Translate this page into:
In situ enrichment and determination of 6 kinds of caine-type anesthetics in cosmetics and rat serum by thin layer chromatography-Raman spectroscopy
⁎Corresponding author. Suihm_9@163.com (Huimin Sui)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
A simple, economical and selective method has been established for simultaneous detection of 6 kinds of caine-type anesthetics in cosmetics and rat serum by in situ enrichment thin layer chromatography (TLC) and Raman spectroscopy. The target caine-type anesthetics, procaine hydrochloride (Pro), tetracaine hydrochloride (Tet), dibucaine (Dib), mepivacaine hydrochloride (Mep), lidocaine hydrochloride (Lid) and ropivacaine hydrochloride (Rop), were separated by TLC. The original spots (or position of Rf equal to that of the standard) of anesthetics on TLC plates were in situ enriched into much smaller ones driven by solvent from different directions, and followed by Raman measurement for quanlitative and quantitative analysis, resulting in the improvement of sensitivity and selectivity. Optimization and standardization of the experimental conditions, the composition and proportion of developing solvent, were conducted. Thus a robust and reliable approach which enabled effective separation, accurate identification and quantification has been developed with the lowest detectable amounts of 0.4, 0.2, 1.2, 1, 1 and 1 μg for Pro, Tet, Dib, Mep, Lid and Rop, respectively. The established method was successfully applied in anesthetics analysis in cosmetics (essence, cream and gel) and rat serum without complex sample pretreatment processes, and the results were verified by HPLC. It turned out that the in situ enrichment TLC-Raman technique was feasible and effective for separation and determination of 6 caine-type anesthetics in biological samples and cosmetics, and would have good prospects for on-site qualitative screening of prohibited goods and adulterants.
Keywords
Thin layer chromatography
In situ enrichment
Raman
Anesthetics
1 Introduction
Local anesthetics are a class of drugs that block the initiation and transmission of sensory nerve impulses in a locally reversible manner (Gartner and Allen, 2022; Miller, 2015; Spinal and Blocks, 2013). The methods of local anesthesia mainly include epidural anesthesia, surface anesthesia, infiltration anesthesia, conduction anesthesia and subarachnoid anesthesia (Williams and Walker, 2014; Neal et al., 2012; Sun et al., 2012; Horlocker, 2011). The commonly used local anesthetics include procaine (Pro), lidocaine (Lid), bupivacaine (Bup), tetracaine (Tet) and dibucaine (Dib), etc. Local anesthetics can be structurally classified into two categories: para-aminobenzoic acid esters (Pro, Tet) and anilides involving Lid, ropivacaine (Rop), Bup, and mepivacaine (Mep).
Local anesthetics play an indispensable and critical role in clinical anesthesia and treatment of acute and chronic pain, but excessive use or improper operation of anesthetics will lead to toxic reaction, thus leading to the occurrence of unexpected medical accidents (El Beheiry, 2020; Jayaram et al., 2019; Yazdi, 2017; Kamel et al., 2015; Onizuka1 et al., 2011). Besides, benzocaine, MS-222 and Pro have been used effectively as general anesthetics for short-term sedation of fish during transport, spawning and stocking operations in aquaculture of many fishery countries, such as America, Canada, Japan, etc. US government sets strict limits on the variety, dosage or residue and withdrawl time of anesthetics (FDA, 2021). There were also many reports on criminal incidients involving the use of anesthetics. Moreover, these components were added into some cosmetics for achieving the effects of anti-wrinkle, anti-aging and local repair. In China, anesthetics were forbidden ingredients in cosmetics specified in the Draft of Safety and Technical Standards for Cosmetics 2022 (STSC 2022) (NIFDC, 2022). Thus, the Chinese government has issued a series of national and industrial standards for anesthetics, such as 《Determination of Prohibited Tetracaine and Its Salts in Cosmetics-Ion Chromatography》 (GB/T 40895–2021) (CNS, 2022), 《Determination of Tricaine, Benzocaine and Quinaldine Residues in Aquatic Products for Export-HPLC/MS/MS method》 (SN/T 5441–2022), 《Methods of Qualitative and Quantitative Analysis for Benzocaine, Lidocaine, Procaine, Amethocaine, Bupivacaine in Case Samples by GC/NPD》 (GA/T 190–1998), etc.
Research works on simultaneous detection of local anesthetics in cosmetics, serum and urine have been described by high-performance liquid chromatography (HPLC) (El Sherbiny and Wahba, 2020; Qin et al., 2010; Gaudreault et al., 2009; Ma et al., 2006; Rifai et al., 2001), gas chromatography (GC) (Abdel-Rehim, 2004; Terada et al., 1996; Hattori et al., 1991), capillary electrophoresis (CE) (Ĺopez Guerrero et al., 2015; Liu et al., 2013), electrochemiluminescence (ECL) (Liu et al., 2013), mass spectrometry (MS)(Lian et al.,2019; Tonooka et al., 2016; Abdel-Rehim, 2004) and fluorescence methods (Ahmadpour and Hosseini, 2019) in the literature. Most studies adopted hyphenated techniques such as GC–MS(Abdel-Rehim, 2004), CE-ECL (Liu et al., 2013) and UPLC-MS(Lian et al.,2019). It is noteworthy that, sample pretreatments or preconcentrations largely determined the sensitivity and accuracy of the methods due to complex components in cosmetics and biological fluids. Liquid-liquid extraction (LLE) (Gaudreault et al., 2009; Ma et al., 2006) and solid-phase extraction (SPE) (Tonooka et al., 2016; Abdel-Rehim, 2004) are the most commonly used sample extraction methods coupled with HPLC and CE. But in general, these methods require large amounts of organinc solvents which have adverse impact on experimenters and environment, long analysis time and high costs. Many researchers have been working on establishing selective sensors by spectroscopic technique to simplify the pretreatment process in complex systems (Hao et al., 2022; Zhang et al., 2022; Zhou et al., 2022). Thus, a simple, economical and reliable assay by means of spectroscopy for simultaneous determination of local anesthetics can be expected.
