Translate this page into:
Meat tenderization mechanism and the impact of plant exogenous proteases: A review
⁎Corresponding author. malinda.madhusankha@gmail.com (G.D.M.P. Madhusankha)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Meat products are considered as one of the most demanding food items from a point of sale perspective and tenderness of meat plays a dominant role in enhancing the meat palatability. The current review describes the meat tenderization mechanism, chemical, physical and conventional protocols to evaluate the extent of meat tenderness and the impact of plant based exogenous proteases. Even though proteolytic enzymes are produced in commercial scale, most of the meat products reach the market without any treatment. Lack of technical knowhow on commercial extractions and inadequate literature on some proteases has been identified as the gaps to be fulfilled.
Keywords
Exogenous
Meat
Plant enzymes
Proteases
Tenderness
1 Introduction
Tenderness of meat is regarded as one of the leading customer perceptions when purchasing meat items. Therefore, a high score for the customer acceptance is needed in selling processed and unprocessed meat products. Out of all the sensory parameters; appearance, juiciness, bitterness, flavor, and taste, tenderness of meat plays a prominent role and is rated as a decisive sensory attribute directly affecting the eating quality of meat.
It has been revealed that the prime grilling steak cuts comprising 10% of the total meat cuts in beef are of relatively more tender (Polkinghorne et al., 2008) while in rabbit meat, the total prime cut percentage is around 30% (Maj et al., 2009). As per the tender meats are concerned, customers are not reluctant to pay more (Shackelford et al., 2001) and it has become a motivational factor for repeated purchasing. According to Miller et al. (2001), approximately 78% of US beef consumers in 5 selected cities are ready to pay premium prices for tender beef steaks. Ramanathan et al. (2020) figures out a loss of $ 7.64 per beef cattle in the US market due to the variations in tenderness. The concept of tenderization is mainly applicable for red meat due to high toughness (Beef, Mutton, Horse, Pork, Buffalo and Lamb) and minimally related with white meats such as chicken and fish (Bekhit et al., 2014). However, despite the meat variety, toughness becomes a physical constraint for the flesh of old animals and the statistical report of Marquer et al. (2015) reveals the drop down of the production of meat from bulls in 7% and increment of veal (age under 8 months) by 4% in European market from 2009 to 2014.
At the point of sale, it is difficult to distinguish tender meat unless the purchaser is an expert or a frequent consumer. Normally, the characteristic colour and odour are concerned as key parameters, when the purchasing decision is made. The fibrous nature of the meat surface indicates the presence of undegraded myofibrillar proteins which makes the meat hard and customers can capture it through visual inspections (Martinez-Arellano et al., 2013). The appearance of a marble structure, especially in red meat is due to the presence of intramuscular fat deposits and they can be identified as tender meat. When light rays strike the meat surface, they get scattered due to the presence of muscle fibres, sarcoplasm, fluids and striations. The scattered light speckles by the fibre bundles can be longitudinally observed through confocal reflection microscopy and tough meat displays intensive peaks (Hughes et al., 2014). In hard meat, the myoglobin content is high and visually they can be distinguished due to their intense red colour. The colour measurements for a* values in red deer meat through chroma meter indicate a negative correlation with tenderness (Wiklund et al., 2010). The initial scientific mediation on identifying the tenderness was the Warner - Bratzler shear force (WBS) and consumers identify meat as more tender when the WBS value is low. The findings of Miller et al. (2001) related to beef samples emphasize the amounts of 30 N and 46 N are the extreme values of customer recognition as tender and tough meat respectively. That means 100% of the beef customers perceive the meat with less than 30 WBS as tender and more than 46 WBS as tough. However, in the meat of wild deer, the two extreme values of WBS are 16 N and 22 N in classification as tender and tough respectively (Lopez-Pedrouso et al., 2019).
The sensory profile of meat is based on three key parameters; visual appearance, in mouth perception of texture and flavour (Font-i-Furnols and Guerrero, 2014). The meat tenderness is positively correlated with intramuscular fat content (IMF) and the juiciness (Lorenzo et al., 2013). Enhanced water retention of tender meat during cooking results in a juicy texture with a favorable mouth feel. In contrast, when tough meat is cooked, they become hard, dry, less palatable and result in chewing and digesting difficulties. The hardness and dryness occur due to the high drip loss during the cooking process of tough meat. A study conducted by Cannata et al. (2010) using pork samples highlight the positive correlation between tenderness and palmitic acid content. Due to the presence of palmitic acid, the off flavour accumulation is low during processing and therefore the flavour profile of tender meat is rich.
The commercial meat industry prefers slaughtering young animals to maintain the palatability, thus reducing the demand for old carcasses. Because, the flesh of old animals becomes hard due to the formation of cross links between the collagen molecules (Lonescu et al., 2008). The slaughtering of young animals is a negative attribute in the industry due to the less output. Under cold storage, hard meat undergoes proteolysis and Koohmaraie (1992) reveals the occurrence of a limited proteolysis in spent meat stored at (0–4 °C) with post mortem aging conditions. However, the cold storage is not sufficient to reduce the toughness of old meat into a consumable level. Since meat tenderness directly influences the quality and the value of the meat, it has become a key research area today.
Most of the literature up to date is focused on the process of meat tenderization, interventions of tenderizations (chemical and physical methods) and exogenous proteases based on their applications. There is a shortage of research evidence on the applicability of exogenous proteases in the meat industry aligning with their chemical structures and optimization of extraction methodologies. Even though there are plenty of potential plant sources to extract proteases, the current applications are limited only for few sources. The reason is, industrial manufacturers are focusing on the availability of raw materials in large scale and technical knowhow on smooth extraction. The active groups present in proteases play a significant role and the tenderizing capacity of some plant based proteases haven’t been experimentally proven yet. Thus, this review will focus on the present findings and express the identified gaps in this field for future investigations.
1.1 Importance and the perception of meat tenderness and toughness to the consumers
According to Meat Standard Australia (MSA) grading system based on the data acquired by consumer observations throughout two decades suggests that the palatability of meat is determined by three main traits; tenderness, flavour and juiciness. A survey conducted by O’Quinn et al. (2018) reveals the overall rejection percentages when the tenderness is acceptable and unacceptable are as 10% and 69% respectively. Out of the 3 traits, the impact of tenderness towards the overall acceptability in beef samples was over 50% in mid-1990′s and dropped down to around 40% recently (Huffman et al., 1996; Chail et al., 2017). The applications of different tenderization mechanisms in recent time has made a significant contribution to drop down the impact of the tenderness on overall acceptability. However, the impact percentage is still high and the protocols to enhance the tenderness while improving the other traits (Flavour and juiciness) are warmly welcome in the modern meat processing industry. Since the eating quality is predominantly driven by the tenderness and thus the tender meats are more liable to customer satisfaction and repeated purchasing (Banović et al., 2009). When the consumers tend to purchase repeatedly, the stability of the meat industry is guaranteed and the manufacturers are motivated to invest in mass scale productions and new product developments.
1.2 Mechanism of meat tenderization
Meat tenderization is a complex process with a number of related sub mechanisms. Degradation of collagen in both quantity and type (Veiseth et al., 2004), reducing the diameter of muscle fibre bundles (Renand et al., 2001), changes in the sarcomere length during rigor mortis (Rhee et al., 2004) and the chemical and structural changes occur during aging are different types of mechanisms aiming at tenderization. Meanwhile tenderization is a continuous process from the birth of the animal and running through the consumption stage.
The related primary mechanisms are protein degradation and protein oxidation. In fact, the protein constituents in muscle fiber are the target components of protease enzymes. Tenderness development depends on the structure, connectivity of the skeletal muscle tissue and the proteolytic enzyme activity on it (MacBride and Parrish, 1977). Moreover, Wang et al. (2013) point out the endogenous enzymatic activity with respect to actomyosin dissociation activated by heat supply. Therefore, dissociation of individual protein types depends on different modes of treatments.
The degradation of meat cuts high in collagen entirely depends on the cooking method and the end -point temperature that they undergo. Light et al. (1985) state the conversion of collagen to gelatin at 80 °C and shrinkage of collagen at 60–70 °C. As a cumulative effect of collagen shrinkage and collagen to gelatin conversion, meat tenderization occurs. However, the excessive increment of temperature to achieve tenderness results in increasing the moisture loss and hardening of myofibrillar proteins which collectively reduce meat tenderness (Barbanti and Pasquini, 2005).
1.3 Evaluation of meat tenderness
The success of the applied meat tenderization methodology is evaluated by different mechanisms. The physical examinations are based on textural analysis and observations through microscopes while sensory analysis can be done by trained panels to evaluate the overall quality based on flavour, aroma, texture, after taste and mouthfeel. In addition, there are conventional physical and chemical protocols to identify and compare the degree of meat tenderness. Measuring the force for shearing, biting, mincing, compressing and stretching are considered as physical methods (Lawrie, 1998) while determining solubility and effect of connective tissue and protein digestion have been identified as chemical methods of analyzing meat tenderization (Mahendrakar et al., 1989).
The evaluation of the meat tenderness is utterly a sensitive process and the measuring criteria have reached the peak with the advancing technology. Many tools have been used to analyse the impact as scanning electron microscopic view, hydroxyproline measurement (Ashie et al., 2002), myofibrillar fragmentation index (Olson and Parrish, 1977) and enzyme activity estimation (Koohmaraie et al., 1988). The colour image processing technique introduced by Tan (2004) is a mechanism to predict the tenderness with the aid of computer vision. The predictions align with the United State Department of Agriculture (USDA) grading system and the pixel value of images is the key predictor. The tenderness evaluation criteria have become sophisticated and Kröger et al. (2006) present their findings in using Dual Energy X-ray Absorptiometry (DEXA) as a feasible mechanism. Therefore, the advancement of technology has resulted in developing methodologies without destructing the muscle structure.
1.4 Factors affecting meat tenderization
The main components of meat are myofibrils; majorly composed of actin, myosin and other accessory proteins, and connective tissue of different sub-types of collagen and elastin (Lana and Zolla, 2016). The chemical structure and the arrangement pattern of meat proteins result in developing a hard texture and degradation of structural meat proteins in order to reduce the toughness of meat is commonly termed as tenderization.
