Translate this page into:
Identification of dimethachlon metabolites and dissipation behavior, processing factor and risk assessment of dimethachlon in rapeseed
⁎Corresponding author. zhangyupinggz@163.com (Yuping Zhang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Two metabolites generated by dimethachlon in rapeseed pods were initially identified. The residue level of dimethachlon and it’s metabolites in rapeseed pods, rapeseed, and oil was determined. The residual change of rapeseed via different process (sun-drying, stir-frying, and pressing) was studied. Risk assessment of intake of dimethachlon from rapeseed oil was evaluated.
Abstract
Pesticides and their transformation products are found in raw agricultural and processed food products, posing risks to human health. Therefore, a thorough investigation of dimethachlon’s dissipation, metabolites, residue levels in crops, and changes during food processing is essential for an accurate dietary risk assessment. This study identified two metabolites, 4-(3,5-dichloroanilino)-4- oxobutanoic acid (DCBAA) and 3,5-dichloroaniline (3,5-DCA), formed from dimethachlon in crops under field conditions. A precise and sensitive analytical method was developed to detect dimethachlon, DCBAA, and 3,5-DCA in rapeseed pods, seeds, and oil. The half-lives of dimethachlon in rapeseed pods at two sites ranged from 1.61 to 1.67 days. During degradation, the maximum residue levels of DCBAA and 3,5-DCA in rapeseed pods reached 38.59 % and 3.47 % of the initial parent compound concentration, respectively. At harvest (day 10), final dimethachlon residues in rapeseed were 0.11 mg/kg and 0.27 mg/kg. Processing rapeseed into oil through sun-drying and stir-frying reduced dimethachlon residues, with processing factors (PF) of 0.69 and 0.64, respectively. However, pressing the seeds concentrated dimethachlon in the oil, with a PF of 1.92. Notably, DCBAA tended to concentrate during sun-drying but decreased during stir-frying and pressing. Supervised trials indicated that dimethachlon residues in rapeseed posed no long-term dietary risks.
Keywords
Dimethachlon
Metabolites
Rape
Degradation
Processing factors
Risk quotient
1 Introduction
Rapeseed (Brassica napus L.) is the second-largest oil crop globally and is a naturally occurring allotetraploid extensively cultivated for its vegetable oil and animal feed value (Chen et al., 2022). However, the soil-borne fungal plant pathogen Sclerotinia sclerotiorum (Lib.) presents a significant threat, affecting over 400 plant species, including Brassica rapa. This pathogen is responsible for an annual yield loss of 10–20 % in rapeseed (Xie et al., 2023).
Dimethachlon (1-(3,5-dichlorophenyl)pyrrolidine-2,5-dione) is a dicarboximide fungicide that was developed in the 1970s and has proven effective in managing Sclerotinia stem rot as well as various other plant pathogenic fungi (Fujinami et al., 1972). The molecular structure of dimethachlon is presented in Fig. S1 of the Supporting Information. However, research conducted in the early 1980s revealed nephrotoxic effects in rats following oral exposure to dimethachlon (Rankin, 1982; Rankin et al., 1985). Further investigations indicated that dimethachlon does not demonstrate direct toxicity to the kidneys nor is it metabolized into the ultimate nephrotoxic species by the kidneys. Instead, in vivo studies suggest that extrarenal metabolism may play a pivotal role in the manifestation of dimethachlon-induced nephrotoxicity (Henesey & Harvison, 1995; Rankin et al., 1988). Potential contributors to this nephrotoxicity include N-(3,5-dichlorophenyl)-2-hydroxysuccinimide (NDHS), N-(3,5-dichlorophenyl)-2-hydroxysuccinamic acid (2-NDHSA), glucuronide conjugates of dimethachlon metabolites, and a sulfate conjugate of dimethachlon metabolites. Additionally, it has been suggested that N-(3,5-dichlorophenyl) maleimide (NDPM) may serve as the primary nephrotoxic species resulting from in vivo exposure to dimethachlon (Cui et al., 2005; Hong et al., 1999; Hong et al., 1998; Rankin, 2004; Rankin et al., 2001). The molecular structures of these metabolites in mammals are illustrated in Fig. S2 of the Supporting Information.
Dimethachlon is widely employed to combat various plant diseases caused by fungi, including Sclerotia sclerotium, rice brown spot, rice sheath blight, kidney bean stem rot, and Botrytis cinerea (Fujinami et al., 1972; Sun et al., 2018). Considering the emerging concerns regarding potential human health risks associated with dimethachlon, its use has been suspended in Europe, the United States, and Japan. Nevertheless, because of its significant antifungal properties, dimethachlon continues to be extensively used in several countries, including China and India (Zhang et al., 2019). According to the China Pesticide Information Network, 14 registered dimethachlon products have been approved in China, of which five are specifically designated for use on rapeseed.
Several studies have investigated the analytical methods, residue levels, and degradation rates of dimethachlon in various crops and soils. For instance, Gao et al. (2023) reported a half-life for dimethachlon in grapes ranging from 14.3 to 18.1 days, while Sun et al. (2018) found the half-life in rice straw to be between 2.5 and 3.7 days. Li et al. (2023) noted that the half-lives of dimethachlon in soil across three different zones ranged from 0.69 to 0.85 days. In soil, dimethachlon can degrade into several byproducts, including DCBAA, 3,5-DCA, succinic acid, and muconic acid (Zhang et al., 2019; Zhang et al., 2022; Li et al., 2023). Conversely, studies focusing on crops have primarily reported on the residual levels of the parent compound, dimethachlon.
It is important to recognize that pesticides degrade through both abiotic and biological pathways within the environment and during food processes, leading to the formation of various transformation products (Li et al., 2021). Some metabolites may display greater persistence and toxicity than their parent compounds. For instance, fenthion is metabolized into fenthion sulfone and fenthion sulfoxide, both of which are more toxic than the original compound (Lee and Kim, 2020). Many governmental agencies and organizations worldwide have also recognized the importance of assessing the combined residues of parent pesticides and their metabolites to estimate dietary intake from plants (Liu et al., 2021; Yang et al., 2017). The Chinese national standard for 'maximum residue limits for pesticides in food' specifies that the allowable pesticide residue, including thiophanate methyl, spirotetramat, and prochloraz, comprises both the parent compound and its metabolites (GB/T2763-2021, 2021).
As previously mentioned, it has been suggested that extrarenal metabolism might play a critical role in the manifestation of dimethachlon-induced nephrotoxicity in mammals. However, research concerning the metabolites produced by dimethachlon in crops remains insufficient. Rape (Brassica napus L.) is widely cultivated in China, which is the second-largest producer of oilseed rape, contributing 16 % of the global production with a total growing area exceeding 6.7 × 106 hm2 in 2020 (FAOSTAT, 2020). Currently, there is no research focused on the residues and degradation of dimethachlon in rapeseed. Thus, understanding the degradation rate and the metabolites resulting from dimethachlon in rapeseed is vital for developing responsible pesticide usage and assessing dietary risks.
