Translate this page into:
Nanostructured lipid carriers based suppository for enhanced rectal absorption of ondansetron: In vitro and in vivo evaluations
⁎Corresponding authors at: Faculty of Pharmacy and Alternative Medicine, The Islamia University of Bahawalpur, Pakistan. fahad.pervaiz@iub.edu.pk (Fahad Pervaiz), m.buabeid@ajman.ac.ae (Manal Buabeid), gmdogar356@gmail.com (Ghulam Murtaza)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The current research aimed to fabricate ondansetron nanostructured lipid carriers (OND-NLCs) and incorporate them into a suppository base to manage chemotherapy-induced vomiting and nausea, which offer the advantage of both rapid onset and prolonged release. NLCs were fabricated by adopting the solvent diffusion method. The binary lipid mixture of oleic acid (liquid lipid) and lauric acid (solid lipid) were prepared in distinct ratios. The NLCs were characterized concerning the surface charge, size, drug encapsulation efficiency, and surface morphology. In addition, the influence of surfactant, co-surfactant, and lipid on entrapment efficiency and particle size was investigated. Phosphate buffer having pH 7.4 is used for evaluating in vitro drug release by utilizing a dialysis membrane. Various kinetics models were used to estimate the drug release kinetics of fabricated nanostructured lipid carriers. The particle size of the NLCs was calculated between 101 and 378 nm with negative zeta potential on the NLC’s surface. The entrapment efficiency was found between 68 and 87%. Scanning Electron Microscopic analysis showed the spherical shape of nanostructured lipid carriers. The dissolution profile of the ondansetron-loaded NLC suppository depicts biphasic behavior of firstly burst release then slow release was observed. The diffusion controlled release was evident from kinetic modeling. The succeeding step comprehended the fabrication and characterization of NLC-based suppositories utilizing NLC formulations that demonstrated the combined advantage of rapid onset, prolonged release, and better in vivo bioavailability as compared to control suppository.
Keywords
Nanostructured lipid carriers
Ondansetron
Oleic acid
Lauric acid
Rectal suppository
Emesis
1 Introduction
In pharmaceutical sciences, targeted and controlled drug delivery system is considered to be most demanding research areas. New dimensions have been opened by preparing colloidal systems of drug delivery, for instance, micelles, liposomes, and nanoparticles (zur Mühlen et al., 1998; Hu et al., 2006). Nanoparticles have supremacy over other conventional drug delivery systems because of particular characteristics like large surface area, small particle size, and the tendency to change their surface properties. Among these, nanostructured lipid transporters are the most innovative transporters that have drawn in the most brilliant consideration because of their remarkable properties like their limited size distribution, biocompatibility, and various potential routes of administration (Azevedo et al., 2021; Kanojia et al., 2021; Naseri et al., 2015). Moreover, NLCs, similar to some other novel drug conveyance frameworks, offer different advantages when contrasted with ordinary carrier systems, for example, site-specific delivery of a drug, controlled drug release, enhanced stability of the drug, reduced toxicity, the tendency of the introduction of both lipophilic and hydrophilic drugs, the possibility of scale-up and lyophilization, preservation of chemicals that are liable to degradation and possibility of sterilization (Selvamuthukumar and Velmurugan, 2012). However, NLCs have overcome some shortcomings of solid lipid nanoparticles like the low capability of drug loading, the expulsion of the active ingredient after the polymer transition during storage, comparatively high water content, and unpredictable drug release (Huguet-Casquero et al., 2020; Sabzichi et al., 2017; Shirodkar et al., 2019). Furthermore, nanoparticle research has been dramatically enhanced by using dual or multiple drug loading to augment treatment efficacy. For example, nanoparticles loaded with dual and multiple drugs have been applied to treat diseases like breast cancer and neuronal disorders (Angelova and Angelov, 2017; Gao et al., 2020; Tapeinos et al., 2017).
NLCs have enhanced the applications and eliminated the limitation of other lipid-based carriers as solid lipid nanoparticles. NLCs have augmented stability as it exists in the solid-state on room temperature which is difficult to achieve in these types of emulsion lipid formulation. Liposomes require costly large-scale manufacturing techniques, which can be replaced with less expensive NLCs (Beloqui et al., 2017). Nanostructured lipid carriers are aqueous colloidal dispersion systems and their matrix consists of biodegradable lipids. NLCs are commonly fabricated by high shear homogenization, micro emulsion-based formulation, solvent emulsification/evaporation technique, supercritical fluid technology, double emulsion method, solvent injection method, spray drying technique, and ultra-sonication technique (Hu et al., 2021; Mozaffar et al., 2021; Khan et al., 2021). The solvent evaporation technique is commonly used to prepare liposomes and polymeric nanoparticles and is used for the formulation of nanostructured lipid carriers. It has some benefits over other preparation methods as it is easy to handle and fast in production without using any sophisticated equipment. In this technique, water-soluble organic phase blended with liquid phase contains lipophilic material, and precipitation of lipids in hydrophilic phase is carried out by solvent evaporation nanoparticle dispersion (Garud et al., 2012; Khosa et al., 2018; Almousallam et al., 2015).
The rectal route has received much attention in recent years because it is the more suitable and reliable route of systemic administration of drugs. It offers the same absorption possibilities as the oral route. Drugs administered through the rectal route circumvent the hepatic first-pass effect, improving the bioavailability of many drugs. Besides, the decline in risk of infection, the rectal route gives ease and convenience, resulting in increased patient compliance. With the increase of solubility of drugs in the adjuvants and vehicles, drug absorption via the rectal route also increases (Abdelbary and Fahmy, 2009).
Ondansetron (OND) is the serotonin (5-hydroxy tryptamine) subtype (5HT3) receptor antagonist and is sparingly soluble in aqueous media. It is used in gastrointestinal disorders and pregnancy to inhibit nausea and vomiting (Bodner and White, 1991). It is mainly used in adjuvant chemotherapy to manage chemotherapy-induced vomiting and nausea, which are common adverse effects of anti-tumor drugs. Gastric stasis and gastroesophageal reflux are also treated by it. It is also effectively used in vomiting associated with migraines and postoperative emesis. OND increases the duodenal peristalsis and increases the upper gastrointestinal tract motility, leading to stimulated small intestinal transit time and gastric emptying time (Leeser and Lip, 1991). OND undergoes broad first-pass metabolism. Due to which its bioavailability in the bloodstream is about 60%. OND has a half-life of about 3–4 h. So, maintaining the therapeutic drug concentration at the target site, it is required to be administered four times daily (Joshi et al., 2012). OND is sparingly soluble in water (0.248 mg/ml), having a logP value of 2.56. The melting point of OND is 223–232 °C and has a polar surface area (PSA) of 39.62 A° (Rajawat et al., 2019).
