Translate this page into:
Hypoglycemic effect and active ingredients screening of Isodon Japonicus based on network pharmacology and experimental validation
⁎Corresponding authors at: College of Pharmacy, Xinxiang Medical University, Xinxiang 453002, Henan Province, PR China. 171048@xxmu.edu.cn (Yang Pengfei), 13373750625@163.com (Zhang Nan), xuejintao@xxmu.edu.cn (Xue Jintao)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
Abstract
Abstract
The ingredient-target-pathway relationship was studied by network pharmacology. 11 hypoglycemic components, 12 targets and 130 pathways were found. Cell test and animal experiments were used to confirm the hypoglycemic effects.
Abstract
Isodon Japonicus (IJ) has historically been widely used as herbal functional food. To date, there has been no publication regarding the hypoglycemic mechanism of IJ. This study was to study the hypoglycemic ingredients and mechanisms of IJ. The network pharmacology method was used to explore the ingredient-target-pathway relationship. There were 11 hypoglycemic ingredients, 12 hypoglycemic targets, and 130 signaling pathways for IJ on diabetes. To confirm the hypoglycemic impacts, cell tests and animal experiments were jointly carried out. With the human umbilical vein endothelial cells (HUVECs) treated by water extract (0.06–2.00 g/L) and alcohol extract (0.06–2.00 g/L) of IJ, the cell viability in the water extract group (0.06–2.00 g/L) and the alcohol extract group (0.25–2.00 g/L) showed significant viability compared to that of the model group (P < 0.05), respectively. Animal experiments showed that both the water extract and the alcohol extract of IJ could lower diabetic rats' blood glucose levels compared to the model group (P < 0.05). This study proposed an effective method to explore the potential hypoglycemic ingredients, targets, signaling pathways, and pharmacological effects for IJ on diabetes. This strategy may be useful for other herbs to explore the active ingredients and pharmacological mechanism.
Keywords
Isodon Japonicus
Network pharmacology
Diabetes
Hypoglycemic ingredient
Mechanism
- FBG
-
fasting-blood glucose
- GO
-
Gene Ontology
- HMGCR
-
3-hydroxy-3-methylglutaryl-coenzyme A reductase
- HUVECs
-
Human umbilical vein endothelial cells
- IJ
-
Isodon Japonicus
- ITGAL
-
integrin α-L
- ITGB2
-
integrin β-2
- PGR
-
progesterone receptor
- SD
-
standard deviation
Abbreviations
1 Introduction
Isodon Japonicus (IJ), known as ‘Xiang-Cha-Cai’ in Chinese, is the dry aerial part of Isodon japonicus (Burm.f.) H.Hara (Isodon genus, Lamiaceae family) (Sun et al., 2019). It is widely distributed in China and other Asian countries (Han et al., 2004). In China, IJ has historically been widely used as a cold dish (young parts) and herbal functional food with a variety of pharmacological effects, including treating gastritis and hepatitis, clearing heat and detoxifying, and invigorating the spleen and activating blood (Kim et al., 2011, Hwang et al., 2012, Hai et al., 2015). In Japan, it is traditionally known as a stomachic treatment for anorexia and dyspepsia (Hai et al., 2015, Matsumoto et al., 2017).
The chemical substances contained in IJ are primarily diterpenoids. In addition, it also contains triterpenoids, flavonoids, lignans, steroids, etc. With the advantage of the main origin area and continuous study of our institute, more than 50 ingredients had been isolated from IJ by our institute, including 2 new compounds (15α, 20β-Dihydroxy-6β-methoxy-6, 7-seco-6, 20-epoxy-1, 7-olide-ent- kaur-16-ene and 6β-Acetoxy-1α, 7β, 11β, 15β-tetrahydroxy-7α, 20-epoxy-ent-kaur-16-ene) and 8 compounds (Rabdosichuanin C, Rabdosinate, Longirabdolide C, Eriocaside A, Parvifoline G, Taihangexcisoidesin C, Dayecrystal A and hyptadienic acid) which were found in Isodon Japonicus for the first time (Yan et al., 2009, Yan et al., 2010, Yan et al., 2010, Xie et al., 2011, Hou et al., 2012, Liang et al., 2012, Liu et al., 2013, Hai et al., 2015). Recent pharmacological studies on IJ showed that it has anti-tumor, anti-inflammation, anti-bacteriostasis, anti-allergy, and anti-oxidation properties (Kim et al., 2004, Hai et al., 2015, Matsumoto and Watanabe 2020, Xing et al., 2020).
After cardiovascular disease and cancer, diabetes has become the third leading non-communicable disease in recent years. The incidence of diabetes was at an alarming rate, and it was becoming a higher and higher concerning issue of global health. High blood glucose levels were considered as a core feature and a high-risk factor of diabetes (Niu et al., 2017, Wang et al., 2019). Diabetes, with long-term hyperglycemia, could lead to a series of problems, including stroke, coronary heart disease, and even organ failure (Schmidt 2018). At present, many studies have highlighted traditional herbs in the treatment of diabetes (Gao et al., 2019, Sun et al., 2019, Jintao et al., 2020). Sun Jiayi et al found rosmarinic acid and its substructures (caffeic acid and 3,4-dihydroxyphenyllactic acid) of IJ showed significant inhibitory activity to amyloid β and human islet amyloid polypeptide, and good antioxidant activity on the therapy of diabetes (Sun et al., 2019). In our previous study, Isodon rubescens, a plant of the same genus with IJ, had revealed good hypoglycemic effect on diabetes (Jintao et al., 2020). As IJ contains many active ingredients, screening hypoglycaemic ingredients and revealing hypoglycaemic mechanisms is particularly difficult. As systems biology, computational biology, multi-direction pharmacology, computational biology, network analysis, and other technologies are all integrated in network pharmacology, network pharmacology could explore the relationship between traditional herbs and disease, and screen targets and signaling pathways from a holistic perspective to improve drug discovery efficiency (Zhang et al., 2019, Huang et al., 2020, Yuan et al., 2022). So, network pharmacology would be a useful tool for studying traditional herbs with multiple components, multiple targets and multiple mechanisms.