In this sense, Raman spectroscopy is an effective analytical tool that satisfies the features of nondestructive determination, handy operation and fingerprint vibration information of target molecules (Cialla-May et al., 2022; Porcu et al., 2022; Scarpitti et al., 2022; Shin et al., 2022). Additionally, thin layer chromatography (TLC) is an important and economical experiment technique for rapid separation and qualitative analysis for small quantities of substances (Chepngeno et al., 2022; Yang et al., 2022). Our group has been working on in situ TLC-Raman technique for identification of illegal drug addition (Liang et al., 2022a, 2022b; Li et al., 2018; Li et al., 2018). TLC-Raman, a simple hyphenated technique with the features of low costs, high separation efficiency and strong fingerprint, has been employed in identification of Chinese Medicine extracts and adulterant detection in dietary supplements (Zhu et al., 2014).
This manuscript describes a facile and selective in situ enrichment TLC-Raman method for simultaneous determination of Pro, Tet, Dib, Mep, Lid and Rop in cosmetics and rat serum with simple sample pretreatment and experimental operation. The original spots deposited on TLC plates can be manually moved and concentrated into much smaller spots driven by solvent from different directions, followed with Raman measurement, resulting in improvement of detection sensitivity and specificity. The method was characterized in terms of linearity, accuracy, reproducibility, specificity and universality.
2 Materials and methods
2.1 Materials
Tetracaine hydrochloride (CAS 136–47-0), procaine hydrochloride (CAS 51–05-8), ropivacaine hydrochloride (CAS 132112–35-7), lidocaine hydrochloride (CAS 6108–05-0), mepivacaine hydrochloride (CAS 1722–62-9) and dibucaine (CAS 85–79-0) were purchased from Shanghai Jingchun Biochemical Technology Co., Ltd. TLC silica gel 60F254 (SKU 1055540001) was purchased from Millipore (Canada) Ltd. Cyclohexane and methanol were supplied by Fuchen (Tianjin) Chemical Reagent Co., Ltd. Triethylamine was obtained from Tianjin Kemio Chemical Reagent Co., Ltd. Cosmetics, namely INTRAL Redness Relief Soothing Serum (Darphin, 5 mL, REF.D320-43, UK, abbreviated as essence) was purchased from Darphin Official Flagship Store (Taobao (China) Software Co., Ltd). Mild Exfoliating Moisturizing Gel (Doctor Li, 100 g) was purchased from local cosmetics store. Cemoy Revival cream (10 mL) were obtained from Internet (Nicomama App, Hangzhou Zhicong Network Technology Co., Ltd). The above reagents were stored in refrigerator at 4℃. All these chemicals were analytical-grade reagents and used directly without further purification. The purified water purchased from Wahaha Group Co., Ltd was used throughout the experiment.
2.2 Preparation of anesthetics standard solutions
The standard solution of each anesthetic and their mixed standard solution were prepared with methanol. All solutions were stored at 4℃ for further use.
2.3 Preparation of rat serum samples
15 SD male rats were selected and raised in the common experimental environment. All rats were allowed to drink water through free access, and the rats were placed in humid cages for 2 weeks. On the last day, all rats should be fasting for 12 h before orbital blood collection. Next, blood was dripped into the 10 mL EP tube and stewed for 30 min at room temperature, then centrifuged at 4 °C, 3000 rpm for 5 min. The supernatant was stored at −80 °C.
Before use, the rat serum samples were thawed in an ice-water bath. 1 mL rat serum was added with 3 mL methanol, then vortexed for 1 min, ultrasonically dispersed for 5 min and finally centrifuged at 12000 rpm for 10 min. The supernatant was directly used for spotting as negative sample. The simulated positive serum samples were prepared as follows, negative rat serum was spiked with the mixed standard solution and treated as above, making the final concentrations were 2 mg mL−1 for Pro, Tet, Dib and 8 mg mL−1 for Mep, Lid, Rop, respectively.
2.4 Preparation of cosmetics samples
1.0 g of essence (gel) was accurately weighed and placed in 15 mL centrifuge tube, vortexed for 30 s with 2 mL methanol, diluted to 10 mL with methanol, and ultrasonically extracted for 20 min, then centrifuged at 5000 rpm for 5 min. 1.0 g of cream was accurately weighed and placed in 15 mL centrifuge tube, vortexed for 1 min with 8 mL 1% trichloroacetic acid and 2 mL methanol, centrifuged at 5000 rpm for 5 min. The supernatant and subnatant were directly used as negative samples for spotting in our study. The positive samples were prepared by adding mixed standard solution to cosmetics in the centrifuge tube and then pre-treated following the above steps. The final concentrations added in the centrifuge tube were 0.5 mg mL−1 for Pro, Tet and Dib, and 2 mg mL−1 for Mep, Lid and Rop.
