Translate this page into:
In silico, in vitro antioxidant and density functional theory based structure activity relationship studies of plant polyphenolics as prominent natural antioxidants
⁎Corresponding author. rammohan4ever@gmail.com (Aluru Rammohan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
In the present study, different extracts of stem bark of Shorea tumbuggaia and roots of Syzygium alternifolium were screened for total phenolic content (TPC), total flavonoid content (TFC) and in vitro DPPH radical scavenging activity to isolate the natural antioxidants. The bioassay-guided fraction of stem bark of Shorea tumbuggaia yielded three compounds, piceatannol (ST-1), resveratrol-12-C-β-d-glucopyranoside (ST-2) and hopeaphenol (ST-3). Similarly, a systematic phytochemical examination of extracts of Syzygium alternifolium roots had resulted five compounds, 5,7,8,5′-tetramethoxy-3′,4′-methylenedioxyflavone (SA-1), 5-hydroxy-4′,7-dimethoxy-6,8-di-C-methylflavone (SA-2), quercetin 7-methylether (SA-3), kaempferol 7,4′-dimethylether 3-O-β-d-glucopyranoside (SA-4) and taxifolin 3-O-α-l-rhamnopyranoside (SA-5). Structures of the isolates were established using UV, IR, Mass, 1H and 13C NMR spectral studies. Density Functional Theory (DFT) calculations, Molecular docking and Lipinski rule of five were conducted to explored the antioxidant activity of isolated compounds. SA-3 (37.52 µg/mL) and ST-1 (42.43 µg/mL) showed highest IC50 values compared to the Ascorbic acid (45.97 µg/mL) and have highest electron affinities (EA eV) along with smallest HOMO-LUMO energy gap. Molecular docking and binding affinity studies with NADPH and SPSB2 proteins, revealed the prominent antioxidant activity of SA-3 and ST-1 with molecular interaction besides promising solvation energies. Hence, the two compounds maybe useful for the treatment of oxidative damage related diseases.
Keywords
Antioxidants
DFT
Flavonoids
Radical Scavenging activity
Shorea tumbuggaia
Syzygium alternifolium
1 Introduction
Imbalance cell redox reactions are the origin for the formation of free radicals or reactive oxygen species (ROS) such as superoxide (O-•), peroxide (•OOH), singlet oxygens (O2), hydroxyl radical (•OH) and nitic oxide (NO•) in human tissue cells during the metabolic process. In addition, food habits, life style process and environmental factors are may be other reasons for over production of ROS (Snezhkina et al., 2019; Zhou et al., 2016). These ROS in high concentration may capable to oxidize biomolecules such as proteins, fats, lipids, carbohydrates and nucleic acids which are present in human organism (Pelaez-Jaramillo et al., 2019; Young and Woodside, 2001). Conversely, lead to endogenous oxidative stress related disease such as hypertension, cancers, digestive ulcers, rheumatoid arthritis, non-inflammatory cancers, cardiovascular risks, aging and neurological disorders (Hertog, 1993; Nita and Grzybowski, 2016; Reaven and Witztum, 1996). Antioxidants are the substances which sustaining the balanced metabolic process by inhibiting detoxification chains of ROS (Anbudhasan et al., 2014). Consumption of dietary foods like vegetables, nuts, fruits, beverages, tea, coffee beans, leafy vegetables and traditional medicinal herbs which are rich in natural antioxidants can prevent such kinds of oxidative damages related diseases (Ariza et al., 2018; Battino et al., 2019; Pietta, 2000). Several synthetic drugs are also available in the pharmaceutical market to treat these oxidative damage related diseases, but they may result several adverse effects on human health due to their inherent toxicity (Saito et al., 2003). Hence, novel antioxidants are needed to identify from the natural source owing to prominent role in human life.
In recent years, the discovery and development of natural antioxidants from traditional plant sources is a great deal of attention due to their prominent role in the control or prevention of oxidation process and associated diseases (Battino et al., 2019; Rajaram et al., 2019). Plants are rich source of polyphenolics such as flavonoids, anthocyanins, pro-anthocyanins, carotenoids, catechins, caffeats, cinnamic acid derivatives, chalcones, stilbenes, tocopherols and certain types of nitrogenous compounds which enhance the quality of life through antioxidant principles (Li et al., 2014; Velioglu et al., 1998). Flavonoids are major plant constituents and few are reported as versatile natural antioxidants such as chalconaringenin, naringenin, kaempferol and quercetin derivatives, xanthohumol, isoxanthohumol, ginko flavone glycoside, silymarin, catechin and anthocyanin derivatives (Cao et al., 1997; Kashima, 1999). In view of the importance of medicinal plants and their phytoconstituents, the present work intended to the isolation of potent antioxidants from rare, endemic and endangered medicinal plants namely Shorea tumbuggaia (Dipterocarpaceae) and Syzygium alternifolium (Myrtaceae). These plants were widely used in the traditional medicine and showed interesting biological properties, for example, ulcers, rheumatic pain (Babu et al., 2016), antihyperglycemic activity (Kasetti et al., 2012), dysentery, diarrhea and cholera (Chitravadivu et al., 2009).
2 Materials and methods
2.1 Chemicals
Chemicals and organic solvents with analytical grade were purchased from Merck and used without further purification. Thin Layer Chromatography (TLC) was performed by using precoated silica gel 60 F254 plates. Column chromatography was executed on silica gel (Acme) finer than 200 mesh (0.08 mm thickness). Melting points were determined using a Kofler hot stage apparatus and are uncorrected. EI mass spectra were obtained on Nermag R10-10 mass spectrometer, ESI-TOFMS and ESI-MS/MS spectra were recorded in positive mode on an API Q-STAR PULSA i of Applied Biosystems. UV spectra were measured in MeOH on a Shimazdu UV-1800 spectrophotometer. IR spectra on a Bruker AlphaEco ATR-FTIR spectrophotometer, and 1H and 13C NMR spectra on Bruker Avance spectrometer operating at 400, 300 for 1H and 100, 75 MHz for 13C, respectively using CDCl3, DMSO‑d6 and Me2CO-d6 with TMS as an internal standard. The UV absorbance was determined by using a Techcomp VIS-7200A visible spectrophotometer.
