Translate this page into:
New thieno[2,3-b]pyridine-based compounds: Synthesis, molecular modelling, antibacterial and antifungal activities
⁎Corresponding author. nmmohamed@uqu.edu.sa (Nashwa M. El-Metwaly) n_elmetwaly00@yahoo.com (Nashwa M. El-Metwaly)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
New thieno[2,3-b]pyridine clubbed various thiazole ring systems were synthesized by the reaction of 2-(1-(3-amino-4,6-dimethylthieno[2,3-b]pyridin-2-yl)ethylidene)hydrazine-1-carbothioamide with chloroacetone, phenacyl chloride, and chloroacetic acid. The molecular modeling of the synthesized compounds using DFT/B3LYP methodology revealed that all have a low HOMO and LUMO energies, −4.85 - −5.52 and −2.79 - −3.62, respectively, where the compound 10 has the highest values. The targeting thienopyridine analogues with various thiazole moieties 3–10 was assessed in order to create new antimicrobial agents and compared with ampicillin, gentamicin and miconazole as reference antibacterial and antifungal drugs. Compounds 8–10 exhibited potent antimicrobial activity against Gram positive S. aureus, Gram Gram negative E. coli bacteria, and C. albicans (antifungal), with IC50 (18.9 ± 0.63––24.3 ± 0.74 µg/mL), (14.2 ± 0.41––19.5 ± 0.64 µg/mL), and (19.2 ± 0.58–––23.4 ± 0.65 µg/mL), respectively. Furthermore, Molecular docking stimulation on MOE program was applied to expect the effect and interactions of the newly thienopyridine analogues and E. coli DNA gyrase B as it expressed by PDB ID: 1AJ6.
Keywords
Thieno[2,3-b]pyridine
Chloroacetone
Fukui’s indices
S. aureus
PDB ID: 1AJ6
1 Introduction
Many medicinal chemists are attentive in the exciting arena of heterocyclic compounds due to the broad spectrum of biological effects they demonstrates (Acharya, Bhavsar, Jethava, Patel, & Patel, 2021; Belen’kii, Gazieva, Evdokimenkova, & Soboleva, 2020; Martins et al., 2015). Even though heterocyclic chemistry research has made tremendous strides, efforts have been done to discover novel biologically potent heterocycles (Bashir, Bano, Ijaz, & Chaudhary, 2015; Heravi & Sadjadi, 2009; Huo, Li, Shi, & Li, 2022). Numerous five-membered heterocyclic analogues exist, and they frequently have biological functions (Plescia, Maggio, Daidone, & Raffa, 2021; Zayda, Abdel-Rahman, & El-Essawy, 2020), for instance pyridines and thiophenes (Arlan, Marjani, Javahershenas, & Khalafy, 2021; ARTUNÇ & MENZEK, 2022; Karki et al., 2015; LS Kishbaugh, 2016; Shehab, Abdellattif, & Mouneir, 2018; Zhao et al., 2022). The chemistry of pyridine derivatives, especially fused ring analogues, played a significant role among the heterocyclic compounds due to their great diverse biological activities such as herbicides, bactericides, fungicides, insecticides, and pharmaceuticals(Garcı́a-Tojal et al., 2001; Kanthecha, Bhatt, & Patel, 2019; Kim et al., 2004; Patil, Sethy, Sameena, & Shailaja, 2013). Among pyridine derivatives, the synthesis of thieno[2,3-b]pyridine derivatives have been developed rapidly using different methods because of their various applications such as dyes, agrochemical and substantial biological activity (Altalbawy, 2013; Dotsenko, Buryi, Lukina, & Krivokolysko, 2020; Ershov, Shishlikova, Ievlev, Belikov, & Maksimova, 2019; Naguib & El-Nassan, 2016). Meanwhile, thiophene and its derivatives, alternatively, are significant category of bioactive analogues since they reveal an extensive variety of biological activities, including antioxidant, anti-inflammatory, antibacterial, anticancer, analgesic, and CNS-related (Chan et al., 2017; da Cruz et al., 2021; El-Sharkawy, El-Sayed, & Zaki, 2012; Shah & Verma, 2019; Sidorenko et al., 2018). Thiophene can combine with a variety of heterocyclic nuclei to create novel compounds with enhanced biological properties. Thienopyridines hold a special place among these substances (Sanad & Mekky, 2020b; van Rensburg et al., 2017). Alternatively, the family of thieno[2,3-b]pyridine compounds exhibited high anticancer activity against difference human cell lines, already including HepG2 and MCF-7. The remarkable activity was attributed to the enriched lipophilicity and enhanced interactions with different cellular enzymes (Abuelhassan et al., 2022; Arabshahi et al., 2015; Dotsenko et al., 2020; Mohareb, Mohamed, & Ibrahim, 2022; Pervan et al., 2022). Moreover, thienopyridine based compounds played a vital role as anti-platelet drugs, e.g., clopidogrel, that were used to prevent blood clot in patients suffering from acute coronary syndromes (ACS) or at stroke risk (Binsaleh et al., 2018). Also, the thieno[2,3-b]pyridines demonstrated impressive anti-inflammatory (Liu et al., 2013), antiparasitic (Masch et al., 2019), antivirus (Amorim et al., 2017), antifungal (Abuelhassan et al., 2022; Kumar et al., 2013) and antidiabetic (Bahekar et al., 2007) activities. Eventually, in comparison with commercial antibiotic drugs as penicillin and streptomycin, thienopyridine derivatives showed very good in vitro antibacterial effectiveness toward several Gram’s positive and negative strains, such as Bacillus cereus, Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, Staphylococcus epidermidis, and Klebsiella pneumoniae (Abuelhassan et al., 2022; Al-Trawneh et al., 2011; Kumar et al., 2013; Sanad & Mekky, 2020a; Sanad, Mekky, Said, & Elneairy, 2021). Additionally, the reported thiosemicarbazide effectively suppressed the activity of Staphylococcus aureus DNA gyrase, with an IC50 value of 14.59 µM, according to research recently conducted in quest of different antimicrobial drugs (Paneth et al., 2016). Therefore, the manuscript aims to synthesize and assess the antimicrobial activity of new thieno[2,3-b]pyridines 3–10 in order to evaluate effective antimicrobial agents based on the biological applications of thieno[2,3-b]pyridine derivatives mentioned above. Molecular modeling and docking simulation were also performed.
2 Experimental
2.1 General remarks
Melting points were determined on a Gallenkamp electric device. The infrared spectra were obtained using a Thermo Scientific Nicolet iS10 FTIR spectrometer. The 1H NMR spectra (500 MHz) and the 13C NMR spectra (125 MHz) were recorded in DMSO‑d6 using a JEOL spectrometer (500 MHz). The mass spectra were obtained using a Quadrupole GC–MS (DSQII) mass spectrometer at a setting of 70 eV. The elemental analyses of C, H, and N were measured on a Perkin Elmer 2400 analyzer. 2-Chloro-4,6-dimethylnicotinonitrile (1) was purchased from Fisher Scientific (CAS: 14237–71-9).
2.2 Synthesis of 2-acetyl-3-amino-4,6-dimethylthieno[2,3-b]pyridine (3)
A mixture of 2-mercaptonicotinonitrile derivative 2 (1.64 g, 0.01 mol) and chloroacetone (0.92 mL, 0.01 mol) in 30 mL acetone containing potassium carbonate (1.40 g) was refluxed for 4 h. After cooling, the mixture was poured on to crushed ice. The formed solid was collected and crystallized from ethanol.
Yellow crystals; yield = 84 %; m.p. = 176–177 °C, Lit. m.p. 177–178 °C (Yassin, 2009). Retention factor = 0.54 (petroleum ether:AcOEt = 1:1). IR ( /cm−1): 3327, 3183 (NH2), 1620 (C = O). 1H NMR (δ/ppm): 2.45 (s, 3H, CH3), 2.55 (s, 3H, CH3), 2.75 (s, 3H, CH3), 6.95 (s, 2H, NH2-D2O exchangeable), 6.85 (s, 1H, C5-pyridine). MS (EI, m/z): 220 (100.00%), 205 (66.14%), 177 (46.11), 150 (16.28%), 133 (9.08%). Anal. Calcd. C11H12N2OS (220.07): C, 59.98; H, 5.49; N, 12.72 %. Found: C, 60.10; H, 5.45; N, 12.79 %.
2.3 Synthesis of 2-(1-(3-amino-4,6-dimethylthieno[2,3-b]pyridin-2-yl)-ethylidene)hydrazine-1-carbothioamide (4)
A mixture of thienopyridine compound 3 (2.20 g, 0.01 mol) and thiosemicarbazide (0.91 g, 0.01 mol) in absolute ethanol (15 mL) and 1 mL conc. HCl was refluxed for 2 h. The produced solid upon cooling was collected to give the corresponding thiosemicarbazone 4.
Orange powder; yield = 72 %; m.p. = 195–196 °C. Retention factor = 0.68 (petroleum ether:AcOEt = 1:1). IR ( /cm−1): 3332, 3274, 3287 (–NH2 and N–H), 1627 (C = N), 1031 (C = S). 1H NMR (δ/ppm): 2.29 (s, 3H, CH3), 2.55 (s, 3H, CH3), 2.74 (s, 3H, CH3), 7.06 (s, 1H, C5-pyridine), 7.15 (s, 2H, NH2-D2O exchangeable), 7.82 (s, 2H, NH2-D2O exchangeable),), 11.07 (s, 1H, N–H-D2O exchangeable). 13C NMR (δ/ppm): 14.92, 19.71, 24.08, 119.47, 121.88, 123.14, 126.37, 144.65, 151.58, 160.23, 181.11. MS (EI, m/z): 293 (62.75%), 263 (100.00%), 249 (31.80%), 222 (65.78%). Anal. Calcd. for C12H15N5S2 (293.08): C, 49.12; H, 5.15; N, 23.87%. Found: C, 49.5; H, 5.20; N, 23.92 %.
2.4 Synthesis of 4,6-dimethyl-2-(1-(2-(4-substitutedthiazol-2-yl)hydrazono)-ethyl) thieno[2,3-b]pyridin-3-amines 5 and 6
A suspension of thiosemicarbazone compound 4 (1.46 g, 0.005 mol) and chloroacetone (0.46 mL, 0.005 mol) or phenacyl chloride (0.77 g, 0.005 mol) in ethanol was refluxed for 6 h. The mixture was poured into ice-cold water and then neutralized by Na2CO3. The separated solid was collected and crystallized from ethanol to produce the corresponding thienopyridine-thiazole hybrids 5 and 6, respectively.
2.4.1 4,6-dimethyl-2-(1-(2-(4-methylthiazol-2-yl)hydrazono)-ethyl)thieno[2,3-b]pyridin-3-amine (5)
Red powder; yield = 73 %; m.p. = 290–291 °C. Retention factor = 0.43 (petroleum ether:AcOEt = 1:2). IR ( /cm−1): 3421, 3289 (NH2 and N–H), 1600 (C = N). 1H NMR (δ/ppm): 2.25 (s, 3H, CH3), 2.33 (s, 3H, CH3), 2.51 (s, 3H, CH3), 2.72 (s, 3H, CH3), 6.28 (s, 1H, C5-thiazole), 6.88 (s, 1H, C5-pyridine), 7.48 (s, 2H, NH2-D2O exchangeable), 8.62 (s, 1H, N–H-D2O exchangeable). 13C NMR (δ/ppm): 14.87, 16.07, 19.70, 24.13, 104.64, 120.25, 121.81, 123.20, 127.58, 144.47, 147.10, 150.77, 159.94, 166.34. MS (EI, m/z): 331 (42.15%), 263 (100.00%), 248 (81.77%), 222 (71.79%), 128 (24.64%), 104 (17.70%), 69 (19.14%). Anal. Calcd. for C15H17N5S2 (331.09): C, 54.36; H, 5.17; N, 21.13 %. Found: C, 54.16; H, 5.28; N, 21.23 %.