2.5 TLC separation and in situ enrichment of anesthetics
The test solutions were pipetted with 2 uL quantitative capillary and spotted on the silica gel thin layer plate. Then the spotted thin layer plate was pre-saturated for 15 min with 5 mL cyclohexane-triethylamine (v:v = 7:3) solution as the developing solvent in the chromatographic tank. After the developing solvent was fully expanded, the spots were marked under 254 nm UV light irradiation and the Rf values were then calculated. Finally, the spots were enriched in situ with methanol and measured directly by Raman spectrometer with 532 nm excitation wavelength on TLC plates. The sampling volumes were 2 μL for standarad and rat serum samples, 4 μL for cosmetics samples.
2.6 Instruments and measurement
Raman spectra were measured with the Thermo Scientific DXR3xi Raman Imaging Microscope equipped with an excitation laser wavelength of 532 nm (laser power: 10.0 mW) at room temperature. All Raman spectra were recorded by a 50 × microscope objective. The laser power, exposure time and scanning times of target anesthetics were different and listed in Table S1. The pinhole was 25 μm. The obtained Raman spectra were baseline corrected by NGS LabSpec 5, and plotted with Origin 8.5. Other instruments included BYLAB three by ultraviolet analyzer (Beijing Bingyang Technology Co., Ltd, 254 and 365 nm), CNC ultrasonic cleaner (KQ-500DE, Kunshan Ultrasonic Instrument Co., Ltd), High speed centrifuge (TG16, Shanghai Huxiangyi Centrifuge Instrument Co., Ltd), vortex mixer (Qun'an Instrument Co., Ltd), 2 μL quantitative spotting capillary (Tianjin Enlida Technology Co., Ltd) and chromatographic tank (Shanghai Huake Laboratory Equipment Co., Ltd). The HPLC system was Aglient 1260 (Palo, Alto, CA, USA) equipped with a sample injection pumb, an automatic sampler, an ultraviolet (UV) detector and a thermostat. Detailed HPLC conditions refer to the Supporting Information.
3 Results and discussion
3.1 Selection of developing solvent
Different developing systems were explored for separation of six kinds of caine-type anesthetics, including chloroform–methanol, chloroform–methanol-cyclohexane, cyclohexane-methanol, cyclohexane-methanol-aqueous ammonia, chloroform-acetone-methanol–water, ethyl acetate–methanol-aqueous ammonia, ethyl acetate–methanol-diethylamine, cyclohexane-diethylamine and cyclohexane-triethylamine systems. The six analytes can not be mutually separated except using cyclohexane-triethylamine system. Optimization on the proportion of developing solvent (5:5, 6:4, 13:7, 7:3, 3:1 and 4:1) was conducted (Fig. S1). The separation effect and spot shapes of cyclohexane-triethylamine system (7:3) were better, thus finally it was used as the developing agent in this experiment. In Fig. 1, the six anesthetics were separated effectively with Rf values of 0.09 (Pro), 017 (Tet), 0.41 (Dib), 0.50 (Mep), 0.62 (Lid) and 0.73 (Rop), respectively. The Rf values of the analytes were recorded and the mixed standard solution was spotted on every TLC plate, so that the TLC spots could be traced when they were invisible under UV irradiation.
TLC plates under 254 nm UV light irradiation for separation of six anesthetics with Rf values of 0.09 (Pro), 017 (Tet), 0.41 (Dib), 0.50 (Mep), 0.62 (Lid) and 0.73 (Rop), respectively. Developing system: cyclohexane-triethylamine system (7:3). The concentrations were 2 mg mL−1 for Pro, Tet, Dib, and 5 mg mL−1 for Mep, Lid, Rop, respectively.
3.2 In situ enrichment and Raman spectra of spots
After TLC separation, the Raman spectra of spots were directly obtained. However, the sensitivity was still largely limited to the visibility of TLC spots. In order to improve the detection sensitivity, an in situ enrichment procedure was applied (Fig. 2). The spots could be manually concentrated to much smaller points through the promotion of methanol with spotting capillary from different directions. Even the originally invisible spots became visible and detectable. As expected, the Raman responses were improved dramatically, especially for Tet, Mep and Rop (Fig. 3), which benefited from in situ enrichment. Thus, the Raman spectra of six anesthetics were all collected on concentrated sample spots, which were basically consistent with intrinsic Raman signals of their solids. Furthermore, the Raman spectra were distinguishable which was attributed to the specificity of Raman spectroscopy. The strong peaks at 1609 cm−1 for Pro and 1611 cm−1 for Tet were assigned to ring C = C stretching vibration. The bands at 1701 cm−1 (Pro) and 1692 cm−1 (Tet) corresponded to the C = O stretching vibration. The peaks observed at 1275 cm−1 (Pro) and 1279 cm−1 (Tet) were from C-O stretching[Sherlin et al., 2018]. For Lid, the very strong one observed at 1265 cm−1 corresponded mainly to CH bending and C–C stretching. At 1597 cm−1, the peak was assigned to HNC scissoring and NC stretching modes. At 2930 cm−1, there was a very intense line in the Raman spectrum, which was due to the methylene CH2 sretching in the two ethyl groups[Badawi et al., 2015]. For Mep and Rop, there was more abundant fingerprint information compared with the others.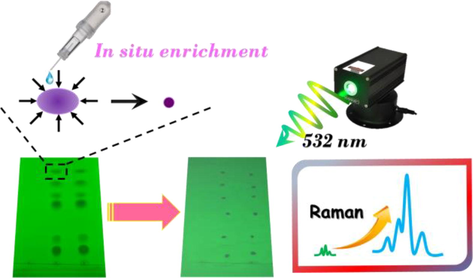
Sketch of in situ enrichment and Raman measurement of spots on TLC plates.
