Translate this page into:
Gold nanoparticles/tetraaminophenyl porphyrin functionalized multiwalled carbon nanotubes nanocomposites modified glassy carbon electrode for the simultaneous determination of p-acetaminophen and p-aminophenol
⁎Corresponding authors. weiyl2000@163.com (Yuli Wei), xbsfda123@126.com (Wu Yang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A novel electrochemical platform was designed and prepared for simultaneous determination of p-acetaminophen (AMP) and p-aminophenol (AP) by combining the excellent conductivity and electrocatalytic activities of tetraaminophenyl porphyrin functionalized multi-walled carbon nanotubes (CNTs-CONH-TAPP) and gold nanoparticles (AuNPs). The as-synthesized CNTs-CONH-TAPP composites were characterized by Fourier transform infrared spectroscopy, X-ray photoelectron spectroscopy and scanning electron microscope. The incisive oxidation current responses of AMP and AP at the modified electrode promised a sensitive and selective simultaneous determination of AMP and AP. Under optimized conditions, the peak currents were directly proportional to the concentrations of AMP and AP over the ranges of 4.5–500 μmol L−1 and 0.08–60 μmol L−1, respectively, and the limits of detection were 0.44 μmol L−1 for AMP and 0.025 μmol L−1 for AP(S/N = 3) respectively. The proposed modified electrode showed excellent selectivity, reproducibility and long-term stability and could be applied in simultaneous determination of p-acetaminophen and p-aminophenol in real samples.
Keywords
Functionalized multi-walled carbon nanotubes
Tetraaminophenyl porphyrin
Gold nanoparticles
p-Acetaminophen
p-Aminophenol
1 Introduction
p-Acetaminophen (paracetamol or N-acetyl-p-aminophenol, AMP) is widely used as analgesic and antipyretic drug. Notably, it is a promising ingredient in the reduction of moderate pain associated with backache, headache, arthritis and postoperative pain (Amiri-Aref et al., 2014). But overuse of AMP can lead to accumulation of toxic metabolites, which may cause some severe diseases, such as hepatoxicity, nephrotoxicity (Mazer and Perrone, 2008), skin rashes and pancreatitis (Prabakar and Narayanan, 2007). Moreover, paracetamol is one of the most-used ingested drugs in self-poisoning (Tortet et al., 2017; Li et al., 2017). p-Aminophenol(AP) is a widely-used industrial raw material and important intermediate in different areas, such as sulfur and azo dyes, rubber, feeding-stuff, petroleum, and photography, and so on. (Filik et al., 2008; Khan et al., 2006; Wang et al., 1994). As a result, a lot of AP may enter the environment as a contamination. Hence, it is widely distributed in the ecological environment, especially in water bodies (Kaur and Srivastava, 2014).
The industrial synthesis of paracetamol occurs through the reaction of acetylation of 4-aminophenol with acetic anhydride (Németh et al., 2008; Roy, 2002). p-Aminophenol is the main impurity of paracetamol containing pharmaceuticals (Németh et al., 2008), and has been reported to have nephrotoxicity and teratogenic effects (Forshed et al., 2002; Yesilada et al., 1991; Song and Chen, 2001). This impurity may arise from degradation of paracetamol or exist in the final product by the presence of the reagent (Németh et al., 2008; Roy, 2002). So, to control the drug quality, the development of a simple, precise and accurate method for the simultaneous determination of AMP and AP in acetaminophen formulations is very important.
Until now, various methods have been developed for AMP and/or AP determination, such as titrametry (Kumar and Letha, 1997; Burgot et al., 1997), high performance liquid chromatography (Wyszecka-Kaszuba et al., 2003; Issa et al., 2012; El-Kommos et al., 2012; Abdelaleem et al., 2015), LC-MA/MA (Esterhuizen-Londt et al., 2016), spectrophotometry, (Moreira et al., 2005; Murtaza et al., 2011) chemililuminescence (Easwaramoorthy et al., 2001), capillary electrophoresis (Capella-Peir’o et al., 2006; Chu et al., 2008), spectrofluorimetry (Madrakia et al., 2009; Liu et al., 2017), flow injection analysis (Santos et al., 2015, 2020), and so on. However, these methods are time-consuming and require relatively expensive instrumentation, so they are not suitable for on-site usage. On the other hand, electrochemical techniques based on chemically modified electrodes, because of their fast response, high sensitivity, high selectivity, simple operation and cheap instrument (Chen and Chatterjee, 2013), have widely used in the determination of trace level of analytes (Wang, 2006). Therefore, it is interesting to build an accurate and sensitive electrochemical method for simultaneously detecting AMP and AP.
Porphyrin is a heterocyclic organic macromolecule which contains four pyrrole rings connected via the methine bridges (Biesaga et al., 2000; Gross et al., 2011). Nowadays, porphyrin and its derivates have been used in various fields such as optical devices, chemical sensors and electro-catalysis due to its extraordinary electron transfer and photo luminescent properties (Suijkerbuijk and Gebbink, 2008; Kubendhiran et al., 2017). Especially, as a kind of electron donor, they are very suitable for electron devices and catalytic applications because of its extensively conjugated two-dimensional π-system and special electrochemical properties (Fang et al., 2014; Mase et al., 2013; Su et al., 2010). Porphyrin derivatives can act as sensing materials to show excellent electrocatalytic activity and it enables them to be potential candidates for development of electrochemical sensors (Hassani et al., 2011; Rajith et al., 2011).
Multi-walled carbon nanotubes (MWCNTs) have attracted attention due to their exciting properties, including the mechanical, optical, chemical and electrical (Wong et al., 2015). The carbon atoms in the sidewall of the MWCNTs are dominated by sp2, and have a large number of highly delimited electrons system, these π electrons can react with other π-containing compounds to obtain organic noncovalent chemically modified carbon nanotubes by π-π interaction. For instance, Murakami et al. (2003) synthesized porphyrin-carbon nanotube complexes with tetraphenylporphyrin zinc and single-walled carbon nanotubes by means of a π-π bond. These organic noncovalent chemically modified carbon nanotubes complexes could be ultrasonically dispersed very easily. The above results show that the formation of π-π complex is one of effective methods for functionalizing CNTs, while the use of amino to modify carboxylate CNTs can further improve stability of the functionalized CNTs and endue them more potentials. Tetraaminophenyl porphyrin (TAPP) is a good electron donor, while carbon nanotubes are strong electron acceptors, which ensure excellent conductivity of the resulting tetraaminophenyl porphyrin-carbon nanotube complex. Moreover, its π structure is beneficial to improve the selectivity of aromatic target molecule recognition. In this present paper, an amino-porphyrin functionalized MWCNTs composite(CNTs-CONH-TAPP) was prepared by the reaction of tetraaminophenyl porphyrin and acylchloride functionalized multi-walled carbon nanotubes and by employing its synergistic effect with excellent conductivity and high electrocatalytical activity of gold nanoparticles(AuNPs) (Wang J.W. et al., 2009) a novel electrochemical sensing platform based on AuNPs/CNTs-CONH-TAPP/GCE modified electrode was constructed for the simultaneous determination of AMP and AP. Big formal potential separation of 252 mV ensured a high analytical selectivity. The proposed method was applied to detect AMP and AP in real sample successfully.
2 Experimental
2.1 Reagents and apparatus
4-Acetamidophenol(AMP) and 4-aminophenol(AP) were purchased from Aladdin. MWCNTs were purchased from Shenzhen Nanotech Port Co., Ltd. Disodium hydrogen phosphate dodecahydrate (Na2HPO4·12H2O) and sodium dihydrogen phosphate dihydrate (NaH2PO4·2H2O) were obtained from Yantai Shuangshuang Chemical Co., Ltd. All other reagents were of analytical grade and double distilled water was used throughout the experiments. Paracetamol Dispersible Tablets were purchased from local drugstore and water sample was collected in Yellow River (Lanzhou).
