Translate this page into:
Identification of anti-tumor constituents from toad skin and toad venom by UPLC-QTOF/MS in-depth chemical profiling combined with bioactivity-based molecular networking
⁎Corresponding authors at: Institute of Interdisciplinary Integrative Medicine Research, Shanghai University of Traditional Chinese Medicine, Shanghai, 201203, China. wdzhangy@hotmail.com (Weidong Zhang), catheline620@163.com (Ji Ye)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Toad skin (TS) and toad venom (TV) are two different traditional Chinese medicine (TCM) prepared from Bufo bufo gargarizans Cantor and Bufo melanostictus Schneider. Both of them were used in the treatment of ulcers, and now they are regarded as animal drugs with anti-tumor activity. However, the comparison analysis between their compositions and anti-tumor active ingredients has not been elucidated clearly. In this study, technology of ultra-performance liquid chromatography coupled with tandem quadrupole time-of-flight mass spectrometry (UPLC-QTOF/MS) and bioactivity-based molecular networking were applied to investigate the anti-tumor active ingredients of TS and TV. A method of collision energies-relied mass spectrometry combined with molecular networking was initially developed for identifying their chemical components. A combination of Cytoscape and molecular networking was then employed to analyze the prototype constitutes of TS absorbed in rat serum. In addition, cytotoxicity test was applied to evaluate their anti-proliferative activities against human gastric cancer (GC) cells. As a result, a total of 162 compounds were tentatively characterized, and four structural types existed in both TS and TV, including bufogenins, bufotoxins, indolealkylamines (IAAs), and acyl-arginine. Cyclic dipeptides could only be detected in TS, and the total number of bufogenins and bufotoxins in TV was higher than that of TS. Both of them significantly inhibited the viability of SGC7901 cells, and 70% methanol eluate of TS was the most potential active fraction. 44 prototype compounds in TS were identified, from which three types of potential tumor cytotoxic components, including bufogenins, IAAs, and cyclic dipeptides, were predicted. In human GC cells SGC7901, a mixture of nine bufogenins, including bufotalin, cinobufotalin, gamabufotalin, telocinobufagin, cinobufagin, bufarenogin, arenobufagin, hellebrigenin, and desacetylcinobufotalin, was conducted as bioactive components, which exerted a significant inhibitory effect on the cell viability of SGC7901 cells with a half maximal inhibitory concentration (IC50) value of 0.23 (0.19–0.27, 95%CI) μg/mL. Comparison of their chemical composition and anti-tumor activity reveals that TS might be able to partially replace TV, whilst bufogenins are the potential active ingredients.
Keywords
Toad skin
Toad venom
UPLC-QTOF/MS
Molecular networking
Anti-tumor activity
- ANOVA
-
analysis of variance
- BPI
-
base peak intensity
- CAS
-
chemical abstracts service
- CCK-8
-
cell counting kit-8
- CE
-
collision energy
- DDA
-
data-dependent acquisition
- DIA
-
data-independent acquisition
- DMSO
-
dimethyl sulfoxide
- FBS
-
fetal bovine serum
- GC
-
gastric cancer
- IAAs
-
indolealkylamines
- IC50
-
half maximal inhibitory concentration
- LC-MS
-
liquid chromatography mass spectrometry
- LC-MS/MS
-
liquid chromatography tandem mass spectrometry
- MRM
-
multiple reaction monitoring
- MS
-
mass spectrometry
- MS/MS
-
tandem mass spectrometry
- OD
-
optical density
- PBS
-
phosphate buffer solution
- RDA
-
retro diels–alder reaction
- RII
-
relative ion intensity
- RPMI 1640
-
Roswell Park Memorial Institute 1640
- TCM
-
traditional Chinese medicine
- TS
-
toad skin
- TV
-
toad venom
- UPLC-QTOF/MS
-
ultra-performance liquid chromatography coupled with tandem quadrupole time-of-flight mass spectrometry
Abbreviations
1 Introduction
Traditional Chinese medicine (TCM) has been used to treat a myriad of ailments, and its therapeutic effects have been reinterpreted through the lens of modern science (Huang et al., 2021; Ma et al., 2016). Identification and screening of the bioactive components of TCM are of great significance for illustrating their underlying multi-components, multi-targets, and multi-pathways synergy effects (Cai et al., 2022; Gao et al., 2022). Therefore, to characterize the components and interpretation of corresponding biological activities in TCM could not only disclose the potential active substances with diverse skeleton structures, but also occupy an essential position as the foundation for quality control and development of the new drug.
Metabolite profiling (Mi et al., 2019) and multi-component (Cheng et al., 2019) identification are two critical steps for exploring the therapeutic basis of botanical or animal-orient drugs, which help to promote their modernization and globalization. The widespread application of analytical techniques, especially the rapid development of liquid chromatography tandem mass spectrometry (LC-MS) (Beccaria and Cabooter, 2020; Liu et al., 2022), has made comprehensive investigation easy to realize. Ultra-high performance liquid chromatography coupled with tandem quadrupole-time-of-flight mass spectrometry (UPLC-QTOF/MS) is an effective tool to rapidly characterize the chemical constituents of TCM (Li et al., 2022; Mahana et al., 2022) with excellent resolution, remarkable sensitivity, and outstanding separation performance (Huang et al., 2022; Wang et al., 2022). Data-independent acquisition (DIA) (Rice and Belani, 2022) and data-dependent acquisition (DDA) (Wang et al., 2019) scan modes have become two effective approaches for structural characterization with the advantages of high coverage in peak capacity and exposure, as well as the distinct correlation of precursor and product ions. Meanwhile, molecular networking minimized the laborious work of processing and interpreting large quantities of MS2 spectrum in complex matrices (Zhang et al., 2021). However, inadequate nodes or clusters in liquid chromatography tandem mass spectrometry (LC-MS/MS) molecular networking could result in the loss of information during the process of deciphering their structures. This inadequacy is typically caused by unstable relative ion intensity (RII) features in DDA scans, which are highly influenced by collision-induced dissociation (Afoullouss et al., 2022). RII has been reported to have a close relationship with the chemical compounds, the inherent stability of which can reflect their diverse skeletal structures. Collision energy (CE), one of the most influential factors of RII, plays a vital role in the spectral interpretation of TCM (Guan et al., 2021; Song et al., 2019). Therefore, to obtain as much composition information as possible from LC-MS/MS molecular networking, a multi-stage process of collision energy acquisition needs to be further optimized.
Exploring the pharmacodynamic material basis of TCM is a challenging task because of the indistinct correlation between the chemicals and their biological activities (Liu et al., 2019). The investigation of prototype constituents as well as experimental screening executed at the animal, tissue, cell, or biomolecule levels were developed to accelerate the discovery of potential active compounds. However, the individual use of each above methods often fails to screen active ingredients targeted for a specific disease. A more comprehensive and targeted screening method is urgently needed. Recently, a bioassay-guided screening method, comprising both serum pharmacochemistry and cell-based screening, has been extensively applied for potential active constituent investigation and established a direct linkage from ingredients in TCM to diseases (Shao et al., 2022; Yin et al., 2022). The bioactivity-based molecular networking, which contains bioassay-guided screening method and molecular networking, has provided a prospect for deciphering the bioactive profile of TCM with the advantage of celerity and convenience.
Toad venom (TV) is prepared from the secretion of the gland behind the ear of Bufo bufo gargarizans Cantor and Bufo melanostictus Schneider (National Pharmacopoeia Commission, 2020). It was first recorded in a medical book named Theory of Medicinal Properties (Yao Xing Lun), written by a Chinese doctor Quan Zhen in the 7th century, which was supposed to treat ulcers on the head of children (Wu and Song, 1998). In the 2020 edition of Pharmacopoeia of the People's Republic of China, TV was approved for reducing inflammatory episodes, relieving pain, ameliorating brain malfunction, and modulating gastrointestinal motility disorder (Committee, 2020). The clinical trial has found that TV has significant anti-cancer activity against human cancers, such as hepatocellular carcinoma, nonsmall-cell lung cancer, and pancreatic cancer, with preventing disease progression or lessening tumoral volume (Meng et al., 2009). Experimental studies have shown that it could inhibit the proliferation of human gastric cancer (GC) cells via suppressing the Wnt/β-Catenin signaling pathway (Wang et al., 2020). Toad skin (TS) was also prepared from the above two types of toads (Wu and Song, 1998), which was the dried whole skin processed by removing the toxic substance from the lump, according to a medical book called Ben Cao Shi Yi written by a herbologist named Zangqi Chen in AD 739. It was used for similar clinical practice with TV but not listed in 2020 edition of the Chinese Pharmacopoeia. Experimental studies have indicated that TS significantly inhibited the growth and proliferation of human hepatocellular carcinoma, lung adenocarcinoma, and gastric neoplasm cell (Qi et al., 2011; Qi et al., 2008; Wang et al., 2009). Numerous studies of the above two animal-orient natural products revealed that bufadienolides and indolealkylamines (IAAs) were the main active ingredients for their antitumor effects (Qi et al., 2011; Li et al., 2021). However, the comparative analysis of their compositions still needs to be improved.
