Translate this page into:
Molecular docking of phenolic compounds and screening of antioxidant and antidiabetic potential of Olea europaea L. Ethanolic leaves extract
⁎Corresponding author. S.Chigurupati@qu.edu.sa (Sridevi Chigurupati)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Oxidative stress has a crucial role in diabetic pathophysiology, therefore consuming naturally derived antioxidants as a remedial target. This study examines the naturally occurring antioxidant and antidiabetic of Olea europaea L. ethanolic leaves extract. Olea europaea L. leaves were macerated (OLE) by using absolute ethanol. Phytochemical and physiochemical analysis of OLE was screened using standard methods. The antioxidant effects were examined by DPPH (1, 1-diphenyl-2-picrylhydrazil) radical scavenging assay. In vitro antidiabetic was assayed by α-amylase enzyme inhibition study. Ethanolic extraction of OLE by maceration technique, 10% yield. Loss on drying, foreign organic matters and total ash value of OLE showed 2%, 0.2% and 16.5%, respectively. Phytochemical test on OLE confirmed saponin, flavonoid, glycoside, tannin, phenol and carbohydrate presences. The total phenolic and flavonoid contents of OLE is 490 mg GAE/g and 855 mg RUE/g of extract, respectively. OLE (IC50 38.37 ± 0.26 µg/ml) showed functional DPPH scavenging assay comparable to ascorbic acid (IC50 30.37 ± 0.17 µg/ml). In the alpha-amylase inhibitory activity, Acarbose showed an IC50 value of 20.06 ± 0.19 µg/ml, while OLE portrayed an IC50 value of 37.99 ± 0.15 µg/ml. The kinetic studies revealed that all samples at high concentrations reacted within a very short time, and a steady state was reached almost immediately. The lowest concentration showed slow kinetic behaviour implied longer periods before the constant state was reached. Molecular docking studies evidenced that most of the phenolic compounds of OLE interact with the active site of Human pancreatic α-amylase through the hydrogen bonding and hydrophobic interaction confirming the alpha-amylase inhibitory effect. The results suggest that Olea europaea L. has been a conceivable natural bioactive source as an antioxidant and an antidiabetic agent.
Keywords
Olea europaea L.
Phytochemical
Molecular docking
Antioxidant
Antidiabetic
1 Introduction
Numerous recently discovered natural products have an indispensable part in preventing and treating diseases in humans, and attributable to their efficacy is the treatment of choice contrasted with synthetic drugs (Salah et al., 2017). Olea europaea L. (olive tree) belong to the Oleaceae family. It is acknowledged as a good food source in olive oil and is well known for its various commercial, nutritious, and therapeutic uses (Soni et al., 2006). Olive leaves are disregarded as reasonable raw material and have been broadly used in both the Mediterranean and European herbal medications (Abd El-Rahman, 2016). Olive leaves are traditionally used in treating hypertension, infectious diseases and hyperglycaemia (El and Karakaya, 2009). According to Candar et al. (2018), ADDQOL-19 (Audit of Diabetes-Dependent Quality of Life), the assessment was ussed on 453 diabetic diagnosed individuals. 12.5% of olive leaves was favourable herbal medications among diabetic patients. The phenolic compounds found in Olea europaea L. are summarised in Table 1 (Benincasa et al., 2019).***
Phenolic compound reported found within Olea europaea L.
Chemical structure
Oleuropein
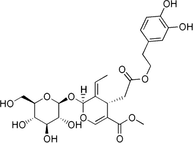
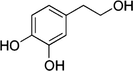
Hydroxytyrosol
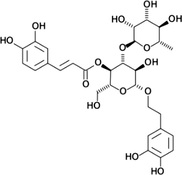
Verbascoside
Apigenin-7-glucoside
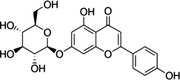
Luteolin-7-glucoside
For instance, numerous diseases, for example, malignancies, hyperlipidaemia, and hypertension, are triggered by free radicals (Betteridge, 2000). Oxidative stress imbalance oxygen free radicals that attack organic particles, including DNA, lipids and proteins. Notable oxidative stress markers are lipid hydroperoxides, isoprostan and ubiquinol-10. Human physiology able to synthesises antioxidants, for instance, catalase, to prevent cellular damage caused by free radicals. Antioxidants scavenge free radicals and restore damaged elements by releasing hydrogen molecules to the molecules or affixing themselves to the host organism's DNA (Lobo et al., 2010; Pham-Huy, He, & Pham-Huy, 2008).
