, B. N., de Vrese, M., Schrezenmeir, J., 2018. Cardioprotective and lipid lowering effects Tabebuia Impetiginosa (Lapacho Tea) on male rats fed a high fat and fructose diet. J. Obes. Nutr. Disord.: JOND-131. Doi, 10, 2577–2244.
- Melanogenesis inhibition of β-lapachone, a natural product from Tabebuia avellanedae, with effective in vivo lightening potency. Arch. Dermatol. Res.. 2015;307:229-238.
- [Google Scholar]
- Larvicidal activity of the active constituent isolated from Tabebuia avellanedae bark and structurally related derivatives against three mosquito species. J. Agric. Food Chem.. 2013;61:10741-10745.
- [Google Scholar]
- Kim, U.H., Lee, G.S., Lee, G.T., Lee, G.G., 2015. Cosmetic composition for skin whitening comprising Tabebuia avellanedae extract with tyrosinase activity and melanin biosynthesis inhibitory effect.Repub. Korean Kongkae Taeho Kongbo, KR 2015004962 A 20150114.
- Comparative study of antiulcer activity of methanolic extracts of wattakaka volubilis (Linn. F.) Staf and Tabebuia Rosea (Bertol.) Dc in rats. Int. J. Pharm Sci. Res.. 2013;4(12):4625.
- [Google Scholar]
- Micellar Electrokinetic Chromatography (MEKC) Separation of Furanonaphthoquinones form Tabebuia impetiginosa. Chem. Pharm. Bull.. 2000;48:873-875.
- [Google Scholar]
- Cyclopentene dialdehydes from Tabebuia impetiginosa. Phytochemistry. 2000;53:869-872.
- [Google Scholar]
- Traditional herbal medicines - a review. J. Rheumatol. Arthr. Res.. 2018;5:611-614.
- [Google Scholar]
- In vitro and in vivo wound healing-promoting activities of β-lapachone. Am. J. Physiol. Cell Physiol.. 2008;295:931-943.
- [Google Scholar]
- Analgesic and anti-inflammatory effects in animal models of an ethanolic extract of Taheebo, the inner bark of Tabebuia avellanedae. Mol. Med. Rep.. 2012;6:791-796.
- [Google Scholar]
- Lee, S.Y., 2017. Cosmetic composition for promoting skin beneficial microorganism. Repub. Korea, KR 1798505 B1 20171117.
- Differential iridoid production as revealed by a diversity panel of 84 cultivated and wild blueberry species. Plos One. 2017;12:e0179417
- [Google Scholar]
- Genotoxic effects of Tabebuia impetiginosa (Mart. Ex DC.) Standl. (Lamiales, Bignoniaceae) extract in Wistar rats. Genet. Mol. Biol.. 2012;35:498-502.
- [Google Scholar]
- Antibacterial activity of Caesaria Sylvestris, Schinus Terebinthifolius and Tabebuia Avellanedae-three native brazilian tree species. Pubvet. 2013;7:2088-2188.
- [Google Scholar]
- Plant sources, extraction methods, and uses of squalene. Int. J. Agron.. 2018;2018:1-13.
- [Google Scholar]
- Healing Power of Pau D’Arco. Lotus Press; 1998.
- Evaluation of Cytotoxic and Mutagenic Activities of Tabebuia aurea (Silva Manso) Benth. and Hook. f. ex S. Moore. IOSR J. Pharm.. 2019;9:62-69.
- [Google Scholar]
- Mabberley, D.J., 2008. The Plant Book: A portable dictionary of plants, their classification and uses.
- In vitro activity of Brazilian medicinal plants, naturally occurring naphthoquinones and their analogues, against methicillin-resistant Staphylococcus aureus. Int. J. Antimicrob. Agents. 2003;21:279-284.
- [Google Scholar]
- A study on phytochemical analysis, antioxidant and larvicidal activity of dried flowers of Tabebuia rosea. J. Chem. Pharm. Res.. 2015;7:693-698.
