Translate this page into:
Influence of nickel powders on burning behaviors of single-based propellant in variable-pressure and constant-pressure combustion conditions
⁎Corresponding authors at: No.200, Xiaolingwei, Xuanwu District, Nanjing 210094, China. liuzhitao331@163.com (Zhitao Liu), liaoxin331@163.com (Xin Liao)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Single-based propellants containing 0.4 wt%, 0.8 wt% and 1.6 wt% nickel powders(NPs) were prepared and investigated in closed bomb tester and transparent combustion chamber, which can provide variable-pressure and constant-pressure conditions for propellant burning, respectively. In closed bomb tester, propellants burned to a pressure above 5–220 MPa. The results showed that burning rate of propellants was increased by 19.0 %, 12.5 % and 11.9 % in 5–8 MPa, 8–16 MPa and 20–200 MPa, when 1.6 wt% NPs used in propellant. Meanwhile, propellants burning in combustion chamber were with a constant pressure of 1.0 MPa, 1.5 MPa, 2.0 MPa, 3.0 MPa and 5.0 MPa. The burning rate of propellants can be increased by NPs in 1.0–5.0 MPa. While the catalytic efficiency(Z) had high dependence on NPs contents and burning pressure. At 5.0 MPa, 0.4 wt%, 0.8 wt% and 1.6 wt% of NPs enhanced burning rate of propellants by 42 %, 44 % and 68 % than that of original propellants. In pressure below 3.0 MPa, burning rate of propellants cannot be increased with 0.4 wt% and 0.8 wt% NPs, but that can be enhanced with 1.6 wt% NPs, suggested that higher NPs content and higher pressure were conducive to catalytic effect, which was significant with the pressure above 5 MPa. Furthermore, after NPs incorporating into propellant, the distance from flame zone to propellant burning surface was significantly reduced. And the potential catalytic mechanism was proposed.
Keywords
Nickel powders
Propellants
Combustion catalyst
Burning rate
1Introduction
Improving the combustion performance of solid rocket and gun propellants are always an important aspect for researchers (Pang et al., 2016) (Shen et al., 2020) (Dokhan et al., 2002). The use of combustion catalysts is one of the important ways to tailor the burning behaviors of propellants, that include modifying propellant burning rate and dependence on pressure and initial temperature (Denisyuk et al., 2021) (Verma and Ramakrishna, 2013) (Jayaraman et al., 2011). The modified combustion properties made it possible to significantly improve performance of rocket and gun weapons. Therefore, a lot of studies have been devoted to the investigation of the influence of catalysts on the combustion of various propellants and the mechanism of catalytic effects (Denisyuk et al., 2021) (Yadav et al., 2021).
At present, a variety of transition metal powders, including aluminum(Al) particles (Deluca, 2018) (Sergienko et al., 2019) (Shen et al., 2021), magnesium(Mg) particles (Yartys et al., 2019) (Huang et al., 2013) (Abd et al., 2016) and boron(B) particles (Sergienko et al., 2019) (Perez et al., 2014) (Yuan et al., 2021), have been introduced into rocket propellants to enhance heat generating and specific impulse. According to previous reports (Jiang and Li, 2006) (Yuan et al., 2019) (Athwawale et al., 2004) (Jiang et al., 2006) (Ma et al., 2015) (Yuan et al., 2016), nickel metal powders are relatively weak in energetic respects, but showed high catalytic activity for combustion of rocket propellant. In Jiang’s work (Jiang and Li, 2006), 2 wt% nano-nickel powders were incorporated into AP/RDX/Al/HTPB composite propellants, which exhibited increased burning rate and decreased pressure exponential in the pressure range of 2–10 MPa. Yuan et al. (Yuan et al., 2019)added nano-nickel powders in CL-20/Al-CMDB propellants, then investigated the combustion performance of propellant in 4–10 MPa, 8–20 MPa and 15–20 MPa. Athawale et al. (Athwawale et al., 2004)reported the burning rate results of Ni-based fuel-rich propellants with hydroxyl terminated polybutadiene(HTPB) and double-based(DB) matrix as binder in the pressure range of 1.0–8.8 MPa. The nickel powder was with 20 wt%∼40 wt% content in the propellants. In work (Yuan et al., 2016), Yuan et al. studied the burning behaviors of RDX/Al-CMDB propellant with nano-nickel in 12–22 MPa, 16–22 MPa and 8–22 MPa. As reported previously, the nickel powder is attractive to rocket propellant, including CMDB and HTPB propellant, and can significantly promote the combustion. However, nickel powders have been still almost unexplored in gun propellant composition so far. Furthermore, nickel oxides particles and nickel salt (Sharma et al., 2015) (Ma et al., 2010) (Ma et al., 2011) (Wei et al., 2009) have been reported as combustion catalysts in both rocket and gun propellants. Sharma (Sharma et al., 2015) investigated the catalytic effect of biosynthesized NiO nanoparticles for the thermal decomposition kinetics of ammonium perchlorate(AP), which is typical oxidizing agent in CMDB propellant. The first exothermic peak of AP is clearly shifted forward. In Ma’s work (Ma et al., 2010) (Ma et al., 2011), a series of nickel salt(NiFe2O4, NiCO3, Ni(NO3)2, Ni(OH)2, NiC2O4) were added in triethylene glycol dinitrate(TEGDN) gun propellants. And the results showed nickel carbonate(NiCO3) and nickel oxalate(NiC2O4) increased propellant burning rate by more than 8 % and 6.3 % in 50-150Mpa. Most works are conducted in the aspects of exploration of experimental rules, but there is a relative scarcity of in-depth mechanistic explanations. While in Wei's work (Wei et al., 2009), the investigation was carried out from the mechanism aspect. Wei (Wei et al., 2009) systematically studied the effect of NiO on the thermal decomposition of NC/TEGDN gun propellant, and proposed the potential catalytic mechanism, which can provide a theoretical reference for more relevant researches.
In these previous reports, nickel powders used as combustion catalyst in field of rocket propellants have been fully studied and exhibited a well catalytic. Most of researches have been focused on the effect of nickel powder on combustion properties of propellants in pressure below ∼ 25 MPa (Yuan et al., 2017) (Hou et al., 2021). However, catalytic combustion at higher pressures has been less explored. Compared with rocket propellants, typical gun propellants including single-based, double-based, triple-based and RDX propellants, burn usually in pressure above 300 MPa (Shen et al., 2020) (Ma et al., 2010) (Oberle, 2001). And the relevant studies of various catalysts should be suggested to perform in relatively high-pressure conditions. Due to the diversity of propellant formulations, few researches focused on single-based gun propellant and corresponding catalysts effect.
As one of important groups of gun propellant, typical single-based propellant consisting of more than 85 wt% nitrocellulose(NC) are featured with smokeless, high-mechanical strength, stable-combustion and low-erosion (Chen et al., 2014) (Fu et al., 2017) (Yu et al., 2020). They are widely used as propellant charges in various caliber guns in past few decades (Liu et al., 2012) (Brochu et al., 2013) (Wu et al., 2006). However, compared with other propellants including double-based, triple-based and RDX propellants, low burning rate and energy density of single-based propellant limit its further applications in some modern gun weapons (Chen, 2014) (Fu et al., 2017) (Yu et al., 2020). To resolve it, rational tailoring burning behaviors of propellants can result in enhanced work efficiency of propellant and increasing muzzle velocity of projectile (Ma et al., 2010) (Ma et al., 2011). Therefore, using nickel powder as catalyst is a potential way to improve performance of single-based gun propellants. Moreover, despite some researches focusing on catalytic combustion of nickel powders in HTPB, DB, CMDB rocket propellants, the catalytic effect of nickel metal powders used in single-based gun propellant has rarely been discussed in the open literature in detail so far.
