Translate this page into:
Jute stick extract assisted hydrothermal synthesis of zinc oxide nanoflakes and their enhanced photocatalytic and antibacterial efficacy
⁎Corresponding authors at: Department of Chemistry, Jagannath University, Dhaka 1100, Bangladesh (M.Z. Hossain), Interdisciplinary Research Center for Hydrogen and Energy Storage (IRC-HES), King Fahd University of Petroleum & Minerals, Dhahran 31261, Saudi Arabia (M.A. Aziz). maziz@kfupm.edu.sa (Md. Abdul Aziz), zamir@chem.jnu.ac.bd (Muhammad Zamir Hossain)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Hot extraction of jute stick. A green hydrothermal approach to synthesize ZnO nanoflakes using jute stick extract. Prepared jute extrcact assisted ZnO nanoflakes showed a significant dye degradation. 98 % efficiency in MB degradation was calcualted after 8 h of UV light irradiation. Prepared jute supported ZnO exhibited 19 mm of zone inhibition against E. coli.
Abstract
In this paper, we used green and hydrothermal methodology to prepare zinc oxide (ZnO) nanoflakes (NFs) with jute stick extract (J–ZnO NFs) as growth substrate. The prepared materials were characterized using different analytical techniques including ultraviolet–visible spectroscopy (UV–vis), X-ray diffraction (XRD), field emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM), thermogravimetric analysis (TGA), fourier transform infrared (FTIR) spectroscopy, and X-ray photoelectron spectroscopy (XPS). The characteristic absorption peak for ZnO NFs and J–ZnO NFs were observed from the UV–vis spectrum at 373 and 368 nm respectively. The hexagonal wurtzite crystal structure of ZnO NFs and J–ZnO NFs was confirmed by XRD analysis. FESEM and TEM analyses of synthesized J–ZnO NFs confirmed their NFs shape and collectively flower-like structure formation by the assembly of NFs of J–ZnO on cellulose of jute stick extract substrate. The FTIR analysis revealed the functional groups of jute stick extract biomolecules, mainly cellulose, are responsible for the formation of collectivel flower like J–ZnO NFs structure. The XPS analysis revealed the surface and chemical compositions (Zn, C, and O) of J–ZnO NFs. The photocatalytic performance of ZnO NFs and J–ZnO NFs samples was carried out by the degradation of methylene blue (MB) dye solution under UV light irradiation. The degradation efficiency of ZnO NFs and J–ZnO NFs was obtained 79 % and 89 %, respectively, for 5 h. Notably, the degradation efficiency of the J–ZnO NFs was 98 % after 8 h of irradiation, which is very inspiring. The both NFs exhibited first-order kinetics with MB photodegradation. We also examined the possible antibacterial activity of both samples against Escherichia coli (E. coli) pathogens, which demonstrated a significant result with a 17 mm and 19 mm zone of inhibition by ZnO NFs and J–ZnO NFs respectively.
Keywords
Jute stick extract
Green hydrothermal approach
ZnO nanoflakes
Photocatalytic dye degradation
Antibacterial assay
1 Introduction
The growing urbanization and the rise of companies producing things like paper and ink, textiles, leather, and other things, which causes water pollution hence harms humans and aquatic life. The most frequent water pollutants are azo dyes (Okeke et al., 2020, Weldegebrieal, 2020). They are mostly employed as colorants in the textile industry, a significant amount of the dyes in a range of 10–15 %, even up to approximately 50 % in the case of a class of dyes called reactive dyes, may not be fixed by the fibers during dyeing and end up in the waste stream. Even very little amounts of dye (less than 1 ppm) can generate extremely vivid colors, alter transparency, influence water–gas solubility, and negatively impact aquatic life by reducing sunlight penetration, limiting photosynthesis, causing an oxygen shortage and cause cancer in both humans and natural sources (Weldegebrieal, 2020, Park et al., 2021, Harikishore et al., 2014).
Microorganisms are a typical component of the organic compounds found in wastewater that can have an impact on human health. In addition, a disease that has a substantial economic impact on rice crops globally is bacterial leaf blight. The final stage in the treatment of wastewater is frequently the elimination of harmful bacteria (Zendehnam et al., 2018). Hence, the development of effective remediation procedures for water-borne infections such Shiga toxin-producing by E. coli has stimulated scientific interest as a result of the emergence of innovative biocidal or disinfecting chemicals to replace conventional antibiotics. Chemical treatments have been widely employed to tackle these illnesses, but they pollute the environment and breed resistant strains. As a result, the creation of biocompatible, safe, and environmentally friendly nanomaterials has become a pressing issue in nanotechnology research (Rambabu et al., 2021, Ogunyemi et al., 2019, Kalpana et al., 2018, Weldegebrieal, 2020). Simultaneous elimination of organic pollutants found in wastewater streams, such as phenolic compounds, dyes, pesticides, microorganisms, and so on, would efficiently replace existing staged treatment approaches (Weldegebrieal, 2020, Ahammed et al., 2020). The need for a substance with antimicrobial and photocatalytic activity is extremely high.
For dye removal from wastewater, a variety of procedures have been used, including adsorption, biological treatment, and heterogeneous photocatalysis. In addition, the use of bacteria in biological wastewater treatment is limited due to the greater chemical oxygen demand of the effluent, and most colors are poisonous to the microbial cell. Adsorption by heterogeneous nanomaterials is similarly restricted, and desorption characteristics are severely lacking. Photocatalysis has several benefits over the adsorption technique. The greater surface area to volume ratio of nanomaterials makes them more reactive and has stronger antibacterial activity (Kaliraj et al., 2019, Balcha et al., 2016, Rambabu et al., 2021, Zendehnam et al., 2018).
These photocatalyst and antibacterial agents for water treatment can generally be split into two groups: organic and inorganic. Particularly at high temperatures and/or pressures, organic materials are frequently less stable than inorganic ones. Since the last ten years, inorganic materials, such as metal and metal oxide nanomaterials, have drawn a lot of attention due to their ability to withstand harsh processing conditions and the fact that they are typically regarded as being safe for both humans and animals. Ag, Au, Cu, CuO, TiO2, and ZnO are examples of inorganic nanomaterials with strong photocatalytic and antibacterial properties (Rambabu et al., 2021, Zendehnam et al., 2018).
ZnO nanomaterials are of special interest among inorganic nanomaterials because they are a simple, affordable, and safe substance for humans and animals to employ. In addition, ZnO nanomaterial is a semiconductor with an excitation energy of 60 meV and has a bandgap of 3.3 eV, which is near equivalent to TiO2 (3.2 eV for anatase), a material with exceptional characteristics. Therefore, ZnO nanomaterials has a wide range of applications, including functional optical devices, ultraviolet photodetectors, gas sensors, varistors, solar cells, ultraviolet laser diodes, hydrogen generation, and ion insertion batteries. It has also been used in the manufacturing of cosmetics and medicine delivery in the healthcare and pharmaceutical industries (Bhuyan et al., 2016, Shi et al., 2014, Borysiewicz, 2019, Kolodziejczak-Radzimska and Jesionowski, 2014, Ashar et al., 2021). ZnO nanomaterials have great potential as photocatalysts for water purification and antibacterial agents due to their high physical and chemical stability, excellent optoelectronic capabilities, photocatalytic activity due to high electron mobility, exceptional antibacterial function, and strong UV and infrared adsorption. The unusual antibacterial, antifungal, wound-healing, and UV filtering properties of ZnO nanomaterials, along with their high catalytic and photochemical activity, have drawn scientists' attention (Kalpana et al., 2018, Moghaddas et al., 2020, Elumalai and Velmurugan, 2015, Qi et al., 2017). However, because of their enormous surface area and high surface energy, ZnO nanmaterials combine quickly. It is necessary to use dispersed coatings or ZnO nanomaterials growth on template substrates instead (Yang et al., 2016, Abdalkarim et al., 2018, Ong, Ng and Mohammad, 2018).
