Translate this page into:
Isotherms, kinetics, and thermodynamics of boron adsorption on fibrous polymeric chelator containing glycidol moiety optimized with response surface method
⁎Corresponding authors. haruna_18002856@utp.edu.my (Haruna Kolawole Afolabi), mahmoudeithar@cheme.utm.my (Mohamed Mahmoud Nasef), nahadi.sapiaa@utp.edu.my (Nik Abdul Hadi Md Nordin)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
A fibrous boron chelator containing glycidol moiety (PE/PP-g-PVAm-G) was prepared by radiation induced grafting of N-vinylformamide (NVF) onto polyethylene/polypropylene (PE/PP) non-woven sheet followed by hydrolysis and immobilization of glycidol moiety. The glycidol density was controlled by optimization of the reaction parameters using the Box-Behnken design of response surface methodology (RSM). The properties of the PE/PP-g-PVAm-G were evaluated using Fourier transform infrared (FTIR) spectroscopy, field emission scanning electron microscopy (FESEM) and energy dispersive x-ray (EDX) analysis, X-ray diffraction (XRD) and thermal gravimetric analysis (TGA). A maximum glycidol density yield of 5.0 mmol·g−1 was obtained with 11.8 vol%, 78.9 °C and 109.4 min for glycidol concentration, reaction temperature and time, respectively. The isotherms, kinetics, and thermodynamic behavior of boron adsorption on the optimized chelator were investigated. The boron adsorption was pH-dependent and attained a maximum adsorption capacity of 25.7 mg·g−1. The equilibrium isotherm proceeded by Redlich–Peterson model whereas the kinetics was best expressed by the pseudo-second-order equation. The thermodynamic analysis revealed that boron adsorption is endothermic and spontaneous. The fibrous chelator demonstrated high boron selectivity and strong resistance to foreign ions with uncompromised regeneration efficiency after five adsorption/desorption cycles. The PE/PP-g-PVAm-G chelator seems to be very promising for boron removal from aqueous media.
Keywords
Polyvinylamine grafted PE/PP
Fibrous polymeric chelator
Boron-selective adsorption
Glycidol density
Box-Behnken design
1 Introduction
Boron is a pollutant to surface, ground and wastewater that has been subjected to various decontamination investigations. Boron has never been elementally found in nature, but it is instead naturally existing in form of complex compounds of sodium or calcium borates such as tinkal (Na2O.2B2O3·10H2O), ulexite (Na2O.2CaO.5B2O3·16H2O) and colemanite (2CaO.3B2O3·5H2O) and its concentration in different water bodies is in the range of 5–100 ppm. The rise in boron pollution is caused by the widespread use of its compounds in industrial operations for borosilicate glass, ceramic glazes, porcelain enamels, pharmaceutical products, pesticides, and agricultural markets (Ezechi et al., 2012; Lin et al., 2021; Tang et al., 2017a,b). The leakage of boron to different water bodies poses very serious environmental problems and toxic effects to humans, plants, and animals. Boron limits for agricultural water is set between 0.3 and 1 ppm and that of drinking water stands maximally at 2.4 ppm according to WHO, whereas that of wastewater was set to few milligrams per liter depending on disposal regulations in various countries (Ezechi et al., 2012; Güler et al., 2015; Herschy, 2012; Wolska and Bryjak, 2013). Thus, effective boron removal methods are in high demand for wastewaters de-boronation to meet the environmental regulations and wastewater disposal standards.
Currently, there is no unique technique to satisfactorily remove boron from wastewater (Recepoğlu et al., 2018), and practiced methods include chemical precipitation (B. Wang et al., 2014), nanofiltration (Landi et al., 2019), reverse osmosis (Zhe Wang et al., 2021), electro-deionization, electrodialysis (Ezechi et al., 2012), electrocoagulation (Chen et al., 2020), and ion-exchange (Ezechi et al., 2012; Güler et al., 2015; Kabay et al., 2008; Najid et al., 2021). Among all, ion-exchange method is one of the most appealing methods for effective removal of boron from wastewater to low concentrations conforming to guidelines and disposal criteria around the world (Dydo and Turek, 2013). This technique relies on the selective ability of N-methyl-D-glucamine (NMDG)-containing resins having multiple alcoholic hydroxyl groups that react with borate to form chelate complexes (Ince et al., 2013). Its operational requirements are quite simple, and it's mostly applicable to solutions with lower boron concentrations. The chemistry of boron has shown that polymers bearing molecules with vicinal diols (e.g. glucamine or glycidol functionality) have the ability to bind boric acid by forming either neutral boron esters or borate type of complex compounds in aqueous solutions by esterification reaction (Kamcev et al., 2019; Sanfeliu et al., 2018). As a result, partial esterification could result in mono-chelate of 1:1 boron: polyol complexes while complete esterification may create a bis-chelate 1:2 boron: polyol complexes in Fig. 1. The former complex is labile and quickly hydrolyzed to their original components in solution, whereas the later is indissociable and much more thermodynamically stable in water (Kamcev et al., 2019; Sanfeliu et al., 2018). However, these resins are challenged by some drawbacks such as slow kinetics, considerable loss in capacity after regeneration by acid treatment and are economically costly.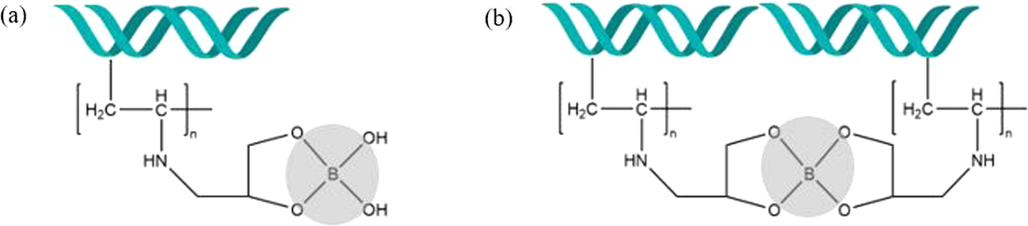
Schematic mechanism representation of: (a) mono-chelate and (b) bis-chelate ester complex formed during boron adsorption on glycidol-containing adsorbent.
Exploration of new chelating adsorbents (boron chelator) with better properties and performance toward the selectivity of boron is imperative. Surface modification of polymeric substrates by graft copolymerization has been frequently used to incur NMDG moieties to develop alternative boron selective chelators with improved chemical and structural properties. Notably, fibrous polymeric chelators with NMDG prepared by radiation induced graft copolymerization (RIGC) have demonstrated improved properties and higher boron chelation performance compared to granular commercial resins (Hoshina et al., 2021; Ting et al., 2020). This is due to their higher functional group density and the associated higher wettability in boron solutions allowing not only stronger affinity and enhanced diffusion but also rapid interaction of boron with functional groups (Nasef et al., 2014; Recepoğlu et al., 2017; Recepoğlu et al., 2018; Tang et al., 2017a). Nonetheless, the performance of NMDG is still not satisfactory and there is room to improve the adsorption capacity and the kinetics by incorporation of alternative functional groups such as glycidol.