Thus, dimethachlon has been reported as potentially hazardous to human health, and its metabolites in vivo exhibit greater toxicity than the parent compound. Current research on the analysis of dimethachlon in crops primarily focuses on the parent compound. This study employed ultra-high-performance liquid chromatography–high-resolution mass spectrometry (UHPLC-HRMS, Q-Exactive Orbitrap) to screen and identify metabolites generated by dimethachlon in rapeseed pods under open field conditions. Subsequently, an accurate quantitative method was developed based on UHPLC–HRMS for the simultaneous determination of dimethachlon and its metabolites, 4-(3,5-dichloroanilino)-4-oxobutanoic acid (DCBAA) and 3,5-dichloroaniline (3,5-DCA), in rapeseed pods, rapeseed, and rapeseed oil. The study also investigated the degradation and metabolite conversion of dimethachlon in rapeseed pods. Additionally, the residue levels of the three target analytes in rapeseed from two typical planting regions in China were evaluated, and processing factors (PFs) for rapeseed were determined through three processing steps: sun-drying, stir-frying, and pressing. The data obtained from this study may help assess the dietary risks associated with dimethachlon exposure through the consumption of rapeseed oil.
2 Materials and methods
2.1 Chemicals and reagents
The standard dimethachlon (98.8 %) used in this study was obtained from Dr. Ehrenstorfer GmbH (Augsburg, Germany). The standard DCBAA (purity 99.5 %) was provided by Dichun Biological Technology Co., Ltd. (Nanjing, China), while 3,5-DCA (purity 99.0 %) was procured from Tanmo Quality Assurance Standards Center (Beijing, China). The wettable powder (WP) containing 40 % dimethachlon was sourced from Henlida Biotechnology Co., Ltd. (Shandong, China).
Chromatographic-grade acetonitrile was acquired from Thermo Fisher Scientific (Waltham, Massachusetts). Analytical-grade acetonitrile, anhydrous magnesium sulfate (MgSO4), sodium chloride (NaCl), and acetic acid were obtained from Jinshan Chemical Reagent Co. (Chengdu, China). Octadecylsilane (C18, 50 μm) and graphitized carbon black (GCB, 120–400 mesh) were sourced from Agela Technologies (Tianjin, China). Nylon syringe filters (0.22 μm) were procured from Peak Sharp Technologies (Beijing, China), and distilled water was obtained from the Watson Group (Hong Kong, China).
2.2 Field trials
The field experiments for rapeseed were conducted at two distinct sites: Guiyang, Guizhou, China, which exhibits a subtropical monsoon humid climate at coordinates 106°7′–107°17′E and 26°11′–27°22′N, located in the Yunnan-Guizhou Plateau; and Changsha, Hunan, China (characterized by a humid subtropical monsoon climate at coordinates 111°53′–114°15′E and 27°51′–28°41′N), situated in the middle and lower reaches of the Yangtze Valley Plain. The field trials took place from April 2022 to May 2022. The temperature during the field experiments in Guiyang (from April 18 to May 9, 2022) and Changsha (from April 26 to May 17, 2022) is presented in Fig. S3 in the Supporting Information.
In this study, rapeseed pods were treated with 40 % dimethachlon WP at the recommended high dose of 900 g.a.i./ha, using a backpack sprayer. The application was performed twice, with the second application occurring one week after the first. Each experimental plot covered an area of 50 m2, and the experiment was replicated three times. Rapeseed pods, weighing approximately 0.5 kg, were randomly collected from each plot at time zero (2 h post-application, after the spraying solution had dried) and at intervals of 1, 2, 3, 5, 7, 10, and 14 days following the last application. The collected pods were minced and mixed thoroughly, and about 150 g of the sample was taken and stored at −18 °C until further analysis. To determine the terminal residue of dimethachlon in rapeseed at the harvest stage (maturity stage of rapeseed), samples were obtained by shelling rapeseed pods from the field, weighing approximately 2 kg, on days 10 and 14 from both sites. These samples were then ground and mixed thoroughly, and about 150 g of the sample was taken using the quartering method and stored at −18 °C until further analysis.
2.3 Metabolite screening
2.3.1 Extraction of rapeseed pod samples
For comprehensive extraction of possible metabolites, a homogenized sample weighing 5 g of rapeseed pod was accurately measured into a 50-mL centrifuge tube. To this, 2.5 mL of water was added, followed by a waiting period of 30 min. Thereafter, 20.0 mL of acetonitrile was introduced to the tube, and the mixture was vortexed for 10 min to ensure thorough mixing. Next, 2 g of NaCl and 4 g of anhydrous MgSO4 were added, followed by an additional 5-minute vortex to aid in further extraction. The sample was then subjected to sonication for 10 min. Following sonication, the resultant extracts underwent centrifugation for 6 min at 8000 rpm. Finally, 1 mL of the supernatant was filtered using a 0.22 μm syringe filter in preparation for subsequent UHPLC-HRMS analysis.
2.3.2 Instrumental conditions for metabolite screening
The screening of dimethachlon's metabolites was conducted using UHPLC-HRMS with an Exactive Orbitrap MS in Full MS/dd-MS2 mode, coupled with Compound Discoverer 3.3 software (Thermo Fisher Scientific, Waltham, Massachusetts). The instrument conditions, including the MS and MS2 acquisition parameters, are detailed in Table S1 of the Supporting Information. For analysis, the ion mass error was defined as (measured ionic mass − theoretical ionic mass)/theoretical ionic mass and was set to be ≤|5| ppm. Each test sample was analyzed in triplicate and separately in either positive or negative polarity mode.
2.4 Samples extract of quantitative analysis of dimethachlon and its metabolites
2.4.1 Extraction and purification procedure for rapeseed pod and rapeseed
To ensure accurate analysis of the residues of dimethachlon and its two metabolites, a homogenized sample weighing 5 g of rapeseed pod or rapeseed was precisely measured and placed into a 50-mL centrifuge tube. Subsequently, 20.0 mL of a 1 % acetic acid-acetonitrile solution was added to the tube, followed by a 10-min vortex to ensure thorough mixing. To this mixture, 1.5 g of NaCl and 2 g of anhydrous MgSO4 were added, and the sample was sonicated for 10 min. Following sonication, the extracts were centrifuged for 5 min at 6000 rpm. After centrifugation, 1.0 mL of the upper layer was carefully transferred to a 2 mL microcentrifuge tube containing 50 mg of C18. The supernatant was then vortexed for 30 s. Subsequently, the supernatant was filtered using a 0.22 μm syringe filter in preparation for UHPLC-HRMS analysis. This residue analysis method was optimized and established based on a modified QuEChERS method for the extraction and purification of carbendazim from rapeseed (Li et al., 2020).
2.4.2 Extraction and purification procedure for rapeseed oil
For the extraction process, a precisely weighed homogenized sample of 2.5 g of rapeseed oil was placed into a 50-mL centrifuge tube. Subsequently, 10.0 mL of 1 % acetic acid-acetonitrile solution was added to the tube, followed by a 10-minute vortex to ensure thorough mixing. To this mixture, 1 g of anhydrous MgSO4 was added, and the sample underwent sonication for 10 min. After sonication, the extracts were centrifuged for 5 min at 6000 rpm. The resulting sample was then stored at −18 °C for 6–7 h until the oil solidified. Once solidified, 1.0 mL of the upper layer was carefully transferred into a 2 mL microcentrifuge tube containing 50 mg of C18 sorbent. The supernatant was vortexed for 30 s after undergoing centrifugation at 6000 rpm for 2 min. Finally, the supernatant was filtered using a 0.22 μm syringe filter in preparation for further analysis. This residue analysis method was optimized and established by modifying the QuEChERS method for the extraction and purification of phenolic compounds from rapeseed oil (Song et al., 2019).
