Translate this page into:
Green fabrication, characterization and antimicrobial activities of AgO/Ag/carboxymethyl chitosan- graphene oxide films
⁎Corresponding author. xd0929@163.com (Dong Xie)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Antimicrobial AgO/Ag/carboxymethyl chitosan-graphene oxide (AgO/Ag/CGO) films were synthesized by UV-light-assisted reduction followed by ionic crosslinking method. The structure of the AgO/Ag/CGO films were explored by X-ray diffraction (XRD), field-emission scanning electron microscope (FE-SEM), Transmission electron microscopy (TEM), X-ray photoelectron spectroscopy (XPS), ultraviolet–visible spectroscopy (UV–vis), and Fourier-transform infrared (FTIR) analysis. We have also tested the cytotoxicity and cell viability of AgO/Ag/CGO3. The results of cytotoxicity of AgO/Ag/CGO3 on HepG2 cells suggests that the nanocomposite is not cytotoxic. Additionally, thermal stability, swelling ratio, antimicrobial activity of the AgO/Ag/CGO films were evaluated in detail, which revealed that the addition of GO improves the thermal stability and swelling ratio of AgO/Ag/CGO films. Of the three AgO/Ag/CGO films, the AgO/Ag/CGO3 exhibited better antimicrobial activity and higher inhibition rate against E. coli (89.2%) and S. aureus (93.4%). Therefore, the simple and green method is potential candidate for prepare of Ag-based antimicrobial film materials.
Keywords
UV-light-assisted reduction
Silver
Silver oxide
Carboxymethyl chitosan
Graphene oxide
Antimicrobial
1 Introduction
Carboxymethyl chitosan (CMCS) as a novel amphoteric biopolymer, has been extensively applied in drug encapsulation, food, tissue engineering, cosmetic, antioxidant, and antibacterial fields owing to its properties of non-toxic, antimicrobial effect, biodegradable and biocompatible, required to meet many special conditions (Jie et al., 2022; Hao et al., 2022; Zhang et al., 2021; Shariatinia, 2018; Olanipekun et al., 2021). So, CMCS-base hybrid materials have obtained much attention in last few years (See Scheme 1).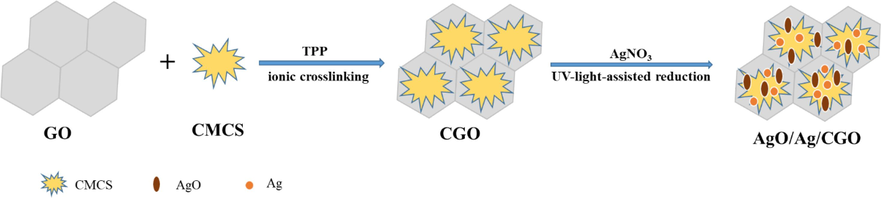
Schematic illustration of the AgO/Ag/CGO films.
Recently, the combination of mineral nanoparticles clay (Eddarai et al., 2022; Benucci et al., 2018; Biswas et al., 2021; Kausar et al., 2019; Lewandowska et al., 2014a; Lewandowska et al., 2014b), hydroxyapatite (Baskar et al., 2022; Ali et al., 2022;Furtos et al., 2013), graphene oxide (Azeman et al., 2022; Rostamian et al., 2022; Cao et al., 2022), metallic oxide and metallic such as zinc oxide (He et al., 2022; Wardana et al., 2022; Gasti et al., 2022) and Ag (Gao et al., 2022) with CMCS has been reported. Among them, GO has many advantages such as its large special surface area, hydrogen bonding, and simplicity of fabrication process, and has obtained a great interest in various fields. Additionally, GO can improve the mechanical property of CMCS-based hybrid materials. In addition, Ag or AgO is another promising material to increase the antibacterial activity of CMCS, and has a wide application in clothing, pharmaceutical products, and medical devices due to its low toxicity and unique physical and chemical properties. There are several reports about CS-Ag, CS/Ag-WPU, GO/CMCS, and CMCS-AgO-Ag, nanocomposites, which have been applied in various areas including wound dressing (Zhou et al., 2021), food packaging (Cheng et al., 2021), water treatment (Su et al., 2021), and drug delivery system (Xu et al., 2020). Luo et al. (Luo et al., 2019) prepared graphene oxide/carboxymethyl chitosan aerogels via vacuum-assisted self-assembly and used for heavy metal adsorption. Huang et al. (Huang et al., 2017) synthesized the chitosan–nanosilver hybrids by in situ reduce method using glucose as the reducing agent. Pan et al. (Pan et al., 2020) prepared nanocomposite antibacterial films by in situ generation of silver nanoparticles and electrodeposition of carboxylate chitosan. Rasoulzadehzali et al. prepared the antibacterial chitosan/graphene oxide-Ag (CS-GO-Ag) bio-nanocomposite hydrogel beads for controlled release of doxorubicin (Rasoulzadehzali et al., 2018). However, there have been few reports on the preparation of antibacterial films by applying the antibacterial properties of silver ions (Ag2+) by a simple and green method.
To address this need, this work prepared the AgO/Ag/CGO nanocomposites films by a UV-light-assisted reduction and ionic crosslinking method. Then, the material was characterized by TEM, XPS, FE-SEM, XRD, and FTIR spectroscopy. The particle size distribution of the prepared Ag-NPs at different UV irradiation times were indicated by TEM. Moreover, we explored the thermal stability property and antimicrobial activity of the AgO/Ag/CGO nanocomposites films. We raise that the AgO/Ag/CGO nanocomposites films has an excellent antimicrobial and thermal stability property. As a consequence, it will be an efficient strategy to prepare novel nanocomposite antimicrobial materials.
2 Materials and methods
2.1 Materials and chemicals
Carboxymethyl chitosan (CMCS, 80 % of substitution degree), sodium hydroxide (NaOH, 96 %), Graphene oxide (GO) nanosheets were synthesized by the modified Hummers method, triphosphoric acid (99.8 %, TPP), silver nitrate (AgNO3, 99.98 %), gelatin (C13H18O2, ≥98 %), ethanol (C2H5OH, 99 %) and deionized water were all purchased from Macklin (Shanghai, China). All the reagents were of analytical grade.
2.2 Preparation of Ag nanoparticles
The Ag nanoparticles was synthesized by UV-light-assisted reduction. Simply, 0.4 g of gelatin was added to 20 mL deionized water in a flask under stirring; then the aqueous AgNO3 was added dropwise to the clear gelatin solution; the mixed solution (Ag+/gel-sol) was placed into the UV reactor for UV irradiation at different times (1, 6, 12, 24 h) at room temperature to obtain the UV irradiated Ag nanoparticles.
