Translate this page into:
Magnetically separable ZnO/ZnFe2O4 and ZnO/CoFe2O4 photocatalysts supported onto nitrogen doped graphene for photocatalytic degradation of toxic dyes
⁎Corresponding author at: School of Chemistry, Faculty of Basic Sciences, Shoolini University, Solan, Himachal Pradesh 173212, India. pardeepchem@gmail.com (Pardeep Singh)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Advanced oxidation processes (AOPs) counting heterogeneous photocatalysis has confirmed as one of the preeminent method for waste water remediation. In the present work, we have successfully fabricated novel visible-light-driven nitrogen-doped graphene (NG) supported magnetic ZnO/ZnFe2O4 (ZnO/ZF/NG) and ZnO/CoFe2O4 (ZnO/CF/NG) nanocomposites. ZnO synthesized via direct precipitation method. Hydrothermal method was used for the preparation of nitrogen-doped graphene supported magnetic ZnO/ZF (ZnO/ZnFe2O4) and ZnO/CF (ZnO/CoFe2O4) nanocomposites. The procured materials were scrutinized by assorted characterizations to acquire information on their chemical composition, crystalline structure and photosensitive properties. The absorption and photocatalytic performance of photocatalysts were studied via UV–Visible spectra. Photodegradation performance of the synthesized nanocomposites was estimated toward mineralization of methyl orange (MO) and malachite green (MG) dyes in aqueous solution. The high surface area of ZnO/ZF/NG and ZnO/CF/NG was suitable for adsorptive removal of MO and MG dyes. The photodegradation performance of heterojunction photocatalysts was superior to bare photocatalyst in 140 min under visible-light irradiation. Spectrophotometer, GC–MS (Gas chromatography–mass spectrometry) elucidation was carried out to expose the possible intermediates formed. Both ZnO/ZF/NG and ZnO/CF/NG were rapidly isolated from the aqueous phase by applying an external magnetic field in 20 sec and 2 min, respectively. The photocatalytic performance and stability of ZnO/ZF/NG and ZnO/CF/NG nanocomposites were confirmed by conducting 10 consecutive regeneration cycles. Owing to recyclability of ZnO/ZF/NG and ZnO/CF/NG, these heterogeneous nanocomposites might be used as cost-effective for treatment of discarded water. The observations endorse that the synthesized ternary heterogeneous nanocomposites facilitates wastewater decontamination using photocatalytic technology.
Keywords
ZnFe2O4
CoFe2O4
Nitrogen doped graphene
Supported photocatalysis Magnetic separation
Dye mineralization
Recycle efficiency
1 Introduction
Day by day, textiles and printing industries produced a high amount of unconsumed dyes that are settled into the waters. In response, innovation in technology has led to introduction of many toxic waste materials to the environment (Zarrabi et al., 2019; Shandilya et al., 2018a,b; Sudhaik et al., 2018a,b). The immense damage to the aquatic environment was generated by the presence of dyes and pigments in water (Rahman et al., 2016; Prado and Costa, 2009; Raizada et al., 2019; El-Daly et al., 2015). Many factors responsible for coloration of wastewaters are mainly toxicity, chemical oxygen demand (COD), bad smell, high biochemical oxygen demand (BOD), originated from the contaminants (Sudhaik et al., 2018a,b; Gautam et al., 2017; Rahman and Asiri, 2015). Nowadays, for elimination of organic dyes, from different marine environs physicochemical procedures like chemical oxidation, coagulation, membrane separation, photocatalysis, aerobic biological treatments, flocculation, filtration, ozone treatment, chemical oxidation, reverse osmosis, and adsorption have been used. Through these methods, fractional degradation of organic dye and production of a large volume of toxic sludge lead to disposal problem Hasija et al., 2019; Priya et al., 2016; Sharma et al., 2013). Thus for the complete degradation of an organic dye from wastewater, a more capable treatment method is needed (Valtchev et al., 2013; Jamwal et al., 2015; Hasnat et al., 2014).
As a solution to these problems, semiconductor photocatalysis has attracted much attention (Sharma et al., 2019). Underneath ambient conditions and illumination of sunlight, this process is a “green” technology for disintegrating water into hydrogen and oxygen, inactivating viruses and/or entirely excluding all kinds of contaminants (Raizada et al., 2017; Singh et al., 2020; Pare et al., 2009; Yuan et al., 2017; Gu et al., 2013; Rahman et al., 2018). Semiconductor photocatalysis can be fulfilled in the presence of light when band gap of a semiconductor is less than the photonic energy, the valence band electrons are excited to the empty conduction band leading to the creation of electrons-hole pairs. After this, superoxide and hydroxyl radicals are produced by reacting with electrons and holes reacting with oxygen and hydroxyl group, respectively, in water. Thus the reactive oxygen species OH•, •O2− helps the degrading of organic dye contaminants existing in water (Gupta et al., 2014; Raizada et al., 2017; Nasr et al., 2016; Singh et al., 2017a,b; Shandilya et al., 2018a,b; Rahman et al., 2017a,b; Yamamoto et al., 2013).
Spinel Ferrites have drawn huge attention because of its electrochemical performance, catalytic, excellent chemical stability, narrow band gap and easy availability. Among spinel ferrites, ZF and CF are two mostly utilized magnetic photocatalysts because of their low production expenses, good chemical stability, high coercivity and high Curie temperature (Akhter et al., 2017; Hegazy et al., 2019; P. Singh et al., 2017; R. Singh et al., 2017). For CF, the band gap and positioning of conduction band (CB) and valence band (VB) with respect to redox potential scale are 1.67 eV, 0.14 V and 1.90 V, respectively and 1.90 eV, −1.54 V and 0.38 V for ZF. Therefore, the researchers used the same as photocatalyst because of unreliable benefits of CF and ZF. It is like a challenge to get high photocatalytic performance by exploiting ferrite material alone, due to room temperature ferromagnetism makes magnetically gather these photocatalysts again and fast combination of photogenerated electron-hole pairs (Gautam et al., 2016; Wang et al., 2014; Hussain et al., 2017; Zhu et al., 2016; Jiang et al., 2014). An effective photocatalyst is not proven by the use of ferrite material alone. Thus, even when copper ferrite and zinc ferrite are used alone, photocatalytic degradation is less dominant than the adsorption process (Hong et al., 2016; Raizada et al., 2016; Singh et al., 2020; Chen et al., 2018). Numerous heterojunctions of CF have been reported such as CF/graphene (Gan et al., 2015), TiO2/CF (Li et al., 2012), and CF/CuS (Kamranifar et al., 2019) to improve its photodegradation activity. In current years, to construct ZF composites, many efforts have been employed for remarkable magnetic and optical properties, such as g-C3N4/ZF (Chen et al., 2016), ZnO/ZF (Guo et al., 2014) ZF/TiO2 (Zhang et al., 2012). A promising approach to boost photocatalytic activity for degrading organic pollutant has been clearly described by these results which point out the introduction of ZF photocatalyst (Dutta et al., 2019; Nada et al., 2017; Singh et al., 2017a,b; Guo et al., 2016).
Meanwhile survey on photocatalytic activity of ZnO explored that it is being used in research laboratory and manufacturing. Thus, Zinc oxide used as a mineral semiconductor because of its 3.30 eV direct band gap along with 60 meV of large exciting binding energy at room temperature. Under the ultraviolet irradiation, ZnO can be used as a photocatalyst effectively, because of its wide band gap. In spite of these outstanding advantages, large direct band gap (3.30 eV) of ZnO reports controlled response for major segment of the solar spectrum, while low degree of electron-hole pair separation, acidic/basic medium prompted corrosion and deactivation during the photocatalytic reactions hampers their extended utility (Rahman et al., 2017a,b; Kavitha and Kumar, 2019). Forming a ZnO heterojunction such as WO3/ZnO (Liu et al., 2014), TiO2/ZnO (Qin et al., 2019), BiOCl/ZnO (Chang et al., 2019) has been displayed the photocatalytic activity and stability of ZnO.
Improved photocatalytic activity and photocatalyst stability, graphene and its conformity are widely used for wastewater treatment. Graphene is a two-dimensional carbon nano-filler with one atom thick planner sheet of sp2 carbon atoms that are densely packed in hexagonal honeycomb crystal lattice. Graphene has high chemical stability, large theoretical surface area (2630 m2 g−1), mechanical flexibility (1060 GPa), and spacious delocalized π-bonds which increase its conductivity (3000 W m−1 K−1) and stability (Nasr et al., 2018; Shandilya et al., 2019; Xue et al., 2015). For enhancement in better performance of graphene, doping with a heteroatom, such as N or S increase the pseudo capacitance by deploying its chemical reactivity and electronic properties. NG possess strong chemical stability, rapid electron/ion transport network, large specific surface area and short ion diffusion length. Thus under visible light irradiation NG demonstrate superior photocatalytic activity and excellent adsorption capacity toward organic dyes (Jiang et al., 2017).
