Translate this page into:
Neuroprotective effects of sonochemical- synthesized SiO2 nanoparticles in vivo models of ischemic/reperfusion injury in stroke
⁎Corresponding author at: No. 99W Huaihai Rd, Xuzhou 221004, Jiangsu Province, China. xye.xmu@yahoo.com (Xinchun Ye)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Cerebral ischemic injury is one of the debilitating diseases that showed inflammation plays an essential role in aggravating ischemic damage. After synthesizing silica nanoparticles (SiO2 NPs) by sonochemical method, serum parameters in the presence of different concentrations of SiO2 NPs are measured for toxicity assay. Rats were separated randomly into control, ischemia/reperfusion, and ischemia/reperfusion + SiO2 NPs groups. Transient forebrain ischemia induced with bilateral occlusion of both common carotid arteries followed by 60minuts of reperfusion. SiO2 NPs were administered (500 mg/kg/day p.o.) 21 days before ischemia/reperfusion time. Animals sacrificed and frontal cortex and hippocampal tissues used to determine malondialdehyde (MDA) level, nitric oxide (NO), glutathione (GSH) levels, an essential antioxidant, superoxide dismutase (SOD), alterations in the level of cytokines, TNFα, IL-1β, MCP-1, and phosphor Ik-кB. We also revealed the involvement of NF-κB downregulation by using western blotting. We reported on a histological investigation. The results showed that SiO2 NPs with a diameter of around 50 nm in dose of 500 mg/kg didn't change the level of liver enzyme (including ALT, AST and ALP) and hematological parameters. 500 mg/kg SiO2 NPs showed significant effects on remission of behavioral impairment. Ischemia/reperfusion oxidative injury in the rat hippocampus demonstrated a significant increase in MDA, TNFα, MCP-1, IL-1β, phosphor Ik-кB, NO levels, and a significant decrease in GSH contents and SOD activities in the hippocampal tissue compared to the control group. Pretreatment of ischemic rats with SiO2 NPs decreased the elevated levels of MDA, TNFα, MCP-1, IL-1β, phosphor Ik-кB, and NO levels. A significant alteration observed in SOD activities and GSH content results between treated and untreated ischemia/reperfusion brains in rats. Decreased protein level of NF-κB also measured in SiO2 NPs-treated animals. Untreated ischemia/reperfusion brains had significantly decreased in number of cells in CA1 hippocampus, nevertheless SiO2 NPs increase the normal cell and decrease the neurodegeneration in hippocampus but it was not significant alteration. SiO2 NPs reduced the damage caused by cerebral ischemia/reperfusion in rats and its molecular mechanism attributed to the downregulation of NF-κB signaling pathway.
Keywords
SiO2
Nanoparticles
Ischemic/reperfusion
NF-κB Signaling pathway
1 Introduction
Stroke is a significant cause of death or disability. Under permanent severe ischemia, brain cells cannot survive. Rechanneling occluded arteries through thrombolysis or thrombectomy is a common therapeutic strategy. Nevertheless, these procedures are associated with ischemia/reperfusion injury. Ischemia/reperfusion injury is often inevitable after stroke and can lead to different molecular cascades that break down the blood–brain barrier, which exacerbates brain damage (Pan et al., 2007; Fusco et al., 2019). Recent evidence showed how inflammation developed the side effect of brain injuries. It demonstrated that the leading causes of cerebral ischemia/reperfusion are the excessive formation of free radicals, cascading inflammatory reactions, overloading of calcium within neurocytes, and cytotoxic effect of excitatory amino acids. These factors are released quickly after brain injuries. The primary cause of cerebral injury exacerbation is acute inflammatory cascade reactions (Lin et al., 2016; Crack and Wong, 2008; Afshari et al., 2021).
In experimental stroke, models showed several transcription factors are activated. Nuclear factor-kappa B (NF-κB) and activator protein-1 (AP-1) were important transcript factors that regulate a huge number of inflammatory factors. NF-κB may be the most vital factor among the transcription which induce the initiation of inflammation and regulation of molecules related to inflammation (Harari and Liao, 2010; Kopitar-Jerala, 2015). Therefore, the downregulation of NF-κB has a vital role in inflammatory disease.
Silica nanoparticles (SiO2 NPs) have hydrophilic nature, regular spherical morphology, and high specific surface area with high thermal and mechanical properties. Besides, they have been used in medicine because of lack of toxicity and biocompatibility (Downing and Jain, 2020). Recent studies showed SiO2 NPs had the potential to target the brain (Oberdörster et al., 2004).
However, doubts remain regarding the effects of SiO2 NPs on the inflammatory response during cerebral ischemia. This study investigated SiO2 NPs on cerebral ischemia/reperfusion inflammatory response and surveyed anti-inflammatory mechanisms.
2 Material and methods
2.1 Experimental animals
Male Wister animals were weighted 270–300 at eight weeks of age. These animals were housed in cages with free access to water and food and 12 h cycles of light/dark.
2.2 Synthesis of SiO2 NPs
2.2.1 Sonochemical-mediated synthesis of SiO2 NPs
SiO2 NPs synthesized by the sonochemical-based method. In this method, two mM tetraethyl orthosilicate (TEOS: Si(OC2H5)4 was dissolved in 100 mL deoxygenated distilled water. Afterward, ultrasonic irradiation (Ti horn, 25 kHz, five h under 1.8 atm of argon) was applied, and 12 mL ammonia was added dropwise to the SiO2 precursor solutions at 50 °C, bubbling in the presence of N2 aged for one h. The SiO2 NPs were isolated from the solutions by centrifugation at 12,000 rpm for 15 min, washed with distilled water several times, then dried in an oven at 100 °C for two h (Kim et al., 2016).
