Translate this page into:
In situ growth of Co3O4 nano-dodecahedeons on In2O3 hexagonal prisms for toluene catalytic combustion
⁎Corresponding author. tangzhicheng@licp.cas.cn (Zhicheng Tang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In this paper, in situ growth of Co3O4 nano-dodecahedra on In2O3 hexagonal prisms were synthesized via pyrolysis of ZIF-67/MIL-68. Interestingly, the amount of Co3O4 dodecahedra on In2O3 hexagonal prisms was regularly regulated and controlled. In detail, four Co3O4/In2O3 catalysts with various Co/In molar ratio were prepared, including Co4In1 (Co/In molar ratio was 4:1), Co2In1 (Co/In molar ratio was 2:1), Co1In1 (Co/In molar ratio was 1:1), Co0.5In1 (Co/In molar ratio was 0.5:1). The catalytic performance of Co3O4/In2O3 catalysts was systematically investigated for toluene combustion. It could be noted that the Co2In1 sample exhibited the superior catalytic performance, and the temperatures for 90% toluene conversion (T90) was 182 °C. Furthermore, the toluene conversion of Co2In1 sample had no significant decrease at 178 °C for 15 h, indicating that it presented superior stability for toluene oxidation reaction. Through various characterizations, it was verified that the Co/In molar ratio of Co3O4/In2O3 catalyst could obviously alter the surface atomic ratio of Co3+/(Co3+ + Co2+), BET surface area, the number of surface adsorbed oxygen, the interaction between In2O3 and Co3O4 of CoInOx catalysts and so on. The lots of surface adsorbed oxygen, strong interaction between In2O3 and Co3O4 would promote the catalytic oxidation of toluene. Especially, we discovered that the catalytic activity of Co3O4/In2O3 was obviously improved with the increase of Co3+/(Co3+ + Co2+) surface atomic ratio.
Keywords
In-situ growth
ZIF-67/MIL-68
Co3O4/In2O3
Co/In molar ratio
Toluene catalytic combustion
1 Introduction
Volatile organic compounds (VOCs) are an important class of air pollutants because they are precursors to smog and ozone, causing toxicity and malodorous (Kim and Shim, 2010; Zhao et al., 2018; Zhao et al., 2019). Meanwhile, it is a class of compounds which includes carbon-based chemicals. Inhaling high concentrations of VOCs could affect the respiratory system and could also cause blood diseases and cancer. To reduce the negative impacts on environment and human health, VOCs are worldwide supervised (Han et al., 2018; Liu et al., 2014; Kampa and Castanas, 2008; Garcia et al., 2010; Jones, 1999). It is important to develop effective materials and methods to remove VOCs. Among those conventional control processes, adsorptionbased techniques are only really suitable for controlling of highly dilute VOCs emissions (Malhautier et al., 2014; Li et al., 2011; Kołodziej and Łojewska, 2005). Absorption and membrane separation are costly (Tokumura et al., 2008; Ruddy and Carroll, 1993). Biological degradation is generally selective, concentration and temperature-sensitive, and effective only for low-weight and highly soluble hydrocarbons (Santos et al., 2007). According to the literature, catalytic combustion is regarded as one of the most effective methods because it avoids repeated contamination and operates at lower temperatures (Wu et al., 2004; Everaert and Baeyens, 2004; He et al., 2009; Papaefthimiou et al., 1997; Li et al., 2009; Tang et al., 2014; Delimaris and Ioannides, 2008).
Metal oxides are regarded as an effective catalyst, which could trigger the reaction for catalytic oxidation of VOCs at low temperature. Co3O4 has a typical spinel structure of Co2+Co3+O4, in which Co3+ is in an octahedral coordination, Co2+ is in tetrahedral coordination structure, and O2– is cubic dense. The high activity of Co3O4 may be due to the low ΔH of O2 vaporization (Wang et al., 2017). Therefore, among the most common metal oxides, cobalt oxide is the most widely used for catalytic oxidation material (Han et al., 2018; Konsolakis et al., 2017). As recently reported, appropriate incorporation of metal oxides may be more active and thermally stable than the single oxides. For example, MnxCo3-xO4 (Zhao et al., 2019), Co3-xCuxO4; (Feng and Zheng, 2012) ZnxCo1-xCo2O4 (Marcos et al., 2013) and NiOx, CrOx and Bi2O3 modified Co3O4 (Yan et al., 2003; Zhao et al., 2012; Lou et al., 2011) exhibited higher activity than pure Co3O4. In addition, Lou et al. prepared In2O3/Co3O4 catalysts for ultralowtemperature CO oxidation by simultaneously tuning the CO adsorption strength and oxygen activation over a Co3O4 surface, which could completely convert CO to CO2 at temperatures as low as −105 ℃ compared to 40 ℃ over pure Co3O4. The doping of In2O3 significantly promoted the catalytic performance of Co3O4 for CO oxidation (Lou et al., 2014). According to a series of characterization, it could be inferred that the doping of In2O3 induced the structural defects, modified the surface electronic structure, and promoted the redox ability of Co3O4, which tuned the adsorption strength of CO and oxygen activation simultaneously (Lou et al., 2014).
Recently, metal organic frameworks (MOFs) have gained extensive consideration to synthesize porous materials (Cai et al., 2015). MOFs has been studied as potential application in various fields owing to the unique architectures and versatile functionalities (Wang et al., 2015; Koo et al., 2017). Zeolitic imidzolate frameworks (ZIFs) are a highly ordered porous soilds (Luo et al., 2018; Ding et al., 2017; Tsai and Langner, 2016). ZIF-67 has been considered a typical ZIF species (Shi et al., 2011). Furthermore, MIL-n materials are a class of MOFs derived from trivalent metal cations such as Al3+, Cr3+, V3+, In3+ or Ga3+ and carboxylic acid groups (Jin et al., 2015). Compared with activated carbon and zeolite, MOFs materials have many advantages, including huge specific surface area, order pore structure, diverse pore surface functional group and surface potential energy. Thus, the application of MOFs materials to VOCs field has board prospects (Yang et al., 2012). As we know; almost few research on the application of Co3O4/In2O3 with different Co/In molar ratio synthesized by pyrolysis of ZIF-67/MIL-68 as the catalyst supports for VOCs.
In the work, four samples of Co3O4/In2O3 with different Co/In molar ratio were synthesized. Moreover, the catalytic performance was investigated by toluene combustion. The effect of morphology was extensively characterized on the catalytic performance.