2.5 Instrumental conditions of quantitative analysis of dimethachlon and its metabolites
Table S2 in the supporting information details the conditions of the UHPLC-HRMS, equipped with an APCI source, used for the analysis of dimethachlon, DCBAA, and 3,5-DCA in t-SIM mode. For both dimethachlon and DCBAA, the precise ionic mass of the parent ion [M − H]− was established at m/z values of 241.9781 and 259.9887, respectively. For 3,5-DCA, the ionic mass of the parent ion [M + H]+ was set to m/z 161.98718 for quantitative analysis. The retention times for dimethachlon, DCBAA, and 3,5-DCA were found to be 3.38, 2.87, and 5.02 min, respectively.
2.6 Processing factor study
In the PF study, rapeseed samples harvested from the Hunan field trial on day 10 were selected for analysis. The final residues of dimethachlon, DCBAA, and 3,5-DCA were quantified as 0.27 mg/kg, 0.30 mg/kg, and <0.04 mg/kg, respectively, based on three repeated tests.
The processing of rapeseed into oil typically involves sun drying, stir-frying, and pressing. In our study, the rapeseed samples underwent the following processing steps: 1. Sun-drying: The rapeseed samples were spread out and sun-dried for 8 h. 2. Stir-frying: The dried rapeseed samples were stir-fried for 10 min in an iron pot over gentle heat. The heat was subsequently turned off, and stirring continued until the temperature of the rapeseed dropped to approximately 30 °C. 3. Pressing: The fried rapeseed samples were pressed using the Hanhuang BOZY-01G automatic intelligent small multi-functional oil press. Before and after processing, relevant samples were frozen at −18 °C for further analysis.
In the PF study, rapeseed harvested from the Guizhou field trial, which exhibited lower dimethachlon residue levels compared to those from Hunan, underwent a different procedure. Specifically, this rapeseed was treated with 40 % dimethachlon wettable powder (WP). To achieve this, 0.015 g of 40 % dimethachlon WP was dissolved in 250 mL of tap water and uniformly applied to the rapeseed via spraying. The dimethachlon-contaminated rapeseed was then left to dry in a cool, dark environment for approximately 5 h to allow for analyte absorption. Following this process, the concentration of dimethachlon in the rapeseed was determined to be 4.57 mg/kg.
Consequently, for the PFs study, two concentration groups were established: 1. The low-concentration group consisted of rapeseed with a dimethachlon concentration of 0.27 mg/kg, sourced from the field trial. 2. The high-concentration group comprised rapeseed artificially spiked with dimethachlon in the laboratory, yielding a concentration of 4.57 mg/kg.
2.7 Data analysis
2.7.1 Matrix effect
The matrix effect (ME) was determined using Eq. (1), where ME represents the ME, Smatrix denotes the slopes of the calibration curves of the matrix, and Ssolvent denotes the slopes of the calibration curves of pure solvent.
The evaluation of the ME was categorized into three levels (Besil et al., 2007):
|ME| ≤ 20 % was classified as a weak ME or no ME.
|ME| between 20 % and 50 % was considered a moderate ME.
|ME| greater than 50 % was regarded as a strong ME.
2.7.2 The half-life of degradation
Most pesticides generally conform to the primary kinetic model of degradation, typically utilizing first-order kinetic equations and regression analysis, as outlined (Montemurro et al., 2002). In these equations, C0 (mg/kg) and Ct (mg/kg) represent the initial and time t (days) concentrations of dimethachlon, respectively, while k indicates the degradation rate constant. The half-life (t1/2, day) can be calculated using Eq. (3).
2.7.3 The processing factors
The PF is an essential metric for evaluating the impact and transformation of pesticide residues during food processing (Lopez-Blanco et al., 2018). It is computed by comparing the residue levels in the processed commodity to those in the raw agricultural commodity, as outlined in Eq. (4) (OECD, 2008).
PF values <1, referred to as reduction factors, indicate a decrease in residue concentration within the processed material. Conversely, PF values exceeding 1, known as concentration factors, suggest an enrichment effect resulting from the processing steps (BfR, 2023).
2.7.4 Risk quotient of dietary
The mean dietary exposure values were utilized to estimate pesticide intake and evaluate long-term risks. The risk quotient (RQ) was computed as the ratio of the estimated intake based on pesticide residues detected in specific food items to the legally permitted daily intake, as expressed in Eq. (6) (Cámara et al., 2020). Additionally, the Non-Dietary Exposure Index (NEDI) was determined using the Supervised Trials Median Residue corrected for processing factors (STMR-Pi) in accordance with Eq. (5).
An RQ of <100 % suggests that the residual pesticide levels in food products are unlikely to pose unreasonable adverse effects on the general population. In contrast, an RQ exceeding 100 % implies that pesticide residues may present a risk to human health, with higher RQ values signifying greater risk (Chen et al., 2019b).
Statistical analyses were conducted using IBM SPSS 16.0 and Microsoft Excel 2016. Differences were assessed using a one-way analysis of variance (ANOVA), with a p-value of less than 0.05 considered statistically significant.