All electrochemical experiments were carried out on a CHI660 B electrochemical workstation (Shanghai, China) equipped with a three-electrode cell with a glassy carbon electrode (GCE, 3 mm diameter) or a modified GCE as the working electrode, a saturated calomel electrode (SCE) as the reference electrode and a platinum sheet as the counter electrode. Scanning electron microscopy (SEM) images were determined with a JSM−6701 F field emission scanning electron microscope (Japanese Electron Optics Company). Fourier transform infrared spectra (FTIR) of the samples were recorded in the spectral range of 4000 - 400 cm-1 using the Nicolet 6700 Infrared Spectrometer (Thermo Scientific Co., USA). Elemental chemical states on the surface of the materials were measured by ESCALAB 250Xi X-ray photoelectron spectroscopy (XPS, Thermo, USA).
2.2 Preparation of tetraaminophenyl porphyrin and acryl chloride-functionalized CNTs
Tetraaminophenyl porphyrin (TAPP) was synthesized via a literature procedure (Bettelheim et al., 1987). The MWCNTs were purified by refluxing the as-received MWCNTs in concentrated nitric acid for 36 h at 80 °C (Pumera and Iwai, 2009). Carboxyl-functionalized CNTs (CNTs-COOH) were prepared by treating the purified MWCNTs with a concentrated nitric/sulfuric acid (1:3, v/v) mixture solution at 50 °C for 6 h. When cooled to room temperature, the suspension was filtered, washed with water several times until the aqueous solution reached neutral pH, and then dried under vacuum at 50 °C (Zhang et al., 2015). Acryl chloride-functionalized CNTs (CNTs-COCl) were prepared by suspending 100 mg of CNTs-COOH into a mixture of thionyl chloride (SOCl2) and dimethylformamide (DMF) (20:1, v/v) at 70 °C for 24 h. The suspension cooled to room temperature was centrifuged and washed with anhydrous tetrahydrofuran several times and dried to obtain anhydrous CNTs-COCl (Shen et al., 2007).
2.3 Synthesis of carbon nanotubes/tetraaminophenyl porphyrin nanocomposites
The nanocomposites were prepared using a modified reference method (Gromov et al., 2015). Typical preparation was as follows: 10 mg of TAPP and 20 mg of CNTs-COCl were added to 20 mL of anhydrous DMF solution. The mixture was reacted at 100 °C under nitrogen atmosphere for 96 h, and then treated using 20 mL of ammonia at 70 °C for 24 h. Finally, the resulted CNTs-CONH-TAPP nanocomposite dispersion was filtrated, washed with ultrapure water and dried in a vacuum at 50 °C overnight.
2.4 Fabrication of the AuNPs/CNTs-CONH-TAPP/GCE
Prior to modification, the glassy carbon electrode (GCE) was polished with 0.05 μm alumina powder slurries to get a mirror-like surface, then sonicated sequentially in ethanol/ultrapure water mixture(1:1, v/v) and double distilled water, and allowed to dry in N2 atmosphere at room temperature. Afterwards, 2 mg of CNTs-CONH-TAPP nanocomposites was dispersed in 1 mL of DMF solution. 6 μL of the CNTs-CONH-TAPP nanocomposite suspension was dropped on the surface of the bare glassy carbon electrode and dried under an infrared lamp for 10 min. To electrochemically deposit AuNPs on the surface of the CNTs-CONH-TAPP/GCE, the CNTs-CONH-TAPP/GCE was immersed in 0.2 g L−1 HAuCl4 solution and treated at the constant potential of −0.2 V for 90 s. Finally, the electrode was rinsed with distilled water and dried under an infrared lamp for later use. The whole preparation process is shown in Scheme 1.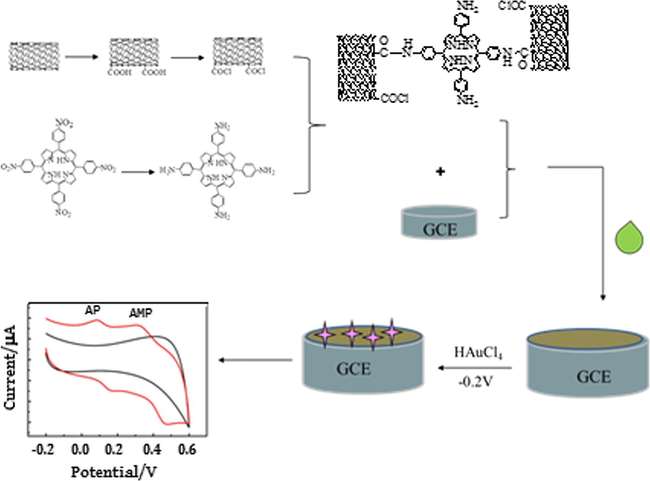
Preparation procedure of the modified electrode.
2.5 Electrochemical measurements
The AuNPs/CNTs-CONH-TAPP/GCE was used as working electrode in a three-electrode electrochemical cell. The experiments were carried out by studying the cyclic voltammetric behavior of the p-acetaminophen and p-aminophenol at a potential range of from −0.2 to 0.6 V.
The DPV was performed at potential range of from 0.0 V to 0.6 V, with a pulse width of 0.05 s, pulse period of 0.5 s and potential increment of 4 mV.
3 Results and discussion
3.1 Characterization of the electrode materials and the modified electrodes
IR spectra of the prepared CNTs-COOH(a), CNTs-COCl(b), TAPP(c) and CNTs-CONH-TAPP(d) are shown in Fig. 1. CNTs-COOH showed an obvious —OH stretching vibration band at 3363 cm−1 and a C⚌O stretching vibration band at 1709 cm−1 (curve a). CNTs-COCl showed a sharp C⚌O stretching band at 1701 cm−1 and a weak C—Cl stretching band at 623 cm−1 respectively (curve b). TAPP showed characteristic absorption bands at 1612 cm−1, 1506 cm−1, 1450 cm−1, respectively assigned to benzene ring and pyrrole ring skeleton vibration peaks, and the bands at 3448 cm−1 and 3355 cm−1 ascribed to primary amine stretching vibration peaks (curve c). After reaction of CNTs-COCl and TAPP to form CNTs-CONH-TAPP, the stretching vibration of the -CONH- at 3444 cm−1, C-N stretching vibration at 1247 cm−1 and -NH- bending vibration at 687 cm−1 appeared (curve d). These results indicated that the CNTs-CONH-TAPP nanocomposites had been successfully fabricated.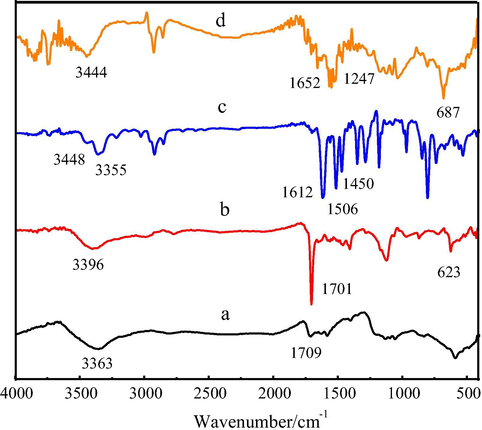
FT-IR spectra of (a) TAPP, (b)CNTs-COOH, (c) CNTs-COCl and (d) CNTs-CONH-TAPP.