Diabetes mellitus (DM) is a well-known metabolic health disorder with both micro- as well as macro-vascular difficulties, resulting in mortality and morbidity. It is contemplated a 20% foremost cause of death worldwide (American Diabetes Association, 2014). There are different diabetic categories, namely Type-1, −2, as well as gestational diabetes. Type-1 diabetes, also known as juvenile-onset or insulin-dependent diabetes, whereby the body autoimmunity harms the insulin-producing (islet, or islets of Langerhans) cells (Alberti and Zimmet, 1998). As for Type-2 diabetes, the human body is unable to use generated insulin effectively. Gestational diabetes is a high or above-average blood sugar level commonly seen in pregnant women without having a diabetic history. To restrain diabetic progression, drugs sourced from medicinal plants are used in therapeutic settings (Alarcon-Aguilar et al., 2002). Medicinal plants are favoured over synthetic drugs due to their toxicity and side effects (Pari and Umamaheswari, 2000). Hence, these plants can inhibit alpha-amylase and –glucosidase is further studies to develop a cure for DM. Degradation of the dietary starch proceeds rapidly and leads to elevated PPHG (postprandial hyperglycemia). It has been shown that activity of HPA (human pancreatic α-amylase) in the small intestine correlates to an increase in postprandial glucose levels, the control of which is therefore an important aspect in treatment of type-2 diabetes (Kajaria et al., 2013) Inhibitors of pancreatic α-amylase delay carbohydrate digestion causing a reduction in the rate of glucose absorption and lowering the postprandial serum glucose levels. In 1970 s, it was realized that inhibition of all or some of the intestinal disaccharidases and pancreatic α-amylase by inhibitors could regulate the absorption of carbohydrate and these inhibitors could be used therapeutically in the oral treatment of the noninsulin-dependent diabetes mellitus ie., type-2 diabetes (Vijan et al.,1997).
In the present study, we focussed to research on the phytochemical and physicochemical properties of Olea europaea L. ethanolic leaves extract. Also, evaluate the extract antioxidant and antidiabetic properties and to perform molecular docking of phenolic compounds that support the pancreatic alpha-amylase inhibition.
2 Materials and methods
2.1 Reagents
Acarbose and alpha-amylase, and Folin–Ciocâlteu reagent were obtained from Sigma- Aldrich Corporations, USA. Ethanol, gallic acid, sodium carbonate and sodium phosphate buffer were purchased from Fouz Chemical Company, Saudi. DNS (3, 5-Dinitrosalicylic acid) and phosphate buffer were attained from Merck Millipore Corporation, USA. DPPH (2,2-diphenyl-1-picrylhydrazyl) was obtained from Cayman Chemical Company, USA. The remaining chemicals or reagents were of analytical grade.
2.2 Plant sample collection
Olea europaea L. matured leaves were gathered from Al-sqeer farms in the Qassim region, Saudi Arabia. The plant specimen was authenticated at the Department of Pharmacognosy, Qassim University, Kingdom of Saudi Arabia (Ref. No.: QA/FOP/03).
2.3 Preparation of plant extract
The plant leaves were cleaned thoroughly in lukewarm water and dried under shade. The leaves were ground and macerated accordingly. For maceration, the ratio of absolute ethanol and ground leaves, 3:1. Thus, 200 g ground leaves were added to 600 ml absolute ethanol in tightly sealed Erlenmeyer flasks (vessels). The mixture was macerated at 100 rpm on the shaker for approximate five days at room temperature. The supernatant liquid was filtered through a muslin cloth. Subsequently, the rotary evaporated at 70°c. The extract (OLE) was transferred to a china dish, kept on a water bath at 60°c, and freeze-dried (Murugan and Parimelazhagan, 2014).
2.4 Physicochemical and phytochemical studies
The physicochemical analysis was studied on OLE using the standard methods (Roy et al., 2013; Sharma et al., 2013).
In brief, for the total ash value method, the crucible weight was taken and recorded. The powdered sample material (2 g) was added to the pre-weighed crucible. Then, the sample was heated at 650°-700 °C until most carbons were burnt off, forming ash or white coloured powder. The sample was left to cool. Subsequently, the ash weight was taken. The total ash percentage was calculated as follows, Ash value %=[(Weight of crucible (g) + Dry ash(g))-Weight of empty crucible (g)]/Weight of sample taken (g) × 100
Loss on Drying (Gravimetric method), powdered sample material (1.5 g) was added into a flat thin porcelain dish and allowed to dry at 100°c in the oven. The sample weight was taken 5 min intervals after cooling until the consecutive weights did not vary by more than 0.5 mg. The loss identified the moisture content upon drying in terms of grams (g), calculated as follows, Moisture content (g) = Initial weight of the powdered sample(g)–Final sample weight after drying (g)
Foreign organic material method, powdered sample material (100 g) was spread on cleaned white cloth. The sample was inspected for any foreign organic material, including the remaining stem or other plant parts to be removed. Then, the sample powder was weighed after most of the foreign organic was removed, calculated as follows, Foreign organic matter(%) = [Sample weight difference(g)/Initial sample weight(g)]x100
Phytochemical analysis was carried out for the plant extracts as per the standard procedures. Qualitative tests on the sample were used to distinguish constituent presence, including carbohydrates, phenols, flavonoids, anthocyanins, proteins, saponins, glycosides, tannins and gums (Chigurupati et al., 2017; Majid et al., 2015; Roopashree et al., 2008).