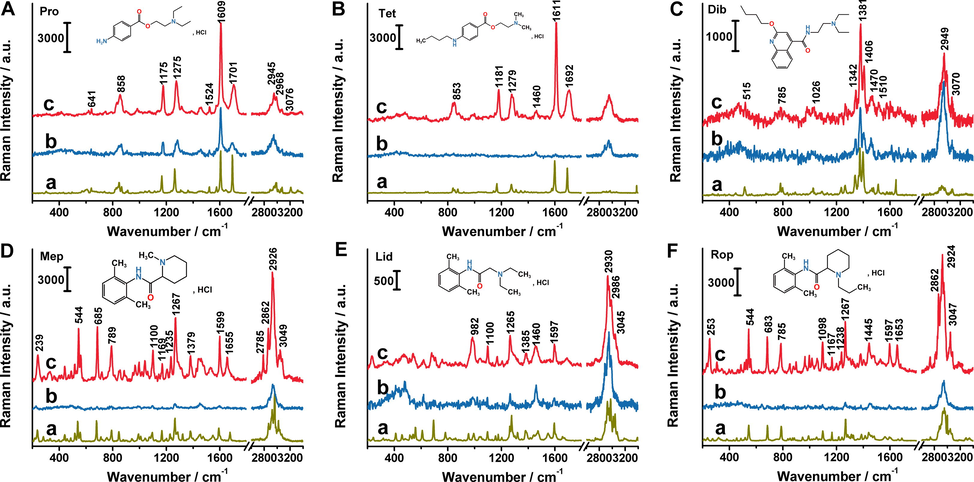
Raman spectra of anesthetics solid (a), before (b) and after (c) in situ enrichment. The concentrations were 2 mg mL−1 for Pro (A), Tet (B), Dib (C), and 5 mg mL−1 for Mep (D), Lid (E),Rop (F), respectively.
3.3 Concentration dependent Raman spectra of anesthetics
A series of anesthetic standard solutions in different concentrations were spotted on a TLC plate and the spots were in situ enriched under 254 nm UV light irradiation following by Raman measurements under 532 nm excitation wavelength. The TLC plates of anesthetics before and after in situ enrichment were shown in Fig. S2. Representative concentration dependent Raman spectra from enriched spots of anesthetics and the plot of Raman intensity versus spotting amounts were illustrated in Fig. 4. The Raman spectra were first baselined then analyzed by characteristic peak’s intensity of each anesthetic, 1609 (Pro), 1611 (Tet), 1381 (Dib), 1267 (Mep), 1265 (Lid) and 1267 cm−1 (Rop), respectively. As can be seen from Fig. 4, the intensities of characteristic bands decreased gradually with the anesthetics concentrations coming down. The variation tendencies of Raman intensities with the sampling amounts of anesthetics were investigated. The error bars were made with at least five sets of data which represented the precision. Finally, after linear fitting by Origin 8.5, the linear equations, the correponding linear range and the minimum detection amounts for Pro, Tet, Dib, Mep, Lid and Rop were listed in Table 1.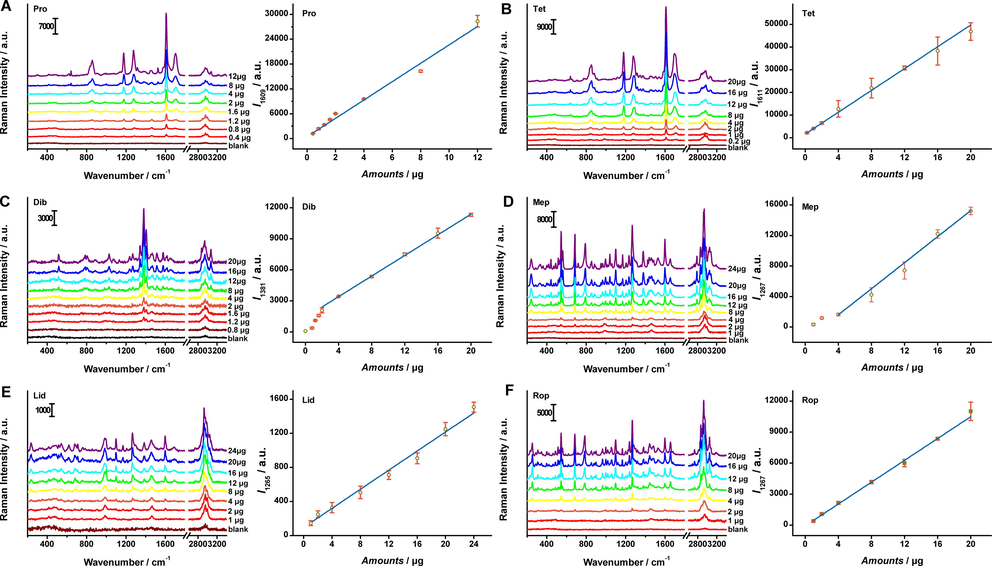
Representative concentration-dependent Raman spectra of the anesthetics. The Raman intensities at 1609 (Pro), 1611 (Tet), 1381 (Dib), 1267(Mep), 1265(Lid) and 1267 (Rop) cm−1 versus the target amounts sampled on TLC plates. Each error bar indicated the standard deviation of the Raman intensity. The error bars were made with at least five sets of data.