Various techniques such as thermal evaporation, hydrothermal process, chemical vapor deposition, sol–gel synthesis, and solution growth have been used for the synthesis of ZnO nanomaterials (Ullah et al., 2019). One of them, the hydrothermal process, was developed by imitating the natural evolution of specific ores. It is a soft chemical synthesis approach. It may be used to develop a variety of single crystals, make ultrafine agglomerated or less agglomerated crystalline ceramic powders, complete some organic processes, treat some organic waste products that are hazardous to human health, or sinter some ceramic materials at a low temperature. In addition, hydrothermal synthesis has a number of advantages over other synthetic techniques, such as one-step synthesis without high-temperature calculations and milling, low aggregation levels, narrow crystallite size distributions, high purity, and excellent particle morphology and size control (Yang and Park, 2019, Basnet and Chatterjee, 2020, Varadavenkatesan et al., 2019, Darr et al., 2017, Alam et al., 2021, Nayem and Hossain, 2021).
Biological substrates are used to generate ZnO nanomaterials using green techniques. These approaches have substantial advantages since the extracts help to produce well organized ZnO nanomaterials and boost their antibacterial activity while also functioning as reducing and stabilizing agents (Borysiewicz, 2019). Additionally, a straightforward chemical precipitation method is provided to create clusters of ZnO nanorods that resemble flowers employing spherical cellulose nanocrystals (with cellulose II crystal structure) as the growth substrate (Yang et al., 2016). In the literature, there are many published reports interms of ZnO composites based on cellulose (Lizundia et al., 2016, Abdalkarim et al., 2018, Lefatshe et al., 2017). Therefore, searching for a green cellulose sources are important for ZnO/cellulose research. In this regard, green materilas like jute stick extract as growth substrate for ZnO would be worthwhile for the enrichment of ZnO/cellulose research area.
The jute stick is a common raw material for starting a fire for cooking. It is an agricultural byproduct that yields 2.5 times more jute fiber by weight. Carbohydrates are the primary component of jute sticks (i. e., cellulose, hemicellulose, and lignin). It contains a trace quantity of fat (1.9 %) and ash content (1 %), in addition to carbohydrates. Similarly, the percentages of hemicellulose (22 %), cellulose (40.8 %), and lignin (40.8 %). Jute sticks are thermally stable at 250 °C and contain a lot of carbon (44 %) and oxygen (46–49 %). As a result, the jute stick is a cheap, easily accessible, renewable, and environmentally friendly supply of carbon and oxygen that can be used as a growth substrate and alternative of cellulose to produce well organized ZnO nanomaterials (Aziz et al., 2020).
In this experiment, we follow the hydrothermal strategy to prepare the jute stick extract-assisted ZnO NFs using a jute stick extract as a growth substrate and alternative of cellulose. The prepared material was characterized using different analytical techniques including UV–vis, XRD, SEM, TEM, FTIR, TGA, XPS etc. The introduction of a potential formation mechanism for the production of sheet-like NFs collectively flower shape was also presented. Jute extract's cellulose and Zn2+ are thought to combine strongly electrostatically to produce J–ZnO NFs that resemble flowers. To corroborate the formation, the finished sheet-like J–ZnO NFs were thoroughly characterized. Lastly, the antibacterial properties of these prospective biological materials were assessed. We also evaluated the photocatalytic activity of the prepared NFs against MB dye under UV light conditions and antibacterial activity against E. coli pathogens.
2 Experimnetal
2.1 Materials
Zinc acetate dihydrate (Zn(CH3COO)2·2H2O), sodium hydroxide (NaOH), and methylene blue (MB) are all analytical grades and are used without further purification. All the chemicals were purchased from Sigma Aldrich. The jute sticks were collected from the remote village of Harriaghope in the Jessore district of Bangladesh. Distilled water (DW) collected from Active Fine Chemicals was used for the whole experiment.
2.2 Collection of jute extract
The gathered dried jute sticks were broken up into small pieces and processed through a blender into a powder. 1.50 g of the jute sticks powder was added to 75 mL of DW water in a Teflon tube. The Teflon tube, along with the mixture, was then placed into a sealed hydrothermal reactor and heat treatment was performed at 140 °C for 3 h in an electric furnace. The heat-treated aqueous solution of jute powder was filtered, and the extracted filtrate was stored in the refrigerator for further analysis (Mrabet et al., 2017).
2.3 Nano zinc oxide synthesis
Based on the literature (Abinaya et al., 2016) with little modification, for the synthesis of ZnO NFs (Scheme 1A), 0.40 g of Zn(CH3COO)2·2H2O was mixed with 20 mL of DW with vigorous stirring for 15 min to get a homogeneous solution. 0.36 g of NaOH was also mixed with 20 mL of DW for a homogeneous solution. Then the homogeneous solution of NaOH was dropwise added to the solution of Zn(CH3COO)2·2H2O under continuous stirring conditions. The mixture was then vigorously stirred for 1 h, and next transfered in a teflon tube and sealed in a hydrothermal reactor for heat treatment at 170 °C for 5 h in an electric oven. After 5 h of reaction, we took out the reactor and cooled it in room temperature water. After cooling, the synthesized NFs were several times washed with water. Finally the washed NFs were dried in an oven at 120 °C for 6 h and used for further analysis.
Schematic representation for the step-wise hydrothermal synthesis of (A) ZnO NFs and (B) J–ZnO NFs.
Similarly, jute extract assisted product (J–ZnO NFs) were synthesized (Scheme 1B); while 0.40 g of Zn(CH3COO)2·2H2O was mixed with 20 mL of aqueous jute extract with vigorous stirring for 15 min to get a homogeneous solution. With the help of vigorous stirring for 15 min, 0.36 g of NaOH was also mixed with 20 mL of DW to produce a homogeneous solution. Then the homogeneous solution of NaOH was added dropwise into the solution of Zn(CH3COO)2·2H2O under continuous stirring. The mixture was then vigorously stirred for 1 h. The mixture was then transfered in the Teflon tube and sealed in a hydrothermal reactor and heated in an electric furnace for 5 h at 170 °C. The reactor was cooled to room temperature water after 5 h of reaction. After cooling, DW water was used to repeatedly wash the produced (J–ZnO NFs). The cleaned J–ZnO NFs was then used for additional examination after being dried in an oven for 6 h at 120 °C.
2.4 Characterization of the synthesized products
For jute stick extract, ZnO NFs and J–ZnO NFs, UV–vis spectra were recorded in the range of 200–800 nm with a resolution of 0.5 nm using a SHIMADZU UV-1800 double-beam spectrophotometer (Shimadzu Corporation, Kyoto, Japan). UV–vis spectra were acquired using 3 mL of all sample contents in a quartz cuvette with a 1 cm path length. As a baseline adjustment, distilled water was used for UV–vis analysis. X-ray diffraction (XRD, Rigaku Miniflex-II diffractometer) was used to determine the crystallinity of the produced NFs. The diffractometer was set at a wavelength of 0.15416 nm, a current of 10 mA, and a voltage of 30 kV. A field emission scanning electron microscope was used to examine the surface morphology of the produced nanoparticles (FESEM, TESCAN-LYRA-3, Czech Republic). Transmission electron microscopy (TEM) (JEM-2011; JEOL) was used to obtain the TEM images, and the selected area electron diffraction (SAED) pattern was taken using the same instrument. Thermogravimetric analysis (TGA) was used with a Mettler Toledo (TGA1 star system) analyzer to evaluate the weight loss of the products as a function of temperature in order to determine their stability. In order to study the possibility of chemical interaction between ZnO and jute stick extract as well as functional groups of the jute stick extract on the surface of the ZnO NFs, Fourier transformation infrared (FTIR) spectra of KBr pellet were collected using a JASCO FT/IR680 spectrometer. To determine the elemental makeup of the generated J–ZnO NFs, X-ray photoelectron spectroscopy (XPS) with an Al-K-alpha monochromatic X-ray source was used (ESCALAB-250Xi XPS-Microprobe, Thermo-Scientific, USA). For the BE (binding energy) calibration, the 284 eV BE of the C 1s peak was employed.
2.5 Antivacterials assay
2.5.1 Maintenanace of bacterial culture
On tryptone soya agar media, the bacterial strain (E. coli) was freshly subcultured. One loopful of a colony was selected from a freshly cultured plate, and it was used to inoculate 9 mL of tryptone soya broth. The mixture was then incubated in an incubator at 37 °C overnight to achieve the turbidity of the 0.5 McFarland Standard (1.5108 CFU/ml). To achieve a bacterial cell concentration of 1.5105 CFU/mL, the bacterial culture was serially diluted in normal saline (Bauer et al.,1966).
