Translate this page into:
Nonhydrolytic sol-gel in-situ synthesis of high performance MgAl2O4/C adsorbent materials
⁎Corresponding authors at: National Engineering Research Center for Domestic & Building Ceramics, Jingdezhen Ceramic University, Jingdezhen 333000, China. guofeng@jci.edu.cn (Guo Feng), jiangweihui@jci.edu.cn (Weihui Jiang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
MgAl2O4/C composite was prepared via a facile low-temperature non-hydrolytic sol-gel (NHSG) route, using Mg powder, Al wire and isopropanol as raw materials. The influence of types of magnesium source and heat treatment temperature on the synthesis and adsorption properties were investigated, and the adsorption mechanism was also studied. The results showed that the amorphous MgAl2O4 formed at < 600 °C, and it crystallized at 700 °C. No impurity phase appeared in the samples calcined at 700–1300 °C, which was attributed to MgAl2O4 crystallized directly from Mg(Al(OiPr)4)2. The uniform-doped carbon in MgAl2O4/C composites came in-situ from the organic groups in Mg(Al(OiPr)4)2. MgAl2O4/C showed a superior adsorption capacity for Congo red (CR). The bimetallic alkoxides structure was favorable for high adsorption property, and the adsorption property of amorphous MgAl2O4/C was significantly superior to that of its crystalline counterpart. The adsorption kinetics data was fitted with the pseudo-second-order model, while the Langmuir isotherm model could well descript the adsorption isotherm behavior, and the maximum adsorption capacity for CR was 5690 mg/g. The high adsorption capacity was attributed to the Lewis acid-base reaction and the electrostatic interactions between the anionic dye CR and MgAl2O4/C surface as well as the in-situ carbon, and amorphous state.
Keywords
Nonhydrolytic sol-gel
MgAl2O4
Congo red
Removal
Adsorption
Adsorption capacity
- NHSG
-
non-hydrolytic sol-gel
- CR
-
Congo red
- HSG
-
hydrolytic sol-gel method
Abbreviations
1 Introduction
Synthetic dyes are widely applied as raw materials in many industries, e.g., in textile, leather tanning, plastic, papermaking, rubber and printing (Vidhu et al., 2014). They provide rich color for people's lives and make important contributions to industrial development and social progress. However, it should be noticed that synthetic dyes have caused serious adverse effects on the water environment. Large quantities of dyestuffs are directly discharged into natural water without meeting the discharging standards. They can change the color of water, reduce sunlight penetration and affect the growth of animals and plants in water resource. Worse, most of the dyes are toxic, even carcinogenic and teratogenicity (Hussain et al., 2020), which poses great threats to human health and other organisms. Therefore, it is an urgent task to developing efficient techniques to eliminate dyes in wastewater before releasing to environment. Numerous methods have been used for the removal of dyes from wastewater, including enzyme degradation (Kaur et al., 2021), adsorption (Ahmad et al., 2020), ion exchange process (Cseri et al., 2021), Fenton reaction (Jain, 2018) and photocatalytic oxidation (Gao et al., 2022), etc. Among these methods, adsorption method has considered to be a fairly successful separation method for wastewater purification, because of its excellent ability to remove almost any type of dyestuff, high removal percentage, easy operation, require no complex equipment or without posing secondary pollution generation (Santhoshkumar et al., 2022). It is well-known that the identity of adsorbent is crucial to the process of alleviating dyestuff in wastewater. Therefore, developing efficient adsorbents is of great importance for the removal of dye molecules from aqueous solutions. So far, several types of adsorbents, including activated carbon (Ma et al., 2020), waste material or naturally abundant biomass (Dovi et al., 2021), clay and minerals (Zhou et al., 2021), metal (hydro) oxide (Kataria et al., 2017), metal-organic frameworks (MOFs) (Guo et al., 2020), have been investigated for the removal of dyes from polluted water. The waste materials, such as peanut husk (Dovi et al., 2021), pineapple peel (Dai et al., 2020), may have lower cost, but adsorbent prepared by them usually suffer from poor adsorption capacity. The merits belonging to MOFs are the high BET surface area, abundant intramolecular pores, thus having attracted much attentions in the fields of separation and adsorption. But its disadvantages, such as high preparation cost, complicated process, are remain unsolved.
In recently years, metal oxides have emerged as promising materials in the area of dyes removal, since their low toxicity, abundant Lewis acid sites and environmentally friendly (Cao et al., 2021, Zhang et al., 2016b). One of the most attractive metal oxides is MgAl2O4 (Guo et al., 2018). It is the unique compound in the Al2O3-MgO binary system. Owing to its excellent properties such as high melting point, good optical properties, high chemical stability and temperature stability (Ganesh et al., 2013), MgAl2O4 has been extensively used in refractory, transparent ceramic, organic dye adsorption, catalyst and catalyst support (Ganesh et al., 2004).
To date, many efforts have been devoted to the preparation of MgAl2O4 adsorbent. For example, H. Liu et al. (Liu et al., 2022) prepared MgAl2O4 nanoparticles by polyacrylamide gel method with an adsorption capacity of 89 mg/g for Congo red. J. Tian et al. (Tian et al., 2015) synthesized MgAl2O4 spinel powders with hierarchical porous structures via a hard template process with a maximum adsorption capacity (845 mg/g) for Congo red. X. Guo et al. (Guo et al., 2018) synthesized MgAl2O4 spinel with continuous mesostructured skeletons and interconnected macropores, which exhibited good adsorption ability for Congo red (2721 mg/g).
However, the adsorption capacity of the above mentioned MgAl2O4 is poor. Therefore, how to obtain high-efficiency adsorbent for organic dye removal is in urgent need.