- [Google Scholar]
- Metabolomic profiling and biological investigation of Tabebuia Aurea (Silva Manso) leaves, family Bignoniaceae. Nat. Prod. Res. 2019:1-6.
- [Google Scholar]
- Tabebuia aurea decreases hyperalgesia and neuronal injury induced by snake venom. J. Ethnopharmacol.. 2019;233:131-140.
- [Google Scholar]
- Guayin: an unusual oxalactone dibenzxanthone from Tabebuia guayacan. J. Chem. Soc. Chem. Commun.. 1975;10:711.
- [Google Scholar]
- Guayacanin—a novel phenolic xanthen derivative from Tabebuia guayacan. J. Chem. Soc. Chem. Commun.. 1974;17:388-389.
- [Google Scholar]
- Anti-pseudomonas aeruginosa drug; to evaluate bactericidal activity of Tabebuia Impetiginosa against pseudomonas aeruginosa and its synergistic effect with common antipseudomonas aeruginosa drug prof. Med. J.. 2018;25:1574-1580.
- [Google Scholar]
- Bioactive dihydroxyfuranonaphthoquinones from the bark of Tabebuia incana AH Gentry (Bignoniaceae) and HPLC analysis of commercial pau d'arco and certified T. incana bark infusions. Acta Amaz.. 2007;37:99-102.
- [Google Scholar]
- Morton, J.F., 1981. Atlas of Medicinal Plants of Middle America. In: Charles, C., Thomas, (Ed.), Springfield, IL, pp. 827–829.
- Análise fitoquímica das folhas de Tabebuia serratifolia (Vahl) Nicholson (Ipê Amarelo) Estação Científica (UNIFAP). 2015;4:33-43.
- [Google Scholar]
- Synthesis and trypanocidal activity of naphthoquinones isolated from Tabebuia and heterocyclic derivatives: a review from an interdisciplnary study. J. Braz. Chem. Soc.. 2001;12:325-338.
- [Google Scholar]
- Mowrey, D.B., 2001. Ancient Herb, Modern Medicine. Mountainwest Institute of Herbal Sciences, Salt Lake City.
- Growth inhibition of estrogen receptor positive human breast cancer cells by Taheebo from the inner bark of Tabebuia avellandae tree. Int. J. Mol. Med.. 2009;24:253-260.
- [Google Scholar]
- Natural plant resources in anti-cancer therapy-A review. Res. Plant Biol.. 2011;1:01-14.
- [Google Scholar]
- Pharmacological properties of specioside from the stem bark of Tabebuia aurea. Rev. Bras. Farmacogn.. 2020;30:118-122.
- [Google Scholar]
- Plantas medicinais comercializadas por raizeiros no Centro de Campo Grande, Mato Grosso do Sul. Rev. Bras. Farmacogn.. 2003;13:83-92.
- [Google Scholar]
- Wound healing properties of stem bark extract of Tabebuia rosea. J. Pharm. Allied Sci.. 2010;7
- [Google Scholar]
- Toxicidade e actividade antiinf lamatoria de Tabebuia avellanedae Lorentz e Griesebach (ipê-roxo) Rev. Fac. Farm. Bioquim. Sao Paulo. 1969;7:47-53.
- [Google Scholar]
- Phenylethanoid Glycosides from the fresh immature legumes of Golden Trumpet Tree (Tabebuia chrysotricha) Bull. Faculty Sci., Univ. Ryukyus. 2015;100:13-19.
- [Google Scholar]
- Chemical structures and biological activities of naphthoquinones from Brazilian Bignoniaceae. Quim. Nova. 1990;13:302-307.
- [Google Scholar]
- Antimycobacterial activity of some Brazilian indigenous medicinal drinks. Rev. de Cienc. Farm. Basica e Apl.. 2009;28:165-169.
