Translate this page into:
Metabolic variations in seaweed, Sargassum polycystum samples subjected to different drying methods via 1H NMR-based metabolomics and their bioactivity in diverse solvent extracts
⁎Corresponding author. m_farhannaza@upm.edu.my (Muhammad Farhan Nazarudin)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Seaweeds are known as excellent sources of unique bioactive metabolites. In the present study, proton nuclear magnetic resonance (1H NMR) combined with principal component analysis (PCA) was used to distinguish the metabolic variations in Brown seaweed, Sargassum polycystum treated under different drying processes. The study also evaluated the phytochemistry, antioxidant, and antimicrobial effects of S. polycystum extracted in different solvents. Mutually under the different drying processes investigated, a total of 12 metabolites were identified from 1H NMR analysis. Freeze drying emerged as the most efficient process that preserved most of the potentially beneficial metabolites in the samples. The results of the qualitative phytochemical screening of differentially dried S. polycystum extracts revealed the presence of various secondary metabolites. The 70% ethanol extract exhibited the highest total phenolic (627 ± 50.81 mg GAE/100 g dried samples) and also displayed the highest DPPH scavenging activity (61.4 ± 0.171%) at the highest concentration (3 mg ml−1) tested. Methanol extract on the other hand contained the highest total antioxidant capacity (121.00 ± 0.003 mmol/g) followed by 70% ethanol extract (120.00 ± 0.001 mmol/g) at concentration of 1.25 mg/mL. The 70% ethanol extract also showed inhibition zone towards all bacteria samples tested compared to others solvent extracts. Based on these results, the identification of metabolites variations using PCA is considered as very useful procedure as a basis to recommend the most efficient processing (drying) method. The potential utilization of the tested Brown seaweed S. polycystum species as a source of antioxidants and antibacterial agents were also highlighted. The commercial cultivation of the species therefore, needs to be encouraged and promoted.
Keywords
Antioxidant
Phytochemistry
Principal component analysis (PCA)
Sargassum polycystum
1 Introduction
Seaweed is a group of marine algae that are sustainable sources of biomass, which are cultivable in Malaysian’s ecological environment. In 2018, seaweed contributed about 30% of total production by weight to the Malaysian fisheries sector (Nor et al., 2019). There are 3 major divisions of seaweed namely; Chlorophyta, Phaeophyta and Rhodophyta. Among these, Phaeophyta or brown seaweed is the most dominant, accounting for approximately 44% of total seaweed species (Asmida et al., 2017). Brown seaweed are commonly found to exhibit yellowish or brown coloration due to presence of xanthophyll pigments known as fucoxanthin and the species is usually found on rocky intertidal shores. The genus of brown marine algae Sargassum belongs to the class Phaeophyceae and is the most prevalent tropical genera, with over 400 species of Sargassum sp. recorded to exist worldwide. Of this number, about 25 species are found in Malaysia (Wong and Phang, 2004; Matanjun et al., 2008). In the west coast of Peninsular Malaysia, Sargassum sp. is available in large quantity as abundant resources that are currently considered to be inexpensive and under-utilized.
In addition to highly valuable nutritional components, seaweed extract also exhibits biological activities resulting from its phytochemical content of bioactive compounds such as antioxidants, anti-cancer agents, antiproliferative, antimicrobial properties, remedies for cardiovascular diseases and ailments such as atherosclerosis, hyperlipidemia, hypertension and thyroid diseases (Ismail, 2017; Kuda et al., 2015; Yuan and Walsh, 2006). Nevertheless, brown seaweed extract is reported to consist higher antioxidant activity compared to red and green seaweed (Al-Amoudi et al., 2009; Kindleysides et al., 2012). This is as the capacity has been proven, of the natural antioxidants abundant in seaweeds that are capable to reduce the risk of chronic diseases harmful to human health, which manifests in the form of ROS (Rajauria et al., 2013).
Reactive oxygen species (ROS) is a phrase used to describe the number of reactive molecules and free radicals which are derive from molecular oxygen. ROS are the components responsible for the response of the immune cells to microbial invasion. It also plays a key role as messenger for normal signaling transduction and cell cycling. ROS are required for proper cell function in term of cell signaling and immune response; however negative cascade might happen in cells if ROS exceed the level of homeostasis leading to oxidative stress in the organism and plant. The negative effects of oxidative stress may be mitigated by antioxidants. Antioxidants help to remove free radicals (ROS), consisting; superoxide (O2–), hydroxyl (HO–) and hydrogen peroxide (H2O2) (Boonchumi et al., 2011).
The genus of brown seaweeds, Sargassum has received attention for its antioxidant potentials (Sowmya et al., 2011). Researchers have reported strong radical scavenging and capacity for singlet oxygen quenching in methanol extracts of different seaweed species and correlated that with their content of polyphenols (Sachindra et al., 2010). Studies by other workers also showed the activity of antioxidants of polyphenols and polysaccharides isolated from seaweeds (Yuan et al., 2005; De Zoysa et al., 2008).
Generally, seaweeds collected for use are washed and dried before being utilized for any nutritional studies or in industrial process, where variations in processing methods have been shown to alter metabolic contents. Therefore, the effects of the drying process prior to extract bioactive ingredients is crucial approach that needs careful consideration, in order to protect the phytochemical content of metabolites (Mediani et al., 2014; Rubinskienė et al., 2015). Various studies have documented that the drying method influences the content of total phenol, total flavonoid and other compounds in Sargassum sp. (Chee et al., 2011; Gupta and Abu-Ghannam, 2011; Norra et al., 2016). Therefore, it is important that such factors are considered during sample processing, including time and cost saving procedure. The commonly employed drying techniques to prepare seaweed are sun drying (Neoh et al., 2016), oven drying (Ling et al., 2015) and freeze drying due to their efficiencies and economical considerations (Müller and Heindl, 2006).
Compared to other medicinal plants, the drying of seaweed takes longer time because it requires cleaned and washed several times due to the concentration of salt in the samples brought over from seawater. In order to assess and monitor metabolite variations under different drying conditions, metabolomics approach is usually one of the suitable methods employed (Kim et al., 2010; Shuib et al., 2011; Verpoorte et al., 2007). Additionally, the effect of varying solvent extraction type, from polar to non-polar during sample processing have also been implicated to influence metabolites variation and yield of active ingredients. Choice of extraction solvent is thus vital, in order to extract metabolites from seaweed that comprises a complex matrix of compounds.
Therefore, the aim of the present study was to distinguish the metabolic variation among different drying methods including air, freeze and oven -drying of S. polycystum seaweed samples using 1H NMR based metabolomics approach in combination with principal component analysis (PCA). When acquired, the information will be useful as the basis for recommendation regarding the suitability of the processing method that preserves the native features and bioactivity by avoiding the degradation of important metabolites. The effect of different extraction solvents (from polar to non-polar; methanol, 70% ethanol, chloroform, acetone and hexane) on total phenolic content (TPC), antioxidant activities [via 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) scavenging assay], total antioxidant capacity (TAC) and antibacterial activities were also investigated.
2 Experimental
2.1 Plant material and samples preparation
S. polycystum samples were collected by hand during low tide (<0.09 m) on reef flats, approximately 100 m from the shore at Teluk Kemang near Port Dickson, Negeri Sembilan, Malaysia (2°26′N, 101°51′E). Collected seaweed samples were cleaned of extraneous materials like epiphytes, sand particles, pebbles and shells using soft bristle brush and thoroughly washed with sea water. The clean samples were then placed in transparent polyethylene bags (inside chilled plastic containers) and transported to the research facilities of the laboratory of Marine Biotechnology, Institute of Bioscience (IBS), Universiti Putra Malaysia (UPM) Serdang, Malaysia. In the laboratory, the samples were further washed thoroughly with tap-, followed by distilled water.
