Translate this page into:
Kosmotropic and chaotropic behavior of hydrated ions in aqueous solutions in terms of expansibility and compressibility parameters
⁎Corresponding author. bnbsk@yahoo.co.uk (Bushra Naseem)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Hydrated ions have fundamental applications in chemical and biological processes. Kosmotropic and chaotropic nature of hydrated ions affect the water structure in solutions depending upon their hydrophobicity or hydrophilicity nature. In present study Kosmotropic and chaotropic behavior of hydrated ions have been explained in terms of volumetric and acoustic parameters like apparent molar volume (Vϕ), expansibility and compressibility factors for aqueous electrolytic solutions provide useful information about interactions among ions and water molecules. Results of Vϕ showed that SO42− ions due to stronger H-bonding with water molecules are termed as kosmotropes while Cl− and HCO3– are chaotropes due to their weaker H-bonding with water molecules. More compressible structure of solutions in the presence of SO42− ions indicated its kosmotropic behavior and comparatively less compressible structure of solutions in the presence of Cl−1 and HCO3– ions renders them chaotropes. Results obtained from expansibility factor showed the dominance of electrostatic interactions over hydrophobic hydration of ions at higher temperatures. Greater values of expansibility factor for SO42− ions as compared to Cl−1 and HCO3– ions renders them kosmotropic ion while later are termed as chaotropes. Hence, thermo-acoustic parameters could be effectively used to describe the hydrogen bonding character of ionic solutions in terms of kosmotropic and chaotropic behavior of solutions.
Keywords
Volumetric parameters
Kosmotropes
Chaotropes
Hydrated ions
Hepler’s constant
Molecular interactions
1 Introduction
Substances producing charged species i.e. cations and anions on dissolving in water are termed as electrolytes. The charged species in the solutions distribute themselves in a regular and uniform way. Dissolved electrolytes or dispersed ionic species in the solutions affect the structure of water by disturbing the extent of hydrogen bonding of water molecules with ions of electrolytes (Rafiee and Frouzesh, 2016) (Zafarani-Moattar and Asadzadeh, 2015) (Dhondge et al., 2014). The ions which promote or enhance the degree of H-bonding in water molecules are termed as kosmotropic ions e.g. sulphate ions etc. On the other hand ions which diminish the water molecule structure by disturbing the H-bonding pattern in water molecules are termed as chaotropic ions, e.g. chloride ions etc. Electrolytes have tremendous medical applications due to their greater solubility in water molecules, as most biological processes occur in aqueous medium (Gupta and Whang, 2007) (Soto et al., 2004).
Water is a highly structured liquid due to an extensive network of hydrogen bonds. Electrolytes dissolved in water dissociate into ions which are hydrated. Large electric field around smaller ions causes dipolar water molecules to rearrange themselves in hydration shell(s) around the ions with structures differing from that in bulk water.
Concepts of “structure making” and “structure breaking” by ions as their effects on the water structure have been generally accepted and applied to the explanation of a variety of phenomena exhibited by electrolyte solutions (Marcus, 2010)
Accumulation behaviour of the ions drastically influences the stabilization or destabilization effects in solutions. As ionic kosmotropes primarily achieve their increased structuring solely within their hydration shell, they partition into the more dense water where they can obtain this hydration water more readily, whereas the ionic chaotropes, by avoiding interference with water's hydrogen-bonded network, tend to clathrate formation within the less dense environment. (Alvarez et al., 2011) (Resa et al., 2007) (George and Sastry 2003). Thus there is agreement with the defining characteristic of an ionic chaotrope which partitions selectively into low-density water whereas a kosmotrope partitions selectively into high-density water. Structured low-density water (by ionic chaotropes) in turn stabilizes the hydration shell around lower molecular mass hydrophobes, as seen in the promoted association of polyene antibiotics by ionic kosmotropes and the stabilization of their solution by ionic chaotropes (Lomesh et al., 2019)
Knowledge about thermodynamic and thermo physical properties of aqueous electrolytic solutions is very important in various chemical and pharmaceutical industries (Nain et al., 2013) (Rajagopal and Edwin Gladson, 2011). Also these properties of electrolytic solutions could be helpful in determining the nature of interactions which exist in their solutions. Interactions occurring in the living organisms are largely affected by the composition of the biological medium in which they are produced. All biological fluids contain electrolytes which exist in the form of ions. Therefore volumetric and acoustic studies of biomolecules in aqueous electrolyte solvents that explain the biomolecule-electrolyte interactions have gained importance now a day (Naseem and Ashraf, 2016) (Rajagopal and Jayabalakrishnan, 2010) (Kapadi, Chavan, and Yemul 1997).
During literature survey it was observed that very few information about the detailed structure including geometrical arrangement of water molecules has been found yet and hence relative importance of ion-water or ion-ion interactions developing due to dissolution of electrolytes in water are difficult to estimate (Omota et al., 2009). Also determination of kosmotropic or chaotropic nature of electrolytes using volumetric and acoustic study has not been carried out yet.
Therefore the present work was aimed to investigate the kosmotropic and chaotropic behavior of hydrated ions in terms of thermo acoustic parameters based upon molecular interactions in solutions using very precise volumetric and acoustic method. Effect of the nature of anions with similar cation, effect of electrolytic concentration and effect of temperature on thermo physical properties of their solutions were observed, which gave information about nature of interactions prevailing in their aqueous solutions which has co related with their respective kosmotropic and chaotropic behavior.
2 Materials and methods
2.1 Materials
Sodium chloride, sodium sulphate, and sodium bicarbonate; product of Sigma-Aldrich were used as received. Doubly distilled and de-ionized water was used for solution preparation. All chemicals exhibit high purity. Specifications of chemicals used in present study have been given in Table 1. Moreover the glassware was carefully washed, cleaned and dried in oven before use.
