Translate this page into:
Microwave assisted synthesis, photoisomerization study and antioxidant activity of a series of N-acylhydrazones
⁎Corresponding author. a.amine@umi.ac.ma (Amina Amine)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
A series of N-acylhydrazone 4(a-f) were prepared by condensation of 2-hydroxybenzohydrazide with substituted benzaldehyde derivatives and catalytic amount of acetic acid via a simple method using microwave-assisted synthesis. Reaction conditions such as temperature (60 °C), irradiation power (150 w), and catalyst were optimized. Target compounds were obtained in prompt time (4–10 min) and with excellent yields reaching 98 %. Structural characterization was accomplished using FTIR, 1H NMR, 13C NMR and EIS-MS spectrometry. Reversible light-induced E/Z isomerization at the C = N bond of the synthetized compounds was achieved as function of time in two solvents: dimethylsulfoxide (DMSO) and methanol. The photodynamical E/Z conversion revealed the influence of the solvent on this process that took about 30 min in methanol and 45 min in DMSO. Moreover, antioxidant activity was evaluated for all the synthetized N-acylhydrazones using the 2,2 Dipheny-1-picrylhydrazyl (DPPH) assays. Results showed promising antioxidant effects for compounds 2-hydroxy-N′-(2-hydroxy-4-(p-tolyldiazenyl)benzylidene)benzohydrazide (4e) and 2-hydroxy-N′-(2-hydroxy-3-methoxy-4-(p-tolyldiazenyl)benzylidene)benzohydrazide (4f) that are combining both the acylhydrazone function and an azo fragment in their structures.
Keywords
Acylhydrazone
Microwave irradiation
Photoisomerization
UV–Visible
Antioxidant activity
1 Introduction
N-acylhydrazones (NAHs) are significants units in chemistry due to their wide-ranging applications. These compounds are considered as important precursors in the preparation of many heterocyclic nucleus such as imidazolines (Zhao et al., 2020), oxadiazoles (Küçük et al. 2022), triazoline and triazoles (Liu et al. 2022). NAHs are also used in coordination chemistry as interesting ligands for many metal cations (Hu et al., 2015) and this ability proved some of them as remarkable selective chemosensors of several metallic cations (Liao et al., 2017) (Aarjane et al., 2020b). In medicinal chemistry, the N-acylhydrazone fragment is one of the privileged units due to its flexibility and to the presence of H-binding sites that give to these compounds the ability to interact with diverse receptors (Welsch et al., 2010). Thus, a wide range of biological properties were revealed by NAHs such as antitumoral (Cui et al., 2010), antimycobacterial (Rohane et al., 2020) antimalarial (dos Santos Filho et al., 2016), antioxidant and numerous other properties (Rollas and Küçükgüzel, 2007) (Socea et al., 2022). Furthermore, NAHs have gained their interest as dynamic responsive molecules to stimuli such as radiation. Upon irradiation, the imine bond in the acylhydrazone unit undergo a reversible light-induced E/Z isomerization making this category of molecules excellent candidates for applications as photoswitches (Van Dijken et al., 2015), optical data storages (Qi et al., 2020), optoelectronic devices and so on. In addition, NAHs undergo a cis/trans arrangement around the carbonyl bond leading to the existence of synperiplanar (sp) and antiperiplanar (ap) conformers (Palla et al., 1986). Another interesting facet of these molecules is the simplicity of their preparation. Indeed, NAH could be readily obtained by a simple condensation between a carbonyl derivative and a hydrazide under mild conditions. Eco-friendly approaches such as microwave assisted synthesis (Andrade and Barros, 2010) or grindstone chemistry method were also used to prepare these kind of compounds and gave satisfying results. (Dos Santos Filho and Pinheiro, 2017).
On the other hand, azobenzenes are molecules with great interest owing to their high stability, their numerous biological properties (Cheng et al., 2021) (Di Martino et al., 2022), in addition to their reversible trans-to-cis isomerization. This interesting property led to involving these compounds as optical switches and molecular machines (Dong et al., 2015) (Choi et al., 2018) (Weis and Wu, 2018). The azo fragment undergoes a reversible light induced trans–cis isomerization of the N = N bond, and usually the reverse isomerization occurs spontaneously in the dark.
In our previous work, we have demonstrated the antibacterial and antioxidant activities of a series of N-acylhydrazones linked to an acridone nucleus, and the role of the acylhydrazone moiety in enhancing the biological responses (Aarjane et al., 2020a). Besides, we have proved the importance of a series of azo Schiff base derived from salicylic aldehyde as antimicrobial and antioxidant agents (Slassi et al., 2019a) (Slassi et al., 2019b) (Slassi et al., 2020) and furthermore the photo-induced isomerization of these compounds (Slassi et al., 2020) (Slassi et al., 2023). To carry on in our approach, we developed new structures that incorporate both azo and N-acylhydrazone fragments. The two units are linked to a salicyl or vaniline nucleus as a central core. The molecules were synthesized following a microwave assisted method and results were compared to those obtained by a conventional one. The light induced E/Z photoisomerization was studied in two polar solvents: methanol and dimethysulfoxide. Results revealed the influence of the solvent on this process that took about 30 min in methanol and 45 min in DMSO. Along with this study, antioxidant activity of the synthetized compounds was evaluated.
