Translate this page into:
Multifunctional Zn0.3Al0.4O4.5 crystals: An efficient photocatalyst for formaldehyde degradation and EBT adsorption
⁎Corresponding authors. umeshfegade@gmail.com (Umesh Fegade), inamuddin@zhcet.ac.in (Inamuddin),
⁎⁎Corresponding author. rjwu@pu.edu.tw (Ren-Jang Wu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The Zn0.3Al0.4O4.5 nanoparticles (ZnAlONPs) with size of 70–90 nm are used as an efficient photocatalyst for formaldehyde (HCHO) degradation and effective adsorbent for the removal of eriochrome black-T (EBT) dye from synthetic aqueous solution. Degradation of HCHO reactions were studied using TiO2 (homemade), TiO2 (P-25) and ZnAlONPs by irradiating under 18 W daylight lamp source for photocatalytic degradation. The HCHO degradation rate is about 67, 76 and 89% for TiO2 (homemade), TiO2 (P25) and ZnAlONPs during 2 h reaction, respectively at initial formaldehyde gas concentration of 20 ppm. Maximum adsorption capacity was optimized by changing the parameters such as pH, EBT concentration and adsorbent dosage. A 200 mg of ZnAlONPs are useable for quick removal of EBT (>95%). Langmuir isotherm model showed a maximum adsorption capacity of 90.90 mgg−1. The ZnAlONPs could be successfully reused upto 5th adsorption/desorption cycle for EBT dye removal from water samples.
Keywords
ZnAlONPs
HCHO degradation
Eriochrome black T
Pseudo second order model
Langmuir isotherm
1 Introduction
Environmental pollution originated by HCHO, is becoming a severe problem in front of scientists and researchers. Among the many chemical contaminants usually found in polluted indoor air, formaldehyde (HCHO) is one of the major species (Zhang et al., 2016). Long term exposure of lower concentration of formaldehyde could cause eye and throat irritation and chest tightness (Tasbihi et al., 2015). Various studies (Ai et al., 2010; Kim et al., 2011; Sekiguchi et al., 2011; Fan et al., 2011; Wan et al., 2011) have presented high conversions of formaldehyde degradation as catalytic oxidation (Kim et al., 2011), high-frequency ultrasound combination with photocatalytic way (Sekiguchi et al., 2011), plasma-catalytic removal method (Fan et al., 2011) and direct circuit corona discharge plasma method (Wan et al., 2011). The photo degradation of HCHO, commonly uses TiO2photocatalytic material in industry. There are several advantages of TiO2 material towards photocatalytic HCHO degradation (Liu et al., 2008); for example high resistant to photo-corrosion, environmentally clean and known to completely oxidize HCHO into CO2 and H2O (Liang et al., 2012; Wang et al., 2012; Fu et al., 2011; Zhu and Wu, 2015).
Dyes have adverse effects on human body as well as aquatic life. These are readily used in textile, printing, dyeing, and related industries which discharge the huge amount of dye-containing wastewater into the environment and generates hazards for human health (Iram et al., 2010; Zhu et al., 2012; Fisli et al., 2014). Very small amount of dye in huge water reduces the photosynthesis efficiency for aquatic life. Dyes are generally thermo and photo stable and possess high resistance to chemical; these qualities increase the serious concern of scientist toward the removal of dyes from the wastewater (Girgis et al., 2015). The various techniques have been applied for removal of dyes from wastewaters (flocculation, coagulation, precipitation, membrane filtration, and adsorption techniques). Among these adsorption method associated with nanoadsorbent widely useful for dyes removal (Kong and Gan, 2012; Deng et al., 2013; Zhang et al., 2014; Zhou et al., 2015; Afkhami et al., 2010). There are some examples about the adsorption application using various adsorbents in recent studies (Van Tran et al., 2019a, 2019b, 2018c, 2019d, 2019e, 2019f, 2019g, 2020). The conventional activated carbon and zeolites have poor adsorption capacity and low dye removal efficiency (Sharma and Lee, 2015, 2017a, 2017b, 2014; Sharma et al., 2010). Thus, improvement of novel nanomaterials having high adsorption ability is still crucial task for scientist (Dehghanian et al., 2016). Sodium 1-[1-hydroxynaothylazo]-6-nitro-2-napthol-4-sulfonate i.e. Eriochrome black T (EBT) is anionic azo dye. It is hazardous (carcinogenic) in case of skin contact and irritant on eye contact. EBT is used in complexometric titrations and for dyeing silk (Dutta et al., 2014; Jethave et al., 2018a, 2018b). In previous years our research group reported the receptor for sensing of ions from wastewater (Fegade et al., 2014a, 2014b, 2014c, 2014d, 2014e, 2015; Jethave and Fegade, 2018; Kondalkar et al., 2018). The current study explored the application of ZnAlONPs for photo-degradation of formaldehyde as well as adsorption of EBT.
