Translate this page into:
LfcinB-Derived Peptides: Specific and punctual change of an amino acid in monomeric and dimeric sequences increase selective cytotoxicity in colon cancer cell lines
⁎Corresponding author. jaegarciaca@unal.edu.co (Javier Eduardo García-Castañeda)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
We observed how a change of specific residues in LfcinB dimeric and palindromic sequences caused a notable increase in the cytotoxic activity against CaCo-2 cells while maintaining or even diminishing the level of the cytotoxic effect against normal fibroblast and HEK-293 cells. In both cases, the IC50 of the peptides was reduced by more than half of the concentration, diminishing the IC50 value from 150 µg/mL (101 µM) (LfcinB (21–25)Pal: RWQWRWQWR) to 60 µg/mL (42.8 µM) for the modified palindromic peptide 5[A] LfcinB (21–25)Pal: RWQWAWQWR and the IC50 from 125 µg/mL (LfcinB (20–30)2: (RRWQWRMKKLG)2-K-Ahx to 58 µg/mL (18 µM) for the modified dimeric peptide 26[F] LfcinB (20–30)2: (RRWQWRFKKLG)2-K-Ahx. The cytotoxic effect of 26[F] LfcinB (20–30)2 and LfcinB (21–25)Pal peptides against colon cancer cell line HCT-116 was greater than the cytotoxic effect of these peptides against Caco-2 cells, suggesting that the cytotoxic effect of these peptides is selective for colon cancer cell lines. The cytotoxic effect of the peptides rapidly caused severe damage to the morphology of CaCo-2 cells, while the morphology of the normal fibroblast and HEK-293 cells was not affected. The dimeric modified peptide 26[F] LfcinB (20–30)2 mainly caused death through apoptotic events. As for the palindromic modified peptide 5[A] LfcinB (21–25)Pal, the cell death was induced by both necrotic and apoptotic pathways. All this suggests that specific modification of a single residue in the peptide sequence can improve the anticancer activity of the original monomeric or dimeric peptides, giving place to new potential molecules for the future development of drugs for use against colorectal carcinoma. Our results show that changes to a residue of the anti-cancer peptide sequence may be considered a versatile, feasible, and invaluable strategy for obtaining promising sequences for developing peptide-based cancer treatments.
Keywords
Bovine Lactoferricin
Colon cancer
Enhanced cytotoxicity
1 Introduction
Colorectal carcinoma is the third most diagnosed cancer and the second leading cause of cancer-related deaths worldwide. For the year 2018, the World Health Organization reported nearly 1.8 million new cases and 880,792 deaths due to this disease (Bray et al., 2018). For those patients with a primary tumour, surgery is the main treatment option, and for those who develop invasive tumours, chemotherapy is the leading approach (Zoetemelk et al., 2019). However, once patients are in remission, it has been estimated that in 40–50% of the cases, the cancer recurs and generally metastatic colorectal carcinoma develops, not only due to a failure of either the surgery or the systemic treatment, where part of the lesion or occult metastasis progresses, but also due to resistance to the chemotherapies used (Su et al., 2015). Therefore, new therapeutic approaches have been studied to improve the efficacy; among them, the use of peptides against this type of cancer represents a breakthrough, because they can directly target tumour cells without affecting normal cells, and their synthesis is relatively easy and fast, and various modifications can be explored for the enhancement of their activity (Shapira et al., 2014).
Of these peptides, bovine lactoferricin (LfcinB), a 25-amino acid peptide generated from the acid hydrolysis of bovine lactoferrin (BFL), not only has exhibited antibacterial, antifungal, and antiviral activity but also has shown cytotoxicity against several types of cancer that is greater than that exhibited by the whole protein, suggesting that this peptide is the active domain(Cutone et al., 2020a; Jenssen, 2005; Pan et al., 2013; Solarte et al., 2017; Vorland et al., 1999; W. et al., 1994). In vivo and in vitro assays have shown that BLF, LfcinB and peptides derived from LfcinB have exhibited anticancer activity against colon cancer(Habib et al., 2013; Maraming et al., 2018), LfcinB had a cytotoxic effect against the cancer cell lines Cal-27 (oral carcinoma), AGS (gastric cancer), Caco-2, HCT-116 (colon cancer), Jurkat, CCRF-CEM (leukemia), MDA-MB-435, MDA-MB-468, MDA-MB-231, MCF-7 (breast cancer), etc. (Baxter et al., 2017; Cutone et al., 2020b; García-Montoya et al., 2012; Pan et al., 2013; Solarte et al., 2015). It has been suggested that the peptide establishes electrostatic interactions between its positively charged residues and the negatively charged molecules in the membrane wall of cancerous cells. In gastric cancer, the peptide induces caspase-dependent apoptosis by inhibition of autophagy at the final step (Baxter et al., 2017; Dennison et al., 2006; Felício et al., 2017; Pan et al., 2013; Riedl et al., 2011). Previous papers have shown that short synthetic peptides derived from LfcinB exhibit higher antibacterial and anticancer activity than the complete sequence and BFL; for example, in breast cancer, multiple peptides with different modifications such as palindromic, polyvalent, and cyclic sequences have exhibited great cytotoxicity and selectivity against breast cancer cells(Barragán-Cárdenas et al., 2020; Guerra et al., 2019; Insuasty-Cepeda et al., 2020; Solarte et al., 2015; Vargas Casanova et al., 2017).
In some of our prior investigations, we evaluated the antibacterial and anticancer effect of the palindromic peptide LfcinB (21–25)Pal: RWQWRWQWR and nine peptides derived from an alanine scan of this sequence, showing that LfcinB (21–25)Pal exhibits greater antibacterial activity against E. coli ATCC 25922, E. faecalis ATCC 29212, S. enteritidis ATCC 13076, P. aeruginosa ATCC 27853, and S. aureus ATCC 25,923 when compared to LfcinB(León-Calvijo et al., 2015; Vargas-Casanova et al., 2019). Also, its cytotoxic effect was evident against oral squamous cell carcinoma (OSCC) CAL-27 and breast cancer MDA-MB-468, MDA-MB-231, and MCF-7 cell lines (Barragán-Cárdenas et al., 2020; Insuasty-Cepeda et al., 2020; Vargas Casanova et al., 2017). On the other hand, the dimeric peptide LfcinB (20–30)2: (RRWQWRMKKLG)2-K-Ahx and five analogous peptides in which position 26 was replaced with amino acids of different chemical natures have also exhibited selective cytotoxicity against breast cancer cell lines MDA-MB-468, MDA-MB-231, and MCF-7 [14,17]. The peptides 5[A] LfcinB (21–25)Pal and 26[F] LfcinB (20–30)2 exhibited the highest selective cytotoxic effect against breast cancer cells, thus being considered to be promising molecules (Insuasty-Cepeda et al., 2020).
These results indicate that these sequences may be cytotoxic against cell lines derived from different types of cancer, suggesting that the activity of these peptides is broad-spectrum. The aim of the present paper is to evaluate the cytotoxic effect of the palindromic peptides LfcinB (21–25)Pal and 5[A] LfcinB (21–25)Pal and dimeric peptides LfcinB (20–30)2 and 26[F] LfcinB (20–30)2 against the colorectal carcinoma CaCo-2 cell line in order to determine whether these molecules also have a cytotoxic effect on colon cancer cell lines and the type of cell death related to this effect (Barragán‐Cárdenas et al., 2020; Huertas Méndez et al., 2017). Peptides 26[F] LfcinB (20–30)2 and LfcinB (21–25)Pal had cytotoxic effects against colorectal carcinoma of Caco-2 and HCT-116 cell lines, the latter being more sensitive to peptides.
