Translate this page into:
HPLC profiling and studies on Copaifera salikounda methanol leaf extract on phenylhydrazine-induced hematotoxicity and oxidative stress in rats
⁎Corresponding author at: Department of Medical Biochemistry, Faculty of Basic Medical Sciences, Alex Ekwueme Federal University Ndufu-Alike, Ebonyi State, Nigeria Chinyere.aloke@funai.edu.ng (Chinyere Aloke)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Anemia is a clinical disorder orchestrated by factors such as drugs, including phenylhydrazine (PHZ). This study explored the protective roles of Copaifera salikounda methanol leaf extract (CSMLE) against PHZ-induced hematotoxicity and oxidative stress in rats. In vitro antioxidants studies of CSMLE using 1,1-diphenyl-2-picryl hydrazyl (DPPH) and ferric reducing antioxidant potential (FRAP) and quantification of CSMLE bioactive compounds using High-Performance-Liquid-Chromatography (HPLC) were done. In vivo, thirty rats in five groups (n = 6) were used. Anemia was induced intraperitoneally in groups 2 to 5 with 40 mg/kg body-weight/day PHZ for two days. Group 1 received normal saline, group 2 (untreated: negative-control), group 3 received 1 ml/kg-body-weight Vit B12 syrup while groups 4 and 5 received 200 mg/kg and 400 mg/kg-body-weight CSMLE, respectively, daily for fourteen days. Result of CSMLE profiling showed luteolin (76.49 ng/Ml) and squalene (38.31 ng/Ml) as the most abundant flavonoid and terpenoid, respectively. In vitro CSMLE demonstrated dose-dependent antioxidant effects on DPPH and FRAP with IC50 of 0.1085 mg/ml and 0.07344 mg/ml (EC50), respectively. In vivo, CSMLE improved the body weight, red blood cell, hematocrit and hemoglobin concentrations, significantly (P < 0.05), reduced serum malondialdehyde and nitic oxide but increased reduced glutathione levels, superoxide dismutase and catalase activities and attenuated the levels of Interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α) in dose-dependent manner in treated groups relative to the negative control. The spleen tissues histology results corroborated biochemical results. The results indicated that CSMLE possesses anti-anemic potentials and attenuate spleenotoxic effects of PHZ.
Keywords
Phenylhydrazine
Oxidative stress
Hematotoxicity
Copaifera salikounda
Spleenotoxic effects
1 Introduction
Globally, hemolytic anemia is a critical public health challenge that affects people of any age (Kambou et al., 2015). Studies had shown that 43% of children and 33% of non-pregnant women globally were anemic, with Africa and South Asia having the highest incidence (Stevens et al., 2013). The prevalence of anemia on the general population world over is on the increase and about 1.62 billion people are estimated to be affected by this disease (Stevens et al., 2015). Anemia is a medical disorder characterized by abnormal red blood cells count, reduced quality or quantity of red blood cells (RBCs). Its impact on health, physical and mental productivity negatively affects the quality of life and culminates in profound economic losses for individuals and countries where anemia is highly prevalent (Diallo et al., 2008). It is manifested by diminution of hemoglobin rate<13 g/dl in male or 12 g/dl in female (Mozos, 2015). About 10 to 20% of the people living in the tropics presents <10 g/dl of hemoglobin. Many people particularly children and pregnant women are exposed to this chronic anemia condition which make them develop pathologies. Different kinds of anemia exist such as aplasic, metabolic, regulatory and hemolytic anemia (Diallo et al., 2008). Hemolytic anemia can be inherited (due to lack of glucose-6-phosphate dehydrogenase) or acquired (due to exposure to hemolytic agents) which culminate in the intra- or extra-vascular damage of red blood cells (RBCs) (Offman et al., 2013). It can also be caused by deficiency of pyruvate dehydrogenase. This enzyme catalyzes the conversion of phosphoenolpyruvate to pyruvate, generating adenosine triphosphate (ATP). Its deficiency culminates in reduction of ATP production and shortened reticulocyte and red cell lifespan because of an inability to maintain the red cell electrochemical gradient and membrane integrity as well as red cell damage and clearance in the spleen (Grace et al, 2015; Zanella et al, 2007).
Exposure to chemicals, in addition to the intake of some drugs, has been seen to alter the lifespan of RBC in the body (Berger, 2007; Kale et al., 2019) and hemolytic disorder (HD) which causes hemolytic anemia is included as a clinical syndrome linked with such intoxication (Awodele et al., 2015; Guillaud et al., 2012). Different chemicals elicit hemolysis through association with sulfhydryl groups, enzymes inhibition, immune mechanisms and fragmentation of RBCs as they move via the platelet (PLT)-fibrin mesh or by unknown or poorly defined mechanisms (Stevens et al., 2015). Phenylhydrazine (PHZ) is a drug that is used for the induction of anemia and studying of anemia mechanisms (Stevens et al., 2015). PHZ is used in the induction of hemolytic anemia because of its interaction with hemoglobin culminating in the generation of hydrogen peroxide which destroys the pigment via the formation of oxidized derivatives and free radicals of the hydrazine. These free radicals result in increased lipid peroxidation promoting membrane damage, diminution in endogenous antioxidant level, altered potassium and calcium permeability, methaemoglobin formation as well as Heins body which induces hemolysis (Paul et al., 2014). Additionally, PHZ causes the activation of immune response which promotes phagocytosis and also inhibit the binding of erythropoietin (EPO) receptors and Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway (JAK-STAT pathway). Furthermore, it causes genotoxic effect via the formation of single strand DNA damage. Due to lipid peroxidation coupled with the production of thiobarbituric acid (TBA)-reactive malondialdehyde, PHZ induces anemia as a result of peroxidation of RBC membrane lipids prompted by the upshot of the autoxidation of the drug and the reaction of membrane lipids and oxygen radicals (Shwetha et al., 2019)
Induction of anemia using PHZ offers experimental model for research into the pathogenesis of HD and the effect of anemia on other physiological conditions or the course of linked disorders (Kale et al., 2019). HD reduces the oxygen transferring capacity of RBCs and increases blood iron which culminates in the alteration of body processes (Awodele et al., 2015; Biswas et al., 2005). Peroxidation of erythrocytic membranes caused by oxidative stress is an important mechanism of hemolysis and direct cause of various blood disorders (Fibach and Rachmilewitz, 2008; Sochaski et al., 2002). PHZ causes oxidative stress which raises reactive oxygen species (ROS) by increasing the concentration of malondialdehyde (MDA), a lipid peroxidation metabolite and lowers antioxidant status, respectively (Luangaram et al., 2007; McMillan et al., 2005). As a strong oxidant, PHZ reactive metabolites phenyldiazene,” “phenylhydrazyl radical,” and “benzenediazonium ion” undergo automatic systematic oxidation that brings about the destruction of erythrocytes by virtue of oxidizing hemoglobin (Berger, 2007). Hence, attenuation of hemoglobin oxidation may be a good strategy against PHZ-induced hemolytic anemia.
Pharmacologists have paid appreciable attention towards the innovation and discovering of therapeutic agents from safe sources owing to the significant functions which traditional medicine could play in prophylaxis and/or treatment of diseases, in addition to improvement of the health status and performance of healthy subjects (Mills and Bone, 2000). Consequently, there is growing interest in the development of therapeutic agents from medicinal plants due to their accessibility, low cost, effectiveness and minimal sides effects (Ijioma et al., 2014). The devastating effects of anemia may be controlled directly by the actions of some phytochemicals or herbs which contain the phytochemicals. The mechanisms of actions of these phytochemicals may be through their antioxidant activity, antagonistic effects on oxidative stress or by autophagy (Trivedi and Pandey, 2021). Also, reports had shown that these phytochemicals may act by reversing chronic inflammation induced by anemia in the elderly population (Macciò and Madeddu, 2012).
Several plants had been harnessed for their therapeutic potentials and among them Copaifera salikounda of the family Caesalpiniaceae (Leguminosae or Fabaceae) is widely used in the treatment of diseases. The seed pod of C. salikounda had been reported to modulate immunological and hematological parameters in experimental rats (Aloke et al., 2019). To our knowledge, the protective potential of C. salikounda methanol leaf extract (CSMLE) against PHZ-induced hemolytic anemia has not been explored. Thus, this study aimed to investigate the possible antioxidant and anti-anemic potentials of CSMLE using in vitro and in vivo assays and to identify the bioactive components contained in the extract that might mediate such potentials.