2 Material and methods
All materials were procured from commercial suppliers (Merck, Sigma-Aldrich). The starting reagents salicylic acid, hydrazine hydrate, acetic acid, benzaldehyde, 2-hydroxybenzaldehyde, 4-hydroxybenzaldehyde and o-vanillin were of analytical grade. Solvents were of analytical grade and were used without further purification. Microwave irradiations were carried out with CEM DiscoverTM apparatus. FTIR spectra were performed on a JASCO FT-IR 4100 spectrometer. UV–visible spectra were registered using SHIMADZU UV-1800 spectrophotometer. 1H and 13CNMR spectra were performed using JNM-ECZ500R/S1 FT NMR SYSTEM (JEOL) Frequency (1H): 500MHZ. Mass spectrometry measurements were performed by (MS-HPLC) Ultimate 3000-Exactive plus THERMO.
2.1 Synthesis
2.1.1 Hydrazide synthesis
Ethyl 2-hydroxybenzoate was prepared from salicylic acid (6 g, 43 mmol) and ethanol (25 ml) with a catalytic quantity of boric acid. The resulting ester (5 g, 30 mmol) was dissolved in ethanol (20 ml) then treated with an excess of hydrazine (3 equiv.). After four hours of stirring at reflux, the reaction mixture was dried under reduced pressure and the resulting crude was dissolved in ethyl acetate and washed with water. The ethyl acetate layer was then dried and solvent was removed to give the hydrazide derivative: 2-hydroxybenzohydrazide. The same reaction was conducted under microwave irradiation (150 W, 80 °C) for 30 min in a quartz tube equipped with a magnetic bar. After solvent evaporation, the crude was treated as described for the conventional method. This MW method led to the hydrazide in similar yield (80 %).
2.1.2 N-acylhydrazones synthesis
Conventional method: In a flask equipped with a refrigerant 2-hydroxybenzohydrazide (5 g, 33 mmol) was mixed with stoichiometric amounts of differently substituted benzaldehyde derivatives using ethanol as solvent and acetic acid in catalytic amount. Reaction mixtures were stirred at reflux four hours to give the hydrazone derivatives (4a-f) as solid products. NAHs 4(a-f) were purified by recrystallization in ethanol.
Microwave-Assisted Method: 2-hydroxybenzohydrazide (1 g, 6.5 mmol) with equimolar amount of a substituted benzaldehyde derivatives were dissolved in 5 ml of ethanol or dichloromethane in a quartz tube equipped with a magnetic bar. To this mixture, glacial acetic acid was added in catalytic amount then the reaction mixture was irradiated for two to ten minutes. The microwave irradiation power was set at 150 W. At the end of the reaction, the obtained product were let to precipitate, they were collected by filtration and then recrystallized from ethanol.
2.1.2.1 N′-benzylidene-2-hydroxybenzohydrazide (4a)
Selected IR bands (KBr): 3200, 3032, 1950, 1628 cm−1. 1H NMR (500 MHz, DMSOd6, 25 °C, TMS): 11.83 (s, 1H), 8.45 (s, 1H), 7.88 (d, 1H, J = 8.0 Hz), 7.71 (d, 2H, J = 6.9 Hz), 7.42 (q, 4H, J = 8.1 Hz), 6.97–6.90 (m, 2H). 13C NMR (126 MHz, DMSOd6, 25 °C, TMS): 165.31, 159.56, 149.24, 134.65, 133.39, 131.32, 130.84, 129.80, 129.41, 129.12, 127.78, 119,49, 116.47, 40.54. MS (ESI), m/z = 239.0851 [M]-.
2.1.2.2 2-hydroxy-N′-(2-hydroxybenzylidene)benzohydrazide (4b)
Selected IR bands (KBr): 3214, 3048, 1918, 1631 cm−1. 1H NMR (500 MHz, DMSOd6, 25 °C, TMS): 11.97 (s, 1H), 11.78 (s, 1H), 8.66 (s, 1H), 7.87 (d, 1H, J = 7.8 Hz), 7.56–7.35 (m, 2H), 7.31–7.23 (m, 1H), 6.93 (dq, 4H, J = 7.8, 7.3 Hz). 13C NMR (126 MHz, DMSOd6, 25 °C, TMS): 165.05, 159.56, 158.06, 134.52, 130.01, 129.13, 119.94, 119.17, 117.85, 117.00, 116.17, 40.05. MS (ESI), m/z = 255.0817 [M]-.