OND is available in the form of oral tablets, solutions, and injections and for intranasal administration. Swallowing the oral tablet is not always convenient for patients with dysphagia, odynophagia, geriatric chemotherapy, and children. This difficulty in swallowing oral medicine might lead to patient noncompliance and can result in ineffective therapy. OND injections are also use unpleasant for some patients due to pain and may lead to overdose. IV injection administers drug dose directly into the bloodstream, resulting in speedy onset, so overdose may occur very rapidly, which needs instant action. During nausea and vomiting in convulsions or uncooperative patients, an injection is impractical or even not possible. In such cases, the rectal route is the best alternative and is now well-accepted (Seager, 1998).
In order to minimize the dosing frequency, there is a need to develop a controlled release system that can attain the improved drug loading and having the ability to give the effective and appropriate drug release trend with no first-pass metabolism of OND. Therefore, the present study aims to develop, characterize, and optimize OND loaded NLC preparation and their incorporation into suppository base to treat nausea and vomiting in unavailability or undesirability of oral route, thus enhancing patient compliance.
2 Material and methods
2.1 Material
Ondansetron was acquired as a generous gift from Consolidates Chemical Laboratories (CCL), Lahore, Pakistan. Oleic acid and lauric acid were purchased from Merck KGaA, Darmstadt, Germany. Chloroform (analytical grade) was bought from Alfa Aesar, Ward Hill, Massachusetts. Tween 80 (Polysorbate 80) and Span 80 were acquired from Acros Organics, Geel – Belgium.
2.2 Preparation of OND loaded NLCs
2.2.1 Preparation of binary lipid mixture
Different ratios of melted solid lipid (lauric acid) and liquid (oleic acid) were used for the preparation of binary lipid mixtures and allotted different formulation codes. Lauric acid was heated above its melting point at 50 °C and then put into oleic acid kept at the same temperature. Homogenous mixing was ensured when the viscosity of the fatty acid was reduced by increasing the temperature of the melting lipid. The melting point was measured by melting the lipid in a heated water bath (Elmasonic S 10H). The melting point of pure lipids was measured and compared with their traditional values to confirm the validity of this method. Individual pure lipid was taken in the Eppendorf tube since the melting point of lipids is low. The water bath was heated to a temperature 5 °C lower than that mentioned in the literature and lipid (in Eppendorf tubes) was dipped into it. The temperature was amplified at a rate of 0.5 °C per minute. The temperature was recorded at which lipid starts melting and this temperature was maintained until the whole lipid melts. The melting point of all formulations was measured by the same procedure (Rehman et al., 2015).
2.2.2 Fabrication of OND loaded NLCs
Solvent diffusion method is used to fabricate OND-NLCs according to the amount stated in Table 1. The drug was completely dissolved in the organic solution and lipid (lauric acid and oleic acid) in 1 ml of ethanol (water-miscible solvent) under slow magnetic stirring (15 rpm) and low temperature in order to evaporate ethanol completely. Then the temperature was kept at 70 °C (above the melting point of lauric acid and oleic acid). Surfactant (tween 80) and co-surfactant (span 80) were solubilized in 5 ml of double distilled water to prepare the aqueous phase and heated to the same temperature of the oil phase and then added to lipid phase under magnetic stirring (1000 rpm) at 60 °C for 1 hr. Cool the formed Nano suspension at room temperature. By decreasing the temperature under such conditions, the lipid droplets solidify, resulting NLCs. Fabricated NLCs were removed from suspension to centrifugal force (Sigma1-14, Germany) that was carried out for 10 min at 30,000 rpm. To re-disperse NLCs, deionized water was added after removing the supernatant. This process was repeated 3 times. After washings, NLCs were recovered by centrifugation and lyophilized to obtain dried NLCs. The samples were frozen for 2 h at −20 °C after adding mannitol as a cryoprotectant, then transported to −80 °C for 22 h. Freeze drying was carried out for 24 h at −47 °C and 0.05 mBar (CHRIST alpha 1–4 LD, UK).
Formulation
Drug (mg)
Lauric acid (mg)
Oleic acid (mg)
Tween 80 (mg)
Span 80 (mg)
Water (ml)
F1
25
50
50
200
100
5
F2
25
60
40
200
100
5
F3
25
70
30
200
100
5
F4
25
80
20
200
100
5
F5
25
90
10
200
100
5
F6
25
60
40
300
–
5
2.2.3 Characterization of OND-NLCs
2.2.3.1 Determination of OND encapsulation efficiency (% E.E.)
The % E.E. of fabricated NLCs was estimated by calculating the free drug in the dispersion medium. Briefly, the drug is dissolved in 100 μl of NLC suspension combined with 9.9 ml ethanol (95%). Centrifugation of resulted suspension was carried out for 5 min at 30,000 rpm to separate Nanostructured lipid carriers. After filtering the supernatant, 310 nm wavelength was used to observe the filtrate by UV spectrophotometry (IRMECO.U2020). The drug concentration was calculated by subtracting the free drug from initial drug. Percentage drug loading was determined by given Equation (1) (Cai et al., 2015).
Encapsulation efficiency of Nanostructured lipid carriers was calculated by the following Equation (2) (Pervaiz et al., 2020).
2.2.3.2 Particle size analysis
Particle size analyzer (Zetasizer ZS-90; Malvern Instruments Ltd; Malvern, U.K.) was used to analyze particle size of OND-NLCs whereas, deionized water was used to dilute NLC suspension (10 times) and the results were executed by means of a 45-mm focus objective and a beam length of 2.4 mm at an angle of 90°. The mean value was obtained by performing the experiment in triplicate for each sample (Rehman et al., 2015).
2.2.3.3 Zeta potential
Zetasizer ZS-90; Malvern Instruments Ltd; Malvern, U.K was employed to calculate the zeta potential of the surface of NLC. To determine the NLC surface charge, where NLC suspension was diluted 10 times deionized water and the measurements were executed and an average of triplicate values was taken (Rehman et al., 2015).
2.2.3.4 Scanning electron microscopy
Morphology of OND-NLCs was observed by field emission scanning electron microscope (JSM-7500F, Jeol, Japan). After placing the sample on it, the grid was introduced into SEM and then scanned between 15 and 30 kV voltages. Best scanning was observed at a magnification of 55,000X at 20.0 kV, and a screen area of 100 nm was taken (Rehman et al., 2015).
2.2.3.5 Fourier Transform Infrared spectroscopy (FTIR)
Fourier Transform Infrared Spectroscopy (FTIR) was adopted to examine the compatibility of formulation components. The spectrum of individual lipid, binary lipid mixtures, drugs, emulsifiers, and drug-loaded NLCs was taken. A small amount of powder was placed on a slit and analyzed by ATR-FTIR spectroscopy (Tensor 27 series, Germany) in the range of 4000–650 cm−1 to record the spectrum.