2 Experimental
2.1 Materials and instrumentation
All the chemicals used in this work were of analytical grade and purchased from Merck, Germany. The MiliQ water (A Grade) was used in every stage of the study. The characterization done by FE-SEM (Bruker S-4800 at 15.0 kV), EDX (500 × 375 Magnitude: 40,000 × HV:15.0 kV), FTIR (Model: FT-IR Bruker) in the range of 500–4000 cm−1 and UV–Visible spectrophotometer (Shimadzu UV-1800) at 25 °C.
2.2 Formaldehyde degradation system
During this study, 200 mg of ZnAlONPs was positioned in reactor with volume of 0.7L. HCHO gas was passed from reactor to test the photo catalytic activity of ZnAlONPs in 18 W daylight lamp of intensity 8.8 mWcm−2 and the remaining concentration of HCHO is calculated after fixed interval (Supplementary Fig. S1). The HCHO degradation rate was determined by Eq. (1):
2.3 Batch adsorption experiment for EBT dye removal
Adsorption behavior of EBT was performed by varying experimental parameters. Optimization of adsorption method is done by varying doses (50–200 mg) of ZnAlONPs into the 50 ml solution of different doses of EBT (50–200 mg) in a 100 ml conical flask. The solution was shaken for 90 min on rotary shaker at 200 rpm. The adsorption of dye is calculated, the aliquot taken at time interval centrifuged at 2000 rpm for 3 min and the supernant solution is used for the measurement of concentration of EBT using spectrophotometer at 489 nm. The concentration of EBT decreased with time due to its adsorption on ZnAlONPs. The adsorption capacity (qe) of nanoparticles for EBT dye was calculated by Eq. (2).
2.4 Kinetic models, adsorption isotherms, separation factor and thermodynamic parameters equations
The pseudo–II–order kinetic model formula
The general form of the Langmuir isotherm is:
After linearization of the Langmuir isotherm, Eq. (4), we obtain
The adsorbed EBT (mg/g) was calculated based on a mass balance equation:
The Freundlich isotherm can be expressed as:
The linearized form of the Freundlich adsorption isotherm equation is as follows
A separation factor as described by Fegade et al. (2014e) (RL), is defined as:
Thermodynamic parameters equation
3 Results and discussion
3.1 Fabrication of ZnAlONPs
ZnAlONPs were fabricated by co-precipitation method followed by heating with stirring (Jethave et al., 2017). Initially 0.75 mol of ZnCl2 and 1.0 mol of AlCl3 were poured into the 300 ml distilled water (DW). Then 50 ml of 1 M NaOH and 30 ml ammonia was added drop wise with constant stirring for two hr at 60 °C. The white precipitates were dried for 4 h and then crushed into powder and again heated in an oven at 400 °C for 4 hr for calcinations. Fig. 1 show FE-SEM images of ZnAlONPs. Fig. 1(a), shows that the synthesized material has nano flake character and image (b) shows the nano flake size upto 70–90 nm. EDX spectrum confirmed the presence of Zn, Al and O in nano flake having 20.81, 10.15 and 69.03%, respectively (Fig. 2). The chemical composition of ZnAlONPs was explored using FTIR analysis and the FTIR spectrum is shown in Fig. 3. The broad band at 3366 cm−1 is assigned to the O—H stretching vibrations of free water (Zawrah and El Kheshen, 2002; Kurien, 2010). The band at 1358 cm−1 corresponds to metal–alloy (Zn–Al) and bands at 774 and 687 cm−1 are attributed to Zn—O bonds while bands at 545 and 511 cm−1 show the presence of the Al—O vibration bands (Zawrah and El Kheshen, 2002; Kurien, 2010) (Fig. 3).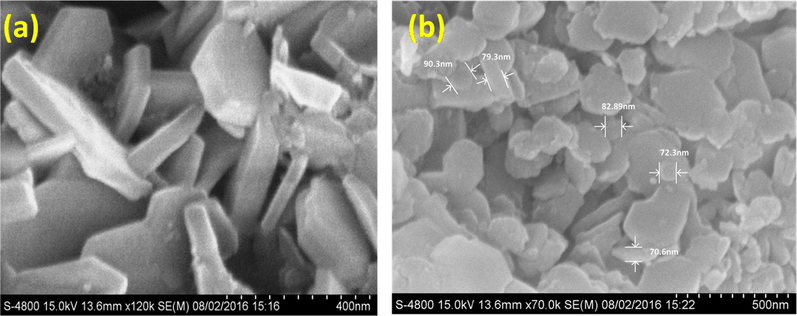
(a) Closed FE-SEM image, and (b) FE-SEM images of nano flake ZnAlONPs with size 70–90 nm.
