Translate this page into:
Heterogeneously catalyzed palm biodiesel production in intensified fruit blender
⁎Corresponding author at: Department of Nuclear Engineering, Faculty of Engineering, Chulalongkorn University, 254 Phayathai Road, Pathumwan, Bangkok 10330, Thailand. Doonyapong.W@chula.ac.th (Doonyapong Wongsawaeng)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
This research investigated palm biodiesel production via solid base-catalyzed transesterification in an intensified kitchen fruit blender. Studied parameters included methanol to palm oil molar ratio, CaO catalyst loading, reaction temperature, and reaction volume. XRD analysis showed a pure CaO phase after calcination while other characteristics illustrated good functionality of the catalyst. An optimal condition was achieved with 15:1 of methanol to palm oil molar ratio, 5 wt% of CaO dosage, 60 °C of reaction temperature, and 1,000 mL of reaction volume, obtaining the highest FAME production of 97.58% and yield efficiency of 1.47 × 10−3 g/J. The FT-IR illustrated an alteration of various functional groups through a reusability experiment demonstrating exceptional activity stability. The catalyst activity was decreased by only 15.8% in the 5th consecutive cycle. Properties of produced biodiesel conformed to international standards. This proved that biodiesel production using the CaO catalyst in the intensified kitchen fruit blender provides a positive effect on reuse, reduces wastewater from the washing process, lowers production costs, and is environmentally responsible to achieve high FAME yield due to the cavitation phenomena inside the reactor to generate fine emulsion of oil and methanol which could be easier to reach the active site of the CaO catalyst.
Keywords
Biodiesel production
Heterogeneous catalyst
Fruit blender
Transesterification
Palm oil
1 Introduction
Biodiesel production has been continuously studied and developed for over 40 years and has been used in many geographic regions as an alternative biofuel to fossil fuels to overcome the problem of price and environmental pollution of diesel fuel. It is very well known that biodiesel offers biodegradability, low toxicity, and reduction of emissions of sulfur dioxide, carbon monoxide, unburned hydrocarbons, as well as the particulate matters which cause the greenhouse effect in the Earth's atmosphere (Atadashi et al., 2013; Farooq et al., 2013). Biodiesel is therefore touted as a cleaner, greener, more environmentally friendly, and sustainable energy than diesel fuel. Typically, in industrial production, biodiesel is produced by transesterification based on homogeneous base or acid catalysts with different feedstocks and alcohols. Due to their low cost, high reaction rates, and mild reaction conditions, potassium hydroxide or sodium hydroxide as homogeneous base catalysts are most commonly applied (Dias et al., 2008; Tabatabaei et al., 2019), requiring only 1–2 h of operation and obtaining a biodiesel yield of more than 98%. Homogeneous acid catalysts, e.g., sulfuric acid, sulphonic acid, and hydrochloric acid require extended time for reaction (over 5 h), high temperatures, as well as high alcohol-to-oil molar ratio (Atadashi et al., 2013). However, the use of both homogeneous catalyst types requires purification processes to remove the catalysts and impurities from biodiesel, which cause a wastewater problem. They also lead to certain problems including reactor corrosion and the difficulty of reusing the catalyst, thus increasing the overall cost of biodiesel production (Helwani et al., 2009b).
At present, the substitution of heterogeneous catalysts has been considered as a green alternative to overcome these limitations on the use of homogeneous catalysts because of easier separation by filtration, high recoverability, long lifetime, insensitivity to the presence of water or free fatty acids (some types of catalysts), less saponification and not requiring the washing process to lessen an environmental issue (Helwani et al., 2009a). A great variety of heterogeneous catalysts have been used in biodiesel production based on vegetable oil feedstocks which include both base and acid such as alkaline earth metal oxides (Liu et al., 2008; Salamatinia et al., 2010), transition metal oxides (Yoo et al., 2010), alkaline metal carbonates (Dai et al., 2020), ion-exchange resins (Chanthon et al., 2021a,b), as well as oxides or metals deposited on supporting materials (Omar and Amin, 2011; Farooq et al., 2013). Heterogeneous basic catalysts exhibit higher activity than solid acid ones, requiring only mild conditions to synthesize biodiesel with the ease of catalyst restoration (Antunes et al., 2008; Ruhul et al., 2015). Alkaline earth oxides have been potentially used in biodiesel production with increasing activity being in the order of BaO > SrO > CaO > MgO (Semwal et al., 2011). However, BaO is not practical because it is soluble in methanol and forms toxic compounds. SrO can strongly react with CO2 and water present in the air to form strontium hydroxide and strontium carbonate and lose its catalytic ability, as well as fully dissolve in the reaction mixture (Yan et al., 2008). CaO and MgO have been broadly studied in transesterification achieving a high fatty acid methyl ester (FAME) content. In particular, CaO has higher strength in basicity and low solubility in methanol (Ruhul et al., 2015). Moreover, commercial CaO is easy to use, has a low price and has been proven to produce high biodiesel yields. It also does not require a complicated synthesis process. Many pieces of literature reported that the use of CaO as a solid catalyst accomplished a 77.3–95% biodiesel yield with various edible oils or even waste cooking oil at the methanol: oil molar ratio of 12:1–15:1, 1–8 wt% catalyst loading and reaction temperature of 60–65 °C within 1–5 h of reaction time (Granados et al., 2007; Liu et al., 2008; Mootabadi et al., 2010; Yoosuk et al., 2010; Maneerung et al., 2016). Therefore, a basic CaO catalyst has been utilized in this research.
In biodiesel production, recently several mixing reactors representing intensification technologies have been employed to increase mass/heat transfer between the two incompatible liquids, namely oil and methanol (Somnuk et al., 2017). These technologies can improve the reaction rate so that the reaction time may be shortened. Microwaves, ultrasonics, microchannel reactors, and hydrodynamic cavitation reactors are examples of intensification technologies used in biodiesel production (Qiu et al., 2010). For the hydrodynamic cavitation reactor, the effect of turbulence (cavitation) in the reactor generates fine emulsions which create large interfacial areas leading to the reaction moving forward (Maddikeri et al., 2014) which results in increased yield. The deliberation issues of these intensification technologies are that some are expensive, have high maintenance costs, have a small reaction volume capacity, and are not suitable for use with solid catalysts. Therefore, the present research utilizes an existing intensification technology based on a high-power kitchen fruit blender to produce biodiesel with a CaO catalyst, employing the cavitation effect combined with a high rotational speed of the impeller, enhancing the mixing efficiency as well as increasing the reaction rate. Due to low cost, availability at a local supermarket, simple operation, and a large chemical reactor volume, Wongjaikham et al. (2021) successfully applied a high-power household fruit blender to produce FAME based on refined palm oil and waste cooking oil with a homogeneous catalyst (NaOH) in a continuous system, obtaining the highest biodiesel yield of 96.81%. The blending vessel was upgraded to a 304 stainless steel reactor to ensure safety and long-term durability. In continuation of the investigation and to take advantage of the heterogeneous catalyst, the current research employed the high-power fruit blender to produce palm biodiesel using a CaO catalyst in a batch system, presenting a novel investigation that has never been carried out by any researcher. The effects of various operating parameters on biodiesel yield and yield efficiency were explored: methanol to oil molar ratio, percentage of catalyst loading, reaction temperature, and reaction mixture volume. The characteristics of the catalyst were studied by Thermogravimetric analysis (TGA), X-ray diffractometer (XRD), Scanning electron microscopy (SEM), N2 adsorption and desorption isotherms, and Fourier-transform infrared (FT-IR) spectroscopy to analyze the thermal stability, phase of crystal structures, morphology, specific surface areas, and chemical functional groups, respectively. The produced biodiesel was evaluated against the EN 14214 and ASTM D 6751 standards.
2 Experimental
2.1 Materials
The commercial refined palm oil of the Morakot brand was procured in a local supermarket in Bangkok with the properties listed in Table 1. Laboratory grade methanol (99.8%) and commercial CaO (99%) were supplied from Kemaus. Methyl heptadecanoate (an internal standard) and n-heptane (99.5%) were purchased from Sigma-Aldrich and Ajax FineChem, respectively. All chemicals were used without further purification.
Property
Value
Fatty acids compositiona
Lauric acid (wt.%)
0.4
Myristic acid (wt.%)
1.1
Palmitic acid (wt.%)
46.1
Stearic acid (wt.%)
4.4
Oleic acid (wt.%)
37.1
Linoleic acid (wt.%)
11.1
Linolenic acid (wt.%)
0.2
Density (g/cm3)b
0.89
Acid value (mg of KOH/g of oil)b
0.21
Kinematic viscosity (40 °C, mm2/s)b
40.21
2.2 Catalyst preparation and characterization
2.2.1 Catalyst preparation
The commercial CaO powder was calcined at 900 °C for 2 h in an open-air furnace and was subsequently stored in a desiccator to prevent activity degradation.
