Translate this page into:
L-cysteine as sustainable and effective sulfur source in the synthesis of diaryl sulfides and heteroarenethiols
⁎Corresponding authors. zh19683@163.com (Hui Zhang), yjliu85@dlut.edu.cn (Yajun Liu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
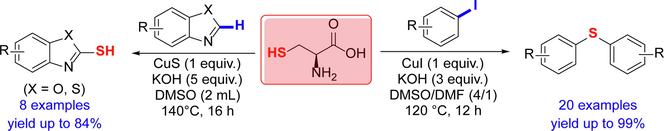
L-cysteine, a natural and essential amino acid, was employed as novel sulfur source in the synthesis of symmetrical diaryl sulfides from a variety of aryl iodides in moderate to excellent yields. A tandem three steps’ reactions including C(sp2)-S bond formation, C(sp3)-S bond cleavage and another C(sp2)-S bond formation were proposed to be involved in this conversion. This protocol was featured by broad substrate scope and good functional group tolerance. In addition, heteroarenes including benzothiazoles and benzoxazoles were successfully converted into the corresponding heteroarenethiols using L-cysteine as C-H mercaptalization reagent.
Keywords
L-cysteine
Sulfur source
C-S coupling
Diaryl sulfide
Heteroarenethiol
1 Introduction
By virtue of the versatile reactivity of sulfur atom, sulfur containing compounds represent a large family of chemicals and widespread in nature and possess various interesting biological activities (Kharasch and Arora, 1976; Milito et al., 2019; Francioso et al., 2020). They also have fundamental application in medicines (Scott and Njardarson, 2018; Zhao et al., 2019), materials (Lim et al., 2015; Mutlu et al., 2019), pesticides (Devendar and Yang, 2017), and so on. As low-valence sulfur compounds, aryl sulfides and aryl thiols are useful substrates for the synthesis of oxidized derivatives such as sulfoxides and sulfones (Rayner, 1995; Chu et al., 2021). Aryl sulfides and aryl thiols are commonly synthesized from aryl halides and arenes with a sulfur source reagent. Common sulfur sources converting aryl halides to aryl sulfides and thiols include elementary sulfur, sodium sulfide, sodium hyposulfide, aliphatic thiols, etc (Liu et al., 2017). In case of other substrates such as α-perfluoroalkyl ketones, sodium sulfinates or arylsulfonyl chlorides are also effective sulfur sources (Chu et al., 2020). Although many synthetic methods for aryl sulfides and aryl thiols have been developed, some of these sulfur sources suffer from several drawbacks such as easily inflammable (e.g., elementary sulfur), instable (e.g., sodium sulfide), smelly (e.g., thiol). Moreover, excess use of non-natural sulfur sources may give rise to environmental and safety issues. In this regards, the upsurge of the concepts of green chemistry led us to pursue a natural biomass as sustainable and safe sulfur source for the synthesis of sulfur containing compounds.
α-Amino acids are a class of small molecules of great importance in living organisms. They are not only basic units for constructing proteins, but also indispensable precusors for the synthesis of fatty acid, glucose and nucleotides through enzyme catalyzed complex biotransformations, such as the well-known tricarboxylic acid cycle and gluconeogenesis pathway. Since α-amino acids are non-toxic and readily available from biomass feedstock, it is very attractive to use them to replace traditional chemical reagents in organic synthesis (Kamanna, 2021). L-Cysteine is a fundamental sulfur source in the biosynthesis of sulfur containing endogenous active substances, such as signal molecules H2S and SO2, in the human body (Stipanuk, 1986; Paul et al., 2018). The biosynthesis of natural products such as antibiotics lincomycin A and sulfur containing amino acid ergothioneine in microorganisms also requires L-cysteines as sulfur soruce (Erdelmeier et al., 2012; Zhang, et al., 2018). L-cysteine is frequently used as sulfur source for the synthesis of inorganic nanomaterials such as Tin sulfide films, SnS nanocrystals and CdS nanowires (Gai et al., 2008; Polivtseva et al., 2016). However, the examples using L-cysteine as sulfur source in organic synthesis are still rare. In 2012, the Erdelmeier group (2012) reported a one-pot synthesis of ergothioneine from brominated imidazoles using L-cysteine as sulfur source (Fig. 1a). Treating 2-bromoimidazole with L-cysteine afforded a L-cysteine conjugate, the C(sp3)-S bond of which was cleaved to provide ergothioneine in the presence of 3-mercaptopropionic acid. Inspired by the examples above, we started to investigate the possibility of applying L-cysteine as sulfur source in the transition metal catalyzed C-S coupling reaction for the synthesis of sulfur containing compounds. Herein, as a continuous work on the development of the synthetic methodology for sulfur containing compounds (Liu et al., 2015; Xue et al., 2017; Xiao et al., 2019), we report a successful application of L-cysteine as effective sulfur source in the synthesis of copper salts mediated symmetrical diaryl sulfides and heteroarenethiols from aryl halides and heteroarenes, respectively (Fig. 1b).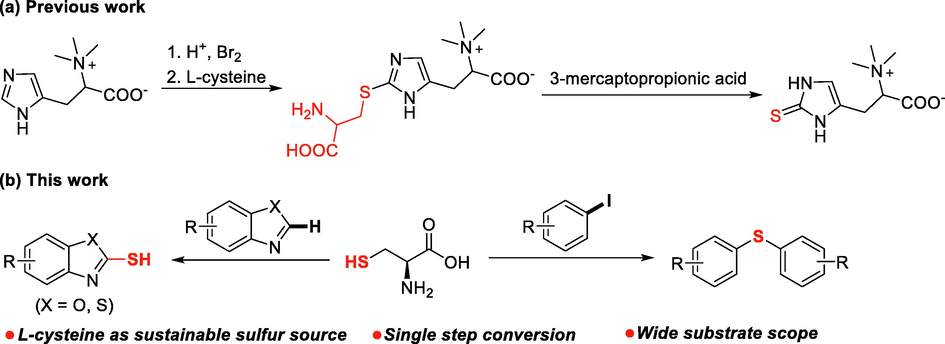
L-cysteine as sulfur source in organic synthesis.
