Translate this page into:
HPLC-MS/MS multiclass determination of steroid hormones in environmental waters after preconcentration on the carbonaceous sorbent HA-C@silica
⁎Corresponding author. antonella.profumo@unipv.it (Antonella Profumo)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In this study, a sensitive and multiclass method has been developed for analysis of three families of steroid hormones, i.e. progestins, oestrogens, androgens, by SPE-HPLC-ESI-MS/MS. The extraction efficiency of thermally condensed humic acids onto silica sorbent (HA-C@silica), here for the first time studied for multiclass enrichment of these sex hormones, was tested in different environmental waters (tap and river water, urban wastewater treatment plant effluent) spiked at the nanograms per litre levels (5–1000 ng L−1). Quantitative adsorption was achieved using 200 mg sorbent for preconcentration of 250–1000 mL sample, at the native pH (pH = 6.5–7.7). Elution was performed by two sequential fractions (methanol followed by acetonitrile), obtaining in all the matrices investigated satisfactory recoveries (71% to 124% for river waters and 71–113% for urban wastewater treatment plant effluent) and RSDs below 15% (n = 3). The high enrichment factors (up to 4000) coupled with high-performance liquid chromatography tandem mass spectrometry quantification (MRM mode) provided low limits of detection and quantification (a few ng L−1), that are suitable for environmental monitoring. Most of the analytes were detected in river water and in wastewater effluent samples (in the ng L−1 concentration range), attesting their environmental diffusion. The proposed method was extended to a fourth class, Glucocorticoids, achieving good results in river samples, by the same SPE cartridge and chromatographic run.
Keywords
Environmental waters
Steroid hormones
Multiclass determination
HPLC-MS
Pollutants
Solid-phase extraction
1 Introduction
Steroid hormones are active compounds involved in almost all vital physiological functions of the body, such as development of sexual characteristics, salt and water balance and metabolism, but also widely employed in medical treatments (Guedes-Alonso et al., 2016). Based on structural differences and affinities, steroid hormones can be divided into four subclasses: oestrogens, androgens, progestogens, corticosteroids (Herrera-Melián et al., 2018). Androgens, the most abundant hormones found in urban wastewater treatment plants (UWWTPs), are used in therapy and growth therapy (Guedes-Alonso et al., 2016; Huysman et al., 2017; Chang et al., 2011); oestrogens and progestins are widely consumed as oral and non-oral contraceptives and in hormone replacement therapy (Guedes-Alonso et al., 2016; Huysman et al., 2017; Chang et al., 2011; Barreiros et al., 2016; Czarny et al., 2017); corticosteroids are used in human medicine for a series of pathologies such as rheumatism, malignant tumours, skin diseases (Huysman et al., 2017; Speltini et al., 2018). Considering steroid hormones’ global extensive usage, their chemical properties, including high octanol–water partition coefficients, low water solubility (Barreiros et al., 2016; Czarny et al., 2017; Snow et al., 2013), and partial removal in UWWTPs (Guedes-Alonso et al., 2016; Iparraguirre et al., 2014; Zou et al., 2014), they are frequently detected in effluents, surface waters and tap water at ng L−1 levels (Chang et al., 2011; Snow et al., 2013; Zou et al., 2014; Vega-Morales et al., 2010; Rui Zhang et al., 2014). Even at these concentrations, they could act as Endocrine Disrupting Compounds (ECDs), causing adverse effects on human health, wildlife and fisheries by interaction with the endocrine system (Vega-Morales et al., 2010; Chang et al., 2011; Barreiros et al., 2016; Czarny et al., 2017; Pailler et al., 2009; Moreira et al., 2011; Kanso et al., 2013; Wang et al., 2016; Ma et al., 2016; Cavaliere et al., 2016). Laboratory and field studies have shown that oestrogenic exposure can cause reproductive disorders that ultimately could lead to reduction of the fertility and even collapse of wild fish populations (Herrera-Melián et al., 2018; Chang et al., 2011; Snow et al., 2013; Kolodziej et al., 2003; Meijide et al., 2016). Hence, the presence of EDCs in surface waters represents nowadays a topic of high concern for national and international organizations and regulatory agencies committed to public and environmental health. The European directives recommend the monitoring of 45 priority substances