2.2.2 Characteristic analyses
The thermal behavior of commercial CaO catalyst was characterized by thermogravimetric analysis (TGA) (Mettler Toledo, TGA/DSC 3+) to determine the optimal calcination temperature range. The catalyst was heated at a heating rate of 15 °C/min from ambient temperature to 1,000 °C in an air atmosphere.
X-ray diffraction (XRD) was employed to identify the crystal structure of the catalyst. The XRD patterns were operated by Bruker AXS Model D8 Discover under 40 kV and 40 mA by using Cu radiation. Data collection was carried out with the diffraction angles 2 from 5° to 80° with a step size of 0.02°.
Scanning electron microscopy (SEM, Jeol JSM-IT500HR) was used to examine the size, shape, and morphology of the calcined and used CaO catalyst at a magnification of up to 20,000 times and an accelerating voltage of 10 kV. Particle size distribution of the CaO catalyst was measured using MALVERN Mastersizer 3000 by measuring 3 replicated random samples. For the used CaO, it was collected from experimental parameters of methanol: oil molar ratio of 15:1, 5 wt% catalyst dosage, 60 °C reaction temperature, and reaction volume of 1,000 mL for 1 h. After that, it was washed with methanol several times, dried at 120 °C for 2 h, and subsequently subjected to the SEM and particle size distribution analyses.
Specific surface areas and porosity were measured by N2 adsorption/desorption isotherms at 77 K based on the Brunauer-Emmett-Teller (BET) and Barrett-Joyner-Halenda (BJH) method, respectively, using Micromeritics – ASAP2060. To acquire the isotherm, the catalyst was degassed under vacuum at 250 °C for 8 h before being analyzed in liquid nitrogen at −196 °C.
Fourier-transform infrared (FT-IR) spectra were determined using a spectrophotometer (Nicolet 6700). The sample was prepared by mixing fine powders of the sample with KBr powder. This experiment revealed different functional groups after each catalyst usage cycle.
2.3 Transesterification procedure and FAME analysis
2.3.1 Transesterification procedure
The high-power fruit blender employed in this research was the same one used in the previous study (Wongjaikham et al., 2021) as depicted in Fig. 1. In brief, it was a 1,200 W Otto brand, model BE127A, with the blending bowl replaced with a 304 stainless steel cylindrical chemical reactor with a maximum liquid handling capacity of 3,000 mL. The chemical reactor was insulated with cotton sheets obtained from a local supermarket to reduce heat loss to the environment and was installed with 4 appropriate baffles inside. The stainless steel impeller originally supplied with the blender was not modified. One side of the reactor had several ports installed with thermocouples and the opposite side was equipped with several discharge ports corresponding to specific reaction mixture volumes.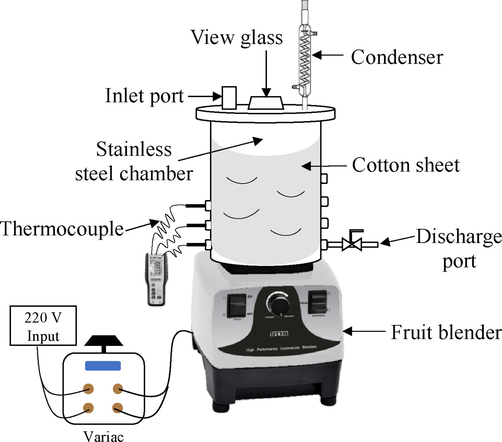
Drawing of modified fruit blender setup.
Initially, the palm oil was slowly poured into the vessel and was solely heated by the motion of the impeller to a temperature of about 70 °C. This step took only approximately 10 min because the impeller operated at a high speed (the variac was adjusted to about 100 V). Methanol and the CaO catalyst were then added to the reactor and all constituents were blended to maintain a reaction temperature of 60 °C (except for the study of the temperature parameter). A variac used to regulate the line voltage to the blender was adjusted intermittently to maintain the desired temperature. Transesterification was carried out for 2 h and samples were collected every 30 min. The studied parameters were methanol to oil molar ratio of 6:1–15:1, catalyst loading of 1–10 wt%, reaction temperature of 50–65 °C, and reaction volume of 1,000–2,000 mL. The samples were centrifuged for 10 min to separate the liquid as the upper layer from the solid as the lower layer. The upper layer consisted of, from bottom to top, the glycerol layer, FAME layer, and methanol layer. The FAME layer was collected for further analysis to determine the FAME yield content. All experiments were repeated 2 times.
2.3.2 FAME yield analysis
FAME yield was determined following the EN 14103 standard (EN14103 2011) using Shimadzu GC 2010 Plus. This GC system was installed with a flame ionization detector and a DB wax capillary column using a helium as a carrier gas. The FAME sample and methyl heptadecanoate were dissolved with n-heptane as a solvent. The 1 μL solution was injected and the temperature was held at 150 °C for 5 min and raised to 190 °C at a rate of 3 °C/min with 5 min holding time. Finally, it was increased to 220 °C at a rate of 3 °C/min and held for 5 min and the detector temperature was 250 °C. FAME yield was evaluated according to Eq. (1):
Yield efficiency was calculated according to Eq. (2) to compare the energy efficiency with other reactors.
2.4 Catalyst reusability
After finishing the transesterification, the reaction mixture was left at room temperature for 4–5 h allowing individual layers to separate. Each liquid layer was removed until only the solid catalyst remained. The used CaO catalyst was washed with methanol several times until the methanol was clear, and it was subsequently heated to about 120 °C for 2 h to completely evaporate the methanol. Transesterification using the recycled catalyst was resumed as described in Section 2.3 with a total of five consecutive cycles.
2.5 Determination of FAME properties
After the production of FAME with the optimal conditions for the highest yield, several properties were determined. Table 2 shows the standards to analyze those properties.
Property
Standard
Density
ISO 4787 (Gülüm and Bilgin, 2017)
Cloud point
ASTM D 2500 (Tesfaye and Katiyar, 2016)
Kinematic viscosity at 40 °C
ASTM D 445 (Gülüm and Bilgin, 2017)
Acid value
AOCS by titration (Bockisch, 1998)
2.6 Statistical analysis
In this work, analysis of variance (ANOVA) was employed to interpret the significant differences. Each parameter was compared within the group which of the values were different from one another at each reaction time. Data were analyzed using Tukey HSD's procedure through the IBM® SPSS® Statistics program version 22.0, at a P-value = 0.05 for decision (see computed P-values in Table S1). The significant differences were defined by the subgroups (a–c).
3 Results and discussion
3.1 Characterization of catalyst
3.1.1 Thermal behavior
In ambient air, calcium oxide can degrade into calcium hydroxide and calcium carbonate in a timespan of hours because of its reactive nature. Fig. 2 demonstrates the weight loss of the uncalcined CaO during the thermal process. The CaO mass was lost in two steps. First, a weight loss of 11% between 400 and 500 °C was due to the decomposition of Ca(OH)2 to eliminate H2O molecules (Wongsaenmai, 2018). Second, at above 650 °C, CaCO3 decomposed into CaO, accounting for the remaining 74.8%. From the TGA result, the calcination temperature should be higher than 800 °C.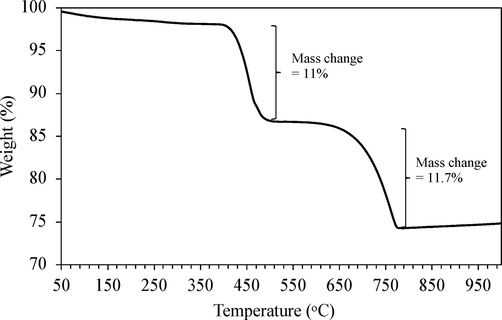
TGA pattern of commercial CaO catalyst.