Fig. 2 shows typical XPS survey spectra and high-resolution elemental scan of N1s of the prepared CNTs- CONH-TAPP. As shown in Fig. 2A, in XPS survey spectra appeared C1s, N1s and O1 characteristic peaks at 284.88 eV, 399.44 eV and 532.86 eV, respectively. The high resolution spectra of N1 s showed that there were four nitrogen-containing functional groups in CNTs-CONH-TAPP, which were —C⚌N (400.44 eV), ⚌CN— (400.31 eV) in the porphyrin ring with similar area, -CONH-(399.94 eV) and —NH2 (399.44 eV), respectively (Fig. 2B). -CONH- binding energy was larger than the NH2 binding energy, mainly because N in —CONH- was adjacent to the electron withdrawing group. In addition, the area ratio of the two peaks is about 1:1, indicating that the two amino groups in the tetraaminophenyl porphyrin were involved in the amidation reaction. The results demonstrated that CNTs-COCl reacted successfully with tetraaminophenyl porphyrin, which was consistent with IR results.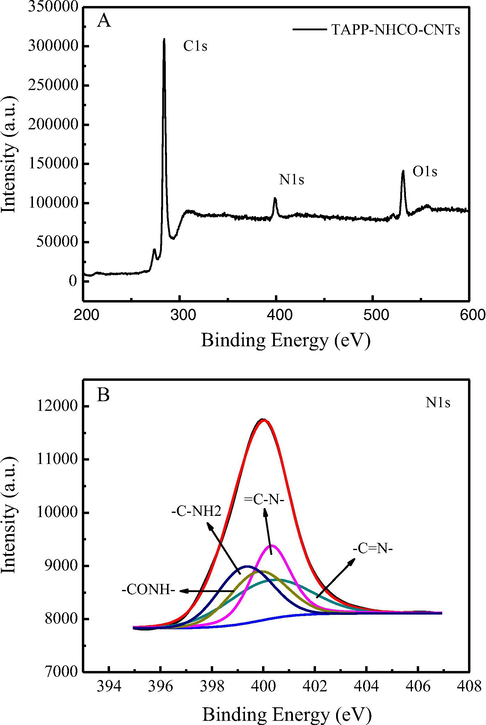
XPS survey spectra of CNTs- CONH-TAPP and high resolution elemental scan of N1s.
The SEM images of the surface of CNTs-CONH-TAPP/GCE and AuNPs/CNTs- CONH-TAPP/GCE were shown in Fig. 3. The SEM image of the CNTs-CONH- TAPP/GCE showed a three-dimensional network structure and this network structure played an important role in electron transfer. In addition, the porous network structure made the electrode surface area and the target molecular adsorption capacity greatly increase to further improve the sensitivity of the determination (Fig. 3A). π-π interaction between CNTs and porphyrin molecules made the composites uniformly distributed and there was not obvious agglomeration of CNTs or porphyrin molecules. Fig. 3B shows that uniform size of AuNPs are well-distributedly dispersed on the walls of the CNTs-CONH-TAPP.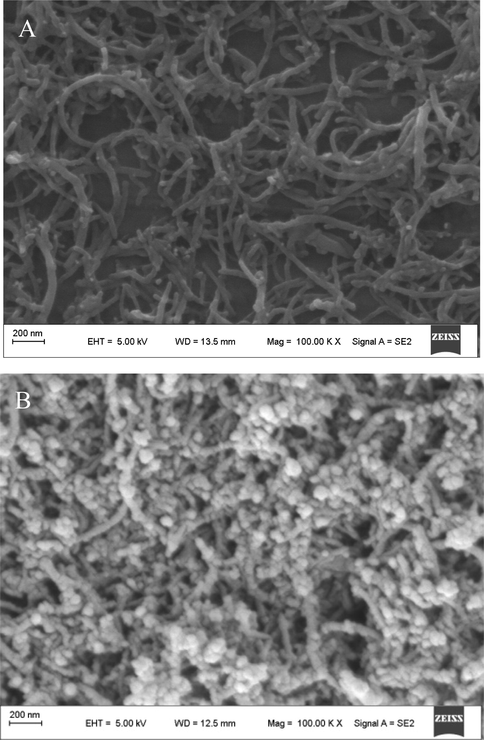
SEM images of CNTs-CONH-TAPP/GCE (A) and AuNPs/CNTs-CONH-TAPP/GCE (B).
3.2 Electrochemical properties of AuNPs/CNTs-CONH-TAPP/GCE
The electrochemical characteristics of the different modified electrodes were investigated by cyclic voltammetry in 0.1 mol L−1 KCl solution containing 1 mmol L−1 Fe(CN)63−/4− at a scan rate of 100 mV s−1. Fig. 4A shows the CVs of GCE(a), TAPP/GCE(b), CNTs-CONH-TAPP/GCE(c) and AuNPs/CNTs-CONH-TAPP/GCE(d). As shown in curve a, a couple of weak redox peaks were observed on the bare GCE. After the electrode was covered by TAPP(b), the redox peaks completely disappeared, indicating that TAPP completely blocked interfacial electron transfer. However, when it was modified with the CNTs-CONH-TAPP nanocomposites (curve c), the currents of redox peaks increased obviously, suggesting that the CNTs-CONH-TAPP nanocomposites could improve the electron transfer on the surface of CNTs-CONH-TAPP/GCE. What’s more, when AuNPs were deposited on the surface of CNTs-CONH-TAPP/GCE(curve d), the redox currents further enhanced.![(A) CVs of bare GCE (a), TAPP/GCE (b), CNTs-CONH-TAPP/GCE (c) and AuNPs/CNTs-CONH-TAPP/GCE (d) in 0.1 mol L−1 KCl containing 1 mmol L−1 Fe(CN)63−/4−. (B) EIS of bare GCE (a), CNTs-CONH-TAPP/GCE (b) and AuNPs/CNTs- CONH-TAPP/GCE (c) in 0.1 mol L−1 KCl containing 5 mmol L−1 [Fe(CN)6]3−/4−. Inset: Amplification of high frequency range.](/content/184/2020/13/1/img/10.1016_j.arabjc.2017.09.008-fig5.png)
(A) CVs of bare GCE (a), TAPP/GCE (b), CNTs-CONH-TAPP/GCE (c) and AuNPs/CNTs-CONH-TAPP/GCE (d) in 0.1 mol L−1 KCl containing 1 mmol L−1 Fe(CN)63−/4−. (B) EIS of bare GCE (a), CNTs-CONH-TAPP/GCE (b) and AuNPs/CNTs- CONH-TAPP/GCE (c) in 0.1 mol L−1 KCl containing 5 mmol L−1 [Fe(CN)6]3−/4−. Inset: Amplification of high frequency range.
Electrochemical impedance spectroscopy (EIS) is usually used to characterize the features of a surface to allow the understanding of chemical transformations and processes associated with the conductive electrode surface. The Nyquist plots were made in 0.1 mol L−1 KCl solution containing 5 mmol L−1 Fe(CN)63−/4− at 100,000–0.01 Hz. In the Nyquist diagram (Fig. 4B), the semicircle diameter of EIS in high frequency range is equal to Ret. The small semicircle at high frequencies and linear part at low frequencies could be observed for bare GCE. The semicircle diameter of the CNTs-CONH-TAPP nanocomposites modified electrode was much smaller than that of bare GCE. This result showed the smart conductivity of the CNTs-CONH-TAPP nanocomposites, which reduced the electron transfer resistance of the GCE. After gold nanoparticles were deposited on the CNTs-CONH-TAPP film surface, it was found clearly that the electron transfer resistance decreased significantly due to the superior conductivity. The above resulted could clearly suggest the success of the assembly of the electrode.