2.2.3.6 X-ray diffraction analysis
X-ray diffraction evaluation was carried out to analyze the crystalline behavior of the NLCs. The X-ray diffraction patterns were analyzed for the drug (OND), lauric acid, oleic acid, and the prepared drug-loaded NLCs. The X-ray diffractometer (Bruker Corporation, Billerica, MA, USA) was employed to record the X-ray diffraction pattern with Cu Kα radiation (λ = 0.1541 nm) in the scan range of 2θ = 15–70° and ultra-fast 1D detector si-strip detector with 160 channels.
2.3 Preparation and estimation of OND NLC-based suppositories
In the suppository base (witepsol) NLC formulations with optimal results of drug loading capacity were designated to be incorporated. The fusion method was used to fabricate OND NLC-based suppositories (Mohamed et al., 2013). Briefly, an accurate weight of suppository base calculated to formulate 2 mg OND suppository was melted at 39 °C and cool at room temperature. Next, accurately weighed OND-loaded NLC dispersion, equivalent to OND suppository, was added to the melted bases resulting in homogenous dispersion. Finally, the suppositories were cooled at room temperature, and to avoid cracking, suppositories were stored at 4 °C. Control suppository was made by suspending the OND with NLC matrix components directly into the suppository base after melting.
2.4 In vitro drug release profile
Dialysis method was adopted to examine the in-vitro release of OND from various NLC loaded suppository formulations. Suppositories samples were carefully positioned in the dialysis membrane of 150kD. The dialysis bag technique has been used previously for such kinds of NLC loaded formulations (Correia et al., 2020). A dissolution test was performed by hanging the dialysis tubing in semiautomatic USP type II apparatus (Pharma Test, Germany). 200 ml of 0.1 M phosphate buffer of pH 7.4 agitated at 50 rpm was used as dissolution medium in which suppositories were placed and maintained at 37 °C for 8 h (Mohamed et al., 2013). After a predetermined time, the interval sample was withdrawn, filtered, and assayed by a U.V. spectrophotometer (IRMECO.U2020) at a wavelength of 310 nm. Each experiment was performed in triplicate, and average values were calculated.
2.5 Analysis of drug release data by applying different kinetic models
Data obtained from dissolution studies of OND NLC-based suppositories were analyzed by applying kinetic models. Different kinetic models were applied to in vitro drug release data to evaluate the mechanism of drug release and trend of release. Regression analysis performed to the in vitro dissolution records and coefficient of zero order (Ramteke et al., 2014), first order (Sun et al., 2017; Sep), Higuchi (Derakhshandeh et al., 2010), and Korsmeyer-Peppas models (Dash et al., 2010), were calculated and related to refer to the order and mechanism of drug release (Raval et al., 2014; Khurram Shahzad et al., 2012).
2.6 In vivo studies
To study the pharmacokinetic considerations of ondansetron, the fabricated NLC based suppositories were compared with market available oral tablets, control suppository, and intravenous infusion.
In vivo evaluation was executed on sixteen healthy rabbits weighing 2.0–2.5 kg. Pharmacy Research Ethics Committee approved the ethics of in vivo characterization, the Islamia University of Bahawalpur (No. 08–2020/PAEC, dated 6th March 2020). The animals were brought one month before in vivo evaluation and were housed in separate cages. The animals were divided randomly into four groups, group A received oral tablets (Onset tablets by Pharmedic Pharma, Pakistan), group B received intravenous infusion (Onset injection by Pharmedic Pharma, Pakistan), and group C received fabricated NLC rectal suppositories and group D was treated with control suppositories. The dose of ondansetron given to animals was equivalent to 2 mg in all three groups. All the animals were kept at room temperature and abstained for 12 h but had free reach to water. In addition, all the animals were provided with fresh green fodder three times a day and kept under identical investigational conditions. After drug administration, 1 ml of blood sample was taken out from the jugular vein of the rabbits at different time intervals (48hrs). A previously established HPLC–U.V. method was used to evaluate the plasma samples (Colthup et al., 1991; VanDenBerg et al., 2000).
Blood samples were extracted by adding 3 ml acetonitrile to 500 µl separated plasma. Then samples were vortexed and the organic layer was separated and evaporated under a stream of nitrogen. The contents after complete evaporation were reconstituted in 100 µl mobile phase and a 20 µl sample was injected in HPLC. HPLC analyses were carried out at room temperature using C18 column. The mobile phase consisted of sodium acetate buffer (pH 4.5) and acetonitrile in 6:4. The mobile phase was run at a flow rate of 1.2 ml per min and the UV detector was set at 305 nm. Peak area was calculated in mAU for quantification of ondansetron in plasma samples.
The analytical data of HPLC were plotted as OND concentration (ng/mL) versus time (h). To access pharmacokinetic parameters, computer-registered software Kinetica 4.1 was used. Non-compartmental analysis NC was performed for the evaluation of kinetic parameters.
2.7 Statistical analysis
One-way ANOVA (Kinetica) was applied using non-compartmental (NC) analyses for comparative bioavailability studies to express the significance of the difference among different data values. The level of significance was set at 0.05.
3 Results and discussion
Binary fatty acid mixtures were utilized to fabricate binary NLCs loaded with OND. In vitro characterization was carried out to evaluate the physicochemical parameters and the drug release pattern.
3.1 Fabrication of binary lipid mixture
Binary lipid mixtures of liquid and solid fatty acids exhibit superior physicochemical characteristics and improved drug entrapment efficiency. However, physical mixing of a liquid fatty acid (oleic acid) is assumed to decrease the melting point of the resulting B.F.s (Binary lipid mixtures). Six formulations were prepared to consist of a binary lipid mixture of lauric acid and oleic acid in the mass ratios of 9:1, 8:1, 7:1, 6:1, 5:1, and 4:1; their melting points are reported in Table 2. Oleic acid existed in liquid form at room temperature and was presumed to lower the melting point of the binary lipid mixture. Sample BL1 resulted in slight (P > 0.05) depression of melting point and was melted at 42.5 °C compared to 43.4 °C of pure lauric acid. By increasing the amount of oleic acid in any of these formulations, the fluidity of lipid nanoparticles also tends to increase.