- [Google Scholar]
- Phytochemical investigation of bioactive plants: Tabebuia serratifolia Nicholson and Tabebuia rosea Bertol (Bignoniaceae) Rev. Bras. Farm. 1999;80:46-48.
- [Google Scholar]
- Novel 6-O-[4-hydroxy-3-methoxybenzoyl] ajugol and know lignans cycloolivid and olivil from Tabebuia Serratifolia-total assignment of 1H and 13C NMR spectra. Rev. Latinoam Quím.. 2001;29:87-99.
- [Google Scholar]
- Cytotoxic effects of Tabebuia rosea oils (leaf and stem bark) Arch. Appl. Sci. Res.. 2010;2:127-130.
- [Google Scholar]
- Ortega, T.E., Stutz de Ortega, L., Spichiger, R., 1989. In: Spichiger, R. (Ed.) Noventa especies forestales del Paraguay. Se-rie especial N° 3 Flora del Paraguay, Conservatoire et Jardin Botaniques de la Ville de Geneva and Missouri, Botanical Garden, pp. 56–57.
- Osawa, S., Haneda, Y., Sawaki, S., Sawaki, S., 2006. Degranulation inhibitor containing Tabebuia impetiginosa bark extract of Tabebuia genus belonging to Bignoniaceae, and its application as skin external preparation. Jpn. Kokai Tokkyo Koho, JP 2006143676 A 20060608.
- Snakebites and ethnobotany in the northwest region of Colombia: Part III: neutralization of the haemorrhagic effect of Bothrops atrox venom. J. Ethnopharmacol.. 2000;73:233-241.
- [Google Scholar]
- Pharmacological activities and mechanisms of natural phenylpropanoid glycosides. Pharmazie. 2003;58:767-775.
- [Google Scholar]
- Stem extract of Tabebuia chrysantha induces apoptosis by targeting sEGFR in Ehrlich Ascites Carcinoma. J. Ethnopharmacol.. 2019;235:219-226.
- [Google Scholar]
- A trimethoxy flavonoid isolated from stem extract of Tabebuia chrysantha suppresses angiogenesis in angiosarcoma. J. Pharm. Pharmacol.. 2020;72:990-999.
- [Google Scholar]
- Selective growth-inhibiting effects of compounds identified in Tabebuia impetiginosa inner bark on human intestinal bacteria. J. Agric. Food Chem.. 2005;53:1152-1157.
- [Google Scholar]
- Antibacterial activity of Tabebuia impetiginosa Martius ex DC (Taheebo) against Helicobacter pylori. J. Ethnopharmacol.. 2006;105:255-262.
- [Google Scholar]
- Antioxidant activity and characterization of volatile constituents of Taheebo (Tabebuia impetiginosa Martius ex DC) J. Agric. Food Chem.. 2003;51:295-300.
- [Google Scholar]
- Oral administration of taheebo (Tabebuia avellanedae Lorentz ex Griseb.) water extract prevents DSS-induced colitis in mice by up-regulating type II T helper immune responses. BMC Complement Altern. Med.. 2017;17:448.
- [Google Scholar]
- Tabetri™ (Tabebuia avellanedae Ethanol Extract) ameliorates atopic dermatitis symptoms in mice. Mediators Inflamm.. 2018;2018:1-11.
- [Google Scholar]
- Tabetri™ (Tabebuia avellanedae Ethanol Extract) ameliorates osteoarthritis symptoms induced by monoiodoacetate through its anti-inflammatory and chondroprotective activities. Mediators Inflamm.. 2017;2017:1-14.
- [Google Scholar]
- Tabebuia avellanedae naphthoquinones: activity against methicillin-resistant staphylococcal strains, cytotoxic activity and in vivo dermal irritability analysis. Ann. Clin. Microbiol. Antimicrob.. 2006;5:1-7.
- [Google Scholar]
- Antiulcer effect of bark extract of Tabebuia avellanedae: activation of cell proliferation in gastric mucosa during the healing process. Phytother. Res.. 2013;27:1067-1073.
