Translate this page into:
Identification and quantitation of NF-κB inhibitory components in weichang'an pill based on UHPLC-QE-MS and spectrum-effect relationship
⁎Corresponding authors. cljuan1258@163.com (Lijuan Chai), zhangpeng@tjutcm.edu.cn (Peng Zhang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Weichang'an pill (WCAP) is a traditional Chinese patent medicine, which is clinically used for the treatment of bowel syndrome and functional dyspepsia such as diarrhea, abdominal distension, and enteritis. So far, quality control studies of WCAP have mainly focused on the determination of chemical composition content, which has little relevance to biological activity and clinical effects. With the aim of identifying the multi-index ingredients with NF-κB inhibitory activities related to WCAP clinical effect, this present work described the chemical profile of WCAP by UHPLC-QE-MS, established the correlated relationship between chromatographic fingerprints and the NF-κB inhibitory activities based on multivariate statistical analysis, including hierarchical clustering analysis (HCA), Pearson correlation analysis, and Partial least squares regression analysis (PLSR). The spectrum-effect relationship analysis indicated 10 compounds, which were ferulic acid, naringin, narirutin, hesperidin, neohesperidin, aloe emodin, emodin, honokiol, magnolol, and physcion, might be the potential NF-κB inhibitory constituents in the pill. The NF-κB inhibitory effects of the ten compounds were verified by in vitro dual luciferase reporting detection system. Considering that the detection index should be representative of more medicinal materials, a rapid and efficient UPLC-DAD method was eventually developed to determine the content of the 13 components. Our findings will provide data support for WCAP quality control and advance the understanding of the quality assessment of traditional Chinese patent medicines.
Keywords
Weichang'an pill
NF-κB inhibitory activity
Spectrum-effect relationship
Quality control of TCM
1 Introduction
WCAP, recorded in the first part of Chinese Pharmacopoeia in the 2020 edition (Commission, 2020), is composed of ten herbs, such as principal herbs Santali Albi Lignum, Aucklandiae Radix, and Magnoliae Officinalis Cortex. All the herbs are crushed to a fine powder, mixed together, and made into water pills. The protected medication has been widely used in China for more than 30 years to treat gastrointestinal diseases including enteritis, diarrhea, bacillary dysentery, stomach aches, abdominal pain, and abdominal distension (Shang et al., 2020; Shi et al., 2018). These diseases are often accompanied by inflammating, for example, enterities is an actue inflammation of ganstrointestinal mocosa, bacilary dysentery is a purulent inflammation of the colon caused by Shigella dysenteriae, manifested as abdominal pain, diarrhea, pus, and bloody stool and other clinical manifestations (Zhang et al., 2020). WCAP methanol extraction could significantly improve the 5-fluorouracil-induced intestinal mucositis mice, the inflammatory factors NF-κB, IL-1β were significant decrease in the IM mice (Chen et al., 2016).
The nuclear factor κB (NF-κB) is one of the most critical regulatory factors involved in inflammation which can induce the gene expression of cytokines and the transcription factors in the regulation of inflammatory responses (Kunnumakkara et al., 2020; Yu et al., 2020). The activation of NF-κB is the decisive factor of many pathological conditions, which makes it a therapeutic target for the clinical treatment of related diseases., NF-κB is a common index in gastrointestinal inflammation effect evaluation. A significant increase of NF-κB was observed in rats with gastritis (Xu et al., 2022), and a decrease of NF-κB p65 was also observed in DSS-induced chronic colitis mice after treatment with asperuloside (Chen et al., 2021). Therefore, compounds with NF-κB inhibition may serve as candidates for quality control indicators of WCAP. Single-component inhibitory activity of NF-κB, such as magnolol and honokiol, have been reported (Chen et al., 2019; Tse et al., 2005), but the overall picture of the NF-κB inhibitory component in WCAP remains unclear. WCAP and its components with NF-κB inhibitory activity were studied by using a TNF-α-induced dual luciferase reporting assay system in HEK 293 cells (Han et al., 2015).
Currently, 41 chemicals from the WACP methanol extract have been identified by HPLC-ESI-MS/MS (Liu et al., 2013). The identified compounds were from Radix et Rhizoma Rhei, Fructus Aurantii, Cortex Magnoliae officinalis, and Radix Aucklandiae, while the chemical compositions of the other six herbs remained unknown. Quality control studies at WCAP have determined the chemical composition content of 14 compounds from five herbs (Zhang et al., 2010; Jing et al., 2012; Zhang et al., 2013). However, there is a lack of systematic studies on the biological activity of WCAP quality control indicators. The composition of WCAP is too complex, and some of the components are difficult to obtain, making it difficult to detect their activities one by one. Spectrum-effect relationship investigations have been proposed as a potential method to predict effective components in complex mixtures and to mirror the internal quality of herbal medicines (Zhu et al., 2016). PLSR and artificial neural network were used to explore the spectrum-effect relationship of Sinomenii Caulis, and 8 potential anti-inflammatory components were screened out from a total of twenty two components (Wang et al., 2019). Other statistical methods such as Pearson correlation analysis have also been widely used to investigate the spectrum-effect relationship (An et al., 2022).
This present work described the identification and quantitation of NF-κB inhibitory components in WCAP based on UHPLC-QE-MS and spectrum-effect relationship. The chemical profile of WCAP was investigated by UHPLC-QE-MS, and 129 chemical constituents were identified from eight herbs. UPLC-UV fingerprints of 16 batches of WCAP were established, and their NF-κB inhibitory activities were detected by the TNF-α-induced dual luciferase reporting assay system in HEK 293 cells. The spectrum-effect relationship indicated 10 compounds, as well as ferulic acid, naringin, narirutin, hesperidin, neohesperidin, aloe emodin, emodin, honokiol, magnolol, physcion, might be potential NF-κB inhibitory constituents and their effects were verified. To cover more medicinal material quality control, a rapid and efficient UPLC-DAD method was subsequently developed to determine the content of the 10 components plus rhein, costunolide and chrysophanol. The result will provide data support for WCAP quality control improvement.
2 Material and methods
2.1 Materials and reagents
Sixteen batches of WCAP were produced by Tianjin Zhongxin Pharmaceutical Group Corporat Lerentang Pharmaceutical Factory, the details of which are given in Table S1. Methanol, acetonitrile and formic acid were purchased from Fisher Scientific and Tianjin Obt Chemical Co., LTD, ferulic acid, narirutin, naringin, hesperidin, neohesperidin, aloe emodin, rhein, emodin, honokiol, costunolide, dehydrocostus lactone, magnolol, chrysophanol, and physcion were obtained from Sichuan Weikeqi Technology Co., Ltd. TNF-α, human embryonic kidney cell 293 (HEK293), NF-κB luciferase reporter plasmid PGL 4.32 and sea kidney luciferase reporter plasmid PRL-TK was purchased from PeproTech.
2.2 Sample extraction
50 mg WCAP powder was immersed in 500 μL 80 % methanol, swirled for 30 s, ultrasonic extraction for 1 h, and centrifuged at 12000 rpm for 15 min. The supernatant was filtered by 0.22 μm microporous filter membrane and then sampled to inject for LC-MS analysis.
An aliquot of 1.0 g of sixteen batches of WCAP powder was immersed in 20 mL methanol, followed ultrasonic extraction at room temperature for 90 min, the mixture was then centrifuged at 13,000 rpm for 10 min. The supernatant was filtered through a 0.45 μm nylon syringe filter and stored at before the UPLC analysis.
2.3 UHPLC-QE-MS analysis
LC-MS/MS analysis was performed on an Agilent ultra-high performance liquid chromatography 1290 UPLC system with a Waters UPLC BEH C18 column (2.1 mm × 100 mm, 1.7 μm). The column temperature was set at 55 ℃ and the sample injection volume was set at 5 μL. The flow rate was set at 0.5 mL/min. The mobile phase consisted of 0.1 % formic acid in water (A) and 0.1 % formic acid in acetonitrile (B). The multi-step linear elution gradient program was as follows: 0 – 11 min, 15 – 75 % B; 11 – 12 min, 75 – 98 % B; 12 – 14 min, 98 – 98 % B; 14 – 14.1 min, 98 – 15 % B; 14.1 – 16 min, 15 – 15 % B.