2.2.2 Transmission electron microscopy (TEM) analysis of SiO2 NPs
The diameter, morphology, and distribution of the synthesized SiO2 NPs by the sonochemical method explored by TEM (Philips GM-30, Germany). One drop of the SiO2 NPs suspensions was dropped on a copper mesh grid and dried at room temperature for this analysis.
2.2.3 Dynamic light scattering (DLS) analysis of SiO2 NPs
The hydrodynamic radius of the synthesized SiO2 NPs at room temperature was determined by DLS, using a Nicomp 380ZLS (Particle Sizing Systems, Port Richey, FL, USA) operating at 535 nm, 50 mW, a frequency of the 200 kHz, and scattering angle of 90°.
2.3 Experimental design
First, we evaluated the toxicity of SiO2 NPs on liver cells and bone marrow. We administered SiO2 NPs orally for 21 days in 100, 250, 500, 750, and 1000 mg/kg to determine the non-toxic dose. On day 21, after the last administration, a blood sample in 2 mL was taken from the central vein of the tail. Blood Sample complete blood count differentiation and aminotransferase test and alkaline phosphatase were analyzed. Flowchart of the procedure is shown in Fig. 1.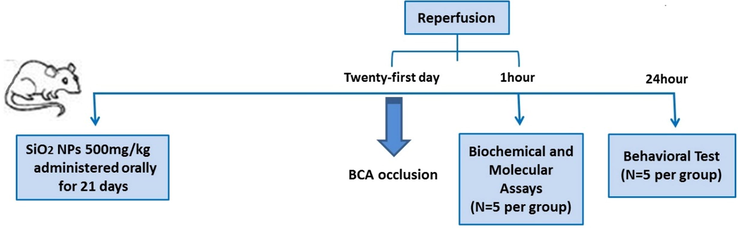
flowchart of the experiment in this study.
Then, the maximum non-toxic dose of SiO2 NPs was administered to animals for 21 days orally. On day 21, a stroke was induced to rat by bilateral common carotid artery occlusion (BCCAO). In the BCCAO model, animals were anesthetized by ketamine (45 mg/kg, i.p.) and xylazine (8 mg/kg), then their necks incised to reveal the two common carotid arteries. Carotid arteries are covered with a nontraumatic clamp for 30 min after separation from the vagal nerves. Sixty minutes were allowed, and the clamp was removed. Twenty-four hours after surgery, behavioural tests were done on treated and untreated stroke groups. The blood sample was taken one hour after surgery. Then animals were decapitated and the entire brain isolated to analyze the acute molecular consequences of SiO2 NPs on ischemic stroke. All experimental groups included five animals. Each goes through only one assessment. Control animals were administered only orally normal saline that was included in each experiment.
2.4 Behavioural test
We carried out a grid walking, hanging wire test, and an assessment of the neurologically modified severity score (mNSS) 24 h following BCCAO to detect post-stroke behavioural deficiencies and their enhancement through SiO2 NPs pretreatment. The grid walking test intended to measure the animal feet at a 2.5 cm high grid floor (45 cm per 45 cm). Animals could move freely on the grid in a 1-minute trial. The number of full traps in which the animal's paw touched the floor counted, and two marks were given. One score was given if the animal pulled its foot quickly without touching the floor (S. Raza, xxxx; Curzon et al., 2011). In the hanging wire test, the rats' neuromuscular abnormality strength was measured. A wire cage lid with duct tape around the border is utilized for this test to keep the mouse from going off the edge. On top of the cage lid, the animal is placed. The lid is gently shaking three times to force the mouse to hold the wires. The lid is then rotated over and over again. The lid is secured at the height of 50–60 cm above a soft underlay, high enough to keep the mouse from jumping down but not high enough to inflict injury if it falls. The time it takes for a person to fall is measured. Time recorded before the animal fell. It took 90 s to cut off time (Gaur et al., 2011; OLIVÁN et al., 2015). We performed mNSS in rats to assess the functions of the engine, reflex, sensory, and balance. Higher scores show more severe injuries according to the methods described (Chen et al., 2008). Blinded researchers recorded and analyzed all behavioural experiments.
2.5 Cytokine levels
The animal's brains were dissected after decapitating rats by anesthesia. The hippocampus and frontal cortex homogenized in a buffer contain TRIS HCL, SDS, DTT, NP40, and glycerol. Then the solution was centrifuged for 5 min, and then the levels of IL-1β and TNF-α in serum were detected with ELISA kits.
2.6 MDA level
Hippocampus and frontal cortex tissue samples were centrifuged for 5 min. Then 100 μl of the supernatant along with 900 μl of distilled water was added to the test tube, and 500 μl of TBA reagent was placed in a boiling water bath for 60 min, after which the tubes were centrifuged again at 4000g for 10 min. Finally, the absorbance of the supernatant was read at 534 nm using a spectrophotometer (UV1600).
2.7 Oxidative stress measurements
All tissues were homogenized in the ice-cold buffer to determine SOD activity. Homogenized samples were centrifuged at 9000 μg for 5 min at 4 °C, and a portion of the supernatant was immediately used for measuring SOD activity (Umg-1 protein) using the SOD assay kit (Sigma, Germany). In short, SOD activity is measured by the auto-oxidation method of pyrogallol.
A Glutathione Assay Kit was used to determine the glutathione level (Sigma-Aldrich, MI, USA). In short, in the 96 well-plate and thoroughly mixed (1.5 mg/mL DTNB, 6 U/mL glutathione reductase, and assay buffer), 10 mL liver homogenate and 150 μl working solution were added. The plate was incubated for 5 min before 50 mL of NADPH solution (0.16 mg/mL) was added to each well. The absorbance spectrophotometrically was measured at 412 nm.
2.8 Nitric oxide
Nitrite level, the rapid oxidation metabolite of NO based on the Griess reaction method, was measured using hippocampus and frontal cortex homogenate supernatant. In short, a mixture of 50 μl of supernatant with 100 μl of Griess reagent (Sigma, USA) was incubated for 30 min at room temperature and, according to the manufacturer's instructions, absorbance was measured at 540 nm by the ELISA reader (Taha, 2003).
