Translate this page into:
Ion exchange recovery of chromium (VI) and manganese (II) from aqueous solutions
⁎Corresponding author. Tel.: +7 391 206 23 17; fax: +7 391 206 21 09. cm2@bk.ru (O.N. Kononova)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Sorption recovery of toxic ions – chromium (VI) and manganese (II) – from aqueous solutions with different acidity (0.001–0.5 M HCl) was investigated on cation and anion exchangers synthesized with long-chained cross-linking agents (LCA). The initial concentrations of Cr(VI) and Mn(II) were 1 g/L and 5 g/L, respectively. It was shown that the resins with LCA possess high ionic permeability due to the elasticity of polymeric skeleton. High selectivity and good kinetic properties of these sorbents allowed to achieve quantitative (∼100%) recovery and separation of manganese (II) and chromium (VI) in counter-current columns, which results in the complete purification of solutions from toxicants (below the maximum permissible limits), whereas the valuable components (chromium and manganese) can be returned back to industrial process.
Keywords
Sorption
Ion exchangers
Long-chained cross-linking agents
Chromium
Manganese
1 Introduction
The continuous development of mining and metal works, steel and metal alloys production, rubber and paint manufacturing, wood processing, production of textiles, fertilizers, etc. entails the increasing problems with waste waters, especially the ones containing heavy metal ions with high toxicity (copper, zinc, lead, chromium, vanadium, manganese, iron, cobalt, nickel, etc.) (Underwood, 1977; Teller, 1989; Fomin, 2010; Gribanov, 2011). Therefore, the issues of purification of sewages from heavy metals, as well as monitoring the contents of heavy metals in discharged waste waters are essential for securing the environmental safety.
In most cases, the sewages of above-mentioned industries simultaneously contain manganese and chromium ions. As an active element of red-ox process, chromium can be discharged both in form of cations Cr3+ and anions of hexavalent chromium. Although the trivalent chromium is not so hazardous, chromium (VI) is carcinogenic and mutagenic toxicant. It was found (Fendorf, 1995) that manganese facilitates the quick oxidation of Cr(III) ions to Cr(VI) ions. These ions have a greater mobility than trivalent chromium, and penetrate rapidly into any environmental object – surface and ground waters and soils, producing hazardous effects on human health and living nature.
In Russian Federation, the official standards for maximum permissible limits (MPL) for chromium and manganese in drinking and domestic water, are 0.05 mg/L and 0.5 mg/L for Cr(VI) and Cr(III), respectively, and 0.1 mg/L for manganese. The EU standards imply 0.5-5 mg/L and 0.1–0.5 mg/L for Cr(III) and Cr(VI), respectively, and 0.5 mg/L for manganese (Fomin, 2010).
Apart from being high-toxic compounds, chromium and manganese are valuable chemical ingredients that should be recycled in industrial processes (Teller, 1989). For example, manganese is used in production of electrochemical manganese and pure vanadium salts (Zhang and Cheng, 2007), and chromium is applied in various kinds of metal processing and chemical manufacturing. It points out to the two principal tasks: to purify waste waters and to recycle the recovered valuable components.
At present, a number of methods are used for purification of waste water, such as chemical treatment, filtration, neutralization, coagulation, membrane separation, electro remediation methods and sorption (Zhang and Cheng, 2007; Gribanov, 2011). Sorption methods provide the opportunity to put the metal salts back to the main process (Zhang and Cheng, 2007; Alexandratos, 2009), and thus are promising for the development of closed-circuit production technologies. For these reasons, many investigations (Cimino et al., 2000; Babel and Kurniavan, 2003; Jimenes et al., 2004; Celik and Demirabas, 2005; Tataeva and Gamzaeva, 2005; Mallick et al., 2006; Namasiuvayan and Sangeetha, 2006; Nameri et al., 2008; Lee et al., 2009; El-Sayed et al., 2011; Singha et al., 2011; Abebaw et al., 2012) are focused on sorption technologies with an application of various sorbents (based on natural raw materials, as a rule). The important advantage of such sorbents is their inexpensiveness. The synthetic organic ion exchangers are not so widely used for waste water purification (Filiz, 2007; Gurgel et al., 2009; Gribanov, 2011), although they surpass the activated carbons and other natural sorbents in mechanical strength, osmotic stability and exchange capacity. Another important feature is that these resins can be regenerated after sorption. The possibility of multiple usages makes these sorbents more cost-effective; yet they are more expensive than activated carbons.