MgAl2O4 is an important oxide that has been fabricated with various methods, including hydrothermal/solvothermal synthesis (Ren et al., 2015), coprecipitation method (Alvar et al., 2010), sol-gel processing (Milani et al., 2021) and non-hydrolytic sol-gel (NHSG) method (Li et al., 2017). Non-hydrolytic sol-gel method (NHSG) is an attractive wet-chemical method in favor of controlling reaction at atomic scale. It is proposed by Corriu date back to 1990′s (Corriu et al., 1992). In contrast to the traditional hydrolytic sol-gel method (HSG), the sol directly condensed to form the metal-oxide-metal bond through polycondensation, instead of hydrolyzing to form metal hydroxides. Hence, there is no need to consider the issue that the inconsistent hydrolysis rates of different metal alkoxides, which simplifies the process greatly. Moreover, the NHSG method allows reaction easy to obtain high homogenous mixed gels with different metal ions, and thus easier to obtain high purity products at lower calcination temperature. Besides, NHSG method is a simple and powerful method to prepare homogeneous mixed oxide xerogels with mesoporous structure (Debecker et al., 2013). Whereas only a few researches have synthesized MgAl2O4 by this method. H. Li et al. (Li et al., 2017) prepared MgAl2O4 nanopowder via NHSG using AlCl3 and MgCl2 as raw materials. MgAl2O4 and MgO coexisted in the as-synthesized sample (calcined at 700–800 °C). The appearance of MgO demonstrated large number of Mg-O-Mg homogeneous condensation occurred, which indicating the use of magnesium or aluminum source needed further optimization.
On the basis of our previous studies (Wu et al., 2022, Jiang et al., 2021a), MgAl2O4/C adsorbent is prepared via NHSG method in this work. In NHSG, the sol does not go through metal alkoxide hydrolysis process but directly turn into gel by the reactants polycondensation. Therefore, NHSG method allows the heterogeneous polycondensation products containing carbon instead of hydroxyl (Debecker et al., 2013). Carbonization of polycondensation products in the heating process is one of the characteristics of NHSG process. Our previous research (Zhao et al., 2020b) revealed that the performance of environmental treatment material (Fe2TiO5) was significantly improved by compounding with in-situ carbon introduced by in situ carbonization of precursors. There is no similar report in the field of preparing MgAl2O4 adsorption materials. Therefore, we designed to prepare MgAl2O4/C adsorbent through the idea that introducing in-situ carbon by NHSG method. To the best of our knowledge, there is no report about MgAl2O4 adsorbent or MgAl2O4/C adsorbent for Congo red prepared via NHSG method. In this work, the influence of types of magnesium source and calcination temperature on the synthesis and properties of the MgAl2O4/C material were systematically investigated. Besides, the present study involved a systematically study of the adsorption mechanism. The as-synthesized sample showed an ultra-high adsorption capacity for Congo red removal from water. In addition, equilibrium and kinetics studies were performed to determine the CR adsorption capability of MgAl2O4/C composite adsorbent materials.
2 Experimental
2.1 Chemical reagents
Magnesium fluoride (MgF2, >99.5 %), magnesium chloride (MgCl2, >99.5 %), aluminum wire (Al, 5 N), isopropanol (CH3CH(OH)CH3, >99.7 %) were purchased from Sinopharm Chemical Reagent Corporation (Shanghai, China). Magnesium powder (Mg, 100–200 mesh), iodine (I2, >99.8 %) and Congo red (C32H22N6Na2O6S2, Ind) was obtained from Aladdin Industrial Corporation (Shanghai, China). The above reagents were used without further purification.
2.2 Samples preparation
The MgAl2O4 sol was prepared by the NHSG method and MgAl2O4/C adsorbent were obtained by a subsequent calcination process. Briefly, 2.160 g aluminum wire and 1.000 g iodine were carefully added to 100 mL isopropanol, followed by refluxed under magnetic stirring at 80 °C for 6 h. As the aluminum wire dissolved, a grey transparent solution gradually formed. Different magnesium source (2.492 g MgF2, 3.818 g MgCl2 or 0.097 g Mg powder) was added into the solution above with molar ratio n(Mg)/n(Al) = 1:2 for samples prepared with different magnesium sources, respectively. Keeping refluxing at 110 °C for 24 h, MgAl2O4 wet gel was obtained. It was further evaporated to form xerogel and dried at 100 °C for 12 h. Finally, the MgAl2O4/C was achieved after calcining at different temperatures (200–1300 °C) for 1 h with a 5 °C /min heating rate. The samples were prepared based on the steps above, as shown in Fig. 1. Fixing Al wire as aluminum source, samples of M1, M2, and M3 were obtained by using MgF2, MgCl2 and Mg powder as magnesium source, respectively.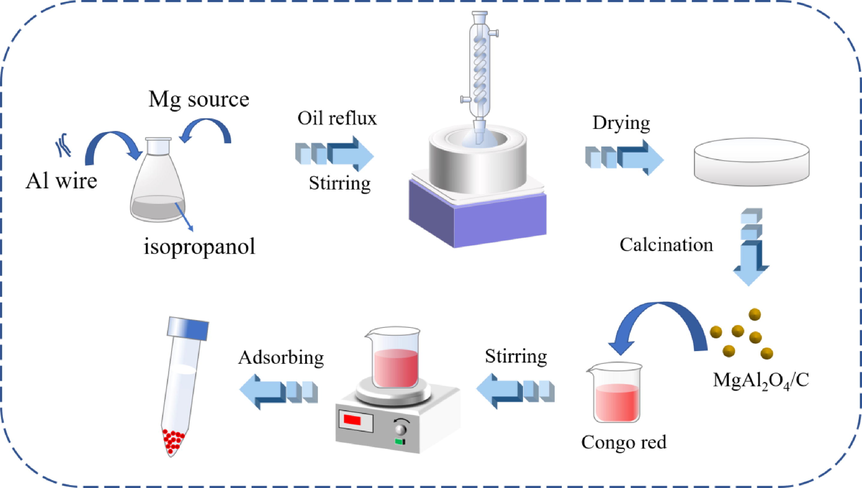
Samples preparation process.