To better understand and evaluate the pharmacological effect and active ingredients of IJ on diabetes, the network pharmacology method was used to explore the ingredient-target-pathway relationship of IJ Integrated with experimental validation, and the hypoglycemic effect of alcohol extract and water extract from IJ was studied by cell tests and animal experiments.
2 Material and methods
2.1 Reagents
Streptozotocin (No. WXBC5204V) was got from Sigma-Aldrich Company (Saint Louis, Missouri, USA). Metformin (No. H31022081) was purchased from Shangyao Xinyi Pharmaceutical Company (Shanghai, China). DMEM low glucose culture medium (No. 31600) was the product of Beijing Solarbio Science & Technology Company (Beijing, China). Ethanol (No. 20170220, purity greater than 99.5%) was supplied by Tian-Jin-Heng-Xing Chemical Reagent Manufacturing Co. Ltd. (Tianjing, China). The glucometer was the product of Changsha Sannuo Biosensor Technology Company (Changsha, China). Cell Counting Kit-8 (No. C0038) was from Shanghai Beyotime Biotechnology Company (Shanghai, China). Water was purified twice. All other reagents were of analytical grade.
2.2 Isodon Japonicus
Isodon Japonicus (IJ), known as ‘Xiang-Cha-Cai’ in Chinese, was the dry aerial parts of Isodon japonicus (Burm.f.) H.Hara in the genus Isodon (family Lamiaceae). The plant name has been confirmed in the Index Kewensis (electronic Plant Information Centre ePIC, Royal Botanic Gardens, Kew, UK: https://www.kew.org/epic) and The Plant List (https://www.theplantlist.org). IJ samples (No. 201807011) were collected from Wanxianshan Mountain, Xinxiang, China. The collection process of IJ samples were followed the Convention on Biological Diversity and the Nagoya Protocol. The IJ samples were identified by Li Chunyan in Aug. 2018. Voucher specimens (No. 201807XY01IJ) were deposited at the School of Pharmacy, Xinxiang Medical University, Xinxiang, China.
Water extract of IJ: 10 g of IJ was decocted in 100 mL of water for 2 h, and then the extract solution was filtrated. With the same procedure, the second decoction was with 80 mL of water for 1.5 h, and the third time with 60 mL of water for 1 h. The filtrate from the above three times was concentrated to 20 mL. So, each 1 mL of extract solution is equivalent to 0.5 g of IJ raw herbs, and the dosage in the following study was calculated on this base.
Alcohol extract of IJ: with the same procedure of water extract, 10 g of IJ was first extracted with 100 mL of 95% ethanol for 2 h by reflux, then second time with 80 mL of 95% ethanol for 1.5 h. The combined filtrate was condensed without ethanol by rotary evaporation. The concentrated solution is fixed to 20 mL (1 mL of extract solution is equivalent to 0.5 g of raw IJ, and the dosage in the following study was calculated on this base).
2.3 Network pharmacology
According to the literature and our previous research, the ingredients of IJ were gathered (Han et al., 2004, Yan et al., 2009, Yan et al., 2010, Yan et al., 2010, Xie et al., 2011, Hou et al., 2012, Liang et al., 2012, Liu et al., 2013, Tanaka et al., 2014, Hai et al., 2015, Liu et al., 2017, Xing et al., 2020, Liu et al., 2021, Liu et al., 2022). Each chemical structure of IJ was drawn with Personalize ChemDraw software (Version 20.0), and converted to inchi and smiles formats with Open Babel GUI software (Version 2.4.1). Then, the inchi and smile files were imported into the Batman-TCM database (https://bionet.ncpsb.org/batman-tcm/index.php/Home/Index/index), TCMSP database (https://www.tcmsp-e.com/) and NPASS database (https://bidd2.nus.edu.sg/NPASS/) to obtain the corresponding targets for each ingredient. Targets of diabetes were obtained from the DrugBank database (Version 5.0, https://www.drugbank.ca/). To select the potential hypoglycemic targets and ingredients of IJ, the intersection analysis was performed between the targets of ingredients and the targets of diabetes. The Z-score was used to show the matching rate between the ingredient and the target.
The hypoglycemic targets were imported to the String database (Version11.0, https://www.string-db.org/) for Gene Ontology (GO) study and KEGG pathway analysis. The biological processes, molecular functions and cellular components were studied with GO enrichment analysis, respectively. Then, a network of the ingredient-target-pathway relationship was constructed with Cytoscape software (Version 3.5).
2.4 Cell test
The human umbilical vein endothelial cells (HUVECs, No. JH-H1285) were provided by the American Type Culture Collection (ATCC). HUVECs were cultured in humidified air at 37 ℃ with 5% CO2, and incubated in DMEM low glucose culture medium supplemented with free serum medium and 5.5 mmol/L glucose solution for 24 h. Then, with the cells at the same passage stage, HUVECs were divided into 5 groups: (I) normal group: it was treated with the normal culture medium; (II) model group: it was cultured in the culture medium containing 33 mmol/L of glucose solutions; (III) positive group: after incubating in the culture medium containing 33 mM of glucose solutions for 48 h, it was treated with 1.0 μmol/L of metformin; (IV) alcohol extract group: with the same procedure with the positive group, it was treated with different concentrations (2.0 g/L, 1.0 g/L, 0.50 g/L, 0.25 g/L, 0.13 g/L and 0.06 g/L) of alcohol extract (instead of metformin), respectively; (V) water extract group: as same with the alcohol extract group, it was treated with the water extract at 2.0 g/L,1.0 g/L, 0.50 g/L, 0.25 g/L, 0.13 g/L, and 0.06 g/L, respectively. After 24 h of treatment, cell viability was analyzed with the Cell Counting Kit-8 method in three independent experiments (Li et al., 2017). In the normal group, cell viability was set to 100% and cell activity was calculated as a percentage of cell viability of the normal group.