The present work is devoted to recovery of chromium (VI) and manganese (II) ions from aqueous solutions on ion exchangers with long-chained cross-linking agents (LCA). Previously we have successfully applied these ion exchangers for purification of manganese sulfate solutions from cobalt ions and for recovery of zinc ions from sewages (Kholmogorov et al., 1997; Kononova et al., 1997). It was shown that the LCA (in contrast to divinylbenzene (DVB), widely used in the synthesis of ion exchangers) improve the osmotic permeability of resins and provide higher elasticity to their matrix, making them mechanically stronger and more selective during the sorption of metal ions.
2 Material and methods
2.1 Characteristics of ion exchangers
For the purposes of our work, we have chosen cation and anion exchangers with LCA. The sorption of manganese (II) was carried out on carboxylic cation exchangers KB-2. These ion exchangers were synthesized on the basis of copolymers of methylacrylate with divinyl esters of diethylene–glycol (DVEDEG), triethylene–glycol (DVETEG), propylene-glycol (DVEPG) and tetravinyl ester of pentaerythritol (TVEPE). The sorption of hexavalent chromium ions was carried out on anion exchangers AN-108. They were also synthesized on the basis of copolymers of methylacrylate aminated with ethylenediamine. The LCA were also DVEDEG, DVETEG, DVEPG and TVEPE. To compare the sorption properties of LCA resins, we also investigated the same trademarks of ion exchangers, but with cross-linking agents DVB and divinylsulfide (DVS).
The functional groups of cation and anion exchangers investigated are the following: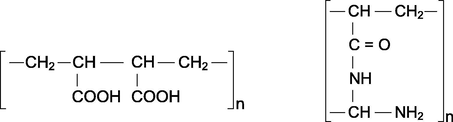
The investigated resins are macroreticular in their physical structure. The use of LCA in the synthesis of carboxylic cation exchangers facilitates better transformation of ester groups into carboxylic groups during hydrolysis because of improved permeability of the formed polymer. The presence of electronegative oxygen atoms in LCA molecules promotes deprotonation of carboxylic groups at the pH range of 3–5, which leads to their increased selectivity (Vdovina et al., 1987).
The use of LCA in the synthesis of anion exchangers aminated with ethylenediamine proceeds with formation of carboxylic groups as side process. The exchange capacity for these groups is ∼0.3–0.8 mmol/g (Tsilipotkina et al., 1990). Therefore, these anion exchangers are expected to show high selectivity during recovery of ions from solutions of complex composition because of amphoteric character of their functional groups.
In addition to the above-mentioned properties, the resins with LCA possess high osmotic stability and mechanical strength (>99%) because of quantitative increase in flexible bonds (–CH2–CH2–) and (–CH2–O–CH2–) in the cross-linking agent molecules, and formation of polymer elastic net (Tager et al., 1990).
The manufacturer for all the resins used is TOKEM (Kemerovo, Russia). The physical–chemical properties of these ion exchangers are presented in Tables 1 and 2.
Trade name
Cross-linking agent (CA)
Quantity of CA (%)
Specific swelling volume in ionic forms (cm3/g)
Exchange capacity in H+-form (mmol/g)
Osmotic stability (%)
H+
Mn2+a
KB-2D
DVB
7
2.3
3.5
10.5
4.9
90–93
KB-2 M
DVEDEG
7
2.9
2.9
11.8
5.6
>98
KB-2 M
DVETEG
7
1.9
3.9
11.9
5.8
>98
KB-2 M
DVEPG
7
2.0
2.3
12.2
5.9
>99
KB-2T
TVEPE
4
2.2
2.3
12.5
6.2
>99
KB-2S
DVS
4
1.9
4.9
8.9
5.1
97–98
Trade name
Cross-linking agent (CA)
Quantity of CA (%)
Specific swelling volume in ionic forms (cm3/g)
Exchange capacity in Cl−-form (mmol/g)
Cl−
Cr(VI)a
AN-108-13
DVB
7
1.7
1.6
4.9
6.5
AN-108-12
DVEDEG
7
3.2
3.1
6.8
5.9
AN-108-11
DVETEG
7
3.0
3.0
6.9
5.8
AN-108-10
DVEPG
7
3.1
2.7
7.1
4.9
AN-108-T
TVEPE
4
2.8
3.1
8.6
3.8
AN-108-S
DVS
4
2.1
1.9
5.1
5.4
Before sorption, the resins were prepared according to the standard methods. Then they were converted to H+-form (cation exchangers) or Cl−-form (anion exchangers) by means of 1 M hydrochloric acid solution.
The acid-base properties of ion exchangers investigated were studied by a potentiometric titration with the glass electrode. Based on the experimental data, we have calculated the average apparent ionization constants of functional groups of ion exchangers (Saldadze and Kopylova, 1980; Soldatov et al., 2004). The constant values are shown in Tables 1 and 2.