Chemicals
Source
CAS No.
Mass fraction purity a
Sodium chloride
Sigma-Aldrich
7647-14-5
0.99
Sodium bicarbonate
Sigma-Aldrich
144-55-8
0.99
Sodium sulphate
Sigma-Aldrich
7757-82-6
0.99
2.2 Methods
Density (d) and sound velocity (u) of aqueous solutions of electrolytes NaCl (0.0342 mol.kg−1–0.1709 mol.kg−1), Na2SO4 (0.0141 mol.kg−1–0.0704 mol.kg−1) and NaHCO3 (0.0238 mol.kg−1–0.1190 mol.kg−1) were measured at temperatures (293.15 K–313.15 K) and at 101 kPa pressure using Anton Paar DSA 5000 M with high precision vibrating tube digital density meter and ultrasound speed measuring device. The instrument has a built-in thermostat to maintain the temperature. The accuracy and repeatability of DSA 5000 M for density are 5 × 10−6 gcm−3 and 5 × 10−6 gcm−3 respectively. Accuracy in Temperature is ± 0.01 K. The sample density is determined by measuring the oscillation frequency of a U-shaped sample tube completely filled with the sample liquid (Omota et al., 2009). The principle of sound velocity measurement is based on propagation time technique. The sample is sandwiched between two piezoelectric ultrasound transducers. One transducer emits sound waves through the sample-filled cavity (frequency around 3 MHz) and the second transducer receives those waves (Dubey et al., 2019). Thus, the sound velocity is obtained by dividing the known distance between transmitter and receiver by the measured propagation time of the sound waves up to 0.5 m s−1 accuracy and 0.1 ms−1repeatability. The weighing of chemicals was done by Wiggin Hauser electronic balance with a precision of ± 0.001 mg and reproducibility of ± 0.005 mg. During experimental work, molal solutions of different concentrations of electrolytes were prepared using following equation.
In present experiment, water was used a solvent for solution preparation.
3 Results and discussion
3.1 Density and ultrasonic velocity measurements
Density and ultrasonic speed data for water and its comparison with literature reported data at different temperatures has given in Table 2, which showed that measured data is in good agreement with literature data. Measured data of density and sound velocity for aqueous solutions of salts at varying temperatures have given in Table 3 and it is obvious from reported data that density of electrolytic solutions in water is higher than that of pure water at respective temperatures. This difference can be attributed to the fact that presence of charges on electrolytic ions in solutions gathers themselves around the water molecules around them causing solution to become dense (Alvarez et al., 2011). Moreover density increases with increasing concentration of salts because of the fact that water structure developed in the presence of electrolyte becomes more prominent at higher concentrations. Density decreases with rise in temperature because at higher temperatures the kinetic energy of the molecules in solutions increases which dominates over binding energy among solution components and hence solution becomes less denser (Roy et al., 2009). The standard uncertainties in molality (m), density (d), sound velocity (u), and temperature (T) and pressure (P) are ±0.0009 mol.kg−1, ±0.66 × 10−6 g.cm−3, ±0.029 m.s−1, ±10−2 K and ±5 kPa respectively. Expanded uncertainties (k = 2) in density and sound velocity are ±1.32 × 10−6 g.cm−3 and ±0.058 m.s−1 respectively
This work
Literature value
T/(K)
do/(g.cm−3)
uo/(m/s−1)
do/(g.cm−3)
uo/(m.s−1)
293.15
0.997258
1482.74
0.997202c
1482.94a
298.15
0.997023
1496.06
0.997031d
1497.00c
303.15
0.996648
1509.59
0.996642a
1509.10b
308.15
0.994058
1519.07
0.994023b
1519.50b
313.15
0.992267
1529.67
0.992213c
1529.30d
m/mol.kg−1
T/K
293.15/K
298.15/K
303.15/K
308.15/K
313.15/K
d/g.cm−3
NaHCO3
0.0238
0.998261
0.997092
0.995678
0.994062
0.991882
0.0476
0.998279
0.997116
0.995684
0.994068
0.991884
0.0714
0.998298
0.997133
0.995691
0.994071
0.991887
0.0952
0.998305
0.997142
0.995695
0.994073
0.991889
0.1190
0.998321
0.997153
0.995701
0.994075
0.991891
NaCl
0.0341
0.998871
0.997666
0.996198
0.994557
0.992304
0.0684
0.999532
0.998272
0.996693
0.994995
0.992579
0.1026
1.000024
0.998806
0.997075
0.995326
0.992797
0.1367
1.000489
0.999381
0.997213
0.995578
0.992882
0.1709
1.000989
0.999872
0.997375
0.995765
0.993013
Na2SO4
0.0141
0.998304
0.997124
0.995703
0.994084
0.991893
0.0282
0.998391
0.997186
0.995731
0.994103
0.991902
0.0423
0.998452
0.997234
0.995751
0.994121
0.991911
0.0563
0.998516
0.997285
0.995769
0.994133
0.991918
0.0704
0.998581
0.997307
0.995775
0.994141
0.991922
u/m.s−1
Water
1482.63
1497.06
1509.57
1519.15
1529.63
NaHCO3
0.0238
1476.81
1482.13
1487.92
1492.23
1497.41
0.0476
1480.26
1486.92
1493.36
1497.46
1503.68
0.0714
1487.71
1501.42
1513.52
1524.01
1532.95
0.0952
1492.49
1506.13
1518.24
1531.85
1543.23
0.1190
1496.44
1509.72
1521.63
1539.95
1547.73
NaCl
0.0341
1255.27
1263.62
1271.17
1277.11
1284.07
0.0684
1256.83
1264.42
1272.02
1279.95
1287.56
0.1026
1258.06
1265.53
1275.88
1286.06
1294.82
0.1367
1260.58
1267.18
1277.59
1287.95
1296.81
0.1709
1263.79
1272.08
1279.05
1289.46
1304.54
Na2SO4
0.0141
1480.74
1498.84
1508.96
1519.18
1527.09
0.0282
1487.81
1501.64
1513.95
1524.91
1533.89
0.0423
1491.78
1503.13
1518.08
1527.01
1535.11
0.0563
1492.49
1506.13
1518.24
1531.85
1543.23
0.0704
1501.87
1539.98
1551.51
1576.54
1593.17
At higher temperatures kinetic energy of components of solutions increases, which renders the solution less dense and hence sound waves passes through solutions more easily at higher temperature. While at higher concentration of salts in their aqueous solutions, due to greater degree of intermolecular interactions, number of free ions moving in solutions decreases which ultimately reduces their hindrance to the passage of sound waves through solutions and hence speed of sound increases (Kant and Sharma, 2013).
