Translate this page into:
Hibiscus sabdariffa synthesized gold nanoparticles ameliorate aluminum chloride induced memory deficits through inhibition of COX-2/BACE-1 mRNA expression in rats
⁎Corresponding author. anadozieso@abuad.edu.ng (Scholastica O. Anadozie)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Alzheimer’s disease (AD) is a major health challenge worldwide, especially among the elderly. The disease is associated with cognitive and memory deficits. This study investigated the effect of Hibiscus sabdariffa synthesized-gold nanoparticles (HS-AuNPs) on AlCl3-induced memory deficits in rats. Forty-two male Wistar rats were divided into six groups (n = 7). Group I served as control. Rats in group II - V were exposed to AlCl3 (100 mg/kg) to induce AD. Group III - V rats were treated with 5 mg/kg donepezil, 5 and 10 mg/kg HS-AuNPs, respectively, for 14 days. Behavioral tests were carried out on the rats on day 28 and 42. At the end of animal experiment, rats were sacrificed and used for various biochemical assays and gene expression. The AD rats showed memory and learning impairment, and these conditions were ameliorated by HS-AuNPs. Significant (p < 0.05) elevation in the activities of acetylcholinesterase, monoamine oxidase and adenosine deaminase, as well as malondialdehyde levels was noted. A significant reduction in the activities of superoxide dismutase (SOD), glutathione peroxidase (GPx) and reduced glutathione (GSH) noted in AlCl3-induced rats were ameliorated by the 5 and 10 mg/kg b.w. doses of HS-AuNPs. In addition, the increased mRNA expression of cyclooxygenase-2 (COX-2) and beta-secretase 1 (BACE-1) caused by AlCl3 were assuaged by the HS-AuNPs treatment. Based on the activities of HS-AuNPs against AlCl3-induced AD, HS-AuNPs could be considered a potential therapeutic agent for managing AD.
Keywords
Aluminum chloride
Alzheimer’s disease
Cyclooxygenase-2
Hibiscus sabdariffa
Gold nanoparticle
1 Introduction
Alzheimer’s disease (AD) is a neurodegenerative disease characterized by the deterioration of the nerve cells and neural connections in the brain's cerebral cortex. Individuals with this disease, usually the older adults are seen with abnormalities in the brain tissues and impairment of cognitive functions (DeTure and Dickson 2019). Pathological features of AD include the formation of amyloid beta (Aβ) plaques and neurofibrillary tangles (DeTure and Dickson 2019). Accumulation of Aβ plaques lead to neurodegeneration which interferes with the synaptic communication between neuron-to-neuron. Tau tangles, on the other hand, block the transport of nutrients and other essential molecules within the neurons (Mietelska-Porowska et al., 2014). Although the pathogenesis of AD is not fully known, Aβ aggregates has been associated with oxidative stress (OS) (Cheignon et al., 2018).
Aluminum is a metallic element primarily found in the air, food, drinking water, pharmaceutical and agrochemical products. Over time, its accumulation in the human body either naturally or occupationally, can lead to organ toxicity (Igbokwe et al., 2019). Aluminum chloride (AlCl3) can cross the blood–brain barrier (BBB), altering the axonal transports and synaptic clefts, thus, making the brain a major target organ for aluminum toxicity (Liaquat et al., 2019). Exposure of experimental animals to AlCl3 could result in behavioral and cognitive deficits, cholinergic neuronal loss, amyloid plaques and neurofibrillary tangles formation, which are pathological features of AD (ELBini-Dhouib et al., 2021). Therefore, AlCl3 was used in the present study to mimic sporadic AD-like disorder.
Alzheimer’s disease poses a great threat to human health and remains incurable till date. Because of the nature of the brain, which is surround by BBB, drug delivery to affected areas in the brain remain difficult and therefore, limits the effective treatment of AD (Dong 2018). The use of drugs such as acetylcholinesterase inhibitors and N-methyl-d-aspartate receptor antagonists have been reported to be less effective. This is because they do not explicitly cure or slow the progression of the disease but only moderately improve cognitive functions and manage the associated symptoms (Breijyeh and Karaman 2020). In addition, these drugs lack tissue-specificity, resulting in adverse effects such as cardiovascular and genitourinary diseases (Ruangritchankul et al., 2021). Therefore, it is necessary to use treatment approach with fewer side effects in managing AD. Medicinal plant-based products are a better option because of their biological activities, availability, fewer side effects, and cost-effectiveness (Roy 2018).
Hibiscus sabdariffa (H. sabdariffa (HS)) commonly called “Roselle” belongs to the Malvaceae family of plant. It is widely grown in tropical and subtropical regions of West Africa, India and Egypt (Riaz and Chopra 2018). The dry red calyces of the plant are used in the preparation of hibiscus tea locally called “zobo” in Nigeria and sorel tea in some parts of Asia. The plant is traditionally used for managing hypertension, diabetes, and obesity (Jalalyazdi et al., 2019, Yusni and Meutia 2020). Its ethnopharmacological properties include anti-inflammatory, anti-cancer, anti-microbial and antioxidant activities (Riaz and Chopra 2018, Izquierdo-Vega et al., 2020). Most medicinal plants lack systemic stability and availability hence, their therapeutic potency is greatly limited (Conte et al., 2016).
Metallic nanoparticles (NPs) have wide range of application in food and cosmetics industries, biomedical and pharmaceuticals (Khan et al., 2019). Gold nanoparticles (AuNPs) among other metallic NPs are efficient in biomedical field because of their size, stability, biocompatibility, large surface area and optical properties (Patra et al., 2018). Unlike the chemical and physical methods of NPs synthesis, a biological approach is ecofriendly, safe, and can be scaled up for larger NPs production (Anadozie et al., 2021). In addition, the plant-based biological synthesis possesses phytochemicals that serve therapeutic purposes and therefore was employed in the present study. Nanoparticles synthesized from H. sabdariffa have been reportedly used in several studies for the management of cancer (Zangeneh and Zangeneh 2020) and diabetes (Bala et al., 2015); however, none has been reported for AD. To the best of our knowledge, our study will be the first to report the synthesis of AuNPs using aqueous extract of H. sabdariffa calyces and its potential in managing AD-like disorder. The present study aimed to investigate the ameliorative potential of H. sabdariffa synthesized-AuNPs against AlCl3-induced memory deficits through the inhibition of COX-2/BACE-1 mRNA expression in rats.
2 Materials and methods
2.1 Chemicals and reagents
Tetrachloroauric acid was procured from Sigma Chemical Co. (St. Louis, MO, USA). Aluminum chloride was procured from Alfa Aersa Belgore, (Germany). Donepezil hydrochloride (Dnpz) was procured from Milpharm labs limited, (United Kingdom). Gene specific primers (cyclooxygense-2 (COX-2), beta-secretase 1 or beta-site amyloid precursor protein cleaving enzyme 1 (BACE-1) and brain-derived neurotrophic factor (BDNF)) for PCR were purchased from Inqaba Biotech, South Africa.
2.2 Plant authentication and extraction
The H. sabdariffa calyces were obtained from a personal garden in Ado Ekiti, Ekiti State, Nigeria. The plant was identified and authenticated at the Forestry Research Institute of Nigeria (FRIN) Ibadan, Nigeria with a reference number 112433. The air-dried HS calyces were blended into fine particles. To a beaker containing 100 mL of distilled water, 10 g of the powder plant material was added, and boiled in a water bath at 80 °C to obtain crude extract, as it is being done traditionally. The crude extract was then filtered and used for the synthesis of AuNPs (Adewale et al., 2020).
2.3 Synthesis and characterization of H. Sabdariffa synthesized gold nanoparticles (HS-AuNPs)
The AuNPs was successfully synthesized using crude extract of H. sabdariffa according to the method described by Anadozie et al., (2022) with slight modification. Briefly, 10 mg/mL of the plant extract was added to 1 mM gold (Au) solution (optimized concentrations). The mixture was continuously stirred at a temperature of 100 °C until a purple color was observed within 10 min. The NPs solution was stirred for additional 20 min without heat, and the formed HS-AuNPs washed twice at a centrifugal speed of 10,000 g for 15 min at 4 °C to remove the uncapped phytochemicals. The AuNPs solution was lyophilized to powder form. The surface plasmon resonance (SPR) of the HS-AuNPs was measured by ultraviolet–visible (UV–vis) spectrophotometer in the range of 350–750 nm. Further characterization of the HS-AuNPs was performed for particle size distribution, stability, surface charge, morphology, crystallinity, and functional groups present on the surface of the AuNPs using dynamic light scattering (DLS), high-resolution transmission electron microscopy (HRTEM) and Fourier transform-infrared spectroscopy (FT-IR).