2 Material and methods
2.1 Chemical reagents
The chemical reagents were purchased from commercial suppliers, and used without further purification.
2.2 Structure characterization
The chemical structure of all synthesized compounds was determined by nuclear magnetic resonance (NMR) spectroscopy and mass spectroscopy. The purity of isolated products was greater than 95%. NMR spectra were recorded at 600 MHz on a Bruker DMX- 600 spectrometer with CDCl3 (7.26 ppm) or DMSO‑d6 (2.50 ppm) as the solvent and tetramethylsilane was used as internal standard. Spin multiplicities are indicated by the following symbols: s (singlet), d (doublet), t (triplet), q (quartet), and m (multiplet). Coupling constants are reported in hertz (Hz). Mass spectra of heteroarenethiols were recorded by GC–MS on QP2010 Ultra (Shimadzu, Japan). Mass spectra of heteroarenethiols were recorded by a UPLC-ESI-MS/MS system. The UPLC-ESI-MS/MS system consisted of an Acquity UPLC system (Waters Corp., MA, USA) coupled to a QTRAP 6500 Plus mass spectrometer (Sciex, Toronto, Canada) equipped with a TurboIonSpray source. Analyst Software1.6.3 was used for data acquisition and data processing.
2.3 General procedure for the synthesis of diaryl sulfides
To a solution of aryl iodide (0.5 mmol) in DMSO /DMF (1.5 mL, v/v = 4/1) in a Schlenk tube, was added L-cysteine (30.3 mg, 0.25 mmol), potassium hydroxide (84.2 mg, 1.5 mmol) and cuprous iodide (95.2 mg, 0.5 mmol). The tube was vacuumed, and backfilled with argon three times consecutively. The reaction mixture was stirred at 120 °C for 12 h. After cooling to room temperature, the reaction mixture was diluted with water and extracted with ethyl acetate three times. The organic layers were combined, washed with water and brine, dried over anhydrous magnesium sulfate and condensed in vacuum. The crude product was further purified by silica column chromatography using petroleum ether/ethyl acetate as eluent. The reported yields were isolated yields.
2.4 General procedure for the synthesis of heteroarenethiols
To a solution of heteroarenes (0.5 mmol) in DMSO (2 mL) in a Schlenk tube, was added L-cysteine (121.0 mg, 1 mmol), potassium hydroxide (140.0 mg, 2.5 mmol) and copper sulfide (47.5 mg, 0.5 mmol). The tube was vacuumed, and backfilled with argon three times consecutively. The reaction mixture was stirred at 140 °C for 16 h. After cooling to room temperature, the reaction mixture was diluted with water and the pH was adjusted to 3 with 5% HCl. The resulted mixture was extracted with ethyl acetate three times. The organic layers were combined, washed with water and brine, dried over anhydrous magnesium sulfate and condensed in vacuum. The crude product was further purified by silica column chromatography using petroleum ether/ethyl acetate as eluent. The reported yields were isolated yields.
