Translate this page into:
High affinity of protocatechuic acid to human serum albumin and modulatory effects against oxidative stress and inflammation in alveolar epithelial cells: Modulation of pulmonary fibrosis
⁎Corresponding authors. 18762043161@163.com (Cheng Huang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Protocatechuic acid (PCA), C7H6O4, has been shown to possess potential antioxidant properties. But its interaction with the main plasma carrier protein, human serum albumin (HSA), as well as its antioxidant mechanism remains largely unknown. It has been shown that induced pulmonary fibrosis can be modulated through mitigating epithelial apoptosis mediated by the prohibition of oxidative stress. Therefore, in this study, the interaction of PCA and HSA was explored by spectroscopy, calorimetry (DSC), as well as molecular docking studies. Also, the protective effects of PCA against lipopolysaccharide (LPS)-induced cytotoxicity and oxidative stress were evaluated by MTT, ROS, ELISA, real-time PCR, and western blot assays. It was shown that under the interaction of HSA with PCA a spontaneous interaction occurs with the contribution of hydrophobic forces, which results in the formation of a stable complex. Cellular assays showed that PCA reduced LPS-induced cytotoxicity in human type II alveolar epithelial cells (AECs) through mitigation of ROS production, release and gene expression of TNf-α and IL-1β as pro-inflammatory mediators, and caspase-3 gene and protein as an apoptotic factor. Also, it was shown that PCA can reduce the expression of NF-κB at the protein level, indicating a possible inhibition of pulmonary fibrosis via regulating the NF-κB signaling pathway. In conclusion, PCA could be a promising therapeutic agent for the control of oxidative stress in AECs which is an important factor in redox modulatory therapy, while its pharmacodynamics can be modulated by interaction with HSA.
Keywords
Human serum albumin
Protocatechuic acid
Interaction
Antioxidant
1 Introduction
Human serum albumin (HSA) is known as a crucial carrier macromolecule for a number of endogenous and exogenous ligands (Forsthuber et al. 2020). In fact, HSA can interact with a wide variety of therapeutic compounds and regulate their pharmacodynamics (Kratz et al. 2014). Exploring the interaction of bioactive compounds with HSA can reveal the characteristics of drug-HSA complexes, as it may furnish important details regarding the structural properties that influence the therapeutic potency of bioactive compounds especially those derived from medicinal plants (Merlino et al. 2023). Interaction with carrier proteins such as HSA could provide useful information about understanding the toxicity of therapeutic agents and the corresponding biodistribution (Varshney et al. 2010; Yu et al. 2022). Therefore, the investigation of the interaction between bioactive molecules and HSA has been a potential research area in different fields, such as life science, biochemistry, and even medicine (Siddiqui et al. 2021).
Promising advancements are becoming apparent in the application of bioactive compounds-derived pharmaceuticals. The various applications of bioactive compounds from plant materials in medicine, such as antioxidant, anticancer, and antibacterial properties, have been investigated over the past few years (Parham et al. 2020; Vuong et al. 2021; Nwozo et al. 2023). Recent research in this field has focused on the use of protocatechuic acid (PCA), C7H6O4, with a molar mass of 154.12 g/mol and a density of 1.54 g/cm3 serves as a potential therapeutic compound (Mert et al. 2023; Zhou et al. 2023). PCA as a dihydroxybenzoic acid belonging to the family of phenolic acid structures, can be used as a potential antioxidant compound with minor serious side effects in the biological system (Khan et al. 2022; Liang et al. 2022; Zhou et al. 2023; Jiang et al. 2023). This potential small molecule then enables us to develop promising platforms against side effects induced by oxidative stress.
Chronic obstructive pulmonary disease (COPD) is a chronic respiratory disorder with systemic symptoms that markedly influence the quality of life of patients, which is linked with airflow obstruction along with lung inflammation and destruction (Stolz et al. 2022). COPD is normally a disease during the aging process and oxidative stress markers and reactive oxygen species (ROS) can alter biological molecules, signaling pathways and molecular functions of antioxidants, which heavily contribute to the pathogenesis of COPD (Dailah et al. 2022). The function of several related cells in COPD patients is changed in the course of this disorder, and the expression of crucial oxidant and antioxidant molecules may be dysregulated (Barnes et al. 2022). Therapeutic compounds such as small molecules may restore the balance of ROS production and affect some aspects of this disease (Dailah et al. 2022).
Although some findings recommend that PCA could be utilized as a therapeutic agent for chronic disease induced by oxidative stress (Abdelrahman et al. 2022; Lee et al. 2022; Kassab et al. 2022), further investigations associated with humans are required. Also, the interaction of PCA and HSA can provide useful information about the regulation of this bioactive molecule in the biological system and its biodistribution.
2 Materials and methods
2.1 Materials and solution preparation
The human pulmonary alveolar epithelial cells (AECs) were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). Human serum albumin and protocatechuic acid were purchased from Sigma–Aldrich Co. (USA). The HSA was dissolved in Tris–HCl buffer solution (50 mM Tris, 150 mM M NaCl, pH 7.4). For protein and cellular assays, protocatechuic acid was dissolved in Tris–HCl buffer solution or cell culture medium, respectively.
2.2 Fluorescence emission spectroscopic study
By fixing the excitation wavelength (280 nm), HSA (2 µM) emission intensity under addition of increasing concentrations of PCA from 1 to 30 µM was read between 300 and 440 nm at three distinct temperatures of 298/ 305, 310 K using a Hitachi fluorescence spectrophotometer F-4600 (Tokyo, Japan). The slit widths for both excitation and emission wavelength were set at 5 nm. The data was then used to determine quenching mechanisms as well as binding and parameter constants. The inner filter effect was also considered for the analysis of fluorescence data based on the previous study (Yeggoni et al. 2022).
2.3 Circular dichroism (CD) study
Alteration of secondary structures of HSA (5 µM) under the addition of a concentration of PCA (30 µM) at room temperature was determined using a CD spectropolarimeter (JASCO, J-815, Tokyo, Japan). CD spectra were read in 200–260 nm ranges with a scan rate of 100 nm/min and cell length of 0.2 cm.