2.9 Western blot measure NF-кB protein
Isolated animal brain tissue was crushed and homogenized in a microtubule with 1 mL of RIPA (Radioimmunoprecipitation Assay) buffer (Abcam, Cambridge, UK) and 1 percent protease inhibitor (Sigma) in a homogenizer device after rinsing with a cold PBS buffer. The supernatant was collected after centrifugation for 15 min at 13000 rpm, and the protein concentration was determined by a BCA kit (Thermo, Pierce, USA). 60μg total protein electrophoresed at a constant voltage of 80 V for 35 min and then 120 V for 45 min on 10 percent polyacrylamide gel. The electrophoresed proteins were then transferred to the Polyvinylidene Fluoride (PVDF) paper, and monoclonal antibodies were incubated at 4° C overnight with NF-κB (Cell Signaling, USA) and beta-actin (β-actin). PBST solution containing 0.1 percent Tween-20 was used to wash the membrane, and then HRP-Rabbit anti-rat secondary antibody (1:10000) was incubated and re-washed against the primary antibody for 190 min on the shaker. Finally, the presence of NF-κB protein in the samples was investigated by adding chemiluminescence ECL (GE Healthcare, Uppsala, Sweden) to the PVDF paper. The negative control sample (without primary antibodies) was also electrophoresed with the same protein concentration. The results of Western blot test were evaluated by Image-Pro plus 6.0 software (Media Cybernetics, Bethesda, MD, USA).
2.10 Nissl staining and histological examination
Histological examination was done 4 days after ischemia/reperfusion. The animals were anesthetized by intraperitoneal injection of ketamine (45 mg/kg), and xylazine (8 mg/kg) and their brains were perfused by heparin (1 unit/g bodyweight). Paraformaldehyde 0% and 4% in 1 M phosphate buffer (pH = 4.7) were tissue stabilized through the heart. After removing the brain from the skull, the samples were kept in 4% paraformaldehyde for 3 days and prepared. Paraffin blocks were fabricated in coronal sections for Nissl staining (Histo Line Laboratories Srl, Milano, MI, Italy). Nissl staining of coronal sections in thickness of 5–10 µm in the distance of 2.3 mm was cut from the back of the leaf and placed on gelatin glass slides, and dried in air. After staining the slides with violet crystal 1%, samples were taken by Leica DM2000 Light microscope (Leica DM2000 Light microscope). Wetzlar, Germany) was examined with a magnification of 400%. Pyramidal cell in the CA1 hippocampus was counted by ImageJ software version 1.40 g (Wayne Rasband, NIH, USA). Only neurons with clear senses and senses were measured (Alipanahzade et al., 2012; Azad et al., 2011).
2.11 Statical analysis
The analysis was conducted using Graphpad Prism 8 (version 8.0; GraphPad, San 142 Diego, CA, USA). All the Value expressed as mean ± SEM. Value differentiation was compromised by one-way, and two-way ANOVA followed by the Tukey test. Significantly considered p < 0.05.
3 Result
3.1 TEM analysis of SiO2 NPs
The SiO2 samples at the nanoscale were analyzed with TEM to explore the presence of SiO2 NPs. As shown in Fig. 2, the non-aggregated grains of SiO2 NPs successfully fabricated through the sonochemical method. Also, the average size of synthesized SiO2 NPs was 25.9 ± 11.07 nm, and they were spherical primarily (43%) or cubical (34%) or hexagonal (17%), as shown in Fig. 2A. DLS study was also done to measure the hydrodynamic radius and zeta potential of synthesized SiO2 NPs. As depicted in Fig. 2B, it indicated that the hydrodynamic radius and zeta potential of SiO2 NPs were about 261.32 ± 47.61 nm (PDI: 0.191) with a zeta potential value of about −37.24 mV, indicating the good colloidal stability of synthesized Nanoparticles.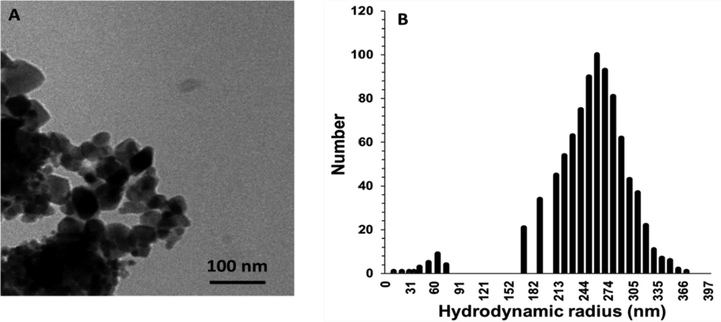
(A) TEM microraphs of synthesized SiO2 NPs through sonochemical methods. (B) The Hydrodynamic radius distribution of the SiO2 NPs determined by the DLS study.
3.2 Toxicity dose of SiO2 NPs
We administered 100, 250, 500, 750, and 1000 mg/kg SiO2 NPs orally and investigated the effect on cell blood count, aminotransferase and alkaline phosphatase enzyme in rats. Table 1 showed the maximum dose of SiO2 NPs, which didn't change the blood count significantly, was 500 mg/kg. In Fig. 3., 1000 mg/kg SiO2 NPs significantly increased ALT (P < 0.01) and ALP (P < 0.05). Abbreviation: WBC(K/μL)White blood cell, RBC(M/μL)Red blood cell., Hb(gr/dL) Hemoglobin., Hct(%)Hematocrit., MCV(fL)Mean corpuscular volume., MCH(pg)Mean corpuscular hemoglobin., PLT(K/μL)Platelet., NEU(%)Neutrophils ., LYM(%)Lymphocyte, Mean ± SEM.
