Translate this page into:
Near-infrared optical properties of Cs2KScF6:Cr3+ phosphor for high-power light-emitting diodes
⁎Corresponding authors. jwan@ymu.edu.cn (Jing Wan), q-zhou@ymu.edu.cn (Qiang Zhou)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A near-infrared phosphor of Cs2KScF6:Cr3+ was introduced. The PL thermal stability of Cs2KScF6:Cr3+ was confirmed. The potential use of Cs2KScF6:Cr3+ in high-power light-emitting diode for night vision imaging was assessed.
Abstract
The recent years have witnessed a great leap for near-infrared (NIR) spectroscopy technology in multifunctional application fields. In this study, a double-perovskite structured Cr3+-doped Cs2KScF6 fluoride NIR phosphor with peak location at 778 nm and full-width at half maximum (FWHM) of 95 nm was synthesized by a co-precipitation method. The integral emission intensity at 423 K retains 70 % of that at room temperature (RT), showing good luminescent thermal stability. Combining the obtained phosphor in an NIR phosphor converted light-emitting diode (NIR pc-LED) device, the output power and photoelectric conversion efficiency can reach 72.28 mW and 6.83 % at 320 mA. By virtue of the good optical performance, clear structural images of different fruits were visualized by using the NIR pc-LED as light source, suggesting the potential of Cs2KScF6:Cr3+ phosphor to be used in high-power LED devices.
Keywords
Near-infrared phosphor
Fluoride
Optical properties
Light-emitting diode
1 Introduction
Over the past decades, NIR spectroscopy technique has been extensively studied and emerged in burgeoning fields such as food composition analysis, biological imaging and plant cultivation due to its strong penetration and harmless to the organism (Rajendran et al., 2021; Liu et al., 2023; Huang et al., 2023). In these practical applications, NIR light sources are entrusted the crucial role (Zhang et al., 2022). In spite of the fact that traditional NIR light sources have a broad spectral region, their large volume and short serving lifetime are the leading cause that enable them to lose competitiveness as NIR light sources for portable spectrometers (Liu et al., 2021; Zhou et al., 2020; Chan et al., 2022). Nowadays, pc-LEDs consist of NIR phosphors and blue LEDs exhibit the peculiar superiorities with respect to compactness, high-efficiency and long-lasting, which are also highly anticipated (Wang et al., 2023; Suo et al., 2022). Nevertheless, the photoelectric properties of NIR pc-LED devices mainly lie on the coated NIR luminescent material. Hence, it is of great significance to develop NIR phosphors with excellent luminous properties for the construction of miniature NIR light sources for pc-LED devices.
Currently, the path to obtain NIR-emitting materials is by introducing rare-earth (i.e., Sm3+, Eu2+, Pr3+) or transition metal (i.e., Ni2+, Mn4+, Cr3+) ions into some oxide or fluoride matrices (Yang et al., 2023; Zhou et al., 2018; Jin et al., 2023; Chen et al., 2023). In particular, the transition metal Cr3+ ion with d3 electron configuration is considered as a promising activator on account of its effective absorption in blue-light regions and tunable spectral position based on crystal field engineering (Song et al., 2023). The emission characteristics of Cr3+ ions basically depend on the crystal field strength of the host material. Generally, broadband NIR emission originated from spin-allowed 4T2 → 4A2 transition could be realized when Cr3+ ion is affected by a weak crystal field, whereas narrowband emission derived from spin-forbidden 2E → 4A2 transition will occur under the influence of a stronger coordination field (Liu et al., 2022; Lin et al., 2017). Based on this observation; a series of thermally stable Cr3+-doped fluoride compounds with wide NIR emission bands were developed. For instance, LiMgAlF6:Cr3+ phosphor shows desirable thermal stability (93.9 % at 423 K), but the output power of the LED devices is only 24.3 mW at 300 mA (Hu et al., 2023). Two types of K3Al/GaF6:Cr3+ NIR phosphors present moderate thermal stability, but the conversion efficiency of the LEDs is less than 1 % (Lee et al., 2020). A typical garnet-structured Na3Al2Li3F12:Cr3+ phosphor has almost zero thermal-quenching (99 % at 423 K), but the NIR pc-LED device received a dissatisfactory output power of 14.3 mW (Huang et al., 2021). These NIR phosphors show relatively high thermal stability, but the photoelectric performances of the LED devices are always unsatisfactory. However, the photoelectric properties of LED devices are also worthy of attention since they are crucial parameters to evaluate the optical performance of LED devices. In order to improve the photoelectric properties, researchers have paid great efforts to explore different Cr3+-activated fluoride NIR phosphors, e.g., K2LiGaF6:Cr3+ (106.1 mW at 300 mA) (Li et al., 2024), Cs2KIn1−yAlyF6:Cr3+ (215.88 mW at 320 mA) (Wang et al., 2023). Even though a large number of Cr3+-activated NIR luminescent materials have been reported, the development of Cr3+-doped NIR phosphor used for high-power LEDs that simultaneously meet good thermal stability and favorable photoelectric properties is still desirable.
In this work, Cs2KScF6 was selected to incorporate Cr3+ ions for the synthesis of NIR phosphor with high thermal stability through a co-precipitation method. The crystal structure, bandgap, micro-morphology, as well as luminescent properties of Cs2KScF6:Cr3+ were thoroughly investigated and characterized. The as-prepared phosphor emits NIR light peaking at 778 nm owing to the weak crystal field. Thanks to the excellent thermal stability of Cs2KScF6:Cr3+ (70 % at 423 K), the NIR pc-LED prototype was fabricated by integrating the obtained NIR phosphor into the InGaN chip. Under the drive current of 320 mA, the high output power of 72.28 mW and the photoelectric conversion efficiency of 6.83 % are achieved. Employing this high-power NIR pc-LED device as light source, the potential of Cs2KScF6:Cr3+ phosphor used for night vision imaging was evaluated.