3.3 Electrochemical behaviors of AMP and AP at different modified electrodes
The electrochemical behaviors of AMP and AP were investigated by cyclic voltammetry (CV). In contrast, CV curves of AuNPs/CNTs-CONH-TAPP/GCE in 0.1 mol L−1 PBS (pH = 7.0) in the presence/absence of AMP and AP at 100 mV s−1 were shown in Fig. 5A. In blank 0.1 mol L−1 PBS (pH = 7.0), there was not any redox peak appeared (curve a). When AP was added (curve b), a pair of well-defined redox peaks were observed at 0.032 and 0.108 V respectively, attributing to the electrochemical redox behavior of AP, while the modified electrode was immersed in AMP solution, a pair of well-defined redox peaks appeared at 0.255 and 0.400 V respectively resulted from redox of AMP (curve c). Notably, the modified electrode immersed in the mixture solution of AP and AMP exhibited two couples of well-defined reduction and oxidation peaks respectively at 0.037 V and 0.108 V for AP and 0.240 V and 0.410 V for AMP. Their formal potentials (Eo′), calculated as the midpoint of anodic and cathodic peak potentials, were 73 mV and 325 mV corresponding to AP and AMP, respectively. Since the oxidation peak of AMP was shifted to a more positive potential, distinct redox peaks were acquired for AMP. The difference between the formal potentials of AMP and AP was found to be 252 mV. Hence, the highly selective simultaneous determination of AMP and AP in PBS buffer became possible with the AuNPs/CNTs-CONH-TAPP/GCE.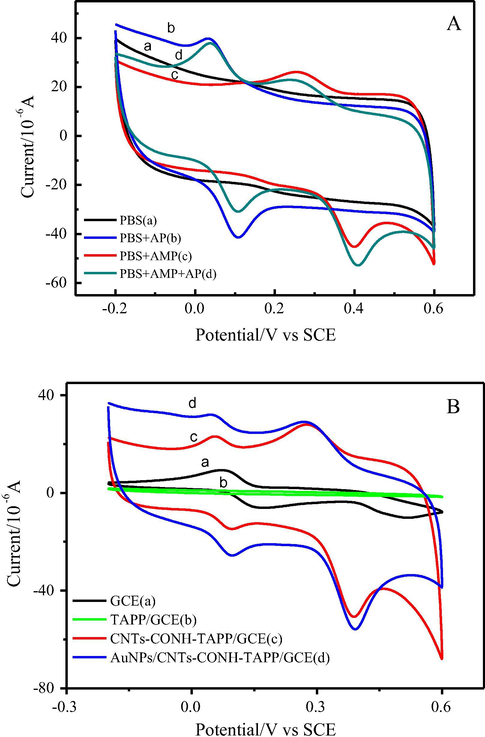
(A) CVs of AuNPs/CNTs-CONH-TAPP/GCE in 0.1 mol L−1 PBS (pH = 7.0) solutions without AMP and AP (a), with AP (b), AMP (c) and AP + AMP (d), respectively, at a scan rate of 100 mV s−1; (B) CVs of bare GCE (a), TAPP/GCE (b), CNTs-CONH-TAPP/GCE (c) and AuNPs/CNTs-CONH-TAPP/GCE (d) in 0.1 mol L−1 PBS (pH = 7.0) containing 0.03 mmol L−1 AP and AMP.
Fig. 5B shows the cyclic voltammograms of the bare GCE(a), TAPP/GCE(b), CNTs-CONH-TAPP/GCE(c) and AuNPs/CNTs-CONH-TAPP/GCE(d) in 0.1 mol L−1 PBS (pH = 7.0) containing 0.03 mmol L−1 AMP and AP. There were a pair of weak and broad redox peaks for AP and no reduction peak appeared for AMP on the bare GCE. TAPP modified glassy carbon electrode showed no redox response. But when CNTs-CONH-TAPP was dropped on the surface of bare GCE electrode, the redox peak currents of AMP and AP obviously increased. The enhancement in peak currents might be ascribed to the high surface area, excellent electrocatalytic activity and good conductivity of the CNTs-CONH-TAPP. Moreover, deposition of AuNPs further enhanced the current responses, indicating that AuNPs showed a good electrocatalytic activity towards redox reaction of AMP and AP on the modified electrode. Two couples of well-separated and strong quasi-reversible redox peaks promised a potential for chemically sensing determination of AMP and AP simultaneously. Curve b did not show any redox response for AMP and AP, which indicated introduction CNTs into TAPP played an important role.
3.4 Optimization of the experimental conditions
Influence of the dripping amount of CNTs-CONH-TAPP of 2 mg mL−1 on the oxidation peak current of 0.03 mmol L−1 AP and AMP were investigated by CV and the results were showed in Fig. S1. The oxidation peak currents of AP and AMP increased obviously with the amount of CNTs-CONH-TAPP suspension from 3.0 to 6.0 μl and then decreased when it further increased. The reason might be that too much amount of CNTs-CONH-TAPP would hinder the electron delivery and the resulting film was easy to fall off. Thus, 6.0 μl was used in further experiment.
In order to acquire the optimal electrochemical performance of the modified electrode, deposition conditions of gold nanoparticles were optimized. Figs. S2–S4 showed that when concentration of HAuCl4 solution, deposition potential and deposition time were 0.2 g L−1, −0.2 V and 90 s respectively, the oxidation peak currents of the two target molecules were the highest. When the deposition time was too long or the concentration of HAuCl4 was too high, the gold nanoparticles would agglomerate so that the effective surface area of the composite would decrease and the current response would reduce.
The effect of pH on the electrochemical behavior of AMP and AP on the modified electrode in 0.1 mol L−1 PBS over the range from 5.5 to 8.5 by DPV (Fig. S5). The oxidation currents gradually increased with increasing pH from 5.5 to 7.0 then decreased. So pH 7.0 was chosen for the subsequent analytical experiments. The oxidation peak potentials (Epa) of AMP and AP shifted linearly toward more negative potential with the pH increasing from 5.5 to 8.5, indicating that protons took part in the electrode reaction. The relationships between the peak potentials and pH could be expressed by the equations: Epa = 0.7777–0.0515pH (R2 = 0.9934) for AMP and Epa = −0.0572pH + 0.5397 (R2 = 0.9969) for AP, respectively. The absolute values of the slopes were close to the theoretical value of 59 mV/pH, indicating that the number of protons was equal to the number of the transferred electrons in the processes (Liu et al., 2011).
The kinetics of the electrode reaction was investigated by studying the effects of the scan rate on the redox peak potentials and currents of AMP and AP at the AuNPs/CNTs-CONH-TAPP/GCE. With the increase of the scan rate, the redox peak current increased gradually and the anode peak and cathode peak shifted to more positive and negative potentials, respectively. This showed that the responses of AMP and AP to the modified electrode were quasi-reversible process. As shown in Fig. S6, the redox peak currents of AMP and AP were linearly proportional to the scan rate (ν) from 40 to 140 mV s−1 with regression equations: Ipa,AMP (μA) = −10.0213–0.4723ν (mV s−1) (R2 = 0.9942), Ipc,AMP(μA) = 8.9086 + 0.2966ν (mV s−1) (R2 = 0.9932) and Ipa,AP(μA) = −3.5505–0.4161ν (mV s−1) (R2 = 0.9971), Ipc,AP(μA) = 4.2329 + 0.4089ν (mV s−1) (R2 = 0.9971), respectively, indicating that the electrochemical process of AMP and AP were adsorption-controlled.
Moreover, as shown in Fig. S7, the anode and cathode peak potentials of AMP and AP had liner relationships with the natural logarithm of scan rates (lnν). The regression equations were expressed as Epa,AMP(V) = 0.3505 + 0.0289lnν (mV s−1) (R2 = 0.9925); Epc,AMP(V) = 0.4318–0.0284lnν (mV s−1) (R2 = 0.9948) and Epa,AP(V) = 0.0626 + 0.0224lnν (mV s−1) (R2 = 1.0000); Epc,AP(V) = 0.1740–0.0184lnν (mV s−1) (R2 = 1.0000), respectively. According to the Laviron’s model (Laviron,1974; Laviron,1979), the electrochemical parameters (n, ks and α) can be calculated by the following formulas:
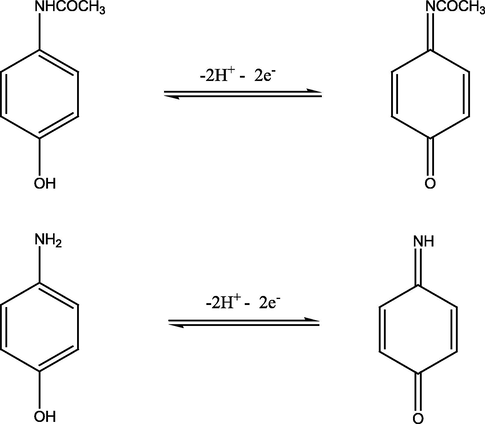
The electrode reaction mechanisms of AMP and AP.
