Translate this page into:
Green synthesis of biodegradable polyurethane and castor oil-based composite for benign transformation of methylene blue
⁎Corresponding authors. misbahsultan@ymail.com (Misbah Sultan), saalissa@pnu.edu.sa (S.A. Alissa), bosalvee@yahoo.com (Munawar Iqbal)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
A series of polyurethane (PU), polyethylene glycol (PEG) and castor oil (CO) (ricin) based foams (PU-PEG, PU-PEG40/CO60, PU-PEG60/CO40 and PU-CO) were prepared and their catalytic activity for removal of methylene blue (MB) dye was evaluated. The prepared foams were characterized by FTIR, SEM and TGA analysis. The foams were porous in nature and the thermal stability was improved with CO incorporation. The PU-CO furnished promising catalytic efficiency and PU-PEG, PU-CO, PU-PEG40/CO60 and PU-PEG60/CO40 removed MB dye completely within 33, 5, 12 and 20 min. The removal of MB dye over foams followed second order kinetic model. The reusability of PU-CO showed stability up to 10 runs. Moreover, the phytotoxicity of the treated dye solution was performed, which reduced significantly after dye solution treatment with prepared foams. The prepared PU-CO also showed biodegradable nature under soil natural conditions. The prepared PU based foams showed excellent catalytic potential for the removal of MB dye along with thermal stability and recyclability, which could be a potential class of materials for the remediation of dyes in effluents.
Keywords
Polyurethane
Ascorbic acid
Castor oil
Catalytic reduction
Methylene blue
Kinetics
1 Introduction
An emergent need of time in materials preparation is to shift the pressure from fossil reserves to natural and renewable resources. The biodegradable polyurethanes by incorporating bioactive moiety as a soft segment is promising to enhance the polyurethanes efficiency (Rane et al., 2015; Remya et al., 2016). At current, the modern world is facing a serious challenge of environmental pollution. Almost every segment of environment has been polluted by the anthropogenic activities. Water is an exquisite and essential element of the environment. Unfortunately, water resources are desperately affected by the addition of pollutants such as dyes. The textile industry is one of the major consumers of water and is consequently one of the major contributors of dyes in water pollution (Daud et al., 2021; Khalid et al., 2021). In addition, different synthetic dyes are used for analytical purposes, radiochemical investigations, biomedical studies and biological staining applications. These dyes are considered as a severe threat because they affect the physical and chemical properties of water even at a very low level of concentration. These are reported as noxious, teratogenic, carcinogenic and difficult to be degraded (Abbas et al., 2018; Iqbal, 2016; Iqbal et al., 2019). Several smart methods have been developed to address this challenge including biological, physical and chemical approaches. However, every proposed method has its own pros and cons (Abbas et al., 2021; Awwad et al., 2021a; Djehaf et al., 2017; Elsherif et al., 2021; Jalal et al., 2021; Minas et al., 2017; Pandey et al., 2020a; Ukpaka et al., 2020).
The reductive degradation of dyes and other organic pollutants is an attractive and efficient approach (Pandey et al., 2020a, b; Pandey et al., 2021; Pandey and Tiwari, 2015). In fact, this is a transformation of hazardous organic compounds into harmless end products. In a number of previous reports, this transformation has been accelerated by the use of different catalysts. These reported catalysts were most often metallic particles like Ag, Au, Pd, CuO, ZnO and TiO2 etc. and different types of clays (Awwad et al., 2021b; Sanda et al., 2021). However, these catalytic materials cannot be considered as an ideal candidate especially for such water treatment processes. As these materials are difficult to recollect and reuse ultimately. On the other hand, they may pose another serious concern to environment if not managed properly. These secondary issues of these materials can be resolved by providing firm supporting matrix like polymeric networks (Awwad et al., 2020; Iqbal and Khera, 2015).
Polyurethane (PUR) is a versatile class of polymers which not only serve as a support but also showed inherent potential to accelerate above mentioned reduction of pollutants in an aqueous system. The diversity of PUR is associated with a wide variety of monomers available for synthesis (Rane et al., 2015; Remya et al., 2016). However, a continuous burden on precious limited resources has directed researchers towards exploitation of renewable bio-based availabilities. An extensively reported reducing agent for reduction of organic pollutants is NaBH4. Unfortunately, it is not considered as a safe reducing agent due to corrosive and hazardous nature of its intermediates and possible byproducts in water. Moreover, its cost becomes an additional burden on the process cost (Inderyas et al., 2020; Iqbal et al., 2017; Khan et al., 2021). Therefore, current study was aimed to employ some safe and economic reducing agent.