2 Results and discussion
2.1 Morphology of the catalysts
Fig. 1a and b was SEM images of ZIF-67/MIL-68 (In) nanocrystals. Obviously, MIL-68 nanocrystals had a regular hexagonal prisms shape with high uniformity, the average size was about 1.7 μm. ZIF-67 presented the appearance of dodecahedron, which grew in situ on the surface of MIL-68 nanocrystals. Fig. 1b-e were SEM images of Co4In1, Co2In1, Co1In1 and Co0.5In1, respectively. After the calcination treatment at 400 °C for 2 h with a heating rate of 1 °C min−1 in air, it was discovered that the mass of Co3O4/In2O3 well inherited the morphology and uniformity of ZIF-67/MIL-68, while a small number of Co3O4 dodecahedra and In2O3 hexagonal prisms collapsed in process of pyrolysis of ZIF-67/MIL-68. Nevertheless, the samples presented concave and plicated appearance. Moreover, the amount of Co3O4 dodecahedra on In2O3 hexagonal prisms was different, notably. The quantity of Co3O4 followed the order: Co4In1 > Co2In1 > Co1In1 > Co0.5In1. Meanwhile, the particle size of Co3O4 nanoparticles of four catalysts were different. Thus, it could be rational reduced that the quantity of Co3O4 affected the the particle size of Co3O4 nanoparticles of four catalysts.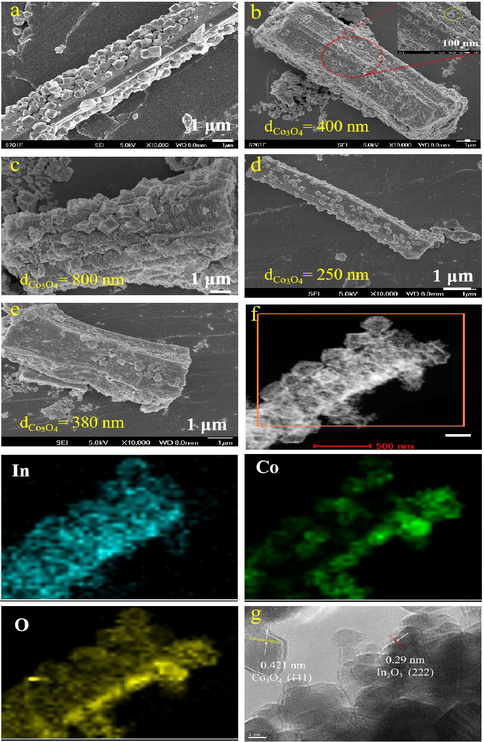
SEM image of the as-prepared ZIF-67/MIL-68(In) (a), Co4In1 (b), Co2In1 (c), Co1In1 (d) and Co0.5In1 (e); TEM image of EDS mapping images of Co2In1 (f) and the HRTEM image of Co2In1 (g).
Fig. 1f showed the elemental mapping image of Co2In1 sample manifested that O element was uniformly distributed in the nanocrystals. Meanwhile, In element was distributed in the hexagonal prisms and dodecahedra while Co element was uniformly distributed in the dodecahedra. It could be inferred the In element on the surface of the hexagonal prisms was migrated or dissolved when in situ growth of Co3O4 nano-dodecahedra on In2O3 hexagonal prisms. Fig. 1g showed the HRTEM image of the as-prepared Co2In1 sample. The Co2In1 showed the Co3O4 (1 1 1) and In2O3 (2 2 2) crystalline plane with lattice distances of 0.421 nm and 0.29 nm, respectively.
2.2 Texture properties of the catalysts
XRD analysis was conducted to study the structural properties of the Co4In1, Co2In1, Co1In1 and Co0.5In1 catalysts. As shown in Fig. 2a, the diffraction peaks at 19.0°, 36.9°, 44.8°, 59.3° and 65.2° were corresponding to the (1 1 1), (3 1 1), (4 0 0), (5 1 1) and (4 4 0) of Co3O4 (PDF#42-1467). The detectable reflections were consistent with standard data of cubic phase Co3O4, indicating that ZIF-67 has been successfully transformed into Co3O4. In addition, The diffraction peaks at 21.5°, 30.6°, 35.4°, 41.8°, 46.7°, 51.0° and 60.6° were corresponding to the (2 1 1), (2 2 2), (4 0 0), (3 3 2), (4 3 1), (4 4 0) and (6 2 2) of In2O3 (PDF#06-0416), suggesting that the MIL-68 has been successfully transformed into In2O3 (Chen et al., 2018). Obviously, with the increase of the molar ratio of In to Co, it could be seen that the diffraction peaks of Co3O4 tended to weak while the diffraction peaks of In2O3 strengthened. Contacted to the SEM, Co3O4 well inherited the dodecahedron morphology of ZIF-67 and In2O3 exhibited the hexagonal prisms morphology of MIL-68, suggesting that the structure of In2O3 and Co3O4 was unbroken. Moreover, the incorporation of In into the Co3O4 structure would inevitably result in lattice distortion due to the difference in ionic radius and electronic state. By applying the Scherrer equation, the crystallite size could be also calculated from the In2O3 (2 2 2) diffraction peak (Luo et al., 2018; Chen et al., 2018; Wei et al., 2010). The results were shown in Table 1. The crystallite size of Co4In1; Co2In1, Co1In1 and Co0.5In1 catalysts was 13.64 nm, 12.14 nm, 13.71 nm and 12.87 nm, respectively.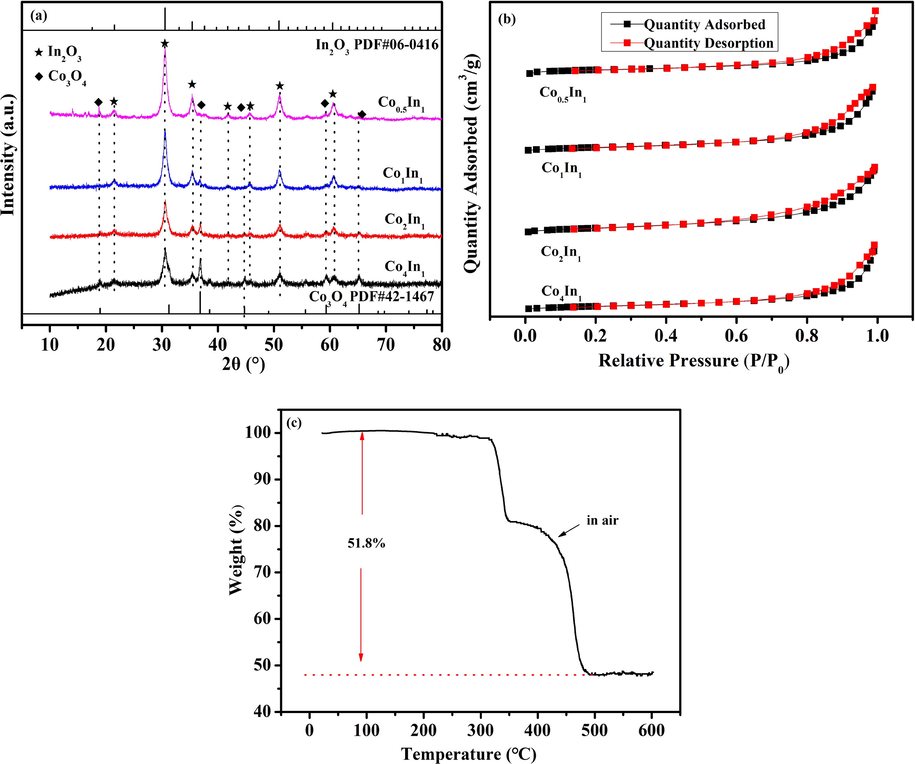
XRD pattern of Co4In1, Co2In1, Co1In1 and Co0.5In1 (a); N2 adsorption–desorption isotherms of Co4In1, Co2In1, Co1In1 and Co0.5In1 (b) and TG curves of the ZIF-67/MIL-68 (2:1) in air (c).