3 Result and discussion
3.1 Structural identification of dimethachlon metabolites in rape
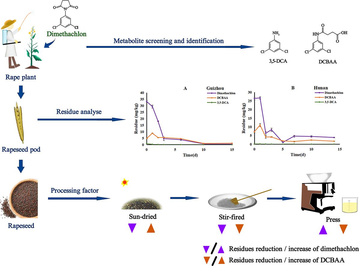
Rapeseed pod samples underwent extraction and analysis according to the methodology outlined in Section 2.3. High-Performance Liquid Chromatography paired with High-Resolution Mass Spectrometry (HPLC/HRMS) is extensively utilized for the identification of pesticide degradation products across various environments. In addition to yielding precise mass numbers, HRMS can collect information through multistage mass spectrometry and hybrid multifunction mass spectrometry (Chen et al., 2019a; Lomheim et al., 2021). Utilizing a comprehensive approach that combined Ultra-High-Performance Liquid Chromatography-High-Resolution Mass Spectrometry (UHPLC-HRMS) analysis (Full-MS/dd-MS2) and data evaluation via Compound Discoverer 3.3 software, two distinct ion peaks at m/z 259.9889 [M − H]− and 161.9874 [M + H]+ were identified. Based on the molecular formulas inferred by the software, along with the specific characteristics of the mass spectrum data, it is plausible to conclude that these ion peaks correspond to DCBAA and 3,5-DCA. It is noteworthy that these compounds have been established as metabolites resulting from dimethachlon in mammalian and soil environments, as reported in previous studies (Li et al., 2023; Rankin, 2004; Zhang et al., 2022). Moreover, it has been documented that both metabolites exhibit reduced nephrotoxicity compared to dimethachlon itself (Rankin, 2004). Standards of DCBAA and 3,5-DCA were subsequently acquired from the market. Using the same analytical method, both the standards and the original samples were analyzed via UHPLC-HRMS. Under both analysis conditions—namely metabolite screening analysis and metabolite quantitative analysis—the retention times of the DCBAA and 3,5-DCA standards aligned with those of the two identified metabolites in the sample. Additionally, the primary and secondary mass spectra of DCBAA and 3,5-DCA standards matched those of the identified metabolites present in the sample. The results, illustrated in Fig. 1, demonstrated congruent features, including identical retention times, isotopic abundances, and accurate mass, between the rapeseed pod extract and the standard solution. This correlation effectively confirmed that the ions detected at 259.9889 m/z and 161.9874 m/z corresponded to the metabolites DCBAA and 3,5-DCA, respectively.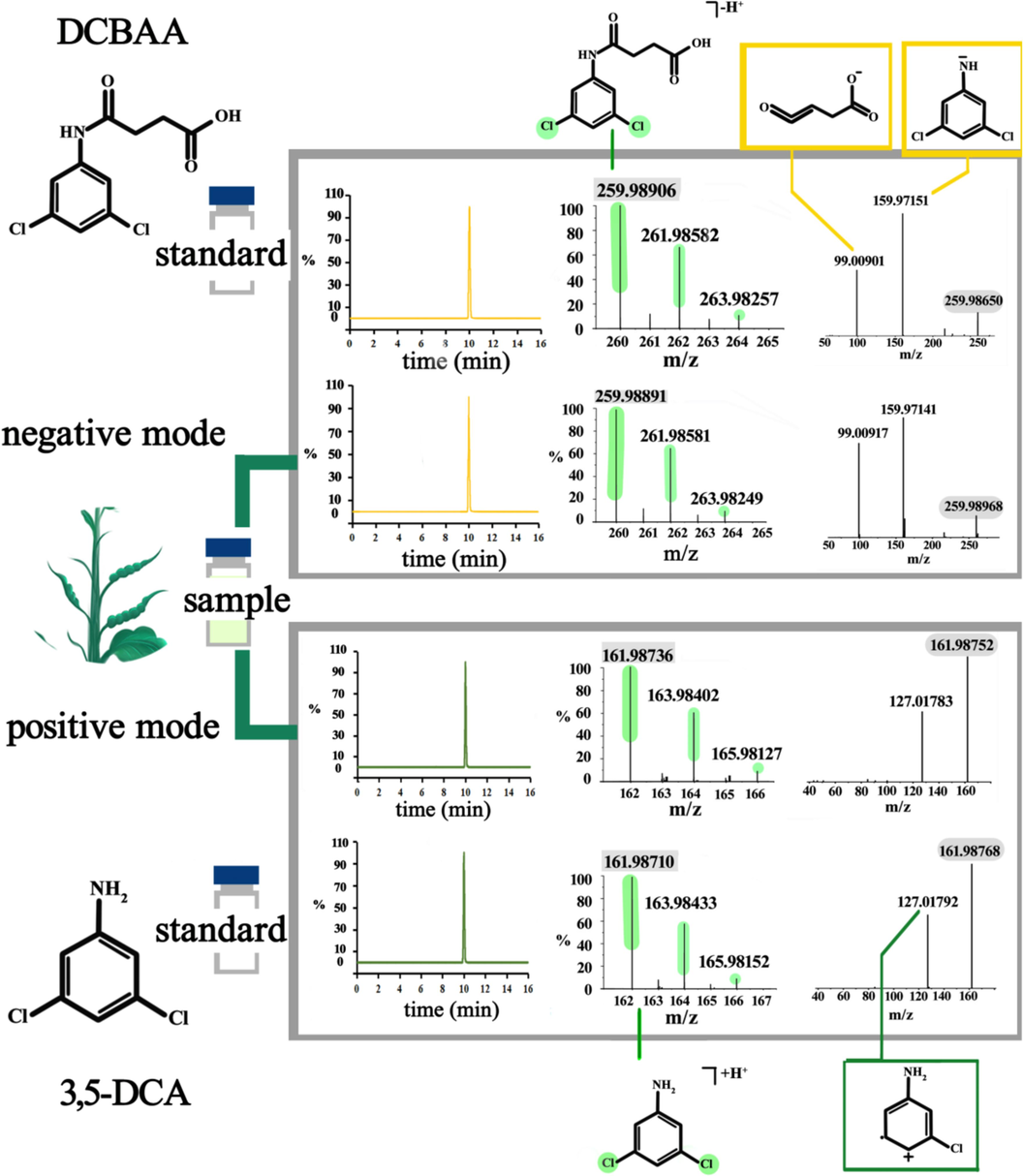
The extracted ion chromatography and mass spectrogram of DCBAA and 3,5-DCA in standard and rapeseed pod extractant.
3.2 Method validation for quantitative analysis
Owing to their high sensitivity and selectivity for complex matrices, HPLC/HRMS techniques are increasingly employed for the quantitative analysis of trace substances in environmental samples (Wang et al., 2024). In this experiment, three chromatographic columns—Hypersil GOLD™ C18, Accucore aQ C18, and Poroshell 120 EC-C18—were utilized to separate the three target analytes. Among these, the Hypersil GOLD™ C18 column exhibited a superior separation effect for all three compounds within a short analysis time of <7 min. Subsequently, various mobile phases were evaluated on the Hypersil GOLD ™ C18 column, with the results indicating that a mixture of water and acetonitrile (V/V = 50:50) provided optimal separation for the target compounds (see Fig. S4 in the Supporting Information). Further investigations were conducted on the peak appearance of the three compounds using ESI and APCI sources under optimized liquid-phase conditions. As illustrated in Fig. S5 of the Supporting Information, the response of 3,5-DCA significantly increased under the APCI source. Therefore, the APCI source was chosen for the quantitative analysis of dimethachlon and its two metabolites, since the response values for all three compounds were essentially uniform.
The performance of the analytical method was assessed using various samples, including blank rapeseed, rapeseed pods, and rapeseed oil. The validation process included several parameters, including linearity, ME, sensitivity, accuracy, and precision. The linearity of dimethachlon and its two metabolites was evaluated by calculating the slope and correlation coefficient (R2) from matrix-matched standard solutions ranging from 0.01 to 2 mg/L. Table 1 presents the regression equations and correlation coefficients for both solvent and matrix standard solutions of dimethachlon and its metabolites. Notably, the standard solutions exhibited excellent linearity, as evidenced by R2 values exceeding 0.999.
Matrices
Analytes
Line equation
Correlation (R2)
LOQs (mg/kg)
LODs (mg/kg)
ME (%)
Acetonitrile
Dimethachlon
y = 29909384.92x + 574818.66
0.9996
−
DCBAA
y = 29556618.84x + 518388.46
0.9999
−
3,5-DCA
y = 16202440.58x + 116473.55
0.9997
−
Rapeseed pod
Dimethachlon
y = 34005569.61x − 85363.59
0.9999
0.04
0.0001
0.28
DCBAA
y = 62115076.32x − 779115.73
0.9994
0.04
0.0001
32.68
3,5-DCA
y = 15923728.93x + 124424.17
0.9998
0.04
0.0002
3.14
Rapeseed
Dimethachlon
y = 25192506.14x − 334452.98
0.9990
0.04
0.0002
−15.77
DCBAA
y = 39360992.75x + 51333.44
0.9994
0.04
0.0001
33.17
3,5-DCA
y = 13194176.92x − 60824.99
0.9999
0.04
0.0002
−18.57
Rapeseed oil
Dimethachlon
y = 48309045.26x + 387959.85
0.9993
0.04
0.0002
−10.53
DCBAA
y = 76300465.18x − 63225.15
0.9997
0.04
0.0003
24.87
3,5-DCA
y = 16166017.31x + 64520.71
1.0000
0.04
0.0002
−7.29
Dimethachlon demonstrated weak MEs in rapeseed pods (0.28 %), rapeseed (−15.77 %), and rapeseed oil (−10.53 %). In contrast, DCBAA exhibited moderate MEs in rapeseed pods (32.68 %), rapeseed (33.17 %), and rapeseed oil (24.87 %). Similarly, 3,5-DCA showed weak MEs across all sample types, with ME values of 3.14 % in rapeseed pods, −18.57 % in rapeseed, and −7.29 % in rapeseed oil. Consequently, to ensure data accuracy, the matrix curve was utilized as the quantitative standard for determining the concentrations of dimethachlon and its two metabolites in the various samples.