According to our previous reports, it is possible that the central region of peptide sequences analogous to the minimal motif of LfcinB (20–25): RRWQWR play an important role in the cytotoxic effect against cancer lines. Richardson et al, reported that LfcinB (20–25) was highly cytotoxic when released into the cytoplasm by fusogenic liposomes in CCRF-CEM and Jurkat cell lines. Vargas et al., (Vargas-Casanova et al., 2019) reported that monomeric, dimeric and tetrameric peptides containing the minimum motif of LfcinB showed a selective cytotoxic effect against breast cancer cell lines. Insuasty et al. (Insuasty-Cepeda et al., 2020) reported that changing the Met of the dimeric LfcinB(20–30)2: (RRWQWRMKKLG)2-Ahx-K sequence for hydrophobic amino acids enhanced the cytotoxic effect against MCF-7 and HTB-132 breast cancer cell lines. Moreover, studies with analogue peptides of a palindromic sequence showed that the palindromic peptides LfcinB (21–25)Pal: RWQWRWQWR and its analogue 5[A] LfcinB (21–25)Pal: RWQWAWQWR exhibited the highest activity against breast cancer cells. In the present investigation, our aim was to determine if these kinds of modifications in active peptides also enhance the cytotoxic effect against colorectal cancer cell line CaCo-2 and at the same time to determine if the residue change influences the type of cell death, the membrane cellular damage, and the mitochondria depolarization.
2 Materials and methods
2.1 Reagents and materials
The cell lines Caco-2, HCT-116 and HEK-293 were purchased from ATCC (Manassas, VA). The primary cell culture of fibroblasts was obtained from the foreskin by the Laboratory of Cellular Physiology of the National University of Colombia, the fibroblasts were cultured and frozen. Some vials were thawed and cultured for up to 7 passes since no transformation/immortalization was done. Fmoc-Arg(Pbf)–OH, Fmoc-Trp(Boc)–OH, Fmoc-Gln(Trt)–OH, Fmoc-Met-OH, Fmoc-Phe-OH, Fmoc-Leu-OH, Fmoc-Gly-OH, Fmoc-Lys(Fmoc)–OH, Fmoc-6-Ahx-OH, Rink amide resin, dicyclohexilcarbodiimide (DCC), and 1-hydroxy-6-Chlorobenzotriazole were purchased from AAPPTec (Louisville, KY, USA). Trifluoroacetic acid (TFA), acetonitrile (ACN), dichloromethane (DCM), N,N-dimethylformamide, ethanodithiol, triisopropylsilane, methanol, acetonitrile, and isopropanol were obtained from Merck (Darmstadt, Germany). SPE SupelcleanTM columns were purchased from Sigma-Aldrich (St. Louis, MO, USA). The CaCo-2 and HEK-293 cell lines were obtained from ATCC® (Manassas, VA, USA); LIVE/DEADTM BacLightTM Viability Kit and Annexin V, Alexa FluorTM 488 conjugate was purchased from Invitrogen (Eugene, Oregon); Mitochondrial Membrane Potential Detection was obtained from BD Biosciences (Torreyana Rd., San Diego, CA 92121, USA). The DMEM culture medium and trypsin were obtained from Sigma-Aldrich (St. Louis, MO, USA). Bovine fetal serum was purchased from Gibco (Waltham, MA, USA).
2.2 Solid-Phase synthesis (SPPS-Fmoc/tBu)
The palindromic and dimeric peptides were manually synthesized employing solid-phase peptide synthesis using the Fmoc/tBu strategy (Vergel Galeano et al., 2014). Briefly, a Rink amide resin with 0.46 meq/g substitution was used as a solid support. An elongation of the peptide sequences was performed by successive steps of (i) deprotection of the alpha-amino group, removing the Fmoc group under basic conditions with 2.5% v/v 4-methyl piperidine (Rodríguez et al., 2020), (ii) activation of the Fmoc-amino acid with DCC and 6-Cl-HOBt in DMF and DCM (2:1 v/v) and adding 2 drops of Triton X-100, and (iii) coupling the amino acid to the growing solid support chain by shaking for 2 h for each couple. The peptides were cleaved from the resin by adding TFA/water/TIS/EDT (92.5/2.5/2.5/2.5 % v/v) and stirring for 8 h. The peptides were precipitated using diethyl ether at −20 °C, centrifuged at 2500 rpm for 5 mins, and diethyl ether remnant was eliminated by evaporation at room temperature. Then the peptides were purified via RP-SPE and characterized by means of RP-HPLC, MALDI-TOF and ESI-QTOF.
2.3 RP-HPLC characterization
For the analysis of the peptides by RP-HPLC, 1 mg/mL was analysed in an Agilent 1260 HPLC. Solvent A: 0.05% TFA in water, solvent B: 0.05% TFA in ACN. As a stationary phase, a Chromolith® C-18 monolithic column (50 × 4.5 mm) was used. Elution gradient program: 5/5/50/100/100/5/5 %B in 0/1/9/9.1/12/12.1/15 min. Flow rate: 2 mL/min, wavelength: 210 nm, injection: 10 μL.
2.4 Peptide purification by RP-SPE
For the purification of the palindromic and dimeric peptides obtained, 5 g RP-SPE columns (particle size: 40–50 um) were used, employing the Insuasty et al. methodology (Insuasty Cepeda et al., 2019). Briefly, the peptide was dissolved in solvent A, and the sample was seeded and then eluted with solutions containing different percentages of solvent B. The fractions containing the pure peptide were collected, evaporated at room temperature, and lyophilized. The final product was characterized via RP-HPLC and stored at 4 °C.
2.5 Mass spectrometry analysis
The molecular weight of the palindromic and dimeric peptides was determined via MALDI-TOF MS in a Bruker microflex mass spectrometer. 1 L of the purified peptide solution (0.5 mg/mL) was mixed with 18 L of matrix (α-cyano-4-hydroxycinnamic acid, 5 mg/mL), and then 1 µL of the mixture was seeded on the plate sample holder. The laser power ranged between 2700 and 3000 V, and 200 laser shots were done.
The MS analysis was realized to establish the oxidation of the indole ring of the Trp residues in the peptides. The peptides (10 µg/mL) were analysed on a Bruker Impact II LC Q-TOF MS spectrometer equipped with an electrospray ionization (ESI) source operating in positive mode. ESI conditions were: End late offset 500 V, capillary voltage 4500 V, dry temperature 220 °C, Nitrogen 8 L/min.
2.6 Cell culture
For CaCo-2 and HCT-116 cells, the medium used was Dulbecco’s Modified Eagle’s Medium (DMEM)/Nutrient Mixture F-12 Ham. For HEK-293 and fibroblast cell lines, the medium used was Roswell Park Memorial Institute (RPMI-1640). For all cell lines, the medium was supplemented with 10% fetal bovine serum (SFB) and 1.5 g/L NaHCO3 and NaOH was added up to pH 7.4, amphotericin (200 μg/mL) and 1% penicillin and streptomycin was added in DMEM medium. All mediums were filtered through a 0.22 μm membrane. Cell culture was maintained in an incubator (Sanyo serial No 11030213) at 37 °C with 5% CO2.
2.7 Viability test by means of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide)
The MTT reagent allows the measurement of cellular metabolic activity as an indicator of cell viability, since metabolically active cells reduce it to formazan whose absorbance is read at 500–600 nm. Briefly, the cells were seeded with a complete medium in 96-well plates at a rate of 10,000 cells and 100 μL per well, and adhesion to the plates was allowed for 24 h. Later, the complete medium was removed, and an incomplete medium was added for synchronization for another 24 h. The cells were then incubated at 37 °C for 2 h with 100 μL of peptide at the evaluated concentrations (200, 100, 50, 25, 12.5, and 6.25 μg/mL). Next, the peptide was removed from the well and 100 μL of incomplete medium with 10% MTT was added and incubated for 4 h. The medium was replaced with 100 μL of isopropanol, and after 30 min of incubation at 37 °C, the absorbance was measured at 575 nm. As a negative control, an incomplete culture medium with 10% MTT was used, and as a positive control, cells without MTT treatment were used (Langan et al., 2016).
