Translate this page into:
Hepatoprotective effects of Pandanus amaryllifolius against carbon tetrachloride (CCl4) induced toxicity: A biochemical and histopathological study
⁎Corresponding author. miqbal@ums.edu.my (Mohammad Iqbal) driqbalmohammad@gmail.com (Mohammad Iqbal)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The purpose of this study was to determine the phytochemical constituents of Pandanus amaryllifolius as well as to evaluate its ability to protect against acute hepatic damage caused by carbon tetrachloride (CCl4) in rats. In animals pre-treated with P.amaryllifolius and intoxicated with CCl4, biochemical parameters such as aspartate aminotransferase (AST), alanine aminotransferase (ALT), glutathione (GSH), catalase (CAT) and malondialdehyde (MDA) were used to assess hepatic damages. Histopathological assessment was also done to evaluate the CCl4 mediated hepatic injury in rats. P.amaryllifolius antioxidant activity was measured using free radical scavenging DPPH (1,1-diphenyl-2-picrylhydrazyl) method. The stable DPPH level was lowered by P. amaryllifolius extract. The value of the half-maximum inhibitory level (IC50) was 46.8 μg/ml. Phytochemical screening of P. amaryllifolius extract showed the presence of tannins, alkaloids, saponins, terpenoids and flavonoids except pholabatannins and cardiac glycosides. The total phenolic content (TPC) was 35.99 ± 0.04 mg GAE/g and total flavonoid content (TFC) was 59.96 ± 0.013 mg CAE/g of extract. P.amaryllifolius pre-treated groups displayed significantly increased catalase antioxidant enzyme activity relative to the CCl4 treated group (57–82%, P < 0.05). P.amaryllifolius was found to moderately reduce serum ALT and AST levels (4–34%, P < 0.05). The formation of MDA due to lipid peroxidation was greatly reduced (29–70%, P < 0.05) when compared to the CCl4 treated group, while GSH was raised in a dosage dependent way (94–100%, P < 0.05). Reduced histological changes in the liver were clear evidence of P. amaryllifolius protective effect. According to the research findings, the antioxidant and free radical scavenging properties of P. amaryllifolius may be responsible for its hepatoprotective benefits.
Keywords
P.amaryllifolius
Carbon tetrachloride (CCl4)
Antioxidant
Hepatoprotective
1 Introduction
The liver is the largest internal organ in the body; weight is approximately 1.2–1.5 kg. The liver plays a very crucial role in protein synthesis, detoxification of drugs and toxins, secretion of biles, excretion of bilirubin, metabolism of hormones and storage as well as the metabolism of fats and carbohydrates (Amol and Tushar, 2013, Al Amin and Menezes, 2021). Liver injury can be caused by many reasons such as toxic chemicals, exposure to antibiotics, carbon tetrachloride (CCl4), thioacetamide (TAA) and excessive alcohol consumption, virus infiltration, drugs or infections (Weber et al., 2003, Anyasor et al., 2020, Basu, 2003). Other than that, a common cause of the liver disease is inflammation which results from the abuse of alcohols, poor diet and even malnutrition (Halliwell, 2012, Wahid et al., 2016, Kilany et al., 2020). This can lead to liver anomalies (Edgar et al., 2015, Basu, 2003, Osman et al., 2021). Most common liver anomalies are cirrhosis, hepatitis, cholestasis, hepatic failure, hepatic encephalopathy and tumours like hepatoma (Weber et al., 2003, Basu, 2003, Li et al., 2015, Gnanaraj et al., 2016). Majority of the anomalies are due to the hyper physiological burden of free radicals, which will cause the imbalance between oxidants and antioxidants in our body (Basu, 2003, Amol and Tushar, 2013, Kahar and Sahoo, 2019).
CCl4 is a potent well-known toxicant which induced hepatic damage through the generation of reactive oxidative stress (Weber et al., 2003, Basu, 2003, Li et al., 2015). CCl4 toxicity results from the bioactivation of cytochrome P-450 system that form toxic reactive trichloromethyl peroxyl radical (CCl*3). This radical further attack on membrane lipids to initiate a chain reaction resulting peroxidation of membrane lipids, leading to hepatocellular damage and carcinoma (Weber et al., 2003, Basu, 2003, Li et al., 2015). Although the use of CCl4 has been restricted because of its toxicity, still it has been extensively used to induce liver toxicity in vivo experimental model system and further to evaluate the therapeutic potential of herbs/purified herbal constituents, drugs and antioxidants (Basu, 2003; Erdemli et al., 2018).