The effects of accumulation potential and accumulation time on the peak currents AMP and AP were also demonstrated by DPV. As shown in Fig. S8A, the oxidation peak currents increased gradually and then decreased from −0.1 to 0.30 V. The most sensitive response was found at 0.1 V. According to the Fig. S8B, when the accumulation time varied from 70 s to 110 s, the oxidation peak currents of AMP and AP increased gradually at first and then decreased, and the maximum value was obtained for 100 s. Therefore, 0.1 V and 100 s were chosen as the optimal accumulation potential and time for simultaneous determination of AMP and AP.
3.5 Chronocoulometry
The electrochemical effective surface areas (A) of bare GCE (a), CNTs-CONH -TAPP/GCE(b) and AuNPs/CNTs-CONH-TAPP/GCE(c) can be determined by chronocoulometry according to Anson equation (Anson, 1964): where Qads is Faradic charge, Qdl is double layer charge which can be eliminated by background subtraction, D is diffusion coefficient, c is concentration of the substrate and A is surface area of the working electrode. Other symbols have their usual meanings. The experiment was firstly carried out in 1 mmol L−1 Fe(CN)63−/4− solution containing 0.1 mol L−1 KCl. As shown in Fig. S9(A, B), the linear regression equations of Q - t1/2 curves on bare GCE(a), CNTs-CONH -TAPP/GCE(b) and AuNPs/CNTs-CONH -TAPP/GCE(c) were Q(mC) = 0.0098t1/2 + 0.0109 (R2 = 0.9926), Q(mC) = 0.0462t1/2 + 0.1621 (R2 = 0.9918) and Q(mC) = 0.0633t1/2 + 0.1500 (R2 = 0.9911), respectively. Based on the slopes of the equations, A was calculated to be 0.0326cm2 for bare GCE, 0.1535 cm2 for CNTs-CONH-TAPP/GCE and 0.2104cm2 for AuNPs/CNTs-CONH-TAPP/GCE. These results indicated that the electrode effective surface area was increased obviously after modification, which could increase the electrochemical active sites, enhance the electrochemical response and decrease the detection limit.
Furthermore, chronocoulometry was also used to determine the saturation absorption capacity for AMP and AP at AuNPs/CNTs-CONH-TAPP/GCE surface in 0.1 mol L−1 PBS (pH = 7.0). As can be seen in Fig. S9(C,D), bigger intercepts and almost same slope were obtained from curves b and c compared with a, which further meant that the oxidation processes of AMP and AP were mainly controlled by adsorption (Ye et al., 2014). The plots of Q versus t1/2 showed good linear relationships and the regression equations could be expressed as Q(mC) = 0.0357t1/2 + 0.1274 (R2 = 0.9924) (in absence of AMP and AP), Q(mC) = 0.0459t1/2 + 0.1869 (R2 = 0.9924) (in presence of 0.03 mmol L−1 AMP) and Q(mC) = 0.0394t1/2 + 0.1703 (R2 = 0.9917) (in presence of 0.03 mmol L−1 AP), respectively. Qads was calculated to be 0.0595mC and 0.0429mC for AMP and AP. As n = 2, A = 0.2104cm2, according to the Laviron equation Qads = nFAΓ∗, the maximum adsorption capacity (Γ∗) of AMP and AP could be obtained as 1.47 × 10−6 and 1.06 × −6 mol cm−2.
3.6 Individual and simultaneous determination of AMP and AP using AuNPs/CNTs -CONH-TAPP/GCE
DPV was performed to investigate the relationship between the peak current and concentration of AMP and AP. The individual determination of AMP or AP in their mixtures was made when the concentration of one species changed while another remained constant. As shown in Fig. 6A, with increasing of AMP concentration its oxidation peak current increased but that of AP almost did not change when concentration of AP was kept 28 μmol L−1. Ipa was linearly proportional to AMP concentration in the range of 4.5 to 500 μmol L−1 with a linear equation I(μA) = −44.8496–0.03774 c (μmol L−1) (R2 = 0.9950) and the limit of detection of 0.44 μmol L−1 (S/N = 3), which was comparable to that at C-Ni/GCE (0.6 μmol L−1) (Wang et al., 2007), GR/GCE (0.6 μmol L−1) (Kang et al., 2010), MWCNT-PDDA-PSS/GE (0.5 μmol L−1) or even lower than that at SWCNTs/nafion modified nitrocellulose/AuNP-PGA/GCE (15.0 μmol L−1) (Lee et al., 2016), AuNP-PGA/SWCNE (1.18 μmol L−1) (Bui et al., 2012), HRP-PEI-SWCNT/GCE (1.36 μmol L−1) (Tertiş et al., 2013), CuO-NiO/GR/GCE (1.33 μmol L−1) (Liu et al., 2016), fCNT-PMG-CE (9.3 μmol L−1) and PMG-fCNT-CE (4.3 μmol L−1) (Barsan et al., 2015), HRP-Ppy-SWCNT/SPE (8.09 μmol L−1) (Tertiş et al., 2013). Similarly, as shown in Fig. 6B, the peak current of AP linearly increased with its concentration increasing from 0.08 to 60 μmol L−1 in presence of 180 μmol L−1 AMP with a linear equation I(μA) = −40.5489–0.278 c (μmol L−1) (R2 = 0.9953) and the detection limit of 0.025 μmol L−1 (S/N = 3), which was lower than that at carbon electrode (1.4 μmol L−1) (Chu et al., 2008), p[NVCzVBSA1]/CFME (1.0 μmol L−1) (Jamal et al., 2004), G-PANI/CPE coupled with droplet-based microfluidic sensor (15.67 μmol L−1) (Rattanarat et al., 2016), or close to that at SWNTs/POAPE (0.06 μmol L−1) (Wang Z. et al., 2009)), graphene–chitosan/GCE (0.057 μmol L−1) (Yin et al., 2010), SPCE (0.0767 μmol L−1) (Lamas-Ardisana et al., 2008). As a method for simultaneous determination of p-acetaminophen and p-aminophenol, the detection limit of the proposed method was also relatively lower than or close to those of some electrochemical methods reported previously, such as DPV with PEDOT/GCE (0.4 μmol L−1 for AMP and 1.2 μmol L−1 for AP) (Mehretie et al., 2011), DPV with CILE (0.5 μmol L−1 for AMP and 0.10 μmol L−1 for AP) (Safavi et al., 2008), DPV with Au/Pd/rGO/GCE (0.30 μmol L−1 for AMP and 0.12 μmol L−1 for AP) (Wang et al., 2017), DPV with GR–PANI/GCE (0.065 μmol L−1 for AP, not reported for AMP) (Fan et al., 2011) and DPV with RGO–TiN/GCE (0.02 μmol L−1 for AMP and 0.013 μmol L−1 for AP) (Kong et al., 2015), and other methods, such as CE with amperometric detection (1.4 μmol L−1 for AMP and AP) (Chu et al., 2008), spectrophotometry (1.2 μmol L−1 for AMP and AP) (Cekic et al., 2005) and CZE equipped with a diode array detector (0.028 μmol L−1 for AMP and 0.10 μmol L−1 for AP) (Pérez-Ruiz et al., 2005). Moreover, the simultaneous determination of AMP and AP was investigated by changing their concentrations simultaneously. As