Formulation Code
Lauric acid: Oleic acid mass/mass
Melting point
BL1
9:1
42.25–42.5 °C
BL2
8:1
41.25–42.0 °C
BL3
7:1
40.75–41.0 °C
BL4
6:1
38.75–39.5 °C
BL5
5:1
37.5–38.0 °C
BL6
4:1
35.25–36.0 °C
A binary lipid mixture of a liquid lipid and solid lipid was prepared to produce a formulation that can show temperature-responsive release based on solid-lipid phase transition (Kang et al., 2010). Below the physiological temperature (37 °C), such formulations should remain solid and tend to melt above 37 °C (Vlad et al., 2007). Thus, BL4 and BL5 were selected as temperature-responsive binary lipid mixture, starting softening at 38 °C, rectum temperature, whereas BL6 formulation starts melting (36.0 °C) below physiological temperature. The result of the study was found in agreement with a study that depicted that combining an oil to a solid lipid system results depression of melting point in a linear fashion. It also showed that an increase in the amount of liquid oil leads to cluster formation of oil and minimizes the stability of nanostructured lipid carriers (Jenning et al., 2000).
3.2 Physiochemical characterization of nanostructured lipid carriers
Six NLCs formulations were prepared for studying physical characterization, chemical stability, drug loading, and release kinetics. Formulation F1 to F6 compared the effect of different binary lipid mixture ratios.
3.2.1 Drug loading and encapsulation efficiency
NLCs exhibit the great entrapment efficiency of OND. In the current research, OND was effectively loaded into fabricated NLCs, and high percentage encapsulation was attained for all samples, as reported in Table 3. Encapsulation efficiency is measured by the indirect method. NLCs were prepared by ultracentrifugation at 30,000 rpm, and the concentration of the drug in the supernatant was calculated. Encapsulation efficacy for all the formulations was found to be from 68% to 87%. A significant (P < 0.05) decrease in encapsulation efficiency from F1 to F6 is due to a decline in oleic acid concentration and a rise in the amount of lauric acid. A sudden and significant (P < 0.05) fall in encapsulation efficiency of the F6 formulation was observed because it contains only one surfactant solution. Use of tween 80 as surfactant results in lowest percentage E.E. (F6) (P < 0.05) as compared to its use in combination with another surfactant that leads to an increase in percentage E.E. (F1) with other. Results also depict that increase in the concentration of tween 80 (F6) did not improve the entrapment efficiencies (Mohamed et al., 2013).
Formulation Code
Drug loading (%)
Drug Entrapment Efficiency (%)
F1
54 ± 6.35
87 ± 3.43
F2
53 ± 7.24
85 ± 3.35
F3
51 ± 5.94
82 ± 3.41
F4
50 ± 5.88
80 ± 3.26
F5
49 ± 5.76
79 ± 2.91
F6
42 ± 4.94
68 ± 2.62
These results also suggest that small nanoparticles have non-significantly (P < 0.05) higher entrapment efficiency. Encapsulation efficiency of hydrophobic drugs shows low encapsulation while hydrophilic drugs are efficiently loaded into the NLCs. Highly hydrophobic drugs like estradiol and hydrocortisone can be encapsulated with 99% and 97% efficiency, while low hydrophobic drug pilocarpine showed encapsulation efficiency of 34–40% (Yang et al., 1999). The drug loading percentage ranged from 42 to 54%, which is exceptional, as more than 50% of drug loading is considered very good (Cai et al., 2015).
3.2.2 Analysis of particle size, zeta potential, and polydispersity index
Effects of altered ratios of binary lipid mixtures and surfactant and co-surfactant concentrations on particle size, zeta potential, and polydispersity index were evaluated. The particle size, polydispersity index, and zeta potential (Z.P.) values of the NLCs developed were observed in the range of 101 to 378 nm, 0.214 to 0.382, and −22.7 to −37.4, correspondingly, as reported in Table 4. Results exhibited that all of the fabricated NLCs formulations were low than 500 nm in size, according to NLC drug delivery (Carvajal-Vidal et al., 2019). The particle size was seen to be significantly (P < 0.05) exaggerated with an inclined in the concentration of lipid at a reduced surfactant amount. When the lauric acid amount upsurges from 50 to 90 mg, the average particle size of the formulation is enhanced. This may be accredited to the surfactant solution's inability to stabilize the emulsion at a poor concentration. A higher surfactant concentration was adequate to stabilize the emulsion even with an increased lipid concentration of 90 mg.
Formulation code
Size (nm) (mean ± S.D.)
Poly-dispersibility Index (mean ± S.D.)
Zeta potential (mean ± S.D.)
F1
101 ± 3.98
0.248 ± 0.017
−37.4 ± 2.59
F2
136 ± 5.35
0.285 ± 0.011
−34.4 ± 2.31
F3
149 ± 5.87
0.346 ± 0.014
−32.2 ± 2.16
F4
178 ± 7.14
0.382 ± 0.015
−23.9 ± 1.62
F5
200 ± 8.16
0.214 ± 0.008
−23.5 ± 1.54
F6
378 ± 14.91
0.248 ± 0.011
−22.7 ± 1.49
It was observed that varying the concentration of oleic acid, and lauric acid affected the NLCs size significantly (P < 0.05). The particle size was found to rise by enhancing the concentration of lauric acid and reducing the concentration of oleic acid, with the amount of surfactant and co-surfactant remaining constant. This reduction in particle size by an increasing amount of oleic acid is due to the increase in viscosity of the oleic acid (Friedrich and Müller-Goymann, 2003).
A finer dispersion can be achieved employing higher energy resulting from longer emulsification time, high production temperature, pressure, more substantial ultrasound power, higher stirring rate, etc. In addition to production parameters, the surfactant blend, the lipid matrix, and the viscosity of the aqueous and lipid phase impact the outcome of the procedure. Likewise, the amount of surfactant used exhibited an insightful influence on the particle size and distribution (Jenning et al., 2000; Li et al., 2006; Schwarz et al., 1994).
The lessening in size of prepared NLCs at more concentration of surfactant is because of an active decrease in interfacial tension among the lipid and aqueous phases, leading to the development of emulsion droplets of small size, cooling leads to smaller nanoparticles. Throughout the process of emulsification, the droplet size reduces under the effect of the enhanced shear, while these droplets tend to form the agglomerates, so that surface energy is decreased. Though, the surfactant molecule stabilizes the emulsion by developing a thick protective layer around the droplets, which revokes them from coalescing. The combination of surfactant and co-surfactant reduces the particle size due to their synergistic effect and prevents particle agglomeration. Therefore, tween 80 and span 80 were selected for NLCs preparation. It is observed that the formulation that stabilized with the surfactant and co-surfactant mixture has a small particle size as compared to the formulation which stabilizes with only the surfactant. The particle size of F6 was observed at 378 nm, in which only tween 80 is used as a surfactant. Thus, surfactant and co-surfactant show a synergistic effect. Surfactant stabilizes the suspension by electrostatic repulsion, whereas span 80 is a nonionic surfactant that stabilizes the suspension by steric effect. As more span 80 is used, particle size decreases. The measurements of the zeta potential depict the stability of the colloidal dispersion (Abdelbary and Fahmy, 2009; Mehnert and Mäder, 2012; Harivardhan Reddy and Murthy, 2005; Lim and Kim, 2002).