The objective of the study was to demonstrate an economic and benign reduction process utilizing renewable resources. This objective was attained by designing a number of targets which are described here. A series of PU based foams was synthesized with polyethylene glycol and castor oil as soft segment. The effect of ingredients was studied in term of physical, structural, morphological and thermal characteristics. Further, catalytic role of prepared foams was evaluated in the reduction of MB dye. Here, reduction process was customized by employing ascorbic acid as an economic and safe reducing agent. The phytotoxicity evaluation of treated dye solution supported benign nature of reductive transformation of MB dye using prepared PU based foams.
2 Material and methods
2.1 Materials
Toluene 2,4-diisocyanate (TDI; 95% purity; analytical grade), Tetraethylenepentamine (TEPA; technical grade) and Ascorbic acid (C₆H₈O₆: 99% purity) were purchased from Sigma Aldrich, and Acros Organics, USA. Polyethylene glycol (PEG, Mw: 1000 g/mole; synthetic grade; OH value: 107–118) was procured from Applichem, Germany. The Methylene blue (MB) dye was collected from a local vendor. Castor oil (CO; 100% natural) was obtained from Hemani International KEPZ Karachi, Pakistan.
2.2 Synthesis and characterization of polyurethane foams
A series of polyurethane foams were prepared by using two different soft segments, i.e., PEG (1000 g/mole) and CO. An already established method of preparation was adopted (Sultan et al., 2018). The PU-CO and PU-PEG have only CO and PEG as soft segment, respectively. Whereas PU-PEG40/CO60 PU-PEG60/CO40 possessed a binary mixture of PEG and CO in 40:60 and 60:40 (%w/w) proportions, correspondingly. In a typical synthesis procedure, firstly, a carefully weighed amount of polyol (1 mol) was heated up to 80 °C on a hot plate for 10mins. to remove any captured moisture. Then calculated amount of deionized water (7% w/w) and TEPA (1 mol) were mixed well in polyol by mechanical stirrer. At last step, a predetermined volume of TDI (2 mol) was added through a disposable syringe with vigorous stirring until reaction mixture was creamed. It was left on shelf for free rising of foam. The reaction was complete in 0.5–2 min. Then samples were kept in oven for 1hr. to cure completely at 80 °C and dried at room temperature before further characterizations and applications.
The prepared samples were cellular apparently. Their physical and chemical characterizations were performed as; solubility in various solvents, density determination according IS0 845 3rd edition 2016 and recording of FTIR spectra by FTIR-ATR spectrometer (Agilent technologies carry 630 USA). Moreover, the samples were analyzed by scanning electron microscope ((SEM FEI Nova Nano SEM 450, USA)) and Thermogravimetric analyzer ((LECO-TGA 701, USA)) for morphological and thermal stability investigations, respectively. The TGA was recorded with a heating rate of 10 °C/min from 25 to 900 °C under nitrogen atmosphere (40 ml/min).
2.3 Reduction of Methylene blue (MB) dye
All the synthesized samples were investigated for their catalytic role in reduction of MB dye in an aqueous solution. The ascorbic acid was selected as a green reducing agent to replace previously applied hazardous reducing agents like NaBH4. In an individual experiment, aqueous solutions of MB (2.5 × 10−5M) and ascorbic acid (0.05 M) were mixed in an equal volume, i.e., 5 ml each. A carefully weighed (0.5 g) piece of each foam sample was added into the mixed solution of dye and reducing agent. Any change in blue color of MB was monitored by UV/Vis spectrophotometer (T90, PG Instruments Ltd) as an indicator of reaction progress.
Further, two parallel sets of blanks were prepared as; blank I which have only MB and ascorbic acid solutions of same volume and concentration, blank II composed of 5 ml of MB and 0.5 g of PU-CO.