2.4.2 4,6-dimethyl-2-(1-(2-(4-phenylthiazol-2-yl)hydrazono)-ethyl)thieno[2,3-b]pyridin-3-amine (6)
Red powder; yield = 66 %; m.p. = 230–331 °C. Retention factor = 0.54 (petroleum ether:AcOEt = 1:2). IR ( /cm−1): 3452, 3297 (NH2 and N–H), 1600 (C = N). 1H NMR (δ/ppm): 1.87 (s, 3H, CH3), 2.32 (s, 3H, CH3), 2.81 (s, 3H, CH3), 7.06 (s, 2H, NH2-D2O exchangeable), 7.12 (s, 1H, C5-pyridine), 7.25 (s, 1H, C5-thiazole), 7.37 (t, J = 7.50 Hz, 2H), 7.51 (t, J = 7.50 Hz, 1H), 7.81 (d, J = 7.50 Hz, 2H), 10.90 (s, 1H, N–H-D2O exchangeable). 13C NMR (δ/ppm): 14.80, 19.59, 24.18, 107.13, 120.33, 122.02, 124.01, 127.16 (2C), 127.69, 128.22, 129.47 (2C), 133.38, 144.54, 147.44, 150.62, 159.78, 170.09. MS (EI, m/z): 393 (30.57%), 376 (22.62%), 361 (23.98%), 265 (55.54%), 250 (100.00%), 220 (78.90%), 222 (32.01%), 176 (33.04%), 177 (86.81%), 134 (40.28%), 105 (16.48%), 77 (19.62%). Anal. Calcd. for C20H19N5S2 (393.11): C, 61.04; H, 4.87; N, 17.80 %. Found: C, 61.24; H, 4.92; N, 17.67 %.
2.5 Synthesis of 2-(2-(1-(3-amino-4,6-dimethylthieno[2,3-b]pyridin-2-yl)ethylidene)hydrazinyl) thiazol-4(5H)-one (7)
To a suspension of thiosemicarbazone derivative 4 (1.46 g, 0.005 mol) and fused sodium acetate (1.0 g) in acetic acid (30 mL), 2-chloroacetic acid (0.70 g, 0.005 mol) was added. The mixture was refluxed for 4 h and then poured into ice-cold water. The obtained solid was subjected to crystallization from ethanol to produce the thienopyridine-thiazolin-4-one hybrid 7.
Orange powder; yield = 78 %; m.p. = 290–291 °C. Retention factor = 0.48 (petroleum ether:AcOEt = 1:2). IR ( /cm−1): 3444, 3289 (NH2 and N–H), 1703 (C = O), 1616 (C = N). 1H NMR (δ/ppm): 2.23 (s, 3H, CH3), 2.41 (s, 3H, CH3), 2.64 (s, 3H, CH3), 3.86 (s, 2H, CH2 thiazolidine), 6.88 (s, 2H, NH2-D2O exchangeable), 7.22 (s, 1H, C5-pyridine), 10.11 (s, 1H, N–H-D2O exchangeable). 13C NMR (δ/ppm): 14.73, 19.64, 24.10, 35.84, 120.39, 122.13, 123.76, 127.85, 144.58, 151.03, 159.67, 161.47, 174.63. MS (EI, m/z): 333 (100.00%), 263 (34.33%), 245 (67.99%), 230 (23.25%), 222 (25.73%), 214 (26.23%), 183 (17.12%), 130 (33.26%), 111 (20.89%), 104 (17.17%). Anal. Calcd. for C14H15N5OS2 (333.07): C, 50.43; H, 4.53; N, 21.00%. Found: C, 50.53; H, 4.47; N, 21.11%.
2.6 Synthesis of thienopyridine-thiazolin-4-one hybrids 8, 9 and 10
Each of the appropriate benzaldehyde derivative (0.003 mol) was added to a solution of thienopyridine-thiazolin-4-one scaffold 7 (1.00 g, 0.003 mol) in acetic acid (20 mL) and fused sodium acetate (0.5 g). The mixture was refluxed for 4 h. The solid that produced upon cooling was collected and crystallized from acetic acid to yield the corresponding thienopyridine-thiazolin-4-one hybrids 8, 9 and 10.
2.6.1 2-(2-(1-(3-amino-4,6-dimethylthieno[2,3-b]pyridin-2-yl)ethylidene)hydrazinyl)-5-(4-methylbenzylidene)thiazol-4(5H)-one (8)
Brown powder; yield = 63 %; m.p. > 330 °C. Retention factor = 0.62 (petroleum ether:AcOEt = 1:2). IR ( /cm−1): 3391, 3307, 3229 (NH2 and N–H), 1687 (C = O), 1621 (C = N). 1H NMR (δ/ppm): 2.29 (s, 3H, CH3), 2.35 (s, 3H, CH3), 2.42 (s, 3H, CH3), 2.68 (s, 3H, CH3), 6.97 (s, 2H, NH2-D2O exchangeable), 7.10 (s, 1H, C5-pyridine), 7.33 (d, J = 8.50 Hz, 2H), 7.57 (d, J = 8.50 Hz, 2H), 7.83 (s, 1H, C = CH), 9.96 (s, 1H, N–H- D2O exchangeable). MS (EI, m/z): 435 (31.48%), 352 (74.69%), 294 (100.00%), 221 (97.73%), 132 (87.37%), 118 (30.78%), 91 (38.94%), 76 (25.61%). Anal. Calcd. for C22H21N5OS2 (435.12): C, 60.67; H, 4.86; N, 16.08 %. Found: C, 60.85; H, 4.93; N, 15.19 %.
2.6.2 2-(2-(1-(3-amino-4,6-dimethylthieno[2,3-b]pyridin-2-yl)ethylidene)hydrazinyl)-5-(4-methoxybenzylidene)thiazol-4(5H)-one (9)
Brown powder; yield = 67 %; m.p. > 330 °C. Retention factor = 0.67 (petroleum ether:AcOEt = 1:2). IR ( /cm−1): 3387, 3296, 3237 (NH2 and N–H), 1779 (C = O), 1619 (C = N). 1H NMR (δ/ppm): 2.35 (s, 3H, CH3), 2.41 (s, 3H, CH3), 2.71 (s, 3H, CH3), 3.80 (s, 3H, OCH3), 6.90 (s, 2H, NH2-D2O exchangeable), 7.04 (d, J = 9.00 Hz, 2H), 7.13 (s, 1H, C5-pyridine), 7.61 (d, J = 9.00 Hz, 2H), 7.78 (s, 1H, C = CH), 9.91 (s, 1H, N–H-D2O exchangeable). MS (EI, m/z): 451 (27.04%), 258 (41.23%), 221 (82.94%), 203 (100.0%), 76 (30.04%). Anal. Calcd. for C22H21N5O2S2 (451.11): C, 58.52; H, 4.69; N, 15.51 %. Found: C, 58.38; H, 4.60; N, 15.61 %.
2.6.3 2-(2-(1-(3-amino-4,6-dimethylthieno[2,3-b]pyridin-2-yl)ethylidene)hydrazinyl)-5-(4-chlorobenzylidene)thiazol-4(5H)-one (10)
Yellowish brown powder; yield = 60 %; m.p. > 330 °C. Retention factor = 0.56 (petroleum ether:AcOEt = 2:3). IR ( /cm−1): 3402, 3310, 3244 (NH2 and N–H), 1691 (C = O), 1622 (C = N). 1H NMR (δ/ppm): 2.36 (s, 3H, CH3), 2.41 (s, 3H, CH3), 2.68 (s, 3H, CH3), 6.96 (s, 2H, NH2-D2O exchangeable), 7.13 (s, 1H, C5-pyridine), 7.57 (d, J = 8.50 Hz, 2H), 7.63 (d, J = 8.50 Hz, 2H), 7.86 (s, 1H, C = CH), 10.00 (s, 1H, N–H-D2O exchangeable). MS (EI, m/z): 455 (18.69%), 262 (24.56%), 221 (61.08%), 205 (33.19%), 76 (100.00%). Anal. Calcd. for C21H18ClN5OS2 (455.06): C, 55.32; H, 3.98; N, 15.36 %. Found: C, 55.11; H, 4.07; N, 15.23 %.
2.7 Computational studies
The studied thienopyridine analogues were geometrically optimized by the DFT/B3LYP/6–311++G (Becke, 1993; Lee, Yang, & Parr, 1988; Perdew & Wang, 1992) incorporated in Gaussian 09 W software (Frisch et al., 2009). The stability of optimized structure of all derivatives was ascertained from obtaining positive frequencies only. The Fukui indices were determined by Materials studio package DMol3 module (BIOVIA, 2017) utilizing the GGA and B3LYP functional with DNP (version 3.5) (Delley, 2006).
2.8 In vitro antimicrobial assay
The antimicrobial effectiveness of all analogues 3–10 was investigated toward panels of two Gram-positive bacteria (Staphylococcus aureus ATCC 29213 and Bacillus subtilis ATCC 6633), two Gram-negative bacteria (Escherichia coli ATCC 25922, Salmonella typhimurium ATCC 14028) and two fungi (Candida albicans ATCC 10231 and Aspergillus fumigatus ATCC 46654) using minimum inhibitory concentration technique (H. Gaffer, Salem, & Marzouk, 2016; H. E. Gaffer & Althagafi, 2020).
2.9 Molecular docking
The molecular docking stimulation was applied to discover the interaction between thienopyridine hybrids and active centers in E. coli DNA gyrase B by utilizing PDB ID: 1AJ6 protein (Eldeab, 2019; Elzahabi, Nossier, Khalifa, Alasfoury, & El-Manawaty, 2018). The new thienopyridine analogues were explored across MOE 2015.10 program. The co-crystallized ligands were initially re-docked into the nominated protein, removing heteroatoms and water, docking ligand atoms in the correct locations using 10 poses, minimization, and docking the ligands. This was done to calculate the root-mean-square deviation. Whoever, If the RMSD values are < 2.0 Å it is recommended for the best scoring position (Ramírez & Caballero, 2018).
3 Results and discussion
3.1 Synthesis of thienopyridine-based compounds
Generally, due to their broad pharmacological activities, thienopyridine analogues have attracted a great deal of attention and have proven successful in one particular antimicrobial area (Abdel-Rahman, Bakhite, & Al-Taifi, 2003). Therefore, our synthetic strategy is based on the synthesis of 2-acetyl-thienopyridine compound 3 (Scheme 1) as building block for various thienopyridine-thiazole hybrids. The synthetic method starts by treatment of 2-chloro-4,6-dimethylnicotinonitrile (1) with thiourea (Yassin, 2009) under reflux in ethanol to produce 2-mercapto-4,6-dimethylnicotinonitrile (2). The mechanism is initiated by nucleophilic attached by sulfur of thiourea at the second position of pyridine ring to form the intermediate (A) which undergoes migration of the chloride ion. The produced isothiouronium chloride intermediate (B) decomposes into the corresponding mercaptopyridine compound 2 and urea. When 2-mercaptopyridine compound 2 was permitted to interact with chloroacetone in acetone and potassium carbonate, 2-acetyl-3-amino-4,6-dimethylthieno[2,3-b]pyridine (3) was obtained exclusively. This is in agreement with the earlier reports (Yassin, 2009). The structure was confirmed based on its compatible spectral data. FTIR spectrum revealed the absorptions of –NH2 function at 3327 and 3183 cm−1 and C = O group at 1620 cm−1. The 1H NMR spectrum showed a singlet at δ 6.95 ppm corresponding to the protons of –NH2 group, a singlet at δ 6.85 ppm corresponding to the proton of C5-H pyridine, beside three singlet signals in the region δ 2.75–2.45 ppm corresponding to the protons of three methyl groups (pyridine-CH3, pyridine-CH3 and –COCH3 groups). Further confirmation about the structure of 3 was also indicated by its mass spectrum which gave molecular ion peak at m/z = 220 (100%) corresponding to its correct molecular weight (C11H12N2OS).![Synthesis of 2-acetyl-3-amino-4,6-dimethylthieno[2,3-b]pyridine (3).](/content/184/2023/16/11/img/10.1016_j.arabjc.2023.105226-fig1.png)
Synthesis of 2-acetyl-3-amino-4,6-dimethylthieno[2,3-b]pyridine (3).