- [Google Scholar]
- Chemical investigation and in vitro antimalarial activity of Tabebuia ochracea ssp. neochrysantha. Int. J. Pharmacogn.. 1997;35:227-231.
- [Google Scholar]
- Actividad antibacteriana de extractos de Phenax rugosus y Tabebuia chrysantha. Biosalud. 2007;6:59-68.
- [Google Scholar]
- A chemical–biological study reveals C9-type iridoids as novel Heat shock protein 90 (Hsp90) inhibitors. J. Med. Chem.. 2013;56:1583-1595.
- [Google Scholar]
- Bioactive properties of Tabebuia impetiginosa-based phytopreparations and phytoformulations: a comparison between extracts and dietary supplements. Molecules. 2015;20:22863-22871.
- [Google Scholar]
- Plowman, T., 1967. Collection #126. Herbarium specimen label data, available online at http://.
- Antifungal activity of Paraguayan plants used in traditional medicine. J. Ethnopharmacol.. 2001;76:93-98.
- [Google Scholar]
- Pott, A., Pott, V.J., 1994. Plantas do pantanal Brasilia: EMBRAPA-SPI, 1994.
- Topical treatment with yellow-ipe extract (Tabebuia aurea) in wound healing by secondary intention in rats. J. Chem. Pharm. Res.. 2016;8:367-373.
- [Google Scholar]
- Chemical examination of the root barks of Jacaranda mimosifolia D. Don. and Tabebuia pentaphylla (Linn) Hemsl. Pharmazie. 1980;35:649.
- [Google Scholar]
- Chemical constituents of stem bark and root heartwood of Tabebuia pentaphylla (Linn.) Hemsl. (Bignoniaceae). Pharmazie. 1980;35:813.
- [Google Scholar]
- Chemical examination of the leaves and stem heartwood of Tebebuia pentaphylla (Linn) Hemsl (Bignoniaceae) J. Indian Chem. Soc.. 1981;58:1122-1123.
- [Google Scholar]
- Comparative studies of the effects of Tabebuia avellanedae bark extract and β-lapachone on the hematopoietic response of tumour-bearing mice. J. Ethnopharmacol.. 2008;117:228-235.
- [Google Scholar]
- Evaluation of anti-ROS and anticancer properties of Tabebuia pallida L. Leaves. Clin. Phytoscience. 2019;5:1-12.
- [Google Scholar]
- In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res. Notes. 2015;8:1-9.
- [Google Scholar]
- An ethnomedicinal, pharmacological and phytochemical review of some Bignoniaceae family plants and a description of Bignoniaceae plants in folk medicinal uses in Bangladesh. Adv. Nat. Appl. Sci.. 2010;4:236-253.
- [Google Scholar]
- Analysis of bioactive constituents from the ethanolic leaf extract of Tabebuia rosea (Bertol.) DC by gas chromatography-mass spectrometry. Int. J. Chem. Tech. Res.. 2011;3:1054-1059.
- [Google Scholar]
- Ramos-Peralta, L., López-López, L.I., Silva-Belmares, S.Y., Zugasti-Cruz, A., Rodríguez-Herrera, R., Aguilar-González, C.N., 2015. Naphthoquinone: Bioactivity and Green Synthesis. The Battle against Microbial Pathogens: Basic Science, Technological Advances and Educational Programs, pp. 542–550.
- Recognition and evaluation of lapachol as an antitumor agent. Cancer Res.. 1968;28:1952-1954.
- [Google Scholar]
- Plant anticancer agents. XII. Isolation and structure elucidation of new cytotoxic quinones from Tabebuia cassinoides. J. Nat. Prod.. 1982;45:600-604.
- [Google Scholar]
- Evaluation of antipyretic, sedative and hypnotic activities of methanol extract of Tabebuia hypoleuca (C. Wright ex Sauvalle) Urb. stems. Bol. Latinoam. Caribe Plantas Med. Aromát.. 2017;16:547-555.