Glycidol is an alternative weakly basic moiety that has been used to prepare boron chelating adsorbents based on substrates of various morphologies that started to receive attention in the past several years as indicated in the summary of previous studies depicted in Table 1 (Luo et al., 2020a; Senkal and Bicak, 2003; Zerze et al., 2013). Particularly, boron polymeric chelators including poly(glycidyl methacrylate (GMA)–methyl methacrylate (MMA)–DVB (divinylbenzene) beads (Senkal and Bicak, 2003), poly(GMA/trimethylolpropane trimethacrylate) nano-beads (Luo et al., 2020a), crosslinked poly (N-vinylformamide) (NVF) microporous beads (Ince et al., 2013), polysulfone membrane (Jin et al., 2019), poly[N-(2,3-dihydroxypropyl)aminostyrene] beads (Nesterov et al., 2013), polyacrylonitrile membrane (Meng et al., 2016), magnetic chitosan microbeads with cis-diols (Oladipo and Gazi, 2016), and microporous DVB cross-linked polystyrene resin (Kluczka et al., 2015), modified with different amine ligands to host glycidol were investigated. Despite such contributions, fibrous polymeric chelators functionalized with glycidol ligands are rarely reported. It was recently our research group explored the preparation of fibrous chelator and its potential application for boron removal for aqueous solution (Afolabi et al., 2021a). PE/PP is a versatile substrate with PE sheath and PP core chosen as inert polymer for the development of fibrous adsorbent with strong radiation resistance, high mechanical integrity with chemical stability, and low cost. The polymeric chelator was prepared by RIGC of NVF onto PE/PP nonwoven sheet followed by hydrolysis of amide to amine and subsequent incorporation of glycidol ligands. However, neither optimization of glycidol content in the chelator nor isothermal, kinetics and thermodynamic behavior of the adsorption were investigated. N-vinyl formamide = NVF, Glycidyl-methacrylate = GMA, Diallyldimethylammonium chloride (DADMAC).
Methods of preparation
Substrates
Monomers
Precursors
qe (mg·g−1)
Refs
Polymerization
Nano-polymer beads
GMA/trimethylolpropane trimethacrylate
Triethylenetetramine, ethylenediamine
23.3
29.2(Luo et al., 2020b)
Co-deposition, functionalization
Polysulfone membrane
–
Dopamine, polyethyleneimine
17.4
(Jin et al., 2019)
Polymerization
Microspheres bead
NVF, DADMAC
Polyvinylamine
43.2
(Ince et al., 2013)
Polymerization
Terpolymer beads
GMA
Ethylenediamine
32.4
(Senkal and Bicak, 2003)
Hydrolysis, amidation
Polyacrylonitrile membrane
–
Hyperbranched polyethylenimine
34.6
(Meng et al., 2016)
Chemical modification
Microporous DVB cross-linked polystyrene resin with amine functional groups
–
Aminostyrene
1.6
(Kluczka et al., 2015)
Nitration, reduction, and functionalization
Poly[N-(2,3-dihydroxypropyl)aminostyrene]
–
Aminostyrene
–
(Nesterov et al., 2013)
Polymerization, hydrolysis, functionalization
Polyethylene/polypropylene
NVF
Polyvinylamine
21.4
(Afolabi et al., 2021b)
To enhance the performance, sustainability, and economy of the reaction, the density of the glycidol must be maximized through optimization of functionalization reaction. This can be carried out using response surface methodology (RSM) which is a combination of mathematical and statistical methods allowing the investigation of various independent variables effects on a dependent variable taking interactive effects of variables on the process performance. Furthermore, RSM enables the development of empirical models and helps in minimizing the number of experiments as well as the use of chemicals, expensive analysis, and time which in turn reduces the costs (Bezerra et al., 2008; Melo et al., 2020). Thus, optimization studies pertaining to the incorporation of functional groups into the chelator precursors to enhance their performance in water and wastewater treatments become appealing (Melo et al., 2020; Moawia et al., 2019; Nallappan et al., 2018).
The objective of this article is to optimize the glycidol immobilization reaction conditions to maximize the glycidol density and to investigate the isotherms, kinetics, and thermodynamics of boron adsorption of the new fibrous chelator. Box Benkhen Design (BBD) in response surface methodology was applied to study the effect of various reaction parameters such as glycidol concentration, reaction time and temperature on the glycidol density. The optimum values for obtaining the maximum glycidol density were determined and the performance was evaluated on batch basis. The adsorption mechanism was also elaborated.
2 Materials and methods
PE/PP non-woven fibrous sheets were procured from Kurashiki Co., Japan. Glycidol (96%) and NVF monomer (98%) were purchased from Sigma Aldrich (Saint Louis, MO, USA). Boric acid, hydrochloric acid (37%), sodium hydroxide and toluene were obtained from Merck (Darmstadt, Germany). Sulfuric acid of 95 to 98% was purchased from R&M chemicals. Hach (Loveland, USA) provided the BoroVer®3 boron reagent powder kit. Deionized water (DI) obtained from the Barnstead nanopores diamond lab water purification system (Thermo Fisher, Waltham, USA) was used for washing and preparation of all solutions. All solvents were reagent grade, and they were used as received.
2.1 Synthesis of PE/PP-g-PVAm-G chelator
Radiation induced grafting was used to modify PE/PP non-woven sheet by placing the samples in PE zipped bags containing N2 gas and sealed thermally. After that, the sample-containing bags were placed on dry ice before being irradiated with an electron beam accelerator (EPS 3000, Nissin High Voltage, Japan) at 25 kGy/pass with an absorbed dose of 300 kGy. The beam current was 10 mA, and the acceleration energy was 2 MeV. The samples irradiated were transferred into evacuated ampoules, and later filled with oxygen-free grafting solutions containing 20 vol% diluted NVF/toluene solution homogenized by bubbling. The ampoules were immersed in a thermostatic bath for a period of 3 h and reaction temperature of 70 °C for the grafting process. The grafted samples were taken from the ampoules and rinsed with methanol after the process a few times before being dried overnight under vacuum at 60 °C. The degree of grafting (DG) was gravimetrically estimated as 120 %. More details on RIG of NVF onto irradiated PE/PP non-woven sheets under various conditions can be found elsewhere in our previous publication (Afolabi et al., 2021a).
The grafted fibrous poly(N-vinyl formamide) (PNVF) chains in PE/PP sheets were hydrolyzed to polyvinylamine (PVAm) under reflux with 2 M solution of NaOH at 80 °C for 4 h in an oil bath. After the reaction, the treated sample was washed and dried at 60 °C under a vacuum oven. The amine content of the PVAm sample was determined by placing 0.5 g of the samples in 10 mL of 1 M HCl for 24 h. 2.5 mL from the residual acidic solution was titrated against 0.1 M NaOH solution in the presence of phenolphthalein (Senkal and Bicak, 2003). The total amine density was estimated as 5.13 mmol·g−1.
2.2 Experimental design for glycidol immobilization
The PVAm chelator precursors were functionalized by dispersing the samples in DI water under stirring. Dropwise additions of glycidol were added to the mixture at room temperature along with continuous stirring before transferring to an oil bath heated at predetermined temperature and reaction time under reflux. The various reaction parameters were varied according to the experimental design for the purpose of optimization to determine the reaction condition with the best response. After the reaction, the samples were washed with an excess amount of DI water and oven-dried at 60 °C in a vacuum to constant weight. Fig. 2 shows a scheme for the grafting of NVF onto PE/PP non-woven sheet, followed by base hydrolysis and the immobilization of glycidol moiety into the PVAm chelator precursor.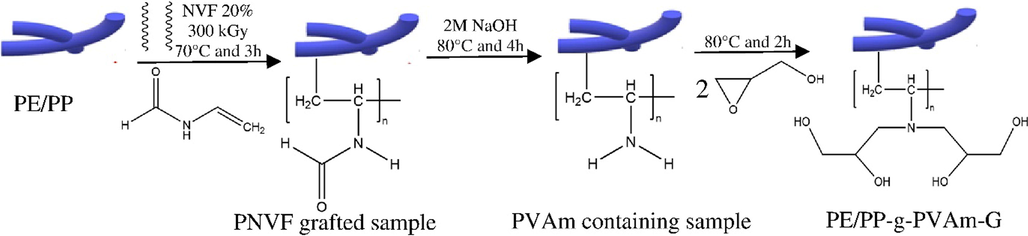
Synthesis scheme for the grafting of NVF onto PE/PP non-woven fiber sheet, base hydrolysis, and the immobilization of glycidol.