On the basis of above discussion it was observed that no one can deduce the recombination of ternary ZnO/ZF/NG and ZnO/CF/NG photocatalyst for better photocatalytic and magnetic recovery performance. The novelty of this work focuses on the evaluation of superior photocatalytic activity of ZnO/ZF/NG and ZnO/CF/NG ternary composite due to following reasons: (i) Band gap of ZF and CF (the band gaps of ZF (Eg = 1.90 eV) and CF (Eg = 1.67 eV) are at lower scale of eV vs Normal hydrogen electrode scale), not suitable for the production of hydroxyl radical and show low absorbance in visible region. Therefore for better photocatalytic activity, we have coupled ZF/CF with ZnO and NG to form ZnO/ZF/NG and ZnO/CF/NG ternary heterostructures, (ii) Magnetic behaviour of ZF and CF: owing to magnetic behaviour of ZF and CF, agglomeration rate of as-prepared ternary nano heterostructure was high. Therefore, ternary nanocomposites ZnO/ZF/NG and ZnO/CF/NG has strong magnetic property which can be easily utilized for separation of charge carriers in photodegradation system (iii) Role of NG: NG provides more surface area to facilitate the better enhancement in photocatalytic activity of loaded magnetic nanoparticle (ZF/CF) and ZnO.
Thus, in this work we have coupled ZnO/CF and ZnO/ZF with NG to improve their photodegradation ability. Malachite dye and Methylene orange dye were opted as target organic dyes to explore the photocatalytic performance of newly synthesized photocatalysts. The degradation kinetics, mechanistic view and recycling efficiency of ZnO/ZF/NG and ZnO/CF/NG were assessed. The influence of adsorption on photo-degradation process was also explored.
2 Experimental
2.1 Chemicals and reagents
Cobalt iron (III) oxide (CAS No. 12052-28-7, Purity 99%), Absolute ethanol (CAS No. 64-17-5, Purity 99.5%), Zinc nitrate (CAS No. 10196-18-6, Purity 98%), Potassium hydroxide (CAS No.1310-58-3, Purity 85%), Iron (III) chloride hexahydrate (CAS No. 10025-77-1, Purity 97%), Zinc chloride (CAS No. 7646-85-7, Purity 99.99%), Polyethylene glycol(PEG) (CAS No. 25322-68-3), Iron nitrate (CAS No. 7782-61-8, Purity 99%), Sodium hydroxide (CAS No. 1310-73-2, Purity 50% in H2O), Citric Acid (CAS No. 77-92-9), Urea (CAS No. 57-13-6), Methylene Orange (MO) (CAS No. 547-58-0, Purity 85%) and Malachite green (MG) (CAS No. 569-64-2) were purchased from Sigma Aldrich, and used devoid of any auxiliary purification.
2.2 Fabrication of ZnO nanoparticles
ZnO nanoparticles were prepared by direct precipitation method. Briefly, an aqueous solution of 0.2 M of zinc nitrate and 0.4 M of KOH were prepared with deionized water respectively. At room temperature, the KOH solution was gradually purred into zinc nitrate solution with constant stirring to form a white suspension. After centrifuging the obtained mixture at 5000 rpm for 20 min and it was washed three times with deionized water and at last with absolute alcohol. Furthermore, the acquired precipitates were calcined at 500 °C for 3 h on a custom made tubular muffle furnace to facilitate formation of ZnO.
2.3 Synthesis of ZF nanoparticles
ZF nanoparticles were prepared in our laboratory by dissolving 4.90 g zinc nitrate and 13.4 g iron nitrate in 50 mL of distilled water. The above solution was poured into aqueous solution of 4.2 g of NaOH in 70 mL distilled water and 3 mL ethylene diamine. Subsequently for achieving complete chelation, this mixed solution was heated at 90 °C for 1 h. Finally, the formed powder was put into an alumina crucible and calcined at 600 °C with heating rate of 10 °C/min for 1 h.
2.4 Synthesis of CF nanoparticles
CF nanoparticles were synthesized by auto combustion method. In brief 1.4 g of cobalt nitrate and 4.04 g of iron nitrate were dissolved in 100 mL deionized water with stoichiometric ratios of 1:2 ([Co]:[Fe]). Then, 0.032 g/mL of citric acid solution was added to the above aqueous mixture under constant magnetic stirring. The molar ratio of cobalt nitrate to citric acid was set to 1:1 for attaining complete combustion process. The mixed aqueous solution was adjusted to pH = 7 using ammonia. After that, the above solution was heated to be converted into a xerogel. After the heating process of died gel, a fluffy powder was formed. Finally as-burnt precursor powder was put into alumina crucible and calcined at 400 °C for 2 h.
2.5 Synthesis of NG nanoparticles
Graphite oxide (GO) was prepared by using modified Hummer’s process. NG was fabricated by using GO as raw material and urea as reducing dopant agent in hydrothermal process. In brief, 30 mL of GO and urea with a mass ratio of 1:30 were mixed in a beaker. This above solution was constantly stirred for 30 min. The resulting aqueous solution was transferred into a Teflon lined stainless steel autoclave, sealed and maintained at 160 °C for 3 h. Then, the temperature of autoclave was naturally low to room temperature and the as-synthesized NG was taking out with a tweezer. Afterwards, as-synthesized NG nanoparticles were dipped into distilled water and washed fully to remove any unreacted urea. For removing surface adsorbed water, NG nanoparticles were blotted with filter paper and freeze-dried under vacuum to get the final products. The final product was named as NG nanoparticles.
2.6 Synthesis of ZnO/ZF/NG and ZnO/CF/NG nanocomposites
Firstly, 0.5 g of CF/ZF and 8.37 g of ZnCl2 were dispersed in 50 mL of PEG (polyethylene glycol, MW-400 g mol−1) followed by 20 min ultrasonication. Well dispersed nanoparticles of CF/ZF and ZnCl2 in PEG solution were obtained due to ultrasonication. The obtained solution was poured into Teflon lined container that had been pre-heated at 150 °C kept in an oven. In another beaker, 0.5 g of NG was dispersed in 20 mL absolute ethanol and then sonicated for 45 min. After this, the above two solutions were mixed followed by dropwise addition of NH4OH with continuous stirring of 6 h. The solution pH was maintained 10.0 utilizing NaOH solution (6 M) followed by addition of 1 g of urea. After this, centrifugation of Reaction mixture was done for 15 min at 14,000 rpm at ambient temperature. The supernatant liquid was decanted off from resultant precipitates. The resultant precipitates were repeatedly washed with deionized water and at last with ethanol. The calcination of the resultant precipitates was performed on alumina crucible at 500 °C for 2 h in a custom made tubular muffle furnace to ease the development of one dimensional ZnO/CF/NG or ZnO/ZF/NG nanostructure. The resultant product was named as ZnO/CF/NG or ZnO/ZF/NG.
2.7 Characterization
The crystalline nature of the products was assessed at room temperature by XRD model Siemens D-5000 diffractometer with Cu Kα radiation (l = 1.5406 Å). Surface morphology was observed by scanning electron microscopy (SEM, Model Nava Nano SEM-45 (USA) system). HRTEM imaging analysis was conducted using Tecnai G2 F20 high resolution transmission electron microscope operated at 200 kV accelerating voltage under vacuum conditions. The functional groups were investigated by Fourier transform infrared (FTIR) spectroscopy (Perkin-Elmer Spectrophotometer Spectrum One) was performed the range of 4000–450 cm. The X-ray photoelectron spectra; (XPS) studies were executed with a photoelectron spectrometer (Thermol scientific Escalab 250Xi) exploiting Al Kα radiation for analyzing the surface components and their valence distributions. The UV–vis diffused reflectance spectra were obtained on UV–vis spectrophotometer (Shimadzu, UV-3600). The magnetic properties of synthesized nanocomposites were measured by a magnetic measurement instrument (MPMS-XL-7, Quantum Design).
2.8 HPLC and LCMS analysis
The degradation fragments during mineralization process were analyzed by high performance liquid chromatography (Water HPLC, Austria) equipped with manual injector and photodiode array detector model. The apparatus was packed with Rheodyne manual injector kit and C18 column (5 μm, 25 cm length and 7 mm diameter). The LC-MS analyses were carried out on JEOL GCMATE II GC–MS with a high resolution data system, double focusing instrument with electrospray ionization (ESI) and C18 column (150 mm × 2 mm) (injection volume 20 μL).
2.9 Evaluation of photocatalytic activity
MO and MG dyes were opted as the target pollutants to examine the photodegradation efficiency of as-synthesized ZnO/ZF/NG and ZnO/CF/NG. The experiments were executed in a modest photocatalytic reactor exploiting a 10 W LED lamp (400 nm < wavelength < 700 nm) as the source of visible light. All degradation experiments were performed under atmospheric conditions with a constant stirring speed of 250 rpm. In the case of MO degradation experiments, 50 mg of photocatalyst was purred into 150 mL of an aqueous MO solution (10 mg/L). The above solution was magnetically agitated in darkness for 30 min to attain adsorption and desorption equilibrium. After this, the dispersion solution was sonicated for 10 min. Then the catalyst containing MO solution was placed under UV irradiation with stirring facility. Throughout the degradation process, 5 mL of solution was removed from mixture and centrifuged it for 15 min in 3500 rpm. Lastly, the supernatant liquid was obtained and the concentration of the dye was determined by UV–vis spectrophotometer. The photocatalytic performance of catalyst was calculated by using following equation (Singh et al., 2013).
