Translate this page into:
Mechanism study of stabilization of double-base propellants by using zeolite stabilizers (nano- and micro-clinoptilolite)
⁎Corresponding author. Tel.: +2 02 22728437, +2 01005776675; fax: +2 02 35728843. mazayed429@yahoo.com (M.A. Zayed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.

Abstract
4% Nano-clinoptilolite considered as a good stabilizer for DBPs. Thermal tests, XRD, AFM and AEM explained surface effect on DBPs stability. Nano-clinoptilolite makes the surface of DBPS more ordered and homogenized. Mechanism of DBP stabilization by clinoptilolite is also explained.
Abstract
The mechanism of stabilization of double-base rocket propellants (DBPs) using inorganic stabilizer (nano- and micro-clinoptilolite) was investigated. The surface structures of the stabilizers, the double-base propellants containing the new stabilizers and the effect of the stabilizers on the surface behavior of propellants and vice versa were checked using XRD and Electron Microscope (AFM and TEM techniques). The results obtained from XRD suggested that the crystalline structure of the new inorganic stabilizers was completely changed when it was introduced into the propellants which may be attributed to the pressing processes of DBPs with stabilizers under very high pressure during their mixture preparation. The results obtained from Atomic Force Electron Microscope (AFM) and TEM indicated that nano-clinoptilolite particles become more regularly arranged on the propellant surface than micro-clinoptilolite which gives the stabilizer a higher ability to absorb more nitrogen oxide. The work aimed chiefly to use zeolite stabilizers for DBPs instead of classically used organic compounds; in order to avoid the harmful and carcinogenic organic products coming from the reaction of NOx gases with these organic stabilizers. This is achieved by studying the thermal behavior of these zeolites via investigation of their surface interaction with NOx gases obtained during stabilization process and suggesting possible interaction mechanism.
Keywords
Nano- and micro-clinoptilolite stabilizers
Double-base rocket propellants
NOx gases
XRD
AFM
TEM
Mechanism
1 Introduction
The nitric esters of double base propellants decomposed at rates dependent on time and temperature. This decomposition corresponds to the rupture of the O–NO2 bonds, and releases nitrogen oxides. Without a stabilizer, the products resulting from the decomposition have a catalytic effect on the decomposition reaction rate. The reaction is controlled when stabilizers are included. In a research work extending over a long period (Macleod and Johnson, 2006; Fryš et al., 2011) researchers investigated the stabilizing effect of various organic substances particularly naphthalene, mononitronaphthalene, N,N-diphenylbenzamide, carbazol, diphenylamine and diphenylnitrosoamine. The role of stabilization of organic stabilizers for double base propellants (DBPs) depends on chemical reactions between the stabilizer and the nitrogen oxide gas evolved through thermal decomposition of the propellants (Macleod and Johnson, 2006; Fryš et al., 2011). These reactions mostly lead to the formation of poisoning compounds which can be avoided by the use of inorganic stabilizers.
Generally the use of inorganic stabilizers for DBPs in the literature is scanty (Wayne et al., 2005), an aluminum silicate molecular sieve was added to propellants that give off gases such as N2, CO2, CO, F2 and NOx during the aging process. The aluminum silicate molecular sieve was selected to have a pore size of less than about 10 Å. This brought about absorption of these undesirable gases during the aging process and thereby prevented degradation of the propellant (Robert, 1976). Zeolites are a large group of natural and synthetic hydrated aluminum silicates. They are characterized by complex three-dimensional structures with large, cage like cavities that can accommodate sodium, calcium or other cations (positively charged atoms or atomic clusters); water molecules; and even small organic molecules. Ions and molecules in the cages can be removed or exchanged without destroying the aluminosilicate framework. Zeolites find wide use as ion-exchange agents, catalysts, and molecular filters in several industrial processes (Tearpock and Bischke, 2002; Perraki and Orfanoudaki, 2004; Penn et al., 2010).
Clinoptilolite is used in many applications such as a chemical sieve, gas absorber, feed additive, food additive, odor control agent and as a water filter for municipal and residential drinking water and aquariums. Its applications are related to a large amount of pore space, high resistance to extreme temperatures and chemically neutral basic structure (Hassan, 2011; Hossein et al., 2008).