2.5.2 Antimicrobial suscceptibility test
For susceptibility testing, the modified disk agar diffusion method as originally described by Bauer (Barry et al., 1970) was used. First, a cotton swab stick was used to evenly streak the final concentration of bacterial broth suspension (1.5105 CFU/ml) across the surface of Mueller Hinton agar plates. Before placing the sample on the agar media surface, the medium's surface was allowed to dry for 3–5 min, but no more than 15 min, to allow for the absorption of extra moisture. Before the plate was seeded, the excess suspension from the swab was eliminated by spinning it against the side of the tube.
Now use a borer to create a hole in the agar surface. Make a sample solution with a 1 mg/mL concentration. Into the borehole, pour 50 μL of the prepared sample solution. A minimum of 30 min of room temperature standing time was given to the plates to ensure optimal diffusion. The positive control was a set of amoxycillin antibiotic discs. Each plate was then incubated for 24 h at 37 °C. The plates were checked after incubation to see if the sample's immediate vicinity had any zone of inhibition.
2.6 Degradation of MB dye under UV light irradiation
Under the influence of UV light, ZnO NFs and J–ZnO NFs photocatalytic degradation capabilities were assessed against MB dye. To a 100 mL aqueous solution containing 10 ppm MB, 50 mg of catalyst was added. The suspension solution was magnetically stirred for one hour in complete darkness prior to irradiation in order to achieve adsorption–desorption equilibrium. In a beaker with a magnetic stirrer and three UV tube lights (20 W of each), the photocatalytic experiment was carried out at room temperature. Light wasn't allowed to enter the reaction medium since a box was covered in aluminum foil. The beaker containing solution was exposed to UV light while it was being stirred at room temperature. The solution was then exposed to radiation for 8 h, and 5 mL of the sample was obtained at regular intervals of 30 min to 5 h for degradation analysis. The catalyst was then removed right away by centrifuging the sample for 15 min at 10,100 rpm. A SHIMADZU UV-1800 double-beam spectrophotometer (Shimadzu Corporation, Kyoto, Japan) was used to measure the deterioration of the derived solutions with a resolution of 0.5 nm between 200 and 800 nm. Then the percent of photocatalytic degradation was calculated based on Eq. (1) (Balcha et al., 2016).
3 Results and discussion
3.1 UV–vis spectra analysis
The structural characterisation of NFs can be assessed by UV–vis spectroscopy. Visual inspection serves as the primary sign of NFs creation, and this change is then measured by UV light at various wavelengths (200 to 800 nm) to find the surface plasmon resonance (SPR). Following the addition of zinc acetate as a precursor and hydrothermal treatment for ZnO NFs in this work, the color of the jute extract was altered to turbid white. After that, UV–vis spectroscopy was used to confirm yet another primary indicator of the formation of ZnO and J–ZnO NFs. A noticeable SPR signal for ZnO NFs occurred at 373 nm, confirming the preparation of ZnO NFs (Sharmila et al., 2019, Chakraborty et al., 2020).
The J–ZnO NFs absorbance spectra were observed at 368 nm, which was characterized spectra for J–ZnO NFs based on previous literature (Sharmila et al., 2019, Chakraborty et al., 2020). Fig. 1 shows the UV–vis spectrum for jute extract (a), ZnO NFs (c), and J–ZnO NFs (b). We also calculated the band gap energy of ZnO NFs and J-ZnO NFs using tau Eq. (2) and it was 3.27 eV and 3.15 eV for ZnO NFs and J-ZnO NFs respectively. Where Eg is the band gap energy, hν is the photon energy, A is a constant, and α refers to the absorption coefficient (Chithra, Sathya and Pushpanathan, 2015).
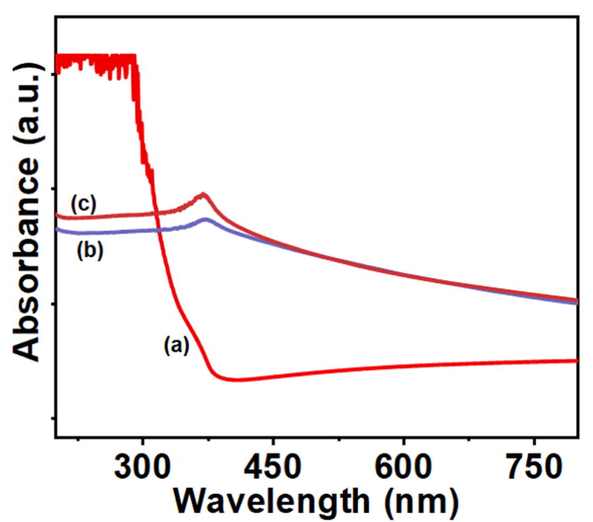
UV–vis spectrum of (a) jute extract, (c) synthesized product without jute extract (ZnO NFs), and (b) synthesized product with jute extract (J–ZnO NFs).
The small change in peak position and band gap energy between ZnO NFs and J-ZnO NFs indicated jute stick extract played an important role in the formation of J-ZnO NFs. Furthermore, the UV–vis spectrum shoulder demonstrates that the produced NFs are efficient against both UVB (290–320 nm) and UVA (320–400 nm) radiations. The biological activity of ZnO nanostructures is also influenced by the UV light action (Ansari et al., 2020).
3.2 XRD analysis
Fig. 2 displays the powder XRD patterns of as-synthesised ZnO NFs and J–ZnO NFs. The ZnO NFs diffraction peaks were observed at 31.6°, 34.3°, 36.2°, 47.5°, 56.6°, 62.8°, 67.8°, 69.1°, 72.46°, and 76.9°, which correspond to the (1 0 0), (0 0 2), (1 0 1), (1 0 2), (1 1 0), (1 0 3), (1 1 2), (2 0 1), (0 0 4), and (2 0 2) planes, respectively. All of the diffraction peaks may also be clearly linked to the hexagonal wurtzite structure of ZnO NFs, which has the P63mc (1 8 6) space group and is generated as a pure phase with highly crystalline peaks. The J-ZnO NFs showed a comparable crystal plane, indicating ZnO NFs was created (Saoud et al., 2015, Alshamsi et al., 2018, Kwon and Kim, 2020, Prasad et al., 2019). Additionally, the highly intense peaks in the XRD spectrum of the J–ZnO NFs demonstrate their highly crystalline nature. We also calculate the average crystallite size of the synthesized ZnO NFs and J-ZnO NFs using Debye–Scherer equation (3) (Hossain et al., 2018, Nayem et al., 2020).

XRD patterns of the product; (a) without jute extract (ZnO) and (b) with jute extract (J–ZnO).