2.4 Reusability
The catalytic stability of best performing sample i.e. PU-CO was assessed by the reusability experiment. A weighed amount of PU-CO (0.5 g) was used for the reduction process according to the procedure described above. The reusability was examined up to 10 runs for reduction of MB. The time required for complete reduction of MB was recorded in each run, separately. The sample of PU-CO used in a typical run was separated after complete reduction of dye, double washed with water and dried in an oven at 50 °C till constant weight. Then, this sample was used again in next run of reduction [18]. This procedure was repeated upto ten successive runs of reduction with same sample of PU-CO and standard solutions of MB and ascorbic acid.
2.5 Phytotoxicity
The role of ascorbic acid as green reducing agent was supported by phytotoxicity evaluation. This particular test was performed for treated and untreated MB solutions with the seeds of Triticum aestivum (wheat). Seedling were raised in two glass petri dishes containing a specific volume of treated water which was a reduced solution of MB and untreated one separately. The germination activity of seeds was observed after 10 days under normal conditions of room temperature 20 ± 3 °C. Their average root length and shoot length were recorded. The number of seeds germinated was noted in both petri plates and germination index (GI) was calculated by following formula:
2.6 Kinetic study
To evaluate the rate constant (k) and order of reaction, kinetic study of most efficient reduction process was carried out at 30 ± 3 °C. The standard models of 1st order and 2nd order kinetics were applied to calculate the regression coefficient (R2) and k accordingly.
2.7 Biodegradation of PU-CO
An initial investigation of biodegradability of PU-CO was performed by simply burying a piece of polymer in soil. After passing 06 months of time period, particular piece was recovered, cleaned and analyzed by FTIR. Any degradation in the structure of polymer was assessed by comparison of FTIR spectra recorded before and after burying it in the soil.
3 Results and discussion
3.1 FTIR analysis of polyurethane foams
The monomers used to synthesize CO and PEG based PU foam were TDI, PEG, TEPA and CO. The FTIR spectra of each monomer and samples were recorded and presented in Fig. 1. These spectra confirmed the synthesis of PU foams as characteristic bands of monomers have disappeared such as –NCO band of TDI, –NH band of TEPA, –OH band of PEG and CO. Moreover, the characteristic bands of polyurethane were observed at 3333–3332 cm−1 and at 1717–1696 cm−1 for —NH and —C⚌O groups of urethane (—NHCOO) linkages in spectra of all synthesized foams [24–26]. The symmetric and anti-symmetric stretching vibrations of —CH generated clear bands between 2900 and 2800 cm−1. Other vibrational modes of the —CH were also observed between 1400 cm−1 and 1300 cm−1. The ether linkage (C—O—C) of PEG based soft segment exhibited multiple bands from 1200 cm−1 to 1000 cm−1. A band around 1510 cm−1 ascribed to the C⚌C of castor oil and aromatic ring of TDI in polymeric chains of PU foams (Li et al., 2016; Rane et al., 2015; Remya et al., 2016).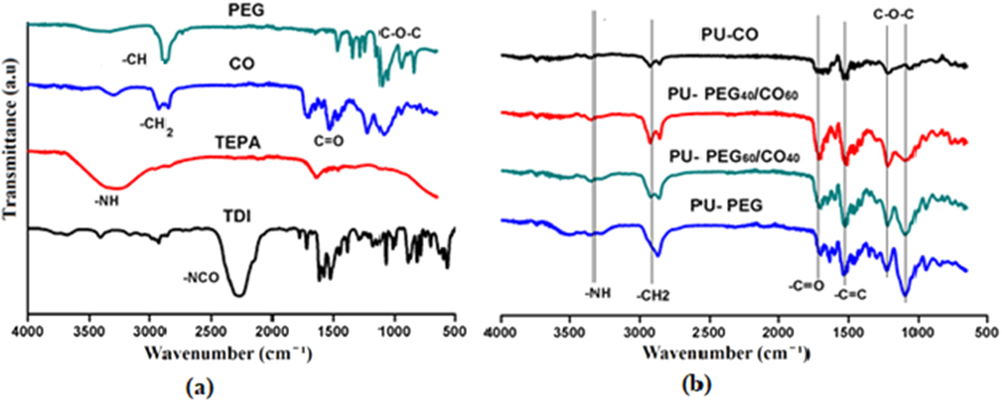
FTIR spectra of (a) monomers; TDI, TEPA, CO and PEG (b) polyurethane foams; PU-PEG, PU-PEG60/CO40, PU-PEG40/CO60 and PU-CO.