A Q Exactive Focus mass spectrometer coupled with Xcalibur software was employed to obtain the MS and MS/MS data based on the IDA acquisition mode. During each acquisition cycle, the mass range was from 100 to 1500, and the top three of every cycle were screened and the corresponding MS/MS data were further acquired. Sheath gas flow rate: 45 Arb, Aux gas flow rate: 15 Arb, Capillary temperature: 400 ℃, Full ms resolution: 70000, MS/MS resolution: 17500, Collision energy: 15/30/45 in NCE mode, Spray Voltage: 4.0 kV (positive) or −3.6 kV (negative).
2.4 UPLC fingerprints
2.4.1 UPLC condition
The prepared samples were injected into a Waters ACQUITY UPLC system (Waters, Milford, MA, USA) with a photodiodearray (PDA) detector. The chromatographic separation was performed with a Waters ACQUITY UPLC BEH C18 column (2.1 mm × 100 mm, 1.7 μm), operated at 35◦C. The flow rate was kept constant at 0.3 mL/min and UV measurements were obtained at 254 nm. The mobile phases were water contain 0.1 % Formic acid (A) and acetonitrile (B) with gradient elution was the following program: 0 – 3 min, 5 – 22.5 % B, 3 – 7 min, 22.5 – 22.5 % B, 7 – 8 min, 22.5 – 47 % B, 8 – 13 min, 47 – 47 % B, 13 – 14 min, 47 – 65 % B, 14 – 19 min, 65 – 65 % B, 19 – 20 min, 65 – 95 % B.
2.4.2 Method validation
The precision was certificated by evaluating six injections of the same working solution, repeatability was determined by the relative standard deviation (RSD) from six working solutions of the same sample, and the stability was analyzed by the same sample using the above-established method at 0, 2, 4, 8, 16, 24 h. The RSDs of precision were calculated based on the relative peak area of each characteristic peak, and the RSDs of repeatability and stability were calculated by mass concentration. Sample 1,530,963 was selected as sample solution for method validation and 13 main peaks were chosen for calculating the RSDs. Accuracy was determined by the recovery test. The mixed standard solutions were added to known amounts of samples, and then the resultant samples were extracted and analyzed by the established UHPLC method. The percentage recoveries were evaluated by calculating the ratio of the detected amount versus added amount.
2.4.3 Establishment and evaluation of fingerprints
To determine a representative chromatographic fingerprints, 16 batches of WCAP were analyzed with the optimal UPLC method. Chemometrics were applied to demonstrate the differences in the 16 batches of WCAP. Similarity analysis was performed by the Similarity Evaluation System for Chromatographic Fingerprints of Traditional Chinese Medicines (version 2012A; Beijing, China). Hierarchical cluster analysis (HCA) of the 16 samples was performed using SIMCA14.1.
2.5 NF-κB inhibitory activity assay
293 T cells were plated 3 × 104/well in 96 well in DMEM supplemented with 10 % FBS, 100 U/mL penicillin, and 100 μL streptomycin at 37.5 °C in a humidified atmosphere containing 5 % CO2.. When the cell reaches about 80 % confluency, the NF-κB luciferase reporter plasmid pGL4.32 (100 ng/well) and the sea chenin luciferase reporter plasmid pRL-TK (9.6 ng/ well) were transfected into cells with PEI (1 mg/mL) transfection reagent and cultured for 24 h (Makó et al., 2010; Gordon et al., 2011). After 24 h culture, TNF-α (10 ng/mL) was added into each well as the model group, Dex (0.01 μM, Dexamethason) + TNF-α (10 ng/mL) as the positive drug group, and each batch of WCAP to be tested (0.001 μg/mL) + TNF-α (10 ng/mL) as the experimental group, a blank control was set and cultured for 6 h. After 6 h incubation at 37.5 °C, cells were washed with PBS and detected by dual luciferase detection system after lysis. The ratio of firefly luciferase activity to sea kidney luciferase activity was calculated to obtain the relative luciferase activity value (Gordon et al., 2011), Each group was set up with six duplicate holes and repeated three times. The NF-κB inhibition activity of 16 batches of methanol extracts from WCAP was determined by Multilabel Plate Reader (Perkins Elmer).
2.6 Spectrum-effect relationship
2.6.1 Pearson correlation analysis
Correlation analysis is a statistical analysis method to study the correlation between two or more random variables. Pearson correlation coefficient is a coefficient to measure the linear relationship between distance variables (Han et al., 2021). The index components of NF-κB inhibitory activity were analyzed by the Pearson correlation coefficient.
2.6.2 Partial least squares regression analysis (PLSR)
PLSR is a multivariate analysis method, which is a linear regression model to find and explain the relationship between a dependent variable and an independent variable. It combines multiple linear regression, canonical correlation analysis, and principal component analysis to overcome multicollinearity caused by multiple independent variables (Chen et al., 2020), and is more comprehensive and comprehensive than the above correlation analysis. In this experiment, PLSR was analyzed using SIMCA14.1.
2.6.3 Statistical analysis
SPSS17.0 was used for statistical analysis, and the results were expressed as mean standard deviation (‾x ± SD). Univariate analysis of variance was used between groups, and the difference was statistically significant when p < 0.05.
3 Results and discussion
3.1 UHPLC-QE-MS profiling of WCAP
A total of 129 compounds were identified from Fructus Aurantii, Cortex Magnoliae officinalis, Radix et Rhizoma Rhei, Radix Aucklandiae, Aquilariae Lignum Resinatum, Jujubae Fructus, Chuanxiong Rhizoma, and Crotonis Semen Pulveratum respectively. It mainly includes flavonoids, phenylpropanoids, alkaloids, etc. The total ion flow diagram of UHPLC-QE-MS is shown in Fig. 1, and the identification results of UHPLC-QE-MS are shown in Table 1 and Table 2. note:*Represents comparison with the standards Cortex Magnoliae officinalis: CMO Fructus Aurantii: FA Aquilariae Lignum Resinatum: ALR Radix et Rhizoma Rhei: RRR Aucklandiae Radix: AR Jujubae Fructus: JF Chuanxiong Rhizoma: CR Crotonis Semen Pulveratum: CSP.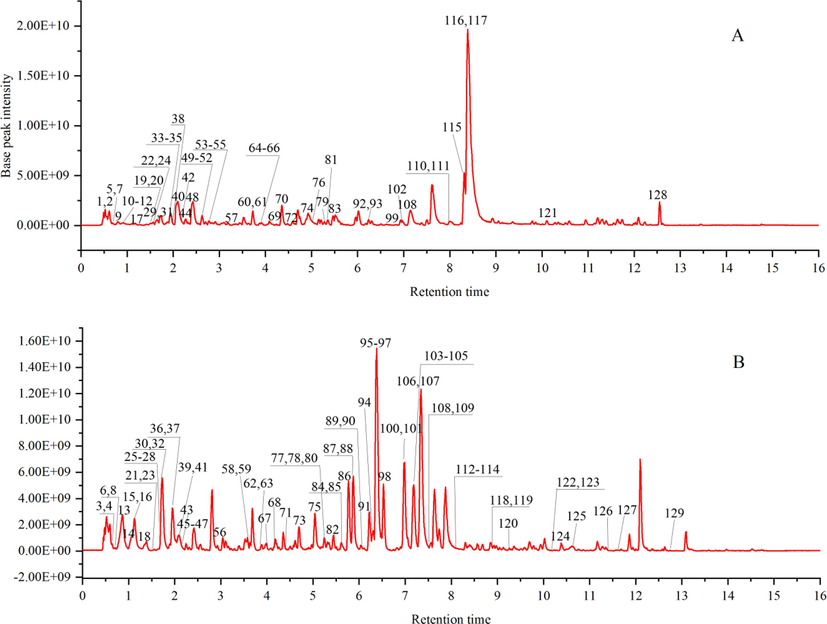
Total ion chromatograms of WCAP by UHPLC-QE-MS, under negative (A) and positive (B) ionization modes.