Recently, phytochemicals herbal medicines attracted more attention, as many herbal plants are used as an alternative medicine for hepatic injury (Amol and Tushar, 2013, Gnanaraj et al., 2016, Osman et al., 2021, Kahar and Sahoo, 2019, Kilany et al., 2020, Osman et al., 2021). Other than fruits and leaves extract, the extract of common vegetables also has been used as an alternative remedy for the liver injury. The common vegetables are Allium sativum (Garlic), Amorphophallus paeoniifolius (Elephant foot yam), Beta vulgaris (beet-root), Brassica oleracea (cabbage), Allium cepa L (Bulbs of the onion) and many other vegetables were studied on CCl4 induced hepatic injury in rats (Sudha et al., 2013).
Pandanus amaryllifolius or screw pine is a plant that looks like a pineapple and has narrow, long, strap-shaped green leaves in a spiral pattern (Ghasemzadeh and Jaafar, 2013). In Malaysia and Indonesia, it is commonly known as Pandan leaf. There are many uses of Pandan leaves in our society. Pandan leaves' sweet and delightful flavour, well-known as a raw flavour source, is extensively utilised in Southeast Asian nations such as Malaysia, Thailand and Indonesia. Because of its high chlorophyll content, Pandan leaves are often used as a culinary colouring. In Malaysia, raw and fresh spices and herbs commonly consumed as salad and are thought to have strong antioxidant activity and have numerous health advantages (Chiabchalard and Nooron, 2015; Chong et al., 2012; Ghasemzadeh and Jaafar, 2013; Reshidan et al., 2019). Pandan leaves possess various bioactive compounds, such as rutin, epicatechin, naringin, catechin, kaempferol, gallic acid, cinamic acid and ferulic acid etc (Ghasemzadeh and Jaafar, 2013). There are also many health benefits of Pandan leaves which have been used traditionally to treat many diseases (Ooi et al., 2014, Ghasemzadeh and Jaafar, 2013). It is used as a cure for pain relief such as headache, ear pain, chest pain and arthritis. Pandan leaves also used in reducing stomach cramps and spasms (Nadaf and Zanan, 2012). It is also believed that the leaves have anti-carcinogenic properties (Ghasemzadeh and Jaafar, 2013). Moreover, it is consumed to reduce fever, improve skin and speed up recuperation of women who have just given birth (Ravindran, 2017). Although, studies have been done on P. amaryllifolius, the hepatoprotective effect of P. amaryllifolius is still lacking. Hence present study aims to determine the phytochemical constituents of P.amaryllifolius as well as its ability to protect against CCl4 induced hepatic damage and toxicity in rats.
2 Experimental
2.1 Chemicals
Gallic acid, hydrogen peroxide (H2O2), reduced glutathione (GSH), cathechin, thiobarbituric acid (TBA), D-aspartic acid, hematoxylin, eosin, Folin-Ciocalteu reagent (FCR), D-alanine, trichloroacetic acid (TCA), 2,4-dinitrophenylhydrazine, carbon terachloride (CCl4), α-ketoglutarate, sulfosalicylic acid (SSA), 5,5-dithio-bis-2-nitrobenzoic acid (DTNB), 2,2-diphenyl-1-picrylhydrazyl (DPPH) and bovine serum albumin (BSA) were acquired from Sigma Aldrich Company, USA.
2.2 Collection of plant sample
The leaves of P. amaryllifolius were purchased fresh at a wet market on Gaya Street in Kota Kinabalu, Sabah, Malaysia. The plant species was identified using its morphology by Mr Johnny Gisil, a botanist at the Universiti Malaysia Sabah's (UMS) Institute of Tropical Biology Conservation (ITBC).
2.3 Extraction of plant sample
The plant leaves and branches were separated from the whole plant and thoroughly cleaned with water and air-dried for 2–3 weeks. After that, the air-dried sample was put in an oven for three days at 35 °C. Then, using a blender the sample were crushed into crude powder and stored at room temperature until further investigation. Using stirring hot plate, powdered plant materials were boiled for 10 min with 1:10 sample-to-distilled-water content ratio, left to cool for an hour at room temperature and then filter using filter paper (Whatman No.1). The filtrate was freeze-dried and stored at −20 °C for further assay.
2.4 Phytochemical screening
The freeze-dried extract was obtained and subjected to six different qualitative tests including tannins, phlobatannins, saponins, flavonoids, cardiac glycosides and terpenoids as described previously (Harborne, 1973).
2.5 Total phenolic content (TPC) determination
The TPC was determined using the technique previously described (Velioglu et al., 1998). The Folin-Ciocalteu reagent (FCR) method used gallic acid as a standard for the determination of the phenol content. In triplicate, six specific concentrations of gallic acid (10, 20, 40, 80, 100, 200 μg/ml) were prepared from a stock solution of 1 mg/ml. At room temperature, FCR aliquot (1.5 ml) was added to each tube and left for 5 min in the dark, followed by Na2CO3 solution (1.5 ml) and further kept in the dark for 90 min. The solutions reaction was measured at 725 nm using a spectrophotometer and the results were stated in mg GAE/g of plant extract.