3.2 Thermogravimetric analysis (TGA) of polyurethane foams
The thermal degradation of PU-PEG, PU-PEG60/CO40, PU-PEG40/CO60, and PU-CO foams was analyzed under identical conditions from room temperature to 900 °C. A comparison of thermograms displaying the weight loss against temperature and respective DTG curves is given in Fig. 2. The thermal degradation of polyurethanes ensues as a result of various physical and chemical phenomena. This degradation is related to a number of factors like nature of hard and soft segment, their molar ratio and the degree of phase separation etc. (Alaa et al., 2015).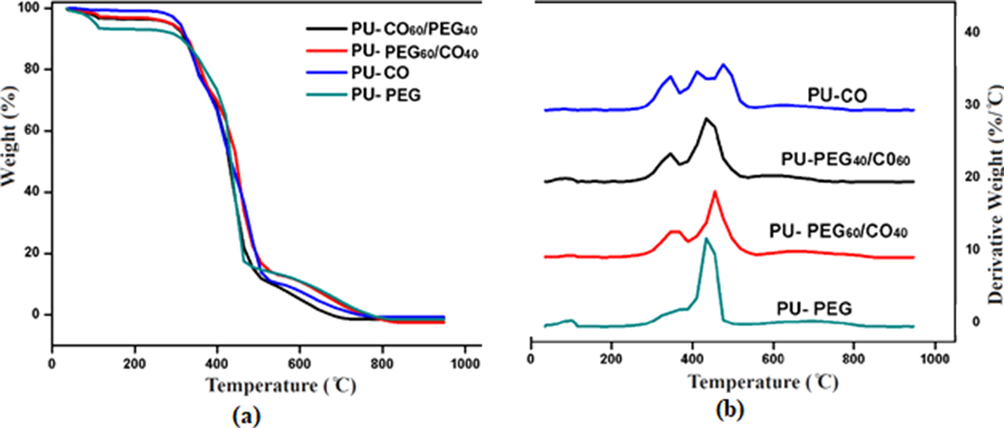
Thermogravimetric analysis (a) TGA thermograms and (b) DTG curves of polyurethane foams; PU-PEG, PU-PEG60/CO40, PU-PEG40/CO60 and PU-CO.
The TGA thermograms of the prepared samples were almost overlapped exhibiting a thermal stability up to 300 °C [30]. The 10% and 70% weight loss of PU-PEG was observed at 310 °C and 450 °C, respectively. While, similar degradation of PU-CO was recorded at 326 °C and 471 °C, respectively. The 50% weight loss of PU-PEG and PU-CO was very close at 430 °C. These thermograms showed an overlapping pattern of degradation. It can be conferred to a similarity shared in hard segment of the synthesized polyurethanes, as hard segment respond earlier in thermal degradation analysis (Qian et al., 2016). The degradation of remaining two samples i.e. PU-CO60/PEG40, PU-CO40/PEG60 containing a combination of soft segments, was perceived between two former samples.
The DTG curves (derivative thermograms) of PU-PEG, PU-PEG40/CO60, PU- PEG60/CO40 and PU-CO are displayed in Fig. 2 (b) these curves showed clear characteristic patterns of degradation for all samples. The PU-PEG exhibited a single step degradation with Tmax around 430 °C which translates a uniformity in bulk structure of the foam. While, PU-CO demonstrated three stage degradation around 350 °C, 415 °C and 480 °C, respectively (Sami et al., 2014). The first degradation corresponds to the initial degradation of hard segments, second degradation within the CO segments and the third, owed to the highly crosslinked areas of polymer originated from crosslinker (TEPA), CO and TDI. The DTG curves of rest of the samples i.e. PU-CO60/PEG40, PU-CO40/PEG60 agreed with their mixed compositions depicting two stages of degradation (Alaa et al., 2015; Li et al., 2016; Liu et al., 2016).