3.2 Apparent and partial molar volume
Apparent molar volume (Vϕ); a volumetric parameter has been calculated using following mathematical relation (Nain and Pal, 2013) (Adebowale and Adebowale, 2007).
Sv is the slope or pair wise interaction parameter.
Calculated data of Vϕ, Voϕ and Sv for electrolyte solutions in water at different temperatures has been summarized in Tables 4 and 5. Graphical representation of variation of apparent molar volume (Vϕ) with concentration (m) of solutions has been shown in Figs. 1-3. The standard uncertainties in molality (m), apparent molar volume (Vϕ), temperature (T) and pressure (P) are ±0.0009 mol.kg−1, ±0.03 cm−3mol−1, ±10−2 K and ±5 kPa respectively. The standard uncertainties in partial molar volume (Vϕo), temperature (T) and pressure (P) are ± 0.77 cm−3mol−1, ±10−2 K and ± 5 kPa respectively.
Electrolytes
m/mol.kg−1
T/K
293.15 K
298.15 K
303.15 K
308.15 K
313.15 K
V ϕ/(cm3.mol−1)
NaHCO3
0.0238
81.87
82.85
83.86
84.20
84.47
0.0476
82.63
83.04
83.98
84.22
84.54
0.0714
82.86
83.20
84.01
84.27
84.54
0.0952
83.11
83.36
84.06
84.31
84.56
0.1190
83.18
83.45
84.07
84.33
84.57
NaCl
0.0341
39.04
40.74
42.99
43.93
46.23
0.0684
39.11
40.78
43.56
44.90
48.51
0.1026
40.76
41.47
44.84
46.25
49.82
0.1367
41.76
41.49
47.27
47.50
51.45
0.1709
42.15
42.00
48.58
48.64
52.16
Na2SO4
0.0141
135.34
137.77
139.97
140.76
142.01
0.0282
135.68
137.87
140.28
141.12
142.26
0.0423
136.41
138.23
140.58
141.26
142.34
0.0563
136.70
138.35
140.76
141.44
142.42
0.0704
136.87
138.84
141.04
141.60
142.51
Electrolyte
T/K
293.15 K
298.15 K
303.15 K
308.15 K
313.15 K
Voϕ/(cm3mol−1)
NaHCO3
81.799
82.725
83.846
84.167
84.474
NaCl
37.902
40.333
40.987
42.639
45.195
Na2SO4
134.97
137.43
139.74
140.64
141.96
Sv/(kg.cm3mol−1)
NaHCO3
13.041
6.3674
2.0722
1.4182
0.871
NaCl
25.943
9.4033
43.515
35.166
43.306
Na2SO4
29.083
18.584
18.653
14.147
8.2294
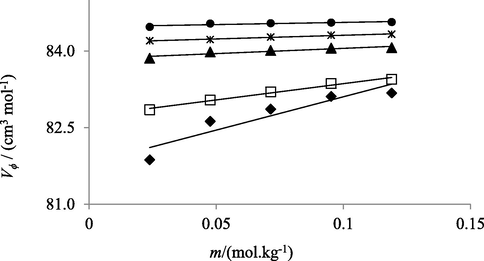
Relation between apparent molar volume (V ϕ) and concentrations (m) of NaHCO3 solutions in water at temperatures 293.15 K (♦), 298.15 K (□), 303.15 K (▲), 308.15 K (*) and 313.15 K (●).
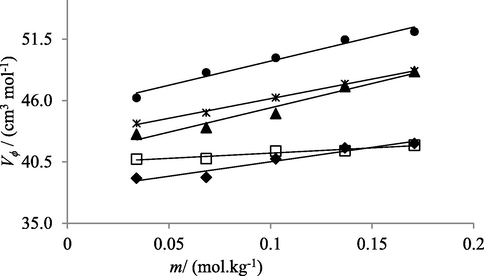
Relation between apparent molar volume (V ϕ) and concentrations (m) of NaCl solutions in water at temperatures 293.15 K (♦), 298.15 K (□), 303.15 K (▲), 308.15 K (*) and 313.15 K (●).
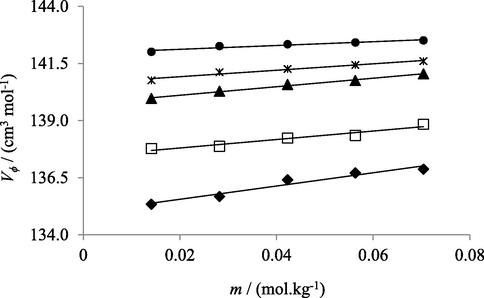
Relation between apparent molar volume (V ϕ) and various concentrations (m) of Na2SO4 solutions in water at temperatures 293.15 K (♦), 298.15 K (□), 303.15 K (▲), 308.15 K (*) and 313.15 K (●).