2.2 Plant materials
The stem bark of Shorea tumbuggaia and roots of Syzygium alternifolium were collected from Seshachalam hill ranges of Andhra Pradesh, India and identified by Dr. K. Madhava Chetty, Assistant Professor and plant taxonomist, Department of Botany, Sri Venkateswara University, Tirupati, India and where voucher specimens (DG-111B & 117) are deposited.
2.3 Preparation of crude extracts for preliminary antioxidant activity studies
50.0 g of shade dried stem bark of Shorea tumbuggaia and roots of Syzgium alternifoium powder material were taken in the thimble and are introduced into the Soxhlet apparatus and then extracted with 500 mL of hexane, acetone and methanol, successively. On evaporation of the solvent yielded the crude extracts. The crude extracts of hexane, acetone and methanol were screened for a) Total Phenolic Content (TPC) b) Total Flavonoid Content (TFC) and c) DPPH radical scavenging activity for their in vitro antioxidant activity.
2.4 Estimation of total phenolic content (TPC)
TPC of the plant extracts was determined using spectrophotometric method (Singleton et al., 1999). To the 1 mL of each plant extract (1 mg/mL), 1 mL of Folin-Ciocalteu’s reagent was added followed by addition of 10 mL of 7% Na2CO3 and 13 mL of sterilized double distilled water. The total mixture was incubated at 25 °C in the dark for about 90 mins. Subsequently, the absorbance was measured at 760 nm and the results were expressed as milligrams of gallic acid equivalents (GAE) per gram of dried sample. The absorbance of all the samples was evaluated in three replications.
2.5 Estimation of Total Flavonoid Content (TFC)
TFC of the plant extracts was resolute with colorimetric method using quercetin as standard (Kim et al., 2003; Rammohan et al., 2015). In this method, 1 mL of each plant extract (1 mg/mL) and 4 mL of distilled water placed in a 10 mL volumetric flask. At zero time, 0.3 mL of 5% NaNO2 and 0.3 mL of 10% AlCl3·6H2O was added. After 5 min, 2 mL of 1 M NaOH was added to the mixture and then make the total volume up to 10 mL with sterilized double distilled water. The absorbance of well mixed sample solutions was measured at 510 nm and the results were stated as milligrams of quercetin equivalents (QE) per gram of dried sample. The absorbance of the experimental samples was measured in three replications.
2.6 DPPH radical scavenging assay
Free radical scavenging activity of plant extracts and the isolated compounds were carried out by using 1, 1-diphenyl-2-picrylhydrazyl radical (DPPH) assay (Morita et al., 2017). 1 mL of test sample with various concentrations (20, 40, 60, 80 & 100 µg/mL) was added to 2.0 mL of 0.1 mM DPPH dissolved in methanol. The mixture was vortexed for 1 min and then incubated for 30 min at room temperature. After incubation time, the absorbance was measured at 517 nm with ascorbic acid as the standard. The radical scavenging activity (RSA) was calculated using the equation where Ac is the absorbance of the control and As is the absorbance of the sample.
2.7 Extraction and isolation of chemical constituents
The shade dried and powdered stem bark (1.8 kg) of S. tumbuggaia was exhaustively extracted with n-hexane, acetone and methanol at room temperature. The hexane extract has not been taken up for further examination as it didn’t show any significant TPC content. The acetone extract (60 g) and the methanol extract (90 g), which have significant phenolic content (TPC) were found to be similar on TLC and hence combined and evaporated in vacuo to give a brownish yellow residue (150 g). It was chromatographed on a silica gel column eluting with n-hexane-ethyl acetate step gradient mixtures. The n-hexane-ethyl acetate, 4:6, 3:7 and 1:9 eluates on concentration afforded three brown solids, were designated as ST-1, ST-2 and ST-3, respectively.
The roots (2.1 kg) of S. alternifolium were shade dried, powdered and exhaustively extracted with n-hexane, acetone and methanol. The hexane extract on concentration under reduced pressure yielded a pale-yellow syrupy residue (10 g) and was purified over a silica gel column using n-hexane-ethyl acetate step gradient mixtures as eluents. The hexane-ethyl acetate (7:3) eluent on concentration yielded a white solid (20 mg) which on crystallization from chloroform gave colourless needles (15 mg) designated as SA-1. The acetone extract was concentrated under reduced pressure to yield a dark green coloured residue (35 g) and it was purified over a silica gel column using n-hexane-ethyl acetate step gradient mixtures. The n-hexane-ethyl acetate eluates 8:2 and 6:4, on concentration afforded two yellow solids which on crystallization from methanol gave pale yellow needles (17 mg) and crystals (18 mg). It was designated as SA-2 and SA-3, respectively. The methanol extract was concentrated under reduced pressure to yield a greenish yellow viscous residue (57 g). It was purified over a silica gel column using n-hexane-ethyl acetate step gradient mixtures. The n-hexane-ethyl acetate eluates 7:3 and 1:1, on evaporation gave two yellow solid which on crystallization from methanol resulted in yellow crystals (17 mg) and colourless needles (15 mg). It was designated as SA-4 and SA-5, respectively.
2.8 Density Functional Theory (DFT) studies
In order to explain the antioxidant activity of all the isolated compounds, the electronic parameters such as Molecular Electrostatic Potential (MEP), HOMO (Highest Occupied Molecular Orbital), LUMO (Lowest Unoccupied Molecular Orbital), their energy gap (ΔE), Ionization Potential (IP) and Electron Affinity (EA) were calculated by using DFT method. The theoretical calculations were performed using the GAMESS-US (Noro et al., 2012; Reddy et al., 2016) line up package with B3LYP (Becke’s three-parameter exchange potential and the Lee-Yang-Parr correlation functional) with standard basis set 6-311G (d,p) and the lowest energy geometry were obtained using the semi-empirical method PM3. The electronic structures along with spin density distribution of HOMO and LUMO were presented in color-coded surface which gives the location of positive and negative electrostatic potentials. The electronic properties were computed and validated using Koopman′s theorem (1934). According to this theorem, IA = −EHOMO, ionization potential is negative to HOMO energy orbital which means to remove an electron from highest occupied orbital. In other way, the capability of HOMO orbital to reduce the free radicals by donating electron. Similarly, electron affinity is computed as, EA = −ELUMO i.e. which means the ability of LUMO orbital to capture a free radical. The energy gap ΔE is the difference between HOMO-LUMO orbitals of the isolated compounds, which specify the capacity of molecule involved in the free radical optimization mechanism (Borges et al., 2015). If the ΔE is small the intramolecular charge transfer is easy, and the molecule participate effectively in radical scavenging reaction (Islam, 2015).