Hematological
parametersControl
SiO2 NPs
100 mg/kgSiO2 NPs250 mg/kg
SiO2 NPs
500 mg/kgSiO2 NPs
750 mg/kgSiO2 NPs
1000 mg/kg
WBC(K/μL)
8.44 ± 0.37
8.31 ± 0.61
8.98 ± 0.83
9.18 ± 0.93
10.38 ± 0.53*
10.86 ± 0.57*
RBC(M/μL)
8.14 ± 1.00
8.08 ± 0.30
0.56 ± 8.34
0.91 ± 8.28
0.81 ± 7.78
0.39 ± 7.18
Hb(gr/dL)
14.19 ± 0.37
13.92 ± 0.58
13.91 ± 0.14
13.62 ± 0.53
13.34 ± 0.86
12.22 ± 0.63*
Hct(%)
38.28 ± 0.82
39.1 ± 1.00
38.7 ± 0.90
37.92 ± 0.99
37.3 ± 0.51
36.92 ± 0.45
MCV(fL)
48.25 ± 0.30
48.54 ± 0.88
48.46 ± 0.41
48.04 ± 0.54
47.7 ± 0.46
47.15 ± 0.82
MCH(pg)
18.14 ± 0.20
18.18 ± 0.66
18.04 ± 0.46
18.02 ± 0.37
17.48 ± 0.43
17.06 ± 0.20*
PLT(K/μL)
768 ± 10.20
762 ± 12.1.00
742 ± 15.70
732 ± 20.50
706 ± 21.70**
709 ± 15.70**
NEU(%)
28.14 ± 0.27
28.28 ± 0.20
28.5 ± 0.30
28.48 ± 0.34
28.8 ± 0.15
28.69 ± 0.13
LYM(%)
67.2 ± 0.82
67 ± 0.89
67.63 ± 1.40
69 ± 0.82
70 ± 1.67
75.33 ± 0.31*
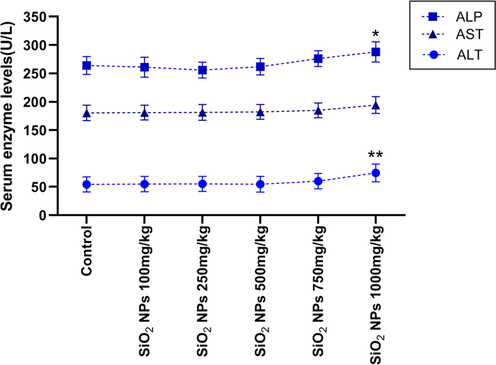
Effect of different doses of SiO2 NPs on the liver enzyme. 1000 mg/kg significantly increased the level of ALP and ALT compared to the control group. (* P < 0.05, ** P < 0.01, One way-ANOVA, post-hoc Tukey).
3.3 Effect of SiO2 NPs on behavioural remission after stroke
We evaluate the effect of pretreatment of the SiO2 NPs on improving motor, reflex, sensory and balance after stroke. As shown in Fig. 4, footfall (F (2, 2) = 1.425, p = 0.0198) (Fig. 4A), severity of neurologic (F(2 , 2) = 1.246, p = 0.0014) (Fig. 4B) and hanging wire test score (F(3 , 3) = 1.207, p = 0.041) (Fig. 4C) were significantly different in untreated and treated animals. Post hoc Tukey test showed footfall (P < 0.05) and severity of neurological score (p < 0.01) significantly decreased in of SiO2 NPs treated compared to untreated groups. Treated animals with SiO2 NPs showed improvement in hanging wire score compared to untreated animals in post-hoc Tukey test (p < 0.05).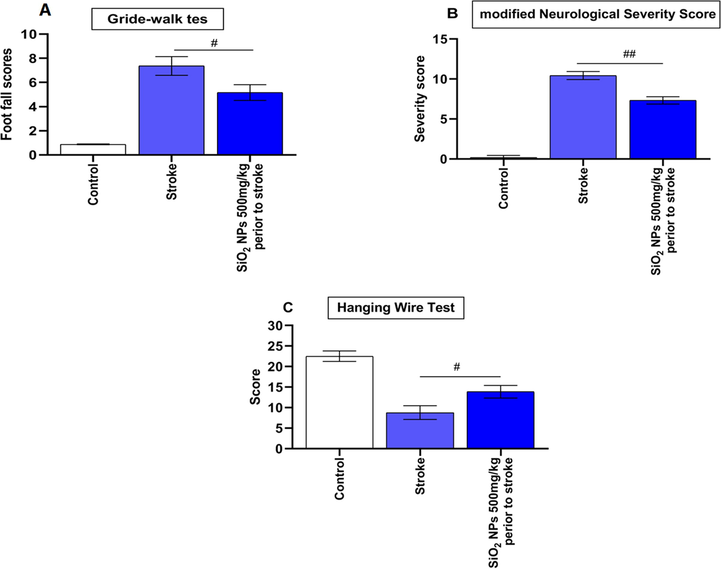
Effect of SiO2 NPs on Gride walk and hanging wire tests and neurologic severity scores. (A) SiO2 NPs treated animals showed a significant decrease in the Gride-walk test a day post-stroke. (B) SiO2 NPs treated animals showed a significant decrease in clinical scores a day post-stroke. (C) Hanging wire test scoring also showed significant improvement in rats receiving SiO2 NPs (# p < 0.05, ## p < 0.01, one-way ANOVA).