and propose a first watch list of substances that may pose a significant risk to or via the aquatic environment (Czarny et al., 2017; Jauković et al., 2017; Sousa et al., 2018). This list includes three oestrogens: estrone (E1), 17β-estradiol (E2) and 17α- ethinylestradiol (EE2) (Huysman et al., 2017; Czarny et al., 2017; Jauković et al., 2017; Decision 495/2015), also cited as contaminants that may require future regulations in Contaminant Candidate List of Environmental Protection Agency (Jauković et al., 2017; United States, 2016). It is evident that the development of fast, reliable and sensitive analytical methods for the determination of steroid hormones in environmental waters is a priority task for the assessment of the concentration levels of these compounds and their related ecological risk. The current analytical methods for determination of steroids essentially rely on liquid chromatography coupled with mass spectrometry which provides a rapid and accurate detection with very low method detection limits (Sousa et al., 2018). The low concentration levels of steroid hormones in surface water and WWTPs, together with the complexity of environmental matrices in which these compounds are dispersed, require a preconcentration step, mainly performed by solid-phase extraction (SPE) using reversed-phase or mixed-mode polymer-based sorbents (Chang et al., 2011; Vega-Morales et al., 2010; Pailler et al., 2009; Jauković et al., 2017; Wang et al., 2016; Ma et al., 2016; Cavaliere et al., 2016; Kolodziej et al., 2003; Kumar et al., 2009; Chang et al., 2009; Zhang and Fent, 2018; Tölgyesi et al., 2010; Kuster et al., 2009). Most methods focused on a single class of hormones, especially oestrogens in view of their evident behaviour as ECDs (Kumar et al., 2009; Alda and Barceló, 2001; LaFleur and Schug, 2011), and only few methods proposed a multiclass analysis (Huysman et al., 2017; Shimko et al., 2019; Chang et al., 2009; Zhang and Fent, 2018; Tölgyesi et al., 2010).
Based on the present state of the art, aim of this work is to develop a sensitive method for simultaneous extraction and determination of steroid hormones in river water and UWWTPs effluent by SPE using a home-made sorbent based on thermally condensed humic acids (HAs) onto micrometric silica (HA-C@silica), followed by high performance liquid chromatography tandem mass spectrometry with electrospray ionization (HPLC-ESI-MS/MS). The characteristics of HA-C@silica as a mixed-mode carbonaceous sorbent, which guaranteed successful results for preconcentration of glucocorticoids (GCs) from environmental waters (Speltini et al., 2018), prompted us to extend the application of this SPE sorbent to environmental determination of other classes of steroid hormones that can be frequently present in the same sample.
In detail, HA-C@silica was tested for multiclass preconcentration of seven sex hormones belonging to three different classes (Fig. 1) - viz. E1, E2, EE2, Testosterone (TST), Progesterone (PROG), 17α-hydroxyprogesterone (H-PROG), medroxyprogesterone acetate (M-PROG) – from tap and river waters, and UWWTP effluents, before and after spiking. After SPE, quantitation was done by HPLC-ESI-MS/MS. The overall performance obtained in terms of recovery, precision, enrichment factor (EF), linearity, matrix effect and sensitivity was discussed. Finally, the analytical procedure has been extended to GCs and it was applied to the environmental monitoring in actual water samples to gain more knowledge on the contamination of natural waters.
The seven sex hormones studied in this paper.
2 Experimental
2.1 Chemicals and materials
All chemicals were reagent grade or higher in quality. Silica microparticles (40–63 μm, surface area 550 m2 g−1, pore volume 0.8 cm3 g−1), humic acids sodium salt (technical grade), formic acid (FA), ammonium hydroxide solution (NH4OH), ammonium fluoride (NH4F), polypropylene tubes and polyethylene frits, high purity steroid hormones standards (E1, E2, EE2, TST, PROG, M-PROG, cortisone (CORT), hydrocortisone (H-CORT), prednisone (PRED), prednisolone (PREDLO), betamethasone (BETA), dexamethasone (DEXA) were supplied by Sigma-Aldrich (Milan, Italy). Analytical grade H-PROG standard was purchased from Steroids (Cologno Monzese, Italy). Analytical grade triamcinolone (TRIAM) and fluocinolone (FLUO) standards were purchased from Farmabios (Groppello Cairoli, Italy). HPLC gradient grade methanol (MeOH), acetonitrile (ACN) and ultrapure water were supplied by VWR (Milan, Italy).