The composition of the muscles; collagen content, intramuscular structure of the connective tissue and post slaughter degradation of the myofibrillar proteins directly affect meat tenderness (Huang et al., 2014). Also, the inherent characteristics of meat such as the type of muscle, pre-slaughter and post-slaughter factors affect meat tenderization (Anderson et al., 2012). The tenderization process begins from the birth of the animal and continues until the consumption stage. The most significant factor under pre slaughtering is the species and in addition, breed, age, sex, feeding and management, genetic influence and stress conditions are affecting the meat tenderization (Nowak, 2011). Specifically, chemical composition, structure and amount of connective tissue depend on the age and the muscle type of the animal (Bolumar et al., 2013).
Post slaughter factors also heavily affect the tenderization process whereas post mortem glycolysis results in reducing the pH of muscle due to the accumulation of lactic acid (White et al., 2006). The changes in the sarcomere length during rigor mortis (Rhee et al., 2004), end point temperature the carcass undergoes and duration of aging (Koohmaraie and Geesink, 2006) directly affect the final texture.
1.5 Conversion of muscle to meat process
Just after the death of the animal, a sequence of processes are taking place in converting the muscles into meat. Muscle to meat conversion expressed in a timeline with weighing factors is depicted in Fig. 1.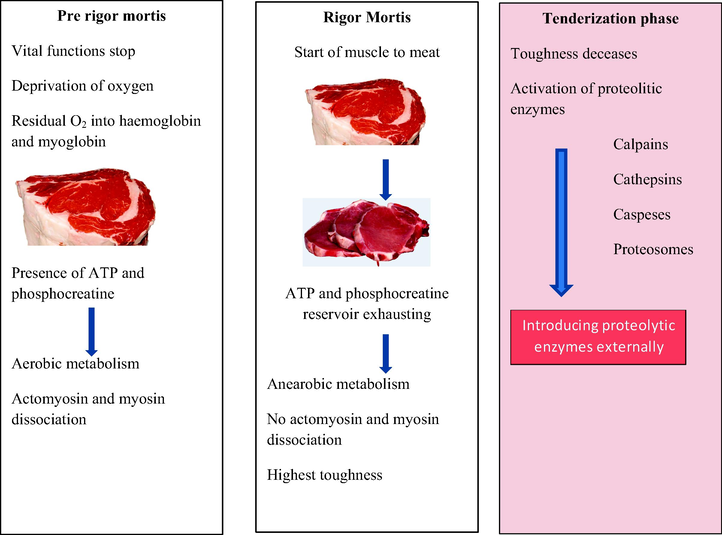
Schematic diagram of conversion of muscle to meat (modified from Lana and Zolla, 2016). The process conversion of muscle to meat in terms of three phases (1. Pre rigor mortis 2. Rigor mortis 3. Tenderization) is illustrated here. Primarily, metabolic reactions and the timeline of activation of muscle proteins are discussed highlighting the best moment to apply exogenous plant proteases.
Briefly, pre rigor mortis phase starts after the death of the animal and blood flow will stop immediately ending all the vital functions. However residual oxygen leads to the aerobic respiration via hemoglobin and myoglobin which carries oxygen to the cells. In this stage, muscular tissues maintain a certain metabolism and the presence of ATP and phosphocreatine will prevent the rigor mortis. This short period of time ends with 3 to 6 h and enters the muscles into the rigor mortis phase.
With the use of residual oxygen, ATP is rapidly used for the glycolysis process and when there is not enough ATP for the metabolism, rigor mortis phase will diminish. Now muscle starts to turn into a meat with the highest toughness which is mitigated by the post rigor mortis and tenderization phase. In the later stage it allows time to develop meat qualities and eating ability. During this time, proteolytic enzymes present in the muscle activate and tenderize the muscle tissues. Now it is probable to enter proteolytic enzymes into the matrix externally which is discussed below.
The degradations in the firmness of meat occur due to the proteolysis of myofibrillar which leads to low molecular weight proteins. This occurs at the N2-line level in muscle tissues which is attributed by the action of lysosomal enzymes, especially cathepsin B and L (Ouali, 1990). During this stage, myosin and actin become more soluble, subsequently increasing the amount of extractable proteins at high salt level by 50–60% between rigor and postmortem for 7–8 days (Valin and Kopp, 1978). Introduction of proteolytic enzymes externally reduces the tenderization time between slaughtering and selling to the customer. This action is highlighted with the recent research work corresponding to the extracted enzymes from popular and underutilized sources. However, the tenderizing time is decided by the producer considering the influence of microorganisms on shelf life. Based on the literature evidence focused on the aging period, it is not well established, but limited to approximately three weeks and characterized with a large variability depending on the species considered and on the typology of the muscle (Lana and Zolla, 2016). Conversion from the pre rigor mortis phase to the rigor mortis phase, an increase in toughness is observed and most of the mechanical tenderization methods are applied at the rigor mortis phase. However, the ideal time to apply the chemical and enzymatic treatments is the tenderization phase.
1.6 Methods of meat tenderization
In order to improve the meat tenderness, various methodologies are applied and based on the mode of application they are classified as mechanical (physical), chemical and enzymatic interventions. A classification of tenderization methodologies is illustrated at Table 1.
Intervention type
Current applications
Significant impact
References
Mechanical
Mechanical aging, shock wave technology, electrical stimulation, pulsed electric field, High pressure processing, Blade tenderization, ultrasound processing, Muscle stretching technology, Freeze - Thaw cycles, contract prevention methods
In addition to improving the tenderness, flavour is also enhanced by mechanical aging by giving a bit salty taste and juiciness. Evidence found on decreasing the cooking loss even though the drip loss is increased.
During the electrical stimulations, the muscle fibres are physically degraded while a slight pH drop can be observed. However, colour changes may occur which lead to customer dissatisfaction. Application of pulsed electric field results in tender meat while preserving the colour and odour. It can be used as a preservative method due to the inactivation and destruction of microbial cells. Most of the mechanical tenderization methods do not leave any toxic residue and therefore the treated meat is generally regarded as safe to consume.
Bolumar et al. (2013), Sorheim and Hildrum (2002), Troy and Kerry (2010), Piao et al. (2015), Bekhit et al. (2014), Hutchison et al. (2014).
Chemical
Infusion, marination and injection with Calcium, Magnesium and Phosphate salts (NaCl, CaCl2, Na3PO4 Potassium lactate)
The use of chemicals as tenderizing agents improve the organoleptic properties such as flavour and aroma to a certain extent.
However, the use of CaCl2 with a high concentration results in bitter, sour and metallic taste. In order to overcome the unfavourable alterations in flavour, it is recommended to use chemical tenderizing agents along with the combinations of maltodextrins and hydrolyzed soy proteins. Significant improvements in water binding ability can be seen by introducing chemical compounds such as Sodium phosphate.Scanga et al. (2000); Yoon et al. (2013)
Enzymatic
Plant proteases (Papain, Bromelain, Ficin, Actinidin Zingibain, Cucumin, etc.) Animal based (Trypsin, Pepsin, Pancreatin, and Chymotrypsin.
Microbial based enzymes (Fungal Amylase, Protease 15, Rhozyme P-11)An endogenous tenderization mechanism is activated in animal flesh and it is controlled by Calpain systems.
The action of exogenous enzymes is temperature dependent and therefore, minimal activity is seen in freezed and cold products. However, there are major differences in tenderized meat quality depending upon the type of the enzyme.
The applications of plant based proteases are further discussed under the review and the high cost of authentic enzymes of microbial and animal based extractions resulted in restricting their usage over plant proteases.Bekhit et al. (2014)
Throughout the previous decade many efficient physical mechanisms have been introduced and the traditional methods were successfully replaced. In comparison with other methods, physical interventions are feasible in industrial scale and minimally alter the sensory attributes. However, the implementation cost is high due the requirement of sophisticated machinery.
The incorporation of chemicals lead to modifications in protein solubility and improving the water holding capacity in order to soften the meat texture (Bhat et al., 2018). Seasoning meat items with Sodium chloride, pepper and lime juice is a conventional practice and Burke and Monahan (2003) had researched on organic acids and citrus juice marinades resulting in enhancing the texture and flavour. Organic acids penetrate into the muscles and reduce the pH significantly. It results in hydrating the protein fractions, weakening the inter- molecularlar bonds to degrade the myofibrillar and connective tissue proteins (Goli et al., 2011). Marination is a slow process where a sufficient time is required to get the maximum output and incorporation of chemicals negatively affects the emerging concept of consuming natural food products.
Of the enzymatic interventions, introducing exogenous proteolytic enzymes into the muscle is a commonly used method. In the context of using meat tenderizing agents, final texture and appearance can differ according to the type, amount of enzyme and the way of introducing it into the meat. Three methods of inserting proteolytic enzymes into meat have been practiced as dipping in a solution of a particular enzyme, pumping the enzyme solution into the blood vessels and rehydration of freeze dried meat (Gerelt et al., 2000). However, the first two methods were found to be unsatisfactory because of the over tenderizing effect. Enzymes which are capable of hydrolyzing the peptide bonds of proteins in meat are known to be proteolytic in nature and according to Tantamacharik et al. (2018), 95% of the exogenous meat tenderization agents in the USA market are from plant origins.
2 Effect of plant based proteolytic enzymes on meat tenderization
Since the plant based exogenous enzymes play a dominant role in meat tenderization, it is important to discuss different proteolytic enzymes; commonly used are papain, bromelain, zingibain, cucumin, ficin, actinidin etc. and other underutilized and unnoticed, but effective agents are available with meat tenderizing effect. These natural tenderizers are extracted from various plant origins such as fruits, vegetables, stems and leaves. Thus, it is valuable to discuss the chemistry and applications of those enzymes with new research findings and to encourage research on the effect of underutilized plants that can be utilized as proteolytic enzyme reservoirs. The chemical structures with respect to active compounds are included in Table 2 and extraction mechanisms of plant proteases are presented in Table 3.