3.3 Morphological analysis by SEM
The specimens were mounted on an aluminum stub and sputter coated with a thin layer of gold to avoid any electrostatic charging during examination by SEM. The micrographs presented in Fig. 3 exposed an open cellular structure of all samples. It can be observed that the porous morphology was varying with the variation in composition of soft segment of polymers. It might be due to increasing —OH groups of CO. In general, this porous and rough morphology supported for binding and ultimate interaction of MB dye and ascorbic acid molecules (Khan et al., 2021).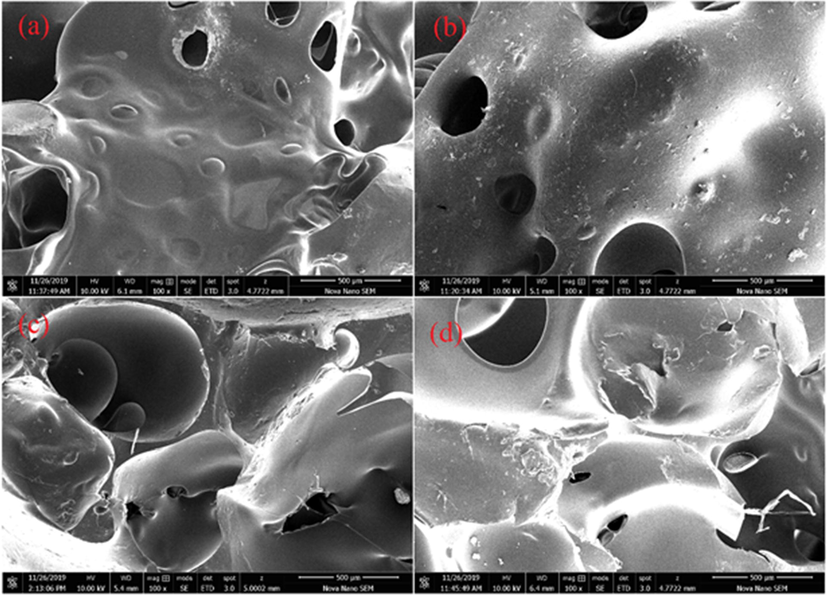
SEM images of polyurethane foams (a) PU-PEG, (b) PU-PEG60/CO40, (c) PU-PEG40/CO60 and (d) PU-CO.
3.4 Reduction of Methylene blue
The reduction of MB was studied to evaluate the catalytic role of foams and results are displayed in Fig. 4. According to these results, alone ascorbic acid was able to reduce only 90% dye in 110 min (Blank I). Conversely, complete reduction of dye was attained within 33 min. in the presence of PU-PEG. Whereas, PU-CO foam showed equivalent performance in just 05 min. The other two samples, PU-PEG40/CO60 and PU-PEG60/CO40 showed similar performance within 12 min. and 20mins, respectively. These results clearly revealed the catalytic role of synthesized foams in reduction process. Furthermore, this role was strengthened with increasing content of CO. Particularly, PU-CO showed excellent performance due to a unique structure of soft segment. The CO offered greater number of hydroxyl groups which generated a highly crosslinked structure of polyurethane. This highly crosslinked porous structure of PU-CO made available a large surface area for MB and ascorbic acid molecules to interact mutually leading to the redox reaction. Moreover, the urethane functional groups containing conjugated sites placed next to the aromatic ring might also facilitated the electrons transfer from ascorbic acid (nucleophile) to MB (electrophile).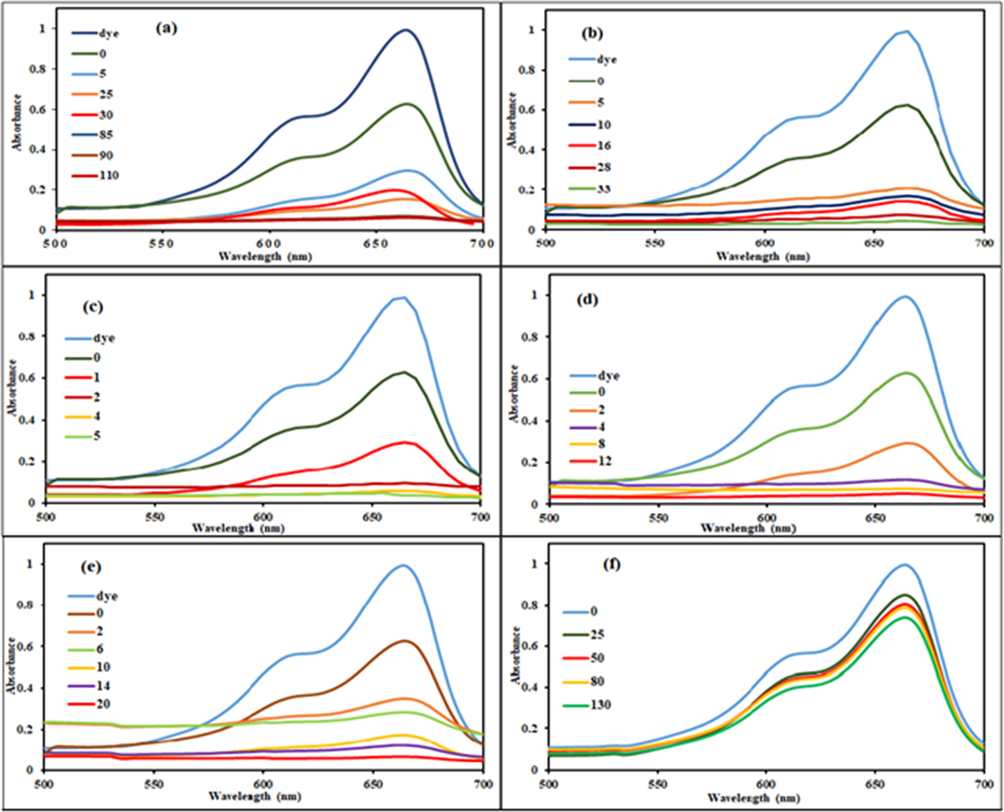
