Translate this page into:
Heat absorption properties of CuO/TiO2/SiO2 trihybrid nanofluids and its potential future direction towards solar thermal applications
⁎Corresponding author at: Institute for Artificial Intelligence and Big Data, Universiti Malaysia Kelantan, City Campus, Pengkalan Chepa, 16100 Kota Bharu, Kelantan, Malaysia. hasyiya@umk.edu.my (Hasyiya Karimah Adli)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
In this study, we investigated the physical properties of CuO/TiO2/SiO2 trihybrid nanofluids. The physical properties that were investigated included density, crystallite size, and surface morphology. The trihybrid nanofluid density was observed to increase at higher volume concentration, with t1 exhibiting the highest density (2.26 gml−1). X-Ray Diffraction (XRD) spectra showed the main diffraction peaks of individual nanoparticles (CuO, TiO2 and SiO2), highlighting the successful formation of trihybrid nanoparticles. The nanofluid's calculated crystallite size showed the formation of smaller trihybrid nanofluid crystallites (5.2 nm) compared to the original nanoparticles. The crystallite size is in good agreement with the SEM surface morphology, which shows the appearance of small particles. The trihybrid solution (t1) had the best thermal properties, based on temperature output, at around 55 °C, as the highest volume concentration of nanofluids was used. The heat absorption of t1 also demonstrated increased temperature output at higher solar radiations with a maximum temperature output at 73 °C under 700 W/m2. This study is the first to report on the thermal properties CuO/TiO2/SiO2 trihybrid nanofluids for future solar thermal application.
Keywords
Trihybrid
Nanofluids
Heat absorption
Temperature output
1 Introduction
Cumulatively, fossil fuels accounted for 84% of the world’s primary energy source in 2019. Fossil fuels also dominated about 40% of global electricity generation in the same year (BP Statistical Review of World Energy, 2020). Global electrical energy consumption is typically high, and the demand is growing rapidly at an alarming rate. In an effort to reduce the dependency on fossil fuels, many countries have turned to renewable energy such as solar energy (Grätzel, 2009). Generating electricity with solar power instead of fossil fuels can dramatically reduce greenhouse gas emissions, particularly carbon dioxide (CO2). Greenhouse gases emitted by fossil fuels have led to increased global temperature and climate change, subsequently contributing to serious environmental and public health issues (Haines et al., 2006).
Solar water heating is currently the most common application of solar energy systems, especially in urban areas. This system is environmentally friendly and harnesses the generated electricity to heat water (Shukla et al., 2013). Basically, this system involves natural solar thermal technology based on simple working principles that require only sunlight to heat the water. The system works by bringing the thermal fluid into contact with a dark surface exposed to sunlight, which then increases the temperature of the fluid (Al-Badi and Albadi, 2012). In a direct system, the fluid may cause the water to heat up directly, or in an indirect system, a heat transfer fluid (e.g., glycol or a water mixture) could be used to pass through some form of heat exchanger (Jamar et al., 2016).
Meanwhile, nanofluids are world-class heat transfer working fluids that have been developed by adding solid nanoparticles to the base fluids (Alawi et al., 2018; Sarafraz et al., 2019). Homogenized mixtures of low-volume-fraction nanoparticles and conventional fluids such as ethylene glycol, glycerine, oil, and water can remarkably increase overall thermal performance (Das et al., 2007; Sarafraz et al., 2016). Due to its potential use in numerous engineering applications, nanofluids are now the focus of an expanding body of research. Additionally, nanofluids are most commonly used in heating and cooling processes prevalently seen in solar cells, solar stills, and other thermal energy storage methods (Khanafer and Vafai, 2018; Sarafraz et al., 2018) and thermal conductivity (Ghalambaz et al., 2020a, 2020b).
Oxide nanofluids such as aluminum oxide (Al2O3), titanium oxide (TiO2), silicon dioxide (SiO2), and copper oxide (CuO) are mostly used in heat transfer applications either as single or hybrid nanofluids, due to their capability to increase heat transfer in the heat transfer system by up to 60% (Azmi et al., 2019). The investigation into various hybrid nanofluid applications have greatly increased (Fikri et al., 2020b). Hybrid or composite nanofluids are an extension of the body of research on single nanofluids. These hybrids can be made via two or more different nanoparticles: either in the form of a mixture or a composite that disperses in liquid (Minea, 2017). Previous works have reported on the superiority of the thermal and rheological properties of hybrid and composite nanofluids over single nanoparticles. Table 1 presents the summary of respective nanofluids for thermal properties characterization.
Particle
Type
Base fluid
Enhancement
References
Al2O3
Single
Ethylene glycol
The thermal conductivity decreased when the EG content in the mixture increased, but increased when the volume concentration of the Al2O3 nanofluids increased
Hamid et al. (2018)
Single
Water and ethylene glycol
The average thermal conductivity enhancement from 2.6 to 12.8% as the percentage of ethylene glycol increases
Chiam et al. (2017)
Single
Ethylene glycol and water
At fixed EG content, the thermal conductivity of nanofluids increased with increasing of temperature
Guo et al. (2018)
SiO2
Single
Ethylene glycol
Viscosity linearly increased with the increased of nanoparticles concentration with maximum enhancement with 1.3905 times
Żyła and Fal (2017)
TiO2
Single
Water, ethylene glycol, paraffin oil
The thermal conductivity increases 22% with the addition of nanoparticles
Sonawane et al. (2015)
Single
Ethylene glycol
Thermal conductivity and viscosity of nanofluids increased with the addition of nanoparticles to base fluids
Khedkar et al. (2016)
CuO
Single
Water, ethylene glycol, engine oil
Thermal conductivity increases 40%, 27% and 19%, for CuO in water, EG and engine oil, respectively
Agarwal et al. (2016)
Single
Water
18% increase in thermal conductivity
Nemale and Waghuley (2016)
Al2O3, TiO2, SiO2 and CuO
Single
Ethylene glycol, water
The thermal conductivity of oxide nanofluids was enhanced up to 40% better than the base fluids (water)
Azmi et al. (2019)
Al2O3 and SiO2
Hybrid
Water
An increase of thermal conductivity with volume fraction and temperature increase
Moldoveanu et al. (2018)
ZnO and TiO2
Hybrid
Ethylene glycol (0.1–3.5 vol%)
Maximum enhancement (32%) at ϕ = 3.5% and 50 °C
Toghraie et al. (2016)
SiO2 and TiO2
Hybrid
Water and ethylene glycol (60:40)
Maximum enhancement of 22.8% obtained at 3.0% volume concentration and 80 °C temperature.