Recovery experiments were performed to evaluate the accuracy and precision of the method at three spiked concentration levels (0.04, 0.4, and 4 mg/kg) in rapeseed pod, rapeseed, and rapeseed oil samples. Each concentration level was replicated five times over three consecutive days. Accuracy and repeatability (intra-day) as well as reproducibility (inter-day) were assessed using the relative standard deviation (RSD). The results presented in Table 2 demonstrate satisfactory recovery of dimethachlon and its metabolites across different concentration levels, with recoveries ranging from 71.42 % to 100.6 % and an inter-day RSD (n = 15) of 0.90 % to 9.88 %. These findings highlight the effectiveness of the method in detecting dimethachlon and its metabolites in rapeseed pod, rapeseed, and rapeseed oil samples. The extracted ion chromatogram of dimethachlon, DCBAA, and 3,5-DCA in a rapeseed pod sample is illustrated in Fig. 2.
Sample
Analytes
Spiked level (mg/kg)
Average recovery, intraday RSD (%, n = 5)
Average recovery, interday RSD (%, n = 15)
Day 1
Day2
Day3
Rapeseed pod
Dimethachlon
0.04
93.83
2.94
103.3
7.08
91.07
2.60
96.06
7.24
0.4
101.2
2.59
97.76
5.34
97.11
4.44
98.69
4.36
4
95.89
6.72
100.8
6.78
97.60
5.57
98.11
6.30
DCBAA
0.04
97.21
2.20
93.71
4.12
95.68
7.21
95.53
4.84
0.4
97.25
3.70
95.44
4.39
90.82
2.75
94.50
4.54
4
98.15
9.50
90.79
6.96
102.5
7.58
97.16
9.14
3,5-DCA
0.04
84.67
4.74
97.41
2.39
99.13
5.94
93.74
8.30
0.4
94.44
1.48
96.38
1.14
96.94
4.55
95.92
2.89
4
96.74
4.32
91.53
6.66
97.02
6.26
95.10
6.04
Rapeseed
Dimethachlon
0.04
98.00
2.56
97.85
2.56
98.58
5.49
98.14
3.52
0.4
95.57
5.05
96.86
0.70
97.92
1.56
96.79
3.34
4
94.89
1.55
100.7
2.26
103.0
1.82
99.52
3.96
DCBAA
0.04
90.64
3.96
81.86
3.84
83.47
1.44
85.32
5.56
0.4
99.67
4.73
91.80
1.68
91.89
2.23
94.45
5.06
4
93.19
6.50
103.2
2.50
105.4
1.29
100.6
6.52
3,5-DCA
0.04
93.47
2.36
100.6
0.66
96.11
1.21
96.71
3.44
0.4
94.55
1.58
98.12
1.09
94.85
2.03
95.84
2.41
4
98.36
3.79
96.44
1.56
96.44
2.49
97.08
2.82
Rapeseed oil
Dimethachlon
0.04
92.57
3.06
85.32
5.65
101.3
8.20
93.07
9.28
0.4
85.24
2.61
94.83
4.74
99.25
9.68
93.11
9.00
4
95.00
6.02
86.44
3.30
89.54
5.32
90.33
6.22
DCBAA
0.04
89.58
2.31
92.43
5.17
99.34
6.40
93.78
6.51
0.4
94.70
2.55
94.63
5.17
93.59
4.21
94.31
4.07
4
89.91
3.51
90.38
5.49
91.67
5.00
90.65
4.48
3,5-DCA
0.04
75.06
5.31
78.88
2.99
73.32
3.62
75.75
4.93
0.4
76.71
5.17
75.79
1.20
76.43
2.12
76.31
3.11
4
71.26
1.14
71.92
0.46
71.06
0.53
71.42
0.90
The extracted ion chromatogram of dimethachlon, DCBAA, and 3,5-DCA in the rapeseed pod sample (A, blank rapeseed pod sample, B, standard solution (0.1 μg/mL), C, matrix-matched standard solution (0.1 μg/mL), and D, rapeseed pod spiked sample (0.4 mg/kg)).
3.3 Degradation dynamics of dimethachlon and formation of metabolites in rapeseed pod
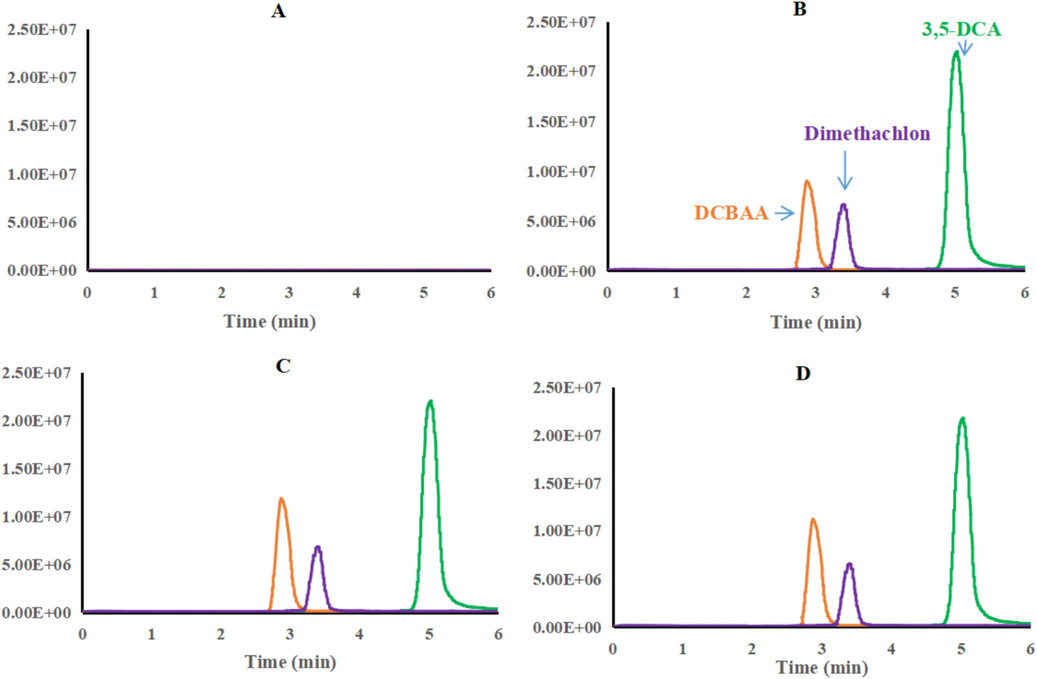