2.3 Preparation of AgO/Ag/CGO films
The AgO/Ag/CGO films was synthesized by two steps. The as-prepared GO was dispersed in deionized water by stirring for 30 min and ultrasonicating for 30 min to obtain the dark brown solution. Firstly, 0.4 g CMCS was slowly dissolved in 20 mL water at 40 °C for 1 h to form a homogeneous solution and 20 mL of GO was added into the CMCS solution. After mixing, 1 mL of TPP solution (1 wt%) was added into above mixed solution and reacted for 2 h under continuous stirring to obtain the CGO solution. Secondly, the Ag/gel-sol (1, 6, 12, 24 h) was added into the CGO solution to react for 4 h to obtain the hydrogels and the resulting sol was cast onto glass plates to obtain the transparent films. The AgO/Ag/CGO films were recorded as AgO/Ag/CGO1, AgO/Ag/CGO2, AgO/Ag/CGO3, AgO/Ag/CGO4.
2.4 Characterizations of AgO/Ag/CGO films
Firstly, Ag/gel-sol samples synthesized under various UV irradiation times were characterized by UV–Vis spectroscopy from 300 to 700 nm. The morphology and microstructure of the CMCS-GO and AgO/Ag/CGO3, films samples were determined by FE-SEM (ZEISS MERLIN) and HRTEM (JEOL-2100F). X-ray diffraction patterns of the CMCS, CGO, and AgO/Ag/CGO3 samples were recorded by XRD (X'Pert PRO Ultima IV) to analyze the phase structure. XPS analyses were investigated by a Thermo Scientific K-ALPHA + spectrometer with all the binding energies referenced to the adventitious carbon at 284.8 eV and the curve was fitted using XPS PEAK 4.1 software. FTIR spectra of CMCS, CGO, CMCS-Gellatin, and AgO/Ag/CGO samples were performed using a FTIR spectrophotometer (INVENIO-S). Raman spectrum of the samples was measured on a confocal Renishaw in Via Reflex Raman spectrometer.
2.5 Thermogravimetric analysis of AgO/Ag/CGO samples
The thermogravimetric analysis of the AgO/Ag/CGO film samples were determined via thermogravimetric analysis (TGA, TG209 F3). Simply, 5~10 mg of sample was placed in an alumina pans and putted into the crucible baskets at a heating rate of 20 °C /min range from 35 °C to 800 °C.
2.6 Water adsorbing ratio test
We detected the water adsorbing ratio of the film samples using a general gravimetric method. In brief, the film samples were putted into distilled water for 24 h at room temperature after weighed (M0). After 24 h, the film samples were taken out and removed the moisture on the surface with filter paper before weighted again (M1). The tests were carried out in triplicate and the average values were recorded. So the water adsorbing ratio AR (%) can be calculated using the following formula:
Among them, M0 is the initial weight of the sample, and M1 is the weight of sample at 24 h.
2.7 Swelling ratio test
The swelling ratio of film samples was conducted in buffer solutions using a general gravimetric method. Briefly, the weighed samples were putted into the buffer solution separately in a shaking water bath at 37 °C. After 24 h, the swelling equilibrium was reached, and the samples were taken out and removed the droplets on their surface with paper towel. Then the samples were again weighed (W1). The swelling ratio (SR) of samples was determined by the Eq. (2).
Among them, W0 is the initial weight of sample, and W1 is the weight of sample at 24 h.
2.8 In vitro cytotoxicity analysis
To evaluate the cytotoxicity of nanocomposite, we tested the cytotoxicity of AgO/Ag/CGO3 on HepG2 cells via the MTT assay, and cell viability was determined after 24 h. The viability of the cultured cell was monitored using a cell MTT assay. Into a 96-well plate100μL Hepg2 cell suspension of a density of 4000 cells/well was filled, and the culture plate was put into an incubator at 37 °C with 5 % CO2 for 24 h. Films samples was sterilized under UV light and thoroughly rinsed with phosphate buffer saline (pH 7.4) and added to the well for incubation for another 24 hours. Then, 20 μL MTT solution was added into each well and incubated for 4 h. Absorbance at 570 nm was measured with a Microplate Reader. The mean optical density (OD) of five wells was used to calculate the percentage of cell activity (CA) as:
2.9 Determination of antibacterial activity of AgO/Ag/CGO films
The antimicrobial activity of AgO/Ag/CGO films against E. coli and S. aureus was investigated by qualitative and quantitative analysis, respectively. The antimicrobial activity of AgO/Ag/CGO films was qualitatively evaluated by disc diffusion assay, and the CGO film was used as negative control. Simply, E. coli as well as S. aureus was evenly spread on a nutrient agar plate medium at 37 °C respectively, and then the AgO/Ag/CGO films slices were placed on the medium. After incubation for 24 h, the bacterial growth was observed and the diameter of the zones of inhibition of these samples were measured. The tests were carried out in three times for each film. In addition, the inhibition rate of AgO/Ag/CGO films on E. coli and S. aureus have been determined by optical density (O.D) method. Simplely, 0.5 g films were immersed in 30 mL of E. coli and S. aureus nutrient agar culture medium, and blank E. coli and S. aureus medium was used as control. These samples were incubated in thermostatic shakers for 12 h at a shaking rate of 50 rpm at 37 °C. Then the absorbance of the culture solution at 600 nm was measured with a UV–visible spectrophotometer. The inhibition ratio (IR) calculation formula is as follows:
Here, A0 is the absorbance before incubation of E. coli; At and Acon are the absorbance of E. coli in the film and the reference sample after incubation for t time.
2.10 In vitro silver-release study
The in vitro silver-release study was carried out by a previously described method, with slightly modification (Hosseini et al., 2013). The initial amount of silver and the amount of silver released from the AgO/Ag/CGO films were evaluated by inductively coupled plasma–optical emission spectrometry (ICP-AES, Iris Intrepid). 20 mg of AgO/Ag/CGO2, AgO/Ag/CGO3, AgO/Ag/CGO4 were separately immersed in 20 mL of phosphate buffer (pH 7.4) and incubated at 37 °C under agitation. At various time intervals (4, 8, 12, 24, 48, and 72 h), samples were centrifuged and 1 mL of release medium were picked up for the analysis and an equal volume of fresh buffer was added into the device to maintain a constant volume. The concentration (ppm) of silver in the release medium was determined by ICP-AES analysis and then the cumulative release amount was calculated.