2.3 Characterization
The phases present in the samples were examined by XRD analysis (D8 Advance, Bruker, Germany) using copper target Kα radiation at 30 mA and 40 kV. The sol-gel transition process, along with MgAl2O4/C before and after adsorbed CR were characterized by Fourier transform infrared (FT-IR) spectroscopy (Nicolet 5700, Thermo, America) in the wavenumber range 400–4000 cm−1. The 27Al solid-state NMR spectrum of MgAl2O4/C was analysed (400 M, Bruker, Switzerland) at a Larmor frequency of 10 KHz. The zeta potential (ζ-potential) of MgAl2O4/C before and after adsorbed CR was studied by a zeta potential analyzer (Zetasizer Nano ZS90, Malvern, England). DTA-TG-MS analysis was conducted by a DTA-TG (STA449C, Netzsch, Germany) coupled with a mass spectrometer (GMBH QMS403C, Netzsch, Germany). The element distribution of as-prepared MgAl2O4/C composite were collected by energy dispersive X-ray spectroscopy (Model 500i, IXRF, America). Transmission electron micrograph (TEM) images were obtained with a JEOL 2010 microscope operated at 200 kV. The surface chemical state of the as-synthesized MgAl2O4/C adsorbent before and after adsorption were collected by X-ray photoelectron spectroscopy (K-Alpha + XPS, Thermo Fisher Scientific, America) with the Al Kα radiation. All spectra were calibrated using the binding energy of C 1 s (284.8 eV) to compensate for the surface charging effects. The avantage software was used to fit the XPS spectra of each relevant element into subcomponents. The nitrogen adsorption experiments of sample calcined at different temperature were conducted on a nitrogen adsorption apparatus (ASAP2020M, Micromeritics, America).
2.4 Adsorption performance experiments
The adsorption kinetic experiments of MgAl2O4/C were obtained on the adsorption of Congo red (CR) in water using batch experiments. Firstly, the MgAl2O4/C adsorbent was added into a glass beaker containing CR solution. The dosage of adsorbent was kept at 1 g/L, and solution pH value was fixed at 7. The beaker was then magnetically stirred with a speed of 500 rpm/min. Subsequently, a certain amount of sample (3 mL) was taken from the solution and centrifuged at different time intervals, and the concentration of the supernatant solution was measured with UV–vis spectrophotometer (Lambda 850, PerkinElmer, America). The adsorption capacity (qt, mg/g) of CR adsorption at time t was calculated by Eq. (1). The isotherm experiments were proceeded in the similar way to the adsorption kinetic experiments. The as-prepared adsorbent was placed into CR solution (500–6000 mg/L), and the adsorbent dosage was kept at 1g/L. Followed by magnetic stirring till reaching the adsorption equilibrium. Subsequently, the mixture was taken out and centrifuged to determine the residual CR concentration. The equilibrium adsorption capacity (qe, mg/g) was calculated according to Eq. (2).
Where C0 (mg/L) is initial concentration of CR; Ct & Ce (mg/L) are CR concentration at time t and equilibrium concentrations; V (L) is the volume of the CR solution; m (g) is mass of the adsorbent.
3 Results and discussion
3.1 Effects of types of magnesium source on the synthesis of MgAl2O4
Fig. 2 shows the XRD patterns of samples series M1-M3 calcined at 400–1300 °C to study the effects of magnesium source. Samples series M1, M2 and M3 shown in Fig. 2(a), (b) and (c) are samples prepared with MgF2, MgCl2 and Mg powders as magnesium source calcined at different temperatures, respectively.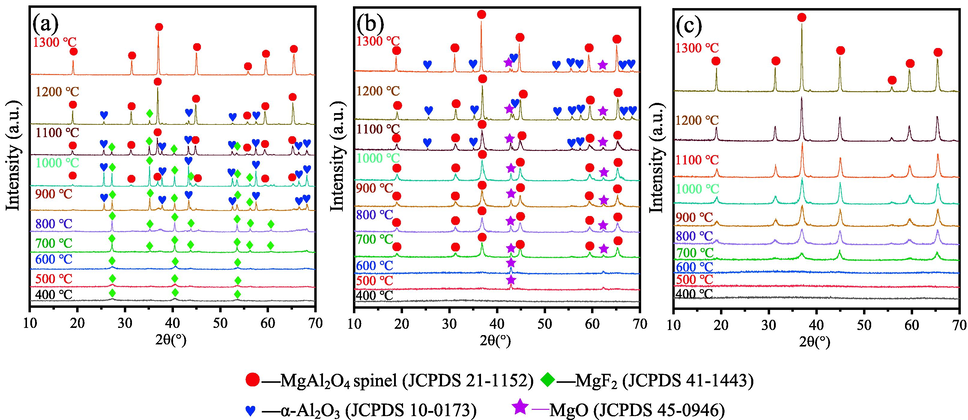
XRD patterns of samples M1(a), M2(b), M3(c) calcined at different temperatures.
As displayed in Fig. 2(a), most MgF2 has not reacted with aluminium source or participated in non-hydrolytic polycondensation reaction in the sol-gel process. This is because MgF2 is dfficult to dissolve in isopropanol, which makes it can not react with aluminium source. After annealing at 900 °C, α-Al2O3 (JCPDS 10–0173) appears in the sample, which is due to the decomposition of aluminium isopropoxide obtained in the reaction between aluminiume and isopropanol (shown in eq. (4)). MgAl2O4 spinel (JCPDS 21–1152) forms at 1000 °C. α-Al2O3 and MgF2 disappear with the temperature further increasing to 1300 °C. Correspondingly, the intensity of the peaks belonging to MgAl2O4 spinel phase increases, which confirms the MgAl2O4 is produced through the reaction between α-Al2O3 and MgF2 (shown in eq.(5)). The presence of diffraction peaks of α-Al2O3 and MgF2 at 900–1200 °C also demonstrates that pure MgAl2O4 phase can not be synthesized before 1300 °C. In summary, the formation of MgAl2O4 in M1 sample mainly due to the solid phase reaction between MgF2 and Al2O3 when using MgF2 as magnesium source, instead of through NHSG route.
When MgCl2 is used as magnesium source, after heat-treatment at 700 °C, the diffraction peaks of MgAl2O4 spinel (JCPDS 21–1152) appear, indicating that the formation of MgAl2O4 spinel crystalline phase. However, the impurity phase of MgO (JCPDS 45–0946) is also observed at 500–1300 °C. The presence of MgO is due to the ionic character of MgCl2. The electrongativity of magnesium and chlorine element in MgCl2 is 1.2 and 3.0, respectively. According to Eq.(6), the ionic character percentage of Mg-Cl bond is calculated to be 55.5 %, which demonstrates that MgCl2 possess relative ionic character. Consequently, the nonhydrolytic polycondensation reaction is difficult to be adequate (Feng et al., 2018). Part of MgCl2 exists in solvent isopropanol in the form of Mg2+ and Cl-, which ultimately lead to the formation of MgO.
In sharp contrast to the XRD results of M1 and M2, only one phase of MgAl2O4(JCPDS 21–1152) can be detected in the sample M3, no impurity peak is observed during the whole temperatures range.