No.
tR(min)
Experimental (m/z)
Ion mode
Error(ppm)
MS/MS
Molecular formula
Identification
Distribution
1
0.59
134.0473
[M−H]-
2.41
119.0350
C5H5N5
Adenine
JF
2*
0.62
169.0141
[M−H]-
0.50
125.0249
C7H6O5
Gallic acid
RRR
3
0.63
168.1020
[M + H]±
−2.83
150.0913;135.0677;119.0897;107.4177
C9H13NO2
Synephrine
FA
4
0.64
152.1071
[M + H]±
−2.89
121.0648
C9H13NO
N-Methyltyramine
FA
5
0.68
483.0781
[M−H]-
1.29
465.1033;439.0866;321.0825;169.0141;151.0398;125.0245
C20H20O14
Gallic acid-O-galloyl-glucoside
RRR
6
0.72
330.1704
[M + H]±
1.24
299.1242;192.1042;177.0547;137.0597
C19H23NO4
Sinomenine
CMO
7*
0.75
153.0191
[M−H]-
0.89
109.0293
C7H6O4
Protocatechuic acid
CR
8
0.75
265.1551
[M + H]±
−0.55
177.0547;145.0496
C14H20N2O3
N-Feruloylputrescine
CMO
9
0.79
785.2503
[M−H]-
−0.13
477.1615;161.0456
C35H46O20
Magnoloside B
CMO
10
0.83
289.0715
[M−H]-
1.79
245.0667;203.0675;137.0245;109.0293
C15H14O6
Cianidanol
RRR
11
0.94
635.0893
[M−H]-
1.37
465.1414;169.0141
C27H24O18
Gallic acid-O-digalloyl-glucoside
RRR
12*
0.94
167.0348
[M−H]-
2.39
152.0354;123.0086;108.0455
C8H8O4
Vanillic acid
FA
13
0.98
313.1591
[M + H]±
−3.03
205.1099;190.0863
C19H22NO3 ±
3,4-Dehydromagnocurarine
CMO
14
1.05
166.1227
[M + H]±
1.72
151.0393;137.0597
C10H15NO
Hordenine
FA
15
1.11
344.1853
[M + H]±
−2.56
299.1473;175.1118;151.0393;137.0597
C20H26NO4 ±
Tembetarine
CMO
16*
1.12
342.1699
[M + H]±
−0.29
297.1118;265.1437
C20H24NO4±
Magnoflorine
CMO
17
1.13
507.1138
[M−H]-
−0.24
345.1557;331.1756;313.0576;169.0141;151.0400;125.0242
C23H24O13
Ferulic acid-O-galloyl-glucoside
RRR
18
1.42
611.1605
[M + H]±
0.90
465.1035;303.0497;153.0548
C27H30O16
Rutin
FA
19
1.43
325.0932
[M−H]-
0.57
235.0609;163.0399;145.0297;119.0503
C15H18O8
p-Coumaric acid-O-glucoside
RRR
20
1.45
623.1991
[M−H]-
1.78
461.2038;315.1082;161.0456;135.0452
C29H36O15
Magnoloside A
CMO
21
1.50
417.1184
[M + H]±
1.04
375.1444;336.1804;255.1743;185.1329
C21H20O9
Daidzin
RRR
22
1.55
729.1461
[M−H]-
0.66
577.1562;381.1353
C37H30O16
Proanthocyanidins B-O-gallate
RRR
23
1.55
451.1230
[M + H]±
−2.20
397.1488;163.0394;289.0703
C21H22O11
Eriodictyol-7-O-Glucoside
FA
24
1.56
441.0823
[M−H]-
0.77
331.1395;289.0749;245.1031;205.0508;203.9948;193.0502;179.0562;169.0141;125.0242
C22H18O10
Epicatechin gallate
RRR
25
1.57
373.1488
[M + H]±
−2.93
211.1321;193.0860
C17H24O9
Syringin
CMO
26
1.67
183.0653
[M + H]±
1.40
155.0702;140.0468;125.0597
C9H10O4
Syringaldehyde
AR
27
1.67
595.1663
[M + H]±
0.56
449.1079;287.0547
C27H30O15
Lonicerin
FA
28*
1.71
195.0655
[M + H]±
2.31
177.0457;149.0598;134.0802;117.0701
C10H10O4
Ferulic acid
FA/CR
29
1.71
541.1362
[M−H]-
2.99
313.1298;227.1293;169.0141;125.0242
C27H26O12
Resveratrol-O-galloyl-glucoside
RRR
30*
1.72
319.1179
[M + H]±
0.43
301.1066;283.0989;255.1020;227.1061;164.0979
C17H18O6
Agarotetrol
ALR
31
1.74
477.1046
[M−H]-
2.71
331.0825;315.1240;313.0562;169.0141;151.0398;125.0242
C22H22O12
p-Coumaric acid-O-galloyl-glucoside
RRR
32
1.79
268.1335
[M + H]±
−1.09
251.1082;219.0287;191.1068
C17H17NO2
Asimilobine
CMO
33
1.87
547.1461
[M−H]-
1.61
233.0450;191.0555;177.0924;169.0141;151.0396;125.0243
C26H28O13
2-(2′-Hydroxypropyl)-5-methyl-7-hydroxychromone-O-galloyl-glucoside
RRR
34*
1.89
861.1887
[M−H]-
1.00
721.1888;465.1038
C42H38O20
Sennoside B
RRR
35
1.91
515.1186
[M−H]-
0.74
353.0879;191.0555;173.0607
C25H24O12
Isochlorogenic acid A
AR
36
1.92
314.1747
[M + H]±
−3.02
269.1363;237.1849;175.1118;107.0856
C19H24NO3 ±
Magnocurarine
CMO
37
1.95
319.1175
[M + H]±
−2.27
301.1076;283.0966;255.1019;227.1089;164.0704
C17H18O6
Aquilarone B
ALR
38
1.98
603.1304
[M−H]-
−7.55
451.1042;289.0714;245.1022;227.0344;205.0348;179.0562;161.0451;137.0242
C28H28O15
Catechin-O-galloyl-glucoside/Epigallocatechin-O-gallate glucoside
RRR
39
2.08
579.1703
[M + H]±
1.16
433.1122;271.0595;253.1797;235.0968
C27H30O14
Rhoifolin
FA
40*
2.08
579.1730
[M−H]-
2.76
271.0616;151.0394;119.0500
C27H32O14
Narirutin
FA
41
2.09
435.1282
[M + H]±
0.47
273.0762;231.1379;195.0289;153.0181
C21H22O10
Naringenin-7-O-glucoside
FA
42
2.18
515.1187
[M−H]-
0.60
353.0878;191.0195;179.0558;173.0448
C25H24O12
Isochlorogenic acid B
AR
43
2.19
181.0497
[M + H]±
1.87
163.0389;145.0496;137.0963
C9H8O4
Caffeic acid
FA
44
2.28
881.1575
[M−H]-
1.09
577.2874;559.2029;541.1334;533.1322;493.1146;467.1194;449.1094;407.1347;381.0740;289.0713;245.0813
C44H34O20
Proanthocyanidins B-O-digallate
RRR
45
2.30
597.1820
[M + H]±
0.09
289.0705;163.1120
C27H32O15
Eriocitrin
FA
46
2.42
465.1390
[M + H]±
−1.40
303.0875;153.0173;151.0754
C22H24O11
Hesperetin 7-O-glucoside
FA
47
2.42
595.2011
[M + H]±
−2.74
449.1437;287.1276;272.1275;254.1826
C28H34O14
Didymin
FA
48*
2.43
609.1823
[M−H]-
0.46
301.0699
C28H34O15
Hesperidin
FA
49
2.52
613.1184
[M−H]-
−1.49
569.2242;443.1700;169.0141;147.0448;125.0242
C29H26O15
Cinnamoyl-O-digalloyl-glucoside
RRR