2.1.2.3 4-hydroxy-N′-(2-hydroxybenzylidene)benzohydrazide (4c)
FTIR (KBr): 3334, 3228, 2315, 1607 cm−1. 1H NMR (500 MHz, DMSOd6, 25 °C, TMS): 11.68 (s, 1H), 9.95 (s, 1H), 8.33 (s, 1H), 7.80 (d, 1H, J = 8.0 Hz), 7.56 (d, 2H, J = 8.0 Hz), 7.41–7.34 (m, 1H), 6.93–6.88 (m, 1H), 6.82 (d, 3H, J = 7.9 Hz). 13C NMR (126 MHz, DMSOd6, 25 °C, TMS): 165.20, 160.19, 159.83, 134.27, 129.63, 128.84, 125.58, 119.37, 117.85, 116.29, 40.01. MS (ESI), m/z = 255.0781 [M]-.
2.1.2.4 2-hydroxy-N′-(2-hydroxy-3-methoxybenzylidene)benzohydrazide (4d)
FTIR bands (KBr): 3348, 3188, 2327, 1614, 1256 cm−1. 1H NMR (500 MHz, DMSOd6, 25 °C, TMS): 11.98 (s, 1H), 11.77 (s, 1H), 8.66 (s, 1H), 7.86 (d, 1H, J = 7.8 Hz), 7.42 (s, 1H), 7.14 (d, 1H, J = 7.8 Hz), 7.01 (d, 1H, J = 8.0 Hz), 6.95 (d, 1H, J = 8.2 Hz), 6.88 (d, 1H, J = 8.3 Hz), 6.84 (t, 1H, J = 7.9 Hz), 3.78 (s, 1H). 13C NMR (126 MHz, DMSOd6, 25 °C, TMS): 166.05, 159.57, 149.35, 148.50, 147.76, 134.54, 129.10, 121.23, 119.63, 117.85, 117.83, 116.17, 114.49, 37.40. MS (ESI), m/z = 285.0875 [M]-.
2.1.2.5 2-hydroxy-N′-(2-hydroxy-4-(p-tolyldiazenyl)benzylidene)benzohydrazide (4e)
FTIR bands (KBr): 3223, 3050, 1940, 1625, 1546 cm−1. 1H NMR (500 MHz, DMSOd6, 25 °C, TMS): 11.31 (s, 1H), 8.61 (s, 1H), 8.42 (s, 1H), 7.83–7.77 (m, 4H), 7.69–7.62 (s, 1H), 7.47–7.41 (m, 4H), 7.15 (s, 1H), 2.35 (s, 1H). 13C NMR (126 MHz, DMSOd6, 25 °C, TMS): 168.04, 160.33, 129.94, 128.11, 120.18, 118.70, 117.93, 116.49, 115.49, 40.52. MS (ESI), m/z = 373.1290 [M]-.
2.1.2.6 2-hydroxy-N′-(2-hydroxy-3-methoxy-4-(p-tolyldiazenyl)benzylidene)benzohydrazide (4f)
FTIR bands (KBr): 3400, 3211, 1900, 1607, 1548 cm−1. 1H NMR (500 MHz, DMSOd6, 25 °C, TMS): 11.89 (s, 1H), 8.78 (s, 1H), 7.87 (d, 3H, J = 8.2 Hz), 7.76 (d, 3H, J = 7.7 Hz), 7.53–7.37 (m, 2H), 7.69–7.62 (s, 1H), 7.37 (d, 1H, J = 7.7 Hz), 6.92 (dd, 2H, J = 8.6 Hz), 3.90 (s, 1H), 2.46 (s, 1H). 13C NMR (126 MHz, DMSOd6, 25 °C, TMS): 159.65, 150.57, 147.82, 145.07, 141.47, 134.51, 130.46, 129.19, 122.83, 119.50, 117.83, 116.34, 56.46, 40.54, 21.51. MS (ESI), m/z = 403.1405 [M]-.
2.2 Photoisomerization
Stock solutions (4.10-6 M) of compounds 4(a-d) were prepared using DMSO as solvent for a first set of experiments and methanol in another set. Samples were exposed to UV radiations at 365 nm with a low-pressure lamp (40 W; model Vilber VL340.BL, light intensity 413 mW/cm2). The reactor was placed 12 cm below the lamp. Samples' spectra were registered as function of time.
2.3 Antioxidant effect
To evaluate the antioxidant capacity of our molecules, we prepared solutions with a concentration of 100 µg/ml in DMSO. Increasing volumes were sampled, supplemented with methanol up to 200 µl, and then mixed with a DPPH solution in methanol (2.4 µg/ml). The prepared samples were stirred and left at ambient temperature in the dark for 30 min. After this incubation period, the absorbance (A) was measured at 517 nm, using methanol as the blank control and ascorbic acid as control. Tests were performed three times, and the IC50 values, which are the required concentration for each compound to inhibit 50 % of the DPPH free radicals, were calculated and presented as the mean value ± standard deviation. IC50 were determined following equation (1).