To further study the NHSG process when using Mg powder and Al wire as raw material, Fig. 3 presents the FT-IR spectra of samples of M3 at different reaction stages: (a) isopropanol; (b) mixtures of Mg powder and Al wire with isopropanol (after refluxing at 110 °C for 24 h); (c) xerogel; (d) powder calcined at 700 °C for 1 h.
FT-IR spectra of (a) (CH3)2CHOH, (b) precursors mixture, (c) xerogel, (d) powder calcined at 700 °C.
The FT-IR spectrum of (a) isopropanol shows absorption peaks at 817 & 952 & 1129 &1162 cm−1, 1310 & 1378 & 1467 cm−1, 2886 & 2973 cm−1, 663 & 3382 cm−1, corresponding to the stretching vibration of C—O bond, the asymmetric stretching vibration of C—H, the asymmetry stretching mode of CH3 groups, the bending vibration and stretching vibration of O—H bond in the isopropanol, respectively (Ermini et al., 2000). These demonstrate a characteristic FT-IR spectrum of isopropanol.
In FT-IR spectrum of (b)mixture of Mg + Al+(CH3)2CHOH (after refluxing at 110 °C for 24 h), the bending vibration at 663 cm−1 ascribing to O—H bond of isopropanol disappears, and the broad bond at 3382 cm−1 assigned to O—H bond becomes weaker. It can be concluded that isopropanol has already reacted. In addition, it is worth mention that the absorption peak located at 817 cm−1 which associated to the C—O stretching vibration belonging to isopropanol shift to a lower wavenumber of 813 cm−1 and became weaker. These indicate the formation of C-(O-Mg) bond (Thanabodeekij et al., 2003). Furthermore, the absorption peaks of 457 and 613 cm−1 are related to the stretching vibration of Al—O and C—(O—Al) (Alinejad et al., 2008). The peak at 613 cm−1 is significantly broader than that of the typical C—(O—Al) bonds (Li et al., 2017), which indicates the existence of Al—O and Mg—O—Al bond. Based on the analysis above, the chemical reaction between Mg, Al and isopropanol is displayed in Eq. (7) (Liu et al., 2013):
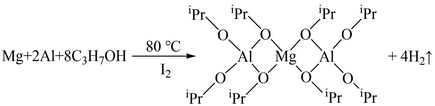
Fig. 3(c) illustrates the FT-IR spectrum of xerogel, it can be seen that the bond associated with the C-(O-Al) at 613 cm−1 become stronger, whereas the peak at 457 cm−1 (Al-O) disappears, demonstrating the further reaction of Al-O bond to form Mg-O-Al bond. It is mainly due to the polycondensation reaction happens between MgAl2(OiPr)8, which further increases the Mg-O-Al bond. The reaction is shown in Eq. (8) (Wu et al., 2022, Turova et al., 2002).

After calcination at 700 °C (Fig. 3(d)), two broad absorption bands appear at 528 and 707 cm−1, corresponding to the vibration of Mg-O-Al network in the MgAl2O4 (Sanjabi et al., 2015). These indicate the formation of MgAl2O4 spinel.
The high-resolution 27Al solid state NMR spectrum of sample M3 obtained at 300 °C is shown in Fig. 4. The peaks located at 66, 37, and 8 ppm can be associated to Al3+ ions in tetrahedral [AlO4], hexahedral [AlO5] and octahedral [AlO6] coordination, respectively (Düvel et al., 2011). These coordination unsaturated Al-species (AlO4 and AlO5) are derived from polycondensation reaction between MgAl2(OiPr)8. It is generally believed that the coordination unsaturated Al-species exhibit high activity (Sarou et al., 2013). Notably, the coordinatively unsaturated Al-species accounted for a large proportion in the MgAl2O4/C composite adsorbent.
27Al NMR of M3-300 adsorbent.
Fig. 5 shows the effect of contact time on the adsorption process of sample M1, M2 and M3 calcined at 300 °C. The initial concentration of CR is 1000 mg/L. The samples obtained at 300 °C are labeled as M1-300, M2-300 and M3-300, respectively. Fig. 6 shows the schematic diagram of the products of gel powder prepared from three magnesium sources after heat treatment at 300 ℃.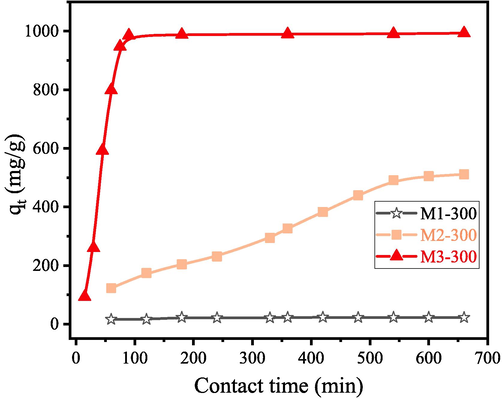
Adsorption capacity of the sample M1-300, M2-300 and M3-300 for CR removal with the change of contact time.

Schematic diagram for the effect of magnesium source on the CR adsorption.
It can be seen from Fig. 5 that the adsorbents obtained from the three different magnesium sources exhibit distinct different adsorption performance for CR solution. Among them, the MgAl2O4 sample prepared with magnesium powder as magnesium source shows the highest adsorption capacity (98.89 mg/g) for CR, followed by the MgCl2 (543.95 mg/g) and the MgF2 (19.51 mg/g). Therefore, it can be speculated that the sample M3 which possesses the bimetallic alkoxides structure is favorable for the adsorption of CR solution. XRD patterns shown in Fig. 2 demonstrate that the three different magnesium sources have different phase transition process. These correspond to the different proceed degree of nonhydrolytic heterogeneous polycondensation reaction, and ultimately lead to their difference in adsorption performance. Sample M1-300 is composed of amorphous aluminium isopropoxide and MgF2, because MgF2 cannot dissolve in isopropoxide. The saturated Al3+ and Mg2+ ions in aluminium isopropoxide and MgF2 lead to poor adsorption performance. However, when using Mg powder as magnesium source, tetracolecular association structure (Wu et al., 2022, Turova et al., 2002) shown in the right part of equation (8) formed in sample M3-300 through the polycondensation between MgAl2(OiPr)8. The Al3+ and Mg2+ irons in tetracolecular association structure are mostly unsaturated, which guarantees the excellent adsorption performances of M3-300. Sample M2-300 contains tetracolecular association structure, amorphous aluminium isopropoxide and MgCl2. The tetracolecular association structure can effectively adsorb CR, while amorphous aluminium isopropoxide and MgCl2 cannot. Therefore, the adsorption performance of M2-300 is at the value between M1-300 and M3-300.