2.5 Animal experiments
Adult male Sprague-Dawley rats (8 weeks), were obtained from the Laboratory Animal Center of Xinxiang Medical University (Henan, China). All rats were maintained in standard conditions with the temperature at 20 ± 2 ℃ and 12 h of light–dark cycles with free access to water and diet. After one week of adaptive feeding, all rats were randomly divided into 6 groups: the normal group, the model group, the positive group, the alcohol extract group and the water extract group. Except for the rats in the normal group, all other rats were fed with the high sugar and fat diet (adding 10% sucrose and 10% lard to the normal diet). Then, after 12 h of fasting with free access to water, diabetic rats were induced with an intraperitoneal injection of freshly prepared streptozotocin (50 mg/kg). The streptozotocin was dissolved at a concentration of 0.5 % (m/v) in 0.1 mol/L citrate buffer (pH 4.5). The rats of normal group were injected with the same dose of sodium citrate-sodium citrate buffer as those in model group. After three days, the fasting-blood glucose (FBG) was measured with the tail vein puncture method. With an FBG value above 11.1 mmol/L, the rat was considered as diabetic rat for the following study (Kamble et al., 2016). The rats of the positive group were administrated with metformin (0.18 g/kg) by gavage; the alcohol and water extract groups were given by gavage the corresponding alcohol extracts or water extracts (2.7 g/kg), respectively. Other rats in the normal group and the model group were treated with the same dose of normal saline. All the above groups were continuously administered for four weeks. At last, the rats were anesthetized with isoflurane and sacrificed by pressing down on the neck and pulling the tail to the rear. The pancreas was collected for Hematoxylin and Eosin Staining.
The experiment was approved by the Animal Ethics Committee of Xinxiang Medical University (XYLL-2020055). All the procedures were performed in accordance with the Animal Ethics protocol, the internationally accepted principles of EU Directive 2010/63/EU, and the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978).
2.6 Statistical analysis
All data were listed as mean ± standard deviations (SD). The statistical difference was analyzed by one-way ANOVAs (2-tailed) via SPSS 13.0 software (SPSS Inc., Chicago, IL, USA). The significance level of P < 0.05 means there was significant difference between two groups, and P < 0.01 represents a very significant difference.
3 Results and discussion
3.1 Hypoglycemic ingredients and targets
A total of 233 ingredients (as listed in Fig. A) of IJ were collected from the related literature and our previous study (Han et al., 2004, Yan et al., 2009, Yan et al., 2010, Yan et al., 2010, Xie et al., 2011, Hou et al., 2012, Liang et al., 2012, Liu et al., 2013, Tanaka et al., 2014, Hai et al., 2015, Liu et al., 2017, Xing et al., 2020, Liu et al., 2021, Liu et al., 2022). For these ingredients, 206 un-repeated targets with 583 frequencies were obtained from the Batman-tcm database, TCMSP database and NPASS database. After intersection analysis of the targets, 12 hypoglycemic targets were got for IJ on diabetes. Then, 11 ingredients (Table 1 and Fig. 1) were related to the above12 hypoglycemic targets (Table 2), so these 11 ingredients were considered as the potential hypoglycemic ingredients of IJ on diabetes. The Z-score value of more than 22 would reflect a good match between compounds and targets. The higher the Z-score, the better the match between the ingredient and the target has. PS: ADRA2A: alpha-2A adrenergic receptor; ADRA1B: alpha-1B adrenergic receptor; AVPR2: vasopressin V2 receptor; CA2: carbonic anhydrase 2; DPP4: dipeptidyl peptidase 4; DRD1: d(1A) dopamine receptor; DRD2: d(2) dopamine receptor; GAA: lysosomal alpha-glucosidase; HMGCR: 3-hydroxy-3-methylglutaryl-coenzyme A reductase; ITGAL: integrin alpha-L; ITGB2: integrin beta-2; PGR: progesterone receptor.
No.
Ingredients
Molecular formula
CAS No.
log P
Target gene (Z'-score)
1
alboatisin A
C20H30O4
1393090–51-1
2.0684
HMGCR (22.37), DRD2 (22.37), DRD1 (22.37), AVPR2 (22.37), ADRA2A (22.37), ADRA1B (22.37)
2
rabdoternin B
C20H28O7
128887–81-0
−0.3060
ITGB2 (80.88), ITGAL (80.88), HMGCR (80.88)
3
enmein
C20H26O6
3776–39-4
1.1954
GAA (22.37), CA2 (22.37)
4
hebeiabinin B
C20H34O5
934832–65-2
1.2243
PGR (122.78)
5
isolushinin G
C22H32O7
1233704–14-7
0.5808
PGR (48.00)
6
eriocasin C
C22H32O6
1254953–48-4
2.0642
PGR (48.00)
7
nervonin I
C24H36O8
1011715–08-4
0.9418
HMGCR (23.00)
8
nervonin D
C28H40O10
1011715–03-9
2.0834
HMGCR (23.00)
9
tormentic acid
C30H48O5
119725–19-8
5.1752
PGR (48.00)
10
β-sitosterol
C29H50O
83–46-5
8.4149
DPP4 (22.37)
11
forrestin C
C26H38O9
/
/
HMGCR (23.00)
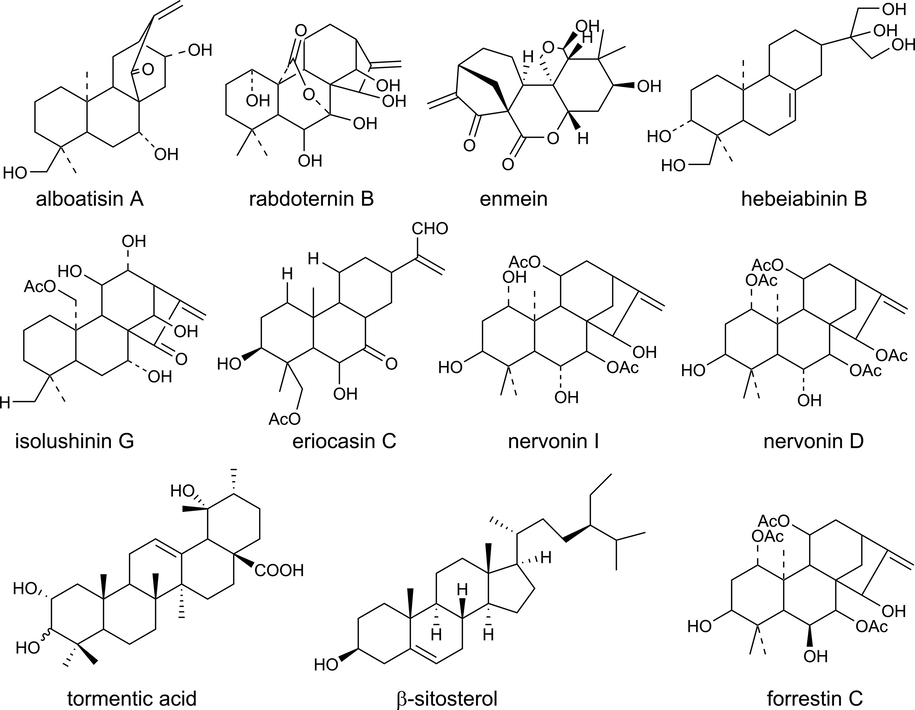
Chemical structural of the hypoglycemic ingredients.