Samples
BET surface areaa (m2 g−1)
Pore volumea (cm3g−1)
Average pore diametera (nm)
Crystallite sizeb (nm)
Co4In1
63.07
0.21
11.3
13.64
Co2In1
75.58
0.26
16.1
12.14
Co1In1
67.07
0.26
15
13.71
Co0.5In1
68.53
0.38
14.9
12.87
The N2 adsorption–desorption isotherms of the Co4In1, Co2In1, Co1In1 and Co0.5In1 catalysts was shown in Fig. 2b. By the IUPAC, the N2 adsorption–desorption isotherms of four catalysts could be divided to type Ⅳ with H3 hysteresis loop, which due to the capillary condensation, the adsorption branch of isotherms at higher relative pressure was inconsistent with the desorption branch. Table 1 showed the average pore diameters, pore volumes and BET surface areas of Co4In1, Co2In1, Co1In1 and Co0.5In1 catalysts. The BET surface areas of the Co4In1, Co2In1, Co1In1 and Co0.5In1 were 63.07, 75.58, 67.07, and 68.53 m2 g−1, respectively. Easy to see, the BET surface areas of four catalysts were almost similar. Thus, the main factor was not the BET surface areas, which affect the catalytic performance. In addition, Co2In1 sample showed the larger average pore diameter (16.1 nm) than that of Co4In1 sample (11.3 nm) Co1In1 (15 nm) and Co0.5In1 sample (14.9 nm). In catalytic oxidation of toluene process, these porous structures could be beneficial for toluene molecules to quickly penetrate into the pores and contact to active sites.
The TG curves of ZIF-67/MIL-68 (4:1) were showed in Fig. 2c. In the temperature range of 310–500 °C, not hard to see, ZIF-67/MIL-68 (2:1) had a sharply weight loss by two step. The total weight loss from 310 °C to 500 °C was 51.8% in the decomposition process. In the temperature range of 310–350 °C, it was concluded that the weight change in the transformation from ZIF-67 to Co3O4, which was regarded as resulting from desorption of surface adsorbed OH, water and decomposition of 2-MIM. In the temperature range of 350–500 °C, the TG curve was well represented by the weight change in the transformation from MIL-68 to In2O3 and the stoichiometry of Co3O4 decompositions, which could be attributed to the stoichiometry of decomposition of 1,4-dicarboxybenzene and lattice oxygen loss from Co3O4. It could be concluded that large amounts of CO2, H2O and NOx would be released in the decomposing process of the ZIF-67/MIL-68.
2.3 Chemical states and redox behavior
The Co 2p, In 3d and O 1s XPS spectra of four catalysts were shown in Fig. 3. In the Co 2p spectrum of four samples, two main peaks at 780 eV and 795 eV were observed, corresponding to Co 2p3/2 and Co 2p1/2, respectively. The fitting peaks at binding energies of 781.2 eV and 796.6 eV were related to Co2+, while another fitting peaks of 780.0 eV and 795.1 eV were related to Co3+ (Bao et al., 2018). Meanwhile, it could be seen that the satellite peaks were located on 786.3 eV and 804.3 eV. Table 2 exhibited the Co3+/(Co3++Co2+) ratios. The relative ratio of Co3+/(Co3++Co2+) that was calculated using the curve-fitted data, which were 0.43, 0.49, 0.41 and 0.44 respectively. It was found that the ratio of Co3+/(Co3++Co2+) conformed the order of Co2In1 > Co0.5In1 > Co4In1 > Co1In1.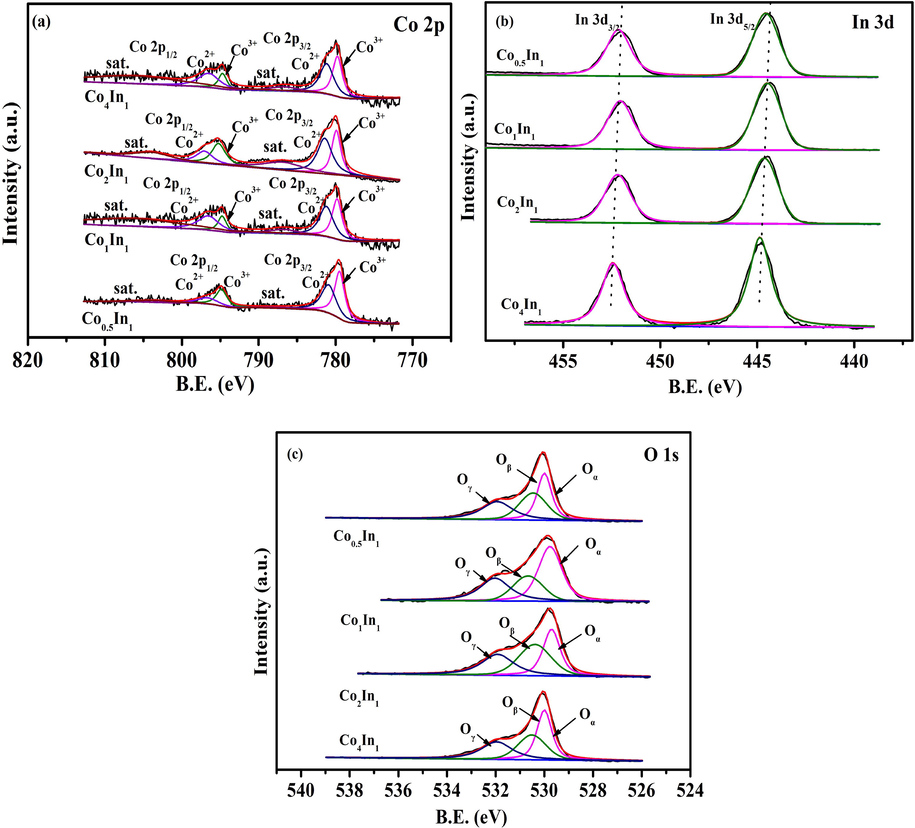
The Co 2p (a), In 3d (b), O 1s (c) XPS spectra of Co4In1, Co2In1, Co1In1 and Co0.5In1 catalysts.
Sample
Co3+/(Co3++Co2+)
Oβ/(Oɑ+Oβ + Oγ)
In 3d3/2/(In 3d5/2 + In 3d3/2)
Co4In1
0.43
0.31
0.45
Co2In1
0.49
0.37
0.45
Co1In1
0.41
0.24
0.45
Co0.5In1
0.44
0.33
0.45
Used Co2In1
0.47
0.35
0.45
Fig. 3b displayed the XPS spectra in the In 3d region of the four samples. Generally, the In 3d peaks were composed of two main spin-orbital lines, In 3d5/2 and In 3d3/2 peaking at 452.1 and 444.6 eV (Lou et al., 2014). The In 3d3/2/(In 3d5/2 + In 3d3/2) ratios were also exhibited in Table 3. The relative ratio of In 3d3/2/(In 3d5/2 + In 3d3/2) that was calculated using the curve-fitted data decreased, which were 0.45, 0.45, 0.45 and 0.45 respectively. It was discovered that the ratio of In 3d3/2/(In 3d5/2 + In 3d3/2) had no difference.