Stock solutions of 1000 μg mL−1 of each steroid hormone were prepared in MeOH and stored in the dark (4 °C). Steroid hormones working solutions of 0.15–1 μg mL−1 were prepared by dilution from a 10 μg mL−1 solution, and they were renewed weekly.
2.2 SPE procedure using HA-C@silica
The SPE procedure was carried out placing in a 3 mL SPE cartridge 200 mg HA-C@silica between two polyethylene frits. HA-C@silica is obtained by a thermal treatment of HAs immobilized onto micrometric silica, as reported in previous work (Speltini et al., 2018). Briefly, HAs (100 mg) were dissolved in 50 mL water in a round-bottom flask, and the suspension obtained by adding 1 g silica was magnetically stirred for 2 min. After water evaporation under vacuum, the resulting brownish material was thermally treated at 600 °C for 1 h under N2 flow into a cylindrical oven (heating ramp 10 °C min−1, cooling ramp 10 °C min−1).
Water samples (500–1000 mL for river water and 250 mL for UWWTP effluent were loaded onto the SPE cartridge at a flow rate of 5 mL min−1, by a multi-channel peristaltic pump (Gilson, Italy) and then the cartridge was dried under vacuum for 5 min. The analytes were eluted (1 mL min−1) with 4 mL MeOH and 4 mL ACN, sequentially. For sample spiking ≤ 25 ng L−1, the extracts were evaporated under N2 flow and reconstituted with 0.25 mL MeOH, prior HPLC-ESI-MS/MS analysis.
2.3 Water samples
Tap water was from the Pavia municipal waterworks. Northern Italy surface waters were collected in Spring and Summer 2018 at 30–50 cm depth, directly in amber glass bottles, from various rivers (Tanaro, Ticino, Staffora), and at the outlet of Pavia UWWTP. Samples were stored in the dark (4 °C) and analyzed within 24 h of collection to check the absence/presence of the target analytes. Staffora river and UWWTP effluent were selected as blank environmental samples due to the absence of all analytes (<MDLs) as verified from recovery tests and quantification by the standard addition method. The physical-chemical parameters of the water samples are reported in Table S1, Electronic Supplementary Material.
2.4 HPLC-ESI-MS/MS analysis
The target analytes were analysed with a HPLC apparatus Agilent 1260 Infinity coupled with an Agilent 6460C MS spectrometer ESI-MS/MS system (Cernusco sul Naviglio, Italy).
The MS spectrometer was tuned up by direct injection of individual analyte solutions (1 mg L−1 in MeOH), and different experimental conditions were investigated to maximize the signal response and increase detection sensitivity. First, the HPLC-ESI-MS/MS conditions proposed in the previous work (Speltini et al., 2018) were applied, but a poor ionization of the target analytes, in particular of oestrogens, was observed. Based on the literature (Shimko et al., 2019; Agilent Application Note), NH4F was selected as additive for the aqueous phase to increase sensitivity.
The MS operating parameters, optimized by Agilent Mass Hunter Source Optimizer Software (Agilent, USA) were: drying gas (N2) temperature 350 °C; drying gas flow 12 L min−1; nebulizer 50 psi; sheath gas temperature 400 °C; sheath gas flow 12 L min−1; capillary voltage 4000 V positive, 3000 V negative; nozzle voltage 0 V positive, 1500 V negative; electron multiplier voltage (EMV) 200 V positive, 0 V negative; cell accelerated voltage 4 V positive, 1 V negative.
The quantitative analysis of the target compounds was performed in multiple-reaction monitoring (MRM) mode, using the two most intense transitions of each compound, as reported in Table 1.