Target
Target gene
Uniprot ID
Ingredients (Z'-score)
3-hydroxy-3-methylglutaryl-coenzyme A reductase
HMGCR
P04035
rabdoternin B (80.88); nervonin D (23.00); nervonin I (23.00); forrestin C (23.00); alboatisin A (22.37)
progesterone receptor
PGR
P06401
hebeiabinin B (122.78); tormentic acid (48.00); eriocasin C (48.00); isolushinin G (48.00)
integrin alpha-L
ITGAL
P20701
rabdoternin B (80.88)
integrin beta-2
ITGB2
P05107
rabdoternin B (80.88)
alpha-1B adrenergic receptor
ADRA1B
P35368
alboatisin A (22.37)
alpha-2A adrenergic receptor
ADRA2A
P08913
alboatisin A (22.37)
vasopressin V2 receptor
AVPR2
P30518
alboatisin A (22.37)
carbonic anhydrase 2
CA2
P00918
enmein (22.37)
dipeptidyl peptidase 4
DPP4
P27487
β-sitosterol (22.37)
d (1A) dopamine receptor
DRD1
P21728
alboatisin A (22.37)
d (2) dopamine receptor
DRD2
P14416
alboatisin A (22.37)
lysosomal alpha-glucosidase
GAA
P10253
enmein (22.37)
As shown in Table 1 and Table 2, the targets, 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR), progesterone receptor (PGR), integrin alpha-L (ITGAL) and integrin beta-2 (ITGB2), played an essential role in the hypoglycemic effect of IJ on diabetes. A study by Takei S. et al showed that when β cells lacked HMGCR, the number of β cells would decrease, and insulin secretion would be damaged, so HMGCR would play a key role in the insulin secretion and the development of β cells (Takei et al., 2020). Picard F. et al found knockdown of PGR could improve glucose tolerance through the proliferation of β cells and secreting higher levels of insulin (Picard et al., 2002). The research of Huang M. et al revealed that the main pathological features of autoimmune diabetes were the recruitment of T cells and its destruction to islet cells, and ITGAL could inhibit the adhesion of T cells to pancreatic islet endothelium (Huang et al., 2005). The study of Barlow S. C. et al displayed that ITGB2 was an important mediator of leukocyte-mediated tissue damage in the development of autoimmune diabetes (Barlow et al., 2004).
3.2 Gene ontology and pathways analysis
Gene Ontology (GO) study and KEGG pathway analysis of the hypoglycemic targets of IJ were analyzed in the String database. The GO study showed that there were 643 biological processes with 884 frequencies (Table A.1), 128 molecular functions with 157 frequencies (Table A.2), and 127 cellular components with 194 frequencies (Table A.3). As indicated in Fig. 2, 17 biological processes occurred at the frequency of more than 4 times, and 5 molecular functions had more than 3 times with 8 cellular components at a frequency of more than 4 times.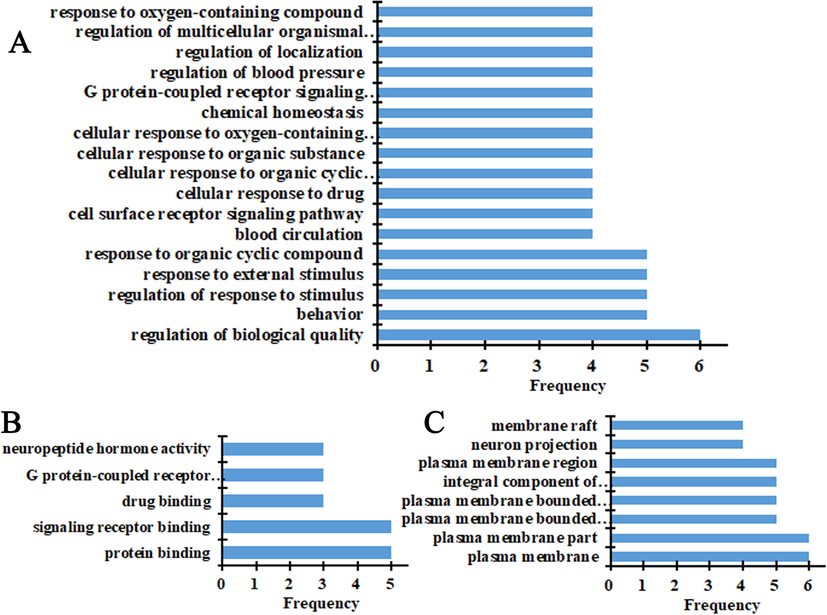
Biological processes (A), cellular components (B), and molecular functions (C) of gene ontology analysis for hypoglycemic targets.