3 Results and discussion
3.1 Characterization of ZnO/ZF/NG and ZnO/CF/NG nanocomposites
3.1.1 Xrd
X-ray diffraction analysis was conducted for the determination of crystal structure and phase purity of the produced samples. The XRD pattern of pure CF, pure ZF, NG, ZnO/ZF/NG and ZnO/CF/NG nanostructures were illustrated in Fig. 1. The crystal plane of pure CF (JCPDS No. 22-1086) with diffraction peaks at 2θ = 30.1°, 35.6°, 43.2°, 57.0° and 62.7° represented (2 2 0), (3 1 1), (4 0 0), (5 1 1) and (4 4 0), respectively (Borgohain et al., 2012). For the planes of pure ZF (JCPDS No. 22-1012), noticeably, all diffraction peak at 2θ = 35.3° and 62.2° were observed which respectively matched exactly with the crystallographic planes of (3 1 1) and (4 4 0); (Huang et al., 2015). The pure nitrogen-doped graphene showed intense peak at 2θ = 26.3° which corresponds to the (0 0 2) crystallographic planes. This diffraction peak confirms that nitrogen atoms have come into the crystal lattice of graphite which produced the increased distance between the graphite layers (Yao et al., 2017). In case of ZnO/ZF/NG nanocomposites, all diffraction peaks correspond to hexagonal phase of zinc oxide (peaks at 2θ = 31.8° and 62.8° can be indexed to the planes of (1 0 0) and (1 0 3) respectively), nitrogen-doped graphene (NG) (peak at 2θ = 26.3° of plane (0 0 2)) and crystal plane of pure ZF (JCPDS No. 22-1012) (peaks at 2θ = 35.3° and 62.2° represented (3 1 1) and (4 4 0) planes) (Sathishkumar et al., 2013). The XRD diffraction pattern of ZnO/CF/NG nanocatalysts showed peaks almost matching with respect to structure of ZnO (peak at 2θ = 56.6° corresponds to (1 1 0) plane), matches with CF (peaks at 2θ = 30.1°, 35.6°, 43.2° and 62.7° corresponds to (2 2 0), (3 1 1), (4 0 0) and (4 4 0) planes) and matches with only one peak of NG nanoparticles (peak at 2θ = 26.3° corresponds to (0 0 2) plane) (Singh et al., 2017a,b). Particle size of nanocomposites were also calculated by Scherrer equation:
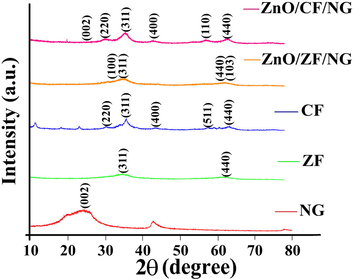
XRD patterns of NG, ZF, CF, ZnO/ZF/NG and ZnO/CF/NG nanocomposites.
3.1.2 TEM and SEM analysis
TEM analysis of NG, ZnO/CF/NG and ZnO/ZF/NG was depicted in Fig. 2(a–f). The curled sheet like structure of NG could be seen in Fig. 2(a). The TEM image of ZnO/CF/NG nanoparticles also revealed that CF nanoparticles (black patches) and ZnO particles (gray patch) have gathered on the surface of NG to form a core–shell like structure. As seen in Fig. 2 the dispersion of both ZF and ZnO was observed onto NG sheet. The d-spacing values were calculated from lattice plane fringes. The measured d-spacing of 0.296 nm resembled to the lattice spacing of (2 2 0) plane of CF. The fringe with d-spacing of 0.476 nm was corresponded to lattice spacing (1 1 1) of cubic nanostructured ZF whilst the lattice fringe of 0.268 nm assigned to the (0 0 2) plane of ZnO (Wang et al., 2017). Thus nanocomposites have a spherical morphology and average size particles.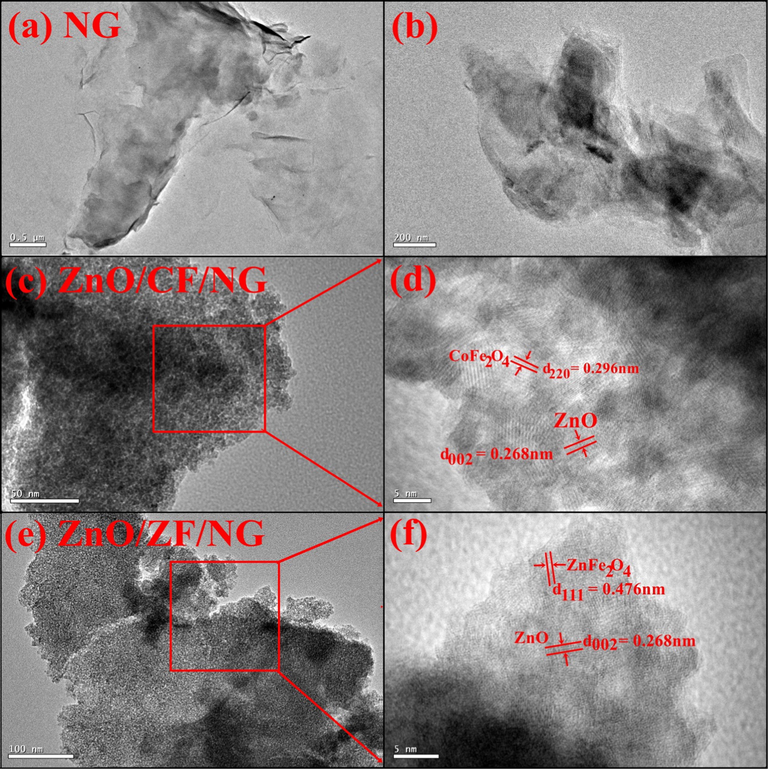
TEM images of (a) NG, (b) High resolution image of NG, (c) ZnO/CF/NG, (d) High resolution image of selected area (c), (e) ZnO/ZF/NG and (f) High resolution image of selected area (e).
The morphology of NG, ZnO/CF/NG and ZnO/ZF/NG was investigated by SEM. As shown in Fig. 3(a–b), NG nanosheet, were loosely loaded like fragments of foam. The loading of CF, ZF, and NG was clearly seen in ZnO/CF/NG and ZnO/ZF/NG. A core-shell structure was seen on the surface of NG by the deposition of CF in ZnO (Sathishkumar et al., 2013). It was also seen that some ZF nanosphere particles collapsed within NG stop the accumulating of particles. It was noted that some ZF nano spheres were compressed within NG and prevent of particles. It was also observed that CF or ZF, ZnO nanoparticles were dispersed on NG surface from Fig. 3(a–b). Also, FESEM image of NG, ZnO/CF/NG and ZnO/ZF/NG observed different shaped agglomerated in Fig. 3(b, d, and f).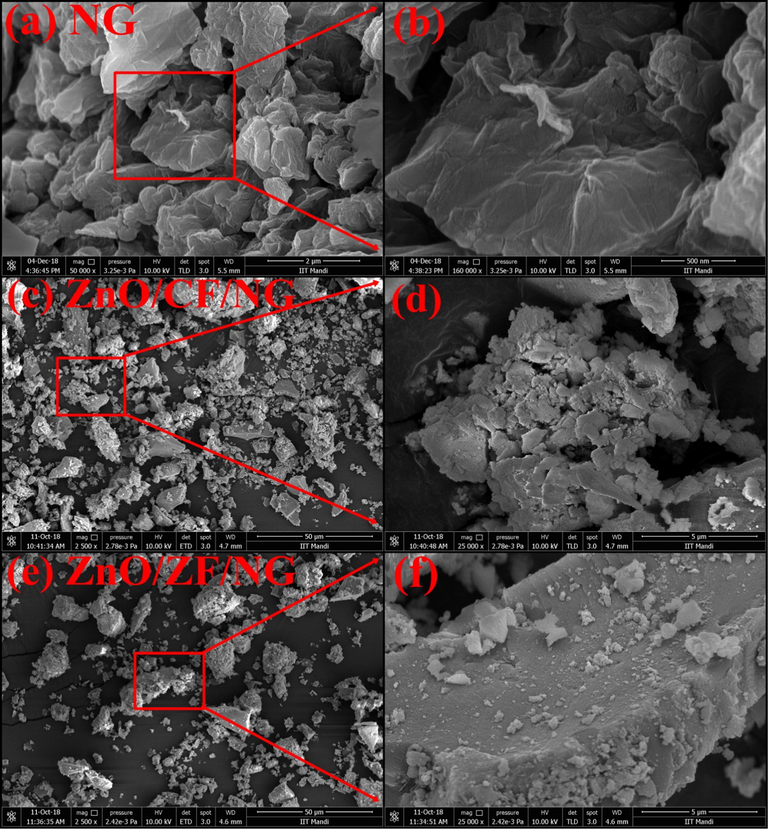
SEM and FESEM images of (a) NG, (b) High resolution image of selected area (a), (c) ZnO/CF/NG, (d) High resolution image of selected area (c), (e) ZnO/ZF/NG and (f) High resolution image of selected area (e).
3.1.3 FT-IR spectra
Fig. S1 displayed FT-IR spectra of ZnO/CF/NG, ZnO/ZF/NG, CF, ZF, and NG. FTIR spectrum of pristine NG depicted typical stretching bands of C⚌C at 1400 cm−1. The other peaks at 1180 cm−1 and 1565 cm−1 corresponded to C—N and C⚌C bond (Singh et al., 2017a,b). FTIR spectrum of CF revealed peaks in the range of 572–435 cm−1 which corresponded to Fe—O bonds (Kachi et al., 2019). Two characteristic peaks at 579 cm−1 and 1480 cm−1 which assigned to metal-oxygen vibration and —OH bending, respectively in CF. The peak observed below at 580 cm−1 ascribed to Zn—O stretching vibration (Zamiri et al., 2017). ZF had two peaks at 600–500 cm−1 and 3450 cm−1 which point out the metal-oxygen vibration and O—H stretching vibration, respectively. In the spectrum of CF/ZnO nanostructure, a wide peak was seen at 3400 cm−1 due to the strong O—H stretching band (Borgohain et al., 2012).