Three variable process parameters viz; concentration of glycidol, reaction time and temperature were optimized using BBD with Design Expert software (Version 10.0.1.0, Stat-Ease, Inc., Minneapolis, USA) for statistical model formation. The yield which is referred to as the glycidol functional group density was set as the system response (dependent variable). The total number of experimental runs was 17 with three-coded levels -1, 0 and + 1 involving three independent variables used for the prediction of optimum yield. Table 2 shows the ranges of the experimental values of independent variables. The glycidol functional group density in PE/PP-g-PVAm-G was calculated with Eq. (1):
Terms
Design variables
Units
Coded levels
−1
0
1
A
Glycidol concentration
vol %
5
10
15
B
Reaction temperature
°C
60
70
80
C
Reaction time
h
1
1.5
2
The experimental ranges selected for the independent variables were 5−15 vol% of glycidol concentration, 60−80 °C for reaction temperature and 1−2 h of reaction time. The independent variables and experiment data from response surface analysis are presented in Table 3. The application of the p-value test to experimental results was used for the establishment of whether the parameter is significant to a 95% level of confidence. The predicted and corresponding experimental values of glycidol functional group density were compared. For the purpose of validating the model adequacy with the experimental data, the analysis of variance (ANOVA) was used. The determination coefficients R2, adjusted R2 and predicted R2 from the analysis were estimated to check the model developed. Consequently, the values predicted by the equation developed from the model were compared with the verification experiments performed at optimum conditions. The independent variables interactions and their corresponding influence on the glycidol functional group density were examined using three-dimensional (3D) response surface plots.
Std
Run
A:Concentration (Vol %)
B:Temperature (°C)
C:Time (h)
Functional group density (mmol·g−1)
Actual
Predicted
5
1
5
70
1
2.09
2.09
2
2
15
60
1.5
3.36
3.40
7
3
5
70
2
2.88
2.98
4
4
15
80
1.5
4.77
4.83
3
5
5
80
1.5
3.89
3.85
6
6
15
70
1
3.25
3.15
13
7
10
70
1.5
3.89
3.96
11
8
10
60
2
3.39
3.35
1
9
5
60
1.5
1.56
1.50
16
10
10
70
1.5
3.91
3.96
15
11
10
70
1.5
4.02
3.96
17
12
10
70
1.5
4.09
3.96
10
13
10
80
1
3.94
3.98
12
14
10
80
2
5.03
4.97
9
15
10
60
1
1.75
1.81
8
16
15
70
2
4.78
4.78
14
17
10
70
1.5
3.88
3.96
2.3 Characterization of PE/PP-g-PVAm-G chelator
The crystallinity and structural properties of PE/PP-g-PVAm-G were analyzed using a Malvern PANalytical X'Pert3 powder diffractometer at 40 mA with a Cu anode material (Kα1 = 1.5406 A°, Kα2 = 1.5444 A°) in the Bragg's angle range of 5−50°. The chemical compositions of PE/PP-g-PVAm-G chelator samples were characterized using Fourier transform infrared (FTIR) spectrophotometer (Nicolet Magna IR-750 spectrometer) composed of diamond ATR with a wavelength ranging from 650 to 4000 cm−1. Field emission scanning electron microscopy (FESEM) was used to determine the morphology using Zeiss Supra 55 VP instrument and energy dispersive x-ray (EDX) spectrometer by Zeiss model EVO LS15 VPSEM was used to provide evidence of the distribution of various elements in the chelator at 15 kV. Thermogravimetric analysis (TGA) was used to test the thermal stability of the chelator using the Mettler Toledo TGA/SDTA 851e analyzer at a heating temperature range of 30–700 °C and a heating rate of 10 °C.
2.4 Batch adsorption experiment, isotherms, and kinetics studies
The adsorption properties of PE/PP-g-PVAm-G chelator were studied to assess its performance. A stock solution of boric acid was prepared in a plastic container by dissolving 5.716 g of H3BO3 in DI water and diluted to 1000 mL. Further solutions of desired concentrations (10−100 mgL−1) were later freshly prepared for each experimental work. The effect of different pH of the solution on the adsorption of boron was evaluated in the range of 2−11 using 0.5 g of the adsorbent in 150 mL of 100 mg/L boric acid solution in plastic Erlenmeyer flasks and the solution is continuously shaken at 30 °C with a speed of 210 rpm. The adsorption isotherms and kinetics study were investigated with different concentrations of boron (10−100 mgL−1) at a fixed fibrous chelator dose of 0.5 g in an Erlenmeyer flask containing 150 mL boric acid solution. The flask was shaken continuously at 210 rpm for 3 h in an incubator shaker. Few aliquots were withdrawn at predetermined time intervals and analyzed. The thermodynamic was studied in a similar condition at various temperatures (298 K, 303 K and 313 K). At the end of each experiment, the residual concentration of boron was spectrophotometrically determined at a wavelength of 610 nm using Shimadzu UV-2600 uv–visible spectrophotometer.
2.5 Analytical method
The boron concentration at equilibrium was measured using the carmine method. The procedure involves the addition of a BoroVer®3 reagent powder pillow into a 75 mL concentrated H2SO4 aqueous solution. The mixture was swirl for 5 min until the powder dissolves completely. 2 mL of the specimen and DI water were accurately pipetted into different 125 mL Erlenmeyer plastic flasks. In each flask, 35 mL of the prepared BoroVer®3/H2SO4 solution was added, swirled, and allowed to react for 30 min. The sample prepared was inserted into the cell holder of the UV–Vis spectrophotometer (Shimadzu UV-2600) at the wavelength of 610 nm. Eq. (2) was used for the estimation of the boron quantity at equilibrium
(mg/g).
2.6 Boron adsorption in the presence of foreign ions
The effect of foreign ions on the adsorption properties of the boron fibrous chelator coexisting in a solution of Na+, Ca2+, Cl- and SO42- were studied. Aqueous solutions containing 300 mgL−1 of each ion and 0.5 g of PE/PP-g-PVAm-G chelator were added to 100 mgL−1 boric acids. The adsorption process was carried out for 3 h at 210 rpm. The quantity of boron adsorbed at equilibrium was then determined.
2.7 Re-usability of PE/PP-g-PVAm-G
The PE/PP-g-PVAm-G chelator was recovered by solvent desorption technique after the adsorption process. It was removed after the adsorption process from the solution and dipped into 1 M HCl solution to strip boron from the chelator, neutralized with 1 M NaOH and water washed. The regenerated PE/PP-g-PVAm-G was then re-applied for the adsorption process. The re-usability efficiency (RE%) was determined according to Eq. (3):
3 Results and discussion
3.1 Fitness of experimental data to model
A mathematical model was developed and analyzed from the experimental data fitted in the software. The BBD analysis used for testing the fitness of the model indicated that the quadratic and linear models were statistically significant with the p-value<0.0001 while the cubic model was found to be aliased and could not be used for modeling as shown in Table 4. Hence, in this study the quadratic model was selected due to its good R2 values (R2 = 0.9954, adjusted R2 = 0.9895 and predicted R2 = 0.9573) suggested. The final equation of the model explaining the mathematical interaction between the three independent variables: A, B and C with the response in the range of the experimental data is represented by Eq. (4).
Sequential
Lack of Fit
Adjusted
Predicted
Source
p-value
p-value
R-Squared
R-Squared
R-Squared
Linear
< 0.0001
0.0043
0.8900
0.8646
0.8194
2FI
0.4178
0.0037
0.9161
0.8658
0.7751
Quadratic
< 0.0001
0.3307
0.9954
0.9895
0.9573
Suggested
Cubic
0.3307
0.9979
0.9916
Aliased
3.2 Statistical analysis
The application of the ANOVA analysis was carried out to evaluate the response surface of the quadratic model and for the determination of the model significance as well as the individual term involved with respect to their p-values. The F-value of the model (169.28) suggests that the model is significant, with only a 0.01 percent chance of this F-value could occur due to noise. Consequently, the high F-value and low Prob > F value 0.0001 give an indication of Fisher’s variance ratio significance as well as confirmation of the quadratic model with a high degree of fitness. Table 5 shows that the various terms A, B, C, AB, AC, BC, A2, B2, and C2 from the model were significant because their Prob > F is <0.0500. The “lack of fit” F-value (1.56) implies that this factor is not significant. There is a good correlation between the predicted and adjusted R2 value while the Adeq precision for measuring the signal to noise ratio of the model is 49.442, suggesting that it can be utilized for navigation of the design space. Thus, these results show that the quadratic model predicted is in good agreement with the responses from the experimental and can therefore be adopted.