2.2 Drying process
The washed seaweeds were divided into 3 groups for drying process, including air-drying at room temperature; freeze drying and vacuum oven-drying. Throughout the drying procedure, all samples were dried until constant weight was attained. The air-dried (AD) samples were exposed to ambient temperature of 25 °C for 6 days. The vacuum oven dried (OD) samples were prepared by spreading samples evenly in a single layer on trays and placed in a vacuum oven (Memmert USA) and dried at 40 ± 5 °C, maintained for 29 h. The freeze dried (FD) samples were prepared by subjecting fresh samples to over- night freezing at −80 °C in a freezer (Thermo Scientific) followed by lyophilizing in a freeze dryer (Labconco FreeZone, USA) until constant weight was attained. All dry samples were subsequently ground into fine powder with a laboratory-scale blender, and then sieved using a 200 µm-sized sieve. The fine powdered samples were collected in screw-capped bottles and stored in refrigerator until further use.
2.3 NMR measurement and multivariate data analysis
A 500 MHz Varian INOVA NMR spectrometer (Varian Inc., Palo Alto, CA, USA), functioning at a frequency of 499.887 MHz at ambient temperature (25 °C) was employed to determine 1H NMR and J-resolved experiments. Samples were prepared according to the method described by Kim et al. (2011). A 50 mg sample was weighed in a 2.0 mL Eppendorf tube. A total of 0.75 mL of a 1:1 mixture of methanol‑d4 and potassium dihydrogen phosphate buffer (pH 6.0) in deuterium oxide containing 0.1% trimethylsilylpropionic acid-d4 sodium salt was added. The mixture was vortexed (1 min), ultrasonicated (20 min) and centrifuged (10,000 rpm, 10 min) at ambient temperature. The supernatant (0.6 mL) was transferred to NMR tube to run NMR analysis by subjecting it to 1H NMR measurement. A pre-saturation sequence was applied to eliminate the residual water signal. The resulting spectra were manually phased and baseline corrected using the Chenomx software (v.5.1, Alberta, Canada) with a consistent setting for all spectra. Spectral intensities were binned by equal width (δ 0.04) corresponding to the region of δ 0.50–10.00. The regions of δ 4.70–4.90 representing water and δ 3.23–3.36 representing residual methanol were excluded.
2D J-resolved experiment was determined for additional support in the metabolite identification. Multivariate data analysis by principal component analysis (PCA) was performed with the SIMCA-P software (v. 13.0, Umetrics, Umeå, Sweden) using scaling based on Pareto. Metabolite identification was determined by comparing the identified metabolite peaks with the Chenomx NMR Suite 7.7 library.
2.4 Samples extraction
The dry brown seaweeds powdered was dissolved with 50 mL methanol, and vortexed for 5 min. This solution was extracted by sonication for 30 min using ultrasonic water bath (Power Sonic 505, Korea) at ambient temperature. The solvent extract was then filtered through Whatmann No. 1 filter paper while the dry residue re-extracted with another 50 mL of methanol for a second round of extraction. The extraction process was subsequently repeated in the same manner for a third round of extraction. The filtered extracts were pooled and evaporated to dryness using a rotary evaporator (N-1001S-WD, with EYELA Oil Bath OSB-2000, Japan) at 30 °C, and stored at −20 °C until further analysis. The described seaweed powder extraction procedure was repeated using other solvents i.e., 70% ethanol, acetone, chloroform and hexane. For each solvent tested, the extraction was replicated six times. The concentrated extracts were stored in amber bottles in a freezer for future utilization.
2.5 Phytochemical screening
Phytochemical examination was carried out for all the extracts according to standard protocols. For terpenoids, extract was dissolved in 2 mL of chloroform and evaporated to dryness. To this, 2 mL of concentrated H2SO4 was added and heated for about 2 min. A greyish color indicated the presence of terpenoids. The presence of steroids was indicated by development of a greenish coloration by mixing extract with 2 mL of chloroform. Then 2 mL of each of concentrated H2SO4 and acetic acid were poured into the mixture. In tests for phenols and tannins, extract was mixed with 2 mL of 2% FeCl3 solution. A blue-green or black coloration indicated the presence of phenols and tannins. Detection for flavonoids was conducted by dilution of extract with 2 mL of NaOH and the mixture turned to intense yellow coloration. Once HCl was added, the solution became colorless. As for saponins, extract was mixed with 5 mL of distilled water in a test tube. This was shaken vigorously. Formation of stable foam was considered as indication for the presence of saponins.
2.6 Total phenolic content (TPC) assay
The phenolic content in S. polycystum was estimated using Folin-Ciocalteu methods as described by Teoh et al. (2013). Approximately 100 μL of Folin-Ciocalteu’s reagent and 80 μL 7.5% (w/v) of sodium carbonate were added to 20 μL of aqueous extract of various concentrations in a well of a 96-well plate. After 30 min of incubation at ambient temperature in the dark, the absorbance was read at 765 nm against blank (solvent without extract) using ELISA plate reader (Thermo multiskan go). Quercetin was used as positive control and a standard curve was plotted using gallic acid (0–1 mg/mL). The results are expressed as milligram gallic acid equivalent (mg GAE)/100-gram dry weight of samples using the following equation: - where,
C: Total phenolic content (mg GAE/g plant extract)
c: concentration of gallic acid (mg/mL)
V: volume of extract (mL)
m: weight of extract
2.7 Antioxidant activity
2.7.1 DPPH radical scavenging
The DPPH radical scavenging activity of extracts was determined in accordance with Navanesan et al. (2015) using 96-well plate with slight modifications. DPPH reagent (0.3 mM) was prepared by weighing 1 mg of DPPH powder and dissolved in 8.4534 mL of methanol. Seaweed extracts and ascorbic acid were prepared and diluted in DMSO to get the stock solution with concentration of 10 mg/mL and 1.0 mg/mL, respectively. A total of 50 μL of various concentrations sample ranging from 0 to 3.0 mg/mL were mixed in 96 wells plate with 150 μL of 0.3 mM DPPH solution in methanol. The assays were performed in triplicates. The mixture was incubated for 30 min in the dark at ambient temperature and the absorbance was measured at 517 nm using ELISA plate reader (Thermo multiskan go). The DPPH radical scavenging activity was calculated by using the following formula: where A0 = absorbance of control, A1 = absorbance of sample.
2.7.2 Total antioxidant capacity
The total antioxidant capacity was determined using the method described by Prieto et al. (1999) with slight modifications. A reagent solution was prepared by mixing 0.6 M sulphuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate. After that, 0.3 mL extracts with a concentration of 1.25 mg/mL was mixed with 3 mL reagent solution. Then, it was incubated at 95 °C for 90 min in a water bath (EYELA Oil Bath OSB-2000, Japan). After samples were cooled at ambient temperature, the absorbance was measured using ELISA plate reader (Thermo multiskan go) against blank at 695 nm. A solvent without extract was used as blank. Quercetin was used as positive control and a standard curve was plotted using ascorbic acid (0–1.25 mg/mL). The total antioxidant activity was calculated as the number of gram equivalent to ascorbic acid.