2.6 Total flavonoid content (TFC) determination
The TFC was done with some modifications using the colorimetric aluminium chloride (AlCl3) method (Zou et al., 2004). In brief, 0.25 ml of the crude extract of 1 mg/mL with 0.75 ml of 1 M NaNO3 was incubated for 6 min. After that, at room temperature 10% AlCl3 (0.15 ml) was added to the solution and left for 5 min in the dark, followed by addition of 4% NaOH (0.5 ml) and dH2O (1.53 ml) and kept further for 5 min before measured at 510 nm with spectrophotometer. Results were stated in mg CAE/g of plant extract.
2.7 Radical scavenging determination (DPPH test)
The approach for determining antioxidant activity was followed as reported before (Hatano et al., 1988). Firstly, 5 mg/mL of plant extract and ascorbic acid solution stock was prepared. Next, eight different concentrations (10, 25, 75, 150, 300, 600, 1200, 2400 µg/ml) of plant extract and ascorbic acid were made in triplicates from the stock solution prepared. Then, distilled water was added to each tube to make 300 µl. After that, to each tube 2.7 ml of DPPH solution was added and shakes using a vortex machine. Prior to measurement the extract was allowed to react with DPPH for 1 h. The reduction of DPPH radical, the change of colour was estimated by using a spectrophotometer to measure the absorption at 517 nm.
2.8 Animals and experimental design
2.8.1 Sprague Dawley rats
Thirty Sprague Dawley rats which weigh between 150 and 300 g and the age between 8 and 12 weeks old were purchased from Faculty of Food Science and Nutrition, Universiti Malaysia Sabah. All the 30 rats were caged in Animal Biosafety Laboratory (ABSL3) in Biotechnology Research Institute (BRI), Universiti Malaysia Sabah (UMS). The rats were kept in a room maintained at 22 ± 2 °C with a 12-h light/dark period under ethical principal according to Animal Ethics Committee (AEC) of the Universiti Malaysia Sabah (UMS). The experiment was conducted in accordance with the guidelines of the UMS AEC (AEC-03/2015). The rats were caged in colony plastic cages and were provided water ad libitum, standard food pellet. The rats were allowed one week to adapt themselves under this environmental condition.
2.8.2 Experimental design
Thirty adult Sprague Dawley male rats weighing between 150 and 300 g, and around 8–12 weeks old and randomly divided into five groups of six animals in each, were taken for the study of the plant extract's effect on histopathological assessment of hepatic damage, antioxidant enzymes and hepatic injury. The group I animals were used as normal controls received saline, the group II animals were given CCl4 (1.0 ml/kg b. wt.) in a 1:1 ratio with corn oil on the 13th and 14th days, and group III-V animals were given plant extract orally in saline (150 and 300 mg/kg b.wt.) respectively, followed by 1 ml/kg b. wt. doses of CCl4 (once a day) on the last two days of 14 days experimental period. Following the last dose of plant extracts all of these animals were sacrificed over a 24-hour period. The rats were firstly anesthetized by using light (C2H5)2O. Then, the liver of the rats was extracted. Extraneous materials were cleaned from the liver and were washed using chilled 0.85% w/v NaCl. After that, the livers were dipped into 0.1 M, pH 7.4 phosphate buffer and stored at −80 °C, for further biochemical studies. For histopathological studies, a small portion of livers from all rats were removed and fixed in 10% neutral formalin buffered solution.
2.9 Collection of blood
The blood sample of each rat was collected from the posterior vena cava. The collection of the blood sample was conducted after cervical dislocation before the heart-beats stop. The blood samples were collected in separated vacutainer blood collection tubes and were centrifuged at 2000 rpm for 30 min to obtain serum for the assay of serum transaminases (AST & ALT).
2.10 Post mitochondrial supernatant (PMS) preparation
The PMS was prepared following previous described method (Mohandas et al., 1984). Firstly, the liver from each group was washed with 0.85% NaCl and homogenized using homogenizer in phosphate buffer (0.1 M, pH 7.4) containing 1.17% KCl before centrifuge it at 2000 rpm for 10 min at 4 °C to separate the homogenate debris. Then the obtained supernatant re-centrifuge again for 30 min at 10,000 g, 4 °C to get the PMS that were used in malondialdehyde and reduced glutathione content assay. The supernatant obtained was kept under −80 °C prior to further analysis.
2.11 Reduced glutathione (GSH) determination
The level of GSH in PMS was done according to previous described method (Jollow et al., 1974). A spectrophotometer (Thermo Spectronic GENESYS 20 model 4001/4, Thermo Electron Scientific Instruments LLC, Madison, WI) was used to measure the yellow colour produced instantly at 412 nm. The obtained results reported in micromoles of reduced glutathione per gram (GSH/gram) of tissues by using a molar extinction coefficient of 1.36 × 103 M−1 cm−1.