3.4 Effect of SiO2 NPs on oxidative stress after stroke
MDA, GSH, SOD, and NO concentrations were computed to indicate oxidative stress in rat brains. One-way ANOVA analysis showed MDA level, GSH, and SOD level were significantly different from the control group in the frontal cortex and hippocampus. As shown in Fig. 5A, Post-hoc Tukey test represents MDA level was significantly higher than the control group. Still, no significant difference was seen between the stroke group and the treated animal in the frontal cortex. However, in the hippocampus, MDA level significantly decreases in the treated group compared to untreated. The level of GSH and SOD was significantly decreased after stoke compare to control group in frontal cortex (p(GSH) < 0.01, p(SOD) < 0.001) and hippocampus (p(GSH) < 0.001, p(SOD) < 0.001) (Fig. 5B and 5C). Treatment with SiO2 NPs only increased the level of the SOD significantly in the frontal cortex compared to untreated rats (p < 0.05) (Fig. 5B and 5C). The level of NO significantly increased after stroke in the frontal cortex (p < 0.001) and the hippocampus (p < 0.001) compared to the control group (Fig. 5D). NO level had significantly decreased after treatment in the hippocampus (p < 0.01) (Fig. 5D).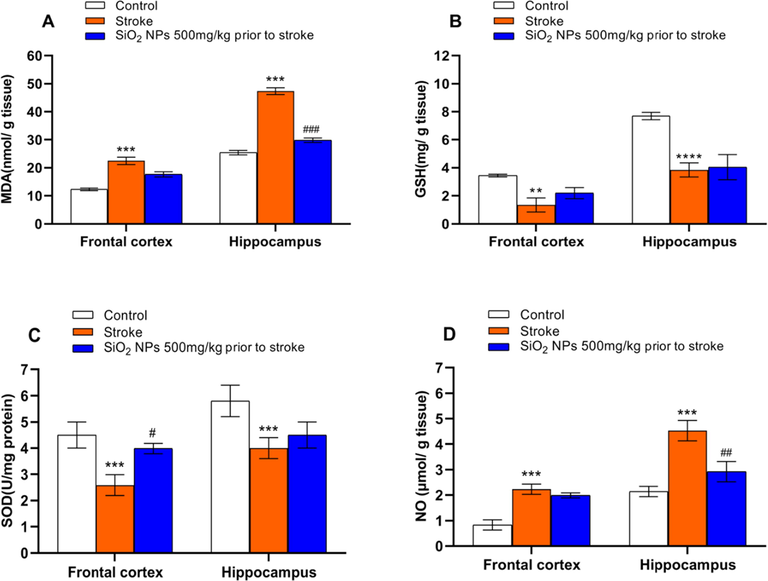
Effect of SiO2 NPs treatment on the oxidative stress factor. MDA (A) and NO (D) significantly increased, and GSH (B) and SOD (C) significantly decreased after stroke in the frontal cortex and hippocampus compared to the control group. (*** p < 0.001, **** p < 0.0001 one-way ANOVA test, post-hoc Tukey) (A) MDA was significantly decreased in the hippocampus of treated animals compared to untreated. (B) Treatment had no significant effect on GSH level in the frontal cortex and hippocampus. (C) SOD level was significantly increased in treatment compared to the untreated group. (D) The treated group had a significantly lower level of NO compared to the untreated group. (# p < 0.05, ## p < 0.01, ### p < 0.001, one-way ANOVA test).
3.5 Effect of SiO2 NPs on inflammatory cytokine after stroke
The inflammatory cytokines level after stroke is shown in Fig. 6A, B, C. Stroke significantly increased the level of TNF-α, IL-1β and MCP-1 in frontal cortex (p(TNF-α) < 0.001, p(IL-1β) < 0.001 and p(MCP-1) < 0.01) and hippocampus (p(TNF-α) < 0.001, p(IL-1β) < 0.001 and p(MCP-1) < 0.01) compare the control group. SiO2 NPs treated group had a significant decrease in TNF-α (p < 0.05), IL-1β (p < 0.01), and MCP-1 (p < 0.05) levels in the hippocampus compared to the untreated group.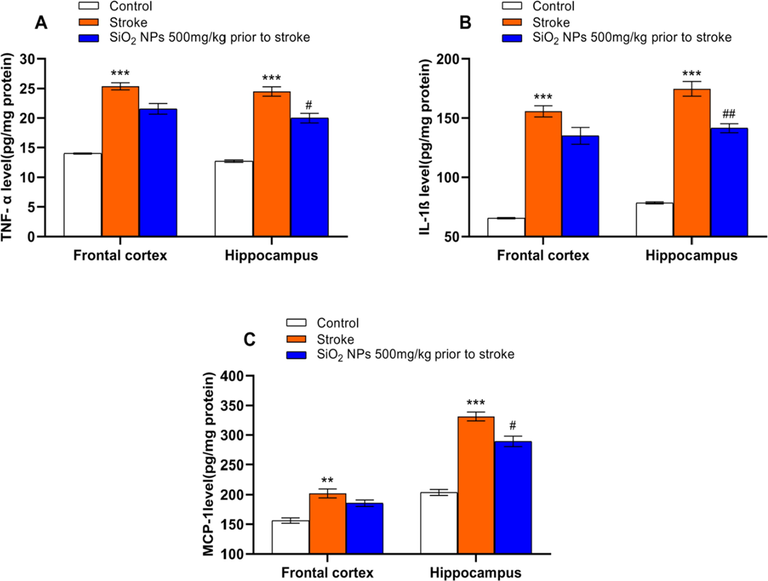
Effect of SiO2 NPs on inflammatory cytokines level. Stroke significantly increased the level of TNF-α (A), IL-1β (B), and MCP-1 (C) compared to the control group (** p < 0.01, *** p < 0.001, one-way ANOVA test, post-hoc Tukey). SiO2 NPs significantly decreased the levels of TNF-α (A), IL-1β (B), and MCP-1 (C) compared to untreated groups. (# p < 0.05, ## p < 0.01, One-way ANOVA test).