Reported data showed that with increasing temperature and concentration of electrolyte in solutions Vϕ increases. According to co-sphere overlap model, for electrolytic solutions, overlap of co-spheres of two ionic species results an increase in volume of solution (Chadha et al., 2016) (Kumar et al., 2016) (Shamil et al., 1989). Vϕ values of solutions provide knowledge about molecular interactions present among components of solutions. Greater values of apparent molar volume at higher temperature are also indicative of stronger molecular interactions in solutions. As formation of cavities or voids in solutions contribute positively to apparent molar volume. Solute molecules (electrolytes) at lower temperature don’t find much space to accommodate themselves in water, while at higher temperature cavities become larger in size facilitating the solute molecules to fit in solvent molecules in a much better way. Resultantly, greater interactions of solute molecules with solvent in solutions are observed (Nain and Pal, 2013). Similarly, at higher concentration of electrolytes greater solute–solvent interactions are observed because water molecules don’t directly attach to the surface of solute molecules but are held via strong hydrogen bonding, thus creating a gap in solutions and as a result increase in apparent molar volume of solutions is observed (Kumar and Kaur, 2012).
The observed order of Vϕ values in salts aqueous solutions with common cation is Na2SO4 > NaHCO3 > NaCl. Such trend showed that in aqueous Na2SO4 solutions intermolecular interactions are maximum and least in aqueous NaCl solutions. SO42− being highly charged shows strongest interactions with water molecules. HCO31− exhibits lower affinity for water owing to its small charge to size ratio but this attraction is far better than Cl−1. Chlorine is less electronegative as compared oxygen and thus produces weaker bonds (Godhani et al., 2013).
The series: F− ≈ SO42− > HPO42− > acetate > Cl− > Br− > NO3– > ClO4− > SCN−, was proposed by Franz Hofmeister in 1888 (Alkschbirs et al., 2015). Hofmeister related phenomena were interpreted based on specific interactions between the ions and water molecules, and their subsequent influence on water hydrogen-bonding network. It had already been confirmed that ions in water could no longer be considered as point charges because ionic radii, shape, and type (cation or anion) could strongly affect the dynamics and energetic of water molecules in their hydration shell. Ion-specific hydration was therefore addressed by grouping the ions into kosmotropes and chaotropes according to their water affinity. The term kosmotropic describes the behavior of ions of small size and high charge density, which tightly bind adjacent water molecules and immobilize them. In contrast, the term chaotropic represents the effect of large bulky ions of low surface charge density, which bind water less strongly than water molecules bind themselves in bulk solution; thus ‘‘freeing up” the water molecules. Accordingly, the charge density of ions determines their water affinity (Millero, 1970). Different forces involved between all solution components, i.e., ion–water (ion hydration), ion–ion (ion pairing), and water–water molecular (hydrogen bonding) interactions. Each of these interactions can contribute (directly or indirectly) to the stability of water structure in solution and are susceptible to the amount and type of ions present (Moghaddam and Thormann, 2019).
In present study, molecular interactions in aqueous electrolytic solutions can also be explored in terms of kosmotropic and chaotropic behavior of electrolytes. Kosmotropes or ions having high charge density exhibit greater degree of intermolecular interactions with solvent (water) molecules than water with itself and therefore weakens the hydrogen bonding in water molecules. Whereas large single charged ions with low charge density are the chaotropes and they develop comparatively weak interactions with water molecules, and resultantly, don’t have profound effect on hydrogen bonding of water molecules present in their surroundings. Therefore, in present study, among electrolytes with same cation SO42− ions exhibit stronger intermolecular interactions(H-bonding) with water molecules and are termed as Kosmotropes while Cl− and HCO3– are classified as chaotropes as they develop weaker H-bonding with surrounding water molecules (Lawal 2006).
From Table 5, it is obvious that Voϕ values are positive; these positive values indicate the dominance of solute–solvent interactions in solution (Sharma et al. 2015). The decreasing trend of Voϕ values in salts solutions is Na2SO4 > NaHCO3 > NaCl indicating highest solute–solvent interactions occur in Na2SO4. This trend can be justified by the fact that presence of charge on oxygen in case of both salts; NaHCO3 and Na2SO4 makes intermolecular interactions stronger than in aqueous NaCl solutions where charge is present on less electronegative element (Cl−). Moreover the SO42− bears double negative charge as compared to HCO31−. Thus solute–solvent interactions are maximum in Na2SO4 and minimum in NaCl (Nain et al., 2013) (Sharma et al. 2015) (Dhondge et al., 2017). Due to greater interactions of SO42− ions with water molecules they are termed as structure maker ions as they strengthen the H-bonds with surrounding water molecules. While Cl− and HCO3– are structure breaker ions as they have a negative effect on structural arrangement of water molecules (Lawal 2006).
3.3 Partial molar expansibility
Partial molar expansibility or temperature derivative of partial molar volume is another volumetric parameter to determine intermolecular interactions in solutions in terms of the nature of structure making or breaking of solute molecules in solutions (Gheorghe et al., 2016). Following general polynomial equation is used to express the variation of Vοϕ with temperature.
In above Eq. (3) a, b, and c are empirical constants and their values have been given in Table 6. Mathematically, Eoϕ is given by following equation (Gheorghe et al., 2016).
The standard uncertainties in temperature (T) and pressure (P) are ± 10−2 K and ± 5 kPa respectively.