Nabil et al. (2018)
Hybrid
Water and ethylene glycol (60:40)
The nanofluid viscosity increased with increased volume concentration and decreased with increased temperature
Hamid et al. (2019) and Guo et al. (2018)
Hybrid
Ethylene glycol and water (40:60)
The maximum enhancement 22.1% at concentration 3.0% and temperature 70 °C
Hamid et al. (2017)
TiO2 and Al2O3
Hybrid
Water
The highest thermal conductivity value of 1.134 W/m K is observed for hybrid nanofluid with mixing ratio of 50:50 at 70 °C, with an average thermal conductivity augmentation of 71% comparative to deionized (DI) water
Wanatasanapan et al. (2020)
Hybrid
Ethylene glycol and water (40:60)
The thermal conductivity is improved by 40.86% at 0.1% volume concentration and 80 °C
Urmi et al. (2020)
Al2O3, TiO2 and SiO2
Trihybrid
Water and Ethylene glycol (60:40)
Reported trihybrid nanofluids remains stable with a concentration ratio of 80%.
Ramadhan et al. (2019)
Trihybrid
Water and Ethylene glycol (60:40)
The maximum enhancement of the heat transfer coefficient for coolant side is observed at 39.7% at 0.3% volume concentration
Ramadhan et al. (2020)
SiO2, Al2O3 and ZrO2
Trihybrid
Deionised water and Ethylene glycol (60:40)
Nanofluid viscosity decreases with increasing liquid temperature and increases with increasing of nanoparticles volume concentration
Safiei et al. (2020)
Thus, it can be summarized that oxides have great promise in solar thermal applications and the hybrid nanofluids have superior thermophysical properties over single nanofluids. Based on the above findings, this study aims to develop a novel, trihybrid nanofluids made up of CuO, TiO2, and SiO2 and to investigate the physical properties of these materials such as density, crystallite size, and surface morphology, as well as temperature output performance. This is the first study to report on the CuO/TiO2/SiO2 trihybrid nanofluid. Thus, this study is the first to report on the thermal properties CuO/TiO2/SiO2 trihybrid nanofluids for future solar thermal application.
2 Experimental method
2.1 Materials preparation
Copper (II) oxide powder (Bendosen) (99.9%, ≤ 160 µm), titanium dioxide (TiO2) powder (R&M chemicals) (≥99.5%, 1 to 150 nm) and silicon dioxide (SiO2) powder (HmbG Chemical) (99.9%, 0–2000 μm) were used without further purification. Initially, each nanofluid from the copper oxide (CuO), titanium dioxide (TiO2), and silicon dioxide (SiO2) nanoparticles was prepared in 2.5 M that were dissolved in ethylene glycol. Then, the mixture was stirred continuously for 3 days and sonicated for 2 h until a homogenized solution was obtained. Each nanofluid was mixed at a 1:3 vol ratio to finally form 100 mL of the (CuO/TiO2/SiO2) trihybrid nanofluids. Different concentrations of the trihybrid nanofluids were prepared in water and ethylene glycol mixture (60:40) which are 0.17, 0.08 and 0.04 M, denoted as t1, t2, and t3 respectively. The overall process is summarized in Fig. A1.
2.2 Physical characterization
The investigation into the physical properties of the prepared (CuO/TiO2/SiO2) trihybrid nanofluids (t1, t2, and t3) was observed via visualization effect (Chakraborty and Panigrahi, 2020). Sedimentation is the most commonly used technique for stability evaluation which based on the formation of sediment at the bottom of the liquid column due to gravity. The longer time taken by the nanofluid for the formation of precipitate is an indication of superior stability of nanofluid. Several researchers have used the sedimentation technique to evaluate nanofluid stability (Chakraborty et al., 2018a, 2018b). The density of the base fluids and the prepared trihybrid nanofluids was checked using a density meter (Radwag Balances and Scales model), and several physical characterizations were conducted via Ultraviolet–visible Spectrophotometer (UV–vis), X-Ray Diffraction (XRD), and Scanning Electron Microscopy (SEM). MeaUV–Vis analysis was carried out using a Spectroquant Pharo 300 UV–vis Spectrometer. This analysis was run 10 days in a row with a wavelength set at 400 nm to determine the absorption and the stability of the trihybrid nanofluid. The crystallite size and the crystalline structure of CuO, TiO2, and SiO2, as well as the t1, t2, and t3 trihybrid were determined via X-ray diffraction (XRD D2 Phaser, Bruker). The surface morphology was observed using a JEOL JSM IT 100 Scanning Electron Microscope (SEM) at 300× magnification.
2.3 Heat absorption performance
t1, t2 and t3 trihybrid nanofluids were exposed to 300 W/m2 artificial solar radiation to investigate the heat absorption performance of each nanofluid, after which t1 was chosen for further analysis on different solar radiation intensities (300 W/m2, 500 W/m2, and 700 W/m2). A dimmer switch was installed in the circuit to regulate the solar radiation. The experiment started with a charging process of 16 min and then a discharging process for another 16 min using a thermocouple. The temperature data was recorded every 2 min in a controlled environment. The temperature was displayed by thermocouple and the information was manually recorded. The experimental setup is shown in Fig. A2, with detailed descriptions given in Table A1.