- [Google Scholar]
- Antinociceptive activity of methanol extract of Tabebuia hypoleuca (C. Wright ex Sauvalle) Urb. stems. Med. Princ. Pract.. 2017;26:368-374.
- [Google Scholar]
- Actividad anti-inflamatoria de los extractos metanólicos de hojas y de tallos de Tabebuia hypoleuca (C. Wright) Urb. J. Pharm. Pharmacogn. Res.. 2015;3:109-117.
- [Google Scholar]
- Tabebuia aurea decreases inflammatory, myotoxic and hemorrhagic activities induced by the venom of Bothrops neuwiedi. J. Ethnopharmacol.. 2014;158:352-357.
- [Google Scholar]
- Rizzini, C., 1988. O livro, o jornal ea tipografia no Brasil, 1500–1822: com um breve estudo geral sobre a informação. Imprensa Oficial do Estado.
- Plants and animals utilized as medicines in the Jaú National Park (JNP), Brazilian Amazon. Phytother. Res.. 2006;20:378-391.
- [Google Scholar]
- Tecoma stans (L.) Juss. ex Kunth (Bignoniaceae): Ethnobotany, phytochemistry and pharmacology. J Pharm Biomed Sci.. 2011;8:1-5.
- [Google Scholar]
- quinones from tecoma-pentaphylla-constitution of tecomaquinone-i and tecomaquinone-ii. Indian J. Chem. B. 1983;22:886-889.
- [Google Scholar]
- Extract of Tabebuia impetiginosa inhibits pancreatic lipase activity and decreases postprandial triglyceride levels in rats. Comp. Biochem. Physiol. Part A. 2008;3:S184.
- [Google Scholar]
- Pharmacological screening of plants recommended by folk medicine as anti-snake venom: I. Analgesic and anti-inflammatory activities. Mem Inst Oswaldo Cruz 1991:203-205.
- [Google Scholar]
- Phytochemical investigation of Tabebuia palmeri. Chem. Nat. Compd.. 2014;49:1039-1042.
- [Google Scholar]
- Primeiras observações com o emprego de lapachol em pacientes humanos portadores de neoplasias malignas. Rev. Inst. Antibiot.. 1980;1:20-61.
- [Google Scholar]
- Biological potential assessment of Tabebuia aurea (Silva Manso) as a source of bioactive molecules for antimicrobial, antiedematogenic and antiradical activity. Rev. Bras. Plantas Med.. 2015;17:1159-1168.
- [Google Scholar]
- Antimicrobial activity of ethanolic extract of leaves of Tabebuia rosea (Bertol.) DC. Asian J. Chem.. 2011;23:3283.
- [Google Scholar]
- Sathiya, M., Muthuchelian, K., 2008. Studies on Phytochemical Profile and Antibacterial Activity of Ethanolic Leaf Extract of Tabebuia rosea (Bertol.) DC. Ethnobotanical leaflets, 2008, 152.
- Antitumor potential of total alkaloid extract from Tabebuia rosea (Bertol.) DC. leaves on MOLT-4 cells in vitro. Nat. Sci.. 2010;8:7.
- [Google Scholar]
- Engineering the biosynthesis of low molecular weight metabolites for quality traits (essential nutrients, health-promoting phytochemicals, volatiles, and aroma compounds) In: Plant Biotechnology and Agriculture. Academic Press; 2012. p. :443-461.
- [Google Scholar]
- Naphthoquinone derivatives and lignans from the Paraguayan crude drug “tayï pytá” (Tabebuia heptaphylla, Bignoniaceae) Z. Naturforsch C. 2003;58:495-501.
- [Google Scholar]
- The Healing Forest. Portland: Dioscorides Press; 1990. p. :107-109.
- Schunke, J., 1993. Collection #14378. Herbarium specimen label data, available online at http://.