2.8 Antibacterial activity
Antibacterial activity was carried out by placing 6 mm diameter of paper disc containing antibiotic onto a Mueller Histon Agar plate where microbes were grown. The bacteria strain was taken from Microbial Culture Collection Unit (UNiCC), Institute of Bioscience, University Putra Malaysia. The microbe culture was standardized to 0.5 McFarland standards, which was approximately 108 cells. Streptomycin was used as standard for each bacterium. The plates were inverted and incubated at 37 °C for 18–24 h, 24–48 h or until sufficient growth had occurred. After incubation, each plate was examined. The diameters of the zones of complete inhibition were measured including the diameter of the disc. Zones were measured to the nearest whole millimeter, using sliding calipers or a ruler, which was held on the back of the inverted petri plate.
2.9 Statistical analysis
All the assays for Antioxidant activities were carried out in six replicates while other assays in triplicates. The values were expressed as mean ± standard deviation. IBM SPSS Statistic 23 was used for the experiment and one-way Analysis of Variances (ANOVA) was used to compare the mean values of intensity. Then the analysis proceeded through Turkey’s multiple range test statistical significance at p ≤ 0.05.
3 Result and discussion
3.1 1H NMR spectra of the dried materials and metabolite identification
Discrimination of the metabolite variations among the air, freeze and oven-dried seaweed samples was done by employing 1H NMR metabolomics analysis. Fig. 1 shows the 1H NMR spectra of seaweed extracts under different drying conditions whereas Fig. 2 shows the 1H NMR spectra with identified metabolites. These metabolites were identified based on the 2D J-resolved (Fig. 1) and comparison with the NMR spectra of the reference compounds measured under the same conditions as extracts. All the spectra showed signals in the aliphatic (δ 0.5–3.0), carbohydrate (δ 3.0–5.5) and aromatic region (δ 5.5–8.0).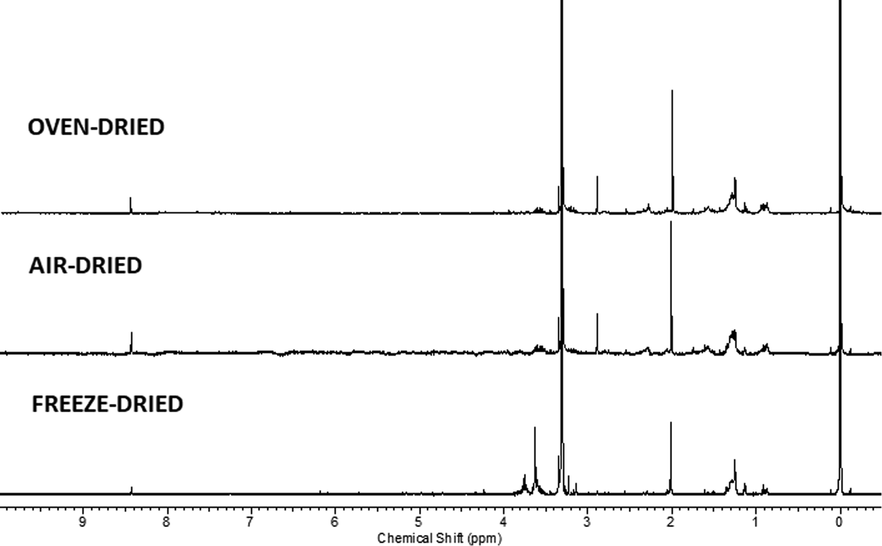
1H NMR spectrum of seaweed at different drying methods.
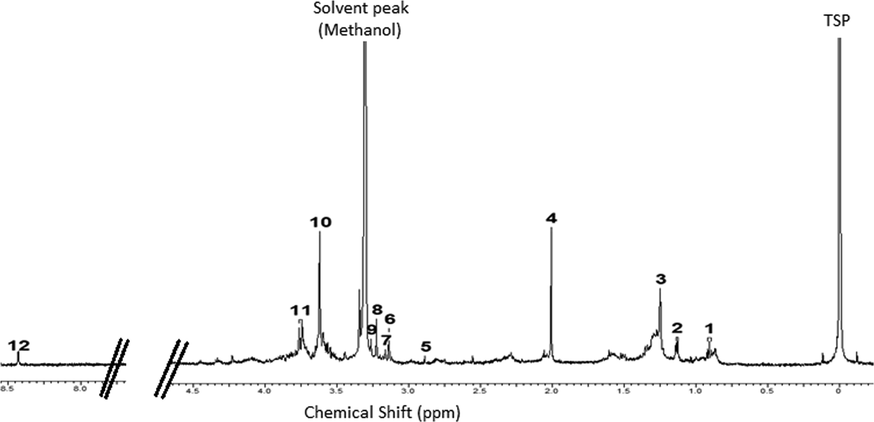
1H NMR spectrum of seaweed.
A total of 12 metabolites were identified and their characteristic signals are shown in Table 1. Valporic acid, propylene glycol, 3-hydroxyisovaleric acid, acetyl aspartic acid and trimethylamine were presence in aliphatic region between δ 0.92 and δ 2.92. Valporic acid and 3-hydroxyisovaleric acid were detectable at δ 0.92 (s) and δ 1.25 (s), respectively. A doublet peak was observed at δ 1.14 (d, J = 5.0 Hz) suggested appearance of propylene glycol. The presence of N-acetyl aspartic acid and trimethylamine were observed at δ 2.00 (s) and δ 2.92 (s), respectively. Malonic acid, dimethylsulfone, choline, betaine, sarcosine and N-acetylglycine were ascribed based on the signals displayed in the carbohydrate region of δ 3.0 – δ 5.5. The presence of malonic acid was confirmed by the peaks at δ 3.14 (s) whereas peaks of dimethylsulfone was detectable at δ 3.15 (s). Choline and betaine were detectable at δ 3.23 (s) and δ 3.26 (s), respectively. A singlet peaks observed at δ 3.62 suggested the presence of sarcosine. Other metabolite detectable in carbohydrate region was N-acetylglycine, which was detected at δ 3.76 (d, J = 10.0 Hz). Characteristic peaks of formic acid were detected at δ 8.43 (s).
Peak no
Metabolite
Chemical shift (multiplicity, J)
1
Valporic acid
0.92 (s)
2
Propylene glycol
1.14 (d, J = 5.0 Hz)
3
3-Hydroxyisovaleric acid
1.25 (s)
4
N-Acetyl-aspartic acid
2.00 (s)
5
Trimethylamine
2.92 (s)
6
Malonic acid
3.14 (s)
7
Dimethyl sulfone
3.15 (s)
8
Choline
3.23 (s)
9
Betaine
3.26 (s)
10
Sarcosine
3.62 (s)
11
N-acetylglycine
3.76 (d, J = 10.0 Hz)
12
Formic acid
8.43 (s)
3.2 Discrimination of metabolites among the three drying methods on seaweed samples
Identification of metabolites variations among the three drying methods i.e. air, freeze and oven-dried seaweed samples was done by principal component analysis (PCA). PCA was employed to cluster the features of the samples and analyze the metabolites that contributed to the variations on the seaweed samples. Air, freeze and oven-dried seaweed samples were clearly discriminated as illustrated in Fig. 2a. PC1 shows the most sample variations, followed by PC2. The first two principal components (PC1 and PC2) cumulatively accounted for 79.4% of the total variations. Separation of air, freeze and oven-dried seaweed samples in score plots were achieved by combining PC1 and PC2. PC1 shows the separation between freeze-dried, air, and oven-dried samples whereas PC2 shows the separation between air-dried, freeze and oven-dried samples.