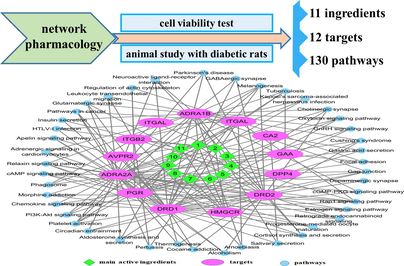
The KEGG Pathways study showed that 130 signaling pathways at 264 frequencies (Table A.4) were related with the hypoglycemic targets of IJ. Fig. 3 showed 16 signaling pathways at a frequency of more than 4 times. To better understand and evaluate the pharmacological mechanism of IJ on diabetes, as illustrated in Fig. 4, a network of 11 hypoglycemic ingredients, 12 hypoglycemic targets, and 43 signaling pathways with more than 3 frequencies were established. This network revealed that the ingredients of IJ could regulate multiple targets, while these targets also could control multiple signaling pathways.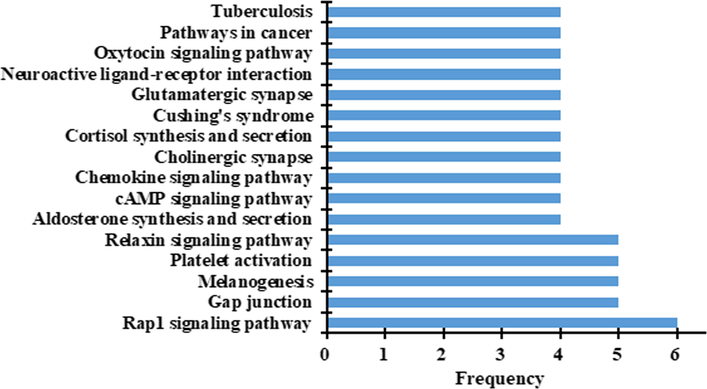
KEGG signaling pathways for hypoglycemic targets of Isodon Japonicus.
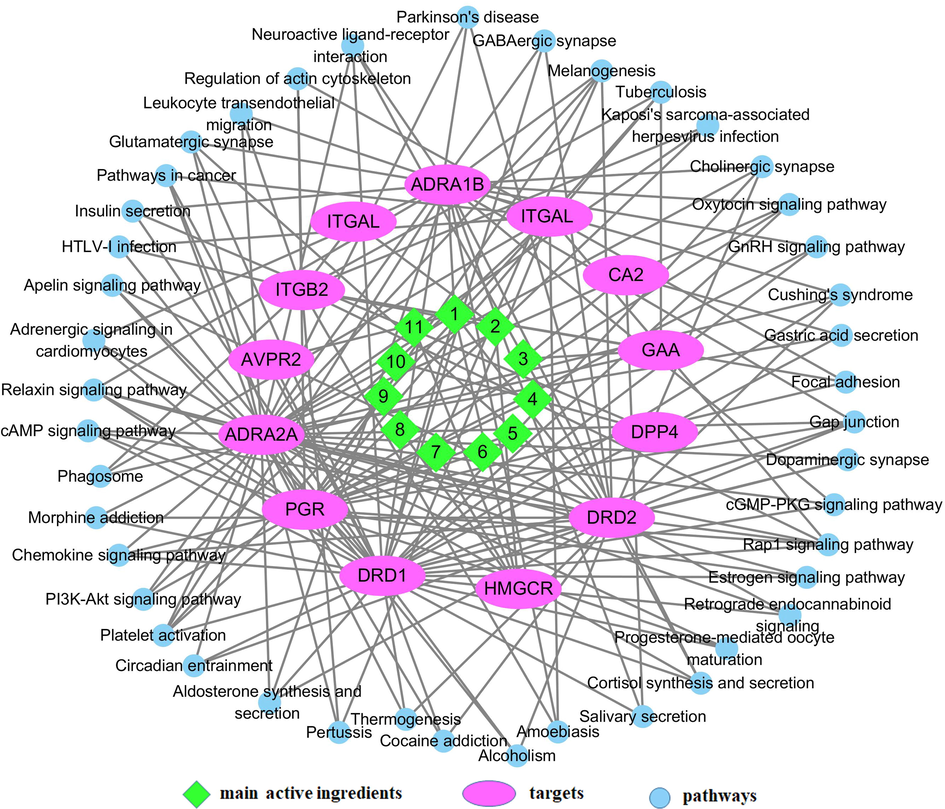
The ingredient-target-pathway network of Isodon Japonicus on diabetes (ingredients No. 1–11 listed in Table 1).
As shown in Fig. 3 and Fig. 4, the signaling pathways, Rap1 signaling pathway, cAMP signaling pathway and Oxytocin signaling pathway, might played an important role in the hypoglycemic effect of IJ on diabetes. Rap1 is a monomeric small GTPase belonging to the Ras family. Ablation of the Rap1 gene would significantly decrease the blood glucose and serum insulin levels, and improve the glucose and insulin resistance (Kaneko et al., 2021). So, the Rap1 signaling pathway could be a very effective mechanism for therapeutic intervention on diabetes. As an important cell signaling molecule, CAMP could adjust the insulin and glucagon secretion of pancreatic β cells and α cells, so the cAMP signaling pathway could regulate blood glucose levels by normalizing the insulin and glucagon secretion (Tengholm and Gylfe 2017). In Oxytocin Signaling Pathway, Oxytocin could significantly decrease the overall blood glucose level through stimulating insulin secretion and increasing plasma insulin concentration (Mohan et al., 2018).