3.2 Effect of calcination temperature on the adsorption performance
Fig. 7(a) illustrates the relationship between adsorption capacity and initial concentration when using sample M3 prepared at different temperatures as adsorbents. The maximal adsorption capacities variation with calcination temperature are shown in Fig. 7(b). The samples calcined at 200–900 °C are labelled as M3-200, M3-300, M3-400, M3-500, M3-600, M3-200, M3-800 and M3-900, respectively.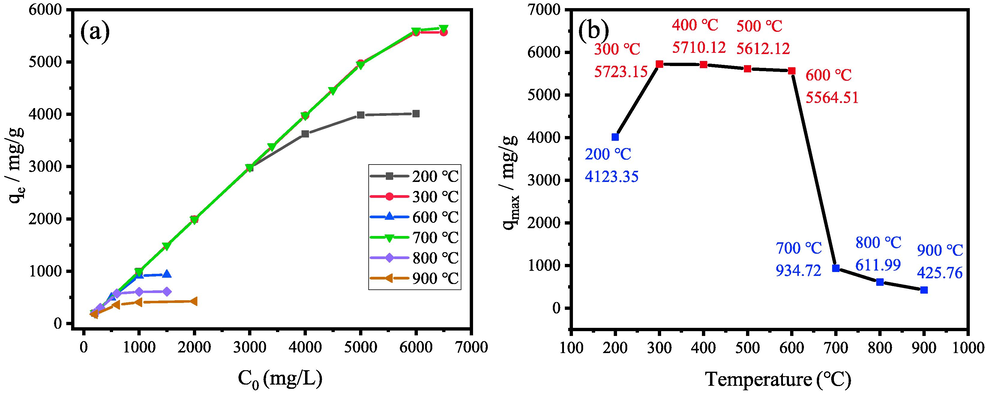
(a) adsorption capacity of the sample M3 calcined at different temperatures for CR removal with the change of initial concentration, (b) maximal adsorption capacity of sample M3 calcined different temperatures.
The results reveal that calcination temperature has a significant influence on the adsorption capacity toward CR solution. It can be seen from Fig. 7(b) that the maximal adsorption capacity is calculated to be 5723.15, 5710.12, and 5612.12, 5564.51 mg/g for M3-300, M3-400, M3-500 and M3-600, respectively. Thereby, M3-300 adsorbent displays the optimum adsorption efficiency among all these samples, which is slightly higher than the adsorption capacity of M3-400, M3-500 and M3-600. Taking M3-300 adsorbent as an example, it can be seen from Fig. 7 (a) that the adsorption capacities qe increase linearly with the increase of initial concentration C0 (at the range of 600–5000 mg/L), the linear growth of adsorption capacities qe is retarded under further increase of initial concentration, and then the adsorption capacities qe gradually become a fixed value (at the range of 5000–6500 mg/L). It reveals that sample M3-300 exhibits excellent adsorption capacity toward CR solution.
However, the maximum adsorption capacity of M3-700, M3-800 and M3-900 decrease significantly with the elevating of temperature. Samples M3-700, M3-800 and M3-900 only have the adsorption capacities of 934.72, 611.99 and 425.76 mg/g. This significant decreasing of adsorption capacities of M3-700, M3-800 and M3-900 is mainly due to the crystallization of MgAl2O4 spinel at 700 °C. There are abundant coordinative-unsaturated Al3+ and Mg2+ in the tetracolecular association structure at the temperatures below 700 °C. In the process of MgAl2O4 crystallization, the unsaturated Al3+ and Mg2+ irons transfer to the saturated Al3+ and Mg2+ in MgAl2O4, which leads to the reduction of adsorption performance.
Meanwhile, it should also be noted that sample M3-200, which is also amorphous, exhibits a lower adsorption capacity than M3-300. In order determine the reason for causing this difference, Fig. 8 shows the DTA-TG-MS curves of the xerogel of M3. There are three stages for the decomposition of xerogel in the air atmosphere. The first stage locates in the temperature ranges from room temperature to 126 °C, which corresponds to a weight loss of 4.79 %. This endothermic peak centered at 76 °C is associated with the further removal of solvent isopropanol and polycondensation by-product isopropyl ether. The second stage involves a wide-broad endothermic peak accompanied by a mass loss of 31.42 %, and the endothermic peak is in the range of 160–600 °C centered at 227 °C. This endothermic peak can be ascribed to the carbonization of the skeleton of residual organic groups in the xerogel and the emission with burning of residual organic groups. After the temperature of 600 °C, the third weight loss stage in the thermogravimetric curve is observed, and it is attributed to the slowly oxide of the carbon-containing group, which lays the foundation for the preparation of MgAl2O4/C composite. These are the reasons that sample M3-300 with the carbonization exhibits the higher adsorption capacity than that of sample M3-200. Besides, thermogravimetry (TG) curve of > 300 °C is shown in Fig. 8(b). The total weight loss varies in the range of 74.34–54.88 wt%. According to the mass of the sample tested and the final weight loss in the test process (Zhao et al., 2020b), the carbon-containing residual content of M3-300 is calculated to be 26.15 wt%.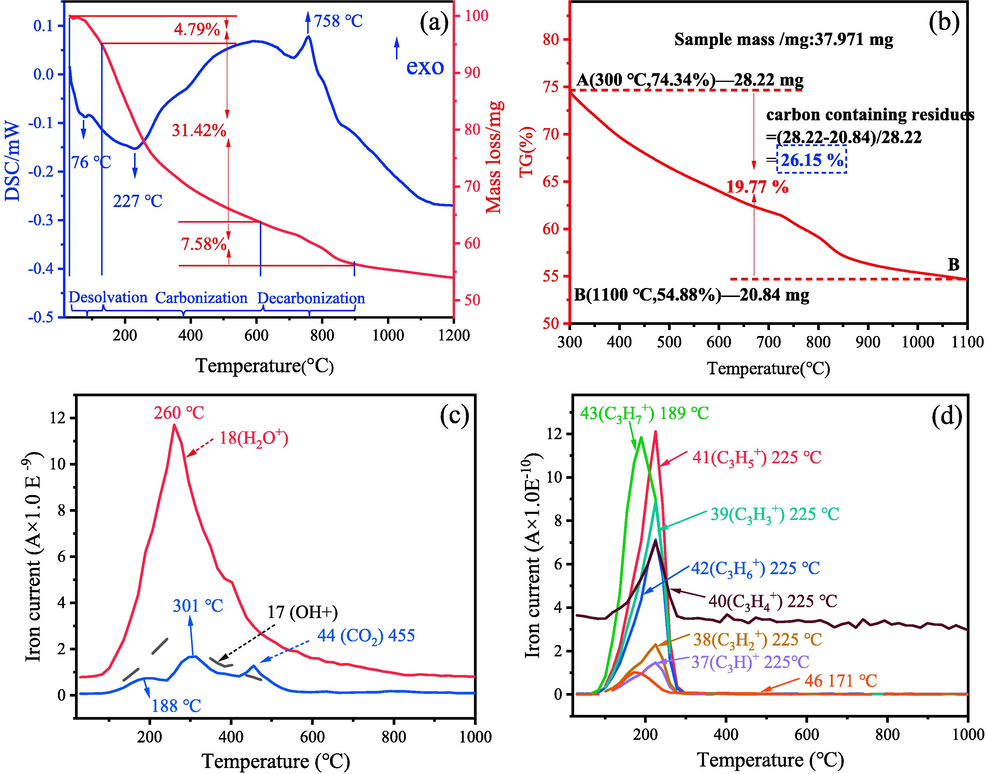
(a) DTA-TG curves of the xerogel, (b) TG curve of the xerogel from 300 °C to 1100 °C, (c-d)MS curves of various products during thermal decompositon.