2-Acetyl-3-amino-4,6-dimethylthieno[2,3-b]pyridine (3) is considered to be a convenient starting compound for the production of many heterocyclic hybrids. Therefore, the reaction of the synthesized 2-acetyl-3-aminothieno[2,3-b]pyridine derivative 3 with thiosemicarbazide was studied. Refluxing of 3 with thiosemicarbazide in ethanol and concentrated HCl for two hours leads unambiguously to the corresponding thiosemicarbazone derivative 4 in 72% yield (Scheme 2). The analytical data of 4 were found identical to the purposed structure. The IR spectrum of 4 lacked the absorption of the carbonyl function, and instead an absorption band of the C = S function was detected at 1031 cm−1. The 1H NMR signals were observed as singlet signals at 11.07, 7.82, 7.15 ppm for the protons of N–H, thioamide-NH2 and thiophene-NH2 groups. The proton of pyridine-C5 was recorded as singlet at 7.06 ppm. The protons of the three methyl groups were recorded as 2.74 (pyridine-CH3, 2.55 (pyridine-CH3) and 2.29 (N = C-CH3) ppm. The mass spectrum indicated the molecular ion peak of the molecular formula C12H15N5S2 at m/z = 293 (24.5%).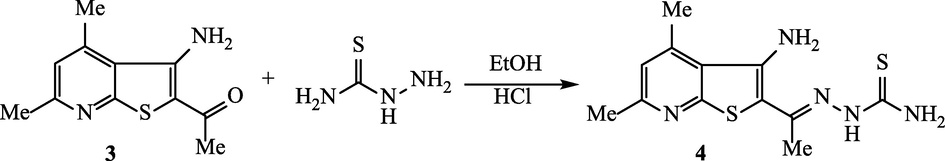
Synthesis of thienopyridine-thiosemicarbazone derivative 4.
An unequivocal support for the structure of thiosemicarbazone compound 4 came from a series of reactions involving the thiourea fragment of the thiosemicarbazone moiety. Thus, the reactivity of the thiosemicarbazone derivative 4 was investigated by the reactions of this compound with some alkylating agents, e.g., chloroacetone, phenacyl chloride and chloroacetic acid. It has been found that the reactions of thiosemicarbazone 4 with chloroacetone or phenacyl chloride were carried out in absolute ethanol to afford 4,6-dimethyl-2-[1-(2-(4-methylthiazol-2-yl)hydrazono)ethyl]-thieno[2,3-b]pyridin-3-amine (5) and 4,6-dimethyl-2-[1-(2-(4-phenylthiazol-2-yl)hydrazono)ethyl]-thieno[2,3-b]-pyridin-3-amine (6), respectively (Scheme 3). Alkylation was assumed to be regioselective relative to the high nucleophilic sulphur atom. The analytical data of compounds 5 and 6 were found to be identical with the suggested cyclic structures proving that the thiol group of the thiosemicarbazone 4 was involved in the cyclization step via alkylation with the α-chloroketone reagent (intermediate C and intermediate D) followed by condensation of the carbonyl group with the amino function of thiosemicarbazone 4. The IR spectra of these products indicated the absence of absorption bands of –NH2, and C = S groups. The 1H NMR spectra of compounds 5 and 6 revealed the proton of thiazole-C5 as singlet signal at 6.28 and 7.25 ppm.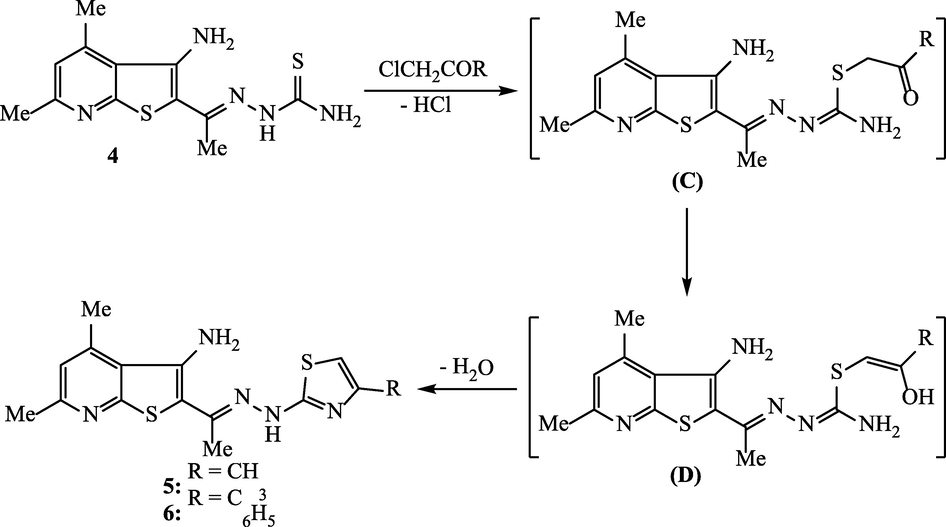
Synthesis of thienopyridine-thiazole hybrids 5 and 6.
A further proof of the structure of the thiosemicarbazone 4 came from its cyclization with chloroacetic acid in the presence of sodium acetate in acetic acid to give 2-[2-(1-(3-amino-4,6-dimethylthieno[2,3-b]pyridin-2-yl)ethylidene)hydrazinyl]-thiazol-4(5H)-one (7) (Scheme 4). The analytical data of 7 were identical to the purposed structure proving that the thiol group is involved in the cyclization step via alkylation with chloroacetic acid (intermediate E) followed by loss of water molecule. The cyclic carbonyl function of the thiazoline-4-one ring was observed at 1703 cm−1 in the IR spectrum. The 1H NMR spectrum of 7 lacked a signal of NH2 group and has a singlet signal of the methylene group at δ = 3.86 ppm. Therefore, it has been shown that the thiosemicarbazone 4 could be considered as a convenient starting material for the synthesis of the condensed polycyclic thieno[2,3-b]pyridine derivatives incorporating substituted thiazole and thiazolinone moieties.
Synthesis of thienopyridine-thiazolin-4-one hybrid 7.
The methylene group of 2-[2-(1-(3-amino-4,6-dimethylthieno[2,3-b]pyridin-2-yl)ethylidene) hydrazinyl]-thiazol-4(5H)-one (7) is proved to be active in Knoevenagel reaction with various substituted benzaldehydes. The reaction was carried out by reflux in acetic acid and sodium acetate to furnish the corresponding 2-(2-(1-(3-amino-4,6-dimethylthieno[2,3-b]pyridin-2-yl)ethylidene)hydrazinyl)-5-(arylidene)thiazol-4(5H)-ones 8, 9, and 10 (Scheme 5). The structures of thienopyridine-thiazolin-4-one hybrids 8–10 were elucidated in the light of their compatible spectral analyses. The IR spectra of hybrids 8, 9 and 10 displayed the absorption of the conjugated carbonyl function at 1687, 1779 and 1691 cm−1, respectively. The olefinic proton (C = CH) was recorded in the 1H NMR spectra as singlet at 7.83, 7.78 and 7.86 ppm.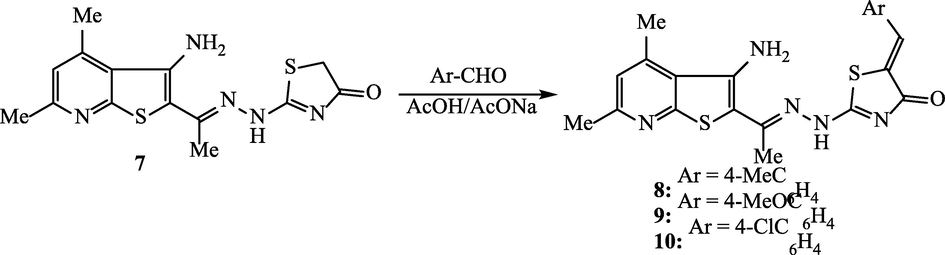
Synthesis of thienopyridine-thiazolin-4-one hybrid 8, 9, and 10.
3.2 Molecular modelling
The parent compound 4, 2-(1-(3-amino-4,6-dimethylthieno[2,3-b]pyridin-2-yl)ethylidene) hydrazine-1-carbothioamide, DFT calculated dihedral data indicated that it has nonplanar configuration in which the two methyl substituents existed in plane with the thienopyridine nucleus, e.g., C7a(thpy)-C3a(thpy)-C4(thpy)-Me4(thpy) = 179.4° and Me6(thpy)-C6(thpy)-N7(thpy)-C7a(thpy) = 179.8°, whereas the amino group was shifted out by ∼ 4.0°, C7a(thpy)-C3a(thpy)–C3(thpy)–NH2(thpy) = 176.1°. Furthermore, the carbothioamide branch was strongly deviated from planarity where for example the azomethine carbon C(Hzn) and nitrogen N2(Hzn) have been slightly moved out the thienopyridine plane, namely, C7a(thpy)-S1(thpy)–C2(thpy)-C(Hzn) = 177.8° and S1(thpy)–C2(thpy)-C(Hzn)–N2(Hzn) = 6.7°, respectively, the correspond thione and methyl groups carbon atoms were strongly shifted out, e.g., C(Hzn)–N2(Hzn)-N1H(Hzn)-CS(Hzn) = -54.8° and C3(thpy)–C2(thpy)-C(Hzn)-Me(Hzn) = 13.4° (Fig. 1) (Table S1).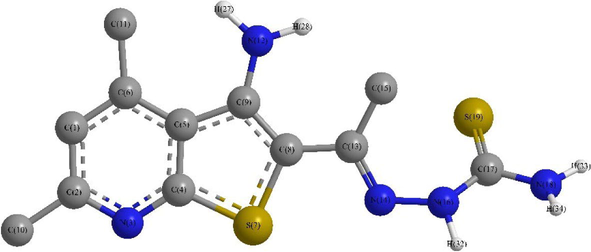
DFT Optimized structure of the parent compound 4.