50*
2.56
579.1731
[M−H]-
1.88
459.0927;271.0611;151.0396
C27H32O14
Naringin
FA
51
2.62
493.1148
[M−H]-
−9.09
331.1393;313.0560;169.0131;151.0028;125.0240
C19H26O15
Gallic acid-O-diglucoside
RRR
52
2.65
461.1082
[M−H]-
1.63
401.0567;313.0561;211.0972;193.0501;169.0139;151.0398
C22H22O11
1-O-Galloyl-6-O-cinnamoyl-glucose
RRR
53
2.80
445.0774
[M−H]-
0.79
325.1294;297.0758;283.1548;269.0450;263.1285;239.1292;235.0612;211.0971;207.0656
C21H18O11
Rhein 8-O-glucoside
RRR
54
2.94
861.1890
[M−H]-
1.34
465.2699
C42H38O20
Sennoside A
RRR
55*
2.97
609.1823
[M−H]-
0.66
301.0706;286.0467
C28H34O15
Neohesperidin
FA
56
3.03
203.0342
[M + H]±
0.89
131.0495;119.0857
C11H6O4
Xanthotoxol
FA
57
3.43
407.1348
[M−H]-
0.60
245.1387;215.0326
C20H24O9
Torachrysone 8-O-glucosid
RRR
58
3.59
271.0967
[M + H]±
2.60
203.1428;159.1167;147.0442;131.0491
C16H14O4
Isoimperatorin
FA
59
3.65
303.0860
[M + H]±
0.12
177.0538;153.0190
C16H14O6
Homoeriodictyol
FA
60*
3.74
271.0612
[M−H]-
0.69
187.0974;151.0031;119.0497
C15H12O5
Naringenin
FA
61
3.74
671.1798
[M−H]-
4.94
509.1069;389.1817;361.0929;227.1293;183.1027;169.0141;151.0396;125.0245
C36H32O13
Resveratrol-O-cinnamoyl-galloyl-glucoside
RRR
62
3.76
315.0863
[M + H]±
1.07
300.1954;285.0757;272.0634;257.1897
C17H14O6
Baicalin 4′,7-dimethyl ether
FA
63
3.87
331.1170
[M + H]±
−3.50
313.1067;149.0598
C18H18O6
3-hydroxy-4′,5,7-trime-thoxyflavanone
FA
64
3.89
517.0978
[M−H]-
−0.72
473.1078;269.0450;225.1128
C24H22O13
Emodin 8-O-6′'-malonylglucoside
RRR
65
3.92
299.1284
[M−H]-
0.32
239.1080
C18H20O4
Magnolignan A or C
CMO
66
3.96
623.1772
[M−H]-
1.12
459.1309;313.0351;307.1193;295.0972;169.0141;163.0400;151.0398;125.0245
C32H32O13
4-(4′-Hydroxyphenyl)-2-butanone-O-cinnamoyl-galloyl-glucoside
RRR
67
4.03
319.1542
[M + H]±
0.56
301.1451;177.0548;162.0914
C18H22O5
Pranferin
FA
68
4.16
237.1850
[M + H]±
−2.11
219.1734;201.1645
C15H24O2
Curcumol
ALR
69
4.32
431.0986
[M−H]-
1.43
269.0452;241.0874;240.0785;225.0921
C21H20O10
Emodin 8-O-glucoside
RRR
70
4.37
241.0866
[M−H]-
0.61
223.0761;197.0970;133.0139
C15H14O3
Randaiol
CMO
71
4.42
249.1486
[M + H]±
1.60
231.1379;203.1798;213.1277;185.1329;157.1015;143.0858
C15H20O3
Santamarine
AR
72
4.60
299.1283
[M−H]-
0.09
239.1073
C18H20O4
Magnolignan A or C
CMO
73
4.72
373.1281
[M + H]±
0.26
358.2371;343.0797;328.1017;315.1583;181.1211
C20H20O7
Isosinensetin
FA
74
4.95
297.0407
[M−H]-
2.53
253.0493;225.0540;210.0332
C16H10O6
6-Methylrhein
RRR
75
5.04
261.1119
[M + H]±
0.50
177.0.0547;149.0960;133.1012
C15H16O4
Meranzin
FA
76
5.08
501.1776
[M−H]-
2.91
337.1448;277.1077;193.0509
C26H30O10
4-(4′-Hydroxyphenyl)-2-butanone-O-feruloyl-glucoside
RRR
77
5.23
267.1017
[M + H]±
1.27
176.0917;161.1326;137.0597
C17H14O3
7-Hydroxy-2-(2-phenylethyl)chromone
ALR
78
5.27
471.2013
[M + H]±
0.63
425.1949;161.1326
C26H30O8
Limonin
FA
79*
5.28
269.0455
[M−H]-
1.86
240.0434;225.0573
C15H10O5
Aloe emodin
RRR
80
5.31
327.1228
[M + H]±
−1.27
221.1898;107.0854
C19H18O5
Qinanone G
ALR
81
5.36
633.1622
[M−H]-
2.27
461.1833;443.1268;313.0718;295.0611;151.0402;125.242
C33H30O13
2,5-Dimethyl-7-hydroxychromone-O-cinnamoyl-galloyl-glucoside
RRR
82
5.43
283.0968
[M + H]±
0.69
192.1099;164.1512
C17H14O4
6,8-Dihydroxy-2-(2-phenylethyl)chromone
ALR
83*
5.53
283.0247
[M−H]-
1.11
257.0459;239.0354
C15H8O6
Rhein
RRR
84
5.60
533.2388
[M + H]±
0.11
369.2067;161.1325
C28H36O10
Nomaline acid
FA
85
5.60
491.2279
[M + H]±
−0.37
449.1595;407.1484;347.1629;329.1216
C26H34O9
Sudachinoid A
FA
86
5.69
473.2167
[M + H]±
−1.79
427.1033;161.1326
C26H32O8
Deacetyl nomilin
FA
87
5.82
343.1172
[M + H]±
0.70
313.1069;285.0761
C19H18O6
6-Demethoxytangeretin
FA
88*
5.87
403.1383
[M + H]±
0.82
388.1144;373.0877;355.0781
C21H22O8
Nobiletin
FA
89
6.09
127.0391
[M + H]±
−3.28
109.1012
C6H6O3
5-Hydroxymethylfurfural
AR
90
6.12
311.1272
[M + H]±
−3.51
203.1797;190.1674;151.1118;121.1011
C19H18O4
6-Methoxy-2-[2-(3′-methoxyphenyl) ethyl] chromone
ALR
91
6.22
341.1380
[M + H]±
0.09
121.0638
C20H20O5
6,7-Dimethoxy-2-[2-(4′-methoxyphenyl) ethyl] chromone
ALR
92
6.25
355.1182
[M−H]-
2.23
311.2230;296.1008;281.0818
C20H20O6
Coniferyl ferulate
CR
93
6.29
171.1390
[M−H]-
0.09
C10H20O2
Decanoic acid
CSP
94*
6.31
193.1222
[M + H]±
1.08
175.1118;147.1168;137.0597;119.0852;105.0701
C12H16O2
Senkyunolide A
CR
95*
6.34
191.1067
[M + H]±
1.39
173.0963;145.1013
C12H14O2
3-n-Butylphathlide
CR
96
6.38
311.1272
[M + H]±
−3.38
220.0725;205.0489;181.0482
C19H18O4
6,7-Dimethoxy-2-(2-phenylethyl) chromone
ALR
97
6.48
419.1335
[M + H]±
−1.63
389.1964;371.1858;361.0899
C 21H 22O 9
Natsudaidain
FA
98*
6.53
373.1281
[M + H]±
0.23
343.0787;325.1429
C20H20O7
Tangeretin
FA
99
6.76
253.0871
[M−H]-
2.53
235.1339;207.0509
C16H14O3
Magnaldehyde D
CMO
100
6.94
389.1234
[M + H]±
1.12
374.1316;359.1842;341.1366
C20H20O8
5-O-Demethylnobiletin
FA
101
6.98
251.1063
[M + H]±