Type of enzyme
Chemical structure
Activeness
Reference
Papain (C19H29N7O6)
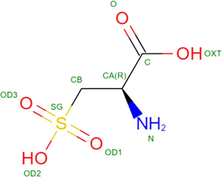
Sulfhydryl group
Papain contains 212 amino acids with a molecular weight of 23,406 DA and exist in the form of single chain. Four disulfide bonds are stabilized in the positions of Gln19, Cys25, His158 and His159. Enzyme shows the best activity in the pH range of 3–9.
RCSB Protein Data Bank Mamboya (2012)
Bromelain (C39H66N2O29)
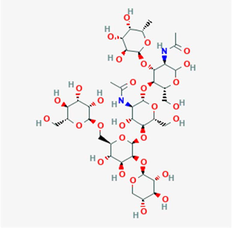
Sulfhydryl group of cysteine amino acid
The two main types of bromelain named stem bromelain (of 285 amino acids) and fruit bromelain consist of molecular weight of 23.40–35.73 kDa and 25–31.00 kDa respectively. The isoelectric point values of them are 9.55 and 4.6 respectively. Optimum enzyme activity over a pH range of 5.0–8.0 and active site affectively cleaves glycyl, alanyl, and leucyl peptide bonds
Pubchem, National Library of Medicine
Ficin (CH2FI2N)
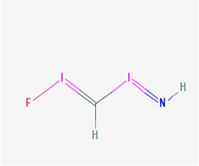
Thiol group
Ficin is known as a thiol protease with two conjugated double bondsand three disulfide bonds which can form 10 isomers and contains a cysteine amino acid at its active site. Ficin is able to cleave proteins at the carboxyl-terminal regions and cysteine majorly causes for the activity.
Pubchem, National Library of Medicine Sattari et al. (2020)
Actinidin (C10H13N)
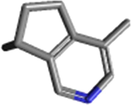
Thiol group
Actinidin consists of 220 amino acids with a molecular weight of about 24 kDa (change in the range of 24 to 33 kDa). Optimum enzyme activity over a pH range of 3.5–5.5 and substrate specificity is high.
Pubchem, National Library of Medicine Gong et al (2020)
Zingibain (GP-II)
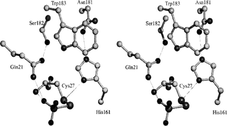
Proline
The enzyme is a 221 amino acid glycoprotein containing two N-linked oligosaccharide chains at Asn99 and Asn156. Proline at the P2 position of the GP-II is the active site which can cleave peptides and proteins. This protein is also stabilized by three disulfide bonds in between Cys24 and Cys65, Cys58 and Cys98, and Cys155 and Cys206.
Choi et al. (1999)
Enzyme
Extraction
Significance
Reference
Cysteine proteases
Potential source
Extraction method
Papain (EC3.4.22.2)
Latex of papaya
Leaves of papaya (Carciya papaya)Aqueous Two-Phase (ATP) extraction system
ATP consisting of 8% (w/w) polyethylene glycol (PEG) and 15% (w/w) NH4)2SO4 acquired the maximum recovery of 88% and 100% purity at pH 5 from papaya latex containing 20–40 mg protein/ml. Extracted papain was free from contamination of other proteins.
Nitsawang et al. (2006).
Ionic Liquid Aqueous Two- Phase (ILATP) extraction technology
ILATP of [CnPy]Cl(n = 2,4,6)-K2HPO4 system resulted high-purity and high active papain. The optimal result of overall desirability was 0.8985 under the conditions of 0.35 g/mL K2HPO4, 0.25 g/mL [C4Py]Cl, pH of 7.87, papain dosage of 2.17 mg/mL at 30℃. The specific activity of papain obtained as 4120.17 U/mg
Zhu and Zhang (2019)
PEG-Based Aqueous Two-Phase Systems (ATPS) using Quaternary Ammonium Ionic Liquids (IL) as adjuvants
ATPS of PEG400+(NH4)2SO4 + H2O is utilized to separate and purify papain from papaya latex adding different IL and the highest papain purity was exhibited from tetramethyl ammonium bromide ([N1111]Br) in the ATPS with 20 wt% (NH4)2SO4 and 20 wt% PEG400 at pH 7.0 and 60 °C. SDS-PAGE analysis showed that addition of 4 wt% IL significantly improved the purification factor (13.51) of crude papain and papain recovery rate was 96.46%.
Yu & Zhang (2020)
Reverse micellar extraction
Cetyltrimethylammonium bromide (CTAB) + Isooctane + hexanol + butanol system is used to optimize forward and backward extraction of papain; efficiency of 61.0% and 90.52% at pH of 11 and 6, Isopropyl Alcohol (%) of 6 and 19.938 and KCl of 20 (% v/v) and 0.729 M respectively.
Prabhu et al. (2017)
Bromelain (EC3.4.22.32)
(EC3.4.22.33)Pineapple (fruit and stem)
Pineapple waste
Pineapple root (Ananas comosus)Aqueous Two-Phase (ATP) extraction
ATP consists of 18% PEG1500 and 14% phosphate at pH 7.0 resulted nearly 228% activity recovery and 4.0-fold increment in purity of bromelain from pineapple fruit (A. comosus L. Merryl). Differential partitioning of bromelain to the polymer rich (top) phase was observed in the PEG/salt system.
Babu et al. (2008)
Reverse micellar extraction
CTAB (150 mM) + iso-octane(80%) + 1-hexanol(5%) + 1-butanol(15%) system resulted 85.0% activity recovery and 4.0 purification fold of bromelain from pineapple core with crude enzyme extract of pH 8.0 with 0.1 M NaCl.
Chaurasiya et al. (2015)
Two-Stage Ceramic Ultrafiltration (TSCU)
TSCU system of ceramic membrane made up of Zirconium Oxide (Surface area of 0.0055 m2 was able to recover 96.8% of bromelain from pineapple crude waste mixture (Ananus comosus L.) performing the feed at pH 7 and temperature of 20 °C.
Nor et al. (2016)
Extraction of crude bromelain extract and stabilizing as cross link aggregates
Crude bromelain was extracted from pineapple core waste and stabilized as cross-linked aggregates for 4 h with 80 mM glutaraldehyde. Enzymatic activity of 87% was recovered by ammonium sulphate (60%, w/v)
Banerjee et al. (2020)
Extraction of bromelain by precipitation with carrageenan
Precipitation method utilized for industrial waste of pineapple (stem and peel) was able to recover 80–90%. Optimum conditions of stem were 4.5 pH for 52.7 mg mL−1 of bromelain in 166.5 µg mL-1 of carrageenan and for crude peel juice; 78.5 mg mL-1 of bromelain in 183.5 µg mL−1 of polysaccharide could obtain 2.7 U mg-1 and 3.1 U mg-1 of proteolytic activity respectively.
Campos et al. (2019)
Zingibain (EC 3.4.22.67)
Ginger rhizome (Zingiber Officanale)
Solvent extraction with an isolation method
Isolation procedure was able to separate two cysteine proteases, GP2 and GP3 from ginger rhizomes (Zingiber officinale) and GP2 is almost identical to ginger protease GPII. Briefly, phosphate buffer of pH 7.0, cysteine and EDTA were basically used and a series of other chemicals were used in the purification process.
Kim et al. (2007)
Extraction from anion exchange chromatography and purification by protein separation method
Cysteine protease (GP) proteins from ginger rhizome were precipitated using ammonium sulfate (40–100%), and subjected to anion-exchange column chromatography of DEAE Toy pearl 650 M with 20 mM phosphate buffer pH 7.0. A new protease enzyme GPii which is similar to GP2 was able to extract from ginger
Nishiyama and Araki (2018)
Ficin (EC 3.4.22.3)
Latex of Fig trees (Ficus glabrata, Ficus anthelmintica, Ficus laurifolia Ficus johannis)
Cation-exchange chromatography
Aqueous dialyzed samples of Ficus carica cv latex used to run on SP-Sepharose fast flow column with pre-equilibrated sodium phosphate buffer at pH 7.0 and identified isoforms of ficin. Latex contained almost 9% of ficin and it had the lowest autolysis and pronounced that this ficin could be used in industry without having any autolysis inhibitors.
Zare et al. (2013)
Precipitation of protein and ion-exchange chromatography
Firstly, ficin protein is precipitated by ammonium sulphate; secondly CM-Sepharose and SP-Sepharose cation-exchange chromatography and thirdly Sephadex G-25 gel filtration chromatography followed to purify ficin from latex of Ficus johannis and identified two ficins. Estimated molecular weight of purified ficin was 25 kDa by SDS-PAGE profile and analyzed proteolytic activity was known to be 254 µM min − 1 mg − 1 with a 31% yield and above 93% purity.
Homaei et al. (2017)
Ion-exchange chromatography
SP-Sepharose packed column with 30 mM citrate buffer of pH 5.0 at a sodium chloride gradient eluted 23.5 yield and could observe optimal 24.2 proteolytic activity and 84 GDU/mg gelatinolytic activity. Isolated alkaline isoform of ficin was identified as ficin 1c based on the available transcriptome data.
Milošević et al.,2020
Ion exchange chromatography and gel filtration
Crude extract of Ficus carica latex used to purify in CM Sepharose column chromatography against acetate buffer by applying dialyzed samples followed by dialysis using DEAE-Sepharose and Sephacryl S-200 against Tris-HCl buffer. Two peroxidase fractions were obtained with recovery folds of 3.05 and 0.79 and 50.2% and 11.32% recovery percentages respectively and molecular weights near 30 KDa
Elsayed et al., 2018
Cloning of ficin gene in E.coli and purification using Ni-sepharose column and gel filtration
A novel method of molecular cloning of ficin gene from leaves of fig trees (Ficus carica cv. Sabz) in all forms of isoforms in E. coli BL21 and purified using the Ni-sepharose column. Optimized conditions expressed as cell density of 1.25, post-induction time 7 hr, 10% (w/v) lactose concentration (at 115 rpm 24 °C) and 0.27 mg/ml purity. The recombinant enzyme production increased by 3 fold from the optimization of RSM.
Sattari, Rigi, and Ghaedmohammadi (2020)
Actinidin (EC 3.4.22.67)
Kiwifruit or gooseberry
Solvent extraction and precipitation method
Seven kiwifruit cultivars of Actinidia chinensis and Actinidia deliciosa species from China were used to isolate actinidin and purified by using ammonium sulfate precipitation and QSFF column electrophoretic homogeneity. SDS-PAGE analysis and zymogram indicated the molecular mass of purified enzyme as approximately 29.0 kDa with the activity of 240.7 U/mg at pH 3.5 and 40 °C. purified actinidin was identified by peptide sequence.