Clinoptilolite is a low field strength zeolite for which the cation specivities Cs+ > Rb+ > > K+ > Na+ > Li+ > H+, and Ba2+ > Sr2+ > Ca2+ > Mg2+ are predicted (Kugbe et al., 2009; Palomino et al., 2008; Xu et al., 2008; Yang et al., 2009; Shang et al., 2009). Charge-balancing of cations present on the surface of very fine-grained clinoptilolite can be replaced by high-molecular weight quaternary amines (Gu et al., 2008), such as hexadecyltrimethylammonium (HDTMA) whereas the internal zeolite cavities remained accessible for small cations. Surfactant modified zeolites (SMZ) absorb , benzene and perchloroethylene (PCE) suggesting that a stable HDTMA bi-layer formed on the external surface of the zeolite. Non-polar organic solutes were absorbed by the organic phase whereas anions were retained on the outward pointing positively charged head groups of the surfactant bi-layer (Gu et al., 2008). Various types of surfactants on clinoptilolite were applied to extract benzene, toluene and xylenes from petrochemical spills (El-Naggar et al., 2008). The modified clinoptilolite exhibited enhanced sorption of cations (Jha and Hayash, 2009; Novosad et al., 2005).
The use of conventional organic stabilizers forms nitrosamines that have toxic and carcinogenic effects. As a result, these conventional stabilizers should be replaced (Bergens and Danielsson, 1995). The use of inorganic stabilizers for double-base propellants can solve this problem (Robert, 1976; Asthana et al., 1992; Dominic and Wilmington, 1975; Sakizci et al., 2011; Zayed et al., 2012). Therefore, in this work clinoptilolite and centralite stabilizers are used in the mechanism of stabilization study.
2 Experimental
2.1 Preparation of clinoptilolite in nano-scale
Clinoptilolite stabilizer was prepared in its nano-form by grinding its micro-form (50–75 μm) (which was purchased from Ostim, Ankara, Turkey) for 8 h with a rate of 400 RPM (Round Per Minute) using a grinder of type (Retsch GMBh & Co.KG, Model 2005, Rheinische Straβe 36, 42781 Haan, Germany), in the Science and Technology Center of Excellence (Ministry of military production, Egypt).
2.2 Preparation of DBP samples
Samples of double-base smokeless powders were manufactured by solvent less process using (3% w/w) centralite I as a reference stabilizer in sample C1, 2.0–4.0% (w/w) nano-clinoptilolite stabilizer in samples S1–S5 and 3% micro-clinoptilolite stabilizer in sample S6.
2.3 Different thermal tests used to check the stability of DBP samples
The classical thermal stability tests (Bergmann – Junk and calorimetric tests) (Johns et al., 2008; Soliman, 2004) were used to evaluate the stability of the prepared DBP samples S1–S6 and C1.
2.3.1 Bergmann – Junk test at 120 °C (B–J)
The B–J test was done on sample pieces with a length of approximately 3–4 mm and a diameter of 2 mm and dried at 45 °C for 16 h. The prepared powder (5 g) was introduced into the test tube and treated as explained by Bergmann – Junk (Johns et al., 2008; Soliman, 2004).
2.3.2 Calorimetric test
Calorimetric test was carried out in calorimetric bomb with a capacity of 450 cc as explained by Hassan (Hassan, 2011; Zayed et al., 2012) to estimate the calorific values of the investigated samples (S1–S6 and C1).
2.4 Different tests used to check the surface structure of stabilizers and DBP samples containing the stabilizers
X-ray Diffraction and electron microscope (both AFM and TEM techniques) analyses were used to check the surface structure of nano-clinoptilolite, micro-clinoptilolite and centralite I stabilizers and DBPs samples containing the stabilizers, and to study the effect of stabilizers on the surface structure of double-base propellants and vice versa (Hassan, 2011).