−3.72
173.0964;160.0511;121.0276
C17H14O2
2-(2-phenylethyl)chromone
ALR
102
7.02
329.1396
[M−H]-
2.27
267.1024;249.1491;239.0714;133.0142
C19H22O5
Magnolignan D
CMO
103
7.08
269.0452
[M−H]-
0.74
241.0875;225.0611;197.0618
C15H10O5
Emodin
RRR
104
7.10
379.1909
[M + H]±
0.05
361.1647;189.0913
C24H26O4
4,5-Dehydrodigestolide
CR
105
7.13
277.1802
[M + H]±
0.71
235.1693;199.0866;177.1273
C17H24O3
Ligustrosone
CR
106
7.33
281.1172
[M + H]±
−2.10
190.0615;151.0383
C18H16O3
6-Methoxy-2-(2-phenylethyl)chromone
ALR
107
7.42
189.0908
[M + H]±
1.14
147.1168;143.0858;133.1011
C12H12O2
3-Butylidenephthalide
CR
108
7.59
191.1067
[M + H]±
1.33
173.0954;145.1013
C12H14O2
Ligustilide
CR
109*
7.62
233.1534
[M + H]±
1.88
215.1427;187.1482;159.1168;145.1013;131.0857
C15H20O2
Costunolide
AR
110
7.82
297.1135
[M−H]-
2.58
249.1492;238.0898;231.9314
C18H18O4
Magnolignan E
CMO
111*
7.98
265.1232
[M−H]-
0.77
249.1488;224.0232;223.0759;209.0457;197.8081
C18H18O2
Honokiol
CMO
112*
8.17
231.1379
[M + H]±
0.30
213.1279;195.1181;185.1317;157.0645;143.0853
C15H18O2
Dehydrocostus lactone
AR
113
8.20
219.1747
[M + H]±
−0.91
201.1636;163.0753
C15H22O
Cyperotundone
ALR
114
8.27
207.1015
[M + H]±
2.20
165.0703;137.0600
C12H14O3
Acetyleugenol
AR
115
8.35
281.1177
[M−H]-
−0.51
263.1071;245.0958;133.0648
C18H18O3
Obovatol
CMO
116
8.56
295.1334
[M−H]-
−0.11
264.1148
C19H20O3
3-OMe-magnolol
CMO
117*
8.74
265.1232
[M−H]-
0.80
247.1129;245.0951;223.0788
C18H18O2
Magnolol
CMO
118
8.93
118.0862
[M + H]±
1.31
59.0483
C5H11NO2
Betaine
CMO
119
8.97
381.2055
[M + H]±
−2.91
335.2002;191.1067;173.0963
C24H28O4
Tokinolide B
CR
120
9.29
235.1693
[M + H]±
1.36
161.1326
C15H22O2
Costic acid
AR
121
10.23
279.1023
[M−H]-
0.84
261.0921;233.1547
C18H16O3
Randainal
CMO
122*
10.27
381.2055
[M + H]±
1.24
191.1069;173.0949;145.1015;105.0701
C24H28O4
Levistilide A
CR
123
10.31
299.1645
[M + H]±
1.70
175.1118;163.0389;119.0854;107.0854
C19H22O3
Auraptene
FA
124
10.45
179.1067
[M + H]±
−2.67
164.0425;151.0753;116.0703;123.0438
C11H14O2
Auraptene
AR
125
10.76
189.0546
[M + H]±
−3.28
131.0491;103.0541
C11H8O3
4′,5′-Dihydropsoralen
FA
126
11.45
413.2330
[M + H]±
0.50
413.2330;223.1882;191.1068
C25H32O5
Wallichilide
CR
127
11.64
163.0389
[M + H]±
0.58
119.0334;107.0487
C9H6O3
7-Hydroxycoumarin
FA/CR
128
12.56
401.2490
[M−H]-
2.24
331.1696
C28H34O2
Piperitylmagnolol
CMO
129
12.75
415.3930
[M + H]±
−7.24
397.3102;367.3212;255.1741;213.1018
C29H50O
β-Sitosterol
FA/CR
NO.
Similarity
NO.
Similarity
S605
0.856
S944
0.980
S826
0.996
S953
0.999
S932
0.996
S954
0.996
S936
0.994
S955
0.936
S938
0.995
S956
0.930
S939
0.996
S963
0.996
S940
0.999
S964
0.996
S942
0.999
S969
0.997
3.2 UPLC fingerprints analyze
3.2.1 Method validation for UPLC fingerprints
The results of the method validation showed that the RSDs of precision and repeatability were below 3.00 %. This means that the UPLC instrument was suitable for fingerprint analysis due to the good precision and repeatability of the UPLC fingerprint method. The RSD values of the major chromatograph peak area at different times were all less than 3.00 %, which indicates that the sample solution was stable within 24 h. The sample recovery rate ranges from 98.83 % to 103.15 %, The RSDs values of precision, reproducibility, stability, and recovery rate are shown in the supporting material (Table S2, S3, S4, S5).
3.2.2 UPLC fingerprints of WCAP samples and identification of common compounds
Import 16 batches of UPLC chromatograms of the main components of WCAP into the Similarity Evaluation System for Chromatographic Fingerprints of Traditional Chinese Medicines, use S1 as the reference spectrum, set a time window of 0.1, and use the median method to generate the control spectrum for fingerprint matching, the chromatographic fingerprints of the 16 batches were shown in Fig. 2A. 14 common peaks were obtained by retention time and reference substance. The UPLC chromatogram of reference substance was present in Fig. 2B, 14 common peaks (1 – 14) were identified as ferulic acid, narirutin, naringin, hesperidin, neohesperidin, aloe emodin, rhein, emodin, honokiol, costunolide, dehydrocostus lactone, magnolol, chrysophanol and physcion, respectively. The similarity calculation results show in Table 3 that the similarity of each batch of samples is greater than 0.90 (except S605). The relative peak areas and relative retention times of common peaks are presented in Table S6 and Table S7.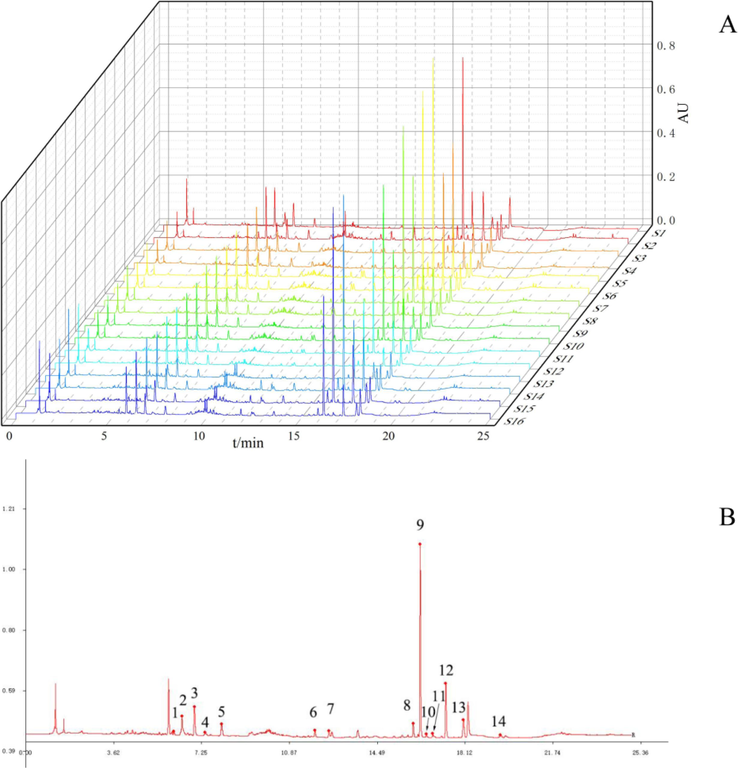
UPLC fingerprints of 16 batches of WCAP methanol extracts (A), WCAP chemical components fingerprints control map1. Ferulic acid; 2. Narirutin; 3. Naringin; 4. Hesperidin; 5. Neohesperidin; 6. Aloe emodin; 7. Rhein; 8. Emodin; 9. Honokiol; 10. Costunolide; 11. Dehydrocostus lactone; 12. Magnolol; 13. Chrysophanol; 14. Physcion (B).