2.12 Lipid peroxidation (LPO) determination
Hepatic LPO in PMS was assessed TBARS (thiobarbituric acid reactive compounds) generation rate given in malondialdehyde (MDA) equivalents as described previously (Buege and Aust, 1978). The aliquot mixture was measured using a spectrophotometer at 535 nm with a molar extinction value of 1.56 × 105 M−1 cm−1. The amount of MDA generated per gram of tissue was measured in nanomoles (nmol).
2.13 Catalase (CAT) activity determination
Catalase activity was carried out using previously described method (Claiborne, 1985) with further slight modification. The changes in absorbance were recorded at 240 nm for every 30 s for 3 min using a spectrophotometer. Other than that, using a molar extinction value of 6.4 × 103 M−1 cm−1, the enzyme activity was estimated as nmol H2O2 consumed/min/mg protein.
2.14 Serum transaminases (AST & ALT) determination
The ALT and AST serum transaminases were determined as previously described method (Reitman and Frankel, 1957). The ALT and AST values were calculated and compared with the normal range of the enzymes in the liver.
2.15 Total protein content determination
The Lowry method using Folin-Ciocalteu reagent (FCR) (Lowry et al., 1951) were used to determine the total protein content and measured at 750 nm using UV–Vis spectrophotometer.
2.16 Histopathological assessment
For histological microscopic assessment, livers from all rats were removed, cleaned and fixed in 10% neutral formalin buffered solution, and then embedded in wax, stain with haematoxylin and eosin (H&E) after cutting into 6 m thick sections (Alturkistan et al., 2016). The stained tissues sections randomly inspected under a microscope with no regard for the animal or group.
2.17 Statistical data analysis
All findings were reported as mean ± standard deviation (SD). The result was analysed for statistical significance by two-way analysis of variance (ANOVA), one-way ANOVA, and a post-hoc Dunnett t-test multiple comparisons using SPSS software (Statistical Package for the Social Sciences). A statistically significant was considered when p-value was <0.05 (p < 0.05).
3 Results
3.1 Phytochemical screening (qualitative analysis)
The phytochemical screening was done to determine the phytochemical constituents in P. amaryllifolius. The result that has been obtained from the phytochemical screening is summarized in Table 1. The sign + indicates the presence of the phytochemical compound, while the sign – indicates the absence of that particular phytochemical compounds.
Phytochemical constituents
Presence (+) or absence (-)
Tannins
+
Phlobatannins
–
Alkaloids
+
Saponins
+
Cardiac Glycosides
–
Terpenoids
+
Flavonoids
+
3.2 Total phenolic content (TPC) and total flavonoid content (TFC)
The obtained results of TPC and TFC were shown in Table 2. The TPC in the P. amaryllifolius was found to be 35.99 ± 0.04 mg GAE/g of dried weight, while the TFC was 59.96 ± 0.013 mg CAE/g of extract. Data presented as Mean ± standard deviation of three replicates.
Sample
Total phenolic content
(mg GAE/g dried weight)Total flavonoid content
(mg CAE/g of extract)
P. amaryllifolius
35.99 ± 0.04
59.96 ± 0.01
3.3 Antioxidant activity using DPPH
The antioxidant activity of free radical scavenging activity of P. amaryllifolius was determined using DPPH assay as shown in Table 3. The antioxidant potential of P. amaryllifolius was measured using ascorbic acid as a reference standard. Data presented as Mean ± standard deviation of three replicates.
Sample
DPPH scavenging activity IC50 value (µg/mL)
Ascorbic Acid
52.5
P.amaryllifolius
46.8
3.4 Body-weight changes in rats
Table 4 shows the changes in body weight in rats from various groups. The body weight of the rats does not differ significantly between the normal, CCl4 alone treated, and pre-treated with plant extracts and treated with CCl4 groups. Data presented as Mean ± standard deviation of six animals (n = 6).
Group
Treatment
Body weight
0th Day
(g)Body weight
7th Day
(g)Body weight
14th Day
(g)
I
Normal saline
235 ± 0.82
242 ± 1.08
250 ± 1.20
II
CCl4 (1 ml/kg b.wt.)
268 ± 0.80
275 ± 1.37
280 ± 1.47
III
Plant extract alone (300 mg/kg b.wt.)