2 Materials and methods
2.1 Drugs and chemicals
Phenylhydrazine and DPPH– (1, 1-diphenyl-1-picrylhydrazyl) were purchased from Sigma-Aldrich Chemical Co. (USA). All other chemicals/solutions/reagents utilized were of standard grade.
2.2 Plant materials and preparation of CSMLE
The fresh leaves of C. salikounda were obtained in April 2020 from Ugwulangwu in Ohaozara LGA, Ebonyi State. This was identified and authenticated by Mr. Alfred Ozioko, a taxonomist at International Centre for Ethnomedicine and Drug Development, Nsukka, Enugu State (ID: InterCEDD/16281) deposited at herbarium in the center. The fresh leaves of C. salikounda were dried and grounded into powder with the aid of a mortar and pestle. The powder obtained weighing 300 g was macerated in 3000 ml of absolute methanol for 72 h at room temperature with intermittent rigorous agitation. It was then filtered three times with muslin cloth and the resulting filtrate was concentrated under atmospheric pressure and room temperature.
2.3 Animals
Male Wistar albino rats weighing 180 to 190 g procured from the animal facility of Alex Ekwueme Federal University Ndufu-Alike, Ebonyi State, Nigeria were used. The rats were kept in standard plastic cages at 25 ± 1 °C and 12 hr dark/12 hr light cycle. They were given pelleted rat growers mash and water ad libitum. Prior to treatment, the rats were allowed to acclimatize for a period of 14 days. The study was carried out following the approval of the Faculty of Basic Medical Sciences Research and Ethics Committee, Alex Ekwueme Federal University Ndufu-Alike with code FBMS/EC/AE/1927. The rats were carefully managed following the approved standard protocol of NIH Guidelines for the Care and Use of Laboratory Animals (NRC, 1985)
2.4 Acute toxicity study
Acute toxicity study was carried out by oral administration of CSMLE at different doses of 500, 1000, 2000, 3000, 4000, and 5000 mg/kg following Karber’s method as described by Emelike et al. (2020)
2.5 Quantitative analysis of phytochemicals
2.5.1 HPLC apparatus and quantification of flavonoids in CSMLE
The methanol leaf extract was quantitatively analyzed to determine the bioactive compounds inherent in it using Agilent 1100 Series LC HPLC equipped with column thermostat, UV detector and a 20 ll injection loop (Agilent Technologies, CA, USA). Furthermore, the separation was obtained chromatographically employing a ZORBAX SB-C18 analytical column (250 mm _ 4.6 mm, 5 mm) at a wavelength of 257 nm. Phosphoric acid–metha nol-acetonitrile (0.01 mol.L–1) was employed as the mobile phase (72:14:14, v/v/v). The process as outline by Wang et al. (2010) and Agilent catalogue 2017 was followed. Into 50 ml centrifuge tube, exactly 3 g of the extract and 12 ml of the extraction solutions were added. These were vigorously shaken for 30 sec using vortex mixer set at high speed. Prior to extraction for about 20 min employing an oscillator on the maximum speed, the mixture’s pH was set to 2.0–2.2. Using 0.22 mm membrane filters (Thermo Fisher Scientific, UK), the supernatants were obtained after centrifugation at 13,000 rmp for 10 min (at 4 _C) and the filtrate (20 ml) was passed into HPLC for further analysis.
2.5.2 HPLC–UV method and quantification of terpenes
The HPLC system is comprised of 2695 Waters HPLC system furnished with a diode array sensor and Sphere Clone 5 µm ODS (2) column (4.6 × 250 mm, Phenomenex, Torrance, CA, USA) with temperature set at 35 °C. The mobile phase of the HPLC is comprised of water having 0.1% phosphoric acid (A) and 100% acetonitrile (B). A straight gradient was carried out with initial 5% B and later increased to 20% B from 0 to 20 min. Thereafter, it was increased to 95% B from 20 to 70 min. and held at 95% B up to 75 min. The UV sensing was regulated at 210 nm and flow rate was 0.75 ml/min while the injection volume was 25 μL. External calibration curve was used in the determination of the concentration of each terpene. Preparation of stock solutions of reference standards was made using concentration of 1.00 mg/mL in methanol and calibration solutions were made by diluting stock solutions with 50% aqueous methanol. There was linear correlation between the peak and concentration in the range of 6.25–100 μg/mL for all the calibration curves (Lü et al., 2020)
2.6 In vitro antioxidant assays
2.6.1 Determination of 1,1, dipheny-2-picrylhydrazyl (DPPH) radical scavenging activities
The DPPH radical scavenging assay was carried out by employing 1,1 diphenyl-2-picrylhydrazyl (DPPH) in accordance with the procedure outlined by Brand-Williams et al. (1995) with slight modifications. Exactly 5 different concentrations of the leaf extract (0.2, 0.4, 0.6, 0.8, and 1 mg/ml) were prepared in methanol (analytical grade). L-ascorbic acid was used as the standard antioxidant and the same concentrations were equally prepared. Into a clean test tube, 1 ml of each of the leaf extract was added followed by addition of 0.5 ml of 0.3 mM DPPH in methanol. The mixture was thoroughly shaken and allowed to stand for 15 min at room temperature in the dark. The baseline consisted of blank solutions made up of the leaf extract solutions (2.5 ml) and 1 ml of methanol. DPPH solution (2.5 ml) and methanol (1 ml) were used as the negative while L-ascorbic acid at the same concentrations as the leaf extracts served as the positive control.
The absorbance values were taken at 517 nm with a spectrophotometer after incubation in the dark. The experiments were carried out three times. The DPPH radical scavenging activity was evaluated with the use of the equation described by Brand-Williams et al. (1995). where As is the absorbance of the sample, and Ac is the absorbance of the control.
The concentration at which the extract was able to mop up 50% (IC50) of the free radicals produced during the reaction of the DPPH antioxidant assay system was computed from a plot of percentage DPPH free radical inhibition versus the extract concentration.
2.6.2 Ferric reducing antioxidant power assay
The procedure as outlined by Oyaizu (1986) with slight modifications was employed in the determination of the reducing power of the extract. A mixture of five different concentrations of methanol extracts (0.2, 0.4, 0.6, 0.8, and 1 mg/ml) and gallic acid at the same concentrations in 2 ml phosphate buffer (0.2 M, pH 6.6) and 2 ml of 1% potassium ferricyanide (K3Fe(CN)6) was made. At 50 °C, the mixture was incubated for 20 min and thereafter 10% trichloroacetic acid (TCA) 2 ml was added. This was followed by centrifuging of the mixture at 1000 revolutions per minute (rpm) for 10 min. Briefly, 2 ml of the supernatant was aspirated and mixed with distilled water (2 ml) and 0.1% ferric chloride (FeCl3) [1 ml]. For each, the experiment was done in triplicate. Thereafter, the absorbance values were measured using spectrophotometer at 700 nm and EC50 determined using Linear Regression analysis and computed by software graph pad prism.
2.7 Experimental design
2.7.1 Induction of anemia
Induction of anemia in the experimental rats was done by intraperitoneal injection of 40 mg/kg body weight of phenylhydrazine in the rats for two consecutive days (Diallo et al., 2008). The hemoglobin (Hb) concentration of the PHZ-induced rats was assessed on the 2nd day and the rats whose Hb concentration was<13 g/dL were considered as anemic and included in this study.
2.7.2 Experimental groups
Thirty (30) male Wistar albino rats divided into five groups of six rats each were used. Anemia was induced in rats except normal group (group I) by intraperitoneal administration of 40 mg/kg of PHZ for 2 days. On the 2nd day, hemoglobin (Hb) level of induced rats was estimated, and the rats injected with PHZ whose Hb concentration < 13 g/dL were considered to be anemic and included in the study. The rats were divided into groups I to V and treated as follows for 14 days.
Group I: Normal rats fed with normal feed and water ad libitum from day 2 for 14 days.
Group II (Negative control): Anemic rats fed with normal feed and water ad libitum from day 2 for 14 days.
Group III (Positive control): Anemic rats treated with vitamin B12 syrup 1 ml/day from day 2 for 14 days.
Group IV: Anemic rats treated with C. salikounda methanol leaf extract (CSMLE) (200 mg/kg) from day 2 for 14 days.
Group V: Anemic rats treated with CSMLE (400 mg/kg) from day 2 for 14 days.
2.7.3 Determination of weight
The weight of the experimental rats was determined using an electronic weighing scale. The weight of the rats was monitored on a daily basis before, during, and after the experiment to know the effect of the extract on the body weight of the experimental rats.