3 Results and discussion
3.1 Synthesis of diaryl sulfides using L-cysteine as sulfur source
Conventionally, symmetrical diaryl sulfides are prepared by transition metal catalyzed C-S coupling reaction of aryl halides with sulfur source including elemental sulfur, thiourea, Na2S KSCN, dithiooxamide and ethanedithiol (Ke et al., 2011; Li et al., 2011; Chen et al., 2012; Li et al., 2012; Kamal et al., 2013; Firouzabadi et al., 2015; Hajipour and Jajarmi, 2016; Yousofvand et al., 2018; Xie et al., 2020; Tamoradi et al., 2020; Ashraf et al., 2020a; Ashraf et al., 2020b; Yarmohammadi et al, 2021; Samanta et al., 2021). In order to assess the feasibility of the C-S coupling reaction using L-cysteine as sulfur source, L-cysteine was applied in the synthesis of symmetrical diaryl sulfide. 4-Iodotoluene (1a, 0.5 mmol) was treated with L-cysteine at 120 °C in DMSO in the presence of KOH as base. Although no reaction was observed in the absence of copper salt, we were delighted to see that 78% yield of the corresponding diaryl sulfide 3a was obtained when 1 equiv. of CuI was added in the reaction system (Entries 1–2, Table 1). Besides diaryl sulfide, 11% yield of 4-methylbenzenethiol was obtained as byproduct (Entry 2, Table 1). A screening of solvents revealed that DMF gave a low yield of diaryl sulfide but no 4-methylbenzenethiol was observed (Entry 3, Table 1). We speculated that DMSO was more effective than DMF in initiating the thiolation of 1a with L-cysteine to give 4-methylbenzenethiol as intermediate, while DMF was able to accelerate the C-S coupling reaction of 4-methylbenzenethiol and 4-iodotoluene to afford final product diaryl sulfide 3a. In fact, many previous reports have revealed DMF was more effective than DMSO in the C-S cross coupling reactions of aryl halides and aryl thiols. [Hajipour, et al., 2014; Venkanna et al., 2014; Jiang et al., 2017] Thus, in order to reduce the production of 4-methylbenzenethiol and improve the yield of diaryl sulfide, a mixture solvent of DMSO and DMF was investigated (Entries 4–6, Table 1). DMSO/DMF (4/1) was the most effective for this conversion as 99% yield of 3a was obtained. Further increasing the proportion of DMF in the solvent lowered the reaction conversion rate. The yield decreased either at lower temperature or at lower loading of copper salt (Entry 5, Table 1). Generally, cuprous salts exhibited higher efficiency than cupric salts to promote this transformation (Entries 7–13, Table 1). Cupric salts including CuCl2, CuSO4, CuS, Cu(OAc)2, Cu(NO3)2 and Cu(OH)2 provided lower yields. Screening of bases revealed that KOH was the most effective base in this conversion (Entries 14–18, Table 1). A variety of different bases, such as NaOH, Ca(OH)2, LiOH·H2O, K2CO3 and NaOAc, afforded lower yields than KOH. In summary, the optimized condition for this conversion is as follows: 4-iodotoluene (0.5 mmol), L-cysteine (0.5 equiv.), CuI (1 equiv.), KOH (3 equiv.), DMSO/DMF (v/v = 4/1, 1.5 mL), 120 °C, 12 h.