Samples
Reaction temperature (°C)
Ea (kJ mol−1)
T50
T90
Co4In1
176
208
132
Co2In1
159
182
88
Co1In1
167
224
136
Co0.5In1
164
200
130
Co3O4
244
260
/
Fig. 3c showed the XPS spectra of O 1s. The O 1s XPS of four catalysts could be fitted by three peaks. The peaks at binding energies of around 529.6, 530.6, 532.1 eV was assigned to the surface lattice oxygen in the lattice oxygen (Oɑ), chemical adsorption oxygen (Oβ), adsorbed water/OH (Oγ), respectively. It was generally accepted that the charge on the oxide ions was significantly influenced by their surrounding chemical environment, and the nature of dopant ions would decide the shifts of O 1s binding energy to either side (Shang et al., 2017). It was rationally deduced that there existed the strong interaction of two oxides in Co3O4/In2O3. Furthermore, the chemical adsorption oxygen species played a significance part in catalytic oxidation. The more Oβ species exhibited, the better catalytic activity was discovered. Table 2 exhibited the Oβ/(Oɑ + Oβ + Oγ) ratio. For Co4In1, Co2In1, Co1In1 and Co0.5In1, the surface atomic ratio of Oβ/(Oɑ + Oβ + Oγ) were 0.31, 0.37, 0.24 and 0.33, respectively.
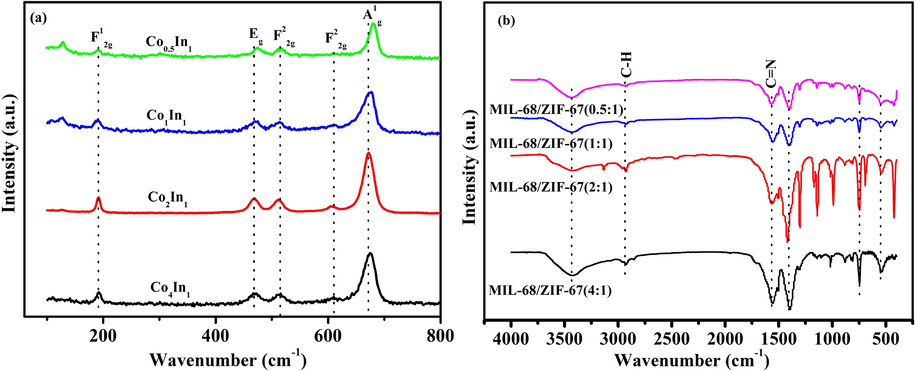
The Raman spectra of four samples were shown in Fig. 4a. Five distinct peaks were located at 192, 477, 521, 613 and 678 cm−1, which were consistent with the position and width of Co3O4 (Bahlawane et al., 2009). The band at 192 cm−1 was attributed to the characteristics of tetrahedral sites (CoO4), corresponding to F2g1 symmetry. The band at 678 cm−1, corresponding to the A1g mode, was attributed to the octahedral sites (CoO6) in the O7h symmetry. The weak band at 613 cm−1 was associated with the F2g2 mode, the Raman modes at 477 cm−1 and 521 cm−1 was respectively symmetries of F2g2 and Eg mode, which resulted from the vibration of tetrahedral and octahedral sites. Nevertheless, no position and width was consistent with the spinel of In2O3. Compared with Co4In1, Co1In1 and Co0.5In1 catalysts, the strongest symmetry, A1g, in the Co2In1 sample slightly shifted to lower wave-numbers as well as described red shift. Generally speaking, red shift narrow peaks indicated that the sample had a highly defective structure. It was vitally significant to activate absorbed oxygen molecules (Ren et al., 2018).
Characteristic functional groups of the synthesized ZIF-67/MIL-68 were verified by FT-IR spectra. The adsorption peaks in the range of 500–4000 cm−1 for ZIF-67/MIL-68 (4:1, 2:1, 1:1, 0.5:1) were shown in Fig. 4b. It was well known that ZIF-67 were derived from the stretching ligand of 2-MIM, and the peaks at 1568 cm−1 and 2936 cm−1 were assigned to the stretching modes of the C=N and C-H bonds in 2-MIM. It was obvious that the strength of bonds tended to decrease from ZIF-67/MIL-68 (4:1) to ZIF-67/MIL-68 (0.5:1). It was verified that bonds strength was proportional to the amount of 2-MIM added.
The oxidation–reduction properties of the four samples were studied by H2-TPR. Fig. 5 illustrated the results of the four samples. It was observed that there were four reduction peaks. The first weak peak centered at 100–130 °C could be related to active surface oxygen species (Xie and Shen, 2009; Davies et al., 2006). The second reduction peak should be the reduction of Co3O4 to CoO (Co3O4 + H2 → 3CoO + H2O), and the third reduction peak should be the reduction of CoO to metallic cobalt (3CoO + 3H2 → 3Co + 3H2O) (Ji et al., 2009). The fourth weak peak could be assigned to the reduction process of In2O3 to metallic indium for the case of In2O3 reducation. As shown in Fig. 5, it was observed that the different peak position of these four samples was caused by different moral ratio of Co to In. Compared to other catalysts, the active surface oxygen (peak I) Co3+ reduction (peak II) Co2+ reduction (peak III) and of Co2In1 catalyst shifted to lower temperatures at l01 °C and 368 °C, respectively. The shift of these reduction peaks was generally recognized as a sign of the improved reducibility of Co3+ to Co2+. Meanwhile, Co2In1 catalyst owned the more active surface oxygen. Nevertheless, the In3+ reduction peak (peak IV) shifted to a higher temperature around 700 °C. The shifted of peak locations indicated the presence of strong interactions between cobalt oxide and indium oxide (Ma et al., 2018). In general, a catalyst with small crystallite size showed superior reduction capacity. Thus, the Co2In1 sample displayed excellent reducibility due to smallest crystallite size. Because of more adsorbed oxygen species, it could clearly be noticed that the Co2In1 catalyst might exhibit superior catalyst activity.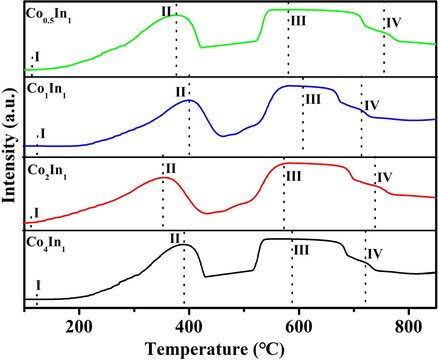
H2-TPR profiles of different catalysts.