The metabolites in the air, freeze and oven-dried seaweed samples which were illustrated using PCA are shown in the loading line plot for PC1 (Fig. 3b) and PC2 (Fig. 3c). As shown in the loading line plot for PC1 (Fig. 2 b), N-acetylglysine, sarcosine, betaine, choline, dimethylsulfone and malonic acid were the most abundant in freeze-dried samples compared to air and oven-dried. Loading line plot for PC2 (Fig. 3c) illustrated that air-dried seaweed samples contained higher level of betaine, dimethylsulfone and acetyl-aspartic acid compared to oven-dried seaweed samples. Formic acid, trimethylamine, 3-hydroxyisovaleric acid, propylene glycol and valporic acid level were found higher in oven-dried than freeze-dried samples. Sargassum sp. is highly regarded due to its active metabolites that exhibit numerous functions in biological activities. Changes in environmental patterns and processing methods may alter metabolic content. For instance, decreased metabolites level in the air-dried samples was expected due to degradation by air oxidation and thermal effects. Residual oxidative enzymes during the drying process may have influenced the decreased level of metabolites in the samples, as explained by Mediani et al. (2013).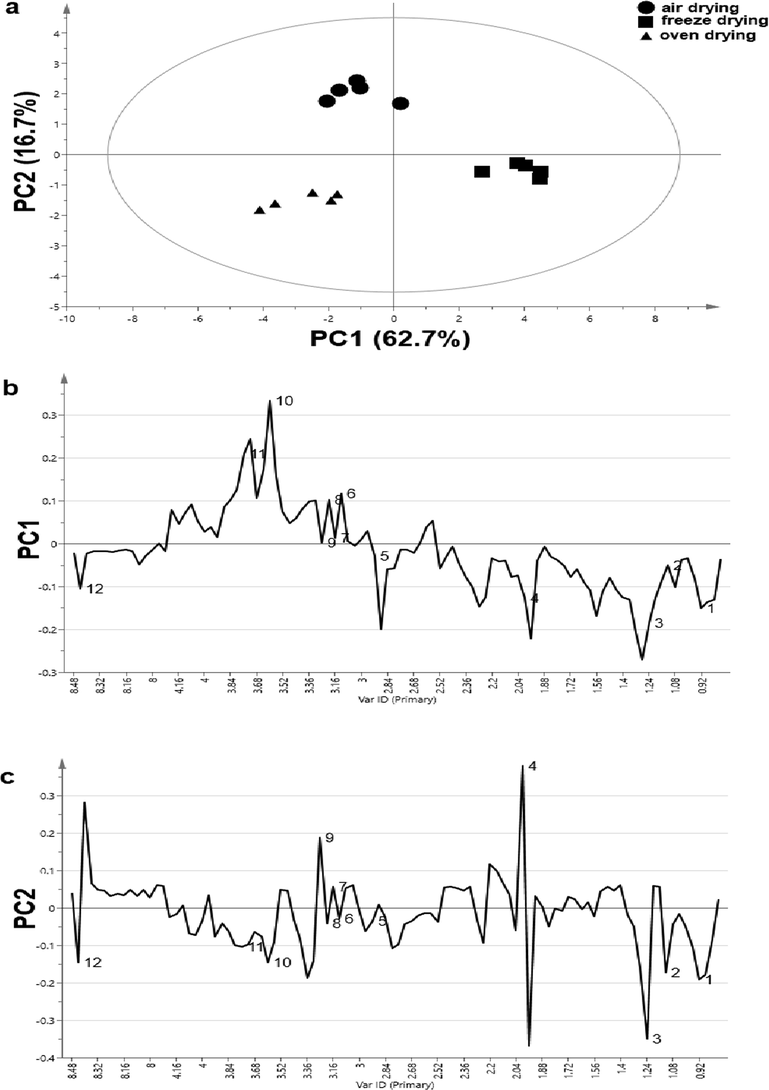
(a) PCA score plot (component 1 vs component 2); (b) Loading line plot of component 1 of 1H NMR data for comparison among the air, freeze and oven-dried seaweed samples and (c) Loading line plot of component 2 of 1H NMR data for comparison among the air, freeze and oven-dried seaweed samples. For the interpretation of the numbers assigned to the metabolites in the loading line plot, reference is made to Table 1.
Oven-drying of plant samples at 44.5 °C has been reported as one of the easiest and rapid thermal processing methods that could preserve phytochemicals (Azwanida, 2015; Mediani et al., 2012). Unsurprisingly, formic acid, trimethylamine, 3-hydroxyisovaleric acid, propylene glycol and valporic acid were observed in oven-dried samples. It has been reported that freeze drying method can avoid degradation of metabolites in samples due to the low temperature applied (Kim et al., 2011). Therefore, several metabolites such as N-acetylglysine, sarcosine, betaine, choline, dimethylsulfone and malonic acid were present in large quantities in the freeze dried samples. It was observed therefore that freeze drying was the most efficient process to preserve most of the potentially beneficial metabolites in the samples, hence formed the basis for recommendation as the most suitable processing method.
3.3 Phytochemical screening
By preliminary phytochemical screening, seven different chemical compounds (steroid, phenol, tannins, saponins, flavonoids, terpenoids and glycosides) were investigated in five different extracts (Table 2). Of the (5 × 7 = 35) tests for presence or absence of the above compounds, a total of 27 tested positive, whereas only eight gave negative results. The 27 positive results include steroid, saponins and glycosides to be present in all five solvent extracts tested. Steroid, saponins and terpenoids showed the maximum presence in all five different extracts. Next to this was phenol in 4 extracts. Among the five different extracts, methanol extract revealed the presence of maximum number of 7 compounds followed by chloroform and acetone extracts at 6 compounds each. The 70% ethanol extract revealed five compounds and hexane extract showed only three compounds. The results of phytochemical investigation of various extracts of S. polycystum revealed the presence of various secondary metabolites at varied degrees. Generally, seaweeds are known as medicinal plants, rich in metabolites and have been extensively studied and used in the pharmaceutical industry. (−) absent; (+) presence.
Metabolites
Methanol
70% Ethanol
Chloroform
Hexane
Acetone
Steroid
+
+
+
+
+
Phenol
+
+
+
–
+
Tannins
+
+
–
–
+
Saponins
+
+
+
+
+
Flavonoids
+
–
+
–
–
Terpenoids
+
+
+
+
+
Glycosides
+
–
+
–
+
In the present study, the presence of tannins in all extracts of S. polycystum was demonstrated. Many tannin-based drugs are used in medicine as astringent (Jeeva et al., 2012). Tannins are used in the production of leather and ink and also for treating wounds and burns (Tiwari et al., 2011; Oludare and Bamidele, 2015). Tannins have also been found to have antimicrobial properties as they are able to bind to adhesives, play roles in enzyme inhibition, substrate deprivation and membrane distruption (Guo et al., 2018). Saponins have specific biological activities such as anticancer, antiinflammatory, antimicrobial and antioxidant properties (Sidana et al., 2016; Güçlü-Ustündag and Mazza, 2007). Saponin also has the property of precipitating and coagulating red blood cells (Yadav and Agarwala, 2011). Flavonoids are hydroxylated phenolic substances that are reported for their response during antioxidant activity (Sytar et al., 2018). Flavonoid activity is probably due to their ability for scavenging hydroxyl radicals, superoxide anion radicals and lipids peroxy radicals are vital for diseases prevention related to oxidative damage of cell, membrane and protein. Steroids have been reported to possess antibacterial properties and they are very important compounds due to their relationship with compounds like sex hormones (Yadav & Agarwala, 2011). The presence of steroids in all extracts was also shown in the present study. Glycosides are non-carbohydrate moiety known to be used as food additives, prebiotic and biopreservatives (Cuaves et al., 2015). Terpenoids have been reported to possess cytotoxicity against a variety of cancer cells and cancer prophylactic (Huang et al., 2012).