Reduction of MB (2.5 × 10−5 M) by (a) only 5 ml (0.5 M) of ascorbic acid (b) 5 ml (0.05 M) of ascorbic acid and 0.5 g PU-PEG (c) 5 ml (0.05 M) of ascorbic acid and 0.5 g of PU-CO (d) 5 ml (0.05 M) of ascorbic acid and 0.5 g of PU- PEG40/CO60 (e) 5 ml (0.05 M) of ascorbic acid and 0.5 g of PU- PEG60/CO40 (f) only 0.5 g PU-CO.
Formerly, PU foam has been praised as an excellent, economic and efficient adsorbent for removal of various dyes [38]. However, the catalytic role of PU foam in reduction of dye was observed in current study. To evaluate the adsorption process, another experiment was conducted with Blank II. It was only composed of MB dye solution and PU-CO. The results shown in Fig. 4 (f) indicated a negligible adsorption of dye molecules. The PU-CO removed merely 8 % MB in 120 min. This slight adsorption of dye molecules on the surface of polymer might be aiding in the reduction process.
3.5 Reusability
The assessment of reusability of best performing sample i.e. PU-CO was carried out up to 10 runs. A weighed amount of PU-CO was used for the reduction process according to the procedure described above. The mandatory time for complete reduction process was observed with UV–Vis spectrophotometer. At the end of the first run, used PU-CO was separated, double washed, and dried in an oven for 30 min at 50 °C till constant weight for the next run. The results displayed in Fig. 5 showed almost a constant catalytic efficiency of PU-CO up to 10 runs of reduction. The time required for complete reduction of MB was almost same for all successive runs. A slight variation in 8th and 9th run may be considered as an experimental or personal handling deviation. Hence, no poisoning or deactivation of catalyst was observed during investigated runs. These observations strongly supported the stability of this PU-CO foam as catalyst (Inderyas et al., 2020).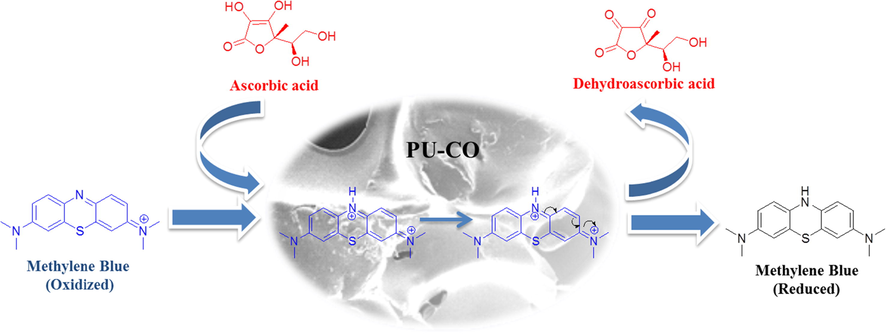
Proposed mechanism for reduction of MB with ascorbic acid in presence of PU-CO.