2.4.1 X-ray diffraction analysis
The composition of the investigated DBP samples and stabilizers used (Clinoptilolite and Centralite I) was determined by the X-ray diffraction technique (XRD) using PHILIPS PW 3710/31 diffractometer, scintillation counter, Cu-target tube and Ni filter at 40 kV and 30 mA. This instrument is connected to a computer system using APD program and PDF-2 database for mineral identification. The scan covered a 2-theta range of 5–80° with a step size of 0.02° and a 0.5-s count time per step. The slits used consisted of 1-degree fixed divergence and anti-scatter slits and a 0.2-mm receiving slit.
2.4.2 Electron microscope analysis
Two different techniques of electron microscope (Transmission Electron Microscope, TEM JOEL JSM-1230 and Atomic Force Microscope, AFM, JEOL JXA-840A) were used to determine the grain size of clinoptilolite and showing its surface structure. On the other hand only AFM technique was used to show the surface structure of nano-clinoptilolite and DBP samples containing (weight/weight, w/w%) 3% nano-clinoptilolite, 3% micro-clinoptilolite and 3% centralite I, and to know the effect of each stabilizer on the surface structure of the propellant samples and vice versa.
3 Results and discussion
The evaluation and determination of the mechanism of stabilization of clinoptilolite for DBPs depend on a comparative study of thermal and surface behavior of these inorganic stabilizers with the results of the classical stabilizer (N,N-diethyldiphenyl urea) (C1 = 3% w/w). The compositions of the tested DBPs samples are given in Table 1. (NC = Nitrocellulose, NG = Nitroglycerine, DBP = Dibutylphthalate, DNT = Dinitrotoluene).
Sample no.
NC %
NG %
DBP %
DNT %
Lead stearate %
Carbon black %
Stabilizer type
Stabilizer %
Grain-size of stabilizer
S1
59
31
5
2.7
2
0.3
Clinoptilolite
4.0
30 nano
S2
59
31
5
2.7
2
0.3
Clinoptilolite
3.5
30 nano
S3
59
31
5
2.7
2
0.3
Clinoptilolite
3.0
30 nano
S4
59
31
5
2.7
2
0.3
Clinoptilolite
3.0
30 nano
S5
59
31
5
2.7
2
0.3
Clinoptilolite
2.5
30 nano
S6
59
31
5
2.7
2
0.3
Clinoptilolite
2.0
2 μm
C1
59
31
5
2.7
2
0.3
Centralite I
3.0
2 μm
3.1 Classical thermal stability tests for double-base propellant samples
Bergmann – Junk test (B–J) and Calorimetric classical thermal test (Johns et al., 2008; Soliman, 2004) results for the double-base propellant samples containing different stabilizers percents are given in Table 2, in which mL NaOH (0.05 N) is equivalent to the amount of NOx gases evolved during stabilization thermal test process. The quantitative stability results (B–J); indicated that nano-clinoptilolite percentages were divided into two groups according to their stabilizing effect in comparison with classical stabilizer. The first group includes S1 = 4%, S2 = 3.5% e and S3 = 3% nano-clinoptilolite, which have a higher stabilizing effect (6.6, 7 and 7 mL of NaOH equivalent to evolved NOx gases respectively) than the classical one (7.2 mL of NaOH). The second group includes S4 = 2.5% and S5 = 2% nano-clinoptilolite which have a lower stabilizing effect (8.5 and 9 mL NaOH respectively) than the classical stabilizer. 3% Micro-clinoptilolite has the same stabilizing effect (7.2 mL NaOH) as the classical stabilizer. This means that, 4% nano-clinoptilolite sample has shown the best stabilizing effect among all the percentages of nano-clinoptilolite and classical stabilizer. N.B. mL NaOH = [NOx gases].
Sample no.
Stabilizer type
Stabilizer %
Bergmann at 120 °C (mL of 0.05 N NaOH)
Calorific value (Cal g−1)
Grain size of stabilizer
S1
Nano-clinoptilolite
4.0
6.6
875
30 nm
S2
Nano-clinoptilolite
3.5
7.0
853
30 nm
S3
Nano-clinoptilolite
3.0
7.0
863
30 nm
S4
Nano-clinoptilolite
2.5
8.5
615
30 nm
S5
Nano-clinoptilolite
2.0
9.0
844
30 nm
S6
Micro- clinoptilolite
3.0
7.2
845
2 μm
C1
Micro- centralite I
3.0
7.2
735
2 μm
The calorific values of samples S1, S2, S3, S5 and S6 (875, 853, 863, 844 and 845 Cal g−1 respectively) are higher than that of the sample containing classical stabilizer (735 Cal g−1) and only sample S4 has lower calorific value (615 Cal g−1) than the classical stabilizer. Therefore, these values confirm that, sample four still has the highest stabilization effects on DBPs.