2.2 Reagents and solutions
The initial stock solutions of manganese (II) and chromium (VI) with concentration 0.5 mol/L were prepared by dissolution of accurately weighed quantities of MnCl2·4H2O (analytical grade) and K2CrO4 (analytical grade) in distilled water. The working solutions with concentrations to chromium 1 g/L (0.02 mol/L) and to manganese 5 g/L (0.09 mol/L) were prepared from the stock solutions by diluting with distilled water.
We have used manganese (II) and chromium (VI) solutions in hydrochloric acid with concentrations 0.001, 0.1 and 0.5 mol/L.
2.3 Apparatus and analytical procedures
The concentrations of Mn(II) and Cr(VI) in solutions after sorption were determined by flame AAS method (model Saturn-2, Russia).
The initial concentrations and acidity of the solutions were chosen with an intention to make the experiment closer to industrial conditions, i.e. we have simulated the composition and initial conditions for manganese and chromium of rinse and waste water of metallurgical enterprises.
2.4 Batch studies
The sorption of chromium (VI) and manganese (II) was studied under batch experiment conditions at (20 ± 1) °С. The batch experiments were carried out at resin masses of 0.1–0.2 g and volume of contacting solution 10.0–20.0 mL. The equilibrium time determined by separate tests was about 24 h.
The sorption ability of the resins investigated was estimated by means of recovery degree (R, %) and distribution coefficient (D, L/g), calculated as follows:
The content of manganese or chromium in the resin phase was calculated from difference between initial and equilibrium concentrations in solution, taking into account the resin mass and volume of contacting solution.
The sorption isotherms were plotted by varying the molar ratio of resins to the amounts of sorbed ions in contacting solution. Then, based on the obtained curves, we have calculated the apparent constants of ion exchange equilibrium according to the law of mass action (Helfferich, 1962; Kokotov and Pasechnik, 1979).
The kinetics of sorption of manganese (II) and chromium (VI) ions on ion exchangers investigated was studied by the “limited bath” method (Helfferich, 1962; Kokotov and Pasechnik, 1979) and diffusion coefficients of Mn}}(II) and Cr(VI) ( cm2/s) were calculated. The kinetic experiment procedure is described below.
2.5 Dynamic studies
The simultaneous sorption of manganese (II) and chromium (VI) from aqueous solutions and their subsequent separation was carried out under dynamic conditions. The experiment started in two glass counter-current columns with cation and anion exchanger (20 mm diameter, 200 mm height, 150 mL bed height, 45 mL of the resin). The flow rate was 100 mL/h. The solution came into the lower part of the columns and was taken out by drainage from the upper part of the columns. The resins were loaded from above and unloaded from below with portions of ∼15 mL every 3 h.
After the sorption, the saturated resins were washed with distilled water (the volume ratio of ion exchanger and water was 1:4). The next operation was elution of metal ions from the resins. Chromium (VI) was eluted from anion exchanger with 1 M NaOH solution. After the elution, the anion exchanger was regenerated by 2 M NaCl solution. Manganese (II) was eluted from cation exchanger with 2 M HCl solution. The subsequent regeneration of the cation exchanger was not required. The volume ratio of the resin and eluent was 1:1.
Based on results of dynamic experiments, we have calculated the recovery degree of manganese or chromium (
as follows:
2.6 “Limited bath” method for sorption kinetics of manganese and chromium
The quantities of preswollen resin (0.1 g) were stirred with 10.0 mL of solutions investigated at (20 ± 1) °С over a period of 30 s to 24 h. The suspensions were intensively stirred (more than 800 rev/min). After a certain time period, the resins and solutions were quickly separated and the concentrations of Mn(II) and Cr(VI) were determined in solutions. Then exchange degree (F) was calculated from:
According to the Boyd’s method (Helfferich, 1962; Kokotov and Pasechnik, 1979), the kinetic coefficient B was calculated from:
The data obtained were plotted as function Bt = f(t). If the process is controlled by gel diffusion (Helfferich, 1962; Kokotov and Pasechnik, 1979), this function should be linear. After that, the diffusion coefficients (
) were calculated according to the following equation:
We also calculated the half-exchange time (t1/2) at F = 0.5.
2.7 Results processing
All the results obtained were statistically processed by standard methods (Harris, 2007): the average from 4 parallel runs was measured, and then the variance, standard deviation and confidence interval were calculated using Student’s t at a confidence level of 0.95. The average experimental error was below 6%.