Zhang et al. (2017)
Solvent extraction with a buffer
Actinidin extracted from kiwi fruit showed optimal extraction conditions at pH 7.0 of most appropriate buffer as sodium phosphate buffer, 2.5 min of extraction time, 1:0.5 in the ratio of fruit:buffer (mg/v) extraction percentage, and 30 min of incubation time.
Al-Zubaidy,(2017)
Solvent extraction with EDTA
Green and gold kiwifruit extracts were reacted with EDTA and sodium metabisulfite at pH 6 of potassium phosphate buffer. Molecular weights from SDS-PAGE analysis observed as 24 and 27 kDa from main proteins in kiwifruit. Protein activity of green and gold types reported as 2–7 and 1–10 mg/mL.
Chao (2016)
Serine protease
Fruits of cucumis plant (Cucumis trigonus Roxb)
Unripe fruits of Cucumis pubescens
Solvent extraction
Plant extract was prepared using Cucumis trigonus Roxb species of melon in a 10% sodium chloride solution and decinormal sodium hydroxide or hydrochloric acid at a suitable pH level. Filtered solution was reacted with a phosphate buffer (pH 7.0) and 80% yield was obtained at the best temperature of 20–25 °C. Cucumin in powder form had prepared by a sequence of reagents.
Hujjatullah and Baloch (1970).
Cucumin
2.1 1 Papain
The history of papain dates back to 1950s and frequent recorded data is available on its applications to tenderize meat. Papain is a proteolytic enzyme extracted from Carica papaya (Poulter and Caygill, 1985) and the enzyme is employed to improve the tenderization of meat by acting on the structural component of muscles (Gracey, 1981). The application is sharpened by the combined action of papain, chymopapain and papaya peptidase-A (Maiti et al., 2008). The action of papain enzyme is multidisciplinary whereas it is commercially used to reduce the shrinkage of wool products, remove stains from fabrics, to produce toothpaste, beer and cosmetic products (Nadaraja, 2010). However, the tenderization effect of papain on meat is extensively pronounced in literature.
A comparative study conducted by Ashie et al. (2002) elucidate the significant reduction of shear force occurred in meat treated with papain under storage conditions (5 °C) for 2 weeks. It gives a hint on papain’s ability to act in chilled conditions. The tenderization potential is comparatively high in papain and therefore a minute amount of enzyme is sufficient for the activity which is a plus point in commercial usage. The heat stability of papain is comparatively high, enabling it to function in a broad temperature range without inactivation and leads to changes in the product texture even after cooking (Homaei et al., 2010). However, due to the broad specificity of papain, an indiscriminate breakdown of all the proteins (Collagen and miofibrilla) may occur and result in over tenderization with a mushy texture (Islam and Molinar-Toribio, 2013). Therefore, the marination with papain is preferred to be done in controlled conditions.
Papain acts dominantly on mucoproteins and collagen of connective tissue and collagen suspensions are converted into compact gels as a result (Lonescu et al., 2008). Even though the tenderness is increased by papain, the decrease of juiciness and imparting a bitter taste due to bitter peptides formed by proteolytic degradation restrict the use of papain in premium quality meat industry (Gerelt et al., 2000). The over tenderization of meat due to papain can be controlled by adding ascorbic acid and Ockerman et al. (1993) reveals that the activity of papain can be totally suppressed by incorporating ascorbic acid with a concentration of 2.5 mM. However, the excess use of ascorbic acid may cause off -odours and off flavours.
Most recently, some emerging technologies have been combined with papain enzymes to enhance meat tenderization. High-intensity ultrasonic radiation, experimented with papain by Barekat & Soltanizadeh (2017) has revealed that highest tenderness and proteolytic activity were found with the combined treatment of papain and ultrasonic power of 100 W for 20 min. Schenkova et al. (2007) has researched the combination of papain and high isostatic pressure on tenderization of beef meat and pressurization to 100 MPa with papain has led to a significant increase of meat tenderness. More evidence proved by Ma et al. (2019) highlight a significant increment of tenderization in yak meat could be obtained at 50 MPa/15 min with injecting papain (80 U/ml). The uniform distribution of the enzyme throughout the matrix is facilitated by the combined emerging technology and results in enhancing the tenderization capacity.
2.1.1 Chemical structure of papain
Papain is a sulfhydryl protease with the molecular weight of 23,000 Da and 3 disulphide bridges are prominent in the structure. Comparatively it is a small protein consisting of 212 amino acids (Lowe, 1976). Kamphuis et al. (1984) has demonstrated the three dimensional structure for papain and in the pH range of 5–8 and at 65 °C temperature papain has shown a broad-spectrum enzymatic activity (Smith and Hong-Shum, 2003). Specifically, papain is more reactive on amino acids with aromatic side chains such as Phenylalanine and Tyrosine at the P2 position (Berger and Schechter, 1970).
Since the enzyme can be inactivated in extreme conditions only, for example at 900 mpa, 80 °C for 22 min, this results in continuing the tenderization process even after cooking meat (Yeom, Zhang and Dunne, 1999). The same study further suggests the possibility of inactivating papain by applying a pulsed electric field where the researchers have observed the breakdown of α- helical structure through Circular Dichroism (CD) analysis.
2.1.2 Bromelain
Bromelain is extracted from pineapple (Ananas comosus) and highly concentrated in stems and fruits whereas also reported to be found in pineapple waste (Cores, peels and leaves) in minute amounts (Sri Watanapongse et al., 2000). Stem bromelain, annanain and comosain are proteolytic enzymes extracted from pineapple stem while fruit bromelain is from pineapple fruit juice (Rowan et al., 1990). Since stem bromelain is extracted as a combination of protease enzymes, purification of stem bromelain is done by removing other components (annanain and comosain) through chromatographic methods (Feijoo-Siota and Villa, 2011; Napper et al., 1994) followed by isolation of stem bromelain by precipitation and centrifugation (Devakate et al., 2009). Sunantha and Saroat (2011) state that the maximum protease activity recovery of bromelain from pineapple peel is observed by using a two-phase aqueous system (18% PEG- 600 and 17% MgSO4). Bromelain is used to increase the extraction yield and gel strength of gelatin extracted from bovin skin, and scanning electron microscopic observations highlight the presence of large particle size, dense and irregular network (Ahmad et al., 2020) in extracted gelatin samples incubated with bromelain.
A research conducted with chunks of chicken, beef and squid marinated with bromelain extracts reported a reduction of firmness by >61% (Sunantha and Saroat, 2011). Incorporation of bromelain on meat samples result in decreasing the pH and increasing the Water Holding Capacity (Manohar et al., 2016). Bromelain acts on myofibrillar proteins and collagen to provide tenderizing effect in order to enhance the sensory parameters (Sullivan and Calkins, 2010). However, the marination of meat with bromelain having a broad specificity results in over tenderization and mushy texture (Ha, Bekhit and Carne, 2014). Therefore, the over tenderization of meat leaving mushy spots is a key drawback of bromelain and pear fruit protease has been identified as an ideal alternative to overcome the defect (Nam et al., 2016).
2.1.3 Chemical structure of bromelain
Bromelain comprises 285 amino acids (Arshad et al., 2014) with a highest percentage of alanine and glycine while histidine and methionine reported to be the least (Ota et al., 1964). Murachi et al. (1964) identified the presence of 2.1% carbohydrate proving that bromelain is a glycoprotein and the concept was further confirmed with the isolation of 30 mg of glycoprotein by 1 g of bromelain using gel filtration (Murachi et al., 1967). In addition to the presence of thiol groups, bromelain is a combination of phosphates, peroxidases, cellulases, glycoproteins and protease inhibitors (Bhattacharyya, 2008). The recent study by Ramli et al. (2018) provides noteworthy details on the two chemical structures of stem bromelain (EC 3.4.22.32) and fruit bromelain (EC 3.4.22.33), their difference, and specificity etc., thus authors do not expect to repeat it here.
2.1.4 Ficin
The plants belonging to the Ficus family (Ficus glabrata, Ficus anthelmintica, Ficus laurifolia) are the key sources of extracting ficin (Gaughran, 1976) whereas there is a recorded history of its usage as a meat tenderizer (Kang and Rice, 1970; Solov’ev and Krakova, 1973). However, fig fruit is the prominent source and its latex consists of 10 proteases (Kramer and Whitaker, 1964) and the extraction is recommended in the pH range of 5–7 and the 65 °C of optimum temperature. Further, the tenderizing effect of Ficin has been experimented and a sufficient proteolytic activity found on actomyosin complex and collagen (Cormier et al., 1989). Gel electrophoresis is used to evaluate the effect of ficin on meat tenderizing and Ramezani et al. (2003) have discovered that the solubility of meat proteins increases after marinating with ficin.
Ficin has the ability to act on elastin at low temperatures (Around 20 °C) and displays a less reactivity on collagen and myofibrillar proteins (Singh et al., 2019). However, the changes in water holding capacity and the meat tenderizing effect of samples treated with ficin has been experimented by Ramezani et al. (2003) through gel electrophoresis. Furthermore, Gaughran (1976) states that the optimal pH for the reactivity differs for elastin and collagen as 5.5 and 7.0 respectively. The activity of ficin is relatively higher in salt soluble fractions of meat (Kang and Rice, 1970). Since most of the meat based recipes are included with a considerable proportion of salt, ficin’s activity on salt soluble fraction adds value to enhance the palatability of the final product.
A comparative study conducted by Ramezani et al. (2003) using two samples of ficin tenderized meat and cysteine- modified soy proteins to produce bologna reveals that the meat sample treated with ficin shows a disappearance of several protein bands and increment of the solubility of meat proteins. The same study further states the substantial increment of the Water holding capacity (WHC), emulsion stability and texture of both samples. The results of the Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) elucidate the impact of ficin on Nitrogen Solubility Index (NSI) and degradation of specific proteins. According to Varnam and Sutherland (1995), heavy chains of myosin are mostly affected by ficin while C- Proteins and α-actinin are also partially degraded.