In addition, the emissions of the MS curves of xerogel shown in Fig. 8(c)(d) present high concentration emissions and low concentration emissions, respectively. These emissions include C3H+ (225 °C), C3H2+ (225 °C), C3H3+ (225 °C), C3H4+ (225 °C),C3H5+ (225 °C), C3H6+ (225 °C), H2O+ (260 °C), OH–(252 °C)and CO2+ (188 °C & 301 °C & 455 °C), respectively·H2O+ (260 °C) shows the strongest emission in the temperature ranges from 120 to 600 °C, and OH– (252 °C) also exhibit a high emission in the range of 120–500 °C. These two points suggest that a great number of O and H originated from residual organic group releasing during the heat treatment of the xerogel, which ensures in situ C residue to form MgAl2O4/C composite material.
The formation mechanism of OH– from the residual organic group can be explained as follows. In contrast to MgAl2O4 crystal phase, M3 sample calcined at the temperatures < 600 °C has an amorphous structure, the amorphous MgAl2O4 are composed of abundant coordinative-unsaturated Mg2+ and Al3+. Both Mg2+ and Al3+ are strong Lewis acid, they can coordinate strongly with oxygen-containing groups. This process makes oxygen-containing groups undergo strong polarization during calcination and decompose into various free radicals or radical association compounds (Martra et al., 2000, Suh et al., 1992). The emission of H2O+ can be attributed to the H contained in isopropyl combined with OH–, then ultimately caused the carbonization of residual organic group. In addition, as can be seen from Fig. 8(c), the group of C3H7+ emission occurs at 189 °C. It is worth mention that a series group of C3H+, C3H2+, C3H3+, C3H4+, C3H5+ and C3H6+ emit at 225 °C, which are the isopropyl group (-C3H7) that have lost hydrogen with a higher emitting concentration. These are the specific reasons ensure the residual of carbon in the system.
As presented in Fig. 8(d), the continuous escape of C3H4+ after 300 °C demonstrated that in-situ carbon in the MgAl2O4/C may exist in the form of C3H4+. During the adsorption process, the existence of C3H4+ in the MgAl2O4 adsorbent is benefit to improve the wetting process between organic pollutants and adsorbent, leading to the promoting of the adsorption, and carbon itself is also a commonly used adsorbent. Based on the analysis above, the groups emission-carbonization process in the heating process of xerogel is shown in Fig. 9.
Schematic illustration of the whole transition process of xerogel during heating process.
3.3 Adsorption kinetics
The contact time dependence of the adsorption capacity at different initial concentrations is shown in Fig. 10. The adsorbent shows a very fast adsorption rate, when the initial concentration of CR solution is 500, 1000 and 2000 mg/L. Its time required is only 20, 120 and 270 min, respectively. The quick adsorption is ascribed to the abundant sorption sites on the surface of MgAl2O4/C adsorbent material.
Contact time dependence of the adsorption capacity at different initial concentrations.
To further analyze the adsorption mechanism, the kinetics of CR adsorption on the MgAl2O4/C composite were investigated by pseudo-first-order (PFO) and pseudo-second-order (PSO) kinetics models. The corresponding linear equations of the two kinetic models are shown in Eq. (9) and (10), respectively. Fig. 11 illustrates the fitted straight lines, and Table 1 displays the values of kinetic parameters calculated according to the two equations.
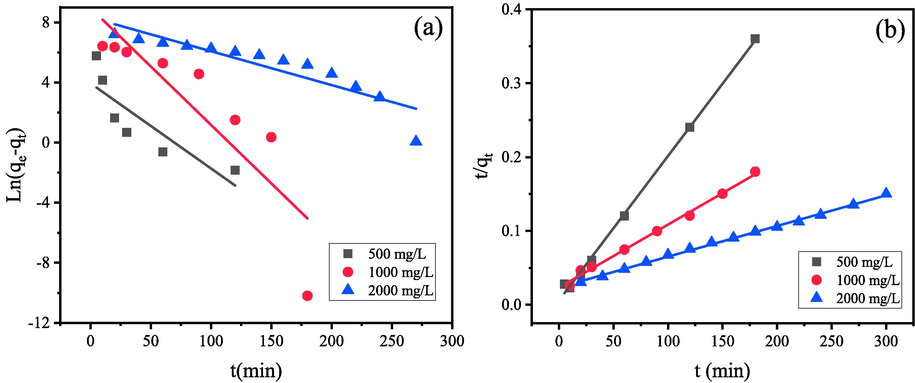
(a)linearly fitted kinetic curves for CR adsorption according to the linear equations for pseudo-first-order, (b) pseudo-second-order kinetics.