2.7.4 Collection of blood sample
After the last administration of CSMLE on the 14th day, blood samples of about 3 – 4 ml were collected through retro-orbital bleeding into labeled EDTA for hematological parameter and plane tubes for other biochemical parameters. The samples were centrifuged using a standard centrifuge at the speed of 12 000 rpm for 10 min and the sera obtained for biochemical analysis.
2.7.5 Determination of packed cell volume (PCV) level
On the 2nd day of PHZ administration, the packed cell volume levels of the treated rats were estimated using micro-hematocrit reader.
2.7.6 Determination of hematological parameters
The hematological parameters such as the red blood cell count (RBC), white blood cell count (WBC), hemoglobin concentration (HGB), mean capsulated hemoglobin (MCH), mean corpuscular volume (MCV) and platelets (PLT) were determined with the use of an automatic counter (Sysmex K21, Tokyo, Japan), following the procedure outlined by Dacie et al. (2001).
2.7.7 Determination of cytokines concentration
The serum concentrations of tumor necrosis factor‐α (TNF‐α), interleukin‐1 beta (IL‐1β), interleukin-4 (IL‐4), and interferon gamma (IFN-γ) were determined employing an ELISA‐based kit for rats according to the manufacturer's instructions.
2.7.8 Assessment of oxidative stress markers and antioxidant status
The serum concentration of malondialdehyde (MDA) was evaluated spectrophotometrically by estimation of thiobarbituric acid reactive substance (TBARS) as explained by Buege and Aust (1978), while nitric oxide (NO) was carried out in accordance with the procedure outlined by Titheradge (1998). The serum activity of superoxide dismutase (SOD) was assayed following the procedure described by Fridovich and Mc-Cord (1969). In this method, the inhibition of autoxidation of epinephrine (pH 10.2) at 30 °C was employed. Briefly, 20 μL of the sample and 2.5 ml of 0.05 M carbonate buffer (pH 10.2) were mixed together and allowed to equilibrate in the spectrophotometer. Thereafter, freshly prepared 0.3 ml of 0.3 mM adrenaline was added and mixed by inversion. The increase in absorbance at 480 nm was observed spectrophotometrically at 30 s intervals for 150 s. The specific activity of SOD was expressed in Units/mg protein. The activity of Catalase (CAT) in serum was assayed according to the procedure described by Sinha (1972). Briefly, 20 μL of the sample was added in 2 ml of 30 mmol/L hydrogen peroxide (H2O2) in 50 mmol/L potassium phosphate buffer (pH 7.0) to initiate the reaction. Enzyme units were considered as mmol/L of consumed H2O2 per min g or m. The activity of glutathione peroxidase was determined following the method described by Rotruck et al. (1973). The serum concentration of reduced glutathione (GSH) was assessed by the method described by Ellman (1959). The principle of this method involves the oxidation of GSH by the sulfhydryl reagent 5,5′-dithio-bis (2-nitrobenzoic acid) (DTNB) to form the yellow derivative 5′-thio-2-nitrobenzoic acid (TNB), measurable at 412 nm. Briefly, 2.3 ml of potassium phosphate [0.2 M, pH 7.6] buffer was taken in the cell and/or cuvette followed by addition of 0.2 ml of sample. To the sample solution, 0.5 ml [DTNB] (0.001 M) in a buffer was added. The absorbance of reaction product in the cuvette was read after 5 min at 412 nm using Shimadzu 1601 UV/Visible double bean spectrophotometer. The chromophoric product produced from the reaction with the reduced glutathione, 2-nitro-5-thiobenzoic acid has a molar absorption at 412 nm. Thus, reduced GSH is proportional to the absorbance at 412 nm.
2.8 Histopathology
The spleen samples were harvested and washed using normal saline. Thereafter, they were immediately transferred to neutral buffered 10% (v/v) formalin solution for preservation and storage for at least 48 h. The preserved samples were dehydrated using graded ethanol. This was followed by clearing using xylene and then implantation in paraffin. Precisely, 5 μm of the processed spleen was made using rotary microtome followed by staining using hematoxylin and eosin (H and E). The stained sections of the processed spleen tissues were mounted for microscopic examination and any alterations in their histopathology were observed. Photomicrographs of these sections were taken with MoticTM light microscope camera with 5.0 megapixels resolution quality and histopathological alterations noted.
2.9 Statistical analysis
The analysis of data was carried out using IBM-SPSS Statistics software version 20 (IBM Corp., Armonk, Ny). Data was expressed as Mean ± SEM. One-way analysis of variance (ANOVA) with Tukey post-hoc test was used for the statistical tests. p < 0.05 was considered as the statistical level of significance.
3 Results
3.1 Result of the percentage yield of the extract
A yield of 102.5 g of the extract was obtained as semi-solid brownish-black residue. The percentage yield of the methanol extract obtained was 34.2 %
3.2 Result of the acute toxicity test
The result of acute toxicity test indicates that no mortality was recorded with the administration of the extract even at the highest dose of 5000 mg/kg. The different doses had no toxic effect on the normal behavior of the rats. Thus, 200 and 400 mg doses per day were chosen.
3.3 Result of HPLC quantitative analysis of flavonoid compounds present in CSMLE
The result of HPLC quantitative analysis of flavonoid compounds present in CSMLE is presented in Table 1. The result indicates that five flavonoid compounds were identified in CSMLE. These flavonoids in their order of abundance include: Luteolin (76.49 ng/Ml) > Caffeic Acid (9.83 ng/Ml) > Chlorogenic Acid (5.76 ng/Ml) > Apigenin (2.22 ng/Ml) > Quercetin (1.28 ng/Ml). The chromatogram graph (Fig. 1) showed five different peaks corresponding to the five identified compounds with Luteolin having the highest peak. The structures of the identified flavonoids are also depicted in Fig. 2. Values are arithmetic mean ± SEM (standard error of mean), n = 3
Compound
Conc ng/Ml
Caffeic Acid
9.83 ± 0.012
Luteolin
76.49 ± 0.006
Apigenin
2.22 ± 0.000
Quercetin
1.28 ± 0.005
Chlorogenic Acid
5.76 ± 0.012
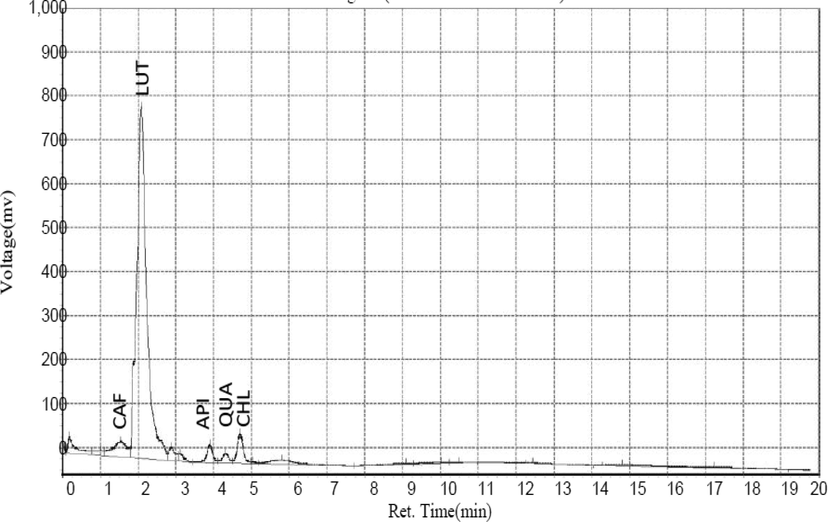
Chromatogram of identified flavonoids corresponding to five different peaks.