3 Results and discussion
3.1 Synthesis
The synthetized acylhydrazones were prepared from salicylic acid following the three-step route illustrated in Fig. 1. 2-hydroxybenzohydrazide (3) was obtained in good yield from ethyl 2-hydroxybenzoate (2) and hydrazine hydrate in ethanol. Finally, N-acylhydrazones (4) were prepared by a coupling reaction between 2-hydroxybenzohydrazide and different natural and substituted aldehydes to afford the final compounds in interesting yields (Fig. 1). For this last step, we used a microwave-assisted synthesis method and results were compared to those obtained by a conventional synthetic method.
Synthetic route to N-acylhydrazone derivatives from salicylic acid.
With the conventional method, reactions were conducted at reflux in ethanol for four hours and compounds 4(a-f) were obtained in 69–80 % yields. Conversely, for the chemistry method, reaction conditions such as solvent, temperature and reaction time were optimized.
Reactions were carried out in ethanol and in dichloromethane at ambient temperature, 30 °C and 60 °C, the irradiation power was set at 80, 100 and 150 W, while the irradiation duration was varied from 2 to 10 min. Results allowed us to conclude that the optimal conditions were to run reactions under an irradiation power of 150 W and a temperature of 60 °C. Results summarized in Table 1 show that compounds 4(a-f) were afforded in excellent yields (95–98 %) with both solvent. Reactions conducted in DCM were achieved in 4 min while those in ethanol required 10 min to be completed.
Reaction conditions: MW (150 W, 60 °C, EtOH)
Compound
4a
4b
4c
4d
4e
4f
Reaction time (min)
10
10
10
10
10
10
Yield (%)
98
97
98
98
95
95
Reaction conditions: MW (150 W, 60 °C, DCM)
Compound
4a
4b
4c
4d
4e
4f
Reaction time (min)
4
4
4
4
4
4
Yield (%)
98
97
98
98
96
97
Reaction conditions: EtOH, reflux
Compound
4a
4b
4c
4d
4e
4f
Reaction time (min)
240
240
240
240
240
240
Yield (%)
80
78
69
72
70
71
Interestingly, microwave assisted synthesis was proved to be a simple, effective and prompt method for the preparation of compounds 4(a-f) and DCM was demonstrated better solvent for the reaction. In a previous work (Andrade and Barros, 2010) compound 4a was prepared with a comparable method using microwave assisted synthesis by reacting hydrazide with benzaldehyde in absence of solvent and catalysts but the reaction conditions were set to 200 W under 4.1 bar and a temperature of 201 °C. Authors obtained the resulting product 4a in 7 min with 89 % yield. Comparing to those results, we can conclude that we developed an effective method using a lower power (150 W) and a lower temperature (60 °C). This synthetic pathway permitted us to prepare the target compounds in higher yields (98 %) in less time.
Structures of the synthetized products were established using various spectral analyses, including FTIR, 1H NMR, 13C NMR, and mass spectrometry. Relevant spectral data are provided as supplementary material (Fig. S1-S18).
In FTIR spectra of compounds (4a-f), the imine function in the N-acylhydrazone fragment revealed a characteristic band at 1600–1611 cm−1 range, and the carbonyl group revealed a signal at 1680 cm−1. In compounds 4(e-f), the azo unit gave characteristic absorption bands in the region of 1530–1490 cm−1.
In 1H NMR, HC = N protons appear as singlets in the 8.78 to 8.33 ppm region, the amide protons produce a broad singlets signal in the 11.89 to 11.68 ppm range and aromatic protons resonate in general as doublets or multiplets in the 7.86–6.3 ppm area. Methoxy group resonates as sharp singlet at 3.78 ppm. The 13C NMR spectra also reveal signals corresponding to the imine (C = N) and amide (–NHCO) moieties at 159.65–166.05 ppm and 159.83–145.07 ppm respectively. The –OCH3 group resonates at 56.37 ppm.