242 ± 1.08
268 ± 1.28
275 ± 1.37
IV
Plant dose 1 (150 mg/kg b.wt.) + CCl4
229 ± 1.04
237 ± 1.80
242 ± 1.08
V
Plant dose 2 (300 mg/kg b.wt.) + CCl4
256 ± 1.02
272 ± 1.08
277 ± 1.39
3.5 Hepatoprotection
As shown in Table 5, in the CCl4 intoxicated rats, the serum levels of AST and ALT drastically increased when compared to normal control rats. The significant reduction in the AST and ALT after treatment of animals with P. amaryllifolius extract proven that it has hepatoprotection ability against hepatotoxin, CCl4. Moderate decrease in the ALT and AST in groups treated with P. amaryllifolius extract alone inferred that aqueous extract of P. amaryllifolius can heal the injury caused by the CCl4. Data presented as Mean ± standard deviation of six animals (n = 6). * Significantly differ from the corresponding values for normal control (*p < 0.05).
Group
Treatment
AST
(IU/I)ALT
(IU/I)
I
Normal
137.2 ± 0.15
77.2 ± 0.12
II
CCl4 (1 ml/kg b.wt.)
557.2 ± 0.10*
537.2 ± 0.14*
III
Plant extract alone (300 mg/kg b.wt.)
117.2 ± 0.17
69.2 ± 0.12
IV
Plant dose 1 (150 mg/kg b.wt.) + CCl4
533.2 ± 0.14#
517 ± 1.05#
V
Plant dose 2 (300 mg/kg b.wt.) + CCl4
397.2 ± 0.29#
357.2 ± 0.10#
3.6 Antioxidant status in the liver
Table 6 shows the antioxidant enzyme status such as glutathione reductase (GSH), lipid peroxidation (LPO) and catalase (CAT) in the liver. In the CCl4 intoxicated rats, the level of LPO is significantly increased while, the level of the GSH and CAT significantly decreased. The aqueous extract of P. amaryllifolius has shown maximum protection, in which the level of GSH and CAT increased to the value similar to the normal control group, while the level of LPO decreased in the group pre-treated with plant extract 150 mg/kg body weight and 300 mg/kg body weight. This proves that, the aqueous extract of P. amaryllifolius has hepatoprotection effect against CCl4 induced liver injury. P. amaryllifolius extract can restore the value of the LPO, GSH and CAT. Data presented as Mean ± standard deviation of six animals (n = 6). * Significantly differ from the corresponding values for normal control (*p < 0.05).
Group
Treatment
GSH
(µmol/g)LPO
(nmol MDA/g tissue)CAT
(µmol/min/mg protein)
I
Normal
3.57 ± 0.11
19.85 ± 0.30
14.88 ± 0.01
II
CCl4 (1 ml/kg b.wt.)
1.77 ± 0.14*
35.77 ± 0.50*
8.01 ± 0.01 *
III
Plant extract alone (300 mg/kg b.wt.)
3.88 ± 0.10
19.21 ± 0.30
13.02 ± 0.02
IV
Plant dose 1 (150 mg/kg b.wt.) + CCl4
3.39 ± 0.08#
30.07 ± 0.80#
8.50 ± 0.19#
V
Plant dose 2 (300 mg/kg b.wt.) + CCl4
3.58 ± 0.10#
21.93 ± 0.94#
12.25 ± 0.1#
3.7 Histopathological assessment
Histopathological assessment was done to support the data that obtained from biochemical studies and to illustrate the morphological changes in normal, CCl4 induced and plant extract treated rats. The results of the observation are shown as Fig. 1. Fig. 1A, shows normal liver cell under 100X magnification. The normal liver has a distinct nucleus and normal hepatocytes. Meanwhile, Fig. 1B, CCl4 alone treated group shows fatty changes, mild necrosis in hepatocytes and inflammation. The arrow labelled shown fatty changes and inflammation. Fig. 1C, shows P. amaryllifolius alone treated group with normal hepatocytes and distinct nucleus. Fig. 1D and E, shows the restoration of the liver, especially the hepatocytes after the pre-treatment of animals with P. amaryllifolius. The arrow labelled shows the improvement of fatty changes and inflammation.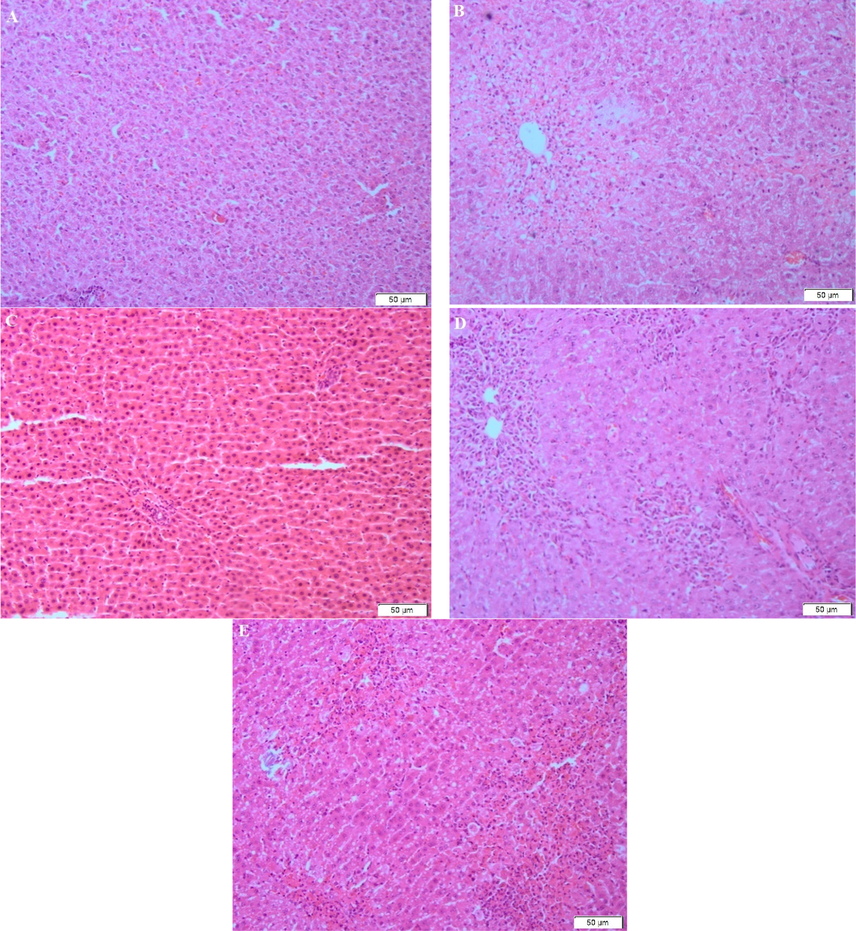