The Z.P. is the electric charge on the particle's surface, which generates an electric barrier and acts as a “repulsive barrier ”in the stabilization method. Higher surface charges increase the stability of the formulation, just like the charges at interface combat coalescence of particles. The zeta potential of all fabricated NLCs containing OND was in the acceptable range of −37.4 mV to −22.7 mV, which is a prerequisite for the long-term stability of NLCs dispersion (Clogston and Patri, 2011). The Z.P. of all formulations (F1–F6) indicated that these formulations would form mild to moderately stable colloidal dispersions.
3.2.3 Morphology of NLC
The shape of NLCs entrapping OND is illustrated in Fig. 1. Imaging analysis revealed that these particles are oval-shaped but not perfectly round. OND loaded NLCs have appeared in two forms. One form of NLCs had a uniform surface as dark color. Another type of NLCs was visualized as having a rough surface due to the drug layer on the surface of lipid crystallinity with time. The surface drug layer may lead to drug expulsion (zur Mühlen et al., 1998; Mohamed et al., 2013; Carvajal-Vidal et al., 2019; Müller et al., 2000).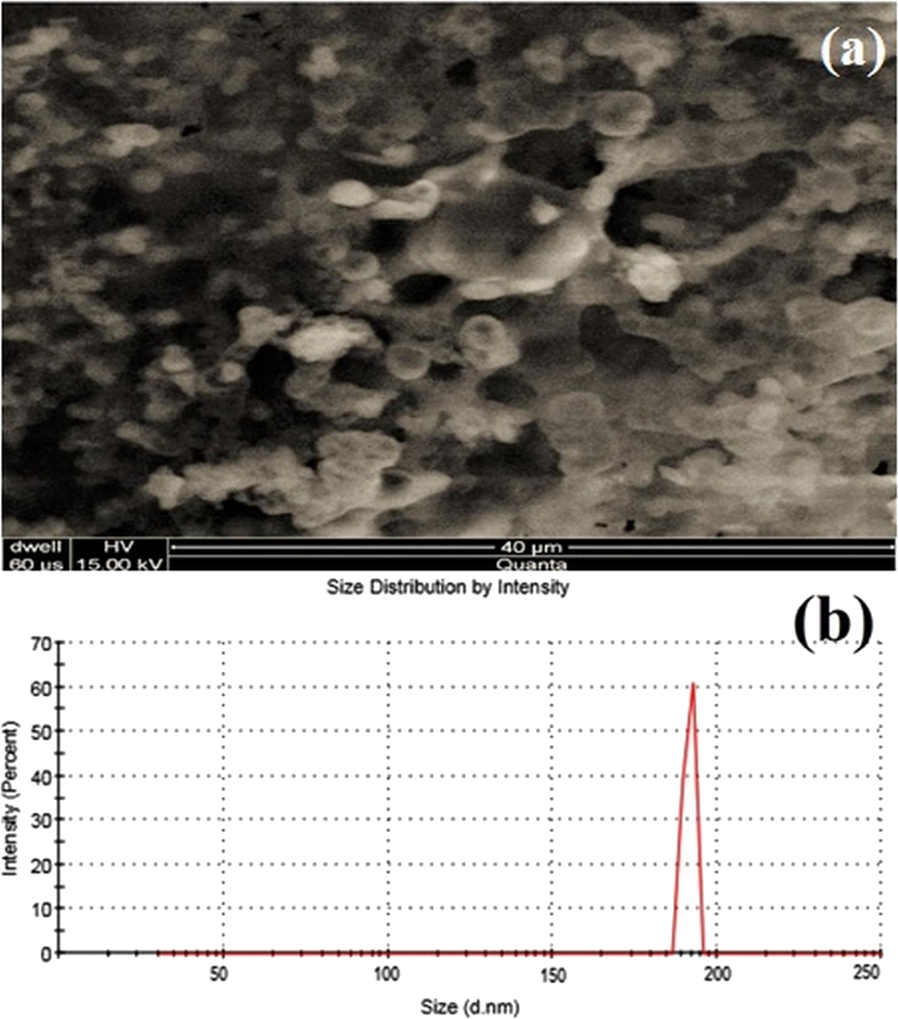
Scanning electron micrographs of ondansetron-loaded NLCs (a), size, and polydispersity graph of F5 (b).
3.2.4 Chemical stability of ondansetron and polymers in NLCs
The chemical stability of drug ondansetron and all ingredients used in NLCs formulation were confirmed by FTIR analysis as illustrated in Fig. 3. Ondansetron FTIR spectra displayed a characteristic peak at 1628 cm−1 of a carbonyl group and N.H. stretching absorption region in 3500–3220 cm−1, as illustrated in Fig. 2. The results are comparable to another study in which structural peaks were observed at the same region (Swain et al., 2009). The characteristic absorption peaks of OND were visible in the FTIR spectra of drug-loaded NLCs with basically no change of frequency and shape of ondansetron hydrochloride bands, which leads to the conclusion that the OND was present physically in fabricated NLCs without any chemical interaction. (Rehman et al., 2015; Teaima et al., 2020) Prepared NLCs using lauric acid and oleic acid reported that the drug remains stable in the NLC formulation.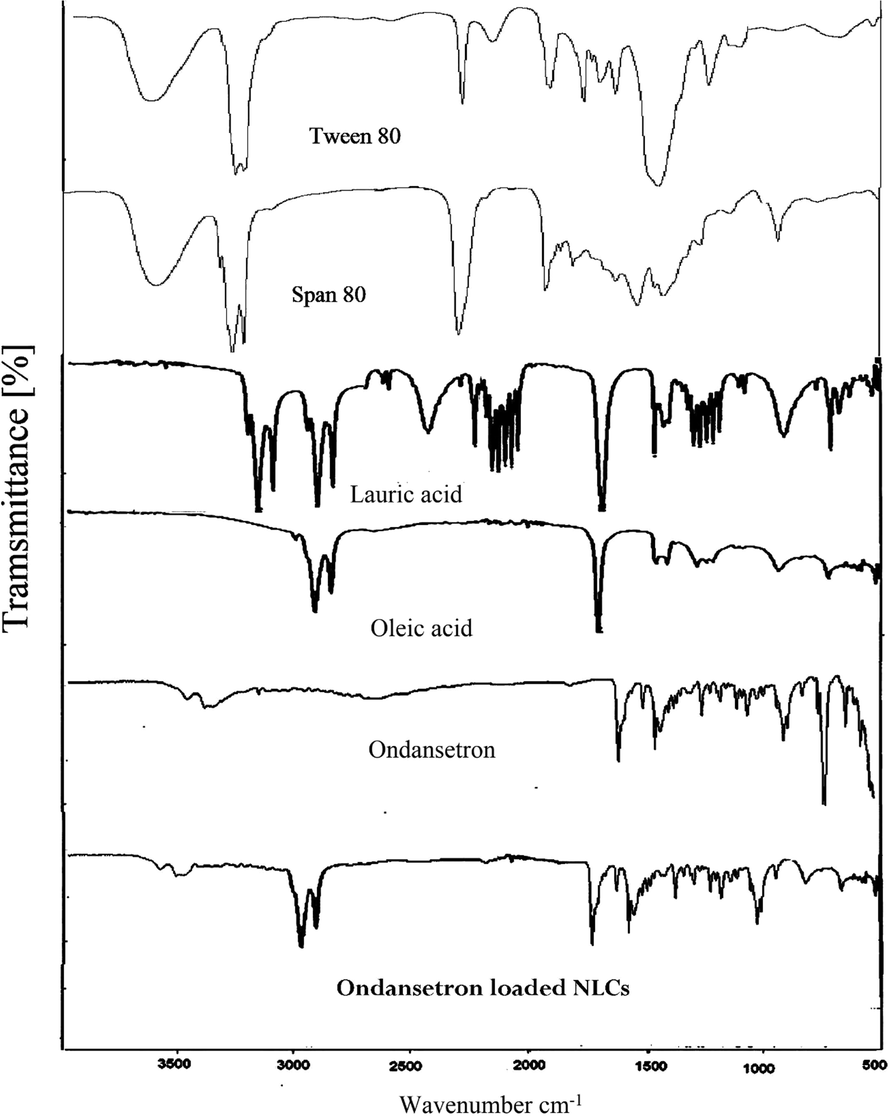
FTIR spectra of lauric acid, oleic acid, ondansetron and ondansetron-loaded NLCs.