2.9 Protein preparation
Crystal structures of NADH (PDBID: 1k4u) (You et al., 2017) and SPSB2 (PDBID: 3emw) (Kami et al., 2002) were retrieved from Protein Data Bank (PDB). Co-crystal ligands, water and ions were removed and hydrogens were added, energy minimization was employed using steepest descent algorithms (Guex and Peitsh, 1997).
2.10 Ligand preparation
Isolated flavonoids were drawn and 3D optimized, hydrogens were added and energy minimized using MarvinSkech. The refined and energy minimized molecules were used for docking studies. Lipinski rule was computed using Molsoft molecular property prediction.
2.11 Molecular docking
AutoDock Vina 4.05 was used to dock into the active site of target proteins (Trott and Olson, 2010; Wolf, 2009). Ligand molecules were uploaded and energy minimized with universal force field using conjugate-gradient algorithm with 200 run iterations and converted as PDBQT files. Docking grid box prepared and positioned to cover the binding pockets according to the location of key residues. The grid box and size were followed: for SH3 domain was center (X) = −1.46251269576, center (Y) = 5.26906821151, center (Z) = −1.16382932524, size (X) = 26.1067726284, size (Y) = 19.4079249122, size (Z) = 26.5021901293, for SPSB2 was center (X) = 11.4873857579, center (Y) = 28.5127526796, center (Z) = 55.8625055939, size (X) = 23.7570462719, size (Y) = 24.9365557258, size (Z) = 20.4731834919 and exhaustiveness was set at 8. Lamarckian genetic algorithm was used and docking parameters were set as follows: the number of individuals in the population was 150, maximum number of energy evaluations was 25,000, maximum number of generations was 27,000, top individual to survive to next generation was 1, gene mutation rate was 0.02, crossover rate was 0.8, Cauchy beta was 1.0 and, genetic algorithm window size was set to 10.0. The best docked ligand conformations were saved, and the bond angles, bond lengths, and hydrogen bonding.
2.12 Born interaction energies and binding affinities
Generalized Born/Volume Integral (GB/VI) implicit solvent methods implemented in MOE determined binding affinities of lead molecules (Bhaskar et al, 2016). Van Der Waals, Coulomb electrostatic interactions and implement solvent interactions are termed as Born interaction energy which determined non-bonded interactions between the ligands and the protein. During the binding affinity assessment, ignored draining energies, solvent molecules of the ligand and the protein. London scoring method was represented in kcal/mol unit for the binding affinity calculations. The binding pocket residues and the ligands were kept as flexible where binding pocket was kept as rigid to tether restraints to discourage gross movements. Energy minimization for the protein-ligand complex was employed and calculated binding affinity.
3 Statistical analysis
All the experiments were performed in triplicate (n = 3). The Statistical data were analyzed by using ANOVA software and expressed as mean ± SD. The values p < 0.05 were considered as significant.
4 Results
4.1 TPC and TFC studies
The phenolic content may contribute directly to the antioxidant activity. The total phenolic content in n-hexane, acetone and methanol extracts of Shorea tumbuggaia and Syzygium alternifolium were determined spectrophotometrically according to the Folin–Ciocalteu method and calculated as gallic acid equivalents (GAE). Total phenolic content of acetone (STA) and methanol (STM) extracts of S. tumbuggaia are 62.56 ± 0.9 and 64.67 ± 0.8 µg/mL of gallic acid equivalents, respectively. The result shows that the phenolic content present in both acetone and methanol extracts are significant and almost similar. In the case of S. alternifolium, it was found that the acetone extract has the higher TPC (SAA, 84.39 ± 1.2 µg/mL of GAEs), followed by methanol extract (SAM, 54.63 ± 1.2 µg/mL of GAEs) and hexane extract (SAH, 44.33 ± 1.4 µg/mL of GAEs). TFC of extracts of S. tumbuggaia and S. alternifolium is presented in Fig. 1, and measured in catechin equivalence (CE). Highest flavonoid content was found in SAA (132.28 ± 1.6 µg/mL) followed by STM (62.47 ± 1.1 µg/mL) whereas lowest content (10.21 ± 1.5 µg/mL) was found in STH. The extras STA, SAM and SAH showed moderate TFC results in 56.55 ± 1.2, 56.45 ± 0.9 and 41.14 ± 1.6 µg/mL, respectively. Comparison of both plant extracts there is no correlation observed between TPC and TFC results. Among all extracts, STH extract showed least TP and TF contents, and hence did not consider for further work.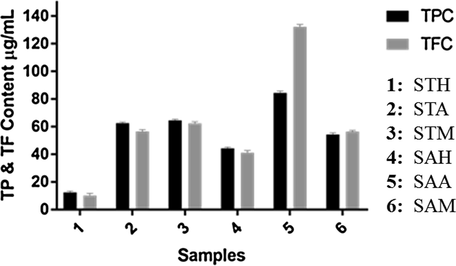
Total phenol content and total flavonoid content in different extracts of Shorea tumbuggaia (ST) and Syzygium alternifolium (SA).
4.2 Free radical scavenging activities and isolation of bioactive compounds
The DPPH free radical is a stable free radical, which has been widely accepted as a tool for estimating free radical-scavenging activities of antioxidants (Cao et al. 1996). The radical-scavenging activities of the acetone and methanol extracts of the stem bark of S. tumbuggaia (STA & STM) and, n-hexane (SAH), acetone (SAA) and methanol (SAM) of S. alternifolium roots were estimated by comparing the percentage inhibition of formation of DPPH radicals with that of vitamin C shown in Fig. 2A. The antioxidant potential of extracts in DPPH assay linearly correlated to its total phenolic compounds. It is found that the antioxidant activity of S. alternifolium root extracts increased proportionally to the phenolic content with a linear relationship between DPPH values. And the high level of antioxidant capacity is may be due to the maximum amount of total phenolic contents (TPC). In view of the presence of significant amount of phenolic contents and DPPH radical scavenging activities of extracts, except the n-hexane extract (STH) of S. tumbuggaia remain all the five extracts were considered for further phytochemical examination individually.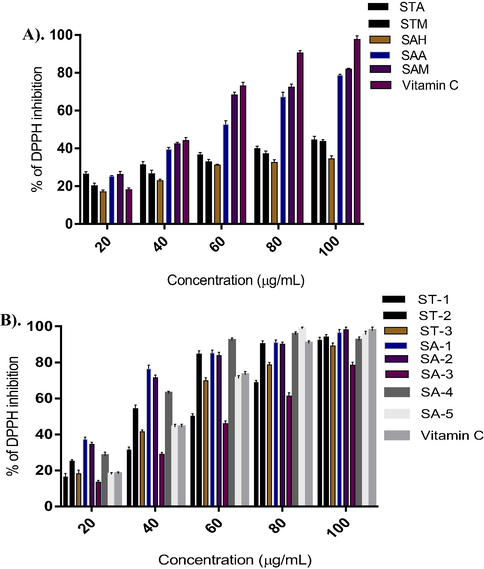
(A) DPPH radical scavenging activity of different extracts; (B) isolates of Shorea tumbuggaia (ST) and Syzygium alternifolium (SA) compared with standard Vitamin C.