3.3 Cell viability analysis
Cell viability of human umbilical vein endothelial cells (HUVECs) was showed in Fig. 5. Compared to the cell viability of the normal group which was set to 100% of viability, the viability of the model group significantly decreased to 75.96 % ± 3.07% (P < 0.01), so it indicates the cell viability treated by the high concentration of glucose solutions (33 mmol/L) would be greatly reduced. Compared with the cell viability of the model group, the alcohol extract at the concentration from 0.25 g/L − 2.00 g/L and the water extract at 0.06 g/L − 2.00 g/L could significantly increase the viability (P < 0.01), respectively. Especially, the 0.13 g/L and 0.25 g/L of water extracts would make the viability to be higher than that of the positive group (P < 0.01). However, the viability of alcohol extract at 0.06 g/L − 2.00 g/L was lower than that of positive group, while the viability of water extract at 0.06 g/L − 0.50 g/L were higher than that of the positive group. In summary, the results of the cell test showed that both the water extract and the alcohol extract of IJ had a protective effect on the cell viability under high glucose treatment, and the water extract, especially at the concentrations of 0.13 g/L and 0.25 g/L, showed higher efficiency than the alcohol extract.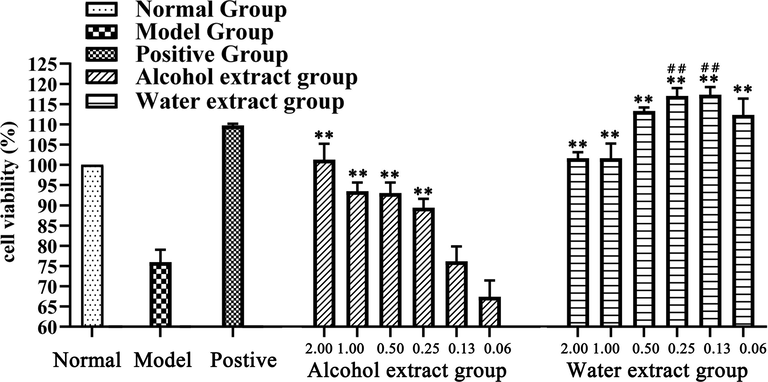
Cell viability treated with the extracts of Isodon Japonicus (n = 3, data = mean ± SD). **: P < 0.01 in comparison with model group; ##: P < 0.01 in comparison with positive group.
3.4 Hypoglycemic effect on diabetic rats
As listed in Table 3, the fasting-blood glucose (FBG) of each group after modeling, except for that of the normal group, was more than 16.6 mmol/L, and it increased significantly (P < 0.05). As shown in Fig. 6A, the pancreatic tissues of the normal rat were observed to be clear and intact, and the islet cells were arranged neatly in good shape. After modeling (Fig. 6B), vacuole-like changes appeared in disorganized form, and the number of pancreatic cells in the diabetic rats decreased with sparse distribution. So, the results of Table 3 and Fig. 6 indicated that the experimental diabetic rat model was successfully induced. As shown in Table 3, after administration with IJ for 4 weeks, compared with the model group, the FBG of the water extract group and the alcohol extract group decreased significantly (P < 0.05), respectively. Moreover, for both the water extract group and the alcohol extract group, there was no significant difference with the positive group. So, both the water extract and the alcohol extract of IJ had a good hypoglycemic effect for diabetic rats. PS: *: P < 0.05 in comparison with normal group after modeling. #: P < 0.05 in comparison before and after modeling for each group, respectively. &: P < 0.05 in comparison with model group after 4 weeks of administration.
Group
N
Blood glucose (mmol/L)
Before modeling
After modeling
4 weeks of administration
Normal group
6
5.1 ± 1.2
4.6 ± 1.0
5.5 ± 1.2&
Model group
6
4.8 ± 17
20.9 ± 5.9*#
21.4 ± 4.9
Positive group
6
4.5 ± 1.6
19.8 ± 5.1*#
6.9 ± 3.0&
Alcohol extract group
6
5.9 ± 1.1
20.1 ± 6.3*#
6.5 ± 3.4&
Water extract group
6
5.6 ± 0.9
18.5 ± 5.7*#
5.9 ± 1.8&
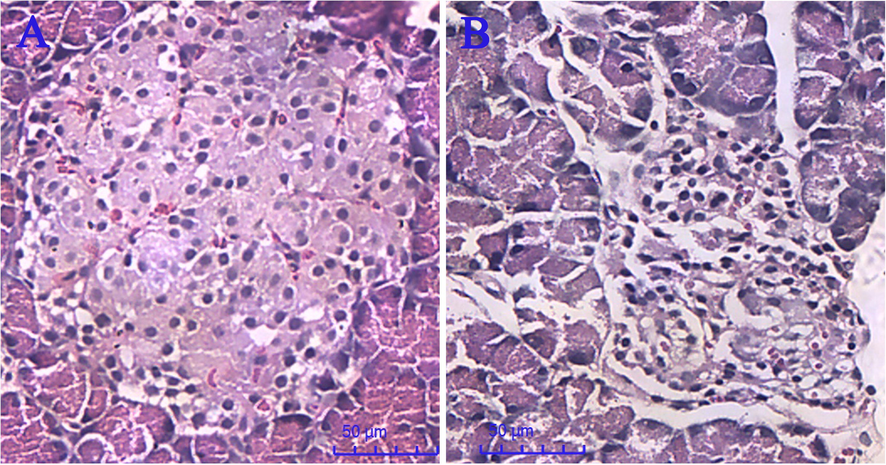
Pancreatic morphology of normal rats (A) and diabetic rats (B).