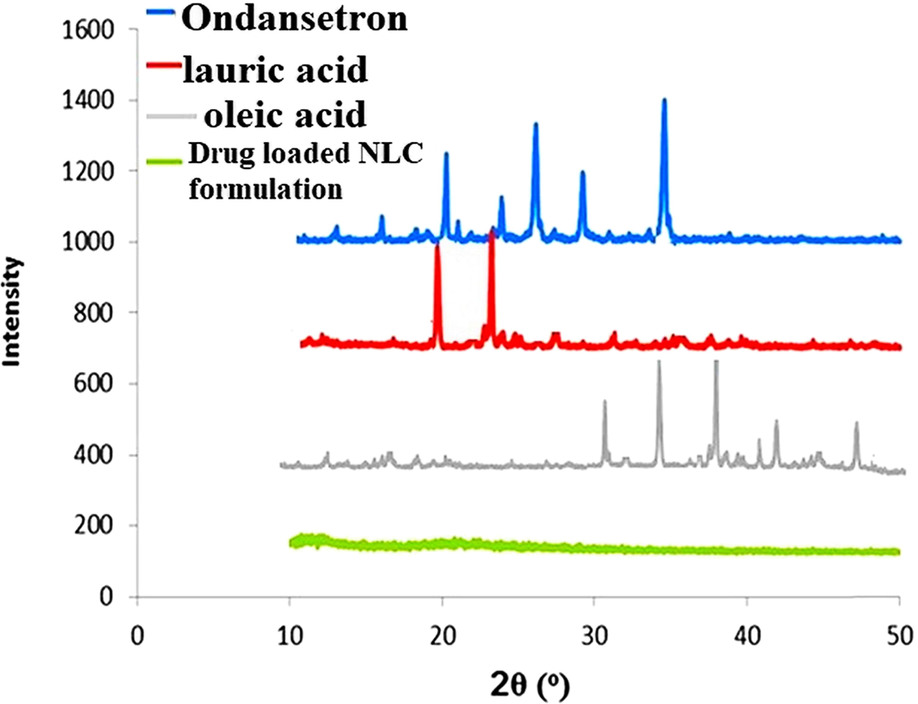
X-ray diffraction analysis of ondansetron, lauric acid, oleic acid, and ondansetron-loaded NLCs.
3.2.5 X-ray diffraction analysis
X-ray diffraction is efficiently utilized to analyze the crystallinity of NLCs. The remarkable difference between diffraction patterns of bulk lipid matrix and drug-loaded NLC, lipids gave sharp peaks which are almost absent in NLCs diffractograms (Fig. 3). Diffraction peaks for ondansetron appeared at 2 theta in the range 19.2°, 22.4°, 25.1°, 29.6°, and 32.1° which indicates its good crystalline nature. Characteristic crystalline diffraction peaks for lauric acid and oleic acid were appeared at 2 theta in the range 20-30° and 30-50°, respectively. NLC formulations possess crystal arrangement is less ordered as compared to pure lipid materials. The amorphous form indicate high loading of drug. Moreover, it is revealed that formulations of NLCs donot exist in crystalline state as disappearance of OND peaks were observed (Abdelbary and Fahmy, 2009; Rehman et al., 2015; Zare et al., 2018).