shown in Fig. 7, the peak currents of AMP and AP were linear with increasing AMP and AP concentrations and corresponding linear equations were I(μA) = −61.1655–0.0544 c (μmol L−1) (R2 = 0.9960) and I(μA) = −46.8382–0.3201c (μmol L−1) (R2 = 0.9945), respectively. And the linear ranges for AMP and AP were 50 to 348 μmol L−1 and 4 to 42 μmol L−1, respectively. It can be seen that individual or simultaneous determination of AMP and AP on AuNPs/CNTs-CONH-TAPP/GCE could be achieved with high sensitivity and selectivity. These results demonstrated that AuNPs/CNTs-CONH-TAPP/GCE were a promising candidate for the simultaneous determination of AMP and AP. In order to show the real application possibilities of the proposed method for determining of AMP and AP, the detection (S/N = 3) and quantification (S/N = 10) limits have also been calculated in real matrix (commercially available Paracetamol Dispersible Tablets and Yellow River water) and it was found that the sensitivity was acceptable (Table S1).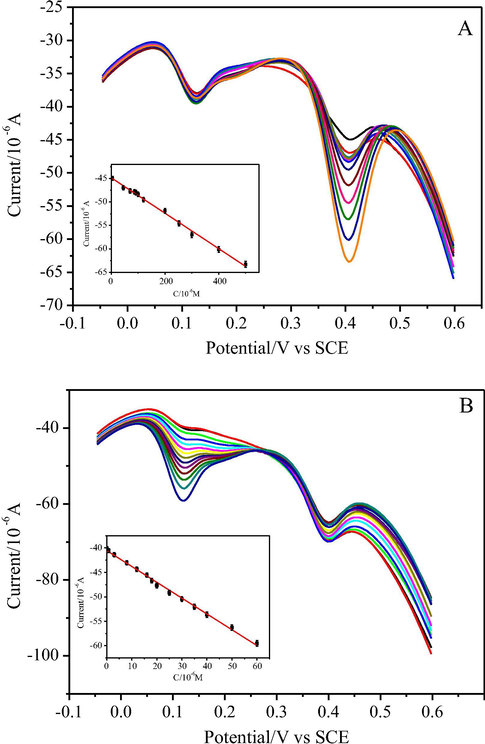
DPVs at AuNPs/CNTs-CONH-TAPP/GCE modified electrode in 0.1 mol L−1 PBS (pH = 7.0) containing 28 µmol L−1 AP and different concentrations of AMP: 4.5, 45.2, 70, 86, 92, 100, 120, 200, 252, 300, 400, 500 µmol L−1 (from up to down) (A) and containing 180 µmol L−1 AMP and different concentrations of AP: 0.8, 3, 8, 12, 16, 18, 20, 25, 30, 35, 40, 50, 60 µmol L−1 (from up to down) (B). Inset: Calibration plots of the anodic peak current versus concentrations of AMP and AP.
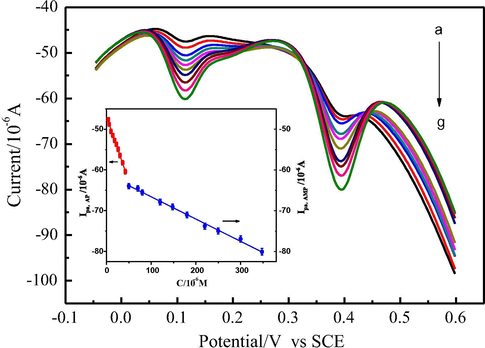
DPVs at AuNPs/CNTs-CONH-TAPP/GCE modified electrode in 0.1 mol L−1 PBS (pH = 7.0) containing different concentrations of AMP: 50, 70, 80, 120, 148, 180, 220, 250, 300, 348 μmol L−1 and AP: 4, 6, 10, 14, 18, 22, 26, 30, 36, 42 μmol L−1 respectively (from a to g). Inset: Calibration plots of the anodic peak current versus concentration of AMP and AP.
3.7 Reproducibility, stability and interferences
The reproducibility of the AuNPs/CNTs-CONH-TAPP/GCE was estimated by fabricating four electrodes independently and using them to determine 120 μmol L−1 AMP and 35 μmol L−1 AP. The relative standard deviation (RSD) was 1.93% and 3.37%, respectively, indicating an excellent reproducibility. Repeatability of the modified electrode was investigated and the relative standard deviation (RSD) of the sensor response to 120 μmol L−1 AMP and 35 μmol L−1 AP was 3.3% and 2.2% for ten successive measurements. After each measurement the modified electrode was immersed 0.1 mol L−1 blank PBS buffer and was CV scanned for 10 cycles to remove adsorbed contaminant. The result indicated the sensor had a good repeatability.
The anti-interference ability of the AuNPs/CNTs-CONH-TAPP/GCE was evaluated by adding potential interfering substances into 0.1 mol L−1 PBS (pH = 7.0) containing 120 μmol L−1 AMP and 35 μmol L−1 AP. The results illustrated that 50-fold K+, Ca2+, Mg2+, Cl−, glucose, citric acid, L-ascorbic acid, phenol, hydroquinone, dopamine, salicylic acid and caffeine did not interfere the detection of AMP and AP (Fig. S10). These results demonstrated that the modified electrode had a good anti-interference ability toward the determination of AMP and AP. The good selectivity was attributed to π-π interaction between the porphyrin ring and the analyte molecules and hydrogen bond interaction between hydroxyl and amino groups of AMP and AP molecules and carbonyl groups of the functionalized carbon nanotubes.
3.8 Application in real samples analysis
In order to evaluate the feasibility of the modified electrode, the proposed method was employed for assaying AMP and AP in Paracetamol Dispersible Tablets and Yellow River water under the optimal conditions with standard addition method. Prior to determination, four tablets of Paracetamol Dispersible Tablets were finely pulverized in a mortar and accurately weighed. The sample was extracted with 60 mL of anhydrous ethanol for 1 h in an ultrasonic bath, and then it was filtered and the filtrate was collected and diluted to 100 mL with deionized water. After the above sample solution was diluted 250 times with 0.1 mol L−1 PBS (pH = 7.0), the resulting solution was transferred to electrolytic cell for the determination using DPV. Water sample was first filtrated with a 0.45 μmol L−1 membrane filter and then injected in 0.1 mol L−1 PBS (pH = 7.0) solution for DPV measurement. The results were presented in Table 1. It can be seen that AMP and AP in mixtures could be satisfactorily detected with the recoveries in the range from 97.8% to 109.1% for AMP, and from 94.4% to 103.8% for AP, additionally, the RSD (n = 3) was less than 5.0%.
Samples
Detected (μmol L−1)
Add (μmol L−1)
Found (μmol L−1)
Recovery (%)
RSD (%)
AMP
AP
AMP
AP
AMP
AP
AMP
AP
AMP
AP
Paracetamol dispersible tablets
110.6
–
160
26.4
280.7
27.4
109.1
103.8
3.12
2.45
180
30.4
295.5
28.7
104.4
94.4
1.19
4.67
Yellow river water
–
–
160
26.4
165.9
25.8
103.7
97.7
4.86
1.23
180
30.4
176.1
29.4
97.8
96.7
1.21
3.67
The results show that tablet matrix does not have any interference on the simultaneous determination of the analytes. The amount of AMP in each pharmaceutical tablet was found to be 104.4 mg with 4.4% error, showing a good agreement with the content of AMP given by the manufacturer.
These results indicated that the proposed electrode could be efficiently applied to AMP and AP detection in real samples.
As seen in Table 1, p-aminophenol was not found in any of the pharmaceutical samples. The amount of p-aminophenol determined by The United States, British and Chinese Pharmacopoeias is limited to 0.005% (w/w) in paracetamol containing products. The limits of p-aminophenol may change depending on the formulation and dosage. p-Aminophenol is limited to 0.1% (w/w) in the monograph of paracetamol tablets present in British Pharmacopeia (Sornchaithawatwong et al., 2010). In this way, the proposed sensor can achieve the limit concentration of p-aminophenol in a pharmaceutical tablets of 400 mg using appropriate dilutions for sample preparation.