2.8 Evaluation of the integrity of the cytoplasmic membrane using SYTO9/PI
The cells with complete medium were seeded and synchronized in boxes of 24 wells at a concentration of 4x 104 cells/well in a volume of 400 μL/well, and adhesion to the plate was allowed for 24 h (Robertson et al., 2019). Then the complete medium was replaced with incomplete medium for synchronization of the cells for an additional 24 h. Subsequently, the culture medium was replaced with 400 μL of a solution containing the dimeric and palindromic peptides to be evaluated at a concentration equivalent to its IC50 and incubated for 2 or 24 h. The cells were harvested by adding 200 μL of trypsin and incubating for 10 min. The trypsin was quenched with 200 μL of complete medium, and the cells were centrifuged at 2500 rpm for 10 min. The supernatant was discarded, and the pellet was washed with 100 μL PBS and centrifuged under the same conditions previously described. The supernatant was discarded, and the cells were stained with 30 μL of a solution from the LIVE/DEAD® FungaLigthTM commercial kit (Invitrogen) containing the mixture of fluorochromes (0.5 μL of SYTO9 and/or 0.5 μL of propidium iodide (PI) with 99 μL of PBS). Subsequently, the cells were incubated at room temperature in the dark for 20 min and centrifuged, and the supernatant was discarded. The pellet was suspended in 100 μL of PBS and analysed via flow cytometry in a BD AccuriTM C6 device. The events were recorded using the green channel (FL1) on the abscissa axis and the red (FL3) on the ordinate axis. Negative control: untreated cells marked with both fluorophores; positive control: cells treated with actinomycin D 10 μg/mL for 24 h. To define the working population, a control of untreated and unstained cells was used. Two controls were used to compensate: (i) untreated cells stained only with PI, and (ii) untreated cells stained only with Syto9.
2.9 Determination of the type of cell death (Apoptosis/Necrosis)
The cells were seeded and synchronized in boxes of 24 wells at a concentration of 4 × 104 cells/well in 400 μL/well, adhesion and synchronization were allowed in the same way as in the previous section, and the culture medium was replaced with medium containing the dimeric and palindromic peptides to be evaluated and incubated for 2 or 24 h. Subsequently, the cells were harvested with trypsin, centrifuged at 2500 rpm for 10 min, washed with PBS, and suspended in 10 μL of staining buffer with the fluorochromes (10 mM Hepes pH 7.4; 10 mM NaCl and 2.5 mM CaCl2 containing 1 μL of PI fluorochrome and 1 μL of Annexin V). Cells with the fluorochromes were incubated at 37 °C in the dark for 15 min and suspended in 80 μL of staining buffer without fluorochromes for analysis via flow cytometry in a BD AccuriTM C6 device. The positive control of the necrosis was cells treated with EDTA 15 mM for 60 min and apoptotic: cells treated with actinomycin D at 15 μM for 24 h. Negative control: cells without treatment; compensation controls: (i) cells stained only with Annexin V, and (ii) only with PI; population control: unstained and untreated cells.
2.10 Determination of mitochondrial membrane depolarization
For this test, the MitoProbeTM JC-1 Assay kit (M34152 from Termofisher, Waltham, MA, USA) was used according to the supplier’s recommendations. The cells seeded and synchronized in a box of 24 wells (4 × 104 cells/well) were incubated with 400 μL of peptide to be evaluated at 2 and 24 h. The cells were then trypsinized and collected by centrifugation at 400g × 5 min. The resulting pellet was stained by adding 100 μL of JC1 “working solution” (1:100 JCI solution reconstituted in DMSO: buffer assay 1 μL) and incubated at 37 °C for 20 min. Subsequently, the cells were washed twice with 1 μL buffer assay and finally resuspended in 100 μL of 1 × buffer assay for reading on the BD AccuriTM C6 cytometer. Negative control: cells without treatment; positive control: cells treated with 15 μM actinomycin D for 24 h.
3 Results
3.1 Peptide synthesis and characterization
To identify if specific changes in the sequence would enhance the cytotoxic effect of an active peptide, four peptides were synthesized: (i) the dimeric peptide LfcinB (20–30)2: (RRWQWRMKKLG)2-Ahx-K and its analogue 26[F] LfcinB (20–30)2: (RRWQWRFKKLG)2-Ahx-K, where 26Met is replaced with 26Phe, and (ii) the palindromic peptide LfcinB (21–25)Pal: RWQWRWQWR and its analogue 5[A] LfcinB (21–25)Pal: RWQWAWQWR, where 5Arg is replaced with 5Ala. The peptides were obtained via manually SPPS/Fmoc-tBu, purified by means of RP-SPE, and characterized using RP-HPLC, LC-MS and MALDI-TOF MS. The cytotoxic effect of the peptides was evaluated against the human colorectal cancer cell line CaCo-2 and the non-tumorigenic human cell line HEK- 293 and a primary culture of foreskin fibroblasts (Table 1).
Code: Sequence
Characterization
Cytotoxic effect
RP-HPLC
MSb
IC50 (µM / µg/mL)
tR (min)
Puritya (%)
CaCo-2
HCT-116
HEK-293
Fibroblast
LfcinB (20–30)2: (RRWQWRMKKLG)2-K-Ahx
5.6
91
3312.2
38/125
ND
60/200
>200
26[F] LfcinB (20–30)2: (RRWQWRFKKLG)2-K-Ahx
4.3
95
3342.5
18/58
7/23
33/110
36/121
LfcinB (21–25)Pal: RWQWRWQWR
6.4
91
1485.1
101/150
40/59
>200
>200
5[A] LfcinB (21–25)Pal: RWQWAWQWR
6.7
92
1401.9
43/60
ND
>200
>200
The peptides obtained showed high synthetic viability since most of the amino acids were quantitatively incorporated in a single step and the crude peptide yields were between 90 and 100%, while the pure peptide yields were around 50%. The peptides were obtained with a purity>90%, and the value of m/z for [M + H]+ is in accordance with the theoretically expected molecular weight. In all cases, MS analysis shows that there is no presence of undesirable species that may result from oxidation of the indole ring of Trp residues (Figure S1–S4, supplementary material).
3.2 Peptides cytotoxic effect against tumorigenic and non-tumorigenic cells
The MTT assays showed that the four peptides exhibited a concentration-dependent cytotoxic effect against the CaCo-2 colon cancer line, reaching values close to 20% viability at the maximum concentration evaluated in all cases (Fig. 1). In addition, the cytotoxic effect of peptides in the non-tumorigenic HEK-293 cell line and the fibroblasts was considerably lower with respect to the CaCo-2 cell line, with values>50% viability in most cases.![Cytotoxic effect of A. Palindromic peptide LfcinB (21–25)Pal, B. Palindromic modified peptide 5[A] LfcinB (21–25)Pal, C. Dimeric peptide LfcinB (20–30)2 and D. Dimeric modified peptide 26[F] LfcinB (20–30)2 against CaCo-2, HEK-293, and fibroblast cell lines. The cells were treated with the peptides for 2 h at 37 °C. The data represent the mean ± SD (n = 3). 2-way ANOVA and Sidak’s multiple comparisons test were used, p ≤ 0.05, showing a) statistically significant differences between CaCo-2 and HEK-293, and b) statistically significant differences between CaCo-2 and fibroblast.](/content/184/2022/15/8/img/10.1016_j.arabjc.2022.103998-fig1.png)
Cytotoxic effect of A. Palindromic peptide LfcinB (21–25)Pal, B. Palindromic modified peptide 5[A] LfcinB (21–25)Pal, C. Dimeric peptide LfcinB (20–30)2 and D. Dimeric modified peptide 26[F] LfcinB (20–30)2 against CaCo-2, HEK-293, and fibroblast cell lines. The cells were treated with the peptides for 2 h at 37 °C. The data represent the mean ± SD (n = 3). 2-way ANOVA and Sidak’s multiple comparisons test were used, p ≤ 0.05, showing a) statistically significant differences between CaCo-2 and HEK-293, and b) statistically significant differences between CaCo-2 and fibroblast.