3.4 Total phenolic content
The total phenolic content (TPC) of the 70% ethanol, methanol, acetone, chlorofom and hexane extracts of S. polycystum were determined, expressed in mg GAE/100 g and shown in Fig. 4. TPC for all solvent extracts was highest at concentration 3 mg/mL, ranging from 218 ± 47.93 mg GAE/100 g in chlorofom extract to 627 ± 50.81 mg GAE/100 g in the 70% ethanol extract. The 70% ethanol extract had highest TPC, which was significantly higher than in methanol extract (484 ± 14.74 mg GAE/100 g), followed by hexane extract (357 ± 153.26 mg GAE/100 g) and acetone extract (238 ± 48.66 mg GAE/g extract), while chloroform extract revealed the least TPC. Results of the present study revealed that in terms of the content of total phenolics, the 70% ethanol and methanol were the favourable extraction solvents, in addition to their hygienic features (Moure et al., 2001). Phenolic compounds are commonly found in plants, including seaweeds (Duan et al., 2006) and largely soluble in polar solvents, as they contain polar phenolic hydroxyl groups. Consequently, polar solvents such as ethanol, methanol and water were more efficient to extract phenolic compounds from seaweed, which comprise complex matrix of compounds including sugar, saponins, glycosides, organic acids, tannins, salts, and mucus (Waterman and Mole, 1994; Cho et al., 2007).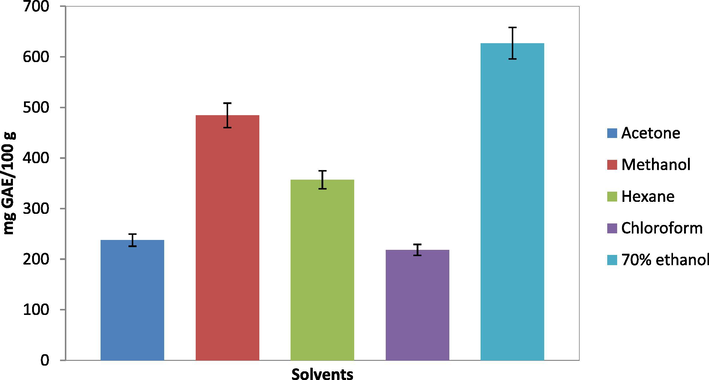
Total phenolic content for Sargassum polycystum crude extract from different solvents expressed in mg GAE/100 g. Data presented are based on the mean of three replicates of each of the solvent systems (acetone, chloroform, hexane, methanol, 70% ethanol) ± standard deviation (SD).
The results of this study clearly demonstrated that different extraction solvents from polar to non polar yield diverse total phenolics content due to differences in their chemical composition and strucutures. Besides, total phenolics content in the present study was much higher compared to previous study on green and red seaweed (Chew et al., 2008). This could be due to phlorotannins, which are mostly found in brown seaweeds (Machu et al., 2015; Chew et al., 2008). Phlorotannins in brown seaweed mainly consist of phloroglucinol, eckol and dieckol with the presence of multiple phenolic groups (Suleria et al., 2015). They are integral structural components of cell walls which play reproductive roles in algal reproduction and exhibit therapeutic properties such as antioxidant, anticancer, anti-aging and anti-inflammatory (Machu et al., 2015).
3.5 Antioxidant activity
3.5.1 DPPH radical scavenging activity assay
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging antioxidant assay has been used extensively as a free radical to evaluate the antioxidant potentials in plant extracts (Duan et al., 2006; Farasat et al., 2014). DPPH assay is considered a valid, accurate, easy, and economical method to evaluate radical scavenging activity of antioxidants, since the radical compound is stable and needs not be generated. The new methodology for evaluation of antiradical efficiency towards DPPH was proposed, which is advantageous over other methods (Sanchez-Moreno et al., 1998). The DPPH method considers not only the antioxidant concentration, but also the time taken for the scavenging reaction to reach a plateau. The results of the evaluation are highly reproducible and comparable to other free radical scavenging methods (Gil et al., 2000). Seaweed with antioxidant molecules are able to reduce the DPPH free radicals by attacking the molecules to add hydrogen atom to it or donate to it an electron, which results in the DPPH solution changing colour from purple to yellow (Indu and Seenivasan, 2013). It has been reported that brown seaweed rich in natural bioactive compounds like carotenoid, fucoxanthin, phenolic and flavonoids have higher antioxidant potentials in comparison with red and green seaweeds (Leelavathi and Prasad, 2014; Cox et al., 2010; Kindleysides et al., 2012). The observation of increase in free radical scavenging activities in a dose-respont manner in the 70% ethanol, methanol, acetone, chloroform and hexane extract from S. polycystum as shown in Fig. 4. The determination of free radical scavenging activity of S. polycystum extract at different concentrations is shown in Fig. 5, where ascorbic acid was used as the standard. All sample tested show antioxidant activities and possessed prominent antioxidant properties.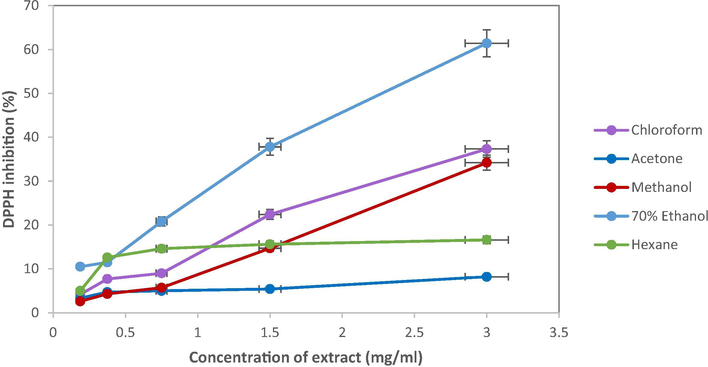
DPPH scavenging activities of S. polycystum at different concentrations (0–3.0 mg/mL) determined spectrophotometrically at 517 nm. Data presented are based on the mean of three replicates in each of the solvent systems (acetone, chloroform, hexane, methanol, 70% ethanol) ± standard deviation (SD).