3 Results and discussion
3.1 Ionic state of chromium (VI) and manganese (II) in aqueous solutions
Although chromium has different oxidation states (from −2 to +6), the compounds of chromium (VI) are the most stable and strong oxidizers (Cotton and Wilkinson, 1969).
It is known (Cotton and Wilkinson, 1969; Lavrukhina and Yukina, 1979) that hexavalent chromium exists in aqueous solutions with different acidity in the form of three anions:
,
and
. In basic solutions, chromium (VI) exists in the form of yellow chromate ion
. With the pH decrease, the solutions become orange, as dichromate ions
are formed. This transformation includes two stages: protonation of chromate ions with formation of hydrochromates
and subsequent dimerization. Therefore, the ionic composition of chromium (VI) solutions can be presented as the following equilibrium:
The formation of chloro-chromate complexes is also possible in hydrochloric acidic solutions (Cotton and Wilkinson, 1969):
Manganese also has many oxidation states (from −3 to +7), however, its stable state is +2. It is known (Cotton and Wilkinson, 1969) that with the increase in oxidation state, manganese tends to form anions and anionic complexes.
Manganese exists in weak acidic solutions mainly in the form of cation Mn2+, whereas its complexes [Mn(H2O)6]2+ and [Mn(H2O)4]2+ are formed in strong acidic solutions (Cotton and Wilkinson, 1969).
3.2 Sorption of chromium (VI) from aqueous solutions on anion exchangers
As mentioned above (see Experimental), the sorption recovery of chromium (VI) was carried out on anion exchangers with LCA. These sorbents are weak basic anion exchangers, which adsorb the transition metal ions through anionic exchange, as well as through additional complex formation at the expense of unshared electron pair of nitrogen atoms of functional groups (Hering, 1967; Saldadze and Kopylova, 1980). That usually leads to increase in selectivity of such sorbents.
At first, we have investigated the Cr(VI) recovery from its individual solutions in dependence on acidity of contacting solution. The results are summarized in Table 3. It can be seen from the data that ion exchangers with LCA possess higher sorption ability compared to anion exchanger AN-108-13 cross-linked by DVB. The latter recovers chromium (VI) up to 40%. Among the anion exchangers with LCA, AN-108-T (cross-linking agent TVEPE) is exceptional. If the other sorbents with LCA recover chromium to about 90%, AN-108-T adsorbs chromium quantitatively (∼100%). The probable reason is its greater exchange capacity, compared to the other anion exchangers with LCA (Table 2).
Trade name
Type of solutiona
R (%)
log D
AN-108-13
A
41 ± 2
2.23 ± 0.11
B
35 ± 2
2.13 ± 0.11
C
26 ± 2
1.93 ± 0.09
AN-108-12
A
85 ± 4
2.93 ± 0.15
B
81 ± 4
2.87 ± 0.14
C
77 ± 4
2.83 ± 0.14
AN-108-11
A
86 ± 4
2.91 ± 0.15
B
83 ± 4
2.90 ± 0.16
C
79 ± 4
2.85 ± 0.16
AN-108-10
A
88 ± 5
2.94 ± 0.15
B
86 ± 4
2.92 ± 0.16
C
81 ± 4
2.78 ± 0.14
AN-108-T
A
98 ± 2
3.41 ± 0.21
B
97 ± 3
3.40 ± 0.19
C
96 ± 4
3.39 ± 0.19
AN-108-S
A
90 ± 5
3.01 ± 0.18
B
88 ± 5
2.99 ± 0.15
C
84 ± 4
2.88 ± 0.14
With the increase in medium acidity, a slight decrease in chromium recovery is observed for all the investigated anion exchangers (Table 3). It can be explained by protonation of nitrogen atoms of functional groups, resulting in reduction of complex-forming abilities of these sorbents. The other probable reason for the decrease in chromium (VI) adsorption with increase in hydrochloric acid concentration may be the competition of chloride ions with the chromium (VI) anions.
For these reasons, it is a matter of practical importance to study the behavior of anion exchangers during chromium sorption in the presence of manganese (II) ions in contacting solutions. For this purpose we have chosen the anion exchanger AN-108-T. The resins AN-108-13 and AN-108-S with the cross-linking agents DVB and DVS, respectively, were taken for a comparison. The results are presented in Table 4.
Trade name
Type of solutiona
R (%)
log D
AN-108-13
A′
38 ± 2
2.21 ± 0.13
B′
32 ± 2
2.11 ± 0.12
C′
21 ± 1
1.85 ± 0.09
AN-108-T
A′
98 ± 2
3.40 ± 0.21
B′
97 ± 3
3.41 ± 0.21
C′
96 ± 4
3.39 ± 0.21
AN-108-S
A′
87 ± 4
2.99 ± 0.18
B′
84 ± 4
2.92 ± 0.17
C′
78 ± 5
2.85 ± 0.14
Similar to the chromium (VI) recovery from its individual solutions, anion exchanger AN-108-T shows exceptional selectivity among the other resins. In contrast to the resins cross-linked with DVB and DVS, the sorption ability of AN-108-T is practically not reduced at the presence of Mn(II) ions in the system.