3.1.4 UV–visible analysis
The optical transmittance absorption properties of the as-prepared CF, ZF and ZnO are examined at room temperature by UV–vis spectroscopy. As shown in Fig. S2a absorption spectrum can be observed in UV–visible range from 200 to 800 nm for ZF, CF, and ZnO. In addition, using the absorption data, the band gap energy can be computed exploiting the Tauc’s equation, given below
3.1.5 XPS analysis
XPS analysis was conducted to explore the chemical components and oxidation states of carbon, oxygen, cobalt, iron, zinc, silver and nitrogen elements. Fig. 4(a–f) exhibits the whole spectra of ZnO/CF/NG and ZnO/ZF/NG nanocomposites. In the case of C1 s, the peaks positioned at 282 eV was assigned to sp2 (sp2C) carbons atom bonded to nitrogen in NG (Mou et al., 2011) (Fig. 4a). In Fig. 4b spectra of O 1s, the peak centred at 527 eV corresponds to lattice oxygen of ZnO, CF and ZF (Wang et al., 2017). In Fig. 4c the peaks at 778 eV and 793 eV were respectively assigned to Co 2p3/2 and Co 2p1/2. These two peaks were representative peaks of Co 2p3. As showed in Fig. 4d the peaks appeared at 708 eV and 722 eV respectively belonged to Fe 2p3/2 and Fe 2p1/2. Introduction of CF in complex was confirmed by the peaks of Co 2p3 and Fe 2p3 (Jing et al., 2016). In Zn 2p3 spectra, the peak at 1019 eV and 1042 eV were respectively assigned to binding energy of Zn 2p3/2 and Zn 2p1/2 (Wang et al., 2017) (Fig. 4e). Moreover, an N 1s, the peak at 397 eV was corresponding to the formation of N—C bonds in NG (Guo et al., 2013) (Fig. 4f).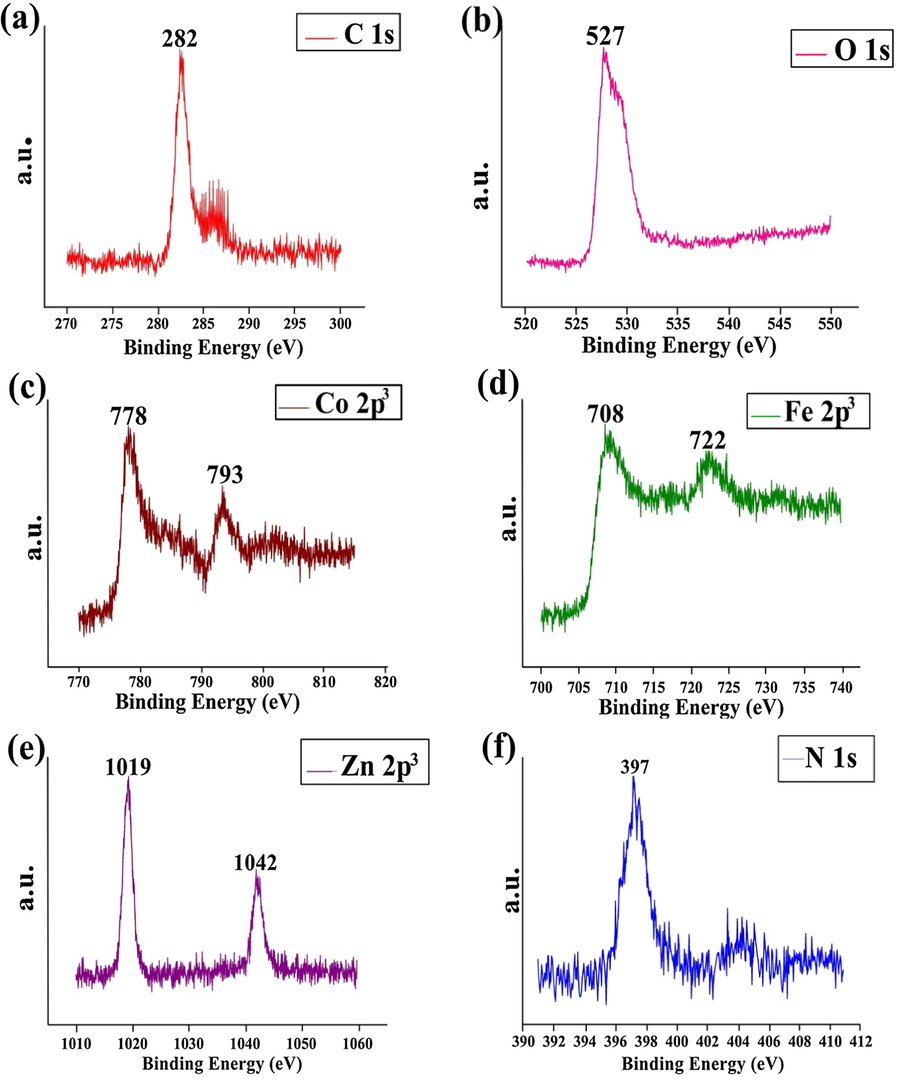
XPS spectra of ZnO/ZF/NG and ZnO/CF/NG showing presence of (a) C 1s, (b) O 1s, (c) Co 2p3, (d) Fe 2p3, (e) Zn 2p3 and (f) N 1s elements.
3.2 Photocatalytic and adsorption removal of MG and MO
The results of the photocatalytic activity of prepared photocatalysts were assessed by the photodegradation and adsorption of malachite green and methyl orange dyes under different conditions as shown in Fig. 5(a–f). Fig. 5(e and f) presents the results of MO and MG photodegradation under 100 W halogen lamp. Both ZnO/ZF/NG and ZnO/CF/NG show significant activity for MO and MG dye photodegradation. For MG photodegradation, following trend was obtained in the reaction time period of 140 min: ZnO/CF/NG (98%) > ZnO/ZF/NG (92%) > CF (50%) > ZnO (49%) > ZF (48%) > NG (30%). In case of MO degradation, removal efficiency had following order for 140 min of photocatalysis: ZnO/CF/NG (99%) > ZnO/ZF/NG (94%) > CF (52%) > ZnO (50%) > ZF (49%) > NG (30%). Ternary photocatalyst ZnO/CF/NG and ZnO/ZF/NG had dominated the photocatalysis. While no degradation in absence of photocatalysts confirmed the stability of MG and MO under visible light (Fig. 5a and b) Moreover, adsorption experiments were also executed to see the effect of adsorption of MG and MO removal. From adsorption experiment, it was concluded that ZnO/ZF/NG, ZnO/CF/NG and NG had significant adsorption ability for MG and MO removal while ZnO, CF, and ZF had removal efficiency below 10%. The integration of NG with ZnO/ZF and ZnO/CF had resulted in significant improvement in adsorption ability of photocatalysts (Fig. 5c and d). The pseudo first order and second order kinetics for adsorption rate (Lu et al., 2014; Singh et al., 2019a) is described by Eqs. (3) and (4):
![Removal of MG and MO under different reaction conditions: Photocatalytic degradation of MG (a), MO (b), removal of MO (c) and MG (d) using adsorption process, Effect of adsorption on photocatalytic removal of MG (e) and MO (f). Reaction conditions: [MO] = 1.5 × 10−5 mol dm−3; [MG] = 1 × 10−5 mol dm−3; [catalyst] = 50 mg/100 mL; pH = 4.0(MO); 6 (MG); reaction time = 70 min (MG) and 140 min (MO), Light Intensity = 750 lx.](/content/184/2020/13/2/img/10.1016_j.arabjc.2019.08.005-fig5.png)
Removal of MG and MO under different reaction conditions: Photocatalytic degradation of MG (a), MO (b), removal of MO (c) and MG (d) using adsorption process, Effect of adsorption on photocatalytic removal of MG (e) and MO (f). Reaction conditions: [MO] = 1.5 × 10−5 mol dm−3; [MG] = 1 × 10−5 mol dm−3; [catalyst] = 50 mg/100 mL; pH = 4.0(MO); 6 (MG); reaction time = 70 min (MG) and 140 min (MO), Light Intensity = 750 lx.