3 Result and discussion
3.1 Characterizations of Ag nanoparticles
In this work, we prepared the Ag nanoparticles by the UV irradiation method and the gelatin was used as a green stabilizer. The in-situ reduction of Ag+ involves radiolysis of aquatic solutions, and then are oxidized to form H+ and OH radicals (OH•) under UV irradiation (Equation (4)), and then the solvated electrons were produced. Then, metallic cations were reduced to the metallic atoms by the solvated electrons. At different UV irradiation times, we observed the color of the Ag+/gel solutions and found that it gradually changed from colorless to yellow, then to brown, and finally dark brown, which suggesting the formation of Ag nanoparticles (Fig. 1a). To further ensure the formation of Ag nanoparticles, the UV–Vis spectroscopy was carried out and the result was shown in Fig. 1b. The appearance of absorption peak of the Ag+/gel solutions at about 420 nm indicated the formation of Ag nanoparticles, and the absorption spectrum peak increased with the increase of UV irradiation times (He et al., 2021; Niu et al., 2020; Nguyen et al., 2020). However, there is no absorption spectrum appearing of the sample prepared without UV irradiation, which indicating that UV irradiation played a crucial role for the synthesis of Ag nanoparticles.
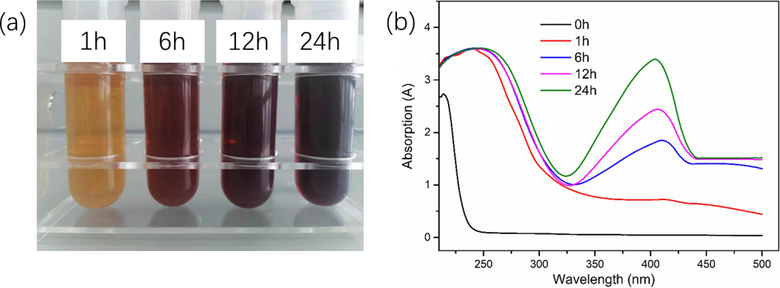
Photograph (a) and UV–Vis spectra (b) of synthesized Ag-NPs in gelatin solution at different UV irradiation times.
3.2 Characterizations of AgO/Ag/CGO films
The morphology of freeze-dried AgO/Ag/CGO films was scrutinized by FE-SEM. In Fig. 2a, b, c, the cross-sectional micromorphologies of CGO shown an open porous network with a smooth surface. The pores distribute randomly but arrange regularly and most of the pore walls are interconnected. This porous structure and abundant hydrophilic polar groups not only facilitates water molecules to enter the network, but resulting in swelling, intercalation, and ion exchange properties. Compared with CGO, the morphology of AgO/Ag/CGO3 has not changed, but we cannot observe the silver and silver oxide particles on the surface of AgO/Ag/CGO3 sample due to the small particle size of silver and silver oxide. The XRD spectrum of the AgO/Ag/CGO3 and its reference samples was determined and the results were shown in Fig. 3a. CMCS showed a broad peak at 20.2° attributing to their anhydrous crystalline states. Compared with CMCS, the diffraction peaks of CGO and AgO/Ag/CGO3 shifted to lower diffraction angle owing to the enhanced interaction between CMCS and GO and the introduction of GO nanosheets destroying the anhydrous crystalline structure of CMCS (Xu et al., 2020). Additionally, the AgO/Ag/CGO3 sample exhibited three new diffraction peaks at 32°, 38° and 46°, which could be well attributed to the (1 1 1) plane of the AgO (JCPDS 43–1038) and (1 1 1), (2 0 0) planes of Ag (JCPDS 04–0783) (Lashin et al., 2021). Ag nanoparticles were prepared by the UV irradiation method, and the in-situ reduction of Ag+ involves radiolysis of aquatic solutions, and then are oxidized to form H+ and OH radicals (OH•) under UV irradiation (Equation (5). AgO is formed by the reaction of Ag2O with OH– or the reaction of Ag with OH (Nwanya et al., 2013; Samsuddin et al., 2017). To further identify the valence state of silver and silver oxide, the X-ray photoelectron spectroscopy of the AgO/Ag/CGO3 was carried out. From the Fig. 3b, we can see that the Ag 3d XPS spectrum of AgO/Ag/CGO3 was fitted to two superimposed doublet. The peaks observed at 367.5 and 373.8 eV were corresponding to Ag0, and peaks at 367.2 eV and 373.2 eV are well attributed to Ag2+. These results are consistent with that of the XRD.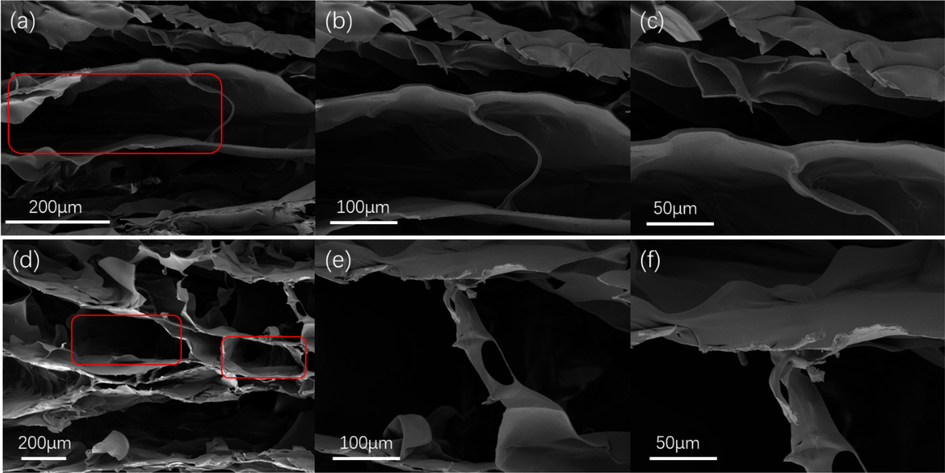
FE-SEM images of (a, b, c) CGO and (d, e, f) AgO/Ag/CGO3 hydrogel samples.
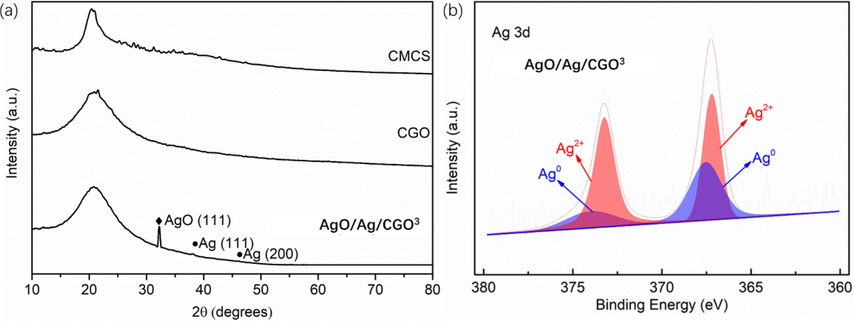
The XRD patterns of CMCS, CGO and AgO/Ag/CGO3 (a); XPS patterns of Ag 3d of AgO/Ag/CGO3 sample (b).