The sorption isotherms of chromium (VI) ions on anion exchanger AN-108-T are presented in Fig. 1. It can be seen from this Figure that the shape of isotherms is the same either at the presence or at the absence of manganese (II) ions in the system. The curves are convex, pointing out to the selectivity of sorption in these systems (Helfferich, 1962; Hering, 1967; Kokotov and Pasechnik, 1979). The apparent constants of ion exchange equilibrium, calculated on the basis of these isotherms, are (2.55 ± 0.15) L/mol in the absence of Mn(II) and (2.51 ± 0.13) L/mol in its presence. For comparison, the values of apparent constants of ion exchange equilibrium on anion exchangers AN-108–13 (DVB) and AN-108-S (DVS) are (1.41 ± 0.08) L/mol and (2.12 ± 0.13) L/mol, respectively, for chromium (VI) recovery from individual solutions. In the presence of manganese (II) ions in the systems, the values of apparent constants are (1.25 ± 0.08) L/mol and (2.05 ± 0.12) L/mol for AN-108-13 and AN-108-S, respectively. The data make visible that the constant values are linked to the selectivity of investigated anion exchangers, as these values determine ion exchange affinity (Helfferich, 1962; Diamond and Whitney, 1968).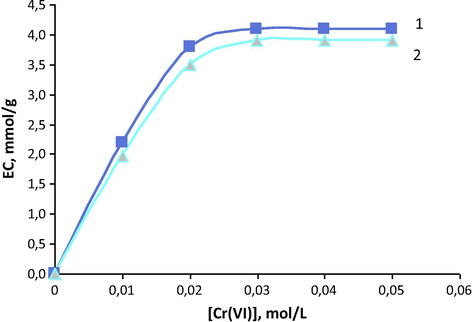
Sorption isotherms of chromium (VI) on anion exchanger AN-108-T from aqueous solutions in the absence (1) and in the presence (2) of manganese (II) C0(HCl) = 0.5 mol/L; C0(Mn) = 5 g/L.
The essential requirement for industrial application of ion exchangers is their good kinetic properties. Therefore, it is a matter of theoretical and practical interest to investigate the kinetics of Cr(VI) ions on anion exchangers. The calculated kinetic parameters are given in Table 5. The data show that kinetics of chromium (VI) sorption from individual solutions (as well as in the presence of Mn(II)) on anion exchanger AN-108-T (TVEPE) compares favorably to the other sorbents. This is especially visible on half-exchange time, which is only 25-28 s for AN-108-T (Table 5) and shows practically no dependence either on acidity, or on composition of solution. This process kinetics is consistent with the above mentioned high permeability of resins with LCA, and makes AN-108-T promising for high quality purification of waste water from hexavalent chromium ions.
Trade name
Individual Cr (VI) solutions
Solutions of Cr (VI) in the presence of Mn (II)
Type of solutiona
(cm2/s)
t1/2 (s)
Type of solutiona
(cm2/s)
t1/2 (s)
AN-108-13
A
0.91
220
A′
0.94
430
B
0.65
–
B′
0.85
–
C
0.51
360
C′
0.82
570
AN-108-T
A
5.67
25
A′
5.64
25
B
5.58
–
B′
5.56
–
C
5.43
25
C′
5.48
28
AN-108-S
A
2.24
115
A′
2.09
310
B
1.87
–
B′
1.31
–
C
1.24
320
C′
1.05
520
Further, we have carried out the desorption of chromium (VI) ions under the batch-experiment conditions from the investigated anion exchangers by means of 1 M NaOH. The chromium (VI) recovery was 68.9% and 82.4% from AN-108-13 (DVB) and AN-108-S (DVS), respectively, whereas about 99% of chromium (VI) was desorbed from AN-108-T (TVEPE).
Based on the obtained results, we have chosen anion exchanger AN-108-T for recovery and separation of Cr(VI) and Mn(II) under dynamic conditions.
3.3 Sorption of manganese (II) from aqueous solutions on cation exchangers
It was mentioned above (see Experimental) that sorption recovery of Mn(II) ions was carried out using carboxylic cation exchangers with LCA. Similar to anion exchangers with LCA, these resins possess high ionic permeability and complex-forming ability at the expense of unshared electron pairs of oxygen atoms of carboxylic groups. Therefore, the sorption of transition metal ions on such cation exchangers proceeds in both ways: by cation exchange and by coordination interaction in dependence on pH of contacting solution.