As shown in Fig. 1, the present study established a robust strategy to screen for biologically active ingredients from TS and TV through UPLC-QTOF/MS and bioactivity-based molecular networking. An integrated approach based on collision energies-relied mass spectrometric combined with the molecular networking was established, and comprehensive chemical structural characterization of constituents in them was performed. A comparison of constituents and activities study was conducted with human GC cell lines SGC7901 and MGC803. Furthermore, the combination method of serum pharmacochemistry and bioassay-guided screening in different fractions of TS was applied, resulting in the elucidation of the bioactive molecular networking, the GC inhibitory profile, as well as the constituents that might play significant roles in therapeutic effects.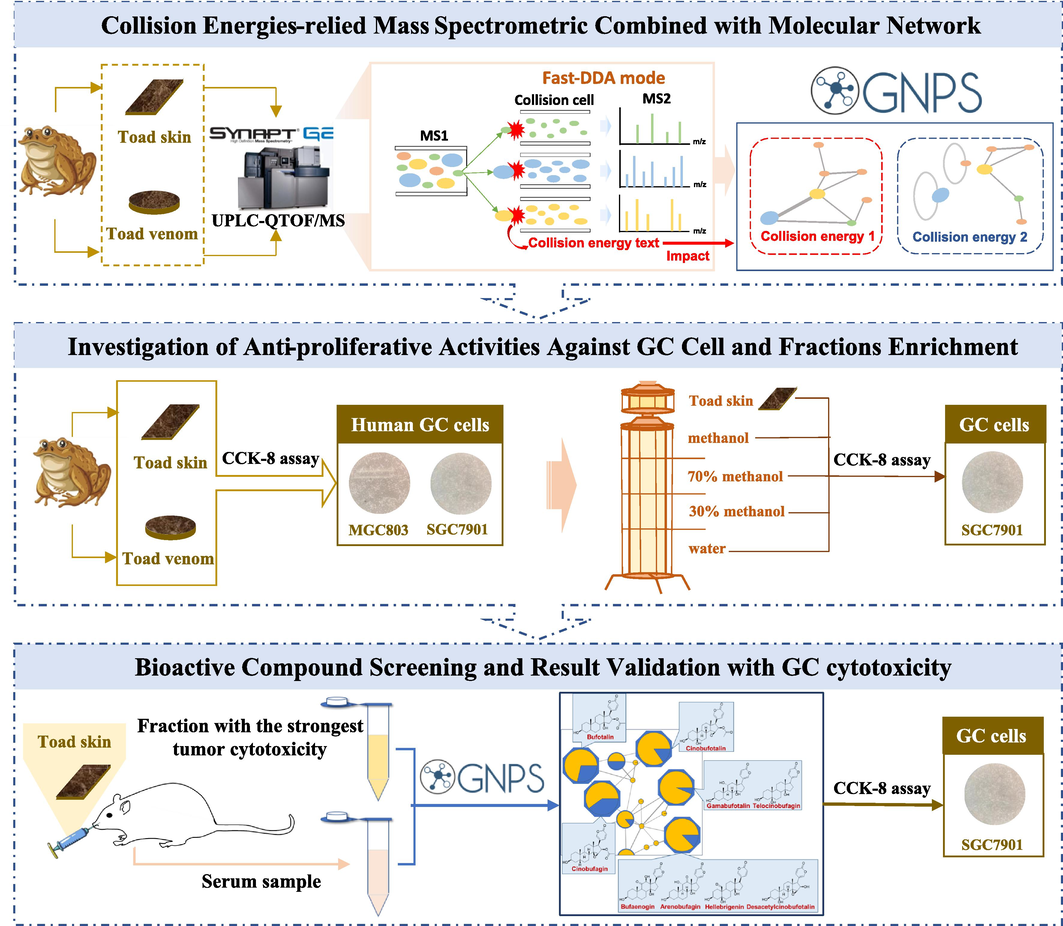
The workflow of anti-tumor constituents screening in toad skin and toad venom by UPLC-QTOF/MS in-depth chemical profiling combined with bioactivity-based molecular networking.
2 Materials and methods
2.1 Reagents
Authentic compounds of bufalin (No. 8090101), glycine betaine (No. 040709), cinobufotalin (No. 8090443), arenobufagin (No. 82051101), desacetylcinobufagin (No. 8101009), gamabufotalin (No. 8060102), uracil (No. 120913), desacetylcinobufotalin (No. 82015001) and hellebrigenin (No. 201112701) were purchased from Shanghai Liding Biotechnology Co. (Shanghai, China). Bufarenogin (No. DST-211101–050) and cinobufagin (No. DST210114-005) were purchased from Chengdu Desite Biotechnology Co. (Chengdu, China). Telocinobufagin (No. 20120726) and bufotalin (No. HB009066198) were provided by Baoji Herbest Biotechnology Co. (Hebei, China). Riboflavin (No. ZM0314BB13) and pantothenic acid (No. 20120417) were provided by Shanghai Yuanye Biotechnology Co. (Shanghai, China). Nicotinmide (No. 130610) was purchased from the Sichuan Weikeqi Biological Technology CO. (Chengdu, Sichuan). Hypoxanthine (No. 120826) was purchased from Chengdu Pufei De Biotech Co. (Chengdu, Sichuan). Resibufogenin (No. 718–9306) was purchased from the National Institute for the Control of Pharmaceutical and Biological Products, NICPBP (Beijing, China). The purity of all reference standards was not less than 98 %.
Toad skin was provided by Anhui China Resources Jinchan Pharmaceutical Co., Ltd. (Huaibei, China). Toad venom was provided by Lei Yun Shang Pharmaceutical Co., Ltd. (Suzhou, China). LC-MS-grade methanol, water, and acetonitrile were provided by Fisher Scientific (Nepean, Ontario). The standard substance of leucine enkephalin was obtained from Sigma-Aldrich (MO, USA). The MCI gel CHP20P was purchased from Mitsubishi Chemical Co. (Tokyo, Japan). The human GC cell line SGC7901 was provided by BeNa Culture Collection (Beijing, China), and human GC cell line MGC803 was provided by Melone Pharmaceutical Co. (Dalian, China). Roswell Park Memorial Institute 1640 (RPMI 1640), fetal bovine serum (FBS), penicillin–streptomycin (10,000 U/mL), trypsin, and phosphate buffer solution (PBS) were obtained from Gibco® (Grand Island, NY, USA). Dimethyl sulfoxide (DMSO) was obtained from Sigma-Aldrich® (St. Louis, MO, USA). Cell counting kit-8 (CCK-8) was purchased from Shanghai Beyotime Biotechnology Co., Ltd. (Shanghai, China).
2.2 Animal experiments
Male Sprague-Dawley (SD) rats (180–220 g) were supplied by Shanghai Xipuer-Bikai Experimental Animal Co. Ltd., Shanghai, China. All animals were raised in an environmentally controlled breeding room (25 ± 2 °C with 55 ± 5 % humidity and equal light/dark periods of 12 h), fed with free access to standard laboratory food and water for 1 week of acclimation, and then fasted for 12 h before dosing. All animal experiments were conducted with the approval of the Institutional Animal Welfare and Ethics Committee of Shanghai University of Traditional Chinese Medicine (License NO. PZSHUTCM220613014).
Thirty-six rats were randomly divided into six groups with six rats per group (Group I was the blank serum group; Groups II–VI, the TS-dosed serum group, were randomly divided into five time-set point groups according to the time of blood collection). TS was dissolved in 0.5 % sodium carboxymethyl cellulose and then ultrasonically mixed for 30 min. The prepared TS suspension was administrated orally to Groups II–VI rats at a dose of 2.16 g/kg. The other six rats in the blank serum group received the equivalent volume of 0.5 % sodium carboxymethyl cellulose in the same way. At 0.25, 0.5, 1, 1.5, and 2 h after administration, blood was collected from the hepatic portal vein and centrifuged at 3500 rpm for 10 min at 4 °C to obtain the supernatant. Then, the supernatant of six rats was merged for each time point. All serum samples obtained were frozen at − 80 °C before analysis.
2.3 Preparation of TS and TV extract
185 g of crude TV were weighed accurately, macerated with ten times volume of 80 % ethanol for one hour, then heated under reflux for one hour and filtered. We heated the residue under reflux with 80 % ethanol, 95 % ethanol, and 95 % ethanol, respectively, for one hour each time, and filtered. All the four filtrates were combined and dried in a vacuum at 45℃, about 114 g of TV extract were obtained. 604 g of dried TS was washed and extracted with 5–15 volumes of water twice. The extraction was concentrated to a relative density of 1.01–1.35 (80℃), and precipitated with 40–70 % ethanol and 70–95 % ethanol, respectively. Filtered, concentrated the filtrate, and then made the concentration dry in vacuum to make 127 g of TS extract.
2.4 Preparation of samples and standard solutions
2.4.1 Preparation of qualitative and quantitative analysis samples
A total of 50 g of TS extract was dissolved in 50 mL of 80 % ethanol and loaded onto MCI gel column (70 × 460 mm). The column was sequentially eluted with water, 30 % methanol, 70 % methanol, and 100 % methanol (v/v, 5 L for each gradient). Each elution was collected as one fraction, respectively. Accurately weighted 1.0 g of each of TS extract, TV extract, and each fraction and extracted by ultrasonicating with 20.0 mL of methanol (power 300 W, frequency 40 kHz) for 30 min, respectively, to prepare the qualitative samples. Diluted samples to 2 mg/mL and centrifuged at 12000 rpm for 10 min for further analysis.
2.4.2 Preparation of antitumor activity samples
A total of 50 g of TS extract was dissolved in 50 mL of 80 % ethanol and loaded onto a MCI gel column (70 × 460 mm). The column was sequentially eluted with water, 30 % methanol, 70 % methanol, and 100 % methanol (v/v, 5 L for each gradient). All the fractions, TS extract and TV extract were employed for anti-tumor activity analysis.