This investigation aimed to elucidate the degradation pattern of dimethachlon and the trends in the formation of its metabolites, assessing three target analytes in rapeseed pod samples collected from open fields. The degradation curves of dimethachlon in rapeseed pod were established by plotting residual concentrations against time, as shown in Fig. 3.Dimethachlon rapidly dissipated in rapeseed pods at both study sites, as illustrated in Fig. 3. The degradation rates reached 83.27 % in Guizhou and 98.09 % in Hunan by day 10 following application. Notably, the degradation trends of dimethachlon at both sites closely align with a first-order model calculation. The degradation equations derived from the rapeseed pod samples revealed distinct patterns: in Guizhou, the equation was represented as C = 36.91e−0.415t with an R2 value of 0.9259 and a half-life (T1/2) of 1.67 days. In contrast, in Hunan, the equation was expressed as C = 29.67e-0.431t with an R2 value of 0.7693, resulting in a half-life (T1/2) of 1.61 days. Thus, the degradation rates of dimethachlon in rapeseed pods from both locations were notably rapid. Statistical analysis revealed no significant difference in the half-lives between the two experimental areas (P < 0.05). Additionally, when compared to previous studies on the degradation rates of dimethachlon in other crops (Gao et al., 2023; Sun et al., 2018), it was observed that dimethachlon dissipated more rapidly in rapeseed pods than in grapes and rice straw, potentially due to differences in geographic environments, climatic conditions, and crop types.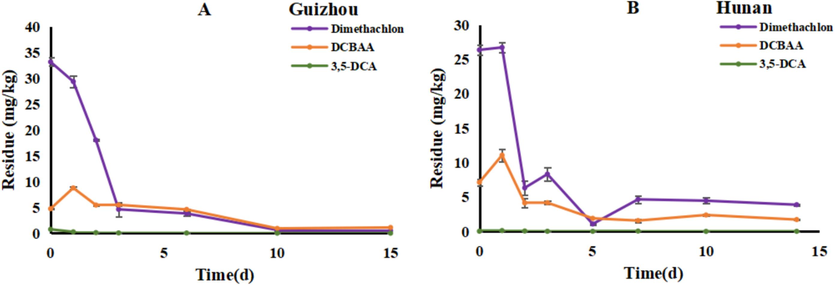
Dynamic curves of dimethachlon and residue of its two metabolites in rapeseed pod.
In Guizhou, the maximum residues of DCBAA and 3,5-DCA on rapeseed pods were recorded as 8.76 mg/kg and 0.76 mg/kg, respectively, after 1 day and 2 h. These values correspond to 24.60 % and 3.47 % of the maximum residual levels of dimethachlon on the rapeseed pods. In comparison, in Hunan, the maximum residues of DCBAA and 3,5-DCA reached 11.07 mg/kg and 0.13 mg/kg, respectively, on the first day, representing 38.59 % and 0.75 % of the maximum residue of dimethachlon. It is evident that DCBAA is the primary metabolite produced in rapeseed following dimethachlon application.
The residual levels of dimethachlon and its two metabolites in rapeseed at the harvest stage are presented in Fig. 4. In Guizhou province, the final residue of dimethachlon in rapeseed after 10 and 15 days was recorded as 0.11 mg/kg and 0.04 mg/kg, respectively. Similarly, in Hunan province, the final residue of dimethachlon in rapeseed after 10 and 14 days was found to be 0.27 mg/kg and 0.14 mg/kg, respectively. Regarding DCBAA, the residue levels in rapeseed after 10 and 15 days in Guizhou province were observed to be 0.18 mg/kg and 0.07 mg/kg, respectively. In Hunan province, the residue of DCBAA in rapeseed after 10 and 14 days was recorded as 0.30 mg/kg and 0.26 mg/kg, respectively. Notably, the residue levels of 3,5-DCA in rapeseed samples from both sites were below the limit of quantification (LOQ), which is 0.04 mg/kg.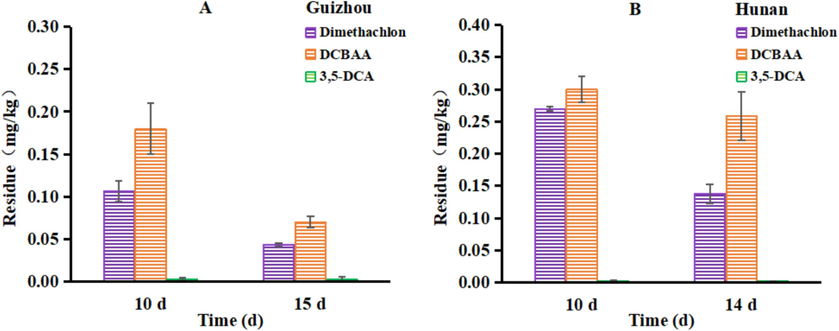
The final residue of dimethachlon and its two metabolites in rapeseed at the harvest stage.
3.4 Effect of sunning, stir-frying, and pressing treatment
This experiment assessed the processing steps involved in converting rapeseed into rapeseed oil, specifically focusing on sunning, stir-frying, and pressing. The concentrations of the three analytes throughout each processing step are illustrated in Fig. 5.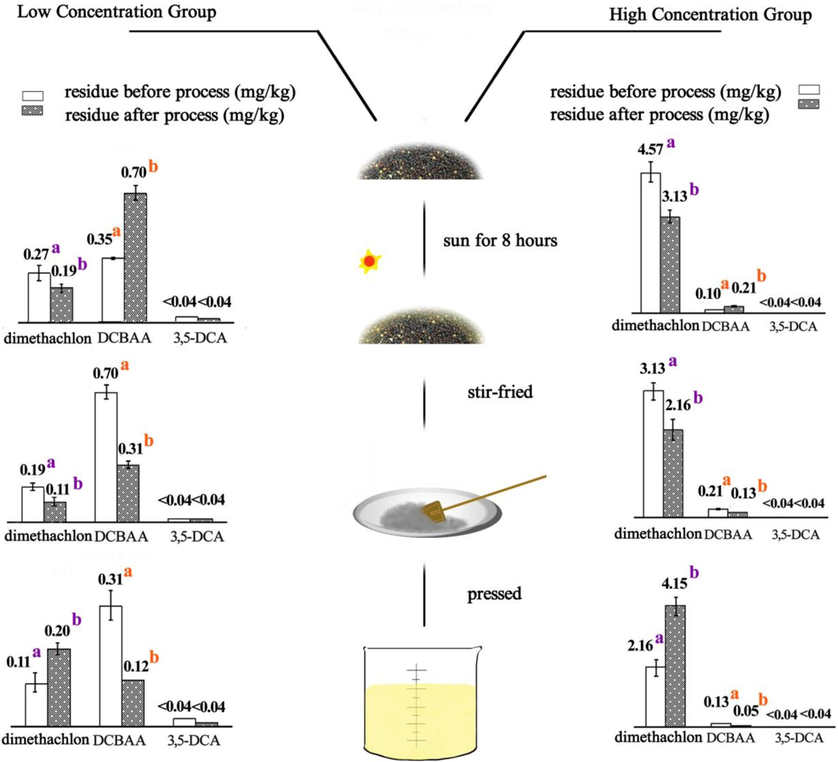
Residues of dimethachlon and its metabolites in samples during processing ab: different letters represent significant differences of residues before and after process at P < 0.05.