Furthermore, the modified peptides exhibited a wider therapeutic window, with statistically significant selectivity between the CaCo-2 cancer line and the non-tumorigenic line HEK-293 and fibroblasts at a concentration range between 50 and 200 µg/mL. At 100 µg/mL, the peptide 5[A] LfcinB (21–25)Pal reached its highest cytotoxic effect, with values close to 20% cell viability for the CaCo-2 cell line, while at this same concentration, the cell viability of the control cell lines was close to 80%, this shows that the replacement of R by A in de sequence induced a greater cytotoxic effect maintaining the selectivity when compared to the original palindrome. In the case of the modified dimeric peptide 26[F] LfcinB (20–30)2, the maximum cytotoxic effect was observed when the cells were treated with the peptide at 100 µg/mL (19% cell viability), while at this same peptide concentration the cell viability of the control cell lines was close to 50%. These results suggest that both modified peptides exert a selective cytotoxic effect in colorectal cancer cell line CaCo-2, and the optimal concentration was 100 µg/mL in all cases. The peptide 5[A] LfcinB (21–25)Pal is more selective than the other peptides evaluated, and the selective cytotoxic effect was in the concentration range between 25 and 200 µg/mL. The peptide 5[A] LfcinB (21–25)Pal exhibited 2.3 times greater activity than the original peptide LfcinB (21–25)Pal, while the peptide 26[F] LfcinB (20–30)2 exhibited 2 times greater activity than the original peptide LfcinB (20–30)2. The palindromic peptides were less toxic to fibroblasts and HEK-293 cells than the dimeric peptides, suggesting that the monomeric peptides are more selective.
Cells treated with the peptides at their IC50 concentration were monitored using an inverted microscope coupled to an AxioCam ICc1 camera (Fig. 2). In the images of the cells without treatment, a normal morphology was observed, according to the characterization previously reported (Gheytanchi et al., 2021; Malakpour-Permlid et al., 2021; Sohrab et al., 2021). The three cell lines evaluated share an epithelial-like morphology, where polygonal and flattened shapes are observed. At two hours of treatment of each of the four evaluated peptides, several morphological changes in colorectal cancer cell line CaCo-2 were observed in all cases; there is an evident compromise of the cytoplasmic membrane: the cells lose their morphology, shrink and the release of the intracellular content can be seen. However, in the case of normal HEK-293 kidney cells treated with the palindromic peptides, it was determined that the cellular morphology is apparently not affected; a conserved epithelial morphology and a cytoplasmic membrane without compromise are observed. In the opposite way, dimeric peptides induced shrinkage on some cells and detritus are present. For primary fibroblasts, the palindromic peptides do not affect the cells since they retain their normal morphology. When cell lines were treated with dimeric peptides, cell detachment and rounded and shrunk cells were observed in fibroblasts; however, these effects were much less severe than those observed with CaCo-2 cells.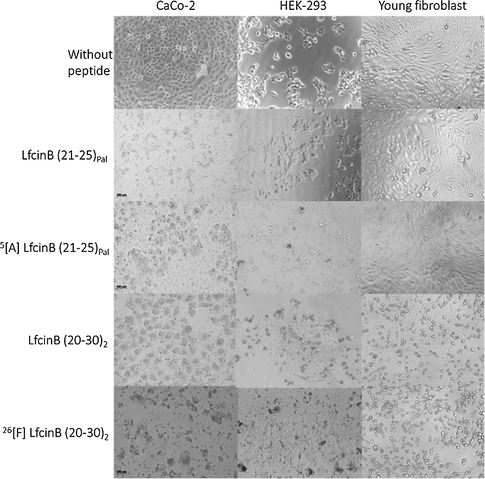
Micrography of CaCo-2, HEK-293 and fibroblast cells treated with dimeric and palindromic peptides at IC50 using an AxioCam ICc1 camera (Scale bar:100 µm).
The cytotoxic effect against the colon cancer cell line HCT-116 of 26[F] LfcinB (20–30)2 and LfcinB (21–25)Pal peptides, which presented the highest and lowest toxicity against Caco-2 cells respectively, was evaluated. IC50 values showed that both peptides had cytotoxic effect against HCT-116 cells and this effect was dependent on peptide concentration (Table 1, Fig. 3B). Comparing the cytotoxic effect of peptides in both cell lines, it is evident that the IC50 values for HCT-116 were about half the IC50 values observed in Caco-2 cells, indicating that HCT-116 cells were more sensitive to peptides. Peptide 26[F] LfcinB (20–30)2 exhibited the greatest cytotoxic effect in both Caco-2 and HCT-116 cell lines at all concentrations evaluated. Like Caco-2, the HCT-116 cells treated with the peptides showed morphological changes at 2 h of treatment, severe damage in the plasma membrane and shrinkage was observed (Fig. 3A).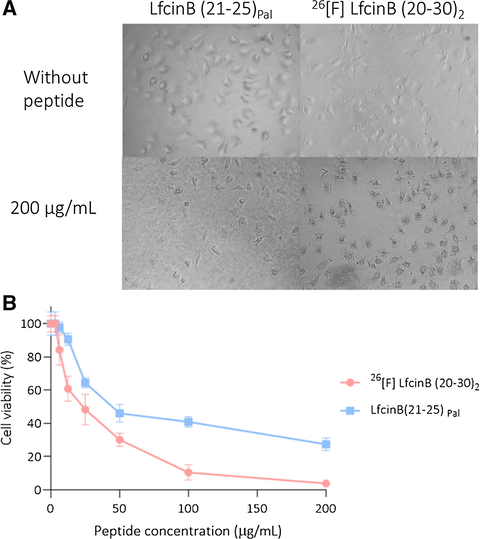
Cytotoxic effect of dimeric and palindromic peptides against HCT-116. The cells were treated with the peptides for 2 h at 37 °C. A. Micrography of cells treated using an AxioCam ICc1 camera. B. Viability reduction of the cell line HCT-116 after treatment. The data represent the mean ± SD (n = 3).
3.3 Type of cell death generated by modified peptides
To establish the type of cell death induced by the modified dimeric and palindromic peptides, cytometry studies were carried out using Annexin V and 7AAD in the presence of peptides LfcinB (21–25)Pal, 5[A] LfcinB (21–25)Pal, 26[F] LfcinB (20–30)2, and LfcinB (20–30)2 at their IC50 concentration (Fig. 4). For cells without treatment, the Q4 quadrant was established as a live cell population with a population cell percentage of 96% in all cases. Actynomicin-D (ActD) 15 μM was used as a control for death, displacing 41.5% of the population to the Q1 quadrant, where the cells were stained only by 7AAD, which indicates a necrotic population.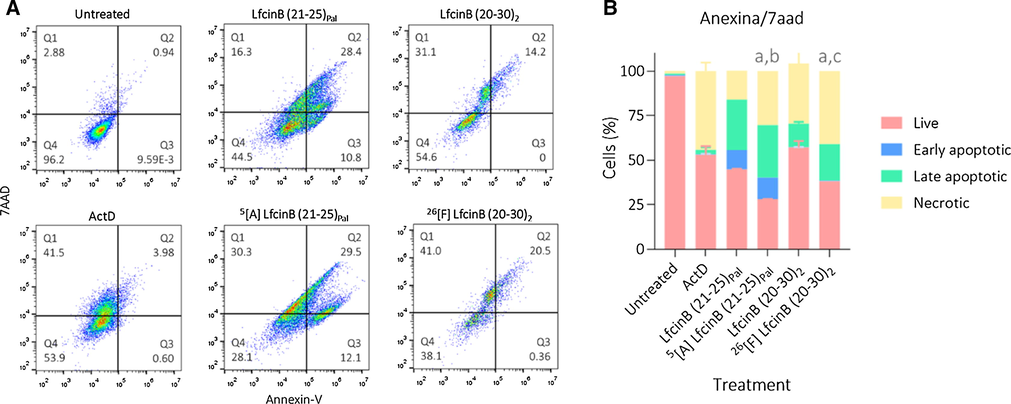
A. Dot-plots of flow cytometry assay in presence of Annexin V and 7AAD fluorophores B. Histogram of percentage of cell events related to live, early, late apoptotic or necrotic population. CaCo-2 cells were treated with palindromic and dimeric peptides in their IC50 concentration. Negative control: untreated cells, vehicle DMEM; dead control: ActD. The data represent the mean ± SE (n = 3). 2-way ANOVA and Sidak’s multiple comparisons test were used, p ≤ 0.05. a) Statistically significant differences of the live population with positive control (ActD). b) Statistically significant differences of the live population with LfcinB (21–25)Pal. c) Statistically significant differences of the live population with LfcinB (20–30)2.