3.2 X-ray diffraction analysis (XRD)
X-ray diffraction analysis is probably the easiest and best mean to identify clinoptilolite and centralite I stabilizer textures and also identify the resulted nature of the double-base propellant samples containing these stabilizers. A representative XRD diffractogram for the stabilizers and the DBP samples containing the stabilizers are shown in Figs. 1–5. The d-spacing and relative peak intensities for clinoptilolite and centralite I stabilizers are listed in Table 3. The ASTM (American Society of Testing Materials) cards were used to identify the stabilizers and DBP samples. Where I an I0 are X-ray diffraction parameters and I/I0 is the X-ray ratio.
XRD for clinoptilolite stabilizer.

XRD for N,N-diethyldiphenyl urea (Centralite I) stabilizer.
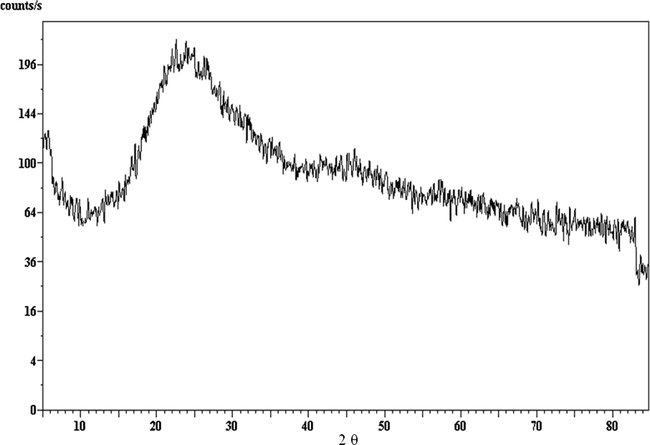
XRD for DBP sample containing 3% nano-clinoptilolite stabilizer.
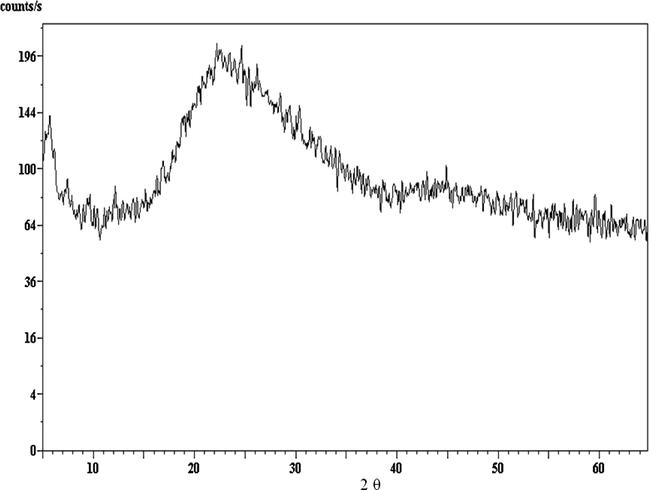
XRD for DBP sample containing 3% micro-clinoptilolite stabilizer.
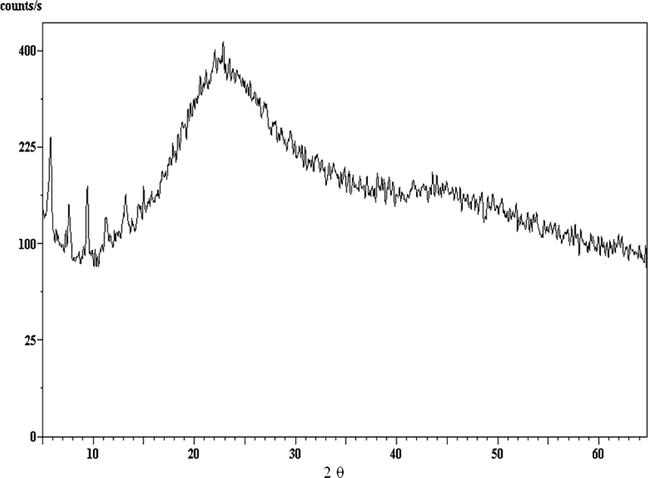
XRD for DBP sample containing 3% centralite I stabilizer.