2.4 Differential scanning calorimetry (DSC) analysis
DSC melting analysis was done employing a VP-DSC microcalorimeter (Micro Cal, Northampton, MA) with a heating rate of 1 °C/min, over the temperature range from 30 to 100 °C. The DSC analyses of HSA (10 μM) in the presence of 30 μM of PCA were carried out and data was processed with the VP-DSC microcalorimeter software to determine the melting temperature (Tm). The DSC analysis was then done based on the previous study (Eskew et al. 2021).
2.5 Molecular docking analysis
A molecular docking study was carried out with Autodock Vina software. Compound-free HSA (PDB id: 1AO6) was downloaded from the protein data bank (https://www.rcsb.org/structure/1ao6), whereas PCA (PubChem CID: 528594) was downloaded from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/compound/Protocatechuic-acid_-TBDMS). The docking format HSA-PCA complex was then determined using AutoDockTools. Docking analysis was done by using a box size of 126 Å × 126 Å × 126 Å with a grid size of 0.375 Å. Si atoms were removed from the PCA compound to perform a docking analysis. From the different complexes deduced from docking, only the complex with the least energy was used for analysis based on a previous study (Faridbod et al. 2011).
2.6 Cell culture
AECs were cultured in RPMI 1640 medium containing fetal bovine serum (10%) and antibiotics (1%) incubated in 5% CO2 at 37 °C. 10 µg/ml LPS and 20 µM PCA for 24 h were used for induction of oxidative stress and protective effect against cytotoxicity, respectively. This data was fixed at our lab and used for further studies. Therefore, for LPS and PCA incubation, the cells were divided into 3 groups. Group (1): Control AECs with no treatment. Group (2): AECs were only incubated with 10 μg/mL LPS for 24 h. Group (3): AECs were incubated with LPS and 20 μM PCA for 24 h. After the incubation time, AECs were harvested and used for further assays.
2.7 MTT assay
Cell cytotoxicity assay was done using conventional MTT assay. In brief, the AECs were cultured onto 96-well plates overnight. The cells were then incubated as explained in section 2.6 for 24 h. Afterward, the cells were added by 10 μL MTT solution at 37 °C for 4 h, replaced by 100 μL DMSO solution and incubated for 15 min. Finally, the absorbance of each well was detected at 570 nm using an ELISA plate reader (RT-2100C Microplate Reader, China).
2.8 Intracellular ROS production assay
DCFDA / H2DCFDA - Cellular ROS Assay Kit (ab113851) was used to assess the generation of ROS based on the provided protocols. Briefly, the cells were seeded and after 24 h, the cells were treated for an additional 24 h. Then, the medium was removed and 100 µL DCFDA probe was added to the cells and kept at 37 °C for 30 min. The fluorescence intensity of samples was read with Ex/Em at 485/528 nm using a fluorescence microplate reader (Bio-Tek Instruments, Winooski, USA).
2.9 ELISA assay
The relative levels of TNF-α and IL-1β in the cell culture supernatant were determined using commercial ELISA kits from Abcam Co. [Human TNF-α ELISA Kit (ab181421)] and [Human IL-1β ELISA Kit (ab214025)], following manufacturer's instructions.
2.10 Real-time PCR analysis
The mRNA expression of IL-1β, TNF-α, and caspase-3 was assessed by real-time PCR based on the procedure reported in the previous study (Ileriturk et al. 2022). Briefly, total RNA was isolated using Trizol Lysis Reagent (Invitrogen, China) and complementary DNA (cDNA) synthesis was performed using cDNA Reverse Transcription Kit (Qiagen GmbH, Hilden, Germany). The samples were mixed with SYBR Green PCR Master Mix (Qiagen GmbH, Hilden, Germany) and then assessed on the Applied Biosystems 7500 real-time PCR device. β-actin expression level was used as an internal control, and relative folds determination was done with the CT 2−ΔΔCT method.
2.11 Western blotting analysis
Western blot analysis was performed similar to the study reported by Yesildag et al. 2022 and Ileriturk et al. 2022. Briefly, 30 μg protein form lysed cells were loaded on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by transferring to the membrane and incubating in 5% BSA, and washing with Tris-buffer saline containing 0.1% Tween 20 after 1 h. The membrane was added to NF-κB, caspase-3, and β-actin primary antibodies (Santa Cruz Biotechnology, Inc., TX) and incubated overnight at 4 °C, followed by washing for 5 min. Goat anti-mouse (1:2000) as a secondary antibody was incubated with IgG-HRP for 2 h at room temperature and then washed. Protein bands were detected using enhanced chemiluminescence.
2.12 Statistical analysis
The data were expressed as mean ± SD of three experiments, and one-way ANOVA, followed by Tukey's post hoc test, was utilized to analyze the data. Statistical difference was reported as significant when P < 0.05.
3 Results and discussion
3.1 Effect of protocatechuic acid (PCA) on HSA fluorescence spectra
A number of molecular interactions between ligands and proteins can result in fluorescence quenching of receptors, such as reaction in the excited state, rearrangement at the molecular level, and complex formation (Sarzehi et al. 2010; Khashkhashi-Moghadam et al. 2022; Siddiqui et al. 2021). The quenching mechanisms are mostly categorized as either dynamic or static quenching, which are classified by their varying behaviors against temperature and viscosity (Bose et al. 2016; Mostafavi et al. 2022; Siddiqui et al. 2021). In this assay, the concentration of HSA was fixed at 2 µM, and the concentrations of PCA were in the range of 1 to 30 µM. The impact of PCA on HSA fluorescence emission intensity at 298 K is displayed in Fig. 1.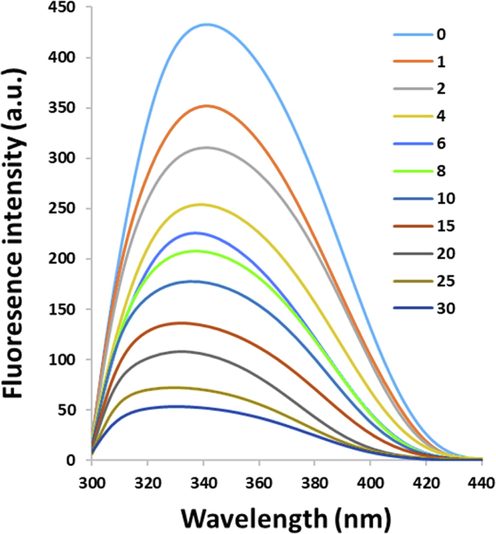
Fluorescence quenching of HSA (2 µM) under the interaction with PCA with increasing concentrations (1–30 µM) at room temperature.