3.3 Surface morphology analysis
The surface morphology of prepared ZnO NFs and J–ZnO NFs was examined by FESEM analysis at various magnifications, and the findings are shown in Fig. 3. The synthesized ZnO (both type with presence of jute extract or without jute extract) exhibits nanoflakes (NFs) type morphology. The average thickness of the prepared NFs was 42.6 nm and 28.7 nm for ZnO NFs and J-ZnO NFs respectively. Whereas, a clear difference in shape can be seen in the FESEM micrographs of the ZnO NFs and J–ZnO NFs. ZnO NFs exhibited irregular morphology while J–ZnO NFs exhibited regular morphology with flower like shape. This demonstrates that the presence of the jute stick extract during hydrothermal treatment for the preparation of J–ZnO NFs had an important role in the nucleation process, resulting in a more defined pattern and less agglomeration. The micrograph hence confirms that the presence of jute stick extract acted as a growth template for flower-like J–ZnO NFs (Soto-Robles et al., 2019, Yang et al., 2016, Lefatshe et al., 2017). The formation of collectively flower like J–ZnO NFs is due to electrostatic interactions between polar biomolecules of jute stick extract and Zn2+ ions (Yang et al., 2016). This NFs-like structure of the ZnO is important for photocatalytic applications as this structure allows light to easily pass through.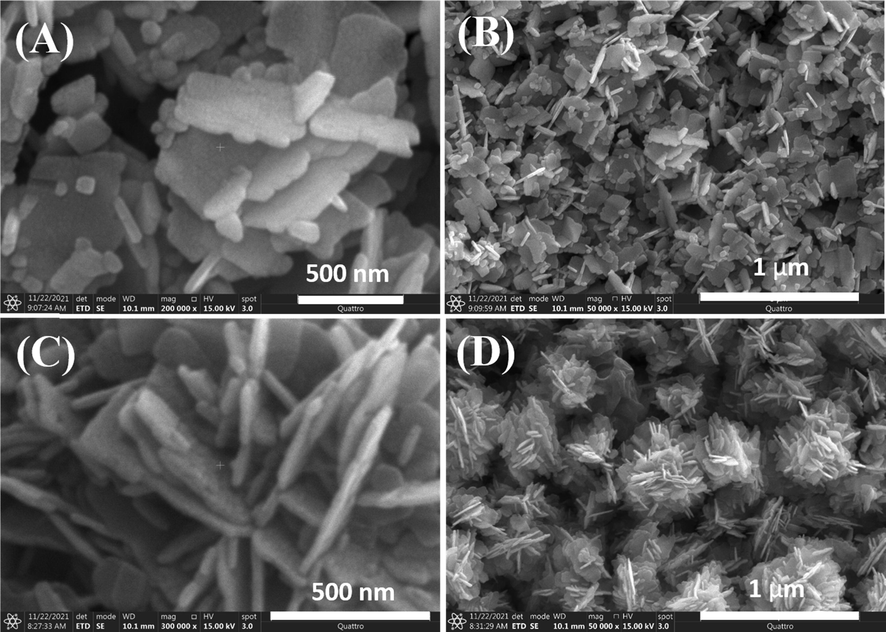
FESEM images of the product synthesized without jute stick extract (ZnO NFs) at different magnifications; (A), (B) and of the product synthesized with jute stick extract (J–ZnO NFs) at different magnifications; (C) and (D).
TEM analysis was carried out for further morphological and particle shape nature investigation of the J–ZnO NFs. In the collective flakes-like structure with an outer diameter of about 100 nm, the TEM image (Fig. 4A) shows the needle shape. A high-resolution TEM picture (HRTEM) of the J–ZnO NFs with an interplanar spacing of less than 0.25 nm is shown in Fig. 4B, demonstrating their high crystalline nature. In agreement with the XRD study of the ZnO hexagonal structure, the selected area electron diffraction (SAED) pattern (Fig. 4C) reveals that the produced J–ZnO NFs crystallized as single crystals with high crystallinity (Kim and Leem, 2021, Greene et al., 2006).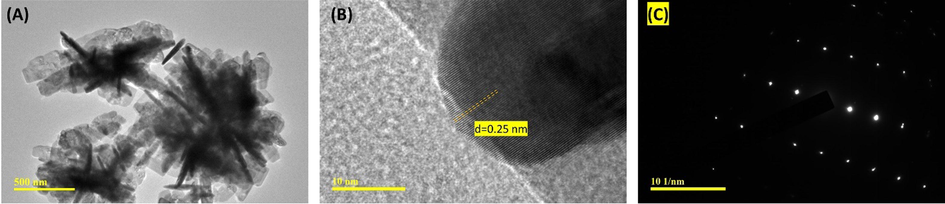
(A) TEM image, and (B) HRTEM image, and (C) SAED pattern of the J–ZnO NFs.
3.4 FTIR spectra analysis
In order to pinpoint the potential biomolecules responsible for the production of J–ZnO NFs, FTIR analysis was done. As can be seen in Fig. 5, the 400–4000 cm−1 range was used to record the FTIR spectra of jute stick extract, J–ZnO NFs, and ZnO NFs. Jute stick extract's spectra (Fig. 5(a)) shows two broad peaks at wavelengths 3436 cm−1 and 2905 cm−1 that correspond to the -O—H stretching vibrations and the -C—H stretching vibration of aromatic alkane (El-Belely et al., 2021, Vijayakumar et al., 2016). The peaks observed at 2324 cm−1 due to the presence of CO2 (Wang et al., 2019). A broad and intense band at 1410 cm−1 depicts the presence of —C—C stretching of the aliphatic groups. The peak at 1628 cm−1 was due to —C⚌C (stretching) of alkane di-substituted. The peak at 1070 cm−1 is assigned to the C—O stretching vibration (El-Belely et al., 2021, Vijayakumar et al., 2016). The spectrum of J–ZnO NFs (Fig. 5c) and ZnO NFs (Fig. 5b) contained a band that was the distinctive signature of the Zn—O bond at 468 cm−1 and 517 cm−1, proving that the substance was in fact zinc oxide. With the exception of a few, all of these peaks were visible in the spectrum of J–ZnO NFs (Fig. 5(c)); however, peaks at 2905 cm−1, 1628 cm−1, and 1410 cm−1 were absent from the spectrum of ZnO NFs (Fig. 5b). The current FTIR data corroborates with the FTIR spectra of ZnO NFs produced from different plant extracts, revealing biomolecules from jute stick extract werepresent on J–ZnO NFs surface (El-Belely et al., 2021, Vijayakumar et al., 2016, Zheng et al., 2015, Varadavenkatesan et al., 2019, Rupa et al., 2018, Bekele et al., 2021, Alayande et al., 2019). This implies that biological molecules of jute stick extract may act as hydrolyzing agents for preparing well organized ZnO NFs. From literature, survey we can assume that the biomolecules might be cellulose (Hospodarova, Singovszka and Stevulova, 2018, Kunusa et al., 2018).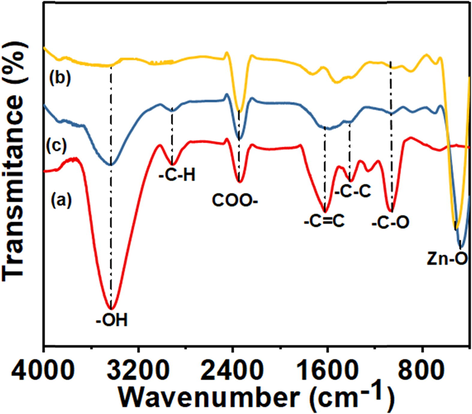
FTIR spectra of (a) jute extract, (b) ZnO NFs, (c) J–ZnO NFs.