In this work, we prepared a series single-based gun propellant samples with contents of 0.4 wt%, 0.8 wt% and 1.6 wt% micron nickel particles(NPs). After that, the combustion properties of the propellants were respectively investigated in closed bomb tester and transparent combustion chamber, which respectively provide variable-pressure and constant-pressure conditions for propellants burning. In the former device, propellant burnt and produced a pressure above 220 MPa. And the latter device would provide initial chamber pressure of 1.0 MPa, 1.5 MPa, 2.0 MPa, 3.0 MPa and 5.0 MPa for propellants burning. In addition, NPs using as commercial product were with more abundant sources and better economical. In the considerations, this work could provide some references for improving combustion of single-based gun propellants to better control their performance in gun weapons.
2 Experimental section
2.1 Materials
Nickel powders(NPs) were both obtained from Chemical Reagent Group of Nanjing University of Science and Technology, with particle sizes of 1-40um. Nitrocellulose were purchased from Sichuan Nitrocell Co., Ltd. (Sichuan, China) with 12.9 wt% nitrogen contents. Absolute Ethanol, acetone and diphenylamine(DPA) were obtained from Sinopharm Chemical Reagent Co., Ltd. (China).
2.2 Preparation process of propellants
In this work, original single-based propellant and single-based propellants with 0.4 wt%, 0.8 wt%, 1.6 wt% NPs replacing nitrocellulose were manufactured by a solvent extrusion technique. The preparation process was as follow: First, the wet nitrocellulose was dried in an oven at 50 °C for 7 days to remove water. Second, dried nitrocellulose, NPs and 225 ml mixed solvent made up of acetone and ethanol(1:1) and 4.5 g DPA were mixed and kneaded in a kneading machine for 2.5 h at 35 °C. After that, propellant dough was obtained, extrude into a propellant strand by single perforating mold and hydraulic machine. Then, the propellant strand was cut into single perforation grains with 4 cm length. Finally, all the grains were dry at 25 °C for 24 h and dried in an oven at 40 °C for 3 days and at 50 °C for 4 days. The formulas of prepared propellants are shown in Table 1.
Propellant samples
Nitrocellulose (wt. %)
Diphenylamine (wt. %)
NPs (wt. %)
NP-1#
98.5
1.5
0
NP-2#
98.1
1.5
0.4
NP-3#
97.7
1.5
0.8
NP-4#
96.9
1.5
1.6
2.3 Experiments
To study variable-pressure burning properties of propellants were performed in a 100 cc closed bomb tester, as shown in Fig. 1. In closed bomb tester, burning propellant grains produced an increasing pressure, that can be controlled by propellants loading density. After that, pressure(p)-time(t) curve would be recorded by a pressure sensor, and be filtered, along with the u-p curves was calculated and presented through a software for calculating the combustion behavior of propellant charge in closed bomb vessel. The calculating processes of burning rate value are based on the following reference equation (Standard, 2005):
where u(mm/s) is burning rate;
(mm) is half value of web size of propellant grain;
(g/cc) is the propellant loading density in closed vessel;
(g/cm3) is the actual propellant density;
(cm3/g) is the covolume value;
is the propellant burn off relative volume, which is calculated by p(t)/Pm;
is the propellant grain shape-coefficient; σ is the relative surface area when the relative volume
is burned off;
(MPa) and
are the maximum burning pressure and ignition powder pressure;
(MPa/s) is the
(MPa/s) value corresponding to the relative volume
burned off. The detailed calculability processes of above equation can refer to study (Zhang, 2014). The burning rate also can be calculated in process referring to study (Kubota, 2015). In this work, nitrocellulose powders were wrapped in rice paper to make an ignition charge to ignite propellant grains. The ignition powders were weight by 0.5000 g ± 0.0005 g with 0.01 g/cc and 0.02 g/cc propellant loading density and by 1.0000 g ± 0.0005 g with 0.20 g/cc propellant loading density. In closed vessel testing, the combustion of each propellant is duplicated twice in loading densities of 0.01 g/cc and 0.02 g/cc at 20 °C, as well as performing once in loading density of 0.2 g/cc at −40 °C, 20 °C and 50 °C, respectively. The duplicating testing performing at three different temperature is to expand the applicability and representativeness of the date, with the consistent tendency in enhanced burning rate observing. Furthermore, propellant grains would be treated in a hot oven at 50 °C and a frozen oven at −40 °C for 4 h before propellants burning test with an initial temperature of 50 °C and −40 °C.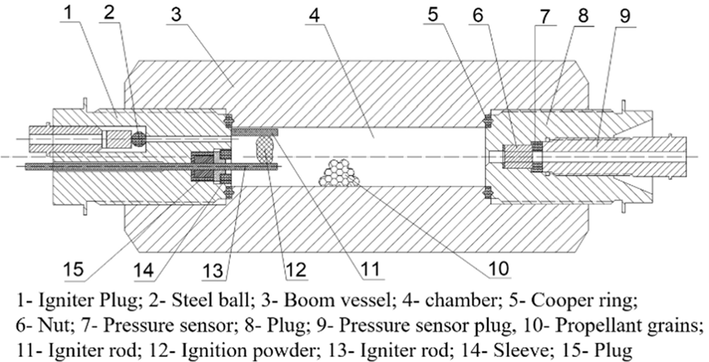
Diagram of the closed bomb tester.
Constant-pressure burning properties of propellants were investigated in a 700 cc transparent combustion chamber, in which nitrogen was pumped into to produce a constant pressure of 1.0 MPa, 1.5 MPa, 2.0 MPa, 3.0 MPa, 5.0 MPa. All single propellant grain was with 4.0 ± 0.1 cm length and 1.26 g ± 0.1 g and would be ignited by Platinum-rhodium ignition wire. After that, the burning process would be recorded by a high-speed camera, which can evaluate burning behavior of tested propellants. In transparent combustion chamber testing, the combustion of each propellant in each tested pressure point are tested twice.
3 Results and discussion
3.1 Morphology characterizations
The appearance of prepared propellants samples with nickel powders(NPs) are shown in Fig. 2. All propellant grains exhibited a smooth surface morphology. Meanwhile, the color of the propellants turned slightly black with increase of NPs contents. Furthermore, microstructure of cross-section of propellants are shown in Fig. 3, in which original single-based propellant sample was mainly composed of nitrocellulose, which kept fibrous morphology, as shown in Fig. 3a. According to Fig. 3(b, c, d), NPs showed smooth spherical appearance with a size distribution below 40um. The SEM image suggested that NPs were well dispersed in the nitrocellulose propellant matrix without agglomeration.
Photographs of prepared propellants with NPs contents of (a) 0 wt%, (b) 0.4 wt%, (c) 0.8 wt%, (d) 1.6 wt%.
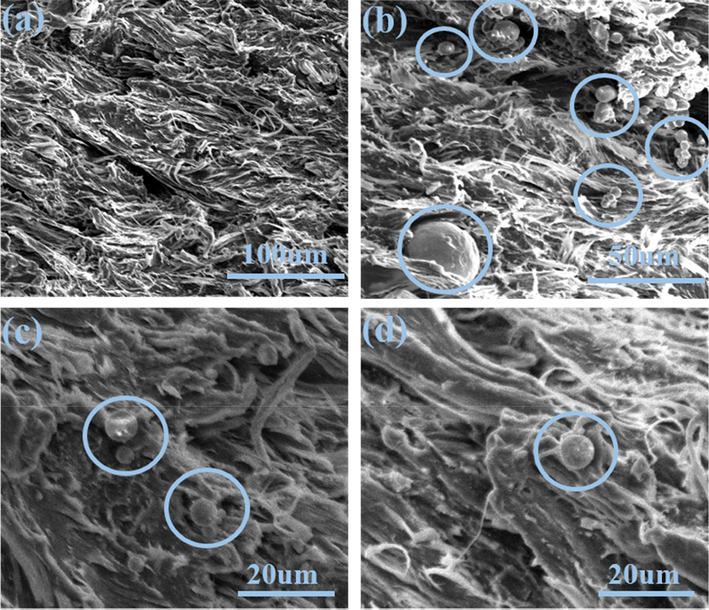
SEM images of (a) original single-based propellants; and (b) (c) (d) modified propellants containing NPs.