It was deduced from Fig. 1 that a progressive quenching in the fluorescence emission intensity was triggered by PCA, associated with a blue shift in wavelength emission maximum (λmax) in the HSA spectra. This data proposes an enhanced hydrophobicity of the environment around the tryptophan residue (Trp-214).
3.2 Effect of protocatechuic acid (PCA) on HSA fluorescence quenching
The quenching parameters were calculated using the Stern-Volmer equation (Farajzadeh-Dehkordi et al. 2023):
The Stern-Volmer plots are exhibited in Fig. 2. It was shown that under the studied concentration range, the data is well-fitted with the Stern-Volmer equation. The determination of KSV values from Stern-Volmer plots were summarized in Table 1. Based on the effect of PCA on fluorescence quenching at each studied temperature (Fig. 2), the data reveals that the KSV values are inversely related to temperature, indicating a possible quenching mechanism of PCA-HSA interaction by static complex formation. Also, kq values were 2.23 × 1013 M−1 s−1, 6.47 × 1013 M−1 s−1, and 0.83 × 1013 M−1 s−1 at 298 K, 305 K, and 310 K, which were much greater than kq values reported for dynamic quenching (1010 M−1 s−1) (Siddiqui et al. 2021).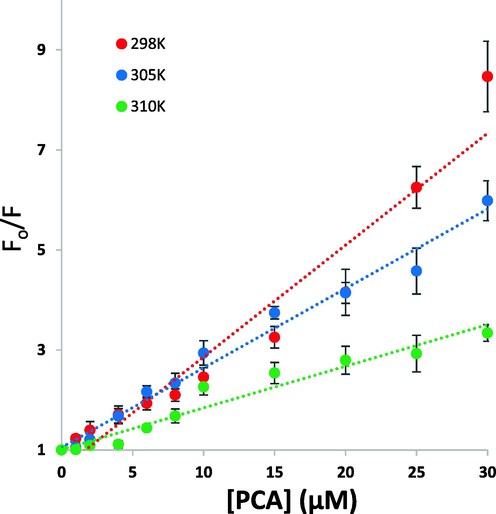
Stern-Volmer plots for the interaction of PCA and HSA at temperatures of 298 K, 305 K, and 310 K for the calculation of quenching constants.
T (K)
Ksv (105 M−1)
kq (1013 M−1 s−1)
logKb
n
ΔH (kJ/mol)
TΔS (kJ/mol)
ΔG (kJ/mol)
298
2.23 ± 0.26
2.23 ± 0.26
5.12 ± 0.21
0.97 ± 0.07
378.65 ± 23.21
407.78 ± 31.24
−29.12 ± 2.34
305
1.58 ± 0.12
1.58 ± 0.12
6.47 ± 0.27
1.25 ± 0.09
378.65 ± 23.21
416.32 ± 31.37
−37.67 ± 2.93
310
0.83 ± 0.07
0.83 ± 0.07
7.72 ± 0.35
1.57 ± 0.09
378.65 ± 23.21
424.34 ± 31.39
−45.68 ± 3.68
3.3 Calculation of binding parameters
When ligands interact with a set of equivalent sites on a protein, the binding parameters, including binding constant (Kb) and the numbers of binding sites (n) can be estimated based on the modified Stern-Volmer equation (Osman et al. 2023):
For the system of PCA and HSA, the Kb and n values at temperatures of 298 K, 305 K, and 310 K were determined based on Fig. 3 and the resultant data were summarized in Table 1. The n value was around 1, which suggested that there can be one class of binding sites on HSA for PCA. Also, it was revealed that the Kb and n values are directly correlated with temperature, as the higher temperature, the binding parameters increase, furnishing a basis for conformational rearrangement of HSA and more favorable interaction with PCA at higher temperature than that in lower ones (Siddiqui et al. 2021). Based on the logKb values it can be revealed that the magnitude of Kb values can be in the range of 105-107, revealing a strong interaction between PCA and HSA. Therefore, it may be suggested that interaction of interaction between PCA and HSA may occur in vivo and PCA probably binds to HSA with a great affinity (Chamani et al. 2005).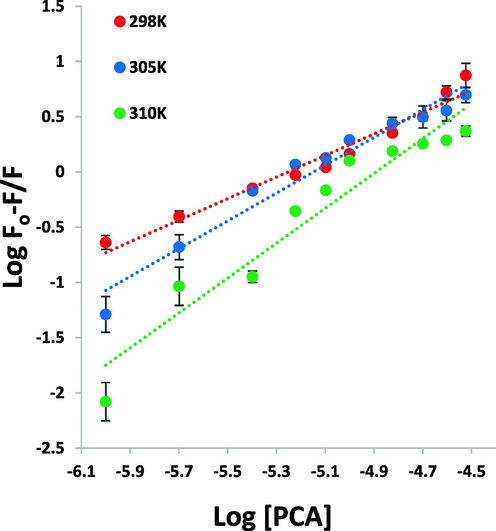
Modified Stern-Volmer plots for the interaction of PCA and HSA at temperatures of 298 K, 305 K, and 310 K for calculation of binding constants.
3.4 Calculation of thermodynamic parameters
The binding mode between PCA and HSA can be determined through the evaluation of thermodynamic parameters (Siddiqui et al. 2021). The interaction bonds between ligands and proteins are electrostatic, hydrogen bonds, van der Waals, and hydrophobic forces. To investigate the interaction between PCA and HSA, the thermodynamic parameters were estimated by using the van’t Hoff plot (Kabir et al. 2023):
Where, Kb is the binding constant, ΔH and ΔS are the enthalpy and entropy changes, respectively, and R is the gas constant. If the variation in ΔH is not significant under the temperature range of 298 K-310 K, the ΔH and ΔS can be calculated from the slope and Y-intercept of van’t Hoff equation (3), respectively (Fig. 4, Table 1). The free energy change (ΔG) can then be calculated based on the following relationship (Wang et al. 2022):
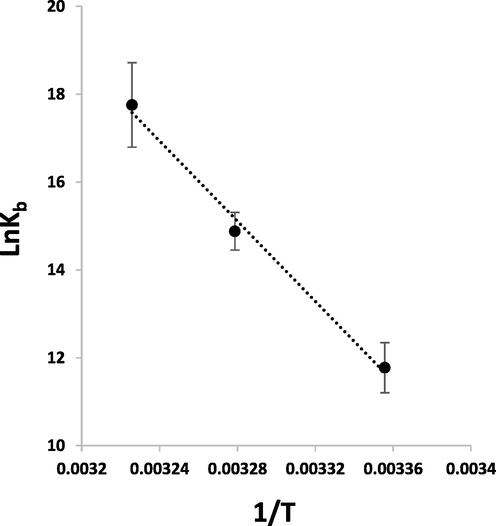
van’t Hoff plot for the interaction of PCA and HSA at temperatures of 298 K, 305 K, and 310 K for calculation of binding constants.