2.4 Experimental animals and design
Forty-two (42) male Wistar rats, weighing between 120 and 150 g were used in the present study. The animals were procured from the animal house, Department of Veterinary Medicine, University of Ibadan, Oyo State, Nigeria. The animals were kept in polypropylene cages and maintained at room temperature (25 ± 2 °C) and relative humidity (55 ± 10 %) with access to a 12-h light/dark cycle. The rats were fed with commercially available rat chow and water ad libitum. The animal experiment was performed following the International and National guidelines for the use of experimental animals.
2.5 Experimental design
After the acclimatization period, animals were randomly divided into six groups comprising of seven animals each. Group I served as control. Rats in group II served as AD control and were orally given 100 mg/kg b.w. AlCl3. Group III rats served as positive control and orally received 100 mg/kg b.w. AlCl3 and treated with 5 mg/kg b.w. Dnpz. Group IV and V orally received 100 mg/kg b.w. AlCl3 and treated with 5 and 10 mg/kg b.w. HS-AuNPs, respectively. Group VI rats orally received 10 mg/kg b.w. HS-AuNPs only. The animal experiment lasted for 42 days where rats in group II – V were exposed to AlCl3. At day 28 and 42 behavioral studies were performed to confirm the induction of AD following the methods described by Shunan et al., (2021) and Bazzari et al., (2019). The predisposition of the experimental rats to AlCl3 (day 1 to 28) exposed the animals to an early signs of sporadic AD. Co-treatment of AlCl3 with Dnpz or HS-AuNPs was done from day 28 to day 42 (Fig. 1). The HS-AuNPs (5 and 10 mg/kg) doses were selected following the preliminary toxicological evaluation performed in our laboratory (data not shown). Treatments (Dnpz and HS-AuNPs were administered to the rats 1 h after AlCl3 intoxication.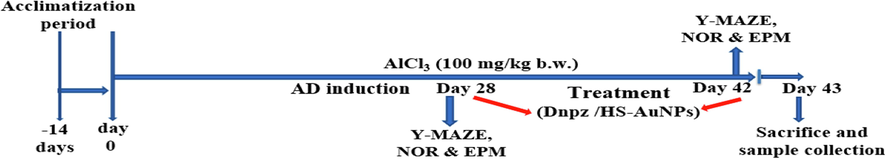
Illustration of the experimental design timeline. AlCl3 - aluminum chloride; AD - Alzheimer’s disease; Dnpz - donepezil; HS-AuNPs - Hibiscus sabdariffa-synthesized gold nanoparticles, NOR - novel object recognition, EPM - elevated plus maze.
2.6 Behavioral test
Behavioral assessment was carried out on the test animals on day 28 and 42 to confirm if AlCl3 initiated memory deficit (one of the symptoms of AD) using the novel object recognition (NOR), Y-maze and elevated plus maze (EPM) tests. The NOR test was performed according to the method described by Antunes and Biala (2012) to assess the learning and recognition memory in AlCl3-induced AD rats. The Y-maze test was performed to examine the functional and cognitive memory of the AD rats following the method of Anadozie et al., (2019). The EPM test was performed following the method of Pellow et al., (1985) to examine test animals’ anxiety levels, memory, and spatial learning. A video tracking device was setup to monitor entries made by each rat in the different neurobehavioral analyses studied. The apparatus was wiped with 70 % ethanol after each test. Time spent by the rats exploring the familiar and novel objects, as well as the discrimination and recognition index were calculated in the NOR test. The number of arm entries and animals’ predisposition toward navigating a less recently visited arm was examined in Y-maze. In EPM, the number of entries into open arms, % open arm entries and % total time spent in open arms were analyzed.
2.7 Preparation of brain tissue homogenate
Twenty-four (24) h after HS-AuNPs treatments, the fasted rats were sacrificed via anesthesia (isoflurane inhalation). The brains were rapidly isolated, mopped of blood stains, and weighed. A little portion of the hippocampus was cut out for mRNA gene expression. The remaining part of the hippocampus and cerebral cortex of the brain were rinsed in cold phosphate buffer (0.1 M, pH 7.4) and subsequently homogenized in four volumes of the phosphate buffer. The tissue homogenate was centrifuged at 10,000 g for 10 min at 4 °C to obtain a clear supernatant for biochemical analyses.
2.8 Measurement of neurological and oxidative biomarkers in the tissue homogenate
Acetylcholinesterase (AChE) and monoamine oxidase (MAO) activities were estimated according to the methods described by Ellman et al., (1961) and Green and Haughton (1961). Adenosine deaminase activity (ADA) was assessed using the method described by Giusti (1974). Lipid peroxidation was performed to determine the level of malondialdehyde (MDA) adducts in the tissue homogenate as described by Ohkawa et al., (1979). Antioxidant activities such as superoxide dismutase (SOD), glutathione peroxidase (GPx) and reduced glutathione (GSH) were evaluated in the tissue homogenate by the methods of Alía et al., (2003), Wendel (1981) and Ellman (1959) respectively.
2.9 Quantitative reverse transcription-polymerase chain reactions (RT-RCR) analysis
2.9.1 Isolation of total RNA
Isolation of total RNA from the hippocampus brain region of the rats was performed using Trizol reagent (Gibco). The RNA quantity (concentration (μg/ml) = 40 × A260) and quality (≥1.8) were measured spectrophotometrically using the ratio A260/A280 (A = absorbance).
2.9.2 Conversion of cDNA
The conversion of DNA-free RNA to cDNA was performed by incubating 40 μg of the total RNA at 37 °C for 1 h using M−MuLV Reverse Transcriptase Kit (NEB) as instructed on the leaflet by the manufacturer. The Snap gene software was used in the present study for primers design.
2.9.3 Polymerase chain reaction amplification
Amplicons were resolved on 1.5 % agarose gel in Tris (RGT reagent)-Borate-EDTA buffer (pH 8.4) and ethidium bromide staining was used for visualization. Quantification of the amplicon bands was done using Image-J software. The following primers of interest were used in this study:
Target
Forward ‘5–3
Reverse ‘5-3′
Base pair
genes
COX-2
TCAGCCATGCAGCAAATCC
GATTCTTGTCAGAAACTCAGGCG
70
BACE-1
CCCCCACAGACGCTCAACAT
GGTGTAGGGCACATACACAGAC
56
BDNF
TTCCCTCAGCTCGCCAC
TCTCACCTGGTGGAACTCAG
28
Β-Actin
CCACCAGTTCGCCATGGAT
CCCACCATCACACCCTGG
42
2.10 Data analysis
Data analysis was performed where applicable using one-way analysis of variance (ANOVA) on GraphPad Prism 8.01, 2018 (GraphPad software, Inc, San Diego, USA), followed by Tukey’s post hoc test. Results were represented as mean ± standard deviation (SD), n = 7 of triplicate readings. Statistical significance at p < 0.05 was considered in all cases.
3 Results
3.1 Preliminary phytochemical screening of H. sabdariffa calyx
The qualitative screening of bioactive metabolites in the crude extract of H. sabdariffa calyces are revealed in Table 1. + signifies the presence of bioactive metabolite.
Phytochemicals
Observation
Alkaloids
+
Flavonoids
+
Glycosides
+
Phenols
+
Saponins
+
Tannins
+
Terpenoids
+
3.2 Gold NPs synthesis
The formation of HS-AuNPs was visually observed for a change in color of the reaction mixture from wine red to purple within 10 min of adding the HS extract to the Au solution.
3.3 Gold NPs characterization
3.3.1 Stability testing of H. sabdariffa synthesized-AuNPs
The SPR and stability testing of the HS-AuNPs was spectrophotometrically measured using UV–vis absorption spectroscopy. The HS-AuNPs maintained a stable absorption maximum (λmax) of 534 nm from the first 1 h to 48 h (Fig. 2, supplementary 1). In addition, no sign of agglomeration was observed in the synthesized HS-AuNPs.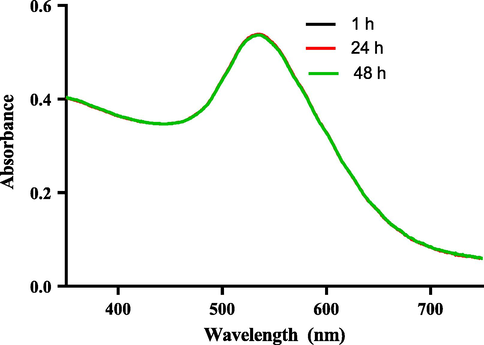
UV–vis absorption spectrum of H. sabdariffa synthesized-AuNPs showing SPR band at different time interval.