Precursor ion* (m/z)
MRM productions (m/z)
Dwell time
Fragmentor Energy (V)
Collision energy (V)
Polarity
E1
269
145.1
15
148
44
Negative
143.1
15
148
60
E2
271
183.3
100
166
50
Negative
143.1
100
166
64
EE2
295
145.1
50
154
44
Negative
143.1
50
154
68
PROG
315
109
15
94
24
Positive
97
15
94
20
H-PROG
331
109.1
15
118
28
Positive
97.1
15
118
24
M-PROG
387
327.4
15
106
8
Positive
123.1
15
106
24
TST
289
109
15
76
24
Positive
97.1
15
76
20
The chromatographic separation was achieved on a Zorbax Eclipse Plus C18 column (4.6 × 100 mm, 3.5 μm), maintained at 25 °C (±0.8 °C), and the injection volume was 5.0 μL. Elution was performed by 1 mM NH4F in the aqueous mobile phase (A) and ACN (B) according to the following program: 30% B for 0.5 min, linear gradient to 85% B in 12 min, maintenance of 85% B for 5 min, washing step with 100% B for 2.5 min. The initial conditions were re-established by 7-min equilibration time (0.5 mL min−1 flow rate, column temperature 25 ± 0.8 °C). A typical MRM chromatogram of a standard solution (150 μg L−1) is reported in Fig. 2.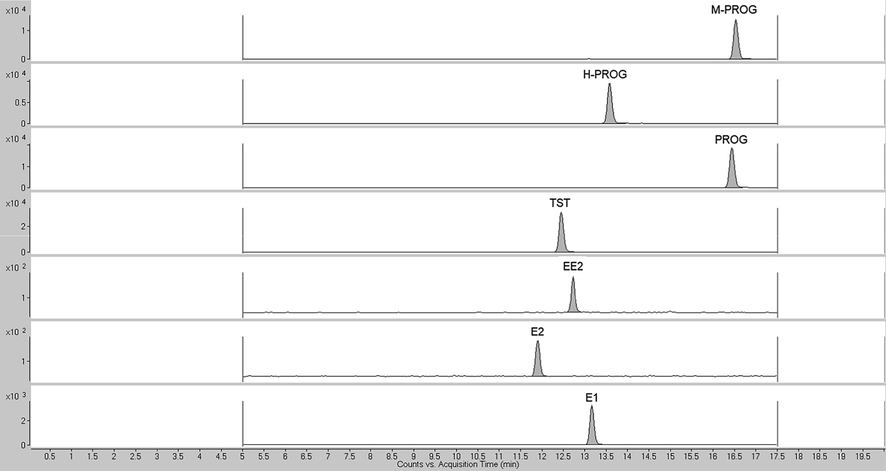
A typical MRM chromatogram of a standard solution of sex hormones (150 μg L−1).
2.5 Analytical evaluation of the SPE/HPLC-ESI-MS/MS procedure
In term of a consistent application of the proposed procedure to multiclass enrichment of steroid hormones from real-world water samples, the entire analytical procedure was within-laboratory evaluated based on the main figures of merit (Speltini et al., 2018; Rui Zhang et al., 2014; Tölgyesi et al., 2010), viz. trueness, precision, linearity, detection and quantification limits.
2.5.1 Trueness and precision
In the absence of certified reference materials, trueness and precision of the method were investigated through recovery tests (Speltini et al., 2018; Dorival-García et al., 2013) performed in tap, river and UWWTP effluent matrices, spiked with each steroid in the range 5–1000 ng L−1 for tap and river waters, and in the range 25–1000-ng L−1 for UWWTP effluent (n = 3). Within-laboratory precision was evaluated by calculating the relative standard deviation (RSD%).
2.5.2 Calibration curves, linearity and matrix effect
The standard addition method on the SPE eluate was chosen for matrix-matched quantification (Speltini et al., 2018; Speltini et al., 2019), in the linear range 10–150 μg L−1. As previously described (Speltini et al., 2018; Speltini et al., 2019); different -small- volumes of a concentrated standard solution were directly added on each SPE eluate by the HPLC autosampler. This approach matches good precision and compensation of matrix effects that are due to those constituents of the matrix retained on the HA-C@silica and eluted with the analytes causing ion suppression or improvement at the electrospray interface, with consequent errors and inaccurate results. To evaluate this matrix effect, the responses obtained in pure methanol were compared with those observed in the SPE eluate (Speltini et al., 2018).
2.5.3 Detection and quantification limits
Instrumental detection limits (IDLs) have been calculated from the matrix-matched calibration curves obtained from a blank pre-concentrated matrix (1000 mL river water and 250 mL UWWTP effluent). In detail, for each analyte IDL was calculated as three times the ratio between the baseline noise away from the peak tail and the regression line slope (Sheehan and Yost, 2015); instrumental quantification limits (IQLs) were calculated as 3.3 times IDLs. Estimated MDLs and MQLs were obtained from IDLs and IQLs, respectively, taking into consideration the EFs (Guedes-Alonso et al., 2016; Barreiros et al., 2016; Speltini et al., 2018; Speltini et al., 2019).