Source
Sum of Squares
DF
Mean Square
F-Value
Prob > F
Remarks
Model
16.18
9
1.80
169.28
< 0.0001
Significant
A-Concentration
4.12
1
4.12
387.72
< 0.0001
B-Temperature
7.16
1
7.16
674.36
< 0.0001
C-Time
3.19
1
3.19
300.11
< 0.0001
AB
0.21
1
0.21
19.92
0.0029
AC
0.14
1
0.14
12.89
0.0089
BC
0.076
1
0.076
7.12
0.0321
A2
0.74
1
0.74
70.01
< 0.0001
B2
0.086
1
0.086
8.08
0.0250
C2
0.35
1
0.35
32.82
0.0007
Residual
0.074
7
0.011
Lack of Fit
0.040
3
0.013
1.56
0.3307
Not significant
Pure Error
0.034
4
8.570E-003
Cor Total
16.26
16
The ANOVA data was used to check the normality of the residuals as well as the predicted and actual responses as illustrated in Fig. 3. The plot of normal probability against the studentized residuals in Fig. 3a shows a linear trend which indicates the error distribution. As such, the model can be said to adequately predict the responses based on the reaction parameters. On the other hand with Fig. 3b showing the plot of predicted value indicate the appropriateness of the model and proper data dispersion (Nallappan et al., 2018).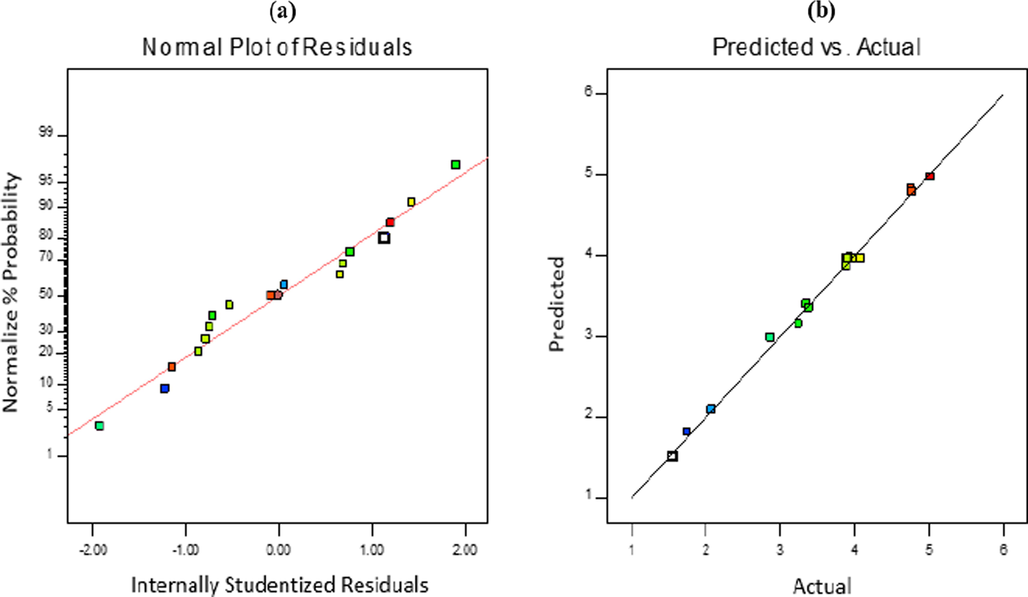
Plot of (a) normal probability vs. studentized residuals and (b) predicted vs. actual numbers of response.
3.3 Effects of independent variables and analysis of response surfaces
The interactions between the independent variables (concentration of glycidol, temperature, and reaction time) and glycidol’s functional density in the fibrous chelator are represented by Fig. 4 as established by the ANOVA analysis. The numerical optimization technique of RSM was used to optimize the variables. The 3D plot of the response surface in Fig. 4a shows the variation of glycidol density with the concentration of glycidol and temperature at a constant reaction time of 90 min. An increase in the concentration of glycidol and reaction temperature results in an increase in glycidol density. A maximum glycidol density was achieved at a concentration of 12.85 vol% and a temperature of 80 °C, respectively. This observation can be ascribed to the temperature-enhanced diffusion of the glycidol moiety into the PVAm fibrous chelator precursor. The 3D surface plot for glycidol density versus reaction time and concentration at a constant temperature of 80 °C is presented in Fig. 4b. Similarly, the glycidol density increased with an increase in both reaction time and concentration. The maximum glycidol density was recorded at a reaction time of 107.36 min and concentration of 14.02 vol% after which there was no further increment achieved because of reaching full functionalization. Fig. 4c shows the 3D plot interaction between the temperature and reaction time at a constant concentration of 12.85 vol%, in which the glycidol density increases with a rise in temperature and reaction time. It can be observed that a maximum glycidol density was achieved when the temperature and reaction time is 77.46 °C and 114.14 min, respectively.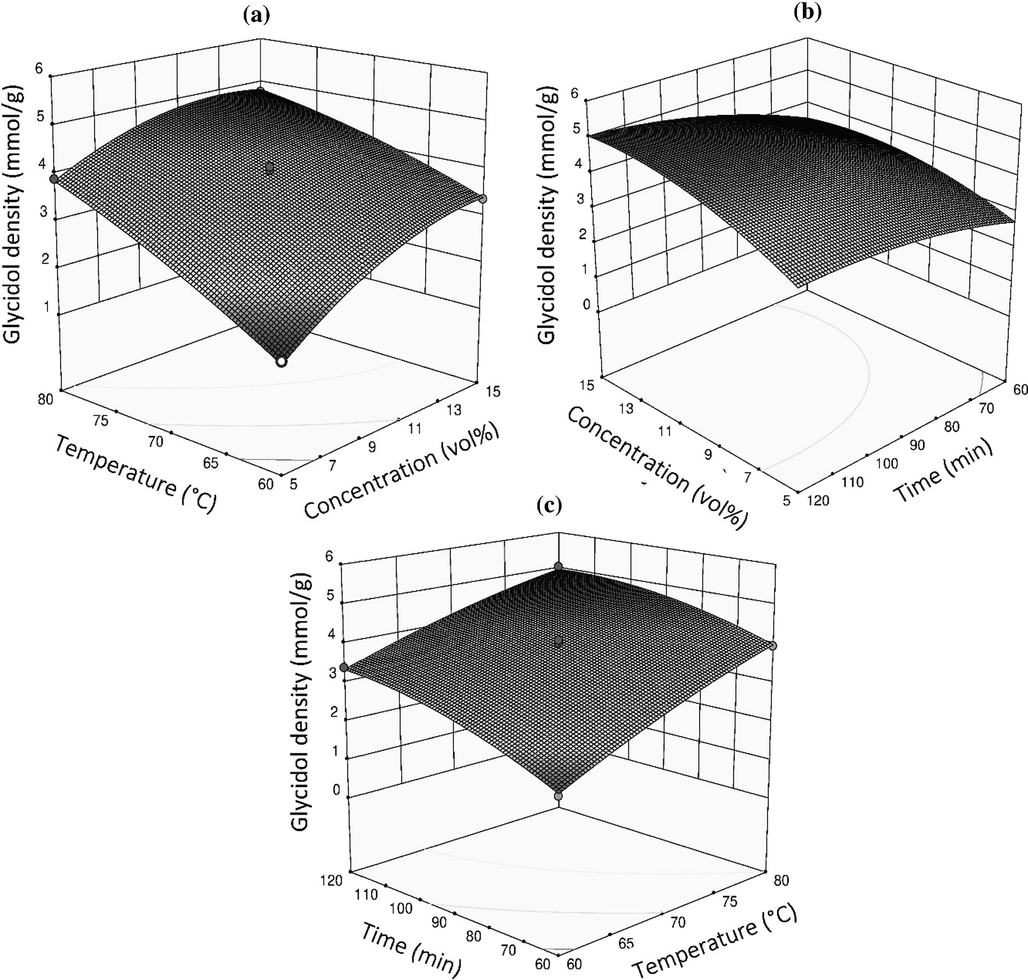
Response surface plots for the effects of: (a) temperature vs. glycidol concentration, (b) reaction time vs. glycidol concentration (c) reaction time vs. temperature on the glycidol density in the chelator.