- Isolation and characterization of kaempferol 3-O-(2''-a-methyl p-coumaryl)-b-d-glucoside from Tabebuia rosea (Flowers) Am. J. Pharm. Tech. Res.. 2016;6:223-231.
- [Google Scholar]
- Tecomaquinone-III: a new quinone from Tabebuia pentaphylla. Phytochemistry. 1988;27(2):632-633.
- [Google Scholar]
- Tabebuialdehydes A-C, cyclopentene dialdehyde derivatives from the roots of Tabebuia rosea. Fitoterapia. 2012;83(8):1456-1459.
- [Google Scholar]
- The antinociceptive effect of the leaves and flowers ethanolic extracts of Tabebuia aurea (Silva Manso) Benth and Hook. F. ex S. Moore. Braz. Arch. Biol. Technol.. 2018;61:1-12.
- [Google Scholar]
- Anti-inflammatory and anti-arthritic activities of 3, 4-dihydro-2, 2-dimethyl-2H-naphthol [1, 2-b] pyran-5, 6-dione (β-lapachone) Inflamm. Res.. 2013;62:107-113.
- [Google Scholar]
- Iridoids and triterpenes of barks of Tabebuia caraiba bignoniaceae stem. Sociedade Brasileira de Química. 2006;39:29.
- [Google Scholar]
- Iridoides, triterpenos e outros constituintes das cascas do caule e flores de Tabebuia caraiba Bignoniaceae. Quím. Nova. 2020;43:399-403.
- [Google Scholar]
- Comparative analysis of the in vitro antioxidant activity of Tabebuia rosea and Tabebuia argentea. J. Pharmacogn. Phytochem.. 2019;8:2673-2677.
- [Google Scholar]
- Inhibitory effects of Tabebuia impetiginosa inner bark extract on platelet aggregation and vascular smooth muscle cell proliferation through suppressions of arachidonic acid liberation and ERK1/2 MAPK activation. J. Ethnopharmacol.. 2006;108:148-151.
- [Google Scholar]
- Modulatory effects of Tabebuia impetiginosa (Lamiales, Bignoniaceae) on doxorubicin-induced somatic mutation and recombination in Drosophila melanogaster. Genet. Mol. Biol.. 2009;32:382-388.
- [Google Scholar]
- HPLC separation and determination of naphtho [2, 3-b] furan-4, 9-diones and related compounds in extracts of Tabebuia avellanedae (Bignoniaceae) J. Chromatogr. A. 1995;693:281-287.
- [Google Scholar]
- High-performance liquid chromatographic separation of some naturally occurring naphtoquinones and anthraquinones. J. Chromatogr. A. 1996;723:206-209.
- [Google Scholar]
- Anti-inflammatory constituents from Tabebuia avellanedae. Fitoterapia. 2012;83:1484-1488.
- [Google Scholar]
- Bioactive phenylpropanoid glycosides from Tabebuia avellanedae. Molecules. 2013;18:7336-7345.
- [Google Scholar]
- Phytochemical studies on Tabebuia argentea. Proc. Natl. Acad. Sci., India. 1982;52:340.
- [Google Scholar]
- Lignan glycosides and phenolic compound glycosides from the branches of Tabebuia chrysotricha. Am. J. Plant Sci.. 2015;6:676-684.
- [Google Scholar]
- Taylor, L., 2005. The healing power of rainforest herbs: A guide to understanding and using herbal medicinals (No. 615.321 T243). SquareOne Publishers.
- Growth inhibitory efficacy and anti–aromatase activity of Tabebuia avellanedae in a model for post–menopausal Luminal A breast cancer. Biomed. Rep.. 2019;11:222-229.
- [Google Scholar]
- Antiulcerogenic activity of bark extract of Tabebuia avellanedae, Lorentz ex Griseb. J. Ethnopharmacol.. 2008;118:455-459.
- [Google Scholar]
- Production of anti-tumour-promoting furano-naphthoquinones in Tabebuia avellanedae cell cultures. Phytochemistry. 1994;36:323-325.