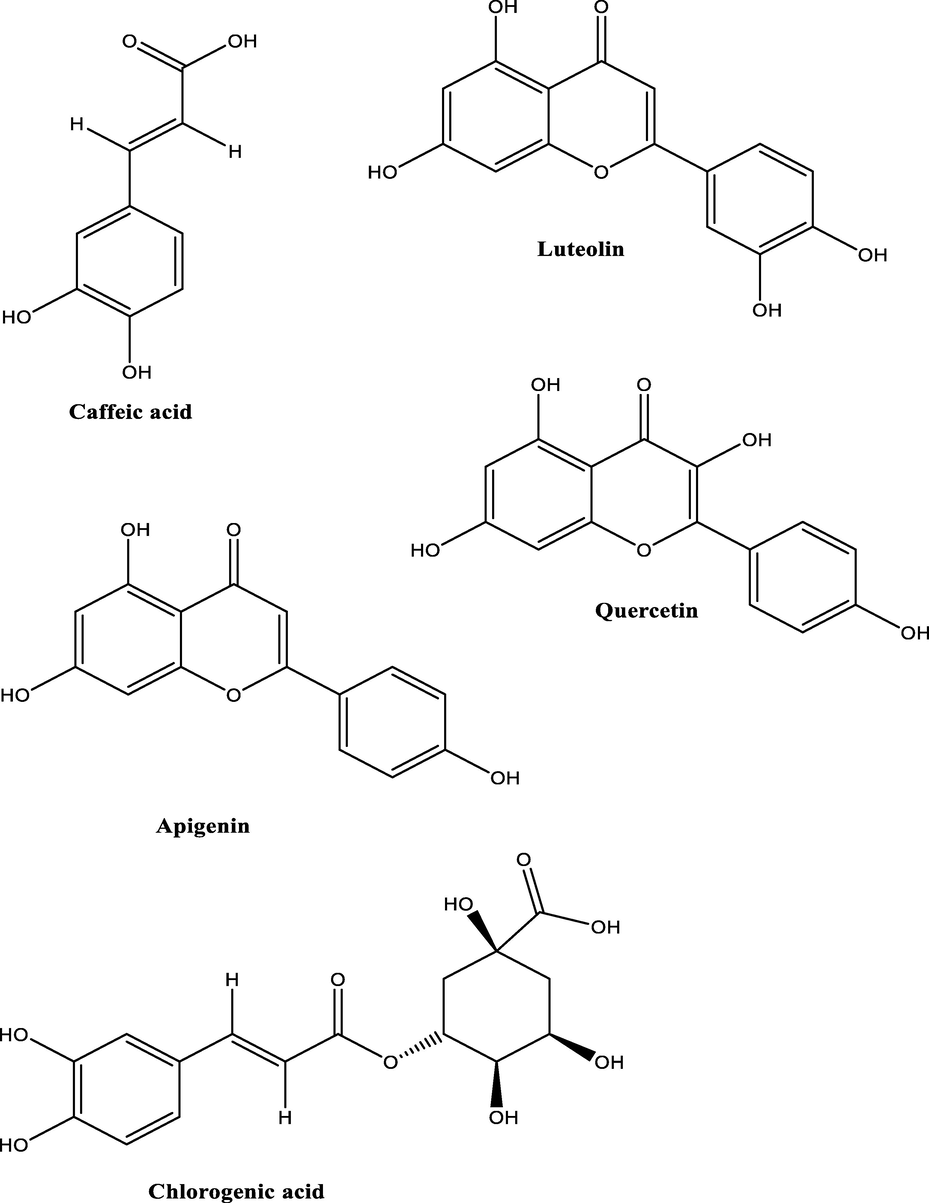
Structures of identified flavonoids.
3.4 Result of HPLC quantitative analysis of terpenoids compounds present in CSMLE
The result of HPLC quantitative analysis of terpenoids compounds present in CSMLE is shown in Table 2. The result showed that six different terpenoids were identified in CSMLE with Squalene (38.31 ng/mL) having the highest concentration. The six compounds correspond to six different peaks in the chromatogram graph (Fig. 3). The six compounds in their order of abundance are: Squalene (38.31 ng/mL) > Geraniol (19.08 ng/mL) > Fusidic Acid (14.02 ng/mL) > Nicaeenin (5.81 ng/mL) > Drimenin (2.69 ng/mL) > Pardinol (1.37 ng/mL). The structures of these terpenoids are shown in Fig. 4. Values are arithmetic mean ± SEM (standard error of mean), n = 3
Compound
Conc ng/mL
Squalene
38.31 ± 0.005
Geraniol
19.08 ± 0.005
Nicaeenin
5.81 ± 0.011
Pardinol
1.37 ± 0.011
Drimenin
2.69 ± 0.005
Fusidic Acid
14.02 ± 0.011
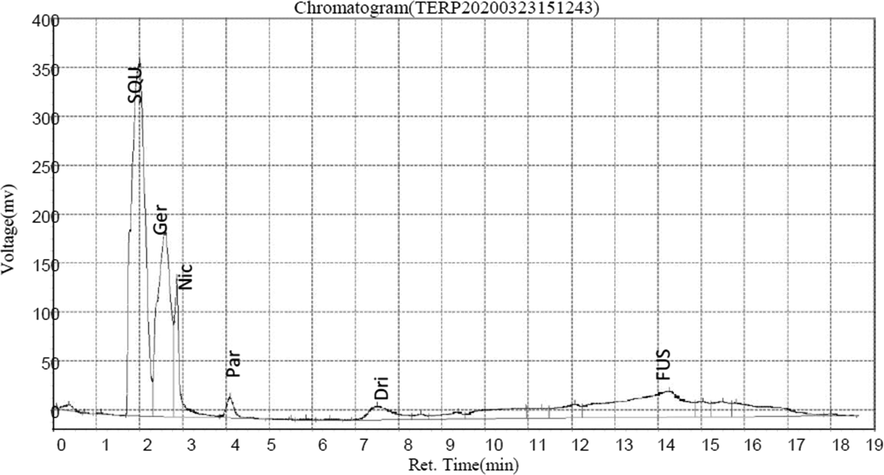
Chromatogram of identified terpenoids corresponding to the different peaks.
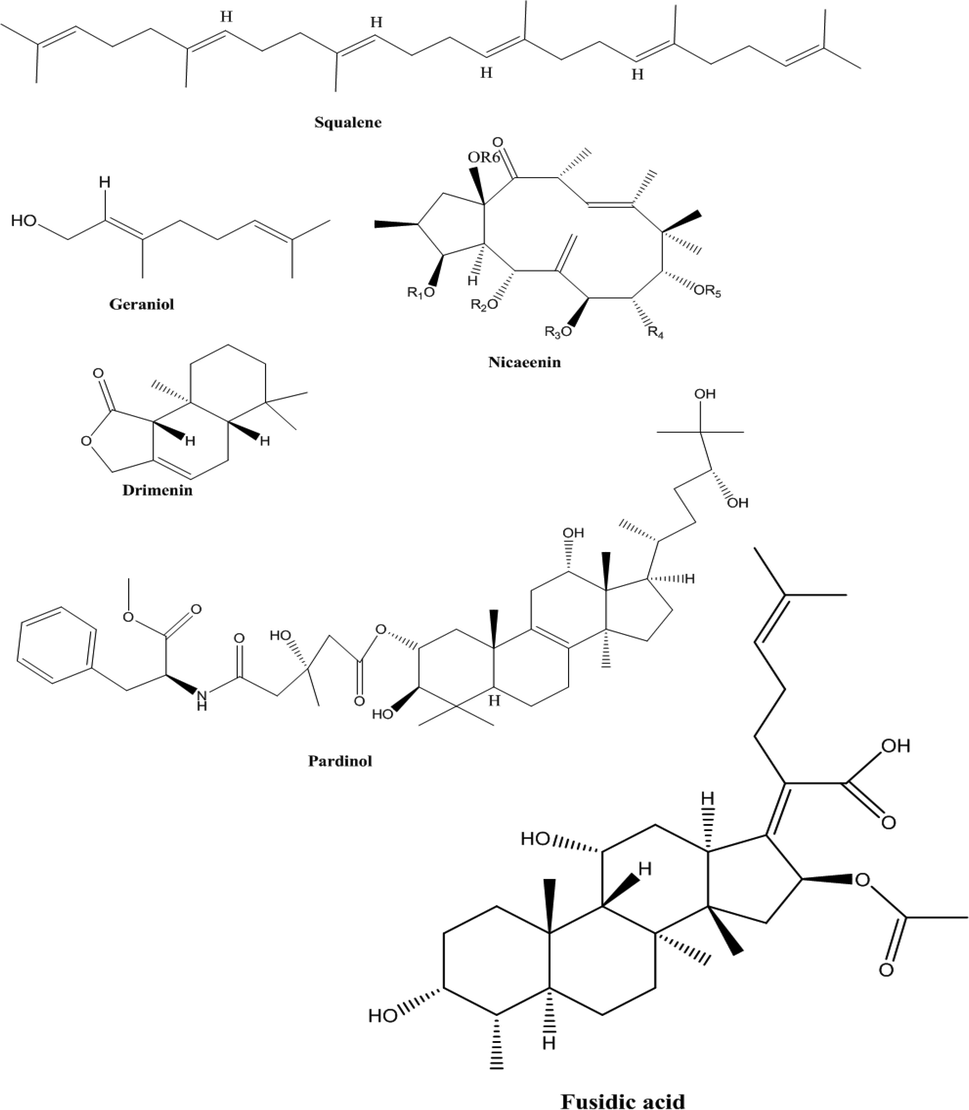
Structures of identified terpenoids.