The TPC result shows that the phenolic content present in acetone and methanol extracts (STA and STM) of Shorea tumbuggaia are significant and almost similar. The TLC profile of both extracts was also found to be same and hence both extracts combined, and purification resulted in three compounds, piceatannol (ST-1), resveratrol-12-C-β-d-glucopyranoside (ST-2) and (+)-hopeaphenol (ST-3). Similarly, a systematic phytochemical examination of extracts of Syzygium alternifolium roots had resulted in five compounds, 5,7,8,5′-tetramethoxy-3′,4′- methylenedioxyflavone (SA-1), 5-hydroxy-4′,7-dimethoxy-6,8-di-C-methylflavone (SA-2), quercetin 7-methylether (SA-3), kaempferol 7,4′-dimethylether 3-O-β-d-glucopyranoside (SA-4) and taxifolin 3-O-α-l-rhamnopyranoside (SA-5). The structures (Fig. 3) of the all the isolated compounds were established using mass, 1H and 13C NMR spectral studies.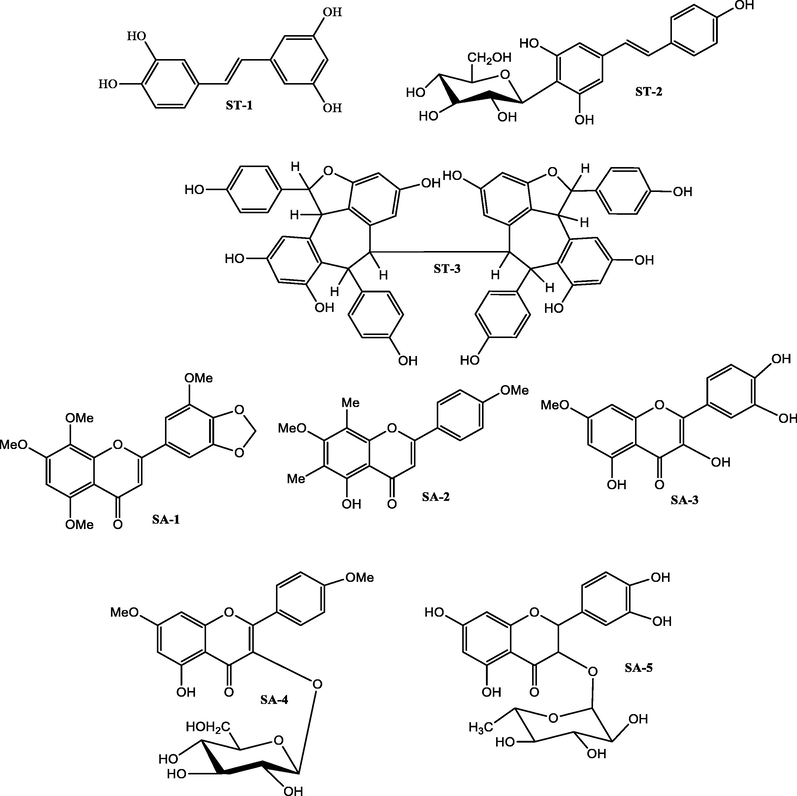
Isolates from the stem bark of Shorea tumbuggaia (ST 1–3) and roots of Syzygium alternifolium (SA 1–5).
The antioxidant activity of the Isolates from Shorea tumbuggaia (ST) and Syzygium alternifolium (SA) was evaluated by DPPH radical scavenging activity with vitamin C as standard as described earlier for the crude extracts (Vide supra), and the results were presented in Fig. 2B. The IC50 values of the isolates from Shorea tumbuggia (ST) showed the highest antioxidant activity for ST-1 followed by ST-3 (tetrameric stilbene) and ST-2 (stilbene derivative) with IC50 values 42.43, 60.16 and 70.47 in μg/mL respectively. The antioxidant activity of piceatannol (ST-1) may be due to the existence of two phenol rings in conjugation with a double bond and ortho dihydroxy phenolic system. The significant antioxidant activity observed for hopeaphenol (ST-3) may be due to the presence of several phenolic moieties which gives stability for the molecular structure. The antioxidant activity associated with reservetrol glycoside (ST-2) may be due to the presence of ortho-dihydroxy system in the glycosidic portion of the molecule. From the Table 1, it is evident that the flavonoids, SA-3 and SA-5, the isolates from Syzygium alternifolium (SA) showed outstanding antioxidant activities with IC50 values (37.52 & 55.33 μg/mL) compared to that of other compounds. The possible polyphenolic oxidation mechanism pathways by DPPH radical shown in Fig. 4.
S.No
Compound
IC50 (μg/mL)
1.
Ascorbic acid
45.97
2.
Picetannol (ST-1)
42.43
3.
Resveratrol-12-C-β-d-Glucopyranoside (ST-2)
70.47
4.
Hopeaphenol (ST-3)
60.16
5.
5,7,8,5′-tetramethoxy-3′,4′-methylenedioxy flavone (SA-1)
100.65
6.
5-hydroxy-4′,7-dimethoxy-6,8-di-C-methyl flavone (SA-2)
95.29
7.
Quercetrin-7-O-methyl ether (SA-3)
37.52
8.
Kaempferol-7,4′-dimethylether-3-O-β-d-glucopyranoside (SA-4)
78.37
9.