-
Test for Tannins:
The appearance of bluish-green coloration on the addition of 2 ml of OLE (1 mg/ml) to 3–4 drops of 5% iron (III) chloride solution indicated the tannin presence.
-
Tests for Flavonoids:
The existence of the flavonoids was confirmed by the appearance of pink color after a few minutes when small pieces of magnesium ribbon and concentrated HCl were combined with OLE.
-
Tests for Glycosides:
OLE was added with 2 ml of acetic acid and 2 ml of chloroform. The mixture was then cooled and Conc. H2SO4 was added. The green color appearance indicated the presence of aglycone, which is a steroidal part of glycosides.
-
Test for Terpenoids:
The terpenoids were indicated via gray color formation when 2 ml of chloroform was added with the 5 ml OLE and then 3 ml of Conc. H2SO4 was added and boiled.
-
Test for Steroids:
Added 5 ml of OLE with 2 ml of H2SO4 concentrated and chloroform. The presence of steroids was shown as red color in the chloroform layer.
-
Test for saponins:
1 ml OLE and 5.0 ml of distilled water were mixed in a test tube and shaken. Then the frothing was mixed with olive oil drops and vigorously shaken. The appearance of foam indicated the presence of saponins.
-
Test for gum:
10 ml OLE (1 mg/ml) was added portion by portion in a slow manner into 25 ml of absolute alcohol and moved lightly. If precipitation occurs, it indicates the point to the gum presence.
-
Test for protein:
2 ml of OLE (1 mg/ml) was added to 2 ml of 4% sodium hydroxide solution. The combination was mixed lightly, then added 1% copper sulfate solution. The protein presence was confirmed by the form of violet color.
-
Test for carbohydrates:
2 ml of OLE (1 mg/ml) was added with about 3–4 drops of Molisch’s reagents and mixed appropriately. Then, added to the solution 2 ml of HCl. The presence of carbohydrates is verified by the appearance of a purple reddish violet ring on the interface zone.
-
Tests for phenol:
2 ml (1 mg/ml) of OLE was prepared then added 5% of ferric chloride solution. The formation of a dark green color indicated phenol presence.
-
Test for Alkaloid:
OLE dissolved in dilute hydrochloric acid and filtered. The presence of alkaloids was confirmed by a yellow-colored precipitate when filtrates of OLE were treated with potassium mercuric iodide (Mayer’s reagent).
2.5 Total phenolic content estimation
The phenolic content was estimated by the Folin–Ciocalteu technique. The samples and Gallic acid (GA) as standard drug (Lins et al., 2018) were prepared at various concentrations (10–1,000 µg/ml). Sample (1 ml), Folin-Ciocalteau reagent (1 ml) and 2.5% sodium carbonate (2 ml) solution were added to the test tube. The mixture was incubated for 30 min at room temperature in dark conditions. The absorbance was taken at 760 nm. The absorbance reading of the samples was calculated and analysed accordingly. The sample's quantifications' phenolic content was determined by a standard curve of GA and was expressed as mg GAE/g (mg of GA equivalent per gram) (Baba and Malik, 2015).
2.6 Total flavonoid content estimation
The flavonoid content was quantified by the aluminium chloride (AlCl3) colourimetric method. The sample and Rutin (RE) as standard drugs were prepared at various concentrations ranging from 10 − 1,000 mg/ml, and 2% aluminium chloride (3 ml) was added. The mixtures were kept for 60 min at room temperature, followed by absorbance taken at 415 nm. The RE calibration curve was plotted accordingly to the absorbance value. The extracts' flavonoid concentration is mg RUE/g (mg of RUE equivalent per gram dry weight) (Baba and Malik, 2015).
2.7 DPPH radical scavenging activity
DPPH solution was formulated using Molyeux and Blois technique with slight modifications for antioxidant assay. The extract and standard ascorbic acid were prepared in different concentrations using absolute ethanol (10–1000 µg/ml). 0.1 mM DPPH solution (0.5 ml) was added to the sample (0.5 ml) and incubated at room temperature in dark conditions for approximate 20 min. Accordingly, the absorbance was measured at 517 nm (Chigurupati et al., 2016). 0.5 ml Absolute ethanol and 0.5 ml DPPH solution are used as control. The percentage (%) of the free radical scavenging inhibitory assay was computed as follows:
% Inhibition = (absorbance control – absorbance sample)/ absorbance control × 100
Absorbance control is the absorbance of the control reaction, and the absorbance sample is the absorbance of the test sample. The sample concentration providing 50% inhibition (IC50) was computed from the plotted graph percentage of radical scavenging versus sample concentration.