3.5 Result of 1,1, dipheny-2-picrylhydrazyl (DPPH) radical scavenging activities.
The result of DPPH radical scavenging activities of CSMLE is shown in Fig. 5. CSMLE exhibited a profound in vitro DPPH radical scavenging potentials in a concentration dependent manner. The standard (ascorbic acid) showed remarkably higher DPPH radical scavenging activities than the extract. The concentration of CSMLE needed to scavenge 50% of the DPPH radicals (IC50) was also evaluated in this study. The IC50 values for CSMLE and ascorbic acid were 0.1085 mg/ml and 0.02779 mg/ml, respectively. The IC50 value of the standard was lower than that of the extract.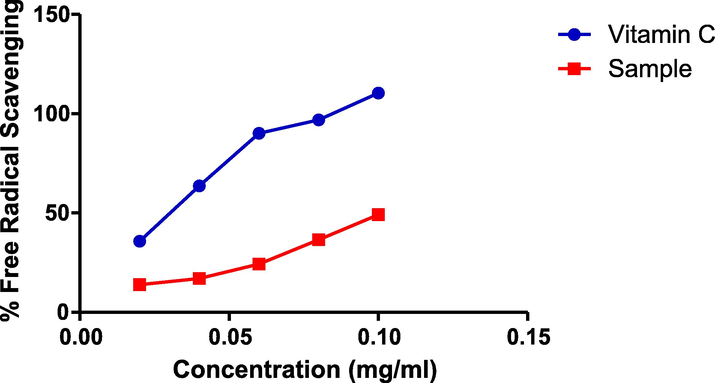
Graph of DPPH assay.
3.6 Result of ferric reducing antioxidant power assay
The result of the ferric reducing antioxidant potential of the extract is presented in Fig. 6. Interestingly, the CSMEL showed concentration dependent increase in absorbance values at a wavelength of 700 nm. At all tested concentrations, the standard (gallic acid) had higher absorbance values than the extract. The EC50 was determined in this study. The EC50 for gallic acid (0.04638 mg/ml) is lower than that of the extract (0.07344 mg/ml)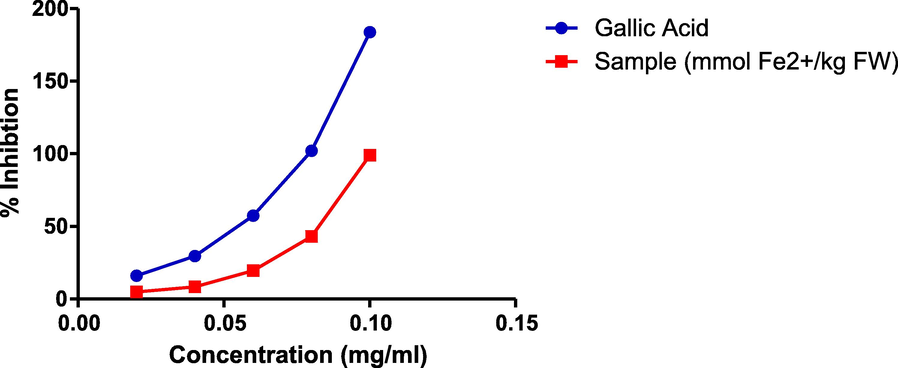
Graph of ferric reducing antioxidant power assay (FRAP assay).
3.7 Effect of CSMLE on the weight of PHZ-induced anemic rats.
The result of the effect of CSMLE on the weight of PHZ-induced anemic rats is shown in Fig. 7. The result showed significant decrease in the weight of the PHZ-induced anemic rats when compared to normal control. However, there was improvement in the weight of the rats on treatment with the extracts relative to the negative control.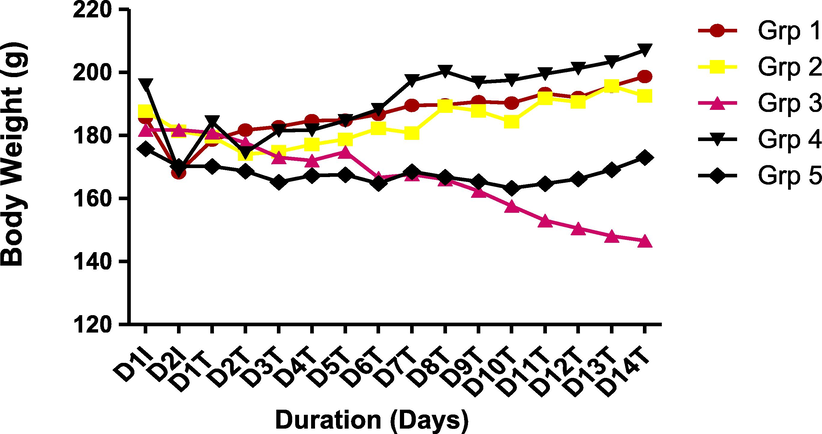
Effect of CSMLE on body weight of PHZ-induced anemic rats.
3.8 Effect of CSMLE on hematological parameters of the PHZ-induced anemic rats.
The result or the effect of CSMLE on hematological parameters of PHZ-induced anemic rat is shown in Table 3. The result revealed that there was significant (p < 0.05) decrease in RBC, HCT, HGB and MCHC in the anemic group II as compared to non-anemic group I. Interestingly, treatment with different doses of the extract (200 and 400 mg/kg) body weight CSMLE) significantly (p < 0.05) improved the levels of RBC, HCT and HGB relative to the untreated group. There was non-significant increase in TWBC in negative control in comparison with the normal control. Values are arithmetic mean ± SEM (standard error of mean), n = 6 *= p < 0.05 versus normal control (group 1); # = p < 0.05 versus anaemic control (group 2). NOTE: TWBC-total white blood cells; RBC-Red blood cells (x1012/L); HCT-Haematocrit (L/L); HGB-Hemoglobin concentration (g/dL); MCV-Mean cell volume (fL); MCH-Mean cell hemoglobin (pg); MCHC-Mean cell hemoglobin concentration (g/dL); PLT-Platelet count (x109/l). Group 1 = normal control, Group 2 = negative control (phenylhyradzine induced anemic group), Group 3 = positive control (anemic rat + Vitamin B 12 syrup 1 ml/day), Group 4 = anemic rat + 200 mg/kg CSMLE, Group 5 = anemic rat + 400 mg/kg CSMLE, CSMLE = C. salikounda Methanol leaf extract.
Groups
Parameters
1
2
3
4
5
TWBC X 109/L
6.73 ± 1.13
6.80 ± 0.41
9.30 ± 1.33
7.43 ± 0.68
5.37 ± 1.04
RBC X 1012/L
6.57 ± 0.03
4.71 ± 0.09*
5.30 ± 0.14*#
5.77 ± 0.20*#
5.82 ± 0.06*#
HCT (L/L)
46.3 ± 0.86
33.30 ± 1.59*
47.43 ± 1.12#
42.40 ± 2.31#
46.67 ± 0.44
HGB (g/dL)
15.50 ± 0.28
10.40 ± 0.53*
15.73 ± 0.35#
14.67 ± 0.88#
15.60 ± 0.06#
MCV (fL)
59.93 ± 0.83
75.63 ± 2.08*
79.23 ± 1.67*
71.37 ± 2.34*
73.90 ± 1.48*
MCH (pg)
23.53 ± 0.31
27.53 ± 0.53*
27.10 ± 0.29*
25.27 ± 0.79#
26.73 ± 0.13*
MCHC (g/dl)
38.40 ± 0.31
36.47 ± 0.31*
34.30 ± 0.58*
35.47 ± 0.33*
36.37 ± 0.91*
PLT X 109/L
920.0 ± 45.41
975.3 ± 67.92
983.0 ± 77.60
880.3 ± 60.07
838.0 ± 46.99
3.9 Results of the effect of CSMLE on cytokines levels of PHZ-induced anemic rats
The results of the cytokines (pro-inflammatory and anti-inflammatory cytokines) are presented in Table 4. The results show that there was a significant (P < 0.05) increase in IFN –ϒ and TNF-α in group 2 when compared to group 1 which served as the normal control. There was significant (P < 0.05) increase in IL-4 in group 4 (anemic rat + 200 mg/kg CSMLE) compared to group 2. There was no significant (P > 0.05) change in IL-1β. Treatment with CSMLE significantly (P < 0.05) decreased the concentrations of IFN-ϒ and TNF-α 4 when compared to negative control. Values are arithmetic mean ± SEM (standard error of mean), n = 6. IL-4 = interleukin-4, IFN-Y = Interferon gamma, IL-1β = interleukin-1beta, TNF-α = tumor necrosis factor alpha. *= significant difference in comparison to group 1 along the column. #= significant difference in comparison to group 2 along the column. Group 1 = normal control, Group 2 = negative control (phenylhyradzine induced anemic group), Group 3 = positive control (anemic rat + Vitamin B 12 syrup 1 ml/day), Group 4 = anemic rat + 200 mg/kg CSMLE, Group 5 = anemic rat + 400 mg/kg CSMLE, CSMLE = C. salikounda Methanol leaf extract.