Taxifolin3-O-α-l-rhamnopyranoside (SA-5)
55.33
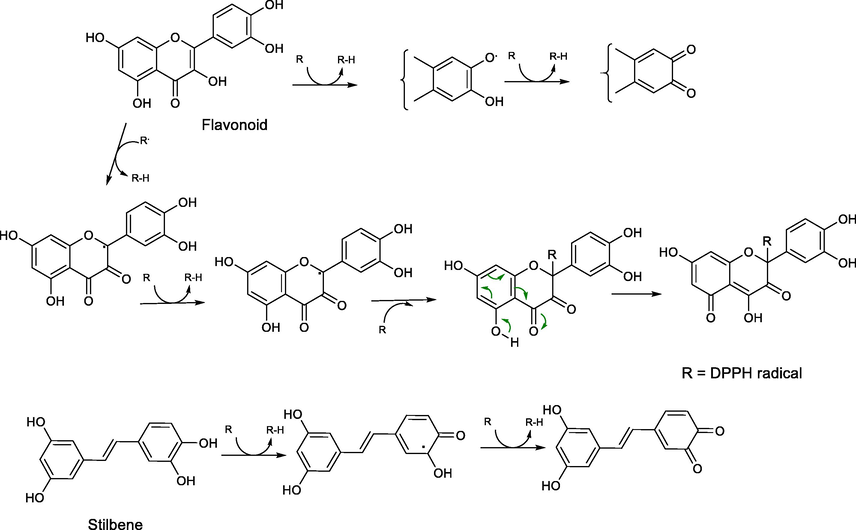
The possible polyphenolic oxidation mechanism pathways by DPPH radical.
4.3 Theoretical and structure activity relationship studies
DFT studies were carried out to attain a better understanding of the antioxidant activity of isolated compounds of plants. The HOMO, LUMO and MEP structures of all the isolated compounds were measured. In addition, the ability of compounds (ST 1–3 and SA 1–5) to transfer hydrogen was calculated in terms of electronic parameters and the results were shown in Table 2. Among the isolated plant phenolics, the compounds ST-1 and SA- 3 were showed smallest HOMO-LUMO energy gap 3.80 and 3.92 eV, respectively. Furthermore, these two compounds have highest electron affinities 1.79 and 1.74 eV, respectively which representing its strongest potential as prominent antioxidants by single electron transfer reactions (in terms of hydrogen free radical donation). Also, SA-1 and SA-5 are the two another flavonoids have moderate antioxidant efficacy owing to finest HOMO-LUMO energy gaps 4.17 and 4.33 ev, respectively with significant electron affinities. Hence, the theoretical result showed that the general decreasing antioxidant trends as follows: ST-1 > SA-3 > SA-1 > SA-5 > SA-2 > ST-3 > SA-4 > ST-2.
Compound
HOMO (eV)
LUMO (eV)
ΔE (eV)
IP (eV)
EA (eV)
ST-1
−5.54
−1.74
3.80
5.54
1.74
ST-2
−5.40
−0.57
4.83
5.40
0.57
ST-3
−5.36
−0.75
4.60
5.36
0.75
SA-1
−5.94
−1.76
4.17
5.94
1.76
SA-2
−5.91
−1.68
4.24
5.91
1.68
SA-3
−5.71
−1.79
3.92
5.71
1.79
SA-4
−6.22
−1.49
4.73
6.22
1.49
SA-5
−5.84
−1.51
4.33
5.84
1.51
4.4 Molecular docking results
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase possesses five cytosolic soluble SH3 domains, some of which implicated in the regulation of oxidase assembly and activity. Of these, Src homology 3 (SH3) domains is presented at the C terminal that specifically associated with PxxP motif of its partner protein as protein-protein interaction and activates the oxidases. Likewise, inducible nitric oxide synthase (iNOS) is one of the isoforms of nitric oxide synthase (NOS) that generates high level of endogenous nitric oxide (NO) during conversion of l-arginine to l-citrulline. SPRY domain is comprising SOCS box protein 2 (SPSB2) which act as negative regulator, binds with iNOS and maintain the lifetime and NO production. However, in this study, we conducted docking studies with SH3 domain and SPSB2 for isolated compounds. In addition, GB/VI interaction energies and binding affinities were calculated that reveal binding energy, binding affinity and critical interactions of leads molecules (Tables 3 and 4). Docking studies exhibited that SA-4, ST-1, SA-2, SA-1 and SA-3 have shown eloquent binding energies of −6.9 kcal/mol, −6.6 kcal/mol, −6.5 kcal/mol, −6.2 kcal/mol and −6.2 kcal/mol and explicated critical interactions with pocket residues of SH3 domain, respectively. SA-4 displayed four bonds, arene-H bond and one polar bond between C ring and Lys502, glucopyranoside formed three bonds with Ala470 and Glu477. ST-1 is stilbenoid which displayed seven interactions, 3 and 4-dihydroxy phenol formed two bonds with Pro473 and Asp475, and one arene-H bond with Glu477. 3 and 5-dihydroxy phenyl exhibited four bonds such as 3-OH bond with Glu477, 5-OH bond with Gln479 and Lys500, and arene-H bond with Phe478. SA-2 formed two bonds such as C ring with Glu477 and A ring with Lys502. SA-1 formed one key arene bond via C ring with Glu477. SA-3 exhibited four bonds: A ring formed arene-H bond with Glu477, O of C ring bonds with Lys502, 3 OH and 4 OH of B ring bonds with Ser499 and Asp482 (Fig. 5). While, in case of SPSB2, SA-1 conferred two bonds such as C ring exhibits one arene-H bond with Trp207 and one bond with Gln209 (Fig. 6). SA-2 showed one arene-H bond via ring A with Trp207. SA-3 exhibited two arene-H bonds with Trp207. ST-3 displayed two bond interactions with Val71 and Gly208.