3.5 Surface composition determination by XPS analysis
Using the XPS method depicted in Fig. 6, the chemical makeup and compositional insight of the produced compounds were evaluated. The survey spectrum of the J–ZnO NFs, shown in Fig. 6A, contains all the predicted elements, including Zn 2p, O 1s, and C 1s. O 1s (531 eV), C 1s (286 eV), Zn 3d (11 eV), Zn 3p (89 eV), Zn 3 s (141 eV), ZnLMM (499 eV), Zn 2p1/2 (1022 eV), and Zn 2p3/2 are the primary XPS peaks in the survey spectrum (1045 eV). These components show the existence of Zn and O atoms alongside C in the J–ZnO NFs' XPS survey spectrum. This led to the conclusion that the produced J–ZnO NFs were chemically pure with some jute stick extract-derived carbon doping. In order to comprehend the oxidation states of these Zn and O elements in the sample, the deconvoluted high-resolution XPS spectra of the Zn 2p (Fig. 6B), O 1s (Fig. 6C), and C 1s (Fig. 6D) were taken. The accentuated peaks were found at 1021.5 and 1045.3 eV, which correspond to the Zn 2p1/2 and Zn 2p3/2, respectively, in Fig. 6B, which shows the deconvolution XPS spectra for Zn 2p. The presence of Zn 2p is highlighted, and the spin–orbit interaction of the Zn 2p components is confirmed. The substantially stronger peak at 1021.5 eV demonstrated that Zn exists in an entirely oxidized state with no neutral nature (Qu et al., 2019). Fig. 6C shows the high-resolution O 1s deconvoluted XPS spectrum, which demonstrates the occurrence of a peak at 531.5 eV, which is mapped to the O 1s. This could be ascribed to the oxygen bound within the wurtzite hexagonal phase of J–ZnO NFs (Qu et al., 2019). The deconvolution of the O1s (Fig. 6C) in the wurtzite ZnO structure is ascribed due to the O2– ions mainly and displays three significant peaks centered at 531.5 eV (i) 532.6 eV (ii), and 533.1 eV (iii). The oxygen atoms coupled with Zn atoms are mostly responsible for the peak at 531.5 eV. The signal at 532.6 eV most likely relates to oxygen adsorbed on the sample surface. Water molecules on the surface are most likely responsible for the peak at 533.1 eV. According to the literature, they are caused by surface imperfections and chemisorbed oxygen (Diallo et al., 2015, Bhirud et al., 2012). Additionally, the intense deconvoluted peaks of C 1s in Fig. 6D at 283.27, 284.76, 286.34, and 288.8 eV might be attributed to the sp3 carbon atoms (C—C), sp2 carbon atoms (C⚌C), —C—OH, and —O—C⚌O species, confirming the hydrocarbon composition formed in the medium. This may be because ZnO NFs made from cellulose (jute stick extract) have carbon on its surface, which was also validated by FTIR spectroscopy (Yang et al., 2016, Geetha et al., 2016, Khan et al., 2019, Diallo et al., 2015, Soto-Robles et al., 2019, Al Marzouqi et al., 2019, El-Belely et al., 2021, Luo et al., 2014, Kayan et al., 2021).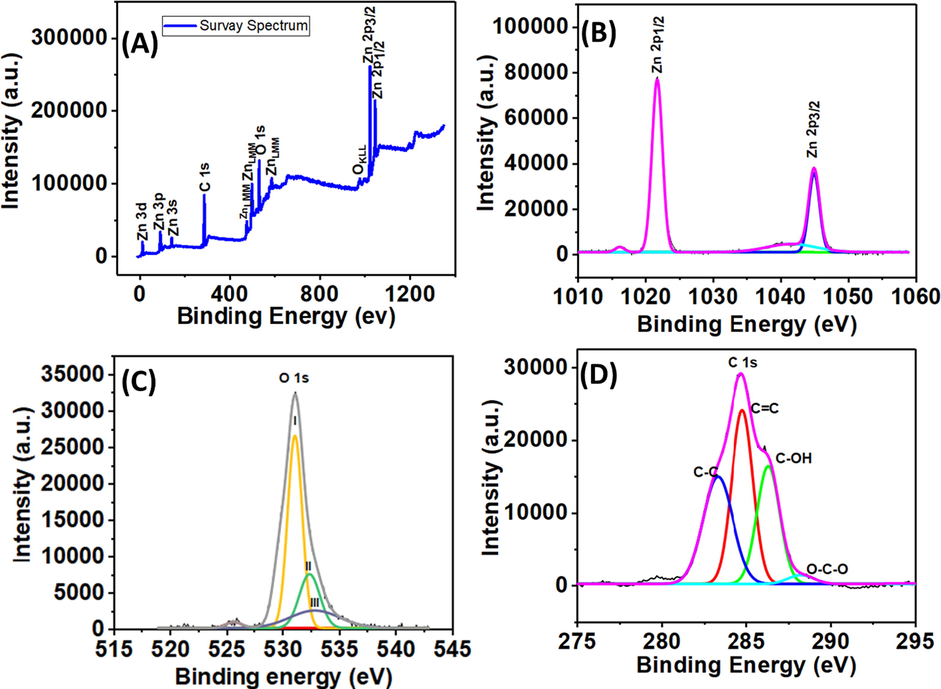
XPS (A) survey spectrum and high-resolution spectrum of (B) Zn 2p, (C) O 1s, and (D) C 1s for the J–ZnO NFs.
3.6 Possible mechanism of J–ZnO NFs formation
From above characterization data, it can say that cellulose from jute stick extract plays an important role in the formation of J–ZnO NFs. From UV–vis analysis, it was observed that cellulose from jute stick extract shifted the peak position and responsible for the lower band gap energy indicating cellulose has an impact on size and shape of the synthesized product. From FESEM it was seen that J–ZnO NFs was forming flower-like shape which indicated cellulose of jute stick extract facilitated the growth of ZnO NFs on cellulose surface to get a flower-like shape. TEM image also clearly confirmed the well organized growth of ZnO NFs on cellulose surface. The XPS and FTIR data confirmed the presence of cellulose on the surface of J–ZnO NFs. The TGA (Fig. S1) also confirmed that J–ZnO NFs were more stable than ZnO NFs and modification of cellulose with J–ZnO NFs. Hence based on characterization data a possible mechanism for the formation of collectively flowers like J–ZnO NFs could be demonstrated. Initially, NaOH converted zinc acetate into Zn(OH)2. Then OH− of alkali again converted Zn(OH)2 into Zn(OH)42− which was then electrostatically attached by cellulose of jute stick extract (positive ion of zinc and negative ion of cellulose due to alkali treatment), resulting in the formation of well organized ZnO NFs on the template of cellulose. In this process NFs of ZnO acts like nuclei on the surface of cellulose template of jute stick extract resulting collectively flower like J–ZnO NFs. The proposed mechanism is shown through the reactions below.
3.7 Degradation of MB dye under UV light irradiation
To study the photocatalytic performance of pure ZnO NFs and J–ZnO NFs under UV light irradiation, MB is used as a target pollutant. It is seen that the intensity of the peak at 664 nm decreases gradually with continuous exposure to irradiation, and the presence of ZnO NFs under UV light (Zhang et al., 2006). Fig. 7A and 7C display the degradation of MB dye using ZnO NFs and J–ZnO NFs. It was found that J–ZnO NFs display stronger photocatalytic performance than that ZnO NFs. The degradation efficiency of ZnO NFs and J–ZnO NFs was 79 and 89 % for 5 h which was obtained from Eq. (1) and shown in Fig. 7B and 7D.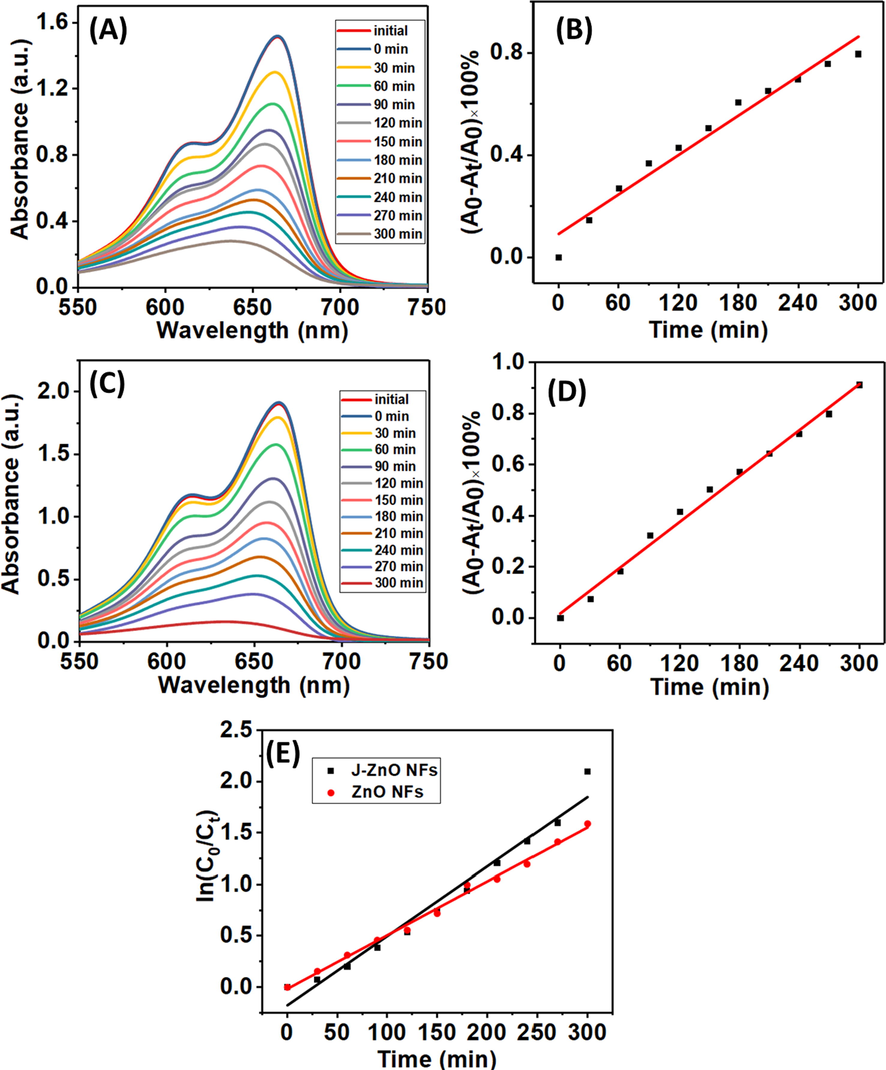
UV–vis spectrum of MB degradation with (A) ZnO NFs and (C) J–ZnO NFs at different times. The efficiency of MB for (B) ZnO NFs and (D) J–ZnO NFs. (E) The kinetic plot of MB degradation.