At first, we have investigated the sorption of Mn(II) ions from individual acidic aqueous solutions. The results are listed in Table 6. The data show that sorption ability of all the cation exchangers with LCA to manganese is rather high. The quantitative recovery of Mn(II) is observed on cation exchangers KB-2M and KB-2T cross-linked with DVEDEG (7%) and TVEPE (4%), respectively. The other resins with LCA and KB-2S adsorb Mn(II) ions on the level >90%. However, the sorption of manganese on cation exchanger KB-2D (cross-linking agent DVB) does not exceed 78–82%.
Trade name
Type of solutiona
R (%)
log D
KB-2D (DVB)
D
82 ± 5
3.82 ± 0.23
E
78 ± 4
3.75 ± 0.22
KB-2M (DVEDEG)
D
∼100
4.26 ± 0.26
E
∼100
4.22 ± 0.25
KB-2M (DVETEG)
D
92 ± 5
4.02 ± 0.24
E
90 ± 5
3.98 ± 0.19
KB-2M (DVEPG)
D
93 ± 5
4.11 ± 0.21
E
90 ± 5
4.06 ± 0.24
KB-2T (TVEPE)
D
∼100
4.25 ± 0.21
E
∼100
4.22 ± 0.25
KB-2S (DVS)
D
94 ± 5
4.07 ± 0.21
E
92 ± 5
4.01 ± 0.19
It should be noted that the decrease in pH of contacting solution does not practically deteriorate the sorption properties of investigated cation exchangers. Therefore, it is a matter of practical interest to study the sorption of manganese (II) from aqueous solutions in presence of hexavalent chromium. For this purpose, we have chosen cation exchangers KB-2M and KB-2T cross-linked with DVEDEG and TVEPE as well as KB-2D and KB-2S. The results are presented in Table 7. It can be seen from these data that cation exchangers with LCA and DVS do not reduce their affinity to manganese (II) ions, whereas KB-2D does not possess such sorption properties. The data clearly show the advantage of resins with LCA in their sorption properties, compared to other sorbents.
Trade name
Type of solutiona
R (%)
log D
KB-2D (DVB)
A′
79 ± 5
3.12 ± 0.19
C′
68 ± 4
2.92 ± 0.17
KB-2 M (DVEDEG)
A′
∼100
4.25 ± 0.22
C′
∼100
4.23 ± 0.25
KB-2T (TVEPE)
A′
∼100
4.24 ± 0.25
C′
∼100
4.23 ± 0.24
KB-2S (DVS)
A′
93 ± 5
4.08 ± 0.23
C′
92 ± 5
4.06 ± 0.24
Further, we have investigated the kinetic properties of cation exchangers with LCA in comparison with KB-2D (DVB). The results are shown in Fig. 2. It follows from these data that cation exchanger KB-2T (TVEPE) compares favorably in its kinetic properties with KB-2M (DVEDEG). That is why KB-2T was taken for further investigation. The coefficient of Mn(II) diffusion for KB-2T is 5.94 · 10−8 сm2/s and half-exchange time is 72 s. For comparison, the diffusions coefficients for KB-2 M (DVEDEG) and KB-2D (DVB) are 3.26 · 10−8 сm2/s and 1.98 · 10−8 сm2/s, and the half-exchange times are 118 s and 320 s, respectively.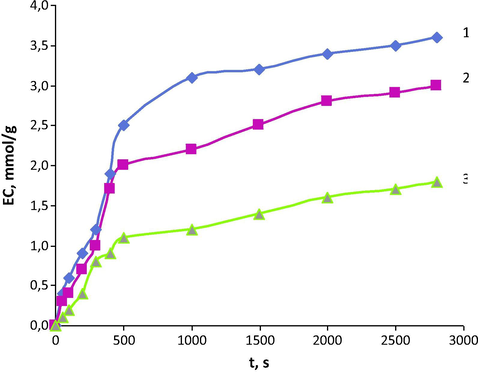
Kinetic curves of Mn (II) sorption on cation exchangers investigated (1) – KB-2T (TVEPE); (2) – KB-2 M (DVEDEG); (3) – KB-2D (DVB); C0(Mn) = 5 g/L; C0(HCl) = 0.5 mol/L.