Entry
[Cu]
Base
Solvent
Yield (%)[b]
1
none
KOH
DMSO
ND
2
CuI
KOH
DMSO
78
3
CuI
KOH
DMF
14
4
CuI
KOH
DMSO/DMF(10/1)
87
5
CuI
KOH
DMSO/DMF(4/1)
99 (51[c], 73[d])
6
CuI
KOH
DMSO/DMF(2/1)
89
7
CuCl
KOH
DMSO/DMF(4/1)
78
8
CuCl2
KOH
DMSO/DMF(4/1)
30
9
CuSO4
KOH
DMSO/DMF(4/1)
60
10
CuS
KOH
DMSO/DMF(4/1)
74
11
Cu(OAc)2·H2O
KOH
DMSO/DMF(4/1)
46
12
Cu(NO3)2
KOH
DMSO/DMF(4/1)
43
13
Cu(OH)2
KOH
DMSO/DMF(4/1)
30
14
CuI
NaOH
DMSO/DMF(4/1)
49
15
CuI
Ca(OH)2
DMSO/DMF(4/1)
76
16
CuI
LiOH·H2O
DMSO/DMF(4/1)
68
17
CuI
K2CO3
DMSO/DMF(4/1)
53
18
CuI
NaOAc
DMSO/DMF(4/1)
42
With the optimized condition in hand, substrate scopes with various aryl iodides as well as heteroaryl iodides have been studied (Fig. 2). Generally, the C-S coupling reaction of (hetero)aryl iodides and L-cysteine afforded the corresponding symmetrical diaryl sulfides (3a–3t) in a good yield. The variation of methyl group at para, meta and ortho-positions gave a yield of 99%, 77% and 90%, respectively. 3,5-Dimethyl and 4-tert-butyl substituted iodobenzenes gave moderate yields of diaryl sulfides. Simple iodobenzene and 1-iodonaphthalene were also smoothly converted into the corresponding diaryl sulfides. Although methoxy group of iodoanisole was slightly demethylated under the optimized condition, moderate to good yields of diaryl sulfides 3h, 3i and 3j were obtained. Iodoanilines afforded relatively low yields, because intermolecular C-N coupling reaction also occurred under the optimized condition (Wang et al., 2010). Electron-deficient iodobenzenes bearing chloro, trifluoromethyl and nitro group were converted into diaryl sulfides in acceptable yields. Several heteroaryl iodides were further tested. Treating iodopyridines and iodothiophene with L-cysteine under the standard condition successfully afforded the corresponding diheteroaryl sulfides. Unfortunately, when aryl bromides and chlorides were employed, very low yields were obtained even at harsher conditions.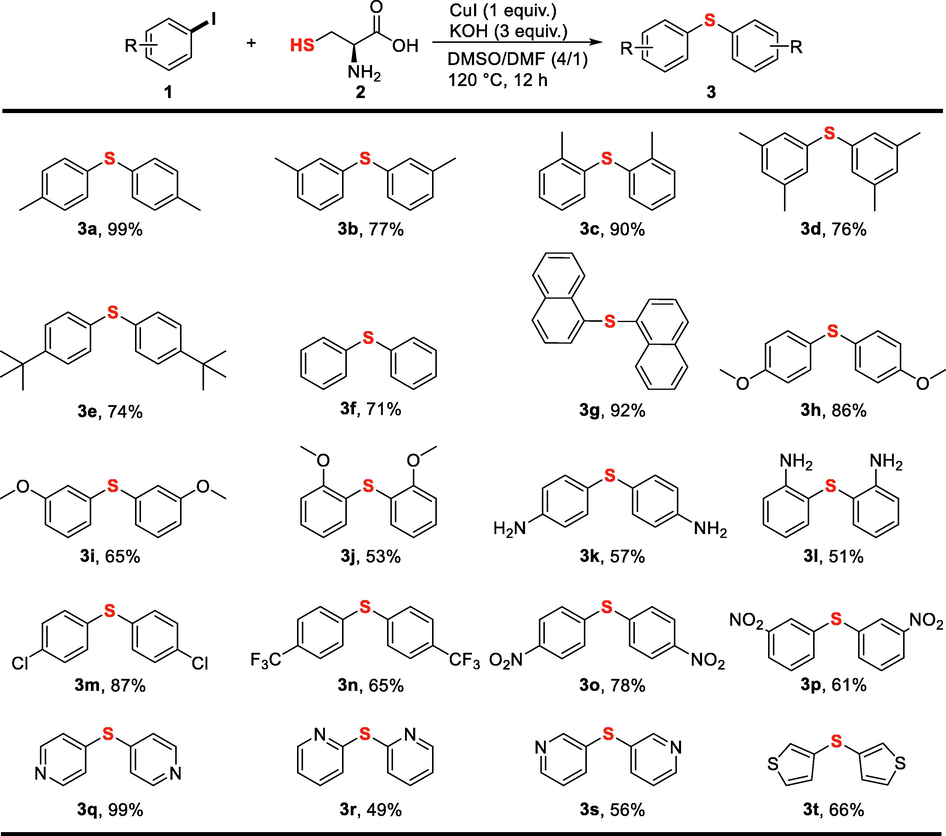
Substrate scope of the synthesis of diaryl sulfides. Reaction conditions: iodobenzenes (0.5 mmol), L-cysteine (0.5 equiv.), CuI (1 equiv.), KOH (3 equiv.), DMSO/DMF (v/v = 4/1, 1.5 mL), 120 °C, 12 h; Isolated yields were presented.
3.2 The formation of diaryl sulfide underwent a multiple C-S bond formation and cleavage process