The pseudo-first-order reaction kinetics represented by the following Eq. (7) governs the photocatalytic degradation of MB under UV irradiation (Chen et al., 2019):
J–ZnO NFs were substantially more active than ZnO NFs. The reason for this is because cellulose from jute stick extract add a lot of surface area, increasing the amount of active sites per square meter and boosting reactivity. Because J–ZnO NFs in the nanocomposite have smaller thickness than ZnO NFs, cellulose can be used to control the creation of new photocatalysts. After 5 h of exposure to radiation in the presence of the J–ZnO NFs, approximately 89 % of the dye had been broken down, indicating high photocatalytic activity that may be caused by the strong electronic interaction between the ZnO and cellulose (Lefatshe et al., 2017). The longer-time degradation of MB using J–ZnO NFs is shown in Fig. 8A. MB dye was 98 % degraded after 480 min under UV light as shown in (Fig. 8B).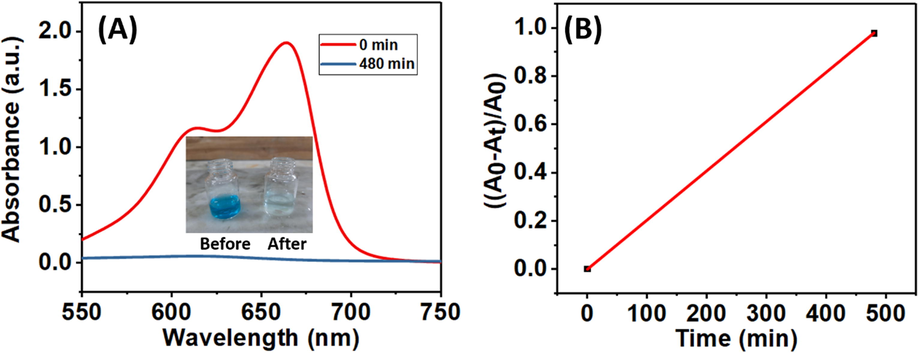
(A) Longer-time degradation of MB using J–ZnO NFs under UV light. Inset shows the image of the color of the MB solution before and after 480 min of degradation. (B) The efficiency of MB after 480 min.
The mechanism of photocatalytic activity can be described as follows: ZnO NFs are utilised as the electron and hole providers during the photodegradation of MB. Electrons (e) from the valence band (VB) are stimulated to the conduction band (CB) and produce holes (h) in the valence band when ZnO NFs are exposed to UV light with an energy equal to or higher than the bandgap (Türkyılmaz et al., 2017, Alshamsi et al., 2018, Georgekutty et al., 2008). Before exposure, a forbidden band separates VB and CB; when energy from UV light is absorbed, VB electrons in J–ZnO NFs will be stimulated to the VB, and CB will become charged. Because of this, these free e/h will attempt to leave the catalyst surface and interact with the H2O solvent and the dissolved molecular oxygen, respectively, to create a variety of reactive oxygen species, such as hydroxyl radicals (—OH), superoxide radicals (O2°), hydrogen peroxide (H2O2), or hydroperoxyl radicals (OOH°) (Türkyılmaz et al., 2017, Alshamsi et al., 2018, Georgekutty et al., 2008). The MB solution would cause the photogenerated electrons to react with the dissolved O2 molecules and produce superoxide anion radicals (O2°) while the holes would react with H2O and produce hydroxyl radicals (OH°), both of which are potent oxidizing agents that would cause the dye to degrade (Türkyılmaz et al., 2017, Alshamsi et al., 2018, Georgekutty et al., 2008). For the enhanced degradation of J-ZnO NFs, we can assume that the electrochemical properties of J-ZnO NFs is increased due to polar functional groups of cellulose from jute stick extract resulting excellent photocatalytic activity (Yang et al., 2016). A possible MB dye degradation mechanism is shown in Fig. 9.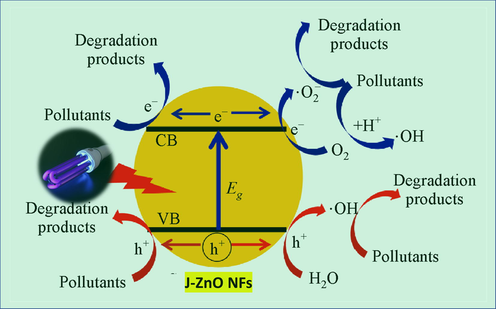
Photocatalytic degradation mechanism of MB against J–ZnO NFs.
3.8 Antibacterial activity assessment
In this study, the disc diffusion method was used to examine the bactericidal activity of jute stick extract-mediated crystalline J–ZnO NFs against E. coli. In comparison to pure ZnO NFs, the antibacterial activity of produced J–ZnO NFs samples was tested against microorganisms. At a concentration of 1 mg/mL, all produced ZnO NFs samples significantly inhibited E. coli growth at 19 mm for J–ZnO NFs (Fig. 10B) and 17 mm for ZnO NFs (Fig. 10A). It was thought that the lipid peroxidation process caused by the production of reactive oxygen species from ZnO NFs was what caused the inhibitory impact. It is also believed that peroxide damages the bacterial cell membrane, causing intracellular contents to leak out and ultimately leading to the death of the bacteria cell. Furthermore, the toxicity in bacterial cells has been linked to the free zinc ion from ZnO NFs. For J-ZnO NFs, it can say that strong interaction between cellulose of jute stick extract and ZnO NFs is responsible for the more reactive oxygen species resulting higher zone of inhibition of J-ZnO NFs than ZnO NFs (Yuvakkumar et al., 2014, Khan et al., 2020). The ZnO nanostructure dispersion enhanced by cellulose polymer, resulting in a greater surface area. It is also hypothesized that microorganisms have a negative charge whereas metal oxides have a positive charge, resulting in a “electromagnetic” attraction between the bacterium and the treated surface (Lefatshe et al., 2017).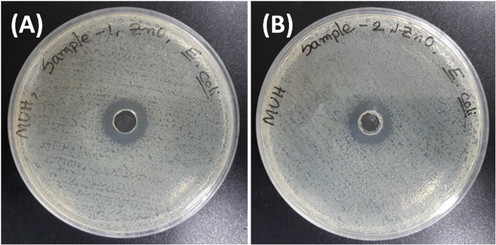
Antibacterial activity towards the E. coli pathogen by (A) ZnO NFs and (B) J–ZnO NFs.
The comparative study of antibacterial activity of J-ZnO NFs against E. Coli with other reported works are summarized in Table 1.