Table 1 tabulates the ΔH and ΔS values estimated for the interaction of PCA and HSA. The negative ΔG values of −29.12 ± 2.34 kJ/mol, −37.67 ± 2.93 kJ/mol, and −45.68 ± 3.68 kJ/mol at temperatures of 298 K, 305 K, and 310 K, summarized in Table 1, indicated the fact that the interaction system is spontaneous. The ΔH value of 378.65 ± 23.21 kJ/mol and TΔS value of 407.78 ± 31.24 kJ/mol indicated that hydrophobic forces are mainly responsible for the interaction of PCA-HSA complex (Siddiqui et al. 2021), which needs docking studies.
3.5 Circular dichroism spectra
To obtain information about the secondary structural changes of HSA, Far-UV CD analysis was done at 298 K. The 1:15 M ratio of HSA to PCA was used and the far-UV CD spectra of HSA in the absence (line blue) and presence (line green) of PCA were shown in Fig. 5. Two negative bands were observed in the far-UV region centered at 208 and 222 nm, revealing the dominance of α-helical structure of HSA due to π → π* and n → π* transfer in peptide bonds (Negrea et al. 2023). With the titration of PCA, the band intensity of minima increased in the CD spectrum relative to the control sample. The CD outcomes were analyzed in terms of mean residue ellipticity (MRE) in degree cm2 dmol−1 based on the following equation (Rao et al. 2020):
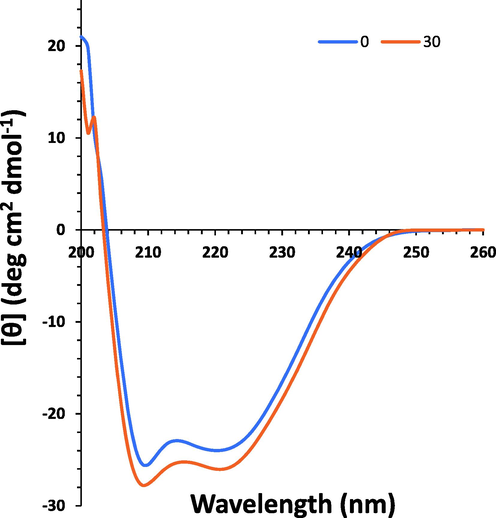
Far-UV CD study for the interaction of PCA (30 µM) and HSA (5 µM) at temperatures of 298 K for exploring the secondary structural changes of the protein.
According to the equations (5) and (6), the α-helix content of HSA was calculated. This content increased from 58.09% for HSA to 61.39% under the addition of PCA to HSA. The increase of α-helix structure revealed that PCA combined with several residues of the polypeptide chain and stabilized their hydrophobic and hydrogen bonds (Song et al. 2021). Generally, the incubation of HSA with PCA resulted in a partial increase in minima, while the position of the minima almost kept unchanged, indicating that the interaction of PCA and HSA triggered some secondary structural changes in HSA, where the α-helical content of HSA elevated.
Therefore, upon interaction of PCA with HSA, the stability of this chiral protein can be increased which can improve the potential application of HSA in different areas (Allenmark et al. 1984).
3.6 Thermo stability analysis of PCA–HSA interaction by DSC
Normally, ligand interaction results in either stabilization or destabilization of the proteins (Abarova et al. 2021; Aricov et al. 2020; Rizzuti et al. 2019). DSC analysis was utilized to explore the impact of PCA on the thermal behavior of HSA. Tm is the main parameter determined from the DSC spectrum that gives detail regarding the impact of ligand interaction on the thermal stability of a biomacromolecule (Naik et al. 2022). Fig. 6 exhibits the DSC thermograms for free HSA and interacted HSA with PCA. It was found that HSA is unfolded in a cooperative manner and gives rise to the formation of an endothermic peak with a Tm value of around 64 °C (337 K). It was observed that under the interaction of PCA, this ligand could lead to partial stabilization of HSA as supported by the elevation in Tm by about 2.0 °C. These findings suggested that the interaction of PCA causes stabilization of the HSA structure as also displayed with the CD data. In fact, the increase in the amount of α-helix content can be a possible reason for increasing the stability and Tm of protein in the presence of PCA.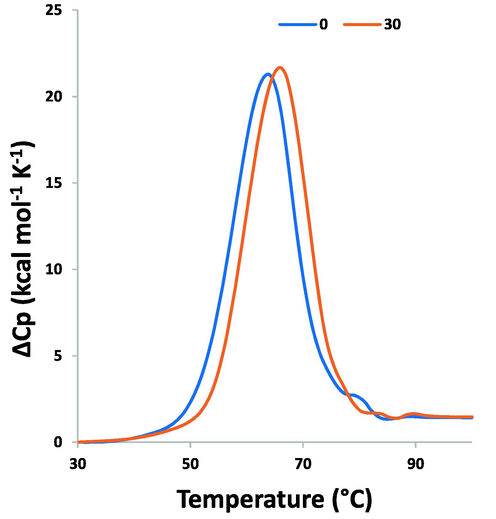
DSC thermogram for the interaction of PCA (30 µM) and HSA (10 µM) for exploring the Tm of protein.