The 4,6-dimethyl-2-(1-(2-(thiazol-2-yl)hydrazineylidene)ethyl)thieno[2,3-b]pyridin-3-amine derivatives 5–7 dihedral angles showed that they have almost planar structure. On comparison with 4, it was observed that the thienopyridine substituents, methyl and amino groups, retained their positions. Similarly, the hydrazone moiety was strongly deviated from planarity where the azomethine carbon retained while its nitrogen atom and methyl substituent were shifted more from the thienopyridine plane, i.e., C7a(thpy)-S1(thpy)–C2(thpy)-C(Hzn) = 176.4–176.7°, S1(thpy)–C2(thpy)-C(Hzn)–N2(Hzn) = 12–14.6°, and C3(thpy)–C2(thpy)-C(Hzn)-Me(Hzn) = 15.8–19.3°, respectively. On contrary, the newly formed thiazole ring was coplanar with the azomethine group, i.e., N2(Hzn)-N1H(Hzn)–C2(thia)-S1(thia) = 0.6–1.0° and N2(Hzn)-N1H(Hzn)–C2(thia)–N3(thia) = 179.1–179.3°. Moreover, the methyl, phenyl or oxo substituent, in 5–7 derivatives, in addition to the benzylidene group in the derivatives 8–10, were allocated in plan of the thiazole ring (Fig. 2) (Table S1).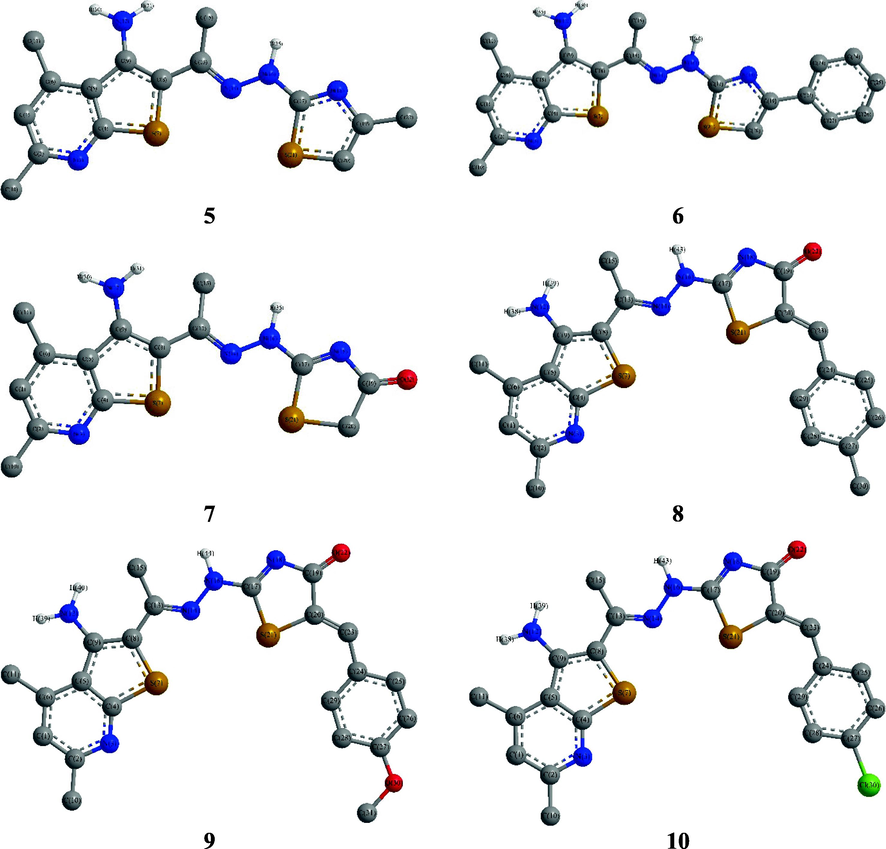
DFT structures of the 5–10 derivatives.
Additionally, the DFT calculated bond lengths and angles were approximately coincided with those reported for similar compounds X-ray single crystal (Dyachenko, Dyachenko, Dorovatovsky, Khrustalev, & Nenajdenko, 2020; Studzińska et al., 2015), where the maximum divergence in lengths was 0.16 Å with 0.051–0.069 Å RMSD while the angles were deviated by 13.3° maximum with 4.94–5.46° RMSD, that may be attributed to the absence of intermolecular columbic interactions in quantum chemical calculations as isolated molecule in gaseous state was considered, however, the factual gained from interacting molecules in solid crystal lattice (Sajan, Joseph, Vijayan, & Karabacak, 2011) (Tables S2-S3).
3.2.1 Frontier molecular orbitals
The HOMO-LUMO configurations and energetic values mainly controlling the molecule electron donation or acceptance (Bulat, Chamorro, Fuentealba, & Toro-Labbe, 2004) where reducing the HOMO-LUMO gap will result in ease intramolecular charge transfer (Makhlouf, Radwan, & Ghazal, 2018; Xavier, Periandy, & Ramalingam, 2015) which may influence the molecule’s biological activity (Bouchoucha, Zaater, Bouacida, Merazig, & Djabbar, 2018). The diagram demonstrated that compound 4 has HOMO spread over the whole molecule and consisted of the π-orbital thienopyridine moiety along with the lone pair of electrons belong to heteroatoms, while their LUMO has been built of the whole molecule π*-orbitals. The thiazolyl thienopyridine derivatives exhibited resemble constructure of the HOMO-LUMO, for instance, the methyl and phenyl derivatives, 5 and 6, presented almost coincided HOMO and LUMO configurations in which the former was built up of the fused and thiazole rings π-orbitals in addition to the lone pair of heteroatoms while the latter represented the π*-orbitals of the whole molecule except the methyl or phenyl substituent. Similarly, the FMO of thiazolone derivatives, 7–10, showed close configurations to those of the previously mentioned but with minor contribution of the benzylidene group in both orbitals. Eventually, the HOMO-LUMO charge transfer occurred in the synthesized derivatives could be mainly defined as π → π* and n → π* transitions (Fig. 3).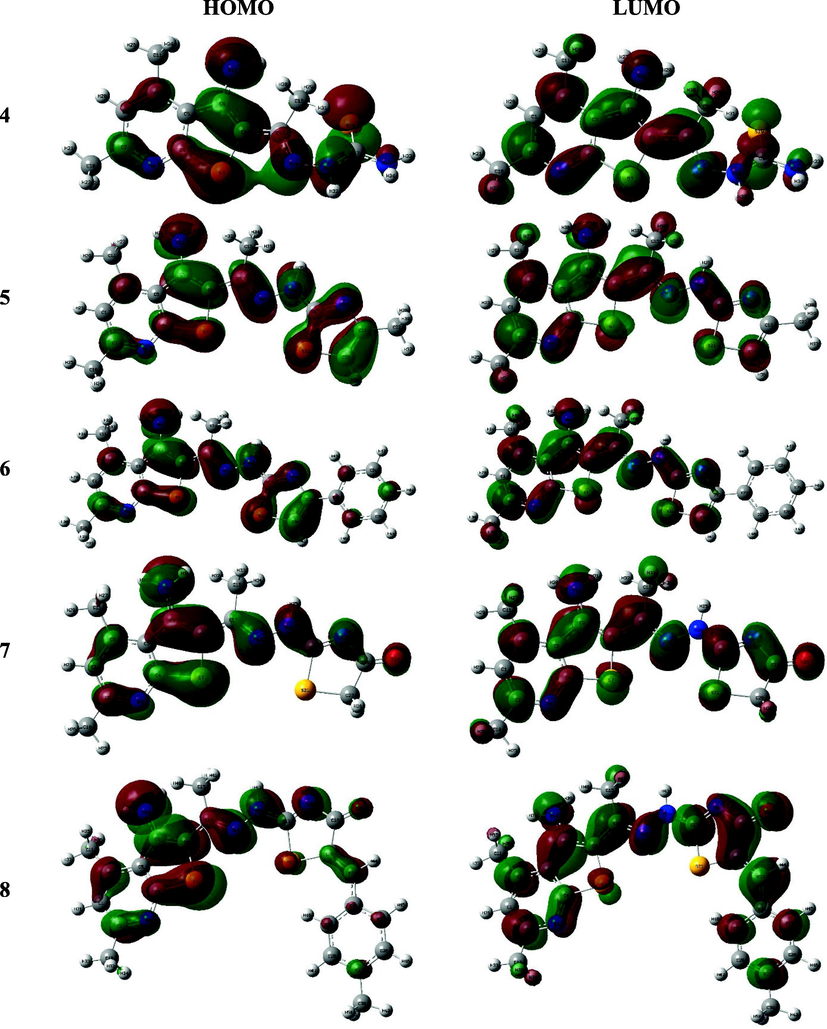
The HOMO and LUMO of the compounds 4–10.
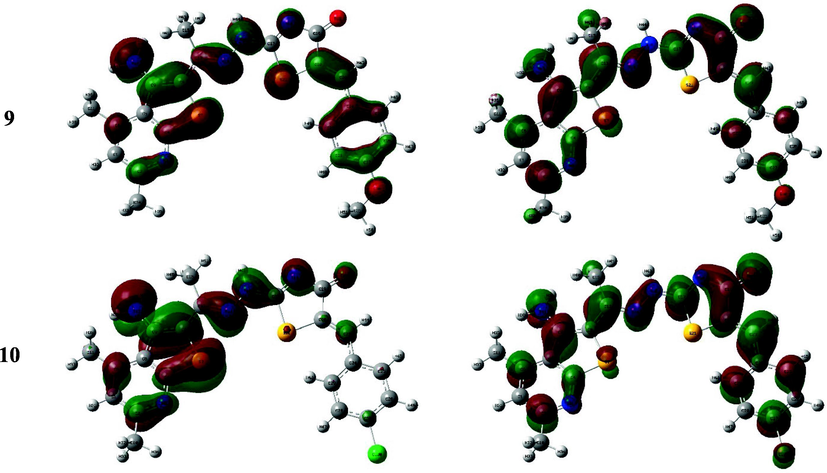
The HOMO and LUMO of the compounds 4–10.
The HOMO (EH) and LUMO (EL) energies were influenced by the abovementioned findings. Even though, the energy data revealed that all the derivatives exhibited close values of the EH and EL, −4.85 - −5.52 and −2.79 – −3.62 eV, respectively. Also, the thiazolone derivatives 7–10 were possessed higher EH and EL than the other compounds 4–6, where the compound 10 exhibited the highest values. However, the ΔEH-L energy gap, ranged from 1.90 to 2.19 eV, indicated that the thiazolone derivatives have lower values than the parent and thiazole derivative and thus, the investigated compounds may be ordered due to ΔEH-L as 10 < 8 < 9 < 6 = 5 < 7 < 4 (Table 1).
Compound
EH
EL
ΔEH-L
χ
η
δ
ω
4
−4.97
−2.79
2.19
3.88
1.09
0.91
6.89
5
−4.85
−2.84
2.01
3.84
1.00
1.00
7.36
6
−4.96
−2.95
2.01
3.95
1.00
1.00
7.78
7
−5.44
−3.32
2.12
4.38
1.06
0.94
9.05
9
−5.37
−3.39
1.97
4.38
0.99
1.01
9.71
8
−5.40
−3.44
1.96
4.42
0.98
1.02
9.98
10
−5.52
−3.62
1.90
4.57
0.95
1.05
10.96
Furthermore, the chemical reactivity parameters like electronegativity (χ), global hardness (η), softness (δ) and electrophilicity (ω), were assessed using the found energies by the formulae (Xavier et al., 2015).
The data revealed that, according to electronegativity (χ), the derivatives whose have the lowest and highest Lewis’s acid character were 5 and 10, 3.84 and 4.57 eV, respectively. Whereas, the global softness (δ) and hardness (η) values designated that compound 4 possessed the maximum electrons receiving and charge transfer ability, 1.09 and 0.91 eV, respectively. Thus, in consistent with hardness, the studied derivatives may be ordered as 10 < 8 < 9 < 6 = 5 < 7 < 4, in agreement with that of ΔEH-L gap (Table 1).
3.2.2 Atomic Mulliken’s charges and Fukui’s indices
The charge transfer within a molecule can be correlated to the Mulliken’s atomic charges (Bhagyasree et al., 2013). Generally, the thienopyridine sulfur S1(thpy) and nitrogen N7(thpy) atoms own close positive charges in all derivatives, 0.366–0.380 and 0.102–0.135, respectively, while the carbon atom that attached to the hydrazone moiety, C2(thpy), groups was bearing negative charge, −0.430 - −0.442, that may be ascribed to participation of the SN atoms in resonating structure of the fused ring and the electron release effect of the hydrazone group to C2(thpy). Moreover, the nitrogen atoms of amino, NH2(thpy), and hydrazone, N2(Hzn) and N1H(Hzn), groups were differentially negatively charged where the former was the highest, −0.697 - −0.713, while the N2(Hzn) has the lowest charge, −0.028 – −0.053 (Table 2). On the other hand, the carbothioamide sulfur and nitrogen atoms in compound 4 have negative charge, SC(Hzn) = -0.062 and NH2(Hzn) = -0.595, respectively. But when they involved in thiazole ring formation in compounds 5–10, the sulfur atom turned to be positively charged, S1(thia) = 0.253–0.380, whereas the nitrogen atom N3(thia), retained small negative charge in the methyl and phenyl derivatives, 5 and 6, respectively, and acquired small positive charge in the thiazolone derivatives, 7–10, 0.010–0.036. The charge conversion of these atoms may be ascribed to the involvement of resonance structure of thiazole ring and electron withdrawing effect of the oxygen of the thiazolone, respectively. Moreover, the oxygen atom of thiazolone ring, O(oxo), in 7 has lower negative charge than in substituted thiazolones 8–10, −0.177 and −0.194 - −0.200, respectively, which might be assigned to the electron releasing effect of the benzylidene substituents (Table 2).