Both the cell test and the animal experiment showed that the ingredients in the water extract and the alcohol extract of IJ had a good hypoglycemic effect on diabetes. Usually, the log P of a chemical ingredient could reflect its solubility in water and ethanol, and its membrane permeability in vivo. More than 3 of log P value means the ingredient had good performance in ethanol, while<0 of log P value indicates good water solubility. The log P value at the range of 0–3 is much better, which means the ingredient could be dissolved in both water and ethanol, and had good performance in cell membrane permeability. Integrated with the results of the cell test, the animal experiment, and the network pharmacology (Table 1), Rabdoternin B of IJ should theoretically have good water solubility, and tormentic acid and β-sitosterol may be mainly existed in alcohol extract of IJ. Other 7 hypoglycemic ingredients, such as alboatisin A, enmein, hebeiabinin B, and so on, were with 0–3 of log P, so this might explain the results that both water extract and the alcohol extract of IJ had a good hypoglycemic effect. However, further study in vivo and in vitro experiments should be carried out to confirm the above results.
4 Conclusion
Historically, IJ has been widely utilized as healthy food and traditional herb. To better understand and evaluate its pharmacological effect and active ingredients on diabetes, the ingredient-target-pathway relationship and hypoglycemic effect of IJ were investigated by network pharmacology, cell tests and animal experiments. Based on network pharmacology method, 11 hypoglycemic ingredients, 12 hypoglycemic targets and 130 signaling pathways were got. The cell test and animal experiment verified the hypoglycemic effect of IJ. The cell viability in water extract group (0.06–2.00 g/L) and alcohol extract group (0.25–2.00 g/L) showed significant viability compared to that of the model group (P < 0.05), respectively. Animal experiments showed that both water extract and alcohol extract of IJ could lower diabetic rats' blood glucose levels when compared to the model group (P < 0.05). Based on network pharmacology technology combined with cell and animal experiments, 11 ingredients may be the main hypoglycemic ingredients, and HMGCR, PGR, ITGAL and ITGB2 may be the potential therapeutic targets. The hypoglycemic mechanisms may be through Rap1 signaling pathway, cAMP signaling pathway, Oxytocin signaling pathway. This study provided some references for our following study. However, to provide support for our findings, further study in vivo and in vitro experiments needed be carried out to confirm the hypoglycemic ingredients, targets and signaling pathways of IJ on diabetes. On the other hand, this strategy may be an effective method for other herbs to explore the active ingredients and pharmacological mechanisms.
Acknowledgments
This work was supported by the Key Research and Promotion Special Projects of Henan Provincial (scientific & technological projects of Henan Province) [grant numbers 222102310566], the Research Foundation of College student innovation project of Henan [grant numbers 202210472019] and the Key Research and Promotion Special Projects of Henan Provincial Science and Technology Department [grant numbers 222102310592]. This work was supported by Vascular remodelling intervention and molecular targeted therapy drug development Innovation team, and Cardiovascular remodelling intervention and molecular targeting drug research and development Key Laboratory. The authors are extremely grateful for the financial support.
Authorship contribution statement
Xue Jintao, Zhang Nan and Yang Pengfei designed this study. Xue Jintao, Lu Yusi, Xiao Xian, Li Chunyan, Wang Canyu, Zhu Huiqing, Liu Xiaolong, Song Liaofan, Zhang Nan, and Yang Pengfei participated in the collecting the samples, acquisition of data, and analysis and interpretation of the data. Xiao Xian and Xue Jintao aided in review, editing and revising the manuscript. All the authors had approved the final version of the manuscript to be submitted.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- CD18 deficiency protects against multiple low-dose streptozotocin-induced diabetes. Am. J. Pathol.. 2004;165:1849-1852.
- [CrossRef] [Google Scholar]
- A systematic review and meta-analysis on the efficacy and safety of traditional Chinese patent medicine Jinqi Jiangtang Tablet in the treatment of type 2 diabetes. Complement. Ther. Med.. 2019;47:102021
- [CrossRef] [Google Scholar]
- Anti-hepatoma activity of a novel compound glaucocalyxin H in vivo and in vitro. AAPS PharmSciTech.. 2015;16:496-504.
- [CrossRef] [Google Scholar]
- A novel cytotoxic oxetane ent-kauranoid from Isodon japonicus. Planta Med.. 2004;70:581-584.
- [CrossRef] [Google Scholar]
- Two New Diterpenoids and Other Constituents from Isodon japonicus. Helv. Chim. Acta.. 2012;7:1175-1180.
- [Google Scholar]
- Integrating Network Pharmacology and Experimental Models to Investigate the Efficacy of Coptidis and Scutellaria Containing Huanglian Jiedu Decoction on Hepatocellular Carcinoma. Am. J. Chin. Med.. 2020;48:161-182.
- [CrossRef] [Google Scholar]
- Alpha L-integrin I domain cyclic peptide antagonist selectively inhibits T cell adhesion to pancreatic islet microvascular endothelium. Am. J. Physiol. Gastrointest. Liver Physiol.. 2005;288:G67-G73.
- [CrossRef] [Google Scholar]
- Study on the immunomodulation effect of Isodon japonicus extract via splenocyte function and NK anti-tumor activity. Int. J. Mol. Sci.. 2012;13:4880-4888.
- [CrossRef] [Google Scholar]
- Network Pharmacological Screening of the Active Ingredients and Hypoglycemic Effect of Isodon rubescens in the Treatment of Diabetes. Planta Med.. 2020;86:556-564.
- [CrossRef] [Google Scholar]
- Effects of Gymnema sylvestre extract on the pharmacokinetics and pharmacodynamics of glimepiride in streptozotocin induced diabetic rats. Chem-Biol. Interact.. 2016;245:30-38.
- [Google Scholar]
- Isodon japonicus inhibits mast cell-mediated immediate-type allergic reactions. Immunopharm. Immunot.. 2004;26:273-284.