3.7 Molecular docking study
The molecular docking study was performed to determine the binding affinity and amino acid residues involved in the interaction of HSA and PCA. Thermodynamic analysis indicated that hydrophobic interactions are the main forces rather than the hydrophilic forces in the formation of HSA-PCA complexes. A rational reason is that dimethyl leads to an enhancement in hydrophobicity of PCA, and strengthens the hydrophobic forces. Generally, PCA molecule (Fig. 7a) after docking analysis (Fig. 7b) existed in a cavity formed by Ile 142 and His 146 (Fig. 7c), which provides a hydrophobic pocket to form alkyl and pi-alkyl interactions with HSA. It was also important to indicate that several other amino acid residues such as Lys 190, Lys 519, Arg 114, Arg, 117, Arg 145, Arg 186, Glu 141, Glu 520, Ser 517, Tyr 138, Leu 115, Leu182, Tyr 161, and Met 123 were located in the vicinity of PCA, suggesting that hydrogen bonding interactions also present in the binding site.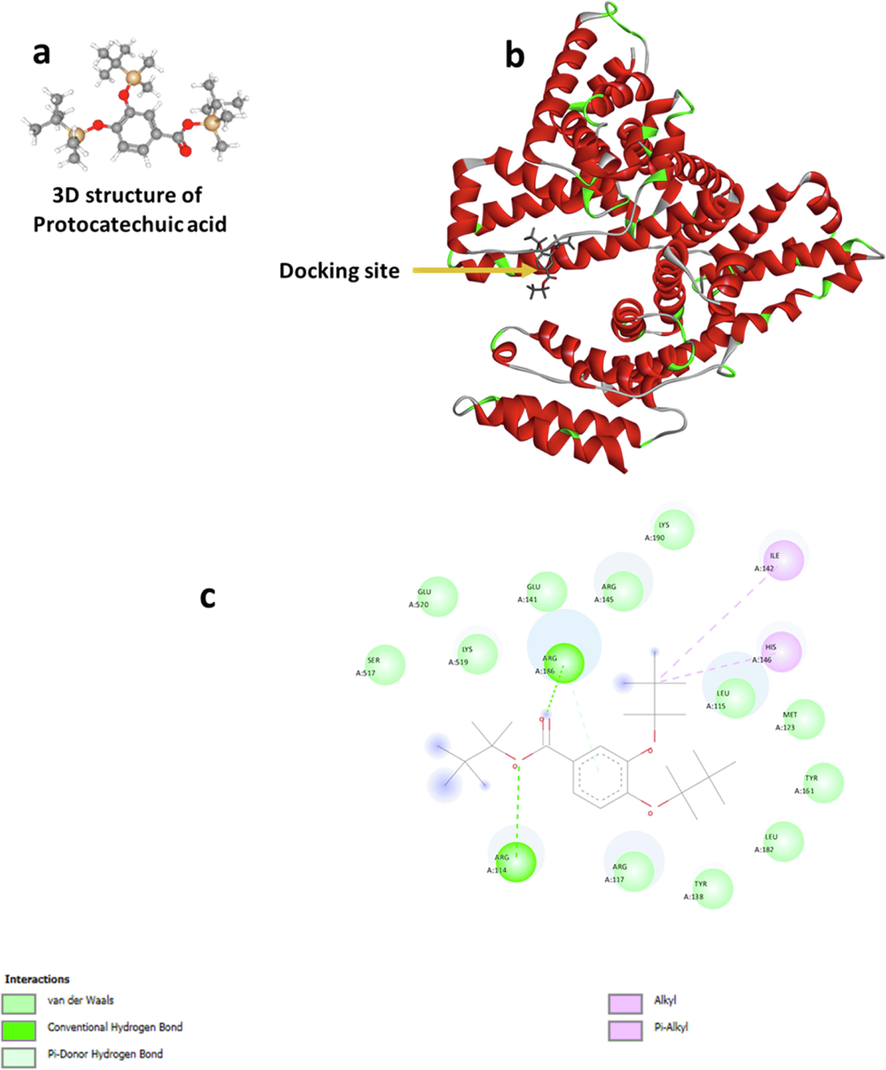
Molecular rocking study of PCA interaction with HSA. (a) The 3D structure of PCA, (b) docking of HSA and PCA, and (c) the amino acid residues involved in the binding site.
3.8 PCA mitigated LPS-induced cytotoxicity and ROS production in AECs
The cells were incubated with LPS (10 µg/ml) for 24 h and it was realized that the cell viability was reduced to around 46.77%±9.47% (***P < 0.001) (Fig. 8a), which was in good agreement with a previous study (Jiang et al. 2020). However, co-incubation of AECs with LPS and PCA (20 µM) regulated the reduction in cell viability induced by LPS and recovered the percentage of cell viability to 82.48%±14.91% (**P < 0.01) (Fig. 8a). This data indicated that PCA can mitigate the triggered cytotoxicity by LPS in AECs.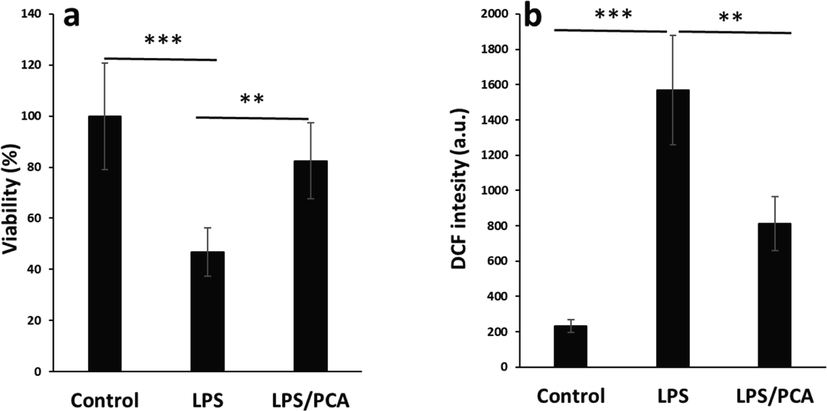
Effects of PCA on the cytotoxicity and ROS production induced by LPS in type II AECs. (a) MTT assay, (b) ROS assay. The cells were treated with LPS (10 µg/mL) or co-incubated with LPS (10 µg/mL) and PCA (20 µM) for 24 h. Data are presented as mean ± SD. **P < 0.01, ***P < 0.001 in comparison with the control group.
It was also shown that incubation of cells with LPS resulted in a significant increase in the ROS production (***P < 0.001), whereas the co-incubation of AECs with LPs and PCA reduced the production of ROS evidenced by DCF intensity (Fig. 8b). This data clearly showed that PCA may modulate the LPS-induced cytotoxicity in AEC cells through the regulation of ROS production (Zhang et al. 2021).