3 Results and discussion
3.1 Physical properties
The formulation of the trihybrid nanofluids is based on the two-step method outlined by Ramadhan et al. (2019), with the difference of metal oxides and solution concentrations used. The copper oxide (CuO), titanium dioxide (TiO2), and silicon dioxide (SiO2) solutions in ethylene glycol were prepared individually before mixing to finally form the (CuO/TiO2/SiO2) trihybrid solution. Fig. A1 shows the image of oxide solution and trihybrids solutions.
Ethylene glycol also acts as an antifreeze when mixed with water to form a solution with a relatively good heat transfer (Peyghambarzadeh et al., 2011). Ethylene glycol (EG) has been reported to minimize the damage and increase the shelf-life of water heating systems (Sundar et al., 2014). According to the literature, mixed base fluids have better heat transfer properties with high thermal conductivity and low viscosity. The use of base-water has limited operated temperature range, high vapour pressure and high corrosivity. Besides, ethylene glycol has higher performance in the convective heat transfer compared to water. Thus, a water-ethylene glycol mixture at a 60:40 ratio was used as the base fluid for this study.
The density of the base fluids used in this study was initially checked to compare the density of distilled water with the distilled water-ethylene glycol mixture, as shown in Table A2. Next, the density of the prepared t1, t2, and t3 trihybrid was measured. It was found that the density increased with increasing nanoparticle concentration. According to the rule of mixtures, the density of good heat transfer fluids increases linearly with volume fraction since the addition of a small fraction of solid nanoparticles to the base liquid will enhance the density of the mixture (Chandrasekar et al., 2012; Tahat and Benim, 2017).
Fig. A3 shows the images of t1, t2 and t3 trihybrid nanofluids with concentrations of 0.17, 0.08 and 0.04 M, respectively, at after preparation (day 1) and at day 10. At room temperature, no sedimentation of particles was observed after the solution was prepared but sedimentation started to form on day 10. The sedimentation was occurred due to the effect of gravity on the particles (Sahid et al., 2017). The suspension of these solid particles in the base fluids reported can enhance the energy transmission in the fluids resulting an increase thermal conductivity properties and heat transfer performance (Ganji et al., 2018; Okonkwo et al., 2019).
3.2 Physical characterization
Fig. A4 displays the X-Ray Diffraction (XRD) patterns of CuO, TiO2, SiO2, and the (CuO/TiO2/SiO2) trihybrid collected within a Bragg’s angle (2θ) between 10° and 90°. The diffraction pattern in Fig. A4(a) is identical to the single-phase monoclinic CuO observed at 2θ ≈ 32° and 35°, indicating the good crystallinity of the copper oxide nanoparticles. All diffraction peaks of CuO at (32, 35, 38, 48, 53, 58, 61 and 67) ° are in good agreement with the JCPDS card NO. 48-1548 (Siddiqui et al., 2018). Next, the peak of the SiO2 nanoparticles was observed at 2θ ≈ 24° reported in amorphous phase (Yuvakkumar et al., 2014; Nayak et al., 2019), while the sharp peak of TiO2 nanoparticles at 2θ ≈ 25°, 38°, and 48° confirm the presence of the anatase phase in concordance with the JCPDS card no.: 21-1272 and 211,276 database (Li et al., 2014; Almashhori et al., 2020; Antić et al., 2012). Meanwhile, the XRD spectrum of the (CuO/TiO2/SiO2) trihybrid (Fig. A4d) shows the appearance of the main diffraction peaks from the individual nanoparticles (CuO, TiO2 and SiO2), highlighting the formation of the trihybrid nanoparticles. The diffraction peaks were observed at 2θ ≈ 25°, 35°, 38°, 48°, and 54°.
The crystallite size of the nanoparticles was identified from XRD patterns according to the peak position (Zak et al., 2012). The crystallite size of the nanofluids was evaluated by measuring the FWHM of the strongest peak (Mohammadi et al., 2017). The average crystallite size was computed using the Debye–Scherrer formula below (Deraz and Abd-Elkader, 2014): where D is the crystallite size (nm), k is a constant equal to 0.9, λ is the wavelength of X-ray radiation (0.15406 nm), β is the full-width at half maximum (FWHM) of the peak (in radians), and θ is peak position (in radians).
Based on the FWHM values, the calculated average crystallite size of CuO, SiO2, TiO2, and the trihybrid was 26.4 nm, 0.6 nm, 14.4 nm, and 5.2 nm, respectively. From the results obtained (Table A3), it can be concluded that the trihybrid particles had a smaller crystallite size (5.2 nm) compared to their original nanoparticles.
From Fig. A5(a), the surface morphology of the CuO nanoparticles is spherical and nearly uniform in size, with a similar shape to that reported previously (Ranjbar-Karimi et al., 2010). This spherical nanoparticle form is favorable for the heat transfer of absorbed solar energy within the nanofluid and can increase solar absorption capacity (Subramaniyan et al., 2018). Meanwhile, Fig. A5 (b) and (c) show the images of the SiO2 and TiO2 nanoparticles that are also similar in shape to that reported by Ramadhan et al. (2019). The surface morphology of CuO/TiO2/SiO2 in Fig. A5(d) appear similar in shape to that of the (CuO, TiO2 and SiO2) original nanoparticles, proving the formation of the trihybrid nanoparticles although the size of CuO changed after the trihybrid formation. The spherical, nearly uniform size, and rock-like shape, as well as the smaller crystallite particles, prove that the nanoparticles are loose agglomerates (Akilu et al., 2017).