3.6 Effect of SiO2 NPs on the expression of pNF-кB protein and Phospho-IкBα level
We used western blot to show the anti-inflammatory of SiO2 NPs mediated by pNF-кB protein. As shown in Fig. 7A, a significant difference seen in expression pNF-κB (F (4, 4) = 1.255, p = 0.03) protein. The treated group with SiO2 NPs compared to the untreated had a significantly lower level of pNF-кB (p < 0.05) protein. SiO2 NPs treated groups had a significant decrease in Phospho-IкBα (p < 0.05) levels in comparing untreated groups (Fig. 7B).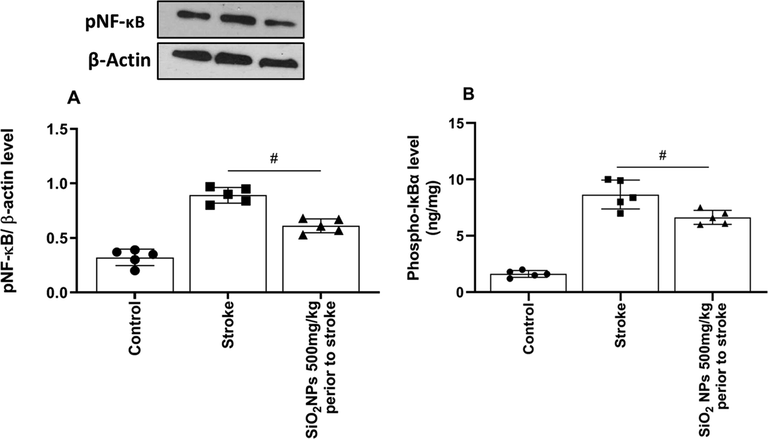
Effect of SiO2 NPs on expression of pNF-кB and Phospho-IкBα protein in western blot. pNF-кB (A) and Phospho-IкBα (B) protein expression level significantly decreased in treated animals (# p < 0.05, One-way ANOVA, post-hoc Tukey).
3.7 Effect of SiO2 NPs on the histological change in the hippocampus
In the control group, all cells in the hippocampus were healthy, and the nucleus was wholly distinct and darker than the cytoplasm (Fig. 8A, B, C). Many cells were seen in the CA1 region. in the untreated stroke group, the number of cells decreased significantly and was darker than the control group cells in the CA1 region. The shape of cells changed from round to spindle-shaped and triangular in the control group with undetected and degenerated nuclei. In the stroke group treated with SiO2 NPs, a slight increase in the number of healthy cells in the hippocampus was observed with reduced neurodegeneration, which was not statistically significant (Fig. 9). They had lower cell death and higher cell density than the untreated ischemic group.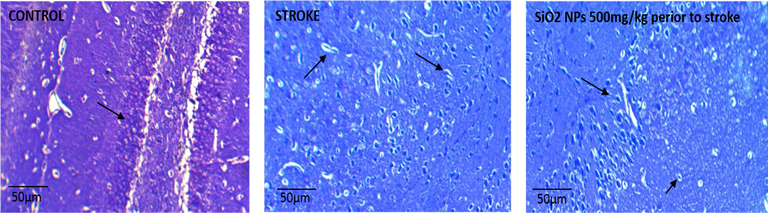
Photomicrograph of the CA1 region of the rat hippocampus in the experimental groups by Nissl staining Zoom × 4 (A) Control group (healthy neurons and healthy nuclei and euchromatin). (B) Stroke group (necrotic, degenerate and pyknoses cells with heterochromatin nucleus). (C) SiO2 NPs 500 mg/kg + Stroke (decrease of necrotic cells).
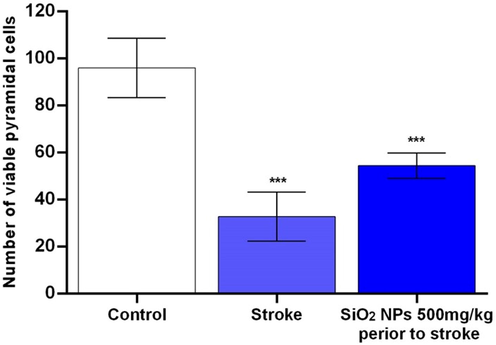
Effect of SiO2 NPs on number of CA1 pyramidal cells in rat models of ischemic/reperfusion injury in stroke *P-value ≤ 0.05, statistically different from control group. Data are mean ± SD.
4 Discussion
Our result showed that oral administration of SiO2 NPs improved behavioural impairment after ischemia/reperfusion stroke. In ischemia/reperfusion stroke brain, neutrophil migration and microglial activation facilitated inflammatory cascades. The inflammatory cascades begin the neuroinflammation process, which then causes more cellular damage and neuronal function loss (Hailer, 2008). A Previous report showed silica nanoparticles deposited in the frontal cortex and hippocampus (Wu et al., 2011), and our result demonstrated SiO2 NPs reduced cytokines in the hippocampus of stroke animals. Increment of viable cells in the hippocampus and decrement of neurodegeneration after administration of SiO2 NPs suggested that the therapeutic effect of SiO2 NPs on ischemia/reperfusion stroke may conduct through an anti-inflammatory effect.
Although silica nanomaterials had been extensively researched, there is consensus on silica toxicity as hemolytic potential and cytotoxicity. (Möller and Bein, 2019). In contrast, amorphous silica has been considered by the International Agency for Research on Cancer (IARC) as an ingredient that has insufficient evidence of carcinogenicity and is generally accredited as safe by the food and drug administration (FDA) for inclusion in food additives and packaging. It means that high levels of human exposure stand without any risk affirmation (Kim et al., 2018). SiO2 nanoparticles have been used as an imaging agent and therapeutic delivery cargo since the 2000s (Baeza et al., 2016). Further, to assess the potential for accumulation-based toxicity, the route of administration and chronic exposure is essential and more research is needed in this area (Fu et al., 2013). Our result showed oral administration of 500 mg/kg SiO2 NPs for 21 days had no hemolytic and cytotoxic effect.