Pseudo first order kinetics
k1(min−1)
qe(mg/g)
R2
MG
MO
MG
MO
MG
MO
ZnO/CF/NG
0.0179 ± 0.0011
0.0171 ± 0.0010
64.00
65.12
0.91 ± 0.10
0.92 ± 0.11
ZnO/ZF/NG
0.0173 ± 0.0012
0.0174 ± 0.0013
62.56
66.00
0.93 ± 0.12
0.90 ± 0.13
NG
0.0171 ± 0.0014
0.0175 ± 0.0012
63.00
67.10
0.90 ± 0.13
0.91 ± 0.14
Pseudo second order kinetics
k2(g/(mg min)
qe(mg/g)
R2
MG
MO
MG
MO
MG
MO
ZnO/CF/NG
0.00025 ± 0.0013
0.00017 ± 0.0011
67.40
64.30
0.99 ± 0.11
0.96 ± 0.12
ZnO/ZF/NG
0.00015 ± 0.0010
0.00011 ± 0.0015
65.00
65.29
0.96 ± 0.14
0.97 ± 0.13
NG
0.00005 ± 0.0011
0.000078 ± 0.0014
66.00
66.00
0.97 ± 0.15
0.98 ± 0.12
Initial reaction pH plays a crucial role in both adsorption and photocatalysis of dyes onto adsorbent surface (Table 2). In present work, pH was varied from 2 to 9. The adsorption of MG was maximum at pH 7, while during MO adsorption, the removal rate was higher at pH 4. Moreover, zeta potential analysis was used to find the pH of zero point charge. The pHzpc values of ZnO/CF/NG, ZnO/ZF/NG and NG was found to be 6.7, 6.7, and 6.7. Above pHzpc, the adsorbent surface was negatively charged and facilitated the adsorption of cationic MG dye. On the other side, below pHzpc, positively charged surface of adsorbent facilitated the adsorption of anion MO dye.
pH
Removal efficiency of ZnO/CF/NG (%)
Removal efficiency of ZnO/ZF/NG (%)
Removal efficiency of NG (%)
MG
MO
MG
MO
MG
MO
4
15
33
12
34
16
31
6
25
25
20
25
22
30
7
34
17
34
18
30
28
8
36
10
37
10
32
25
9
36
10
37
9
32
22
3.3 Synergy between adsorption and photocatalysis and mineralization of dyes
It is well documented that photocatalytic activity of the photocatalysts is greatly impressed by the adsorption of organic contaminants onto photocatalysts surface (Shandilya et al., 2018a,b). Therefore, the photodegradation of MG and MB was subjected to two different reaction criteria: (i) adsorption followed by photocatalysis and (ii) concurrent adsorption with photocatalysis (A + P) process. From Fig. 5, it is obvious that A + P processes are more efficient for photocatalytic degradation of MO and MG dye. During A-P process, the excessive adsorption of dye molecules onto photocatalysts surface acted as screen for visible light and overall photoactivity was reduced; while in case of A + P, adsorbed dye molecules were concurrently photodegraded by photocatalysts under halogen lamp. Thus, it was concluded that ZnO/ZF/NG/A + P and ZnO/CF/NG/A + P were efficient for photocatalytic degradation of MG and MO degradation. Further studies were carried out with ZnO/ZF/NG and ZnO/CF/NG photocatalysts under simultaneous and adsorption. The degradation kinetics MG and MO was investigated using Eq. (6) (Raizada et al., 2014a,b).
The linearity of −ln(C/C0) versus time (t) plot describes pseudo first order kinetics controls degradation process. The rate constants, 0.034 and 0.032 min−1 was obtained for MG degradation using ZnO/ZF/NG (R2, 0.96) and ZnO/CF/NG (R2, 0.98) photocatalyst, respectively (Table 3); while ZnO/ZF/NG (R2, 0.97) and ZnO/CF/NG (R2, 0.98) had rate constants, of 0.26 and 0.29 min−1, respectively, for MO photodegradation (see Table 4). The dye degradation involves both decolorization and breaking of aromatic compounds into simpler inorganic moieties. To confirm the breaking of aromatic compounds, COD removal experiments were performed. 99% and 98% of COD was removed during MG mineralization process using ZnO/ZF/NG and ZnO/CF/NG respectively, in 140 min; while in the case of MO mineralization, ZnO/ZF/NG and ZnO/CF/NG had 99% and 98% of respective COD removal in 300 min (Fig. 6a and b).
Dye (Pollutants)
Photo-catalyst
R2
k (min−1)
MG
ZnO/ZF/NG
0.96
0.034
ZnO/CF/NG
0.98
0.032
MO
ZnO/ZF/NG
0.97
0.026
ZnO/CF/NG
0.98
0.029
S. no.
Composite
Targeted dye
Source of light
Photodegradation efficiency
Degradation time
References
1.
ZnO/CF
Direct Blue 71
150 W, Tungsten halogen lamp
100%
5 h
Sathishkumar et al., 2013
2.
CF/Graphene
Methylene Blue (MB)
Tungsten halogen lamp
100%
3 h
Gan et al., 2015
3.
CF/ZnO
Acid Violet, Acid Brown
UV–Visible
76%
63%100 min
Ferdosi et al., 2019
4.
CF/ZnO
Phenolphthalein
UV radiation
89%
1 h
Borgohain et al., 2012
5.
ZF/FeFe2O4/ZnO
Azo Textile Dye F3B
UV Radiation
90%
140 min
6.
Ag3PO4/ZF
MB, Rhodamine B (Rh B)
LED light
100%
60 min
7.
ZF/Graphene
Rh B, MB, MO
Visible light
100%
120 min
Lu et al., 2014
8.
Fe2O4/ZnO/ZF
Rh B, MO
Visible light
95.2%
52.3%60 min
9.
ZnO/ZF/NG
MG, MO
Halogen lamp
92%
94%
140 min
10.
ZnO/CF/NG
MG, MO
Halogen lamp
98%
99%
140 min
![(a) COD removal during MO mineralization, (b) COD removal during MG mineralization. Scavenging experiment during ZnO/ZF/NG assisted photocatalysis for (c) MG and (d) MO degradation. Scavenging experiment during ZnO/CF/NG assisted photocatalysis for (e) MG and (f) MO degradation. Reaction conditions: [MO] = 1.5 × 10−5 mol dm−3; [MG] = 1 × 10−5 mol dm−3; [catalyst] = 50 mg/100 mL; pH = 4.0(MO); 6 (MG); reaction time = 70 min (MG) and 140 min (MO), [IPA] = 1.0 × 10−5 mol dm−3, [BZQ] = 2.0 × 10−5 mol dm−3, [ Cr(VI)] = 1.0 × 10−5 mol dm−3; [AO] = 1.5 × 10−5 mol dm−3, Light Intensity = 750 lx.](/content/184/2020/13/2/img/10.1016_j.arabjc.2019.08.005-fig6.png)
(a) COD removal during MO mineralization, (b) COD removal during MG mineralization. Scavenging experiment during ZnO/ZF/NG assisted photocatalysis for (c) MG and (d) MO degradation. Scavenging experiment during ZnO/CF/NG assisted photocatalysis for (e) MG and (f) MO degradation. Reaction conditions: [MO] = 1.5 × 10−5 mol dm−3; [MG] = 1 × 10−5 mol dm−3; [catalyst] = 50 mg/100 mL; pH = 4.0(MO); 6 (MG); reaction time = 70 min (MG) and 140 min (MO), [IPA] = 1.0 × 10−5 mol dm−3, [BZQ] = 2.0 × 10−5 mol dm−3, [ Cr(VI)] = 1.0 × 10−5 mol dm−3; [AO] = 1.5 × 10−5 mol dm−3, Light Intensity = 750 lx.
3.4 Detection of reactive species and possible mechanism for photocatalytic degradation
As evidenced from previous work, photocatalytic degradation of dyes was mainly due to presence of reactive oxidative species like electrons (e−CB), holes (h+VB), hydroxyl radicals (OH• and superoxide radicals (O2•−). In present work, Cr(VI) ion, ammonium oxalate(AO), isopropyl alcohol (IPA), and benzoquinone (BZQ) were respectively used as trapping agents for e−CB, h+VB, OH•, and O2•− scavenger (He et al., 2014; Pare et al., 2008). During scavenging experiment for ZnO/ZF/NG, removal efficiency was decreased to 16% (MO dye) and 15% (MG dye) with addition of IPA and BZQ to the reaction solution. While in case of ZnO/CF/NG, 17% and 18% of removal efficiency was found for addition of IPA and BZQ scavengers, respectively (Fig. 6c and d). On the other hand, AO and Cr (VI) had no significant effect on photodegradation of MO and MG dyes under halogen lamp (Fig. 6c and d). The scavenging experiments confirmed the presence of OH• and O2•− as main reactive species in MO and MG photodegradation (Fig. 6e and f).