Clinoptilolite Zeolite
Centralite I
d(Å)
I/I0
d(Å)
I/Io
8.92
100
8.40
50
7.97
4
7.69
100
6.78
4
6.24
60
5.15
8
5.29
40
4.65
14
4.92
80
4.35
2
4.66
80
3.96
55
4.20
40
3.90
55
4.09
70
3.55
6
3.78
70
3.48
4
3.65
50
3.32
4
3.42
40
3.17
14
3.18
60
3.12
16
2.98
40
3.07
8
2.58
50
2.974
80
2.52
40
2.793
15
2.46
10
2.728
35
2.39
10
2.719
16
2.33
40
2.09
30
1.83
30
The zeolite sample which is shown in Fig. 1 composed mainly of clinoptilolite of ASTM Card No. (22-1236). Clinoptilolite is iso-structure with heulandites (Hossein et al., 2008), therefore XRD analysis of the two phases is nearly identical which makes the differentiation difficult. These two zeolite phases are distinguished by the differences in thermal stability, Si/Al ratio and cation content.
Centralite I sample is shown in Fig. 2; it composed mainly of N,N-diethyldiphenyl urea ASTM Card No. (3–0042) (Zayed et al., 2012).
Repetitive XRD data of DBP Samples containing different clinoptilolite ratios are shown in Figs. 3–5 and the d-spacing values for pure clinoptilolite and centralite samples are given in Table 3. They appear to be of amorphous shapes; so they do not give any pattern, and this indicated that the crystalline structures (crystallinity Fig. 1) of the new stabilizers (nano-clinoptilolite and micro-clinoptilolite) and of classical one (Fig. 2) were not observed in the samples (S1–S6) diffractograms (Figs. 3–5). This may be attributed to the pressing processes of DBPs with stabilizers under very high pressure during their mixture preparation (Hassan, 2011; Zayed et al., 2012) and the adsorption of nitric oxides by the new stabilizers which cause changes in the crystal structure of nano- or micro-clinoptilolite stabilizers (Figs. 1 and 2 and Table 3).
3.3 Electron microscope analysis
The clinoptilolite samples in nano and micro sizes were prepared by the milling technique (Hassan, 2011; Zayed et al., 2012) and checked by two electron microscope tools (AFM and TEM). The data obtained are shown in Figs. 6–11 and Table 4; which will show the role of electron microscope analysis in clarifying the surface behavior of these inorganic stabilizers and their role in stabilizing the process of DBPs.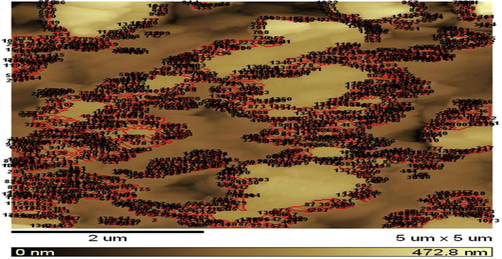
AFM of nano-clinoptilolite.
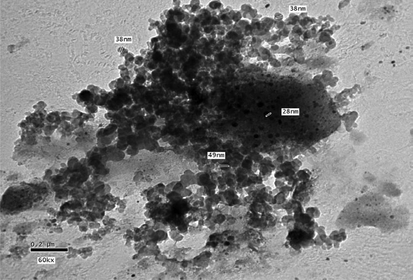
TEM of nano-clinoptilolite.

TEM of micro-clinoptilolite.
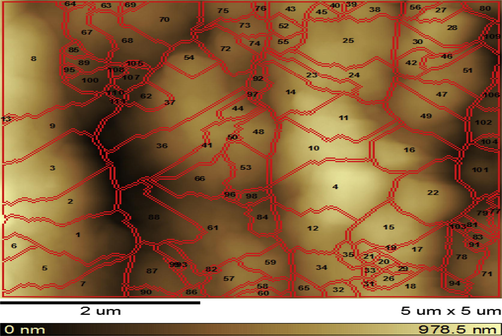
AFM structure of DBP sample stabilized by 3% nano-clinoptilolite.