3 Results and discussion
3.1 Solid-phase extraction on HA-C@silica
As described in Section 2.2, this work focuses on multiclass enrichment of three classes of steroid hormones, using HA-C@silica, a mixed-mode sorbent material recently successfully applied by this research group for preconcentration of GCs (Speltini et al., 2018) and benzotriazoles and benzothiazoles (Speltini et al., 2019) from environmental waters. Due to its peculiar characteristics – viz. the presence of sp2 hybridized carbon and polar groups- HA-C@silica was here tested for the extraction of oestrogens, progestins and androgens. It was investigated firstly in tap water samples (500 mL) enriched with 1 μg L−1 of each compound. Based on literature data about the commercial SPE cartridges (Pailler et al., 2009; Moreira et al., 2011; Jauković et al., 2017; Kumar et al., 2009; Kuster et al., 2009; Chen et al., 2012), the extraction was carried out at the sample native pH and under acidic conditions (pH 3). Generally (data shown in Table S2, Supplementary material), the pH influence was not so pronounced considering the RSDs, although higher extraction efficiencies were achieved for all analytes at native pH values. Therefore, the extraction was done with no pH adjustment.
In these preliminary tests, elution was performed with MeOH (4 mL), which provided quantitative recovery for all analytes except for PROG (50%). Hence, further tests were done in the same experimental conditions (500 mL tap water spiked with 1 μg L−1 of each hormone) to assess the performance of other eluents: MeOH + 2% v/v NH4OH (alkaline solvent), MeOH + 0.2% v/v FA (acidic solvent) and ACN (aprotic solvent). Alkaline and acidic MeOH did not lead to improvements in the elution of PROG, while the aprotic solvent provided quantitative recovery for this compound, which indeed does not present —OH groups in the skeleton. On the other hand, ACN provided lower recovery than ones obtained with MeOH for the other compounds, as reported in Table S3, Supplementary material. By these evidences, it seemed clear that the SPE procedure has to be performed with elution in two sequential steps, using 4 mL MeOH followed by 4 mL ACN.
HA-C@silica was then tested first in a river water sample not containing the analytes (blank sample from Staffora river) and on the same sample after spiking (500 mL enriched at 25–600 ng L−1) to verify the recovery. As it is shown in Table 2, good performance was obtained at the different concentrations, with recovery slightly lower for PROG. The highest EF (4000) was achieved by preconcentrating 1 L of river water enriched with 5 ng L−1, obtaining satisfying recoveries also at the lowest concentration level for at least three consecutive extractions (71–124%). RSDs < 15%, n = 3.
Mean recovery (%)
River water (500 mL)
UWWTP effluent (250 mL)
Spike (ng L−1)
600
300
25
5b
1000
300
25
E1
120
79
81
101
76
95
95
E2
124
91
109
120
89
87
90
EE2
105
86
106
104
71
89
75
PROG
74
72
75
71
79
106
109
H-PROG
115
103
117
105
91
107
113
M-PROG
79
81
84
91
93
103
112
TST
120
83
115
111
83
85
92
The SPE procedure was then evaluated on a more complex aqueous matrix, that is UWWTP effluent (physical-chemical parameters are reported in Table S1, Electronic Supplementary Material). In this matrix, accurate recoveries were achieved processing a sample volume of 250 mL at the three concentration levels investigated (25, 300 and 1000 ng L−1), for all contaminants (see Table 2).
These results collected on different water matrices demonstrate the real applicability of HA-C@silica for preconcentration of different classes of sex hormones in environmental waters at low nanograms per litre levels, with results comparable to those reported in the most recent literature (Zhang and Fent, 2018).
3.2 HA-C@silica SPE followed by HPLC-ESI-MS/MS: Figures of merit
3.2.1 Trueness and precision
Trueness, investigated by recovery tests as the percentage of analyte extracted in relation to the spiking level, was satisfactory. As reported in Table 2, the mean recoveries (%) obtained in raw river water (500–1000 mL) at environmentally significant concentrations ranged from 71% to 124% for the target analytes. Quantitative extraction for all the analytes (71–113%) was also obtained in UWWTP matrix (250 mL).