Electrolytes
a/(cm3mol−1)
b/(cm3mol−1K−1)
c/(cm3mol−1K−2)
NaCl
266.51
−1.8241
0.0036
Na2SO4
−933.64
6.7359
−0.0105
NaHCO3
−492.61
3.6662
−0.0058
Obtained positive values of Eoϕ for aqueous salts solutions have been given in Table 7. Reported data showed that partial molar expansibility decreases with rising temperature for both NaCl and NaHCO3 solutions at all studied temperatures because hydrated water molecules in the outermost shell of both above mentioned salts increases due to stronger electrostatic interactions among ions of salts and water molecules releasing very less water molecules into bulk even at higher temperature and give rise to smaller values of Eoϕ, indicative of absence of “caging effect” and predominance of electrostriction changes in solution over structural hydration of solute (NaCl and NaHCO3) in aqueous solutions (Sharma et al., 2016) (Rafiee and Frouzesh, 2016) (Melinder, 2010). Eoϕ values increase with increasing temperature for aqueous Na2SO4 solutions which indicates that structural hydrophobic hydration is dominant over hydration of solute molecules with water molecules by strong electrostatic interactions (H-bonding). At higher temperature, water molecules get discharge from loose secondary solvation shell of solute molecules, causing an increase in volume of solution. Therefore, Eoϕ values become positive and indicate the presence of ‘caging or packing effect’ of solute (Na2SO4) molecules. It means that Na2SO4 molecules occupy the interstitial spaces in water molecular network, which shows the structure making/hydrophobic character of Na2SO4 solutions. The standard uncertainties in partial molar expansion (Eϕo), temperature (T) and pressure (P) are ±0.008 cm−3mol−1K−1, ±10−2 K and ±5 kPa respectively.
T/K
Eoϕ/cm3.mol−1. K−1
(∂Eoϕ/∂T)/cm3mol−1K−2
NaCl
293.15
0.28658
298.15
0.32258
303.15
0.35858
0.0072
308.15
0.39458
313.15
0.43058
NaHCO3
293.15
0.26566
298.15
0.20766
303.15
0.14966
−0.0116
308.15
0.09166
313.15
0.02946
Na2SO4
293.15
0.57975
298.15
0.47475
303.15
0.36975
−0.0210
308.15
0.26475
313.15
0.15975
3.4 Hepler’s constant
Hepler proposed a mathematical relation to determine the tendency of solute molecules to promote or disrupt the structure of solvent molecules surrounding them. Hepler’s constant can be calculated as follows (Melinder, 2010) (Nain and Pal, 2013).
The sign of Hepler’s constant, (∂Eoϕ/∂T)P defines whether a solute in solution is structure maker or breaker. Positive and equal to or close to zero values of (∂Eoϕ /∂T)P indicate the structure promoting tendency of solute molecules. While for structure breaker solutes (∂Eoϕ/∂T)P values are negative. Calculated values of (∂Eoϕ/∂T)P for these salts in their aqueous solutions have been given in Table 7. Results showed that (∂Eoϕ/∂T)P values are negative for aqueous NaCl and NaHCO3 solutions indicating their ability to break structured water molecule in their surroundings. This can be explained as single charged ions (like Cl− and HCO3–) having low charge density develop weaker intermolecular interactions with solvent (water) molecules and affect the network of hydrogen bonding in surrounding water to a very little degree. Such ions are called chaotropes or structure breaker ions (Yan et al., 2016) (Roy et al., 2009). On the other hand, small or multiple-charged ions like SO42− ions, develop stronger interactions with water molecules due to their high charge density and are capable to strengthen the H-bonding in water molecules. Also positive value of (∂Eoϕ/∂T) for aqueous Na2SO4 solutions is indicative of structure making ability of Na2SO4 in water. Obtained results are according to classification of ions in Hofmeister series as kosmotropes and chaotropes where Cl− and HCO3– are chaotropes while SO42− ions are kosmotropes (Miranda‐Quintana and Smiatek, 2020).
3.5 Apparent and partial molar isentropic compressibility
Apparent molar isentropic compressibility (Kϕ) gives insight about molecular interactions in terms of compressibility of solutions. Mathematical equation for calculating Kϕ values is as follows (Iloukhani et al., 2005).
β is adiabatic compressibility of salts solutions, βso is adiabatic compressibility of pure solvent, i.e. water and is calculated using following equation.
Calculated Kϕ values for aqueous salts solutions have been given in Table 8. Results showed that Kϕ values are negative for all studied solutions which show that portion of solution occupied by hydrated solute molecules is less compressible indicating the presence of strong electrostatic intermolecular interactions than in bulk solution (Iloukhani et al., 2005) (Godhani et al., 2013). Calculated Kϕ values for presently studied salts in solutions decrease in order i.e. Na2SO4 > NaCl > NaHCO3. The standard uncertainties in molality (m), apparent molar isentropic compressibility (Kϕ), temperature (T) and pressure (P) are ±0.0009 mol.kg−1, ±0.02 × 10−4 cm−3mol−1Pa−1, ±10−2 K and ±5 kPa respectively.
Electrolytes
m/(mol.kg−1)
T/K
293.15 K
298.15 K
303.15 K
308.15 K
313.15 K
Kϕ/(cm3.mol−1Pa−1)
NaHCO3
0.0238
−0.50621
−0.51183
−0.51815
−0.5176
−0.5153
0.0476
−0.18486
−0.34804
−0.3468
−0.0487
−0.1654
0.0714
−0.124
−0.25155
−0.1171
−0.6657
−0.8743
0.0952
−0.0411
−0.14829
−0.1307
−0.5566
−0.7192
0.1190
−0.1985
−0.1814
−0.119
−0.4614
−1.0609
NaCl
0.0341
−51.443
−49.822
−48.526
−47.447
−46.193
0.0684
−25.946
−25.242
−24.626
−24.053
–23.481
0.1026
−17.715
−17.653
−17.529
−17.48
−17.206
0.1367
−13.486
−13.44
−13.303
−13.399
−13.268
0.1709
−10.911
−10.858
−10.715
−10.942
−10.714
Na2SO4
0.0141
−125.62
−127.14
−125.73
−125.43
−123.36
0.0282
−64.058
−63.866
−63.584
−63.553
−62.706
0.0423
−43.078
−42.588
−42.746
−42.446
−41.76
0.0563
–32.298
–32.166
−31.982
–32.219
–32.01
0.0704
−26.512
−28.335
−28.072
−29.022
−29.136
Least compressible structures having greater Kϕ values shows stronger intermolecular interactions and vice versa. In terms of kosmotropic and chaotropic ions, SO42− ions due to their greater hydration and enhanced degree of H-bonding with water molecules are termed as kosmotropes. While, Cl−1 and HCO3– ions due to comparatively weaker H- bonding, lose their hydration sheath having less Kϕ values and are more compressible. Therefore, these ions are known as chaotropes (Godhani et al., 2013).