A = Normal saline control, B = CCl4 (1 ml/kg b.wt.), C = P. amaryllifolius extract (300 mg/kg b.wt.), D = P. amaryllifolius (150 mg/kg b.wt.) with CCl4 (1 ml/kg b.wt.), E = P. amaryllifolius (300 mg/kg b.wt.) with CCl4 (1 ml/kg b.wt.). Magnifications 100X.
4 Discussion
This study was undertaken to evaluate the phytochemical constitution of P.amaryllifolius and their ability to protect against CCl4 induced hepatic damage and toxicity in rats. CCl4 is a potent well-known toxicant which induced hepatic damage through the generation of reactive oxidative stress (Weber et al., 2003, Basu, 2003, Li et al., 2015). CCl4 toxicity results from the bioactivation of cytochrome P-450 system that form toxic reactive trichloromethyl peroxyl radical (CCl*3). This radical further attack on membrane lipids to initiate a chain reaction resulting peroxidation of membrane lipids, leading to hepatocellular damage and carcinoma (Weber et al., 2003, Basu, 2003, Li et al., 2015). The present study revealed that CCl4 intoxication in rats increased the level of AST, ALT and MDA which was accompanied by a decrease in hepatic GSH and catalase activity. Liver damage was also analysed by histopathological observation. However, most of these detrimental effects were restored after pretreatment of animals with P.amaryllifolius.
P.amaryllifolius leaves has also been tested for its phytochemical constituents. Earlier reports showed the presence of various bioactive compounds such as rutin, epicatechin, naringin, catechin, kaempferol, gallic acid, cinamic acid and ferulic acid etc (Ghasemzadeh and Jaafar, 2013). These compounds are purported to have strong antioxidant activity and have numerous health advantages (Chiabchalard and Nooron, 2015; Chong et al., 2012; Ghasemzadeh and Jaafar, 2013; Reshidan et al., 2019). In accordance with that phytochemical screening of P.amaryllifolius provide evidence for the presence of flavonoids, alkaloids, saponins, terpenoids and tannins. The leaves extract of P.amaryllifolius exhibited high content of both phenolics and flavonoids. The presence of these secondary metabolites demonstrates that P.amaryllifolius has high antioxidant activity and is likely to have contributed to hepatoprotective activity by inhibiting oxidative stress.
Body-weight of the rats was recorded to monitor the health condition of the rats. Based on the data tabulated, there was an increase in the body-weight of the rats. This can be inferred that all the rats used were healthy. Based on the initial and final weights, there was no significant difference among all the groups compared. This supports the previous study stating that body weight and liver index did not differ in the control and CCl4 treated group as well as plant extract treated groups (Lala et al., 2021).
Serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are two main important biochemical markers that play an important role in the measurement of liver damage (Li et al., 2015). Normal control rats exhibits a normal range of serum AST and ALT. The level of the serum enzymes elevated drastically in the CCl4 induced rats, compared to the normal rats which shows that the liver is damaged (Chatterjee et al., 2011). The hepatoprotective effect of aqueous extract of P. amaryllifolius is shown in Table 5, in which the extracts able to reduce the elevated level of the AST and ALT serum when treated with two different doses of extract 150 mg/kg b.wt and 300 mg/kg b.wt. This revealed that the P. amaryllifolius extract can heal the damages induced by the CCl4.