Compounds
Interaction
Biding energy (kcal/mol)
Binding affinity (kcal/mol)
RMSD (Å)
Strain (kcal/mol)
Solvation (kcal/mol)
Protein–Ligand
SA-1
Glu477—arene
−6.2
−6.1
0.6
3.5
−19.7
SA-2
Gu477—arene
Lys502—arene−6.5
−5.3
0.1
1.6
−17.9
SA-3
Glu477—arene
Asp482—OH
Ser499—OH−6.2
−4.5
0.4
12.5
−15.5
SA-4
Ala470—OH
Glu477—OH
Glu477—OH
Lys502—O
Lys502—arene−6.9
−5.4
0.7
34.2
−13.7
SA-5
Glu472—OH
Glu474—OH−5.8
−3.9
1.0
16.1
−13.1
ST-1
Pro473—OH
Asp475—OH
Glu477—arene
Glu477—OH
Phe478—arene
Gln479—OH
Lys500—OH−6.6
−4.6
0.3
14.4
−21.0
ST-2
Glu469—OH
Thr471—OH
Asp475—OH
Asn491—OH−5.7
−4.1
1.2
29.0
−16.7
ST-3
Glu474—OH
Asp475—OH
Glu496—OH−5.3
−2.0
0.1
13.5
−6.7
Compounds
Interaction
Biding energy (kcal/mol)
Binding affinity (kcal/mol)
RMSD (Å)
Strain (kcal/mol)
Solvation (kcal/mol)
Protein–Ligand
SA-1
Trp207—arene
Gln209—O−6.4
−4.9
0.5
3.8
−16.8
SA-2
Trp207—arene
−6.0
−4.4
0.3
2.5
−14.4
SA-3
Trp207—arene
Trp207—OH−6.4
−4.8
0.1
1.4
−16.8
SA-4
Pro70—OH
Tyr20—O−5.7
−4.7
0.7
11.1
−17.3
SA-5
Pro70—OH
Gly208—OH−5.7
−4.7
0.2
8.5
−17.3
ST-1
Pro70—OH
Gly208—OH−5.5
−5.0
0.3
3.6
−16.5
ST-2
Thr102—arene
Val206—OH
Gly208—OH−5.7
−4.4
0.2
6.2
−14.6
ST-3
Val71—arene
Val71—OH−6.0
−6.5
0.5
9.4
−26.4
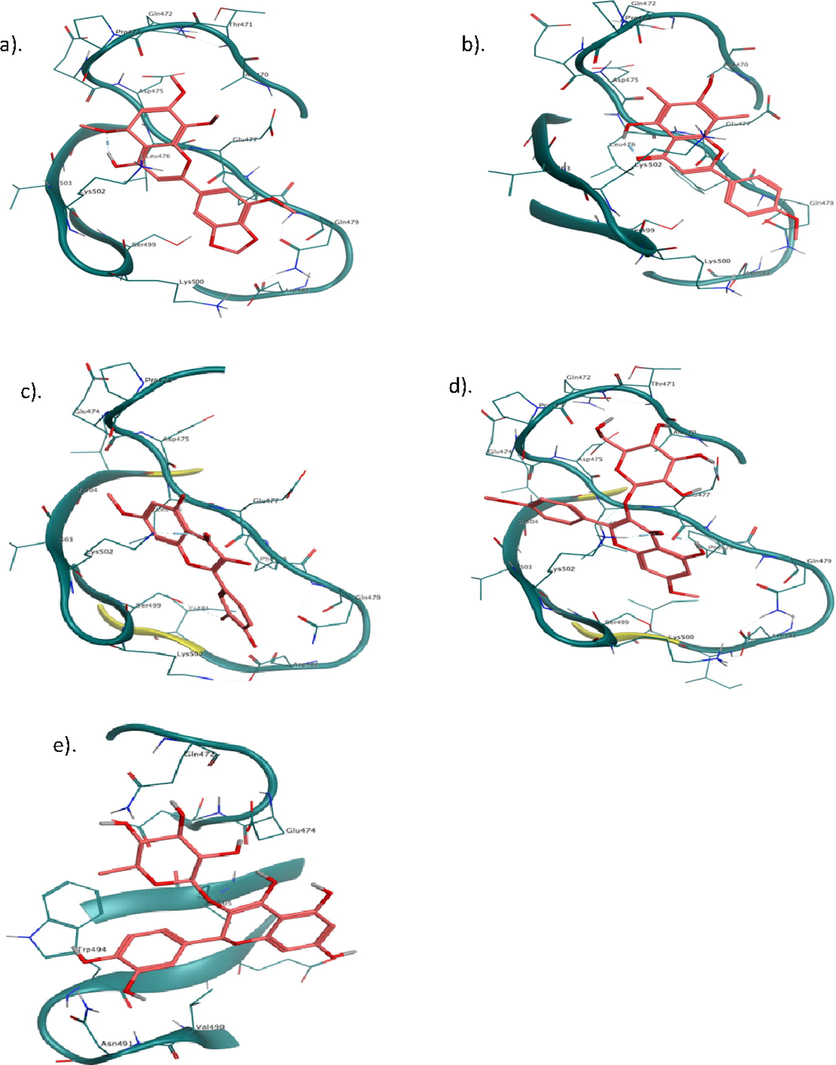
Binding interactions of isolated compounds (a) SA-1, (b). SA-2, (c) SA-3, (d) SA-4 and (e) ST-1 with active site residues of SH3 domain of NADH.
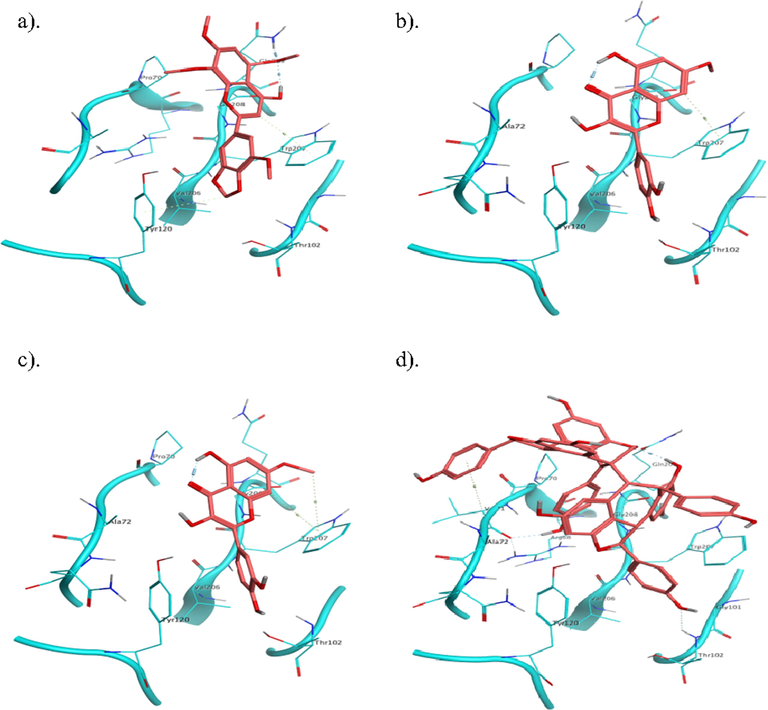
Binding interactions of isolated compounds (a) SA-1, (b) SA-2, (c) SA-3 and (d) ST-3 with active site residues of SPSB2.