2.8 Alpha-Amylase inhibitory activity
Extract and standard Acarbose were prepared in various concentrations using absolute ethanol (10–1,000 µg/ml). Accordingly, the samples (0.5 ml) were added to 0.5 mg/ml alpha-amylase solution (0.5 ml) prepared in 0.2 mM phosphate buffer (pH 6.9). Then, the mixtures were incubated at 25 °C for 10 min. 1% Starch solution (0.5 ml) prepared in 0.02 M sodium phosphate buffer is added then incubated for another 10 min at 25 °C. DNS (1 ml) is added and bring to boil for approximate 5 min. The tubes were cooled at room temperature. Distilled water (10 ml) was added accordingly. Control contains all the same solution mixture except the sample. Subsequently, absorbance was measured at 540 nm (Noreen et al., 2017). The sample's enzymatic inhibitory activity for the antidiabetic assay was computed as follows: %Inhibition=(absorbance control – absorbance sample)/absorbance controlx100
Absorbance control is the absorbance of the control reaction, and the absorbance sample is the absorbance of the test sample. The sample concentration providing 50% inhibition (IC50) was computed from the plotted graph percentage of enzyme inhibition versus sample concentration.
2.9 Kinetic analysis
Kinetic analysis of free radical scavenging activity was carried out by the modified Levenberg–Marquardt method (Benmehdi et al., 2017). In brief, the DPPH Stock solution was prepared by dissolving 1 mg of DPPH in 25 ml of methanol and stored in the dark at room temperature. Simultaneously, 5 mg of extract was dissolved in 4 ml methanol and from this, different concentrations were prepared (0.6250; 0.3125; 0.1562; 0.0780 mg/ml) to perform kinetic studies. A mixed equal volume of stock solution with extract solution (1:1 ratio) and absorbance was noted every 0.5 min until it became constant. The spectrophotometric data were taken until the disappearance of DPPH in the presence of the crude extracts occurred. The remaining (DPPH.) present was calculated using the following equation: %(DPPH.)=[(DPPH)]t=T/[(DPPH.]t=0)X100
[(DPPH.]t=0 represents the initial concentration of DPPH; [(DPPH.]t=T represents the concentration of DPPH at a steady-state
2.10 Statistical analyses
The samples were evaluated individually in triplicate, and data were shown as mean ± SEM (Standard error mean). The IC50 value for the antidiabetic assay was computed by Graph Pad Prism Software (Version 5.03) by a non-linear regression graph calculated between the percentages of enzyme inhibition versus sample concentration. Similarly, the IC50 values for the antioxidant assay were computed by Graph Pad Prism Software (Version 5.03) by non-linear regression graph calculated between percentages of radical scavenging versus sample concentration. With p < 0.001, the difference in the IC50 between Ascorbic acid and OLE is significant for both antioxidant and antidiabetic studies. The kinetic analysis is implemented in Origin v.6.0 Programme for Windows.
2.11 Molecular docking
The X-ray crystal structure of the human pancreatic α-amylase in complex with mini-montbretin A (PDBID: 5E0F) was downloaded from the Research Collaboratory for Structural Bioinformatics (RCSB) protein data bank (available online: https://www.rcsb.org/structure/) (Alam et al., 2019). The structures of Phenolic compounds of Olea europaea L. were retrieved from the PubChem database (Kim et al., 2019). The mini-montbretin A, and the water atoms were deleted, while the nonpolar hydrogens were merged. The protein and ligands were prepared and optimised by using AutoDock Tool (ADT), bundled with the MGLTools package (version 1.5.6) (Morris et al., 2009) to add charges, polar hydrogen atoms, and set up rotatable bonds. At that moment, ADT was operated to make PDBQT files for the protein and ligands. The molecular docking was performed using AutoDock Vinav1.1.2 (Kim et al., 2019) in PyRx 30.8. The active binding site of the α-amylase was chosen as the grid centre and obtained by removing the ligand. The centre of grid box dimensions was selected to include all atoms of the ligand set. (Kalinowsky et al., 2018). The site of the grid box in α-amylase was set at −7.946, 10.438, and −21.863 Å (for ×, y and z) by means of a grid of 40, 40, and 40 points (for ×, y and z). Then, we created a configuration text file (config.txt) to run AutoDock Vina 1.1.2. This configuration file involves the receptor and ligand PDBQT file and the centre grid box coordinates. The docking scores were resulted in the generated .log files. The output docking scores were defined as affinity binding (Kcal/mol). The various protein–ligand interactions, such as hydrogen bonds, cation − π bonds, π − π bonds, and alkyl-π interactions between amino acids of the active sites and compounds were studied. The ligands protein interactions were created by using the Discovery Studio 2020 client. (Dassault Systèmes BIOVIA, 2020).
3 Results
3.1 Phytochemical screening
In the current study (Table 2), the OLE phytochemical screening showed the presence of saponin, flavonoid, glycoside, tannin, phenol, and carbohydrate are inferring that blood glucose lessening the plant extract activity because of these phytoconstituents.
Phytochemical constituents
OLE
Saponin
+
Flavonoid
+
Gum
–
Tannin
+
Glycoside
+
Protein
–
Phenol
+
Carbohydrate
+
Starch
-
Flavanoid
+
Alkaloids
+
The total ash value commonly used characterise the purity or quality of crude extract, mostly in powder form. It excludes all traces of organic substances in vegetable drugs. After burning, the ash content of crude extracts the resulting residue due to naturally inorganic salt presences. The total ash value and foreign organic matter of OLE was 16.5 % and 0.2 %, respectively. As for loss on drying (Gravimetric method), the moisture content was 2 g.