number
NF-κB inhibitory activity
number
NF-κB inhibitory activity
S605
2476.99 ± 958.91
S944
3389.04 ± 852.86
S826
3383.59 ± 962.67
S953
4156.92 ± 464.89
S932
2408.56 ± 624.58
S954
2906.45 ± 837.65
S936
3385.08 ± 890.89
S955
4752.11 ± 860.41
S938
3415.84 ± 1022.92
S956
3039.59 ± 1082.51
S939
3499.08 ± 671.74
S963
4414.87 ± 667.42
S940
3735.95 ± 562.79
S964
3380.62 ± 556.82
S942
4393.00 ± 808.91
S969
3015.55 ± 409.27
Control
18.06 ± 2.80
Model
5717.10 ± 705.58
Dex
2504.64 ± 362.80
3.3 Hierarchical cluster analysis (HCA)
HCA was used to distinguish WCAP from different years by generating different clusters according to the similarity of fingerprints, and the result of HCA was shown in Fig. 3A. The result indicated that the samples were divided into two groups, S605 in group 1 and the rest of sample in group 2. The sum of the common peak areas of the 16 batches was made into a bar plot, which is shown in Fig. 3B. It can be seen from the bar chart and HCA results that the classification is related to the sum of the contents of the compounds.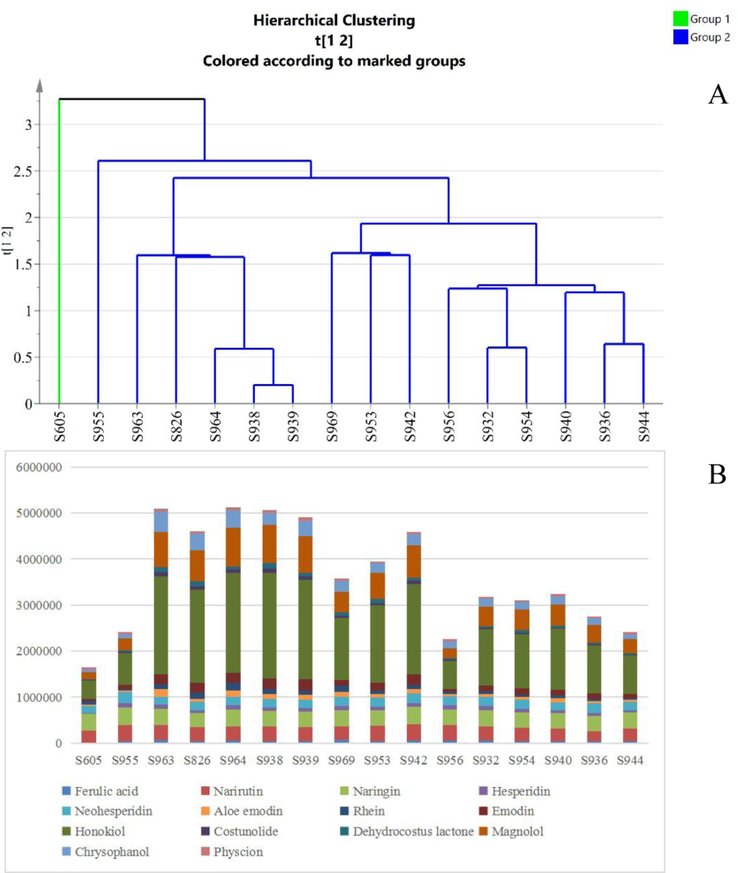
Hierarchical cluster analysis of WCAP methanol extracts (A), Bar chart of the sum of peak areas in 16 batches of WCAP (B).
3.4 NF-κB inhibitory activity test
NF-κB inhibitory activity was determined from the above 16 batches of WCAP methanol extract using the method of "2.5″. As shown in Table 3 and Fig. 4, the model group induced by TNF-α (10 ng/mL) significantly increased NF-κB activity. Compared with the Model group, each batch of samples significantly inhibited NF-κB inhibitory activity. Though S605 has the lowest sum of peak areas, its NF-κB inhibitory activity was not the weakest, which might be due to some strong activity coming from a low content compound.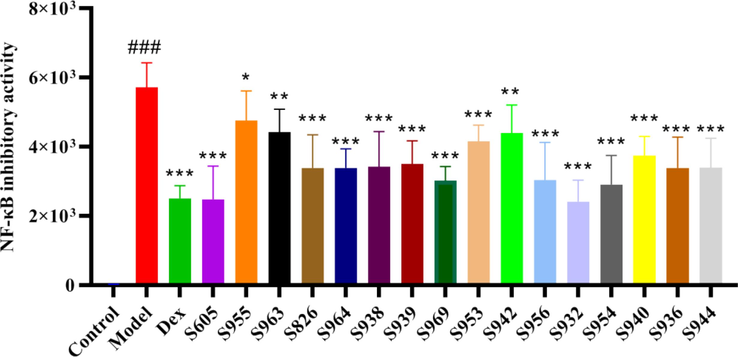
NF-κB inhibitory activity of 16 batches WCAP methanol extracts ### p < 0.001 VS Control, * p < 0.05 VS Model, ** p < 0.01 VS Model, *** p < 0.001 VS Model.
3.5 Correlation analysis
The Pearson correlation analysis of NF-κB inhibitory activity and chemical components of the correlation analysis result is shown in Fig. 5A. The values range from −1 to 1. The larger the absolute value, the darker the color, and the smaller the absolute value, the weaker the correlation. An absolute value of 0.0 – 0.2 is a very weak correlation, 0.2 – 0.4 is a common correlation, 0.4 – 0.6 is a strong correlation. The Pearson correlation coefficients of each compound are shown in Table 4, The correlation analysis showed that the correlation coefficient with NF-κB inhibitory activity was classified narirutin (0.51), hesperidin (0.43), hesperidin (0.37), naringin (0.34), aloe emodin (0.34), ferulic acid (0.33), and honokiol (0.33), emodin (0.33), magnolol (0.32), and physcion (0.31). This analysis concluded that the flavonoid components, narirutin, naringin, hesperidin, and neohesperidin, made the greatest contribution to the inhibitory activity of NF-κB. Lignans and rhubarb anthraquinones are secondary, while the sesquiterpene components xylide and dehydroxylinol NF-κB contributed minor. The rhein correlation coefficient of −0.01 indicates that there is no obvious contribution to the inhibitory activity of NF-κB.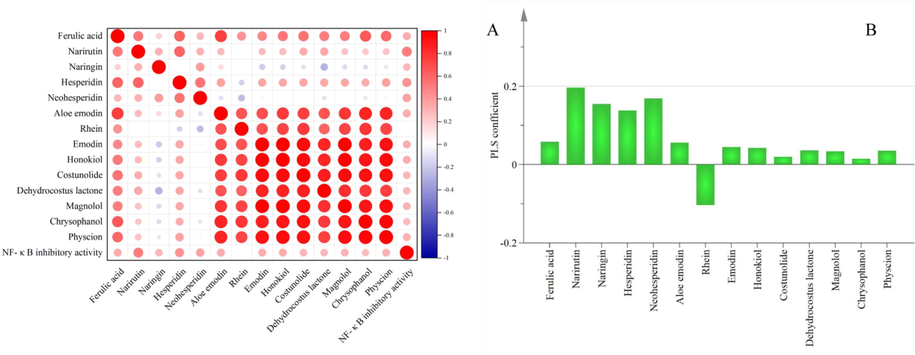
Pearson correlation results between potential quality markers in all WCAP methanol extracts (A), PLS variation coefficients of 14 chromatographic peaks with NF-κB inhibitory activity (B).