- [CrossRef] [Google Scholar]
- Anti-neuroinflammatory activity of Kamebakaurin from Isodon japonicus via inhibition of c-Jun NH(2)-terminal kinase and p38 mitogen-activated protein kinase pathway in activated microglial cells. J. Pharmacol. Sci.. 2011;116:296-308.
- [CrossRef] [Google Scholar]
- Circular RNA hsa_circ_0003575 regulates oxLDL induced vascular endothelial cells proliferation and angiogenesis. Biomed. Pharmacother.. 2017;95:1514-1519.
- [CrossRef] [Google Scholar]
- Isolation, structural elucidation, and cytotoxicity of three new ent-kaurane diterpenoids from Isodon japonica var. glaucocalyx. Planta Med.. 2012;78:589-596.
- [CrossRef] [Google Scholar]
- Isolation and identification of a new ent-kaurane diterpenoid from the leaves of Isodon japonica. Nat. Prod. Res.. 2013;27:1388-1392.
- [CrossRef] [Google Scholar]
- Study on mechanism of matrine in treatment of COVID-19 combined with liver injury by network pharmacology and molecular docking technology. Drug Deliv.. 2021;28:325-342.
- [CrossRef] [Google Scholar]
- Hypoglycemic Effect and Experimental Validation of Scutellariae Radix based on Network Pharmacology and Molecular Docking. Processes.. 2022;10:2553.
- [Google Scholar]
- Diterpenoids from Isodon species: an update. Nat. Prod. Rep.. 2017;34:1090-1140.
- [CrossRef] [Google Scholar]
- Antimutagenic activity of ent-kaurane diterpenoids from the aerial parts of Isodon japonicus. Tetrahedron Lett.. 2017;58:3574-3578.
- [CrossRef] [Google Scholar]
- Isolation and structure elucidation of constituents of Citrus limon, Isodon japonicus, and Lansium domesticum as the cancer prevention agents. Genes Environ.. 2020;42:17.
- [CrossRef] [Google Scholar]
- Oxytocin is present in islets and plays a role in beta-cell function and survival. Peptides.. 2018;100:260-268.
- [CrossRef] [Google Scholar]
- In Vitro Antioxidant activities and anti-diabetic effect of a polysaccharide from Schisandra sphenanthera in rats with type 2 diabetes. Int. J. Biol. Macromol.. 2017;94:154-160.
- [CrossRef] [Google Scholar]
- Progesterone receptor knockout mice have an improved glucose homeostasis secondary to beta -cell proliferation. Proc. Natl. Acad. Sci. U S A.. 2002;99:15644-15648.
- [CrossRef] [Google Scholar]
- Highlighting Diabetes Mellitus: The Epidemic Continues. Arterioscler. Thromb. Vasc. Biol.. 2018;38:e1-e8.
- [CrossRef] [Google Scholar]
- Sun, J.Y., Jiang, G.D. and Shigemori, H. 2019. Inhibitory Activity on Amyloid Aggregation of Rosmarinic Acid and Its Substructures from Isodon japonicus. Nat Prod Commun. 14, 1934578X1984303. https://doi.org/10.1177/1934578X19843039.
- β-Cell-Specific Deletion of HMG-CoA (3-hydroxy-3-methylglutaryl-coenzyme A) Reductase Causes Overt Diabetes due to Reduction of β-Cell Mass and Impaired Insulin Secretion. Diabetes.. 2020;69:2352-2363.
- [CrossRef] [Google Scholar]
- Hikiokoshins A-I, diterpenes from the leaves of Isodon japonicus. Phytochemistry.. 2014;102:205-210.
- [CrossRef] [Google Scholar]
- cAMP signalling in insulin and glucagon secretion. Diabetes Obes. Metab.. 2017;19(Suppl 1):42-53.
- [CrossRef] [Google Scholar]
- Associations between stressful life events and diabetes: Findings from the China Kadoorie Biobank study of 500,000 adults. J. Diabetes Investig.. 2019;10:1215-1222.
- [CrossRef] [Google Scholar]
- Two new diterpenoids and other constituents from Isodon rubescens. Fitoterapia.. 2011;82:726-730.
- [CrossRef] [Google Scholar]
- Anti-Inflammatory ent-Kaurane Diterpenoids from Isodon serra. J. Nat. Prod.. 2020;83:2844-2853.
- [CrossRef] [Google Scholar]
- Chemical constituents of Isodon nervosus and their cytotoxicity. J. Asian Nat. Prod. Res.. 2009;11:326-331.
- [CrossRef] [Google Scholar]
- 6beta-Acet-oxy-1alpha,7beta,11beta,15beta-tetra-hydr-oxy-7alpha,20-ep-oxy-ent-kau r-16-ene. Acta Crystallogr. E.. 2010;66:o295
- [CrossRef] [Google Scholar]
- 15alpha,20beta-Dihydr-oxy-6beta-meth-oxy-6,7-seco-6,20-ep-oxy-1,7-olide-ent-kaur- 16-ene. Acta Crystallogr. E.. 2010;66:o930
- [CrossRef] [Google Scholar]
- Progress and Prospects of Research Ideas and Methods in the Network Pharmacology of Traditional Chinese Medicine. J. Pharm. Pharm. Sci.. 2022;25:218-226.
- [CrossRef] [Google Scholar]
- Network Pharmacology Databases for Traditional Chinese Medicine: Review and Assessment. Front. Pharmacol.. 2019;10:123.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Fig. A: The active ingredients of Isodon Japonicus. Table A.1: The biological processes of the hypoglycemic targets for Isodon Japonicus. Table A.2: The molecular functions of the hypoglycemic targets for Isodon Japonicus. Table A.3: The cellular components of the hypoglycemic targets for Isodon Japonicus. Table A.4: the KEGG results of the hypoglycemic targets for Isodon Japonicus.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105108.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1
Supplementary data 2
Supplementary data 2
Supplementary data 3
Supplementary data 3
Supplementary data 4
Supplementary data 4
Supplementary data 5
Supplementary data 5