3.2 Total phenol and flavonoid contents
The flavonoid content was denoted in Rutin equivalent; the macerated ethanolic extract of O. europaea exhibited the flavonoid content of 855 mg RUE/g. As for phenolic content, it was estimated using Folin-Ciocalteu reagent in terms of Gallic acid equivalent. The macerated ethanolic extract of O. europaea has total phenolic content of 490 mg GAE/g.
3.3 Antioxidant assay
The DPPH assay is based on the measurement of the scavenging capacity of antioxidants towards it. The odd electron of nitrogen atom in DPPH is reduced by receiving a hydrogen atom from antioxidants to the corresponding hydrazine (Sahoo et al., 2013). As depicted in Fig. 1, OLE (IC50 ± SEM: 38.37 ± 0.26 µg/ml) portrayed antioxidant activity comparable to the ascorbic acid (IC50 ± SEM: 30.37 ± 0.17 µg/ml).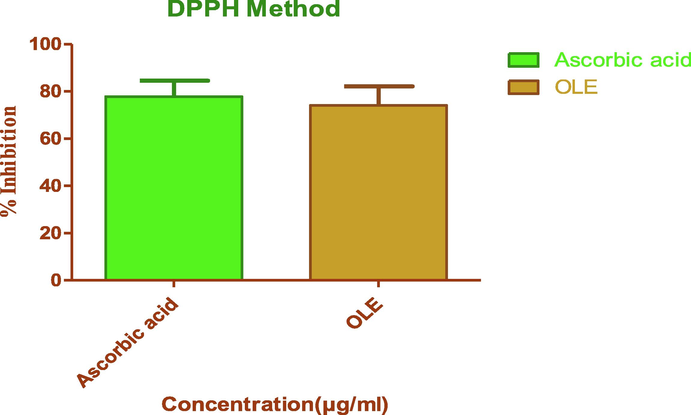
DPPH radical scavenging effects of OLE and standard, Ascorbic acid (n = 3).
3.4 Kinetic analysis
Kinetic analysis of OLE was carried out to calculate the scavenging activity of DPPH· as a function of time. In this study, the effect of the concentration of ethanolic extracts (0.625; 0.312; 0.156 and 0.078 mg/ml) on scavenging free radicals was studied. Free DPPH· radical is relatively stable for more than 110 min at 25 °C in the reaction medium. So, we allowed the evaluation of the free radical scavenging activity of the extracts within that time. The free radical trapping was measured by calculating the remaining DPPH· concentration at the end of the reaction.
With the help of kinetic study, the steady-state time, half-life (t1/2) and the remaining percentage DPPH were calculated for different concentrations of extract (Table 3). The results showed clearly that the ethanolic extract exhibited a significant antioxidant effect the steady-state time were 5.5; 16.1; 117.1 and 20.6 min at extract concentration of 0.625; 0.312; 0.156 and 0.078 mg/ml respectively. Further, we calculated the remaining DPPH· concentration (9.7; 20.0; 28.4 and 33.4 %). The effectiveness of extract was expressed by half-life t1/2, which was shorter when concentration increases.
Concentration(%w/v)
Steady-state time (min)
Half-life (min)
(t1/2)Remaining (DPPH·) (%)
0.078
20.6
17.6
33.4
0.156
17.1
10
28.4
0.312
16.1
9
20.0
0.625
5.5
1.5
9.7
3.5 In vitro antidiabetic assay
As depicted in Table 4, the standard Acarbose and OLE were examined at various concentrations ranging from 10 − 1,000 µg/ml. In the alpha-amylase inhibitory activity, Acarbose and OLE showed enzymatic inhibition with an IC50 value of 20.06 ± 0.19 µg/ml and IC50 value of 37.99 ± 0.15 µg/ml, respectively. Note a SEM using Graph Pad prism 5 (n = 3).
Concentration
(µg/ml)% of inhibition of alpha-amylase
OLE
Acarbose
10
27.42
29.03
25
44.35
42.74
50
59.68
71.77
100
69.35
83.06
250
78.23
89.52
500
83.06
91.13
1000
95.16
94.35
IC50 ± SEM
37.99 ± 0.15
20.06 ± 0.19
3.6 Molecular docking studies
Upon conducting molecular docking studies, the most of the reported phenolic compounds of OLE showed good binding affinity against the human pancreatic α-amylase (HPA) (Table 5) (Sabbah et al., 2010).The docking scores for cynaroside and apigenin 7-O-glucoside are − 9.80 kcal/mol and − 9.70 kcal/mol, respectively while the docking scores for oleuropein and verbacoside are – 8.20 kcal/mol and − 8.10 kcal/mol, respectively. The binding interactions of the phenolic compounds of OLE versus Acarbose share hydrogen bonds with E233, D300, and H305 and π - π stacking with W56. The hydroxyl groups of flavone glycosides form H-bond with Q63, D197, and E233, and the aromatic ring of cynaroside induces π – anion with D300. The ligand–protein interactions of oleuropein and acteoside are similar to flavone glycosides (Fig. 3). The oleuropein and acteoside have extra H-bond with H305.