Linear equation
R2
Lniear range (μg)
The minimum detection amounts (μg)
Pro
y = 2215.813703x + 475.446698
0.9814
0.4–12
0.4
Tet
y = 2407.470763x + 1601.561254
0.9991
0.2–20
0.2
Dib
y = 497.453912x + 1421.64032
0.9991
2–20
1.2
Mep
y = 853.670341x-1804.914295
0.9978
4–20
1
Lid
y = 55.983634x + 95.907192
0.9905
1–24
1
Rop
y = 527.144531x-56.809454
0.9989
1–20
1
In Fig. S2, the minimum amounts visible to the naked eyes were about 1.2, 2, 1.2, 2, 8 and 8 μg for Pro, Tet, Dib, Mep, Lid and Rop by TLC. Combining in situ enrichment TLC with Raman scattering technique, the minimum detection amounts were optimized to 0.4, 0.2, 1.2, 1, 1 and 1 μg, respectively. Additionally, the accuracy of identification can be further guaranteed by uniqueness of molecular vibration spectrum. Thus, our method not only utilized the TLC predominance of convenient sepration for mixtures, but also exhibited the superiority in fingerpringting of Raman spectroscopy.
3.4 Determination of cosmetics samples
Cosmetics samples were tested by means of standard addition method for exploring application fields of the proposed protocol. Image of TLC plate and corresponding Raman responses of spiked samples (essence, gel and cream) were presented in Fig. 5. For the negative sample of gel, an interference spot with the same Rf value as Pro appeared, but no Raman signal was observed. There were no background interferences observed from both TLC image and Raman spectra of negative samples (Fig. S3). Anesthetics between spiked samples and standard solution achieved consistency on the spots positions. Then we calculated the amounts of anesthetics via Raman intensity of characteristic peaks and their linear equations. As shown in Table 2, the calculated amounts of each anesthetic in this work coincided with true addition amounts basically. The recoveries were between 91.5% and 110.9%, the RSD values were all within 11%. Accordingly, our approach based on in situ concentration TLC and Raman scattering was proved to be feasible for anesthetics determination precisely and conveniently.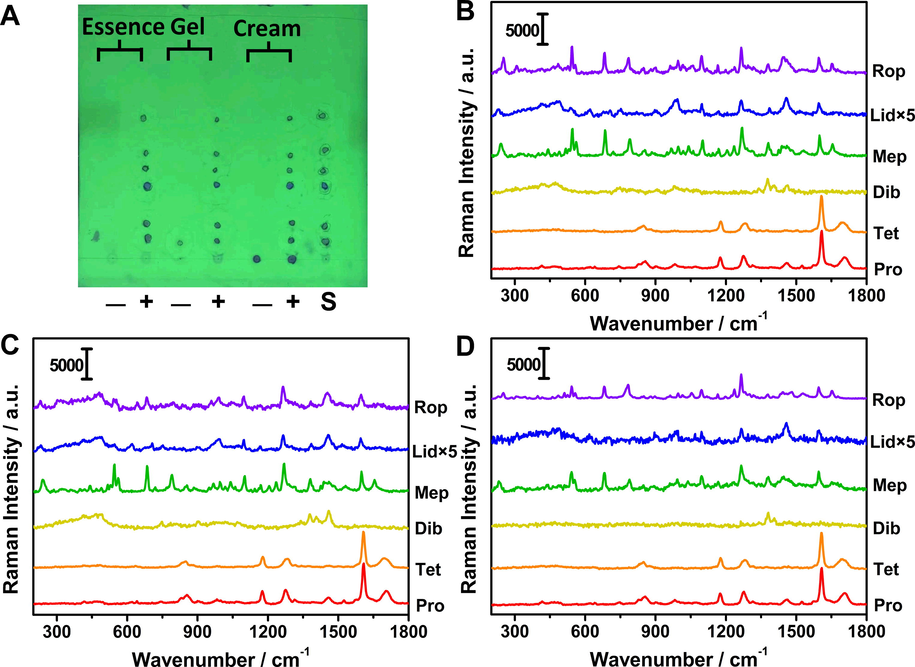
Image of TLC plate (A) and corresponding Raman responses of negative and spiked samples with essence (B), gel (C) and cream (D). The Raman intensity of lidocaine was multiplied by 5 times. “S” means “the mixed standard solution”. “+” and “-” represent positive and negative samples.
Added amounts (μg)
Measured amounts (μg)
Recovery(%)
RSD(%)
Essence
Pro
2
2.13 ± 0.12
106.5
11
Tet
2
1.87 ± 0.20
93.5
11
Dib
2
2.22 ± 0.21
110.9
9.5
Mep
8
7.76 ± 0.45
97.0
5.8
Lid
8
8.10 ± 0.41
101.2
5.1
Rop
8
8.29 ± 0.30
103.6
3.7
Cream
Pro
2
1.91 ± 0.05
95.5
2.7
Tet
2
1.86 ± 0.19
93.0
11
Dib
2
2.10 ± 0.15
105.0
7.2
Mep
8
7.32 ± 0.24
91.5
3.3
Lid
8
7.78 ± 0.46
97.2
6.0
Rop
8
7.80 ± 0.44
97.5
5.7
Gel
Pro
2
2.19 ± 0.07
109.8
3.2
Tet
2
1.85 ± 0.16
92.5
8.7
Dib
2
2.14 ± 0.17
110.0
8.0
Mep
8
7.66 ± 0.46
95.8
6.1
Lid
8
7.66 ± 0.45
95.8
5.9
Rop
8
7.44 ± 0.51
93.0
6.9
As stated above, LLE and SPE are commonly used for extraction of anesthetics in cosmetics samples, which have adverse impact on experimenters and environment due to large amounts of organinc solvents, long analysis time and high costs. In our study, the treatments and separation processes for cosmetics were comparatively simpler and more affordable. Besides, the method would have great potential for on-site qualitative screening of anesthetics if portable Raman spectrometer was used.