3.2 Variable-pressure burning properties
Variable-pressure burning properties of propellants samples were performed with a strand 100 cc closed bomb tester. Burning rate(u) and burning pressure (p) of propellant are represented by Vieille’s law (Shen et al., 2020) (Oberle, 2001) (Kubota, 2015). According to Equation(1):
Where a is the burning rate coefficient, which is a constant that depends on the chemical composition and the initial propellant temperature; and n is pressure exponent of the burning rate. To further investigate the combustion behavior of propellants, dynamic vivacity (L) and relative pressure (B) were calculated (Shen et al., 2020) (Oberle, 2001) (Kubota, 2015), according to Equations (2) and (3):
Fig. 4 showed p-t, u-p and L-B curves of NPs propellants with 0.01 g/cc and 0.02 g/cc propellants loading density at 20 °C. And Fig. 5, Fig. 6 and Fig. 7 showed p-t, u-p and L-B curves of prepared propellant with 0.20 g/cc loading density at 20 °C. Obviously, with the increase of NPs contents, propellants showed higher burning rate in 5–8 MPa(Fig. 4b), 8–16 MPa(Fig. 4e), and 20–200 MPa(Fig. 6). When pressure ranged from 5 MPa to 8 MPa(0.01 g/cc loading density), the maximum burning rate of 0 wt% NPs propellant and 1.6 wt% NPs propellant reached to 2.1 cm/s and 2.5 cm/s, respectively; and the burning rate increased 19.0 %. When pressure ranged from 8 MPa to 16 MPa(0.02 g/cc loading density), burning rate of propellant with 0 wt% NPs and 1.6 wt% NPs arrived at 1.6 cm/s and 1.8 cm/s, respectively; the burning rate increased 12.5 %. The high burning rate of propellant in 0.01 g/cc loading density can be attributed to high burning rate of ignition powder that empirically provided ignition pressure of 4.5 MPa and affects the calculated value of propellants burning rate. Furthermore, when pressure ranged from 20 MPa to 180 MPa(0.20 g/cc loading density), 1.6 wt% NPs propellant showed burning rate of 11.3 cm/s at 20 °C, 11.3 cm/s at 50 °C, 10.0cms at −40 °C. While the 0 wt% NPs propellant showed the values of 10.1 cm/s at 20 °C, 10.5 cm/s at 50 °C, 8.5 cm/s at 40 °C over the same burning pressure range. More specifically, the burning rate of 1.6 wt% NPs propellant was increased by 11.9 %, 7.6 % and 17.6 % at 20 °C, 50 °C, −40 °C, respectively. The above results suggested that the burning rate of single-based propellants can be significantly increased by NPs in the variable-pressure of 5–200 MPa, with a widely initial temperature of −40 °C to 50 °C.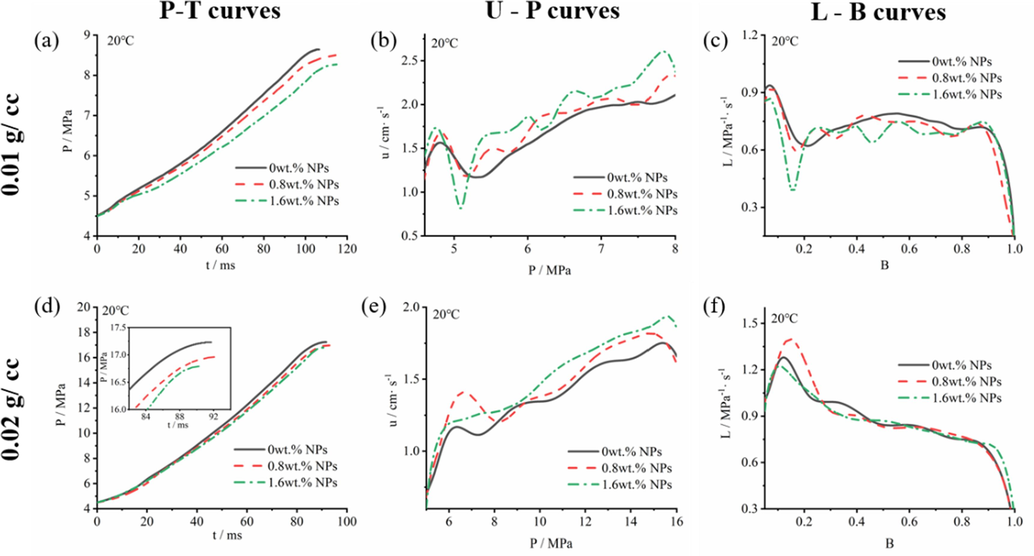
The (a)p-t, (b)u-p and (c)L-B curves of propellants containing NPs in 0.01 g/cc loading density; (d)p-t, (e)u-p and (f)L-B curves in 0.02 g/cc loading density in closed bomb performing at 20 °C.
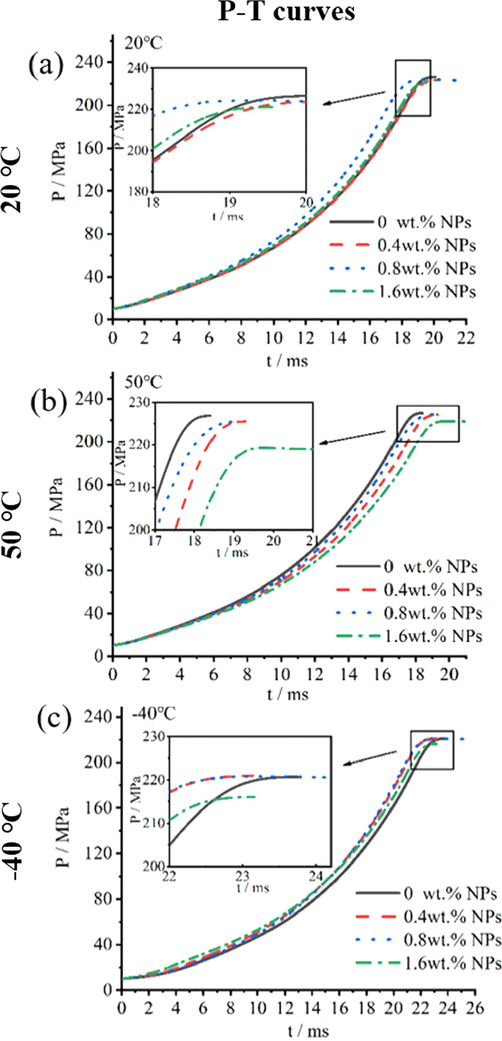
The p-t curves of propellant containing NPs in 0.20 g/cc loading density in closed bomb performing at (a)20 °C, (b)50 °C and (c)-40 °C.