3.1.2 XRD results
The XRD patterns of fresh commercial CaO and the calcined CaO at 900 °C are shown in Fig. 3. The XRD patterns of uncalcined commercial CaO illustrated in Fig. 3(a) show the main characteristic peaks at 18.0° and 34.1° and minor characteristic peaks positioned at 28.7°, 47.1°, 50.8°, 54.4°, 59.4°, 62.6°, and 71.7° which are attributed to the calcium hydroxide (Ca(OH)2) phase. These peaks were identified using Card No. 00-044-1481. Other peaks at 32.2°, 37.4°, 54.4°, and 64.4° were identified as calcium oxide (Card No. 00-043-1001). The small peaks such as at 29.4°, 39.4°, and 43.2° correspond to the calcite (CaCO3) phase of the catalyst (Card No. 01-086-5294). As depicted in Fig. 3(b), the primary peaks assigned to the CaO phase were observed at 2
= 32.2°, 37.4°, 53.9°, 64.2°, 67.4°, and 79.7°, indicating that CaCO3 was completely converted into CaO after the calcination (Yoosuk et al., 2010; Rezaei et al., 2013). Calcium hydroxide caused by water absorption was also almost eliminated.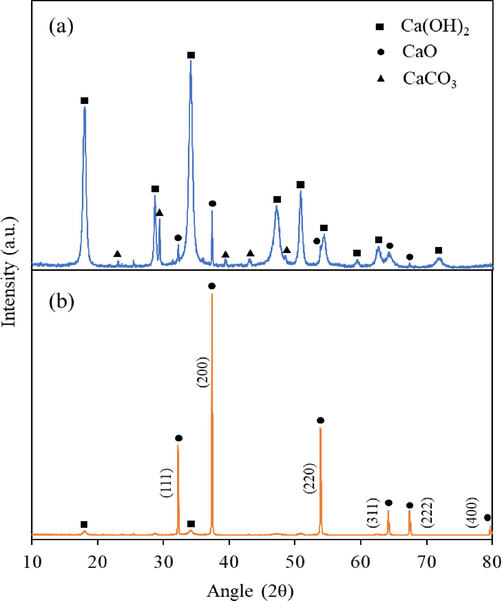
XRD patterns of (a) fresh commercial CaO and (b) calcined CaO at 900 °C.
3.1.3 SEM investigation
Fig. 4 shows the SEM micrographs of CaO before and after transesterification. Before transesterification, Fig. 4(a) and (b) revealed the roughly round shape of CaO particles which were interconnected and agglomerated. This microstructural feature offered pores and a high surface area enabling the oil to transfer to and interact with active sites inside the catalyst (Zarubica et al., 2015). After transesterification, the SEM micrographs of used CaO particles shown in Fig. 4(c) and (d) displayed a different microstructure from the fresh one, having a stacked flake-shaped and sharp structure which could be due to the reaction mixture coverage (Choedkiatsakul et al., 2013). As illustrated in Fig. 4(e), the particle size distribution of the fresh CaO was quite uniform, and the majority of particle size scaled from 2 to 20 µm while after the transesterification process, the used CaO exhibited heterogeneous particle distribution in the range of 0.3–800 µm. A large fraction of particle size fell within the range of 1–50 µm.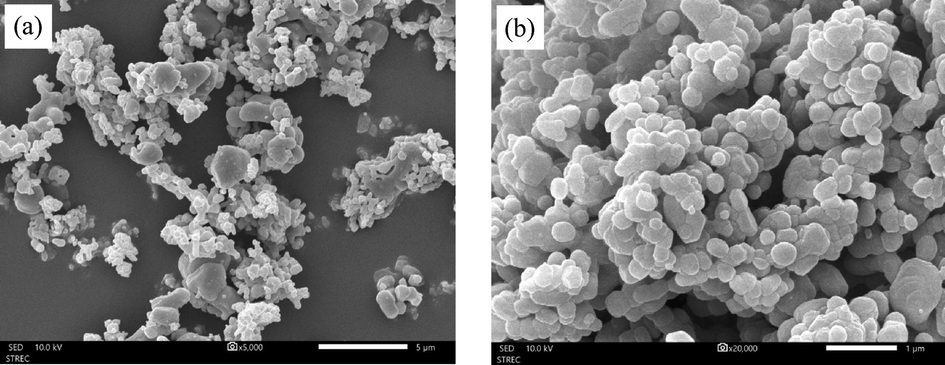
SEM micrographs of (a and b) fresh CaO calcined at 900 °C, (c and d) used CaO, and (e) particle size distribution.
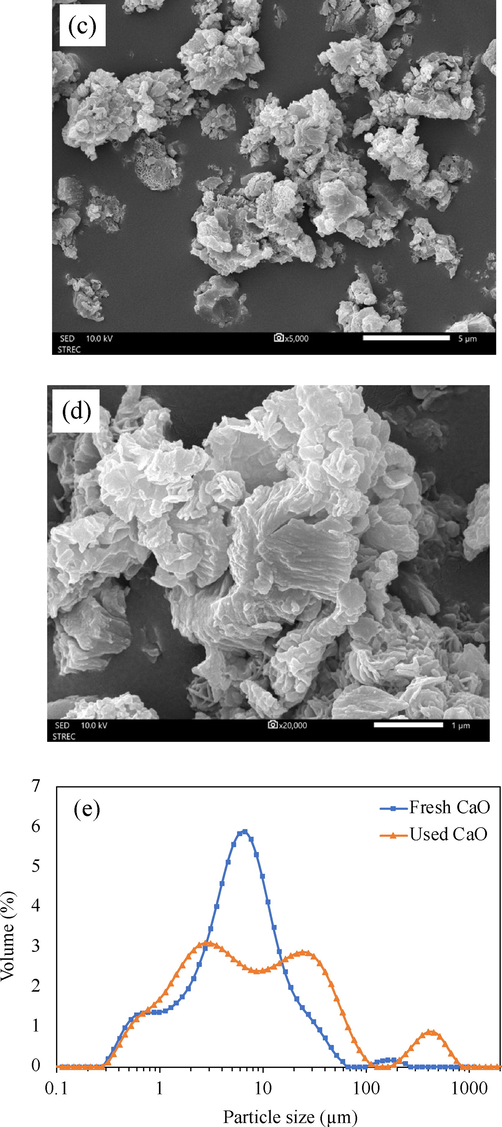
SEM micrographs of (a and b) fresh CaO calcined at 900 °C, (c and d) used CaO, and (e) particle size distribution.
3.1.4 Specific surface area and porosity of CaO
Fig. 5(a) depicts an adsorption isotherm for the calcined CaO catalyst. The isotherm is categorized as type IV according to the IUPAC classification which can be considered a mesoporous material. The specific surface area, pore volume and average pore size are displayed in Table 3. The catalyst has a low specific surface area which could be attributed to the high calcination temperature employed for FAME production (Sousa et al., 2016). Based on the BJH method, the CaO catalyst had pore volume of 0.0122 cm3/g and an average pore diameter of 17.73 nm which is consistent with pore size distribution as shown in Fig. 5(b).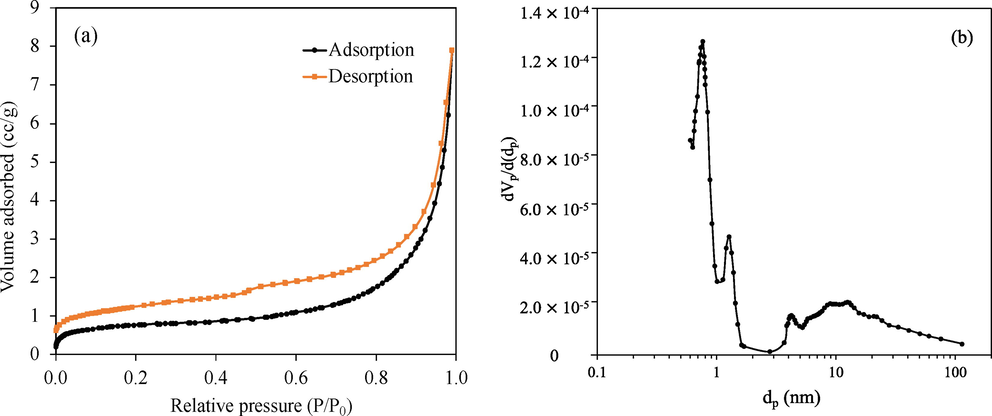
(a) Nitrogen adsorption/desorption isotherms and (b) pore size distribution of CaO.