C0/mg/L
qe (exp) / mg/g
Pseudo-first order
Pseudo-second order
qe cal
k1*10-2
R2
qe cal
K2*10-2
R2
500
499.63
138.15
5.67
0.728
510.20
0.066
0.997
1000
998.84
1284.55
7.78
0.765
1137.76
0.0033
0.993
2000
1997.65
2497.78
2.25
0.821
2375.21
0.0007
0.997
Where k1 (min−1) and k2 (g/mg·min) are rate constants of pseudo-first and second-order models, respectively.
As observed, the straight lines in Fig. 11(b) show a better match with the experimental data points compared with those in Fig. 11(a). According to Table 1, it can be seen that experimental results agree well with the PSO kinetic model. Because the R2 (correlation coefficients) values for this model are closer to 1 (0.993–0.997), and the R2 calculated from PFO model is much lower (0.728–0.821). In addition, the as-measured experimental adsorption capacity data [qe(exp)] can match better to the calculated adsorption capacity values [qe(cal)] obtained by PSO model than that from PFO kinetic model. Therefore, it can be seen that the adsorption process of MgAl2O4/C fitted better with the PSO model, and it is reasonable to conclude that chemical adsorption is probably-one of the rate-limiting steps.
3.4 Adsorption isotherms
The adsorption isotherms were used to reveal the relationship between the amount of CR adsorbed and the constant equilibrium concentration of CR in the liquid phase. The adsorption isotherms of MgAl2O4/C composite are shown in Fig. 12. The equilibrium adsorption quantity increases with the increase of equilibrium concentrations and then reaches adsorption saturation.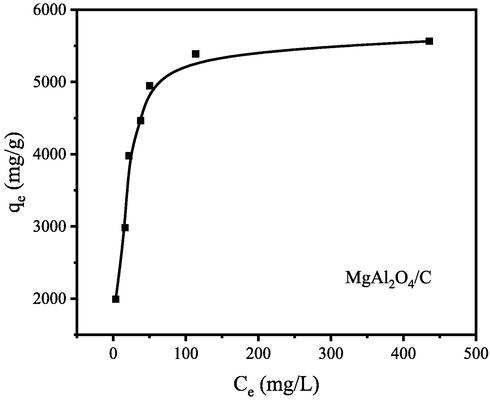
Equilibrium isotherms of MgAl2O4/C for CR adsorption. (Initial CR concentration = 2000–6000 mg/L, adsorbent dose = 1 g/L, T = 30 °C).
To attain profound information about the adsorption behavior and mechanism, two well-known adsorption isotherms have been selected to analyze the experimental adsorption results. They are Langmuir and Freundlich isotherm models. The linearized forms of the two models can be expressed by Eqs. (11) and (12), respectively. The Langmuir model is used to described monolayer adsorption, and it assumes the adsorption occurs on the homogenous surface of adsorbent. The Freundlich model stands for the heterogeneous adsorption and multilayer adsorption patterns.
Where KL (L/mg) is the Langmuir adsorption constant, KF is the Freundlich constant, and n is the adsorption intensity factor.
Fig. 13 illustrates the fitted results using isotherm equations, and the determined parameters of both isotherm models are presented in Table 2. As shown in Fig. 13 and Table 2, the Langmuir model fitted better to the adsorption data. Because the Langmuir model gave higher correlation coefficients (R2 = 0.9996) than that of the Freundlich equations (R2 = 8057), so the CR is adsorbed on the surface of MgAl2O4/C composite in a monolayer coverage.
Corresponding linearly fitted isotherms: Langmuir isotherm (a) and Freundlich isotherm (b).
Langmuir isotherm
Freundlich isotherm
KL (L/mg)
qm (mg/g)
R2
KF (L/mg)
n
R2
0.1085
5690.19
0.9996
1777.41
4.5149
0.8057
The as-prepared MgAl2O4/C with outstanding adsorption capability is superior to other previously reported magnesium oxide based and alumina based metal (hydro)oxides adsorbents shown in Table 3.
Adsorbent type
Adsorption capacity (mg/g)
Reference
MgAl2O4/C
5690
this work
MgO nanofiber
4802
Yu et al., 2018
20 % MgO-SiO2
4000
Hu et al., 2018
MgO hollow microspheres
3022
Dai et al., 2018
MgAl2O4
2721
Guo et al., 2018
MgO nanoparticles
2375
Zhang et al., 2019
MgO sphere
1928
Ahmad et al., 2019
Porous MgO
1638
Zhao et al., 2020a
Mesoporous γ-Al2O3 nanofibers
1323
Li et al., 2021
Flower-like Mg/Fe-LDO
1250
Mubarak, 2021
Fe2O3·Al2O3 composite
941
Jiang et al., 2021b
porous MgAl2O4
845
Tian et al., 2015
Al2O3@ZnO
714
Zheng et al., 2019
MgO/GO
684
Guo et al., 2021
γ-Al2O3/ZnFe2O4
413
Sun et al., 2018
g-Al2O3
370
AlSalihi et al., 2022
Mg–Al-LDH
305
Sriram et al., 2020
MgAl2O4 nanoparticles
89
Liu et al., 2022
3.5 Adsorption mechanisms analysis
FT-IR was used to further investigate the interaction between MgAl2O4/C composite adsorbent and CR in the adsorption process. The spectra of MgAl2O4/C, CR and CR-adsorbed MgAl2O4/C are performed and displayed in Fig. 14. For MgAl2O4/C before adsorption (curve (a) in Fig. 14), two strong vibration bonds centered at around 528 and 707 cm−1 appear. These bonds correspond to the characteristic bond of Al-O-Mg vibrations from MgAl2O4/C (Dash et al., 2017).
(a)FT-IR spectra of MgAl2O4/C, (b) MgAl2O4/C after CR adsorption and (c) pure CR.