Compd
Docking Scores (kcal/mol)
Interacting Residues
Acarbose
−8.10
K200, E233, D300, H305
Verbacoside
−8.20
W59, K200, E233, H305
Apigenin 7-O-glucoside
−9.70
W59, Q63, D197, E233
Cynaroside
−9.80
W59, Q63, D197, E233, D300
Hydroxytyrosol
−5.90
D197, E233, H305
Oleuropein
−8.20
W59, Q63, D197, E233, D300, H305
4 Discussions
DM is a notable chronic disease depicted by hyperglycaemia attributable to irregularities in insulin action, secretion, or both (Saltiel and Kahn, 2001). Potential phytocompounds that proficient at taking action on multiple disease-related drug targets in conjunction with fewer unfavourable effects for the diabetic treatments (Nicolle et al., 2011). Phytocompounds originated from diverse remedial plants, for instance, flavonoids, phenols, alkaloids, saponins, tannins, terpenoids, glycolipids, glycosides, anthocyanins and carotenoids, have been known to possess productive antidiabetic activity (Rex et al., 2018). In numerous findings, flavonoids and polyphenols exhibit blood glucose inhibition by enhancing the GLUT-2 expression within the pancreatic beta cells and GLUT-4 translocation expression (Nisar et al., 2018).
Oxidative stress and inflammation are associated with the pancreas, linked to the progression and the pathogenesis of diabetes. The free radicals produced from protein glycosylation and hyperglycemia-induced glucose autoxidation has an imperative role in DM pathogenesis (Chen et al., 2015; Stirban et al., 2014). According to Dal et al. (2016) and Gothai et al. (2016), most plant extracts or naturally derived phytocompounds have portrayed pancreatic beta-cell protective effects attributable to their antioxidant properties. Correspondingly, OLE has antioxidant attributes within the present study, signifying that the antioxidants may affect its antidiabetic action in digestive enzymes, alpha-amylase.
The OLE extract displayed a comparable enzymatic inhibitory assay against alpha-amylase. Plant-derived phytocompounds affect glucose metabolism by lessening alpha-amylase activity, intensifying insulin secretion and counter-react, inhibiting apoptosis, inhibiting gluconeogenesis, and enhancing pancreatic beta-cell proliferation (Sayem et al., 2018). These phytocompounds mechanisms give an in-depth comprehension of antidiabetic drug designs. In the present study, OLE displayed comparative alpha-amylase inhibition with the standard drug, Acarbose.
The molecular docking of the Phenolic compounds of Olea europaea L against the crystal structure of the human pancreatic α-amylase (HPA) showed that the flavone glycosides such as cynaroside and apigenin 7-O-glucoside are the most potent binding affinity against HPA. The more negative docking scores is the more favorable binding affinity (Sabbah et al., 2010). the docking scores for cynaroside and apigenin 7-O-glucoside are − 9.80 kcal/mol and − 9.70 kcal/mol, respectively. In addition, oleuropein and acteoside showed good binding energy values against HPA. the docking scores for oleuropein and verbacoside are – 8.20 kcal/mol and − 8.10 kcal/mol, respectively. Besides, we studied the binding interactions of Phenolic compounds of Olea europaea L. against HPA to identify their inhibitory mechanism. Hydrophilic residues mostly surround the surface of the binding site of HPA protein. The structure of HPA protein with mini-montbretin A molecule exposed that the binding site of HPA encloses mostly by hydrophilic residues: W59, Q63, H101, Y151, R195, D197, K200, H201, E233, E240, I253, N298, D300, H305, and D356 (Williams et al., 2015). The majority of compounds of OLE interact with the active site of HPA through the hydrogen bonding with Q63, D197, E233, D300, and H305, and hydrophobic interaction with W59. Therefore, these docking scores and the ligand protein interactions were involved in stabilizing the complex of OLE - HPA confirming the inhibitory effect of compounds of OLE for HPA.
In summary, Fig. 2 showed that the kinetic profile of DPPH scavenging activity of ethanolic extracts. Immediately after adding the extracts to the reaction medium, the absorbance of DPPH·at 517 nm dropped significantly due to the decrease of DPPH· concentration in the medium. From the kinetic behaviour, it is observed that practically all samples at high concentrations reacted within a very short time, and a steady state was reached almost immediately. On the other hand, the lowest concentration showed slow kinetic behaviour implied longer periods before the constant state was reached (Benmehdi et al., 2017; Nenadis and Tsimidou, 2002; Savatovic et al., 2012).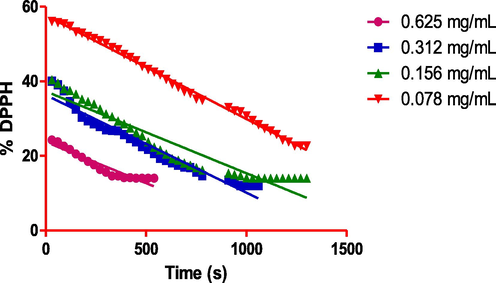
Reaction kinetics of OLE with DPPH· radical.