In order to investigate the plausible mechanism of L-cysteine as sulfur source and the effect of amino group and carboxylic group of L-cysteine involved in this developed protocol for the synthesis of symmetrical diaryl sulfides, several control experiments were carried out. In the absence of L-cysteine, 3a was not observed in the reaction mixture, suggesting that the sulfur atom in 3a was derived from L-cysteine (Fig. 3a). Treating 4-iodotoluene (1a) with 3-mercaptopropionic acid (4) under standard condition predominately afforded sulfide 5 in 78% yield, along with the production of 3a in 13% yield after 12 h. It should be noted that, when the reaction time was extended to 24 h, the yield of 3a increased to 30% and the yield of 5 decreased to 65%, suggesting a slow conversion from 5 into 3a (Fig. 3b). The reaction of 4-iodotoluene (1a) with 2-aminoethanethiol (6) gave 84% yield of 3a, 5% yield of 2-(p-tolylthio)ethan-1-amine (7) (Fig. 3c). It was obvious that L-cysteine was a better sulfur source than either 3-mercaptopropionic acid or 2-aminoethanethiol. On the other hand, these results indicated that the aryl alkyl sulfide may be formed as an intermediate from aryl iodide and L-cysteine, and then reacted with another molecule of aryl iodide to yield diaryl sulfide. In order to verify this hypotheses, commercially available S-phenyl L-cysteine (8) was used to react with iodobenzene (1f) and, as expected, this reaction gave 87% yield of diaryl sulfide 3f (Fig. 3d). Furthermore, we found that 8 was converted into benzenethiol 9 in 90% yield under standard condition, indicating that diaryl sulfide 3f was formed through C-S coupling reaction of 9 and another molecule of 1f (Fig. 3e).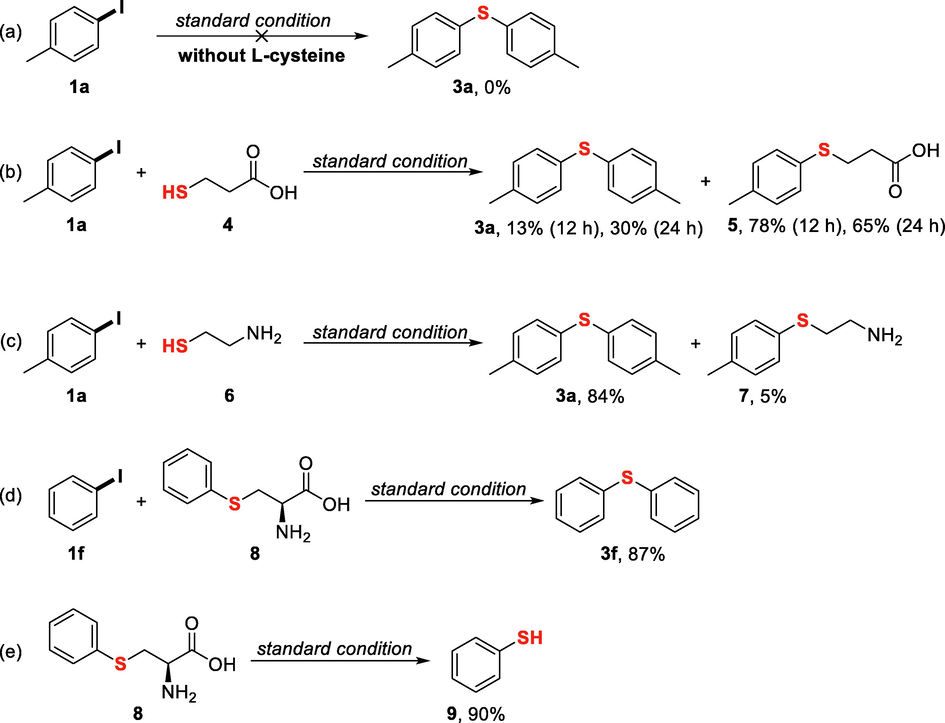
Control experiments. Standard reaction conditions: CuI (1 equiv.), KOH (3 equiv.), DMSO/DMF (v/v = 4/1, 1.5 mL), 120 °C, 12 h; 0.5 equiv of sulfur sources 4 and 6 were used in (b) and (c), respectively; 1 equiv. of 8 was used in (d).
Based on these results of control reactions, taking iodobenzene (1f) as example, a plausible reaction pathway involving double C(sp2)-S bond formations and a C(sp3)-S bond cleavage was proposed as Fig. 4. Like common copper catalyzed C-S coupling reactions, oxidative addition occurred between 1f and copper salt afforded a copper complex, and the subsequent ligand exchange by L-cysteine and final reductive elimination completed the first C(sp2)-S bond formation to provide thioether 8. [Deng et al., 2004] Intermediate 8 was then converted into benzenethiol 9 via C(sp3)-S bond cleavage, which was initiated through an intramolecular nucleophilic attack by the terminal amine and carboxylate under basic condition (Johnston et al., 1986). Amino group and carboxylic group possibly have synergistic effect on promoting the C(sp3)-S cleavage as both 4 and 6 exhibited less efficiency than L-cysteine in this conversion. Benzenethiol 9 was further coupled with another molecule of 1f to afford the final diaryl sulfide 3f through the second C(sp2)-S bond formation.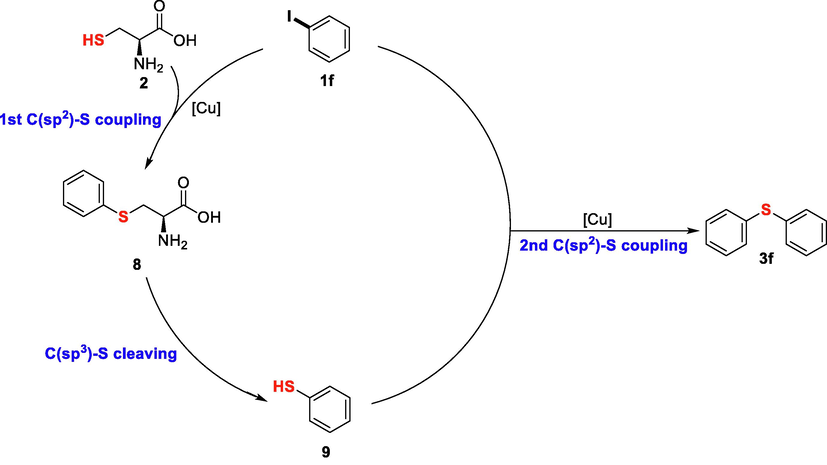
Plausible reaction pathway.