Physicochemical property
Value
Specific surface area (m2/g)
2.75
Pore volume (cm3/g)
0.0122
Adsorption average pore diameter (nm)
17.73
3.2 Effects of various parameters on FAME production
3.2.1 Methanol to oil molar ratio
To study the influence of methanol: oil molar ratio, the reaction with 1 wt% catalyst loading, 60 °C reaction temperature, and 1,000 mL reaction volume was performed at the ratio of 6:1, 9:1, 12:1, and 15:1 with the results illustrated in Fig. 6. The methanol: oil molar ratio of 15:1 achieved the highest FAME yield of 72.7% within 120 min, while for the other molar ratios, the FAME yield increased from 6:1 to 12:1 but presented less significant differences among the yield profiles. Low molar ratios resulted in low FAME yields because the methanol content was insufficient to react with the oil. According to the literature, excess methanol is usually required to shift the transesterification equilibrium to the product side. The higher methanol content could reduce the reaction mixture viscosity, facilitating the transesterification rate to produce more biodiesel (Chanthon et al., 2021a,b). The molar ratio higher than 15:1 was not tested in this study because several studies have shown that much higher molar ratios than 15:1 such as 18:1, 20:1, or even 25:1 did not contribute to the reaction and may even reduce biodiesel yields because more methanol in the reaction mixture can reduce the oil concentration to lower the transesterification rate. Importantly, the excessive use of methanol increases the cost of biodiesel production unnecessarily. Therefore, the use of the 15:1 M ratio is appropriate and consistent with many studies. When comparing the accumulated energy consumption of FAME production after 2 h of operation, it was found that the ratios of 6:1, 9:1, 12:1, and 15:1 consumed energy of 0.189, 0.193, 0.198, and 0.201 kWh, respectively, indicating that the higher the methanol: oil molar ratio, the higher the energy consumption. As observed in the process of mixing methanol with the CaO catalyst, the temperature of the mixture remained relatively reduced which might be due to calcium methoxide formation. Although CaO is slightly soluble in methanol (0.035% at 60 °C), calcium methoxide can be formed on its surface in a suspension form (Gryglewicz 1999). This causes a small decrease in the reaction mixture temperature. When adding this “cold” mixture to the heated oil, the temperature of the oil-methanol mixture dropped sharply, requiring a higher voltage to agitate the liquid mixture to maintain the reaction temperature of 60 °C. Thus, a higher methanol content resulted in slightly more energy consumption. This phenomenon is different from homogeneous transesterification because during mixing methanol with catalysts such as NaOH, heat was generated due to NaOH being soluble in methanol. In addition, transesterification based on heterogeneous catalysts is unlike the case of homogeneous ones. Most homogeneous catalysts such as NaOH and KOH require the optimal molar ratio of 6:1, while heterogeneous ones require a higher ratio since the catalyst is present as a different phase from the reactants.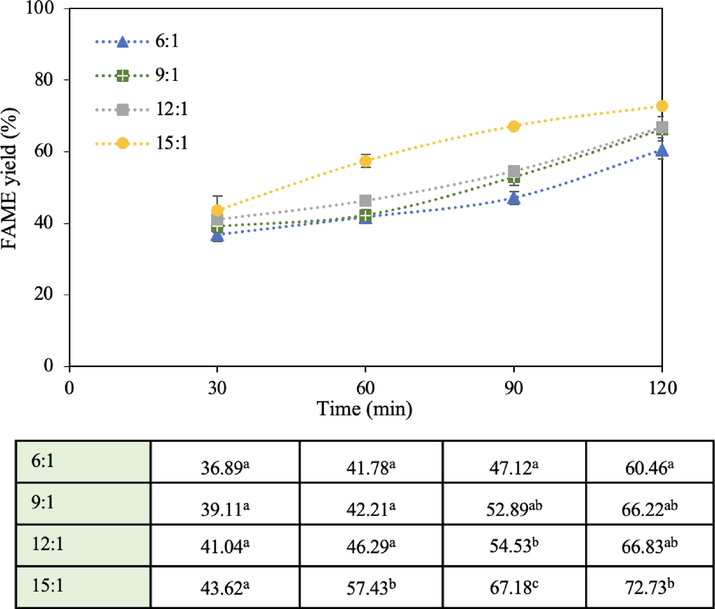
Effect of methanol to oil molar ratio on FAME yield (1 wt% catalyst loading, 60 °C reaction temperature, and 1,000 mL reaction volume).
3.2.2 Catalyst loading
The weight percentage of a catalyst plays a significant role in biodiesel yield. It was investigated from 1 to 10 wt% (based on the weight of oil) at 60 °C with methanol: oil molar ratio of 15:1 and reaction volume of 1,000 mL for 2 h. The results are presented in Fig. 7 which is observed that an increase in catalyst loading from 1 to 5 wt% resulted in an increasing FAME yield from 57.4 to 96.9% at 1 h with a decrease in FAME yield for 10 wt% after 1 h. With a low catalyst concentration (1 wt%), a small number of active sites of calcium oxide was offered which was inadequate to drive transesterification. When increasing the catalyst concentration to 5 wt%, the highest yield was obtained due to a sufficient number of active sites on the catalyst. However, the 10 wt% case presented an excessive concentration leading to a decreased FAME yield because the excessive catalyst thickened the reaction mixture. The interaction between oil and methanol became restricted and the movement of the catalyst and reactants in the solution was also poor. This finding is consistent with the investigation of Maneerung et al. that used ground chicken manure as a catalyst for biodiesel production. The viscosity of the reaction mixture at different catalyst dosages was measured and it was found that increasing the concentration of the catalyst from 7.5 to 20 wt% caused an elevation in the viscosity from 41 to 53 mm2/s (Maneerung et al., 2016). Additionally, the maximum catalyst concentration of 10 wt% can promote the side reaction (saponification) caused by water as a by-product from the mixture of CaO and methanol (Masood et al., 2012; Prasertsit et al., 2014), thus reducing the yield. In terms of energy consumption, the higher viscosity required the fruit blender to draw a higher electrical power to agitate the liquid mixture and to maintain the desired reaction temperature at 60 °C. The energy consumption of each catalyst weight percentage (1, 5, and 10%) was 0.201, 0.205, and 0.214 kWh, respectively, after 2 h. The use of higher power is undesirable in terms of cost and sustainability. Based on these findings, 5 wt% was selected as the optimal value.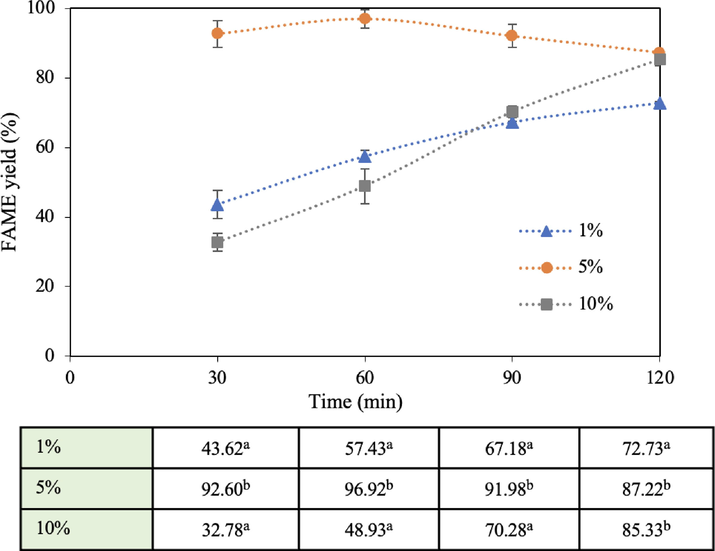
Effect of catalyst loading on FAME yield (60 °C reaction temperature, 15:1 methanol: oil molar ratio, and 1,000 mL reaction volume).
It is worth noting that at a 5 wt% catalyst dosage, the FAME yield increased from 92.6 to 96.9% with an increase in the reaction time from 30 to 60 min, which was not much different. However, after 2 h of reaction time, the biodiesel yield was reduced to 87.2%. This may be due to a reverse reaction occurring or the more biodiesel product being dissolved in the glycerol phase. At 1 and 10 wt% catalyst concentrations, the behavioral profiles of FAME yield gradually increased over time, possibly due to the low number of active sites of the catalyst in the case of 1 wt% and the high viscosity of the solid–liquid mixture in the case of 10 wt% causing a slow transesterification rate.