3.2 UV–visible absorption and photoisomerization study
The UV–visible spectra of 4(a-d) were monitored at room temperature in the region 200–800 nm in two solvents: methanol (Fig. 2) and DMSO (Fig. 3). All the N-acylhydrazone compounds 4(a-d) exhibit three main absorption bands, the first one appeared in the visible region with a weak intensity and was attributed to n-π* transition characteristic of the N-acylhydrazone fragment. This band appeared at 392–438 nm in methanol and 384–456 nm in DMSO. The spectra of 4(a-d) display two other bands assigned to π-π* transitions at 288–324 and 298–344 nm in MeOH, and at 270–300 and 312–328 nm in DMSO. We noticed also that the presence of the methoxy group in the structure of compound 4d affects the absorption bands of both n-π* and π-π* transitions in either MeOH or DMSO solutions, resulting in a significant redshift.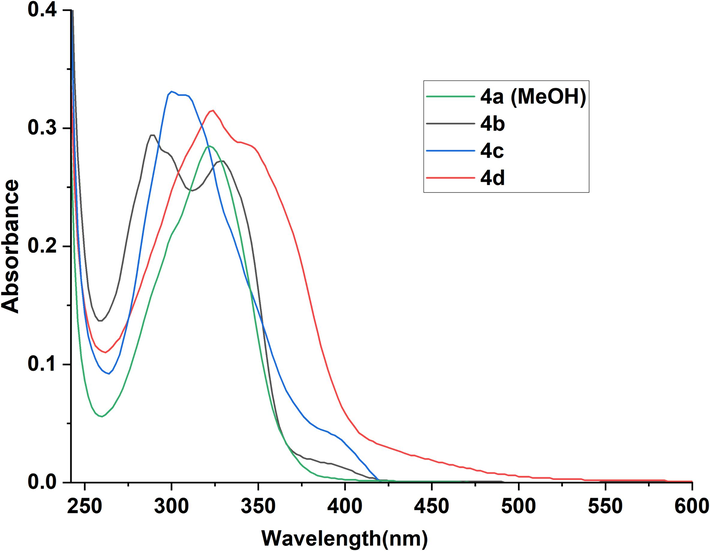
Absorption spectra of N-acylhydrazones 4(a-d) (4.10-6 M) in MeOH.
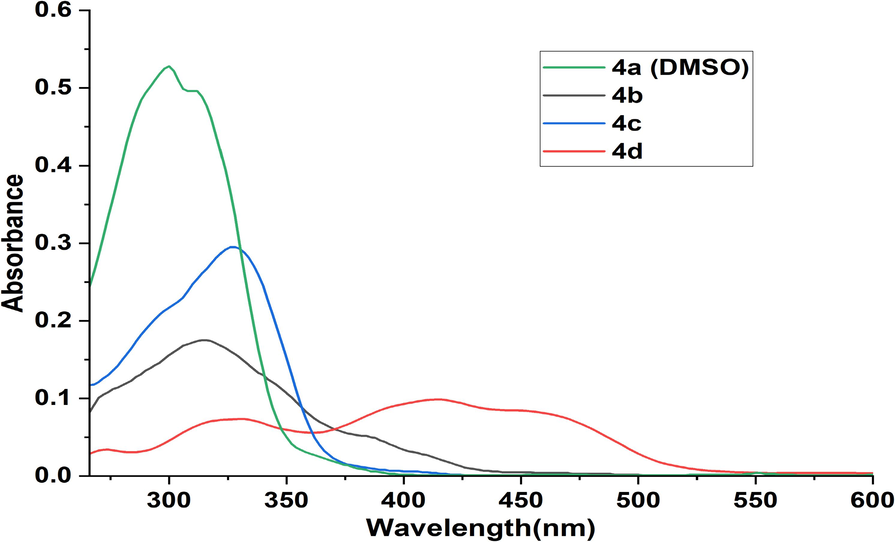
Absorption spectra of N-acylhydrazones 4(a-d) (4.10-6 M) in DMSO.
The solvent nature has a notable effect on the absorbance of each molecule (Karmakar et al., 2021). In effect, changing methanol with DMSO induced a decrease in the absorbance of compounds 4b and 4c, which have a hydroxyl group on the phenyl ring while the absence of this group in 4a led to an increase in absorbance around 320 nm. At the same time, we noticed the decrease of the band at 328 nm for compound 4d and the enhancement of absorbance of the band at 456 nm. These spectra changes are probably due to hydrogen bond formation between the solvent and the hydroxyl or NH groups.