3.3 Synthesis of heteroarenethiols using L-cysteine as sulfur source
C-H mercaptalization with a proper thiosurrogate represesents an attractive way to prepare heteroarenethiols from heteroarenes. Several thiolsurrogates such as elementary sulfur and aliphatic dithiols were reported to convert benzothiazoles and benzoxazoles into the corresponding heteroarenethiols (Popov et al., 2009; Yan et al., 2017; Xiao et al., 2019). In order to test whether L-cysteine is a usable sulfur source in C-H mercaptalization, we next tried to apply L-cysteine in the synthesis of heteroarenethiols. Benzothiazole (11a) was used as a model substrate and treated with L-cysteine in the presence of copper salt and base in DMSO. Preliminary condition screening revealed that CuS was effective in promoting this C-H mercaptalization reaction. As shown in Table 2, both copper salt and L-cysteine were indispensable for this conversion (Entries 1–2, Table 2). With loading 1 equiv. of CuI and 1 equiv. of L-cysteine, 46% yield of 2-mercaptothiozole (12a) was obtained, indicating that L-cysteine functioned as thiol source in this conversion (Entry 3, Table 2). A screening of copper salts revealed that CuS was the most effective in this conversion (Entries 4–6, Table 2). Considering that CuS was significantly more effective that other copper salts, it is possible that CuS provided additional sulfur source in the mercaptalization of benzothiazole. Reducing the loading amount of CuS significantly decreased the yields (Entries 7–8, Table 2). Further increasing the amount of L-cysteine to 2 equiv. improved the yield to 84%, along with the recovery of 10% of benzothiazole (Entry 9, Table 2). It is noteworthy that no byproduct was found by TLC in these reactions. Further condition screening revealed that either less loading amount of KOH or lowering the reaction temperature led to the lower yields (Entries 10–11, Table 2). Therefore, the optimized condition for C-H mercaptalization of benzothiazole was as follows: benzothiazole (0.5 mmol), L-cysteine (2 equiv.), KOH (5 equiv.), CuS (1 equiv.), DMSO (2 mL), 140 °C, 16 h, under argon.
Entry
[Cu] (equiv.)
L-cysteine (equiv.)
Bases (equiv.)
Temp (°C)
Yield[b] (%)
1
–
1
KOH (5)
140
ND
2
CuI (1)
–
KOH (5)
140
ND
3
CuI (1)
1
KOH (5)
140
46
4
CuSO4 (1)
1
KOH (5)
140
27
5
CuO (1)
1
KOH (5)
140
35
6
CuS (1)
1
KOH (5)
140
62
7
CuS (0.5)
1
KOH (5)
140
49
8
CuS (0.1)
1
KOH (5)
140
7
9
CuS (1)
2
KOH (5)
140
84
10
CuS (1)
2
KOH (3)
140
64
11
CuS (1)
2
KOH (5)
130
44
The substrate scope was further broadened by using benzothiazoles and benzoxazoles bearing a variety of functional groups (Fig. 5). Using L-cysteine as sulfur source, all benzothiazoles and benzoxazoles were successfully converted into the corresponding thiolated products in satisfying yields. Functional groups including halides, amino and nitro groups were well compatible with this reaction system.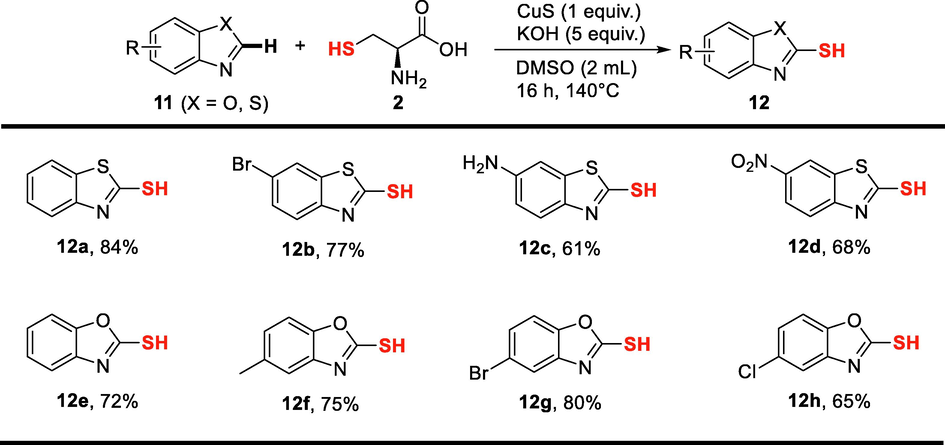
Substrate scope of the synthesis of heteroarenethiols. Reaction conditions: benzothiazole or benzoxazole (0.5 mmol), L-cysteine (2 equiv.), KOH (5 equiv.), CuS (1 equiv.), DMSO (2 mL), 140 °C, 16 h, under argon; Isolated yields were presented.