3.4 Experimental validation of glycidol density
The predicted value of glycidol density from the numerical optimization (model) was validated under a chosen optimum parameter of 11.8 vol%, 78.9 °C and 109.4 min for glycidol concentration, temperature, and reaction time, respectively in triplicate runs. It is worth mentioning that the desirability of the response for the selected parameters is 1, as such the validation experiment performed was found to be 4.98 mmol·g−1. This value agrees well with the 4.97 mmol·g−1 glycidol density predicted with negligible deviation. Thus, this confirms a successful optimization of the glycidol density in the fibrous chelator using BBD despite the use of three levels for the independent parameters’ values in this study.
3.5 Properties of PE/PP-g-PVAm-G
The diffraction pattern of the PE/PP-g-PVAm-G chelator is shown in Fig. 5a. Five major crystalline peaks from the PE and PP substrate were observed to agree with previous studies (Afolabi et al., 2021a; Furukawa et al., 2006). This indicates that the chelator maintained its structural integrity required for the boron adsorption. The infra-red spectrum of the PE/PP-g-PVAm-G is presented in Fig. 5b. The band at 1032 cm−1 depicts the C—O stretching vibration of glycidol epoxy ring-opened linkage while the peak at 1244 cm−1 can be ascribed to amine's C—N stretching vibration. The peaks located at 2849 and 2916 cm−1 are associated with CH2 antisymmetric and symmetric stretching of the PE/PP skeletal structure (Hayashi et al., 2018). The very broad and strong peak around 3323 cm−1 shows the presence of the O—H group for chelation in the material. These characteristics peaks are associated with chelating chelator for boron (Hoshina et al., 2021). Results of chemical changes resulting from grafting and hydrolysis were discussed in our previous publication (Afolabi et al., 2021a). The PE/PP-g-PVAm-G chelator's thermal stability is revealed by TGA. Fig. 5c depicts three stages of weight loss. The first weight loss in the temperature range of 32−140 °C is corresponding to the dehydration of the bound water molecules caused by the imparted hydrophilic character resulting from incorporation of glycidol moiety. The second weight loss was at approximately 220−385 °C which can be ascribed to the PVAm grafts depolymerization. The third weight loss (385−480 °C) denotes a typical PE/PP substrate thermal decomposition transition (Xu et al., 2019). The FESEM image (Fig. 5d) corresponds to the structure of the PE/PP-g-PVAm-G with no apparent damage in the PE/PP non-woven fibrous sheet. The EDX mapping of PE/PP-g-PVAm-G results indicates that the fibrous chelator mainly contains C (76.9%), O (18.3%) and N (4.8%) elements. The rich distribution of O indicates a random functionalization and excellent dispersion of functional groups throughout the polymeric structure, thereby making it suitable for the adsorption of boron.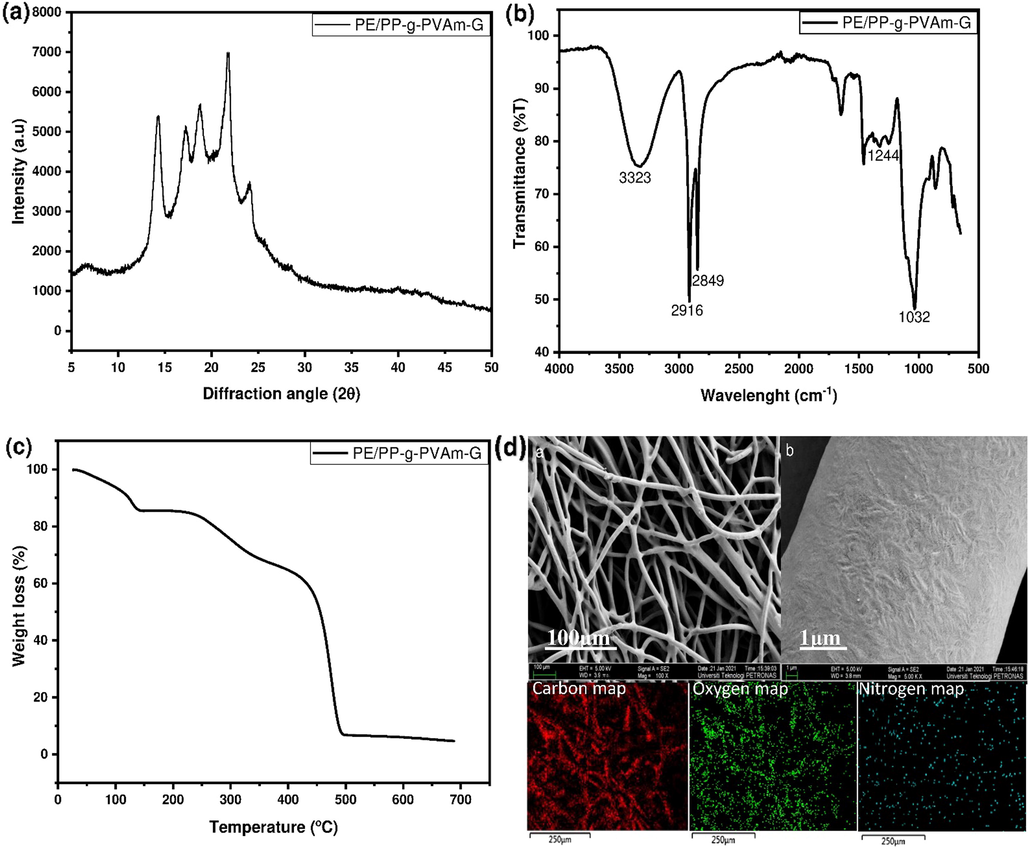
(a) XRD pattern, (b) FTIR spectrum, (c) TGA thermogram and (d) FESEM-EDX mapping of PE/PP-g-PVAm-G.
3.6 Exploring the factors influencing the experiment
3.6.1 Effect of pH
The distribution and quantity of each boron anion in the aqueous solution varies at different pH levels, likewise the forms at which they exist is complex. As a result, investigating boron-adsorbent complexation in various acid-base conditions is crucial. The influence of different pH solution ranges on the adsorption capacity of boron was investigated to determine the optimum pH value. The adsorption capacity of the chelator was found to initially increase from pH 2 until pH 7 beyond which it decreases as depicted from Fig. 6 illustrating the variation of adsorption capacity with pH of the treated solution. The increase in adsorption capacity with the increase in pH value can be attributed to the fact that fewer protons become available in the solution to compete with the boron ion for binding sites (glycidol) on the adsorbent surface as the pH increases (Oladipo and Gazi, 2016). At neutral pH, the adsorption capacity reaches its maximum value (21.4 mg·g−1) in which formation of bis-chelate ester complex is predominant. Further increase in pH values (pH great than 7) leads to a decrease in adsorption capacity due to competition between borate ions and the growing amount of hydroxyl groups in the solution. This behaviour can be explained by the fact that boric acid, B(OH)3 is an extremely weak, monobasic acid (pKa = 9.23) that acts as a Lewis acid by accepting hydroxyl groups in water to form B(OH)4− species, rather than donating proton (Sanfeliu et al., 2012). Similar result was reported for adsorption of boron by boron-selective adsorbent with low-cost materials (Sanfeliu et al., 2012), and N-methylglucamine-type cellulose derivatives (Inukai et al., 2004). A comparison of the chelator in this study with different boron-selective adsorbents and commercial resins performance reported in literature are summarized in Table 6. Te = Equilibrium time.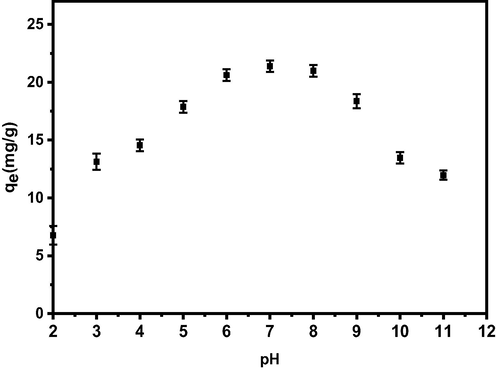
Adsorption of boron by PE/PP-g-PVAm-G chelator as a function of pH.