2.4.3 Preparation of standard solutions
Stock solutions of 18 reference compounds were prepared individually by dissolving each authentic compound in methanol at a concentration of 1 mg/mL. Then, all the stock solutions were diluted with methanol to 0.05 mg/mL before injection for qualitative analysis. The standard stock solutions of bufotalin, cinobufotalin, gamabufotalin, telocinobufagin, cinobufagin, bufarenogin, arenobufagin, hellebrigenin and desacetylcinobufotalin were then mixed and diluted to serial concentrations of 800, 400, 200, 100, 50, 25, 12.5, 6.25, 3.13, and 1.06 μg/mL for quantitative analysis.
2.4.4 Preparation of serum samples
Each aliquot of 200 μL serum sample was added 1 mL of methanol, vortex-mixed for 5 min, and then centrifuged at 13,000 rpm for 10 min at 4 °C. The supernatant was transferred to another clean tube and evaporated to dryness under nitrogen at 30 °C. The residue was dissolved with 200 μL of methanol and vortex-mixed for 1 min for further analysis. All obtained test solutions were centrifuged at 13,000 rpm for 10 min, and the supernatant was stored at 4 °C before analysis.
2.5 UPLC- QTOF/MS analysis
The chromatographic separation of TS and TV was carried out using a Waters Acquity UPLC I-class system (Waters, Milford, MA, USA), equipped with a binary pump, an auto-sampler, a degasser and a thermostarted column compartment. The separation of TV and TS sample was performed on an Agilent Zorbax Eclipse Plus C18 column (2.1 mm × 150 mm, 1.8 μm, Agilent Corp), with the column temperature maintained at 35℃. The mobile phase consisted of 0.1 % formic acid in both water (A) and acetonitrile (B), the flow rate was 0.3 mL/min. For qualitative analysis, the gradient elution program examination was optimized as follows: 0–2 min, 5–5 % B; 2–4 min, 5–13 % B; 4–14 min, 13–30 % B; 14–17 min, 30–45 % B; 17–23 min, 45–95 % B; 23–27 min, 95–95 % B; 27–29 min, 95–5 % B; 29–30 min, 5 %-5% B. For quantitative analysis, the gradient elution program examination was set as follows: 0–2 min, 5–5 % B; 2–3 min, 5–28 % B; 3–6 min, 28–28 % B; 6–11 min, 28–35 % B; 11–17 min, 35–39 % B; 17–18 min, 35–95 % B; 18–20 min, 95–95 % B; 20–21 min, 95 %-5% B; 21–22 min 5 %-5% B. Inject volume was set to 1 μL.
Mass spectrometry detection was performed on the SYNAPT G2-Si HDMS system, equipped with an electrospray ionization source (Waters Corp., Manchester, UK). Data acquisition was progressed in positive ionization mode through fast-DDA and multiple reaction monitoring (MRM), respectively. Mass spectrometry conditions were ultimately set as follows: dry gas flow rate, 800 L/h; dry gas temperature, 400℃; ion source temperature, 120℃; capillary voltage, 3.0 kV; cone voltage, 40 V; source offset, 80 V; cone gas flow, 50 L/h. The parameters in fast-DDA mode were set as follows: mass scan range, m/z 50–2000 Da; MS and MS/MS scan rate, 0.2 s; maximum number of ions for MS/MS from a single MS scan, 5; dynamic peak exclusion on; the acquire and then exclude time, 6 s.
To obtain much better ionization efficiency, three tandem mass spectrometry (MS/MS) CE ranges were examined, including 0–40 V and 40–80 V (CE1), 20–60 V and 60–100 V (CE2), 40–80 V and 80–120 V (CE3), from low mass collision energy to high mass collision energy (Fig.S1). The parameters in MRM mode were set as follows: mass scan range, m/z 100–600 Da; mass spectrometry (MS) and MS/MS scan rate, 0.2 s; the retention time, precursor ion, main product ions, cone voltage and collision energy of the tested components were shown in Table S1. Real-time data were calibrated using an external reference (LockSpray™) by the constant infusion of a leucine enkephalin solution at a concentration of 200 pg/μL, with the lock masses at m/z 556.2771 in positive ionization mode, at a flow rate of 5 μL/min. Data acquisition was obtained by MassLynx V4.1.
2.6 Database construction of TS and TV
A total of 257 chemical components for all the medicinal components in TS and TV were discovered by literature and databases including Pubmed, Sci-Finder, PubChem, chemical book, and the Web of Science. Each chemical component structure was stored in mol format. The chemical constituents of TS and TV were cataloged in a self-built database containing compound names, Chemical Abstracts Service (CAS) number, chemical structure, molecular formulas, and precise molecular weight information. The database was apt to provide adequate MS and MS/MS ions information for further qualitative analysis.
2.7 Data analysis
Global Natural Products Social Molecular Networking (GNPS) (https://gnps.ucsd.edu/), an open source data processing platform, could be an assistant for the structural characterization of TS and TV. The obtained files in fast-DDA mode were transformed to mzML format and then uploaded to the GNPS platform. For molecular networking parameters, the maximum mass tolerance of the parent ions and fragment ions was set to 0.2 Da and 0.05 Da, respectively. The edges between related MS/MS spectra was filtered by setting the cosine score above 0.7 and the minimum matched fragment ions at six. Similar structural components were aggregated into clusters by means of GNPS platform analysis, and they were visualized by Cytoscape 3.7.1. In order to comprehensively evaluate the effect of collision energy on the production of molecular networking, five evaluation criteria were counted, including nodes, edges, self-loops, clusters and cosine score, which were used as a basis for selecting the most suitable clusters to facilitate analysis in each of the three main structural types existing in TV and TS. The number of clusters and edges reflects the clustering degree of a molecular networking. On the contrary, the number of self-loop nodes reflects the dispersion degree of a molecular networking. The number of nodes, including self-loops and clusters, could be a quantitative standard to evaluate the richness of a molecular networking. The cosine score, a parameter that was able to reflect the similarity between each pair of structurally similar nodes and help to discover new compounds with structural similarities to known compounds, was considered as a standard to evaluate the internal correlation of a molecular networking.
2.8 Cell viability
The human GC cell line MGC803 and SGC7901 were cultured in RPMI 1640, supplemented with 10 % FBS and 1 % penicillin–streptomycin in a humidified CO2 (5 %) incubator at 37 °C. The CCK-8 colorimetric assay, following the manufacturer's instructions, was used to determine the cell viability. Briefly, 100 μL cells were seeded in a 96-well plate at a density of 1 × 105 cells/mL to cultivate for 24 h. MGC803 and SGC7901 were pretreated with extracts of TS and TV at concentrations of 160, 80, 40, 20, 10, and 5 mg/mL for comparison of anti-GC effects. SGC7901 was processed with each fraction of TS and a mixed standard solution of bufogenins at concentrations of 625, 125, 25, 5, 1, and 0.2 μg/mL for bioactive ingredient screening. After 48 h of incubation, the cells were treated with 10 μL of CCK-8 reagent (5 mg/mL) and the absorbance measured at 450 nm by absorbance microplate readers (Bio-Teck, USA).
cell viability (%) =.
2.9 Statistical analysis
The percentage of cell viability was calculated compared to the control untreated cells. Dose-response relationships were obtained and presented as means (±S.E.M.) with a one-way analysis of variance (ANOVA) test and Dunnett’s multiple comparison test. The IC50 values were calculated by fitting the data to a sigmoidal curve, and using a four-parameter logistic model. The values of the blank wells were subtracted from each well of treated and control cells, so that half maximal inhibition of growth (IC50) in comparison to untreated control were calculated, and all the values were reported at 95 % confidence interval. Biological data were calculated by GraphPad Prism 7.00 (GraphPad, La Jolla, CA, USA), and each experiment was repeated for three times.
3 Results
3.1 Optimization of collision energy parameters
Collision energy often generates product ions in MS2 spectra from a given precursor ion, affecting the responses, including the cosine score, numbers of nodes, cluster co-efficient, edges and self-loops nodes, et al., in molecular networking. It has been confirmed that a higher collision energy led to fewer nodes and edges but higher cosine scores in molecular networking, which is similar to previous reports (Afoullouss et al., 2022). The optimization of collision energy, a parameter that shows the ability to influence the molecular network topology, is significant for the interpretation of particular types of chemical structures, especially for the TCM that contains multiple structural types. To trace the most effective collision energy for compounds with diverse skeletal structures, concerns were devoted to constructing the LC-MS/MS molecular networking based on DDA mode via direct correlation between precursor-to-fragment ions in MS2 spectra.