3.5 Determination of rat serum simulated positive samples
In the same way, we applied the approach to rat serum samples. Negative sample, rat serum simulated positive sample and mixed standard solution were spotted on the same TLC plate. As can be seen in inset of Fig. 6, there was a one-to-one correspondence for spot positions between positive sample and mixed stardand solution. There presented no visible spots for the negative sample, and no peaks were detected for negative sample. It was demonstrated that rat serum matrix did not interfere with the identification of anesthetics we studied, which facilitated the detection of targets. The Raman spectra of six anethetics from positive sample and mixed stardard solution were measured and compared in terms of peak position and Raman intensity. For each anesthetic, the Raman intensities of characteristic peaks were substituted into respective standard curve. The calculated amounts and related results were listed in Table 3, which were basically in agreement with added amounts. The recoveries were between 94.6% and 108.1%, the RSD values were all within 12%.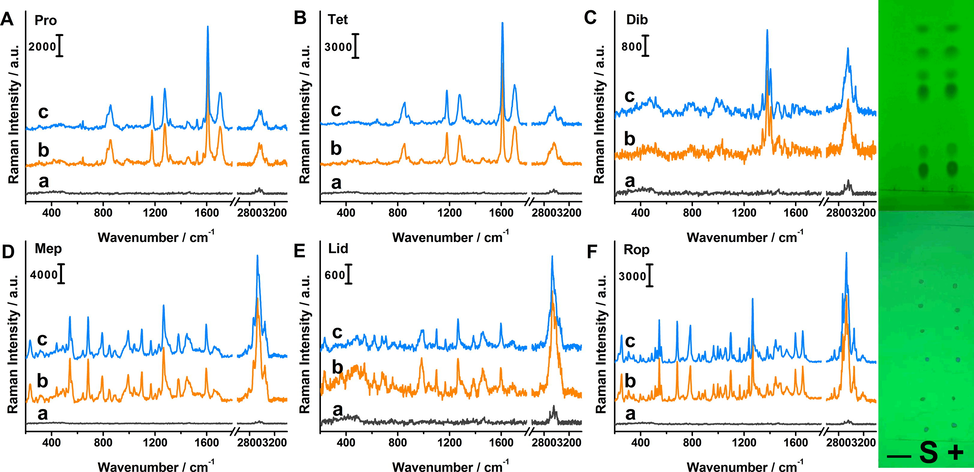
Raman spectra of Pro (A), Tet (B), Dib (C), Mep (D), Lid (E) and Rop (F) for negative rat serum sample (a), standard solution (b) and positive sample (c). The inset images were corresponding TLC plates before and after in situ enrichment. “S” means “the mixed standard solution”. “+” and “-” represent positive sample and rat serum blank sample.
Added amounts (μg)
Measured amounts (μg)
Recovery(%)
RSD(%)
Pro
4
4.25 ± 0.49
106.2
12
Tet
4
4.33 ± 0.06
108.1
1.4
Dib
4
3.86 ± 0.28
96.5
7.3
Mep
16
15.14 ± 0.91
94.6
6.1
Lid
16
16.82 ± 1.52
105.0
9.0
Rop
16
16.70 ± 0.66
104.4
4.0
3.6 Accuracy and reproducibility
To verify the accuracy and reproducibility of the method, the recovery tests on simulated positive samples (rat serum and cosmetics) in different concentrations for anesthetics within the linear range were conducted, obtaining corresonding data in Table 2 and 3. The recovery was calculated through the ratio of measured value to added value. The repetitive experiments were conducted by repeated measurements for more than 5 times, then calculating RSD based on the determined amounts. The results indicated that our approach was feasible for identification and quantification in both rat serum and cosmetics with good accuracy and reproducibility. Additionally, lt can also be seen from error bars of peak intensities in different amounts in Fig. 3 that the method was of acceptable repeatability. The repeatability was also confirmed by the Raman mapping image of TLC spots from Tet standard solution in Fig. S4. Thus, the established method exhibited good accuracy and reproducibility. Notably, spotting and enriching procedures were all conducted manually, so it would inevitably introduce certain deviations. In our future research, automatic TLC sampler can be used for getting narrower sample strips to improve the RSD values. Additionally, more Raman data, even all the Raman mapping data on one spot can be obtained for quantitative analysis to further improve the RSD values at the cost of a certain efficiency.
3.7 Specificity
As shown in Figs. 5 and 6, the spots of simulated positive sample on TLC plate had the same Rf values with that of the mixed standard solution. There were no visible spots appeared on TLC plate for negative sample, nor that discriminable Raman responses. It was indicated that the anesthetics in rat serum and cosmetics were separated and identified effectively without interferrence in our study. Combined with Raman spectroscopy, the specificity was greatly improved due to the fingerprint property of Raman spectra.
3.8 HPLC verification
To further confirm the results of this work, all the cosmetics samples were also analyzed by HPLC method (Fig. S5-S8), and similar results were obtained as shown in Table S2 including HPLC experimental details. The results also proved the accuracy and feasibility of the proposed approach. Although our method was less sensitive than HPLC, the simplicity, selectivity and low costs would make it a promising and practical platform for simple analysis of anesthetics. Meanwhile, we will focus on the enhancement of sensitivity ulteriorly.