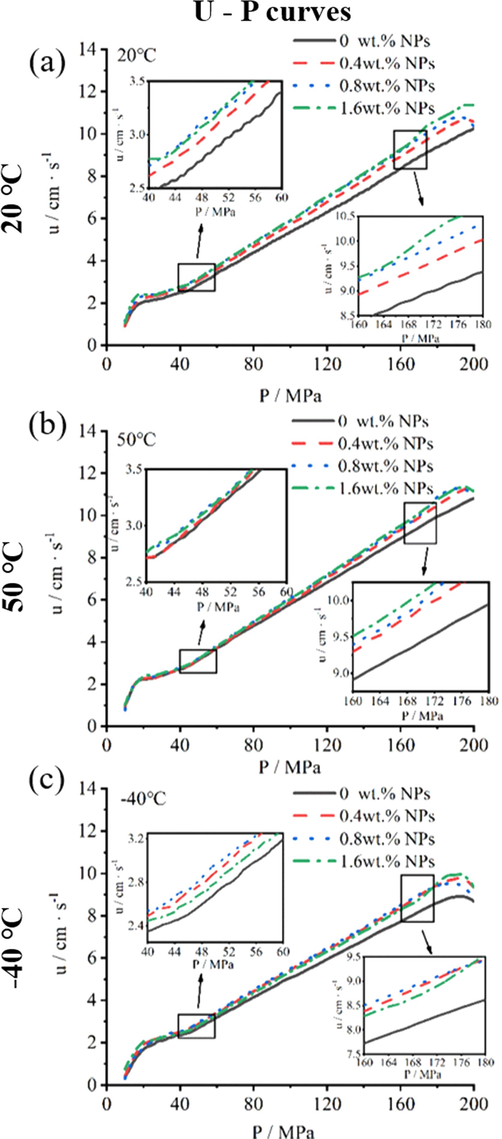
The u-p curves of propellant containing NPs in 0.20 g/cc loading density in closed bomb performing at (a)20 °C, (b)50 °C and (c)-40 °C.
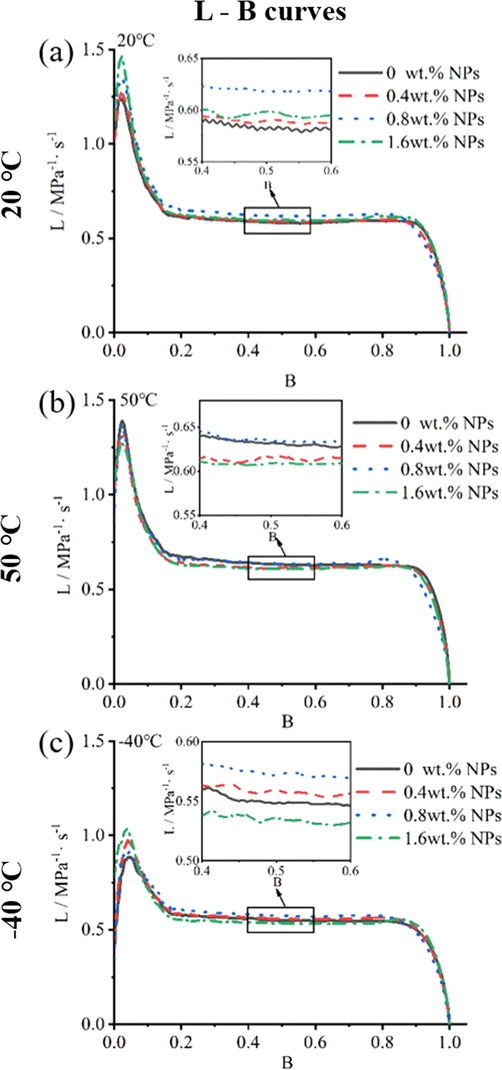
The L-B curves of propellant containing NPs in 0.20 g/cc loading density in closed bomb performing at (a)20 °C, (b)50 °C and (c)-40 °C.
Generally, L-B curves (dynamic vivacity) has been used to assess the propellant grains surface area upon propellants burning process in propellant lot acceptance (Oberle, 2001). According to Fig. 4c, 4f and Fig. 7, the L-B curves of propellants exhibited stable L value of 0.6–0.8 over the same range of B value(0.2–0.8) with 0.01 g/cc and 0.20 g/cc propellant loading density, suggested that the grains surface area behavior are stable during combustion. While the dynamic vivacity of propellants with 0.02 g/cc loading density (Fig. 4f) exhibited a slight burning regressivity of the grains. Meanwhile, the L –B curves tendency of propellants did not change after NPs adding, indicated that the addition of NPs influenced the burning rate but did not vary the burning surface area of propellant grains.
To further study the influence of NPs on the burning behavior of single-based propellants, maximum pressures are shown in Fig. 8. And pressure exponent(n), burning rate coefficient(α) are given in Fig. 9. From equation (1), the burning pressure(p), pressure exponent(n) and burning rate coefficient(α) are closely affected, which suggested that a slight change in the experimentally measured variables will result in a large change in the calculated pressure exponent and combustion rate coefficient (Oberle, 2001) (Kubota, 2015). According to Fig. 8, the maximum pressure gradually decreases as the NPs content increasing. Compared with 0 wt% NPs propellant, the maximum pressure of 1.6 wt% NPs propellants decreased by 0.43 MPa (5.0 %), 0.53 MPa (3.1 %) with 0.01 g/cc and 0.02 g/cc loading density at 20 °C test; and decreased 5.2 MPa (2.3 %), 7.5 MPa (3.3 %), 4.6 MPa (2.1 %) with 0.20 g/cc loading density at 20 °C, 50 °C and −40 °C test, respectively. Meanwhile, the maximum burning pressure of propellants samples were not increased by NPs that phenomenon can prevent higher bore pressure of gun weapons. We thought that the reduced burning pressure to some extent hedge against the higher bore pressure of gun weapons caused by the increasing burning rate of propellant.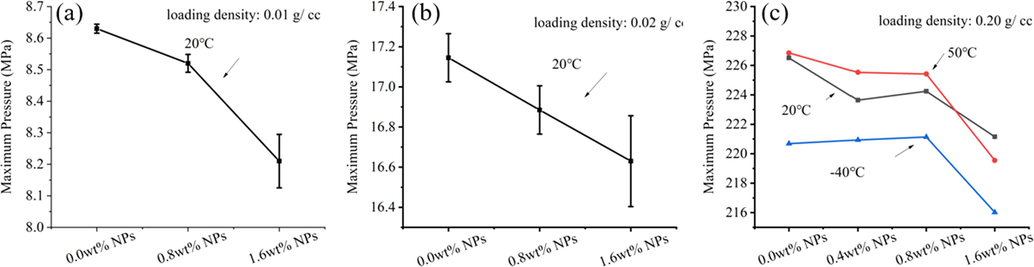
The maximum burning pressure of propellants with NPs in (a)0.01 g/cc (b)0.02 g/cc and (c)0.20 g/cc propellants loading density.
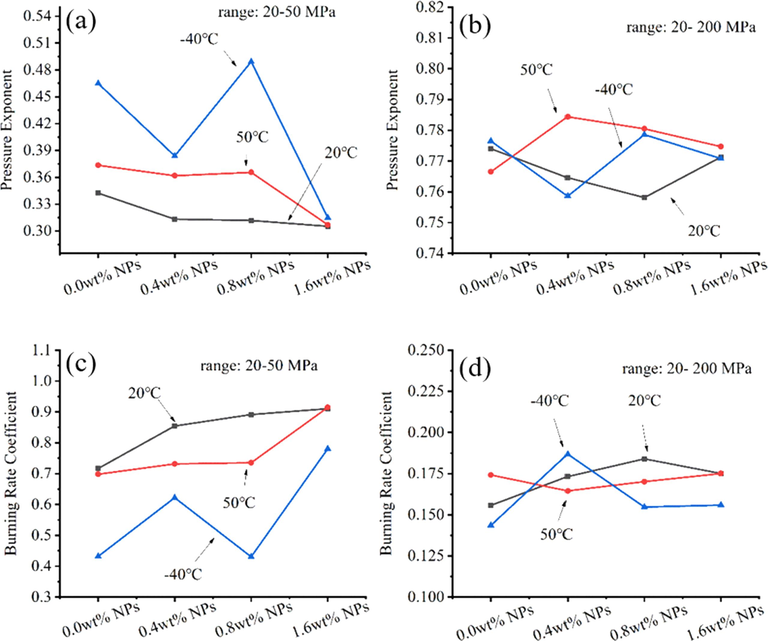
The pressure exponent(n) and burning rate coefficient(α) of propellants with NPs in 0.20 g/cc propellants loading density.