- [Google Scholar]
- Ovipositional responses of the pulse beetle, Bruchus chinensis (Coleoptera: Bruchidae) to extracts and compounds of Capparis decidua. J. Agric. Food Chem.. 2006;54:9747-9751.
- [Google Scholar]
- Velasquez, J., Rojas, L. B., Usubillaga, A., 2004. Antifungal activity of naphtoquinone from Tabebuia serratifolia (Vahl. Nicholson). Ciencia, 12.
- Vidal-Tessier, A. M., Delaveau, P., Champion, B., Jacquemin, H., 1988. Lipophilic quinones of the trunk wood of Tabebuia serratifolia (Vahl.) Nichols. In: Annales pharmaceutiques francaises, Vol. 46, pp. 55–57.
- 4-Aryltetralin lignan and furanonaphtoquinones from Tabebuia palmeri wood. Fitoterapia. 1995;66:281-282.
- [Google Scholar]
- One-step green synthesis of silver nanoparticles using flower extract of Tabebuia argentea Bur. and K. Sch. and their antibacterial activity. Res. J. Pharm. Biol. Chem. Sci.. 2017;8:527-534.
- [Google Scholar]
- Effects of nitric oxide in septic shock. Am. J. Respir. Crit. Care Med.. 2000;161:1781-1785.
- [Google Scholar]
- Isolation of colour components from flowers of Tabebuia argentea: kinetic and adsorption studies on silk yarn. Colorat. Technol.. 2011;127:205-209.
- [Google Scholar]
- The distribution of iridoids in Bignoniaceae. Biochem. Syst. Ecol.. 2000;28:351-366.
- [Google Scholar]
- Structure Determination of New Isomeric Naphtho [2, 3-b] furan-4, 9-diones from Tabebuia avellanedae by the selective-INEPT technique. Helv. Chim. Acta. 1989;72:659-667.
- [Google Scholar]
- Constituents from the bark of Tabebuia impetiginosa. Phytochemistry. 2004;65:2003-2011.
- [Google Scholar]
- Further constituents from the bark of Tabebuia impetiginosa. Phytochemistry. 2005;66:589-597.
- [Google Scholar]
- Constituents from the bark of Tabebuia impetiginosa. Chem. Pharm. Bull.. 2006;54:14-20.
- [Google Scholar]
- Growth inhibition of A549 human lung carcinoma cells by β-lapachone through induction of apoptosis and inhibition of telomerase activity. Int. J. Oncol.. 2005;26:1017-1023.
- [Google Scholar]
- β-lapachone, a quinone isolated from Tabebuia avellanedae, induces apoptosis in HepG2 hepatoma cell line through induction of Bax and activation of caspase. J. Med. Food. 2006;9:161-168.
- [Google Scholar]
- Woo, Y.T., Kim, H.H., Ahn, G.U., Cho, B.G., 2009. Tabebuia avellanedae extract-containing cosmetic composition with anti-inflammatory and skin irritation alleviating effects Repub. Korean Kongkae Taeho Kongbo, KR 2009025497 A 20090311.
- Synthesis and evaluation of bioactive naphthoquinones from the Brazilian medicinal plant, Tabebuia avellanedae. Bioorg. Med. Chem.. 2009;17:6286-6291.
- [Google Scholar]
- Anti-inflammatory cyclopentene derivatives from the inner bark of Tabebuia avellanedae. Fitoterapia. 2016;109:217-223.
- [Google Scholar]
- Iridoid esters from Tabebuia avellanedae and their in vitro anti-inflammatory activities. Planta Med.. 2017;83:164-171.
- [Google Scholar]
- Furanonaphthoquinones from Tabebuia avellanedae induce cell cycle arrest and apoptosis in the human non-small cell lung cancer cell line A549. Phytochem. Lett.. 2015;11:9-17.
- [Google Scholar]