2 Experimental section
Sample Synthesis and LED fabrication. Sc2O3 (Adamas, 99.9 %), CsF (Aladdin, 99.9 %), KF (Aladdin, 99.9 %), HF (Adamas, 40 wt%), Cr(NO3)3·9H2O (Adamas, 99 %) and absolute ethanol (Adamas, 99.7 %) were selected as raw materials and used without additional purification. The precursor of (NH4)3CrF6 was prepared based on the method provided in the literature (Yu et al., 2022). The XRD pattern shown in Fig. S1 of Supplementary Material (SM) demonstrates the pure phase of the precursor. A series of Cs2KScF6:xCr3+ (x = 0.01–0.09) samples were synthesized at RT through a facile co-precipitation method. The specific synthesis procedure is described as follows: firstly, 2.5 mmol of Sc2O3 was weighed and added to a 50 mL plastic tube containing 5 mL of HF solution, and magnetically stirred for 1 h until Sc2O3 was completely dissolved. Then, 12 mmol of KF, 15 mmol of CsF and proportional (NH4)3CrF6 were poured into the above solution in turn and continuously stirred for 8 h. After the reaction, the soluble impurities were removed by washing with absolute ethanol for three times. Finally, the precipitates were dried at 80 °C for 6 h in an oven to obtain the Cs2KScF6:xCr3+ (x = 0.01, 0.03, 0.05, 0.07, 0.09) products.
The LED device was prepared by coating the as-prepared Cs2KScF6:Cr3+ phosphor on the surface of a commercially available blue InGaN chip (San'an Optoelectronics Co., Ltd, ∼ 450 nm, 3 W). To be specific, the phosphor was firstly mixed with a UV structural adhesive (Shenzhen Tegu New Materials Co., Ltd.) at a mass ratio of 1:1, and then coated on the surface of a blue LED chip, finally dried at 120 °C for 1 h for the fabrication of NIR pc-LED device.
Characterization and theoretical computation. The phase purity of Cs2KScF6:xCr3+ samples was substantiated by X-ray diffraction (XRD) patterns obtained from a Bruker D8 Advance powder diffractometer with Cu Kα radiation (λ = 1.5406 Å). The operating voltage, current, scanning rate, angular range, and step size were 40 kV, 40 mA, 10°/min, 10°-70°, and 0.02°, respectively. The powder diffraction data used for Rietveld refinement were tested on the same diffractometer with a scanning rate of 1°/min and an angular range of 5°-120°. The morphology and chemical composition were analyzed from a FEI Nova NanoSEM 450 scanning electron microscope (SEM) equipped with an energy-dispersive spectrometer (EDS) attachment. Electron paramagnetic resonance (EPR) spectra were examined on a Bruker EMXplus-6/1 spectrometer. The X-ray photoelectron spectroscopy (XPS) was collected from a Thermo Scientific K-Alpha XPS system. The internal quantum efficiency (IQE), external quantum efficiency (EQE), as well as decay curves were recorded from a steady-state fluorescence spectrophotometer (FLS1000, Edinburgh Instrument) with an additional integrating sphere. The photoluminescence excitation (PLE) and photoluminescence (PL) spectra were obtained using an AVANTES Avaspec Mini 2048CL-SHB3 fiber spectrophotometer. Thermal quenching behavior was conducted on the same spectrophotometer with a temperature-control instrument and sample cooling/heating stage. The moisture resistance test was carried out in a BPS-50CL constant temperature and humidity chamber with a temperature of 85 °C and a humidity of 85 %. The electroluminescent (EL) spectra of the packed NIR pc-LEDs were executed by an OHSP-350 M LED fast-scan spectrophotometer (Hangzhou Hopoo Light & Color Technology Co., Ltd.). The thermographs and working temperatures of the LED devices recorded under drive current of 200 mA were acquired through a thermal imager (FLIR E8). An industrial camera (Canon XA10E) loaded with natural and NIR modes was used to shoot the demonstration images.
The General Structure Analysis System (GSAS) software was used to perform the Rietveld refinement for XRD data. Visualization for Electronic and Structural Analysis (VESTA Ver. 3.4.4) software was applied to construct crystal structure of the Cs2KScF6 host. Based on the density functional theory (DFT), the electronic structure of Cs2KScF6 were assessed by a CASTEP module of the Materials Studio package. The PBE functional was employed to approximate the exchange–correlation potential. The cutoff energy and k-point mesh were set as 300 eV and 3 × 3 × 3 Monkhorst-Pack grid, respectively. The electronic convergence criterion was10-5 eV, and the atomic positions and lattice parameters were relaxed to 0.02 eV/Å.