The FT-IR spectra of CMCS, CGO, AgO/Ag/CGO1, AgO/Ag/CGO2, AgO/Ag/CGO3, AgO/Ag/CGO4 were investigated to further confirm the formation mechanisms of the AgO/Ag/CGO hydrides. These results were shown in Fig. 4 and Fig.S1. In the characteristic peaks of CMCS, the characteristic peaks at 3305 cm−1 and at 1576 cm−1 attributing to the overlapping stretching vibrations of –NH and –OH and the –NH2 deformation vibration and –COO– asymmetric stretching vibration overlapping, which shift to a lower frequency comparing to the spectra of CGO, suggesting the intermolecular hydrogen bonds between GO and CMCS (Azeman et al., 2022). The peak at 1079 cm−1 is ascribed to the C–O stretching vibration of the secondary hydroxyl groups. Compare with CGO, it shifted to a lower frequency in AgO/Ag/CGO1, AgO/Ag/CGO2, AgO/Ag/CGO3, AgO/Ag/CGO4. This is may be attributed to the attachment of Ag or AgO. Additionally, a peak at around 1420 cm−1 was attributed to the symmetrical stretching vibrations of the carboxyl groups, and the peak shifted to lower frequencies, which suggested that the –COOH groups may also contribute to silver immobilization.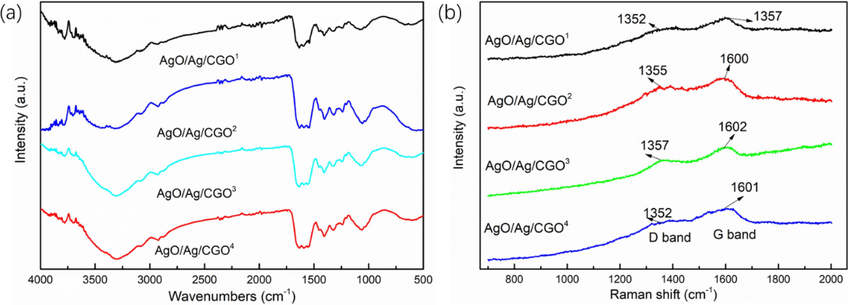
FTIR spectra (a) and Raman spectra (b) of AgO/Ag/CGO1, AgO/Ag/CGO2, AgO/Ag/CGO3 and AgO/Ag/CGO4.
To further determine the chemical composition, the chemical states of the GO, CMCS, CGO and AgO/Ag/CGO3 were investigated with XPS method. The C1s spectrum of CMCS-GO and AgO/Ag/CGO3 exhibits the same peaks with GO and CMCS at 284.1, 285.2, 286.6 and 287.3 eV, respectively (Fig. 5a, b, c, d). From Fig. 5e, N 1s spectrum of CMCS exhibited three peaks at 399.1 eV, 398.5 eV, and 401.3 eV, which attributed to pyrrolic/pyridone (N-5), pyridinic (N-6), and quaternary nitrogen (N-Q), respectively. Similar to CMCS, the CGO and AgO/Ag/CGO3 also presents three peaks attributing to N-5, N-6, and N-Q, respectively (Fig. 4f, g). By the interacting between CMCS and GO, the nitrogen content of N-5 decreases while the nitrogen content of N-6 and N-Q increases, suggesting that the N atoms of N-5 convert to N-6 and N-Q (Xu et al., 2020). Therefore, we can conclude that the CMCS is successfully assembled on the GO nanosheets by the hydrogen-bonding interaction. This result can be further confirmed by Raman spectra. From Fig. 4b h, all samples exhibited two characteristic peaks at about 1355 cm−1 (D band) and 1606 cm−1 (G band), which are the two characteristic peaks of GO.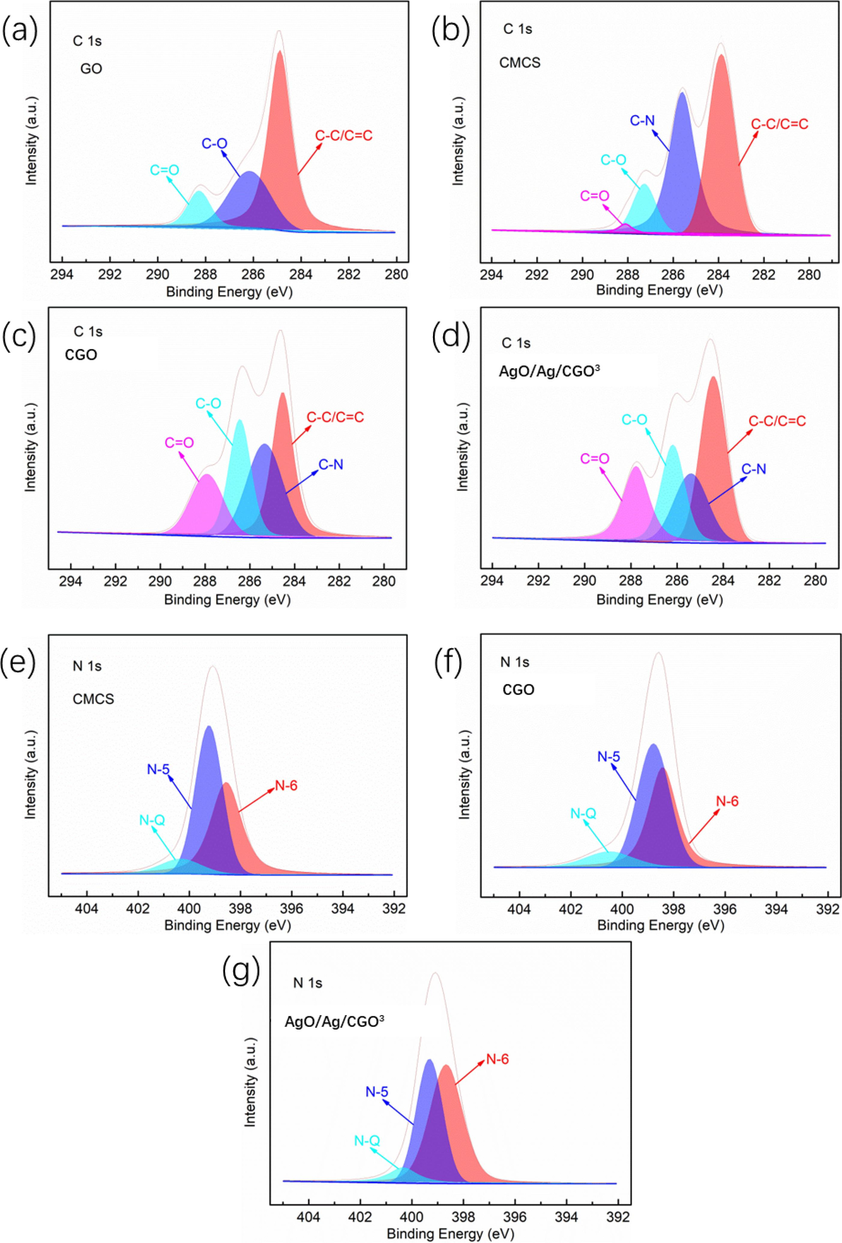
XPS patterns of the surveys of GO, CMCS, CGO and AgO/Ag/CGO3. C1s of GO, CMC, CMCS-GO and AgO/Ag/CGO3 (a–d); N1s of CMCS, CGO and AgO/Ag/CGO3 (e–g).