2.1.5 Chemical structure of ficin
Ficin is categorized as a cysteine proteinase and is reported to occur in multiple forms (Devaraj et al., 2008). Englund (1968) revealed that Ficin is a single chain polypeptide and it was separated into different proteolytic compounds through CM- Cellulose chromatography by Sgarbieri et al. (1964).
2.1.6 Actinidin
The proteolytic enzyme found in gooseberry or kiwi fruit (Actinidia chinensis) is referred as “Actinidin” (Arcus, 1959). Target protein in tenderizing meat is myofibrillar proteins and degrades them into peptides and additionally activates m-calpain in the postmortem ageing process (Ha et al., 2012). Few studies show that actinidin possesses a mild tenderizing effect even at high concentrations, overcoming surface mushiness. Inactivation temperature is relatively low (60 °C), and it helps to control the tenderization process preventing overcooking (Tarté, 2009; Eshamah et al., 2014). This tenderization agent is used in instances where a minimum tenderization effect is required due to the less tenderizing capacity in comparison with other proteolytic enzymes. The fact is confirmed by Lewis and Luh (1988) by analysing the effect of actinidin and papain on beef muscle proteins and observed the less hydrolysis of myofibrillar proteins by actinidin than papain and prevention of over tenderization of steaks in actinidin marinated samples. Moreover, actinidin has the ability to act on a broad optimum pH Range (5–7) (McDowall, 1970) enabling its usage throughout a wide range.
A study conducted by Christensen et al. (2009) elucidate the tenderizing effect of actinidin on pork muscles. According to the study, the Warner- Bratzler (WB) shear force is reduced and tenderness has been increased even though there was no effect on flavour and the juiciness of meat. However, an improvement of organoleptic properties with actinidin treated samples were reported by Aminlari et al. (2009) and the same study reveals the significant improvement of Nitrogen Solubility Index, Water Holding Capacity and texture. The activity of acinidicin can be enhanced significantly by combining with the ultrasonication (Jørgensen et al., 2008). Since the homogeneous mixing of the enzyme in marinated samples is facilitated by ultrasound waves, the tenderizing effect of the combined treatment is comparatively high. Even at uncooked stage (20 °C) with acidic pH conditions, actinidin degrades cattle achilles tendons into collagen sub units chains; ß and ɑ chains and peptide fragments in different sizes (Wada et al., 2004). The finding is really important since it gives a clue on the activity of plant thiol protease on collagen at uncooked conditions and earlier it is believed that thiol proteases cannot act on native collagen and the activity starts with the thermal degradation when cooking (Lawrie, 1998)
The effect of kiwi fruit protease on pork has been studied by Chen et al. (2012) and highlights the significant changes occurred in myofibril fragmentation index, viscosity, particle size and the microstructure. The scanning electron microscopic observations of Samejima et al. (1991) using beef pieces immersed in crude actinidin solutions confirm the changes in microstructure, more prone towards a fragile structure after marination. The increment of springiness, chewiness and reduction of hardness in chicken samples incorporated with actinidin was observed by Bagheri Kakash et al. (2019). The same study further reveals the mechanism of hydrolysis as reducing the length of the peptide chains, thus significantly increasing the lightness.
2.1.7 Chemical structure of actinidin
Actinidin is categorized under the class of plant thiol protease (Glazer and Smith, 1971) due to the presence of a free sulfhydryl group (McDowall, 1970). The molecular weight is 26,000 and two active proteins have been identified through Diethylaminoethyl (DEAE) cellulose chromatography (Boland and Hardman, 1972). The fractionation of actinidin has been done by McDowall (1973) on DEAE- Sephadex A25 and identified the 2 fractions based on the electrophoretic mobility towards the anode of the SDS-PAGE. The conformation of the structure has been identified as almost similar to papain and consisting of a single chain of 220 residues with at least 2 disulfide bridges (Baker, 1977).
2.2 Zingibain
The meat tenderizing ability of ginger (Zingiber officinalae) is due to the Ginger Protease (GP) or Zingibain (Sakasai et al., 1980; Hamazume et al., 1987). The proteolysis of zingibain is heavily applied on collagen than actomyosin and the research conducted by Thompson et al. (1973) proves that zingibain outperforms papain and ficin by its ability to proteolyse more collagen while it has a higher optimum activity temperature (60 °C) than bromelain and optimum pH of 6 (Ha et al., 2012). Thompson et al. (1973) further state that both collagen and actomyosin show a linear increase in proteolysis at 30–50 °C whereas the proteolysis rate of actomyosin drops down steadily at temperatures above 50 °C. The combined proteolysis of both muscle proteins result in significantly more tender meat.
In addition to enhancing the tenderness, zingibain has the ability to sharpen the flavour and juiciness (Naveena et al., 2004) of meat products. Ginger is used as a flavour enhancer in culinary dishes and therefore treating with zingibain gives an additional advantage of enhancing the flavour and aroma. Abuduwaili et al. (2019) state that the application of zingibain in dry cured mutton ham resulted in significantly high fat and protein degradation, increasing the water loss, and shortening the maturation cycle. The same study further reveals the increment of Total Nitrogen (TN), Non-Protein Nitrogen (NPN) and Proteolysis Index (PI) in the samples treated with zingibain. In comparison with other natural tenderizing agents, the commercial use of zingibain is at a minimal level due to its low stability and treating with sodium ascorbate or preparing as ginger protease acetone powders extend the shelf life from 2 days to 18 months at storage of 5 °C (Adulyatham and Owusu-Apenten, 2005). Since ginger is a commercially grown spice, the raw material supply is continuous and therefore, more research should be done to improve the stability of zingibain in order to encourage the commercial usage.
2.3 Cucumin
The fruits of Cucumis trigonus Roxb available in India, Pakistan, Afghanistan and Persia is used to extract cucumin and it is recognized as a proteolytic enzyme for meat tenderization (Hujjatullah and Baloch, 1970). The extractions are done with 10% Sodium chloride and Ammonium sulphate and the outcome is known as cucumin (Mane et al., 2014). The use of cucumin is more popular in traditional villages and has a less impact in commercial applications up to date (Cazarin et al., 2015). There are records on the usage of cucumin to tenderize buffalo meat which is regarded as a tougher meat than beef even though the cholesterol content is comparatively low (Naveena et al., 2004).
Ability to retain the enzymatic activity throughout a wide temperature range (40 °C – 70 °C) is a specialty of cucumin and it activates around pH 5 (Hujjatullah and Baloch, 1970). Moreover, a comparative study conducted by Narayan et al. (2015) to identify the impact of citric acid, cucumin powder and high pressure treatment on the quality of goat meat came up with the conclusion of significant improvement in juiciness, tenderness, flavour and overall acceptability of the meat sample marinated with cucumin powder. Generally, goat meat is widely consumed in rural parts of India and statistical reports highlight that around 41% of goats are slaughtered at the completion of their productive life time. As the meat of the spent animals is tough, the whole production is used for their local consumption only. Therefore, there is a potential of incorporating a protease enzyme like cucumin in order to enhance the commercial usage of goat meat.
3 Comparative effect of the discussed plant proteolytic enzymes
Generally, it is known that enzymatic hydrolysis of meat proteins will increase the solubilization of free amino groups and hydroxyproline. As a result, the integrity of muscle will be loosened and will reduce the shear force or increase the tenderness (Fogle et al., 1982). A comparative study of Ha et al. (2012) show that the enzyme activity (casein hydrolytic activity, collagen hydrolysis capability and meat myofibril protein hydrolysis) of commercially available protease enzymes; papain, bromelain, actinidin and zingibain is significantly different. Zingibain protease has a more capability of hydrolysing meat connective tissue proteins while the actinidin protease is specifically effective at hydrolysing meat myofibrillar proteins.
Nevertheless, the study of Maqsood et al. (2018) on camel meat revealed that papain and bromelain enzymes, even at 100 ppm levels, the degradation of different protein fractions (total protein, sarcoplasmic protein, trichloroacetic acid (TCA)-soluble peptides and soluble collagen) were higher, but compared to meat treated with ficin, textural parameters were lower. The authors suggested that the tenderness of camel meat can be improved enzymatically and essential to optimize the process in terms of enzyme concentrations, method of administration and treatment time. Although there is no considerable difference in optimum pH ranges of the proteolytic enzymes, the Isoelectric Point of actinidin is significantly low (3.00) in contrast to that of papain and ficin (8.75 and 9.00 respectively) (Glazer and Smith, 1971).
Even though numerous researches have been done on the tenderizing capacity of plant proteases, their effect on altering the volatile composition and odour generation is minimally studied. According to Zhao et al. (2020), beef cuts treated with papain resulted in generating amino acids while bromelain produced more aldehydes and ketones. Aldehydes enhance flavour profiles with lack of off odours and therefore, treating with bromelain results in tender meat with more flavour and aroma.
4 Extraction, separation and purification of cysteine proteases
Novel purification methods have been employed for the extraction and purification of crude extract of meat tenderizing enzymes. Commonly used technologies are Aqueous Two-Phase Systems (ATPS), Reverse Micellar Extraction (RME), Precipitation, Ion exchange Chromatography (IEC) and Adsorption. However, each is having both advantages and disadvantages thus, these are currently being employed at a laboratory scale, not sufficiently developed upon large-scale processing. Therefore, given a broad impact, to understand how different technologies have been used for each enzyme extraction, significant findings are summarized in Table 3.
Briefly, ATPS is based on the formation of two immiscible layers by dissolving salts and polymers in aqueous solution. Then extractable protein is distributed among these phases by changing the ionic strength (use buffers), temperature and, pH and depending on the polarity protein is separated effectively. Although it costs low and efficient to eliminate trace contaminants, recovery means of target proteins are obscured. In RME reverse micelles are formed by a polar head inside the aqueous core and nonpolar tail outside intact with solution and fully embedded in a surfactant (Wheelwright (1991). Enzyme is solubilized in an aqueous solution and taken into organic phase; entrapping (forward extraction) and releasing (backward extraction) the target protein into the micelle by manipulating the ion strength, pH and concentration of surfactant. Recovery of target protein is difficult, but the loss of native protein is less and scaling up is convenient. Protein precipitation involves adding different types of salts, polar and nonpolar solvents into plant extracts while varying the solvent temperature and pH. Salting out or precipitation is anchored by decreasing the solubility of proteins and most often, ammonium sulphate is used as the precipitating agent. Additional steps of centrifugation and dialysis are being used as mentioned in Table 3 aiming to remove impurities. IEC is commonly used in the packed column with stationary and mobile phases implementing adsorption mechanisms. The base of adsorption is the use of solid adsorbent to bind with a solute dissolved in a solution and this method is becoming quite popular. Ultrafiltration is suggestable as an alternative option for above mentioned methods because of the non-consumption of solvents and quickness. Ultrasound can be assisted with the micro filtration for better separation.