The unmodified palindromic peptide LfcinB (21–25)Pal showed most of the population in apoptosis, finding that 28.4% were late apoptotic cells and 10.8% early apoptotic cells; similarly, its modified analogue 5[A] LfcinB (21–25)Pal yielded comparable population percentages, with values of 29.5% for late apoptosis and 12.1% for early apoptosis; however, the population in necrosis increased from 16.3% to 30.3% when compared to the original peptide. In contrast, the starting dimeric peptide LfcinB (20–30)2 showed cell death mediated mainly by necrotic events, with 31.1% of the population in the Q1 quadrant corresponding to necrosis and 14.2% of the population stained in quadrant Q2, which is due to late apoptosis. With the modified peptide 26[F] LfcinB (20–30)2, the main type of cell death was the same, except in this case the apoptotic events increased to 20.5% and the necrotic events to 41.0%.
To define the effect of modified dimeric and palindromic peptides on the cytoplasmic membrane, CaCo-2 cells were studied via cytometry using Syto-9 and propidium iodide fluorophores. As a cell death control, actinomycin D was used (Fig. 5).![A. Dot-plots of flow cytometry assay in the presence of PI and SYTO9 fluorophores B. Histogram of percentage of cell events related to live or dead population. CaCo-2 cells were treated with modified palindromic and dimeric peptide at their IC50 concentration. Positive control: Actinomycin-D. The data represent the mean ± SE (n = 3). 2 way ANOVA and Sidak’s multiple comparisons test were used, p ≤ 0.05. a) Statistically significant differences of the dead population with positive control (ActD). b) Statistically significant differences of the dead population with 5[A] LfcinB (21–25)Pal.](/content/184/2022/15/8/img/10.1016_j.arabjc.2022.103998-fig5.png)
A. Dot-plots of flow cytometry assay in the presence of PI and SYTO9 fluorophores B. Histogram of percentage of cell events related to live or dead population. CaCo-2 cells were treated with modified palindromic and dimeric peptide at their IC50 concentration. Positive control: Actinomycin-D. The data represent the mean ± SE (n = 3). 2 way ANOVA and Sidak’s multiple comparisons test were used, p ≤ 0.05. a) Statistically significant differences of the dead population with positive control (ActD). b) Statistically significant differences of the dead population with 5[A] LfcinB (21–25)Pal.
The modified palindromic peptide 5[A] LfcinB (21–25)Pal shifted the population towards the upper quadrant by 17%, while peptide 26[F] LfcinB (20–30)2 did so by 61.5%. These results suggest that the effect of the dimeric peptide compromises the cytoplasmic membrane of the cells to a greater extent than does the palindromic peptide. The results are consistent with those obtained in the Annexin V/7AAD assay, where the dimeric peptide displaces the cell population to a greater extent towards necrotic events, while a greater part of the cell population is seen in early and late apoptosis for the palindromic peptide.
Finally, to evaluate whether the dimeric and palindromic modified peptides generated any effect on the mitochondrial membrane, flow cytometry assays were performed using the cationic JC-1 fluorophore. ActD was used as a positive control (Fig. 6).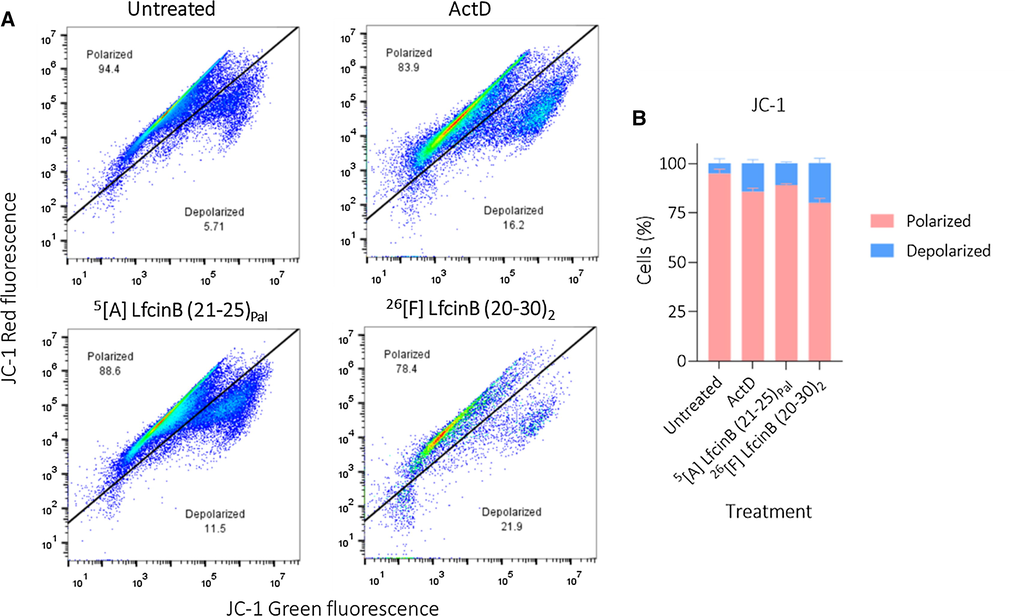
A. Dot-plots of flow cytometry assay in the presence of cationic fluorophore JC-1B. Histogram of percentage of cell events related to population with polarized or depolarized mitochondrial membrane. CaCo-2 cells were treated with modified palindromic and dimeric peptide at their IC50 concentration. Positive control: Actinomycin-D 15 μM. The data represent the mean ± SE (n = 3). 2 way ANOVA and Sidak’s multiple comparisons test were used, p ≤ 0.05. a) Statistically significant differences of the depolarized population with untreated cells. b) Statistically significant differences of the depolarized population with positive control (ActD).
These results show that both the peptides 5[A] LfcinB (21–25)Pal and 26[F] LfcinB (20–30)2 generate significant mitochondrial membrane depolarization. Peptide 5[A] LfcinB (21–25)Pal induced a depolarization of 11.5% and peptide 26[F] LfcinB (20–30)2 a depolarization of 21.9%, which is an even higher value than that generated by ActD (16.2%). This outcome suggests that both peptides involve intrinsic apoptotic events within their type of cell death, which agrees with the results obtained in the Annexin V/7AAD assay, where it was observed that both peptides displace a significant percentage of the population towards the early and late apoptotic quadrants.
4 Discussion
Dimeric peptides LfcinB (20–30)2: (RRWQWRMKKLG)2-Ahx-K and 6[F] LfcinB (20–30)2: (RRWQWRFKKLG)2-Ahx-K, and monomeric peptides LfcinB (21–25)Pal: RWQWRWQWR and 5[A] LfcinB (21–25)Pal: RWQWAWQWR, were obtained as a white powder and were soluble in water. Peptide purities were >90% and presented the expected molecular mass (supplementary material) in all cases.