Antibacterial agent
Zone of iihibition (mm)
Concentration of agent
References
CNC–ZnO
15
1 mg/mL
(Lefatshe et al., 2017)
CNC–ZnO
35 ± 0.25
0.1 mg/ mL
(Elemike, Onwudiwe and Mbonu, 2021)
ZnO-coated cotton
10 ± 0.78
–
(Kalpana et al., 2018)
PCC3/ZnO
16
0.05 g
(Zahiri Oghani, Tahvildari and Nozari, 2021)
J-ZnO NFs
19
1 mg/mL
This work
4 Conclusions
In conclusion, jute stick extract-assisted ZnO NFs with photocatalytic and antibacterial characteristics were made using a straightforward, environmentally friendly hydrothermal approach. Product characterizations were accomplished using UV–vis, XRD, FTIR, TGA, FESEM, TEM, HRTEM, and XPS techniques. It very much confirms that jute extract was used as a template for the growth of J–ZnO NFs. The photocatalytic performance of the NFs was evaluated for the degradation of MB under UV light irradiation. Because biomolecules likely contribute a lot of surface area, which increases the number of active sites per square unit area and leads to increased reactivity, the photocatalytic removal efficiency under 5 h UV light irradiation for J-ZnO NFs was 89 %, which was higher than that of ZnO NFs (79 %). The maximum degradation efficacy of the prepared J–ZnO NFs sample was found at 98 % for 8 h, which indicates almost full degradation of long=persistent and carcinogenic organic dye molecules is possible. The NFs exhibited first-order kinetics with MB. Both the prepared ZnO and J–ZnO NFs also exhibited significant antibacterial activity against the E. coli pathogen. Antibacterial activity against E. coli pathogens resulted in a 19 mm zone of inhibition by J–ZnO NFs. It can be said that this research may offer the most effective catalyst supported by a green technique for the photodegradation of organic contaminants from industrial wastewater, and it is anticipated to encourage its practical applications in other environmental science fields.
Acknowledgments
This research was funded by a research project (Grant No. 37.01.0000.073.05.002.22.519) from the University Grants Commission of Bangladesh. Sample analysis of this research was partly supported from the projects of the Interdisciplinary Research Center for Hydrogen and Energy Storage (IRC-HES), King Fahd University of Petroleum & Minerals, Saudi Arabia (INHE-2105) and King Abdullah City for Atomic and Renewable Energy (KACARE211-RFP-03). MZH would like to acknowledge the Advanced Instruments Center, Jagannath University for analytical support and Waffen Research Lab, Dhaka, Bangaldesh for antibcaterial tests.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Green synthesis of sheet-like cellulose nanocrystal–zinc oxide nanohybrids with multifunctional performance through one-step hydrothermal method. Cellulose. 2018;25(11):6433-6446.
- [Google Scholar]
- Structural and optical characterization and efficacy of hydrothermal synthesized Cu and Ag doped zinc oxide nanoplate bactericides. Mater. Chem. Phys.. 2016;184:172-182.
- [Google Scholar]
- Microwave assisted synthesis of zinc oxide (ZnO) nanoparticles in a noble approach: utilization for antibacterial and photocatalytic activity. SN Appl. Sci.. 2020;2(5):955.
- [Google Scholar]
- A Simple Hydrothermal Protocol for the Synthesis of Zinc Oxide Nanorods. Jagannath Univ. J. Sci.. 2021;7:75-80.
- [Google Scholar]
- Evaluation of microbial inhibition properties of green and chemically synthesized ZnO nanoparticles. Bull. Mater. Sci.. 2019;42(3):101.
- [Google Scholar]
- Hydrothermal Preparation of Silver Doping Zinc Oxide Nanoparticles : Studys, Characterization and Photocatalytic Activity. Orient. J. Chem.. 2018;34:1898-1907.
- [Google Scholar]
- Cinnamomum verum bark extract mediated green synthesis of ZnO nanoparticles and their antibacterial potentiality. Biomolecules. 2020;10(2):336.
- [Google Scholar]
- Integrated hydrothermal assisted green synthesis of ZnO nano discs and their water purification efficiency together with antimicrobial activity. J. Mater. Res. Technol.. 2021;15:6901-6917.
- [Google Scholar]
- Preparation and Utilization of Jute- Derived Carbon : A Short Review. Chem. Rec.. 2020;20:1074-1098.
- [Google Scholar]
- Photocatalytic degradation of methylene blue dye by zinc oxide nanoparticles obtained from precipitation and sol-gel methods. Environ. Sci. Pollut. Res.. 2016;23:25485-25493.
- [Google Scholar]
- An improved single-disk method for testing the antibiotic susceptibility of rapidly-growing pathogens. Am. J. Clin. Pathol.. 1970;53(2):149-158.
- [Google Scholar]
- Structure-directing property and growth mechanism induced by capping agents in nanostructured ZnO during hydrothermal synthesis—A systematic review. Nano-Struct. Nano-Obj.. 2020;22:100426
- [Google Scholar]
- Antibiotic susceptibility testing by a standard single disc method. Am. J. Clin. Pathol.. 1966;451:493-496.
- [Google Scholar]
- Green versus Chemical Precipitation Methods of Preparing Zinc Oxide Nanoparticles and Investigation of Antimicrobial Properties. J. Nanomater.. 2021;2021
- [Google Scholar]
- An eco-friendly, highly stable and efficient nanostructured p-type N-doped ZnO photocatalyst for environmentally benign solar hydrogen production. Green Chem.. 2012;14(10):2790-2798.
- [Google Scholar]
- Facile synthesis and characterization of zinc oxide nanoparticles and studies of their catalytic activity towards ultrasound-assisted degradation of metronidazole. Mater. Lett.. 2016;168:158-162.
- [Google Scholar]
- Averrhoe carrambola fruit extract assisted green synthesis of zno nanoparticles for the photodegradation of congo red dye. Surf. Interfaces. 2020;19:100488
- [Google Scholar]
- Green synthesis of zinc oxide nanoparticles from root extract of Scutellaria baicalensis and its photocatalytic degradation activity using methylene blue. Optik. 2019;184:324-329.
- [Google Scholar]
- Effect of pH on crystal size and photoluminescence property of zno nanoparticles prepared by chemical precipitation method. Acta Metallurgica Sinica (Engl. Lett.). 2015;28(3):394-404.
- [Google Scholar]
- Continuous Hydrothermal Synthesis of Inorganic Nanoparticles: Applications and Future Directions’. Chem. Rev.. 2017;117(17):11125-11238.
- [Google Scholar]
- Green synthesis of ZnO nanoparticles by Aspalathus linearis: Structural & optical properties. J. Alloys Compd.. 2015;646:425-430.
- [Google Scholar]
- Green synthesis of zinc oxide nanoparticles (Zno-nps) using arthrospira platensis (class: Cyanophyceae) and evaluation of their biomedical activities. Nanomaterials. 2021;11(1):359.
- [Google Scholar]
- Facile synthesis of cellulose–ZnO-hybrid nanocomposite using Hibiscus rosa-sinensis leaf extract and their antibacterial activities. Appl. Nanosci. (Switzerland). 2021;11(4):1349-1358.
- [Google Scholar]
- Green synthesis, characterization and antimicrobial activities of zinc oxide nanoparticles from the leaf extract of Azadirachta indica (L.) Appl. Surf. Sci.. 2015;345:329-336.
- [Google Scholar]
- Green mediated synthesis and characterization of ZnO nanoparticles using Euphorbia Jatropa latex as reducing agent’. J. Sci.: Adv. Mater. Devices. 2016;1(3):301-310.
- [Google Scholar]
- A highly efficient Ag-ZnO photocatalyst: Synthesis, properties, and mechanism’. J. Phys. Chem. C. 2008;112(35):13563-13570.
- [Google Scholar]
- Effect of Ag Doping on Antibacterial and Photocatalytic Activity of Nanocrystalline TiO2. Procedia Mater. Sci.. 2014;6:557-566.
- [Google Scholar]
- Characterization of Cellulosic Fibers by FTIR Spectroscopy for Their Further Implementation to Building Materials. Am. J. Anal. Chem.. 2018;09(06):303-310.
- [Google Scholar]
- Synthesis of spherical silver nanoparticles by chemical reduction method. J. Bangladesh Chem. Soc.. 2018;30(2):42-47.
- [Google Scholar]
- Synthesis of panos extract mediated ZnO nano-flowers as photocatalyst for industrial dye degradation by UV illumination. J. Photochem. Photobiol., B. 2019;199:111588
- [Google Scholar]
- Biosynthesis of zinc oxide nanoparticles using culture filtrates of Aspergillus niger: Antimicrobial textiles and dye degradation studies’. OpenNano. 2018;3:48-55.
- [Google Scholar]
- Bio-reduced GO/Pd nanocomposite as an efficient and green synthesized catalyst for hydrogen evolution reaction’. Int. J. Energy Res.. 2021;45(7):11146-11156.
- [Google Scholar]
- Phytogenic Synthesis of Band Gap-Narrowed ZnO Nanoparticles Using the Bulb Extract of Costus woodsonii’. BioNanoScience. 2019;9(2):334-344.