compound
Pearson correlation coefficient
compound
Pearson correlation coefficient
Ferulic acid
0.33
Emodin
0.33
Narirutin
0.51
Honokiol
0.33
Naringin
0.34
Costunolide
0.29
Hesperidin
0.43
Dehydrocostus lactone
Dehydrocostus lactone0.29
Neohesperidin
0.37
Magnolol
0.32
Aloe emodin
0.34
Chrysophanol
0.28
Rhein
−0.01
Physcion
0.31
3.6 Partial least squares regression (PLSR) analysis
As shown in Fig. 5B, the Y regression equation for each explanatory variable is: Y = 0.058X1 + 0.196X2 + 0.154X3 + 0.138X4 + 0.168X5 + 0.055X6-0.104X7 + 0.044X8 + 0.043X9 + 0.019X10 + 0.035X11 + 0.036X12 + 0.015X13 + 0.035X14. The larger the regression coefficient, the more the compound contributed to the NF- κB inhibitory activity. The regression equation shows that the contribution of the compounds to NF-κB inhibitory activity was sorted as naringenin, neohesperidin, naringin, ferulic acid, aloe emodin, emodin, honokiol, magnolol, dehydrocostus lactone, physcion, chrysophanol, rhein.
Thus combining the Pearson correlation analysis and PLSR analysis, 10 compounds are likely to be active components to exert NF-κB inhibitory activity.
3.7 In vitro validation of active components
The NF-κB inhibitory activity was detected for the 10 chemical components. As shown in Fig. 6, except aloe emodin and physcion, the other 8 compounds showed significant activity.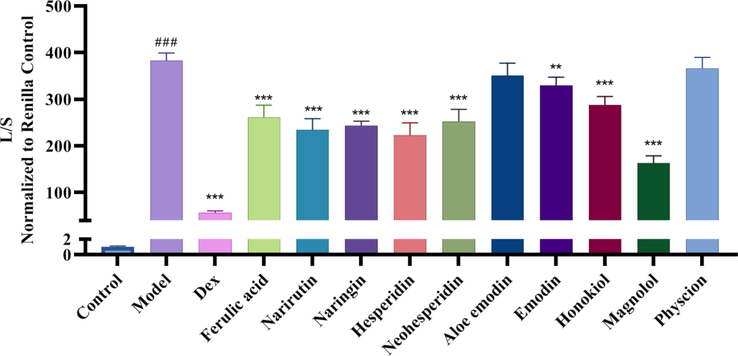
NF-κB inhibitory activity of the 10 compounds in WCAP ### p < 0.001 VS Control, ** p < 0.01 VS Model, *** p < 0.001 VS Model.
3.8 Determination of the main chemical composition of WCAP
A rapid and efficient UPLC-DAD method was developed to determine the content of the 13 components of WCAP was determined, the precision, repeatability, stability, and recoveries (Table S5) met the requirements. The content of three batches of WCAP was determined by this method and the contents of naringenin and naringin were more than 4.3 mg/g, magnolol and honokiol were more than 3.0 mg/g, and the total amount of anthraquinones in rhubarb was more than 1.05 mg/g. The specific values are presented in supporting material Table S8. The chromatogram of S939 is shown in Fig. 7. The proposed method can be applied as a potent tool for the quality control of WCAP. The content overlay map of 13 quantitative components is shown in Fig S1, and the results are basically consistent with the peak area overlay map in 3.3, which can be mutually supported by the clustering analysis results.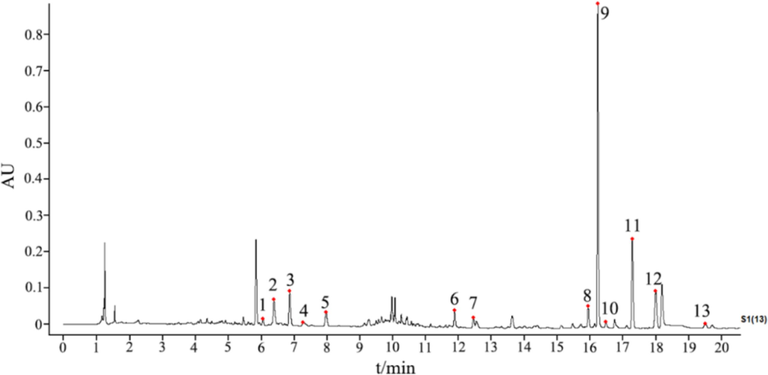
The UPLC chromatogram of WCAP in 254 nm (1. Ferulic acid; 2. Narirutin; 3. Naringin; 4. Hesperidin; 5. Neohesperidin; 6. Aloe emodin; 7. Rhein; 8. Emodin; 9. Honokiol; 10. Costunolide; 11. Magnolol; 12. Chrysophanol; 13. Physcion.).
4 Conclusion and discussion
We identified 129 chemical components from WCAP and the Phthalides derived from Chuanxiong were identified for the first time. 10 compounds are likely to be active components to exert NF-κB inhibitory activity by a spectral activity relationship analysis. A quantitative analysis method was established and indicator component of the Adjuvant medicine Chuanxiong was added. In summary, the comprehensive method combining spectral effect and quantification could provide data support for screening the effect substance basis and the selection of quality control index components for WCAP.
Qualitative analysis was conducted on the methanol extract of WCAP using methods such as UHPLC-QE-MS. A total of 129 chemical components were identified in Table 1, mainly from Aurantii Fructus, Rhei Radix et Rhizoma, Magnoliae Officinalis Cortex, Aucklandiae Radix, Chuanxiong Rhizoma and Aquilariae Lignum Resinatum. However, the identification of components in medicinal materials such as Crotonis Semen Pulveratum, Santali Albi Lignum, and Moschus was relatively rare or not identified, possibly because the higher content of components in them were all volatile components, the components with higher content in Jujubae Fructu sinclude polysaccharides, proteins, and amino acids, which may be due to the unsuitable chromatographic column and mass spectrometry conditions for detecting such components.
The four compounds of rhein, costunolide, dehydrocostus lactone, and chrysophanol were not screened out. At present, it indeed reported that these four compounds alone have NF- κB inhibitory activity and anti-inflammatory activity when administrated as pure compound. 80 μM rhein showed NF- κB inhibition on LPS-induced RAW264.0 cells (Wen et al., 2020), the three compounds of chrysophanol, costunolide, and dehydrocostus lactone have anti-inflammatory activity (Song et al., 2019; He et al., 2019; Yuan et al., 2022). NF-κB plays a crucial role in the main pharmacological effect of WCAP, these four components may contribute less to the NF- κB inhibitory activity in the complicated WCAP, so it was not screened out through the spectral efficiency relationship method. Therefore, these four compounds may not be components of concern for quality control of WCAP.
In the chemometrics research, we found that the cluster analysis results were consistent with the sum of the peak areas. Components including magnolol, honokiol, naringin, narirutin, and neohesperidin accounted for a large proportion in the sum of the peak areas, and thus play a main role in batch classification. In both the cluster analysis and the fingerprint similarity evaluation, S605 was different from the other batches (the similarity is 0.856). S605 was manufactured much earlier than other batches, and the storage time may have an impact on the chemical composition content. The differences in chemical composition may also be caused by the raw herbal material, reminding the importance of fixed medicinal materials and harvesting conditions in the production of TCM.