3.9 PCA decreased LPS-induced release and expression of pro-inflammatory cytokines in AECs
The present study indicated the protective potency of PCA against the ROS production induced by LPS in AECs. We detected that LPS induced cell toxicity by inducing oxidative stress production which was accompanied by an elevation in the level of ROS. In addition, it was found that the expression and the amount of pro-inflammatory cytokines such as TNF-α and IL-1β, were high in the AECs treated with LPS.
It was seen that LPS (10 µg/ml) exposure for 24 h induced the release and overexpression of pro-inflammatory cytokines, such as TNF-α (Fig. 9a, b) and IL-1β (Fig. 9c, d), which could induce the activation of the inflammatory response and apoptosis. TNF is known as a pleiotropic pro-inflammatory cytokine whose activation results in the expression of several pro-inflammatory cytokines, especially IL-1β (Reuter et al. 2010; Shahcheraghi et al. 2023). In the current report, LPS incubation led to a large release of TNF-α and IL-1β (Fig. 9a, c), and the mRNA expression levels of these markers were also elevated (Fig. 9b, d). This data indicated that LPS exposure triggered the expression of pro-inflammatory-associated markers and genes in AECs, and triggered an inflammatory response. However, this study reported that PCA affected the release of TNF-α and IL-1β, as well as the expression levels of TNF-α and IL-1β genes, revealing that PCA could relieve the side effects in inflammatory response induced by LPS stress.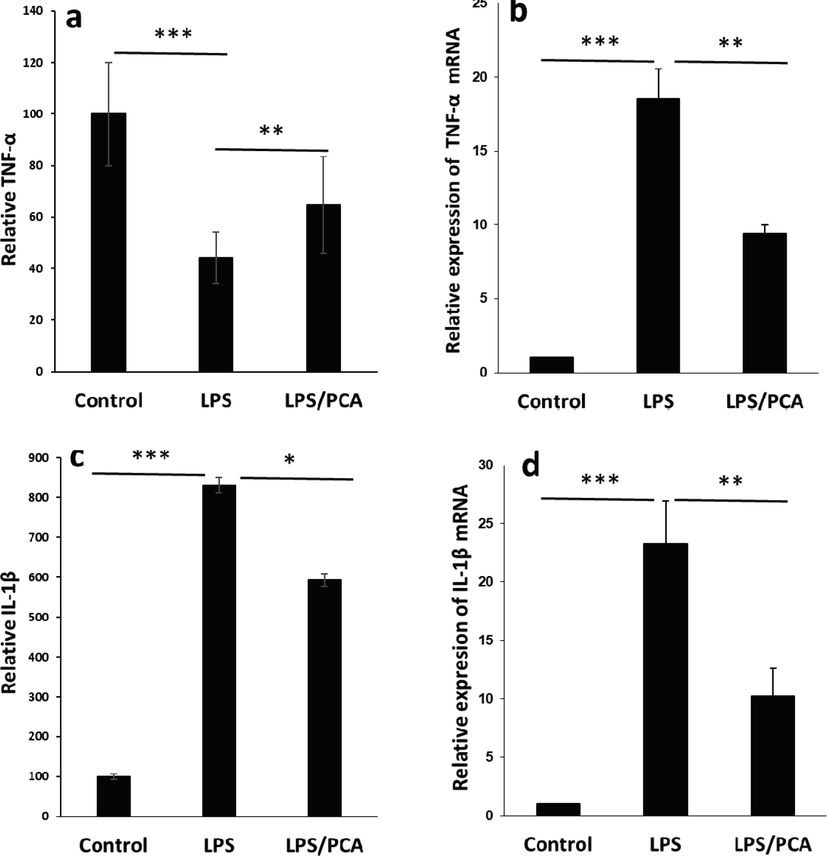
Effects of PCA on the release and expression of pro-inflammatory mediators induced by LPS in type II AECs. (a) Relative TNF-α release assessed by ELISA assay, (b) Relative TNF-α mRNA expression assessed by real-time PCR assay, (c) Relative IL-1β release assessed by ELISA assay, (d) Relative IL-1β mRNA expression assessed by real-time PCR assay. The cells were treated with LPS (10 µg/mL) or co-incubated with LPS (10 µg/mL) and PCA (20 µM) for 24 h. Data are presented as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 in comparison with the control group.
3.10 PCA mitigated LPS-induced activation of apoptotic-related factor and NF-κB
It has been shown that LPS-induced cytotoxicity is derived from the activation of apoptosis (Daldal et al. 2022; Fu et al. 2022). LPS-induced oxidative stress can result in the regulation of apoptotic signaling pathways. Based on the above facts, we explored the expression of caspase-3 at mRNA and protein levels. It was seen that PCA mitigated the expression of caspase-3 mRNA (Fig. 10a) and caspase-3 protein (Fig. 10b) triggered by LPS exposure as evidenced by real-time PCR and western blot analysis, respectively. LPS treatment markedly elevated the level of caspase-3 mRNA and protein, while co-incubation of AECs with LPS and PCA resulted in a significant reduction in the protein and mRNA level of caspase-3. Also, PCA as an antioxidant, can be involved in regulating the cytotoxicity induced by LPS through inactivation of the NF-κB signaling pathways (Zhang et al. 2015). The NF-κB activation is involved in overexpression of pro-inflammatory signaling pathways (Roberti et al. 2022). Wang et al. reported that PCA could inhibit the expression of inflammatory mediators in LPS-triggered BV2 microglia via modulation of NF-κB and MAPK signaling pathways (Wang et al. 2015). Also, this study reported that PCA affects the expression level of NF-κB protein in LPS-treated cells, revealing that PCA could decrease the inflammatory response stimulated by LPS (Fig. 10b).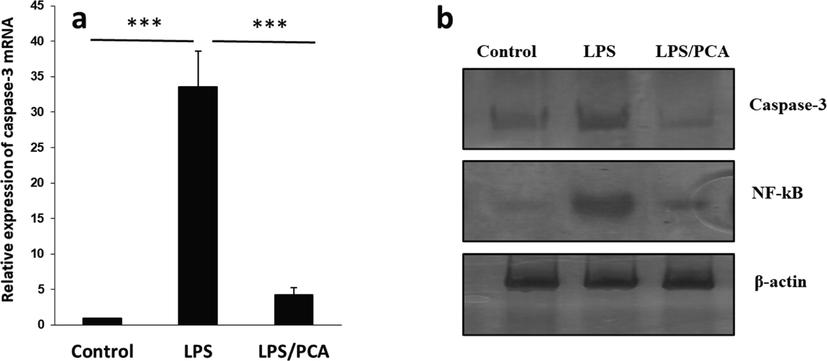
(a) Effects of PCA on the expression of caspase-3 mRNA induced by LPS in type II AECs. (b) Western blot assay for the expression of caspase-3 and NF-κB. The cells were treated with LPS (10 µg/mL) or co-incubated with LPS (10 µg/mL) and PCA (20 µM) for 24 h. Data are presented as mean ± SD. ***P < 0.001 in comparison with the control group.