5 Identified gaps and future trends
Comparative data regarding the required concentrations of proteolytic enzymes to deliver a similar output with respect to meat tenderness is lacking and there is an untouched research field on determining the best tenderizing agents according to the meat variety and the meat cut. Even though a variety of proteolytic enzymes are currently used in small scale, their commercial applications are limited. The chemical structures, mode of application, purification and optimum extraction protocols need to be further investigated and finely tuned.
Although a significant number of researches have been done on papain, bromelain and ficin a very few are published on the optimization of the separation and purification of zingibain and actinidin; noticeably no any recent studies on cucumin. Overall, there’s a small touch on novel extraction methods like ultrasonication and other combined mechanisms which could enhance the efficiency and avoid the usage of solvents (green technology). In an attempt to clarify this, cloning and encapsulation can be suggested to grow and stabilize the isolated cysteine proteases where authors emphasize that it is required to scrutinize deeply and establish new platforms to prepare these protease enzymes for the sake of industrial use.
For the benefit of the industry, a new protocol defined “immobilization” of proteases has become a trend recently. Immobilization of enzymes can improve the features of enzymes (eg: rigidity, intermolecular structure, resistance in unfavorable conditions etc) besides reusing them again and again (Morellon-Sterling et al., 2020). Nevertheless, some scientists discuss additional drawbacks of immobilizations itself (unexpected inactivation and formation of new bonds supporting the enzymes) and suggest cross linking of enzyme aggregates with immobilization using suitable cross linkers to overcome these problems (Banerjee et al, 2020). However, if these tools can be used to explore systematically, then it would result fruitfully in the meat tenderizing industry.
Patel (2015) reveals the tenderization effect of lemongrass volatile oil and there is a potential of incorporating oleoresins and volatile oils to enhance the tenderness of meat products. Since oleoresins contain all the components present in raw plant materials, all the other beneficial effects such as flavour, aroma generation, enhancing antioxidant capacity and natural preservative qualities are imparted in addition to the tenderization effect. The use of oleoresins of plant extracts is a feasible move in the industry due to the convenience in extraction, storage and cost effectiveness. Since the moisture content of oleoresins is negligible, the shelf life is high and the off flavour generation during cooking is minimal as a result of the heat stability of oleoresins. A list of other plant materials which can be used as tenderizing agents is given in Table 4 and it is necessary to research more on alternative sources with an economic feasibility in industrial scale.
Useful plant extracts
Protease activity on meat
Temperature of maximum protease activity
Reference
Grape juice (Vitis vinifera L)
Medium activity (225.86 unit)
50 °C
Koak et al. (2011)
Apple juice (Malus Pumila)
Lower activity (78.29 unit)
60 °C
Koak et al. (2011)
Pears juice (Pyrus serotina L.)
Lower activity (97.75unit)
50 °C
Koak et al. (2011); Han and Chin, 2004
Citrus juice (Family of Citrus)
Lower effect Not quantified
Unknown
Burke and Monahan (2003)
Garcinia (Garcinia gummi-gutta)
Not quantified
Unknown
Traditional tenderizing effect is known, but no literature evidence
Pepper oil (Piper nigrum)
Authors research on pepper oleoresin, revealed a significant effect
Under research stage
Traditional tenderizing effect is known, but no literature evidence
6 Conclusion
The incorporation of proteolytic enzymes with meat products result in reducing their toughness and enhancing the eating quality. However, the use of exogenous proteases should be done under controlled conditions to achieve optimum results and their excessive usage may deteriorate the product quality. Majority of the exogenous enzymes are plant based and mainly extracted through solvents. Furthermore, only a limited number of proteolytic enzymes are commercially manufactured and thus most of the meat producers are unable to afford them. With respect to increasing the usage of meat tenderization agents, it is recommended to research further on extraction and purification mechanisms of minimally utilizing proteolytic enzymes such as zingibain, cucumin, actinidin and other available underutilized plant varieties. Additionally, exploration of food enzyme structures could contribute to the development of the food processing industry by letting understanding the behaviour of the enzymes and, improving the separation and purification of plant enzymes.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Effect of zingibain on the quality characteristics of dry-cured mutton ham. Shipin Kexue/Food Sci.. 2019;40(8):15-21.
- [Google Scholar]
- Stabilization and partial purification of a protease from ginger rhizome (Zingiber offinale Roscoe) J. Food Sci.. 2005;70(3):C231-C234.
- [Google Scholar]
- Extraction, characterization and molecular structure of bovine skin gelatin extracted with plant enzymes bromelain and zingibain. J. Food Sci. Technol. 2020:1-10.
- [Google Scholar]
- Optimizing Extraction Conditions of Actinidin from Kiwifruit (Actinidia deliciosa) Al-Mustansiriyah J. Sci.. 2017;28(3):61-67.
- [Google Scholar]
- Effect of actinidin on the protein solubility, water holding capacity, texture, electrophoretic pattern of beef, and on the quality attributes of a sausage product. J. Food Sci.. 2009;74(3):C221-C226.
- [Google Scholar]
- Profile of biochemical traits influencing tenderness of muscles from the beef round. Meat Sci.. 2012;91:247-254.
- [Google Scholar]
- Bromelain: an overview of industrial application and purification strategies. Appl. Microbiol. Biotechnol.. 2014;98(17):7283-7297.
- [Google Scholar]
- Effects of papain and a microbial enzyme on meat proteins and beef tenderness. J. Food Sci.. 2002;67(6):2138-2142.
- [Google Scholar]
- Influence of cooking conditions on cooking loss and tenderness of raw and marinated chicken breast meat. LWT-Food Sci. Technol.. 2005;38(8):895-901.
- [Google Scholar]
- Liquid–liquid extraction of bromelain and polyphenol oxidase using aqueous two-phase system. Chem. Eng. Process. Process Intensif.. 2008;47(1):83-89.
- [Google Scholar]
- Kinetic study of the effect of kiwi fruit actinidin on various proteins of chicken meat. Food Sci. Technol.. 2019;39(4):980-992.
- [Google Scholar]
- Structure of actinidin: Details of the polypeptide chain conformation and active sitfrom an electron density map at 2· 8 Å resolution. J. Mol. Biol.. 1977;115(3):263-277.
- [Google Scholar]
- Extraction and crosslinking of bromelain aggregates for improved stability and reusability from pineapple processing waste. Int. J. Biol. Macromol. 2020
- [Google Scholar]
- Beef quality perception at the point of purchase: A study from Portugal. Food Qual. Prefer.. 2009;20(4):335-342.
- [Google Scholar]
- Improvement of meat tenderness by simultaneous application of high-intensity ultrasonic radiation and papain treatment. Innovative Food Sci. Emerg. Technol.. 2017;39:223-229.
- [Google Scholar]
- Exogenous proteases for meat tenderization. Crit. Rev. Food Sci. Nutr.. 2014;54(8):1012-1031.
- [Google Scholar]
- Mapping the active site of papain with the aid of peptide substrates and inhibitors. Philos. Trans. Roy. Soc. London Series B, Biol. Sci.. 1970;257(813):249-264.
- [Google Scholar]
- Applied and emerging methods for meat tenderization: A comparative perspective. Compr. Rev. Food Sci. Food Saf.. 2018;17(4):841-859.
- [Google Scholar]
- Kinetic studies on the thiol protease from Actinidia chinensis. FEBS Lett.. 1972;27(2):282-284.
- [Google Scholar]
- New developments in shockwave technologyintended for meat tenderization: Opportunities and challenges. A review. Meat Sci.. 2013;95(4):931-939.
- [Google Scholar]
- The tenderisation of shin beef using a citrus juice marinade. Meat Sci.. 2003;63(2):161-168.
- [Google Scholar]
- Optimization of bromelain isolation from pineapple byproducts by polysaccharide complex formation. Food Hydrocolloids. 2019;87:792-804.
- [Google Scholar]
- Effect of visual marbling on sensory properties and quality traits of pork loin. Meat Sci.. 2010;85(3):428-434.
- [Google Scholar]
- Cazarin, C.B.B., Lima, G.C., da Silva, J.K., Maróstica, M., 2015. Enzymes in meat processing. In: Enzymes in food and beverage processing. CRC, Boca Raton, pp. 337–351.
- Consumer sensory evaluation and chemical composition of beef gluteus medius and triceps brachii steaks from cattle finished on forage or concentrate diets. J. Anim. Sci.. 2017;95(4):1553-1564.
- [Google Scholar]
- Chao, D., 2016. Actinidin: the predominant protease in kiwifruit: a thesis presented in partial fulfilment of the requirements for the degree of Master of Philosophy in Food technology at Massey University, Manawatū, New Zealand (Doctoral dissertation, Massey University).
- Efficacy of reverse micellar extracted fruit bromelain in meat tenderization. J. Food Sci. Technol.. 2015;52(6):870-880.
- [Google Scholar]
- Valuation and characterization of actinidin treatent to Pork. Adv. Mater. Res. Trans Tech Publications Ltd. 2012;554:1258-1261.
- [Google Scholar]
- The 2.1 Å structure of a cysteine protease with proline specificity from ginger rhizome, Zingiberofficinale. Biochemistry. 1999;38(36):11624-11633.
- [Google Scholar]
- Injection of marinade with actinidin increases tenderness of porcine M. biceps femoris and affects myofibrils and connective tissue. J. Sci. Food Agric.. 2009;89(9):1607-1614.
- [Google Scholar]
- Partial purification and properties of proteases from fig (Ficuscarica) callus cultures. Biotechnol. Lett.. 1989;11(11):797-802.