3 Results and discussion
The XRD patterns of a series of Cs2KScF6:xCr3+ (x = 0.01–0.09) samples with various doping concentrations and standard diffraction card (JCPDS No. 21–0851, cubic Fm
m space group) are shown in Fig. 1a, in which all the XRD diffraction peaks are in good agreement with the standard JCPDS card, indicating that the five samples have the same pure phase as the host material and the incorporation of Cr3+ ions does not change the cubic structure of Cs2KScF6 host (Wu et al., 2023). The role of Cr3+ ion has changed with the increasing doping concentration from x = 0.01 to x = 0.09, specifically from the dopant to a key composition of Cs2KScF6 matrix, manifesting that the target Cs2KScF6:xCr3+ (x = 0.01–0.09) phosphors have been synthesized.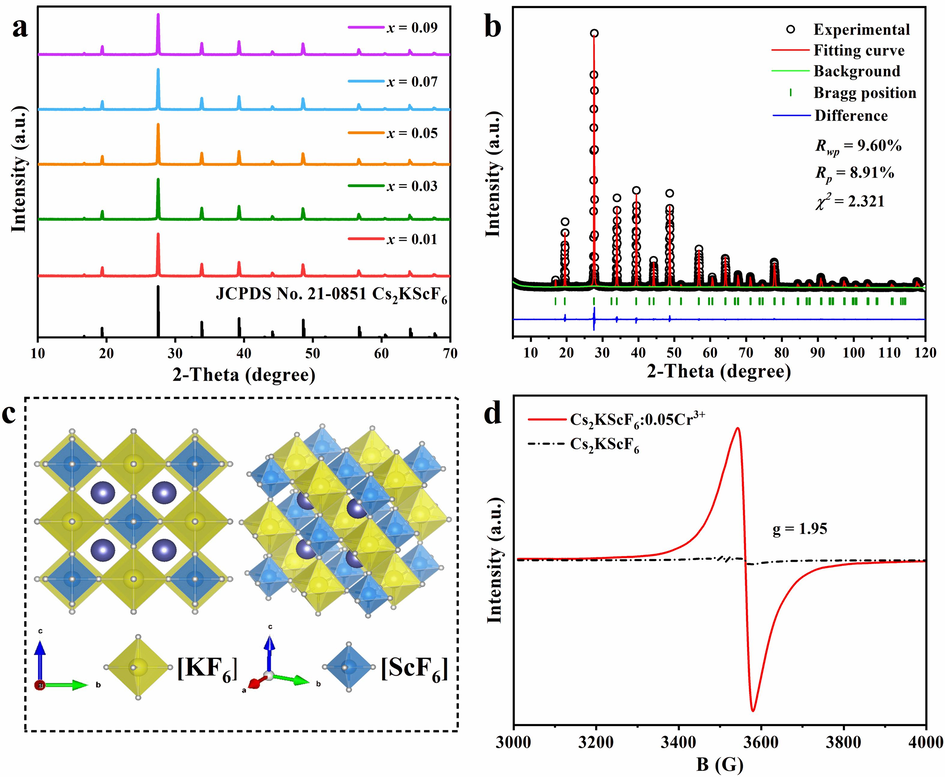
(a) XRD patterns of Cs2KScF6:xCr3+ (x = 0.01–0.09) samples. (b) Refined XRD results of Cs2KScF6:0.05Cr3+ product. (c) Crystal structure diagram of Cs2KScF6 host. (d) EPR spectra of Cs2KScF6:0.05Cr3+ sample and Cs2KScF6 host.
The XRD diffraction data of Cs2KScF6:0.05Cr3+ sample in the angular range of 5-120° are employed to carry out Rietveld structure refinement by the GASA software for verifying the structural changes after incorporating Cr3+ into the host. Fig. 1b shows the structural refinement pattern obtained by the Rietveld method, the reliable residual factors are RWP=9.60 %, RP=8.91 % and χ2 = 2.321 for Cs2KScF6:0.05Cr3+ product, which further indicate that the crystal structure remains unchanged even though Cr3+ ions were introduced (Li et al., 2021). The detailed crystallographic parameters of Cs2KScF6 host and refined parameters of Cs2KScF6:0.05Cr3+ phosphor are listed in Table S1; and the built crystal structure diagram is shown in Fig. 1c. In the cubic structure, Sc3+ and K+ are coordinated with six F- ions to form [ScF6] and [KF6] octahedron cages, while Cs+ is filled in the cavity surrounded by four [ScF6] and four [KF6] octahedrons, they are alternately connected by sharing a common vertex to establish a double-perovskite structure. According to the substitution rule of the same valence state and close ion radius, Cr3+ (r6-coor. = 0.615 Å) tends to occupy the lattice site of Sc3+ (r6-coor. = 0.745 Å) (Qing et al., 2022). Furthermore, the partial replacment of small Cr3+ for large Sc3+ site induces the lattice shrinkage and the decreasing of interplanar spacing, resulting in the unit cell volume of Cs2KScF6:Cr3+ sample being smaller than that of Cs2KScF6 matrix (Zhang et al., 2022). Specifically, the lattice parameter and unit cell volume of the undoped matrix are a = 9.33 Å, and V=812.21 Å3 respectively, while the doped sample has reduced to 9.19 Å and 776.96 Å3 (Table S1).