A comparative study between single and combination collision energy was developed on LC-MS/MS based molecular networking. After data acquisition at three CE settings in DDA manner, the combination of CE1 and CE2 in molecular networking produced much more numbers of nodes, edges, cluster co-efficient and self-loops nodes than each of the single collision energy, with no significant decrease in the average cosine scores. Increasing numbers of nodes in other combination collision energy settings have also been observed in CE2 + CE3 and CE1 + CE3 (Fig. 2A). Thus, it is much more beneficial to choose a combination of collision energy settings rather than single collision energy in mass spectrometry analysis to generate a molecular networking during composition identification.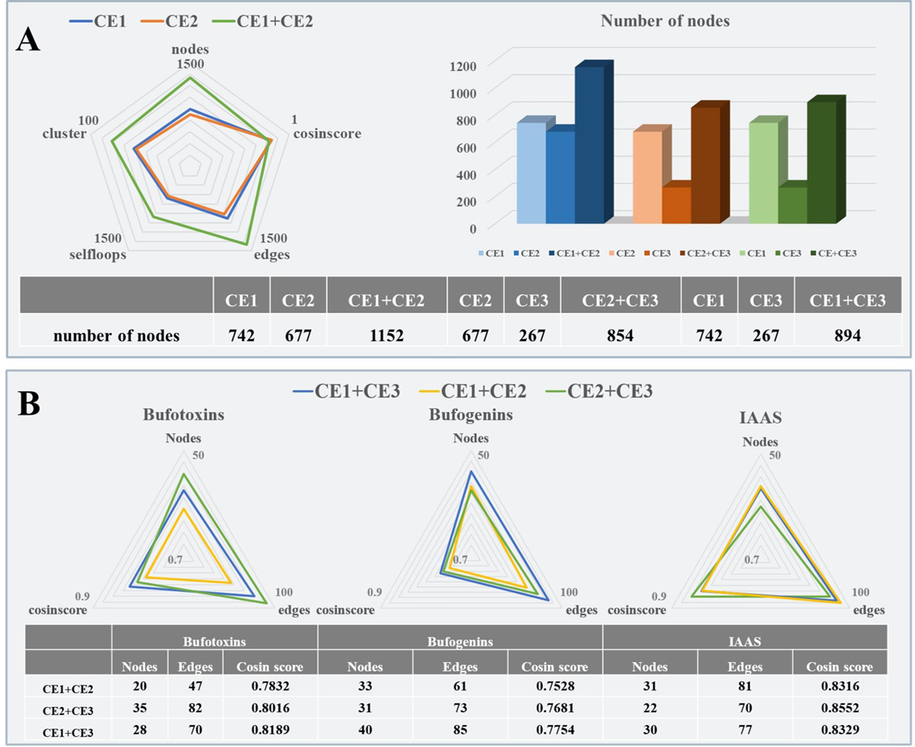
Effect of collision energy on the relative change of responses in molecular networking. (a) Radar graph of collision energy at CE1, CE2 and CE1 + CE2 (left) and bar graph of the single and multiple collision energies (right) effect on relative change of responses in molecular networking. (b) Effect of three different collision energy combinations on richness and clustering ability in molecular networking of bufotoxins (left), bufogenins (middle) and IAAs (right).
The collision energy settings on three main structural types in TS and TV, including bufotoxins, bufogenins and IAAs, were further evaluated by average cosine scores, numbers of nodes and edges. Fig. 2B demonstrates the effect of three different collision energy combinations on richness and clustering ability. For bufotoxins (left radar graph of Fig. 2B), compared with CE1 + CE3 and CE1 + CE2 groups, the MS2 spectra produced with CE2 + CE3 resulted in a higher number of nodes (+75.0 %) in bufotoxin clusters with a higher number of edges (+74.5 %) and the average cosine scores > 0.70. Similarly, as shown in the middle radar graph of Fig. 2B, the responses of nodes and edges numbers in CE1 + CE3 were higher than those in CE1 + CE2 and CE2 + CE3, increasing by + 21.2 % and + 29.0 %, respectively, indicating that these combination collision energies were optimized for cluster of bufogenins. The collision energy for the IAAs cluster was also optimized as CE1 + CE2 (right radar graph of Fig. 2B). Under the optimized combination collision energy settings, an effective LC-MS/MS molecular networking has been established, which provides sufficient nodes for the further structural characterization of compounds in TS and TV.
3.2 Identification of components in TS and TV
A combination of collision energies-relied mass spectrometric and molecular networking was established and then applied to the comprehensive structural characterization of constituents in TS and TV. High mass spectrometry responses were exhibited in positive ion mode, in which both bufadienolides and IAAs could exhibit much better response than in negative ion mode. Abundant and high RII-MS2 fragment ions produced under the optimized collision energies could be used for aggregating the compounds with homogeneous structures in the same cluster of molecular networking. With the help of chemical compound information in literatures, a total of 162 compounds in TS and TV, mainly including bufadienolides, IAAs, acyl-arginine, cyclic dipeptides, and nucleosides, were unambiguously classified and tentatively characterized. A total of eighteen constituents were confirmed by comparing their accurate mass measurements of mass spectra and retention times with reference compounds, and their chemical structures are displayed in Fig. S2. The base peak intensity (BPI) chromatograms of TS and TV are shown in Fig. 3. The detailed information of all chemical compounds, which covers the peak number, retention time, scan mass, formula, selective ion, fragment ion, name and the origin, is described in Table S2.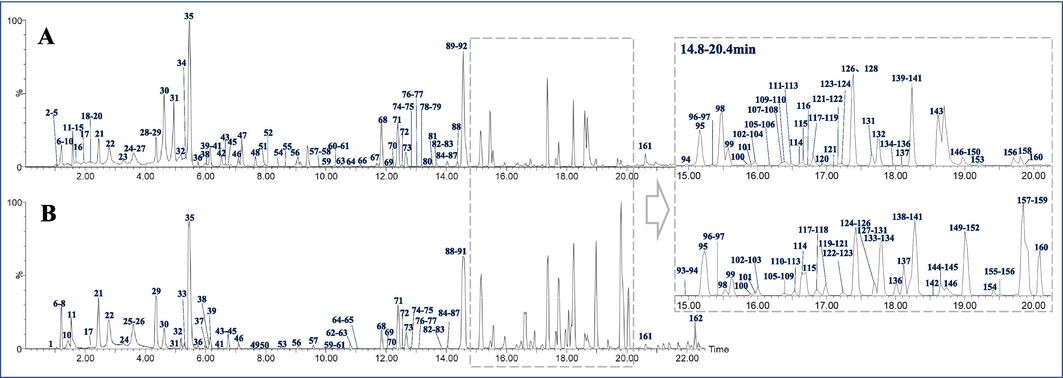
Base peak chromatograms of toad skin (a) and toad venom (b) obtained by UPLC-QTOF/MS in positive ion mode.
3.2.1 Bufadienolides
Bufadienolides, a significant chemical category from TS and TV, exhibited anticancer effects by inducing cell differentiation, cell cycle arrest, and apoptosis (Deng et al., 2020). They are cardiotonic steroids with a unique steroidal A/B cis and C/D cis skeleton with α-pyrone ring at C-17. Free and conjugated types of bufadienolides were commonly seen in toads. The formal one is bufogenins with a hydroxyl group at the C-3 position, whereas the latter one is bufotoxins that are featured by the esterification of the C-3 hydroxyl group to form various amino acid-esters, such as arginine, glutamine, et al(Meng et al., 2016; Hu et al., 2011). Successive losses of H2O, elimination of CO, hydrolysis of ester bond, and retro diels–alder reaction (RDA) cleavage, were considered as the key fragmentation behaviors for bufadienolides(Liu et al., 2010). Due to the presence of different ester bonds substituted at the C-3 hydroxyl group, bufotoxins were apt to be hydrolyzed, yielding MSn fragment ion from corresponding bufogenins, and further experiencing the cleavage of amide bond and neutral losses of NH3, H2O and CO from the acyl-arginine moieties(Cao et al., 2019). Thus, bufogenins and bufotoxins were assembled in two clusters in the molecular networking of TS and TV, which greatly accelerated the dereplication process during structural characterization.
Bufogenins Owing to the similarity of the structural skeleton, the nodes of bufogenins were gathered in one cluster (Fig. 4A). It is worth noting that the initial visible relationship between TS and TV could be seen by the color of the nodes, which facilitates to tracing the source of each compound. The structural illustration procedure of the bufogenins cluster in Fig. 4A was taken as an example. Nodes #445 showed a quasi-molecular ion [M + H]+ at m/z 445.2588 that corresponded to the molecular formula of C26H36O6, with a mass error within 0.67 ppm. As MS2 spectrum shown in Fig. 4B, high molecular weight region of m/z 300–450 under CE1 exhibited a more obvious precursor ion at m/z 445 and a much higher RII of fragment ions than those under CE3, whereas the low molecular weight region of m/z 50–200 under CE3 produced more newer and much higher RII ions than those under CE1. We presumed that the comprehensive information provided under the combined collision energies of CE1 + CE3 was the main reason for manufacturing more nodes and edges numbers for this cluster, without missing the good cosine scores. Product ion at m/z 385.24 was corresponded to the neutral loss of CH3COOH (60 Da) at C-16 position from m/z 445.26. Product ion at m/z 403.18 only existed under CE1, which was 42 Da less than precursor ion, indicating the hydrolysis of ester bond. These fragment ions at m/z 367.23, 349.22 and 331.21 were formed by successive losses of H2O, and they could be further experiencing the elimination of CO, resulting in the product ions at m/z 321.22 and 303.21. Conversely, more fragment ions could be seen under CE3, which was useful for the summarization of the fragmentation routes of the steroid nucleus of this compound. Fragment ion at m/z 253.20, which could only be seen under this collision energy, was due to the elimination of an α-pyrone ring at the C-17 position. It can further undergo fragmentation to yield fragment ions at m/z 131.09 and 119.09 by sequential RDA cleavage, and the two ions can further eliminate CH2 (14 Da) to form a series of signals at m/z 105.07 and 91.05. Fragment ion at m/z 159.12 and 145.10 were also presumed to be the result of RDA cleavage and the subsequently loss of CH2. Node #445 was determined to be bufotalin by comparing their accurate mass measurements, fragment ions, and retention time with the reference compound and its proposed fragmentation pathway was exhibited in Fig. 4C.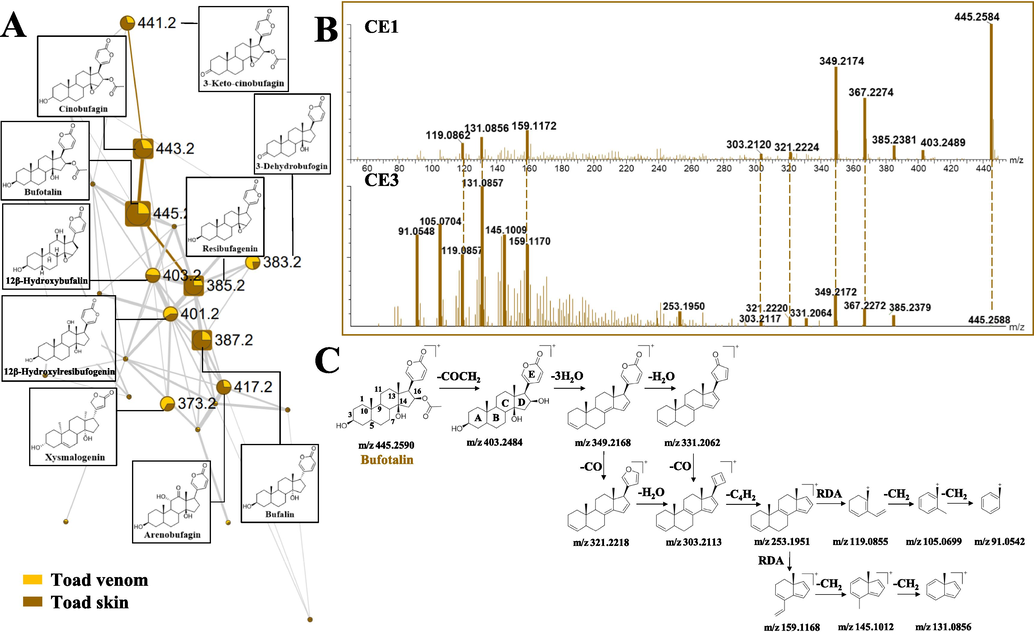
The molecular networking and fragmentation pathway of bufogenins. (A) Molecular networking of bufogenins (yellow means TV, brown means TS, the thickness of the connections between nodes is directly related to the structural similarity). (B) MS/MS spectra of bufotalin in CE1 and CE3. (C) Proposed fragmentation pathway of bufotalin.