From the calculated data of Kϕ, partial molar isentropic compressibility (Koϕ) was calculated using following equation (Sharma et al. 2015)
The standard uncertainties in partial molar isentropic compressibility (Kϕo), temperature (T) and pressure (P) are ±0.35 × 10−4 cm−3mol−1Pa−1, ±10−2 K and ±5 kPa respectively.
Electrolytes
T/K
293.15 K
298.15 K
303.15 K
308.15 K
313.15 K
K°ϕ/(cm3.mol−1Pa−1)
NaHCO3
0.6057
0.6915
0.6252
0.4968
0.651
NaCl
−49.288
−49.288
−49.026
−47.767
−46.026
Na2SO4
−127.41
−127.72
−126.61
−125.88
−123.64
Sk /(kg cm3mol−2kPa−1)
NaHCO3
−6.8713
−6.6646
−7.3607
−10.361
−15.573
NaCl
303.89
254.96
254.41
244.81
254.41
Na2SO4
1635.1
1630.4
1613.4
1593.6
1558.2
3.6 Intermolecular free length (Lf)
Another acoustical parameter, i.e. intermolecular free length (Lf) has been calculated using adiabatic compressibility data of salt’s solutions. Variation in intermolecular length of solution components is a direct measure of strength of interactions present in solutions. Following mathematical relation has been used to calculate Lf values (Iloukhani et al., 2005).
Lf = K. βs1/2 (10)
In above equation, K is Jacobson temperature-dependent constant {K = (93.875 + 0.375. T)0.10−8.
Results reported in Table 10 indicated that as the temperature and concentration of salts in solutions increases, Lf values decreases. With increasing concentration of salts in water, the degree of association of solute molecules with water increases, leading to more compact structure of solution, which results in a decrease in Lf values. In other words the structural readjustments in the solution are proceeding in the direction of less compressible phase (closer packing of the molecules) (Roy et al., 2009) (Liu et al., 2007) (Krakowiak, 2011). Ions in solutions cause significant changes in the structural arrangement of solvent (water) molecules. The difference in their effects lies on the relative strength of their bonding with solvent molecules in solutions. From results it is clear that Na2SO4 in water exhibits maximum intermolecular interactions (H-bonding). Observed order of strength of intermolecular interactions in different aqueous salts solutions is: Na2SO4 > NaCl > NaHCO3. The standard uncertainties in molality (m), intermolecular free length, temperature (T) and pressure (P) are ±0.0009 mol.kg−1, ±0.002 × 10−3 m, ±10−2 K and ±5 kPa respectively.
Electrolyte
m/(mol.kg−1)
293.15 K
298.15 K
303.15 K
308.15 K
313.15 K
Lf × 10−3/(m)
NaHCO3
0.0238
1.0072
9.951
9.847
9.772
9.687
0.0476
1.0047
9.938
9.834
9.728
9.635
0.0714
1.0027
9.921
9.774
9.636
9.527
0.0952
9.987
9.895
9.748
9.608
9.498
0.1190
9.936
9.818
9.726
9.585
9.385
NaCl
0.0341
7.272
7.229
7.183
7.154
7.120
0.0684
7.234
7.178
7.128
7.101
7.059
0.1026
7.158
7.036
6.936
6.853
6.791
0.1367
7.109
6.988
6.892
6.781
6.700
0.1709
7.068
6.952
6.861
6.709
6.660
Na2SO4
0.0141
7.238
7.073
6.988
6.905
6.849
0.0282
7.169
7.046
6.942
6.854
6.789
0.0423
7.13
7.031
6.904
6.835
6.778
0.0563
7.123
7.003
6.902
6.791
6.707
0.0704
7.034
6.698
6.609
6.412
6.293
Enhanced degree of H-bonding in water molecules structure due to SO42− ions shows the structure making ability of SO42− ions in aqueous solutions. While, destructive effect of Cl− and HCO31− ions on the structuring in water molecules renders them chaotropes or structure breaker ions. Moreover, obtained results are also in accordance with Hofmeister series about kosmotropes and chaotropes; where Cl− and HCO3– are chaotropes while SO42− ions are kosmotropes (Smiatek, 2020).
4 Conclusions
Kosmotropic and chaotropic behavior of salts in aqueous solutions have been studied in terms of their volumetric and acoustic parameters at different temperatures. Density and sound velocity of water and aqueous electrolyte solutions were measured at different temperatures. With increasing temperature, density of studied solutions decreases, as at higher temperature, kinetic energy dominates over binding energy making solution less dense. Different volumetric and acoustic parameters were calculated using measured density data. Positive values of apparent molar volume indicated presence of strong molecular interactions in all salt’s solutions which is found responsible for their structure making behavior in solutions. High charge density on SO42− ions makes them more hydrated with water molecules due to greater intermolecular interactions, resulting in greater values of apparent molar volume, and hence are termed as kosmotropes. Cl− and HCO3– ions showed weaker interactions with surrounding water molecules due to their low charge density and do not affect the hydrogen bonding in water molecules and are classified as chaotropes as obvious from results of apparent molar volume. Negative values of Cl− and HCO3– ions and positive value of SO42− ions for Hepler’s constant indicated their chaotropic and kosmotropic behavior in solutions respectively.