In the present study, all the extracts depicted higher tendency to scavenge the DPPH radicals in a dose dependent manner and steadily increased with increase in extract concentrations with DPPH reduction ranging from 8.2 to 61.4% up to concentration of 3 mg/mL except for acetone and hexane extract. At the concentration of 3 mg/mL, the 70% ethanol extract show the highest activity compared to other extract (61.4 ± 0.171%) which suggested that the compound extracted by ethanol possess more potential to scavenge DPPH free radicals due to the higher concentration of phenolic content present in the extract as discussed above. Previous studies reported that ethanolic and aqueous extracts of Sargassum spp. including S. horneri, S. macrocarpum and S. siliquastrum produced more than 60% of DPPH radical scavenging activity (Boonchum et al., 2011; Matsukawa et al., 1997). Meanwhile, chloroform extract (37.3 ± 0.089%) exhibit slightly higher activity than methanol extract (34.2 ± 0.250%) followed by hexane extract which was obtained at 16.6 ± 0.194%). Acetone extract exhibited the least DPPH inhibition (8.2 ± 0.007%) at that concentration while the control (ascorbic acid) attained the half maximal inhibitory concentration (IC50) at concentration of 0.028 mg/mL. The variance in the DPPH radical scavenging activity of each sample studied suggests that the different extracting solvents used may have been affected the radical scavenging activity as the different polarities of each antioxidant compound groups existing in the seaweeds (Marinova and Yanishlieva, 1997). Methanol is the best choices of solvent in the determination of free radical scavenging activity via DPPH assay (Marinova and Batchvarov, 2011), where polyphenols from water were best extracted using polar solvents (Moure et al., 2001). However, the solubility of the antioxidant compound group and phytochemical composition of the solvent used for the seaweed extraction also played crucial roles as demonstrated in the antioxidant activities (Naczk and Shahidi, 2006; Rattaya et al., 2015).
3.5.2 Total antioxidant capacity
Total antioxidant capacity (TAC) used to assess the status of antioxidants in biological samples and to evaluate the antioxidant responses against the free radicals produced. Table 3 shows the TAC of 70% ethanol, methanol, acetone, chlorofom and hexane extract from S. polycystum at concentration of 1.25 mg/mL. It has been shown that different extraction solvents displayed varying amounts of antioxidant capacity. Total antioxidant compound in methanolic extract showed no significant difference from 70% ethanolic extract (p ≤ 0.05) as both had similar TAC value which were 121.00 ± 0.003 mmol/g and 120.00 ± 0.001 mmol/g, respectively. Meanwhile, hexane extract had TAC value at 61.39 ± 0.007 mmol/g followed by chloroform at 68.00 ± 0.002 mmol/g and acetone extract at 46.00 ± 0.000 mmol/g. * Data presented are based on the mean of three replicates each of the solvent systems (acetone, chloroform, hexane, methanol, 70% ethanol) ± standard deviation (SD).
Solvent
TAC (mmol/g)
Chloroform
68.00 ± 0.002
Hexane
61.39 ± 0.007
Methanol
121.00 ± 0.003
Acetone
46.00 ± 0.000
70% Ethanol
120.00 ± 0.001
The results attained on TAC of 70% ethanol extract were supported by the findings from TPC and DPPH radical scavenging activity where it shows phenolic content was highest in 70% of ethanol extract, resulting the highest antioxidant activity in DPPH scavenging assay. Phenolic compounds have an influence on antioxidant activity by chelating metal ions, inhibiting radical formation and refining the antioxidant system (Al-Azzawie and Mohamed-Saiel, 2006). Antioxidant activities of extracts are strongly dependent on the types of solvents used due to metabolites with different polarity exhibiting differing rates of antioxidant potentials (Marinova and Yanishlieva, 1997). Most antioxidant potential metabolites including phenols, tannins and flavonoids can be extracted by polar solvents. Seaweeds have been shown to contain high levels of natural bioactive compounds such as carotenoids, α-tocopherol and chlorophylls and its derivatives, flavonoids and phenolics (Leelavathi and Prasad, 2014). These substances are known to be important free radical scavengers and antioxidants for the prevention of oxidative damage. Specifically, brown seaweeds demonstrate the highest antioxidant activity compared to green and red seaweed species (Cox et al., 2010). This is likely due to their content of fucoxanthin (a pigment belonging to the group of xanthophylls), a carotenoid that is abundant in brown seaweeds and responsible for their antioxidant activities (Airanthi et al., 2011). Although this observation was not noted in the present study, it is likely that the contents and concentrations of bioactive compounds in the extracts of the seaweeds studied may have been affected by the different polarities of the extracting solvents used, as explained by Rattaya et al. (2015). In seaweed extracts which comprise complex matrixes of compound, antioxidant activity would not be closely connected with a specific compound, carotenoid for instance, but with a mixture of compounds as this mixture of compounds can act synergistically (Plaza et al., 2010). Generally, high-polarity solvents like ethanol and methanol are effective in extracting more antioxidant compounds when compared to an intermediate polar solvent, acetone, chloroform and nonpolar solvent, hexane.
3.6 Antibacterial activity
Seaweed contains epiphytic bacteria that are excellent source of natural antimicrobial and antioxidant compounds (Horta, et al., 2014). Many researches have been reported on the antimicrobial activity of Sargassum sp. extract against gram-positive as well as gram-negative bacteria (Patra et al., 2008; Zubia et al., 2008; Yoon et al., 2010). However, differences between results of the other studies could be due to production of bioactive compounds related to the seasons and area, drying methods, organic solvents used for extraction of bioactive compounds and differences in assay methods. The drying stage is crucial because fresh seaweed contains volatile antimicrobials (terpenoid and bromo-ether compounds, hydrogen peroxide and volatile fatty-acids) that may be lost due to high temperature (Mendes et al., 2013). In the present study, antibacterial study of the 70% ethanol, methanol, acetone, chloroform and hexane extract from S. polycystum was tested against Gram-positive bacteria (Staphylococcus aureus (ATCC 43300) and Bacillus subtilis (B29)) and Gram-negative bacteria (Pseudomonas aeruginosa (ATCC 15442) and Escherichia coli (UPMC 25922). The inhibition zone towards selected bacteria were presented in Fig. 6 and Table 4. The 70% of ethanol, acetone, chloroform, and hexane extract showed inhibition zone towards Staphylococcus aureus (ATCC 43300) with moderate inhibition zone between 10 mm and 15 mm while hexane showed antibacterial activity with <10 mm. It was found that 70% of ethanol extract showed inhibition zone towards all bacteria tested compared to other extraction solvents, which suggested that ethanol was the best solvent for extracting the antibacterial active constituents from the S. polycystum. Meanwhile, the absence of zone of inhibition in other extraction solvents (methanol, acetone, chloroform and hexane) may be due to low concentration of antibacterial active metabolites, which cause the resistance towards microorganisms to the seaweed extract. +++ Strong (>15 mm). ++ Moderate (10 mm < x < 15 mm). + Low (<10 mm). – No activity.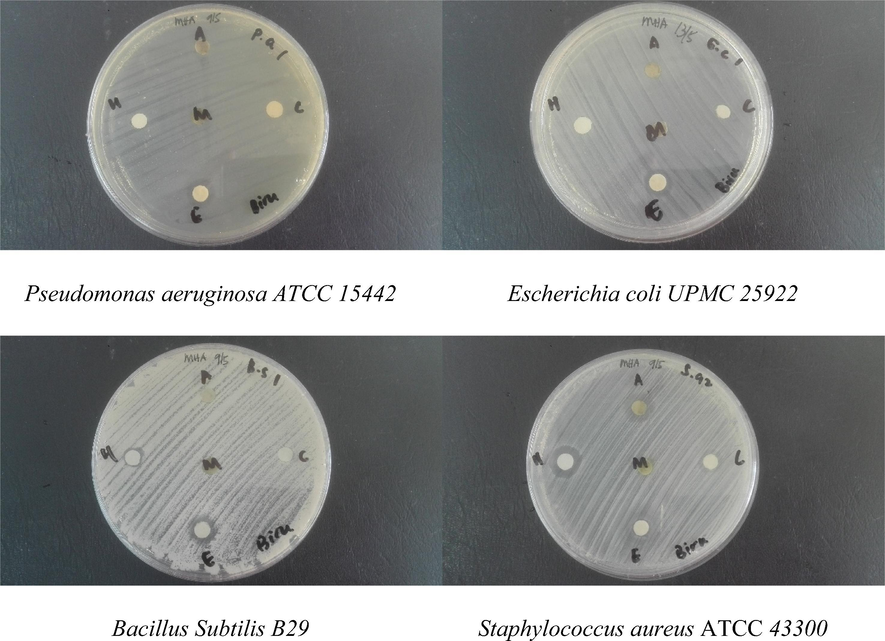
Antibacterial activity of Sargassum sp. with different solvent, M = methanol, H = hexane, A = acetone, C = chloroform and E = 70% ethanol.