Adjacent to the node of bufotalin, node #443 and #385 exhibited high MS/MS spectral similarity, but with 2.0 and 60.0 Da difference in quasi-molecular ion. This indicated that these two nodes were missing a H2 and CH3COOH, respectively, and shared the same cardiotonic steroids. They were tentatively identified as cinobufagin and resibufagenin and were further confirmed by the reference compounds comparison. Node #441 was located adjacent to the node of cinobufagin, and exhibited high MS/MS spectral similarity with 2 Da difference in quasi-molecular ion and fragment ions. This indicated that there might be one more double bond in node #441, compared with cinobufagin. Based on the literature, this node was tentatively identified as 3-keto-cinobufagin (Ma et al., 2010). Similarly, other nodes in the cluster were characterized, and a total of 83 bufogenins were identified.
Bufotoxins Fig. S3A shows the bufotoxins-related compounds that are clustered by their acyl-arginine related skeleton. Node #729 (Peak 97) exhibited an adduct molecular [M + H]+ ion at m/z 729.4074 in MS scan mode, with molecular formula of C38H56N4O10 and a mass error of 0.14 ppm. Its MS/MS spectra and proposed fragmentation behavior were displayed in Fig. S3B. It was apt to yield the product ion at m/z 711.3969 through the loss of H2O. Under high CE3, two high RII fragment ions at m/z 399.22 and m/z 331.20 were found, which corresponded to arenobufagin and suberoyl arginine moieties due to the ɑ-cleavage at C-3 position. The product ion at m/z 399.22 was likely to experience successive losses of H2O, resulting in characteristics fragment ions at m/z 381.21 and 363.20. Meanwhile, fragment ions at m/z 272.15 and m/z 175.12 were produced by the ɑ-cleavage at C-N position and amide bond hydrolysis at m/z 331.1981. The successive losses of 53 and 28 Da could give rise to the fragment ions at m/z 278.15 and 250.16, respectively. Whilst ion at m/z 175.12, corresponding to arginine moiety, could further undergo fragmentation to yield ions at m/z 158.09 and 112.09 by successive loss of amino group and carbonyl group. All of above mass peaks were found in arenobufotoxin, and were confirmed by comparison with the reference compound.
Node #715 was adjacent to the node of arenobufotoxin with a high similarity in fragmentation behaviors. As MS2 spectrum shown in Fig. S4, they displayed the same characteristic fragment ions at m/z 331.20, 278.15, 250.16, 175.12, 158.09, and 112.09, indicating that they might have the same suberoyl arginine moiety. It’s daughter ions at m/z 697.42 and 385.23 were 14 Da less than those ions at m/z 711.39 and 399.22 in MS2 spectra of arenobufotoxin, indicating that the substitute carbonyl group at C ring disappeared. Using the above evidences together with the information from the molecular network, node #715 could be unambiguously identified as gamabufotalintoxin, with the chemical formula of C38H59N4O9 ([M + H]+) and a mass error of −2.66 ppm. Moreover, cluster in this molecular networking showed that node#757 was originated from TV exclusively, and it exhibited high similarity with gamabufotalintoxin in MS2 spectra. However, it showed 42 Da higher in quasi-molecular compared to gamabufotalintoxin, indicating the presence of an acetyl group. According to the previous report, the acetyl group is usually linked to the C-16 position of bufadienolides (Zou et al., 2022). Therefore, we present proposals for identifying this node as bufotoxin using this information and matching it with the in-house database. Similarly, other nodes in the cluster were characterized, and a total of 26 bufotoxins and 19 acyl-arginine were identified.
3.2.2 IAAs
IAAs are biogenic amines and are assigned as linear, β-carboline and quinolin types due to different derivatives of 5-hydroxytryptamine(Zhang et al., 2018). Compounds of this type have been reported to exert anti-tumor effects(Wang et al., 2019). In TS and TV, a total of 10 IAAs were detected, including linear IAAs and quinolin IAAs. Both of these two types of IAAs are gathered in one cluster by molecular networking in Fig. S5A. Node #203 showed the precursor ion at m/z 203.12, which corresponded with the formula C12H14N2O. Two fragment ions at m/z 188.09 and 173.07 corresponded to the successive losses of CH3 (Fig. S5B). The above fragment ion at m/z 173.07 could further undergo the elimination of H2O, yielding the fragment ion at 155.06. By comparison with the literature, this compound was tentatively assigned as dehydrobufotenine, a quinolin type IAAs compound.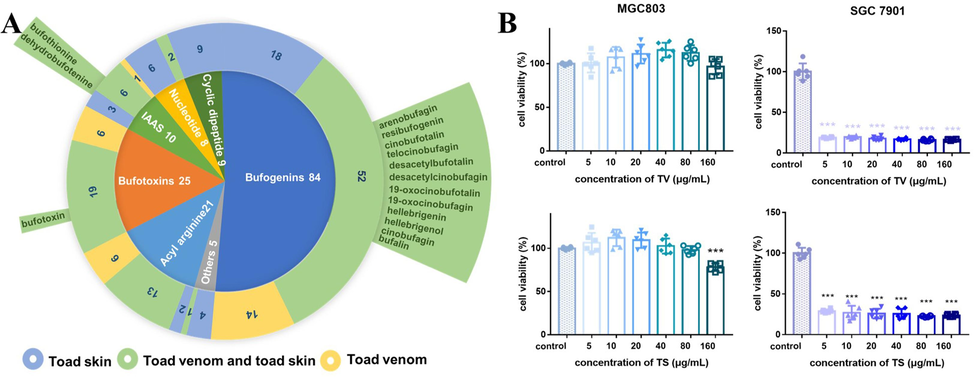
Pie chart of components (A) and bar graph of anti-GC effects (b) of TV and TS. (a) Inner of pie chart shows Proportion of structure type, median exhibits number of sources of each structure types (blue means TS, yellow means TV, green means both TS and TV) and potentially active ingredients from numerous studies are labeled in outer of pie chart. (B) Inhibitory effects of TS and TV on the viability of human GC cell lines SGC7901 and MGC803. Cells were treated with TS or TV extract for 48 h, respectively, and cell growth was measured by microplate reader (n = 6, and the data were present as mean (±S.E.M). ***p < 0.001 vs normal control, by ANOVA test and Dunnett’s multiple comparison test).
Due to the common fragment ions, three connected nodes #219, #326 and #575showed good cosine scores within 0.92, indicating that these compounds displayed similar fragmentation patterns and their structures were in high similarity. As shown in Fig. S5C, the high intensity of diagnostic ion at m/z 160.08 was obtained by the loss of the alkyl amine, demonstrating that these three nodes might be assigned as linear IAAs. The above ion could further generate the common ions at m/z 132.08, m/z 117.06, and m/z 115.05 by loss of CO, CO + CH3, and CO + NH3 residues, respectively. Nodes #219 and 325 showed the quasi-molecular ions at m/z 219.15 and 326.16, corresponding to the formula C13H18N2O and C18H20N3O3, which were 59 Da and 165 Da more than characteristic ion at m/z 160. By comparing the accurate mass measurements of the MS2 spectrum with previous literature report (Li et al., 2022; Davison et al., 2015), node #219 and 326 was tentatively identified as bufotenidine and bufoserotonin C, respectively. Node #575 was supposed to be an unknown linear IAAs compounds, exhibiting the molecular formula of C34H42N2O6 with a mass error of 0.17 ppm. The product ions at m/z 557.29, 539.29, and 521.28 could be explained by the successive losses of H2O from precursor ion. After observation and analysis, it was found that the fragmentation behavior of it exhibited a remarkable similarity with that of bufoserotonin C and bufotoxins, which means the bond on C11-N was cleaved from the ion at m/z 539.29, and then fragment ions at m/z 363.20 and substructure of indole ethyl amine at 160.0762 were produced. The fragment ion at m/z 345.19 was presumed to be generated by expulsion of H2O from the ion at m/z 363.20, with similar fragmentation behavior to that of bufogenins. Taken together, node # 575 could tentatively be speculated as 3-(5- hydroxy tryptophol)- desacetylcinobufotalin, which has not been published before in TS and TV. and the proposed chemical structure was shown in Fig. S5A.