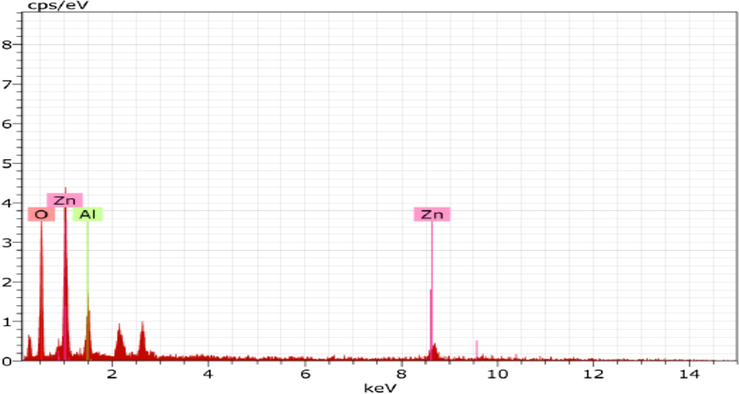
EDX spectrum of the ZnAlONPs.
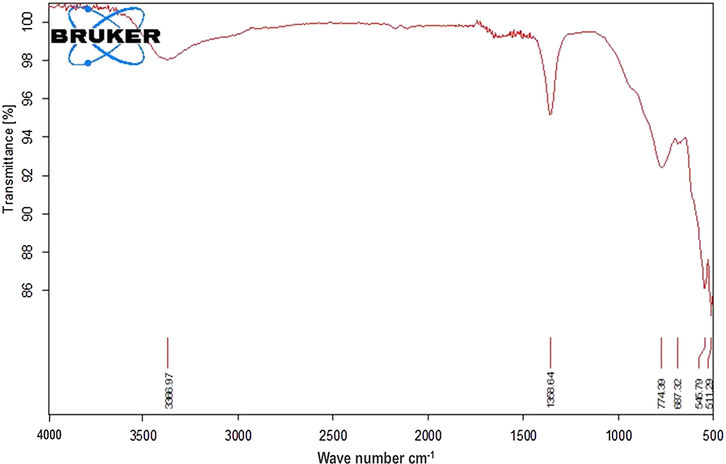
FTIR spectrum of ZnAlONPs.
3.2 Photo-degradation of formaldehyde
The photocatalytic HCHO degradation system and its working are given in supplementary Fig. S1. The photo-degradation of HCHO was investigated quantitatively using TiO2 (homemade), TiO2 (P25) and Zn0.3Al0.4O4.5 under 18 W daylight lamp irradiation and the results are shown in Fig. 4. The degradation rate was found to be 67, 76 and 89% for TiO2 (homemade), TiO2 (P25) and Zn0.3Al0.4O4.5 during 2 h reaction, respectively (Supplimentry information Table S1). The ZnAlONPs showed a maximum photo-degradation efficiency for HCHO than other catalysts because ZnAlONPs have wide range and strong absorption in visible light region than the TiO2 (P25).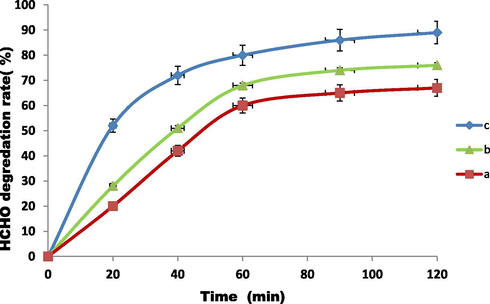
The photo-degradation of 20 ppm HCHO by various photo-catalysts (a) Homemade TiO2 (b) P-25 TiO2 (c) ZnAlONPs under photo radiation.
3.3 Possible mechanism of HCHO degradation
HCHO photo-degradation mechanism as proposed is shown in Fig. 5 and Eqs. (11)–(16). Eq. (11) described the electron excitation and hole formation using photoenergy. Under visible light irradiation of ZnAlONPs the electrons present in valence band are excited and transfer to conduction band and form electron-hole pairs. The Eq. (13) shows the generation of •OH radicals using photocatalyst and Eqs. (14)–(16) shows the degradation of HCHO through the •OH radicals which is previously generated by photocatalyst in equation (13).
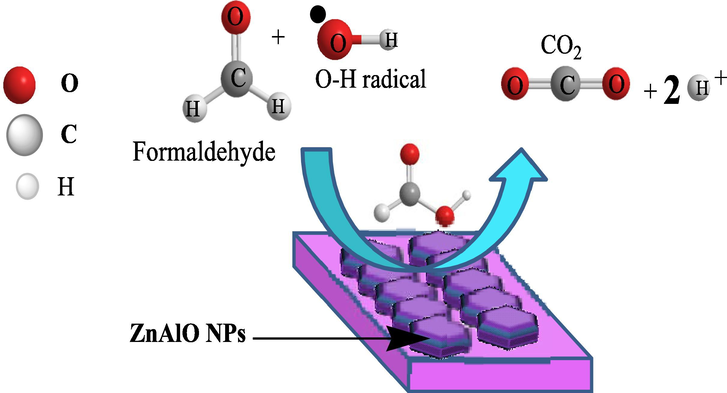
Schematic representation of possible mechanism of HCHO degradation.