Intra- and inter-day precision showed RSDs below 15% in all matrices.
3.2.2 Calibration curves and linearity
The matrix effect showed an average signal suppression of about 20% for all compounds with respect to the response given by the standard solution in pure MeOH (data not shown). A quite good compensation was achieved by the standard addition method operated by the instrument directly on the SPE eluate. Matrix-matched standard calibration showed good linearity in the range of 10–150 μg L−1 for all steroid hormones for river water and UWWTP effluent with correlation coefficients (R2) higher than 0.9937.
3.2.3 Detection and quantification limits
The calculated MDLs are in the range 0.02–0.9 ng L−1 for river water and 0.5–4.1 ng L−1 for UWWTPs effluent. The calculated MQLs are in the range 0.5–3.0 ng L−1 for river water and 1.8–13.7 ng L−1 for UWWTPs effluent. These results highlight that method sensitivity is suitable for environmental monitoring, as proved by ultra-trace determination of hormones in actual samples, described in the following.
3.3 Determination of steroid hormones in real-world samples
Based on the good results obtained by recovery tests, the proposed analytical method was applied to enrichment of real-world surface waters (Tanaro and Ticino rivers) collected in high-density populated areas of Northern Italy, and of UWWTP effluent sampled at the Pavia WWTP upstream the UV treatment. As expected and as reported in Table 3 and Fig. S1, steroid hormones were detected at few nanograms per litre, attesting their environmental diffusion in surface waters. In particular, high levels of oestrogens were found, probably due to their extensive usage and poor metabolization (Huysman et al., 2017; Barreiros et al., 2016). The results highlight the applicability of the proposed SPE coupled to HPLC-ESI-MS/MS method for environmental monitoring of steroid hormones at the low nanograms per litre levels. However, deeper monitoring investigations are required to be more aware about the actual contamination levels of these xenobiotic compounds in the water ecosystems considered.
Concentration (ng L−1)
Tanaro Rivera
Ticino Rivera
UWWTP effluentb
E1
76
14
39
E2
9
34
< MQL
EE2
3
6
15
PROG
4
6
10
H-PROG
6
<MDL
<MDL
M-PROG
6
<MDL
<MDL
TST
6
7
2
3.4 Application of method extended to GCs in river water
The good preconcentration results obtained on HA-C@silica in this work and in the previous one for GCs (Speltini et al., 2018) prompted us to verify the applicability of the HPLC-ESI-MS/MS method for the simultaneous and quantitative determination of the four classes of steroid hormones (oestrogens, progestins, androgens and GCs). For this reason, eight GCs (Speltini et al., 2018) were separated and fragmented in the new chromatographic conditions, proposed in this work (see Electronic Table S4, Supplementary Material).
Recovery tests were then performed in triplicate on the blank river water matrix (1 L) enriched with 5 ng L−1 of the fifteen analytes, obtaining quantitative recoveries (see Table 4). aRSDs < 15%, n = 3.
Mean recovery (%)a
Spike (ng L−1)
5
E1
107
E2
118
EE2
109
PROG
84
H-PROG
106
M-PROG
96
TST
109
CORT
84
H-CORT
87
PRED
84
PREDLO
101
BETA
105
DEXA
102
TRIAM
106
FLUO
89
At the same time, an aliquot of Ticino river sample was analysed; oestrogens, progestins, androgens were confirmed (see Table 3) and among GCs, CORT, H-CORT and DEXA were quantified at concentrations of 2–3 ng L−1, (see Fig. S2, Supplementary Material).
These findings show that all compounds are quantitatively pre-concentrated on the same cartridge and quantified in a single chromatographic run, further widening the method applicability compared to the most recent methods proposed in the literature (Zhang and Fent, 2018).
4 Conclusions
The present paper proposes a sensitive and straightforward method for multi-analyte determination of four classes of steroid hormones in environmental waters. The extraction was performed on HA-C@silica, a homemade mixed-mode carbonaceous sorbent here applied for the first time for multiclass SPE of sex hormones. The developed SPE procedure coupled with HPLC-ESI-MS/MS method provided good recovery and high enrichment factors in raw river water and UWWTP effluent, also at low nanograms per litre levels, and MDLs and MQLs suitable for identification and quantification of the target analytes in real-world samples.