3.3.2 Hydrodynamic size distribution, morphology, and crystallinity of H. sabdariffa synthesized-AuNPs
The DLS measurement revealed an average hydrodynamic diameter of 23.82 ± 0.22 nm and polydispersity index (PDI) of 0.378 ± 0.02 nm for the synthesized HS-AuNPs in liquid medium. In addition, the zeta potential measurement of the HS-AuNPs showed a surface charge of −19.1 ± 0.96 mV. The HRTEM microscopic image revealed mostly spherical-shaped AuNP cores (Fig. 3 A – B). As analyzed by image J, an average size of 12.99 ± 4.04 nm was obtained for the synthesized AuNPs (Fig. 3 C). The SAED image showed the crystalline nature of the HS-AuNPs with a ring pattern corresponding to the Bragg’s reflections ring (1 1 1), (2 0 0), (2 2 0), and (3 1 1) of face centered cubic (fcc) structure of metallic Au, JCPDS no. 04–0784 (Fig. 3 D).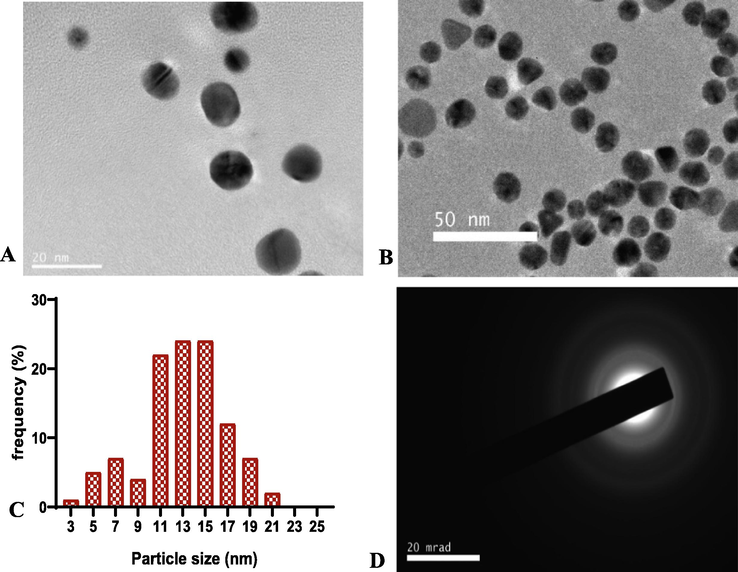
HRTEM micrographs and histogram images of H. sabdariffa synthesized-AuNPs at 20 nm scale bar (A), 50 nm scale bar (B), histogram of particle size distribution (C), SAED image (D).
3.3.3 Functional groups present on the surface of H. sabdariffa plant extract and its synthesized-AuNPs
Table 2 depicts the FTIR absorption values and functional groups present on the surface of HS extract and its synthesized-AuNPs. Two characteristics peaks different from other absorption peaks were observed in both the plant extract and its synthesized-AuNPs (Table 2, supplementary 2). These include the intense peaks at 1633.93 and 1632.70 cm−1 and broad peaks at 3333.13 and 3252.65 cm−1, respectively. HS - Hibiscus sabdariffa, HS-AuNPs - Hibiscus sabdariffa synthesized gold nanoparticles.
HS extract wavelength (cm-1)
HS-AuNPs wavelength (cm-1)
Bonds
Functional groups
3333.13
3252.65
O—H stretch
N—H stretchAlcohols, phenols
Aliphatic secondary
Amines
2106.83
2036.72
C≡C stretching
Alkyne
1633.93
1632.70
C⚌C stretch
Alkene
–
1416.40
O—H bending
Alcohol
1157.26
1082.71
C—O stretch
Aromatic alcohols
1022.68
979.46
=C—O—C stretch
Ethers
3.4 Assessment of behavioral, cognitive, and hippocampal functions of AlCl3-induced AD rats
3.4.1 Novel object recognition (NOR) behavioral test
Fig. 4 shows the effect of HS-AuNPs in enhancing the hippocampal function and recognition memory in AlCl3-induced AD rats using the NOR test. The rats in AlCl3 group performed poorly on day 28 and 42 by significantly (p < 0.05) decreasing the time spent in both familiar and novel object when compared to the control (Fig. 4 (A-B)). The treatments (Dnpz and HS-AuNPs) on day 42 restored the impaired cognitive functions. Similarly, in the novel object preference (% (Fig. 4 C)), the ability of rats in the AlCl3 (AD rats) group to recognize the novel object on days 28 and 42 was significantly (p < 0.05) reduced when compared to the control group. Treatment of the AD rats with HS-AuNPs at 10 mg/kg b.w. significantly (p < 0.05) increased the novel object preference on day 42 when compared to the control, AlCl3, and Dnpz groups, respectively.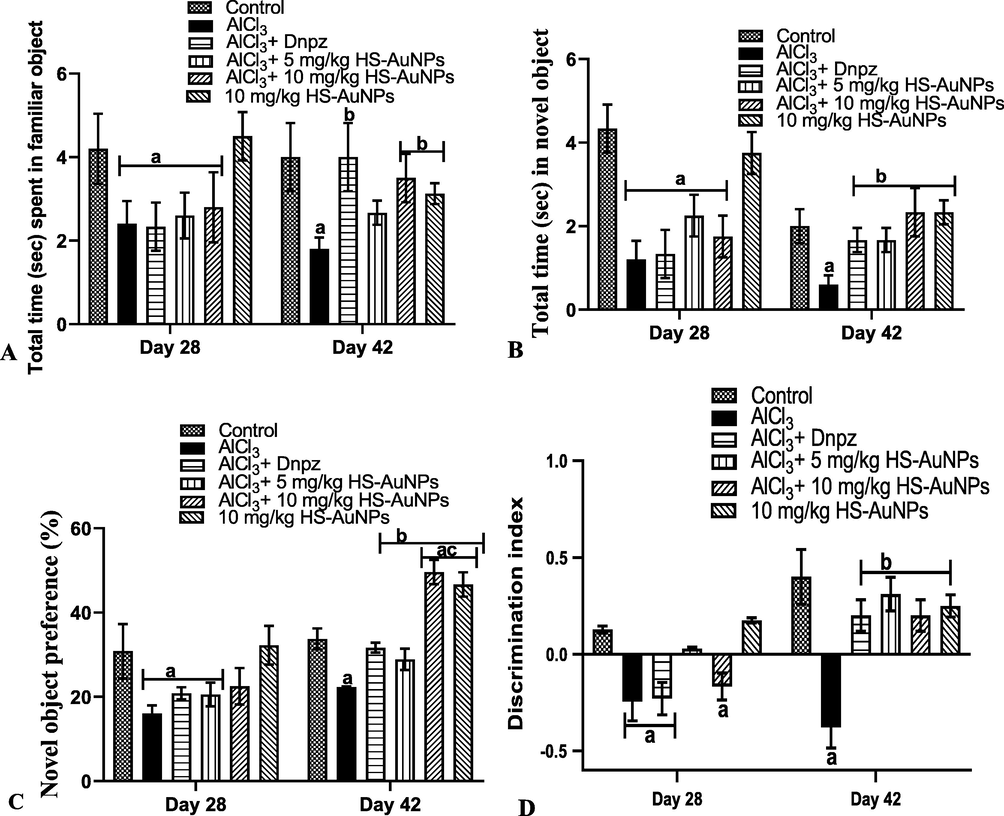
Effect of H. sabdariffa synthesized-AuNPs on the cognitive function test of AlCl3-induced AD rats. Familiar object (A), novel object (B), % novel object preference (C), discrimination index (D). Results are expressed as mean ± SD (n = 7). ap < 0.05 vs control, bp < 0.05 vs AlCl3 group, cp < 0.05 vs Dnpz group.Legend: AlCl3 - aluminum chloride; Dnpz – donepezil; HS-AuNPs- Hibiscus sabdariffa synthesized-gold nanoparticles.
Aluminum-exposed rats performed very poorly in the discrimination index analysis on days 28 and 42 as shown in Fig. 4 D. Statistically, in comparison with the control, the AD rats could not distinguish between familiar and novel objects. Treatment with tested doses of Dnpz and HS-AuNPs on day 42 significantly (p < 0.05) increased the familiar and novel object when compared with the AlCl3 group. The treatment doses of HS-AuNPs exhibited similar result as that of Dnpz group.