The potential to generate reactive oxygen species (ROS) is present in silica nanoparticles. Although overproduction of ROS causes oxidative stress and toxicity, low ROS levels play a role in regulating the physiological functions of living organisms. Moderate levels of ROS can extend the long life of living species. (Obata et al., 2018). Kim et al. showed silica nanoparticles by altering the ROS and oxygen balance represent the diminish in pro-inflammatory of M1 macrophage and increment the anti-inflammatory of M2 macrophage in treatment of rheumatoid arthritis (RA) (Kim et al., 2019).
Inflammation plays an essential role in stroke pathophysiology. Serial cellular events include infiltration of circulating immune cells and activation of brain-resident immune-responsive cells, including microglia and astrocytes, and stroke seen in endothelial cells. (Zhang et al., 2005; Impellizzeri et al., 2019; Khalilzadeh et al., 2018). The initial injury was exacerbated by the subsequent activation of the inflammatory NF-кB signalling pathway (Shih et al., 2015). NF-κB occurs in an inactive form by binding to the suppressor protein of the IкB (inhibitor of nuclear factor kappa B) group (Wang et al., 2012). We found in this study that ischemia/reperfusion injury caused activation of Phospho-IкBα. SiO2 NPs significantly reduced the protein levels of Phospho-IкBα and inhibited phosphorylation and degradation induced by ischemic-reperfusion. Concerning the mechanism underlying attenuation by SiO2 NPs of ischemia/reperfusion induced downregulation of NF-кB.
Delayed nerve cell death after ischemia/reperfusion has been demonstrated in sensitive areas of the central nervous system, such as the CA1 region of the hippocampus (Pulsinelli and Brierley, 1979). Many studies have been performed on the cause of ischemic cell death, but so far, no definitive function has been found that causes delayed neuron death after ischemia. Numerous studies have reported that neurogenesis occurs after ischemia in the CA1 region of the hippocampus (Mori et al., 2009). Conventionally neurogenesis does not appear in the brain's nerve tissue, but in ischemic model animals, new neurons are produced in the sub-ependymal sub-region (Yamashita et al., 2006). New neurons that have developed in the subgranular region of the hippocampus differentiate into granular neurons and become mature neurons (Morris, 1984). Brain nerve cells need oxygen; therefore, adult neurons are more vulnerable to ischemia (Bendel et al., 2005). Pyramidal cells in the CA1 region of the hippocampus are susceptible and react rapidly to pervasive ischemia (Kirino, 1982). These pyramidal cells play a crucial role in learning, and spatial memory and their destruction can cause disorders in this area (Eckenhoff and Rakic, 1988). Tissue lesions due to ischemia result from pathophysiological events that lead to an increase in glutamate concentration, which in turn can stimulate glutamate receptors, especially NMDA, which leads to increased intracellular calcium and eventually causes cellular death (Choi, 1992).
Inflammation plays an essential role in stroke pathophysiology. Serial cellular events include infiltration of circulating immune cells and activation of brain-resident immune-responsive cells, including microglia and astrocytes, and stroke seen in endothelial cells. (Zhang et al., 2005). Glial cells, specifically microglia, are involved in acute inflammation, mediated by NF-κB activation (Godínez-Rubí et al., 2013). Cytotoxic molecules such as NO, oxygen radicals, and cytokines were reported to play a crucial role in releasing microglia (Shi et al., 2019). In the present study, ischemia/reperfusion injury showed a significant increase in pro-inflammatory cytokines, TNF-α, IL-1β, MCP-1, and ROS. SiO2 NPs treatment reduced elevated levels of MDA, TNF-α, MCP-1, IL-1β and NO. A significant alteration between treated and untreated ischemia/reperfusion brains in rats was observed in SOD operations and GSH content results.
5 Conclusion
In conclusion, our study showed that SiO2 NPs administration significantly reduced the effect of cerebral ischemic/reperfusion stroke in a rat model. The neuroprotective effects of SiO2 NPs appear to be associated with the increment of viable cells and the decrement of neurodegeneration in the CA1 hippocampus. This effect is associated with inhibition of NF-кB activation and consequent suppression of inflammatory responses. SiO2 NPs had varied ranges of use and needed more investigation on its properties. Our study had some limintiation so for future investigation we suggest to focus on ROS and their precursor.
Ethics approval and consent to participate
All animal use procedures were carried out under the Regulations of Experimental Animal Administration issued by the State Committee of Science and Technology of the People's Republic of China, with the approval of the Ethics Committee in our university.
Consent for publication
The authors declare consent for application
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
CRediT authorship contribution statement
Chengcheng Cui: Conceptualization, Methodology. Dayong Shen: Conceptualization, Methodology. Dandan Zuo: Conceptualization, Methodology. Xinchun Ye: Supervision, Validation.
Acknowledgements
We thank Dr Xinfeng Liu and Haotao Li for their assistance in discussion and data analysis of this manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Reperfusion injury following cerebral ischemia: pathophysiology MR imaging, and potential therapies. Neuroradiology. 2007;49(2):93-102.
- [Google Scholar]
- N-palmitoylethanolamide-oxazoline protects against middle cerebral artery occlusion injury in diabetic rats by regulating the SIRT1 pathway. Int. J. Mol. Sci.. 2019;20(19):4845.
- [CrossRef] [Google Scholar]
- Ischemia-reperfusion injury in the brain: mechanisms and potential therapeutic strategies. Biochem. Pharmacol. Open Access. 2016;5(4)
- [Google Scholar]
- Modulation of neuro-inflammation and vascular response by oxidative stress following cerebral ischemia-reperfusion injury. Current Med. Chem.. 2008;15(1):1-14.
- [Google Scholar]
- Sumatriptan improves the locomotor activity and neuropathic pain by modulating neuroinflammation in rat model of spinal cord injury. Neurolog. Res.. 2021;43(1):29-39.