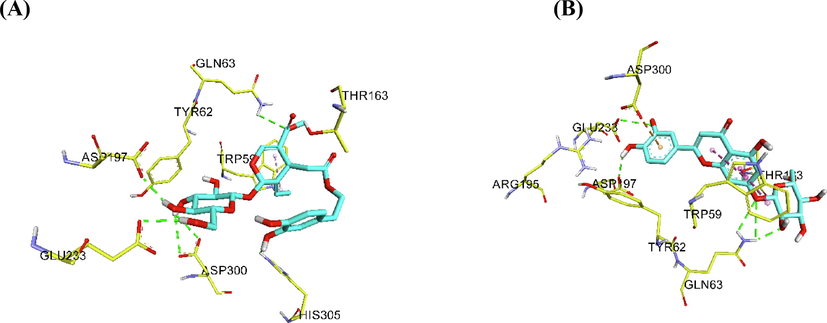
Ligand – protein interactions for A) oleuropein B) cynaroside.
5 Conclusion
This research presented that the OLE possesses flavonoids and phenols that have promising antioxidant effects counter to DPPH. Signifying Olea europaea L. extract may well be utilised to counteract the free radicals. The antidiabetic assay of the extract was examined on the enzymatic inhibitory effects on alpha-amylase. The majority of compounds of OLE interact with the active sites of HPA. This finding proposes a probable action mechanism for Olea europaea L. leaf extract due to inhibition of digestive enzymes. Nevertheless, broad research should be done to isolate and distinguish the active bio-constituents of Olea europaea L. and researched for their therapeutic bioactivity.
Acknowledgements
The researchers would like to thank the Deanship of Scientific Research, Qassim University, for funding the publication of this project.
Formatting of funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The effect of olive leaf extract and Α-Tocopherol on nephroprotective activity in rats. J Nutr Food Sci. 2016;6:479.
- [Google Scholar]
- Evaluation of the hypoglycemic effect of Cucurbita ficifolia Bouché (Cucurbitaceae) in different experimental models. J. Ethnopharmacol.. 2002;82(2-3):185-189.
- [Google Scholar]
- Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. J. Br. Diabet. Assoc.. 1998;15(7):539-553.
- [Google Scholar]
- Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. J. Taibah Univ. Sci.. 2015;9(4):449-454.
- [Google Scholar]
- Eco-friendly extraction and characterisation of nutraceuticals from olive leaves. Molecules. 2019;24(19):3481.
- [Google Scholar]
- Free radical scavenging activity, kinetic behaviour and phytochemical constituents of Aristolochia clematitis L. roots. Arab. J. Chem.. 2017;10:S1402-S1408.
- [Google Scholar]
- The association between quality of life and complementary and alternative medicine use in patients with diabetes mellitus. Complement. Ther. Clin. Pract.. 2018;31:1-6.
- [Google Scholar]
- Chen, L., Chen, R., Wang, H., Liang, F., 2015. Mechanisms linking inflammation to insulin resistance. Int. J. Endocrinol. 2015.
- Chigurupati, S., Mohammad, J.I., Vijayabalan, S., Vaipuri, N.D., Selvarajan, K.K., Nemala, A.R., 2017. Quantitative Estimation And Antimicrobial Potential Of Ethanol Extract Of Durio Zibethinus Murr. Leaves. Asian J. Pharm. Clin. Res. 10, 1–4.
- Identification of novel acetylcholinesterase inhibitors: Indolopyrazoline derivatives and molecular docking studies. Bioorganic Chem.. 2016;67:9-17.
- [Google Scholar]
- Protective effect of antioxidants consumption on diabetes, hepatic and vascular complications: alliance between chemistry and biology. J. Int. Soc. Antioxid. Nutr. Health. 2016;3(1):1.
- [Google Scholar]
- El, S.N., Karakaya, S., 2009. Olive tree (Olea europaea) leaves: potential beneficial effects on human health. Nutr. Rev. 67, 632–638.
- Gothai, S., Ganesan, P., Park, S.-Y., Fakurazi, S., Choi, D.-K., Arulselvan, P., 2016. Natural phyto-bioactive compounds for the treatment of type 2 diabetes: inflammation as a target. Nutrients 8, 461.
- In-vitro α amylase and glycosidase inhibitory effect of ethanolic extract of antiasthmatic drug - Shirishadi. J. Adv. Pharm. Technol. Res.. 2013;4(4):206.
- [CrossRef] [Google Scholar]
- A Diverse Benchmark Based on 3D Matched Molecular Pairs for Validating Scoring Functions. ACS Omega. 2018;3(5):5704-5714.