Groups
Parameters
IL-4 m(pg/mL)
IFN-Y (pg/mL)
IL-1β (ng/mL)
TNF-α (pg/mL)
Group 1
54.68 ± 4.34
13.15 ± 1.06
88.35 ± 10.93
244.44 ± 2.87
Group 2
62.84 ± 0.81
18.91 ± 0.19*
93.25 ± 0.48
359.49 ± 5.43*
Group 3
54.56 ± 2.68
13.02 ± 0.20#
80.18 ± 7.73
234.01 ± 3.16#
Group 4
84.33 ± 3.87#
11.87 ± 0.37#
111.28 ± 2.55
253.63 ± 1.45#
Group 5
76.29 ± 6.39
12.91 ± 0.31#
111.78 ± 16.22
240.51 ± 6.68#
3.10 Effect of CSMLE on oxidative stress markers and antioxidant status
The results or effects of CSMLE on antioxidant values and lipid peroxidation markers of the PHZ-induced anemic rat are presented in Table 5. The results show that there was significant (p < 0.05) decrease in SOD and GSH in the negative control (group 2) as compared to normal control (group I). However, there was significant (p < 0.05) increase in SOD, CAT and GSH in all the groups treated with the extract in comparison with the negative control. MDA and Nitric oxide were significantly (p < 0.05) elevated in negative control relative to the normal control. Interestingly, oral administration of vitamin B 12 syrup 1 ml/day and the extracts at 200 and 400 mg/kg body weight significantly (p < 0.05) attenuated their levels in comparison with the negative control rats. Values are arithmetic mean ± SEM (standard error of mean), n = 6. SOD = Superoxide dismutase, GPX = Glutathione peroxidase, CAT = Catalase, GSH = reduced glutathione, MDA = Malondialdehyde, NO = Nitric oxide. *= significant difference in comparison to group 1 along the column. #= significant difference in comparison to group 2 along the column. Group 1 = normal control, Group 2 = negative control (phenylhyradzine induced anemic group), Group 3 = positive control (anemic rat + Vitamin B 12 syrup 1 ml/day), Group 4 = anemic rat + 200 mg/kg CSMLE, Group 5 = anemic rat + 400 mg/kg CSMLE, CSMLE = C. salikounda Methanol leaf extract.
Groups
Parameters
SOD (U/mg Protein)
GPX (U/mg Protein)
CAT (U/mg Protein)
GSH (U/mg Protein)
MDA (U/mg protein)
NO (U/mg protein)
Group 1
871.87 ± 42.66
160.98 ± 27.39
123.11 ± 0.87
293.83 ± 12.65
1.03 ± 0.06
8.08 ± 1.09
Group 2
777.63 ± 0.53
201.80 ± 0.44
137.37 ± 1.51
105.88 ± 0.29*
1.85 ± 0.08*
13.05 ± 0.67*
Group 3
1203.29 ± 43.59#
161.93 ± 12.65
648.09 ± 6.57#
335.55 ± 1.62#
1.12 ± 0.03#
4.67 ± 0.95#
Group 4
1035.01 ± 50.40#
204.68 ± 32.61
197.67 ± 20.62#
168.77 ± 0.33#
1.23 ± 0.03#
8.59 ± 0.86#
Group 5
1128.45 ± 37.68#
174.25 ± 22.24
334.43 ± 4.90#
134.44 ± 3.74#
1.280.10#
8.00 ± 0.15#
3.11 Histopathology result
The results of the histology sections of the spleen are shown in Fig. 8. The results indicate normal architecture of the spleen in the normal control rats with red pulp (RP), white pulp (WP) and spleenic artery (SA) (Fig. 8A). In the untreated rats (Fig. 8B), the photomicrograph indicates hemorrhage area (HA) and aggregate of inflammatory cells (AIC). However, in the groups treated with the standard drug (Fig. 8C) and extracts Fig. 8 D and E), the result showed moderate/mild focal area of hemorrhage (MFAH) and mild area of fatty change (MFC) indicating improvement in the spleen.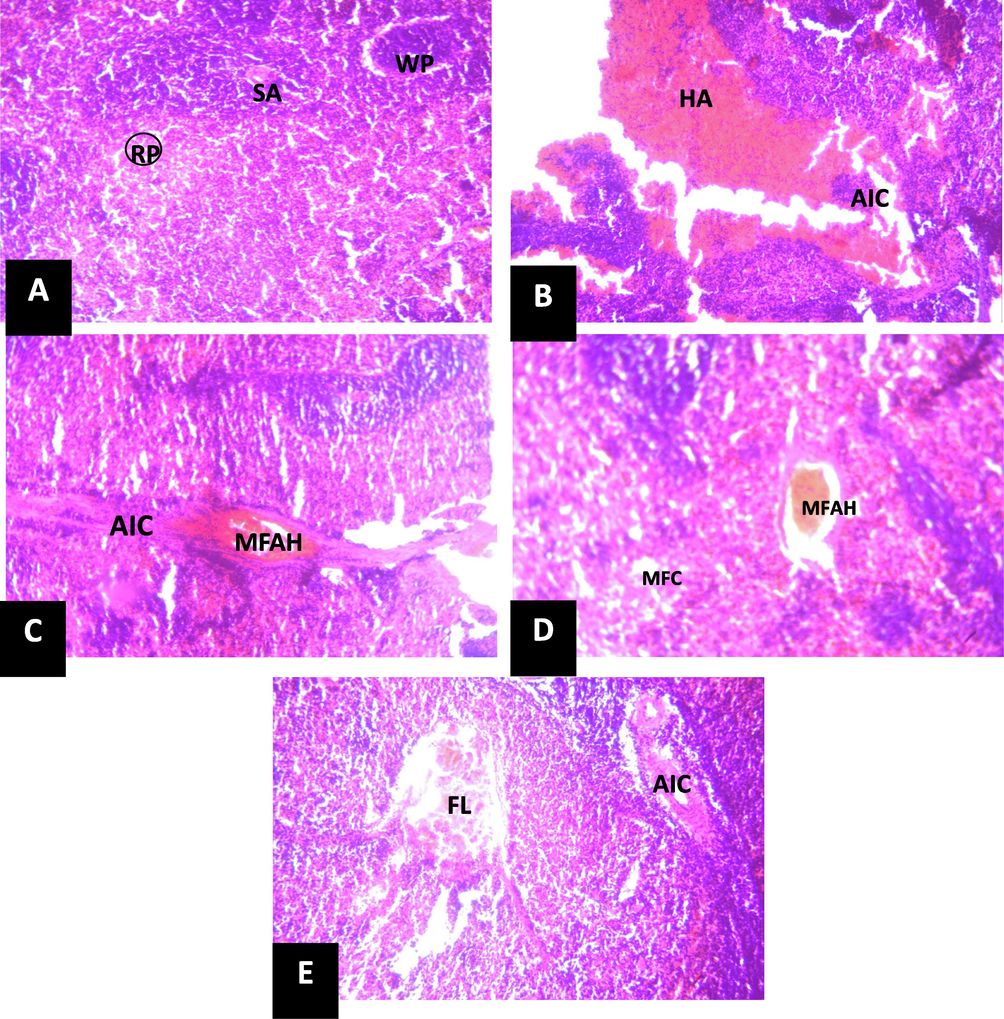
Photomicrograph of section of spleen (X150) (H/E).