The absorption spectra of the trihybrid nanofluid in different volume concentrations were determined using a UV–Vis spectrophotometer at 400 nm for up to 10 days at room temperature to optimize the stability of the nanohybrid solutions. The absorbance of the nanofluids was found to linearly increase with an increase in volume concentration. This trend is in agreement with the Beer-Lambert Law (Sharif et al., 2019). The UV–vis absorption of the 0.05% (t3), 0.1% (t2), and 0.2% (t1) concentrations are shown in Fig. A6. From the UV–vis spectrum, t1 clearly recorded the highest UV–vis absorption, as t1 has the highest concentration of the sample series. The mixing solution was confirmed due to the comparison of spectra made based on the literature (Saidina et al., 2020; Kumar et al., 2020; Adam et al., 2020). Furthermore, the absorbance of the trihybrid nanofluids in different concentrations was monitored after 24 h up until day 10 (240 h). It was found that the absorbance of the CuO/TiO2/SiO2 trihybrid (t1, t2, and t3) decreased over hours (Fig. A7). On day 5, t2 was the most stable trihybrid with only a 10.5% decrease, compared to t3 (60%) and t1 (27.6%). However, nearing 240 h, t1 showed the highest absorption of the series, with only a 62.1% drop in the initial absorbance reading.
3.3 Heat absorption performance analysis
Fig. A8 shows the comparison of solar absorption and the cooling curve of the base fluids, the distilled water (dw), and the mixture of distilled water and ethylene glycol (w:eg), carried out under 15 min charging and 15 min discharging. The results clearly show w:eg having better base fluid properties so it will store heat better than dw.
In a similar method, the heat absorption performance of the (t1, t2, and t3) trihybrid nanofluids in water-ethylene glycol as the base fluid was investigated under 300 W/m2 solar radiation. From the analysis, the temperature gradually increased with time during the charging process and started to decrease during the discharge process (Fig. A9a). Hence, t1 recorded the highest performance due to its highest concentration of nanofluids and thus the best thermal absorber of the series. Under different solar radiation (Fig. A9b), t1 exhibited increasing heat absorption at higher solar radiation (500 W/m2) and 700 W/m2) with a maximum temperature achieved at 73 °C. In summary, the obtained results exhibited improved performance in thermophysical properties, compared to the TiO2-SiO2 hybrid nanofluids, which recorded a maximum temperature of 37 °C under 300 W/m2 solar radiation (Fikri et al. 2020a, 2020b).
4 Conclusion
A trihybrid nanofluid was successfully prepared from CuO, TiO2, and SiO2 and analyzed using XRD, SEM, UV–vis, and thermocouple. t1 recorded the highest density of 2.26 gml−1. The XRD spectra showed the existence of the main diffraction peaks of the individual nanoparticles (CuO, TiO2, and SiO2), proving the formation of the CuO/TiO2/SiO2 trihybrid. The calculated crystallite size and SEM images demonstrated the smaller size of the trihybrid nanoparticles (5.2 nm) compared to the original size of their oxides. The as-prepared trihybrid nanofluid solution (t1) performed stably for 240 h, with only a 62.1% drop in the initial absorbance reading. From the heat absorption performance, t1 recorded the best performance, achieving maximum temperature at 73 °C under 700 W/m2.
Acknowledgement
Author would like to thank Faculty of Bioengineering and Technology, UMK for providing instrumentation facility.
Funding
This study was funded by UMK PRO (Grant No. R/PRO/A1300/01501A/002/2020/00759).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Effect of temperature on the stability and optical properties of SiO2-water nanofluids for hybrid photovoltaic/thermal applications. Appl. Therm. Eng.. 2020;175:115394-115405.
- [CrossRef] [Google Scholar]
- Synthesis, characterization, thermal conductivity and sensitivity of CuO nanofluids. Appl. Therm. Eng.. 2016;102:1024-1036.
- [CrossRef] [Google Scholar]
- Experimental measurements of thermal conductivity and viscosity of ethylene glycol-based hybrid nanofluid with TiO2-CuO/C inclusions. J. Mol. Liq.. 2017;246:396-405.
- [CrossRef] [Google Scholar]
- Thermal conductivity and viscosity models of metallic oxides nanofluids. Int. J. Heat Mass Transf.. 2018;116:1314-1325.
- [CrossRef] [Google Scholar]
- Domestic solar water heating system in Oman: Current status and future prospects. Renew. Sust. Energ. Rev.. 2012;16:5727-5731.
- [CrossRef] [Google Scholar]
- Antibacterial and photocatalytic activities of controllable (anatase/rutile) mixed phase TiO2 nanophotocatalysts synthesized via a microwave-assisted sol–gel method. New J. Chem.. 2020;44(2):562-570.
- [CrossRef] [Google Scholar]
- Multisite luminescence of rare earth doped TiO2 anatase nanoparticles. Mater. Chem. Phys.. 2012;135(2–3):1064-1069.
- [CrossRef] [Google Scholar]
- A review on thermo-physical properties and heat transfer applications of single and hybrid metal oxide nanofluids. J. Mech. Eng. Sci.. 2019;13(2):5182-5211.
- [Google Scholar]
- BP Statistical Review of World Energy, 2020. https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2020-full-report.pdf (accessed 15 October 2020).
- Stability of nanofluid: A review. Appl. Therm. Eng.. 2020;174(115259):1-26.
- [CrossRef] [Google Scholar]
- Thermo-physical properties of Cu-Zn-Al LDH nanofluid and its application in spray cooling. Appl. Therm. Engi.. 2018;141:339-351.
- [CrossRef] [Google Scholar]
- Synthesis of Cu-Al LDH nanofluid and its application in spray cooling heat transfer of a hot steel plate. Powder Technol.. 2018;335:285-300.
- [CrossRef] [Google Scholar]
- Mechanisms proposed through experimental investigations on thermophysical properties and forced convective heat transfer characteristics of various nanofluids–A review. Renew. Sust. Energ. Rev.. 2012;16:3917-3938.