Photoisomerisation is an important property of a molecule that attract great interest due to its diverse applications. This property was studied for NAHs 4(a-d) by evaluating the differences in the UV–Vis spectra of these compounds before and upon UV irradiation as function of time. The disparities in the spectra of compounds 4(a-d) were recorded in two solvents: methanol and DMSO. The resulting UV–visible spectra are displayed in Fig. 4 and Fig. 5. Results analysis allowed us to conclude that all the studied compounds demonstrated effective photochromic behavior.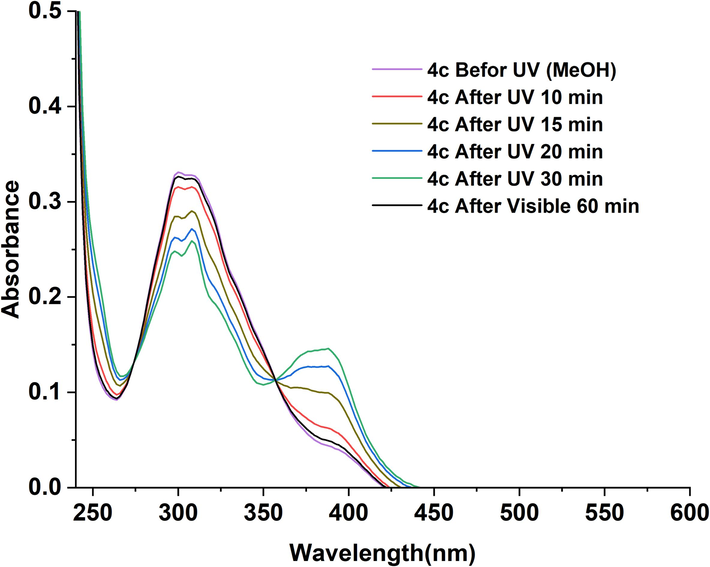
UV–visible spectra of 4(c) in MeOH (4.10-6 M) recorded as function of time before and upon irradiation at 365 nm and after exposure to visible light for 60 min.
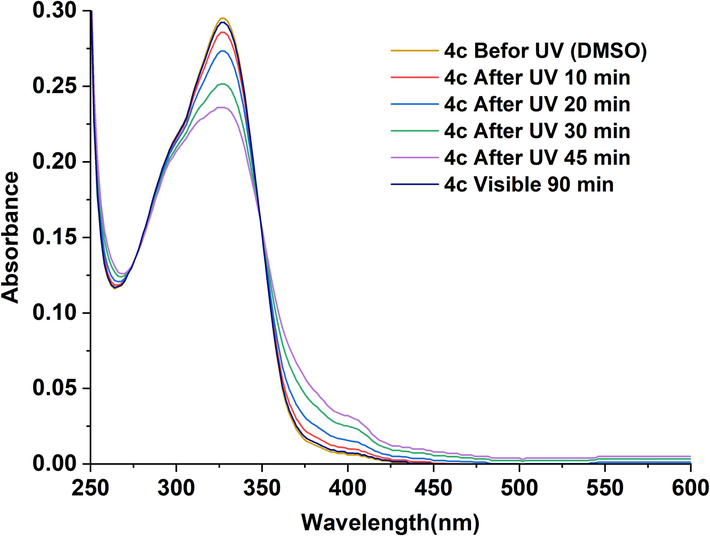
UV–visible spectra of 4(c) in DMSO (4.10-6 M) recorded as function of time before and upon irradiation at 365 nm and after exposure to visible light for 60 min.
In both MeOH and DMSO solvents, compound 4c, upon 10 min of irradiation at 365 nm, exhibited a drastic decrease in the intensity of the λmax in the π–π* band. Simultaneously, the lower energy band of the characteristic n-π* transition of the acylhydrazone group, underwent a hyperchromic shift and, at the same time, a slight red shift (10 nm for DMSO and 6 nm for MeOH). These changes indicated clearly the light-induced trans to cis isomerization of the imine bond in N-acylhydrazones 4(a-d).
E/Z isomerization process strongly depends on the polarity of the solvent and its ability to stabilize the photoinduced transition state since the interactions between the solvent and the solute influence the distribution of isomers during this process (Cordier et al., 2004) (Galić et al., 2012). When a solution of acylhydrazones 4(a-d) in methanol are exposed to UV light, a complete trans to cis isomerization was detected after 30 min. Methanol is a polar protic solvent that can form intermolecular interactions with hydroxyl groups and the hydrazide group of solute compounds through hydrogen bonds. These interactions stabilize the formed isomers and lead to a faster isomerization. However, DMSO, a polar aprotic solvent, can also interact through hydrogen bonds with specific functional groups of the solute inducing stabilization effects distinct from methanol. These interactions might modify the dynamics of the compounds involved in the photoisomerisation process, thereby influencing the rate of isomerization. Consequently, the trans to cis isomerization was achieved in 45 min in DMSO. In addition, the presence of different substituents, including hydroxyl groups (OH) and methoxy (OCH3), affects the intermolecular interactions, thus also affected the isomerization rate.
Immediately after switching off the UV light, as the visible light-return time progressed, we observed a decrease in the intensity of π–π* transition and an increase in the intensity of the n–π* transition. As reported in the spectra presented in Fig. 3 and Fig. 4, the trans form was completely recovered after 60 min in MeOH and 90 min in DMSO. These finding proved the reversible isomerization from cis- to trans-form of 4c compound. The formation of isopiestics points, indicating the coexistence of cis and trans-forms, further confirms the isomerization. Similar results were obtained for compounds 4a, 4b and 4d.