As known, Cr3+ ion has three d-electrons in the unfilled 3d shell with a total spin of S=3/2. When the Cr3+ ion enters the octahedral site and connects with six neighbouring ions to form an [CrF6] octahedron, the ground state 4F term divides into 4A2g, 4T1, and 4T2 sublevels, and then the EPR signals can be observed. As shown in Fig. ld, the sharp EPR signal with g-factor of 1.95 verifies the presence of Cr3+ ions that locate in the octahedral sites (Miao et al., 2021). On the contrary, no EPR peak appears in the blank reference of the Cs2KScF6 matrix. Moreover, the XPS spectrum also confirms the signal that comes from chromium ion in the obtained Cs2KScF6:0.05Cr3+ sample (Fig. 2a). It is well-known that the chromium ions have mutiple valence states, thus the high-resolution spectrum of Cr 2p was used to verify its valance state (Fig. 2b). Apparently, the double peaks at 578.7 eV and 588.8 eV belong to Cr 2p3/2 and Cr 2p1/2 orbits in Cr3+-doped Cs2KScF6 compound, which strongly support the conclusion that chromium ion exists in trivalent state and only Cr3+ ion devotes to the NIR luminescence in this case (Xiahou et al., 2021).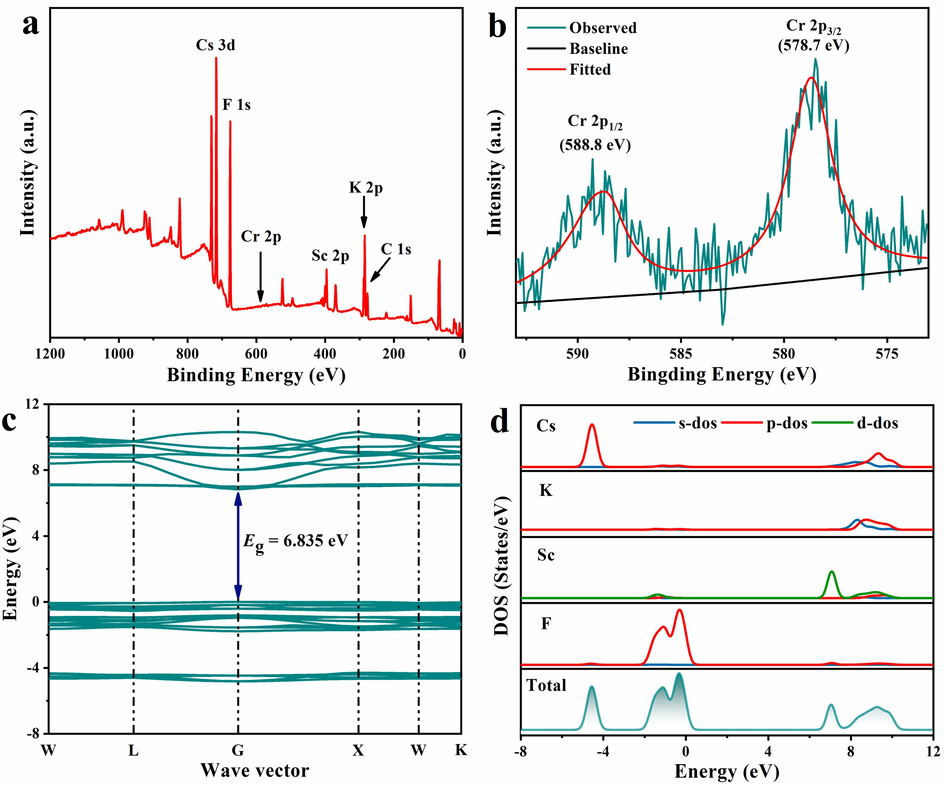
(a) XPS spectrum of Cs2KScF6:0.05Cr3+ phosphor. (b) High-resolution Cr-2p XPS spectrum. (c, d) Calculated band structure, partial and total densities of states of Cs2KScF6 host.
The SEM image exhibits that Cs2KScF6:Cr3+ phosphor crystallizes in regular particulate shape, and the smooth surfaces and an average size of 2 μm imply the high crystallinity of Cs2KScF6:Cr3+ (Fig. S2a). The EDS results and element mapping diagram (Fig. S2b and S2c) demonstrate that Cr3+ ions are uniformly dispersed throughout the sample particles. Based on the first-principles calculations and electron configurations of Cs (5p66s1), K (3p64s1), Sc (3d14s2), F (2s22p5), the electronic structure of Cs2KScF6 host was calculated (Fig. 2c and 2d). The flat valence band and dispersive conduction band indicate that the host material has a direct bandgap, which is beneficial to realizing band-to-band luminescence because of its higher PL transition probability toward the indirect one (Ming et al., 2019). The wide bandgap value (6.835 eV) is not only preferable to incorporate Cr3+ energy levels, but also is effective to hinder thermal ionization and maintain good thermal stability (Chu et al., 2023). The valence band top is mainly dominated by F-2p orbitals; while the conduction band bottom is donated from Sc-3d orbitals, indicating that Sc-3d and F-2p orbitals partially hybridize to form Sc-F bond, and thus affecting luminescent properties of Cs2KScF6:Cr3+ (Fig. 2d) (Nie et al., 2021).