3.4.2 Y-maze behavioral test (memory index)
Table 3 shows a significant (p < 0.05) reduction in the % spontaneous alternation in the AlCl3-induced rats on days 28 and 42 when compared to the control. A similar result was noted in Dnpz and 5 mg/kg b.w. HS-AuNPs treated groups on day 42. The spatial working memory of the AD rats were however, restored by HS-AuNPs treated groups at 10 mg/kg b.w. on day 42 by a significant (p < 0.05) increase in the % spontaneous alternation of rats when compared with the AlCl3 and Dnpz groups, respectively. Results are expressed as mean ± SD (n = 7). ap < 0.05 vs control, bp < 0.05 vs AlCl3 group, cp < 0.05 vs Dnpz group. Legend: AlCl3 - aluminum chloride; Dnpz – donepezil; HS-AuNPs- Hibiscus sabdariffa synthesized-gold nanoparticles.
Groups
Spontaneous alternation (%)
Number of arm entries
28 days
42 days
28 days
42 days
Control
93.3 ± 10.00
90.0 ± 11.54
5.25 ± 1.50
5.25 ± 1.50
AlCl3
49. 0 ± 7.41a
54.6 ± 6.33a
5.60 ± 1.34
5.25 ± 1.26
AlCl3 + Dnpz
39.2 ± 3.29a
47.5 ± 6.45a
4.00 ± 2.00
3.75 ± 0.96
AlCl3 + 5 mg/kg HS-AuNPs
39.5 ± 4.78a
50.0 ± 8.16a
4.67 ± 0.57
4.25 ± 0.96
AlCl3 + 10 mg/kg HS-AuNPs
53.2 ± 9.58a
77.5 ± 6.45bc
5.66 ± 0.50
5.50 ± 1.29
10 mg/kg HS-AuNPs
88.3 ± 9.13bc
85.0 ± 12.91bc
6.75 ± 1.52c
6.50 ± 1.29c
In addition, comparing the AlCl3-exposed rats and the treated groups to the control on days 28 and 42, no significant (p > 0.05) difference was observed in the number of arm entries (locomotor activity), except for the rats treated with 10 mg/kg b.w. HS-AuNPs only where a significant (p < 0.05) increase was observed in the number of arm entries on day 42 when compared with the Dnpz group.
3.4.3 Elevated plus maze (EPM) behavioral test
In the EPM test, a significant (p < 0.05) decrease in the total arm entries was observed in groups exposed to AlCl3 on day 28 when compared with the control group. However, on day 42 no significant (p > 0.05) difference was observed in the AlCl3 group when compared to the control, Dnpz and HS-AuNPs treated groups, respectively. Although not significant, an increase in the total arm entries was observed in the 10 mg/kg b.w. HS-AuNPs when compared to AlCl3 (Fig. 5 A).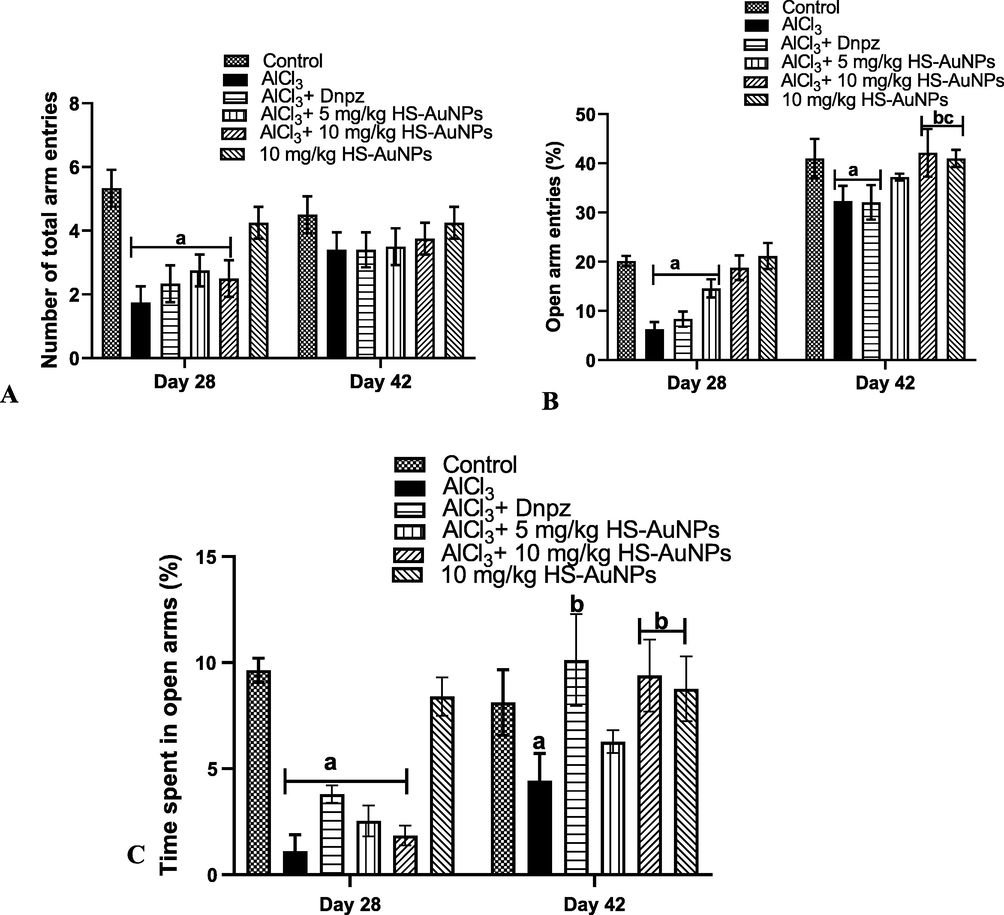
Effect of H. sabdariffa synthesized-AuNPs on the cognitive function test of AlCl3-induced AD rats. Number of total arm entries (A), % open arm entries (B), % time spent on open arms (C) in elevated plus maze test. Results are expressed as mean ± SD (n = 7). ap < 0.05 vs control, bp < 0.05 vs AlCl3 group, cp < 0.05 vs Dnpz group. Legend: AlCl3 - aluminum chloride; Dnpz – donepezil; HS-AuNPs- Hibiscus sabdariffa synthesized-gold nanoparticles.
Similarly, a significant (p < 0.05) decrease in the % open arm entries was observed in the AlCl3 group and Dnpz treated group on days 28 and 42 when compared with the control. Treatment with HS-AuNPs at 10 mg/kg b.w., restored the anxiolytic memory impairment caused by AlCl3 administration with a marked significant (p < 0.05) increase in the % open arm entries on day 42 when compared with the AlCl3 and Dnpz groups, respectively (Fig. 5 B). Also, the administration of AlCl3 in rats caused a significant (p < 0.05) reduction in the total time spent in the open arm (%) at days 28 and 42 when compared with the control (Fig. 5 C). A significant (p < 0.05) increase in the total time spent in open arm (%) was observed in Dnpz and 10 mg/kg b.w. HS-AuNPs at day 42 when compared with the AlCl3 group.