Pseudomonas aeruginosa
ATCC 15442
Escherichia coli
UPMC 25922
Bacillus subtilis
B29
Staphylococcus aureus
ATCC 43300
Acetone
–
–
–
++
Chloroform
–
–
–
++
70% Ethanol
+
+
+
++
Hexane
–
–
+
++
Methanol
–
–
–
–
Streptomycin
+++
+++
+++
+++
The antimicrobial inhibitory activity rely and depends on the solvent used to extract antibacterial compounds within the seaweed. The influence of antioxidant activity of phenolic compounds prevents microbial growth effectively by binding to their cell surfaces and affect metabolism of the bacteria (Glombitza, 1979; Reguant et al., 2000). It is believed that other phytochemicals from S. Polycystum such as flavonoids, tannins and terpenoid which can be extracted by polar solvents, may play crucial roles in antibacterial activity. However, the activity may also depend on the concentration of antibacterial active metabolites.
4 Conclusions
Identification of variations in metabolites between the three drying methods including air, freeze and oven drying of S. polycystum seaweed samples was done by PCA and the results are the basis for recommendation of the most suitable processing method. From our findings, freeze drying is the most efficient process to preserve potentially beneficial metabolites in samples. Besides, from results of phytochemical investigation of various extracts of S. polycystum, the presence was revealed of various secondary metabolites at varied degrees. The 70% ethanol extract exhibited the highest phenolic content, displayed the highest antioxidant activity for DPPH inhibition and TAC and showed inhibition zone towards all bacteria tested. It may, therefore, be concluded that results of the present study indicate that the evaluated seaweed species S. polycystum is potential candidate for cultivation as functional food resources for human consumption, as its extracts are useful in drug discovery and for other industrial applications.
CRediT authorship contribution statement
Muhammad Farhan Nazarudin: Conceptualization, Methodology, Investigation, Formal analysis, Writing - review & editing, Project administration. Anusha Paramisparam: Investigation, Methodology, Formal analysis, Writing - review & editing. Nur Afiqah Khalid: Investigation, Methodology, Formal analysis, Writing - review & editing. Maziah Nazihah Albaz: Investigation, Methodology, Formal analysis, Writing - review & editing. Muhammad Syazwan Shahidan: Investigation, Methodology, Formal analysis, Writing - review & editing. Ina Salwany Md Yasin: Investigation, Resources, Writing - review & editing. Azizul Isha: Investigation, Methodology, Formal analysis, Writing - review & editing. Mazni Abu Zarin: Investigation, Methodology, Formal analysis, Writing - review & editing. Mohammed Aliyu-Paiko: Conceptualization, Methodology, Investigation, Writing - review & editing.
Acknowledgements
Authors wish to acknowledge and thank Universiti Putra Malaysia for providing funds for this research under the Geran Putra grant no. 9578500 and SATREPS-COSMOS Project. Authors also graciously acknowledge the support and assistance of Mrs Khairiyah Hassan, Mr Muhammad Luth Zhafran, Mr Muhammad Hud Irfan, Mr Mohd. Shukri Abu Bakar and Mr. Azhar Baharom for their contributions during sampling, laboratory work and analysis.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Chemical composition and antioxidant activities of Jeddah corniche algae, Saudi Arabia. Saudi J. Biol. Sci.. 2009;16:23-29.
- [Google Scholar]
- Hypoglycemic and antioxidant effect of oleuropein in alloxan-diabetic rabbits. Life Sci.. 2006;78:1371-1377.
- [Google Scholar]
- Comparative antioxidant activity of edible Japanese Brown seaweeds. J. Food Sci.. 2011;76:C104-C111.
- [Google Scholar]
- Biodiversity of macroalgae in Blue Lagoon, the Straits of Malacca, Malaysia and some aspects of changes in species composition. Sains Malaysiana. 2017;46:1-7.
- [Google Scholar]
- A review on the extraction methods use in medicinal plants, principle. Med. Aromat. Plants. 2015;4:1-6.
- [Google Scholar]
- Antioxidant activity of some seaweed from the gulf of Thailand. Int. J. Agric. Biol.. 2011;13:95-99.
- [Google Scholar]
- Extraction and characterisation of alginate from brown seaweeds (Fucales, Phaeophyceae) collected from Port Dickson, Peninsular Malaysia. J. Appl. Phycol.. 2011;23:191-196.
- [Google Scholar]
- Antioxidant activity of three edible seaweeds from two areas in South East Asia. LWT-Food Sci. Technol.. 2008;41:1067-1072.
- [Google Scholar]
- The antioxidant properties of brown seaweed (Sargassum siliquastrum) extracts. J. Med. Food. 2007;10:479-485.
- [Google Scholar]
- An assessment of the antioxidant and antimicrobial activity f six species of edible Irish seaweeds. Int. Food. Res. J.. 2010;17:205-220.
- [Google Scholar]
- Anticoagulant activity of sulfated polysaccharide isolated from fermented brown seaweed Sargassum fulvellum. J. Appl. Phycol.. 2008;20:67-74.
- [Google Scholar]
- Evaluation of antioxidant property of extract and fractions obtained from a red alga, Polysiphonia urceolata. Food Chem.. 2006;95:37-43.
- [Google Scholar]
- Antioxidant activity, total phenolics and flavonoid contents of some edible green seaweeds from Northern coasts of the Persian Gulf, Iranian. J. Pharm. Res.. 2014;13:163-170.
- [Google Scholar]
- Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem.. 2000;48:4581-4589.
- [Google Scholar]
- Antibiotics from algae. In: Hoppe H.A., Levring T., Tanaka Y., eds. Marine Algae in Pharmaceutical Science. Berlin: Walter de Gruyter; 1979. p. :303-342.
- [Google Scholar]
- Saponins: properties, applications and processing. Crit. Rev. Food Sci. Nutr.. 2007;47:231-258.
- [Google Scholar]
- Development of tannin-inspired antimicrobial bioadhesives. Acta Biomater. 2018
- [CrossRef] [Google Scholar]
- Bioactive potential and possible health effects of edible brown seaweeds. Trends Food Sci. Tech.. 2011;22:315-326.
- [Google Scholar]
- Antioxidant and antimicrobial potential of the Bifurcaria bifurcata epiphytic bacteria. Mar. Drugs. 2014;12:1676-1689.
- [Google Scholar]
- Terpenoids: Natural products for cancer therapy. Expert Opin. Invest. Drugs. 2012;21:1801-1818.
- [Google Scholar]
- In vitro antioxidant activity of selected seaweeds from southeast coast of India. Int. J. Pharm. Pharm. Sci.. 2013;5:474-484.