3.2.3 Reaction temperature
The investigation of the effect of the reaction temperature on FAME yield was carried out over three conditions; 50, 60, and 65 °C. The following parameters were constant: methanol: oil molar ratio of 15:1, 5 wt% catalyst dosage and reaction volume of 1,000 mL for 2 h, and the results are displayed in Fig. 8. At 50 °C, the transesterification rate was sluggish resulting in a low FAME yield of 84.8% after 1 h. When the temperature was increased to 60 °C, FAME yield was enhanced to 96.9% at 1 h. The reaction rate rises as the fraction of molecules with high kinetic energy increases at higher temperatures, resulting in a larger yield (Li et al., 2013). Furthermore, mass transfer was enhanced when increasing the reaction temperature due to a higher diffusivity. The increased temperature results in less viscous oil, reducing the mass transfer resistance, allowing for easier mixing with methanol and enhancing the yield. However, the FAME yield slightly dropped to 89.6% at 1 h with the excess temperature of 65 °C. This is because as the boiling point of methanol is 64.7 °C, some of the methanol content must have vaporized, turning into a 3-phase interface (catalyst-oil-methanol), affecting the optimal molar ratio. Although a condenser was installed on top of the chamber, the continuously vaporizing methanol and high methanol content in the vapor phase must have negatively affected the optimal ratio. The finding of the 60 °C optimal temperature was consistent with the generally-accepted transesterification temperature of around 60 °C. The operating conditions at 50 and 65 °C began to plateau at around 85–89% FAME yield while that at 60 °C slightly dropped after 60 min. In terms of the accumulated energy consumption after 2 h running time at 50, 60, and 65 °C, it was 0.183, 0.205, and 0.238 kWh, respectively. The results were as expected, as higher reaction temperatures necessitated higher input power for stirring to maintain the system temperature.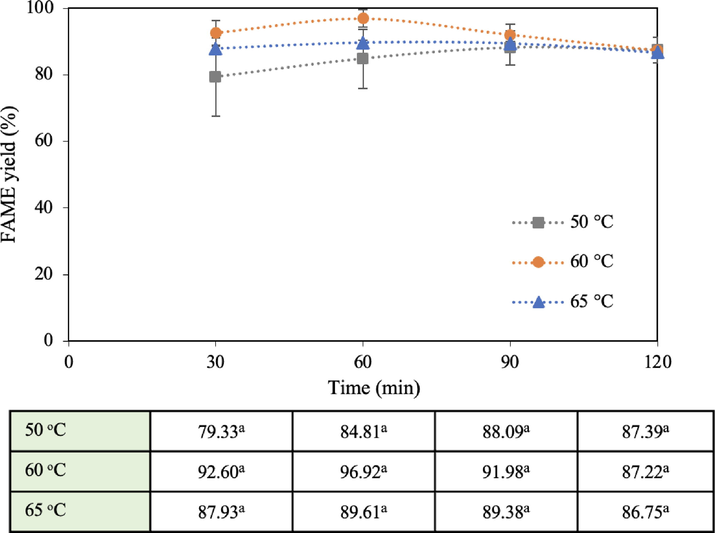
Effect of reaction temperature on FAME yield (15:1 methanol: oil molar ratio, 5 wt% catalyst dosage, and 1,000 mL reaction volume).
3.2.4 Reaction volume
The reaction volume was investigated at 1,000–2,000 mL with a 15:1 methanol: oil molar ratio and the catalyst loading at 5 wt%. The temperature was set at 60 °C. The effect of the reaction volume is shown in Fig. 9. The FAME yield decreased with increasing reactant content from 1,000 to 2,000 mL, but the yields were less significant different. The high-power blender uses the principle of the cavitation phenomenon to mix liquids (in this study) to form an emulsion. The cavitation effect is caused by a pressure drop of the fluid accelerated over the tip of the impeller to create a large number of microbubbles or cavitation bubbles. These bubbles collapse into the surrounding fluid and thoroughly mix the liquid to become a rich emulsion (Chuah et al., 2015). As the volume of the mixture increased (more than 1,000 mL), the mixed reactants at a higher position in the reactor distant away from the impeller in the so-called inactive zone (Kamjam et al., 2021) contain fewer of these tiny bubbles as well as experience less mass and heat transfer, causing a large portion of the liquid to not well-mix resulting in a reduced FAME yield. The accumulated energy consumption after 2 h for 1,000, 1,500, and 2,000 mL was 0.205, 0.240, and 0.252 kWh, respectively, showing that a higher reaction volume required more energy to maintain the system temperature. In terms of cavitation intensity (Kamjam et al., 2021), it was 0.106, 0.084, and 0.066 W/mL corresponding to 1,000, 1,500, and 2,000 mL, respectively, at 1 h of operation, signifying that the larger the volume, the lower the cavitation intensity due to inactive zones at areas away from the impeller tips. The cavitation effect is highly important for this type of high-efficiency blender combined with a high-speed impeller, which is one of the main factors allowing immiscible liquids to mix well in the presence of fine emulsion. To increase mixing effectiveness, the cavitation effect occurring inside the reactor was an extremely powerful phenomenon being able to impact numerous particles at one time. The quickly rotating impeller sufficiently lowered the liquid's pressure less than the vapor pressure, creating a large number of microbubbles. They moved through the tips and trailing edges of the impeller, where they were ripped into the surrounding fluid before collapsing and thoroughly mixing the reaction mixture (Chuah et al., 2015). Therefore, in areas away from the impeller, liquid mixing became poor. There was a report that the energy or shockwaves released from microbubbles collapsing far from a wall (in an unbounded liquid) were particularly concentrated. On the other hand, the energy generated when microbubbles collapsed close to a wall or surface was multiply less intense (Pandit et al., 2021). The cavitation activity, therefore, was influenced by the design of the reactor size and the volume of the liquid. Additionally, because of the high rotational speed of the impeller, quick liquid circulation improves mixing effectiveness and speeds up mass and heat transfer, which in turn increases reaction kinetics. A high impeller speed also increases oil droplets dispersion in the catalyst-methanol phase (Kamjam et al., 2021) to enhance the FAME yield.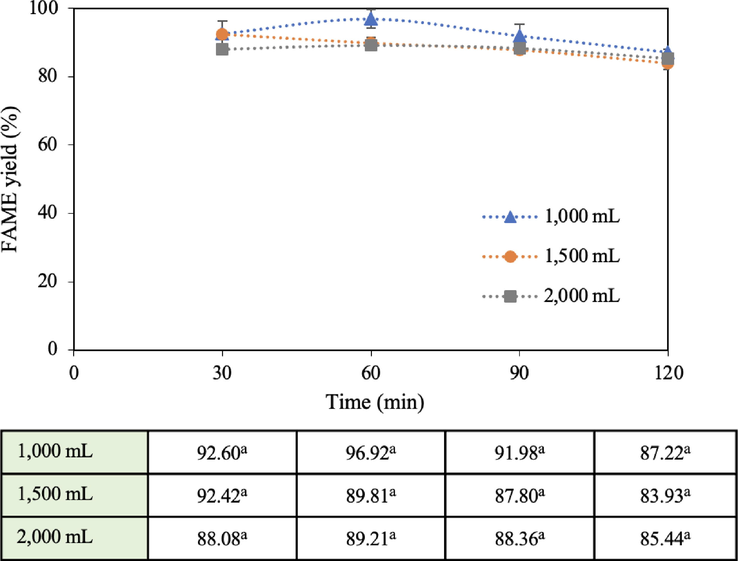
Effect of reaction volume on FAME yield (15:1 methanol: oil molar ratio, 5 wt% catalyst loading, and 60 °C reaction temperature).
The principle for an ideal liquid level in a mixing tank is the ratio between the height of the liquid level (H) and the tank diameter (D) which is 0.8, or any ratio that is close to 1 is sufficient (James 2015). This ratio has been proven that the liquids efficiently mix with improved heat transfer resulting in higher yields. In the present investigation, the H/D ratio of the liquid level (for the 1,000 mL case) to the studied reactor diameter was 8.5/15 = 0.57 which was not close to 0.8. For the case of 2,000 mL, this ratio was 11.5/15 = 0.77 which was closer to 0.8. Wongjaikham et al. stated that their high-powered blender in biodiesel production provided the highest yield at a 2,000 mL reaction volume and 50 mL/min flow rate (Wongjaikham et al., 2021), which is different from the present investigation. They used a base homogeneous catalyst, but the present study employed the heterogeneous one. The heterogeneous system required more mixing degree to overcome the diffusion mass transfer limitation which facilitated the methanol microbubbles to adsorb on the active sites of CaO to form calcium methoxide. Therefore, more cavitation intensity provides more chance for methanol to access the active sites resulting in an accelerated transesterification rate (Poosumas et al., 2016). It should be noted that the appropriate reaction volume of the reactants could vary when using different catalyst types or different particle sizes. In addition, if the ratio is less than unity and if the impeller is properly designed in relation to the reactor diameter, a high degree of mixing can be expected (McFeeters, 2017). The optimal ratio of the impeller diameter to the reactor diameter should be 0.4–0.6. The ratio of the present study is 7.5/15 = 0.5, which is considered appropriate. Also, the aforementioned ideal mixing tank ratios are typical and may not be economically optimal for all processes (Couper et al., 2010). The present research uses the high-power blender with a high impeller speed (maximum of 12,000 rpm) to achieve high efficiency to improve the miscibility of the reactants and the solid catalyst obtaining high FAME yields. Therefore, the H/D ratio had less effect on the yields of the current study.