10 compounds are likely to exert NF-κB inhibitory activity by combining analysis of the Pearson correlation analysis and PLSR. The four active components with regression coefficients greater than 0.1 were narirutin, naringin, hesperidin, and neohesperidin. The first three compounds have been reported to have NF-κB inhibitory activity (Wang et al., 2020; Zhao et al., 2016; Ha et al., 2012). Neohesperidin has a protective effect on DSS induced colitis (Liu et al., 2022), and this paper reports the NF-κB inhibitory activity of neohesperidin for the first time. The present work demonstrated that the spectrum-effect relationship analysis can quickly find the effect components from complex TCM extracts for the screening of quality control biomarkers, which could be considered as a reference to establish the quality control method for other Chinese patent medicines.
Acknowledgements
This study was supported by the Tianjin Committee of Science and Technology of China (22ZYJDSS00040 and 21ZYJDJC00080), Science and Technology Project of Haihe Laboratory of Modern Chinese Medicine(22HHZYSS00007).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Validation of Sennae Folium specification grade classification based on UPLC-Q-TOF/MS spectrum-effect relationship. Arab. J. Chem.. 2022;15(11)
- [Google Scholar]
- Magnolol attenuates the inflammation and enhances phagocytosis through the activation of MAPK, NF-κB signal pathways in vitro and in vivo. Mol. Immunol.. 2019;105:96-106.
- [CrossRef] [Google Scholar]
- Spectrum-effect relationship analysis for antioxidant of Bletilla striata (Thunb.) Reichb. f. based on sparse partial least squares regression. Chin. Wild Plant Resour.. 2020;39(11):1-6.
- [Google Scholar]
- Asperuloside suppressing oxidative stress and inflammation in DSS-induced chronic colitis and RAW 264.7 macrophages via Nrf2/HO-1 and NF-κB pathways. Chem. Biol. Interact.. 2021;344:109512
- [CrossRef] [Google Scholar]
- Protective effect and potential mechanisms of Wei Chang An pill on high-dose 5-fluorouracil-induced intestinal mucositis in mice. J. Ethnopharmacol.. 2016;190:200-211.
- [CrossRef] [Google Scholar]
- Commission, C. P., 2020. Pharmacopoeia of the People’s Republic of China. Volume I. Beijing, China Med. Sci. Press.
- Multiple facets of NF-κB in the heart: to be or not to NF-κB. Circ. Res.. 2011;108(9):1122-1132.
- [CrossRef] [Google Scholar]
- Narirutin fraction from citrus peels attenuates LPS-stimulated inflammatory response through inhibition of NF-κB and MAPKs activation. Food Chem. Toxicol.. 2012;50(10):3498-3504.
- [CrossRef] [Google Scholar]
- Research method of spectrum-effect relationship and its application in traditional Chinese medicine research. Guangzhou Chem. Ind.. 2021;49(10):16-19.
- [Google Scholar]
- Comparative evaluation of different cultivars of Flos Chrysanthemi by an anti-inflammatory-based NF-κB reporter gene assay coupled to UPLC-Q/TOF MS with PCA and ANN. J. Ethnopharmacol.. 2015;174:387-395.
- [CrossRef] [Google Scholar]
- Costunolide inhibits matrix metalloproteinases expression and osteoarthritis via the NF-κB and Wnt/β-catenin signaling pathways. Mol. Med. Rep.. 2019;20(1):312-322.
- [CrossRef] [Google Scholar]
- Quantitation and qualitation of eleven components in Wei Chang An pill by ultra-performance liquid chromatography-UV-tandem mass spectrometry. J. Pharm. Anal.. 2012;32(07):1165-1170.
- [Google Scholar]
- Inflammation, NF-κB, and Chronic Diseases: how are they linked ? Crit. Rev. Immunol.. 2020;40(1):1-39.
- [CrossRef] [Google Scholar]
- Protective effect and mechanism of neohesperidin in mice with DSS-induced ulcerative colitis. Mod. Prev. Med.. 2022;49(24):4505-4512.
- [CrossRef] [Google Scholar]
- Antinociceptive activity and chemical composition of Wei Chang An pill extracts. Pharm. Biol.. 2013;51(6)
- [CrossRef] [Google Scholar]
- Proinflammatory activation pattern of human umbilical vein endothelial cells induced by IL-1β, TNF-α, and LPS. Cytometry A. 2010;77(10):962-970.
- [CrossRef] [Google Scholar]
- Clinical effect of Wei Chang An pill in the treatment of child patients with rotavirus enteritis and its influence on myocardial enzyme spectrum. China J. Pharm. Econ.. 2020;15(11):74-77.
- [Google Scholar]
- Clinical effect evaluation of Wei Chang An pill in treating functional diarrhea. Clin. J. Chin. Med.. 2018;10(33):14-18.
- [Google Scholar]
- Chrysophanol attenuates airway inflammation and remodeling through nuclear factor-kappa B signaling pathway in asthma. Phytother. Res.. 2019;33(10):2702-2713.
- [CrossRef] [Google Scholar]
- Honokiol inhibits TNF-alpha-stimulated NF-kappaB activation and NF-κB-regulated gene expression through suppression of IKK activation. Biochem. Pharmacol.. 2005;70(10):1443-1457.
- [CrossRef] [Google Scholar]
- Function of hesperidin alleviating inflammation and oxidative stress responses in COPD mice might be related to SIRT1/PGC-1α/NF-κB signaling axis. J. Recep. Signal Transduct. Res.. 2020;40(4):388-394.
- [CrossRef] [Google Scholar]
- Identification of anti-inflammatory components in Sinomenii Caulis based on spectrum-effect relationship and chemometric methods. J. Pharm. Biomed. Anal.. 2019;167:38-48.
- [CrossRef] [Google Scholar]
- Rhein attenuates lipopolysaccharide-primed inflammation through NF-κB inhibition in RAW264.7 cells: targeting the PPAR-γ signal pathway. Can. J. Physiol. Pharmacol.. 2020;98(6):357-365.
- [CrossRef] [Google Scholar]
- Mechanism of nonylphenol induced gastric inflammation through NF-κB/NLRP3 signaling pathway. Toxicology. 2022;479:153294
- [CrossRef] [Google Scholar]
- Targeting NF-κB pathway for the therapy of diseases: mechanism and clinical study. Signal Transduct. Target. Ther.. 2020;5(1):209.
- [CrossRef] [Google Scholar]
- Dehydrocostus lactone suppresses dextran sulfate sodium-induced colitis by targeting the IKKα/β-NF-κB and Keap1-Nrf2 signalling pathways. Front. Pharmacol.. 2022;13:817596
- [CrossRef] [Google Scholar]
- Analysis on chemical components in Wei Chang An pill. Drug Eval. Res.. 2010;33(02):116-120.
- [Google Scholar]
- Identification and simultaneous determination of twelve active components in the methanol extract of traditional medicine Wei Chang An pill by HPLC-DAD-ESI-MS/MS. Iran. J. Pharm. Res.. 2013;12(1):15-24.
- [Google Scholar]
- Effects of Wei Chang An pill on Treg/Th17 immune balance in ulcerative colitis model mice. J. Tradit. Chin. Med.. 2020;61(22):1983-1989.
- [Google Scholar]
- Naringin protects against cartilage destruction in osteoarthritis through repression of NF-κB signaling pathway. Inflammation.. 2016;39(1):385-392.
- [CrossRef] [Google Scholar]
- The spectrum-effect relationship-a rational approach to screening effective compounds, reflecting the internal quality of Chinese herbal medicine. Chin. J. Nat. Med.. 2016;14(3):177-184.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105328.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