The peptides evaluated were more cytotoxic against CaCo-2 cells to a greater extent than against HEK-293, so the morphological changes induced by peptide treatment in cancer cells were more visible in them. It has been suggested that the selective cytotoxic effect by cancer cells of cationic peptides such as LfcinB is mediated by electrostatic interaction of the positive side chains of the peptide with negatively charged molecules on the cell surface. It has been established that phosphatidylserine is transferred to the outer leaflet of the plasma membrane of cells undergoing apoptosis, whereas in normal cells it is found on the inner leaflet of the cytoplasmic membrane. Other factors that may contribute to the increased negative charge on the surface of cancer cells is the presence of heparin sulphate and O-glycosylated mucins. While normal cells do not have a high density of negative charge on their surface, this is one of the main factors for the selectivity of these peptides for cancer cells. After the approach of the peptide to the cell surface, the indole rings of the Trp side chains interact with the lipid bilayer causing its disruption leading to cell lysis and/or internalization of the peptide in search of targets at the level of ribosomes or the nucleus (Chan et al., 2006).
To improve the effect of short synthetic peptides against CacO-2 cells, some specific amino acid replacements were made in two peptides with known cytotoxic activity. The specific modification of the central amino acid, for both the dimeric and the palindromic peptides, markedly potentiated the cytotoxic effect against CaCo-2 cells (Fig. 1, Table 1). The dimeric peptide 26[F] LfcinB (20–30)2 showed the greatest cytotoxic effect on both cell lines, the percentage of cell viability of HCT-116 cells was close to zero at 200 μg/mL (Fig. 3B). This result agrees with previous results that showed that polyvalent peptides had higher anticancer activity than monomeric peptides, suggesting that the versatility of the sequences increased anti-cancer activity. It has been suggested that the electrostatic interaction and membrane disruption caused by LfcinB requires peptide aggregation (Chan et al., 2006).
The peptides 26[F] LfcinB (20–30)2 and LfcinB (21–25)Pal had the greatest and lowest cytotoxic effect against Caco-2 cells, respectively (Table 1). These peptides also exerted a cytotoxic effect against the HCT-116 colorectal adenocarcinoma cell line (Fig. 3). The cytotoxic effect was dependent on peptide concentration and the maximum effect was observed when the cells were treated with peptide at 200 μg/mL in all cases. Our results suggest that peptides have selective cytotoxic effect against these colorectal adenocarcinoma cell lines. Peptides induce morphological changes in HCT-116 cells, causing membrane damage, similar to that observed in Caco-2 cells. Our results are in accordance with previous studies; Habib et al. [11] reported that camel lactoferrin (KLF) inhibited the growth of HCT-116 cells in vitro and KLF inhibited DNA damage and exerted antioxidant activity through the elimination of free radicals. Cationic peptide KT2 (NGVQPKYKWWWWWWWW-NH2) inhibited Xenograft tumour growth from HCT116 cells in BLAB/c nu/nu nude athymic mice, suggesting that peptides containing positively charged residues and tryptophan exert anticancer activity against HCT-116 cells (Freiburghaus et al., 2012, 2009; Maraming et al., 2018). On the other hand, peptide 5[A] LfcinB (21–25)Pal showed the highest therapeutic index in all cases, indicating that this peptide was the most selective for cancer cells, suggesting that this peptide may be a promising molecule to be considered as a candidate for development colon cancer treatment.
The replacement of Met with Phe in the 26[F] LfcinB (20–30)2 peptide increased the hydrophobicity of the sequence. Note that the Phe, flanked by two polar amino acids, Arg and Lys, possibly contributed to the amphipaticity of the sequence. On the other hand, the substitution of Arg with Ala in LfcinB (21–25)Pal of the palindromic sequence increased the hydrophobicity the sequence but diminished the net charge and the amphipaticity. The Circular Dichroism (CD) spectra of the LfcinB (20–30)2, LfcinB (21–25)Pal and 5[A] LfcinB (21–25)Pal showed a random coil pattern indicating that peptides do not have secondary structural elements (Barragán-Cárdenas et al., 2020). This is in line with previous reports showing that the CD spectra of LfcinB, LfcinB (20–25), LfcinB (17–31), LfcinB (20–25)4; LfcinB (21–25)Pal; and LfcinB (20–25)Cyc showed a random coil pattern (Huertas Méndez et al., 2017). The CD spectra of LfcinB (21–25)Pal and 5[A] LfcinB (21–25)Pal were similar indicating that the change of Arg to Ala in the sequence did not significantly affect the structure of the peptide(Barragán-Cárdenas et al., 2020).
The peptide LfcinB (21–25)Pal (RWQWRWQWR) has a net charge of +4 and has amphiphilic characteristics. Helical wheel projections (Figure S4, supplementary material) show that the peptide has a charged face formed by Arg residues and the opposite side containing the Gln residues, and these faces are flanked by two faces containing Trp residues. Peptide 5[A]LfcinB (21–25)Pal (RWQWAWQWR) has a net charge of +3 which decreases amphipaticity and increases hydrophobicity. The Ala is in the middle of the sequence, so it does not affect palindromic behaviour. The helical wheel projections show that the Arg residues are at the ends of the sequence and the charged face has a charge of +2, while the opposite faces formed by Gln and the faces formed by Trp do not change, indicating that the substitution of Arg for Ala does not induce significant loss of hydrophilic properties, maintaining amphipathic characteristics. The greater anticancer activity of peptide 5[A]LfcinB (21–25)Pal is possibly related to the length of the Ala side chain, which is shorter than that of Arg, which would favour the interaction of the peptide with the cell surface. Structure-activity studies are required to corroborate this.
In previous reports it was suggested that the amphipaticity is related to the peptide-membrane interactions, which is accordance with the electrostatic interaction between the cationic peptides and the cell membrane. It has been established that phosphatidylserine is transferred to the outer leaflet of the plasma membrane of cells undergoing apoptosis, whereas in normal cells it is found on the inner leaflet of the cytoplasmic membrane. Other factors that may contribute to the increased negative charge on the surface of cancer cells is the presence of heparin sulphate and O-glycosylated mucins (Deslouches and Peter Di, 2017; Hoskin and Ramamoorthy, 2008). Like the proposed mechanism for the antibacterial activity of LfcinB, the interaction between the peptide and the cancer cell would be mediated by the electrostatic interaction between positively charged peptide residues and negatively charged molecules on the cell surface. The electrostatic interaction between the side chains of positively charged residues and the negatively charged molecules (LPS) on the bacterial surface allows the approximation of the peptide allowing the interaction of the side chain of the Trp residues with the lipid bilayer causing its disruption that leads to cell lysis and/or internalization of the peptide (Chan et al., 2006; Farnaud and Evans, 2003).
The first of them was the palindromic peptide LfcinB (21–25)Pal; its apoptotic effect was proven against breast cancer cell lines, MCF-7, MDA-MB-123, and MDA-MB-468 with IC50 between 66 and 135 µM (Barragán‐Cárdenas et al., 2020). Now we can confirm that this peptide also induces cell cytotoxicity against colorectal adenocarcinoma cell line CaCo-2, with an IC50 in the same micromolar range, 101 µM. These results are interesting, because the palindromic arrangement of the minimum motif of LfcinB has a smaller IC50 than that obtained synthetic LfcinB against the colorectal adenocarcinoma cell line HT-29 (148 µM) (Fadnes et al., 2009), which provides a tremendous synthetic advantage, since its length is reduced by two thirds with respect to LfcinB. This palindromic peptide did not show a significant reduction in the cell viability of young fibroblasts and kidney embryo cells HEK-293. At its maximum concentration of 134 µM, the viability was reduced by only 29%, which agrees with the selectivity results previously reported (Barragán‐Cárdenas et al., 2020).