4.5 Bioavailability
The relationship between physiochemical and pharmacokinetic properties were measured using Lipinski rule of five (molecular weight < 1000, H-donor (<10), H-acceptor (<12) and cLogP (<10) and the results were significant (Table S4). Lipinski rule of five revealed all the isolated plant phenolics showed molecular weight was found to be less than 500 (except ST-3) and LogP was found to be below five, hydrogen bond donors and hydrogen bond acceptors also were obeyed. These properties disclosed ADME properties that are beneficial to understand the drug mechanism within the cell environment, for instance, blood brain barrier permeability and interaction with different isoform cytochromes. Thus, the results of Lipinski rule recommended that these polyphenolics could be beneficial as antioxidant and endorsed for further in vivo studies.
5 Discussion
On comparison of Simulation results and the molecular docking parameters with the DPPH experimental results, the radical scavenging activities of isolated compounds were some deviations to the order in relation to hydrogen transfer due to several factors including solubility, polarizability, resonance effects and inductive effects. The capacity of polyphenolics to act as antioxidants depends on their molecular structure, O-H group in specific position on the flavonoid core and how fast it releases of hydrogen radical to involve in radical scavenging activity (Cai et al., 2014; Rice-Evans et al., 1996). The high antioxidant activity of the flavonoid (SA-3) and piceatannol (ST-1) may be attributed to the highly significant contribution of 3′,4′- dihydroxyl groups on the B-ring as well as 3-hydroxy group on the C-ring in SA-3 (Rice-Evans et al., 1996) and the 3,4-diydroxyl groups in conjugation of whole molecule in ST-1 (Piotrowska et al., 2012). In addition, it may thus be assumed that 3′-OH and 4′-OH are primarily involved in H-atom transfer reactions to DPPH in SA-3; and/or that each OH group strongly facilitates H-abstraction from the other by stabilizing the corresponding radical by a combination of electronic and H-bonding effects. This was further supported by the distribution of HOMO orbital in SA-3 (in ring-B and C) and ST-1 (Fig. 7), which denotes the strong antioxidant capabilities (Clare, 1995).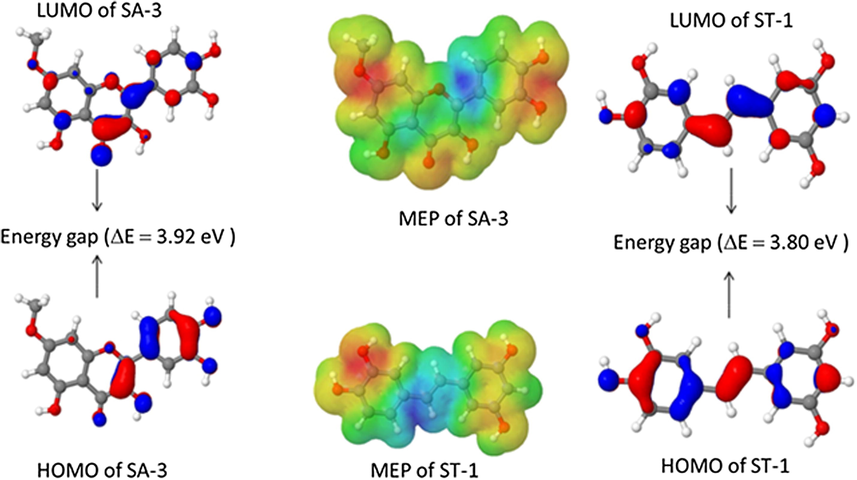
Frontier molecular orbitals of SA-3 and ST-1.
Free radical scavenging activity of dihydroflavonol glycoside i.e., taxifolin 3-O-α-l-rhamnopyranoside (SA-5) was lesser than that of SA-3 as the 3-hydroxyl group in SA-5 was blocked due to glycosylation. In addition, the double bond at C2 and C3 in the C ring is absent, and the protection of the hydroxyl group(s) with sugar moieties are responsible for the lesser free radical-scavenging activity than SA-3 (Burda and Oleszek, 2001). This is further evidenced by the distribution of HOMO orbital only on ring B of SA-5. In case of tetrameric stilbenes ST-3, there is no specific theoretical parameter which supports the experimental results, but it is assumed that the significant antioxidant is may be due to polyphenolic moieties and the stable molecular structure. The less potent antioxidant activity of SA-4, SA-1 and SA-2 in comparison with that of SA-3 and SA-5 must be associated with the absence of ortho-dihydroxyl and 3-OH groups in the former three flavonoid derivatives. These three compounds (SA-4, SA-1 and SA-2) exhibited moderate activities as free-radical scavengers in the DPPH assay, in comparison with reference antioxidant (vitamin C) because of the presence of double bond at C2 and C3 in the C ring and polyoxygenated flavonoid moiety/moieties which have capability to bind free radicals.
6 Conclusions
The two isolates viz., quercetin 7-O-methyl ether (SA-3) and piceatannol (ST-1), showed potent antioxidant properties. Whereas the isolates taxifolin-3-O-α-l-rhamnopyranoside (SA-5) and hopeaphenol (ST-3), showed moderate radical scavenging activity when compared to the other isolates. Hence, the two isolated plant phenolics SA-3 and ST-1, can be explored for their applications in the prevention of free radical related diseases viz., hypertension, anti-inflammatory, cancer, rheumatoid arthritis, diabetes, vascular diseases and hyperplasic disease associated with oxidative damage.
Acknowledgements
The Author AR is thankful to the Ural Federal University, Russia for providing laboratory facilities. The Author DG was grateful to the University Grants Commission (UGC), New Delhi for financial assistance under UGC-MRP programme.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
References
- Bioaccessibility and potential bioavailability of phenolic compounds from achenes as a new target for strawberry breeding programs. Food Chem.. 2018;248:155-165.