The TEM images show that Ag nanoparticles formed by reduction of Ag+ ions using the UV irradiation, and the corresponding particle size distribution of the Ag nanoparticles are shown in Fig. 6. From TEM image, we can see that spherical Ag nanoparticles were distributed homogeneously in the CMCS-GO network. Particle size and size distribution of the Ag nanoparticles in AgO/Ag/CGO1, AgO/Ag/CGO2, AgO/Ag/CGO3, AgO/Ag/CGO4 were measured and the Ag nanoparticles in AgO/Ag/CGO3 exhibited the narrowest size distribution (in the range of 4–5 nm) and the smallest mean diameter (4.72 ± 1.31 nm). This may be because of long time exposure of UV light results in the change of arrangement around the nanoparticle surface. However, AgO/Ag/CGO4 has a higher mean diameter than AgO/Ag/CGO3. This result may be attribute to the gelatin degradation under the long-term UV-irradiation source, which result in Ag nanoparticles could not enveloped in the gelatin framework agglomerate into larger particles. In Fig. 7, the HRTEM image and SAED pattern of AgO/Ag/CGO3 samples further demonstrated that Ag nanoparticles were crystalline in samples. The lattice spacings were identified to be 0.234 nm, which was indexed to the d-spacing of the Ag (1 1 1) plane (JCPDS 04–0783) (Huang et al., 2017; Duan et al., 2020; Biao et al., 2017). The SAED pattern showed periodic diffraction rings, which indicated that the Ag nanoparticles were polycrystalline.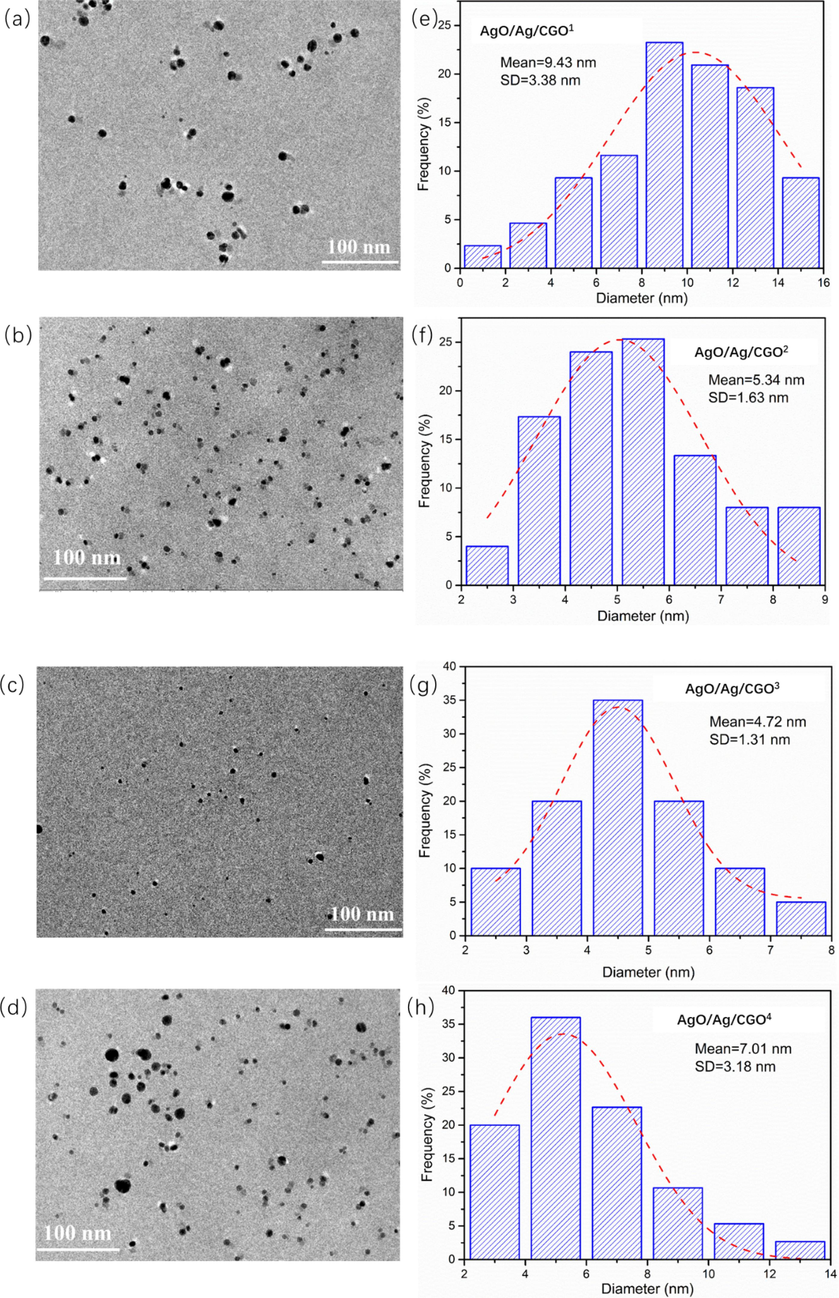
TEM images and corresponding size distributions of fabricated AgO/Ag/CGO1, AgO/Ag/CGO2, AgO/Ag/CGO3 and AgO/Ag/CGO4.
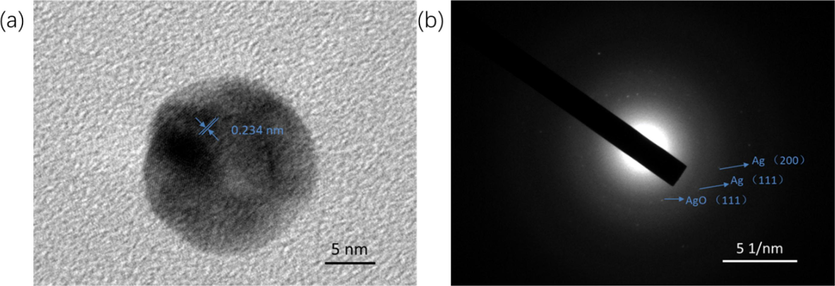
HRTEM image and SADE pattern of AgO/Ag/CGO3.