3.4 Effect of pH, time and adsorbent variation
The effect of pH on dye adsorption using ZnAlONPs was evaluated at various pH ranges 3–10. Fig. 6 shows that percent adsorption enhance from 38 to 96% when the pH varies from 4 to 6. It is due to the protonation on ZnAlONPs. The dissociation constant pKa for EBT is 6.2 and 11.55, consequently EBT present as monovalent anions, at the equilibrium pH (Dehghanian et al., 2016). EBT was protonated and increases repulsion with ZnAlONPs due to similar charges and percent removal decreases at low pH. As demonstrated the experiment by varying the time from 0 to 120 min in supplementary Fig. S2, the maximum adsorption efficiency of EBT was achieved after 60 min and the phenomenon is explained based on that the great numbers of active sites if available but active sites required sufficient contact time for the chemical or physical interaction for adsorption. The influence of ZnAlONPs quantity on adsorption of EBT was examined in batch experiments by adding adsorbent in range of 50–200 mg in 50 mg L-1 dye solution. The results showed that the ZnAlONPs amount is directly proportional to percent adsorption, until it reaches a saturation point. The enhancement in percent adsorption with ZnAlONPs can be attributed to increased surface area and more adsorption sites. Fig. 7 results showed that the adsorption efficiencies (%) increased from 58.51 to 97.75% by increasing adsorbent dose from 50 to 200 mg due to availability of more adsorption sites.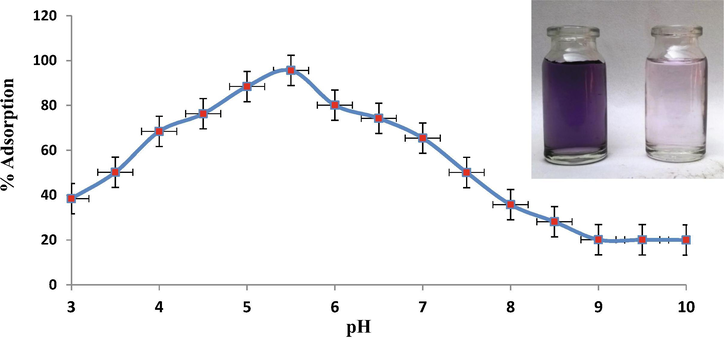
Effect of pH on adsorption of EBT (conditions, ZnAlONPs dose = 200 mg, EBT concentration = 50 mg/L, shaking time = 90 min). Inset shows the color of dye solution before and after treatment.
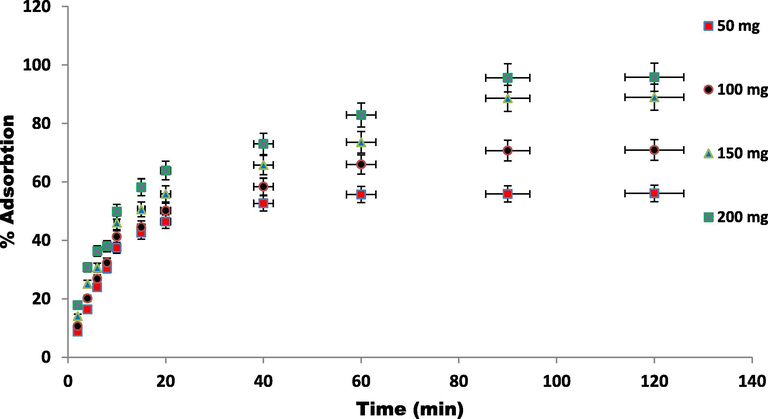
Variation in ZnAlONPs dose at constant EBT concentration (at 25 °C).