Fig. 3a expounds the PLE and PL spectra of Cs2KScF6:0.05Cr3+ phosphor monitored at RT. Two absorption bands located at ∼ 435 and ∼ 657 nm are observed in the PLE spectrum, which correspond to Cr3+:4A2 → 4T1(4F) and 4T2(4F) spin-allowed transitions, respectively (Trueba et al., 2011). The stronger blue excitation band around 435 nm (FWHM=78 nm) perfectly covers the emission band of commercial InGaN chip (∼20 nm) (Qing et al., 2024), indicating the resultant Cs2KScF6:Cr3+ phosphor can be effectively pumped by blue InGaN chip. Moreover, two obvious dips were inspected from the 4A2 → 4T2 transition, which are mainly due to the destructive interference of strongly coupled 4T2 levels on weakly coupled 2E/2T1 levels, resulting in the reduction of excitation peak and the generation of other peaks (Nie et al., 2022). The PL spectrum presents a wide NIR emission band peaking at 778 nm (FWHM=95 nm), which is assignable to the Cr3+:4T2 → 4A2 transition in a weak octahedral crystal field (Adachi, 2020). According to the Tanabe-Sugano diagram (Fig. S3), Cr3+ emits broad NIR emission from 4T2 to 4A2 in a weak coordination field with Dq/B<2.3, whereas produces sharp line or narrowband emission from 2E to 4A2 in a strong coordination environment (Zhou et al., 2022). Referring to the two excitation peak locations of 435 and 657 nm from PLE spectrum, the Dq, B and Dq/B values were calculated to be 1522 cm−1, 875 cm−1 and 1.74 (eqn (S1) and (S2) in the SM), respectively. The above results reveal that Cr3+ ions experience a weak crystal field in Cs2KScF6 to present NIR emission, which is similar to the luminescence behavior of Cr3+-activated fluoride phosphors reported previously (Wang et al., 2023).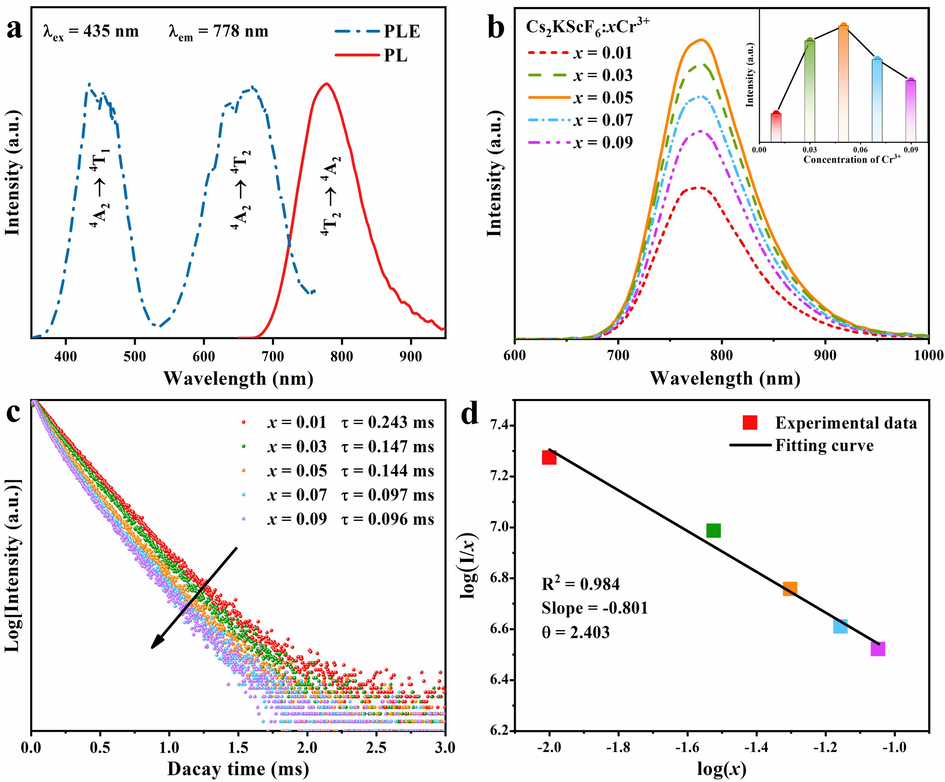
(a) PLE and PL spectra of Cs2KScF6:0.05Cr3+ phosphors recorded at RT. (b) Concentration-dependent PL spectra of Cs2KScF6:xCr3+ (x = 0.01–0.09) samples and the inset presents the relationship between PL intensity and Cr3+ concentration. (c) PL decay curves of Cs2KScF6:xCr3+ phosphors. (d) Relationship between log(I/x) and log(x).
For optimizing the luminescent quality of the as-obtained phosphors, Cs2KScF6:xCr3+ (x = 0.01–0.09) samples with various Cr3+ contents were prepared, and the corresponding PL spectra were measured (Fig. 3b). As the rising of Cr3+ doping content, the integral NIR emission intensity augments continuously until the critical doping level x = 0.05. Affected by concentration quenching effect, the intensity starts to decrease when x value was further increased (Zhang et al., 2022). Thus, the optimal Cr3+ content is fixed as x = 0.05 (the inset in Fig. 3b). The PL decay curves reveal the concentration quenching mode of Cs2KScF6:Cr3+ phosphor (Fig. 3c). All the decay curves live up to a single exponential equation, indicating that Cr3+ ions replaced the single site of Sc3+ in Cs2KScF6 host (Yuan et al., 2023). The average lifetime fitted by eqn (S3) is gradually dropped from 0.243 ms to 0.096 ms with the increase of x value, attributing to the intensified energy transfer among Cr3+-Cr3+ pairs. Based on the Blass formula given in eqn (S4), the critical distance (Rc) between adjacent Cr3+ ions was estimated as 19.51 Å in this case, which indicates multipolar interaction may be the reason for the non-radiative energy transfer in Cs2KScF6:Cr3+ phosphor (Wang et al., 2022). According to the Dexter theory, the fitting results obtained from eqn (S5) of log(I/x) against log(x) are provided (Fig. 3d), the corresponding θ value is equal to 2.403 (close to 3). One conclusion can be drawn that the energy transfer among the nearest or next nearest Cr3+ ions leads to the concentration quenching of Cr3+ in Cs2KScF6 host (Dai et al., 2019). Under such circumstance, the IQE and EQE values of the optimal Cs2KScF6:0.05Cr3+ sample is measured as 47.7 % and 8.25 %, manifesting that its prospect in NIR pc-LED devices. The integrated emission intensity of Cs2KScF6:0.05Cr3+ phosphor can preserve 84.1 % of the initial value after aging for 36 h under a high temperature of 85 ℃ and a high humidity of 85 % conditions, and still remain 74.9 % after being aged for 132 h, indicating that the as-prepared material has strong moisture resistance (Fig. S4).