- [CrossRef] [Google Scholar]
- Thermal conductivity and viscosity of Al2O3 nanofluids for different based ratio of water and ethylene glycol mixture. Exp. Therm. Fluid Sci.. 2017;81:420-429.
- [CrossRef] [Google Scholar]
- Nanofluids: Science and Technology. John Wiley & Sons; 2007.
- Effects of precursor on preparation and properties of nano-crystalline hopcalite particles. Asian J. Chem.. 2014;26:2133-2137.
- [Google Scholar]
- TiO2 -SiO2 nanofluid characterization: Towards efficient with water/ethylene glycol mixture for solar application. IOP Conf. Ser. Mater. Sci. Eng.. 2020;863:1-8.
- [CrossRef] [Google Scholar]
- Effects of heat transfer based water for three square multilayer absorber solar collector. IOP Conf. Ser. Mater. Sci. Eng.. 2020;788(1):012078-012089.
- [CrossRef] [Google Scholar]
- Ganji, D.D., Sabzehmeidani Y., Sedighiamiri A., 2018. Nonlinear systems in heat transfer: Chapter 3—Radiation Heat Transfer New York: Elsevier.
- Free convective melting-solidification heat transfer of nano-encapsulated phase change particles suspensions inside a coaxial pipe. Adv. Powder Technol.. 2020;31(11):4470-4481.
- [CrossRef] [Google Scholar]
- Free convection heat transfer analysis of a suspension of nano–encapsulated phase change materials (NEPCMs) in an inclined porous cavity. Int. J. Therm. Sci.. 2020;157:1-12.
- [CrossRef] [Google Scholar]
- Recent advances in sensitized mesoscopic solar cells. Acc. Chem. Res.. 2009;42:1788-1798.
- [CrossRef] [Google Scholar]
- Experimental investigation of thermal and electrical conductivity of silicon oxide nanofluids in ethylene glycol/water mixture. Int. J. Heat Mass Transf.. 2018;117:280-286.
- [CrossRef] [Google Scholar]
- Climate change and human health: impacts, vulnerability, and mitigation. Lancet. 2006;367:2101-2109.
- [CrossRef] [Google Scholar]
- Heat transfer performance of TiO2–SiO2 nanofluids in a tube with wire coil inserts. Appl. Therm. Eng.. 2019;152:275-286.
- [CrossRef] [Google Scholar]
- Improved thermal conductivity of TiO2–SiO2 hybrid nanofluid in ethylene glycol and water mixture. IOP Conf. Ser. Mater. Sci. Eng.. 2017;1:1-7.
- [CrossRef] [Google Scholar]
- Experimental investigation of thermal conductivity and dynamic viscosity on nanoparticle mixture ratios of TiO2-SiO2 nanofluids. Int. J. Heat Mass Transf.. 2018;116:1143-1152.
- [CrossRef] [Google Scholar]
- A review of water heating system for solar energy applications. Int. Commun. Heat Mass Transf.. 2016;76:178-187.
- [CrossRef] [Google Scholar]
- A review on the applications of nanofluids in solar energy field. Renew. Energy. 2018;123:398-406.
- [CrossRef] [Google Scholar]
- Experimental investigations and theoretical determination of thermal conductivity and viscosity of TiO2–ethylene glycol nanofluid. Int. Commun. Heat Mass Transf.. 2016;73:54-61.
- [CrossRef] [Google Scholar]
- Comparative effectiveness of thermal stability and rheological properties of nanofluid of SiO2–TiO2 nanocomposites for oil field applications. Ind. Eng. Chem. Res.. 2020;59(35):15768-15783.
- [CrossRef] [Google Scholar]
- Generation of oxygen vacancies in visible light activated one-dimensional iodine TiO2 photocatalysts. RSC Adv.. 2014;4(70):36959-36966.
- [CrossRef] [Google Scholar]
- Hybrid nanofluids based on Al2O3, TiO2 and SiO2: numerical evaluation of different approaches. Int. Commun. Heat Mass Transf.. 2017;104:852-860.
- [CrossRef] [Google Scholar]
- TiO2/SiO2 nanofluids as novel inhibitors for the stability of asphaltene particles in crude oil: Mechanistic understanding, screening, modeling, and optimization. J. Mol. Liq.. 2017;238:326-340.
- [CrossRef] [Google Scholar]
- Experimental study on thermal conductivity of stabilized Al2O3 and SiO2 nanofluids and their hybrid. Int. J. Heat Mass Transf.. 2018;127:450-457.
- [CrossRef] [Google Scholar]
- Experimental investigation of heat transfer and friction factor of TiO2-SiO2 nanofluids in water: ethylene glycol mixture. Int. Commun. Heat Mass Transf.. 2018;124:1361-1369.
- [CrossRef] [Google Scholar]
- Comparative assessment of chemical treatments on extraction potential of commercial grade silica from rice husk. Eng. Rep.. 2019;1:1-13.
- [CrossRef] [Google Scholar]
- A novel approach for enhancement of thermal conductivity of CuO/H2O based nanofluids. Appl. Therm. Eng.. 2016;95:271-274.
- [CrossRef] [Google Scholar]
- Comparison of experimental and theoretical methods of obtaining the thermal properties of alumina/iron mono and hybrid nanofluids. J. Mol. Liq.. 2019;292:111377-111394.
- [CrossRef] [Google Scholar]
- Experimental study of heat transfer enhancement using water/ethylene glycol based nanofluids as a new coolant for car radiators. Int. Commun. Heat Mass Transf.. 2011;38:1283-1290.
- [CrossRef] [Google Scholar]
- Heat transfer characteristics of car radiator using tri-hybrid nanocoolant. IOP Conf. Ser. Mater. Sci. Eng.. 2020;863(1):012054-012064.
- [CrossRef] [Google Scholar]
- Investigation on stability of tri-hybrid nanofluids in water-ethylene glycol mixture. IOP Conf. Ser. Mater. Sci. Eng.. 2019;469(1):1-9.