3.6 Phytotoxicity
The Phytotoxicity evaluation helped to confirm the effectiveness and safety of this treatment of dye. It was performed by growing the seeds of Triticum aestivum in treated and untreated MB solutions as shown in Fig. 6. The activity of seeds was observed according to the method previously reported by Bibi et al. (Bibi et al., 2017). The seedlings were raised in glass petri dishes containing a specific volume of treated solution of MB dye and an original solution of MB dye, independently. This analysis was carried out for 10 days under normal conditions of room temperature. The germination index of treated water was 88% while 0% was observed for untreated MB solution. An average root length of 14 cm and shoot length of 9 cm were recorded for seedlings grown in treated water. This Phytotoxicity evaluation revealed an effective and safe transformation of MB in the presence of ascorbic acid and PU-CO (Nouren et al., 2017).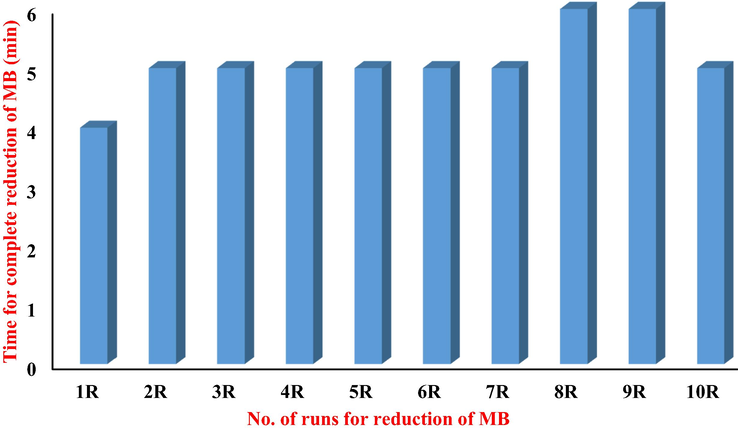
Reusability of PU-CO for reduction of MB dye. Standard conditions for each reduction run: MB dye (5 ml of 2.5 × 10−5 M), 5 ml (0.05 M) of ascorbic acid and 0.5 g of PU-CO.
3.7 Density and solubility of polymers
The solubility and density of PU-CO, PU-PEG40/CO60, PU-PEG60/CO40 and PU-PEG is mentioned in Table 1. An average density of samples was recorded according to a standard method. It was found that PU-CO was of lowest density i.e. 0.29gcm−3. This lowest density might be observed due to an availability of additional –OH groups on CO which contributed in construction of more crosslink networking in polymer. However, the lowest density of PU-CO indicated an offer of enhanced surface area for further processes such as reduction of MB (Inderyas et al., 2020). Swelled polymer: ± Insoluble: -
Sample code
DMF
DMSO
Water
Xylene
Toluene
Density (g/cm3)
PU-CO
±
±
±
-
-
0.29
PU-PEG
±
±
±
-
-
0.36
PEG60/CO40
±
±
±
-
-
0.44
PEG40/CO60
±
±
±
-
-
0.45
A solubility test of all synthesized polymers was carried out in different solvents. According to the results documented in Table 1. Samples were almost completely insoluble in organic non-polar solvents like toluene and xylene. However, they exhibited swelling in water and other polar solvents. This behavior of sample revealed their stable crosslinked structure and resistance toward solvents.
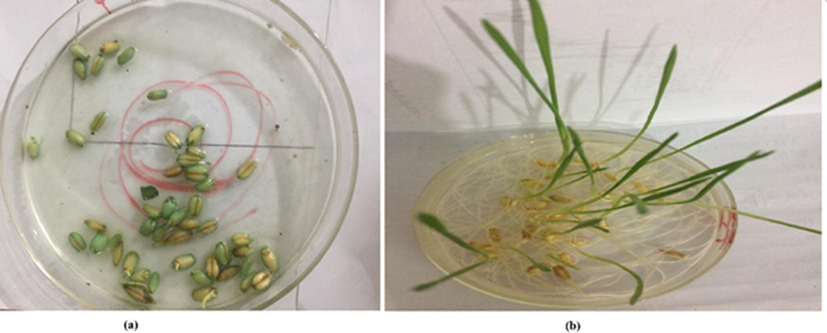
3.8 Kinetic study
To determine the rate constant and order of reaction, kinetic analysis of reduction process was carried out. First order, and second order kinetics models were applied on PU-CO catalyzed reduction reaction and results are presented in Table 2. The highest value of regression coefficient (R2) and apparent rate constant (k) were obtained with 2nd order kinetic model as shown in Fig. 7. Hence, these results concluded that PU-CO catalyzed reduction process of MB with ascorbic acid followed 2nd order kinetics.