AFM structure of DBP sample stabilized by 3% micro-clinoptilolite.
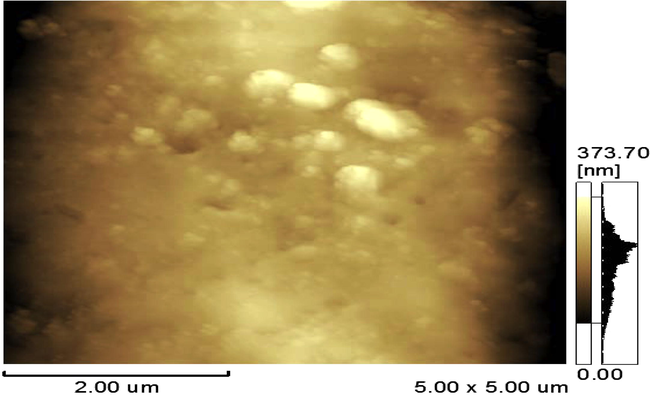
AFM structure of DBP sample stabilized by 3% Centralite I.
Particle no.
Main radius μm
Particle no.
Main radius μm
Particle no.
Main radius μm
1
0.007
32
0.116
63
0.025
2
0.01
33
0.061
64
0.02
3
0.039
34
0.007
65
0.042
4
0.019
35
0.018
66
0.007
5
0.031
36
0.041
67
0.007
6
0.043
37
0.097
68
0.026
7
0.037
38
0.02
69
0.153
8
0.031
39
0.016
70
0.034
9
0.054
40
0.046
71
0.039
10
0.016
41
0.024
72
0.047
11
0.021
42
0.025
73
0.057
12
0.019
43
0.069
74
0.055
13
0.007
44
0.074
75
0.012
14
0.056
45
0.046
76
0.041
15
0.022
46
0.012
77
0.016
16
0.038
47
0.016
78
0.007
17
0.057
48
0.041
79
0.007
18
0.01
49
0.007
80
0.007
19
0.037
50
0.024
81
0.007
20
0.026
51
0.007
82
0.017
21
0.021
52
0.072
83
0.016
22
0.007
53
0.05
84
0.016
23
0.028065
54
0.018
85
0.036
24
0.028102
55
0.047
86
0.041
25
0.028139
56
0.025
87
0.057
26
0.028177
57
0.007
88
0.025
27
0.028214
58
0.013
89
0.007
28
0.028251
59
0.022
90
0.021
29
0.028289
60
0.02
91
0.047
30
0.028326
61
0.007
92
0.022
31
0.028363
62
0.023
93
0.071
From the data in Table 4, the results of Atomic Force Electron Microscope (AFM) (Fig. 7) and Transmission Electron Microscope (TEM) (Fig. 8) of the clinoptilolite samples obtained by milling technique; it is observed that the average grain size (main radius of particles) of this sample is equal to 30 nm, which was calculated from the radius values of nano-clinoptilolite particles that ranged between 7 and 153 nm (Table 4). These data mean that the grain sizes of clinoptilolite samples are in a nano-scale; which is practically used for stabilizing DBP samples (S1–S5) (Table 1). On the other hand the TEM technique is also used to check the grain size of another sample of clinoptilolite (Fig. 9), which is also used as stabilizer for DBP sample S6 in comparison with the nano-scale samples (S1–S5). From Fig. 9, it is clear that this sample has particles of micro-scale sizes. From the AFM (Fig. 7) for nano-clinoptilolite, and scanning electron microscope (Fig. 9) for micro-clinoptilolite, we found a great difference in surface structure between them due to the difference in grain size and also the difference in some physical properties such as the surface area (as the grain size decreases the surface area increases). This means that, the difference in grain size between nano- and micro-clinoptilolite samples leads to a big difference in their stabilizing ability for DBPs.