- [CrossRef] [Google Scholar]
- Sonochemical synthesis, characterization and thermal and optical analysis of CuO nanoparticles. Physica B: Condens. Matter. 2010;405:3096-3100.
- [CrossRef] [Google Scholar]
- Safiei, W., Rahman, M.M., Musfirah, A.H., Maleque, M.A., Singh, R., 2020. Experimental study on dynamic viscosity of aqueous-based nanofluids with an addition of ethylene glycol. IOP Conf. Ser., Mater. Sci. Eng. 788 (1), 012094-012103. IOP Publishing. https://doi.org/10.1088/1757-899X/788/1/012094.
- Experimental investigation on properties of hybrid nanofluids (TiO2 and ZnO) in water–ethylene glycol mixture. J. Mech. Eng. Sci.. 2017;11:3087-3094.
- [Google Scholar]
- Metal oxide nanofluids in electronic cooling: a review. J. Mater. Sci. Mater. Electron.. 2020;31:4381-4398.
- [CrossRef] [Google Scholar]
- Thermal performance and viscosity of biologically produced silver/coconut oil nanofluids. Chem. Biochem. Eng. Q.. 2016;30(4):489-500.
- [Google Scholar]
- Thermal and hydraulic analysis of a rectangular microchannel with gallium-copper oxide nano-suspension. J. Mol. Liq.. 2018;263:382-389.
- [CrossRef] [Google Scholar]
- Thermal assessment of nano-particulate graphene-water/ethylene glycol (W/EG 60:40) nano-suspension in a compact heat exchanger. Energies. 2019;12:1-17.
- [CrossRef] [Google Scholar]
- Energy saving in automotive air conditioning system performance using SiO2/PAG nanolubricants. J. Therm. Anal. Calorim.. 2019;135:1285-1297.
- [CrossRef] [Google Scholar]
- Recent advances in the solar water heating systems: A review. Renew. Sust. Energ. Rev.. 2013;19:173-190.
- [CrossRef] [Google Scholar]
- Studies of structural, optical, and electrical properties associated with defects in sodium-doped copper oxide (CuO/Na) nanostructures. J. Mater. Sci.. 2018;53(12):8826-8843.
- [CrossRef] [Google Scholar]
- Effect of sonication time on enhancement of effective thermal conductivity of nano TiO2–water, ethylene glycol, and paraffin oil nanofluids and models comparisons. J. Exp. Nanosci.. 2015;10(4):310-322.
- [CrossRef] [Google Scholar]
- Investigation on band gap of CuO–ZnO nanocomposite thin films and stability of CuO–ZnO nanofluids. J. Nanoelectron. Optoelectron.. 2018;13:1366-1373.
- [CrossRef] [Google Scholar]
- Thermal conductivity and viscosity of stabilized ethylene glycol and water mixture Al2O3 nanofluids for heat transfer applications: An experimental study. Int. Comm. Heat Mass Transf.. 2014;56:86-95.
- [CrossRef] [Google Scholar]
- Experimental analysis on thermophysical properties of Al2O3/CuO hybrid nano fluid with its effects on flat plate solar collector. Defect Diffus. Forum. 2017;374:148-156.
- [CrossRef] [Google Scholar]
- Measurement of thermal conductivity of ZnO–TiO2/EG hybrid nanofluid. J. Therm. Anal. Calorim. 2016;125(1):527-535.
- [CrossRef] [Google Scholar]
- An experimental investigation on the thermophysical properties of 40% ethylene glycol based TiO2-Al2O3 hybrid nanofluids. Int. Commun. Heat Mass Transf.. 2020;116:104663-104676.
- [CrossRef] [Google Scholar]
- Effect of TiO2-Al2O3 nanoparticle mixing ratio on the thermal conductivity, rheological properties, and dynamic viscosity of water-based hybrid nanofluid. J. Mater. Res. Technol.. 2020;9(6):13781-13792.
- [CrossRef] [Google Scholar]
- High-purity nano silica powder from rice husk using a simple chemical method. J. Exp. Nanosci.. 2014;9:272-281.
- [CrossRef] [Google Scholar]
- Synthesis, magnetic properties and X-ray analysis of Zn0.97X0.03O nanoparticles (X= Mn, Ni, and Co) using Scherrer and size–strain plot methods. Solid State Sci.. 2012;14(4):488-494.
- [CrossRef] [Google Scholar]
- Viscosity, thermal and electrical conductivity of silicon dioxide–ethylene glycol transparent nanofluids: An experimental studies. Thermochimica acta. 2017;650:106-113.
- [CrossRef] [Google Scholar]
Appendix A
See Figs. A1–A9.
Preparation of trihybrid nanofluids (t1, t2, t3) from CuO (A), TiO2 (B) and SiO2 (C).
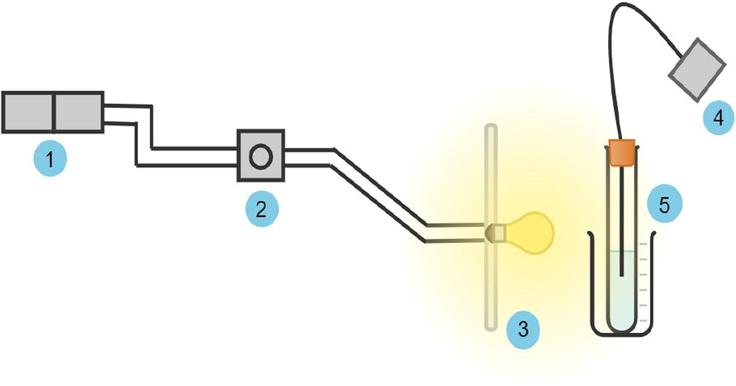
The solar radiation test rig.