3.5 Possible photocatalytic mechanism of ZnO/ZF/NG and ZnO/CF/NG
3.5.1 Type II scheme approach for ZnO/ZF/NG
The detailed mechanistic route of photocatalytic processes was displayed in Fig. 7a. The oxidation potential of VB and reduction potential of CB in ZF were found to be +0.38 V and −1.54 V, respectively, whilst positions of VB maximum and CB minimum of ZnO were +2.7 and −0.5 V (Kachi et al., 2019; Wang et al., 2017). Because of the strong interfacial contact and positioning of CB on redox scale, photoexcited electrons from ZF were shifted to CB of ZnO. On the other hand, the holes from ZnO were moved towards the VB of ZF photocatalyst. Therefore, electron–hole recombination was avoided by the formation of heterojunction-type II. Graphene has already been reported to have a sink behavior for photogenerated electrons (Shandilya et al., 2019). However in this surface assisted photocatalytic mechanism, graphene acted as a surface supported for ZnO and ZF photocatalyst. Due to the negative reduction potential in CB of ZnO, photogenerated electrons reacted with molecular O2 to produce O2•− radicals. The photogenerated holes in VB of ZnO and O2•− radicals were lead to degradation of organic dye and mineralize it into degraded products. The improved photocatalytic performance can be explained by two main reasons as can be concluded from the observations. First, because of the lower band gap of the nanocomposite, higher numbers of photons are absorbed which can raise photocatalytic activity. Second, the interdependent influence of ZnO, NG and ZF semiconductors could prohibit electron–hole pair recombination by charge transfer process.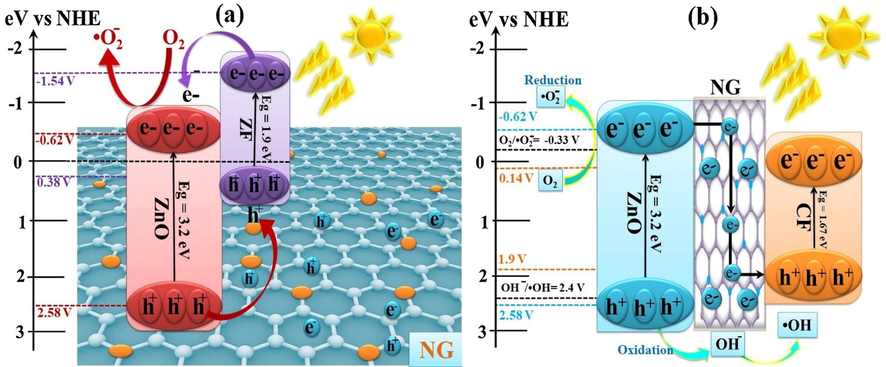
Mechanistic view of photocatalytic degradation of pollutants using (a) ZnO/ZF/NG and (b) ZnO/CF/NG nanocomposites.
3.5.2 Z-scheme approach for ZnO/CF/NG
The two semiconductors which having proper band gap energies assimilating to form heterostructure of Z-scheme type, which improve the separation efficiency of photogenerated charge carriers, and hereby enhance the photocatalytic activity of synthesized system. In Fig. 7b, a proposed schematic shows a possible photocatalytic mechanism of electron and hole formation and their reactions with water and oxygen. The positioning of CB and VB with respect to redox potential scale were 0.14 V and 1.9 V for cobalt ferrite (CF), whilst positions of CB minimum and VB maximum of ZnO were −0.62 V and 2.58 V (Ferdosi et al., 2019; Wang et al., 2017). As the CF has narrower band gap than ZnO and NG can act as ideal electron surface mediator between these two semiconductors due to its superpriorites in photochemical and conductivity properties (Shandilya et al., 2019; Singh et al., 2019b). The band potential of p-type semiconductor CF and n-type semiconductor ZnO ultimately satisfied the requirements of Z-scheme. Under visible light irradiation, transferring photoinduced electrons may happen from the conduction band of ZnO to the valence band of CF. On the other hand, the transferring hole happens from more anodic valence band of ZnO to the valence band of CF. Therefore, the recombination of electron-hole pairs can be hampered in the synthesized composite. Due to the inhibition of electro-hole pair recombination, the number of holes participating in response to oxidation increases. Subsequently, photogenerated electrons and holes can react with the O2 and OH− existence in water to generate superoxide radical anions (•O2−) and hydroxyl radicals (•OH), respectively, for the decomposition of organic dye.
3.6 Identification of degradation fragments during MG and MO mineralization process
In order to confirm formation, the decolourisation of dyes was established the UV–visible spectral with respect to time. The sharp decrease in absorption peaks was observed for both MO (465 nm) and MG (615 nm) with respect to time. The reduction in absorption peaks indicated the decolourisation on selected dyes (Fig. 8a and b).![UV–visible spectra of ZnO/ZF/NG showing degradation of (a) MG and (b) MO during degradation process using HPLC profile of (c) MG and (d) MO during mineralization process, GC–MS analysis of (e) MG after 70 min and (f) MO after 150 min. Reaction conditions: [MO] = 1.5 × 10−5 mol dm−3; [MG] = 1 × 10−5 mol dm−3; [catalyst] = 50 mg/100 mL; pH = 4.0(MO); 6 (MG); reaction time = 70 min (MG) and 140 min (MO), Light Intensity = 750 lx.](/content/184/2020/13/2/img/10.1016_j.arabjc.2019.08.005-fig8.png)
UV–visible spectra of ZnO/ZF/NG showing degradation of (a) MG and (b) MO during degradation process using HPLC profile of (c) MG and (d) MO during mineralization process, GC–MS analysis of (e) MG after 70 min and (f) MO after 150 min. Reaction conditions: [MO] = 1.5 × 10−5 mol dm−3; [MG] = 1 × 10−5 mol dm−3; [catalyst] = 50 mg/100 mL; pH = 4.0(MO); 6 (MG); reaction time = 70 min (MG) and 140 min (MO), Light Intensity = 750 lx.
Moreover, during HPLC analysis, peaks at retention time 8 min (MO) and 7 min (MG) decreased with an increase in reaction time. However, during initial degradation time, a bundle of peaks emerged between retention times 2–4 min and eventually dissipated after 240 min, it revealing the formation of reaction intermediate during degradation process (Fig. 8c and d). The GC–MS analysis was performed for MG and MO after 70 and 140 min of photocatalysis of selected dyes under visible light. For MG degradation, different fragments were observed at m/z ratios of 332 (A), 211 (B), 227 (C), 122 (D) and 131 (E). During degradation first step involves Cl− removal from MG to form fragment with m/z 316. The •OH radical attack resulted in the demethylation with formation of fragment A (m/z, 332). The attack of •OH radicals on central carbon atom and demethylation results in the formation of a fragment with m/z ratio 332 and 302. Moreover, the fragments B (m/z, 211), C (m/z, 227) and D (m/z, 172) were formed by demethylation, nitration and oxidation process. Lastly, these fragments were mineralized into CO2 and H2O. The obtained degradation fragments were also on accordance with previous work reported (Ghaedi et al., 2015, Ai et al., 2010). The GCMS analysis of degraded MO shows fragments F, G, H and I were observed with m/z 320, 306, 322 and 308, respectively. The fragment F was formed by the demethylation of MO dye (Fig. 8e and f). During photocatalysis, OH radical attack mainly caused the formation of fragments with m/z ratio, G, H and I (Yong et al., 2015). The results of GCMS and HPLC demonstrated that no toxic intermediates were identified during the photodegradation process, whereas the complete mineralization of organic dyes MG and MO in carbon dioxide (CO2) and water (H2O) was achieved (Le et al., 2016).
3.7 Magnetic separation and recyclability of ZnO/ZF/NG and ZnO/CF/NG
In addition to photocatalytic activity, recyclability and separation of photocatalyst are very crucial for heterogeneous photocatalysis. We measured the magnetization hysteresis loops at room temperature which are shown in Fig. 9a and c. ZnO/ZF/NG displayed superparamagnetic behavior and was isolated from the reaction solution in 20 s using an external magnetic field (Fig. 9b). The saturation magnetization (Ms) and were found to be 55 emu g−1. The coercivity (Hc) for ZnO/ZF/NG was zero (Fig. 9a). The magnetization hysteresis curve of ZnO/CF/NG reveals a typical ferromagnetic nature having saturation magnetization (Ms) value of about 34 emu g−1 and coercivity (Hc) value of about 12,580 Oe (Fig. 9c). ZnO/CF/NG was magnetically isolated from the solution phase in 2 min under external magnetic field (Fig. 9d). During recycle experiments, photocatalysts were magnetically separated and used for next consecutive cycle. Both ZnO/CF/NG and ZnO/ZF/NG showed significant recycling performance for ten catalytic cycles. After ten cycles, ZnO/ZF/NG had 79% and 80% of removal efficiency for MG and MO degradation, respectively. While 85 and 82% of MG and MO were photodegraded after ten cycles using ZnO/ZF/NG photocatalyst (Fig. 9e and f). The recycle experiments indicated significant stability and recyclability of ZnO/ZF/NG and ZnO/CF/NG photocatalyst for dye degradation. Furthermore, SEM, FTIR and XRD images did not show any significant morphological or chemical changes in ZnO/ZF/NG and ZnO/CF/NG photocatalyst after ten catalytic cycles (Fig. S4). It directed towards the stability of photocatalyst during degradation process.![Hysteresis curve and magnetic separation of ZnO/ZF/NG (a–b), Hysteresis curve and magnetic separation of ZnO/CF/NG (c–d), Recycle efficiency of ZnO/ZF/NG (e) and ZnO/CF/NG (f) for MG removal. Reaction conditions: [MO] = 1.5 × 10−5 mol dm−3; [MG] = 1 × 10−5 mol dm−3; [catalyst] = 50 mg/100 mL; pH = 4.0(MO); 6 (MG); reaction time = 70 min (MG) and 140 min (MO), Light Intensity = 750 lx.](/content/184/2020/13/2/img/10.1016_j.arabjc.2019.08.005-fig9.png)
Hysteresis curve and magnetic separation of ZnO/ZF/NG (a–b), Hysteresis curve and magnetic separation of ZnO/CF/NG (c–d), Recycle efficiency of ZnO/ZF/NG (e) and ZnO/CF/NG (f) for MG removal. Reaction conditions: [MO] = 1.5 × 10−5 mol dm−3; [MG] = 1 × 10−5 mol dm−3; [catalyst] = 50 mg/100 mL; pH = 4.0(MO); 6 (MG); reaction time = 70 min (MG) and 140 min (MO), Light Intensity = 750 lx.