3.5 Relative organ weight of rats AlCl3-induced AD rats
Oral administration of AlCl3 to rats caused a significant (p < 0.05) reduction in the body weight gain of rats when compared with the control. In comparing the various doses of Dnpz and HS-AuNPs to the AlCl3 group, a significant increase was observed (Fig. 6 A).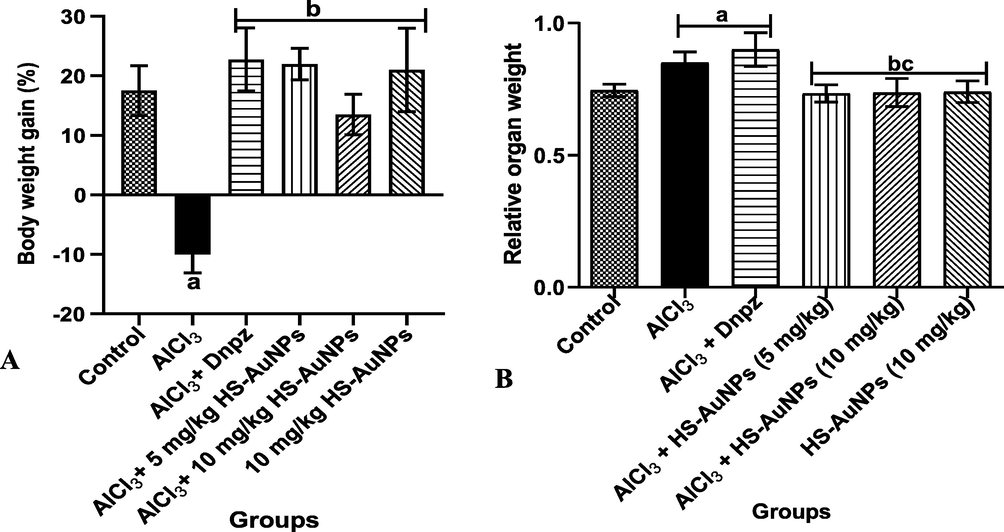
Effect of H. sabdariffa synthesized-AuNPs on the body weight gain (A) and relative brain weight (B) of AlCl3-induced AD rats. Results are expressed as mean ± SD (n = 7). ap < 0.05 vs control, bp < 0.05 vs AlCl3 group, cp < 0.05 vs Dnpz group. Legend: AlCl3 - aluminum chloride; Dnpz – donepezil; HS-AuNPs- Hibiscus sabdariffa synthesized-gold nanoparticles.
In addition, a significant increase in the relative brain weight of rats was observed in the AlCl3 and Dnpz groups when compared with the control (Fig. 6 B). The HS-AuNPs treatment at tested doses were able to ameliorate the effect of AlCl3 on the rat’s brain.
3.6 Biochemical assays
3.6.1 Neuronal acetylcholine inhibitors and neuromodulator indices
The effect of HS-AuNPs on AChE, MAO and ADA activities in AlCl3-intoxicated rats are shown in Fig. 7 (A - C). Oral administration of AlCl3 significantly (p < 0.05) elevated the AChE and MOA activities in AD rats compared with the control (Fig. 7 A and B). Treatment with Dnpz and HS-AuNPs at tested doses were able to attenuate the effect of AlCl3 in the AD rats by significantly (p < 0.05) decreasing the acetylcholine inhibitors activities when compared with the AlCl3 group. A significant (p < 0.05) decrease in the MAO activity was also observed in the AD rats treated with HS-AuNPs at a dose of 10 mg/kg b.w. when compared with the Dnpz group (Fig. 7 B).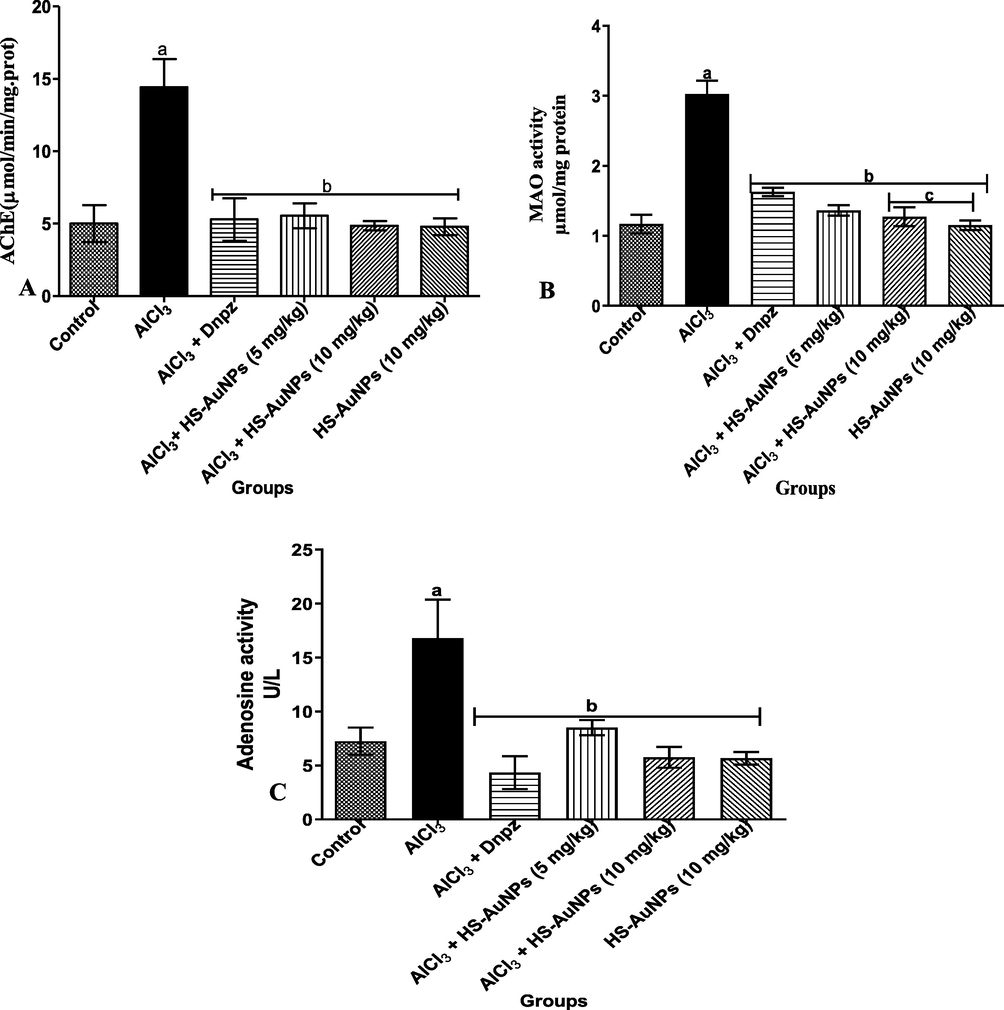
Effect of H. sabdariffa synthesized-AuNPs on acetylcholinesterase (A), monoamine oxidase (B), adenosine deaminase (C) activities in the brain of AlCl3-induced AD rats. Results are expressed as mean ± SD (n = 7). ap < 0.05 vs control, bp < 0.05 vs AlCl3 group, cp < 0.05 vs Dnpz group. Legend: AlCl3 - aluminum chloride; Dnpz – donepezil; HS-AuNPs- Hibiscus sabdariffa synthesized-gold nanoparticles.
As revealed in Fig. 7 C, a significant (p < 0.05) increase in the activity of ADA was observed in the AlCl3 group when compared with the control. Treatment with Dnpz and HS-AuNPs at tested doses significantly (p < 0.05) decreased the activity of ADA when compared with the AlCl3 group.
3.7 Oxidative and antioxidant biomarkers
As displayed in Table 4, oral administration of AlCl3 significantly (p < 0.05) increased the MDA levels in AD rats when compared with the control. Treatment with Dnpz and HS-AuNPs significantly (p < 0.05) reduced the MDA levels when compared to the AlCl3 group. Results are expressed as mean ± SD (n = 7). ap < 0.05 vs control, bp < 0.05 vs AlCl3 group, cp < 0.05 vs Dnpz group. Legend: AlCl3 - aluminum chloride; Dnpz – donepezil; GPx - glutathione peroxidase; GSH - reduced glutathione; HS-AuNPs- Hibiscus sabdariffa synthesized-gold nanoparticles; MDA – malondialdehyde; superoxide dismutase (SOD).
Treatment group
MDA (nm/ mg prot)
SOD (U/mg prot)
GPx(U/mg prot)
GSH(U/mg prot)
Control
0.4 ± 0.03
110.7 ± 3.83
115.4 ± 8.29
67.0 ± 3.48
AlCl3 alone
0.9 ± 0.09a
68.8 ± 6.32 a
63.2 ± 2.92a
44.5 ± 1.76a
AlCl3 + Dnpz
0.4 ± 0. 09b
107.9 ± 1.64b
98.1 ± 4.59ab
65.6 ± 3.01b
AlCl3 + 5 mg/kg HS-AuNPs
0.5 ± 0.08b
106.2 ± 6.23b
94.9 ± 6.31a
58.6 ± 2.37ab
AlCl3 + 10 mg/kg HS-AuNPs
0.4 ± 0.03b
104.4 ± 6.11b
107.9 ± 6.11b
61.2 ± 4.70b
10 mg/kg HS-AuNPs
0.4 ± 0.05b
106.6 ± 2.66b
119.0 ± 2.72bc
62.0 ± 1.57b
Furthermore, the administration of AlCl3 significantly (p < 0.05) reduced the neuronal SOD and GPx activities in the AlCl3 (AD) group when compared with the control. In the GPx activities, Dnpz and 5 mg/kg b.w. HS-AuNPs treated groups showed similar results as the AlCl3 group when compared with the control. Treatment with HS-AuNPs at tested doses mitigate the radical effect of AlCl3 in the rats by a significant (p < 0.05) increase in the SOD and GPx activities (Table 3).