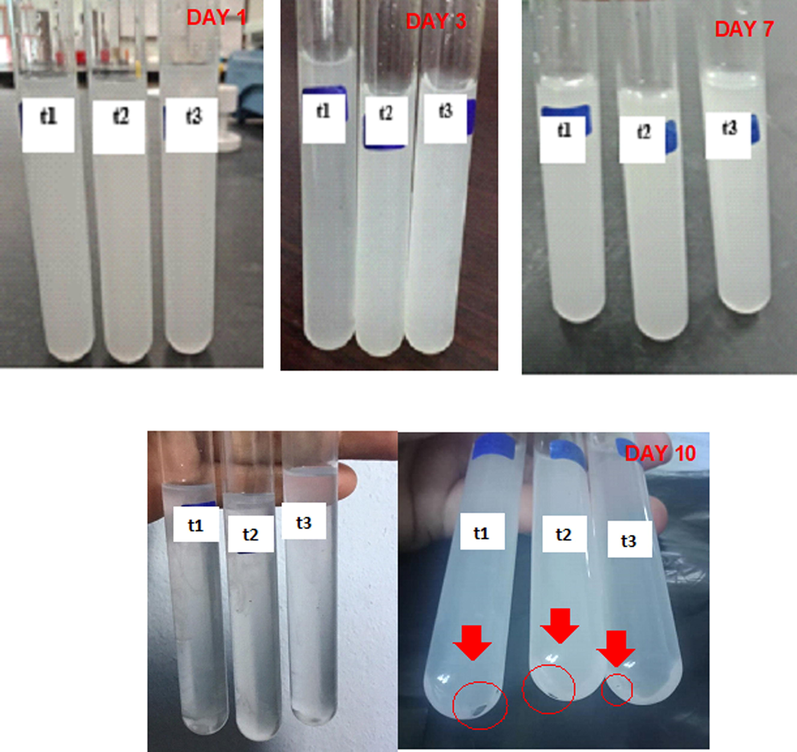
Sedimentation of trihybrid nanofluids from Day 1 until Day 10, which the solutions were kept at room temperature.
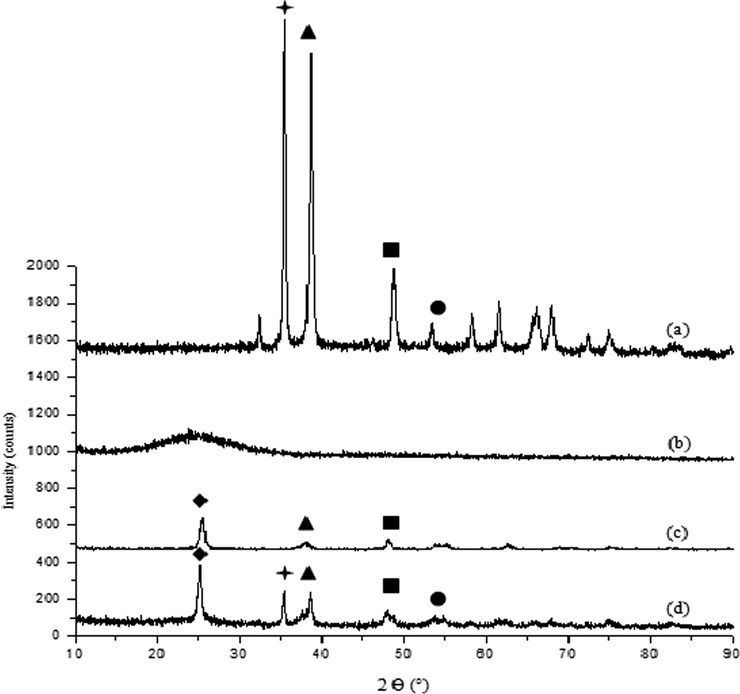
XRD spectrum of (a) CuO, (b) SiO2, (c) TiO2 and (d) trihybrid (CuO/SiO2/TiO2).
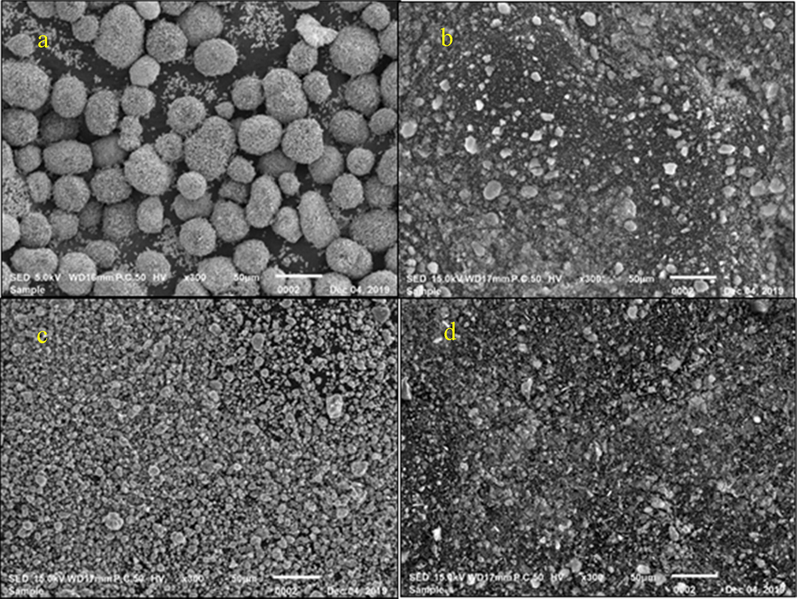
Surface morphology of (a) CuO, (b) SiO2, (c) TiO2 and (d) trihybrid (CuO/SiO2/TiO2) at 300× magnification (50 μm).