AFM Figs. 9–11 show the surface structure of DBP sample stabilized by 3% nano-clinoptilolite, by 3% micro-clinoptilolite and that stabilized by 3% classical stabilizer (Centralite I) respectively. From these figures it is observed that, in general; clinoptilolite makes the surface of double-base propellant more ordered and homogenized as indicated by the regular map of sample surface (Fig. 10); which is greatly different from non-ordered nano-clinoptilolite sample itself (Fig. 7). This ordering and homogeneity of the stabilized DBP sample by 3% nano-clinoptilolite may be attributed to the ordering of nano-clinoptilolite particles on the surface of DBP during the process of preparation. This ordering and homogeneity lead to the high surface area of the sample that adsorbs a higher amount of nitrogen oxides evolved on its surface in an ordered and homogenized shape. But in case of double-base propellant samples stabilized by 3% micro-clinoptilolite; the ordering and homogeneity of the surface structure decrease due to the high grain size of stabilizers. The surface structure of double-base propellant sample stabilized by the (3% w/w) classical stabilizer (Centralite I) showed the lowest ordering and homogeneity, and this may be due to the less stabilizing effect and large grain size of centralite I stabilizer. Finally it is concluded that the nano-size of stabilizer be more effective than the micro-size in DBPs stabilization process.
3.4 Mechanism of stabilization
From the data obtained (Tables 1–4) and Figs. 1–11 of XRD and electron microscope for the stabilizers (nano-clinoptilolite, micro-clinoptilolite and centralite I) and the double-base propellant samples containing these stabilizers, the mechanism of stabilization of the inorganic stabilizer (Clinoptilolite) for DBPs can be deduced. This mechanism can be explained by the first step which included the adsorption (physico- or chemi-sorption) of the evolved NOx via interaction with cationic surface groups to form a stable bi-layer on the external surface of the zeolite. The second one included the partitioning of the evolved NOx gases into the internal cavities of clinoptilolite together. The third one may involve specific binding of absorbed NOx gas radicals with cationic form of zeolite at its internal base structure (Fig. 12). On the other hand centralite (Fig. 11) stabilizing mechanism is only explained by chemical absorption interaction with NOx gases. Therefore, mechanism process depends on the adsorption of nitrogen oxides in the pore openings of clinoptilolite, and/or physical or chemi-ionic surface interaction leading (Fig. 12) to the high stability results at very high temperatures (Hassan, 2011; Dominic and Wilmington, 1975; Sakizci et al., 2011; Zayed et al., 2012). The grain size of inorganic stabilizer played an important role in the stabilization process.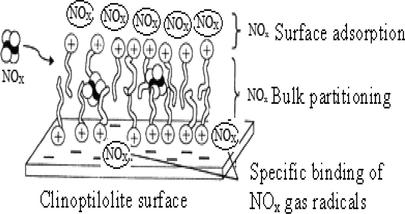
Sketching drawing of (NOx) forming a bi-layer (tail to tail) on the surface of clinoptilolite, NOx partitioning into the bulk and specific base binding of gas radicals.
4 Conclusion
Nano-clinoptilolite can be considered as a good stabilizer for DBPs. The percentage 4% w/w of nano-clinoptilolite is considered as the best between the different percentages of nano-clinoptilolite; because it shows a pronounced stabilizing effect on the propellant sample. The thermal stability tests together with XRD, AFM and AEM give good explanation of surface behavior of these stabilizers on stabilization processes of DBPs.
The mechanism of stabilization of DBPs by using clinoptilolite can be explained by the first step which included the adsorption (physico- or chemi-sorption) of the evolved NOx via interaction with cationic surface groups to form a stable bi-layer on the external surface of the zeolite. The second one included the partitioning of the evolved NOx into the internal cavities of clinoptilolite. The third one involved specific binding of absorbed NOx gas radicals with cationic form of zeolite at its internal base structure (Fig. 12).
Acknowledgment
It is indebted to present our thanks for all societies (Cairo University, Military Production Society and Science and Technology Center of Excellence of EGYPT) who covered financial support of this research and the expense of all measurements.
References
- Thermal behaviour of AP-based CMDB propellants with stabilizers. Defen. Sci. J.. 1992;42(3):201-204.