Since it has been established that charged residues in a peptide are necessary for its activity (Anbanandam et al., 2008), this peptide was evaluated trough an alanine scan on breast cancer cells, showing that the replacement of the central Arg with Ala has a major consequence for the activity, reducing its cytotoxic effect (Barragán-Cárdenas et al., 2020). Contrary to this, the 5[A] LfcinB (21–25)Pal peptide with this specific modification in CaCo-2 cells exerts greater cytotoxic activity with an IC50 of 43 µM, less than half of that calculated for the unmodified palindromic peptide. These results match those where a small peptide, LfcinB (14–31), was modified with multiple amino acid replacements, including Arg with Ala, and were tested against the colorectal adenocarcinoma cell line HT-29, decreasing the IC50 from > 404 µM to a range between 52 and 248 µM(Yang et al., 2002). In terms of selectivity, this specific modification also had a positive outcome. While the viability of young fibroblasts was unaffected, the cytotoxic effect on the HEK-293 cells was diminished, generating an amazing window of selectivity at IC50.
With respect to the form of death induced by the palindromic peptide on CaCo-2 cells, it was fairly like observed in the MCF-7 cells, since in both cell types most of the events belong to the apoptotic process, either early or late (Barragán‐Cárdenas et al., 2020). On comparing this effect to that exerted by the 5[A] LfcinB (21–25)Pal peptide, the apoptotic population remains the same, but the necrotic population doubles and leads to a stronger cytotoxic effect.
On the other hand, we considered the dimeric peptide LfcinB (20–30)2. It was tested on the MCF-7 cell line at the same concentrations here evaluated, and it did not induce any reduction in cell viability, but against HTB-132, another breast cancer cell line, it exerted a cytotoxic effect, with an IC50 of 30 µM (Insuasty-Cepeda et al., 2020). Our results confirm that this peptide has a broad spectrum of activity, since a result similar to the latter one was observed in adenocarcinoma cell line CaCo-2, with an IC50 of 38 µM, mainly related to necrotic events and in a lesser way to early and late apoptotic events. Also, these results support the finding that polyvalent peptides in their dimeric form have a greater effect than in the linear one, since a peptide containing the same sequence plus 4 amino acids LfcinB (17–31) did not exert any effect on the colorectal adenocarcinoma cell line HT-29(Eliassen et al., 2003). LfcinB (20–30) causes prolongation of the S-phase in Caco-2 cells, reducing cell proliferation and prolonged treatment with LfcinB (20–30) causes decreased DNA chain breaks in Caco-2 cells irradiated with UV light (Freiburghaus et al., 2012, 2009).
Synthetically, this peptide is a challenging one, due to its Met residue located in the 26th position. This residue is easily oxidized to methionine sulfoxide, and its activity could be in jeopardy. To overcome this side effect, specific modifications were made through SPPS and evaluated on the MCF-7 cell line, showing that its replacement with Phe caused a stronger cytotoxic effect (Insuasty-Cepeda et al., 2020). To corroborate this, the same specific modification was evaluated on CaCo-2 cells, showing that the 26[F] LfcinB (20–30)2 peptide diminished the IC50 to 18 µM, creating a therapeutic window between concentrations of 100 to 200 µg/mL due to its selectivity. This increase in activity could be caused by the increase in the amphipaticity of the peptide since this position is flanked by the charged amino acids 25Arg and 27Lys. Even though this specific modification did not change the proportion of necrotic cells, there was a significant decrease in living cells, which was accompanied by an increase in late apoptotic events. This result is very interesting, since it shows that the modified dimeric peptide could be a broad-spectrum peptide capable of affecting different types of cancer, so it could contribute to the development of a multifunctional cancer drug in the future.
We found quite promising results against the CaCo-2 cell line, because both peptides involve phenomena of necrosis and early and late apoptosis, which suggests that these peptides could simultaneously have different mechanisms of action. Additionally, the results with the cationic fluorophore JC-1 showed that both peptides involve intrinsic apoptosis events in the type of cell death they generate, since they significantly depolarize the mitochondrial membrane.
5 Conclusions
In summary, we obtained two promising peptides 26[F] LfcinB (20–30)2 and 5[A] LfcinB (21–25)Pal for future in vivo and ex vivo trials to search for new and more selective colon cancer therapeutics with fewer side effects. Peptides are selective against the CaCo-2 cell line, since viabilities greater or near 50% are found at the maximum concentration evaluated for the normal HEK-293 cell line and primary fibroblasts. Peptides 26[F] LfcinB (20–30)2 and LfcinB (21–25)Pal had cytotoxic effect against Caco-2 and HCT-116 cell line, and the 26[F] LfcinB (20–30)2 had the highest cytotoxic effect in both cell lines. The dimeric modified peptide 26[F] LfcinB (20–30)2 has a lower IC50 value than the palindromic 5[A] LfcinB (21–25)Pal; however, the palindromic peptide can be considered as promising because it is more selective, presents a wider therapeutic window and is easier and cheaper to obtain at the synthetic level compared to the dimeric one, since it has fewer amino acids and is a linear sequence.
Funding
This research was funded by MINCIENCIAS grant number Code 66986, RC 845-2019.Project: “OBTENCIÓN DE UN PROTOTIPO PEPTÍDICO PROMISORIO PARA EL DESARROLLO DE UN ME-DICAMENTO DE AMPLIO ESPECTRO PARA EL TRATAMIENTO DEL CÁNCER DE COLON, CUELLO UTERINO Y PRÓSTATA”.
CRediT authorship contribution statement
Andrea Carolina Barragán-Cárdenas: Investigation, Formal analysis, Visualization, Writing – original draft, Writing – review & editing. Diego Sebastián Insuasty-Cepeda: Investigation, Formal analysis, Visualization, Writing – original draft, Writing – review & editing. Karen Johanna Cárdenas-Martínez: Validation, Writing – review & editing. Joel López-Meza: Conceptualization, Methodology, Supervision. Alejandra Ochoa-Zarzosa: Conceptualization, Methodology, Supervision. Adriana Umaña-Pérez: Methodology, Supervision, Validation. Zuly Jenny Rivera-Monroy: Conceptualization, Methodology, Supervision, Funding acquisition, Writing – review & editing. Javier Eduardo García-Castañeda: Conceptualization, Methodology, Supervision, Funding acquisition, Writing – original draft, Writing – review & editing.
Acknowledgements
The authors are most grateful to MINCIENCIAS for the financial support and to the doctoral program of Chemistry Department, and Universidad Nacional de Colombia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Molecular basis for proline- and arginine-rich peptide inhibition of proteasome. J. Mol. Biol.. 2008;384:219-227.
- [CrossRef] [Google Scholar]
- Selective cytotoxic effect against the MDA-MB-468 breast cancer cell line of the antibacterial palindromic peptide derived from bovine lactoferricin. RSC Adv.. 2020;10:17593-17601.
- [CrossRef] [Google Scholar]
- The Nonapeptide RWQWRWQWR: A Promising Molecule for Breast Cancer Therapy. ChemistrySelect. 2020;5:9691-9700.
- [CrossRef] [Google Scholar]
- Tumor cell membrane-targeting cationic antimicrobial peptides: novel insights into mechanisms of action and therapeutic prospects. Cell. Mol. Life Sci.. 2017;74:3809-3825.
- [CrossRef] [Google Scholar]
- Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin.. 2018;68:394-424.
- [CrossRef] [Google Scholar]
- Tryptophan- and arginine-rich antimicrobial peptides: Structures and mechanisms of action. Biophys. Acta - Biomembr Biochim. 2006
- [CrossRef] [Google Scholar]
- Lactoferrin in the Prevention and Treatment of Intestinal Inflammatory Pathologies Associated with Colorectal Cancer Development. Cancers (Basel).. 2020;12:3806.
- [CrossRef] [Google Scholar]
- Lactoferrin’s anti-cancer properties: Safety, selectivity, and wide range of action. Biomolecules 2020
- [CrossRef] [Google Scholar]
- Anticancer alpha-helical peptides and structure/function relationships underpinning their interactions with tumour cell membranes. Curr. Protein Pept. Sci.. 2006;7:487-499.