Acknowledgments
Authors gratefully acknowledge Department of Chemistry, Lahore College for women University Lahore for providing us the necessary chemicals.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The influence of kosmotropic and chaotropic salts on the functional properties of Mucuna pruriens protein isolate. Int. J. Biol. Macromol.. 2007;40(2):119-125.
- [CrossRef] [Google Scholar]
- Effects of some anions of the Hofmeister series on the rheology of cetyltrimethylammonium-salicylate wormlike micelles. Colloids Surfaces A Physicochem. Eng. Asp.. 2015;470:1-7.
- [CrossRef] [Google Scholar]
- Thermophysical properties of binary mixtures of {ionic liquid 2-hydroxy ethylammonium acetate + (water, methanol, or ethanol)} J. Chem. Thermodyn.. 2011;43(7):997-1010.
- [CrossRef] [Google Scholar]
- Densities, Dynamic Viscosities, Speeds of Sound, and Relative Permittivities for Water + Alkanediols (Propane-1,2- and -1,3-diol and Butane-1,2-, -1,3-, -1,4-, and -2,3-Diol) at Different Temperatures. J. Chem. Eng. Data. 2003;48(6):1529-1539.
- [CrossRef] [Google Scholar]
- Interionic interactions of glycine, l-alanine, glycylglycine and phenylalanine in aqueous 1-hexyl-3-methylimidazolium chloride ionic liquid solutions at T = (288.15 to 308.15) K: Volumetric, speed of sound and viscometric measurements. J. Mol. Liq.. 2016;218:68-82.
- [CrossRef] [Google Scholar]
- Physicochemical study of solute-solute and solute-solvent interactions of aqueous binary mixtures of l -alanine methyl ester hydrochloride and l -valine methyl ester hydrochloride at different temperatures: Volumetric and compressional studies. J. Chem. Thermodyn.. 2017;105:217-225.
- [CrossRef] [Google Scholar]
- Volumetric and acoustic study of aqueous binary mixtures of quinine hydrochloride, guanidine hydrochloride and quinic acid at different temperatures. J. Chem. Thermodyn.. 2014;81:34-43.
- [CrossRef] [Google Scholar]
- Acoustic, volumetric and spectral studies of binary liquid mixtures of aliphatic dialkylamine and 2-alkanols at different temperatures. J. Chem. Thermodyn.. 2019;132:1-8.
- [CrossRef] [Google Scholar]
- Partial molar volumes, isentropic compressibilities, and partial molar expansibilities of N-Methylglycine and d-Glucose in aqueous environments at temperatures between (298.15 and 323.15) K. J. Mol. Liq.. 2016;218:515-524.
- [CrossRef] [Google Scholar]
- a., Mehta, J.P., Ultrasonic Studies of molecular interactions in different solvents of 4-Methylumbelliferyl- β -D-glucuronide (4-MUG) at 303K. J. Mol. Liq.. 2013;180:179-186.
- [CrossRef] [Google Scholar]
- Effects of anion and synthesis route on the structure of (La0.9Sr0.1) (Cr0.85Fe0.05Co0.05Ni0.05)O3 - δperovskite and removal of impurity phases. Solid State Ionics. 2007;178(29-30):1617-1626.
- [CrossRef] [Google Scholar]
- Excess molar volumes and speeds of sound of tetrahydrofuran with chloroethanes or chloroethenes at 298.15 K. Phys. Chem. Liq.. 2005;43(4):391-401.
- [CrossRef] [Google Scholar]
- Measurement and modelling of water activity, density, sound velocity, refractive index and viscosity of the Na2MoO4 + poly(ethylene glycol) + H2O system in the temperature range from 313.15 to 333.15 K. Fluid Phase Equilib.. 2020;518:112628
- [CrossRef] [Google Scholar]
- Apparent Molar Volume, Viscometric and Conductance Studies of Sodium Chloride in Different Composition of Lactose. Chem. Sci. Trans.. 2013;2:911-921.
- [CrossRef] [Google Scholar]
- Partial Molar Volumes and Viscosity B Coefficients of Benzyltriethylammonium Chloride in Dimethyl Sulfoxide + Water at Different Temperatures. J. Chem. Eng. Data. 1997;42:548-550.
- [CrossRef] [Google Scholar]
- Apparent molar volumes and compressibilities of tetrabutyl-ammonium bromide in organic solvents. J. Chem. Thermodyn.. 2011;43(6):882-894.
- [CrossRef] [Google Scholar]
- Investigation on molecular interaction of amino acids in antibacterial drug ampicillin solutions with reference to volumetric and compressibility measurements. J. Mol. Liq.. 2012;173:130-136.
- [CrossRef] [Google Scholar]
- Volumetric, acoustic and viscometric behaviour of dipotassium hydrogen phosphate and disodium hydrogen phosphate in aqueous solution of N-acetyl glycine at different temperatures. J. Chem. Thermodyn.. 2016;94:204-220.
- [CrossRef] [Google Scholar]
- Kosmotropes and chaotropes as they affect functionality of a protein isolate. Food Chem.. 2006;95(1):101-107.
- [CrossRef] [Google Scholar]
- Interactions of some amino acids with aqueous N, N-dimethylacetamide solutions at 298.15 and 308.15 K: A volumetric approach. J. Solution Chem.. 2007;36:923-937.