2.4 Catalytic performance of toluene oxidation
2.4.1 Catalytic activity
The catalytic activity curves of Co4In1, Co2In1, Co1In1 and Co0.5In1 and pure Co3O4 catalysts was shown in Fig. 6a, and the temperatures for 50%, 90% and 100% toluene conversion (T50, T90 and T100) of four catalysts were listed in Table 3. It could be observed that the conversions of toluene increased with a rise of the reaction temperature. T50 of Co4In1, Co2In1, Co1In1, Co0.5In1 and Co3O4 catalysts were 176, 159, 167, 164 and 244 °C, while T90 of Co4In1, Co2In1, Co1In1, Co0.5In1 and Co3O4 catalysts were 208, 182, 224, 200 and 260 °C, respectively, indicating that the performance of catalysts was the order of Co2In1 > Co0.5In1 > Co4In1 > Co1In1 > Co3O4 catalysts. It could be inferred that Co3O4/In2O3 catalysts presented the superior catalytic performance due to the presence of In2O3. In addition, the differences distinctly depended on the various moral ratio of Co to In, indicating that the choice of suitable moral ratio played an important role in determining the activity of toluene oxidation. Table 4 summarized the comparation of catalytic activity on these samples and the former literatures for toluene oxidation. For example, Co-Mn (1:1) (T90 = 240 °C) (Luo et al., 2018), Co3O4-0.01 (T90 = 226 °C) (Li et al., 2018), 7.4 Au/Co3O4 microspheres (T90 = 250 °C) (Yang et al., 2014), Co3O4-KIT-6 (T90 = 233 °C) (Du et al., 2012), Co3O4-HT (T90 = 260 °C) (Bai and Li, 2014). Compared to the previous literature, Co2In1 catalyst presented excellent catalytic performance.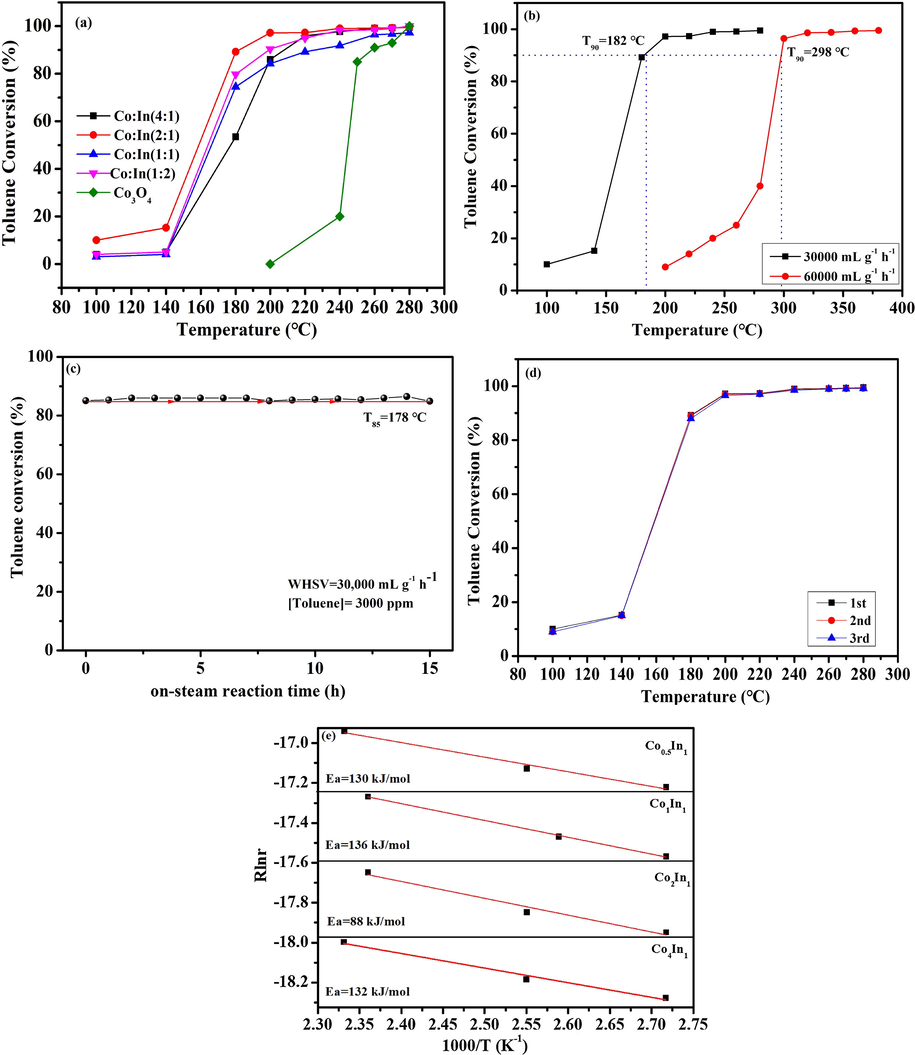
(a) Catalytic performance for toluene oxidation (b) Effect of weight hourly space velocity on toluene oxidation over the Co2In1 sample. (c) Stability tests on Co2In1. (d) The toluene conversion during three consecutives runs using Co2In1 sample. (e) Arrhenius plots of toluene catalytic oxidation reaction over Co4In1, Co2In1, Co1In1 and Co0.5In1 catalysts.
samples
Surface area(m2 g−1)
VOC type
VOC conc. (ppm)
WHSV (mL g-1h−1)
T50% (°C)
T90% (°C)
Ref. no.
Mn-Co (1:1)
27.9
Toluene
500
96,000
226
240
36
Co3O4-0.01
56
Toluene
1000
15,000
217
226
53
7.4 Au/Co3O4 microspheres
22.4
Toluene
1000
20,000
242
250
54
Co3O4-KIT6
102
Toluene
1000
20,000
228
233
55
Co3O4-HT
41.9
Toluene
1000
20,000
241
260
56
Co2In1
75.58
Toluene
3000
30,000
159
182
Present work
Fig. 6b showed the influence of weight hourly space velocity (WHSV) for the toluene reaction over Co2In1 catalyst. Because of the increase of retention time of toluene in the catalyst bed, the complete conversion temperature of toluene increased with the decrease of WHSV from 60,000 to 30000 mL g-1h−1. When the WHSV was 30,000 and 60000 mL g-1h−1, the total conversion temperature (T100) was 182 and 298 °C, respectively. The Co2In1 catalyst exhibited superior catalytic performance at the lower WHSV, suggesting that the WHSV greatly influenced on the performance of Co2In1 catalyst.