- [Google Scholar]
- Purification, characterization, and solvent-induced thermal stabilization of ficin from Ficus carica. J. Agric. Food. Chem.. 2008;56(23):11417-11423.
- [Google Scholar]
- Purification and biochemical characterization of peroxidase isoenzymes from Ficus carica latex. Biocatal. Agric. Biotechnol.. 2018;16:1-9.
- [Google Scholar]
- Antibacterial effects of natural tenderizig enzymes on different strains of Escherichia coli O157: H7 and Listeria monocytogenes on beef. Meat Sci.. 2014;96(4):1494-1500.
- [Google Scholar]
- Studies on ficin. I. Its isolation and characterization. Biochemistry®. 1968;7(1):163-175.
- [Google Scholar]
- Native and biotechnologically engineered plant proteases with industrial applications. Food Bioprocess Technol.. 2011;4(6):1066-1088.
- [Google Scholar]
- Tenderization of beef, effect of enzyme, enzyme level, and cooking method. J. Food Sci.. 1982;47(4):1113-1118.
- [Google Scholar]
- Consumer preference, behavior and perception about meat and meat products: An overview. Meat Sci.. 2014;98(3):361-371.
- [Google Scholar]
- Meat tenderization by proteolytic enzymes after osmotic dehydration. Meat Sci.. 2000;56(3):311-318.
- [Google Scholar]
- Glazer, A.N., Smith, E.L., 1971. Papain and other sulfhydryl proteolytic enzymes. In: Boyer, P.D. (Ed.), The Enzymes. Academic Press, New York, pp. 501–546.
- Mass transfer dynamics during the acidic marination of turkey meat. J. Food Eng.. 2011;104(1):161-168.
- [Google Scholar]
- Comparative efficacy of actinidin from green and gold kiwi fruit extract on in vitro simulated protein digestion of beef Semitendinosus and its myofibrillar protein fraction. Int. J. Food Sci. Technol.. 2020;55(2):742-750.
- [Google Scholar]
- Thornton's meat hygiene (7th edition.). Bailliere Tindall; 1981.
- Characterisation of commercial papain, bromelain, actinidin and zingibain protease preparations and their activities toward meat proteins. Food Chem.. 2012;134(1):95-105.
- [Google Scholar]
- Effects of L-and iso-ascorbic acid on meat proteinhydrolyzing activity of four commercial plants and three microbial protease preparations. Food Chem.. 2014;149:1-9.
- [Google Scholar]
- Positions of disulfide bonds in riboflavin protein of hen egg white. J. Biochem.. 1987;101(1):217-223.
- [Google Scholar]
- Study on Meat Tenderness of a Pretense Extracted from Domestic Pear. Food Sci. Animal Resour.. 2004;24(4):326-328.
- [Google Scholar]
- Cleavage of the calpain inhibitor, calpastatin, during postmortem ageing of beef skeletal muscle. Food Chem.. 2014;148:1-6.
- [Google Scholar]
- Cysteine enhances activity and stability of immobilized papain. Amino Acids. 2010;38(3):937-942.
- [Google Scholar]
- Purification, catalytic, kinetic and thermodynamic characteristics of a novel ficin from Ficus johannis. Biocatal. Agric. Biotechnol.. 2017;10:360-366.
- [CrossRef] [Google Scholar]
- Effect of beef tenderness on consumer satisfaction with steaks consumed in the home and restaurant. J. Anim. Sci.. 1996;74(1):91-97.
- [Google Scholar]
- Proteolytic activity of Cucumis trigonus Roxb. extraction, activity, characteristics. J. Food Sci.. 1970;35(3):276-278.
- [Google Scholar]
- Improving beef meat colour scores at carcass grading. Animal Prod. Sci.. 2014;54(4):422-429.
- [Google Scholar]
- Effect of pelvic suspension on the instrumental meat quality characteristics of red deer (Cervus elaphus) and fallow deer (Dama dama) venison. Meat Sci.. 2014;98(2):104-109.
- [Google Scholar]
- Islam, M.N., Molinar-Toribio, E.M., 2013. Development of a meat tenderizer based on papaya peel. Observación por Pares Basada en Mapas Conceptuales: Una Estrategia para Fomentar el “Scholarship of Teaching and Learning” en la Universidad Tecnológica de Panamá, 24.
- Jørgensen, A.S., Christensen, M., Ertbjerg, P., 2008. Marination with kiwifruit powder followed by power ultrasound tenderizes porcine m. biceps femoris. International Conference of Meat Science and Technology, Cape Town. Sudafrica.
- Structure of papain refined at 1.65 Å resolution. J. Mol. Biol.. 1984;179(2):233-256.
- [Google Scholar]
- Degradation of various meat fractions by tenderizing enzymes. J. Food Sci.. 1970;35:563-565.
- [Google Scholar]
- Factors associated with the tenderness of three bovine muscles. J. Food Sci.. 1988;53(2):407-410.
- [Google Scholar]
- Contribution of postmortem muscle biochemistry to the delivery of consistent meat quality with particular focus on the calpain system. Meat Sci.. 2006;74(1):34-43.
- [Google Scholar]
- Plant collagenase: Unique collagenolytic activity of cysteine proteases from ginger. BBA. 2007;1770:1627-1635.
- [Google Scholar]
- Characterization of a protease from over-matured fruits and development of a tenderizer using an optimization technique. Food Sci. Biotechnol.. 2011;20(2):485-490.
- [Google Scholar]
- Koohmaraie, M., 1992. Role of neutral proteinases in postmortem muscle degradation and meat tenderness. In: Proceeding of Reciprocal Meat Conference, 45 ,pp. 65.
- Ficus enzymes II. Properties of the proteolytic enzymes from the latex of Ficus carica variety Kadota. J. Biol. Chem.. 1964;239(7):2178-2183.
- [Google Scholar]
- Meat tenderness evaluation using dual energy X-ray absorptiometry (DEXA) Comput. Electron. Agric.. 2006;54(2):93-100.
- [Google Scholar]
- Lana, A., Zolla, L., 2016. Proteolysis in meat tenderization from the point of view of each single protein: A proteomic perspective, 147, pp. 85–97.
- Lawrie, R.A., 1998. Lawrie’s Meat Science. Section 10. 3. 4, pp. 244–246
- Application of actinidin from kiwifruit to meat tenderization and characterization of beef muscle protein hydrolysis. J. Food Biochem.. 1988;12(3):147-158.
- [Google Scholar]
- Light, N., Champion, A.E., Voyle, C., Bailey, A.J., 1985. Meat Science. pp 13, 137.
- Lonescu, A., Aprodu, I., Pascaru, G., 2008. Effect of Papain and Bromelain on Muscle and collagen proteins in beef meat. Ann. Univ. Dunarea de Jos of Galati Fascicle VI--Food Technology, 1 (31).
- A proteomic-based approach for the search of biomarkers in Iberian wild deer (Cervus elaphus) as indicators of meat quality. J. Proteomics. 2019;205:103422
- [Google Scholar]
- Influence of muscle type on physicochemical and sensory properties of foal meat. Meat Sci.. 2013;94(1):77-83.
- [Google Scholar]
- 30,000-Dalton component of tender bovine longissimus muscle. J. Food Sci.. 1977;42:1627-1629.
- [Google Scholar]
- Studies on the influence of age of sheep and post-mortem carcass conditioning treatments on muscular collagen content and its thermal ability. J. Food Sci. Technol.. 1989;26(2):102-105.
- [Google Scholar]
- Papain, a plant enzyme of biological importance: a review. Am. J. Biochem. Biotechnol.. 2012;8(2):99-104.
- [Google Scholar]
- Application of natural tenderizers in meat-a review. Agric. Rev.. 2008;29(3):226-230.
- [Google Scholar]
- The effect of crossing New Zealand White with Californian rabbits on growth and slaughter traits. Archives Animal Breeding. 2009;52(2):205-211.
- [Google Scholar]
- Mane, B.G., Mendiratta, S.K., Dhanze, H., 2014. Tenderization of meat and meat products: A detailed review. Food Composition Anal.: Methods Strategies, 95.
- Tenderisation of meat using bromelain from pineapple extract. Int. J. Pharmaceut. Sci. Rev. Res.. 2016;39(1):81-85.
- [Google Scholar]
- Degradation of myofibrillar, sarcoplasmic and connective tissue proteins by plant proteolytic enzymes and their impact on camel meat tenderness. J. Food Sci. Technol.. 2018;55:3427-3438.
- [Google Scholar]
- Marquer, P., Rabade, T., Forti R., 2015. Meat Production Statistics. https://ec.europa.eu/eurostat/statistics-explained/pdfscache/28947.pdf.
- Changes in the physicochemical and sensory characteristics in raw and grilled ovine meat. J. Sci. Food Agric.. 2013;93(7):1743-1750.
- [Google Scholar]
- Tenderization of yak meat by the combination of papain and high-pressure processing treatments. Food Bioprocess Technol.. 2019;12(4):681-693.
- [Google Scholar]
- Anionic proteinase from Actinidia chinensis: preparation and properties of the crystalline enzyme. Eur. J. Biochem.. 1970;14(2):214-221.
- [Google Scholar]
- The action of proteinase A, of Actinidia chinensis on the p-chain of oxidized insulin. BBA. 1973;293:226.
- [Google Scholar]
- Consumer thresholds for establishing the value of beef tenderness. J. Anim. Sci.. 2001;79(12):3062-3068.
- [Google Scholar]
- Isolation, identification, and stability of Ficin 1c isoform from fig latex. New J. Chem.. 2020;44(36):15716-15723.
- [Google Scholar]
- Morellon-Sterling, R., El-Siar, H., Tavano, O.L., Berenguer-Murcia, Á., Fernández-Lafuente, R., 2020. Ficin: A protease extract with relevance in biotechnology and biocatalysis. Int. J. Biol. Macromol.
- Purification and physical characterization of stem bromelain*. Biochemistry. 1964;3(1):48-55.
- [Google Scholar]
- Evidence for glycoprotein nature of stem bromelain. Isolation of a glycopeptide*. Biochemistry. 1967;6(12):3730-3736.