3.3 Thermogravimetric analysis of AgO/Ag/CGO samples
The thermal properties of the AgO/Ag/CGO1, AgO/Ag/CGO2, AgO/Ag/CGO3, AgO/Ag/CGO4, were determined by TGA, as shown in Fig. 8a. We can observed that, in the temperature range of 35–500 °C, the weight loss of all samples could be divided into three stages. In the first stage, the all samples exhibited a weight loss from 35 °C to 120 °C, which attributing to the removal of physical adsorption and bound water. In the second stage, it exhibited a rapid weight loss from 120 °C to 250 °C, corresponding to the decomposition of functional groups on the polymer chain and the degree of reduction of each sample are difference. Notably, the decomposition temperature of the AgO/Ag/CGO1, AgO/Ag/CGO2, AgO/Ag/CGO3, AgO/Ag/CGO4 all samples was occurred at approximate 250~330 °C and lose their weight slowly. In addition, we can observed that the AgO/Ag/CGO2 exhibited the biggest starting decomposition temperature, which suggest that the AgO/Ag/CGO2 exhibited a higher better thermal stability than the other samples. This is may be due to the gelatin degradation under the 12 h or 24 h UV-irradiation source, which destroy the network structure of the compound resulting in the decrease of thermal stability. In addition, Ag2O first formed and it is not stability when the short UV-irradiation source. So the AgO/Ag/CGO2 has a lower thermal stability than AgO/Ag/CGO1.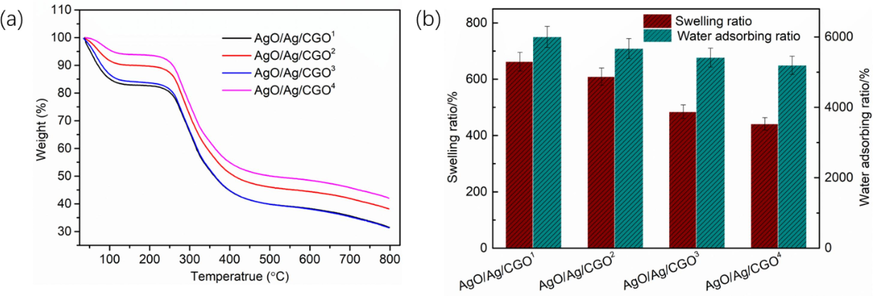
(a) TGA curves and (b) Water adsorbing ratio in water and swelling behavior in PBS 7.4 of AgO/Ag/CGO1, AgO/Ag/CGO2, AgO/Ag/CGO3 and AgO/Ag/CGO4.
3.4 Water adsorbing ratio and swelling ratio test
The water adsorbing ratio of the AgO/Ag/CGO1, AgO/Ag/CGO2, AgO/Ag/CGO3, AgO/Ag/CGO4 was tested, as shown in Fig. 8b. We can observed that the four samples exhibited high water absorption ratio in H2O and swelling ratio in pH = 7.4 PBS. This is because of the existence of GO nanosheets creates more free spaces and higher porosity in the CMCS polymeric matrix. In addition, the interactions between GO and CMCS macromolecules also can enhance the water adsorbing ratio. Notably, the water absorption ratio and swelling ratio decreased with the increasing of the UV irradiation times, which resulted in the decrease of the amount of Ag+ ions. But the amount of Ag and AgO nanoparticles increased. This is due to the dispersion of Ag+ ions inside the polymer- networks, which can cause repulsion of networks and ultimately result in an improved swelling behavior of the system. So the water absorption ratio and swelling ratio decreased with the decreasing of the amount Ag + ions (Gils et al., 2010). In addition, the amount of Ag and AgO nanoparticles increases with the increasing of the UV irradiation times, which indicated a more cross-linking point in the polymer-network, which prevent the aqueous medium from entering the polymer-network. So the water absorption ratio and swelling ratio decreased with the increasing of UV irradiation times (Xu et al., 2020).
3.5 Antimicrobial activities and cytotoxicity of AgO/Ag/CGO films
The antibacterial activity of the AgO/Ag/CGO films was studied the inhibition rings and inhibition rate against E. coli and S. aureus. The inhibition rings of the bacteria colonies on AgO/Ag/CGO2, AgO/Ag/CGO3 and AgO/Ag/CGO4 films were presented in Fig. 9(a and b), and their diameters were 4.2 ± 0.1 mm, 5.2 ± 0.1 mm and 4.8 ± 0.2 mm against E.coli and 4.7 ± 0.2 mm, 5.4 ± 0.1 mm and 4.9 ± 0.2 mm against S. aureus, respectively. We can observe that the AgO/Ag/CGO2, AgO/Ag/CGO3 and AgO/Ag/CGO4 films all exhibited excellent antimicrobial activities, which attributed to the presence of AgO/Ag in the films. Compared to AgO/Ag/CGO2 and AgO/Ag/CGO4 films, the AgO/Ag/CGO3 film showed better antimicrobial activities. This is because of the narrowest size distribution (in the range of 4–5 nm) and the smallest mean diameter (4.72 ± 1.31 nm) of AgO/Ag/CGO3 film. In addition, the AgO/Ag/CGO3 showed stable and continuous silver release with no obvious initial burst. What is more, the AgO/Ag/CGO3 film showed a higher cumulative release of silver (Fig. 10). Therefore, the presence of AgO/Ag enhanced antimicrobial ability of CMCS-based nanocomposite significantly. In addition, the bacteriostatic ability of the AgO/Ag/CGO films is determined by the inhibition rate. From Fig. 9(c and d), we can find that the inhibition rate of AgO/Ag/CGO3 on E. coli (89.2 %) and S. aureus (93.4 %) is higher than other samples. These results are consistent with that of bacteriostatic zone test. The robust antimicrobial activity of the film could additionally reduce the soft rotting of fresh produce and strengthen the safety of consumption. This would reduce microorganism spoilage which devastates fresh fruit and vegetables after harvest and might even reduce foodborne illness outbreaks when it was made as packaging materials. Generally, antibacterial properties of AgO/Ag nanoparticles may be described based on following mechanisms(Prabhu and Poulose, 2012; Pandey and Ramontja, 2016): (1) AgO/Ag nanoparticles react with phosphorus and sulfur groups of bacterial DNA, which result in the disruption of DNA replication, (2) the Ag ions released from Ag nanoparticles penetrate into bacteria that lead to the disruption of DNA replication and ATP production by damaging the peptidoglycan, DNA, and protein (3) AgO/Ag nanoparticles attached to the surface of bacteria's membrane, which disturb its permeability and respiration ability during their interaction cell wall of bacteria. The cytotoxicity were evaluated using in vitro studies with a human pancreatic cancer cell line (HepG2) to evaluate the biosafety of the film. Biocompatibility is crucial for the film to be applied in the packing material. So we have tested the cytotoxicity of AgO/Ag/CGO3 on HepG2 cells via the MTT assay, and cell viability was determined after 24 h. The obtained results are presented in Fig. S2. It is obvious from the result, we can concluded that there is no significant change in the HepG2 cell after 24 h incubation with 1 to 10 μg mL−1. In each concentration of the sample, over 90 % of the cells stayed alive, which suggests that the nanocomposite is not cytotoxic. And this antimicrobial AgO/Ag/CGO films can be applied in food packaging materials or controlled-release carrier material.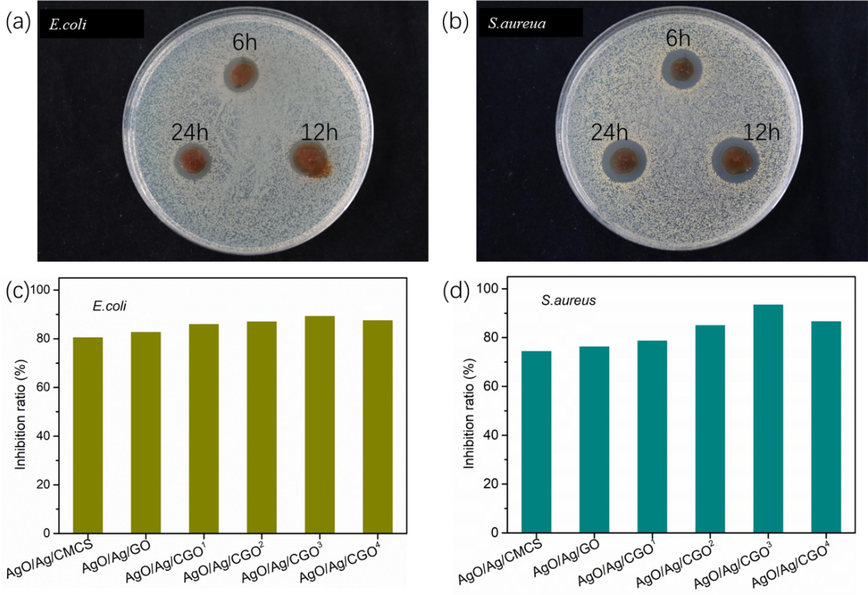
Photograph of the inhibition zone of the AgO/Ag/CGO2, AgO/Ag/CGO3 and AgO/Ag/CGO4 against (a) E.coli and (b) S.aureua and bacteriostatic rate of the films against (c) E.coli and (d) S.aureua.