In addition, as shown in Fig. 9a, with the increase of NPs contents, the pressure exponent(n) of propellants exhibited a decreasing trend in 20–50 MPa at 20 °C and 50 °C. The decreased pressure exponent(n) indicated more stable combustion under varying burning pressure condition (Zuo et al., 2022). From Fig. 9c; over the same pressure range at 20 °C and 50 °C, the burning rate coefficient(α) of NPs propellants gradually increased as the NPs contents increasing. Furthermore, the large fluctuations of pressure exponent(n) and burning rate coefficient(α) of 0.8 wt% NPs propellant at −40 °C can be result from the unstable combustion, which attributed to poor mechanical properties of propellant at cool temperatures. Moreover, as shown in Fig. 9b and Fig. 9d, pressure exponent(n) ranged from 0.76 to 0.79, and burning rate coefficient(α) ranged from 0.14 to 0.19 in the pressure from 20 MPa to 200 MPa. The results indicated that NPs had positive enhancing effect on propellants burning rate and had minor effect on the average pressure exponent(n) and burning rate coefficient(α) of propellant from 20 MPa to 200 MPa.
3.3 Constant-pressure burning properties
Constant-pressure burning properties of nickel powders(NPs) propellants were investigated by a transparent combustion chamber which filled nitrogen with constant pressure of 1.0 MPa, 1.5 MPa, 2.0 MPa, 3.0 MPa, 5.0 MPa. Fig. 10a and Fig. 10b showed respectively the burning rate and catalytic efficiency of propellant samples. The relationships between burning rate(u) and pressure(p) have been also calculated according to Equation(1). Meanwhile, the catalytic efficiency(Z) was evaluated by Equation (4) (Denisyuk et al., 2018):
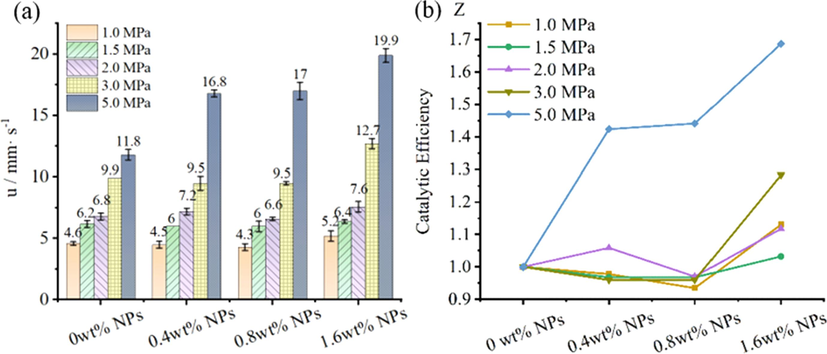
(a) Burning rate of NPs propellants and (b) Catalytic efficiency of NPs propellants in pressure of 1.0 MPa, 1.5 MPa, 2.0 MPa, 3.0 MPa, 5.0 MPa.
Where uadd and u0 are the burning rates of NPs propellants and propellant without NPs, respectively.
As seen from Fig. 10a, with increasing of initial chamber pressure, burning rate(u) of propellants were obviously enhanced. Meanwhile, according to Fig. 10b, the catalytic efficiency(Z) had high dependence on NPs contents and pressure. At 5 MPa, the 0.4 wt%, 0.8 wt% and 1.6 wt% NPs in propellant can both enhance burning rate, with catalytic efficiency(Z) of 1.42, 1.44 and 1.68, respectively. It indicated that a little NPs can increase burning rate in a higher pressure. On the other hand, when pressure was below 3.0 MPa, only 1.6 wt% NPs propellants had obviously catalytic effect, with maximum Z value of 1.28 at 3.0 MPa. Over the same pressure range, adding 0.4 wt% and 0.8 wt% NPs cannot increase propellant burning rate, with Z value between 0.93 and 1.06, suggested that the less NPs content and lower pressure were not conducive to improve burning rate. The result is consistent with the phenomenon in work (Ma et al., 2015), in which nickel additives has no significant effect on the burning rate of RDX-CMDB propellant at 1 MPa. In pressure of 1.0–5.0 MPa, the propellant with 1.6 wt% NPs exhibited the highest catalytic efficiency(Z) at 5.0 MPa, with value of 1.69, indicated that catalysis effect of NPs was significant with a high pressure.
To further investigate the dependence of the burning rates on initial chamber pressure, the pressure exponent(n) and burn rate coefficient(α) were fitted according to Equation (1). As seen from Fig. 11, despite higher catalytic efficiency with the increase of NPs content, the pressure exponent of propellants was enhanced, indicated that combustion depends on burning pressure. In general knowledge, pressure exponent(n) was affected by many factors. In work (Oberle, 2001), 2 wt% nano nickel powders decrease pressure exponent(n) of AP/RDX/Al/HTPB propellants. In work (Athwawale et al., 2004), 20 wt%-40 %wt% micron nickel powders decrease pressure exponent(n) of HTPB propellants, but that increase the pressure exponent(n) of GAP-based and DB matrix-based propellants. The disparate results in above studies suggested that pressure exponents(n) were affected by much aspects, such as particles sizes of catalyst, chemical composition of propellant and flame temperature. In this work, Ni powders exhibited higher the catalytic efficiency as the increasing pressure in closed bomb(∼200 MPa) and at constant 5 MPa in transparent combustion chamber(1 MPa, 1.5 MPa, 2 MPa, 3 MPa and 5 MPa). Previous reported that higher burning pressure can leaded to the enhanced temperature in burning surface, which could promote the melting of the metal powder and its oxide, along with their vapor flows out (Athwawale et al., 2004) (Kubota, 2015), which increase the catalytic interface and efficiency. And the increased pressure dependence of the catalytic effect may be due to the relatively lager size of Ni particles. It is notable that the high-pressure-exponent is detrimental for rocket propellants burning (Kubota, 2015). While it might partially increase burning progression for gun propellant and offset the expansion of the volume in gun chamber during projectile launching, and thus improve the work efficiency of propellant gases.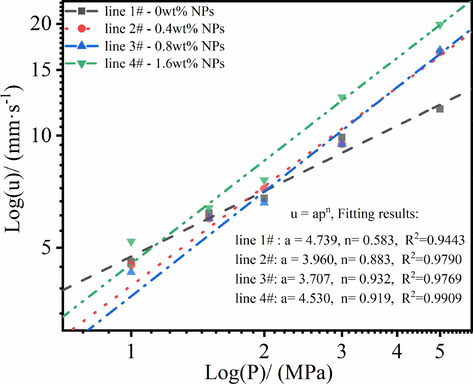
Dependence of the burning rates on initial chamber pressure (1.0–5.0 MPa).
3.4 Observation of flame structure
The overall flame propagation processes of prepared propellants were recorded with a high-speed camera in transparent combustion chamber. The flame sequence photographs of propellants combustion obtained at 1.0 MPa and 5.0 MPa are shown in the Figs. 12 and 13, respectively. All propellants samples can burn in parallel basically. At 1.0 MPa, the region above the burning surface of 0 wt% NPs propellant was dark. The phenomenon is consistent with Kubota’s observation (Kubota, 1978) in DB propellant burning at 12 atm. The flame of propellant without catalyst are almost no visible in relatively low pressure. This is due to the low velocity of NO2
NO
N2 process (Kubota, 2015). While with increase content of NPs, the NPs propellants had a flame surface with more thickness and more brightness. In addition, at 5.0 MPa, the combustion of all the samples became more intense than that at 1.0 MPa, as well the brightness zone of flame was closer to the propellant burning surface, indicating the above NO2
NO
N2 process accelerating and that the combustion wave structure was modified not only by the presence of Ni catalyst, but also by the increasing pressure. Furthermore, as seen from Fig. 14, some burning nickel particles were away from propellant grains surface, which is consistent with the typical combustion phenomenon of metal particles (Yuan et al., 2021) (Jiang et al., 2006) that burning metal powders will liquefy and vaporize and away from burning surface when temperature reach their melting and boiling point (Kubota, 2015).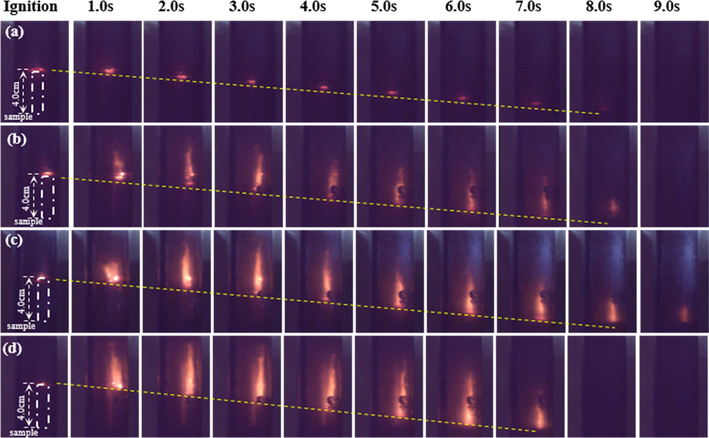
Flame propagation images sequences of propellant strands at 1.0Mpa: (a) 0 wt%; (b) 0.4 wt%; (c) 0.8 wt%; (d) 1.6 wt% NPs propellants.