- [Google Scholar]
- Development of novel HER2 inhibitors against gastric cancer derived from flavonoid source of Syzygium alternifolium through molecular dynamics and pharmacophore-based screening. Drug Des. Devel. Ther.. 2016;10:3611.
- [Google Scholar]
- Relevance of functional foods in the Mediterranean diet: the role of olive oil, berries and honey in the prevention of cancer and cardiovascular diseases. Crit. Rev. Food Sci. Nutr.. 2019;59(6):893-920.
- [Google Scholar]
- Antibacterial efficacy of fractions and compounds from Indigofera barberi: identification of DNA gyrase B inhibitors through pharmacophore based virtual screening. Proc. Biochem.. 2016;51(12):2208-2221.
- [Google Scholar]
- The antioxidant properties of salicylate derivatives: a possible new mechanism of anti-inflammatory activity. Bioorg. Med. Chem. Lett.. 2015;25(21):4808-4811.
- [Google Scholar]
- Antioxidant and antiradical activities of flavonoids. J. Agri. Food Chem.. 2001;49(6):2774-2779.
- [Google Scholar]
- Characterization and density functional theory study of the antioxidant activity of quercetin and its sugar-containing analogues. Eur. Food Res. Technol.. 2014;238(1):121-128.
- [Google Scholar]
- Antioxidant capacity of tea and common vegetables. J. Agri. Food Chem.. 1996;44(11):3426-3431.
- [Google Scholar]
- Antioxidant and prooxidant behavior of flavonoids: structure-activity relationships. Free Radical. Bio. Med.. 1997;22(5):749-760.
- [Google Scholar]
- Antimicrobial activity of Laehiums prepared by herbal venders, South India. Am. Eur. J. Sci. Res.. 2009;4(3):142-147.
- [Google Scholar]
- Charge transfer complexes and frontier orbital energies in QSAR: a congeneric series of electron acceptors. J. Mol. Struc: Theochem.. 1995;337(2):139-150.
- [Google Scholar]
- SWISS-MODEL and the swiss-PDB viewer: an environment for comparative protein modeling. Electrophoresis. 1997;15:2714.
- [Google Scholar]
- Content of potentially anticarcinogenic flavonoids of tea infusions, wines, and fruit juices. J. Agric. Food Chem.. 1993;41(8):1242-1246.
- [Google Scholar]
- Investigation of comparative shielding of Morin against oxidative damage by radicals: a DFT study. Cogent. Chem.. 2015;1(1):1078272.
- [Google Scholar]
- Diverse recognition of non-PxxP peptide ligands by the SH3 domains from p67(phox), Grb2 and Pex13p. EMBO J.. 2002;21(16):4268-4276.
- [Google Scholar]
- Cinnamic acid as one of the antidiabetic active principle (s) from the seeds of Syzygium alternifolium. Food Chem. Toxicol.. 2012;50(5):1425-1431.
- [Google Scholar]
- Effects of catechins on superoxide and hydroxyl radical. Chem. Pharm. Bull.. 1999;47(2):279-283.
- [Google Scholar]
- Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem.. 2003;81(3):321-326.
- [Google Scholar]
- Uber die Zuordnung von Wellenfunktionen und Eigenwerten zu den einzelnen Elektronen eines Atoms. Physica. 1934;1(1–6):104-113.
- [Google Scholar]
- Research progress of natural antioxidants in foods for the treatment of diseases. Food Sci. Human Wellness. 2014;3(3–4):110-116.
- [Google Scholar]
- Antioxidant capacity of blueberry extracts: peroxyl radical scavenging and inhibition of plasma lipid oxidation induced by multiple oxidants. J. Berry Res.. 2017;7(1):1-9.
- [Google Scholar]
- The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid. Med. Cell. Longev.. 2016;2016
- [CrossRef] [Google Scholar]
- Segmented contracted basis sets for atoms H through Xe: Sapporo-(DK)-nZP sets (n= D, T, Q) Theor. Chem. Acc.. 2012;131(2):1124.
- [Google Scholar]
- Impact of a formulation containing unusual polyunsaturated fatty acids, trace elements, polyphenols and plant sterols on insulin resistance and associated disturbances. Diabetes Ther. 2019:1-17.
- [Google Scholar]
- Biological activity of piceatannol: leaving the shadow of resveratrol. Mutat. Res.. 2012;750(1):60-82.
- [Google Scholar]
- Plant-based dietary patterns, plant foods, and age-related cognitive decline. Adv. Nutr.. 2019;10(Supplement 4):S422-S436.
- [Google Scholar]
- Structure elucidation and antioxidant activity of the phenolic compounds from Rhynchosia suaveolens. Nat. Prod. Commun.. 2015;10(4) 1934578X1501000418
- [Google Scholar]
- Oxidized low-density lipoproteins in atherogenesis: role of dietary modification. Annu. Rev. Nutr.. 1996;16(1):51-71.
- [Google Scholar]
- Synthesis, antimicrobial activity and advances in structure-activity relationships (SARs) of novel tri-substituted thiazole derivatives. Eur. J. Med. Chem.. 2016;123:508-513.
- [Google Scholar]
- Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med.. 1996;20(7):933-956.
- [Google Scholar]
- Cytotoxicity and apoptosis induction by butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) Anticancer Res.. 2003;23(6C):4693-4701.
- [Google Scholar]
- [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol.. 1999;299:152-178.
- [Google Scholar]
- ROS Generation and antioxidant defense systems in normal and malignant cells. Oxid. Med. Cell. Longev.. 2019;2019
- [CrossRef] [Google Scholar]
- AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem.. 2010;31(2):455-461.
- [Google Scholar]
- Antioxidant activity bon total phenolics in selected fruits, vegetables and grain products. J. Agr. Food Chem.. 1998;46(10):4113-4117.
- [Google Scholar]
- PyRx. C&EN. 2009;87:31.
- Crystal structure of SPSB2 in complex with a rational designed RGD-containing cyclic peptide inhibitor of SPSB2-iNOS interaction. Biochem. Biophys. Res. Commun.. 2017;489(3):346-352.
- [Google Scholar]
- Alcoholic beverage consumption and chronic diseases. Int. J. Environ. Res. Publ. Health. 2016;13(6):522.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2019.12.017.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