4 Conclusion
In conclusion, we employed L-cysteine as sulfur source and developed novel C-S coupling strategies for the synthesis of symmetrical diaryl sulfides from aryl halides. Brief mechanistic study revealed that a tandem C(sp2)-S coupling and C(sp3)-S cleaving reactions were possibly involved in this conversion. In addition, we also successfully applied L-cysteine in the C-H mercaptalization of benzothiazoles and benzoxazoles. Since L-cysteine is an attractive choice of sulfur source because of the safety and sustainability, more synthetic applications of L-cysteine in the preparation of sulfur containing compounds could be expected in the future.
Acknowledgement
This work was supported by Natural Science Foundation of Liaoning Province of China [Grant No. 2019-ZD-0453, 2020 MS 105], Fundamental Research Funds for the Central Universities [Grant No. DUT20LK20], and the Career Development Support Plan for Young and Middle-aged Teachers in Shenyang Pharmaceutical University (Grant No. ZQN2021007).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Glycerol Cu(II) complex supported on Fe3O4 magnetic nanoparticles: A new and highly efficient reusable catalyst for the formation of aryl-sulfur and aryl-oxygen bonds. Catal. Lett.. 2020;150:1128-1141.
- [CrossRef] [Google Scholar]
- Trisaminomethane–cobalt complex supported on Fe3O4 magnetic nanoparticles as an efficient recoverable nanocatalyst for oxidation of sulfides and C-S coupling reactions. Appl. Organomet. Chem.. 2020;34:e5260.
- [CrossRef] [Google Scholar]
- Efficient copper-catalyzed double S-arylation of aryl halides with sodium sulfide in Peg-400. Phosphorus Sulfur Silicon Relat. Elem.. 2012;187:1284-1290.
- [CrossRef] [Google Scholar]
- Desulfonylation via radical process: recent developments in organic synthesis. Chem. Rev.. 2021;121:12548-12680.
- [CrossRef] [Google Scholar]
- Synthesis of di(hetero)aryl sulfides by defluorinative sulfenylation of polyfluoroalkyl ketones with sodium sulfinates or arylsulfonyl chlorides. Chem. Commun.. 2020;2020(56):8699-8702.
- [CrossRef] [Google Scholar]
- CuI-catalyzed coupling reactions of aryl iodides and bromides with thiols promoted by amino acid ligands. Synlett. 2004;2004:1254-1258.
- [CrossRef] [Google Scholar]
- Cysteine as a sustainable sulfur reagent for the protecting-group-free synthesis of sulfur-containing amino acids: biomimetic synthesis of L-ergothioneine in water. Green Chem.. 2012;14:2256.
- [CrossRef] [Google Scholar]
- Dithiooxamide as an effective sulfur surrogate for odorless high-yielding carbon–sulfur bond formation in wet PEG200 as an eco-friendly, safe, and recoverable solvent. Eur. J. Org. Chem.. 2015;2015:2914-2920.
- [CrossRef] [Google Scholar]
- Chemistry and biochemistry of sulfur natural compounds: Key intermediates of metabolism and redox biology. Oxid. Med. Cell. Longev.. 2020;2020:8294158.
- [CrossRef] [Google Scholar]
- Solvothermal synthesis of CdS nanowires using L-cysteine as sulfur source and their characterization. Appl. Phys. A. 2008;91:69-72.
- [CrossRef] [Google Scholar]
- Highly efficient and reusable polystyrene-supported copper(II) catalytic system for S-arylation of potassium thiocyanate by aryl halides in water. Appl. Organomet. Chem.. 2016;30:566-570.
- [CrossRef] [Google Scholar]
- Highly efficient and magnetically separable nano-CuFe2O4 catalyzed S-arylation of thiourea by aryl/heteroaryl halides. Chin. Chem. Lett.. 2014;25:1382-1386.
- [CrossRef] [Google Scholar]
- Room-temperature arylation of thiols: breakthrough with aryl chlorides. Angew. Chem. Int. Ed.. 2017;56:874-879.
- [CrossRef] [Google Scholar]
- Some observations on the synthesis of the mercapturic acid conjugate of paracetamol. J. Chem. Res. Synopses. 1986;10:386-387.
- [Google Scholar]
- Copper oxide nanoparticles supported on graphene oxide-catalyzed S-arylation: An efficient and ligand-free synthesis of aryl sulfides. Adv. Synth. Catal.. 2013;355:2297-2307.
- [CrossRef] [Google Scholar]
- Amino acids and peptides organocatalysts: A brief overview on its evolution and applications in organic asymmetric synthesis. Curr. Organocatalysis. 2021;8:126-146.
- [CrossRef] [Google Scholar]
- An efficient copper-catalyzed carbon−sulfur bond formation protocol in water. Org. Lett.. 2011;2011(13):454-457.
- [CrossRef] [Google Scholar]
- Chemistry of biologically active sulfur compounds. Phosphor. Sulfur Silicon Relat Elem.. 1976;2:1-50.
- [CrossRef] [Google Scholar]
- Efficient copper(I)-catalyzed S-arylation of KSCN with aryl halides in PEG-400. Chinese J. Chem.. 2012;30:651-655.
- [CrossRef] [Google Scholar]
- Recent approaches for the direct Use of elemental sulfur in the synthesis and processing of advanced materials. Angew. Chem. Int. Ed.. 2015;46:3249-3258.