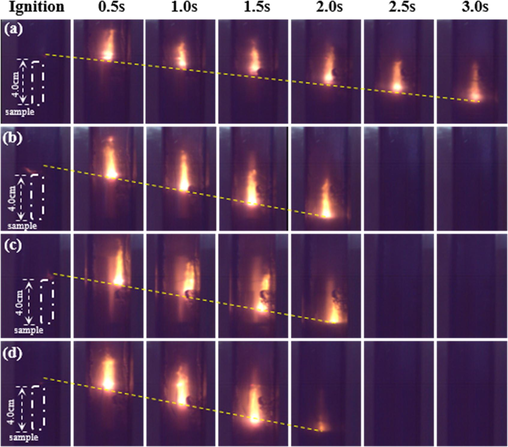
Flame propagation images sequences of propellant strands at 5.0Mpa: (a) 0 wt%; (b) 0.4 wt%; (c) 0.8 wt%; (d) 1.6 wt% NPs propellants.

Combustion flame structures of propellants at 5Mpa: (a) 0 wt%; (b) 0.4 wt%; (c) 0.8 wt%; (d) 1.6 wt% NPs propellants.
3.5 Discussion of combustion mechanism
Due to high density, reactivity, and appropriate melting point, Ni and its oxide have been extensively studied as catalysts for promoting the combustion of Al powders (Vummidi et al., 2010) (Liang et al., 2020) (Lee et al., 2020), B powders (Ma et al., 2022) (Yan et al., 2023), AP particles (Elbasuney et al., 2023) (Tan et al., 2004), and CMDB rocket propellants (Athwawale et al., 2004) (Jiang et al., 2006) (Divekar et al., 2001). In this work, Ni powders also exhibited well-catalytic effect on the burning rate of single-base gun propellant, and obvious modified the propellant flame structure. Based on the previous studies, the catalytic mechanisms of nickel powder on the propellant were considered from following perspectives:
As shown in Fig. 15a, it is well-known that typical flame structure of homogeneous propellant containing R-ONO2 group composes of five zones, which are heat conduction zone, solid-phase reaction zone, fizz zone, dark zone, and flame zone (Athwawale et al., 2004) (Kubota, 2015), respectively. The flame zone involving NO
N2 process is primarily responsible for the heat generating during propellant combustion, but which is constrained by the reaction velocity of NO2
NO occurring in fizz/dark zone (Kubota, 2015).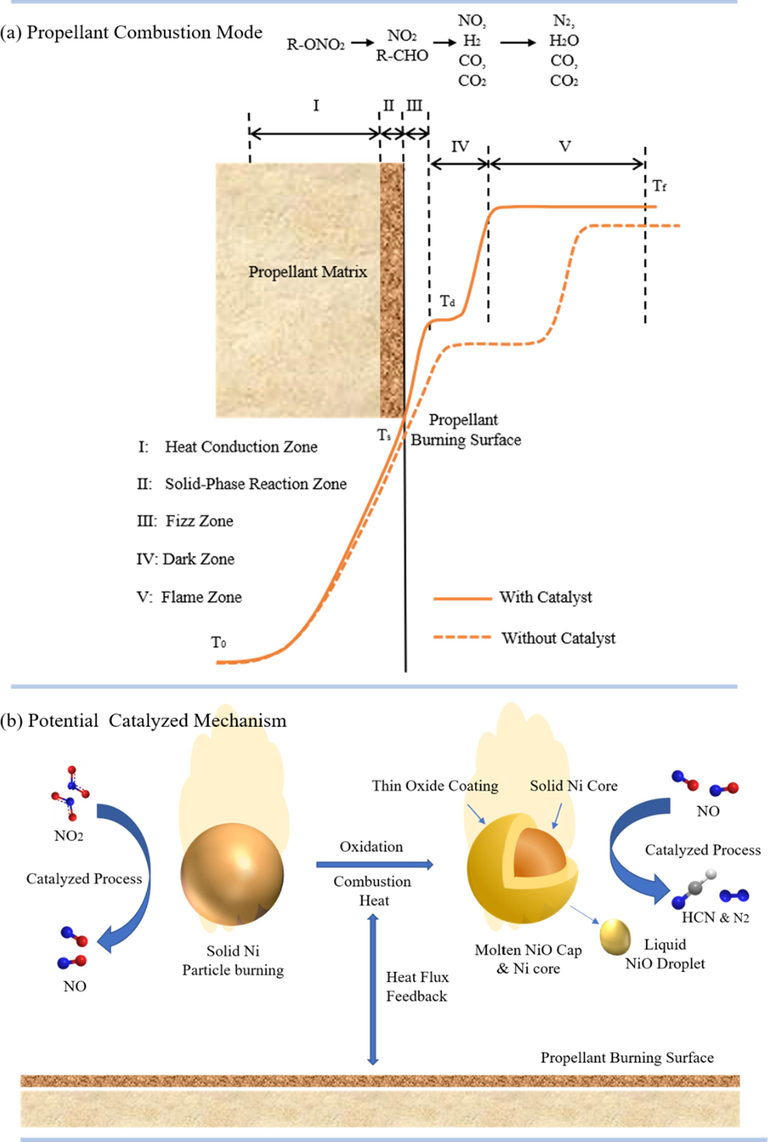
(a) The typical flame structure of homogeneous propellant containing R-ONO2 group (Kubota, 2015); (b) The proposed catalyzed combustion mechanism of Ni powders for propellant in this work.
The flame structure observation in this study revealed that the inclusion of Ni powders resulted in a noticeable reduction in the distance of the fizz/dark zone, as well as a significant increase in the length of the luminous flame zone for single-based propellant. The phenomenon can be explained on basis of Kubota’s theory (Kubota, 2015) (Kubota, 1978), that Ni powders promote the reduction reactions of NO2 → NO in propellant fizz/dark zone. The promoted NO2 reduction reaction by Ni can possibly be explained on the partially filled d-shell to accept electron and a high magnetism to increase the mobility of the electrons (Elbasuney et al., 2023). Due to the electron-deficient state of the Ni atom surface, it can adsorb gas phase substances which have excess electrons, and form complexes with them, thereby facilitating the corresponding heterogeneous catalysis occurring in metal/gas interface (Tan et al., 2004) (Behrens, 2014).
Subsequently, Ni powders melted, burn and converted into NiO product (Liang et al., 2020), with heat generating in the process, which is similar to the typical combustion of Al, Mg and B powders, but released comparatively less heat (Athwawale et al., 2004). Compared with uncatalyzed propellant, the combustion heat of metal powders and the increased length of flame zone could both result in higher conductive/radiative heat flux feedback from flame zone to burning surface (Athwawale et al., 2004) (Divekar et al., 2001).