4 Conclusions
The interaction of PCA with an important plasma protein, HSA, was evaluated using spectroscopic and computational analyses. Also, the antioxidant properties of PCA against LPS-induced cytotoxicity and ROS production were assessed by different cellular and molecular assays. The motivation of this study was to investigate the interaction of a potential antioxidant bioactive compound, PCA, with HSA and also reveal the antioxidant signaling pathway regulated by PCA. It was shown that PCA strongly interacted with HSA based on a static quenching mechanism, and the interaction was mediated by the dominance of hydrophobic bonds. PCA partially increased the percentage of α-helix content of HSA as well as Tm of protein. PCA at the interacting site of HSA interacted with some hydrophobic amino acid residues. Also, PCA mitigated LPS-induced cytotoxicity in AECs through the reduction of ROS, and expression of pro-inflammatory and pro-apoptosis mediators mediated by inactivating the NF-κB signaling pathway. In conclusion, it can be deduced that PCA interacts strongly with HSA and shows antioxidant potential, which needs further investigations in future studies.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Protocatechuic acid protects against thioacetamide-induced chronic liver injury and encephalopathy in mice via modulating mTOR, p53 and the IL-6/IL-17/IL-23 immunoinflammatory pathway. Toxicol. Appl. Pharmacol.. 2022 Apr;1(440):115931
- [Google Scholar]
- Some applications of chiral liquid affinity chromatography using bovine serum albumin as a stationary phase. Prep. Biochem. Biotech.. 1984 Jun 1;14(2):139-147.
- [Google Scholar]
- Interaction of piroxicam with bovine serum albumin investigated by spectroscopic, calorimetric and computational molecular methods. J. Biomol. Struct. Dyn.. 2020 Jun 12;38(9):2659-2671.
- [Google Scholar]
- Oxidative stress in chronic obstructive pulmonary disease. Antioxidants. 2022 May 13;11(5):965.
- [Google Scholar]
- Interaction of tea polyphenols with serum albumins: A fluorescence spectroscopic analysis. J. Lumin.. 2016 Jan;1(169):220-226.
- [Google Scholar]
- Structural changes in β-lactoglobulin by conjugation with three different kinds of carboxymethyl cyclodextrins. Thermochim. Acta. 2005 Jul 1;432(1):106-111.
- [Google Scholar]
- Therapeutic potential of small molecules targeting oxidative stress in the treatment of chronic obstructive pulmonary disease (COPD): A comprehensive review. Molecules. 2022 Aug 28;27(17):5542.
- [Google Scholar]
- Rituximab attenuated lipopolysaccharide-induced oxidative cytotoxicity, apoptosis, and inflammation in the human retina cells via modulating the TRPM2 signaling pathways. Ocul. Immunol. Inflamm.. 2022 Aug 18;30(6):1315-1328.
- [Google Scholar]
- Ligand binding to natural and modified human serum albumin. Anal. Biochem.. 2021 Jan;1(612):113843
- [Google Scholar]
- Insights into the binding interaction of Reactive Yellow 145 with human serum albumin from a biophysics point of view. J. Mol. Liq.. 2023 Jan;1(369):120800
- [Google Scholar]
- Interaction study of pioglitazone with albumin by fluorescence spectroscopy and molecular docking. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2011 Jan 1;78(1):96-101.
- [Google Scholar]
- Albumin is the major carrier protein for PFOS, PFOA, PFHxS, PFNA and PFDA in human plasma. Environ. Int.. 2020 Apr;1(137):105324
- [Google Scholar]
- Zearalenone promotes LPS-induced oxidative stress, endoplasmic reticulum stress, and accelerates bovine mammary epithelial cell apoptosis. Int. J. Mol. Sci.. 2022 Sep 18;23(18):10925.
- [Google Scholar]
- Evaluation of protective effects of quercetin against cypermethrin-induced lung toxicity in rats via oxidative stress, inflammation, apoptosis, autophagy, and endoplasmic reticulum stress pathway. Environ. Toxicol.. 2022 Nov;37(11):2639-2650.
- [Google Scholar]
- Jiang SQ, Chen ZL, Zhang S, Ye JL, Wang YB. Protective effects of protocatechuic acid on growth performance, intestinal barrier and antioxidant capacity in broilers challenged with lipopolysaccharide. animal. 2023 Jan 1;17(1):100693.
- Procyanidin B2 suppresses lipopolysaccharides-induced inflammation and apoptosis in human type II alveolar epithelial cells and lung fibroblasts. J. Interferon Cytokine Res.. 2020 Jan 1;40(1):54-63.
- [Google Scholar]
- Characterization of Climbazole-Bovine serum albumin interaction by experimental and in silico approaches. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2023 Mar;5(288):122197
- [Google Scholar]
- Protocatechuic acid abrogates oxidative insults, inflammation, and apoptosis in liver and kidney associated with monosodium glutamate intoxication in rats. Environ. Sci. Pollut. Res.. 2022 Feb;1:1-4.
- [Google Scholar]
- Pharmacological postconditioning by protocatechuic acid attenuates brain injury in ischemia-reperfusion (I/R) mice model: Implications of nuclear factor erythroid-2-related factor pathway. Neuroscience. 2022 May;21(491):23-31.
- [Google Scholar]
- Novel perspective into the interaction behavior study of the cyanidin with human serum albumin-holo transferrin complex: Spectroscopic, calorimetric and molecular modeling approaches. J. Mol. Liq.. 2022 Jun;15(356):119042
- [Google Scholar]
- A clinical update of using albumin as a drug vehicle—A commentary. J. Control. Release. 2014 Sep;28(190):331-336.