- [CrossRef] [Google Scholar]
- Physicochemical approach to study the solute-solute and solute-solvent interactions of drug Levofloxacin hemihydrate in aqueous sorbitol solutions at different temperatures: Volumetric, acoustic and conductance studies. J. Mol. Liq.. 2019;283:133-146.
- [CrossRef] [Google Scholar]
- Effect of ions on the structure of water. Pure Appl. Chem.. 2010;82:1889-1899.
- [CrossRef] [Google Scholar]
- Properties and other aspects of aqueous solutions used for single phase and ice slurry applications. Int. J. Refrig.. 2010;33(8):1506-1512.
- [CrossRef] [Google Scholar]
- The Apparent and Partial Molal Volume of Aqueous Sodium ChlorideSolutions. J. Phys. Chem.. 1970;74:356-362.
- [Google Scholar]
- Theoretical Insights into Specific Ion Effects and Strong-Weak Acid-Base Rules for Ions in Solution: Deriving the Law of Matching Solvent Affinities from First Principles. ChemPhysChem. 2020;21(23):2605-2617.
- [CrossRef] [Google Scholar]
- The Hofmeister series: Specific ion effects in aqueous polymer solutions. J. Colloid Interface Sci.. 2019;555:615-635.
- [CrossRef] [Google Scholar]
- Study of solute-solute and solute-solvent interactions of l-methionine in aqueous-sucrose solutions at different temperatures. J. Chem. Thermodyn.. 2013;58:101-109.
- [CrossRef] [Google Scholar]
- Study of solute-solute and solute-solvent interactions of l-threonine in aqueous-glucose solutions at different temperatures by using volumetric and viscometric methods. J. Chem. Thermodyn.. 2013;60:98-104.
- [CrossRef] [Google Scholar]
- Volumetric behavior of nitroimidazoles in binary solvent mixtures. J. Mol. Liq.. 2016;224:377-386.
- [CrossRef] [Google Scholar]
- Effect of temperature on density, sound velocity, and their derived properties for the binary systems glycerol with water or alcohols. J. Chem. Thermodyn.. 2017;109:124-136.
- [CrossRef] [Google Scholar]
- Densities and derived properties of water, 1,4-dioxane and dimethyl sulfoxide binary and ternary systems at temperatures from 293.15 K to 313.15 K. Rev. Roum. Chim.. 2009;54:63-73.
- [Google Scholar]
- The study of thermodynamic properties of the ternary (1-ethyl-3-methylimidazolium hydrogen sulfate + lithium chloride + water) system and corresponding binary systems at different temperatures and ambient pressure. J. Chem. Thermodyn.. 2016;102:95-104.
- [CrossRef] [Google Scholar]
- Interactions of potassium fluoride with coexistent components in water-dimethyl sulfoxide mixed solvent at different temperatures. Thermochim. Acta. 2011;525(1-2):197-205.
- [CrossRef] [Google Scholar]
- Volumetric, ultrasonic speed, and viscometric studies of salbutamol sulphate in aqueous methanol solution at different temperatures. J. Chem. Thermodyn.. 2010;42(8):984-993.
- [CrossRef] [Google Scholar]
- Influence of temperature on ultrasonic velocity measurements of ethanol+water+1-propanol mixtures. J. Therm. Anal. Calorim.. 2007;87(1):237-245.
- [CrossRef] [Google Scholar]
- Partial molar volumes and viscosity B-coefficients of nicotinamide in aqueous resorcinol solutions at T = (298.15, 308.15, and 318.15) K. Int. J. Thermophys.. 2009;30:515-528.
- [CrossRef] [Google Scholar]
- Structural functions of taste in 5-membered ring structures. Food Chem.. 1989;32(3):171-188.
- [CrossRef] [Google Scholar]
- Effect of N-acetylglycine on volumetric and acoustic behaviour of aqueous tetrabutylammonium iodide solutions at different temperatures. J. Chem. Thermodyn.. 2016;96:143-152.
- [CrossRef] [Google Scholar]
- Study of solute-solute and solute-solvent interactions of N-acetyl glycine in aqueous d-fructose solutions at different temperatures. Thermochim. Acta. 2015;607:1-8.
- [CrossRef] [Google Scholar]
- Theoretical and computational insight into solvent and specific ion effects for polyelectrolytes: The importance of local molecular interactions. Molecules. 2020;25:21-23.
- [CrossRef] [Google Scholar]
- Thermodynamics of diglycine and triglycine in aqueous NaCl solutions: Apparent molar volume, isentropic compressibility, and refractive index. J. Solution Chem.. 2004;33(1):11-21.
- [CrossRef] [Google Scholar]
- Water activity, density, sound velocity, refractive index and viscosity of the (NH4)6Mo7O24 + poly(ethylene glycol) + H2O system in the temperature range from 313.15 to 333.15 K: Experiment and modelling. J. Chem. Thermodyn.. 2020;142:105986.
- [CrossRef] [Google Scholar]
- Volumetric and conductometric studies on the interactions of dipeptides with potassium perfluoroalkanesulfonate in aqueous solution at different temperatures. J. Chem. Thermodyn.. 2016;93:172-178.
- [CrossRef] [Google Scholar]
- Effect of 1-carboxymethyl-3-methylimidazolium chloride, [HOOCMMIM][Cl], ionic liquid on volumetric, acoustic and transport behavior of aqueous solutions of l-serine and l-threonine at T = 298.15 K. J. Mol. Liq.. 2015;202:79-85.
- [CrossRef] [Google Scholar]
- Vapor-liquid equilibria, density and sound velocity measurements of (water or methanol or ethanol + 1,3-propanediol) binary systems at different temperatures. Thermochim. Acta. 2016;642:111-123.
- [CrossRef] [Google Scholar]