Adsorbents material
Morphology
Ligands
Te (min)
qe (mg·g−1)
pH
References
Nylon-6 based adsorbent
Fibrous
NMDG
120
12.4
7
(Ikeda et al., 2011)
Terpolymer-based
Bead
Glycidol
30
17.3
6.5
(Gazi & Bicak, 2007)
Cellulose
Fibrous
NMDG
> 60
12.1
8.7
(Inukai et al., 2004)
Diaion CRB −03
Granules
NMDG
> 60
11.6
7
(Ting et al., 2016)
Chitosan
Bead
NMDG
400
20.4
7
(Wu et al., 2019)
Terpolymer-based
Bead
Glycidol
80
32.4
6.6
(Senkal & Bicak, 2003)
Nylon-6 based adsorbent
Fibrous
NMDG
30
13.8
7
(Ting et al., 2016)
Polyacrylonitrile
Nanofibrous
NMDG
100
5.5
6–7
(J. Wang et al. (2014b))
PP/PE based adsorbent
Fibrous
Glycidol
60
21.4
7
This study
3.6.2 Adsorption equilibrium isotherms
Adsorption isotherm relates the quantity of adsorbate adsorbed onto the fibrous chelator to the quantity of adsorbate remaining in the solution once the adsorption process has reached equilibrium. The isotherm parameter values provide information about the chelator surface characteristic, as well as its affinity for the adsorbate, and are crucial for optimizing the chelator usage (Asare et al., 2021). Here, the adsorption isotherms examination is to demonstrate how the PE/PP-g-PVAm-G and the boron adsorbate are behaving when in contact with the solution. The fitting of adsorption isotherm data to various adsorption isotherm models is a critical step in determining the most appropriate adsorption isotherm model for design purposes. The amount of boron adsorbed onto PE/PP-g-PVAm-G was studied using six different adsorption isotherms including two parameters (Langmuir, Freundlich, and Temkin) and three parameters (Redlich-Peterson, Toth, and Sips) equations. Although linear isotherms have been utilized to describe adsorption systems in many studies, however, non-linear isotherms were used in this work for more accuracy. The adsorption isotherms were established based on certain assumptions. As a result, linearizing them would undermine the assumptions that influence their development and could lead to an erroneous solution. The non-linear form of the isotherms, despite its complexity, gives a remarkable and precise mathematical approach to compute the isotherm parameters. Hence, to prevent inaccuracy caused by linearization, the parameters of the various isotherms in this study were obtained with the non-linear technique.
The Langmuir isotherm model is representing the monolayer coverage of boron on the chelator surface at a constant temperature. It hints towards the homogeneity of the surface and assumes that the application of intermolecular forces by the chemical surface of unsaturated atoms does not extend furthermore than the adsorbed molecule diameter (Jin et al., 2019). This model also assumes that all adsorption sites have the same energy properties, and that adsorption takes place on a structurally homogeneous surface. The Langmuir equation has the following expression:
The Freundlich adsorption isotherm model portrays the heterogeneity of the surface and the adsorbed molecule’s multilayer adsorption. Therefore, it is generally used for heterogeneous systems, and the amount of adsorbed molecule is the aggregation of all the adsorption sites (each having bond energy) (Luo et al., 2020b). The equation of Freundlich isotherm can be expressed in Eq. (7).
The Temkin isotherm is derived on the assumption that the decrease in adsorption heat is linear rather than logarithmic and that the adsorption process is characterized by a uniform distribution of binding energies at the chelator surface. The non-linear equation of Temkin isotherm can be represented by:
The Sips isotherm eliminates the limiting deficiencies associated with both Langmuir and Freundlich isotherms by combining them into a single equation (Asare et al., 2021). The Sips isotherm is expressed as:
The Toth isotherm is a three-parameter isotherm that can be used for describing the adsorption behavior on a heterogeneous surface. The isotherm can describe how heterogeneous surface adsorption at low and high concentration boundaries of a particular adsorbate behaves. The Toth isotherm is written as follows:
Redlich-Peterson isotherm can be referred to as hybrid isotherm which features both Langmuir and Freundlich isotherms, with the existence of three parameters in the empirical equation (Foo and Hameed, 2010). Thus, due to its versatility, this model is applied for both homogenous and heterogeneous systems. The Redlich-Peterson isotherm can be expressed using Eq. (11).
where (mg/L) is the concentration at equilibrium in the liquid phase, and (mg·g−1) is the adsorption capacity of boron at equilibrium, while (mg−1) and (Lg−1) are Redlich-Peterson adsorption isotherm constants. The isotherm exponent (g) values are ranging between 0 and 1. Different studies have proven Redlich-Peterson isotherm to be more accurate when compared with Langmuir and Freundlich isotherms due to the three unknown parameters involved in it and thus, both isotherms’ derivations can be achieved from Eq. (11) (Foo and Hameed, 2010).
Fig. 7 represents the boron adsorption equilibrium isotherms for the PE/PP-g-PVAm-G chelator. The values of various constants and fitting data including the correlation coefficient R2, and χ2 are also listed in Table 7. According to the estimated data from the various models of the two-parameter isotherms, the Langmuir correlation coefficient R2 is 0.9799, which is greater than the values for the other two isotherms. Moreover, it shows the least χ2 (0.0092) value. This data suggests that monolayer adsorption occurred on a homogeneous surface during the adsorption process. The monolayer saturation capacity (qm) for boron on PE/PP-g-PVAm-G was estimated as 25.7 mg·g−1 while the RL values estimated were found to fall between 0 and 1 which gives an indication that the adsorption of boron is favorable (Kipçak and Özdemir, 2012; Oladipo and Gazi, 2016). Consequently, in the Freundlich isotherm model, the 1/n value obtained is below one. This suggests that the adsorption process followed a favorable normal Langmuir isotherm as indicated by the RL values. The adsorption process is not represented by Temkin isotherm due to its correlation coefficient value, however, the heat of adsorption RT/bT was estimated as 11 kJ mol−1. The typical range of bonding energy for ion-exchange mechanisms has been reported in the range of 8–16 kJ mol−1 (Baker, 2009; Wei et al., 2011).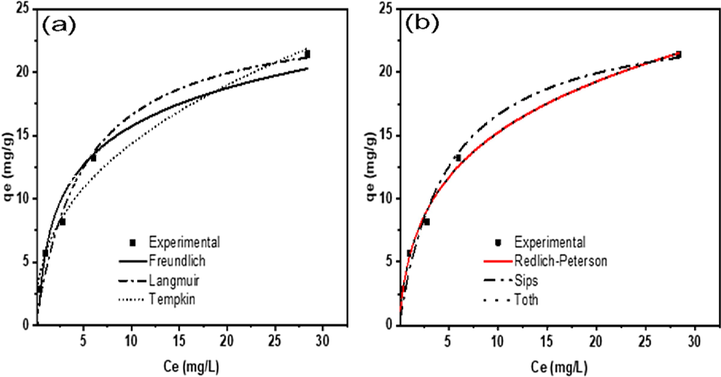
Plots of: (a) two-parameter and (b) three-parameter isotherms of boron adsorption by PE/PP-g-PVAm-G.