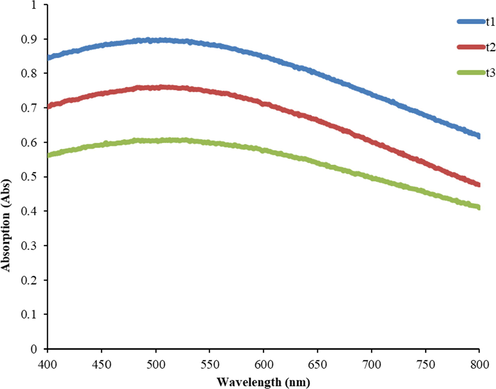
UV–vis absorption of trihybrid nanofluids of t1, t2 and t3.
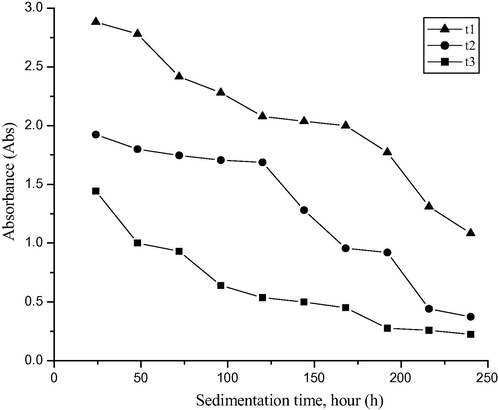
The absorbance of trihybrid nanofluids up to 240 h.
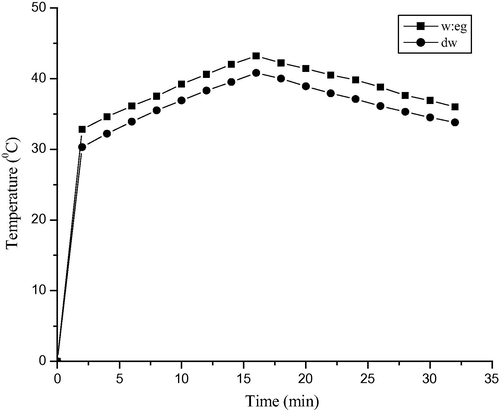
Solar absorption and cooling curve of base fluids with solar radiation of 300 W/m2.
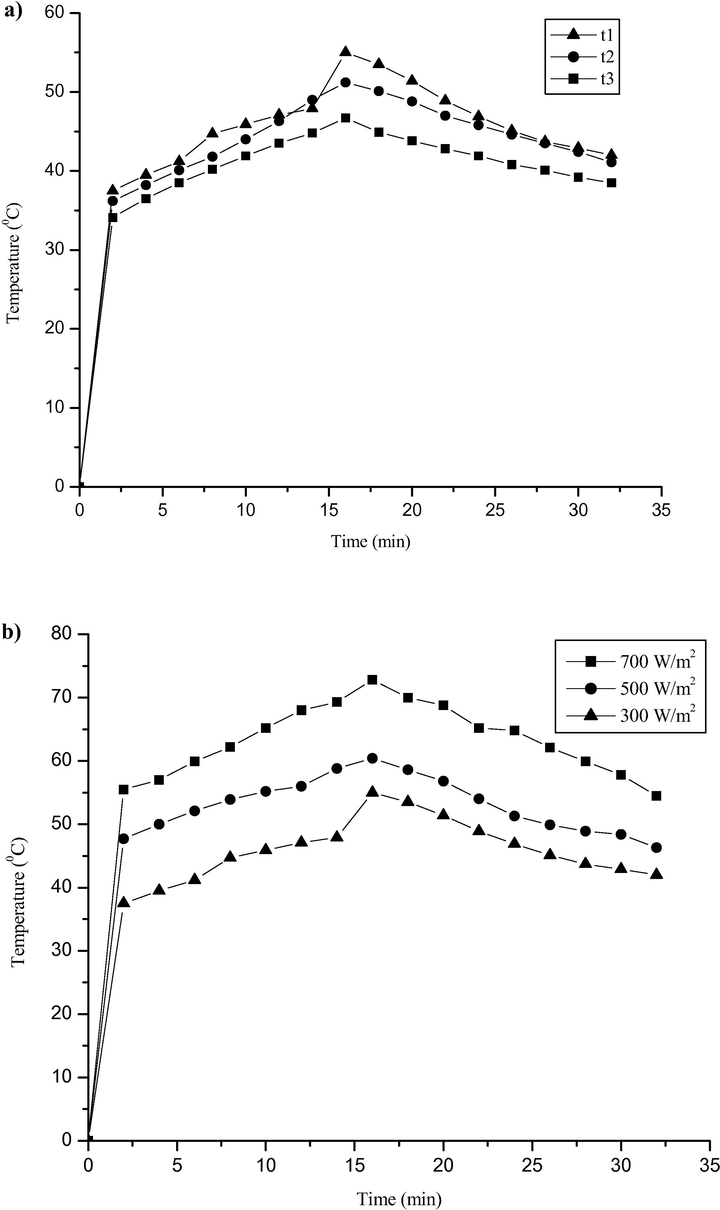
Temperature output a) of the absorber based on t1, t2 and t3, b) of t1 with solar radiation of 300, 500 and 700 W/m2 respectively.
See Tables A1–A3.
Number
Specification
Description
1
Switch Socket Outlet (Data Logger & Spotlight Dimmer)
SSO
2
Setting up with pyranometer (300 & 700 W/m2)
Dimmer
3
Halogen 500 W
Spotlight
4
K-type
Thermocouple
5
100 mL
Test tube
Samples
Density (g ml−1)
Distilled water
0.99
Water-Ethylene Glycol (60:40)
1.69
t3 trihybrid nanofluids
1.86
t2 trihybrid nanofluids
2.06
t1 trihybrid nanofluids
2.26
Nanoparticles
CuO
SiO2
TiO2
Trihybrid
Peak position
35.47
24.30
25.49
25.10
FWHM
0.32
14.28
0.57
1.57
Crystallites size (nm)
26.41
0.57
14.39
5.19