Another important piece of information that can be extracted from Fig. 9 is the aspect of reaction time. The longer the running time, the greater the yield drop across all volumes. Fig. 9 shows that the best reaction time was 1 h and that the FAME yield declined afterward. This may be because the FAME can be dissolved in the glycerol by-product phase (Zhou et al., 2006). In addition, the longer the operating time, the more electricity was consumed, which negatively affected the cost of biodiesel production. The quick reaction time of this study strongly favors the economics of biodiesel production. This finding conforms to Lani’s work (Lani et al., 2017) who reported that the FAME yield rose as the reaction time increased from 30 to 90 min and slightly decreased after 120 min. The authors indicated that the early step of the transesterification process was slow owing to the difficulties of mixing and dispersing alcohol into oil, because of the immiscibility of the two-phase liquid system. Prasertsit and the group (Prasertsit et al., 2014) used AR-grade CaO to produce biodiesel via conventional stirring, requiring over 6 h to obtain 82% of the FAME yield. Operations with low methanol: oil molar ratios and low solid catalyst concentrations may provide high biodiesel yields, but with a trade-off of longer reaction times. Tahvildari et al. used a 7:1 methanol: oil molar ratio with a catalyst concentration of 3% based on mechanical stirring for 4 h to complete the process achieving a 92% FAME yield (Tahvildari et al., 2015). The present research used a high-power blender providing a high blending speed. The initial mixing of the reactants was so well that the FAME yield in the first 30 min was almost as high as that at 1 h of reaction time, with a difference of only 4.3% (for 1,000 mL). Therefore, using a very high rotational speed device can significantly shorten the reaction time.
3.3 Catalyst reusability
The reusability of the catalyst was investigated for 5 cycles of repeated use under the optimal conditions of 15:1 methanol: oil molar ratio, 60 °C reaction temperature, 5 wt% catalyst, 1,000 mL reaction volume, and 1 h reaction time. As shown in Fig. 10, FAME yields decreased slightly with each additional cycle. In the 1st cycle, the FAME yield was 97.58% and after the 3rd and 5th cycles, the yield was slightly reduced. FAME yield reduction of 15.8% was observed over the 5th cycle compared to the 1st cycle. It should be noted that about 5% of the catalyst mass was lost after every washing step. The decrease in the yields with repeated use was probably due to the mass loss of the catalyst or the charge in the surface composition of the catalyst during each cycle. Methanol was used to wash the used CaO causing it to encounter water/moisture/air converting the CaO to Ca(OH)2. To prove this assumption, fresh CaO was immersed in methanol for 0.5 h, then dried and subjected to the same transesterification experimental conditions. The results showed that after 1 h the yield was 92.34%, which was close to the yield in the second cycle. Therefore, Ca(OH)2 formation could slightly affect the FAME yield reduction. CaO transformation after each transesterification cycle was subjected to the FT-IR analysis with the results depicted in Fig. 11. The primary absorption peak at the wavenumber 3642 cm−1 represents the OH stretching from hydration adsorption on the catalyst surface, indicating the existence of OH groups isolated on calcium. The —OH is a byproduct of the carbonation process (Galván-Ruiz et al., 2009). The broad band at around 3300–3600 cm−1 and the peak positioned at 1631 cm−1 indicate the presence of moisture (H2O) on the catalyst surface from the adsorption (Memon et al., 2015). As can be seen that the more cycle the catalyst underwent, the more water the catalyst absorbed on the surface, especially in the 4th and 5th cycles. It could be derived from the water byproduct of the calcium methoxide generation during CaO catalyzed transesterification (Masood et al., 2012). The absorption band in the region of 400–500 cm−1 corresponds to the Ca—O group representing calcium oxide (Galván-Ruiz et al., 2009). The relative absorption peaks at 1411, 1080, and 862 cm−1 belong to the three different vibration modes of C—O bonds of the carbonate group (CO32–) (Khachani et al., 2014). This result implies that CaCO3 is present at a low concentration in the catalyst after the 3rd transesterification. However, after the 4th and 5th transesterification, there was an obvious increase in the carbonate peaks because of CO2 adsorption on the surface of CaO, turning into CaCO3, thus reducing the FAME yield. There are small peaks at around 2800–3000 cm−1 attributed to the contamination of the aliphatic group (CH2) from the biodiesel product. Another hypothesis for the yield drop in several cycles of catalyst usage is the active site on the catalyst surface being obscured by the reaction mixture adsorption during the transesterification process, such as those peaks between 1100 and 1300 cm−1 which were unidentified peaks appearing in the 4th and 5th cycles (Choedkiatsakul et al., 2013). The SEM micrographs of the used CaO catalyst also supported the hypothesis of surface coverage from the reaction mixture.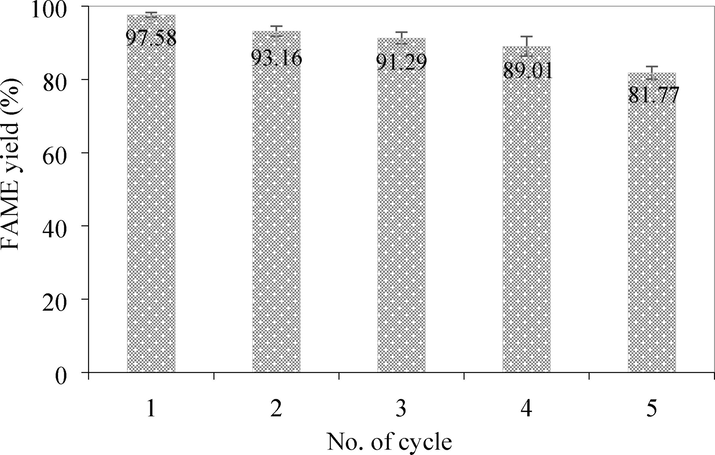
FAME yield after each catalyst reuse cycle (15:1 methanol: oil molar ratio, 60 °C reaction temperature, 5 wt% catalyst, 1,000 mL reaction volume, and 1 h reaction time).
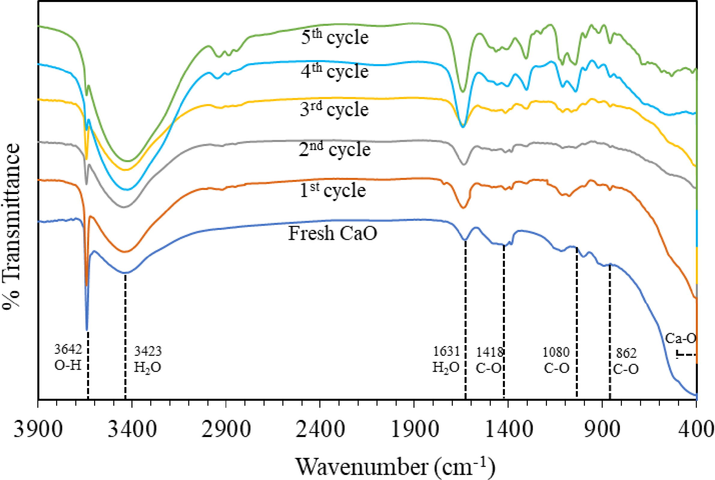
FT-IR spectra of fresh CaO and used CaO after each reaction cycle.
Nevertheless, some pieces of the literature indicated that the recalcination method decreased the FAME yield more than methanol washing without calcination. Recalcination in the reusability process could result in a reduction of calcium oxide activity owing to the decreased surface area and elevated extraction of calcium by methanol (Rezaei et al., 2013). The present work used methanol washing only, which was useful in terms of reducing the recalcination time.
3.4 FAME properties
Properties of the produced biodiesel (15:1 methanol: oil molar ratio, 60 °C reaction temperature, 5 wt% catalyst, 1,000 mL reaction volume, and 1 h reaction time) such as density, cloud point, kinematic viscosity, and acidity were reported in Table 4 and these properties comply with both European (EN 14214) and American (ASTM D6751) standards. Noted that performing the experiment at a high methanol to oil molar ratio resulted in biodiesel with quite low density, cloud point, and kinematic viscosity, but the values were still within the acceptable range. The acid value was low as well compared to other studies using the CaO catalyst which was about 0.3 (Niju et al., 2016; Miyuranga et al., 2023). The low acid value is beneficial for long-term storage and use in diesel engines because of a reduced chance of engine corrosion.
Property
Synthesized biodiesel
Standard
EN 14214
ASTM D6751
FAME yield (%)
97.58 ± 0.63
96.5
96.5
Density (g/cm3) at 40 °C
0.85 ± 0.01
0.85–0.90
0.86–0.90
Cloud point (°C)
10 ± 0.10
–
−3–12
Kinematic viscosity
(40 °C, mm2/s)3.57 ± 0.01
3.5–5.0
1.9–6.0
Acid value(mg of KOH/g of oil)
0.17 ± 0.01
0.5
0.5
3.5 Yield efficiency
The yield efficiency or energy efficiency is one of the parameters indicating the economic worthiness of biodiesel production. It is evaluated based on the energy consumption, produced FAME yield and reaction time. If biodiesel produced from any technology has a high yield efficiency, it suggests that the technology should be worth the investment due to low production costs and low energy consumption. The yield efficiency of the synthesized biodiesel compared with other studies employing different reactor technologies is stated in Table 5. The yield efficiency of the present work was 14.68 × 10−4 g/J. When compared with other studies using different intensified reactors and conventional methods (mechanical stirrer) based on heterogeneous catalysts, the yield efficiency of the present work was higher.