3.2.3 Other types
In addition to the two groups mentioned above, 19 compounds were detected. 12 nucleoside and 4 others were unambiguously characterized by comparison with the publications(Zhang et al., 2018; Li et al., 2022). Three new cyclic dipeptides were tentatively identified by matching with online database of GNPS platform. Fig. S6 shows molecular networking, fragmentation behaviors and prediction verse actual MSn spectrum of three small cyclic peptides. The elimination of the side chains, the cleavage of amide bonds as well as the neutral losses of NH3, H2O and CO were the main fragmentation behaviors of cyclic peptides, and node #227 was taken as an example to explain it. It showed a quasi-molecular [M + H]+ ion at m/z 227.13 in the full mass spectra scan. A series of fragment ions at m/z 199.14, 182.11, 181.13, 153.14 and 136.11 were the consequence of losses or successive losses of H2O, CO and NH3. The alkane side chain was also apt to be expelled, giving rise to fragment ion at m/z 170.07. After that, two cleavages of amide bonds occurred, yielding the typical ion at m/z 114.05. After searching the online database of the GNPS platform, this node was predicted to be cyclo(Leu-Hpro). According to their high similarity in fragmentation behaviors and daughter ions, the other two nodes were tentatively assigned as cyclo(Leu-Pro) and cyclo(Phe-Hpro).
3.3 Analysis and activity evaluation of TS and TV extract
With the help of LC-MS molecular networking, a number of 162 compounds were characterized, including 135 in TS and 120 in TV (Fig. S7). The component distribution is shown in Fig. 5A, the pie chart in the center exhibits the distribution of all component types, the donut in the middle indicates the source of each type, and the sector outermost displayed all of components in toad that were reported to have antitumor effects. Four structural types of bufogenins, bufotoxins, IAAs and acyl-arginine existed in both TS and TV, whereas cyclic dipeptides could only be detected in TS. The total number of bufogenins and bufotoxins in TV was higher than that of TS. Of these compounds in both TV and TS, 15 have showed significant anti-tumor activities according to the previously reported literatures(Wu et al., 2020; Li et al., 2019; Pan et al., 2020; Wang et al., 2019; Yang et al., 2021).
The inhibitory effect of TS and TV on the viability of GC cells MGC803 and SGC7901 is shown in Fig. 5B, which provides a reference for the evaluation of pharmacodynamic differences between them. Both TS and TV significantly inhibited the viability of SGC7901 cells, whereas neither of them exhibited inhibitory effects on MGC803 cells. High similarity in characteristic constituents and activities between TV and TS makes it possible for them to substitute each other in clinical applications.
3.4 Activity evaluation of different fractions in TS
TV and TS showed the similar anti-tumor activities. Moreover, there was a high similarity in characteristic constituents between them. Of the total 162 compounds, 93 common components were found in both TV and TS. Bufadienolides, IAAs, acyl-arginine, and nucleoside type compounds can be found in both of them, whilst cyclic dipeptides could only be detected in TS. TS has a greater number and more structure types of ingredients, and was used in the following studies. TS was eluted with 100 % water, 30 % methanol, 70 % methanol and 100 % methanol, respectively. Base peak chromatograms of each obtained by UPLC-QTOF/MS are shown in Fig. S8. To discover potential antitumor active ingredients, the cytotoxicity of each fraction was measured in SGC7901 cells (Fig. 6). It was found that three fractions showed cytotoxicity except for the water-eluted fraction (mainly including nucleosides and acyl-arginine) (Fig. 6A). Meanwhile, the 70 % methanol eluate fraction (mainly contained IAAs, small cyclic peptides and bufogenins) exhibited the strongest tumor cytotoxicity with an IC50 value of 1.31 (0.70–2.31, 95 %CI) μg/mL (Fig. 6C). Notable, 30 % methanol eluted fraction (mainly including IAAs and small cyclic peptides) and 100 % (mainly including bufogenins) methanol eluted fraction showed a slightly inhibitory activity on the viability of SGC7901 cell with IC50 value of 33.96 (23.36–49.26, 95 %CI) μg/mL (Fig. 6B) and 16.24 (9.42–27.10, 95 %CI) μg/mL (Fig. 6D).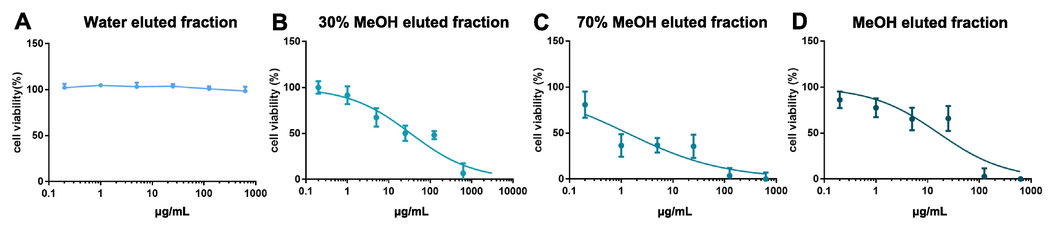
Effect of each TS fraction on SGC7901 cells growth(A–D). Inhibitory activity was evaluated in cell by measuring the rate of decrease in fluorescence intensity. To set the Y-axis, all data were normalized to the mean value of the control group. SGC7901 cells were treated with each fraction of TS for 48 h, and cell growth was measured by microplate reader (n = 6, and the data were present as mean (±S.E.M).
3.5 Identification of prototype constituents absorbed in rat serum
It was proposed that the constituents absorbed into the blood might be the potential material basis of pharmacodynamics(Han et al., 2020). The endogenous matrix remains the major factor affecting the identification of prototypes. In our previous work, a combination of LC-MS molecular networking and Cytoscape software has been used to rapidly eliminate the interference of endogenous compounds in a complex system and successfully applied to produce a clean and concise molecular networking of prototype compounds(Zhang et al., 2023). Herein, the molecular networking among blank serum (pink nodes), TS-treated serum (red nodes) and TS sample (blue nodes) was processed with the aid of Cytpscape software, yielding six individual clusters containing three endogenous interference components clusters, one unabsorbed TS components cluster, one absorbed prototypes cluster and one metabolites cluster (Fig. 7). The nodes in prototypes cluster, which corresponded to the prototype components absorbed in rat, were used for further analysis.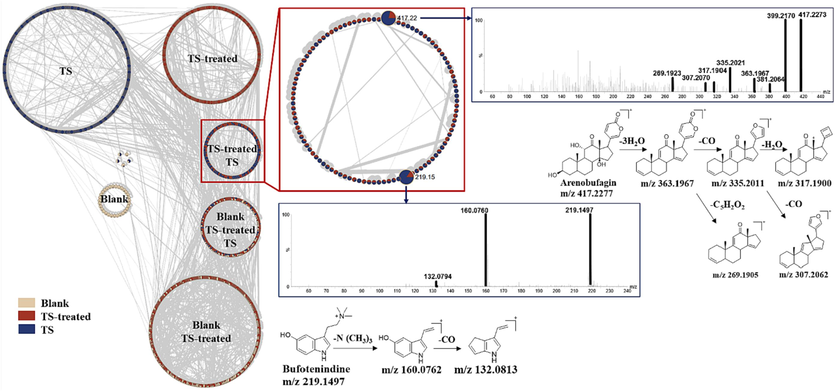
Rapid extraction of prototype components in molecular networking by means of cytoscape, identification of arenobufagin and bufotenidine in TS-treated sample.
Two nodes #417 and #219 were taken as examples to elucidate the identification procedure of prototypes. Node #417 (tR = 14.71) displayed a strong molecular ion [M + H]+ at m/z 417.23. A series of fragment ions at m/z 399.22, 381.21, and 363.20 were generated from the successive losses of H2O. Fragment ion at 363.20 could further experience the elimination of an α-pyrone ring at the C-17 position and two continuous losses of CO, yielding the product ions at m/z 269.19, 335.20 and 307.21, respectively. It was tentatively identified as arenobufagin through comparison with a previous literature report and was further confirmed through comparison with the MS/MS spectra and retention time of the standard compound (Zhang et al., 2016). Node #219 exhibited a quasi-molecular ion of m/z 219.1497 (C13H18N2O, with a mass error of 4.7 ppm) at 3.53 min. Fragment ions at m/z 160.08 and 132.08 are thought to be formed by the typical loss of trimethylamine moiety and the subsequent elimination of CO. Thus, node # 219 was tentatively identified as bufotenidine. A total of 44 prototypes were identified, including 28 bufogenins, 4 IAAs, 4 acyl-arginine, 4 cyclic dipeptides, 2 nucleoside, and 2 vitamins. The detailed information on all prototype compounds, which covers the peak number, retention time, scan mass, formula, selective ion, fragment ion, name, and origin is summarized in Table S3.