Moreover, this work gives new evidences of the versatility of HA-C@silica, therefore further work is ongoing to widen HA-C@silica application in the sample treatment field, for both extraction and clean-up of trace analytes in complex matrices.
Acknowledgments
The Authors are grateful to Prof. Lorenzo Malavasi (Department. Chemistry, University of Pavia) for the experimental support.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Application of microwave-assisted extraction and ultra-high performance liquid chromatography–tandem mass spectrometry for the analysis of sex hormones and corticosteroids in sewage sludge samples. Anal. Bioanal. Chem.. 2016;408(24):6833-6844.
- [Google Scholar]
- Study on the removal of hormones from domestic wastewaters with lab-scale constructed wetlands with different substrates and flow directions. Environ. Sci. Pollut. Res.. 2018;25:20374-20384.
- [Google Scholar]
- Development and validation of an ultra-high performance liquid chromatographic high resolution Q-Orbitrap mass spectrometric method for the simultaneous determination of steroidal endocrine disrupting compounds in aquatic matrices. Analytica Chimica Acta. 2017;984:140-150.
- [Google Scholar]
- Occurrence of androgens and progestogens in wastewater treatment plants and receiving river waters: Comparison to estrogens. Water Res.. 2011;45:732-740.
- [Google Scholar]
- Analysis of 17-β-estradiol and 17-α-ethinylestradiol in biological and environmental matrices — A review. Microchem. J.. 2016;126:243-262.
- [Google Scholar]
- The impact of estrogens on aquatic organisms and methods for their determination. Crit. Rev. Environ. Sci. Technol.. 2017;47:909-963.
- [Google Scholar]
- Thermally condensed humic acids onto silica as SPE for effective enrichment of glucocorticoids from environmental waters followed by HPLC-HESI-MS/MS. J. Chromatogr. A. 2018;1540:38-46.
- [Google Scholar]
- Sensitive and simplified analysis of natural and synthetic steroids in water and solids using on-line solid-phase extraction and microwave-assisted solvent extraction coupled to liquid chromatography tandem mass spectrometry atmospheric pressure photoionization. Anal. Bioanal. Chem.. 2013;405:1759-1771.
- [Google Scholar]
- Matrix effect during the membrane-assisted solvent extraction coupled to liquid chromatography tandem mass spectrometry for the determination of a variety of endocrine disrupting compounds in wastewater. J. Chromatogr. A. 2014;1356:163-170.
- [Google Scholar]
- Hollow-fiber-supported liquid-phase microextraction using an ionic liquid as the extractant for the preconcentration of bisphenol A, 17-β-estradiol, estrone and diethylstilbestrol from water samples with HPLC detection. Water Sci. Technol.. 2014;69(5):128-135.
- [Google Scholar]
- Determination of alkylphenol polyethoxylates, bisphenol-A,17α-ethynylestradiol and 17β-estradiol and its metabolites in sewage samples by SPE and LC/MS/MS. J. Hazard. Mater.. 2010;183:701-711.
- [Google Scholar]
- Ionic liquid foam floatation coupled with ionic liquid dispersive liquid–liquid microextraction for the separation and determination of estrogens in water samples by high-performance liquid chromatography with fluorescence detection. J. Sep. Sci.. 2014;37:3133-3141.
- [Google Scholar]
- Solid phase extraction coupled to liquid chromatography-tandem mass spectrometry analysis of sulfonamides, tetracyclines, analgesics and hormones in surface water and wastewater in Luxembourg. Sci. Total Environ.. 2009;407:4736-4743.
- [Google Scholar]
- Determination of endocrine-disrupting compounds in waters from Rio das Velhas, Brazil, by liquid chromatography/high resolution mass spectrometry (ESI-LC-IT-TOF/MS) Environ. Technol.. 2011;32:1409-1417.
- [Google Scholar]
- Immunosensors for estradiol and ethinylestradiol based on new synthetic estrogen derivatives: application to wastewater analysis. Anal. Chem.. 2013;85:2397-2404.