Studied reactor
Condition
Yield efficiency
(
10−4 g/J)Reference
Heterogeneous catalyst
Modified fruit blender
97.58% yield at 15:1, 5 wt% CaO,
60 °C, 1 h, batch process14.68
This work
Mechanical stirrer
76.76% yield at 15:1, 5 wt% CaO,
60 °C, 2 h, 1 L volume, batch process2.88
This work
Homogenizer
80% yield at 9:1, 10 wt% CaO,
60 °C, 2 h, batch process8.27
Laosuttiwong et al. (2018)
Ultrasonic
80% yield at 9:1, 10 wt% CaO,
60 °C, 2 h, batch process4.65
Laosuttiwong et al. (2018)
Mechanical stirrer
80% yield at 9:1, 10 wt% CaO,
60 °C, 4 h, batch process3.27
Laosuttiwong et al. (2018)
Homogeneous catalyst
Household food blender
96.8% yield at 6:1, 1 wt% NaOH,
60 °C, continuous process21.1
Wongjaikham et al. (2021)
Hand blender
96.6% yield at 6:1, 0.8 wt% NaOH, 60 °C, continuous process
18.0
Kamjam et al. (2021)
Homogenizer
87% yield at 6:1, 1 wt% NaOH,
60 °C, 3 min, batch process169.46
Laosuttiwong et al. (2018)
Hydrodynamic cavitation
98.1% yield at 6:1, 1 wt% NaOH,
60 °C, 15 min, batch process12.5
Chuah et al. (2015)
Mechanical stirrer
98% yield at 6:1, 1 wt% NaOH,
60 °C, 90 min, batch process1.5
Chuah et al. (2015)
To present a clear yield efficiency comparison between the modified fruit blender and a conventional mechanical stirrer, an ad-hoc batch experiment was conducted using a mechanical stirrer under the same experimental condition (except for the stirring speed) as follows: methanol: oil molar ratio of 15:1, 5 wt% of CaO heterogeneous catalyst, reaction volume of 1,000 mL (performed in a 3.5 L beaker), 60 °C, and reaction time of 2 h. A magnetic bar was used with a stirring speed of 600 rpm. At a stirring speed higher than this, the magnetic bar frequently tended to slip. The FAME yield efficiency of the modified fruit blender was evaluated to be 5 times higher than that of a conventional mechanical stirrer. Laosuttiwong and the group (Laosuttiwong et al., 2018) investigated biodiesel production from refined palm oil and CaO employing various intensified reactors: homogenizer, ultrasonic and mechanical stirrer. The FAME yield and yield efficiency of biodiesel produced from a molar ratio of oil: methanol of 1:9, catalyst concentration of 10 wt%, and 60 °C reaction temperature were reported. The homogenizer and 20 kHz ultrasonic took 2 h to reach the yields of over 80%, obtaining the yield efficiency of 8.27 × 10−4 and 4.65 × 10−4 g/J for the homogenizer and ultrasonic, respectively, while the yield efficiency of the mechanical stirring reactor was 3.27 × 10−4 g/J at 80% FAME yield for the reaction time of 4 h. The yield efficiency of the utilized fruit blender was as much as 1.8, 3.2, and 4.5-fold greater than that of the homogenizer, ultrasonic, and mechanical stirrer, respectively.
The yield efficiency of biodiesel production based on homogeneous catalysts was higher. Wongjaikham et al. (2021), Kamjam et al. (2021), Laosuttiwong et al. (2018) and Chuah et al. (2015) performed biodiesel production using NaOH as a catalyst employing the high-power household fruit blender (the same one used in the present work), hand blender, homogenizer, hydrodynamic cavitation, and mechanical stirrer, respectively, and the reported yield efficiency was 21.1, 18.0, 169.46, 12.5, and 1.5 × 10−4 g/J, respectively. Transesterification using homogeneous catalysts requires less energy as the reactants are in two phases, compared to three phases for the heterogeneous catalysts.
The satisfied transesterification condition of this study based on the heterogeneous base catalyst required a higher fraction of methanol and catalyst loading than homogeneous ones as reported in several pieces of literature (Laosuttiwong et al., 2018; Kamjam et al., 2021; Wongjaikham et al., 2021). However, adding a greater methanol fraction can lower the use of the catalyst in the process. This also accelerates transesterification toward the product based on Le Chatelier’s principle (Li et al., 2013), resulting in more biodiesel. The feedstock oil could be fully transformed into biodiesel. Unreacted methanol can be extracted and recycled by evaporation. Although the reaction time is relatively longer than that of homogeneous catalysts based on various intensified reactors, it does not require too much time to purify the product, for example, separation and washing. If the CaO catalyst can be used commercially, simple filtration is a possible way to recycle the catalyst and decrease the cost, as well as reduce environmental impact compared to homogeneous catalysts that generally have to be discarded and neutralized after a single use. The heterogeneous solid base catalyst also exhibits a longer catalyst lifetime and better stability than current homogeneous catalysts. The calculated yield efficiency of the studied system shows an advantage in terms of lower energy requirement, offering an opportunity for economic and environmentally friendly biodiesel production on a community scale.
4 Conclusions
Biodiesel production was developed to mitigate the problem of fossil diesel fuel concerning greenhouse gas emissions and various toxic dust. The current study effectively illustrates the use of the affordable kitchen fruit blender for transesterification of refined palm oil and commercial calcium oxide in a batch regimen. The catalyst was calcined in an open atmosphere at 900 °C to remove moisture and CO2 before use. The highest FAME yield of 97.58% was obtained using the satisfactory parameters of methanol: oil molar ratio of 15:1, CaO catalyst dosage of 5 wt%, reaction temperature of 60 °C, and reaction volume of 1,000 mL. The yield efficiency showed 14.68 × 10−4 g/J being higher than those of other publications. The reusability evaluation of the catalyst demonstrated exceptional activity stability. The properties of synthesized FAMEs complied with EN 14214 and ASTM D6751 standards. The use of the heterogeneous catalyst for biodiesel production not only provides an ecologically beneficial and cost-effective technique but also contributes to the reduction of biodiesel production costs.
Acknowledgments
This research is supported by Ratchadapisek Somphot Fund for Postdoctoral Fellowship and Ratchadaphiseksomphot Endowment Fund [RCU_B_64_03_21], Chulalongkorn University. D. Wongsawaeng, K. Ngaosuwan, W. Kiatkittipong, and S. Assabumrungrat also would like to acknowledge the supports from the Research Chair Grant of National Science and Technology Development Agency (NSTDA) and the NSRF via the Program Management Unit from Human Resources & Institutional Development, Research and Innovation (Grant number B05F640085).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Transesterification of soybean oil with methanol catalyzed by basic solids. Catal. Today. 2008;133–135:548-554.
- [Google Scholar]
- The effects of catalysts in biodiesel production: A review. J. Ind. Eng. Chem.. 2013;19:14-26.
- [CrossRef] [Google Scholar]
- Chapter 9 – Analytical Methods. Fats and Oils Handbook. AOCS Press; 1998. p. :803-808.
- A review of catalyst and multifunctional reactor development for sustainable biodiesel production. ScienceAsia. 2021;47:531.
- [CrossRef] [Google Scholar]
- High-efficiency biodiesel production using rotating tube reactor: New insight of operating parameters on hydrodynamic regime and biodiesel yield. Renew. Sustain. Energy Rev.. 2021;151:111430
- [CrossRef] [Google Scholar]
- Application of heterogeneous catalysts for transesterification of refined palm oil in ultrasound-assisted reactor. Fuel Process. Technol.. 2013;111:22-28.
- [CrossRef] [Google Scholar]
- Intensification of biodiesel synthesis from waste cooking oil (Palm Olein) in a Hydrodynamic Cavitation Reactor: Effect of operating parameters on methyl ester conversion. Chem. Eng. Process.. 2015;95:235-240.
- [CrossRef] [Google Scholar]
- 10 – Mixing and agitation. In: Chemical Process Equipment (Revised Second Edition). Boston: Gulf Professional Publishing:; 2010. p. :273-324.
- [Google Scholar]
- Natural soil and lithium carbonate as economical solid-base catalysts for biodiesel production. Energy Rep.. 2020;6:2743-2750.