- [Google Scholar]
- Protocatechuic acid protects hepatocytes against hydrogen peroxide-induced oxidative stress. Curr. Res. Food Sci.. 2022 Jan;1(5):222-227.
- [Google Scholar]
- Injectable protocatechuic acid based composite hydrogel with hemostatic and antioxidant properties for skin regeneration. Mater. Des.. 2022 Oct;1(222):111109
- [Google Scholar]
- Metallodrug binding to serum albumin: Lessons from biophysical and structural studies. Coord. Chem. Rev.. 2023 Apr;1(480):215026
- [Google Scholar]
- Effects of protocatechuic acid against cisplatin-induced neurotoxicity in rat brains: an experimental study. Int. J. Neurosci.. 2022 Nov;16:1.
- [Google Scholar]
- Evaluation of interaction between Ponceau 4R (P4R) and trypsin using kinetic, spectroscopic, and molecular dynamics simulation methods. J. Mol. Liq.. 2022 Sep;15(362):119761
- [Google Scholar]
- Elucidating the binding mechanism of an antimigraine agent with a model protein: insights from molecular spectroscopic, calorimetric and computational approaches. J. Biomol. Struct. Dyn.. 2022 Mar;14:1-6.
- [Google Scholar]
- Spectroscopic studies on binding of ibuprofen and drotaverine with bovine serum albumin. J. Photochem. Photobiol. A Chem.. 2023 Apr;1(438):114512
- [Google Scholar]
- Antioxidant, phytochemical, and therapeutic properties of medicinal plants: a review. Int. J. Food Prop.. 2023 Dec 31;26(1):359-388.
- [Google Scholar]
- Perception of the interaction behavior between pepsin and the antimicrobial drug secnidazole with combined experimental spectroscopy and computer-aided techniques. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2023 Jan;9:122336
- [Google Scholar]
- Antioxidant, antimicrobial and antiviral properties of herbal materials. Antioxidants. 2020 Dec 21;9(12):1309.
- [Google Scholar]
- Mechanistic and conformational studies on the interaction of human serum albumin with rhodamine B by NMR, spectroscopic and molecular modeling methods. J. Mol. Liq.. 2020 Oct;10(316):113889
- [Google Scholar]
- Oxidative stress, inflammation, and cancer: how are they linked? Free Radic. Biol. Med.. 2010 Dec 1;49(11):1603-1616.
- [Google Scholar]
- Warfarin increases thermal resistance of albumin through stabilization of the protein lobe that includes its binding site. Arch. Biochem. Biophys.. 2019 Nov;15(676):108123
- [Google Scholar]
- NF-κB signaling and inflammation—Drug repurposing to treat inflammatory disorders? Biology. 2022 Feb 26;11(3):372.
- [Google Scholar]
- Investigation on the interaction between tamoxifen and human holo-transferrin: determination of the binding mechanism by fluorescence quenching, resonance light scattering and circular dichroism methods. Int. J. Biol. Macromol.. 2010 Nov 1;47(4):558-569.
- [Google Scholar]
- Resveratrol regulates inflammation and improves oxidative stress via Nrf2 signaling pathway: Therapeutic and biotechnological prospects. Phytother. Res. 2023 Feb 8
- [Google Scholar]
- Siddiqui S, Ameen F, ur Rehman S, Sarwar T, Tabish M. Studying the interaction of drug/ligand with serum albumin. Journal of Molecular Liquids. 2021 Aug 15;336:116200.
- Interaction of Bis-guanidinium acetates surfactants with bovine serum albumin evaluated by spectroscopy. Tenside Surfactant Deterg.. 2021 May 1;58(3):187-194.
- [Google Scholar]
- Towards the elimination of chronic obstructive pulmonary disease: a Lancet Commission. Lancet. 2022 Sep 17;400(10356):921-972.
- [Google Scholar]
- Ligand binding strategies of human serum albumin: how can the cargo be utilized? Chirality: Pharmacol. Biol. Chem. Consequences Mol. Asymmetry. 2010 Jan;22(1):77-87.
- [Google Scholar]
- Natural products and their derivatives with antibacterial, antioxidant and anticancer activities. Antibiotics. 2021 Jan 13;10(1):70.
- [Google Scholar]
- Wang L, Liang YS, Wu ZB, Liu YS, Xiao YH, Hu T, Gao R, Fang J, Liu J, ping Wu A. Exploring the interaction between Cry1Ac protein and Zn2+, Cd2+ metal ions by fluorescence quenching and molecular docking approaches. Chemosphere. 2022 Jun 1;297:134105.
- Protocatechuic acid inhibits inflammatory responses in LPS-stimulated BV2 microglia via NF-κB and MAPKs signaling pathways. Neurochem. Res.. 2015 Aug;40:1655-1660.
- [Google Scholar]
- Chebulinic and chebulagic acid binding with serum proteins: biophysical and molecular docking approach. J. Biomol. Struct. Dyn.. 2022 Apr;1:1-6.
- [Google Scholar]
- Evaluation of oxidative stress, inflammation, apoptosis, oxidative DNA damage and metalloproteinases in the lungs of rats treated with cadmium and carvacrol. Mol. Biol. Rep.. 2022;49(2):1201-1211.
- [Google Scholar]
- Yu L, Hua Z, Luo X, Zhao T, Liu Y. Systematic interaction of plasma albumin with the efficacy of chemotherapeutic drugs. Biochimica et Biophysica Acta (BBA)-Reviews on Cancer. 2022 Jan 1;1877(1):188655.
- Antioxidant effects of protocatechuic acid and protocatechuic aldehyde: old wine in a new bottle. Evid. Based Complement. Alternat. Med.. 2021 Nov;8:2021.
- [Google Scholar]
- Protective effects of protocatechuic acid on acute lung injury induced by lipopolysaccharide in mice via p38MAPK and NF-κB signal pathways. Int. Immunopharmacol.. 2015 May 1;26(1):229-236.
- [Google Scholar]
- Protocatechuic acid-mediated injectable antioxidant hydrogels facilitate wound healing. Compos. B Eng.. 2023 Feb;1(250):110451
- [Google Scholar]