In addition, a significant (p < 0.05) decrease in the neuronal GSH activity was observed in the AlCl3 group when compared with the control. The 5 mg/kg b.w. HS-AuNPs treated group exhibited similar result as the AlCl3 group. The HS-AuNPs treatment at both doses significantly ameliorated the reduced GSH activity instigated by AlCl3 administration.
3.8 mRNA expression
The polymerase chain reaction analyses of COX-2, BACE-1 and BDNF mRNA expression in the hippocampus of AD rats were evaluated as shown in Fig. 8 A-D.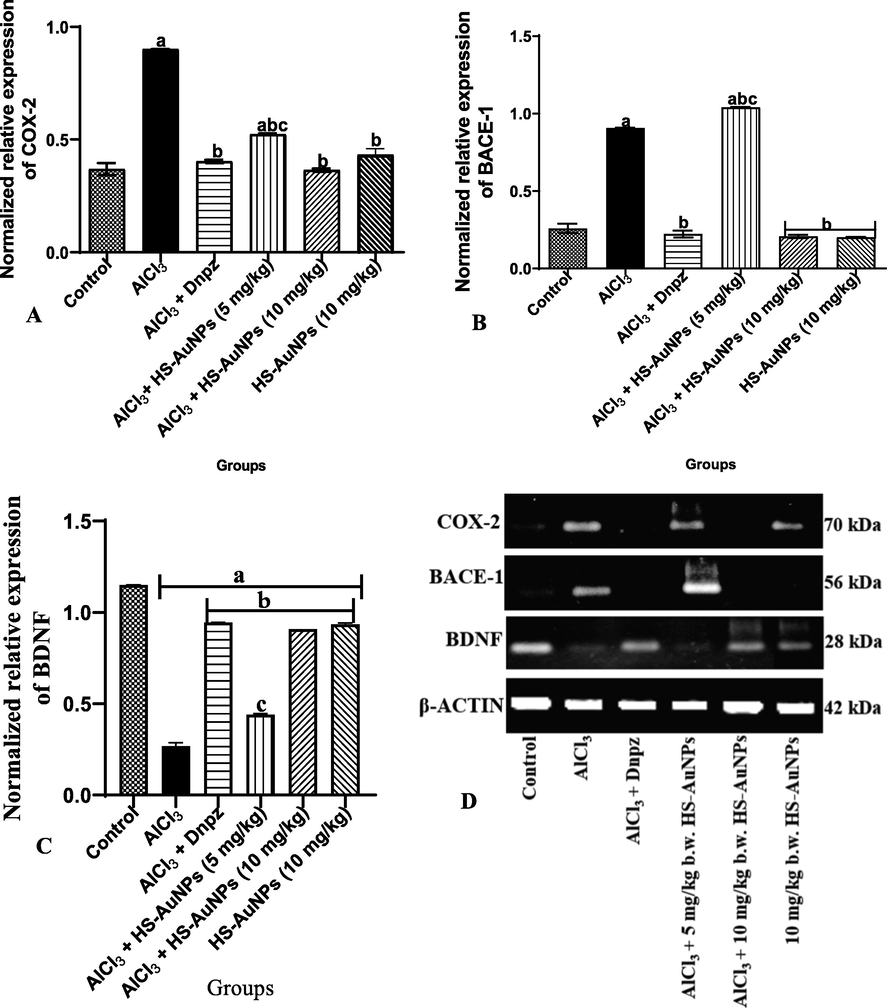
Expression pattern of COX-2 (A), BACE-1 (B) BDNF (C) and pictorial representation of the band density of agarose gel electrophoresis of the RT-PCR analysis of COX-2, BACE-1, BDNF and β actin (D) genes in control, AlCl3 and treatment groups. Each bar represents control normalized relative expression (gene/ β-actin). Results are expressed as mean ± SD. ap < 0.05 vs control, bp < 0.05 vs AlCl3 group, cp < 0.05 vs Dnpz group. Legend: AlCl3 - aluminum chloride; Dnpz – donepezil; HS-AuNPs- Hibiscus sabdariffa synthesized-gold nanoparticles.
Oral administration of AlCl3 significantly (p < 0.05) increase mRNA expression of COX-2 and BACE-1 in the hippocampus of AD rats (AlCl3 group) and 5 mg/kg b.w., HS-AuNPs treated group when compared with the control (Fig. 8 A – B and D). The AD rats treated with Dnpz and HS-AuNPs at tested doses significantly (p < 0.05) decreased mRNA expressions of COX-2 and BACE-1 when compared with AlCl3 group (Fig. 8 A – B, and D). A significant (p < 0.05) increase in the BACE-1 mRNA expression was observed in the 5 mg/kg b.w HS-AuNPs when compared with the AlCl3 group (Fig. 8 B). Statistically, a significant (p < 0.05) increase in the mRNA expression of COX-2 and BACE-1 were observed in the 5 mg/kg b.w HS-AuNPs when compared with the Dnpz group (Fig. 8 A – B, and D).
A significant (p < 0.05) difference was noted in the mRNA expression of BDNF in AlCl3 (AD rats), Dnpz and HS-AuNPs treated groups when compared with the control (Fig. 8 C and D). Increased mRNA expression of BDNF was noted in the Dnpz and HS-AuNPs treated groups when compared with the AlCl3 group. The target mRNA genes were normalized against β-actin as revealed by the band density of the RT-PCR (Fig. 8 D).
4 Discussion
Alzheimer’s disease remains the most common cause of dementia, accounting for about 70 % of the 57.4 million cases worldwide. This figure is estimated to be a threefold increase by 2050 (He et al., 2016, Nichols and Vos 2021). Alzheimer’s disease is reportedly linked to OS (Zhao and Zhao 2013). Aluminum chloride alters several signaling pathways in the brain, thereby, inducing OS, neuroinflammation and cognitive deficits (Justin-Thenmozhi et al., 2018, ELBini-Dhouib et al., 2021). Therefore, in the present study, we considered AlCl3 a suitable neurotoxicant for inducing AD-like disorder in rats.
The successful synthesis of HS-AuNP could be attributed to the presence of alkaloids, polyphenolics, saponins and glycosides in the crude extract of H. sabdariffa calyx. These phytochemicals have been known for their pharmacological properties (Sinha 2019, Rajput et al., 2022), which could be responsible for the ameliorative potential of the HS-AuNPs. In the present study, AuNPs were synthesized using crude extract of H. sabdariffa calyces. The phytochemicals in the plant extract serves to reduce the Au3+ to Au0 and likewise as capping and therapeutic agents (Anadozie et al., 2021). The observed color change in the reaction mixture on adding the plant extract to the Au solution indicates the successful synthesis of HS-AuNPs, which is attributed to the characteristic peak of the SPR (Bawazeer et al., 2021). The SPR absorption maxima of the HS-AuNPs, as revealed by the UV–vis spectroscopy measurement (Fig. 2), suggest that phytochemicals present in the H. sabdariffa extract was able to reduce Au3+ to Au0.
The hydrodynamic size and PDI value obtained in the present study suggests that the particle in solution was uniformly distributed without agglomeration. These parameters also determine the cellular uptake of the AuNPs (Danaei et al., 2018). The particle size measured on DLS was much larger than the HRTEM measurement. This is because DLS measures NPs in liquid suspension (Mourdikoudis et al., 2018). The size and shape of NPs enhances the delivery of drug across the BBB. Nanoparticles of<100 nm easily crosses the BBB (Saraiva et al., 2016). The small size and mostly spherical-shaped AuNPs revealed by the HRTEM images of this study could be responsible for the passage of the HS-AuNPs across the BBB. Unlike the peripheral barriers, BBB accommodate only smaller-sized particles because of the tight junctions (zonulae occludentes) in between them (Danaei et al., 2018). Medium-sized AuNPs in the range of 15 nm are the most efficient in crossing the BBB (Ohta et al., 2020). It is worthy of note that the HS-AuNPs produced in our study was within this range.