During the sun-drying process, the residue of dimethachlon decreased by 29.63 % and 31.51 % in the low- and high-concentration groups, respectively. The associated PFs were determined to be 0.70 and 0.68. In contrast, the levels of DCBAA residue increased by 133 % and 110 % during sun-drying for the low- and high-treatment groups, respectively, with PFs of 2.33 and 2.10. Notably, the residue of 3,5-DCA remained below 0.04 mg/kg both before and after sun-drying. Statistical analysis of the residues of dimethachlon and DCBAA before and after processing revealed that sun-drying significantly reduces dimethachlon while increasing metabolite DCBAA (P<0.05).
In the stir-frying phase of rapeseed processing, the residue of dimethachlon decreased by 42.11 % and 30.99 % in the low- and high-concentration groups, respectively, with corresponding PFs of 0.58 and 0.69. Additionally, DCBAA residue decreased by 55.71 % and 38.10 % during pressing for the low- and high-treatment groups, respectively, with PFs of 0.44 and 0.62. Similar to the sun-drying process, the residue of 3,5-DCA remained below 0.04 mg/kg both before and after stir-frying. Statistical analysis indicated that stir-frying significantly reduces both dimethachlon and DCBAA (P < 0.05).
During the pressing process of rapeseed, the residue of dimethachlon increased by 81.82 % and 101.39 % in the low- and high-concentration groups, respectively, with PFs calculated at 1.82 and 2.01. Meanwhile, DCBAA residue decreased by 61.29 % and 61.54 %, with PFs of 0.39 and 0.38 for the low- and high-concentration groups, respectively. As observed in the previous processing steps, the residue of 3,5-DCA remained below 0.04 mg/kg both before and after pressing. Statistical analysis indicated that pressing significantly reduces DCBAA while increasing dimethachlon (P < 0.05).
3.5 Dietary intake risk assessment
According to the Dietary Guidelines for Chinese Residents (2022), the recommended daily intake of oil for a balanced diet for Chinese residents is between 25 and 30 g, with a maximum limit set at 30 g (Chinese Nutrition Society, 2022). The average weights for males and females aged 20 to 50 are typically 63 kg and 56 kg, respectively. In our study, the PF for dimethachlon was determined to be 0.85, based on the mean values from both low and high-concentration groups.
On day 10 of the trial, the median residue levels of dimethachlon, DCBAA, and 3,5-DCA in rapeseed were measured at 0.195 mg/kg, 0.254 mg/kg, and <0.04 mg/kg, respectively. Consequently, the total median residual value (expressed as dimethachlon equivalent and including dimethachlon, DCBAA, and 3,5-DAC) was calculated to be 0.43 mg/kg. The ADI for dimethachlon is established at 0.0013 mg/kg body weight/day (GB/T2763-2021, 2021). Based on the dietary risk assessment formulated using the provided equations, when the residual value is 0.43 mg/kg, the NEDI is assessed to be 0.00020 mg/kg body weight/day for girls and 0.00017 mg/kg body weight/day for boys. Consequently, the RQ for females and males is calculated to be 15.06 % and 13.39 %, respectively. Given that the RQs are below 100 %, this indicates that the dietary intake risk associated with dimethachlon in rapeseed oil is within acceptablelimits. Our study assessed the dietary intake risk based on total residue values, including dimethachlon, DCBAA, and 3,5-DAC, to ensure a conservative risk estimate, demonstrating that the RQs were less than 16 %, significantly lower than the threshold of 100 %. It can be concluded that rapeseed oil is safe for consumption when dimethachlon is used appropriately, adhering to the recommended dosage and application frequency.
4 Conclusion
In this study, it was observed that the metabolites DCBAA and 3,5-DCA were generated in samples following the application of dimethachlon to rapeseed under open field conditions. A sensitive analytical method utilizing UHPLC-HRMS was successfully developed for the determination of dimethachlon and its metabolites, DCBAA and 3,5-DCA, in rapeseed pods, rapeseed, and rapeseed oil, achieving satisfactory precision and accuracy. Analysis of samples collected under open field conditions revealed that the dissipation of dimethachlon on rapeseed pods occurred relatively rapidly, with a degradation rate exceeding 83.27 % within 10 days. Moreover, during the processing of rapeseed into oil, sun-drying was shown to reduce dimethachlon residue, while DCBAA displayed a tendency to accumulate. Subsequently, residues of both dimethachlon and DCBAA were found to decrease during the stir-frying process. Interestingly, it was observed that the residue of dimethachlon tended to accumulate in the oil during the pressing process. Based on supervised trials with residue values adjusted for PFs of dimethachlon, the dietary intake risk associated with dimethachlon in rapeseed oil was determined to be at an acceptable level for human health.
This study focused solely on determining the residues of dimethachlon and its two metabolites in rapeseed from Guizhou and Hunan, and the limited number of experimental sites may have resulted in inadequate monitoring of residual values across diverse natural environments and typical planting regions. Therefore, future research should include four additional experimental sites that represent typical rapeseed planting regions from north to south to better evaluate the residual levels of dimethachlon and its metabolites in rapeseed. Furthermore, it is essential to investigate whether the metabolites DCBAA and 3,5-DCA could be produced in other crops.
CRediT authorship contribution statement
Lianhong Mou: Writing – original draft, Methodology, Formal analysis, Data curation, Investigation, Visualization. Ling Wu: Methodology, Formal analysis, Data curation, Investigation, Validation, Writing – review & editing. Ling Liu: Methodology, Formal analysis, Data curation, Investigation, Validation, Writing – review & editing. Yu Xiang: Methodology, Validation. Deyu Hu: Funding acquisition, Conceptualization, Supervision, Visualization. Yuping Zhang: Writing – review & editing, Supervision, Project administration, Methodology, Funding acquisition, Conceptualization.
Acknowledgments
The authors thank the National Key Research and Development Program of China [No. 2021YFD1700104], the National Natural Science Foundation of China [Nos. 32260693 and 21866009], the Program of Introducing Talents of Discipline to Universities of China [111 Program, D20023], and the Frontiers Science Center for Asymmetric Synthesis and Medicinal Molecules, Department of Education, Guizhou Province [Qianjiaohe KY number (2020)004] for financial support.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Matrix effects and interferences of different citrus fruits coextractives in pesticide residue analysis using ultra high-performance liquid chromatography-high resolution mass spectrometry. J. Agric. Food Chem.. 2007;65(23):4819-4829.
- [CrossRef] [Google Scholar]
- BfR, 2023. EU database on processing factors. <https://www.bfr.bund.de/cm/349/eu-database-on-processing-factors.pdf> (Accessed 1 December 2023).
- Removal residues of pesticides in apricot, peach and orange processed and dietary exposure assessment. Food Chem.. 2020;325:126936
- [CrossRef] [Google Scholar]
- Dissipation behavior, residue distribution and dietary risk assessment of field-incurred boscalid and pyraclostrobin in grape and grape field soil via MWCNTs-based QuEChERS using an RRLC-QqQ-MS/MS technique. Food Chem.. 2019;274:291-297.