Reduced glutathione (GSH) is important for the detoxification of CCl4′s harmful metabolites (Chatterjee et al., 2011). Based on the present study, this is evidence that the level of GSH significantly depleted in group treated with CCl4 alone. The level was raised moderately with the treatment of animals with P. amaryllifolius extract.
Lipid peroxidation (LPO) is the oxidative damage of polyunsaturated lipids (Prabha and Annapoorani, 2009). Peroxidation involves the reaction of oxygen and lipids to form free radicals and peroxides which induces the cell and organelle damages. In this study, the MDA content increased significantly in the liver of CCl4 alone treated rats. The content of MDA in the liver decreased moderately when animals were pre-treated with the extract of P. amaryllifolius. The decrease in the MDA level indicated the inhibition of the lipid peroxidation as well as enhancement of anti-oxidative defence mechanism to prevent the free radical formation and generation, which will induce the oxidative damages.
Catalase (CAT) is an anti-oxidative enzyme that presents in almost all tissues, however it can be found abundant in liver and red blood cell (Palanivel et al., 2008). Catalase protects the tissues against free hydroxyl radicals which are highly reactive (Palanivel et al., 2008). In the present study, the catalase activity in the liver was significantly decreased in CCl4 alone treated rats. Meanwhile, the treatment of P. amaryllifolius extracts at a dosage level of 150 mg/kg and 300 mg/kg body weight restored the catalase activity by increasing the value back to normal.
Histopathological studies are needed to support biochemical studies (Prakash et al., 2008). Rats treated with CCl4 alone shown severe hepatotoxicity such as the appearance of the centrilobular necrosis, fatty changes and damage of the hepatocytes. The normal saline-treated control rats showed normal cytoplasm, prominent nucleus as well as visible central veins. The sections of the liver of animals pre-treated with aqueous extract of leaves of P. amaryllifolius and treated with CCl4 reveals hepatoprotective activity. Almost negligible damage to a few hepatocytes was observed.
5 Conclusion
In conclusion, P. amaryllifolius has been demonstrated to contain several important phytochemical constituents such as saponin, tannins, alkaloids, flavonoids and terpenoids. P. amaryllifolius has relatively high antioxidant activity, flavonoid content and phenolic contents. Other than that, P. amaryllifolius has been proved to have hepatoprotective activity against CCl4 intoxication in rats via inhibiting oxidative stress.
Acknowledgements
Financial support for this research was provided by UMS Priority Area Scheme SBK0192-SKK-2015. Authors are also thankful to the Director of Biotechnology Research Institute, Universiti Malaysia Sabah, Dr. Zarina Amin, for support and encouragement.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Histological stains: A literature review and case study. Glob. J. Health Sci.. 2016;8:72-79.
- [Google Scholar]
- Carbon tetrachloride toxicity. In: StatPearls [Internet]. Treasure Island (FL): Stat Pearls Publishing; 2021.
- [Google Scholar]
- Antioxidant and hepatoprotective activity of an ethanol extract of Piper cubeba fruit. Intl J. Res. Dev. Pharm. Life Sci.. 2013;2:321-329.
- [Google Scholar]
- Hepatoprotective and haematological effects of Justica secunda Vahi leaves on carbon tetrachloride induced toxicity in rats. Biotech. Histochem.. 2020;95:349-359.
- [Google Scholar]
- Carbon tetrachloride induced lipid peroxidation: eicosanoid formation and their regulation by antioxidant nutrients. Toxicol.. 2003;189(1-2):113-127.
- [Google Scholar]
- Hepatoprotective effect of the ethanolic extract of Calocybe indica on mice with CCl4 hepatic intoxication. Intl. J. Pharm. Tech. Res.. 2011;3:2162-2168.
- [Google Scholar]
- Antihyperglycemic effects of Pandanus amaryllifolius Roxb. Leaf extract. Pharmacogn. Mag.. 2015;11(41):117.
- [CrossRef] [Google Scholar]
- In vitro evaluation of Pandanus amaryllifolius ethanol extract for induction of cell death on non-hormone dependant breast adenocarcinoma MDA-MB-231 cell via apoptosis. BMC Complement. Altern Med.. 2012;12:134.
- [Google Scholar]
- Catalase activity. In: CRC handbook of methods for oxygen radical research. 1985. p. :283-284.
- [Google Scholar]
- Pyrrolizidine alkaloids: potential role in the etiology of cancers, pulmonary hypertension, congenital anomalies, and liver disease. Chem. Res. Toxicol.. 2015;28(1):4-20.