- [CrossRef] [Google Scholar]
- Copper(II)-catalyzed single-step synthesis of aryl thiols from aryl halides and 1,2-ethanedithiol. Adv. Synth. Catal.. 2015;357:2205-2212.
- [CrossRef] [Google Scholar]
- Transition-metal-catalyzed synthesis of phenols and aryl thiols. Beilstein J. Org. Chem.. 2017;13:589-611.
- [CrossRef] [Google Scholar]
- A highly efficient method for the copper-catalyzed selective synthesis of diaryl chalcogenides from easily available chalcogen sources. Eur. J. Org. Chem.. 2011;2011:7331-7338.
- [CrossRef] [Google Scholar]
- Natural sulfur-containing compounds: An alternative therapeutic strategy against liver fibrosis. Cells. 2019;8:1356.
- [CrossRef] [Google Scholar]
- Sulfur Chemistry in Polymer and Materials Science. Macromol. Rapid Commun.. 2019;40:1800650.
- [CrossRef] [Google Scholar]
- Cysteine metabolism in neuronal redox homeostasis. Trends Pharmacol. Sci.. 2018;39:513-524.
- [CrossRef] [Google Scholar]
- Tin sulfide films by spray pyrolysis technique using L-cysteine as a novel sulfur source. Phys. Status Solidi C. 2016;13:18-23.
- [CrossRef] [Google Scholar]
- In situ generation and trapping of aryllithium and arylpotassium species by halogen, sulfur, and carbon electrophiles. J. Org. Chem.. 2009;74:8309-8313.
- [CrossRef] [Google Scholar]
- Synthesis of thiols, sulfides, sulfoxides and sulfones. Contemp. Org. Synth.. 1995;2:409-440.
- [CrossRef] [Google Scholar]
- Analysis of US FDA-approved drugs containing sulfur atoms. Top Curr. Chem. (Z). 2018;376:5.
- [CrossRef] [Google Scholar]
- Metabolism of sulfur-containing amino acids. Ann. Rev. Nutr.. 1986;6:179-209.
- [CrossRef] [Google Scholar]
- Copper(0) nanoparticles immobilized on SBA-15: A versatile recyclable heterogeneous catalyst for solvent and ligand free C-S coupling reaction from diverse substrates. Micropor. Mesopor. Mater.. 2021;323:111198.
- [CrossRef] [Google Scholar]
- C−C and C−S coupling catalyzed by supported Cu(II) on nano CoFe2O4. ChemistrySelect. 2020;5:5077-5081.
- [CrossRef] [Google Scholar]
- Catalytic C-S cross-coupling reactions employing Ni complexes of pyrrole-based pincer ligands. ACS Catal.. 2014;4:2941-2950.
- [CrossRef] [Google Scholar]
- Recent progress in copper-catalyzed C-N coupling reactions. Chin. J. Org. Chem.. 2010;30:181-199.
- [Google Scholar]
- Metal-free C-H mercaptalization of benzothiazoles and benzoxazoles using 1,3-propanedithiol as thiol source. Beilstein J. Org. Chem.. 2019;2019(15):279-284.
- [CrossRef] [Google Scholar]
- L-Tyrosine-Pd complex supported on Fe3O4 magnetic nanoparticles: A new catalyst for C-C coupling and synthesis of sulfides. Appl. Organomet. Chem.. 2020;2020(34):e5256.
- [CrossRef] [Google Scholar]
- Copper-catalyzed direct synthesis of aryl thiols from aryl iodides using sodium sulfide aided by catalytic 1,2-ethanedithiol. Synlett. 2017;28:2272-2276.
- [CrossRef] [Google Scholar]
- Elemental sulfur as a sulfuration agent in the copper-catalyzed C-H bond thiolation of electron-deficient arenes. Org. Biomol. Chem.. 2017;15:8276-8279.
- [CrossRef] [Google Scholar]
- Copper based on diaminonaphthalene-coated magnetic nanoparticles as robust catalysts for catalytic oxidation reactions and C-S cross-coupling reactions. RSC Adv.. 2021;11:9366-9380.
- [CrossRef] [Google Scholar]
- Synthesis of Ni(II)-3,5-dichloro-2-hydroxybenzenesulfonyl chloride supported SBA-15 and its application as a nanoreactor catalyst for the synthesis of diaryl sulfides via reaction of aryl halides with thiourea or S8. J. Porous Mater.. 2018;25:1349-1358.
- [CrossRef] [Google Scholar]
- Biosynthesis of lincosamide antibiotics: reactions associated with degradation and detoxification pathways play a constructive role. Acc. Chem. Res.. 2018;51:1496-1506.
- [CrossRef] [Google Scholar]
- Pharmaceutical and medicinal significance of sulfur (SVI)-containing motifs for drug discovery: A critical review. Eur. J. Med. Chem.. 2019;162:679-734.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.103896.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