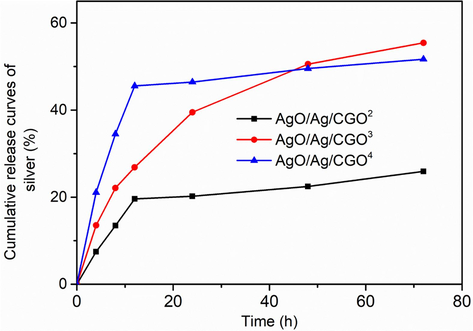
In vitro silver release time profiles of AgO/Ag/CGO2, AgO/Ag/CGO3, AgO/Ag/CGO4.
3.6 In vitro silver-release study
The in vitro release profiles of silver from the AgO/Ag/CGO2, AgO/Ag/CGO3, AgO/Ag/CGO4 were assessed and the results are presented in Fig. 10. As shown in Fig. 10, AgO/Ag/CGO2 exhibited an initial burst release followed by subsequent slower release. The release of silver reaches a maximum of 25.9 % in pH 7.4 PBS. The initial burst release of silver may be attributed to the rapid dissolution of free AgNPs suspended in the phosphate buffer, as well as the discrete AgNPs attached on the surface of CGO. The AgO/Ag/CGO3 showed stable and continuous silver release with no obvious initial burst. This is because that AgNPs were well dispersed in AgO/Ag/CGO3 and they firmly anchored and embedded inside the CGO-polymer network. In addition, the strong interactions between silver and CGO prevented the fast dissolution of AgNPs to silver ions and the diffusion of silver ions into phosphate buffer, allowing AgO/Ag/CGO3 to release silver in a controlled way. In sharp contrast, AgO/Ag/CGO4 showed very fast silver release in the early stage and the release rate slowed. The initial burst release may be due to the rapid dissolution of free AgNPs, which were suspended in the phosphate buffer or attached on the surface of CGO. In addition, gelatin molecules degrade into the small fragments under the long-term UV-irradiation source. When many gelatin molecules degrade into the small fragments at a high irradiation time, some Ag-NPs that cannot be enveloped in the gelatin framework agglomerate into larger particles on the surface of CGO. Thus, the initial burst release was obvious. Therefore, AgO/Ag/CGO3 had much better performance in retaining silver for prolonged periods. Besides, in the case of AgO/Ag/CGO3 is not cytotoxic and has excellent antimicrobial. This may help expand its potential applications in food packaging materials or controlled-release carrier material.
4 Conclusion
In summary, antimicrobial AgO/Ag/CGO films were synthesized successfully by UV irradiation and ionic crosslinking method. Benefiting from the green and simple method, the Ag nanoparticles with a small size (4.72 ± 1.31 nm) was successfully synthesized, which will benefit to the antimicrobial of AgO/Ag/CGO films. Our study found that, the addition of GO improve the thermal stability and swelling ratio of AgO/Ag/CGO films. The results of cytotoxicity of AgO/Ag/CGO3 on HepG2 cells suggests that the nanocomposite is not cytotoxic, and the cell viabilities were still higher than 80 %. Furthermore, we concluded that the AgO/Ag/CGO3 exhibited better antimicrobial activity than AgO/Ag/CGO2 and AgO/Ag/CGO4 from the result of bacteriostatic zone test and the inhibition rate test (E. coli 89.2 % and S. aureus 93.4 %). We also obtained that AgO/Ag/CGO3 exhibited stable and continuous silver release and better performance in retaining silver for prolonged periods owing to the strong coordination and electrostatic interactions between CGO and silver. Consequently, the simple and green method to prepare Ag-based antimicrobial films is promising and meaningful.
CRediT authorship contribution statement
Chen Li: Methodology, Investigation, Validation, Data curation, Writing – original draft. Ke Wang: Conceptualization. Fayong Li: Software. Dong Xie: Conceptualization, Supervision.
Acknowledgements
This research was supported by the GDAS' Project of Science and Technology Development (nos. 2021GDASYL-20210103040); GDAS' Project of Science and Technology Development (nos. 2022GDASZH-2022010110); Guangzhou Science and Technology Project (nos. 202002030172).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- HPMC crosslinked chitosan/hydroxyapatite scaffolds containing Lemongrass oil for potential bone tissue engineering applications. Arab. J. Chem. 2022103850
- [Google Scholar]
- Carboxymethyl chitosan/graphene oxide/silver nanotriangles nanohybrid as the sensing materials for the enhancement of ammonia localized surface plasmon resonance sensor. Opt. Laser Technol.. 2022;148:107789
- [Google Scholar]
- Eggshell derived nano-hydroxyapatite incorporated carboxymethyl chitosan scaffold for dentine regeneration: a laboratory investigation. Int. Endod. J.. 2022;55:89-102.