- [CrossRef] [Google Scholar]
- Antimicrobial peptides with selective antitumor mechanisms: prospect for anticancer applications. Oncotarget. 2017;8:46635.
- [CrossRef] [Google Scholar]
- Enhanced antitumour activity of 15-residue bovine lactoferricin derivatives containing bulky aromatic amino acids and lipophilic N-terminal modifications. J. Pept. Sci.. 2003;9:510-517.
- [CrossRef] [Google Scholar]
- The anticancer activity of lytic peptides is inhibited by heparan sulfate on the surface of the tumor cells. BMC Cancer. 2009;9:1-13.
- [CrossRef] [Google Scholar]
- Lactoferrin - A multifunctional protein with antimicrobial properties. Mol. Immunol. 2003
- [CrossRef] [Google Scholar]
- Peptides with dual antimicrobial and anticancer activities. Front. Chem.. 2017;5:1-9.
- [CrossRef] [Google Scholar]
- Lactoferricin treatment decreases the rate of cell proliferation of a human colon cancer cell line. J. Dairy Sci.. 2009;92:2477-2484.
- [CrossRef] [Google Scholar]
- Reduction of ultraviolet light-induced DNA damage in human colon cancer cells treated with a lactoferrin-derived peptide. J. Dairy Sci.. 2012;95:5552-5560.
- [CrossRef] [Google Scholar]
- Lactoferrin a multiple bioactive protein: an overview. Biochim. Biophys. Acta. 2012;1820:226-236.
- [CrossRef] [Google Scholar]
- Morphological and molecular characteristics of spheroid formation in HT-29 and Caco-2 colorectal cancer cell lines. Cancer Cell Int.. 2021;21
- [CrossRef] [Google Scholar]
- The tetrameric peptide LfcinB (20–25)4 derived from bovine lactoferricin induces apoptosis in the MCF-7 breast cancer cell line. RSC Adv.. 2019;9:20497-20504.
- [CrossRef] [Google Scholar]
- Camel milk lactoferrin reduces the proliferation of colorectal cancer cells and exerts antioxidant and DNA damage inhibitory activities. Food Chem.. 2013;141:148-152.
- [CrossRef] [Google Scholar]
- Studies on anticancer activities of antimicrobial peptides. Biochim. Biophys. Acta - Biomembr.. 2008;1778:357-375.
- [CrossRef] [Google Scholar]
- Synthetic Peptides Derived from Bovine Lactoferricin Exhibit Antimicrobial Activity against E. coli ATCC 11775, S. maltophilia ATCC 13636 and S. enteritidis ATCC 13076. Molecules. 2017;22:1-10.
- [CrossRef] [Google Scholar]
- Peptides Derived from (RRWQWRMKKLG)2-K-Ahx Induce Selective Cellular Death in Breast Cancer Cell Lines through Apoptotic Pathway. Int. J. Mol. Sci.. 2020;21:1-13.
- [CrossRef] [Google Scholar]
- Synthetic Peptide Purification via Solid-Phase Extraction with Gradient Elution: A Simple, Economical, Fast, and Efficient Methodology. Molecules. 2019;24
- [CrossRef] [Google Scholar]
- Anti herpes simplex virus activity of lactoferrin/lactoferricin – an example of antiviral activity of antimicrobial protein/peptide. Cell. Mol. Life Sci.. 2005;62:3002-3013.
- [CrossRef] [Google Scholar]
- Synchronization of mammalian cell cultures by serum deprivation. In: Methods in Molecular Biology. Humana Press Inc.; 2016. p. :97-105.
- [CrossRef] [Google Scholar]
- Antibacterial activity of synthetic peptides derived from lactoferricin against Escherichia coli ATCC 25922 and Enterococcus faecalis ATCC 29212. Biomed Res. Int.. 2015;2015
- [CrossRef] [Google Scholar]
- Identification of extracellular matrix proteins secreted by human dermal fibroblasts cultured in 3D electrospun scaffolds. Sci. Reports. 2021;111(11):1-18.
- [CrossRef] [Google Scholar]
- Antitumor Ability of KT2 Peptide Derived from Leukocyte Peptide of Crocodile Against Human HCT116 Colon Cancer Xenografts. In Vivo. 2018;32:1137-1144.
- [CrossRef] [Google Scholar]
- Bovine lactoferricin B induces apoptosis of human gastric cancer cell line AGS by inhibition of autophagy at a late stage. J. Dairy Sci.. 2013;96:7511-7520.
- [CrossRef] [Google Scholar]
- Membrane-active host defense peptides - Challenges and perspectives for the development of novel anticancer drugs. Chem. Phys. Lipids. 2011;164:766-781.
- [CrossRef] [Google Scholar]
- Optimisation of the protocol for the liVE/DEAD®BacLightTM bacterial viability kit for rapid determination of bacterial load. Front. Microbiol.. 2019;10:801.
- [CrossRef] [Google Scholar]
- Efficient Fmoc Group Removal Using Diluted 4-Methylpiperidine: An Alternative for a Less-Polluting SPPS-Fmoc/tBu Protocol. Int. J. Pept. Res. Ther.. 2020;26:585-587.
- [CrossRef] [Google Scholar]
- Peptides for diagnosis and treatment of colorectal cancer. Curr. Med. Chem.. 2014;21:2410-2416.
- [CrossRef] [Google Scholar]
- Designing and evaluation of MERS-CoV siRNAs in HEK-293 cell line. J. Infect. Public Health. 2021;14:238-243.
- [CrossRef] [Google Scholar]
- A tetrameric peptide derived from bovine lactoferricin as a potential therapeutic tool for oral squamous cell carcinoma: A preclinical model. PLoS ONE. 2017;12:e0174707
- [CrossRef] [Google Scholar]
- A Tetrameric Peptide Derived from Bovine Lactoferricin Exhibits Specific Cytotoxic Effects against Oral Squamous-Cell Carcinoma Cell Lines. Biomed Res. Int.. 2015;2015:1-13.
- [CrossRef] [Google Scholar]
- Anticancer bioactive peptides suppress human colorectal tumor cell growth and induce apoptosis via modulating the PARP-p53-Mcl-1 signaling pathway. Acta Pharmacol. Sin.. 2015;36:1514.
- [CrossRef] [Google Scholar]
- Synergistic bactericide and antibiotic effects of dimeric, tetrameric, or palindromic peptides containing the RWQWR motif against Gram-positive and Gram-negative strains. RSC Adv.. 2019;9:7239-7245.
- [CrossRef] [Google Scholar]
- Antibacterial Synthetic Peptides Derived from Bovine Lactoferricin Exhibit Cytotoxic Effect against MDA-MB-468 and MDA-MB-231 Breast Cancer Cell Lines. Molecules. 2017;22:1-11.
- [CrossRef] [Google Scholar]
- Efficient synthesis of peptides with 4-methylpiperidine as Fmoc removal reagent by solid phase synthesis. J. Mex. Chem. Soc.. 2014;58:386-392.
- [Google Scholar]
- Antibacterial effects of lactoferricin B. Scand. J. Infect. Dis.. 1999;31:179-184.
- [CrossRef] [Google Scholar]
- W., B., K., Y., H., W., M., Takase, N., T., S., S., M., Tomita, 1994. Antifungal properties of lactoferricin B, a peptide derived from the N-terminal region of bovine lactoferrin. Lett. Appl. Microbiol. https://doi.org/10.3/JQUERY-UI.JS.
- Enhanced antitumor activity and selectivity of lactoferrin-derived peptides. J. Pept. Res.. 2002;60:187-197.
- [CrossRef] [Google Scholar]
- Short-term 3D culture systems of various complexity for treatment optimization of colorectal carcinoma. Sci. Reports. 2019;91(9):1-14.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.103998.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