- [Google Scholar]
- Decomposition of diphenylamine in nitrocellulose based propellants I. Optimization of a numerical model to concentration-time data for diphenylamine and its primary degradation products determined by liquid chromatography with dual amperometric detection. Talan. 1995;42(2):83-171.
- [Google Scholar]
- A. Dominic, D.E. Wilmington, Composite modified double base propellant with metal oxide stabilizer, United States Patent 3905846 Berta (1975).
- Two-step method for preparation of NaA–X zeolite blend from fly ash for removal of cesium ions. J. Hazard. Mater.. 2008;154:963-972.
- [Google Scholar]
- Utilization of new non-toxic substances as stabilizers for nitrocellulose-based propellants. Propell. Explos. Pyrotech.. 2011;36(4):347-355.
- [Google Scholar]
- Effect of copper cation on the adsorption of nitrosamines in zeolite. Solid State Sci.. 2008;10:1658-1665.
- [Google Scholar]
- H.S. Hassan, Enhancement of stabilizers properties for double-base propellants using nano-scale inorganic compounds, M.Sc. thesis, Cairo University, Egypt, 2011.
- Ion exchange behavior of zeolites A and P synthesized using natural clinoptilolite. Iran J. Chemist Chem. Eng.. 2008;27:111-117.
- [Google Scholar]
- Modification on natural clinoptilolite zeolite for its retention capacity. J. Hazard. Mater.. 2009;169(1–3):29-35.
- [Google Scholar]
- Identification of homemade inorganic explosives by ion chromatographic analysis of post-blast residues. J. Chromatog. A. 2008;1182:205-214.
- [Google Scholar]
- Synthesis of Linde type A zeolite–goethite nanocomposite as an adsorbent for cationic and anionic pollutants. J. Hazard. Mater.. 2009;164:929-935.
- [Google Scholar]
- Frontline and factory: comparative perspectives on the chemical industry at war, 1914–1924. Archimedes. 2006;16:203-219.
- [Google Scholar]
- Characterization of modified clinoptilolite. J. Raditional Nucl. Chem.. 2005;165(5):287-294.
- [Google Scholar]
- Thermodynamics of hydrogen adsorption on the zeolite Ca–Y. Catal. Today. 2008;138:249-252.
- [Google Scholar]
- Maximizing ammonium nitrogen removal from solution using different zeolites. Enviro. Qual. Art.. 2010;39(4):1478-1485.
- [Google Scholar]
- Mineralogical study of zeolites from Pentalofos area, Thrace, Greece. Appl. Clay Sci.. 2004;25:9-16.
- [Google Scholar]
- J.B Robert, Aluminum silicate stabilizer in gas producing propellants, USA patent No 3979236 (1976).
- Thermal and SO2 adsorption properties of some clays from Turkey. J. Therm. Aanl. Calorim.. 2011;103(2):435-441.
- [Google Scholar]
- Study on adsorption of N2 and O2 by magnesium (II)-exchanged zeolite A. J. Alloy Comp.. 2009;478:L5-L7.
- [Google Scholar]
- Action of a new material on storage of single base propellant. Propell. Explos. Pyrotech.. 2004;10(4):105-107.
- [Google Scholar]
- Applied Subsurface Geological Mapping with Structural Methods. New Jersey: Prentice Hall; 2002.
- F. Wayne, C.M. Hugh, J.O. Donald, Ammonium nitrate propellants and methods for preparing the same, US Patent No. 6913661 (2005).
- Adsorption separation of carbon dioxide, methane, and nitrogen on Hβ and Na-exchanged β-zeolite. J. Nat. Gas Chem.. 2008;17:391-396.
- [Google Scholar]
- Adsorption of nitrogen oxides by the moisture-saturated zeolites in gas stream. J. Hazard. Mater.. 2009;162:866-873.
- [Google Scholar]
- Enhancement of stabilizing properties of double-base propellants using nano-scale inorganic compounds. J. Harad. Mater.. 2012;227–228:274-279.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.arabjc.2013.08.021.
Appendix A
Supplementary data
Supplementary data 1
Supplementary data 1
Supplementary material.
Supplementary data 2
Supplementary data 2
Supplementary material.
Supplementary data 3
Supplementary data 3
Supplementary material.