Fig. 3 contains the isotherms of manganese (II) sorption on cation exchanger KB-2T (TVEPE) in absence and in presence of chromium (VI) ions. The convex curves indicate the selectivity of sorbents, facilitated by good ionic permeability of KB-2T polymeric skeleton.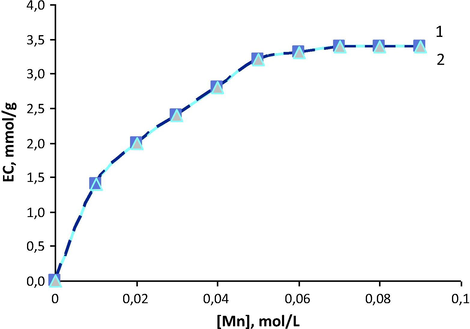
Sorption isotherms of manganese (II) on cation exchanger KB-2T (TVEPE) from aqueous solutions in the absence (1) and in the presence (2) of chromium (VI) C0(Cr) = 1 g/L; C0(HCl) = 0.5 mol/L.
Then we have carried out desorption of manganese from cation exchanger KB-2T by 2 M HCl under batch-experiment conditions. As a result, the complete manganese desorption was achieved.
Based on the obtained results, we recommend the cation exchanger KB-2T (TVEPE) for subsequent recovery and separation of Mn(II) and Cr(VI) ions under dynamic conditions.
3.4 Simultaneous sorption of chromium (VI) and manganese (II) from aqueous solutions and their subsequent separation in columns
It was mentioned above (see Experimental) that sorption and separation of Mn(II) и Cr(VI) were carried out in two counter-current glass columns. One column was loaded with cation exchanger KB-2T (TVEPE) for manganese sorption and the other column contained anion exchanger AN-108-T (TVEPE) for recovery of chromium (VI). The operation of columns and their parameters is described in Section 2.5.
The initial solution (1 L) containing manganese (II) and chromium (VI) with concentrations 5 g/L and 1 g/L, respectively, as well as hydrochloric acid (0.5 mol/L) was passed through the column with cation exchanger. The breakthrough capacity to manganese was 2.1 mmol/g, and the recovery degree of manganese (II) ions was more than 99.8%. The remaining manganese concentration in purified solution was less than MPL, which is 0.1 mg/L (by the standards of Russian Federation).
Then the solution entered the column with anion exchanger to recover Cr(VI) ions and the cation exchanger was washed with distilled water (∼200 mL), and after that the elution of manganese was carried out. For this purpose, 2 M HCl solution (∼50 mL) was passed through the column. After the complete desorption, the contents of manganese (II) in eluate were 5.2–5.3 g.
The breakthrough capacity of anion exchanger to chromium was 1.2 mmol/g, the recovery of hexavalent chromium was more than 99.9%. The remaining concentration of chromium in purified aqueous solution was below the limits of its analytical determination by AAS method, i.e. less than MPL (0.05 mg/L according to the standards of Russian Federation).
After sorption of chromium, the anion exchanger was washed with distilled water (∼200 mL) and then the elution of chromium was carried out with 1 M NaOH (∼50 mL). The chromium desorption was quantitative, i.e. on the level ∼100%.
Therefore, the high ionic permeability of ion exchangers synthesized with LCA facilitates high quality purification of aqueous solutions from chromium (VI) and manganese (II) ions. The achieved separation of these ions is promising for recycling them back to technological process.
4 Conclusions
-
We investigated the sorption recovery of chromium (VI) and manganese (II) from individual aqueous solutions as well as from solutions with their simultaneous presence on some ion exchangers synthesized with LCA. The sorption properties of these resins were compared with the characteristics of sorbents, cross-linked by DVB and DVS. It was shown that ion exchangers with LCA possess higher ionic permeability and facilitate the quantitative (∼100%) recovery of Mn(II) and Cr(VI).
-
The simultaneous sorption of Mn(II) and Cr(VI) followed by their complete separation was accomplished in counter-current columns on ion exchangers with LCA. The purified aqueous solution does not contain toxicants. The valuable components – manganese and chromium – can be sent back to the technological process.
References
- Adsorption of Mn (II) ions from wastewater using activated carbon obtained from Birbira (Melita Ferrginea) leaves. Global J. Sci. Frontier Res. Chem.. 2012;12:4-12.
- [Google Scholar]
- Ion-exchange resins: a retrospective from industrial and engineering chemistry research. Ind. Eng, Chem, Res.. 2009;48:388-398.
- [Google Scholar]
- Low cost adsorbents for heavy metals uptake from contaminated water, a review. J. Hazard. Mater. B. 2003;97:219-243.
- [Google Scholar]
- Removal of heavy metal ions from aqueous solutions via adsorption onto modified lignin wastes. Energy Sources. 2005;27:1167-1177.
- [Google Scholar]
- Removal of toxic cations and Cr (VI) from aqueous solutions by hazelnut shell. Water Research. 2000;34:2955-2962.