3.3 Antioxidant activity.
The anti-radical activity is demonstrated by the aptitude of a substance to neutralize free radicals, which are unstable molecules produced naturally in the body or introduced by external factors. This characteristic contributes to protecting cells against oxidative damage associated with health benefits, including the prevention of chronic diseases. Several methods are employed to assess anti-radical activity in in vitro tests (Abd Alkareem and Waheed, 2022) (Bale, 2022). In our study, antioxidant assays were conducted following the 2,2 Dipheny-1-picrylhydrazyl (DPPH) radical scavenging test reported in literature [Kedare et al., 2011), (Hani et al., 2023) and using ascorbic acid as reference. Half-maximal inhibitory concentrations (IC50) are summarized in Table 2. Data show that compounds 4e and 4f exhibited a significant capacity for scavenging DPPH free radicals although it is less than the ascorbic acid. However, molecules 4a-d revealed a very high IC50 values, making it challenging to determine whether their activity is very low or null compared to 4e and 4f.
Compound
Ar
CI50 (µg/ml)
4a
H
˃100
4b
2-OH
˃100
4c
4-OH
˃100
4d
2-hydroxy-3-methoxy
˃100
4e
2-hydroxy-5-(p-tolydiazenyl)benzylidene
73,94 ± 1,63
4f
2-hydroxy-3-methoxy-5-(p-tolydiazenyl)benzylidene
38,09 ± 1,74
Ascorbic acid
2,09 ± 0,12
Molecules possessing OH or NH mobile protons groups in their structures are probable scavengers of reactive oxygen species (ROS) and several studies have demonstrated the relation between radical scavenging property and the facility of an H-atom transfer, mostly for the hydroxyl group, to free radicals (Santos-Sanchez et al., 2019).
For our compounds, this promising activity is not only due to the presence of the OH group that is generally known to be the active group for DPPH scavenging but can be attributed to the presence of the azobenzene group. In effect, this later induces a substantial conjugation in the structure that offers to the system various resonance structures and consequently high stability of these molecules. We noticed also that the presence a donating group such as –OCH3 on the central core of the molecule was favorable for this activity.
4 Conclusion
N-acylhydrazones 4(a-f) were prepared using microwave-assisted synthesis. Reaction conditions such as temperature, irradiation power and reaction time were optimized. Excellent yields (98 %) were reached under MW irradiation (150 W) at 60 °C, the reactions were achieved in 4 min in DCM and in 10 min in ethanol. UV–Visible spectra of compounds 4(a-d) were recorded in two polar solvents: methanol and DMSO. The observed disparities in absorbance and wavelengths depended on the polarity of each solvent and also on the substituents in the studied NAHs. Reversible light-induced E/Z isomerization at the C = N bond of compounds 4(a-d) was achieved as function of time in DMSO and in methanol. The effect of the solvent on the photoisomerization of compounds 4(a-d) was revealed through the important changes in UV–Visible spectra. MeOH shows a quicker photoisomerization of trans to cis form (30 min) compared to DMSO (45 min) with different kinetics in the recovery of the trans form, that approve the significant solvent influence on the photoisomerization process. Furthermore, antioxidant activity was investigated for all the synthetized N-acylhydrazones using the DPPH assays. Results showed negative responses for compounds 4(a-d) but promising antioxidant effects for compounds 4e and 4f that are combining both the acylhydrazone function and an azo fragment in their structures. This later is offering to the system various resonance structures and consequently high stability of these molecules. We noticed also that the presence a donating group such as –OCH3 on the central core of the molecule was favorable for this activity.
CRediT authorship contribution statement
Yahya Boubekri: Formal analysis, Investigation, Writing – original draft, Data curation. Siham Slassi: Formal analysis, Investigation, Writing – original draft. Mohammed Aarjane: Formal analysis. Bouchra Tazi: Resources. Amina Amine: Methodology, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Synthesis, antibacterial evaluation, in silico ADMET and molecular docking studies of new N-acylhydrazone derivatives from acridone. Arab. J. Chem.. 2020;13:6236-6245.
- [CrossRef] [Google Scholar]
- Novel highly selective and sensitive fluorescent sensor for copper detection based on N-acylhydrazone acridone derivative. J. Mol. Struct.. 2020;1199:126990
- [CrossRef] [Google Scholar]
- Formation, characterization and antioxidant study of mixed ligand complexes derived from succinyl chloride. Chem. Methodol.. 2022;6:914-928.
- [CrossRef] [Google Scholar]
- Fast synthesis of N-acylhydrazones employing a microwave assisted neat protocol. J. Comb. Chem.. 2010;12:245-247.
- [CrossRef] [Google Scholar]
- Bale, A.T., 2022. Synthesis and Biological Study of Substituted 2 ” -H ydoxy , 2 ”, 4 ” - dichloro Chalcones and Their Co (II), Cu (II) and Ni (II) Complexes for Their Antioxidant and Antimicrobial Potentials 5, 94–103.