AMP and AP were not detected in Yellow River water sample, demonstrating that AMP and AP contents in the tested sample was below the detection limit of the present method. Notably, HPLC with porous graphitized carbon (PGC) column (Monsera and Darghouth, 2002) also did not find their existence.
4 Conclusions
A sensitive and selective electrochemical sensor based on AuNPs/CNTs-CONH -TAPP/GCE was constructed successfully for the simultaneous determination of p-acetaminophen and p-aminophenol in this paper and corresponding electrode reaction mechanism was proposed. The sensor displayed a wide linear range, low detection limit, excellent reproducibility, selectivity and long-term stability. Therefore, the present method could be efficiently used for the determination of p-acetaminophen and p-aminophenol in real samples.
Acknowledgements
The authors are very grateful to National Natural Science Foundation of China (Grant No. 21665024) and Key Lab of Eco-Environment Related Polymer Materials of MOE for their financial supports.
References
- HPTLC and RP-HPLC methods for simultaneous determination of paracetamol and pamabrom in presence of their potential impurities. J. Pharm. Biomed Anal.. 2015;114:22-27.
- [Google Scholar]
- A highly sensitive electrochemical sensor for simultaneous voltammetric determination of noradrenaline, acetaminophen, xanthine and caffeine based on a flavonoid nanostructured modified glassy carbon electrode. Sen. Actuat. Part B. 2014;192:634-641.
- [Google Scholar]
- Application of potentiostatic current integration to the study of the adsorption of cobaIt(lll)—(ethylenedinitrilo) tetraacetate on mercury electrodes. Anal. Chem.. 1964;36:932-934.
- [Google Scholar]
- New electrode architectures based on poly(methylene green) and functionalized carbon nanotubes: characterization and application to detection of acetaminophen and pyridoxine. J. Electroanal. Chem.. 2015;736:8-15.
- [Google Scholar]
- Electrochemical polymerization of amino-, pyrrole-, and hydroxy-substituted tetraphenylporphyrins. Inorg. Chem.. 1987;26:1009-1017.
- [Google Scholar]
- Determination of acetaminophen by thermometric titrimetry. Anal. Chim. Acta. 1997;343:125-128.
- [Google Scholar]
- Determination of acetaminophen by electrochemical co-deposition of glutamic acid and gold nanoparticles. Sens. Actuat. Part B. 2012;174:318-324.
- [Google Scholar]
- Optimization of a capillary zone electrophoresis method by using a central composite factorial design for the determination of codeine and paracetamol in pharmaceuticals. J. Chromatogr. Part B. 2006;839:95-101.
- [Google Scholar]
- Simultaneous Spectrophotometric determination of paracetamol and p-aminophenol in pharmaceutical products with tiron using dissolved oxygen as oxidant. J. Anal. Chem.. 2005;60:1019-1023.
- [Google Scholar]
- Nanomaterials based electrochemical sensors for biomedical applications. Chem. Soc. Rev.. 2013;42:5425-5438.
- [Google Scholar]
- Rapid determination of acetaminophen and p-aminophenol in pharmaceutical formulations using miniaturized capillary electrophoresis with amperometric detection. Anal. Chim. Acta. 2008;606:246-251.
- [Google Scholar]
- Chemiluminescence detection of paracetamol by a luminol-permanganate based reaction. Anal. Chim. Acta. 2001;439:95-100.
- [Google Scholar]
- Selective reversed phase high perforrmance liquid chromatography for the simultaneous determination of some pharmaceutical binary mixtures containing nsaids. J. Liquid Chromatogr. Relat. Technol.. 2012;35:2188-2202.
- [Google Scholar]
- LC–MS/MS method development for quantitative analysis of acetaminophen uptake by the aquatic fungus Mucor hiemalis. Ecotox. Environ. Safe.. 2016;128:230-235.
- [Google Scholar]
- Graphene–polyaniline composite film modified electrode for voltammetric determination of 4-aminophenol. Sens. Actuat. B. 2011;157:669-674.
- [Google Scholar]
- Impact of substituents and nonplanarity on nickel and copperporphyrin electrochemistry: first observation of a CuII/CuIII reactionin nonaqueous media. Inorg. Chem.. 2014;53:10772-10778.
- [Google Scholar]
- Development of an optical fibre reflectance sensor for p-aminophenol detection based on immobilised bis-8-hydroxyquinoline. Talanta. 2008;77:103-109.
- [Google Scholar]
- NMR and Bayesian regularized neural network regression for impurity determination of 4-aminophenol. J. Pharm. Biomed. Anal.. 2002;29:495-505.
- [Google Scholar]
- Covalent amino-functionalisation of single-wall carbon nanotubes. J. Mater. Chem.. 2015;15:3334-3339.
- [Google Scholar]
- Nickel (II) tetraphenylporphyrin modified surfaces via electrografting of an aryldiazonium salt. Electrochem. Commun.. 2011;13:1236-1239.
- [Google Scholar]
- Self-assembly of insoluble porphyrins on Au(111) under aqueous electrochemical control. Langmuir. 2011;27:14828-14833.
- [Google Scholar]
- Simultaneous determination of paracetamol, caffeine, domperidone, ergotamine tartrate, propyphenazone, and drotaverine HCl by high performance liquid chromatography. J. Liquid Chromatogr. Relat. Technol.. 2012;35:2148-2161.
- [Google Scholar]
- Conductive copolymer-modified carbon fibre microelectrodes: electrode characterisation and electrochemical detection of p-aminophenol. Sens. Actuat. B Chem.. 2004;97:59-66.
- [Google Scholar]
- A graphene-based electrochemical sensor for sensitive detection of paracetamol. Talanta. 2010;81:754-759.
- [Google Scholar]
- Simultaneous electrochemical determination of nanomolar concentrations of aminophenol isomers using nanocrystalline zirconosilicate modified carbon paste electrode. Electrochim. Acta. 2014;141:61-71.
- [Google Scholar]
- Degradation of 4-aminophenol by newly isolated Pseudomonas sp. strain ST-4. Enzyme Microb. Technol.. 2006;38:10-13.
- [Google Scholar]
- Facile green synthesis of graphene–titanium nitride hybrid nanostructure for the simultaneous determination of acetaminophen and 4-aminophenol. Sens. Actuators B:Chem.. 2015;213:397-403.
- [Google Scholar]
- Green reduction of reduced graphene oxide with nickel tetraphenyl porphyrin nanocomposite modified electrode for enhanced electrochemical determination of environmentally pollutant nitrobenzene. J. Colloid Interf. Sci.. 2017;497:207-216.
- [Google Scholar]
- Determination of paracetamol in pure form and in dosage forms using N,N-dibromo dimethylhydantoin. J. Pharm. Biomed. Anal.. 1997;15:1725-1728.
- [Google Scholar]
- Adsorption, autoinhibition and autocatalysis in polarography and in linear potential sweep voltammetry. Electroanal. Chem. Interf. Electrochem.. 1974;52:355-393.
- [Google Scholar]
- General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem.. 1979;101:19-28.
- [Google Scholar]
- Multiwalled carbon nanotube modified screen-printed electrodes for the detection of p-aminophenol: Optimisation and application in alkaline phosphatase-based assays. Anal. Chim. Acta. 2008;615:30-38.
- [Google Scholar]
- Determination of acetaminophen using functional paper- based electrochemical devices. Sens. Actuat. Part B. 2016;232:514-522.
- [Google Scholar]
- Photosensitized degradation of acetaminophen in natural organic matter solutions: The role of triplet states and oxygen. Water Res.. 2017;109:266-273.