3.6 Evaluation of activity compounds
Mass spectrometry informatization of constituents in TS-treated serum as well as 70 % methanol eluted fraction were mapped to the molecular networking, and shared nodes were rapidly extracted. Therefore, the bioactive molecular networking of TS was established as shown in Fig. 7. Sixteen shared nodes containing a large number of bufogenins were marked, which have been predicted as potential anti-GC active ingredients. To clarify the inhibitory effect of bufogenins in TS on SGC7901 cells, the content of 9 major bufogenins, including bufotalin, cinobufotalin, gamabufotalin, telocinobufagin, cinobufagin, bufarenogin, arenobufagin, hellebrigenin and desacetylcinobufotalin, was determined with UPLC-QTOF-MS/MS in MRM positive ionization mode (Table S4). A mixture of bufogenins was prepared according to the quantitative results and showed strong inhibitory activity against SGC901 cells with an IC50 value of 0.23 (0.19–0.27, 95 %CI) μg/mL (Fig. 8).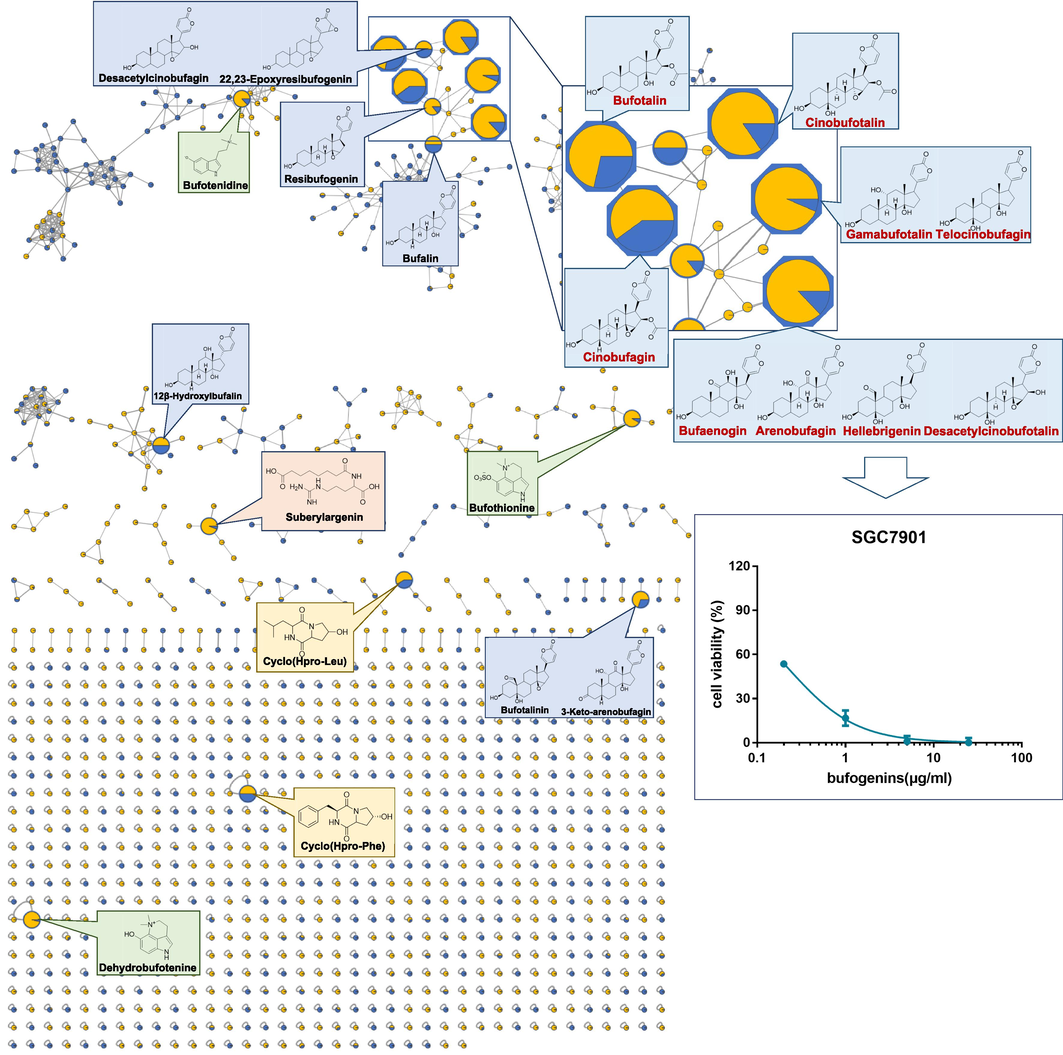
Molecular networking of prototypical components in serum samples and 70% methanol eluted fraction in TS (blue means bufogenins, green means IAAs, yellow means cyclic dipeptides, pink means acyi-argnines), and GC cytotoxicity of nine of major bufogenins.
4 Discussion
TS and TV are two different natural products with similar clinical applications in the treatment of cancer, while the comparison analysis between their compositions has not been elucidated. In this study, a comprehensive and robust strategy for deciphering the bioactive components of TS and TV was established through UPLC-QTOF/MS and bioactivity-based molecular networking. Parameters of collision energy were optimized to enrich the information of UPLC-QTOF/MS molecular networking. Therefore, this optimized method was applied in comprehensively investigating and comparing the chemical profiles of TV and TS.
A total of 162 compounds were speculated and identified, including bufogenins, bufotoxins, IAAS, cyclic peptides, nucleoside and others. In addition, it was found that both TS and TV exhibited significant inhibition on SGC7901 cells viability but not on MGC803 cells. The high similarity in characteristic constituents and activities between TV and TS makes it possible for them to substitute each other in clinical applications. TS has a greater number and more structural types of ingredients compared to TV. Therefore, it was used to screen active ingredients by serum pharmacochemistry as well as cell-based screening. 70 % methanol eluate fractions showed the greatest cytotoxicity to SGC7901 cells with an IC50 value of 1.31 (0.70–2.31, 95 %CI) μg/mL, and 44 prototypical components in serum samples of TS were tentatively identified. Ingredients that contained in not only TS-treated serum but also 70 % methanol eluate fraction were rapidly predicted with the platform of Cytoscape combined with molecular networking, and the bioactive molecular networking of TS was established. There are three types of compounds contained in it, including bufogenins, IAAs, and cyclic dipeptides, and they are considered potential biomarker. Moreover, 30 % methanol eluate fraction of TS, which mainly contained IAAs and cyclic dipeptides without bufogenins, only slightly inhibited the viability of the SGC7901 cell. All of above results suggested that bufogenins might be the primary active components for TS to inhibit the viability of tumor cells. Hence, the content of nine major bufogenins, including bufotalin, cinobufotalin, gamabufotalin, telocinobufagin, cinobufagin, bufarenogin, arenobufagin, hellebrigenin and desacetylcinobufotalin, contained in bioactive molecular networking was quantified and a mixture of them showed strong inhibition of SGC7901 cell viability with IC50 value of 0.23 (0.19–0.27, 95 %CI) μg/mL, indicating that bufogenins were the main active component of TS to inhibit the viability of tumor cells. This is consistent with the extensive literature reporting (Qi et al., 2011c). Furthermore, previous studies have demonstrated that bufothionine (an IAAs) displayed anti-tumor properties (Kong et al., 2021; Wang et al., 2019), and there were some cancer cell binding peptides in TS (Wang et al., 2021). However, it still needs experimental confirmation. This LC-MS/MS molecular networking study fully interpreted the chemicals and pharmacological activity differences between TS and TV as well as correctly predicted the bioactive components that are responsible for the anti-tumor activity for the first time. More significantly, the integrated strategy can serve as a practical example and establish a new workflow for systematically exploring the potential bioactive components in TCM.
5 Conclusion
This study established a comprehensive and robust strategy for deciphering the bioactive components of TS and TV through UPLC-QTOF/MS in-depth chemical profiling combined with bioactivity-based molecular networking. It was proved that there was no significant difference in chemical composition and toxicity to GC cells between TS and TV, whilst bufogenins might be the potential antitumor ingredients.
6 Authorship contribution statement
Study conception and design: W.D.Z., J.Y.; Acquisition of data: R.X.L.; Analysis and interpretation of data: R.X.L., Y.H.Z; Drafting of manuscript: R.X.L.; Critical revision: J.Y, H.W.Z., Y.H.Z.; Assisted experiments: R.J.Z., X.K.X., Y.H.S, X.L., C.M., F.H., L.W., R.W.Z. All authors approved its final version and agreed to be accountable for all aspects of the work.
Acknowledgements
This research was funded by the National Key Research and Development Program of China (2022YFC3502000), National Natural Science Foundation of China (82274172, 82141203), Shanghai Municipal Science and Technology Major Project (ZD2021CY001), Three-year Action Plan for Shanghai TCM Development and Inheritance Program [ZY(2021-2023)-0401], Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (ZYYCXTDD-202004).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Optimization of LC-MS2 data acquisition parameters for molecular network applied to marine natural products. Metabolites. 2022;12:245.
- [CrossRef] [Google Scholar]
- Current developments in LC-MS for pharmaceutical analysis. Analyst. 2020;145:1129-1157.
- [CrossRef] [Google Scholar]
- Integrated metabolomics and network pharmacology to reveal the therapeutic mechanism of Dingkun Pill on polycystic ovary syndrome. J. Ethnopharmacol.. 2022;295:115442
- [CrossRef] [Google Scholar]
- Chemical profile and multicomponent quantitative analysis for the quality evaluation of toad venom from different origins. Molecules. 2019;24:3595.
- [CrossRef] [Google Scholar]
- Hybrid multidimensional data acquisition and data processing strategy for comprehensive characterization of known, unknown and isomeric compounds from the compound Dan Zhi Tablet by UPLC-TWIMS-QTOFMS. RSC Adv.. 2019;9:8714-8727.
- [CrossRef] [Google Scholar]
- Alkaloids from the Traditional Chinese Medicine ChanSu: synthesis-enabled structural reassignment of bufopyramide to bufoserotonin C. Org. Biomol. Chem.. 2015;13:7911-7914.