Sr. no.
Name of kinetic model
Mathematical form
Regression coefficient (R2)
Rate Constant (k)
PU-CO
PU-CO
1.
Zero order
Co-Ct=kt
0.7369
0.1033 mol dm-3 min-1
2.
1st order
ln(Ct/Co)=kt
0.9322
0.5591 min-1
3.
2nd order
1/Ct=kt+1/Ct
0.9789
5.2731mol-1 dm3 min-1
Germination of seeds of Triticum aestivum (a) in pure MB untreated solution, (b) in reduced MB with ascorbic acid in presence of PU-CO.
3.9 Biodegradation of PU-CO
One of the objectives to design this whole study was to exploit the bio based soft segment in the polyurethane. Above results supported the advantages of this incorporation and PU-CO was found best in performance among all others. Additionally, natural degradation of PU-CO was evaluated by burying a piece in soil. The FTIR spectra of polymer before and after burying is given in Fig. 8. It clearly depicted a strong structural change during a period of six months. The important regions of spectra including —NH, —CH, —C⚌O and C—O—C bands (encircled in Fig. 9) showed a pronounced change in shape and intensity at their respective positions. This evidence is enough to demonstrate the biodegradable nature of PU-CO. It could be accredited to the bio based soft segment, i.e., CO which possibly underwent an attack of soil microbes. Hence, these observations reflected an initial milestone to develop biodegradable polyurethanes. In view promising catalytic efficiency and biodegradable nature of PU-CO material could be a valuable material for the remediation of dyes in textile wastewater and there is also need of eco-benign and efficient materials for the treatment of effluents responsible for environmental pollution (Abulude et al., 2018; Adetutu et al., 2020; Chokor, 2021; Deeba et al., 2019; Palutoglu et al., 2018; Sasmaz et al., 2018; Sasmaz et al., 2019; Yildirim and Sasmaz, 2017).
Linear plot of 1/Ct versus time (min) for PU-CO catalyzed reduction process of MB.
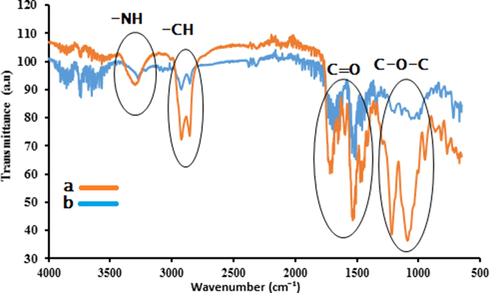
FTIR spectra of PU-CO (a) before burying in soil (b) after 06 months of soil burial.
4 Conclusion
In this study. a series of PUR foams was prepared with pure and mixed PEG and CO based soft segments. They were employed for transformation of MB dye in presence of ascorbic acid. The FTIR spectra of foams confirmed their designed synthesis. A porous morphology of foams was recorded by SEM imaging. The thermal stability of PUR was improved with incorporation of CO. The lowest density of PU-CO was accredited to an enhanced availability of —OH groups in the soft segment. Furthermore, the PU-CO offered best catalytic role in the reduction of MB. This reduction process followed 2nd order kinetic model. An investigation of the reusability of PU-CO established a stable and active role of foam up to 10 runs of reduction process. Moreover, the safe and benign transformation of MB was confirmed by phytotoxicity evaluation of the treated and untreated water. A simple soil burial method demonstrated significant biodegradation of PU-CO when traced by FTIR. Hence, it can be concluded that these findings supported a green synthesis of PUR foams and their catalytic potential for economic and benign transformation of MB and other related pollutants along with an economic and safe replacement of NaBH4.
Acknowledgment
This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.