- Advances in application of azobenzene as a trigger in biomedicine: molecular design and spontaneous assembly. Adv. Mater.. 2021;33:1-42.
- [CrossRef] [Google Scholar]
- Azobenzene molecular machine: light-induced wringing gel fabricated from asymmetric macrogelator. ACS Macro Lett.. 2018;7:576-581.
- [CrossRef] [Google Scholar]
- Salicylaldehyde benzoyl hydrazone: Isomerization due to water. A structural analysis using a combination of NMR, IR, and theoretical investigations. Struct. Chem.. 2004;15:295-307.
- [CrossRef] [Google Scholar]
- New class of potent antitumor acylhydrazone derivatives containing furan. Eur. J. Med. Chem.. 2010;45:5576-5584.
- [CrossRef] [Google Scholar]
- Red-shifting azobenzene photoswitches for in vivo use. Acc. Chem. Res.. 2015;48:2662-2670.
- [CrossRef] [Google Scholar]
- Conjugation of N-acylhydrazone and 1,2,4-oxadiazole leads to the identification of active antimalarial agents. Bioorganic Med. Chem.. 2016;24:5693-5701.
- [CrossRef] [Google Scholar]
- Stereoselective, solvent free, highly efficient synthesis of aldo- and keto-: N -acylhydrazones applying grindstone chemistry. Green Chem.. 2017;19:2212-2224.
- [CrossRef] [Google Scholar]
- Structural investigation of aroylhydrazones in dimethylsulphoxide/water mixtures. Spectrochim. Acta - Part A Mol Biomol. Spectrosc.. 2012;95:347-353.
- [CrossRef] [Google Scholar]
- Study of some heterocyclic compounds made from a new 4(3H)-quinazolinone and their biological and antioxidant activities. Chem. Methodol.. 2023;7:372-382.
- [CrossRef] [Google Scholar]
- Acylhydrazone based fluorescent chemosensor for zinc in aqueous solution with high selectivity and sensitivity. Sensors Actuators, B Chem.. 2015;208:581-587.
- [CrossRef] [Google Scholar]
- Solvatochromism: a tool for solvent discretion for UV-Vis spectroscopic studies. Appl. Spectrosc. Rev.. 2021;56:513-529.
- [CrossRef] [Google Scholar]
- A novel acylhydrazone-based derivative as dual-mode chemosensor for Al3+, Zn2+ and Fe3+ and its applications in cell imaging. Sensors Actuators, B Chem.. 2017;244:914-921.
- [CrossRef] [Google Scholar]
- Conformational behaviour and E/Z isomerization of N-acyl and N-aroylhydrazones. Tetrahedron. 1986;42:3649-3654.
- [CrossRef] [Google Scholar]
- Stimuli-responsive behavior of naphthyl acylhydrazone derivative and its application in information security protection. Spectrochim. Acta - Part A Mol. Biomol. Spectrosc.. 2020;242:118768
- [CrossRef] [Google Scholar]
- Synthesis and in vitro antimycobacterial potential of novel hydrazones of eugenol. Arab. J. Chem.. 2020;13:4495-4504.
- [CrossRef] [Google Scholar]
- Biological activities of hydrazone derivatives. Molecules. 2007;12:1910-1939.
- [CrossRef] [Google Scholar]
- Synthesis, crystal structure, DFT calculations, Hirshfeld surfaces, and antibacterial activities of schiff base based on imidazole. J. Mol. Struct.. 2019;1197:547-554.
- [CrossRef] [Google Scholar]
- Imidazole and azo-based schiff bases ligands as highly active antifungal and antioxidant components. Heteroat. Chem.. 2019;2019
- [CrossRef] [Google Scholar]
- New copper(II) and zinc(II) complexes based on azo Schiff base ligand: Synthesis, crystal structure, photoisomerization study and antibacterial activity. Appl. Organomet. Chem.. 2020;34:1-10.
- [CrossRef] [Google Scholar]
- Acylhydrazones and their biological activity: a review. Molecules. 2022;27
- [CrossRef] [Google Scholar]
- Acylhydrazones as widely tunable photoswitches. J. Am. Chem. Soc.. 2015;137:14982-14991.
- [CrossRef] [Google Scholar]
- Light-switchable azobenzene-containing macromolecules: from UV to near infrared. Macromol. Rapid Commun.. 2018;39:1-12.
- [CrossRef] [Google Scholar]
- Privileged scaffolds for library design and drug discovery. Curr. Opin. Chem. Biol.. 2010;14:347-361.
- [CrossRef] [Google Scholar]
- [3+2] Cycloaddition of Trifluoromethylated N-Acylhydrazones with Azomethine Ylides: Synthesis of Trifluoromethylated Imidazolidines. Asian J. Org. Chem.. 2020;9:1036-1039.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2024.105913.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