The polyphenolic compounds present in the plant extract as revealed by the FTIR measurement could be responsible for the reduction of Au+ to Au°. The unique peaks shown on the FT-IR chromatogram (supplementary 1) corresponds to the OH, –NH and C⚌C groups, which denotes the presence of polyphenolic compounds and alkaloids (Singh et al., 2018). Therefore, suggesting their roles in the reduction and stabilization of the HS-AuNPs. Ahmed and Mustafa (2020) reported that a shift in the FT-IR absorption bands could be because of the involvement of plant phytochemicals as reducing agents in the synthesis of NPs. In this study, the slight change in absorption bands of the HS-AuNPs indicates the functional groups responsible for the reduction and formation of the AuNPs.
Memory and learning process are key players in assessing behavioral patterns in AD patients. Therefore, in this study, neurobehavioral assessment was performed on the AlCl3-induced AD rats to assess the degree of neurological, motor, and functional damage caused by oral administration of AlCl3. Aluminum chloride can cross the BBB, causing cognitive impairment and neuronal damage (Shunan et al., 2021). In this study, oral administration of AlCl3 caused cognitive impairment and neurobehavioral deficits in the rats on days 28 and 42 (Table 2, Figs. 4 and 5). These changes were however, reversed on day 42 by HS-AuNPs treatment at doses of 5 and 10 mg/kg b.w. The findings of the present study suggest that the HS-AuNPs is site specific and could have cross the BBB to exert it therapeutic activities. This can be attributed to the small-sized AuNPs obtained in this study. Our findings is similar to the previously reported study by Singh et al., (2018) where nanoEGCG reversed the impaired recognition memory caused by AlCl3 administration.
The significant reduction in the % weight gain of rats caused by AlCl3 administration was reversed in the AD rats treated with HS-AuNPs at doses of 5 and 10 mg/kg b.w. Also, AlCl3 caused an increase in the size and relative brain weight of rats, whereas the HS-AuNPs treated groups restored the relative brain weight of rats comparably to the control. The increased relative brain weight of rat observed in this study could be linked to AlCl3 toxicity resulting from the long-term exposure of rats to AlCl3.
Acetylcholine (ACh) is a cholinergic neurotransmitter responsible for various cognitive functions such as learning, memory, and cognition. Acetylcholinesterase is an enzyme responsible for ACh hydrolysis and a good indicator for cholinergic neuronal damage (Anadozie et al., 2019). In the present study, the elevated AChE activity caused by oral administration of AlCl3 was significantly reduced by HS-AuNPs at doses of 5 and 10 mg/kg b.w., thus, suggesting that HS-AuNPs could increase the levels of acetylcholine and promote cognitive functions. The findings of this study correlate with the previously reported study by Auti and Kulkarni (2019) where AlCl3-induced rats displayed elevated AChE levels when compared to control rats.
Monoamine oxidase is a neuronal enzyme responsible for catalyzing the oxidative deamination of monoamine neurotransmitters such as norepinephrine, dopamine, and serotonin to produce peroxide (H2O2) (Behl et al., 2021). Previous studies have demonstrated that memory decline in an aging adult is linked to a reduced level of biogenic amine neurotransmitters in the brain (El-Baz et al., 2021, Gasiorowska et al., 2021). In the present study, AlCl3 significantly increased MAO activity, which could reduce monoamine neurotransmitters levels and subsequently upsurge the generation of OS in the AD brain (Oboh et al., 2020). Treatment with HS-AuNPs at tested doses reversed this action by decreasing the activity of MAO.
Adenosine deaminase is a neuromodulator most predominant in the brain and lymphoid tissues (Sauer et al., 2017). In the central nervous system, the release of biogenic amines neurotransmitters is controlled by adenosine (Shen et al., 2018). In the present study, HS-AuNPs was able to modulate the elevated ADA activity in the brain of AlCl3-induced rats.
The brain utilizes high amount of oxygen for its daily activities and therefore, most vulnerable to OS (Stefanatos and Sanz 2018). In the present study, oral administration of AlCl3 increased the levels of MDA and reduced the antioxidant system (SOD, GSH and GPx) which can be linked to cognitive impairment and OS. Treatment with HS-AuNPs however, reversed the oxidative damage induced by the AlCl3. Our findings correlate with the previously reported study by Shunan et al., (2021) where AlCl3-induced AD was linked to an increased activity of oxidative marker and a reduction in the antioxidant systems.
The brain’s functioning can be monitored by the expression of COX-2, BACE-1 and BDNF. Cyclooxygenase-2 is closely associated with neuroinflammatory disorders, and its expression is upregulated in AD (Tyagi et al., 2020). It is an early neuronal gene highly expressed in hippocampus and cortical glutamatergic neurons (Syed et al., 2015). In the present study, oral administration of AlCl3 increased the mRNA expression of COX-2 in the hippocampi of the AD rats. The HS-AuNPs especially at 10 mg/kg b.w. was able to downregulate the mRNA expression of COX-2.
Beta-secretase 1 is a major target for AD therapy. Its activity in an AD patient's brain is relatively high. Also, its cleavage of APP is a rate-limiting step in Aβ production (Hampel et al., 2021). The findings of the present study showed that oral administration of AlCl3 significantly upregulated the mRNA expression of BACE-1 in the hippocampi of the rats. The HS-AuNPs at 10 mg/kg b. w. however, modulated the expression of BACE-1 gene. The HS-AuNPs could have block the enzymatic cleavage of the amyloid beta precursor protein (AβPP) thereby inhibiting the expression of BACE-1. Similar observation has been reported in the study by Cai et al., (2018), where Berberis vulgaris extract decreased Aβ levels and inhibit the mRNA expression of BACE-1.
Brain-derived neurotrophic factor (BDNF) plays a vital role in regulating neuronal survival and neuroplasticity in the brain, as well as promoting growth, differentiation, and repair of neurons (Lin and Huang 2020). In this study, oral administration of AlCl3 subsequently initiated OS by decreasing the mRNA expression of BDNF in the hippocampi of the rats. However, the AD rats treated with HS-AuNPs was able to reverse the changes in the mRNA expression of this gene. This, therefore, suggest the therapeutic role of HS-AuNPs and its ability to cross the BBB, which could be related to the size and shape of the AuNPs.
5 Conclusion
The present study evaluated the effect of HS-AuNPs on AD rats. The results of this study showed that HS-AuNPs could improve the memory and cognitive functions of AlCl3-induced AD rats, as well as ameliorate the neuronal damage caused by AlCl3. The 10 mg/kg HS-AuNPs was more effective than the 5 mg/kg HS-AuNPs. The therapeutic effect of HS-AuNPs could be because of its ability to penetrate the BBB and prevent the aggregation of Aβ plaque. The result of the present study suggests that HS-AuNPs could be a promising treatment approach for managing AD. The study recommends drug loading and release activities of the HS-AuNPs to further support the current study’s findings.
Authors contribution
SOA conceived and designed the experiments, SOA and DOE drafted the manuscript, DOE, JIJ and IZ performed the experiment, OBA and OBA performed the data analysis, JNO and SR reviewed the manuscript, FOA and CCI All authors contributed significantly to this manuscript, read, and gave final approval of the version to be published.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Biological synthesis of gold and silver nanoparticles using leaf extracts of Crassocephalum rubens and their comparative in vitro antioxidant activities. Heliyon. 2020;6:e05501.
- [Google Scholar]
- Green synthesis of silver nanoparticles mediated by traditionally used medicinal plants in Sudan. Int. Nano Lett.. 2020;10:1-14.
- [CrossRef] [Google Scholar]
- Effect of grape antioxidant dietary fiber on the total antioxidant capacity and the activity of liver antioxidant enzymes in rats. Nutr. Res.. 2003;23:1251-1267.
- [Google Scholar]
- Prevention of short-term memory impairment by Bryophyllum pinnatum (Lam.) Oken and its effect on acetylcholinesterase changes in CCl4-induced neurotoxicity in rats. J. Basic Clin. Physiol. Pharmacol.. 2019;30
- [CrossRef] [Google Scholar]
- In vitro anti-oxidant and cytotoxic activities of gold nanoparticles synthesized from an aqueous extract of the Xylopia aethiopica fruit. Nanotechnology 2021
- [CrossRef] [Google Scholar]
- Synthesis of gold nanoparticles using extract of Carica papaya fruit: Evaluation of its antioxidant properties and effect on colorectal and breast cancer cells. Biocatal. Agric. Biotechnol.. 2022;42:102348
- [CrossRef] [Google Scholar]
- The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn. Process.. 2012;13:93-110.