- [Google Scholar]
- Monitoring of endocrine-disrupting compounds in surface water and sediments of the three gorges Reservoir Region China. Arch. Environ. Contam. Toxicol.. 2016;71(4):509-517.
- [Google Scholar]
- Parent and conjugated estrogens and progestogens in surface water of the Santa Ana river: determination, occurrence and risk assessment. Environ. Toxicol. Chem.. 2016;35(11):2657-2664.
- [Google Scholar]
- Multiresidue analysis of endocrine-disrupting compounds and perfluorinated sulfates and carboxylic acids in sediments by ultra-high-performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. A. 2016;1438:133-142.
- [Google Scholar]
- Quantification of steroid hormones with heromonal properties in municipal wastewater effluent. Environ. Contam. Toxicol.. 2003;22:2622-2629.
- [Google Scholar]
- Effects of waterborne exposure to 17β-estradiol and 4-tert-octylphenol on early life stages of the South American cichlid fish Cichlasoma dimerus. Ecotoxicol. Environ. Saf.. 2016;124:82-90.
- [Google Scholar]
- Determination of sterols and steroid hormones in surface water and wastewater using liquid chromatography-atmospheric pressure chemical ionization-mass spectrometry. Microchem. J.. 2017;135:39-47.
- [Google Scholar]
- A review on environmental monitoring of water organic pollutants identified by EU guidelines. J. Hazard. Mater.. 2018;344:146-162.
- [Google Scholar]
- Decision 495/2015/EU of 20 March 2015 establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council, Off. J. Eur. Union 78 (2015) 40–42.
- United States Environmental Protection Agency (US EPA), Contaminant Candidate List (CCL) and Regulatory Determination, https://www.epa.gov/ccl/chemical-contaminants-ccl-4 2016 (accessed 24.03.17).
- Rapid determination of free and conjugated estrogen in different water matrices by liquid chromatography–tandem mass spectrometry. Chemosphere. 2009;77:1440-1446.
- [Google Scholar]
- Determination and source apportionment of five classes of steroid hormones in urban rivers. Environ. Sci. Technol.. 2009;43:7691-7698.
- [Google Scholar]
- Determination of two progestin metabolites 17α-hydroxypregnanolone and pregnanediol) and different classes of steroids (androgens, estrogens, corticosteroids, progestins) in rivers and wastewaters by high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) Sci. Total Environ.. 2018;610–611:1164-1172.
- [Google Scholar]
- Simultaneous determination of corticosteroids, androgens and progesterone in river by liquid chromatography/tandem mass spectrometry. Chemosphere. 2010;78:972-979.
- [Google Scholar]
- Analysis of phytoestrogens, progestogens and estrogens in environmental waters from Rio de Janeiro (Brazil) Environ. Int.. 2009;35(7):997-1003.
- [CrossRef] [Google Scholar]
- Review of analytical methods for the determination of estrogens and progestogens in waste waters. Fresenius J. Anal. Chem.. 2001;371:437-447.
- [Google Scholar]
- A review of separation methods for the determination of estrogens and plastics-derived estrogen mimics from aqueous systems. Anal. Chim. Acta. 2011;696:6-26.
- [Google Scholar]
- A pilot wastewater-based epidemiology assessment of anabolic steroid use in Queensland, Australia. Drug Test Anal.. 2019;11:937-949.
- [Google Scholar]
- Agilent Application Note, Improved Analysis of Trace Hormones in Drinking Water by LC/MS/MS (EPA 539) using the Agilent 6460 Triple Quadrupole LC/MS.
- Analysis of quinolone antibiotic derivatives in sewage sludge samples by liquid chromatography–tandem mass spectrometry: comparison of the efficiency of three extraction techniques. Talanta. 2013;106:104.
- [Google Scholar]
- Humic acids pyrolyzed onto silica microparticles for solid-phase extraction of benzotriazoles and benzothiazoles from environmental waters. Chromatographia. 2019;82:1275-1283.
- [Google Scholar]
- Sheehan, T.L., Yost, R.A., 2015. Spectroscopy solution for material analysis.
- Ultra-high performance liquid chromatography/tandem mass spectrometry determination of feminizing chemicals in river water, sediment and tissue pretreated using disk-type solid-phase extraction and matrix solid-phase dispersion. Talanta. 2012;89:237-245.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2019.10.009.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