- [Google Scholar]
- Can crocin play a preventive role in Wistar rats with carbon tetrachloride-induced nephrotoxicity? Iran J. Basic Med. Sci.. 2018;21:382-387.
- [Google Scholar]
- Profiling of phenolic compounds and antioxidant activity and anticancer activities of Pandanus amaryllifolius Roxb extract from different locations in Malaysia. BMC Compl. Alt. Med.. 2013;13:341.
- [Google Scholar]
- Hepatoprotective and immunosuppressive effect of Synedrella nodiflora L., on carbon tetrachloride-intoxicated rats. J. Env. Pathol. Toxicol. Oncol.. 2016;35:29-42.
- [Google Scholar]
- Free radicals and antioxidants: updating a personal view. Nutrition Reviews. 2012;70:257-265.
- [Google Scholar]
- Phytochemical methods. London: Chapman and Hall; 1973.
- Two new flavonoids and other constituents in licorice root: their relative astringency and radical scavenging effects. Chem. Phar. Bull.. 1988;36(6):2090-2097.
- [Google Scholar]
- Acetaminophen-induced hepatic necrosis II. Role of covalent binding in vivo. J. Phar. Exp. Thera.. 1974;187:195-202.
- [Google Scholar]
- Ameliorative effect of Homalium zeylancium against carbon tetrachloride induced oxidative stress and liver injury in rats. 2019. Biomed. Pharmacothera.. 2019;111:305-314.
- [Google Scholar]
- Tribulus terrestris ameliorates carbon tetrachloride induced hepatotoxicity in male rats through suppression of oxidative stress and inflammation. Envoron. Sci. Pollut Res Int.. 2020;27(20):24967-24981.
- [Google Scholar]
- Lala, V., Goyal, A., Bansal, P., Minter, D.A., 2021. Liver Function Tests. In: Stat Pearls [Internet]. Treasure Island (FL): Stat Pearls Publishing, 2021.
- The role of oxidative stress and antioxidants in liver diseases. Intl. J. Mol. Sci.. 2015;16(11):26087-26124.
- [Google Scholar]
- Protein measurement with the folin phenol reagent. J. Biol. Chem.. 1951;193(1):265-275.
- [Google Scholar]
- Differential distribution of glutathione and glutathione related enzymes in rabbit kidney: Possible implications in analgesic neuropathy. Cancer Res.. 1984;44:5086-5091.
- [Google Scholar]
- Economical Importance of Indian Pandanus Species. In: Indian Pandanaceae - an overview. New Delhi: Springer; 2012.
- [Google Scholar]
- Purification and characterization of new antiviral protein from the leaves of Pandanus amaryllifolius (Pandanaceae) Int. J. Biochem. Cell Biol.. 2014;36:1440-1446.
- [Google Scholar]
- Osman, A., Enam, M.K., Silothy, M., 2021. Alleviation of carbon tetrachloride induced hepatocellular damage and oxidative stress in rats by Anabaena oryzae phycocyanin. J. Food. Biochem. Jan; 45(1):e13562. Doi:10.1111/jfbc.13562. Epub 20020. Nov 13.
- Hepatoprotective and antioxidant effect of Pisonia aculeate against CCl4 induced hepatic damage in rats. Sci. Pharm.. 2008;76:203-215.
- [Google Scholar]
- Hepatoprotective effect of Cynodon dactylon on CCl4 induced experimental mice. J. Biol. Sci.. 2009;17:24-34.
- [Google Scholar]
- Hepatoprotective activity of leaves of Rhododendron arboretum in CCl4 induced hepatotoxicity in rats. J. Med. Plants Res.. 2008;2:315-320.
- [Google Scholar]
- The encyclopaedia of herbs and spices. Boston: CABI; 2017.
- The effects of Pandanus amaryllifolius (Roxb.) leaf extracts on fructose induced metabolic syndrome in rat model. BMC Complement. Altern Med.. 2019;19:232.
- [Google Scholar]
- A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol.. 1957;28(1):56-63.
- [Google Scholar]
- A review on hepatoprotective activity of commonly consumed vegetables. Der. Pharm. Lett.. 2013;5:290-304.
- [Google Scholar]
- Antioxidant and total phenolics in selected fruits, vegetables and grain products. J. Agric. Food. 1998;31:140-148.
- [Google Scholar]
- Hepatoprotective activity of ethanolic extract of Salix subserrata against CCl4 induced chronic hepatotoxicity in rats. BMC Compl. Alt. Med.. 2016;16:263.
- [Google Scholar]
- Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Critical Rev. Toxicol.. 2003;33:105-136.
- [Google Scholar]
- Antioxidant activity of a flavonoid rich extract of Hypericum perforatum L. in vitro. J. Agric. Food Chem.. 2004;52(16):5032-5039.
- [Google Scholar]