3.12 Discussion
Medicinal plants are good sources of remedial preparations employed for management of diverse health challenges that might be life threatening to both animals and humans. Anemia is a disorder suffered by many people, particularly females from developing countries and its causes are multifactorial. Sequel to this, the exploration for new anti-anemics is, thus, regarded as a captivating research subject by different scientists as well as pharmacologists and pathologists (Gheith and El-Mahmoudy, 2018)
The search for an anti-anemic preparation calls for a suitable experimental anemia model. PHZ-induced hemolytic anemia in rats has been used as an acute model for hemolytic anemia study that is considered a good rapid tool for an investigational study. The ingestion of many chemicals including the intake of some drugs has been linked with RBC damage (Jain et al., 2020) and hemolytic anemia is one of the clinical conditions involving intoxication (Dogan, 2018; Dalar et al., 2018). The lysis of RBC by chemicals can occur via different mechanisms viz: interaction with sulfhydryl groups, the inhibition of various enzymes, immune mechanisms, and the breakdown of erythrocytes as they pass through the platelet–fibrin mesh or by unknown or poorly defined mechanisms. PHZ induces the production of ROS, lipids peroxidation and oxidative damage of spectrin in the membrane skeleton. Thus, its mechanistic injury seems to be predicated on the oxidative alterations to RBC proteins (Banerjee et al., 2020; Berger, 2007)
In this study, HPLC analysis of the leaf extract was carried out to identify and quantify the different bioactive components inherent in it. Robust evidences suggest that these bioactive compounds in plant products account for their diverse medicinal and therapeutic efficacy against several disease conditions (Dahibhate et al., 2019; Denaro et al., 2020; Mickymaray, 2019). The presence of caffeic acid, luteolin, apigenin, chlorogenic acid, quercetin, squalene, geraniol, nicaeenin, pardinol, drimenin and fusidic acid in CSMLE (Figs. 1–4) indicate that the extract may have remarkable medicinal and therapeutic effects when fully harnessed. Accumulating evidences indicate that squalene has antioxidant, antimicrobial, anti-hyper-cholesterolemia, anticarcinogenic, radioprotective, cardioprotective and cytoprotective potentials (Narayan et al., 2010; Reddy and Couvreur, 2009). Previous studies have shown that quercetin (a flavonoid compound) identified in CSMLE has strong antioxidant effect even at a minute quantity (Kanimozhi et al., 2017; Lin and Zhou, 2018). The antioxidant effect of chlorogenic acid had been reported in literature (Wu, 2007). Luteolin seen in different kinds of vegetables and fruits is a flavone subclass of flavonoids (Fu et al., 2006, Kure et al., 2016; Lv et al., 2009). Mounting evidences have shown that luteolin possess anti-inflammatory, antioxidative, antidiabetic, antimicrobial and anticancer potentials in vivo (Androutsopoulos and Spandidos, 2013; Francisco et al., 2014; Kim et al., 2014, Lu et al., 2012; Meng et al., 2016). As a result of its diverse biological actions and pharmacological potentials, luteolin has been widely employed in the fields of medicine and functional foods (Dong et al., 2018; Dong and Xiao, 2017). Consequently, the high concentrations of luteolin, squalene and chlorogenic acid and other bioactive compounds seen in a relatively smaller amounts in CSMLE could have been responsible individually or synergistically in eliciting the biological activities of the extract against the hematoxicity, biochemical changes and oxidative stress induced in the rats. Consistent with our study, the protective effect of quercetin and chlorogenic acid (constituents of our extract) against hematotoxicity have been reported in literature (Alam et al., 2017; Cheng et al., 2017).
To ascertain whether the bioactive compounds identified in CSMLE could elicit antioxidant effect, we carried out in vitro antioxidant assay using DPPH and FRAP. In DPPH antioxidant assay, an increase in decolouration at a low concentration of CSMLE was seen depicting the efficacy of the antioxidant activity of CSMLE against the free radicals generated in the assay. Interestingly, this gave rise to IC50 of 0.1085 mg/ml which is impressive and indicates that CSMLE at 0.1085 mg/ml was able to mop up 50% of the free radicals produced during the reaction of the DPPH antioxidant assay system. This is lower than the IC50 of many plant extracts like Caesalpinia volkensii, Vernonia lasiopus, and Acacia hockii reported to have antioxidant activity via DPPH assay method (Guchu et al., 2020).
Furthermore, the ferric reducing antioxidant potential (FRAP) assay result was comparable to the result of DPPH assay for the extract as we observed a dose dependent increase in absorbance due to the antioxidant activity of the extract in the FRAP assay that is comparable to the absorbance of pure gallic acid employed as standard in the study. This profoundly indicates that CSMLE possesses antioxidant propensities which enables it to mop up free radicals in the assay, stabilized and further terminated the free radical’s chain reactions. Remarkedly, this low IC50 for DPPH and increase in absorbance for the FRAP assay are strong indications that CSMLE possesses strong antioxidant activity. Hence, we further ascertain this antioxidant potential of CSMLE via in vivo studies in animal model.
The acute toxicity study (LD50) of the CSMLE indicated that no deaths were recorded amongst the rats, even at 5000 mg/kg indicating no signs of toxicity during the investigation and hence leaves extract might be safe for both human and animal consumption.
The alteration in the body weight of rats administered CSMLE is depicted in Fig. 7. The results indicated a remarkable diminution in the body weights of the rats after induction of anemia with PHZ probably due to the effect of oxidative damages (erythrocytes hemolysis) (Muhammad et al., 2020). However, there was remarkable improvement in the body weight of the groups treated with the extract at different doses in respect to the weight of the rats administered PHZ only. Our finding is consistent with previous studies in which body weight of rats profoundly reduced after anemia induction with PHZ but was improved after treatment with plants extracts for 14 days (Muhammad et al., 2020; Shende et al., 2017). The improvement in body weight of the groups treated with CSMLE after fourteen days might be due to reversal of oxidative damage by CSMLE. The reversal of this oxidative damage might be due to the action of the different flavonoids and terpenoids in the extract (Ogbe et al., 2010).
Previous study had reported a strong relationship between RBC, Hb, PCV, and red cell indices (MCV, MCH, and MCHC) in both humans and rats (Agbor et al., 2005). There is similarity between animals and humans and diminution in the values of Hb, RBC, and PCV is indicative of anemia (Ashaolu et al., 2011). There is often diminution in MCHC (MCHC - the amount of Hb per unit erythrocyte volume) in hemolytic anemia or elevation in the case of massive intravascular hemolysis. In hemolytic anemia, the value of MCV (MCV - average volume of the erythrocyte) is often elevated due to reticulocytosis likewise, the value of MCH (MCH - the average amount of Hb per cell) (Prasad et al., 2018). The results of hematological indices as shown in Table 3 indicated that there was remarkable decrease in PCV value two days after induction of anemia with PHZ in groups 2 to 5 relative to the normal control which is an indication that the rats were anemic. The Hb and PCV of the anemic rats significantly decreased in comparison with the normal control. However, on treatment with CSMLE, there was remarkable improvement of their values near to values in normal control when compared with the untreated anemic group (negative control) consistent with the results of previous author (Gheith and El-Mahmoudy, 2018). The elevation in PCV level might be ascribed to the body’s response to low PCV values culminating in the production of blood cells so as to prevent deprivation of oxygen in the circulation. The antioxidant flavonoids and terpenoids inherent in the extract might be accountable for this anti-anemic efficacy by reversing the action of the drug, PHZ. Mounting evidence had indicated that phenylhydrazine causes oxidative damage to red blood cells by forming ROS (Banerjee et al., 2020), thus the reduction in the value of RBC in the rats after induction. However, its value was significantly elevated after treatment with CSMLE and this might be ascribed to the rich antioxidant bioactive compounds found in the extract that perhaps reversed the damaging action of phenylhydrazine. Consequently, the extract might have probably caused the stimulation of the stem cells to produce new blood cells via erythropoiesis (Suzanne et al., 2020). These results are consistent with the report on Justicia carnea leaves extract (Onyeabo et al., 2017) and Tectona grandis (Diallo et al., 2008) which elevated Hb concentration and RBC after PHZ induction (Diallo et al., 2008). The significant increase in Hb concentration of the anemic rats treated with the extract at doses of 200 and 400 mg/kg is an indication of the efficacy of the extract as blood booster. This indicates that the rats recuperated from the anemia on treatment with CSMLE which could have stimulated the haemopoietic pathway. The improvement in the hematological parameters exhibited by CSMLE may be due to different flavonoids and terpenoids inherent in it. Treatment with CSMLE may indicate that the extract has the potentials to stimulate the erythropoietic factors that have a direct effect on the production of blood in the bone marrow. Erythropoietin is implicated in erythropoiesis as a maturation factor that elevates the number of erythropoietin-sensitive committed stem cells in the bone marrow, which is changed to red blood cells and in turn to mature erythrocytes (Prasad et al., 2018). There was insignificant increase in WBC and platelet counts in negative control relative to normal control. The elevation in WBC might be to the rat’s immune response that probably assume that the cause of the anemia might be due to infection or disease and thus increases WBC production to fight such infections (Okonkwo et al., 2015). During a foreign attack on the system by pathogens, there is usually elevation in WBC counts and the physiological response of the system will be to boost the body’s defense mechanisms (Eyong et al., 2004). However, treatment with CSMLE attenuated WBC and platelets in groups 4 and 5.