3.3 Characterization of OND loaded NLC based suppositories
3.3.1 Appearance
The fabricated suppositories are well-fabricated and white or creamy-white with a smooth, shining surface, as illustrated in Fig. 4. No cracks, fissures, or contraction holes were seen after slicing the suppositories longitudinally. The size of suppositories was 28 mm in length and 9 mm in width, which is pediatric size, as shown in Fig. 4.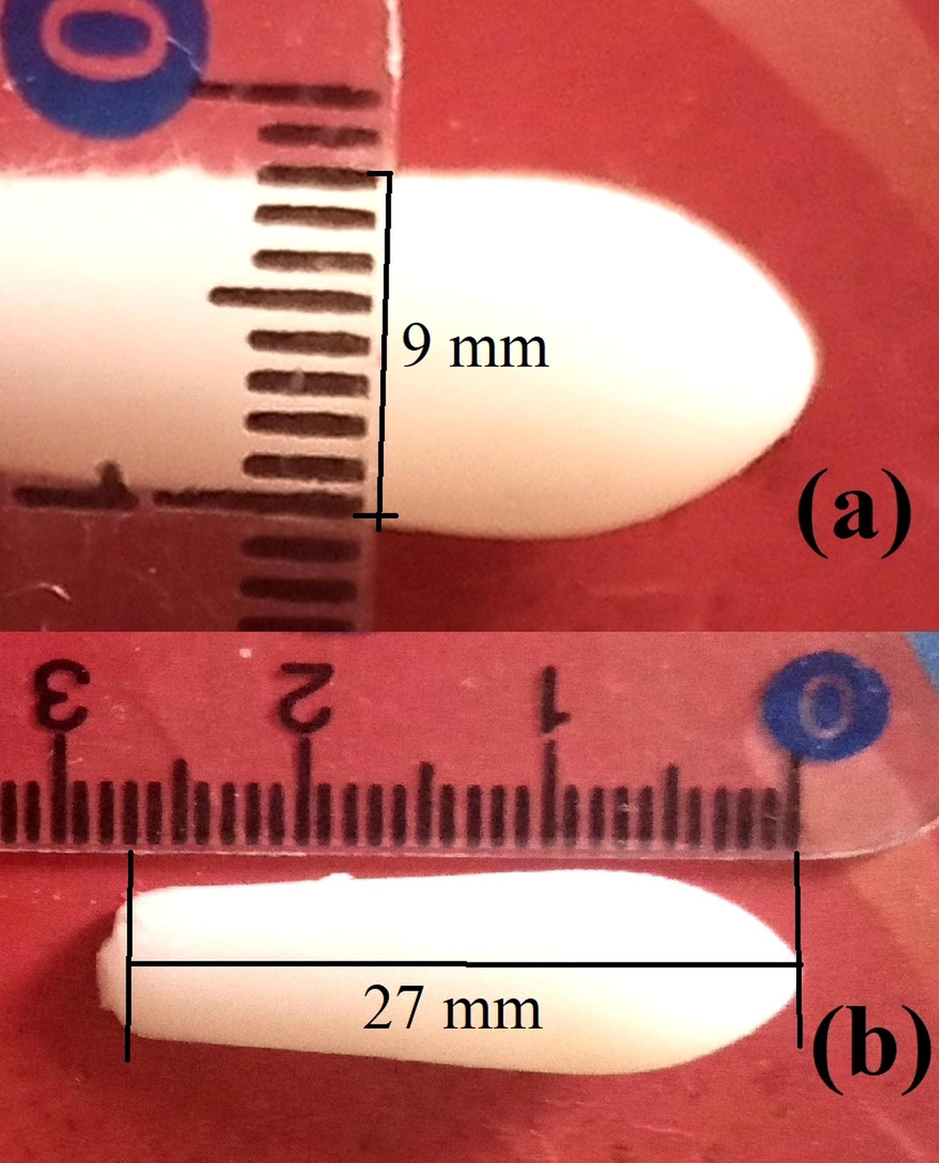
Optical image of NLC based rectal suppositories of ondansetron with scale.
3.3.2 Melting range test
OND-NLC suppositories, each containing 2 mg OND, were formulated using the fusion method, and the melting range was determined (Gold et al., 1988). Witepsol suppositories possess an optimal melting point (35.3–38.3 °C), resulting in a higher release of OND, whereas, the high drug release from the base was observed by lowering the melting point. The optimal melting point of witepsol suppository led to OND release from NLC based suppositories to be nearly consistent with OND invitro release from NLC (Aulton and Taylor, 2017; Lootvoet et al., 1992).
3.3.3 In vitro release profile of OND loaded NLC based suppositories
The dissolution profile of OND loaded NLC suppository depicts biphasic behavior of initial abrupt release, followed by the slow release as illustrated in Fig. 5. Generally, all OND-NLC formulations exhibited a fast release in the starting 2 h that extended almost half of the total OND released from every sample, which may be because of drug-enriched shell surrounding the particles. This portion of the drug is immediately released from formulation because of the short path length of diffusion. This was chased by the extended release of OND due to the slow diffusion of the lipophilic drug from the lipid matrix (Abdelbary and Fahmy, 2009; Lobovkina et al., 2011). A previous study also indicates an initial fast release for 6 h and sustained release 24 h from NLCs (Correia et al., 2020).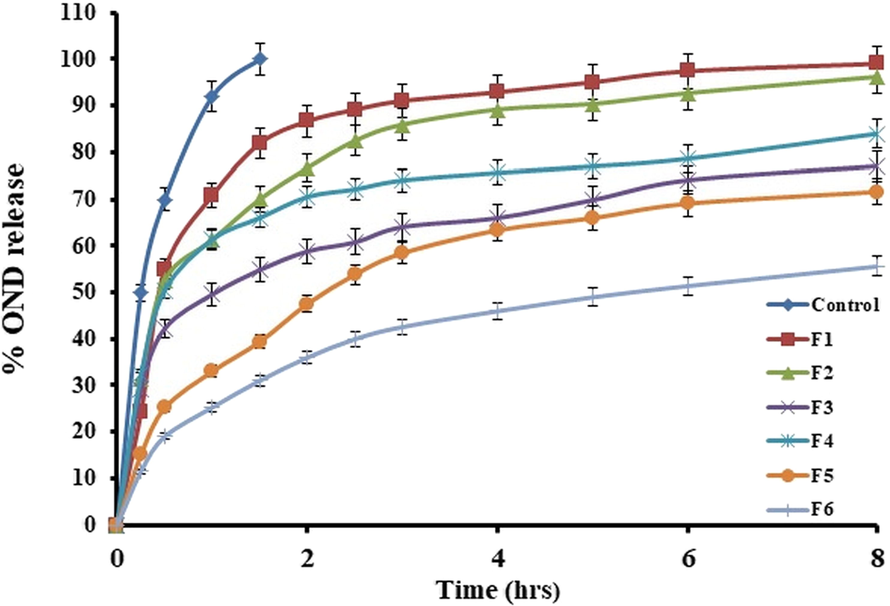
The release profile of ondansetron from different NLCs based suppository formulations.
The release rate from NLCs can be influenced by various parameters that could help to fast release. These complex factors are large surface area, a short diffusion distance for the drug (i.e., the release of drug existing on the outer surface of the nanoparticle), low viscosity in the matrix, high diffusion coefficient due to a small molecular size (Abdelbary and Fahmy, 2009).
In all formulations, more than 70% OND was released in 8 h. The results showed that the F1, F2, F3, F4, F5, and F6 formulations released 99%, 96%, 77%, 84%, 71%, and 51% of the drug, respectively, in almost 8 h. By comparing the release results of NLC formulations, F1 depicted greater release extents, which could be due to the minor particle size of the F1 formulation (101 nm). The available surface area enhances with the decline in particle size, leading to a rise in drug release (zur Mühlen et al., 1998). Formulation F6 has no span 80 but contains more tween 80 (300 mg) as a surfactant which may lead to delayed and retarded release from the NLCs are tween 80 with higher concentration provides extra stability to NLCs.
Some previous reports have observed that the creation of crystals could suppress the drug release from NLCs because the proportion of drugs encapsulated in crystals is not released. However, most of the drug was released in this study due to liquid oleic acid that reduced the crystal formation, and the drug was present in an amorphous form in NLCs (Jenning et al., 2000).