4 Conclusions
A simple and selective TLC-Raman analytical method combined with in situ enrichment had been developed to be applied to simultaneously determine 6 kinds of caine-type anesthetics in cosmetics and rat serum. The whole experimental processes were convenient and low cost by TLC separation followed with in situ concentration and Raman operation. The detection sensitivity was improved by in situ enrichment of original sample spots with solvent, which reducing the restriction of spots visibility on TLC detection sensitivity to a certain extent. Furthermore, the selectivity was guaranteed and enhanced by unique Raman fingerprint vibrational information. The quantitative analysis of anesthetics based on TLC-Raman hyphenated technique was implemented compared with our previous identification of illegally added drugs. In conclusion, the methodology could be available for simple and selective identification and quantitation of caine-type anesthetics in cosmetics and biological samples. In addition, the TLC-Raman technique with in situ enrichment for determination of real samples has enormous potential to become a handy and effective approach for unambiguous testing of drug abuse in various complex systems.
CRediT authorship contribution statement
Chao-Yang Zhao: Methodology, Investigation, Formal analysis, Writing – original draft. Xinyue Ma: Investigation. Jialin Zang: Software, Resources. Tingting Liu: Validation. Huiyu Wang: Visualization. Shuang Fu: Data curation. Cuiyan Han: Supervision. Huimin Sui: Conceptualization, Methodology, Writing – original draft.
Acknowledgements
This work was supported by grants from the Fundamental Research Funds for Education Department of Heilongjiang Province [2021-KYYWF-0347]; Young Elite Scientist Sponsorship Program by Heilongjiang Province [2022QNTJ019].
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- New trend in sample preparation: on-line microextraction in packed syringe for liquid and gas chromatography applications I. Determination of local anaesthetics in human plasma samples using gas chromatography–mass spectrometry. J. Chromatogr. B. 2004;801:317-321.
- [CrossRef] [Google Scholar]
- A molecularly imprinted modified CdSeS/ZnS core–shell quantum dot embedded glass slide for highly selective and sensitive solid phase optosensing of trace amounts of lidocaine in biological samples. Anal. Methods. 2019;11:851-859.
- [CrossRef] [Google Scholar]
- The conformational stability, solvation and the assignments of the experimental infrared, Raman, 1H and 13C NMR spectra of the local anesthetic drug lidocaine. Spectrochim. Acta A. 2015;142:382-391.
- [CrossRef] [Google Scholar]
- Baobab pulp authenticity and quality control by multi-imaging high-performance thin-layer chromatography. Food Chem.. 2022;390:133108
- [CrossRef] [Google Scholar]
- Raman spectroscopy and imaging in bioanalytics. Anal. Chem.. 2022;94:86-119.
- [CrossRef] [Google Scholar]
- CNS. Determination of prohibited tetracaine and its salts in cosmetics—Ion chromatography, Chinese National Standards: GB/T 40895-2021, 2022-06-01. https://std.samr.gov.cn/gb/gbQuery.
- El Beheiry, H., 2020. Local Anesthetics, eds. Springer, Singapore, pp. 95–102. https://doi.org/10.1007/978-981-15-3591-8_4.
- Studying the effect of vasopressors on therapeutic drug monitoring of two local anesthetics using hybrid micelle liquid chromatography as an analysis tool. J. Chromatogr. B. 2020;1154:122277
- [CrossRef] [Google Scholar]
- FDA. Fish and Fishery Products Hazards and Controls Guidance Fourth Edition. 2021. Available online: https://www.fda.gov/media/80637/download (accessed on 9 March 2022).
- Gartner, J.A., Allen, B.F.S., 2022. Local Anesthetics, in: D.A. Edwards, P. Gulur, C.M. Sobey (Eds.), Hospitalized Chronic Pain Patient. Springer, Cham, pp. 167–173. https://doi.org/10.1007/978-3-031-08376-1_31.
- High-performance liquid chromatography using UV detection for the simultaneous quantification of ropivacaine and bupivacaine in human plasma. Ther. Drug. Monit.. 2009;31:753-757.
- [CrossRef] [Google Scholar]
- Ĺopez Guerrero, M.d.M., Hernández-Mesa, M., Cruces-Blanco, C., García-Campaña, A.M., 2015. On-line preconcentration strategy for the simultaneous quantification of three local anesthetics in human urine using CZE. Electrophoresis 36, 2961–2967. http://doi.org/10.1002/elps.201500081.
- Serum metal ion-induced cross-linking of photoelectrochemical peptides and circulating proteins for evaluating cardiac ischemia/Reperfusion. ACS Sens.. 2022;7:775-783.
- [CrossRef] [Google Scholar]
- Determination of local anaesthetics in body fluids by gas chromatography with surface ionization detection. J. Chromatogr.. 1991;564:278-282.
- [CrossRef] [Google Scholar]
- Complications of regional anesthesia and acute pain management. Anesthesiol. Clin.. 2011;29:257-278.
- [CrossRef] [Google Scholar]
- Chondrotoxic effects of local anesthetics on human knee articular cartilage: a systematic review. PM&R. 2019;11:379-400.
- [CrossRef] [Google Scholar]
- Kamel, I., Trehan, G., Barnette, R., 2015. Intralipid therapy for inadvertent peripheral nervous system blockade resulting from local anesthetic overdose. Case Re. Anesthesiol. 486543. http://doi.org/10.1155/2015/486543.
- Rapid detection of six glucocorticoids added illegally to dietary supplements by c ombining TLC with spot-concentrated Raman scattering. Molecules. 2018;23(7):1504.