Langmuir
Freundlich
Temkin
KL = 0.203
KF = 5.18
AT = 3.68
qm = 25.7
1/n = 0.403
RT/bT = 11
R2 = 0.9799
R2 = 0.9528
R2 = 0.9659
χ2 = 0.0092
χ2 = 0.191
χ2 = 2.423
From Table 8, the correlation coefficient R2 of the three-parameter isotherms is higher than all the two-parameter isotherms. This is suggesting the applicability of three-parameter isotherm models to represent the adsorption equilibrium of boron by PE/PP-g-PVAm-G chelator. The correlation coefficients in Redlich-Peterson, Toth and Sips are 0.9953, 0.9951 and 0.9858, respectively. The Redlich-Peterson and Toth adsorption model showed the highest R2 value and leads to the best regression coefficients. Similar result was observed by Benzaoui et al. (2018). More so, both the Redlich-Peterson and Toth isotherm constant values i.e., g and t are not far from unity, indicating that it is approaching the Langmuir isotherm but not the Freundlich isotherm. Thus, Langmuir is a special case of Redlich-Peterson when the constant g approaches unity (Ayawei et al., 2017; Ding et al., 2017). The value of the Sips isotherm constant (1/ns) also approaches unity too, suggesting that boron uptake by PE/PP-g-PVAm-G occurs on a homogenous surface. This finding is consistent with the two-parameter isotherm analysis. Comparable results were reported for nanofibrous chelator containing NMDG counterpart for boron removal (Nallappan et al., 2019).
Redlich-Peterson
Toth
Sips
KR = 11.57
Kt = 1.29
Ks = 5.52
AR = 1.21
qt = 6.63
qs = 24.85
g = 0.74
t = 0.97
1/ns = 1.103
R2 = 0.9953
R2 = 0.9951
R2 = 0.9858
χ2 = 0.0003
χ2 = 0.477
χ2 = 1.429
3.6.3 Adsorption kinetics
The adsorption kinetics show how the adsorption process changes over time. Thus, the latter is a critical variable that must be considered. The pseudo-first-order, pseudo-second-order, and intraparticle diffusion models were considered for the determination of the suitable model as well as for the explanation of the experimental data mechanism. The pseudo-first-order adsorption model theorized that the rate is proportional to the number of the unoccupied adsorption sites and is expressed as:
The pseudo-second-order rate expression is used to explain the chemisorption that involves valence forces through the exchange or sharing of electron as ion exchange and covalent forces between the adsorbent and adsorbate. The second-order rate expression is given below:
Fig. 8 shows the kinetic models with the impact of contact time on the removal of boron by PE/PP-g-PVAm-G at different concentrations and contact time. During the process of adsorption, the chelator was able to adsorb 70–85% of the saturated adsorption capacity within 30 min, and the chelator reaches the saturated adsorption capacity within 60 min because PE/PP-g-PVAm-G is rich in polyols. Henceforth, a substantial amount of boron diffused to the surface of the chelator at the start of the adsorption reaction, and the polyols and boron were continually complexed. As boron continuously occupies the adsorption sites, the adsorption rate is reduced until all of the adsorption sites are filled up, and the amount adsorbed tended to become saturated and constant (Luo et al., 2020b). Table 9 shows the data for pseudo-first-order rate constants k1, pseudo-second-order rate constants k2, calculated equilibrium adsorption capacity
(cal), R2, and χ2 for better understanding of the adsorption mechanism such as mass transfer and chemical reaction. The kinetics fitting results for the models indicate that the process does not follow pseudo-first-order kinetics. The pseudo-second-order kinetic best fit the experimental data as it provides the highest R2, and lowest χ2 values at different initial concentrations than the pseudo-first-order kinetic. This is because the pseudo-second-order kinetic
values calculated corroborate more with the experimental results. Thus, the consistency of the ion exchange adsorption process with pseudo-second-order kinetic model further shows that the complexation between B(OH)3 and the polyol groups from the chelator is controlled by chemical interaction (Lyu et al., 2017; Uba et al., 2020).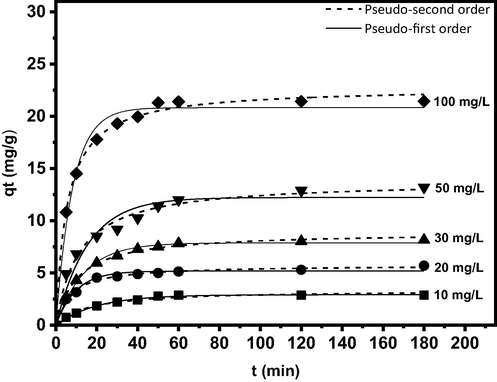
The Kinetic models plots for boron adsorption by the PE/PP-g-PVAm-G.
Kinetic models
Parameters
10 mg/L
20 mg/L
30 mg/L
50 mg/L
100 mg/L
Pseudo-first order
qe,(calc) (mg·g−1)
2.51
5.18
7.38
11.23
20.03
k1 (min−1)
0.050
0.103
0.073
0.064
0.12
R2
0.9811
0.9776
0.9936
0.9488
0.9872
χ2
0.022
0.077
0.053
0.972
0.759
Pseudo-second order
qe,(calc) (mg·g−1)
3.05
5.77
8.90
13.82
22.76
k2 (g·mg−1·min−1)
0.019
0.025
0.011
0.064
0.0081
R2
0.9945
0.9932
0.9959
0.9872
0.9964
χ2
0.007
0.0231
0.0337
0.243
0.190
Intraparticle diffusion
kp (mg·g−1·min1/2)
0.190
0.26
0.399
0.659
0.732
C (mg·g−1)
0.94
2.83
3.89
5.33
13.94
R2
0.7066
0.7477
0.7298
0.796
0.6607
χ2
0.407
0.509
0.823
1.133
1.80
3.6.4 Mechanism of boron removal by PE/PP-g-PVAm-G
The mechanism involved in the boron removal by PE/PP-g-PVAm-G was evaluated with Eq. (14). The intraparticle diffusion model was adopted in this context. It is based on the theory that the fraction of boron removed depends on the chelating diffusivity of PE/PP-g-PVAm-G and the radius of boron. The intra-particle diffusion model expression is given as:
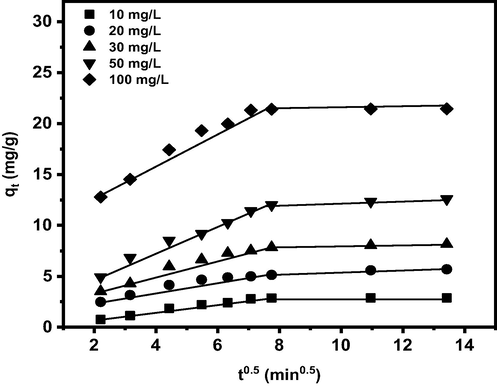
The intra-particle diffusion plots of boron adsorption by the PE/PP-g-PVAm-G.
3.6.5 Adsorption thermodynamics studies
The evaluation of thermodynamic parameters such as enthalpy (ΔHo), Gibbs free energy (ΔGo), and entropy (ΔSo) changes was carried out to comprehend the nature and feasibility of the adsorption process. This can be achieved from the changes in temperature (T) and thermodynamic equilibrium constant (KC). They were calculated from the following equations:
From the intercept and slope of the plot from lnKC versus 1/T, ΔHo and ΔSo values were calculated and summarized in Table 10. The negative values of ΔG° indicate that the boron adsorption on PE/PP-g-PVAm-G is spontaneous, and the free energy value swings. Not only that but also ΔG° became more negative as temperature increased, implying a greater driving force and hence a bigger adsorption capacity at higher temperatures which further demonstrates that boron adsorption is spontaneous and thermodynamically favorable (Lyu et al., 2017). The positive values of ΔHo (5.541 kJ mol−1) confirm the endothermic nature of adsorption, while the positive value of ΔSo (25.829 J mol−1 K−1) suggests an increased degree of randomness at the solid–liquid interface during the process of boron adsorption onto PE/PP-g-PVAm-G. Similar observation was reported for boron removal from solution (Zijie Wang et al., 2021).