3.5 Kinetic models, adsorption isotherms, separation factor and thermodynamic parameters
To determine the controlling mechanism of dye adsorption from aqueous solution, the adsorption kinetics of EBT on ZnAlONPs was analyzed using kinetic models proposed by Ho and McKay (1999a, 1999b). The kinetic study confirmed the pseudo second-order (Fig. 8 and Table 1). The results obtained for adsorption of EBT were analyzed by the use of well known Langmuir (Dada, 2012; Langmuir, 1916, 1918) and Freundlich (Dada, 2012; Freundlich and Heller, 1939) models. The isotherms for EBT were obtained for ZnAlONPs at the solution pH of 5.5. The Figs. 9 and 10 represent the graph obtained by the adsorption isotherms and the calculated parameters are shown in Table 2. It is clear that the Langmuir is preeminent fitted than Freundlich of EBT adsorption. So, it is concluded that the above study may be a homogeneous monolayer adsorption. The separation factor (RL) (Weber and Chakravorti, 1974) for EBT dye fall in between 0 and 1 which shows that dye adsorption on nano flake is a favorable adsorption process (Fig. 11). The study of temperature illustrated whether the process is exothermic or endothermic. The variation of dye EBT adsorbed on ZnAlONPs as function of solution temperature (Fegade et al., 2014a) is shown in Fig. 12. The ΔG determined is −7.08 kJ mol−1. The negative ΔG specifies the spontaneous nature of process between adsorbent for the dyes (Banerjee and Chattopadhyaya, 2017; Deniz, 2013; Murcia-Salvador et al., 2019).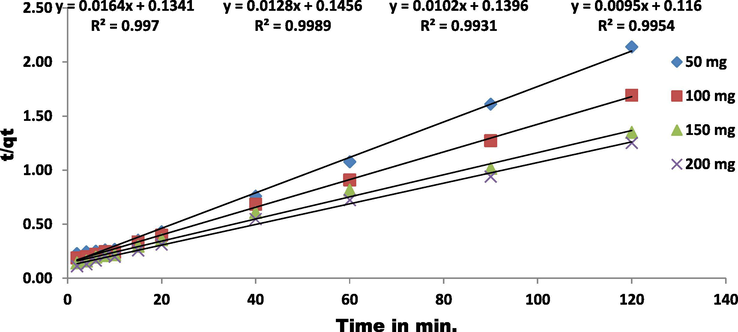
Kinetic plots of EBT onto ZnAlONPs at various primary EBT concentrations.
Parameters
Concentration (mg)
50
100
150
200
Kad g mg-1 min−1
0.045
0.094
0.089
0.083
qe (mg/g)
62.5
83.33
100
111.11
R2
0.997
0.998
0.993
0.995
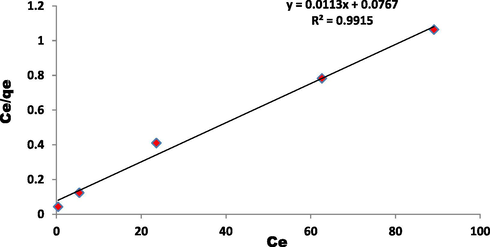
Linearization of the Langmuir isotherm. Conditions: pH 5.5, and 25 °C.
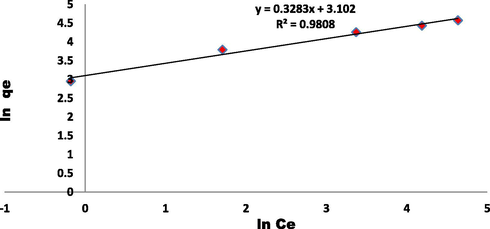
Freundlich plot for the adsorption of EBT onto ZnAlONPs at 25 °C and pH 5.5.
Langmuir
Freundlich
KL
13.157
log Kf
1.347
qmax = KL/αL
90.909
1/n
0.328
R2
0.991
R2
0.98
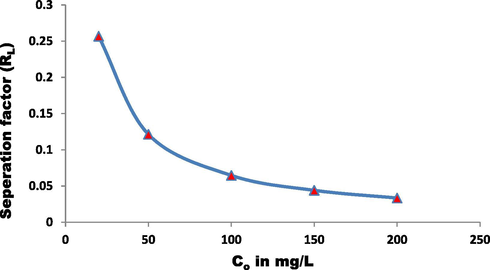
RL of the adsorption of EBT onto ZnAlONPs at 25 °C. (C0 = initial EBT dye concentration).
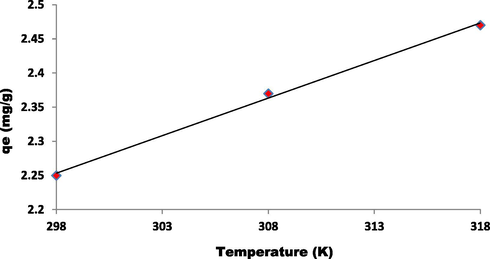
Effect of temperature on qe of dye EBT using ZnAlONPs.