The temperature-dependent PL spectra of Cs2KScF6:0.05Cr3+ measured from 298 K to 473 K are depicted in Fig. 4a, in which the PL intensity continuously diminishes along with the temperature goes up. It could be observed that 70 % of initial intensity is preserved at 423 K, strongly suggesting that the Cs2KScF6:Cr3+ phosphor possesses good luminous thermal stability (the red dot in Fig. 4b). Besides, the red-shift tendency of spectral position appears as elevated temperature, which could be traced to lattice expansion and decreasing crystal field strength at higher temperatures (Fig. S5) (Liu et al., 2022; Zhang et al., 2023). The FWHM enlarges significantly from 95 nm to 111 nm (the blue dot in Fig. 4b), which is imputed to intensified electron–phonon coupling (EPC) effect that takes the Huang-Rhys factor (S) as an evaluation parameter. Fig. 4c shows the fitting relationship of FWHM2 versus 2kT referring to eqn (S6), a trustworthy correlation coefficient of R2 = 0.987 and S value of 1.68 indicate that Cs2KScF6:Cr3+ phosphor is subjected to a relatively low EPC effect to resist non-radiative relaxation process, as a result, achieving well-pleasing PL thermal stability.![(a) Temperature-dependent PL spectra of Cs2KScF6:0.05Cr3+ phosphor from 298 K to 473 K. (b) The relationship between temperature and relative PL intensity, FWHM value. (c, d) The fitting results of FWHM2 versus 2kT and ln[(I0/IT)-1] versus 1/kT. (e) Configurational coordinate diagram for transition mechanism of Cr3+ ions.](/content/184/2024/17/9/img/10.1016_j.arabjc.2024.105921-fig4.png)
(a) Temperature-dependent PL spectra of Cs2KScF6:0.05Cr3+ phosphor from 298 K to 473 K. (b) The relationship between temperature and relative PL intensity, FWHM value. (c, d) The fitting results of FWHM2 versus 2kT and ln[(I0/IT)-1] versus 1/kT. (e) Configurational coordinate diagram for transition mechanism of Cr3+ ions.
A configuration coordinate diagram (Fig. 4e) could be used to illustrate the thermal-quenching mechanism of Cs2KScF6:Cr3+ phosphor. The energy difference from the bottom of 4T2 state to the intersection point of 4A2 and 4T2 levels (C point) is determined as the activation energy (ΔE). The electrons of 4A2 level are excited to the high-energy 4T2 level under blue light illumination, and then most of them will return to the low-energy state in the form of NIR emission. If the temperature continues to rise, the electrons in the 4T2 excited state could be further thermally excited to C point and then directly fall back to the ground state through non-radiative transition. During this transition process, energy consumption happens because of thermal activation and multi-phonon effects. To further explain the thermal quenching behavior of the Cs2KScF6:Cr3+ phosphor, the ΔE value was calculated by eqn (S7) (Li and Zhong, 2022). As shown in Fig. 4d, a linear relationship with a slope of −0.340 is plotted by employing ln[(I0/IT)-1] as a function of 1/kT, and the ΔE value is estimated to be 0.340 eV. Large activation energy demonstrates that the energy barrier is difficult to be crossed to induce non-radiative relaxation, resulting in the desirable PL thermal stability of Cs2KScF6:Cr3+ phosphor. Therefore, the large ΔE value contributes to reducing the non-radiative transition probability and inducing the low thermal quenching, and finally achiving a favorable PL thermal stability (Yan et al., 2022).
Given the excellent PL properties as well as low thermal-quenching behavior of Cs2KScF6:Cr3+ phosphor, an NIR pc-LED prototype was fabricated to evaluate the device performance of the synthesized material. Fig. 5a shows the EL spectra of the NIR pc-LED device measured at various drive currents ranging from 20 mA to 320 mA. In the whole spectra range, a primary NIR emission that comes from Cs2KScF6:Cr3+ phosphor and a weak blue emission that originates from InGaN chip can be clearly observed. Moreover, the growing drive current boosts a stable rise in NIR emission intensity without saturation, demonstrating that the Cs2KScF6:Cr3+ phosphor has great potential for high-power NIR pc-LED application. Fig. 5b presents the NIR output power and photoelectric conversion efficiency of NIR pc-LED devices against drive currents. Increasing the drive current from 20 mA to 320 mA, the output power monotonously enhances from 4.70 mW to 72.28 mW, superior to photoelectric performance of many reported NIR pc-LED devices (Table S2). Meanwhile, owing to the efficiency degradation of the blue LED chip, the conversion efficiency changes from 8.86 % to 6.83 %, which is also higher than most NIR phosphors reported so far (Table S2). For confirming the stability of the as-obtained phosphor in high-power LEDs, its dynamic process was monitored in real-time using an NIR thermal imager. The corresponding thermographs and working temperatures of the NIR pc-LED device operated at 200 mA for different irradiation times are presented in Fig. S6 and Fig. 5c, respectively. The operating temperature fluctuates around 42 °C at a constant current of 200 mA, which shows that the phosphors have good thermal conductivity and stability, the potential application for Cs2KScF6:Cr3+ phosphor could be found in high-power pc-LEDs.
(a) EL spectra of the fabricated NIR pc-LED measured at various drive currents in the range of 20–320 mA. (b) NIR output power and photoelectric conversion efficiency of NIR pc-LED device under different drive currents. (c) Working temperatures of NIR pc-LED device operating at 200 mA for different irradiation times. (d) Photographs of the fruits under natural and NIR light irradiation.