The phenomenon about different on catalytic activity of four Co3O4/In2O3 catalysts might be explained by catalysts synthesis and characterization. From N2 adsorption–desorption analysis, the Co2In1 catalyst exhibited larger specific surface area, thus it showed better catalytic activity. Furthermore, the higher surface Co3+ species and surface chemical adsorbed oxygen of Co2In1 catalyst were advantageous to the reaction of toluene oxidation. Thus, the combination of these effects usually leads to the superior catalytic activity of Co2In1 catalyst in reaction of toluene oxidation. According to XRD, XPS and Raman, cobalt oxide spinel-type crystallites included both Co2+ in tetrahedral and Co3+ in octahedral positions. Active centers of oxidation were formed in octahedral positions, it could be benificial to form oxygen vacancies in electron transferred progress, which boost absorption of toluene molecules in the catalytic oxidation cycle (Ma et al., 2018). Thus Co3+ which located at a relatively opened coordination position could be a centre of oxygen adsorption and formation of active oxygen species which were a precondition for catalytic oxidation. The Co2In1 catalyst had the higher surface Co3+ species, thus it exhibited superior catalytic activity. The surface chemical adsorbed oxygen played an extremely important role on toluene oxidation. It could be observed that the Co2In1 catalyst owned the more surface Oβ species and Oβ/(Oα + Oβ + Oγ) atomic ratios was directly proportional to catalytic activity. Therefore, this was a reason that Co2In1 catalyst possessed excellent catalytic activity in reaction of toluene oxidation. In addition, a weak reduction peak was observed from H2-TPR, it attested the molecular oxygen species adsorbed on the oxygen vacancies, which consistent with XPS. The oxygen vacancy could active the O2 and promote catalytic oxidation, simultaneously. Furthermore, the strong interactions between cobalt oxide and indium oxide influenced the catalytic activity in toluene catalytic oxidation. Besides, the Co2In1 catalyst had smallest crystallite size, leading to strong reducibility. Low temperature reducibility could enhance the catalytic performance of this catalyst. All of these factors determined that the Co2In1 catalyst presented excellent catalytic activity for toluene catalytic oxidation.
2.4.2 Stability
The suitability of a catalyst for VOCs catalytic combustion was influenced by a limited or ideally negligible ability to yield by-products coming from an incomplete conversion of the feed. At lower reaction temperatures, although no CO was noticed, significant amounts of benzoic acid benzaldehyde and benzyl alcohol were detected. However, catalysts showed a comparable excellent selectivity to CO2 (100%) at the temperature for 100% toluene conversion. Furthermore, the best Co2In1 catalyst was selected to evaluate its thermal stability, and the results were exhibited in Fig. 6. The thermal lifetime experiments of the Co2In1 were investigated and shown in Fig. 6c. It was obvious that the curve fluctuates in 85% ranges. Hence, the Co2In1 catalyst at 178 °C was stable over the period of 15 h, perfectly. In summary, Co2In1 catalyst had excellent thermal stability. As shown in Fig. 6d, it was discovered that there was almost no differences between the three curves, suggesting that the Co2In1 catalyst presented similar catalytic activity. Therefore, it was concluded that the Co2In1 catalyst had favorable performance in the catalytic cycling for toluene combustion.
It was well known that the rate of chemical reaction was associated with the activation energy. By reducing the activation energy, some slow reactions would be promoted. The Arrhenius plots for toluene oxidation over three samples reaction were studied. The detail calculation method was shown in supporting information. As shown in Fig. 6e, all profiles exhibited excellent linear relations between ln r and 1000/T and the Ea of the Co2In1 catalysts were listed in Table 3. The Co2In1 catalyst had the lower Ea (Ea = 88 kJ mol−1) than Co4In1 catalyst (Ea = 132 kJ mol−1), Co1In1 catalyst (Ea = 136 kJ mol−1) and Co0.5In1 catalyst (Ea = 130 kJ mol−1). It was obvious that the catalytic activities followed an inverse trend with respect to the Ea values. Thus, the results confirmed that the Co2In1 exhibited the superior catalytic activity.
2.4.3 The texture and chemical properties after toluene oxidation reaction
XPS results of Co2In1 catalyst after three consecutive runs (used Co2In1) were presented in Fig. 7. The Co 2p XPS results of the catalyst could also be curve-fitted into 2 components, which were characteristic of Co3+ (low energy) and Co2+ (high energy). The above work confirmed that the ratio of Co3+/(Co3++Co2+) could influence the catalytic activity of catalyst. It was discovered that there was no difference between the fitting peaks of fresh and used Co2In1 catalyst at binding energies. Furthermore, according to Table 2, the ratios of Co3+/(Co3++Co2+) were 0.49 and 0.47, indicating that Co3+ cations were the dominant species in Co3O4 spinel oxide. As shown in Fig. 7b, the O 1s XPS of used Co2In1 catalyst could be fitted by three peaks as well. The peaks at binding energies of around 529.6, 530.6, 532.1 eV was assigned to the surface lattice oxygen in the lattice oxygen (Oɑ), chemical adsorption oxygen (Oβ), adsorbed water/OH (Oγ), respectively. According to Table 3, the ratios of Oβ/(Oɑ+Oβ + Oγ) were 0.37 and 0.35, indicating that the surface adsorption oxygen was not much less. Thus, the Co2In1 catalyst had favorable performance in the catalytic cycling of toluene oxidation.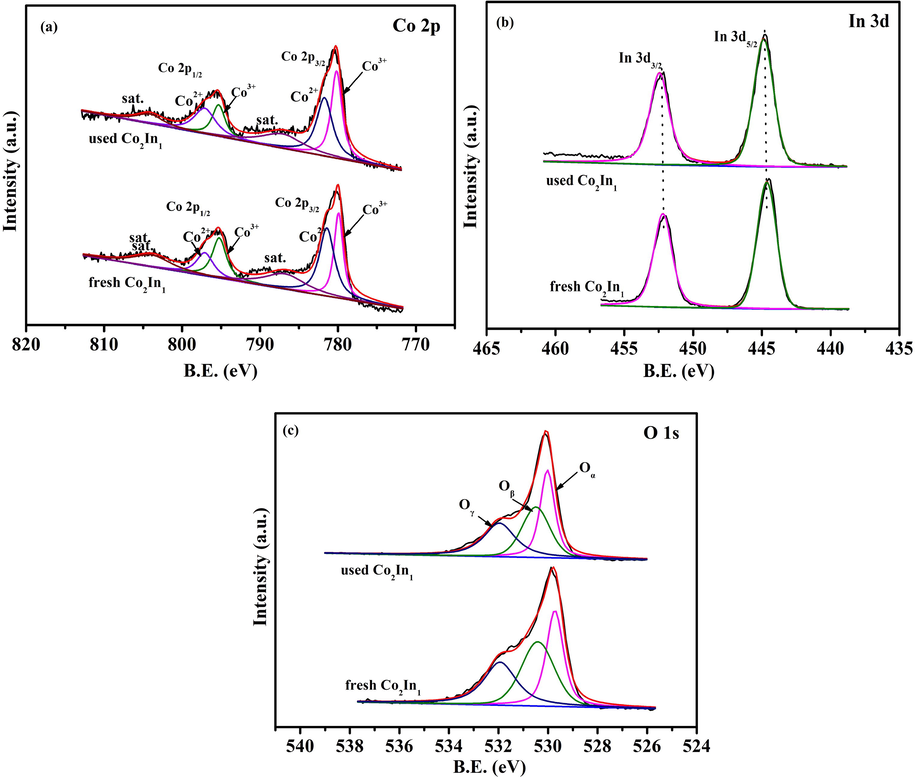
Co 2p, In 3d and O 1s XPS spectra of Co2In1 catalyst before and after reaction.