3.6 Reuse of ZnAlONPs by acidic cleaning
To explore the reuse ability of ZnAlONPs the dye adsorbed material converted in to desorbed material using HCl (0.1 M) and repeated upto five cycles. It is confirmed that the percent adsorption of ZnAlONPs is higher than 93% after the five reuse cycles (Fig. 13).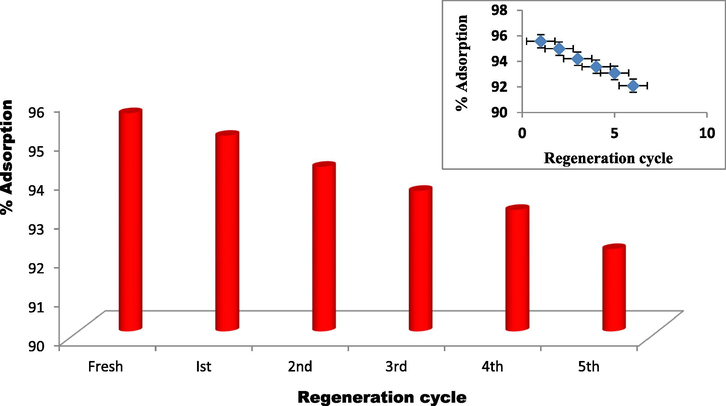
Effect of reuse of ZnAlONPs by acid clean on adsorption.
4 Conclusion
The ZnAlONPs showed the rapidest HCHO decomposition efficiency rate than TiO2 catalysts. The ZnAlONPs synthesized by easy and economic way for EBT adsorption. The degradation of HCHO was studied on a variety of TiO2 (home made), TiO2 (P-25) and ZnAlONPs under photo irradiation. The HCHO degradation rate is about 67, 76 and 89% for TiO2 (home made), TiO2 (P25) and ZnAlONPs during 2 h reaction, respectively at 20 ppm initial formaldehyde concentration. The small amount 200 mg of the proposed ZnAlONPs is applicable for quick removal of EBT (>95%). From the above results we can say that the synthesized nano flake will be the effective candidate for the HCHO photocatalysis as well as for EBT adsorption.
Acknowledgement
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, under grant no. KEP-54-130-38. The authors, therefore, acknowledge with thanks DSR for technical and financial support.
References
- Modified maghemite nanoparticles as an efficient adsorbent for removing some cationic dyes from aqueous solution. Desalination. 2010;263:240-248.
- [CrossRef] [Google Scholar]
- Photocatalytic removal of NO and HCHO over nanocrystalline Zn2SnO4 microcubes for indoor air purification. J. Hazard. Mater.. 2010;179:141-150.
- [CrossRef] [Google Scholar]
- Adsorption characteristics for the removal of a toxic dye, tartrazine from aqueous solutions by a low cost agricultural by-product. Arab. J. Chem.. 2017;10:S1629-S1638.
- [CrossRef] [Google Scholar]
- Langmuir, Freundlich, Temkin and Dubinin-Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR J. Appl. Chem.. 2012;3:38-45.
- [CrossRef] [Google Scholar]
- A random forest approach for predicting the removal of Congo red from aqueous solutions by adsorption onto tin sulfide nanoparticles loaded on activated carbon. Desalin. Water Treat.. 2016;57:9272-9285.
- [CrossRef] [Google Scholar]
- Simultaneous removal of Cd(II) and ionic dyes from aqueous solution using magnetic graphene oxide nanocomposite as an adsorbent. Chem. Eng. J.. 2013;226:189-200.
- [CrossRef] [Google Scholar]
- Adsorption properties of low-cost biomaterial derived from Prunus amygdalus L. for dye removal from water. Sci. World J.. 2013;9
- [CrossRef] [Google Scholar]
- High adsorption capacity for cationic dye removal and antibacterial properties of sonochemically synthesized Ag2WO4 nanorods. Eur. J. Inorg. Chem.. 2014;2014:5724-5732.
- [CrossRef] [Google Scholar]
- The roles of various plasma species in the plasma and plasma-catalytic removal of low-concentration formaldehyde in air. J. Hazard. Mater.. 2011;196:380-385.
- [CrossRef] [Google Scholar]
- A chemosensor showing discriminating fluorescent response for highly selective and nanomolar detection of Cu2+ and Zn2+ and its application in molecular logic gate. Anal. Chim. Acta.. 2015;872:63-69.
- [CrossRef] [Google Scholar]
- Colorimetric and fluorescent “on-off” chemosensor for Cu2+ in semi-aqueous medium. Sensors Actuators, B Chem.. 2014;202:924-928.
- [CrossRef] [Google Scholar]
- “Turn-on” fluorescent dipodal chemosensor for nano-molar detection of Zn2+: application in living cells imaging. Talanta. 2014;125:418-424.
- [CrossRef] [Google Scholar]
- A selective and discriminating noncyclic receptor for HSO4- ion recognition. RSC Adv.. 2014;4:15288-15292.
- [CrossRef] [Google Scholar]
- Highly selective and sensitive receptor for Fe3+ probing. Spectrochim. Acta - Part A Mol. Biomol. Spectrosc.. 2014;121:569-574.