- [Google Scholar]
- Kim, S., Chen, J., Cheng, T., Gindulyte, A., He, J., He, S., Li, Q., Shoemaker, B.A., Thiessen, P.A., Yu, B., 2019. PubChem 2019 update: improved access to chemical data. Nucleic Acids Res. 47, D1102–D1109.
- Lins, P.G., Pugine, S.M.P., Scatolini, A.M. and de Melo, M.P., 2018. In vitro antioxidant activity of olive leaf extract (Olea europaea L.) and its protective effect on oxidative damage in human erythrocytes. Heliyon, 4(9), e00805.
- Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev.. 2010;4(8):118.
- [CrossRef] [Google Scholar]
- Studies on phytochemical, antioxidant, anti-inflammatory, and analgesic activities of Euphorbia dracunculoides. BMC Complementary and Alternative Medicine. 2015;15(1):349-363.
- [Google Scholar]
- AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem.. 2009;30(16):2785-2791.
- [Google Scholar]
- Comparative evaluation of different extraction methods for antioxidant and anti-inflammatory properties from Osbeckia parvifolia Arn.–An in vitro approach. J. King Saud Univ.-Sci.. 2014;26(4):267-275.
- [Google Scholar]
- Observations on the estimation of scavenging activity of phenolic compounds using rapid 1, 1-diphenyl-2-picrylhydrazyl (DPPH•) tests. J. Am. Oil Chem. Soc.. 2002;79(12):1191.
- [CrossRef] [Google Scholar]
- Flavonoids as promising lead compounds in type 2 diabetes mellitus: molecules of interest and structure-activity relationship. Curr. Med. Chem.. 2011;18:2661-2672.
- [Google Scholar]
- Chemical Components and Biological Activities of the Genus Phyllanthus: A Review of the Recent Literature. Molecules. 2018;23(10):2567.
- [CrossRef] [Google Scholar]
- Synthesis of alpha amylase inhibitors based on privileged indole scaffold. Bioorganic Chem.. 2017;72:248-255.
- [Google Scholar]
- Antihyperglycaemic activity of Musa sapientum flowers: effect on lipid peroxidation in alloxan diabetic rats. Phytother. Res.. 2000;14(2):136-138.
- [Google Scholar]
- Free Radicals, Antioxidants in Disease and Health. Int. J. Biomed. Sci. IJBS. 2008;4:89-96.
- [Google Scholar]
- Phytochemicals as a potential source for antimicrobial, anti-oxidant and wound healing-A review. MOJ Biorg Org Chem. 2018;2:61-70.
- [Google Scholar]
- Antibacterial activity of antipsoriatic herbs: Cassia tora, Momordica charantia, and Calendula officinalis. International Journal of Applied Research in Natural products. 2008;1(3):20-28.
- [Google Scholar]
- Pharmacognostic and preliminary phytochemical investigation of whole plant extract of cuscuta reflexa growing on different host plants. Int J Phytopharm.. 2013;4:190-194.
- [Google Scholar]
- Docking studies on isoform-specific inhibition of phosphoinositide-3-kinases. J. Chem. Inf. Model.. 2010;50(10):1887-1898.
- [Google Scholar]
- Phytochemical investigation and in vitro antioxidant activity of an indigenous medicinal plant Alpinia nigra BL Burtt. Asian Pac. J. Trop. Biomed.. 2013;3(11):871-876.
- [Google Scholar]
- Anti-diabetic activity and oxidative stress improvement of Tunisian Gerboui olive leaves extract on alloxan induced diabetic rats. J Mater. 2017;8:1359-1364.
- [Google Scholar]
- Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414(6865):799-806.
- [Google Scholar]
- Kinetic behaviour of DPPH radical scavenging activity of tomato waste extracts. J. Serbian Chem. Soc.. 2012;77(10):1381-1389.
- [Google Scholar]
- Action of phytochemicals on insulin signaling pathways accelerating glucose transporter (GLUT4) protein translocation. Molecules. 2018;23(2):258.
- [CrossRef] [Google Scholar]
- Study of physico-chemical properties of drug and physiological variation in leaves of andrographis paniculata (burmf.) nees. Acta Chim Pharm Indica. 2013;3:52-64.
- [Google Scholar]
- Safety assessment of aqueous olive pulp extract as an antioxidant or antimicrobial agent in foods. Food Chem. Toxicol.. 2006;44(7):903-915.
- [Google Scholar]
- Vascular effects of advanced glycation endproducts: clinical effects and molecular mechanisms. Mol. Metab.. 2014;3(2):94-108.
- [Google Scholar]
- Estimated benefits of glycemic control in microvascular complications in type 2 diabetes. Ann. Intern. Med.. 1997;127:788-795.
- [Google Scholar]
- The amylase inhibitor montbretin A reveals a new glycosidase inhibition motif. Nat. Chem. Biol.. 2015;11:691-696.
- [Google Scholar]