- [Google Scholar]
- Antibacterial activities of zinc oxide and Mn-doped zinc oxide synthesized using Melastoma malabathricum (L.) leaf extract. Bioprocess Biosyst. Eng.. 2020;43(8):1499-1508.
- [Google Scholar]
- Crystallization of ZnO thin films via thermal dissipation annealing method for high-performance UV photodetector with ultrahigh response speed’. Sci. Rep.. 2021;11(1):382.
- [Google Scholar]
- Zinc oxide-from synthesis to application: A review. Materials. 2014;7(4):2833-2881.
- [Google Scholar]
- FTIR, XRD and SEM Analysis of Microcrystalline Cellulose (MCC) Fibers from Corncorbs in Alkaline Treatment. J. Phys. Conf. Ser.. 2018;1028(1):012199
- [Google Scholar]
- Silver-doped ZnO for photocatalytic degradation of methylene blue. Korean J. Chem. Eng.. 2020;37(7):1226-1232.
- [Google Scholar]
- Extraction of nanocellulose and in-situ casting of ZnO/cellulose nanocomposite with enhanced photocatalytic and antibacterial activity. Carbohydr. Polym.. 2017;164:301-308.
- [Google Scholar]
- Increased functional properties and thermal stability of flexible cellulose nanocrystal/ZnO films. Carbohydr. Polym.. 2016;136:250-258.
- [Google Scholar]
- In situ green synthesis of Au nanoparticles onto polydopamine-functionalized graphene for catalytic reduction of nitrophenol’. RSC Adv.. 2014;4(110):64816-64824.
- [Google Scholar]
- A green approach to the microwave-assisted synthesis of flower-like ZnO nanostructures for reduction of Cr(VI) Toxicol. Environ. Chem.. 2019;101(1–2):1-12.
- [Google Scholar]
- Biosynthesis of pure zinc oxide nanoparticles using Quince seed mucilage for photocatalytic dye degradation. J. Alloys Compd.. 2020;821:153519
- [Google Scholar]
- Phenolic extracts obtained from thermally treated secondary varieties of dates: Antimicrobial and antioxidant properties. LWT - Food Sci. Technol.. 2017;79:416-422.
- [Google Scholar]
- Enhanced photodegradation of methylene blue by Ag- ZnO nanocomposites’. J. Bangladesh Chem. Soc.. 2021;33(1):57-60.
- [Google Scholar]
- Green Synthesis of Gold and Silver Nanoparticles by Using Amorphophallus paeoniifolius Tuber Extract and Evaluation of Their Antibacterial Activity. Molecules. 2020;25(20):4773.
- [Google Scholar]
- Green synthesis of zinc oxide nanoparticles using different plant extracts and their antibacterial activity against Xanthomonas oryzae pv. oryzae’. Artif. Cells Nanomed. Biotechnol.. 2019;47(1):341-352.
- [Google Scholar]
- Impact of Cu doping on ZnO nanoparticles phyto-chemically synthesized for improved antibacterial and photocatalytic activities’. J. Nanopart. Res.. 2020;22(9):272.
- [Google Scholar]
- A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications’. Renew. Sustain. Energy Rev.. 2018;81:536-551.
- [Google Scholar]
- Synthesis of zinc oxide nanoparticles from Gynostemma pentaphyllum extracts and assessment of photocatalytic properties through malachite green dye decolorization under UV illumination-A Green Approach’. Optik. 2021;239:166249
- [Google Scholar]
- Bio-inspired green synthesis of zinc oxide nanoparticles using Abelmoschus esculentus mucilage and selective degradation of cationic dye pollutants’. J. Phys. Chem. Solids. 2019;127:265-274.
- [Google Scholar]
- Review on the improvement of the photocatalytic and antibacterial activities of ZnO’. J. Alloys Compd.. 2017;727:792-820.
- [Google Scholar]
- Graphene-Modified ZnO Nanostructures for Low-Temperature NO2 Sensing’. ACS Omega. 2019;4(2):4221-4232.
- [Google Scholar]
- Green synthesis of zinc oxide nanoparticles using Phoenix dactylifera waste as bioreductant for effective dye degradation and antibacterial performance in wastewater treatment’. J. Hazard. Mater.. 2021;402:123560
- [Google Scholar]
- Synthesis of zinc oxide nanoparticles from immature fruits of Rubus coreanus and its catalytic activity for degradation of industrial dye’. Optik. 2018;172:1179-1186.
- [Google Scholar]
- Synthesis of supported silver nano-spheres on zinc oxide nanorods for visible light photocatalytic applications’. Mater. Res. Bull.. 2015;63:134-140.
- [Google Scholar]
- Green synthesis of ZnO nanoparticles using Tecoma castanifolia leaf extract: Characterization and evaluation of its antioxidant, bactericidal and anticancer activities’. Microchem. J.. 2019;145:578-587.
- [Google Scholar]
- Synthesis, antibacterial activity, antibacterial mechanism and food applications of ZnO nanoparticles: A review. Food Addit. Contaminants - Part A Chem. Anal. Control Exp. Risk Assess.. 2014;31(2):173-186.
- [Google Scholar]
- Study on the effect of the concentration of Hibiscus sabdariffa extract on the green synthesis of ZnO nanoparticles’. Results Phys.. 2019;15:102807
- [Google Scholar]
- Photocatalytic efficiencies of Ni, Mn, Fe and Ag doped ZnO nanostructures synthesized by hydrothermal method: The synergistic/antagonistic effect between ZnO and metals’. J. Photochem. Photobiol., A. 2017;341:39-50.
- [Google Scholar]
- Green synthesis of catalytic Zinc Oxide nano - flowers and their bacterial infection therapy’. Organomet. Chem.. 2019;34:e5298
- [Google Scholar]
- Photocatalytic degradation of Rhodamine B by zinc oxide nanoparticles synthesized using the leaf extract of Cyanometra ramiflora’. J. Photochem. Photobiol., B. 2019;199:111621
- [Google Scholar]
- Laurus nobilis leaf extract mediated green synthesis of ZnO nanoparticles: Characterization and biomedical applications. Biomed. Pharmacother.. 2016;84:1213-1222.
- [Google Scholar]
- Enhancing long-term biodegradability and UV-shielding performances of transparent polylactic acid nanocomposite films by adding cellulose nanocrystal-zinc oxide hybrids. Int. J. Biol. Macromol.. 2019;141:893-905.
- [Google Scholar]
- Synthesis method, antibacterial and photocatalytic activity of ZnO nanoparticles for azo dyes in wastewater treatment: A review’. Inorg. Chem. Commun.. 2020;120:108140
- [Google Scholar]
- Flower-like zinc oxide nanorod clusters grown on spherical cellulose nanocrystals via simple chemical precipitation method’. Cellulose. 2016;23(3):1871-1884.
- [Google Scholar]
- Conventional and microwave hydrothermal synthesis and application of functional materials: A review’. Materials. 2019;12(7):1177.
- [Google Scholar]
- Novel green synthetic strategy to prepare ZnO nanocrystals using rambutan (Nephelium lappaceum L.) peel extract and its antibacterial applications’. Mater. Sci. Eng., C. 2014;41:17-27.
- [Google Scholar]
- Novel Antibacterial Food Packaging Based on Chitosan Loaded ZnO Nano Particles Prepared by Green Synthesis from Nettle Leaf Extract. J. Inorg. Organomet. Polym. Mater.. 2021;31(1):43-54.
- [Google Scholar]
- Employing cold atmospheric plasma (Ar, He) on Ag thin film and their influences on surface morphology and anti-bacterial activity of silver films for water treatment. Int. Nano Lett.. 2018;8(3):157-164.
- [Google Scholar]
- Large-scale synthesis of β-MnO2 nanorods and their rapid and efficient catalytic oxidation of methylene blue dye. Catal. Commun.. 2006;7(6):408-412.
- [Google Scholar]
- Green biosynthesis and characterization of zinc oxide nanoparticles using Corymbia citriodora leaf extract and their photocatalytic activity. Green Chem. Lett. Rev.. 2015;8(2):59-63.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104265.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