Atom
4
5
6
7
8
9
10
S1(thpy)
0.368
0.366
0.366
0.380
0.376
0.376
0.378
C2(thpy)
−0.430
−0.438
−0.439
−0.441
−0.442
−0.440
−0.441
C3(thpy)
0.181
0.192
0.193
0.198
0.196
0.196
0.197
C3a(thpy)
0.323
0.321
0.321
0.319
0.320
0.320
0.319
C4(thpy)
0.402
0.400
0.401
0.397
0.404
0.397
0.397
C5(thpy)
−0.504
−0.503
−0.502
−0.465
−0.499
−0.465
−0.464
C6(thpy)
0.099
0.098
0.098
0.105
0.099
0.105
0.106
N7(thpy)
0.132
0.134
0.134
0.105
0.135
0.102
0.104
C7a(thpy)
−0.665
−0.663
−0.662
−0.656
−0.659
−0.656
−0.655
NH2(thpy)
−0.697
−0.713
−0.712
−0.705
−0.706
−0.706
−0.704
Me4(thpy)
−0.897
−0.899
−0.898
−0.898
−0.898
−0.898
−0.897
Me6(thpy)
−0.828
−0.828
−0.827
−0.839
−0.826
−0.840
−0.839
C(Hzn)
0.296
0.226
0.229
0.257
0.252
0.257
0.262
Me(Hzn)
−0.886
−0.895
−0.893
−0.887
−0.888
−0.885
−0.884
N2(Hzn)
−0.053
−0.032
−0.028
−0.036
−0.031
−0.032
−0.036
N1H(Hzn)
−0.366
−0.299
−0.299
−0.290
−0.299
−0.301
−0.298
CS(Hzn)
−0.151
SC(Hzn)
−0.062
NH2(Hzn)
−0.595
S1(thia)
0.338
0.348
0.318
0.259
0.253
0.259
C2(thia)
−0.265
−0.324
−0.198
−0.233
−0.233
−0.228
N3(thia)
−0.028
−0.031
0.010
0.033
0.033
0.036
C4(thia)
0.372
0.305
0.035
−0.016
−0.016
−0.011
C5(thia)
−0.720
−0.730
−0.828
−0.243
−0.238
−0.238
Me(thia)
−0.814
C1(Phthia)
0.365
C4(Phthia)
−0.254
O(oxo)
−0.177
−0.198
−0.200
−0.194
C(Bnzl)
−0.275
−0.288
−0.276
C1(PhBnzl)
0.523
0.549
0.541
C4(PhBnzl)
0.458
0.306
−0.128
Me(PhBnzl)
−0.862
OMe(PhBnzl)
−0.222
MeO(PhBnzl)
−0.612
Cl(PhBnzl)
−0.020
In addition, the atomic Fukui’s reactivity indices,
,
and
, to nucleophilic, electrophilic and radical attacks, respectively, were evaluated (El Adnani et al., 2013; Messali et al., 2018; Mi, Xiao, & Chen, 2015; Olasunkanmi, Obot, & Ebenso, 2016). The nucleophilic attack Fukui’s indices (
) of the carbothioamide derivative 4 disclosed that the thienopyridine sulfur S1(thpy) was the most labile atom followed by the carbothioamide sulfur and nitrogen atoms, SC(Hzn) and N2(Hzn), respectively. However, the methyl and phenyl thiazole derivatives 5 and 6, respectively, showed matched order of the most active atoms which is N2(Hzn) > S1(thpy) > C6(thpy) > C4(thpy). Although, the thiazolone derivatives 7–10 presented different pattern of the active sites but they were agreed in that thiazolone oxygen, O(oxo), was the most active one (Table 3).
4
5
6
7
8
9
10
atom
atom
atom
atom
atom
atom
atom
SC(Hzn)
0.12
S1(thia)
0.08
S1(thia)
0.07
S1(thpy)
0.10
S1(thpy)
0.08
S1(thia)
0.05
S1(thpy)
0.09
S1(thpy)
0.10
C5(thia)
0.06
C5(thia)
0.06
NH2(thpy)
0.08
NH2(thpy)
0.07
NH2(thpy)
0.05
NH2(thpy)
0.07
NH2(thpy)
0.08
NH2(thpy)
0.06
NH2(thpy)
0.05
C2(thpy)
0.06
C2(thpy)
0.05
S1(thpy)
0.05
C2(thpy)
0.06
N2(Hzn)
0.06
N1H(Hzn)
0.06
N1H(Hzn)
0.05
O(oxo)
0.06
C3(thpy)
0.05
O(oxo)
0.04
N2(Hzn)
0.05
C2(thpy)
0.05
S1(thpy)
0.05
S1(thpy)
0.05
N2(Hzn)
0.06
N2(Hzn)
0.05
C3(thpy)
0.04
C3(thpy)
0.05
atom
atom
atom
atom
atom
atom
atom
S1(thpy)
0.07
N2(Hzn)
0.06
N2(Hzn)
0.06
O(oxo)
0.06
O(oxo)
0.06
O(oxo)
0.06
O(oxo)
0.07
SC(Hzn)
0.07
S1(thpy)
0.06
S1(thpy)
0.06
S1(thpy)
0.06
C(Hzn)
0.04
C(Hzn)
0.04
Cl(PhBnzl)
0.05
N2(Hzn)
0.07
C6(thpy)
0.06
C6(thpy)
0.05
N2(Hzn)
0.06
C(Bnzl)
0.04
S1(thpy)
0.04
C(Bnzl)
0.05
C6(thpy)
0.06
C4(thpy)
0.05
C4(thpy)
0.05
C6(thpy)
0.05
C6(thpy)
0.04
C6(thpy)
0.04
C(Hzn)
0.04
C4(thpy)
0.06
N7(thpy)
0.04
C3(thpy)
0.04
C(Hzn)
0.05
S1(thpy)
0.04
N2(Hzn)
0.04
C4(thia)
0.04
atom
S+/S-
atom
S+/S-
atom
S+/S-
atom
S+/S-
atom
S+/S-
atom
S+/S-
Atom
S+/S-
C3a(thpy)
7.00
C3a(thpy)
5.00
C3a(thpy)
4.60
C3a(thpy)
4.00
C2(thia)
3.56
C3a(thpy)
3.75
C1(PhBnzl)
4.00
C(Hzn)
2.65
C4(thpy)
2.23
C1(Phthia)
4.00
C(Hzn)
3.25
C3a(thpy)
3.50
C2(thia)
3.10
C5(thia)
3.75
C4(thpy)
2.39
N2(Hzn)
2.13
C4(thpy)
2.35
C2(thia)
2.23
C(Hzn)
2.80
C(Hzn)
2.75
C2(thia)
3.50
N7(thpy)
1.78
N7(thpy)
1.91
N2(Hzn)
2.30
C4(thpy)
1.82
C4(thia)
2.62
C4(thia)
2.46
C2(PhBnzl)
3.40
Me4(thpy)
1.78
Me4(thpy)
1.75
N7(thpy)
2.00
Me(Hzn)
1.75
C2(PhBnzl)
1.71
C4(thpy)
2.00
C4(thia)
2.79
atom
S-/S+
atom
S-/S+
atom
S-/S+
atom
S-/S+
atom
S-/S+
atom
S-/S+
Atom
S-/S+
N1H(Hzn)
2.67
N1H(Hzn)
2.29
C2(Phthia)
2.60
C2(thpy)
2.86
C2(thpy)
3.50
C1(PhBnzl)
4.25
C2(thpy)
5.50
NH2(thpy)
2.23
S1(thia)
1.97
N1H(Hzn)
2.32
N1H(Hzn)
2.43
N1H(Hzn)
2.83
C5(thia)
4.14
S1(thpy)
3.07
C2(thpy)
1.80
C4(thia)
1.95
S1(thia)
2.09
NH2(thpy)
2.22
NH2(thpy)
2.23
N1H(Hzn)
2.67
NH2(thpy)
2.43
SC(Hzn)
1.72
NH2(thpy)
1.90
C4(thia)
2.00
S1(thpy)
1.76
S1(thpy)
2.13
S1(thia)
2.21
N1H(Hzn)
2.36
S1(thpy)
1.42
C5(thia)
1.78
C5(thia)
1.87
N3(thia)
1.17
N2(Hzn)
1.25
C2(thpy)
2.20
N2(Hzn)
1.93
Alternatively, the electrophilic attack Fukui’s indices ( ) of the methyl and phenyl derivatives 5 and 6, offered coincided patterns of the highly susceptible atoms wherein the thiazole S1(thia) and C5(thia) atoms inhabited the first two positions pursued by NH2(thpy), N1H(Hzn) and S1(thpy), respectively. In thiazolone derivatives 7–10, the thienopyridine sulfur, S1(thpy), amino nitrogen, NH2(thpy) and carbon, C2(thpy), were the most active sites, respectively, except in compound 9, in which the thiazolone sulfur atom was the most labile one.
To overcome the inaccurate prediction of the nucleophilic and electrophilic attack active sites via Fukui’s indices, the relative electrophilicity and nucleophilicity descriptors, and , respectively, were estimated (R. Roy, de Proft, & Geerlings, 1998; R. Roy, Krishnamurti, Geerlings, & Pal, 1998; R. K. Roy, Pal, & Hirao, 1999), where , and δ is global softness. The relative nucleophilicity descriptors data, , of the investigated derivatives offered different patterns of the most susceptible atoms but in compounds 4–7 and 9, the most active one was the thienopyridine carbon C3a(thpy) while the derivatives 8 and 10 showed that the thiazole carbon, C2(thia), and benzylidene carbon, C1(PhBnzl), were the first active atoms, respectively.
Alternatively, the relative electrophilicity descriptors, , data of the compound 4 and 5 displayed resemble patterns wherein the azomethine nitrogen, N1H(Hzn), occupied the first place while in derivative 6 the phenyl thiazole carbon C2(Phthia) existed on the top and followed by the azomethine nitrogen, respectively. Whilst in 7, 8 and 10, the thienopyridine carbon, C2(thpy), was the most susceptible site followed by the N1H(Hzn) except in the last where the S1(thpy) was in the second position (Table 3).
3.3 In vitro antimicrobial assay
The synthesized derivatives 3–10 antibacterial effectiveness were screened toward bacterial strains Streptococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, and Escherichia coli. The prepared analogues effectiveness has been matched with the references, ampicillin and gentamicin for Gram (+ve) and Gram (-ve) bacteria, correspondingly. All of analogues and reference were established using a concentration of 5 > g/mL and recorded in Table 4. The synthesized thienopyridine derivatives 8–10 having arylidine thiazolone moiety revealed significant inhibitory effect (18.9 ± 0.63––24.3 ± 0.74 µg/mL) on Gram (+ve) S. aureus, appropriate inhibition with (14.2 ± 0.41––19.5 ± 0.64 µg/mL) on Gram (-ve) E. coli, in comparison with ampicillin and gentamicin, respectively. Temporarily, some pervious thinopyridine derivatives have hydrazinyl-acetamide moiety inhibition effectiveness towards Gram (-ve)strains, particularly towards S. aureus, with an inhibition zone of 19 mm and an MIC value of 15.63 µg/mL (Mohi El-Deen, Abd El-Meguid, Hasabelnaby, Karam, & Nossier, 2019). Where, thienopyridine compound 4 containing thiosemicarbazone arm displayed higher inhibition (17.2 ± 0.49 µg/mL) on S. aureus and (19.1 ± 0.61 µg/mL) on B. subtilis as Gram (+ve) bacterial strains. Meanwhile, thieno-pyridine derivative 7 with hydrazinyl thiazolone moiety demonstrated proper inhibition (26.8 ± 0.58 µg/mL) toward B. subtilis. Though, derivatives 5, 6 with methyl and phenyl thiazoles displayed lower inhibitions against both of gram (+ve and –ve) strains, and thienopyridine compound 3 exhibited the lowest inhibition than the other analogues. Moreover, the title derivatives 3–10 were screened also toward antifungal strains A. fumigatus and C. albicans in contrast to Miconazole as a reference. Only, thienopyridine derivative 8–10 have arylidine thiazolone moiety discovered higher inhibitory (19.2 ± 0.58––23.4 ± 0.65 µg/mL) over C. albicans. Furthermore, the synthesized derivatives 3–10 were not displayed any remarkable inhibitions against A. fumigatus. Ampicillin reference for Gram(+ve), Gentamicin reference for Gram (–ve) bacteria, and Miconazole reference for antifungal agent. Experiments were achieved in triplicate and results are characterized by mean ± S.D.