- [CrossRef] [Google Scholar]
- Comparison of the performance of different homogeneous alkali catalysts during transesterification of waste and virgin oils and evaluation of biodiesel quality. Fuel. 2008;87:3572-3578.
- [CrossRef] [Google Scholar]
- Fat and Oil Derivates – Fatty Acid Methyl Esters (FAME), Determination of Ester and Linolenic Acid Methyl Ester Contents. Brussels: European Committee for Standardization; 2011.
- Biodiesel production from waste cooking oil using bifunctional heterogeneous solid catalysts. J. Clean. Prod.. 2013;59:131-140.
- [CrossRef] [Google Scholar]
- Characterization of calcium carbonate, calcium oxide, and calcium hydroxide as starting point to the improvement of lime for their use in construction. J. Mater. Civ. Eng.. 2009;21:694-698.
- [CrossRef] [Google Scholar]
- Biodiesel from sunflower oil by using activated calcium oxide. Appl. Catal. B. 2007;73:317-326.
- [CrossRef] [Google Scholar]
- Rapeseed oil methyl esters preparation using heterogeneous catalysts. Bioresour. Technol.. 1999;70:249-253.
- [CrossRef] [Google Scholar]
- Measurements and empirical correlations in predicting biodiesel-diesel blends’ viscosity and density. Fuel. 2017;199:567-577.
- [CrossRef] [Google Scholar]
- Technologies for production of biodiesel focusing on green catalytic techniques: A review. Fuel Process. Technol.. 2009;90:1502-1514.
- [Google Scholar]
- Solid heterogeneous catalysts for transesterification of triglycerides with methanol: A review. Appl. Catal. A. 2009;363:1-10.
- [CrossRef] [Google Scholar]
- James, B., 2015. Mixing 101: Optimal Tank Design. Retrieved 20 March, 2022, from https://dynamixinc.com/optimal-tank-design/.
- Continuous biodiesel production based on hand blender technology for sustainable household utilization. J. Clean. Prod.. 2021;297:126737
- [CrossRef] [Google Scholar]
- Non-isothermal kinetic and thermodynamic studies of the dehydroxylation process of synthetic calcium hydroxide Ca(OH)2. J. Mater. Environ. Sci.. 2014;5(2):615-624.
- [Google Scholar]
- Parametric study on the transesterification reaction by using CaO/Silica catalyst. Chem. Eng. Trans.. 2017;56:601-606.
- [Google Scholar]
- Performance comparison of different cavitation reactors for biodiesel production via transesterification of palm oil. J. Clean. Prod.. 2018;205:1094-1101.
- [CrossRef] [Google Scholar]
- A stirring packed-bed reactor to enhance the esterification–transesterification in biodiesel production by lowering mass-transfer resistance. Chem. Eng. J.. 2013;234:9-15.
- [CrossRef] [Google Scholar]
- Transesterification of soybean oil to biodiesel using CaO as a solid base catalyst. Fuel. 2008;87:216-221.
- [CrossRef] [Google Scholar]
- Intensified synthesis of biodiesel using hydrodynamic cavitation reactors based on the interesterification of waste cooking oil. Fuel. 2014;137:285-292.
- [Google Scholar]
- Sustainable biodiesel production via transesterification of waste cooking oil by using CaO catalysts prepared from chicken manure. Energ. Conver. Manage.. 2016;123:487-497.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of calcium methoxide as heterogeneous catalyst for trimethylolpropane esters conversion reaction. Appl. Catal. A. 2012;425–426:184-190.
- [CrossRef] [Google Scholar]
- McFeeters, M., 2017. Vessel geometry considerations for bottom-mount magnetically driven agitators in the biopharmaceutical industry.
- Development of composite PCMs by incorporation of paraffin into various building materials. Materials (Basel). 2015;8:499-518.
- [CrossRef] [Google Scholar]
- Biodiesel production through the transesterification of waste cooking oil over typical heterogeneous base or acid catalysts. Journal. 2023;13
- [CrossRef] [Google Scholar]
- Ultrasonic-assisted biodiesel production process from palm oil using alkaline earth metal oxides as the heterogeneous catalysts. Fuel. 2010;89:1818-1825.
- [CrossRef] [Google Scholar]
- Enhancement of biodiesel synthesis over highly active CaO derived from natural white bivalve clam shell. Arab. J. Chem.. 2016;9:633-639.
- [CrossRef] [Google Scholar]
- Biodiesel production from waste cooking oil over alkaline modified zirconia catalyst. Fuel Process. Technol.. 2011;92:2397-2405.
- [CrossRef] [Google Scholar]
- Estimation of chemical and physical effects of cavitation by analysis of cavitating single bubble dynamics. Ultrason. Sonochem.. 2021;77:105677
- [CrossRef] [Google Scholar]
- Role of ultrasonic irradiation on transesterification of palm oil using calcium oxide as a solid base catalyst. Energ. Conver. Manage.. 2016;120:62-70.
- [CrossRef] [Google Scholar]
- Use of calcium oxide in palm oil methyl ester production. Songklanakarin J. Sci. Technol.. 2014;36:195-200.
- [Google Scholar]
- Process intensification technologies in continuous biodiesel production. Chem. Eng. Process.. 2010;49:323-330.
- [Google Scholar]
- Optimization of biodiesel production using waste mussel shell catalyst. Fuel. 2013;109:534-541.
- [CrossRef] [Google Scholar]
- State of the art of biodiesel production processes: a review of the heterogeneous catalyst. RSC Adv.. 2015;5:101023-101044.
- [CrossRef] [Google Scholar]
- An entirely renewable biofuel production from used palm oil with supercritical ethanol at low molar ratio. Braz. J. Chem. Eng.. 2017;34:1023-1034.
- [Google Scholar]
- Optimization of ultrasonic-assisted heterogeneous biodiesel production from palm oil: A response surface methodology approach. Fuel Process. Technol.. 2010;91:441-448.
- [CrossRef] [Google Scholar]
- Biodiesel production using heterogeneous catalysts. Bioresour. Technol.. 2011;102:2151-2161.
- [CrossRef] [Google Scholar]
- Effects of mixing technologies on continuous methyl ester production: Comparison of using plug flow, static mixer, and ultrasound clamp. Energ. Conver. Manage.. 2017;140:91-97.
- [Google Scholar]
- Performance of CaO from different sources as a catalyst precursor in soybean oil transesterification: Kinetics and leaching evaluation. J. Environ. Chem. Eng.. 2016;4:1970-1977.
- [CrossRef] [Google Scholar]
- Reactor technologies for biodiesel production and processing: A review. Prog. Energy Combust. Sci.. 2019;74:239-303.
- [CrossRef] [Google Scholar]
- The study of CaO and MgO heterogenic nano-catalyst coupling on transesterification reaction efficacy in the production of biodiesel from recycled cooking oil. J. Environ. Health Sci. Eng.. 2015;13:73.
- [CrossRef] [Google Scholar]
- Microwave assisted synthesis of biodiesel from soybean oil: Effect of poly (lactic acid)-oligomer on cold flow properties, IC engine performance and emission characteristics. Fuel. 2016;170:107-114.
- [CrossRef] [Google Scholar]
- Low-cost alternative biodiesel production apparatus based on household food blender for continuous biodiesel production for small communities. Sci. Rep.. 2021;11:13827.
- [CrossRef] [Google Scholar]
- Characterization of calcium oxide derived from cockle shells for carbon dioxide capture. SNRU J. Sci. Technol.. 2018;10:32-36.
- [Google Scholar]
- Supported CaO catalysts used in the transesterification of rapeseed oil for the purpose of biodiesel production. Energy Fuel. 2008;22:646-651.
- [CrossRef] [Google Scholar]
- Synthesis of biodiesel from rapeseed oil using supercritical methanol with metal oxide catalysts. Bioresour. Technol.. 2010;101:8686-8689.
- [CrossRef] [Google Scholar]
- Modification of calcite by hydration–dehydration method for heterogeneous biodiesel production process: The effects of water on properties and activity. Chem. Eng. J.. 2010;162:135-141.
- [CrossRef] [Google Scholar]
- Solid base-catalysed transesterification of sunflower oil. An essential oxidation state/composition of cao-based catalyst and optimisation of selected process parameters. Oxid. Commun.. 2015;38:183-200.
- [Google Scholar]
- Solubility of multicomponent systems in the biodiesel production by transesterification of Jatropha curcas L. Oil with methanol. J. Chem. Eng. Data. 2006;51:1130-1135.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary material to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105273.
Appendix A
Supplementary material
The following are the Supplementary material to this article:Supplementary data 1
Supplementary data 1