4 Conclusion
In summary, ZnO/ZF/NG and ZnO/CF/NG nanocomposites with enhanced photocatalytic activity were successfully fabricated and characterized. Nitrogen-doped graphene acted as a sink material that eventually led to reduction in charge carrier recombination and increase in photodegradation activity of nanocomposites. ZnO/ZF/NG nanocomposite possessed simple type-II heterojunction whereas ZnO/CF/NG nanocomposite followed z-scheme path for dye degradation and ensued significant diminution in photogenerated electron-hole pair recombination. A proposed mechanistic view of the nanocomposites was given with the generation of radicals that clearly exposed the degradation process of dye. Furthermore, ZnO/ZF/NG and ZnO/CF/NG nanocomposites exhibited higher degradation ability in the exclusion of dyes by generating reactive species such as electrons, holes, superoxides and hydroxyl radicals. Hydroxyl radicals (OH•) and holes (h+VB) were the main reactive species responsible for the degradation of dyes. ZnO/ZF/NG and ZnO/CF/NG nanocomposites displayed 99% and 94% removal efficiency for MO degradation respectively, whereas 98% and 92% removal efficiency was observed for MG dye degradation. It was concluded from the results that photodegradation efficiency of ZnO/ZF/NG nanocomposite was higher than ZnO/CF/NG nanocomposite for MO and MG eradication. Both, ZnO/ZF/NG and ZnO/CF/NG nanocomposites were effortlessly recovered by magnet. On the basis of outcomes of this work, ZnO/ZF/NG and ZnO/CF/NG nanocomposites are expected to be promising as a magnetically mendable visible-light driven photocatalyst for dyes mineralization. Recycling experiments of these ternary heterogeneous photocatalyst in wastewater revealed that there was an negligible diminution in photodegradation ability in successive 10 cycles. Overall, this paper demonstrates systematic design of ZnO/ZF/NG and ZnO/CF/NG heterogeneous visible light driven photocatalyst possess realistic stability that enhances their superior photocatalytic performance, recyclability and applicability for wastewater mitigation.
References
- Activated carbon/CoFe2O4 composites: facile synthesis, magnetic performance and their potential application for the removal of malachite green from water. Chem. Eng.. 2010;J.156:243-249.
- [Google Scholar]
- Fabrication of hydrazine sensor based on silica-coated Fe2O3 magnetic nanoparticles prepared by a rapid microwave irradiation method. J. Alloy Compd.. 2017;698:921-929.
- [Google Scholar]
- A facile synthesis of nanocrystalline CoFe2O4 embedded one-dimensional ZnO hetero-structure and its use in photocatalysis. J. Mol. Catal. A-Chem.. 2012;363:495-500.
- [Google Scholar]
- Study on highly efficient BiOCl/ZnO pn heterojunction: Synthesis, characterization and visible-light-excited photocatalytic activity. J. Mol. Struct.. 2019;1183:209-216.
- [Google Scholar]
- Facile synthesis of highly efficient graphitic-C3N4/ZnFe2O4 heterostructures enhanced visible-light photocatalysis for spiramycin degradation. J. Photochem. Photobiol. A. 2016;328:24-32.
- [Google Scholar]
- Nanostructures confined self-assembled in biomimetic nanochannels for enhancing the sensitivity of biological molecules response. J. Mater. Sci. – Mater. El.. 2018;29:19757-19767.
- [Google Scholar]
- Review on advances in photocatalytic water disinfection utilizing graphene and graphene derivatives-based nanocomposites. J. Environ Chem. Eng.. 2019;1; 7(3):103132
- [Google Scholar]
- Fluorescence quenching of perylene DBPI dye by colloidal low-dimensional gold nanoparticles. J. Fluoresc.. 2015;25:973-978.
- [Google Scholar]
- Investigation the photocatalytic activity of CoFe2O4/ZnO and CoFe2O4/ZnO/Ag nanocomposites for purification of dye pollutants. Sep. Purif. Technol.. 2019;211:35-39.
- [Google Scholar]
- Hydrothermal synthesis of magnetic CoFe2O4/graphene nanocomposites with improved photocatalytic activity. Appl. Surf. Sci.. 2015;351:140-147.
- [Google Scholar]
- a. Superparamagnetic MnFe2O4 dispersed over graphitic carbon sand composite and bentonite as magnetically recoverable photocatalyst for antibiotic mineralization. Sep. Purif. Technol.. 2017;172:498-511.
- [Google Scholar]
- Solar photocatalytic mineralization of antibiotics using magnetically separable NiFe2O4 supported onto graphene sand composite and bentonite. J. Water Process Eng.. 2016;14:86-100.
- [Google Scholar]
- Modeling of competitive ultrasonic assisted removal of the dyes–Methylene blue and Safranin-O using Fe3O4 nanoparticles. Chem. Eng. J.. 2015;268:28-37.
- [Google Scholar]
- Unseeded organotemplate-free hydrothermal synthesis of heteroatomic MFI zeolite poly-nanocrystallites. J. Mater. Chem. A.. 2013;1:2453-2460.
- [Google Scholar]
- Synthesis and characterization of nitrogen-doped graphene hydrogels by hydrothermal route with urea as reducing-doping agents. J. Mater. Chem. A.. 2013;1(6):2248-2255.
- [Google Scholar]
- High-efficiency sono-solar-induced degradation of organic dye by the piezophototronic/photocatalytic coupling effect of FeS/ZnO nanoarrays. Nanotechnology. 2016;27:375704
- [Google Scholar]
- Visible-light-driven photocatalytic properties of ZnO/ZnFe2O4 core/shell nanocable arrays. Appl. Catal. B – Environ.. 2014;160:408-414.
- [Google Scholar]
- Pectin–cerium (IV) tungstate nanocomposite and its adsorptional activity for removal of methylene blue dye. Int. J. Environ. Sci. Technol.. 2014;11:2015-2024.
- [Google Scholar]
- Recent advances in noble metal free doped graphitic carbon nitride based nanohybrids for photocatalysis of organic contaminants in water: a review. Appl. Mater. Today. 2019;15:494-524.
- [Google Scholar]
- Copper-immobilized platinum electrocatalyst for the effective reduction of nitrate in a low conductive medium: Mechanism, adsorption thermodynamics and stability. Appl. Catal. A-Gen.. 2014;478:259-266.
- [Google Scholar]
- Enhanced photodegradation activity of methyl orange over Z-scheme type MoO3–g-C3N4 composite under visible light irradiation. RSC Adv.. 2014;4:13610-13619.
- [Google Scholar]
- Preparation, characterization and photocatalytic evaluation of aluminum doped metal ferrites. Ceram. Int.. 2019;45:7318-7327.
- [Google Scholar]
- High piezo-photocatalytic efficiency of CuS/ZnO nanowires using both solar and mechanical energy for degrading organic dye. ACS Appl. Mater. Inter.. 2016;8:21302-21314.
- [Google Scholar]
- Synthesis of magnetic CoFe2O4/g-C3N4 composite and its enhancement of photocatalytic ability under visible-light. Colloids Surf. A. 2015;478:71-80.
- [Google Scholar]
- Ultrasensitive and selective 4-aminophenol chemical sensor development based on nickel oxide nanoparticles decorated carbon nanotube nanocomposites for green environment. J. Environ. Sci.. 2017;53:27-38.
- [Google Scholar]
- Twin-tail surfactant peculiarity in superficial fabrication of semiconductor quantum dots: toward structural, optical, and electrical features. J. Phys. Chem. C. 2015;119:5062-5073.
- [Google Scholar]
- Controllable fabrication of CuO nanostructure by hydrothermal method and its properties. Appl. Surf. Sci.. 2014;311:602-608.
- [Google Scholar]
- Nitrogen-doped graphene hydrogels as potential adsorbents and photocatalysts for environmental remediation. Chem. Eng. J.. 2017;327:751-763.
- [Google Scholar]
- Novel magnetic CoFe2O4/Ag/Ag3VO4 composites: highly efficient visible light photocatalytic and antibacterial activity. Appl. Catal. B-Environ.. 2016;199:11-22.
- [Google Scholar]
- Cobalt Ferrite Nanoparticles: preparation, characterization and salinized with 3-aminopropyl triethoxysilane. Energy Proc.. 2019;157:1353-1365.
- [Google Scholar]
- Synthesis and characterizations of a novel CoFe2O4@ CuS magnetic nanocomposite and investigation of its efficiency for photocatalytic degradation of penicillin G antibiotic in simulated wastewater. J. Hazard. Mater.. 2019;366:545-555.
- [Google Scholar]
- A review on plasmonic Au-ZnO heterojunction photocatalysts: Preparation, modifications and related charge carrier dynamics. Mater. Sci. Semicond. Process.. 2019;93:59-91.
- [Google Scholar]
- Toxicity removal assessments related to degradation pathways of azo dyes: toward an optimization of electro-Fenton treatment. Chemosphere. 2016;161:308-318.
- [Google Scholar]
- A novel magnetically separable TiO2/CoFe2O4 nanofiber with high photocatalytic activity under UV–vis light. Mater. Res. Bull.. 2012;47:333-337.
- [Google Scholar]
- Investigation of WO3/ZnO thin-film heterojunction-based Schottky diodes for H2 gas sensing. Int. J. Hydrogen Energy. 2014;39:10313-10319.