In addition, according to Wei’s investigation (Wei et al., 2009), nickel oxide(NiO) has also shown to be as catalyst for the thermal decomposition of NC/TEGDN propellant. In Wei’s work, NiO nanoparticles can lead to the decrease intensities in NO, H2O, CO2 signals, along with the increase intensities in CO, N2, HCHO and HCN signals in TG-MS analysis (Wei et al., 2009). And thus; the presence of NiO in propellant burning is indicated to enhance the conversion rate of NO more rapidly in flame zone. Fig. 15b shows the possibly catalytic mechanism involved in this work. All of the above process could potentially cause to the increased velocity in gas reaction and enhanced burning surface temperature, and thus accelerated thermal decomposition of propellant matrix and burning rate.
4 Conclusions
In this work, single-based propellants containing 0.4 wt%, 0.8 wt% and 1.6 wt% nickel powders(NPs) have been prepared and investigated in a closed bomb tester and a transparent combustion chamber, which provided variable-pressure and constant-pressure combustion condition, respectively. The SEM images exhibited NPs can well dispersed in propellant matrix. And the prepared NPs propellants have higher burning rate than the original single-based propellant in various pressure ranges and combustion conditions. The flame propagation images were recorded by high-speed camera in transparent combustion chamber. All propellants samples can stably burn in parallel. The propellants with NPs had thicker and brighter flame surface than original propellants. The main conclusions are as follows:
In variable-pressure combustion condition, propellants burned in closed bomb tester to a pressure above 8 MPa, 16 MPa and 220 MPa, with propellants loading density of 0.01 g/cc, 0.02 g/cc, 0.20 g/cc, respectively. The results showed that the burning rate of propellants was significantly enhanced by NPs, which content was 0.4 wt%, 0.8 wt% and 1.6 wt% in propellants. The Propellants with 1.6 wt% NPs exhibited highest burning rate in prepared propellant samples. Compared with original single-based propellant, 1.6 wt% NPs respectively increased the maximum burning rate by 19.0 %, 12.5 % and 11.9 % in 5–8 MPa, 8–16 MPa and 20–200 MPa. The above results suggested that the burning rate of single-based propellants can be significantly increased by NPs in the variable-pressure of 5–200 MPa, with a widely initial temperature of −40 °C to 50 °C. Meanwhile, the maximum burning pressure of propellants samples were not increased by NPs that phenomenon has potential to hedge against the higher bore pressure of gun weapons caused by the increasing burning rate of propellants. The maximum burning pressure of 1.6 wt% NPs propellants decreased by 0.43 MPa (5.0 %), 0.53 MPa (3.1 %) and 5.2 MPa (2.3 %) than 0 wt% NPs propellant in 0.01 g/cc, 0.02 g/cc and 0.20 g/cc propellant loading density at 20 °C. In addition, pressure exponent(n) decreased and burning rate coefficient(a) increased in 20–50 MPa after adding NPs. In 20–200 MPa, pressure exponent(n) ranged from 0.76 to 0.79 and burning rate coefficient(α) ranged from 0.14 to 0.19.
In constant-pressure combustion condition, propellants burned in transparent combustion chamber, which filled nitrogen with constant pressure of 1.0 MPa, 1.5 MPa, 2.0 MPa, 3.0 MPa, 5.0 MPa. The results showed NPs could increase burning rate of propellants in 1.0–5.0 MPa. While the catalytic efficiency(Z) had high dependence on NPs contents and burning pressure. At 5 MPa, the 0.4 wt%, 0.8 wt% and 1.6 wt% NPs in propellant can both enhance burning rate, with Z value of 1.42, 1.44 and 1.68, respectively. In pressure below 3.0 MPa, adding 1.6 wt% NPs in propellant had catalytic, with maximum Z value of 1.28 at 3.0 MPa; While adding 0.4 wt% and 0.8 wt% NPs cannot increase burning rate, with Z value between 0.93 and 1.06, suggested that the high NPs content and high pressure were conducive to improve burning rate. The catalysis effect of NPs was significant with the pressure above 5 MPa.
Acknowledgements
The authors acknowledge the support of the instrument and equipment fund of the Key Laboratory of Special Energy, Ministry of Education, Nanjing University of Science and Technology.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abd El-Shafey I. Ahmed, A. A. Ali, A. M. El-Masry, S. M. Tawfik. Development of polyurethane-based solid propellants using nanocomposite materials, Vol. 41, Issue2, April 2016, Pages 286-294. https://doi.org/10.1002/prep.201500182.
- Burning rate studies of metal powder(Ti, Ni)-based fuel-rich propellants. J. Energ. Mater.. 2004;22(2):pp.
- [CrossRef] [Google Scholar]
- M. Behrens. Heterogeneous Catalysis of CO2 Conversion to Methanol on Copper Surfaces. ANGEW. CHEM. INT. EDIT. Vol. 53, Issue 45. November 3, 2014. Pages 12022- 12024. https://doi.org/10.1002/anie.201409282.
- Toward high-performance, hreener, and low vulnerability munitions with the RIGHTTRAC technology demonstrator program. Int. J. Energ. Mater. Chem. Propul.. 2013;13(1):7-36.
- [CrossRef] [Google Scholar]
- Y. Chen, M. Chen, L. Zhang, R. Zhao, J. Wen. Combustion characteristic parameter testing and calculating of single-base propellant. Advanced Materials Research, Vol. 1006-1007, August 2014, Pages, 185-187. https://doi.org/10.4028/www.scientific.net/AMR.1006-1007.185.
- Overview of Al-based nanoenergetic ingredients for solid rocket propulsion. Defence Technol.. 2018;14(5):357-365.
- [CrossRef] [Google Scholar]
- Effect of carbon nanotubes on the catalysis of propellant combustion. Dokl. Chem.. 2018;483:301-303.
- [CrossRef] [Google Scholar]
- Energetic materials combustion catalysis: necessary conditions for implementation. Propellants Explos. Pyrotech.. 2021;46(1):90-98.
- [CrossRef] [Google Scholar]
- C. N. Divekar, S. N. Asthana, H. Singh. Studies on Combustion of Metallized RDX-Based Composite Modified Double-Base Propellants. Journal of Propulsion and Power. Vol. 17, No. 1. January- February 2001. https://doi.org/10.2514/2.5707.
- A. Dokhan, E. W. Price, J. M. Seitzman, R. K. Sigman. The Effects of bimodal aluminum with ultrafine aluminum on the burning rates of solid propellants, proceedings of the combustion institute, 2002, Vol. 29, No. 2, Issue 2, Pages 2939–2946. https://doi.org/10.1016/S1540-7489(02)80359-5 1540-7489.
- Facile fabrication and catalytic activity of nickel to ferric oxide nanoparticles for ammonium perchlorate decomposition. Braz. J. Chem. Eng. 2023
- [CrossRef] [Google Scholar]
- Effect of RDX on Combustion performance of modified single base propellant. Chinese J. Energ. Mater.. 2017;25(2):161-166.
- [CrossRef] [Google Scholar]
- X. Hou, M. Zhang, F. Zhang, S. Liu, H. Li, Y. Zuo, Y. Jiang, R. Li, F. Zhao, Research progress of green combustion catalysts for DB/CMDB propellants. Vol. 44, Issue 3, 2021, Pages 271-283. DOI: 10.14077/j.issn.1007-7812.202012024.
- Analysis of the aluminum reaction efficiency in a hydro-reactive fuel propellant used for a water ramjet. Combust. Explos. Shock Waves. 2013;49:541-547.
- [CrossRef] [Google Scholar]
- K. Jayaraman, K. V. Anand, S. R. Chakravarthy, R. Sarathi. Effect of nano-aluminum in plateau-burning and catalyzed composite solid propellant combustion. Combustion and Flame, 2011, Vol. 156, Issue 8, 2011, Pages 1662–1673. https://doi.org/10.1016/j.combustflame.2009.03.014.