Benefiting from the strong penetration of NIR light and the excellent luminescent properties of the developed NIR phosphor, the application demonstrations were implemented in night vision by using the LED device manufactured with Cs2KScF6:Cr3+ phosphor as light source. Fig. 5d displays images of the fruits taken by an industrial camera under natural and NIR modes. Compared with the colorful fruit photos taken in natural light, when lighting the NIR pc-LED lamp, the recognizable black-and-white photos of fruit that cannot be observed at night were also captured by the NIR camera. The above demonstration results confirm application potential of the Cs2KScF6:Cr3+ phosphor for high-power LED devices in the field of night vision.
4 Conclusion
In summary, a fluoride Cs2KScF6:Cr3+ NIR-emitting phosphor with double-perovskite structure was presented in this work. The Cs2KScF6:Cr3+ sample launches NIR light peaking at 778 nm (FWHM=95 nm) from 4T2 → 4A2 spin-allowed transition of Cr3+ activators. In view of the weak EPC effect, Cs2KScF6:Cr3+ phosphor shows good anti-thermal quenching behavior (70 % at 423 K). Coating the obtained phosphor on the surface of the InGaN chip to fabricate NIR pc-LED device, high output power of 72.28 mW and photoelectric conversion efficiency of 6.83 % at 320 mA drive current were achieved. This device was used as a light source to evaluate its prospect in night vision imaging, indicating the Cs2KScF6:Cr3+ phosphor have great potential in high-power LED devices.
CRediT authorship contribution statement
Lingxiang Chu: Writing – original draft, Methodology, Investigation, Data curation. Enbei Yang: Writing – review & editing. Han Ma: Writing – review & editing. Jing Wan: Writing – review & editing, Supervision, Conceptualization. Tao Yang: Writing – review & editing. Yi Qin: Writing – review & editing. Yanqing Ye: Writing – review & editing. Qiang Zhou: Writing – review & editing, Supervision, Resources, Project administration. Zhengliang Wang: Writing – review & editing.
Acknowledgments
The authors appreciate the financial support from the National Natural Science Foundation of China (22365034 and 22065039).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Racah parameter ratio C/B for the 3d3 -configuration ions like Mn4+ and Cr3+ in the Tanabe-Sugano Diagram. ECS J. Solid State Sci. Technol.. 2020;9:066003
- [Google Scholar]
- The optical research progress of nanophosphors composed of transition elements in the fourth period of near-infrared windows I and II for deep-tissue theranostics. Nanoscale. 2022;14:7123-7136.
- [Google Scholar]
- Stable polyhedron-cluster-based rigid fluoride framework toward zero-thermal-quenching near-infrared emission for prolonged LED applications. Inorg. Chem.. 2023;62:7964-7975.
- [Google Scholar]
- Near-infrared luminescence and high thermal stability of Rb2NaScF6:Cr3+ phosphor for spectroscopy applications. Mater. Adv.. 2023;4:4583-4589.
- [Google Scholar]
- Broad band emission near-infrared material Mg3Ga2GeO8:Cr3+: substitution of Ga-In, structural modification, luminescence property and application for high efficiency LED. J. Alloy. Compd.. 2019;806:926-938.
- [Google Scholar]
- A highly thermo-stable far-red LiMgAlF6:Cr3+ phosphor for plant-growth lighting. J. Lumin.. 2023;263:120095
- [Google Scholar]
- An efficient garnet-structured Na3Al2Li3F12:Cr3+ phosphor with excellent photoluminescence thermal stability for near-infrared LEDs. Chem. Eng. J.. 2021;426:131332
- [Google Scholar]
- Near-infrared windows I and II phosphors for theranostic applications: Spectroscopy, bioimaging, and light-emitting diode photobiomodulation. Adv. Opt. Mater.. 2023;11:2202061.
- [Google Scholar]
- A new persistent luminescence phosphor of ZnGa2O4:Ni2+ for the second near-infrared transparency window. J. Alloy. Compd.. 2023;931:167491
- [Google Scholar]
- Chromium(III)-doped fluoride phosphors with broadband infrared emission for light-emitting diodes. Inorg. Chem.. 2020;59:376-385.
- [Google Scholar]
- Y. Li, J. Zuo, T. Zheng, S. Yang, P. He, J. Zhang, S. Liu, F. Yang, J. Peng, X. Ye, Mn4+, Cr3+ co-doped K2LiGaF6 phosphors with bright near-infrared luminescence, Adv. Opt. Mater. (2024) 2400171.
- Thermally stable CaLu2Mg2Si3O12:Cr3+ phosphors for NIR LEDs. Adv. Opt. Mater.. 2021;9:2100388.
- [Google Scholar]
- Highly efficient broadband near-infrared luminescence with zero-thermal-quenching in garnet Y3In2Ga3O12:Cr3+ phosphors. Chem. Mater.. 2022;34:8418-8426.
- [Google Scholar]
- Site occupancy and near-infrared luminescence in Ca3Ga2Ge3O12:Cr3+ persistent phosphor. Adv. Opt. Mater.. 2017;5:1700227.
- [Google Scholar]
- Simultaneous broadening and enhancement of Cr3+ photoluminescence in LiIn2SbO6 by chemical unit cosubstitution: Night-vision and near-infrared spectroscopy detection applications. Angew. Chem. Int. Ed.. 2021;60:14644-14649.
- [Google Scholar]
- Valence conversion and site reconstruction in near-infrared-emitting chromium-activated garnet for simultaneous enhancement of quantum efficiency and thermal stability. Light Sci. Appl.. 2023;12:248-260.
- [Google Scholar]
- Structural rigidity control toward Cr3+-based broadband near-infrared luminescence with enhanced thermal stability. Chem. Mater.. 2022;34:1376-1384.