3.3.4 Kinetic modeling of drug release
Different pharmacokinetic models were applied to calculate drug release manner from nanostructured lipid carriers based on suppository. The mechanism and rate of drug release were evaluated by performing kinetic modeling for all formulations, as reported in Table 5. All fabricated samples were best fitted with the Korsmeyer-Peppas model as their values of the regression coefficient (R2) and “n” exponent were between the range of 0.9182–0.9905 and 0.201–0.384, correspondingly. Korsmeyer-Peppas model exhibited the drug release, where “n” (diffusion exponent) defines the procedure of drug release as values for the release exponent (n) were observed to be beneath 0.5, which calculate the diffusion-controlled release from fabricated NLCs and. These are supported by a previous study which also that Fickian diffusion from NLCs (Correia et al., 2020). The correlation coefficient (R2) value was also evaluated to be greater for the Higuchi model. Hence, all formulations were also following the Higuchi model, which demonstrates drug release is diffusion-controlled; although, R2 values did not obey the model flawlessly. It is concluded from the above results that drug release from NLCs was diffusion controlled (Rehman et al., 2015; Mohamed et al., 2013; Elbialy et al., 2015; Barzegar-Jalali et al., 2008).
Formulations
Zero-order
First-order
Higuchi
Korsmeyer-Peppas
R2
R2
R2
R2
N
F1
0.5053
0.9764
0.7467
0.9182
0.244
F2
0.4414
0.7670
0.7103
0.9693
0.250
F3
0.5081
0.5231
0.7964
0.9905
0.238
F4
0.8818
0.6109
0.8330
0.9655
0.201
F5
0.3430
0.8462
0.9406
0.9763
0.384
F6
0.3541
0.7072
0.9504
0.9864
0.384
3.3.5 In vivo pharmacokinetic study
HPLC method was validated before application to the pharmacokinetic studies. Precision accuracy was calculated for intraassay and interassay. The lower limit of quantification was found to be 1 ng/ml and sample recovery was up to 95%. The CV% for intraassay and interassay was found to be within 0.7–5.7%, which is in the acceptable range. Peak areas were calculated for estimation of plasma levels and plasma levels were then plotted against time.
The calculated mean plasma concentration against time for drug achieved after administering oral tablets, IV, and fabricated NLC suppositories is illustrated in Fig. 6. Furthermore, the average pharmacokinetic parameters of all three groups are given in Table 6. The results indicate that the extent of absorption (Cmax: 56.99 ng ml−1) of the drug from an NLC-suppository is more than the control suppository and attains a maximum concentration in eight hours (VanDenBerg et al., 2000). Cmax of NLC loaded suppository is comparable with oral tablet and suppository are only to employed if the oral route is not available, which justifies its importance. The MRT of OND-loaded NLC-suppositories is significantly higher than control suppository confirming the sustained release pattern of NLC suppository (DeVane, 2003). The half-life of the NLC loaded suppository was longer than that of oral, IV and control formulations, indicating the developed formulation's sustained release behavior. AUC of the NLC loaded suppository of OND was higher than that of a control suppository and oral formulation, indicating higher bioavailability by NLC formulation (Teaima et al., 2020). The higher bioavailability of NLC loaded suppository attained makes it more suitable for administration. Overall pharmacokinetic parameters of rectally administered NLC loaded suppository indicates its applicability as an alternative to oral route. *P-value of comparison between control and NLC suppositories. Note. All values are expressed as mean ± S.D. (n = 3). The value of “p” less than 0.05 was considered significant.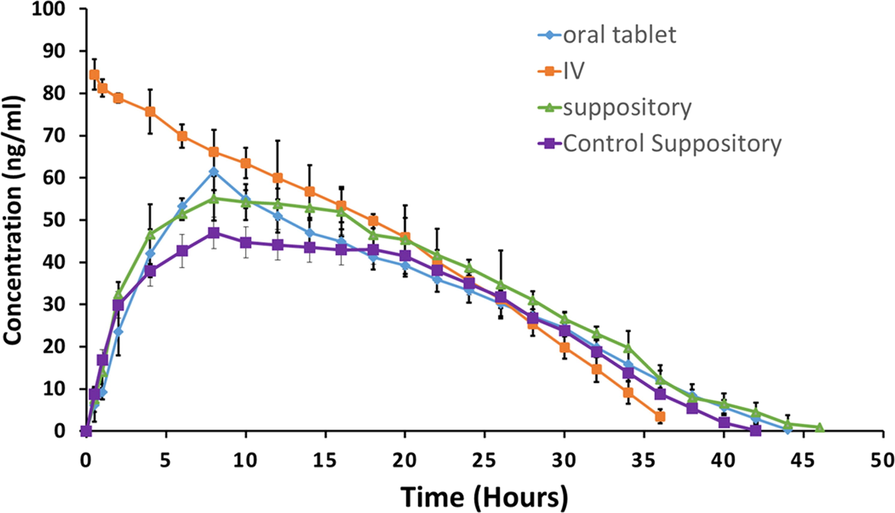
Rabbit plasma concentrations of ondansetron after administration of the fabricated NLC based suppositories, market available oral tablets, and intravenous infusion.
Pharmacokinetic parameters
IV
Oral
NLC Suppositories
Control Suppositories
p-value*
Cmax (ng ml−1)
84.45 ± 2.91
61.44 ± 3.77
55.12 ± 3.75
46.76 ± 2.67
0.008
Tmax (h)
0.5 ± 0.29
8 ± 2.31
8 ± 1.15
8 ± 0.19
1.271
t1/2 (h)
0.75 ± 0.46
0.99 ± 0.44
1.74 ± 0.32
0.72 ± 0.79
0.0003
AUC 0–∞ (ng ml-1h)
1668.05 ± 3.06
1347.14 ± 4.16
1478.29 ± 3.84
1152.25 ± 2.63
0.0014
MRT (h)
13.32 ± 0.96
16.68 ± 0.79
17.12 ± 0.73
14.63 ± 0.41
0.009
4 Conclusion
It was observed that the solvent diffusion technique was successfully implemented to incorporate the antiemetic drug ondansetron into NLCs. The encapsulation efficacy for formulation F1 was found to be about 87% whereas, drug loading was 54%. The in vitro release tests proved the prolongation of the drug release time, where OND-loaded NLC based Suppositories showed slower release than the OND solution-based suppository (control). In vitro release profile followed Higuchi and Korsmeyer-Peppas, whereas Higuchi and Korsmeyer-Peppas kinetics predicted release mechanism as Fickian diffusion. The NLCs based OND suppositories as a resultant dosage form would successfully offer the route by which the OND- loaded NLCs would be administered rectally, enhancing rectal absorption of OND when compared with the control suppository.
Acknowledgments
This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-Track Path of Research Funding Program.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.