- [CrossRef] [Google Scholar]
- Rapid detection of four chemical components added illegally in slimming health food with TLC situ Raman spectroscopy. Spectrosc. Spect. Anal.. 2018;30:830-836.
- [CrossRef] [Google Scholar]
- Determination of 10 kinds of caine-type prohibited ingredients in cosmetics by ultra-performance liquid chromatography-differential mobility spectrometry-mass spectrometry. Chinese J. Anal. Chem.. 2019;47:756-764.
- [CrossRef] [Google Scholar]
- Rapid limit test of seven pesticide residues in tea based on the combination of TLC and Raman imaging microscopy. Molecules. 2022;27(16):5151.
- [CrossRef] [Google Scholar]
- Rapid detection of five estrogens added illegally to dietary supplements by combining TLC with Raman imaging microscope. Molecules. 2022;27(9):2650.
- [CrossRef] [Google Scholar]
- Separation and determination of anesthetics by capillary electrophoresis with mixed micelles of sodium dodecyl sulfate and Tween 20 using electrochemiluminescence detection. Luminescence. 2013;28:673-678.
- [CrossRef] [Google Scholar]
- Liquid-phase microextraction combined with high-performance liquid chromatography for the determination of local anaesthetics in human urine. J. Pharmaceut. Biomed.. 2006;40:128-135.
- [CrossRef] [Google Scholar]
- Miller’s anesthesia (8th ed.). Philadelphia: Elsevier/Saunders; 2015.
- American society of regional anesthesia and pain medicine checklist for managing local anesthetic systemic toxicity: 2012 version. Reg. Anesth. Pain Med.. 2012;37:16-18.
- [CrossRef] [Google Scholar]
- NIFDC. Safety and Technical Standards for Cosmetics 2022 (Draft), National Institutes for Food and Drug Control, 2022-03-31. https://cosmetic.chemlinked.com/database/view/1374.
- Onizuka1, S., Yonaha, T., Tsuneyoshi, I., 2011. Local anesthetics with high lipophilicity are toxic, while local anesthetics with low pka induce more apoptosis in human leukemia cells. J. Anesthe. Clinic. Res. 2, 1000116. https://doi.org/10.4172/2155-6148.1000116.
- Rapid in situ detection of THC and CBD in Cannabis sativa L. by 1064 nm Raman spectroscopy. Anal. Chem.. 2022;94:10435-10442.
- [CrossRef] [Google Scholar]
- Simultaneous determination of procaine, lidocaine, ropivacaine, tetracaine and bupivacaine in human plasma by high-performance liquid chromatography. J. Chromatogr. B. 2010;878:1185-1189.
- [CrossRef] [Google Scholar]
- Simultaneous measurement of plasma ropivacaine and bupivacaine concentrations by HPLC with UV detection. Ther. Drug. Monit.. 2001;23:182-186.
- [CrossRef] [Google Scholar]
- In vitro imaging of lycopene delivery to prostate cancer cells. Anal. Chem.. 2022;94:5106-5112.
- [CrossRef] [Google Scholar]
- Büchi's model based analysis of local anesthetic action in procaine hydrochloride: Vibrational spectroscopic approach. Spectrochim. Acta A. 2018;205:55-65.
- [CrossRef] [Google Scholar]
- Quantitative chemical imaging of bone tissue for intraoperative and diagnostic applications. Anal. Chem.. 2022;94:3791-3799.
- [CrossRef] [Google Scholar]
- Maternal and fetal physiology and anesthesia (5th ed.). McGraw-Hill: New York; 2013.
- Perioperative systemic lidocaine for postoperative analgesia and recovery after abdominal surgery. Dis. Colon. Rectum.. 2012;55:1183-1194.
- [CrossRef] [Google Scholar]
- Determination of ester-type local anesthetic drugs (procaine, tetracaine, and T-caine) in human serum by wide-bore capillary gas chromatography with nitrogen-phosphorus detection. J. Anal. Toxicol.. 1996;20:318-322.
- [CrossRef] [Google Scholar]
- Sensitive liquid chromatography/tandem mass spectrometry method for the simultaneous determination of nine local anesthetic drugs. Forensic Sci. Int.. 2016;265:182-185.
- [CrossRef] [Google Scholar]
- A nomogram for calculating the maximum dose of local anaesthetic. Anaesthesia. 2014;69:847-853.
- [CrossRef] [Google Scholar]
- Rapid analysis of differential chemical compositions of Poria cocos using thin-layer chromatography spray ionization-mass spectrometry. Analyst. 2022;147:3072-3080.
- [CrossRef] [Google Scholar]
- Yazdi, C.A., 2017. Local Anesthetics, Eds. Springer, Cham, pp 347–356. https://doi.org/10.1007/978-3-319-50141-3_45.
- Neurogenesis and proliferation of neural stem/progenitor cells conferred by artesunate via FOXO3a/p27Kip1 axis in mouse stroke model. Mol. Neurobiol.. 2022;59:4718-4729.
- [CrossRef] [Google Scholar]
- Usefulness of enzyme-free and enzyme-resistant detection of complement component 5 to evaluate acute myocardial infarction. Sensor Actuat. B-Chem.. 2022;369:132315
- [CrossRef] [Google Scholar]
- Rapid on-site TLC–SERS detection of four antidiabetes drugs used as adulterants in botanical dietary supplements. Anal. Bioanal. Chem.. 2014;406:1877-1884.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105121.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