- [CrossRef] [Google Scholar]
- The flavonoid biosynthesis and regulation in Brassica napus: a review. Int. J. Mol. Sci.. 2022;24(1)
- [CrossRef] [Google Scholar]
- Degradation products and pathway of ethiprole in water and soil. Water Res.. 2019;161:531-539.
- [CrossRef] [Google Scholar]
- Chinese Nutrition Society, 2022. The dietary guideline for chinese. People's med. publishing house. Beijing, Part I: Dietary guidelines for the general population.
- Metabolism of the nephrotoxicant N-(3,5-Dichlorophenyl)succinimide in rats: evidence for bioactivation through alcohol-O-glucuronidation and O-sulfation. Chem. Res. Toxicol.. 2005;18(6):991-1003.
- [CrossRef] [Google Scholar]
- FAOSTAT. 2020. FAO statistics division. [2024-01-29] <https://www.fao.org/faostat/en/#data/QCL>.
- Studies on biological activity of cyclic imide compounds. part II. antimicrobial activity of i-phenylpyrrolidine-2,5diones and related compounds. Agric. Biol. Chem.. 1972;36(2):318-323.
- [CrossRef] [Google Scholar]
- Dissipation, residue, and dietary risk assessment of dimethachlon in grapes. Environ. Sci. Pollut. Res.. 2023;30(39):91199-91206.
- [CrossRef] [Google Scholar]
- GB/T2763-2021., 2021. National food safety standard- maximum residue limits for pesticides in food GB 2763-2021. <https://www.sdtdata.com/fx/fmoa/tsLibCard.doView> (Accessed 1 December 2023).
- Potential metabolism and cytotoxicity of N-(3,5-dichlorophenyl)succinimide and its hepatic metabolites in isolated rat renal cortical tubule cells. Toxicology. 1995;104(1–3):9-16.
- [CrossRef] [Google Scholar]
- Gender differences in acute N-(3,5-dichlorophenyl)-2-hydroxysuccinimide (NDHS) and N-(3,5-dichlorophenyl)-2-hydroxysuccinamic acid (2-NDHSA) nephrotoxicity in Fischer 344 rats. J. Toxicol. Environ. Health. Part A: Curr. Issues. 1998;54(8):613-632.
- [CrossRef] [Google Scholar]
- The role of glucuronidation in N-(3,5-dichlorophenyl)succinimide (NDPS) nephrotoxicity: nephrotoxic potential of NDPS and NDPS metabolites in Gunn, Wistar, and Fischer 344 rats. Toxicol. Appl. Pharmacol.. 1999;154(2):170-180.
- [CrossRef] [Google Scholar]
- Simultaneous analysis of fenthion and its five metabolites in produce using ultra-high performance liquid chromatography-tandem mass spectrometry. Molecules. 2020;25(8):1938.
- [CrossRef] [Google Scholar]
- Transfer assessment of carbendazim residues from rapeseed to oil production determined by HPLC–MS/MS. J. Environ. Sci. Health b.. 2020;55(8):726-731.
- [CrossRef] [Google Scholar]
- Identification and quantification of dimethachlon degradation products in soils and their effects on soil enzyme activities. J. Agric. Food Chem.. 2023;71(4):1852-1861.
- [CrossRef] [Google Scholar]
- The present situation of pesticide residues in China and their removal and transformation during food processing. Food Chem.. 2021;354:129552
- [CrossRef] [Google Scholar]
- Determination, dissipation dynamics, terminal residues and dietary risk assessment of thiophanate-methyl and its metabolite carbendazim in cowpeas collected from different locations in China under field conditions. J. Sci. Food Agric.. 2021;13:5498-5507.
- [CrossRef] [Google Scholar]
- Identification of a fully dechlorinated product of chlordecone in soil microcosms and enrichment cultures. Environ. Sci. Technol. Lett.. 2021;8(8):662-667.
- [CrossRef] [Google Scholar]
- Experimental and theoretical determination of pesticide processing factors to model their behavior during virgin olive oil production. Food Chem.. 2018;239:9-16.
- [CrossRef] [Google Scholar]
- Chlorpyrifos decline curves and residue levels from different commercial formulations applied to oranges. J. Agric. Food Chem.. 2002;50(21):5975-5980.
- [CrossRef] [Google Scholar]
- OECD, 2008. Series on testing and assessment No 96: guidance document on magnitude of pesticide residues in processed commodities. <https://one.oecd.org/document/env/jm/mono(2008)23/en/pdf> (Accessed 1 December 2023).
- Nephrotoxicity following acute administration of N-(3,5-dichlorophenyl)succinimide in rats. Toxicology. 1982;23(1):21-31.
- [CrossRef] [Google Scholar]
- Nephrotoxicity induced by C- and N-arylsuccinimides. J. Toxicol. Environ. Health Part b: Critical Reviews. 2004;7(5):399-416.
- [CrossRef] [Google Scholar]
- Acute nephrotoxicity of N-phenyl and N-(monochlorophenyl)succinimides in Fischer 344 and Sprague-Dawley rats. Toxicology. 1985;34(4):299-308.
- [CrossRef] [Google Scholar]
- Nephrotoxicity of N-(3,5-dichlorophenyl)succinimide metabolites in vivo and in vitro. Toxicol. Appl. Pharmacol.. 1988;96(3):405-416.
- [CrossRef] [Google Scholar]
- In vitro nephrotoxicity induced by N-(3,5-dichlorophe-nyl)succinimide (NDPS) metabolites in isolated renal cortical cells from male and female Fischer 344 rats: evidence for a nephrotoxic sulfate conjugate metabolite. Toxicology. 2001;163(2–3):73-82.
- [CrossRef] [Google Scholar]
- Development and validation of a QuEChERS-LC-MS/MS method for the analysis of phenolic compounds in rapeseed oil. J. Agric. Food Chem.. 2019;67(14):4105-4112.
- [CrossRef] [Google Scholar]
- Residual level of dimethachlon in rice-paddy field system and cooked rice determined by gas chromatography with electron capture detector. Biomed. Chromatogr.. 2018;32(7):e4226.
- [Google Scholar]
- Multi-pesticide residue screening, identification, and quantification analysis in various fruits and vegetables by UHPLC-Q Exactive HRMS. Anal. Methods 2024
- [CrossRef] [Google Scholar]
- Screening of microRNAs and target genes involved in Sclerotinia sclerotiorum (Lib.) infection in Brassica napus L. BMC Plant Biol.. 2023;23(1)
- [CrossRef] [Google Scholar]
- Determination of dinotefuran and its metabolites in orange pulp, orange peel, and whole orange using liquid chromatography-tandem mass spectrometry. J. AOAC Int.. 2017;100(5):1551-1558.
- [CrossRef] [Google Scholar]
- Degradation of dimethachlon by a newly isolated bacterium paenarthrobacter sp. strain JH-1 relieves its toxicity against chlorella ellipsoidea. Environ. Res.. 2022;208:112706
- [CrossRef] [Google Scholar]
- Removal of dimethachlon from soils using immobilized cells and enzymes of a novel potential degrader providencia stuartii JD. J. Hazard. Mater.. 2019;378:120606
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2024.106030.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