- [CrossRef] [Google Scholar]
- Molecular mechanisms of bufadienolides and their novel strategies for cancer treatment. Eur. J. Pharmacol.. 2020;887:173379
- [CrossRef] [Google Scholar]
- Integrated metabolomics and network pharmacology revealed Hong-Hua-Xiao-Yao tablet's effect of mediating hormone synthesis in the treatment of mammary gland hyperplasia. Front. Pharmacol.. 2022;13:788019
- [CrossRef] [Google Scholar]
- Full collision energy ramp-MS2 spectrum in structural analysis relying on MS/MS. Anal. Chem.. 2021;93:15381-15389.
- [CrossRef] [Google Scholar]
- Chinmedomics, a new strategy for evaluating the therapeutic efficacy of herbal medicines. Pharmacol. Ther.. 2020;216:107680
- [CrossRef] [Google Scholar]
- Comprehensive chemical analysis of Venenum Bufonis by using liquid chromatography/electrospray ionization tandem mass spectrometry. J. Pharm. Biomed. Anal.. 2011;56:210-220.
- [CrossRef] [Google Scholar]
- Comparative pharmacokinetic study of the five anti-inflammatory active ingredients of Inula cappa in a normal and an LPS-induced inflammatory cell model. Front. Pharmacol.. 2022;13:981112
- [CrossRef] [Google Scholar]
- Traditional Chinese Medicine (TCM) in the treatment of COVID-19 and other viral infections: Efficacies and mechanisms. Pharmacol. Ther.. 2021;225:107843
- [CrossRef] [Google Scholar]
- Bufothionine induces autophagy in H22 hepatoma-bearing mice by inhibiting JAK2/STAT3 pathway, a possible anti-cancer mechanism of cinobufacini. J. Ethnopharmacol.. 2021;270:113848
- [CrossRef] [Google Scholar]
- Toad venom: A comprehensive review of chemical constituents, anticancer activities, and mechanisms. Arch. Pharm.. 2021;354:e2100060.
- [Google Scholar]
- Differentially expressed gene profile and relevant pathways of the traditional Chinese medicine cinobufotalin on MCF-7 breast cancer cells. Mol. Med. Rep.. 2019;19:4256-4270.
- [CrossRef] [Google Scholar]
- An integrated strategy to delineate the chemical and dynamic metabolic profile of Huachansu tablets in rat serum based on UPLC-ESI-QTOF/MSE. J. Pharm. Biomed. Anal.. 2022;218:114866
- [CrossRef] [Google Scholar]
- Metabolome and transcriptome association analysis revealed key factors involved in melatonin mediated cadmium-stress tolerance in cotton. Front. Plant Sci.. 2022;13:995205
- [CrossRef] [Google Scholar]
- A strategy for screening bioactive components from natural products based on two-dimensional cell membrane chromatography and component-knockout approach. J. Chromatogr. A. 2019;1601:171-177.
- [CrossRef] [Google Scholar]
- Systematic screening and characterization of novel bufadienolides from toad skin using ultra-performance liquid chromatography/electrospray ionization quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom.. 2010;24:667-678.
- [CrossRef] [Google Scholar]
- Comparative study on brain pharmacokinetics of Buyang Huanwu Decoction in normal and cerebral ischemia rats using brain microdialysis combined with LC-MS/MS. Chin. Herb. Med.. 2022;14:630-637.
- [CrossRef] [Google Scholar]
- Efficient isolation and purification of five products from microbial biotransformation of cinobufagin by high-speed counter-current chromatography. J. Sep. Sci.. 2010;33:2272-2277.
- [CrossRef] [Google Scholar]
- Traditional Chinese medicine: potential approaches from modern dynamical complexity theories. Front. Med.. 2016;10:28-32.
- [CrossRef] [Google Scholar]
- Metabolomics combined to chemometrics reveals the putative alpha-glucosidase and alpha-amylase inhibitory metabolites of ground cherry (Physalis pruinosa L.) Food Res. Int.. 2022;161:111903
- [CrossRef] [Google Scholar]
- Pilot study of huachansu in patients with hepatocellular carcinoma, nonsmall-cell lung cancer, or pancreatic cancer. Cancer. 2009;115:5309-5318.
- [CrossRef] [Google Scholar]
- Chemical profiling and cytotoxicity assay of bufadienolides in toad venom and toad skin. J. Ethnopharmacol.. 2016;187:74-82.
- [CrossRef] [Google Scholar]
- Metabolite profiling of traditional Chinese medicine formula Dan Zhi Tablet: An integrated strategy based on UPLC-QTOF/MS combined with multivariate statistical analysis. J. Pharm. Biomed. Anal.. 2019;164:70-85.
- [CrossRef] [Google Scholar]
- National Pharmacopoeia Commission, 2020. Chinese Pharmacopoeia. China medical science and technology press, Beijing.
- Bufalin exerts antitumor effects in neuroblastoma via the induction of reactive oxygen species-mediated apoptosis by targeting the electron transport chain. Int. J. Mol. Med.. 2020;46:2137-2149.
- [CrossRef] [Google Scholar]
- Apoptosis-inducing effect of cinobufacini, Bufo bufo gargarizans Cantor skin extract, on human hepatoma cell line BEL-7402. Drug Discov. Ther.. 2008;2:339-343.
- [Google Scholar]
- Antitumor activity of extracts and compounds from the skin of the toad Bufo bufo gargarizans Cantor. Int. Immunopharmacol.. 2011;11:342-349.
- [CrossRef] [Google Scholar]
- Optimizing data-independent acquisition (DIA) spectral library workflows for serum proteomics studies. Proteomics. 2022;22:e2200125.
- [Google Scholar]
- Identification of the active compounds and functional mechanisms of Jinshui Huanxian formula in pulmonary fibrosis by integrating serum pharmacochemistry with network pharmacology. Phytomedicine. 2022;102:154177
- [CrossRef] [Google Scholar]
- Retention time and optimal collision energy advance structural annotation relied on LC-MS/MS: An application in metabolite identification of an antidementia agent namely echinacoside. Anal. Chem.. 2019;91:15040-15048.
- [CrossRef] [Google Scholar]
- Involvement of caspase-3 activity and survivin downregulation in cinobufocini-induced apoptosis in A 549 cells. Exp. Biol. Med. (Maywood). 2009;234:566-572.
- [CrossRef] [Google Scholar]
- Cinobufacini inhibits colon cancer invasion and metastasis via suppressing Wnt/beta-catenin signaling pathway and EMT. Am. J. Chin. Med.. 2020;48:703-718.
- [CrossRef] [Google Scholar]
- Cell affinity screening combined with nanoLC-MS/MS based peptidomics for identifying cancer cell binding peptides from Bufo Bufo gargarizans. J. Pharm. Biomed. Anal.. 2021;206:114354
- [CrossRef] [Google Scholar]
- Bufothionine exerts anti-cancer activities in gastric cancer through Pim3. Life Sci.. 2019;232:116615
- [CrossRef] [Google Scholar]
- A novel hybrid scan approach enabling the ion-mobility separation and the alternate data-dependent and data-independent acquisitions (HDDIDDA): Its combination with off-line two-dimensional liquid chromatography for comprehensively characterizing the multicomponents from Compound Danshen Dripping Pill. Anal. Chim. Acta. 2022;1193:339320
- [CrossRef] [Google Scholar]
- In-depth profiling, characterization, and comparison of the ginsenosides among three different parts (the root, stem leaf, and flower bud) of Panax quinquefolius L. by ultra-high performance liquid chromatography/quadrupole-Orbitrap mass spectrometry. Anal. Bioanal. Chem.. 2019;411:7817-7829.
- [CrossRef] [Google Scholar]
- Wu, Y.G., Song, L.R., 1998. Chinese Materia Medica. Shanghai Scientific and Technical Publishers. Shanghai.
- Identification of antitumor constituents in toad venom by spectrum-effect relationship analysis and investigation on its pharmacologic mechanism. Molecules. 2020;25:4269.
- [CrossRef] [Google Scholar]
- Resibufogenin suppresses triple-negative breast cancer angiogenesis by blocking VEGFR2-mediated signaling pathway. Front. Pharmacol.. 2021;12:682735
- [CrossRef] [Google Scholar]
- Prediction of the mechanism of Dachengqi Decoction treating colorectal cancer based on the analysis method of “ into serum components -action target-key pathway”. J. Ethnopharmacol.. 2022;293:115286
- [CrossRef] [Google Scholar]
- Separation and characterization of bufadienolides in toad skin using two-dimensional normal-phase liquid chromatography × reversed-phase liquid chromatography coupled with mass spectrometry. J Chromatogr B.. 2016;1026:67-74.
- [CrossRef] [Google Scholar]
- Comparative analysis of hydrophilic ingredients in toad skin and toad venom using the UHPLC-HR-MS/MS and UPLC-QqQ-MS/MS methods together with the anti-inflammatory evaluation of indolealkylamines. Molecules. 2018;24:86.
- [CrossRef] [Google Scholar]
- An integrated approach for structural characterization of Gui Ling Ji by traveling wave ion mobility mass spectrometry and molecular network. RSC Adv.. 2021;11:15546-15556.
- [CrossRef] [Google Scholar]
- A novel strategy integrating gas phase fractionation with staggered mass range and LC-MS/MS molecular network for comprehensive metabolites profiling of Gui Ling Ji in rats. J. Pharm. Biomed. Anal.. 2023;222:115092
- [CrossRef] [Google Scholar]
- Bufadienolides originated from toad source and their anti-inflammatory activity. Front. Pharmacol.. 2022;13:1044027.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105427.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