- [Google Scholar]
- Advanced Inorganic Chemistry. A Comprehensive Text. Moscow: Mir; 1969. pp. 229–260
- Selectivity of ion exchangers in diluted and concentrated solutions. In: Marinsky J.A., ed. Ion exchange. A Series of Advances. Moscow: Mir; 1968. p. :174-275.
- [Google Scholar]
- Removal of Zn (II), Cd (II) and Mn (II) from aqueous solutions by adsorption on maize stalks. The Malaysian J. Anal. Sci.. 2011;15:8-21.
- [Google Scholar]
- Extraction of Mn (II) from aqueous hydrochloric acid solutions into Alamine 336- m-xylene system. Hydrometallurgy. 2007;87:58-62.
- [Google Scholar]
- Water: a Control of Chemical, Bacterial and Radiation Safety according to International Standards. Moscow: Protector; 2010. pp. 1008
- Gribanov, E.N., 2011. Sorption preconcentration and determination of manganese (II), chromium (III) and vanadium (IV) in natural and waste water. PhD-Thesis, Moscow. (in Russian).
- Adsorption of chromium (VI) ion from aqueous solution by succinylated mercerized cellulose functionalized with quaternary ammonium groups. Bioresource Tech.. 2009;100:3214-3220.
- [Google Scholar]
- Quantitative Chemical Analysis. New York: Freeman; 2007. pp. 828
- Ion Exchange. New York: McGraw Hill; 1962. pp. 624
- Chelate-forming Ion Exchangers. Berlin: Akademie-Verlag; 1967. p. :268. (in German)
- Heavy metals removal from wastewater by the natural zeolite scolerite – temperature and pH influence in single- metal solutions. Qimica Nova. 2004;27:734-738.
- [Google Scholar]
- Ion exchange purification of manganese sulfate solutions from cobalt. Hydrometallurgy. 1997;45:261-269.
- [Google Scholar]
- Ion Exchange Equilibrium and Kinetics of Ion Exchange. Khimiya: Leningrad; 1979. p. :336. (in Russian)
- Study of sorption properties of the cation exchangers KB-2M with macroreticular structure for recovery of zinc ions from sewage and rinsing water. Acta Hydrochim. Hydrobiol.. 1997;25:206-212. (in German)
- [Google Scholar]
- The Analytical Chemistry of Chromium. Moscow: Nauka; 1979. p. :434. (in Russian)
- Removal of Mn (II) from aqueous solutions using manganese-coated sand samples. J. Chem. Eng. Data. 2009;54:1823-1828.
- [Google Scholar]
- Adsorption of hexavalent chromium on manganese nodule leached residue obtained from NH3 – SO2 – leaching. J. Colloid Interface Sci.. 2006;297:419-425.
- [Google Scholar]
- Removal of chromium (VI) by ZnCl2 activated coir pith carbon. Toxic. Environ. Chem.. 2006;88:219-233.
- [Google Scholar]
- Adsorption of hexavalent chromium from aqueous solution by wheat bran. Int. J. Environ. Sci. Tech.. 2008;5:161-168.
- [Google Scholar]
- Complex-forming Ion Exchangers. Khimiya: Moscow; 1980. p. :402. (in Russian)
- Cr (VI) ions removal from aqueous solutions using natural adsorbents – FTIR studies. J. Environ. Prot.. 2011;2:729-735.
- [Google Scholar]
- Influence of length of cross-linking agent molecules and of their nature on structure of polymeric sorbents. Vysokomolekularnye Soedineniya A. 1990;32:727-732. (in Russian)
- [Google Scholar]
- Tataeva, S.D., Gamzaeva, U.G., 2005. Method for preconcentration and determination of chromium and manganese ions in biosubstrates. Patent of Russian Federation, No. 2 292 545 from 21.03.2005.
- Heavy metals in soil and their environmental significance. Adv. Soil Sci.. 1989;9:113-142.
- [Google Scholar]
- Synthesis and structure of ion exchangers on the basis of methylacrylate and divinyl ester of diethylene-glycol. Vysokomolekularnye Soedineniya A. 1990;32:36-40. (in Russian)
- [Google Scholar]
- Trace Elements in Human and Animal Nutrition. New York: Academic Press; 1977. p. :545.
- Structure and properties of carboxylic cation exchanger KB-2. Plasticheskie Massy. 1987;8:24-26. (in Russian)
- [Google Scholar]
- Manganese metallurgy review. Part II. Manganese separation and recovery from solution. Hydrometallurgy. 2007;89:160-177.
- [Google Scholar]