Organism Entry
Gram-positive bacteria
Gram-negative bacteria
Fungi
S. aureus
B. subtilis
S. typhimurium
E. coli
C. albicans
A. fumigatus
3
13.6 ± 0.37
12.7 ± 0.28
11.6 ± 0.22
9.9 ± 0.33
14.9 ± 0.72
15.8 ± 0.38
4
17.2 ± 0.49
19.1 ± 0.61
11.4 ± 0.36
10.3 ± 0.22
18.2 ± 0.26
11.2 ± 0.24
5
16.6 ± 0.17
18.9 ± 0.29
11.3 ± 0.45
8.9 ± 0.41
12.9 ± 0.63
13.4 ± 0.65
6
13.5 ± 0.06
14.3 ± 0.36
10.2 ± 0.31
11.7 ± 0.53
16.3 ± 0.15
14.3 ± 0.58
7
19.5 ± 0.62
26.8 ± 0.15
12.3 ± 0.25
12.8 ± 0.19
12.6 ± 0.38
10.9 ± 0.40
8
24.3 ± 0.74
21.4 ± 0.03
18.7 ± 0.68
19.5 ± 0.64
19.2 ± 0.58
15.7 ± 0.19
9
18.9 ± 0.63
17.9 ± 0.49
11.4 ± 0.27
14.2 ± 0.41
23.4 ± 0.65
11.9 ± 0.34
10
19.5 ± 0.44
29.8 ± 0.58
12.3 ± 0.25
16.6 ± 0.19
22.3 ± 0.25
12.3 ± 0.07
Ampicillin
23.6 ± 0.23
32.5 ± 0.20
–
–
–
–
Gentamicin
–
–
17.6 ± 0.37
20.4 ± 0.21
–
–
Miconazole
–
–
–
–
27.6 ± 0.24
25.2 ± 0.33
3.3.1 Structure-activity relationship
Thienopyridine derivative 8 has p-methyl on the arylidine thiazolone moiety and displayed eminent inhibition (24.3 ± 0.74 µg/mL) towards S. aureus compared to ampicillin reference (23.6 ± 0.23 µg/mL) which may led to amazing enhancements in the inhibition effectiveness upon the existence of a methyl group (methylation effect) (Naclerio, Karanja, Opoku‐Temeng, & Sintim, 2019), rather than the substituted chlorine atom in derivative 10 that showed good inhibition, more than derivative 9 which was substituted by a methoxy group (Asiri et al., 2021). Whereas, the presence of the thiosemicarbazone moiety showed respectable inhibitions towards both of S. aureus and B. subtilis as Gram (+ve) bacterial strains, as in thieno-pyridine 4 due to thiosemicarbazone, which showed remarkable inhibition as mentioned in several literatures (Azam, Warad, Al‐Resayes, Siddiqui, & Oves, 2013; Hashem, Amr, Nossier, Elsayed, & Azmy, 2020; Tehranchian, Akbarzadeh, Fazeli, Jamalifar, & Shafiee, 2005). Meanwhile, thienopyridine-thiazole hybrid 5 has a methyl-thiazole branch that displayed moderate inhibition, while hybrid 6 has a phenyl-thiazole branch that demonstrated weak reactivity rather than the rest of the derivatives even more than hybrid 3 that contain acetyl group, this may be attributed to the isolation of the thiazole moiety from the conjugation with the thieno-pyridine nucleus, which led to the formation of two separate parts that revealed weak activities. Moreover, thienopyridine derivative 7 with the hydrazinyl thiazol-4-one moiety demonstrated appropriate inhibition due to the thiazol-4-one ring, displayed promising lipid peroxidation inhibitors (Narasimhan, Kumar, & Sharma, 2010).
Eventually, the Multiple Linear Regression methodology (MLR) (Worachartcheewan, Nantasenamat, Isarankura-Na-Ayudhya, Prachayasittikul, & Prachayasittikul, 2011), built-in OriginPro program (OriginLab, 2018), was employed to explore the relationship between the anticancer activity (IC50), as dependent variable, and the quantum chemical calculation parameters (EH, EL, ΔEH-L, χ and δ), as independent variables (Table 5). The data presented that the HOMO-LUMO gap coefficients have negative sign which denotes that the increase in such parameter will lead to decrease in the activity. Moreover, the cell lines showed good regression coefficients, R2 = 0. 8747–0. 9962, with standard deviation, SD = 0.26–4.66.
Organism
Intercept
EH
EL
ΔEH-L
χ
δ
R2
SD
S. aureus
−1.02
1.08
5.09
−4.63
5.09
5.09
0.9726
1.35
B. subtilis
−1.02
−3.44
3.85
−6.07
1.11
4.88
0.8747
4.66
S. typhimurium
0.30
−0.12
−1.48
−5.58
−1.48
−1.48
0.9559
1.45
E. coli
1.84
−1.21
−9.19
−3.80
−9.19
−9.19
0.9931
0.75
C. albicans
1.93
−0.15
−9.66
2.17
−9.66
−9.66
0.9264
2.81
A. fumigatus
0.95
−0.86
−4.76
−2.97
−4.76
−4.76
0.9962
0.26
3.3.2 Escherichia coli DNA gyrase inhibition
The inhibition of DNA gyrase has been proposed as a potential technique for producing antimicrobial drugs that can combat antimicrobial resistance. As a result, the most active antibacterial analogues 7, 8, 9, and 10 were chosen to compare their in vitro inhibitory activity against DNA gyrase from Escherichia coli to novobiocin (Novo) as a reference inhibitor. Table 6 shows that the thieno-pyridine analogue 7 with the hydrazinyl thiazolone moiety produced mild inhibition (IC50 = 8.27 ± 0.43 g/mL). Meanwhile, p-methyl analogue 8 demonstrated acceptable inhibition action (IC50 = 6.46 ± 0.01 M), p-methoxy analogue 9 had the most active inhibitory activity (IC50 = 3.19 ± 0.56 M), and Novo demonstrated inhibition (IC50 = 4.31 ± 0.61 M). Moreover, p-chloro analogue 10 showed promising inhibition activity (IC50 = 5.83 ± 0.29 µM) near that of the inhibition value of Novo. From the compounds’ IC50 values, it was clear that analogues 8–10 possess the presence of thienopyridine-thiazolin-4-one with p-methyl, p-methoxy, and p-chloro moieties, respectively in their structure supported inhibition activity against DNA gyrase.
Analogues
(IC50 µM)
7
8.27 ± 0.43
8
6.46 ± 0.01
9
3.19 ± 0.56
10
5.83 ± 0.29
Novo (Ref.)
4.31 ± 0.61
3.4 Molecular docking
A crucial phase in drug design, molecular docking is one of the utmost significant tool drug design since it can provide insight into the interaction types of new molecules in the appropriate target protein (Eldeab, 2019; Elzahabi et al., 2018). As pervious literatures, many researches were proceeded to discover the interaction between thienopyridine hybrids and active centers in E. coli DNA gyrase B by utilizing PDB ID: 1AJ6 (Mohi El-Deen et al., 2019; Mohi El-Deen, Abd El-Meguid, Karam, Nossier, & Ahmed, 2020; Mohi El-Deen, Nossier, & Karam, 2022) Herein, we have applied MOE program to expect the effect and interactions between the newly synthesized derivatives and E. coli DNA gyrase B as it expressed by PDB ID: 1AJ6. Table 7 revealed the docking results for the synthesized thieno-pyridine derivatives, including the binding energy score (S), route main square (Rmsd), types and sites of contacts between ligands and 1AJ6 amino-acids, and intermolecular distances (Å) in contrast to Gentamicin reference that displayed one H- donor interaction between O8 of the hydroxyl group on the pyran ring with Ala 100 (2.83 Å), through good binding scores S = -7.0352 kcal/mol over Rmsd = 1.4938 (Figure S1). In comparison to Gentamicin, Acetyl-amino-thienopyridine 3 displayed lower binding score S = -5.5340 kcal/mol with rmsd = 1.2748, through H-donor between S7 of thiophene ring with ASP 73(3.67 Å), H-acceptor between O14 of acetyl group with Gly77 (2.96 Å) (Figure S2). Though, thiosemicarbazone derivative 4 revealed the Rmsd value = 0.8304 that reflect binding energy S = -6.3868 kcal/mol, H-donor between N 16 of with thiosemicarbazone moiety and Asp 73(3.06 Å), and π-H between the pyridine ring with Ile78 (4.31 Å) as in figure S3. However, thiazolyl-thienopyridine 5 with 4-methyl moiety shown poor binding energy value S = -6.5783 kcal/mol over rmsd = 1.1762 through only one H- donor between S 21 of thiazole ring and Asp 73(3.14 Å) (Fig. 4).
No.
S (affinity score) (kcal/mol)
Rmsd (refine unit)
E_conf
Ligand interactions
Interactions
Distance (Å)
3
−5.5340
1.2748
−1.7530
S 7 with ASP 73
O 14 with Gly77H-donor
H-acceptor3.67
2.96
4
−6.3868
0.8304
−50.3914
N 16 with Asp 73
6-ring with Ile78H-donor
π-H3.06
4.31
5
−6.5783
1.1762
25.4519
S 21 with Asp 73
H-donor
3.14
6
−5.3856
1.4216
46.1311
N 16 with Glu 131
S 21 with Glu 131
N18 with Arg 168H-donor
H-donor
H-acceptor3.55
3.68
3.38
7
−6.5561
1.2101
−1.4224
N 14 with Asn 46
5-ring with Ile 78H-acceptor
π-H3.15
4.18
8
−7.2009
1.4516
19.3507
S 7 with Gly 77
S 7 with His 136
5-ring with His 136H-donor
H-donor
H-π3.21
4.33
4.23
9
−6.8076
1.3977
19.4038
5-ring with Ile 78
π-H
3.71
10
−6.3842
0.9785
74.6188
N 18 with Gly 77
H-acceptor
3.29
Gentamicin
−7.0352
1.4938
179.9333
O 8 with Ala100
H-donor
2.83
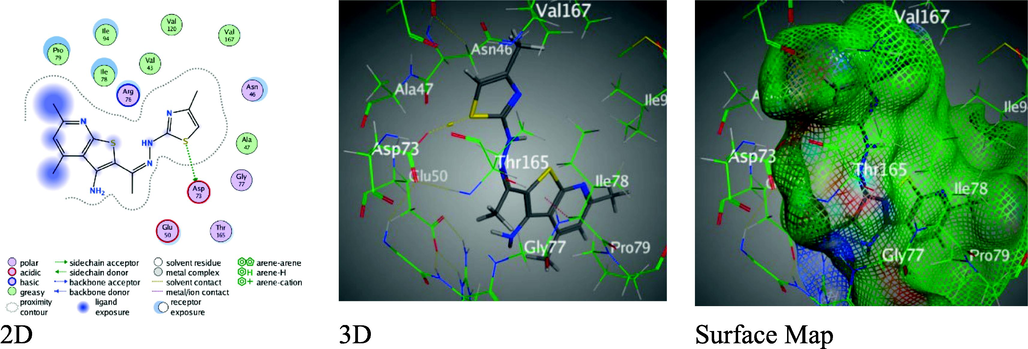
Binding interactions between derivative 5 and 1AJ6 amino-acids.