- [Google Scholar]
- Broadband short-wave infrared light-emitting diodes based on Cr3+-doped LiScGeO4 phosphor. ACS Appl. Mater. Interfaces.. 2021;13:36011-36019.
- [Google Scholar]
- An ultra-high yield of spherical K2NaScF6:Mn4+ red phosphor and its application in ultra-wide color gamut liquid crystal displays. J. Mater. Chem. C. 2019;7:7237-7248.
- [Google Scholar]
- Cr3+-activated Na3X2Li3F12 (X = Al, Ga, or in) garnet phosphors with broadband NIR emission and high luminescence efficiency for potential biomedical application. J. Mater. Chem. C. 2021;9:15230-15241.
- [Google Scholar]
- Ultra-broad NIR emission from two-site Cr3+ occupation in fluoride phosphor via composition-driven structural transformation. Mater. Today Chem.. 2022;26:101164
- [Google Scholar]
- Luminescence properties of Cr3+-doped near-infrared emissive fluoroyttrates for light-emitting diodes. Dalton Trans.. 2022;51:14214-14220.
- [Google Scholar]
- Rational design for broad near-infrared emission from a two-sited Rb2LiAlF6:Cr3+ phosphor with high efficiency and thermal stability for spectroscopic applications. Inorg. Chem. Front.. 2024;11:2718-2725.
- [Google Scholar]
- Chromium ion pair luminescence: A strategy in broadband near-infrared light-emitting diode design. J. Am. Chem. Soc.. 2021;143:19058-19066.
- [Google Scholar]
- Ultra-broad near-infrared emitting phosphor LiInF4:Cr3+ with extremely weak crystal field. Inorg. Chem.. 2023;62:11112-11120.
- [Google Scholar]
- Rapid nondestructive detection enabled by an ultra-broadband NIR pc-LED. Laser & Photonics Rev.. 2022;16:2200012.
- [Google Scholar]
- Cr3+-doped fluorides and oxides: role of internal fields and limitations of the Tanabe-Sugano approach. J. Phys. Chem. a.. 2011;115:13399-13406.
- [Google Scholar]
- K2LiScF6:Cr3+/Mn4+ fluoride phosphor with enhanced broadband emission for NIR pc-LEDs. Dalton Trans.. 2023;52:11071-11078.
- [Google Scholar]
- Ion substitution strategy toward high-efficiency near-infrared photoluminescence of Cs2KIn1–yAlyF6:Cr3+ solid solutions. J. Phys. Chem. Lett.. 2023;14:1371-1378.
- [Google Scholar]
- Two-site occupation in Cr3+-activated BaIn2(P2O7)2 phosphor for broadband near-infrared thermometry and LED applications. Mater. Res. Bull.. 2023;163:112222
- [Google Scholar]
- Design, luminescence properties and applications of Cr3+-doped ScTaO4: A broadband near-infrared phosphor. Dalton Trans.. 2022;51:16325-16335.
- [Google Scholar]
- The co-optimization of efficiency and emission bandwidth in GSAGG:Cr3+ NIR ceramic phosphors. Ceram. Int.. 2023;49:21688-21694.
- [Google Scholar]
- Local structure regulation in near-infrared persistent phosphor of ZnGa2O4:Cr3+ to fabricate natural-light rechargeable optical thermometer. ACS Appl. Electron. Mater.. 2021;3:3789-3803.
- [Google Scholar]
- Photoluminescence properties of AScSi2O6:Cr3+ (A = Na and Li) phosphors with high efficiency and thermal stability for near-infrared phosphor-converted light-emitting diode light sources. ACS Appl. Mater. Interface.. 2022;14:8179-8190.
- [Google Scholar]
- Recent progress in inorganic afterglow materials: Mechanisms, persistent luminescent properties, modulating methods, and bioimaging applications. Adv. Opt. Mater.. 2023;11:2202382.
- [Google Scholar]
- Green HF-free synthetic route to the high-efficiency K2NaGaF6:Cr3+ phosphor and its NIR-LED application toward veins imaging. ACS Sustain. Chem. Eng.. 2022;10:8022-8030.
- [Google Scholar]
- La3Ga5SiO14:Cr3+: A broadband near-infrared phosphor induced by the two-site occupation of Cr3+. Ceram. Int.. 2023;49:18000-18006.
- [Google Scholar]
- Highly distorted Cr3+-doped fluoroantimonate with high absorption efficiency for multifunctional near-infrared spectroscopy applications. Mater. Today Chem.. 2022;26:101194
- [Google Scholar]
- Thermally robust broadband near-infrared luminescence in the NaGaP2O7:Cr3+ phosphor. ACS Appl. Opt. Mater.. 2023;1:85-93.
- [Google Scholar]
- A novel Cr3+-activated far-red titanate phosphor: synthesis, luminescence enhancement and application prospect. Mater. Today Chem.. 2022;24:100835
- [Google Scholar]
- Blue LED-pumped intense short-wave infrared luminescence based on Cr3+-Yb3+-co-doped phosphors. Light Sci. Appl.. 2022;11:136-148.
- [Google Scholar]
- Crystal-field induced tuning of luminescence properties of Na3GaxAl1−xF6:Cr3+ phosphors with good thermal stability for NIR LEDs. Dalton Trans.. 2022;51:10965-10972.
- [Google Scholar]
- Mn2+ and Mn4+ red phosphors: Synthesis, luminescence and applications in WLEDs. A review. J. Mater. Chem. C. 2018;6:2652-2671.
- [Google Scholar]
- An ultraviolet-visible and near-infrared-responded broadband NIR phosphor and its NIR spectroscopy application. Adv. Opt. Mater.. 2020;8:1902003.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2024.105921.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