2.4.4 Proposed reaction mechanism
According to the above results and literature (Han et al., 2018; Kamal et al., 2016), chemically adsorbed oxygen played an important role and Co3+ transformed to Co2+ in the catalytic reaction of toluene combustion. Thus, Langmuir-Hinshelwood (L-H) mechanism was considered for the toluene oxidation process, preferentially. The L-H mechanism assumed that the reaction occurred between the adsorbed molecular, including the adsorbed VOCs and the adsorbed oxygen species. Therefore, both the VOCs and oxygen molecule must be adsorbed on the surface of the catalyst. Toluene oxidation reaction path could be carried out in a continuous step. As shown in Fig. 8, the toluene molecule was adsorbed on the surface of catalysts, and reacted with the chemical adsorbed oxygen to form benzaldehydic species, eventually, formed CO2 and H2O. At the same time, the catalysts produced oxygen vacancies, and then O2 molecular would replenish in the reaction gas to form the new chemical adsorbed oxygen. So, it completed the redox cycle.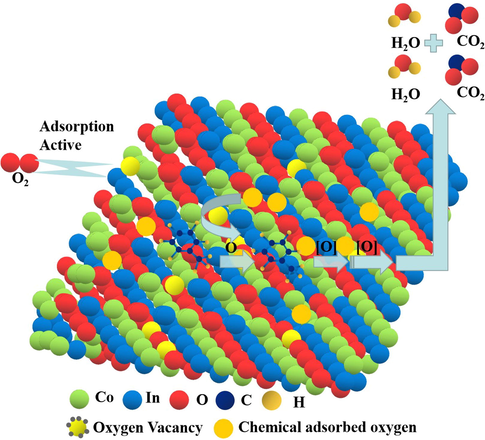
Schematic of toluene combustion on Co3O4/In2O3.
3 Conclusion
In summary, we synthesized a series of Co3O4/In2O3 catalysts with different Co/In molar ratio through the pyrolysis of ZIF-67/MIL-68, and the influence of Co/In molar ratio of Co3O4/In2O3 catalysts on the toluene oxidation was studied. From SEM image, the amount of in situ growth of Co3O4 nano-dodecahedra on In2O3 hexagonal prisms was diverse, obviously. Among the four Co3O4/In2O3 catalysts, the Co2In1 catalyst exhibited superior catalytic activity. The temperature for 90% toluene conversion (T90) of Co2In1 catalyst was 182 °C. Meanwhile, the Co2In1 catalyst also exhibited good cycling at three consecutive runs and the excellent long-term thermal stability. Through various characterizations, it was concluded that the excellent catalytic performance of Co2In1 catalyst was attributed to the higher atomic ratio of Co3+/(Co3++Co2+) on the surface, lots of surface adsorbed oxygen, larger specific area and minimum crystallite size. In addition, this novel strategy could also open a door for the application of MOF materials to VOCs removal.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21707145, 51908535), Key Science and Technology Program of Lanzhou City (2018-RC-65), Major Science and Technology Projects in Inner Mongolia Autonomous Region, Province Natural Science Foundation of GanSu (18JR3RA383), the Science and Technology Program of Chengguan district, Lanzhou city (2019JSCX0042).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Tailoring the properties and the reactivity of the spinel cobalt oxide. Phys. Chem. Chem. Phys.. 2009;11:9224-9232.
- [Google Scholar]
- Positive Effects of K+ Ions on Three-Dimensional Mesoporous Ag/ Co3O4 Catalyst for HCHO Oxidation. ACS Catal.. 2014;4:2753-2762.
- [Google Scholar]
- Surface-nucleated heterogeneous growth of zeolitic imidazolate framework-A unique precursor towards catalytic ceramic membranes: Synthesis, characterization and organics degradation. Chem. Eng. J.. 2018;353:69-79.
- [Google Scholar]
- Catalytic combustion of 1,2-dichlorobenzene at low temperature over Mn-modified Co3O4 catalysts. Appl. Catal. B: Environ.. 2015;166–167:393-405.
- [Google Scholar]
- In situ pyrolysis of Ce-MOF to prepare CeO2 catalyst with obviously improved catalytic performance for toluene combustion. Chem. Eng. J.. 2018;344:469-479.
- [Google Scholar]
- Nanocrystalline cobalt oxide: a catalyst for selective alkane oxidation under ambient conditions. Chem. Commun.. 2006;32:3417-3419.
- [Google Scholar]
- VOC oxidation over MnOx-CeO2 catalysts prepared by a combustion method. Appl. Catal. B: Environ.. 2008;84:303-312.
- [Google Scholar]
- Pd nanoparticles supported on N-doped porous carbons derived from ZIF-67: Enhanced catalytic performance in phenol hydrogenation. J. Ind. Eng. Chem.. 2017;46:258-265.
- [Google Scholar]
- Three-dimensional mesoporous manganese oxides and cobalt oxides: High-efficiency catalysts for the removal of toluene and carbon monoxide. Microporous Mesoporous Mater.. 2012;162:199-206.
- [Google Scholar]
- Catalytic combustion of volatile organic compounds. J. Hazard. Mater.. 2004;109:113-139.
- [Google Scholar]
- Copper Ion Enhanced Synthesis of nanostructured cobalt oxide catalyst for oxidation of methane. ChemCatChem. 2012;4:1551-1554.
- [Google Scholar]
- Deep oxidation of volatile organic compounds using ordered cobalt oxides prepared by a nanocasting route. Appl. Catal. A: Gen.. 2010;386:16-27.
- [Google Scholar]
- Outstanding Water-resistance Pd-Co nanoparticles functionalized mesoporous carbon catalyst for CO catalytic oxidation at room temperature. Chemistryselect. 2018;3:6601-6610.
- [Google Scholar]
- Morphology-controlled synthesis of 3D, mesoporous, rosette-like CeCoOx catalysts by pyrolysis of Ce[Co(CN)6] and application for the catalytic combustion of toluene. Nanoscale. 2018;10:21307-21319.
- [Google Scholar]
- Comparative Studies on Porous Material-Supported Pd Catalysts for Catalytic Oxidation of Benzene, Toluene, and Ethyl Acetate. Ind. Eng. Chem. Res.. 2009;48:6930-6936.
- [Google Scholar]
- Comparative Study on the Formation and Reduction of Bulk and Al2O3-Supported Cobalt Oxides by H2-TPR Technique. J. Phys. Chem. C. 2009;113:7186-7199.
- [Google Scholar]
- MIL-68 (In) nano-rods for the removal of Congo red dye from aqueous solution. J. Colloid Interface Sci.. 2015;453:270-275.
- [Google Scholar]
- Catalytic oxidation of volatile organic compounds (VOCs)-a review. Atmos. Environ.. 2016;140:117-134.