- Research on the combustion properties of propellants with low content of nano metal powders. Propellants Explos. Pyrotech.. April 2006;31(2):139-147.
- [CrossRef] [Google Scholar]
- Laser ignition and combustion properties of composite propellant containing nanometal powders. AIAA J.. July 2006;44(7)
- [CrossRef] [Google Scholar]
- Research on the combustion properties of propellants with low content of nano metal powders. Propellants Explos. Pyrotech.. April 2006;31(2):139-147.
- [CrossRef] [Google Scholar]
- Role of Additives in Combustion Waves and effect on stable combustion limit of double-base propellants. Propellants Explos. Pyrotech.. 1978;3(6):163-168.
- [CrossRef] [Google Scholar]
- N. Kubota. Propellants and explosives: thermochemical aspects of combustion. March 2015. Wiley‐VCH Verlag GmbH & Co. KGaA. DOI:10.1002/9783527693481.
- N. Kubota. Propellants and Explosives: Thermochemical Aspects of Combustion. Third, Revised and Updated Edition. Publisher: Wiley-VCH. 2015. Pages:17-20. EPdf ISBN: 978-3-527-69351-1.
- Ignition of nickel coated aluminum agglomerates using shock tube. Combust. Flame. November 2020;221:160-169.
- [CrossRef] [Google Scholar]
- Improve the interfacial adhesion, corrosion resistance and combustion properties of aluminum powder by modification of nickel and dopamine. Appl. Surf. Sci.. April 2020;508(1):144790
- [CrossRef] [Google Scholar]
- D. Liu, Z. Zhao, Y. Yu, Y. Zhou, X. Lu, L. Zhang. Experiments on the combustion characteristics of deterrent-coated propellants and their application in traveling charge propulsion, Combustion Science and Technology, Vol. 184, Issue 2, 2012. https://doi.org/10.1080/00102202.2011.625372.
- Effects of nickel powder on combustion performance of RDX-CMDB propellant. Initiators & Pyrotechnics. 2015;04:47-49.
- [CrossRef] [Google Scholar]
- F. Ma, J. Zhao, P. Du, X. Liao, Z. Wang. Influences of catalysts on burning rate of TEGDN propellant. Chinese Journal of Explosives & Propellants, 2010, 33(04):63-65+69. DOI:10.14077/j.issn.1007-7812.2010.04.016.
- F. Ma, X. Liao, Z. Wang. Effect of catalyst on combustion property of TEGDN propellant charge. Journal of Ballistics, 2011, 23(01):9-12+22.
- Nickel improving the combustion of boron powder. Thermochim. Acta. December 2022;718:179368
- [CrossRef] [Google Scholar]
- Oberle, William F.. “Dynamic Vivacity and Its Application to Conventional and Electrothermal-Chemical (ETC) Closed Chamber Results.” (2001). DOI:10.21236/ada398117.
- Effects of different nano-sized metal oxide catalysts on the properties of composite solid propellants. Combust. Sci. Technol.. 2016;188(3):315-328.
- [CrossRef] [Google Scholar]
- Boron nanoparticles with high hydrogen loading: mechanism for B-H binding and potential for improved combustibility and specific impulse. ACS Appl. Mater. Interfaces. 2014;6(11):8513-8525.
- [CrossRef] [Google Scholar]
- Burning characteristics of the HMX/CL-20/AP/Polyvinyltetrazole binder/Al solid propellants loaded with nanometals. Propellants Explos. Pyrotech.. 2019;44(2):217-223.
- [CrossRef] [Google Scholar]
- J.K.Sharma, Pratibha Srivastava, Gurdip Singh, M. Shaheer Akhtar, S.Ameen. Biosynthesized NiO nanoparticles: potential catalyst for ammonium perchlorate and composite solid propellants. Ceramics International. Vol. 41, Issue 1, Part B, January 2015, Pages 1573-1578. https://doi.org/10.1016/j.ceramint.2014.09.093.
- Tuning the thermal, mechanical, and combustion properties of NC-TEGDN-RDX propellants via incorporation of graphene nanoplates. J. Energ. Mater.. 2020;38(3):326-335.
- [CrossRef] [Google Scholar]
- Combustion behavior of composite solid propellant reinforced with Al-based alloy fuel. Mater. Lett.. December 2021;304(1):130608
- [CrossRef] [Google Scholar]
- National Military Standard of China, 703.1 method: Closed bomb vessel testing, Differential pressure method, GJB/770B-2005-Test method of propellant, 2005 (in Chinese).
- L. Tan, Q. Li, Y. Yang, et al. Study on the preparation and catalytic characteristics of nano-nickel powder. Journal of Solid Rocket Technology. Vol.27 No .3 2004.
- Effect of specific surface area of aluminum on composite solid propellant burning. J. Propul. Power. 2013;29(5):1200-1206.
- [CrossRef] [Google Scholar]
- S. L. Vummidi, Y. Aly, M. Schoenitz, et al. Characterization of fine nickel-coated aluminum powder as potential fuel additive. Journal of Propulsion and Power. Vol. 26, No. 3. May- June 2010. https://doi.org/10.2514/1.47092.
- Study on the catalytic effect of NiO nanoparticles on the thermal decomposition of TEGDN/NC propellant. J. Hazard. Mater.. 2009;168:838-842.
- [CrossRef] [Google Scholar]
- Optimum design for propellant charge of 105 mm gun-launched missile. J. Ballistics. 2006;18(2):60-63.
- [Google Scholar]
- Recent advances in catalytic combustion of AP-based composite solid propellants. Defence Technol.. 2021;17(3):1013-1031.
- [CrossRef] [Google Scholar]
- Study on combustion performance of boron powder promoted by nickel oxide. Thermochim. Acta. August 2023;726:179558
- [CrossRef] [Google Scholar]
- Magnesium based materials for hydrogen based energy storage: past, present and future. Int. J. Hydrogen Energy. 2019;44(15):7809-7859.
- [CrossRef] [Google Scholar]
- A. Yu, B. Xu, B. Wang, X. Liao. Effect of ternary mixed solvents on the single-based propellants with high nitrogen content, Chinese Journal of Energetic Materials, 2020, Vol. 28, Issue 3, Pages 235-241. https://kns.cnki.net/kcms/detail/detail.aspx?FileName=HNCL202003010&DbName=CJFQ2020.
- Z. Yuan, F. Zhao, Y. Yang, X. Song, H. Gao, S. Xu, Comparative investigation of different nano-metal materials on combustion properties of DB and CMDB propellants. Vol. 16, Issue 3, 2017, Pages 219-229. DOI: 10.1615/IntJEnergeticMaterialsChemProp.2018024804.
- Z. Yuan, J. Li, H. Shun, J. Zhang, X. Song, H. Gong, F. Zhao. Effect of nano⁃Ni on overall properties of Al⁃CMDB and RDX/Al⁃CMDB propellants. Chinese Journal of Energetic Materials, Vol. 27, Issue 9, 2019 Pages, 729-734. DOI:10.11943/CJEM2018351.
- Combustion and agglomeration characteristics of boron particles in boron-containing fuel-rich propellant. Combust. Flame. October 2021;232:111551
- [CrossRef] [Google Scholar]
- Effect of nano-nickel powder on combustion properties of Al-CMDB and Cl-20-CMDB propellants. Chinese J. Explos. Propell.. 2016;39(5):99-103.
- [CrossRef] [Google Scholar]
- X. Zhang. Interior Ballistics of Guns. Publishers: Beijing Institute of Technology Press. Beijing. 2014. Pages 41-45. ISBN 978-7-5640-8780-7.
- Thermal decomposition and combustion behavior of solid propellant containing Si-based composites. Combust. Flame. June 2022;240:111959
- [CrossRef] [Google Scholar]