- [Google Scholar]
- High sensitive simultaneous determination of hydroquinone and catechol based on graphene /BMIMPF6 nanocomposite modified electrode. Sens. Actuat. B: Chem.. 2011;157:540-546.
- [Google Scholar]
- Electrochemical preparation of nickel and copper oxides-decorated graphene composite for simultaneous determination of dopamine, acetaminophen and tryptophan. Talanta. 2016;146:114-121.
- [Google Scholar]
- Fluorescence Turn-off-on probe based on polypyrrole/graphene quantum composites for selective and sensitive detection of paracetamol and ascorbic acid. Bioelectron. Biosens. 2017
- [CrossRef] [Google Scholar]
- Second - order advantage applied to simultaneous spectrofluorimetric determination of paracetamol and mefenamic acid in urine samples. Anal. Chim. Acta. 2009;645:25-29.
- [Google Scholar]
- Efficient two-electron reduction of dioxygen to hydrogen peroxide with one-electron reductants with a small over potential catalyzed by a cobalt chlorin complex. J. Am. Chem. Soc.. 2013;135:2800-2808.
- [Google Scholar]
- Acetaminophen-induced nephrotoxicity: Pathophysiology, clinical manifestations, and management. J. Med. Toxicol.. 2008;4:2-6.
- [Google Scholar]
- Simultaneous determination of N-acetyl-p- aminophenol and p-aminophenol with poly(3,4-ethylenedioxythiophene) modified glassy carbon electrode. Talanta. 2011;85:1376-1382.
- [Google Scholar]
- Simultaneous LC determination of paracetamol and related compounds in pharmaceutical formulations using a carbon-based column. J. Pharm. Biomed. Anal.. 2002;27:851-860.
- [Google Scholar]
- Direct determination of paracetamol in powdered pharmaceutical samples by fluorescence spectroscopy. Anal. Chim. Acta. 2005;539:257-261.
- [Google Scholar]
- Noncovalent porphyrin-functionalized single-walled carbon nanotubes in solution and the formation of porphyrin–nanotube nanocomposites. Chem. Phys. Lett.. 2003;378:481-485.
- [Google Scholar]
- Development of a UV-spectrophotometric method for the simultaneous determination of aspirin and paracetamol in tablets. Sci. Res. Essays. 2011;6:417-421.
- [Google Scholar]
- Determination of paracetamol and its main impurity 4-aminophenol in analgesic preparations by micellar electrokinetic chromatography. J. Pharm. Biomed. Anal.. 2008;47:746-749.
- [Google Scholar]
- Migration behaviour and separation of acetaminophen and p-aminophenol in capillary zone electrophoresis: Analysis of drugs based on acetaminophen. J. Pharm. Biomed. Anal.. 2005;38:87-93.
- [Google Scholar]
- Amperometric determination of paracetomol by a surface modified cobalt hexacyanoferrate graphite wax composite electrode. Talanta. 2007;72:1818-1827.
- [Google Scholar]
- Multicomponent metallic impurities and their influence upon the electrochemistry of carbon n anotubes. J. Phys. Chem., Part C. 2009;113:4401-4405.
- [Google Scholar]
- Mechanistic study for the facile oxidation of trimethoprim on amanganese porphyrin incorporated glassy carbon electrode. J. Phys. Chem. C. 2011;115:21858-21864.
- [Google Scholar]
- Graphene-polyaniline modified electrochemical droplet-based microfluidic sensor for high-throughput determination of 4-aminophenol. Anal. Chim. Acta. 2016;925:51-60.
- [Google Scholar]
- A selective and sensitive method for simultaneous determination of traces of paracetamol and p-aminophenol in pharmaceuticals using carbon ionic liquid electrode. Electroanalysis. 2008;20:2158-2162.
- [Google Scholar]
- Flow injection simultaneous determination of acetaminophen and tramadol in pharmaceutical and biological samples using multiple pulse amperometric detection with a boron-doped diamond electrode. Diamond Rel. Mater.. 2015;60:1-8.
- [Google Scholar]
- Flow injection analysis system with electrochemical detection for the simultaneous determination of nanomolar levels of acetaminophen and codeine. J. Chem. Arab.. 2020;13(1):335-345.
- [CrossRef] [Google Scholar]
- Thermo-physical properties of epoxy nanocomposites reinforced with amino- functionalized multi-walled carbon nanotubes. Compos. Part A. 2007;38:1331-1336.
- [Google Scholar]
- p-Aminophenol-induced liver toxicity: Tentative evidence of a role for acetaminophen. J. Biochem. Mol. Toxicol.. 2001;15:34-40.
- [Google Scholar]
- Simultaneous determination of paracetamol and its main degradation product in generic paracetamol tablets using reverse-phase HPLC. J. Health Res.. 2010;24:103-106.
- [Google Scholar]
- Molecular electrocatalysis for oxygen reduction by cobaltporphyrins adsorbed at liquid/liquid interfaces. J. Am. Chem. Soc.. 2010;132:2655-2662.
- [Google Scholar]
- Merging porphyrins with organometallics: Synthesis and applications. Angew. Chem. Int. Ed.. 2008;47:7396-7421.
- [Google Scholar]
- Carbon based electrodes modified with horseradish peroxidase immobilized in conducting polymers for acetaminophen analysis. Sensors (Basel). 2013;13:4841-4854.
- [Google Scholar]
- Adsorptive elimination of paracetamol from physiological solutions: interaction with MFI-type zeolite. Microp. Mesop. Mater.. 2017;252:188-196.
- [Google Scholar]
- Electrochemical sensor based on palladium-reduced graphene oxide modified with gold nanoparticles for simultaneous determination of acetaminophen and 4-aminophenol. Talanta 2017
- [CrossRef] [Google Scholar]
- Anaerobic biodegradability of cellulose and hemicellulose in excavated refuse samples using a biochemical say. J. Ind. Microbiol.. 1994;13:147-153.
- [Google Scholar]
- Carbon-coated nickel magnetic nanoparticles modified electrodes as a sensor for determination of acetaminophen. Sens. Actuators B. 2007;123:495-500.
- [Google Scholar]
- Analytical Electrochemistry (third ed.). New Jersey: John Wiley & Sons; 2006.
- Electrodeposition of gold nanoparticles on indium/tin oxide electrode for fabrication of a disposable hydrogen peroxide biosensor. Talanta. 2009;77:1454-1459.
- [Google Scholar]
- Fabrication of the single-wall carbon nanotube compound polymer film electrode and the simultaneous electrochemical behavior of aminophenol isomers. Electrochim. Acta. 2009;54:7531-7535.
- [Google Scholar]
- Development and application of an electrochemical sensor modified with multi-walled carbon nanotubes and graphene oxide for the sensitive and selective detection of tetracycline. J. Electroanal. Chem.. 2015;757:250-257.
- [Google Scholar]
- Determination of 4-aminophenol impurities in multicomponent analgesic preparations by HPLC with amperometric detection. J. Pharm. Biomed. Anal.. 2003;32:1081-10086.
- [Google Scholar]
- Study of the voltammetric behavior of jatrorrhizine and its sensitive determination at electrochemical pretreatment glassy carbon electrode. Talanta. 2014;126:38-45.
- [Google Scholar]
- Second derivative spectrophotometric determination of p-aminophenol in the presence of paracetamol. Anal. Lett.. 1991;24:129-138.
- [Google Scholar]
- Electrochemical behavior and voltammetric determination of 4-aminophenol based on graphene–chitosan composite film modified glassy carbon electrode. Electrochim. Acta. 2010;55:7102-7108.
- [Google Scholar]
- Development and application of tetrabromobisphenol A imprinted electrochemical sensor based on graphene/carbon nanotubes three-dimensional nanocomposites modified carbon electrode. Talanta. 2015;134:435-442.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.arabjc.2017.09.008.
Appendix A
Supplementary material
Supplementary data 1
Supplementary data 1