- [Google Scholar]
- Rapid, microwave assisted, and one pot synthesis of magnetic palladium–CoFe2O4–graphene composite nanosheets and their applications as recyclable catalysts. Part. Part. Syst. Char.. 2014;31:245-251.
- [Google Scholar]
- Forming mechanism of nitrogen doped graphene prepared by thermal solid-state reaction of graphite oxide and urea. Appl. Surf. Sci.. 2011;258(5):1704-1710.
- [Google Scholar]
- Mesoporous ZnFe2O4@ TiO2 nanofibers prepared by electrospinning coupled to PECVD as highly performing photocatalytic materials. J. Phys. Chem. C.. 2017;121:24669-24677.
- [Google Scholar]
- Enhanced visible-light photocatalytic performance of electrospun rGO/TiO2 composite nanofibers. J. Phys. Chem. C.. 2016;121:261-269.
- [Google Scholar]
- Recent progress on titanium dioxide nanomaterials for photocatalytic applications. Chem. Sus. Chem.. 2018;11:3023-3047.
- [Google Scholar]
- ZnO assisted photocatalytic degradation of acridine orange in aqueous solution using visible irradiation. Desalination. 2008;232:80-90.
- [Google Scholar]
- Artificial light assisted photocatalytic degradation of lissamine fast yellow dye in ZnO suspension in a slurry batch reactor. Indian J. Chem.-A. 2009;48:1364-1369.
- [Google Scholar]
- Photocatalytic decouloration of malachite green dye by application of TiO2 nanotubes. J. Hazard. Mater.. 2009;169:297-301.
- [Google Scholar]
- Photocatalytic mineralization and degradation kinetics of ampicillin and oxytetracycline antibiotics using graphene sand composite and chitosan supported BiOCl. J. Mol. Catal. A-Chem.. 2016;423:400-413.
- [Google Scholar]
- Fabrication and enhanced photocatalytic property of TiO2-ZnO composite photocatalysts. Mat. Lett.. 2019;240:84-87.
- [Google Scholar]
- Cu-loaded ZSM-5 zeolites: An ultra-sensitive phenolic sensor development for environmental safety. J. Ind. Eng. Chem.. 2018;61:304-313.
- [Google Scholar]
- Fabrication of a selective 4-amino phenol sensor based on H-ZSM-5 zeolites deposited silver electrodes. RSC Adv.. 2016;6:48435-48444.
- [Google Scholar]
- Fabrication of selective chemical sensor with ternary ZnO/SnO2/Yb2O3 nanoparticles. Talanta. 2017;170:215-223.
- [Google Scholar]
- Hydrazine sensors development based on a glassy carbon electrode modified with a nanostructured TiO2 films by electrochemical approach. Microchim. Acta. 2017;184:2123-2129.
- [Google Scholar]
- Fabrication of highly sensitive ethanol sensor based on doped nanostructure materials using tiny chips. RSC Adv.. 2015;5:63252-63263.
- [Google Scholar]
- Kinetics of photocatalytic mineralization of oxytetracycline and ampicillin using activated carbon supported ZnO/ZnWO. Desalin. Water Treat.. 2017;79:204-213.
- [Google Scholar]
- Solar light induced photodegradation of oxytetracycline using Zr doped TiO2/CaO based nanocomposite, Indian. J. Chem.. 2016;55:803-809.
- [Google Scholar]
- Zero valent iron-brick grain nanocomposite for enhanced solar-Fenton removal of malachite green. Sep. Purif. Technol.. 2014;133:429-437.
- [Google Scholar]
- Solar photocatalytic activity of nano-ZnO supported on activated carbon or brick grain particles: role of adsorption in dye degradation. Appl. Catal. A.. 2014;486:159-169.
- [Google Scholar]
- Fabrication of Ag3VO4 decorated phosphorus and sulphur co-doped graphitic carbon nitride as high-dispersed photocatalyst for phenol mineralization and E.Coli disinfection. Sep. Purif. Technol.. 2019;212:887-900.
- [Google Scholar]
- ZnO supported CoFe2O4 nanophotocatalysts for the mineralization of Direct Blue 71 in aqueous environments. J. Hazard. Mater.. 2013;252:171-179.
- [Google Scholar]
- Fabrication of fluorine doped graphene and SmVO4 based dispersed and adsorptive photocatalyst for abatement of phenolic compounds from water and bacterial disinfection. J. Clean. Prod.. 2018;203:386-399.
- [Google Scholar]
- Islanding of EuVO4 on high-dispersed fluorine doped few layered graphene sheets for efficient photocatalytic mineralization of phenolic compounds and bacterial disinfection. J. Taiwan Inst. Chem. Eng.. 2018;93:528-542.
- [Google Scholar]
- GdVO4 modified fluorine doped graphene nanosheets as dispersed photocatalyst for mitigation of phenolic compounds in aqueous environment and bacterial disinfection. Sep. Purif. Technol.. 2019;210:804-816.
- [Google Scholar]
- Recent advances in enhanced photocatalytic activity of bismuth oxyhalides for efficient photocatalysis of organic pollutants in water: a review. J. Ind. Eng. Chem.. 2019;75(28):1-20.
- [Google Scholar]
- Graphene bentonite supported ZnFe2O4 as super-paramagnetic photocatalyst for antibiotic degradation. Adv. Mater. Lett.. 2017;8:229-238.
- [Google Scholar]
- Photocatalytic mineralization of antibiotics using 60% WO3/BiOCl stacked to graphene sand composite and chitosan. Arab. J. Chem.. 2019;12:4627-4645.
- [CrossRef] [Google Scholar]
- Preparation of BSA-ZnWO4 nanocomposites with enhanced adsorptional photocatalytic activity for methylene blue degradation. Int. J. Photoenergy. 2013;2013:7.
- [CrossRef] [Google Scholar]
- Enhanced photocatalytic activity and stability of AgBr/BiOBr/graphene heterojunction for phenol degradation under visible light. J. Saudi Chem. Soc.. 2019;23(5):586-599.
- [Google Scholar]
- Review on various strategies for enhancing photocatalytic activity of graphene based nanocomposites for water purification. Arab. J. Chem.. 2020;13:3498-3520.
- [CrossRef] [Google Scholar]
- Nitrogen doped graphene nickel ferrite magnetic photocatalyst for the visible light degradation of methylene blue. Acta Chim. Slov.. 2017;64:170-178.
- [Google Scholar]
- Preparation, characterization and Cr(VI) adsorption behavior study of poly(acrylic acid) grafted Ficus carica bast fiber. Adv. Mat. Lett.. 2013;4(4):271-276.
- [Google Scholar]
- Review on fabrication of graphitic carbon nitride based efficient nanocomposites for photodegradation of aqueous phase organic pollutants. J. Ind. Eng. Chem.. 2018;67:28-51.
- [Google Scholar]
- Magnetically recoverable graphitic carbon nitride and NiFe2O4 based magnetic photocatalyst for degradation of oxytetracycline antibiotic in simulated wastewater under solar light. J. Environ. Chem. Eng.. 2018;6:3874-3883.
- [Google Scholar]
- Tailored crystalline microporous materials by post-synthesis modification. Chem. Soc. Rev.. 2013;42:263-290.
- [Google Scholar]
- Electrospinning direct synthesis of magnetic ZnFe2O4/ZnO multi-porous nanotubes with enhanced photocatalytic activity. Appl. Surf. Sci.. 2017;396:780-790.
- [Google Scholar]
- Fabrication of nanostructured CuO films by electrodeposition and their photocatalytic properties. Appl. Surf. Sci.. 2014;317:414-421.
- [Google Scholar]
- Piezo-potential enhanced photocatalytic degradation of organic dye using ZnO nanowires. Nano Energy. 2015;13:414-422.
- [Google Scholar]
- Effects of reaction temperature on the photocatalytic activity of photo-SCR of NO with NH3 over a TiO2 photocatalyst. Catal. Sci. Technol.. 2013;3:1771-1775.
- [Google Scholar]
- Three-dimensional carbon-coated ZnFe2O4 nanospheres/nitrogen-doped graphene aerogels as anode for lithium-ion batteries. Ceram. Int.. 2017;43:1022-1028.
- [Google Scholar]
- Photodegradation of malachite green under simulated and natural irradiation: kinetics, products, and pathways. J. Hazard. Mater.. 2015;285:127-136.
- [Google Scholar]
- One-pot construction of Fe/ZSM-5 zeolites for the selective catalytic reduction of nitrogen oxides by ammonia. Catal. Sci. Technol.. 2017;7:3036-3044.
- [Google Scholar]
- Optical and magnetic properties of ZnO/ZnFe2O4 nanocomposite. Mater. Chem. Phys.. 2017;192:330-338.
- [Google Scholar]
- Solar-light-driven photodegradation of organic dyes on sono-dispersed ZnO nanoparticles over graphene oxide: Sono vs. conventional catalyst design. Sep. Purif. Technol.. 2019;211:738-752.
- [Google Scholar]
- Enhanced photovoltaic effect of TiO2-based composite ZnFe2O4/TiO2. J. Photochem. Photobiol. A. 2012;233:15-19.
- [Google Scholar]
- Novel multifunctional NiFe2O4/ZnO hybrids for dye removal by adsorption, photocatalysis and magnetic separation. Appl. Surf. Sci.. 2016;369:1-10.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2019.08.005.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