- [CrossRef] [Google Scholar]
- Fluorescent and chromogenic receptor bearing amine and hydroxyl functionality for iron (III) detection in aqueous solution. J. Fluoresc.. 2014;24:675-681.
- [CrossRef] [Google Scholar]
- Fisli, A., Yusuf, S., Krisnandi, Y.K., Gunlazuardi, J., 2014. Preparation and characterization of magnetite-silica nano-composite as adsorbents for removal of methylene blue dyes from environmental water samples. In: Adv. Mater. Res., Trans Tech Publ, 2014, pp. 525–531.
- The adsorption of cis- and trans-Azobenzene. J. Am. Chem. Soc.. 1939;61:2228-2230.
- [CrossRef] [Google Scholar]
- Photocatalytic degradation of low concentration formaldehyde and simultaneous elimination of ozone by-product using palladium modified TiO2 films under UV254+185 nm irradiation. Appl. Catal. B Environ.. 2011;105:220-228.
- [CrossRef] [Google Scholar]
- Cobalt ferrite nanotubes and porous nanorods for dye removal. Adv. Nano Res.. 2015;3:111-121.
- [CrossRef] [Google Scholar]
- Pseudo-second order model for sorption processes. Process Biochem.. 1999;34:451-465.
- [CrossRef] [Google Scholar]
- Comparative sorption kinetic studies of dye and aromatic compounds onto fly ash. J. Environ. Sci. Heal. - Part A Toxic./Hazard. Subst. Environ. Eng.. 1999;34:1179-1204.
- [CrossRef] [Google Scholar]
- Adsorption and magnetic removal of neutral red dye from aqueous solution using Fe3O4 hollow nanospheres. J. Hazard. Mater.. 2010;181:1039-1050.
- [CrossRef] [Google Scholar]
- Facile synthesis of lead doped zinc-aluminum oxide nanoparticles (LD-ZAO-NPs) for efficient adsorption of anionic dye: Kinetic, isotherm and thermodynamic behaviors. J. Ind. Eng. Chem.. 2017;53
- [CrossRef] [Google Scholar]
- Design and synthesis of Zn0.3Fe0.45O3 nanoparticle for efficient removal of Congo red dye and its kinetic and isotherm investigation. Int. J. Ind. Chem.. 2018;9:85-97.
- [CrossRef] [Google Scholar]
- An multifunction Zn0.3Mn0.4O4 nanospheres for carbon dioxide reduction to methane via photocatalysis and reused after fifth cycles for phosphate adsorption. J. Environ Chem. Eng. 2018
- [Google Scholar]
- Dye pollutants removal from waste water using metal oxide nanoparticle embedded activated carbon: an immobilization study. J. Dispers. Sci. Technol.. 2018;40:563-573.
- [CrossRef] [Google Scholar]
- A study on HCHO oxidation characteristics at room temperature using a Pt/TiO2 catalyst. Appl. Catal. A Gen.. 2011;398:96-103.
- [CrossRef] [Google Scholar]
- Experimental investigation on phosphate adsorption, mechanism and desorption properties of Mn-Zn-Ti oxide trimetal alloy nanocomposite. J. Dispers. Sci. Technol.. 2018;39:1635-1643.
- [CrossRef] [Google Scholar]
- Design and synthesis of magnetic nanoparticles augmented microcapsule with catalytic and magnetic bifunctionalities for dye removal. Chem. Eng. J.. 2012;197:350-358.
- [CrossRef] [Google Scholar]
- Kurien, S., 2010. Analysis of FTIR spectra of nanoparticles of MgAl2O4, SrAl2O4, and NiAl2O4. http://shodhganga.inflibnet.ac.in:8080/jspui/bitstream/10603/468/10/10_chapter4.pdf.
- The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc.. 1916;38:2221-2295.
- [CrossRef] [Google Scholar]
- The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc.. 1918;40:1361-1403.
- [CrossRef] [Google Scholar]
- Photo-catalytic degradation of gaseous formaldehyde by TiO2/UV, Ag/TiO2/UV and Ce/TiO2/UV. Build. Environ.. 2012;51:345-350.
- [CrossRef] [Google Scholar]
- TiO2 hydrosols with high activity for photocatalytic degradation of formaldehyde in a gaseous phase. J. Hazard. Mater.. 2008;152:347-355.
- [CrossRef] [Google Scholar]
- Adsorption of Direct Blue 78 using chitosan and cyclodextrins as adsorbents. Polymers (Basel). 2019;11
- [CrossRef] [Google Scholar]
- Synergistic effects of high-frequency ultrasound on photocatalytic degradation of aldehydes and their intermediates using TiO2 suspension in water. Ultrason. Sonochem.. 2011;18:158-163.