Peroxidation of lipid is one of the main aftermaths of increased production of ROS in physiological system culminating in generation of malondialdehyde (MDA) (Cui et al., 2018; Misra et al., 2017). Hence, MDA is a marker for an increment in the oxidation of lipids as a result of high levels of free radicals in the form of ROS and in turn oxidative stress in the body. MDA is thus a remarkable oxidative stress marker in the body and has been shown to be involved in the etiology of various ailments (Cui et al., 2018; Saleh et al., 2018). In the present study, MDA and nitric oxide (NO) were elevated in the serum of PHZ-induced rats but were significantly reduced in the rats treated with CSMLE in comparison to the negative control which might probably be attributed to high antioxidant power of the extract due to the presence of terpenoids and flavonoids compounds. This result was similar to the results of obtained from feeding PHZ-induced anemic rats with beetroot pomace biscuit (Abdo et al., 2021) and dried beet green (Elaby and Ali, 2018), respectively in previous studies.
In order to maintain redox balance and to shield the body against the harmful effects of ROS and reactive nitrogen species (RNS), the biological system has evolved a complex antioxidant system to circumvent the obnoxious effects of oxidative stress (Rahman et al., 2012). The sources of the body’s antioxidant defense may be endogenous and/or exogenous (Umar et al., 2012]. Nutrients such as β-carotene, L-ascorbic acid (vitamin C), α-tocopherol, and tocotrienols (vitamin E) obtained from dietary foods are good examples of exogenous sources of antioxidants (Kocyigit and Selek, 2016) while enzymes such as superoxide dismutase (SOD), glutathione peroxidase (GPx), glutathione reductase, and catalase (CAT) enzymes, which mop up or scavenge free radicals are endogenous sources (Ighodaro and Akinloye, 2018; He et al., 2017). Presently, synthetic antioxidants like propyl gallate (PG), butylated hydroxyanisole (BHA), and butylated hydroxytoluene (BHT) employed in the management of oxidative stress, have been linked with deleterious health challenges including liver damages and malignancies. Besides, they have minimal efficacy in animal models and humans (Gandomi et al., 2014). Consequently, there is increasing quest for substitution of synthetic antioxidants with naturally occurring antioxidants from plants because of their safety, accessibility, and affordability (Lourenço et al., 2019; Saxena et al., 2012).
The result of this study indicates remarkable decrease in the activities of SOD and CAT and the concentration of GSH in PHZ-induced rats in comparison with the normal control. However, administration of the CSMLE significantly increased the activities of SOD, CAT and GSH when compared with negative control in consonance with the results of previous study (Milad et al., 2020; Paul et al., 2014). Literature had revealed that medicinal plants contain active principles responsible for their antioxidant activity (Moriasi et al., 2020). Quantitative phytochemical profiling of CSMLE using HPLC indicates that the extract contains different flavonoids and terpenoids possessing antioxidant potentials which might have contributed to its antioxidant efficacy. The antioxidant effect of these bioactive compounds could be via reductive and oxidative capacities that allow absorption and counteracting effects of free radicals (Elzaawely and Tawata, 2012). Several of these metabolites from plants are enriched with remarkable reductive potentials that are accountable for lower incidences of death and suffering due to oxidative stress-related diseases (Kibiti and Afolayan, 2015). Thus, the antioxidant efficacy of CSMLE could be ascribed to the presence of these bioactive components
Induction of oxidative stress on erythrocytes and membrane lipid oxidation has been reported as among the mechanisms by which phenylhydrazine induces anemia (Banerjee et al., 2020). Physiologically, biological systems respond to stress stimuli to facilitate defense and survival. Cytokines have diverse effect on biological processes and have been linked with the pathogenesis of many diseases, especially immunoinflammatory conditions (Musaya et al 2015). In our study, PHZ administration induces oxidative stress and causes increase in the concentrations of the proinflammatory cytokines which is in tandem with the report of Zangeneh et al. (2017). Ershler (2003) had earlier reported that increased levels of proinflammatory cytokines such as IL-1, TNF-apha, IL-6, and other immune mediators, particularly interferon-gamma (IFN-γ) are linked with anemia development via direct inhibition of erythropoietin or by interference of normal iron metabolism. Cytokines are implicated in the regulation of erythropoiesis. In addition, tumor necrosis factor (TNF) and IL-6 had been revealed to have negative effect on erythropoiesis (Ershler, 2003). IFN-γ has been reported to inhibit bone marrow proliferation, involved in the pathogenesis of aplastic anemia and its overexpression in mice results in chronic anemia (Musaya et al., 2015). The mechanistic action of IFN-γ in anemia induction might be via disturbance in metabolism of iron through upregulating the expression of the iron transporter DMT-1, which increases iron influx and sequestration into cells (Stijlemans et al., 2008; Stijlemans et al., 2010). In our study, the concentrations of IFN-γ, and TNF were significantly elevated on PHZ administration in comparison to the negative control similar to the result obtained by Zangeneh et al. (2017). However, treatment with CSMLE significantly reduced their levels near to the normal control when compared to the negative control comparable to the result of previous authors (Zangeneh et al., 2017). This effect could be as a result of the presence of different flavonoids and terpenoids present in the extract.
Robust evidence indicates that the spleen is implicated in the mobilization or recycling of red blood cells and immune function (Kim et al., 2012). Some chemicals or drugs such as PHZ had been reported to cause diminution of spleen functions (Sheth et al., 2021). Asplenia and hyposplenia referred to as a non-functioning spleen and a partially functioning spleen, respectively might occur due to congenital defect, injury due to trauma, ailments like sickle cell anemia and usage of some drugs such as PHZ. These medical situations may bring about a mild elevation of circulating WBCs and platelets, reduced response to certain vaccines and elevation in the vulnerability to infection (Jia and Pamer, 2009). The causes of anemia can be grouped into four categories which include diminished synthesis of hemoglobin, increased breakdown of hemoglobin or blood loss and splenic pooling (Kasper et al., 2004). The histological sections of the spleen tissues of the experimental rats in this study indicate that the normal control shows normal red pulp, white pulp and spleenic artery (Fig. 8) consistent with the findings of Sheth et al. (2021) in which normal architecture of spleen showing presence of normal features of red pulp, white pulp and adequate lymphoid cellular population was observed in normal control rats. This indicates that the spleen is intact and not altered as the red and white pulps are responsible for the different functions of the spleen (Lewis et al., 2019). The negative control (given PHZ only) comparatively shows severe degeneration with severe hemorrhagic area (HA), and moderate aggregate of inflammatory cells (AIC) within the hemorrhagic area. This might be an indication of toxic effect of PHZ which might have altered the function of the spleen. However, groups administered the extracts show mild focal area of hemorrhage (MFAH), with mild area of fatty change (MFC), no inflammation seen (group 4) focal loss of spleenic tissue (FL), with moderate aggregate of inflammatory cells (AIC) around the spleenic artery (group 5) indicating amelioration of the spleen damage. Our result is similar to the result obtained by Sheth et al. (2021) in which Raktavardhak kadha restored the pathological alterations of the spleen of PHZ-induced anemic rats near to normal This is an indication that the extract is probably potent in protecting the spleen.
4 Conclusion
The data obtained from this study indicate that CSMLE has considerable anti-anemic potential against PHZ-induced hemolytic anemia in experimental rat model. The results revealed that the extract increased the levels of HGB, RBC, HCT and the activities of antioxidant enzymes while there was diminution in lipid peroxidation and immunological parameters. The results of the biochemical findings were also corroborated by the histological findings as CSMLE ameliorated spleenotoxic effect of PHZ on the experimental rats. These effects exerted by CSMLE were probably attributed to the different flavonoids and terpenoids inherent in it. Further studies are required to establish the molecular aspect of this interaction and to develop suitable formulations to ensure maximum bioavailability and therapeutic efficacy and equally to explore the anti-anemic potential of CSMLE in the development of a novel herbal delivery system.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.