- [Google Scholar]
- Catalytic combustion of VOCs over a series of manganese oxide catalysts. Appl. Catal. B: Environ.. 2010;98:180-185.
- [Google Scholar]
- Optimization of structured catalyst carriers for VOC combustion. Catal. Today. 2005;105:378-384.
- [Google Scholar]
- Effect of cobalt loading on the solid state properties and ethyl acetate oxidation performance of cobalt-cerium mixed oxides. J. Colloid. Interf. Sci.. 2017;496:141-149.
- [Google Scholar]
- Nanoscale PdO catalyst functionalized Co3O4 hollow nanocages using MOF templates for selective detection of acetone molecules in exhaled breath. ACS Appl. Mater. Inter.. 2017;9:8201-8210.
- [Google Scholar]
- Surface modification of coconut shell based activated carbon for the improvement of hydrophobic VOC removal. J. Hazard. Mater.. 2011;192:683-690.
- [Google Scholar]
- Catalytic combustion of VOCs on non-noble metal catalysts. Catal. Today. 2009;148:81-87.
- [Google Scholar]
- Fabrication of mesoporous Co3O4 oxides by acid treatment and their catalytic performances for toluene oxidation. Appl. Catal. A: Gen.. 2018;550:67-76.
- [Google Scholar]
- Mesoporous Co3O4-supported gold nanocatalysts: Highly active for the oxidation of carbon monoxide, benzene, toluene, and o-xylene. J. Catal.. 2014;309:408-418.
- [Google Scholar]
- The effects of Bi2O3 on the CO oxidation over Co3O4. Catal. Today. 2011;175:610-614.
- [Google Scholar]
- Promoting Effects of In2O3 on Co3O4 for CO Oxidation: Tuning O2 Activation and CO Adsorption Strength Simultaneously. ACS Catal.. 2014;4:4143-4152.
- [Google Scholar]
- Ultralow-temperature CO oxidation on an In2O3-Co3O4 catalyst: a strategy to tune CO adsorption strength and oxygen activation simultaneously. Chem. Commun.. 2014;50:6835-6838.
- [Google Scholar]
- Insights into the high performance of Mn-Co oxides derived from metal-organic frameworks for total toluene oxidation. J. Hazard. Mater.. 2018;349:119-127.
- [Google Scholar]
- Indium-doped Co3O4 nanorods for catalytic oxidation of CO and C3H6 towards diesel exhaust. Appl. Catal. B: Environ.. 2018;222:44-58.
- [Google Scholar]
- Kinetic characterization of toluene biodegradation by Rhodococcus erythropolis: towards a rationale for microflora enhancement in bioreactors devoted to air treatment. Chem. Eng. J.. 2014;247:199-204.
- [Google Scholar]
- Control of the Interphases Formation Degree in Co3O4/ZnO Catalysts. ChemCatChem. 2013;5:1431-1440.
- [Google Scholar]
- Combustion of non-halogenated volatile organic compounds over group VIII metal catalysts. Appl. Catal. B: Environ.. 1997;13:175-184.
- [Google Scholar]
- Controllable synthesis of 3D hierarchical Co3O4 nanocatalysts with various morphologies for the catalytic oxidation of toluene. J. Mater. Chem. A.. 2018;6:498-509.
- [Google Scholar]
- Treatment of Wet Process Hardboard Plant VOC Emissions by a Pilot Scale Biological System. Biochem. Eng. J.. 2007;37:261-270.
- [Google Scholar]
- Activity and stability of Co3O4-based catalysts for soot oxidation: The enhanced effect of Bi2O3 on activation and transfer of oxygen. Appl. Catal. B: Environ.. 2017;209:33-44.
- [Google Scholar]
- Synthesis of ZIF-8 and ZIF-67 by steam-assisted conversion and an investigation of their tribological behaviors. Angew. Chem. Int. Ed.. 2011;50:672-675.
- [Google Scholar]
- Porous Mn-Co mixed oxide nanorod as a novel catalyst with enhanced catalytic activity for removal of VOCs. Catal. Commun.. 2014;56:134-138.
- [Google Scholar]
- Chemical Absorption Process for Degradation of VOC Gas Using Heterogeneous Gas-Liquid Photocatalytic Oxidation: Toluene Degradation by Photo-Fenton Reaction. Chemosphere. 2008;73:768-775.
- [Google Scholar]
- The effect of synthesis temperature on the particle size of nano-ZIF-8. Micropor. Mesopor. Mater.. 2016;221:8-13.
- [Google Scholar]
- Nanoscale Co-based catalysts for low-temperature CO oxidation. Catal. Sci. Technol.. 2015;5:1014-1020.
- [Google Scholar]
- Geometrical-site-dependent catalytic activity of ordered mesoporous Co-based spinel for benzene oxidation: In situ DRIFTS Study coupled with Raman and XAFS spectroscopy. ACS Catal.. 2017;7:1626-1636.
- [Google Scholar]
- A cost-effective supercapacitor material of ultrahigh specific capacitances: spinel nickel cobaltite aerogels from an epoxide-driven sol-gel process. Adv. Mater.. 2010;22:347-351.
- [Google Scholar]
- Removal of azo-dye Acid Red B (ARB) by adsorption and catalytic combustion using magnetic CuFe2O4 powder. Appl. Catal. B: Environ.. 2004;48:49-56.
- [Google Scholar]
- Morphology control of cobalt oxide nanocrystals for promoting their catalytic performance. Nanoscale.. 2009;1:50-60.
- [Google Scholar]
- Catalytic decomposition of N2O over MxCo1-xCo2O4 (M = Ni, Mg) spinel oxides. Appl. Catal. B: Environ.. 2003;45:85-90.
- [Google Scholar]
- Porous Cube-Aggregated Co3O4 Microsphere-Supported Gold Nanoparticles for Oxidation of Carbon Monoxide and Toluene. ChemSusChem. 2014;7:1745-1754.
- [Google Scholar]
- Probing the adsorption performance of the hybrid porous MIL-68 (Al): a synergic combination of experimental and modelling tools. J. Mater. Chem.. 2012;22:10210-10220.
- [Google Scholar]
- Solvothermal systhesis of size-controlled porous Co3O4 nanostructure catalyst and their catalytic properties for VOCs removal. Chemistryselect. 2018;3:10408-10417.
- [Google Scholar]
- Controlled porous hollow Co3O4 polyhedral nanocages derived from metal-organic frameworks (MOFs) for toluene catalytic oxidation. Mol. Catal.. 2019;463:77-86.
- [Google Scholar]
- Carefully design hollow MnxCo3-xO4 polyhedron derived by Mn@Co-ZIFs and applied to catalytic oxidation of VOCs. Cryst. Growth Des.. 2019;19:6207-6217.
- [Google Scholar]
- MOx (M = Mn, Fe, Ni or Cr) improved supported Co3O4 catalysts on ceria–zirconia nanoparticulate for CO preferential oxidation in H2-rich gases. Appl. Catal. B: Environ.. 2012;115–116:53-62.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2020.01.014.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1