Temperature (°C)
ΔG° (kJ mol−1)
ΔHo (kJ mol−1)
ΔSo (J mol−1 K−1)
293
−2.005
303
−2.331
5.541
25.829
313
−2.518
3.6.6 Boron adsorption in the presence of foreign ions
The presence of a large number of inorganic salts which are ionizable into various ions in aqueous solutions can reduce the chelator's adsorption capacity in surface water, industrial wastewater, and subsurface water sources. As a result, studying the selectivity properties of PE/PP-g-PVAm-G towards anions and cations in solutions is important. The adsorption selectivity study was conducted in the presence of foreign ions such as Na+, Ca2+, Cl- and SO42-. The sources of the various ions used in this study were NaCl, CaCl2, KCl, and Na2SO4. The experiments revealed that, in the presence of boric acid, these foreign ions are also absorbed. The highest loading is observed for Ca2+ ions (1.67 mg·g−1) as shown in Fig. 10. The foreign ion adsorption observed could be due to the precipitation of their hydroxides and the weak basicity of the tertiary amino group from the polymeric substrate (Gazi and Shahmohammadi, 2012). Fortunately, adsorption of those coexisting ions does not show a significant decrease in the boron loading capacity of PE/PP-g-PVAm-G. This may be due to the coordination capability of the polyol of the chelator. Not only that but may also be due to the influence of the inorganic salt ions occupying some of PE/PP-g-PVAm-G active sites, resulting in a modest reduction in boron adsorption capacity. Therefore, the chelator can be said to have good boron selectivity as well as anti-interference properties.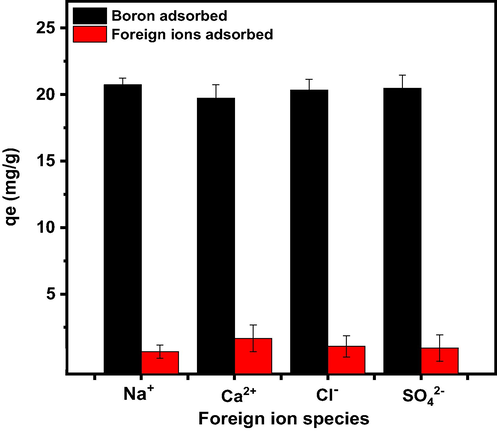
Adsorption capacities of the PE/PP-g-PVAm-G for boron and coexisting ions in simulated water.
3.7 Re-usability study
The regeneration cycle result for the elution process is presented in Fig. 11. The result showed a trivial increase in capacity loss with a decrease in adsorptive efficiency after five adsorption/desorption cycles as evidenced by Fig. 11. This observation can be attributed to the fact that there is a decrease in active sites of PE/PP-g-PVAm-G. The decline in efficiency is <3% which indicates that the PE/PP-g-PVAm-G has a high recyclability and nearly maintains a constant regeneration efficiency after 5 cycles. The result implied that PE/PP-g-PVAm-G could be successfully regenerated as an effective adsorbing material for removing boron from contaminated solutions.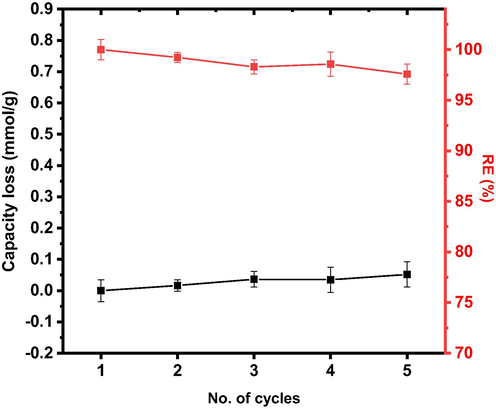
Re-usability of PE/PP-g-PVAm-G for boron adsorption.
After the PE/PP-g-PVAm-G chelator is subjected to 5 cycles of adsorption–desorption, the changes in mass before and after adsorption was observed to be negligible. The infrared spectroscopy comparison of PE/PP-g-PVAm-G before and after the 5 cycles is displayed (Fig. 12). The results reveal that the PE/PP-g-PVAm-G functional groups remained unaltered after the 5 cycles of adsorption–desorption. This gives an indication that the fibrous chelator has a promising regeneration stability. To make the process green, the recovery of boron and the acid used to strip it should be considered to reach the pure water discharge. The recovered acid and boron can be further used.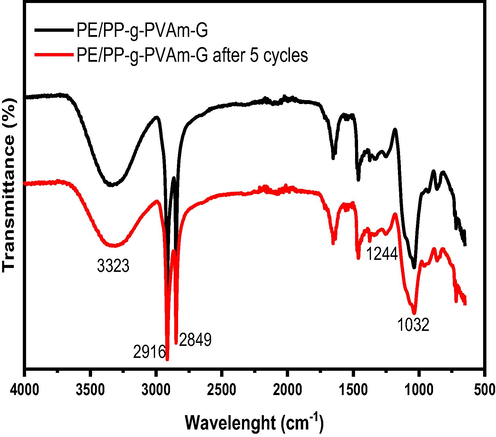
IR of PE/PP-g-PVAm-G before and after 5 cycles.
4 Conclusions
PE/PP-g-PVAm-G fibrous chelator containing cis-diol groups was successfully prepared by RIGC of NVF onto PP/PE non-woven sheet followed by conversion of polyamide grafts to polyamine and subsequent chemical treatments with glycidol and tested for boron adsorption from aqueous solution. The reaction variables were optimized using RSM to maximize the glycidol density on the chelator. A maximum glycidol density yield of ∼5.0 mmol·g−1 was obtained at 11.8 vol%, 78.9 °C, and 109.4 min for glycidol concentration, temperature, and time, respectively. The FT-IR, SEM, EDX, DSC, and TGA analysis of the adsorbents revealed convincing proof of chemical treatment and covalent immobilization of the glycidol groups onto PVAm. The boron adsorption data was best represented by the Langmuir from the two-parameter isotherm, suggesting that the adsorption process occurs on a homogenous surface by monolayer adsorption with a maximum adsorption capacity of 25.7 mg·g−1. The three-parameter isotherm model correlation coefficient was higher than all the two-parameter isotherms. Thus, suggesting their applicability and gives an indication that the Langmuir is a special case of Redlich-Peterson. The adsorption process kinetics revealed that the uptake of boron followed a second-order kinetic path. This implies that the process occurred by chemical attachment of the boron to the polyol groups of PE/PP-g-PVAm-G as a chelator. The spontaneous removal process was found to be endothermic with ΔHo of 5.541 kJ mol−1 and random in nature with ΔSo of 25.829 J mol−1 K−1. Na+, Ca2+, Cl- and SO42- ions had little effect on the PE/PP-g-PVAm-G adsorption capacity of boron. The fibrous chelator has a good recyclability and virtually maintained a steady regeneration efficiency after 5 cycles. The results demonstrated that PE/PP-g-PVAm-G can be used as an alternative adsorbent in terms of adsorption capacity, selectivity, and re-usability for removing boron from aqueous solutions.
Acknowledgments
The authors are appreciative of the financial support provided by Universiti Teknologi Petronas through the grant of Y-UTP with cost center 015LC0-065 and 015LC0-310. The Malaysian Nuclear Agency’s invaluable technical assistance during the radiation grafting processes is well appreciated.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
The raw/processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.