- [CrossRef] [Google Scholar]
- Cd(II) removal and recovery enhancement by using acrylamide-titanium nanocomposite as an adsorbent. Appl. Surf. Sci.. 2014;313:624-632.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of anionic/nonionic surfactant-interceded iron-doped TiO2 to enhance sorbent/photo-catalytic properties. J. Solid State Chem.. 2015;229:1-9.
- [CrossRef] [Google Scholar]
- Growth of TiO2 nano-wall on activated carbon fibers for enhancing the photocatalytic oxidation of benzene in aqueous phase. Catal. Today.. 2017;287:113-121.
- [CrossRef] [Google Scholar]
- Photocatalytic reduction of carbon dioxide to methanol using nickel-loaded TiO2 supported on activated carbon fiber. Catal. Today. 2017;298:158-167.
- [CrossRef] [Google Scholar]
- Iron doped phenolic resin based activated carbon micro and nanoparticles by milling: synthesis, characterization and application in arsenic removal. Chem. Eng. Sci.. 2010;65:3591-3601.
- [CrossRef] [Google Scholar]
- A short review on photocatalytic degradation of formaldehyde. J. Nanosci. Nanotechnol.. 2015;15:6386-6396.
- [CrossRef] [Google Scholar]
- A hollow mesoporous carbon from metal-organic framework for robust adsorbability of ibuprofen drug in water. R. Soc. Open Sci.. 2019;6
- [CrossRef] [Google Scholar]
- Combined minimum-run resolution IV and central composite design for optimized removal of the tetracycline drug over metal-organic framework-templated porous carbon. Molecules. 2019;24:1887.
- [CrossRef] [Google Scholar]
- Facile synthesis of manganese oxide-embedded mesoporous carbons and their adsorbability towards methylene blue. Chemosphere. 2019;227:455-461.
- [CrossRef] [Google Scholar]
- MIL-53 (Fe) derived magnetic porous carbon as a robust adsorbent for the removal of phenolic compounds under the optimized conditions. J. Environ. Chem. Eng.. 2019;8:102902
- [CrossRef] [Google Scholar]
- Effect of thermolysis condition on characteristics and nonsteroidal anti-inflammatory drugs (NSAIDs) absorbability of Fe-MIL-88B-derived mesoporous carbons. J. Environ. Chem. Eng.. 2019;7:103356
- [CrossRef] [Google Scholar]
- Application of Fe-based metal-organic framework and its pyrolysis products for sulfonamide treatment. Environ. Sci. Pollut. Res.. 2019;26:28106-28126.
- [CrossRef] [Google Scholar]
- Tunable synthesis of mesoporous carbons from Fe3O(BDC)3 for chloramphenicol antibiotic remediation. Nanomaterials. 2019;9:237.
- [CrossRef] [Google Scholar]
- Optimization, equilibrium, adsorption behavior and role of surface functional groups on graphene oxide-based nanocomposite towards diclofenac drug. J. Environ. Sci. 2020
- [CrossRef] [Google Scholar]
- Removal of low-concentration formaldehyde in air by DC corona discharge plasma. Chem. Eng. J.. 2011;171:314-319.
- [CrossRef] [Google Scholar]
- TiO2 nanoparticles with increased surface hydroxyl groups and their improved photocatalytic activity. Catal. Commun.. 2012;19:96-99.
- [CrossRef] [Google Scholar]
- Pore and solid diffusion models for fixed-bed adsorbers. AIChE J.. 1974;20:228-238.
- [CrossRef] [Google Scholar]
- Synthesis and characterisation of nanocrystalline MgAl2O4 ceramic powders by use of molten salts. Br. Ceram. Trans.. 2002;101:71-74.
- [CrossRef] [Google Scholar]
- A dual function magnetic nanomaterial modified with lysine for removal of organic dyes from water solution. Chem. Eng. J.. 2014;239:250-256.
- [Google Scholar]
- Photocatalytic degradation of formaldehyde by nanostructured TiO2 composite films. J. Exp. Nanosci.. 2016;11:185-196.
- [CrossRef] [Google Scholar]
- One-step synthesis of amino-functionalized attapulgite clay nanoparticles adsorbent by hydrothermal carbonization of chitosan for removal of methylene blue from wastewater. Colloids Surf. A Physicochem. Eng. Asp.. 2015;470:248-257.
- [CrossRef] [Google Scholar]
- Highly efficient removal of organic dyes from waste water using hierarchical NiO spheres with high surface area. J. Phys. Chem. C. 2012;116:6873-6878.
- [CrossRef] [Google Scholar]
- The degradation of formaldehyde using a Pt@TiO2 nanoparticles in presence of visible light irradiation at room temperature. J. Taiwan Inst. Chem. Eng.. 2015;50:276-281.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2020.04.002.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