- [CrossRef] [Google Scholar]
- Neuroprotective Effect of Cardamom oil against aluminum induced neurotoxicity in rats. Front. Neurol.. 2019;10
- [CrossRef] [Google Scholar]
- Green synthesis of zinc oxide nanoparticles using Hibiscus subdariffa leaf extract: effect of temperature on synthesis, anti-bacterial activity and anti-diabetic activity. RSC Adv.. 2015;5:4993-5003.
- [Google Scholar]
- Punica granatum peel extracts mediated the green synthesis of gold nanoparticles and their detailed in vivo biological activities. Green Process. Synth,. 2021;10:882-892.
- [CrossRef] [Google Scholar]
- Chenodeoxycholic acid ameliorates AlCl(3)-induced Alzheimer's disease neurotoxicity and cognitive deterioration via enhanced insulin signaling in rats. Molecules. 2019;24
- [CrossRef] [Google Scholar]
- Role of monoamine oxidase activity in alzheimer’s disease: an insight into the therapeutic potential of inhibitors. Molecules. 2021;26:3724.
- [Google Scholar]
- Comprehensive review on alzheimer's disease: causes and treatment. Molecules (Basel, Switzerland).. 2020;25:5789.
- [CrossRef] [Google Scholar]
- Berberine alleviates amyloid-beta pathology in the brain of APP/PS1 transgenic mice via inhibiting β/γ-Secretases activity and enhancing α-secretases. Curr. Alzheimer Res.. 2018;15:1045-1052.
- [CrossRef] [Google Scholar]
- Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol.. 2018;14:450-464.
- [CrossRef] [Google Scholar]
- New therapeutic potentials of nanosized phytomedicine. J. Nanosci. Nanotechnol.. 2016;16:8176-8187.
- [Google Scholar]
- Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10:57.
- [Google Scholar]
- The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener.. 2019;14:32.
- [CrossRef] [Google Scholar]
- Current strategies for brain drug delivery. Theranostics.. 2018;8:1481-1493.
- [CrossRef] [Google Scholar]
- Dunalialla salina microalgae and its isolated zeaxanthin mitigate age-related dementia in rats: modulation of neurotransmission and amyloid-β protein. Toxicol. Rep.. 2021;8:1899-1908.
- [CrossRef] [Google Scholar]
- Curcumin attenuated neurotoxicity in sporadic animal model of Alzheimer’s disease. Molecules. 2021;26:3011.
- [Google Scholar]
- A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol.. 1961;7:88-95.
- [CrossRef] [Google Scholar]
- The Biology and pathobiology of glutamatergic, cholinergic, and dopaminergic signaling in the aging brain. Front. Aging Neurosci.. 2021;13
- [CrossRef] [Google Scholar]
- Adenosine Deaminase. In: Bergmeyer H.U., ed. Methods of Enzymatic Analysis (second edition). Academic Press; 1974. p. :1092-1099.
- [Google Scholar]
- A colorimetric method for the estimation of monoamine oxidase. Biochem. J. 1961;78:172.
- [Google Scholar]
- An aging world: 2015. DC: United States Census Bureau Washington; 2016.
- Aluminium toxicosis: a review of toxic actions and effects. Interdiscip. Toxicol.. 2019;12:45-70.
- [CrossRef] [Google Scholar]
- Organic acids from roselle (Hibiscus sabdariffa L.)-A Brief review of its pharmacological effects. Biomedicines.. 2020;8:100.
- [CrossRef] [Google Scholar]
- Effect of Hibiscus sabdariffa on blood pressure in patients with stage 1 hypertension. J. Adv. Pharma. Technol. Res.. 2019;10:107-111.
- [CrossRef] [Google Scholar]
- Attenuation of aluminum chloride-induced neuroinflammation and caspase activation through the AKT/GSK-3β pathway by hesperidin in wistar rats. Neurotox. Res.. 2018;34:463-476.
- [Google Scholar]
- Nanoparticles: Properties, applications and toxicities. Arab. J. Chem.. 2019;12:908-931.
- [CrossRef] [Google Scholar]
- Acute aluminum chloride toxicity revisited: Study on DNA damage and histopathological, biochemical and neurochemical alterations in rat brain. Life Sci.. 2019;217:202-211.
- [Google Scholar]
- Brain-derived neurotrophic factor and mental disorders. Biomed. J.. 2020;43:134-142.
- [CrossRef] [Google Scholar]
- Tau protein modifications and interactions: their role in function and dysfunction. Int. J. Mol. Sci.. 2014;15:4671-4713.
- [Google Scholar]
- Characterization techniques for nanoparticles: comparison and complementarity upon studying nanoparticle properties. Nanoscale. 2018;10:12871-12934.
- [Google Scholar]
- The estimation of the global prevalence of dementia from 1990–2019 and forecasted prevalence through 2050: an analysis for the global burden of disease (GBD) study 2019. Alzheimers Dement.. 2021;17:e051496.
- [CrossRef] [Google Scholar]
- Characterization and neuroprotective properties of alkaloid extract of Vernonia amygdalina Delile in experimental models of Alzheimer’s disease. Drug Chem. Toxicol. 2020:1-10.
- [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95:351-358.
- [Google Scholar]
- Investigating the optimum size of nanoparticles for their delivery into the brain assisted by focused ultrasound-induced blood-brain barrier opening. Sci. Rep.. 2020;10:18220.
- [CrossRef] [Google Scholar]
- Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnol.. 2018;16:71.
- [CrossRef] [Google Scholar]
- Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods. 1985;14:149-167.
- [CrossRef] [Google Scholar]
- Pharmacological activities and toxicities of alkaloids on human health. Mater. Today:. Proc.. 2022;48:1407-1415.
- [CrossRef] [Google Scholar]
- A review on phytochemistry and therapeutic uses of Hibiscus sabdariffa L. Biomed. Pharmacother.. 2018;102:575-586.
- [Google Scholar]
- Role of medicinal plants against Alzheimer’s disease. Int. J. Complement. Altern. Med.. 2018;11:205-208.
- [Google Scholar]
- Adverse drug reactions of acetylcholinesterase inhibitors in older people living with dementia: a comprehensive literature review. Ther. Clin. Risk Manag.. 2021;17:927.
- [Google Scholar]
- Nanoparticle-mediated brain drug delivery: Overcoming blood–brain barrier to treat neurodegenerative diseases. J. Control. Release. 2016;235:34-47.
- [CrossRef] [Google Scholar]
- Alterations in the brain adenosine metabolism cause behavioral and neurological impairment in ADA-deficient mice and patients. Sci. Rep.. 2017;7:40136.
- [CrossRef] [Google Scholar]
- Adenosine actions on oligodendroglia and myelination in autism spectrum disorder. Front. Cell. Neurosci.. 2018;12
- [CrossRef] [Google Scholar]
- Neuroprotective effect of Betalain against AlCl3-induced Alzheimer's disease in Sprague Dawley rats via putative modulation of oxidative stress and nuclear factor kappa B (NF-κB) signaling pathway. Biomed. Pharmacother.. 2021;137:111369
- [CrossRef] [Google Scholar]
- EGCG nanoparticles attenuate aluminum chloride induced neurobehavioral deficits, beta amyloid and tau pathology in a rat Model of Alzheimer’s disease. Front. Aging Neurosci.. 2018;10
- [CrossRef] [Google Scholar]
- ‘Green’ synthesis of metals and their oxide nanoparticles: applications for environmental remediation. J. Nanobiotechnol.. 2018;16:84.
- [CrossRef] [Google Scholar]
- Pharmacological importance of polyphenols: a review. Int. Res. J. Pharm.. 2019;10:13-23.
- [Google Scholar]
- Cyclooxygenase I and II inhibitors distinctly enhance hippocampal-and cortex-dependent cognitive functions in mice. Mol. Med. Rep.. 2015;12:7649-7656.
- [Google Scholar]
- Integrated pathways of COX-2 and mTOR: roles in cell sensing and Alzheimer’s disease. Front. Neurosci.. 2020;14
- [CrossRef] [Google Scholar]
- Action mechanism of rosella (Hibiscus sabdariffa L.) used to treat metabolic syndrome in elderly women. Evid.-Based Complement. Altern. Med.. 2020;2020:5351318.
- [Google Scholar]
- Novel green synthesis of Hibiscus sabdariffa flower extract conjugated gold nanoparticles with excellent anti-acute myeloid leukemia effect in comparison to daunorubicin in a leukemic rodent model. Appl. Organomet. Chem.. 2020;34:e5271.
- [Google Scholar]
- Oxidative stress and the pathogenesis of Alzheimer's disease. Oxid. Med. Cell. Longev.. 2013;2013:316523
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.104604.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