But, thiazolyl-thienopyridine derivative 6 with 4-phenyl moiety revealed the lowest binding energy value S = -5.3856 kcal/mol through Rmsd = 1.4216 came from three H-bonds, two H-donors were arisen between Glu 131 with both of N16 of hydrazonyl moiety (3.55 Å) and S21 of thiazole ring (3.68 Å), one H-acceptor between N18 of thiazole moiety with Arg 168 (3.38 Å) (Figure S4). Though, thieno-pyridine derivative 7 have thiazolone ring was demonstrated two unlike interactions, H-acceptor between N 14 of hydrazonyl moiety and Asn 46(3.15 Å), π-H binding between thiophene ring with Ile 78(4.18 Å), and Rmsd = 1.2101 with binding score S = -6.5561 kcal/mol (Figure S5). Meanwhile, arylidine derivative 8 showed higher binding scores S = -7.2009 kcal/mol more that the reference’s score with Rmsd = 1.4516 over two H-donors between S7of thiophene ring with of Gly 77 and His 136 through 3.21 and 4.33 Å, respectively. Also, derivative 8 displayed H-π binding between one of the methyl on the pyridine ring with the phenyl ring of His 136 over 4.23 Å (Fig. 5).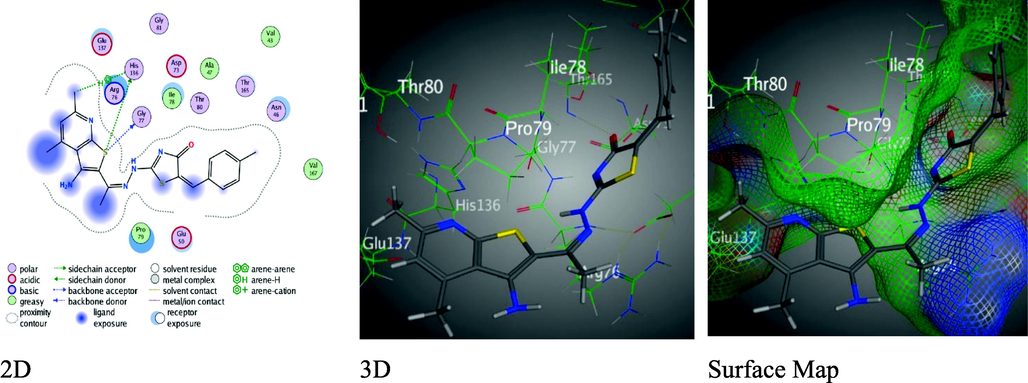
Binding interactions between derivative 8 and 1AJ6 amino-acids.
Moreover, p-methoxy derivative 9 presented one π-H binding among benzene moiety with Ile 78 (3.71 Å), through poor binding scores S = -6.8076 kcal/mol with Rmsd = 1.3977 (Fig. 6).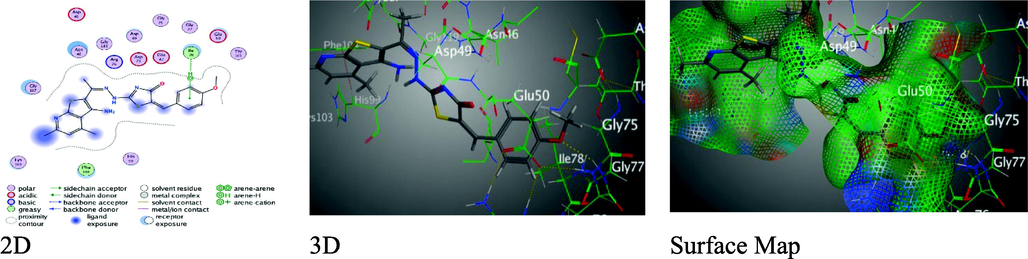
Binding interactions between derivative 9 and 1AJ6 amino-acids.
Furthermore, p-chloro derivative 10 offered one H-acceptor interaction between N18 of thiazole ring with Gly 77 (3.29 Å), through poor binding scores S = -6.3842 kcal/mol(Ismail, Abdulwahab, Nossier, El Menofy, & Abdelkhalek, 2020) with good Rmsd = 0.9785 (Figure S6).
Finally, the stimulating reasonable docking experiment is used as a last step to predict how much the ligand interactions change as they interact with different analogues. A network of hydrogen bonding, H-π, and π-H interactions between the chemical structures of the different heterocyclic nucleuses with different amino acids of 1AJ6 was explained and represented through 2D and 3D images. The most of the synthesized analogues that were docked into the 1AJ6 pockets may be seen as a fork surrounded by polar and anon-polar residues (Asp 73, Ile78, Gly77, and His 136). Additionally, in relation to the formed derivatives, thieno-pyridine analogues 5, 7, 8, and 9 recorded acceptable binding scores toward the amino acids of 1AJ6, which somewhat nearest with the outcomes of the antibacterial activities.
4 Conclusion
A series of thieno[2,3-b]pyridine-thiazole hybrids were obtained by heterocyclization of 2-(1-(3-amino-4,6-dimethylthieno[2,3-b]pyridin-2-yl)ethylidene)hydrazine-1-carbothioamide with chloroacetone, phenacyl chloride, and chloroacetic acid. The investigated thieno-pyridine compounds DFT calculated structure designated that all were deviated from planar conformation. The HOMO-LUMO energy gap of studied derivatives were low, 1.90–2.19 eV, following the order 10 < 8 < 9 < 6 < 5 < 7 < 4. The antibacterial inhibition of the prepared thieno-pyridine analogues 8–10 exhibited effective antimicrobial activity against Gram positive S. aureus and Gram negative E. coli, and C. albicans (antifungal), with inhibition activities at (18.9 ± 0.63––24.3 ± 0.74 µg/mL), (14.2 ± 0.41––19.5 ± 0.64 µg/mL), and (19.2 ± 0.58––23.4 ± 0.65 µg/mL), respectively. Meanwhile, thieno-pyridine 4 contain thiosemicarbazone arm was exhibited respectable inhibition (17.2 ± 0.49 µg/mL) on S. aureus and (19.1 ± 0.61 µg/mL) on B. subtilis. Whoever, thieno-pyridine derivative 7 with hydrazinyl thiazolone moiety demonstrated appropriate inhibition (26.8 ± 0.58 µg/mL) toward B. subtilis. Additionally, molecular docking stimulation between the newly synthesized thieno-pyridine analogues and the E. coli DNA gyrase’s protein demonstrated that analogues 5, 7, 8, and 9 recorded adequate binding scores.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Synthesis and antimicrobial testing of some new S-substituted-thiopyridines, thienopyridines, pyridothienopyrimidines and pyridothienotriazines. Pharmazie. 2003;58(6):372-377.
- [Google Scholar]
- Synthesis, photophysical properties and biological activities of some new thienylpyridines, thienylthieno[2.3-b]pyridines and related fused heterocyclic compounds. J. Heterocycl. Chem. 2022
- [Google Scholar]
- A review on development of bio-active thiosemicarbazide derivatives: Recent advances. J. Mol. Struct.. 2021;1226:129268
- [Google Scholar]
- Synthesis and antimicrobial evaluation of some novel bis-α, β-unsaturated ketones, nicotinonitrile, 1, 2-dihydropyridine-3-carbonitrile, fused thieno[2, 3-b] pyridine and pyrazolo[3,4-b]pyridine derivatives. Int. J. Mol. Sci.. 2013;14(2):2967-2979.
- [Google Scholar]
- Synthesis and biological evaluation of tetracyclic thienopyridones as antibacterial and antitumor agents. Bioorg. Med. Chem.. 2011;19(8):2541-2548.
- [Google Scholar]
- Thieno[2,3-b] pyridine derivatives: a new class of antiviral drugs against Mayaro virus. Arch. Virol. 2017;162(6):1577-1587.
- [Google Scholar]
- A synthesis, in silico, in vitro and in vivo study of thieno[2,3-b] pyridine anticancer analogues. Med. Chem. Comm.. 2015;6(11):1987-1997.
- [Google Scholar]
- Recent developments in the synthesis of polysubstituted pyridines via multicomponent reactions using nanocatalysts. New J. Chem.. 2021;45(28):12328-12345.
- [Google Scholar]
- Synthesis and reactions of di (thiophen-2-yl) alkane diones: Cyclocondensation. Turkish J. Chem.. 2022;46(5):1397-1404.
- [Google Scholar]
- Design, synthesis and antimicrobial activity of novel 2-aminothiophene containing cyclic and heterocyclic moieties. Bioorg. Med. Chem. Lett.. 2021;44:128117
- [Google Scholar]
- Synthesis, physicochemical properties, and in vitro antibacterial screening of palladium (II) complexes derived from thiosemicarbazone. Chem. Biodiver.. 2013;10(6):1109-1119.
- [Google Scholar]
- Synthesis and antidiabetic activity of 2, 5-disubstituted-3-imidazol-2-yl-pyrrolo[2,3-b]pyridines and thieno[2,3-b]pyridines. Bioorg. Med. Chem.. 2007;15(21):6782-6795.
- [Google Scholar]
- Recent developments and biological activities of N-substituted carbazole derivatives: a review. Molecules. 2015;20(8):13496-13517.
- [Google Scholar]
- Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys.. 1993;98(7):5648-5652.
- [Google Scholar]
- Belen’kii, L. I., Gazieva, G. A., Evdokimenkova, Y. B., Soboleva, N. O., 2020. The literature of heterocyclic chemistry, part XVIII, 2018. Adv. Heterocycl. Chem. 132, 385-468.
- Vibrational spectroscopic (FT-IR, FT-Raman, (1) H NMR and UV) investigations and computational study of 5-nitro-2-(4-nitrobenzyl) benzoxazole. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2013;102:99-113.
- [Google Scholar]
- Thieno[2,3-b]pyridine derivatives are potent anti-platelet drugs, inhibiting platelet activation, aggregation and showing synergy with aspirin. Eur. J. Med. Chem.. 2018;143:1997-2004.
- [Google Scholar]
- BIOVIA, D. S. (2017). Materials Studio.
- Synthesis and characterization of new complexes of nickel (II), palladium (II) and platinum(II) with derived sulfonamide ligand: Structure, DFT study, antibacterial and cytotoxicity activities. J. Mol. Struct.. 2018;1161:345-355.
- [Google Scholar]
- Condensation of frontier molecular orbital Fukui functions. J. Phys. Chem.. 2004;108(2):342-349.
- [Google Scholar]
- Thiophene antibacterials that allosterically stabilize DNA-cleavage complexes with DNA gyrase. Proc. Natl. Acad. Sci.. 2017;114(22):E4492-E4500.
- [Google Scholar]
- Thiophene-based compounds with potential anti-inflammatory activity. Pharm.. 2021;14(7):692.
- [Google Scholar]
- Ground-state enthalpies: evaluation of electronic structure approaches with emphasis on the density functional method. J. Phys. Chem.. 2006;110(50):13632-13639.
- [Google Scholar]
- Recent advances in the chemistry of thieno [2, 3-b] pyridines 1. Methods of synthesis of thieno[2,3-b]pyridines. Russian. Chem. Bull.. 2020;69(10):1829-1858.
- [Google Scholar]
- One-pot synthesis of thieno[2,3-b]pyridine and pyrido[3′,2′: 4, 5]thieno[3,2-d]pyrimidine derivatives. Russ. J. Org. Chem.. 2020;56(6):974-982.
- [Google Scholar]
- DFT theoretical study of 7-R-3methylquinoxalin-2(1H)-thiones (RH;CH3;Cl) as corrosion inhibitors in hydrochloric acid. Corros. Sci.. 2013;68:223-230.
- [Google Scholar]
- Ecofriendly microwave assisted synthesis of some new pyridine glycosides. Nucleosides Nucleotides Nucleic Acids. 2019;38(7):509-520.