- [Google Scholar]
- Chitosan/clay nanocomposite films as supports for enzyme immobilization: An innovative green approach for winemaking applications. Food Hydrocolloid.. 2018;74:124-131.
- [Google Scholar]
- Synthesis, characterization and antibacterial study on the chitosan-functionalized Ag nanoparticles. Mater. Sci. Eng. C. 2017;76:73-80.
- [Google Scholar]
- Chitosan–clay composites for wastewater treatment: a state-of-the-art review. ACS EST Water. 2021;1:1055-1085.
- [Google Scholar]
- Preparation and properties of O-chitosan quaternary ammonium salt/polyvinyl alcohol/graphene oxide dual self-healing hydrogel. Carbohyd. Polym. 2022119318
- [Google Scholar]
- Facile synthesis of chitosan/Ag-waterborne polyurethane composite films with a high stability and controllable water resistance for potential application in antibacterial materials. J. Mater. Res. Technol.. 2021;15:5316-5325.
- [Google Scholar]
- Facile synthesis of Ag NPs@ MIL-100 (Fe)/guar gum hybrid hydrogel as a versatile photocatalyst for wastewater remediation: photocatalytic degradation, water/oil separation and bacterial inactivation. Carbohyd. Polym.. 2020;230:115642
- [Google Scholar]
- Chitosan-kaolinite clay composite as durable coating material for slow release NPK fertilizer. Int. J. Biol. Macromol.. 2022;195:424-432.
- [Google Scholar]
- New composite bone cement based on hydroxyapatite and nanosilver. Particul. Sci. Technol.. 2013;31:392-398.
- [Google Scholar]
- Preparation of quaternized chitosan/Ag composite nanogels in inverse miniemulsions for durable and antimicrobial cotton fabrics. Carbohyd. Polym.. 2022;278:118935
- [Google Scholar]
- Chitosan/pullulan based films incorporated with clove essential oil loaded chitosan-ZnO hybrid nanoparticles for active food packaging. Carbohyd. Polym.. 2022;277:118866
- [Google Scholar]
- Designing of silver nanoparticles in gum arabic based semi-IPN hydrogel. Int. J. Biol. Macromol.. 2010;46:237-244.
- [Google Scholar]
- Carboxymethyl chitosan-based hydrogels containing fibroblast growth factors for triggering diabetic wound healing. Carbohyd. Polym. 2022119336
- [Google Scholar]
- UV-assisted deposition of antibacterial Ag–tannic acid nanocomposite coating. ACS Appl. Mater. Interfaces. 2021;17:20708-20717.
- [Google Scholar]
- Fabrication and properties of novel chitosan/ZnO composite bioplastic. Cellul.. 2022;29:233-243.
- [Google Scholar]
- Two-step method for encapsulation of oregano essential oil in chitosan nanoparticles: preparation, characterization and in vitro release study. Carbohyd. Polym.. 2013;95:50-56.
- [Google Scholar]
- In situ green synthesis of antimicrobial carboxymethyl chitosan–nanosilver hybrids with controlled silver release. Int. J. Nanomed.. 2017;12:3181.
- [Google Scholar]
- High internal phase emulsions stabilized solely by carboxymethyl chitosan. Food Hydrocolloid.. 2022;127:107554
- [Google Scholar]
- Preparation and characterization of chitosan/clay composite for direct Rose FRN dye removal from aqueous media: comparison of linear and non-linear regression methods. J. Mater. Res. Technol.. 2019;8:1161-1174.
- [Google Scholar]
- Antimicrobial and in vitro cytotoxic efficacy of biogenic silver nanoparticles (Ag-NPs) fabricated by callus extract of Solanum incanum L. Biomolecules. 2021;11:341.
- [Google Scholar]
- Characterization of chitosan composites with various clays. Int. J. Biol. Macromols.. 2014;65:534-541.
- [Google Scholar]
- Mechanical and morphological studies of chitosan/clay composites. Mol. Cryst. Liq. Cryst.. 2014;590:193-198.
- [Google Scholar]
- Novel graphene oxide/carboxymethyl chitosan aerogels via vacuum-assisted self-assembly for heavy metal adsorption capacity. Colloid. Surf. A. 2019;578:123584
- [Google Scholar]
- Fast and simple synthesis of triangular silver nanoparticles under the assistance of light. Colloid. Surf. A. 2020;594:124659
- [Google Scholar]
- UV-light-assisted preparation of MoO3−x/Ag NPs film and investigation on the SERS performance. J. Mater. Sci.. 2020;55:8868-8880.
- [Google Scholar]
- Structural and optical properties of chemical bath deposited silver oxide thin films: role of deposition time. Adv. Mater. Sci. Eng. 2013450820
- [Google Scholar]
- Comparative studies of chitosan and carboxymethyl chitosan doped with nickel and copper: Characterization and antibacterial potential. Int. J. Biol. Macromol.. 2021;183:1971-1977.
- [Google Scholar]
- In situ generation of silver nanoparticles and nanocomposite films based on electrodeposition of carboxylated chitosan. Carbohyd. Polym.. 2020;242:116391
- [Google Scholar]
- Sodium alginate stabilized silver nanoparticles–silica nanohybrid and their antibacterial characteristics. Int. J. Boil. Macromol.. 2016;93:712-723.
- [Google Scholar]
- Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett.. 2012;2:32.
- [Google Scholar]
- Introducing a bio sorbent for removal of methylene blue dye based on flexible poly (glycerol sebacate)/chitosan/graphene oxide ecofriendly nanocomposites. Chemosphere. 2022;289:133219
- [Google Scholar]
- Loading effect of Ag/AgO on the photocatalytic performance of ZnO rods. Appl. Phys. A. 2017;123:1-12.
- [Google Scholar]
- Carboxymethyl chitosan: Properties and biomedical applications. Int. J. Boil. Macromol.. 2018;120:1406-1419.
- [Google Scholar]
- Preparation and characterization of graphene oxide/O-carboxymethyl chitosan (GO/CMC) composite and its unsymmetrical dimethylhydrazine (UDMH) adsorption performance from wastewater. Environ. Technol. 2021:1-12.
- [Google Scholar]
- The antifungal effect against Penicillium italicum and characterization of fruit coating from chitosan/ZnO nanoparticle/Indonesian sandalwood essential oil composites. Food Packaging Shelf. 2022;32:100849
- [Google Scholar]
- A novel Ag/AgO/carboxymethyl chitosan bacteriostatic hydrogel for drug delivery. Mater. Res. Express. 2020;7:085403
- [Google Scholar]
- High-mechanical strength carboxymethyl chitosan-based hydrogel film for antibacterial wound dressing. Carbohyd. Polym.. 2021;256:117590
- [Google Scholar]
- Construction of chitosan/Ag nanocomposite sponges and their properties. Int. J. Biol. Macromol.. 2021;192:272-277.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105380.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