- [Google Scholar]
- Nadaraja, U.D., 2010. The extraction of papain enzyme from sekakaiand exotica papaya and its effect on the aged egg layer chicken meat texture. School of Food Science and Nutrition, University of Malaysia Sabha, pp. 1–28.
- Identification and functional characterization of cysteine protease from nine pear cultivars (Pyrus pyrifolia) Int. J. Food Prop.. 2016;19(7):1631-1644.
- [Google Scholar]
- Purification and characterization of multiple forms of the pineapplestem-derived cysteine proteinases ananain and comosain. Biochem. J.. 1994;301:727-735.
- [Google Scholar]
- Effects of citric acid, cucumis powder and pressure cooking on quality attributes of goat meat curry. J. Food Sci. Technol.. 2015;52(3):1772-1777.
- [Google Scholar]
- Tenderization of buffalo meat using plant proteases from Cucumis trigonus Roxb (Kachri) and Zingiber officinale roscoe (Ginger rhizome) Meat Sci.. 2004;68(3):363-369.
- [Google Scholar]
- Substrate Profiling of Cysteine Protease from Zingiber officinale. Food Biotechnol.. 2018;32(2):148-161.
- [Google Scholar]
- Purification of papain from Carica papaya latex: Aqueous two-phase extraction versus two-step salt precipitation. Enzyme Microb. Technol.. 2006;39(5):1103-1107.
- [Google Scholar]
- Separation of bromelain from crude pineapple waste mixture by a two-stage ceramic ultrafiltration process. Food Bioprod. Process.. 2016;98:142-150.
- [CrossRef] [Google Scholar]
- Enzymes in tenderization of meat-the system of calpains and other systems-a review. Polish J. Food Nutrition Sci.. 2011;61(4):231-237.
- [Google Scholar]
- Inhibition of papain in meat by potato protein or ascorbic acid. J. Food Sci.. 1993;58:1265-1268.
- [Google Scholar]
- Relationship of myofibril fragmentation index to measures of beef steak tenderness. J. Food Sci.. 1977;42(2):506-509.
- [Google Scholar]
- Preparation and chemical properties of purified stem and fruit bromelains*. Biochem. J.. 1964;3(2):180-185.
- [Google Scholar]
- Evaluation of the contribution of tenderness, juiciness, and flavor to the overall consumer beef eating experience. Transl. Animal Sci.. 2018;2(1):26-36.
- [Google Scholar]
- Meat tenderization: possible causes and mechanisms. A review. J. Muscle Foods. 1990;1(2):129-165.
- [Google Scholar]
- Plant essential oils and allied volatile fractions as multifunctional additives in meat and fish-based food products: a review. Food Additives Contaminants: Part A. 2015;32(7):1049-1064.
- [Google Scholar]
- Comparison of carcass and sensory traits and free amino acid contents among quality grades in loin and rump of Korean cattle steer. Asian-Australasian J. Animal Sci.. 2015;28(11):1629.
- [Google Scholar]
- Development of a commercial system to apply the Meat Standards Australia grading model to optimise the return on eating quality in a beef supply chain. Aust. J. Exp. Agric.. 2008;48(11):1451-1458.
- [Google Scholar]
- Production and utilization of papain-a proteolytic enzyme from Carica papaya L. Tropical. Sci.. 1985;25:123-137.
- [Google Scholar]
- Prabhu, A.A., Chityala, S., Garg, Y., Venkata Dasu, V., 2017. Reverse micellar extraction of papain with cationic detergent based system: an optimization approach. Preparative Biochem. Biotechnol. 47(3), pp. 236–244.
- Biochemical changes of postmortem meat during the aging process and strategies to improve the meat quality. In: Meat Quality Analysis. Academic Press; 2020. p. :67-80.
- [Google Scholar]
- Effect of chemically modified soy proteins and ficin-tenderized meat on the quality attributes of sausage. J. Food Sci.. 2003;68(1):85-88.
- [Google Scholar]
- Comparative structural analysis of fruit and stem bromelain from Ananas comosus. Food Chem.. 2018;266:183-191.
- [Google Scholar]
- Relationships between muscle characteristics and meat quality traits of young Charolais bulls. Meat Sci.. 2001;59(1):49-60.
- [Google Scholar]
- Variation in palatability and biochemical traits within and among eleven beef muscles. J. Anim. Sci.. 2004;82(2):534-550.
- [Google Scholar]
- Reduction in elasticity of Kamaboko (boiled fish-paste) by some additives containing protease. Japanese Soc. Food Sci. Technol.. 1980;27(8):371-376.
- [Google Scholar]
- Hydrolysis of muscle proteins by actinidin (kiwifruits protease) Nippon Shokuhin Kogyo Gakkaishi. 1991;38(9):817-821.
- [Google Scholar]
- The first report on molecular cloning, functional expression, purification, and statistical optimization of Escherichia coli-derived recombinant Ficin from Iranian fig tree (Ficus carica cv. Sabz) Int. J. Biol. Macromol.. 2020;165:2126-2135.
- [Google Scholar]
- Palatability of beef steaks marinated with solutions of calcium chloride, phosphate, and (or) beef-flavoring. Meat Sci.. 2000;55(4):397-401.
- [Google Scholar]
- Schenkova, N., Šikulová, M., Jelenikova, J., Pipek, P., Houška, M., Marek, M., 2007. Influence of high isostatic pressure and papain treatment on the quality of beef meat. High Pressure Res. 27(1), pp. 163–168.
- Sgarbieri, V.C., Gupte, S.M., Kramer, D.E., Whitaker, J.R., 1964. Ficus enzymes I. Separation of the proteolytic enzymes of Ficus carica and Ficus glabrata latices. J. Biol. Chem. 239(7), pp. 2170–2177.
- Shackelford, S.D., Wheeler, T.L., Meade, M.K., Reagan, J.O., Byrnes, B.L., Koohmaraie, M., 2001. Consumer impressions of Tender Select beef. J. Animal Sci. 79 (10), pp. 2605–2614.
- Gases (second ed.). Food Additives Data Book; 2003. p. :581-596.
- Solov’ev, V.I., Krakova, V.Z., 1973. Enzyme activity of ficin preparations from different sources and their effect on meat. J. Appl. Microbiol. Biochem. 7, pp. 158–164.
- Muscle stretching techniques for improving meat tenderness. Trends Food Sci. Technol.. 2002;13(4):127-135.
- [Google Scholar]
- Thermal Inactivation Kinetics of Bromelain in Pineapple Juice. J. Am. Soc. Agric. Eng.. 2000;43(6):1703-1708.
- [Google Scholar]
- Application of exogenous enzymes to beef muscle of high and low-connective tissuee. Meat Sci.. 2010;85(4):730-734.
- [Google Scholar]
- Sunantha, K., Saroat, R., 2011. Application of bromelain extract for muscle foods tenderization. Food Nutrition Sci. 2(5), pp. 393–401.
- Use of Plant Proteolytic Enzymes for Meat Processing. Biotechnol. Appl. Plant Proteolytic Enzymes 2018:43-67.
- [Google Scholar]
- Tarté, R. (Ed.)., 2009. Ingredients in meat products properties, functionality and applications. Springer Science & Business Media, pp. 1–23.
- Ginger rhizome: A new source of proteolytic enzyme. J. Food Sci.. 1973;38(4):652-655.
- [Google Scholar]
- Consumer perception and the role of science in the meat industry. Meat Sci.. 2010;86(1):214-226.
- [Google Scholar]
- Valin, C., Kopp, J., 1978. Effects of technological factors on tenderness of beef meat [slaughtering, chilling of the carcass, temperature and time of storage, cooking]. Industries Alimentaires et Agricoles (France).
- Meat and Meat Products. London: Chapman and Hall; 1995. p. :64-65.
- Factors regulating lamb longissimus tenderness are affected by age at slaughter. Meat Sci.. 2004;68(4):635-640.
- [Google Scholar]
- The solubilization of unheated cattle achilles tendon with actinidin under neutral and acidic conditions. Food Sci. Technol. Res.. 2004;10(1):35-37.
- [Google Scholar]
- Changes in actomyosin dissociation and endogenous enzyme activities during heating and their relationship with duck meat tenderness. Food Chem.. 2013;141(2):675-679.
- [Google Scholar]
- Wheelwright, S.M., 1991. Protein Purification. Design and Scale up of Downstream Processing. John Wiley & Sons, Inc
- White, A., O’sullivan, A., Troy, D.J., O’Neill, E.E., 2006. Manipulation of the pre-rigor glycolytic behaviour of bovine M. longissimus dorsi in order to identify causes of inconsistencies in tenderness. Meat Sci. 73(1), pp. 151–156.
- Seasonal variation in red deer (Cervus elaphus) venison (M. longissimus dorsi) drip loss, calpain activity, colour and tenderness. Meat Sci.. 2010;86(3):720-727.
- [Google Scholar]
- Inactivation of papain by pulsed electric fields in a continuous system. Food Chem.. 1999;67(1):53-59.
- [Google Scholar]
- Effects of cooking methods and chemical tenderizers on survival of Escherichia coli O157: H7 in ground beef patties. Meat Sci.. 2013;95(2):317-322.
- [Google Scholar]
- Separation and purification of papain crude extract from papaya latex using quaternary ammonium ionic liquids as adjuvants in PEG-based aqueous two-phase systems. Food Anal. Methods. 2020;13:1462-1474.
- [Google Scholar]
- Purification and autolysis of the ficin isoforms from fig (Ficus carica cv. Sabz) latex. Phytochemistry. 2013;87:16-22.
- [Google Scholar]
- Characterization of actinidin from Chinese kiwifruit cultivars and its applications in meat tenderization and production of angiotensin I-converting enzyme (ACE) inhibitory peptides. LWT. 2017;78:1-7.
- [Google Scholar]
- Influence of proteolytic enzyme treatment on the changes in volatile compounds and odors of beef longissimus dorsi. Food Chem.. 2020;333:127549
- [Google Scholar]
- Optimization of [CnPy] Cl (n= 2, 4, 6) ionic liquid aqueous two-phase system extraction of papain using response surface methodology with Box-Behnken design. Process Biochem.. 2019;77:113-121.
- [Google Scholar]