- [Google Scholar]
- Uses of 2-amino-5,6-dihydro-4h-cyclopenta[b] thiophene-3-carbonitrile in the synthesis of heterocyclic compounds with anticonvulsant, behavioral and CNS antidepressant activities. Int. Res. J. Pure Appl. Chem.. 2012;2(1):91.
- [Google Scholar]
- Anticancer evaluation and molecular modeling of multi-targeted kinase inhibitors based pyrido [2, 3-d] pyrimidine scaffold. J. Enzyme Inhib. Med. Chem.. 2018;33(1):546-557.
- [Google Scholar]
- DIPEA catalyzed step-by-step synthesis and photophysical properties of thieno[2,3-b] pyridine derivatives. Tetrahedron. 2019;75(34):130465
- [Google Scholar]
- Frisch, M., Trucks, G., Schlegel, H., Scuseria, G., Robb, M., Cheeseman, J., Scalmani, G., Barone, V., Mennucci, B., Petersson, G., 2009. Gaussian 09, Revision A. 1. Wallingford, CT, USA: Gaussian.
- Synthesis of new azobenzene dyes clubbed with thiazolidinone moiety and their applications. Pigm. Resin Technol.. 2020;49(3):207-214.
- [Google Scholar]
- Synthesis of 4-hydroxy coumarin dyes and their applications. Pigm. Resin Technol.. 2016;45(5):320-329.
- [Google Scholar]
- Garcı́a-Tojal, J., Garcı́a-Orad, A., Dı́az, A. A., Serra, J. L., Urtiaga, M. K., Arriortua, M. a. I., Rojo, T., 2001. Biological activity of complexes derived from pyridine-2-carbaldehyde thiosemicarbazone: structure of [Co(C7H7N4S)2][NCS]. J. Inorg. Biochem. 84(3-4), 271-278.
- Synthesis, antimicrobial activity and molecular docking of novel thiourea derivatives tagged with thiadiazole, imidazole and triazine moieties as potential DNA gyrase and topoisomerase IV inhibitors. Molecules. 2020;25(12):2766.
- [Google Scholar]
- Recent advances in the application of the Sonogashira method in the synthesis of heterocyclic compounds. Tetrahedron. 2009;65(37):7761-7775.
- [Google Scholar]
- Huo, H., Li, G., Shi, B., Li, J., 2022. Recent advances on synthesis and biological activities of C-17 aza-heterocycle derived steroids. Bioorg. Med. Chem. 116882.
- Synthesis of novel 2-aminobenzothiazole derivatives as potential antimicrobial agents with dual DNA gyrase/topoisomerase IV inhibition. Bioorg. Chem.. 2020;94:103437
- [Google Scholar]
- Synthesis, characterization and biological activities of imidazo[1,2-a] pyridine based gold (III) metal complexes. Heliyon. 2019;5(6):e01968.
- [Google Scholar]
- Synthesis and biological activity of 2, 4-di-p-phenolyl-6-2-furanyl-pyridine as a potent topoisomerase II poison. Eur. J. Med. Chem.. 2015;90:360-378.
- [Google Scholar]
- Synthesis and biological activity of novel substituted pyridines and purines containing 2, 4-thiazolidinedione. Eur. J. Med. Chem.. 2004;39(5):433-447.
- [Google Scholar]
- DBU promoted facile synthesis of new thieno[2,3-b] pyridine/quinoline derivatives and their antimicrobial evaluation. J. Heterocycl. Chem.. 2013;50(S1):E131-E135.
- [Google Scholar]
- Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev.. 1988;37(2):785-789.
- [Google Scholar]
- Synthesis, preliminary structure–activity relationships, and in vitro biological evaluation of 6-aryl-3-amino-thieno[2,3-b] pyridine derivatives as potential anti-inflammatory agents. Bioorg. Med. Chem. Lett.. 2013;23(8):2349-2352.
- [Google Scholar]
- LS Kishbaugh, T., 2016. Pyridines and Imidazopyridines with medicinal significance. Curr. Top. Med. Chem. 16(28), 3274-3302.
- Experimental and DFT insights into molecular structure and optical properties of new chalcones as promising photosensitizers towards solar cell applications. Appl. Surf. Sci.. 2018;452:337-351.
- [Google Scholar]
- Heterocyclic anticancer compounds: recent advances and the paradigm shift towards the use of nanomedicine’s tool box. Molecules. 2015;20(9):16852-16891.
- [Google Scholar]
- Structure–activity relationships in a series of antiplasmodial thieno [2, 3-b] pyridines. Malaria J.. 2019;18(1):1-10.
- [Google Scholar]
- A new schiff base derivative as an effective corrosion inhibitor for mild steel in acidic media: Experimental and computer simulations studies. J. Mol. Struct.. 2018;1168:39-48.
- [Google Scholar]
- Theoretical evaluation of corrosion inhibition performance of three antipyrine compounds. Comput. Theor. Chem.. 2015;1072:7-14.
- [Google Scholar]
- Design, synthesis, molecular docking and biological studies of 4H-benzo [d][1, 3] oxazin-4-one derivatives and their uses in heterocyclic synthesis. ChemistrySelect. 2022;7(21):e202200325.
- [Google Scholar]
- Synthesis, docking studies, and in vitro evaluation of some novel thienopyridines and fused thienopyridine–quinolines as antibacterial agents and DNA gyrase inhibitors. Molecules. 2019;24(20):3650.
- [Google Scholar]
- Synthesis and biological evaluation of new pyridothienopyrimidine derivatives as antibacterial agents and escherichia coli topoisomerase II inhibitors. Antibiotics. 2020;9(10):695.
- [Google Scholar]
- New quinazolin-4 (3 H)-one derivatives incorporating hydrazone and pyrazole scaffolds as antimicrobial agents targeting DNA gyraze enzyme. Sci. Pharm.. 2022;90(3):52.
- [Google Scholar]
- Antibacterial small molecules that potently inhibit Staphylococcus aureus lipoteichoic acid biosynthesis. Chem. Med. Chem.. 2019;14(10):1000-1004.
- [Google Scholar]
- Synthesis of new thieno[2,3-b] pyridine derivatives as pim-1 inhibitors. J. Enzyme Inhib. Med. Chem.. 2016;31(6):1718-1725.
- [Google Scholar]
- Biological activities of hydrazide derivatives in the new millennium. Acta Pharm. Sci.. 2010;52(2)
- [Google Scholar]
- Adsorption and corrosion inhibition properties of N-{n-[1-R-5-(quinoxalin-6-yl)-4,5-dihydropyrazol-3-yl]phenyl} methanesulfonamides on mild steel in 1 M HCl: experimental and theoretical studies. RSC Adv.. 2016;6(90):86782-86797.
- [Google Scholar]
- OriginLab. (2018). OriginPro (Version 2018): OriginLab Corporation Northampton, MA.
- Biological evaluation and molecular modelling study of thiosemicarbazide derivatives as bacterial type IIA topoisomerases inhibitors. J. Enzyme Inhib. Med. Chem.. 2016;31(1):14-22.
- [Google Scholar]
- Pair-distribution function and its coupling-constant average for the spin-polarized electron gas. Phys. Rev.. 1992;46(20):12947-12954.
- [Google Scholar]
- Novel thieno[2,3-b] pyridine anticancer compound lowers cancer stem cell fraction inducing shift of lipid to glucose metabolism. Int. J. Mol. Sci.. 2022;23(19):11457.
- [Google Scholar]
- 4-(3H)-quinazolinones N-3 substituted with a five membered heterocycle: A promising scaffold towards bioactive molecules. Eur. J. Med. Chem.. 2021;213:113070
- [Google Scholar]
- Is it reliable to take the molecular docking top scoring position as the best solution without considering available structural data? Molecules. 2018;23(5):1038.
- [Google Scholar]
- Roy, R., de Proft, F. d., Geerlings, P., 1998. Site of protonation in aniline and substituted anilines in the gas phase: a study via the local hard and soft acids and bases concept. J. Phys. Chem. 102(35), 7035-7040.
- Local softness and hardness based reactivity descriptors for predicting intra-and intermolecular reactivity sequences: carbonyl compounds. J. Phys. Chem.. 1998;102(21):3746-3755.
- [Google Scholar]
- On non-negativity of Fukui function indices. J. Phys. Chem.. 1999;110(17):8236-8245.
- [Google Scholar]
- Natural bond orbital analysis, electronic structure, non-linear properties and vibrational spectral analysis of L-histidinium bromide monohydrate: a density functional theory. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2011;81(1):85-98.
- [Google Scholar]
- Novel Nicotinonitriles and Thieno [2, 3-b] pyridines as potent biofilm and COX-2 inhibitors: Synthesis, in vitro and in silico studies. ChemistrySelect. 2020;5(28):8494-8503.
- [Google Scholar]
- Synthesis, cytotoxicity and in vitro antibacterial screening of novel hydrazones bearing thienopyridine moiety as potent COX-2 inhibitors. J. Iranian Chem. Soc.. 2020;17(12):3299-3315.
- [Google Scholar]
- New thieno[2,3-b]pyridine-fused[1,2,4]triazolo[4,3-a]pyrimidinone hybrids as potential MRSA and VRE inhibitors. Mendeleev Commun.. 2021;31(3):370-372.
- [Google Scholar]
- Synthesis of thiophene derivatives and their anti-microbial, antioxidant, anticorrosion and anticancer activity. BMC Chem.. 2019;13(1):1-13.
- [Google Scholar]
- Heterocyclization of polarized system: synthesis, antioxidant and anti-inflammatory 4-(pyridin-3-yl)-6-(thiophen-2-yl) pyrimidine-2-thiol derivatives. Chem. Cent. J.. 2018;12(1):1-8.
- [Google Scholar]
- Sidorenko, A. Y., Kravtsova, A., Wärnå, J., Aho, A., Heinmaa, I., Il’ina, I., Ardashov, O., Volcho, K., Salakhutdinov, N., Murzin, D. Y., 2018. Preparation of octahydro-2H-chromen-4-ol with analgesic activity from isopulegol and thiophene-2-carbaldehyde in the presence of acid-modified clays. Mol. Catal. 453, 139-148.
- 2-Allylaminothiazole and 2-allylaminodihydrothiazole derivatives: synthesis, characterization, and evaluation of bioactivity. Monatsh. Chem.. 2015;146(10):1673-1679.
- [Google Scholar]
- Synthesis and antibacterial activity of 1-[1,2,4-triazol-3-yl] and 1-[1,3, 4-thiadiazol-2-yl]-3-methylthio-6,7-dihydrobenzo [c] thiophen-4 (5H) ones. Bioorg. Med. Chem. Lett.. 2005;15(4):1023-1025.
- [Google Scholar]
- Synthesis and antiproliferative activity of 2-chlorophenyl carboxamide thienopyridines. Bioorg. Med. Chem. Lett.. 2017;27(2):135-138.
- [Google Scholar]
- Predicting the free radical scavenging activity of curcumin derivatives. Chemom. Intell. Lab. Syst.. 2011;109(2):207-216.
- [Google Scholar]
- NBO, conformational, NLO, HOMO–LUMO, NMR and electronic spectral study on 1-phenyl-1-propanol by quantum computational methods. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2015;137:306-320.
- [Google Scholar]
- Synthesis, reactions and biological activity of 2-substituted 3-cyano-4, 6-dimethylpyridine derivatives. Chem. Heterocycl. Compd.. 2009;45(1):35-41.
- [Google Scholar]
- Synthesis and antibacterial activities of different five-membered heterocyclic rings incorporated with pyridothienopyrimidine. ACS Omega. 2020;5(11):6163-6168.
- [Google Scholar]
- Design, synthesis, and biological activity evaluation of 2-(benzo[b]thiophen-2-yl)-4-phenyl-4,5-dihydrooxazole derivatives as broad-spectrum antifungal agents. Eur. J. Med. Chem.. 2022;228:113987
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105226.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