- [Google Scholar]
- Biochemical composition of some Egyptian seaweeds with potent nutritive and antioxidant properties. Food Sci. Technol, Campinas. 2017;37:294-302.
- [Google Scholar]
- Preliminary phytochemical studies on some selected seaweeds from Gulf of Mannar, India. Asian Pac. J. Trop. Biomed.. 2012;2:S30-S33.
- [Google Scholar]
- NMR-based plant metabolomics: Where do we stand, where do we go? Trends Biotechnol.. 2011;29:267-275.
- [Google Scholar]
- Inhibition of fish oil oxidation and the radical scavenging activity of New Zealand seaweed extracts. Food Chem.. 2012;133:1624-1631.
- [Google Scholar]
- Induction of the superoxide anion radical scavenging capacity of dried ‘funori’Gloiopeltis furcata by Lactobacillus plantarum S-SU1 fermentation. Food Funct.. 2015;6:2535-2541.
- [Google Scholar]
- Evaluation of antioxidant properties of marine seaweed samples by DPPH method. Int J. Pure Appl. Biosci.. 2014;2:132-137.
- [Google Scholar]
- Effect of different drying techniques on the phytochemical content and antioxidant activity of Kappaphycus alvarezii. J. Appl. Phycol.. 2015;27:1717-1723.
- [Google Scholar]
- Phenolic content and antioxidant capacity in algal food products. Molecules. 2015;20:1118-1133.
- [Google Scholar]
- Antioxidative activity of extracts from selected species of the family Lamiaceae in sunflower oil. Food Chem.. 1997;58:245-248.
- [Google Scholar]
- Antioxidant activities and phenolics content of eight species of seaweeds from north Borneo. J. Appl. Phycol.. 2008;20:367-373.
- [Google Scholar]
- Matsukawa, R., Dubinsky, Z., kishimoto, E., Masakki, K., Masuda, Y., Takeuchi, T.C., 1997. A comparison of screening methods for antioxidant activity in seaweeds. J. Appl. Phycol. 9, 29–35.
- Effects of different drying methods and storage time on free radical scavenging activity and total phenolic content of cosmos caudatus. Antioxidants. 2014;3:358-370.
- [Google Scholar]
- Mediani, A., Abas, F., Khatib, A., Maulidiani, Shaari, K., Choi, Y.H., Lajis, N.H., 2012. 1H-NMR-based metabolomics approach to understanding the drying effects on the phytochemicals in Cosmos caudatus. Food Res. Int. 49, 763–770
- Cosmos Caudatus as a potential source of polyphenolic compounds: Optimisation of oven drying conditions and characterisation of its functional properties. Molecules. 2013;18:10452-10464.
- [Google Scholar]
- Moure, A., Franco, D., Sineiro, J., Domı́nguez, H., Núñez, M.J., Lema, J.M., 2001. Antioxidant activity of extracts from Gevuina avellana and Rosa rubiginosa defatted seeds. Food Res. Int. 34, 103–109.
- Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J. Pharm. Biomed.. 2006;41:1523-1542.
- [Google Scholar]
- Evaluation of selected biological capacities of Baeckea frutescens. BMC Complement. Altern. Med.. 2015;15:1-8.
- [Google Scholar]
- Comparative study of drying methods on chemical constituents of Malaysian red seaweed. Dry Technol.. 2016;1–22
- [CrossRef] [Google Scholar]
- A value chain analysis of Malaysia’s seaweed industry. J. Appl. Phycol. 2019
- [CrossRef] [Google Scholar]
- Effects of drying methods, solvent extraction and particle size of Malaysian brown seaweed, Sargassum sp. on the total phenolic and free radical scavenging activity. Int. Food Res. J.. 2016;23:1558-1563.
- [Google Scholar]
- Evaluation of antioxidant and antimicrobial activity of seaweed (Sargassum sp.) extract: a study on inhibition of glutathione-s-transferase activity. Turk. J. Biol.. 2008;32:119-125.
- [Google Scholar]
- Screening for bioactive compounds from algae. J. Pharmaceut. Biomed.. 2010;51:450-455.
- [Google Scholar]
- Spectrophotometric quantification of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application of vitamin E. Anal. Biochem.. 1999;269:337-341.
- [Google Scholar]
- Antimicrobial, antioxidant and free radical scavenging capacity of brown seaweed Himanthalia Elongata from western coast of Ireland. J. Food Biochem.. 2013;37:322-335.
- [Google Scholar]
- Extraction, antioxidative, and antimicrobial activities of brown seaweed extracts, Turbinaria ornata and Sargassum polycystum, grown in Thailand. Int. Aquat. Res.. 2015;7:1-16.
- [Google Scholar]
- Influence of phenolic compounds on the physiology of Oenococcus oeni from wine. J. Appl. Microbiol.. 2000;88:1065-1071.
- [Google Scholar]
- Effect of drying methods on the chemical composition and colour of peppermint (Mentha × piperita L.) leaves. Zemdirbyste-Agriculture. 2015;102:223-228.
- [Google Scholar]
- Radical scavenging and singlet oxygen quenching activity of extracts from Indian seaweeds. J. Food Sci. Technol.. 2010;47:94-99.
- [Google Scholar]
- Shuib, N.H., Shaari, K., Khatib, A., Maulidiani, Kneer, R., Zareen, S., Raof, S.M., Lajis, N.H., Neto, V., 2011. Discrimination of young and mature leaves of Melicope ptelefolia using 1H NMR and multivariate data analysis. Food Chem. 126, 640–645.
- Insights from the structural analysis of protein heterodimer interfaces. Bioinformation. 2011;6:137-143.
- [Google Scholar]
- Marine-based nutraceuticals: An innovative trend in the food and supplement industries. Mar. Drugs. 2015;13:6336-6351.
- [Google Scholar]
- Comparative analysis of bioactive phenolic compounds composition from 26 medicinal plants. Saudi. J Biol. Sci. 2018;25:631-641.
- [Google Scholar]
- Antioxidant capacity, cytotoxicity, and acute oral toxicity of Gynura bicolor. Evid. Based Complementary Altern. Med. 2013:1-10.
- [Google Scholar]
- Phytochemical screening and extraction: a review. Internationale Pharmaceutica Sciencia. 2011;1:98-106.
- [Google Scholar]
- Analysis of Phenolic Plant Metabolites. Oxford: Blackwell Scientific Publications; 1994.
- Biomass production of two Sargassum species at Cape Rachado, Malaysia. Hydrobiologia. 2004;512:79-88.
- [Google Scholar]
- Antimicrobial activity of the Sargassum fulvellum ethanol extract and the effect of temperature and pH on their activity. Korean J. Food Sci. Technol.. 2010;42:155-159.
- [Google Scholar]
- Preparation and in vitro antioxidant activity of κ-carrageenan oligosaccharides and their oversulfated, acetylated, and phosphorylated derivates. Carbohydr. Res.. 2005;340:685-692.
- [Google Scholar]
- Antioxidant and antiproliferative activities of extracts from a variety of edible seaweeds. Food Chem. Toxicol.. 2006;44:1144-1150.
- [Google Scholar]
- Alginate, mannitol, phenolic compounds and biological activities of two range-extending brown algae, Sargassum mangarevense and Turbinaria ornata (Phaeophyta: Fucales), from Tahiti (French Polynesia) J. Appl. Phycol.. 2008;20:1033-1043.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2020.09.002.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