- [Google Scholar]
- NF-κB and innate immunity in ischemic stroke. Ann. New York Acad. Sci.. 2010;1207:32.
- [Google Scholar]
- Innate immune response in brain NF-kappa B signaling and cystatins. Front. Mol. Neurosci.. 2015;8:73.
- [Google Scholar]
- M.A. Downing, P.K. Jain, Mesoporous silica nanoparticles: synthesis, properties, and biomedical applications, Nanoparticles for Biomedical Applications, Elsevier2020, pp. 267–281.
- Translocation of inhaled ultrafine particles to the brain. Inhalation Toxicol.. 2004;16(6-7):437-445.
- [Google Scholar]
- Sonochemical synthesis of silica particles and their size control. Appl. Surf. Sci.. 2016;380:305-308.
- [Google Scholar]
- S. Raza, NEUROPROTECTIVE EFFECT OF MEDICINAL HERBS ON CEREBRAL ISCHEMIA IN MALE WISTAR RATS.
- P. Curzon, M. Zhang, R.J. Radek, G.B. Fox, The behavioral assessment of sensorimotor processes in the mouse: acoustic startle, sensory gating, locomotor activity, rotarod, and beam walking, (2011).
- V. Gaur, A. Aggarwal, A. Kumar, Possible nitric oxide mechanism in the protective effect of hesperidin against ischemic reperfusion cerebral injury in rats, (2011).
- Comparative study of behavioural tests in the SOD1G93A mouse model of amyotrophic lateral sclerosis. Experim. Anim.. 2015;64(2):147-153.
- [Google Scholar]
- S.F. Chen, C.W. Hsu, W.H. Huang, J.Y. Wang, Post-injury baicalein improves histological and functional outcomes and reduces inflammatory cytokines after experimental traumatic brain injury, British J. Pharmacol. 155(8) (2008) 1279–96.
- Effect of transforming growth factor alpha of dentyte jyrus neurons and pyramidal cells of CA1 subfiled of hippocampus following ischemia-reperfusion in Rats. J. Gorgan Univ. Med. Sci.. 2012;14(3):26-32.
- [Google Scholar]
- Neuroprotective effects of carnosic acid in an experimental model of Alzheimer’s disease in rats. Cell J. (Yakhteh). 2011;13(1):39.
- [Google Scholar]
- Immunosuppression after traumatic or ischemic CNS damage: it is neuroprotective and illuminates the role of microglial cells. Prog. Neurobiol.. 2008;84(3):211-233.
- [Google Scholar]
- Neurotoxicity of silica nanoparticles: brain localization and dopaminergic neurons damage pathways. ACS Nano. 2011;5(6):4476-4489.
- [Google Scholar]
- Degradable drug carriers: vanishing mesoporous silica nanoparticles. Chem. Mater.. 2019;31(12):4364-4378.
- [Google Scholar]
- Silica exposure and work-relatedness evaluation for occupational cancer in Korea. Ann. Occupat. Environ. Med.. 2018;30(1):4.
- [Google Scholar]
- Recent advances in mesoporous silica nanoparticles for antitumor therapy: our contribution. Biomater. Sci.. 2016;4(5):803-813.
- [Google Scholar]
- The absorption, distribution, excretion and toxicity of mesoporous silica nanoparticles in mice following different exposure routes. Biomaterials. 2013;34(10):2565-2575.
- [Google Scholar]
- Early-life exposure to low-dose oxidants can increase longevity via microbiome remodelling in Drosophila. Nat. Commun.. 2018;9(1):1-12.
- [Google Scholar]
- Synergistic oxygen generation and reactive oxygen species scavenging by manganese ferrite/ceria co-decorated nanoparticles for rheumatoid arthritis treatment. ACS Nano. 2019;13(3):3206-3217.
- [Google Scholar]
- Neuronal activation of NF-κB contributes to cell death in cerebral ischemia. J. Cerebral Blood Flow Metab.. 2005;25(1):30-40.
- [Google Scholar]
- The neuroprotective effects of micronized PEA (PEA-m) formulation on diabetic peripheral neuropathy in mice. FASEB J.. 2019;33(10):11364-11380.
- [Google Scholar]
- The protective effects of sumatriptan on vincristine-induced peripheral neuropathy in a rat model. Neurotoxicology. 2018;67:279-286.
- [Google Scholar]
- NF-kappaB signaling pathways in neurological inflammation: a mini review. Front. Mol. Neurosci.. 2015;8:77.
- [Google Scholar]
- Protection by silibinin against experimental ischemic stroke: up-regulated pAkt, pmTOR, HIF-1α and Bcl-2, down-regulated Bax NF-κB expression. Neurosci. Lett.. 2012;529(1):45-50.
- [Google Scholar]
- A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke. 1979;10(3):267-272.
- [Google Scholar]
- Phenotype analysis and quantification of proliferating cells in the cortical gray matter of the adult rat. Acta histochemica et cytochemica. 2009;42(1):1-8.
- [Google Scholar]
- Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J. Neurosci.. 2006;26(24):6627-6636.
- [Google Scholar]
- Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods. 1984;11(1):47-60.
- [Google Scholar]
- Reappearance of hippocampal CA1 neurons after ischemia is associated with recovery of learning and memory. J. Cereb. Blood Flow Metab.. 2005;25(12):1586-1595.
- [Google Scholar]
- Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res.. 1982;239(1):57-69.
- [Google Scholar]
- Nature and fate of proliferative cells in the hippocampal dentate gyrus during the life span of the rhesus monkey. J. Neurosci.. 1988;8(8):2729-2747.
- [Google Scholar]
- Nitric oxide donors as neuroprotective agents after an ischemic stroke-related inflammatory reaction. Oxid. Med. Cell. Longevity. 2013;2013:1-16.
- [Google Scholar]