In the IR spectrum of CR (curve (c) in Fig. 14), the adsorption peak appears at 3459 cm−1 is ascribed to the stretching vibration of N—H bond (Cao et al., 2021). The peak at 1548 cm−1 can be attributed to the stretching vibration of azo group (—N⚌N—) (Cao et al., 2021). The peak at 1616 cm−1 is associated with C⚌C stretching vibration in benzene (Wang et al., 2013), and the peaks at 1226, 1178 and 1062 cm−1 are ascribed to the stretching vibration of S⚌O in sulfonate group (—SO32-) (Cao et al., 2021). In comparison with the FT-IR spectrum of the pure MgAl2O4/C and CR, it is noticed that several obvious changes appear after adsorption. The —NH2 group peak (at 3459 cm−1) shifts to a lower position (3425 cm−1). It merges with the OH stretching vibration bond of the MgAl2O4/C (3384 cm−1). It can be explained by the Lewis acid-base interaction between Mg2+ and Al3+ on the MgAl2O4 and —NH2 on CR molecules. According to Pearson‘ s hard-soft acid-base (HSAB) principle, Mg2+ and Al3+ are both hard Lewis acid site, and —NH2 is belong to a hard Lewis base site. They tend to combine with each other to form more stable bonds (Wang et al., 2020). Furthermore, after adsorption on the MgAl2O4/C, it is evident that the sharp bond at 1062 cm−1 which relate to S⚌O stretching vibration occurs at a lower wavenumber of 1048 cm−1. The shift of S⚌O bond, which ascribe to the characteristic peak of sulfonate group (—SO32-) in CR molecules, indicates that the stability of S⚌O bond on CR decreases. The results above verify that Congo red molecules have successfully adsorbed onto the surface of MgAl2O4/C.
XPS measurement was performed to further study the surface chemical states and surface composition of MgAl2O4/C adsorbent before and after adsorption of CR. The XPS test results are shown in Fig. 15. The XPS survey profile of MgAl2O4/C reveals four major sets of peaks corresponding to Mg 1 s, Al 2p, O 1 s and C 1 s. In addition, no other impurity peaks are detected. The carbon peak is attributed to the residual carbon in the sample and hydrocarbons from the XPS instrument (Ansari et al., 2019).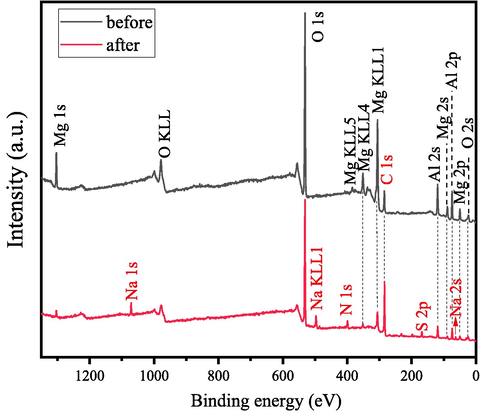
XPS spectra of samples.
As shown in Fig. 15, CR-adsorbed MgAl2O4/C exhibits obvious Na, N and S peaks besides to MgAl2O4/C relative peaks. This confirms the existence of CR onto MgAl2O4/C. No other significant changes are observed in the spectra after CR adsorption, which suggests that no secondary pollutant is generated during the adsorption process (Beheshti et al., 2018).
For more specific insight comparisons before and after CR adsorption, high-resolution XPS spectra of Mg 1 s, Al 2p, N 1 s and S 2p, collected from adsorbents, pure CR powders and CR-adsorbed adsorbent were carefully recorded. The results are shown in Fig. 16. As shown in Fig. 16(a), for the Mg 1 s and Al 2p spectra, the peaks located at 1303.73 eV and 74.43 eV are shown in the MgAl2O4/C, and both of them shift to higher binding energy positions (1304.38 eV and 74.73 eV) after CR adsorption, indicating some charge transfer to the Mg and Al during the process of adsorption (Carley et al., 1996).The N 1s peak of CR can be divided into two peaks, and they are 399.45 eV (C⚌N) and 401.88 eV (—NH2) (Xiong et al., 2021).After the adsorption of CR onto MgAl2O4/C, the peak position of-NH2 shifts to a higher binding energy position, suggesting that the N containing groups are responsible for strong chemical bonding which can lead to electron density change (Aoopngan et al., 2019).
XPS spectra of (a) Mg 1s, (b) Al 2p on the surface of MgAl2O4/C before and after removal of CR, (c) N 1s, and (d) S 2p on the surface of CR and MgAl2O4/C after CR adsorption.
Furthermore, S 2p spectra of CR (Fig. 16d) can be divided into two peaks. Peaks of S1 (168.45 eV) and S2(169.74 eV) are related to S 2p1/2 and S 2p3/2 of sulfonic group (-SO32-) of CR molecules. After CR adsorption onto the surface of MgAl2O4/C, the S 2p peaks shift to lower binding energies (168.34 and 169.60 eV) slightly, further suggesting that electrostatic attraction occurs via the -SO32- groups of Congo red (Aoopngan et al., 2019).
Here zeta potential analysis is applied to study the surface charge of the adsorbent in aqueous solution at pH = 7. Fig. 17 illustrates the change of zeta potential value before and after CR adsorption. It is clear that the adsorbent obtained at 700 °C possesses electropositive surface, with the zeta potential values of 7.62 mV. After adsorption of CR, the zeta potential of MgAl2O4/C adsorbent changed from positive to negative. As known, Congo red is polar molecules, it exists in the form of anions and cations in both alkaline and acidic aqueous. Its isoelectronic point is about 3. Therefore, when adsorbent MgAl2O4/C is dispersed in CR aqueous at pH = 7, the negatively charged CR can be adsorbed by the positive charged MgAl2O4/C due to the electrostatic attraction.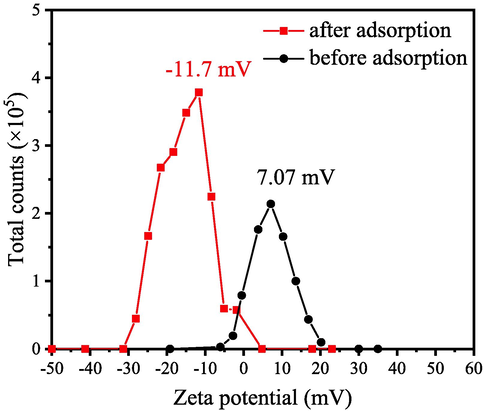
Zeta potentials of sample prepared at 600 °C before (black) and after CR adsorption (red).
The N2 adsorption–desorption isotherms of samples calcined at different temperatures are presented in Fig. 18. Table 4 lists corresponding characterized data. The samples prepared at 200, 300, 600 and 700 °C are M3-200, M3-300, M3-600 and M3-700, respectively.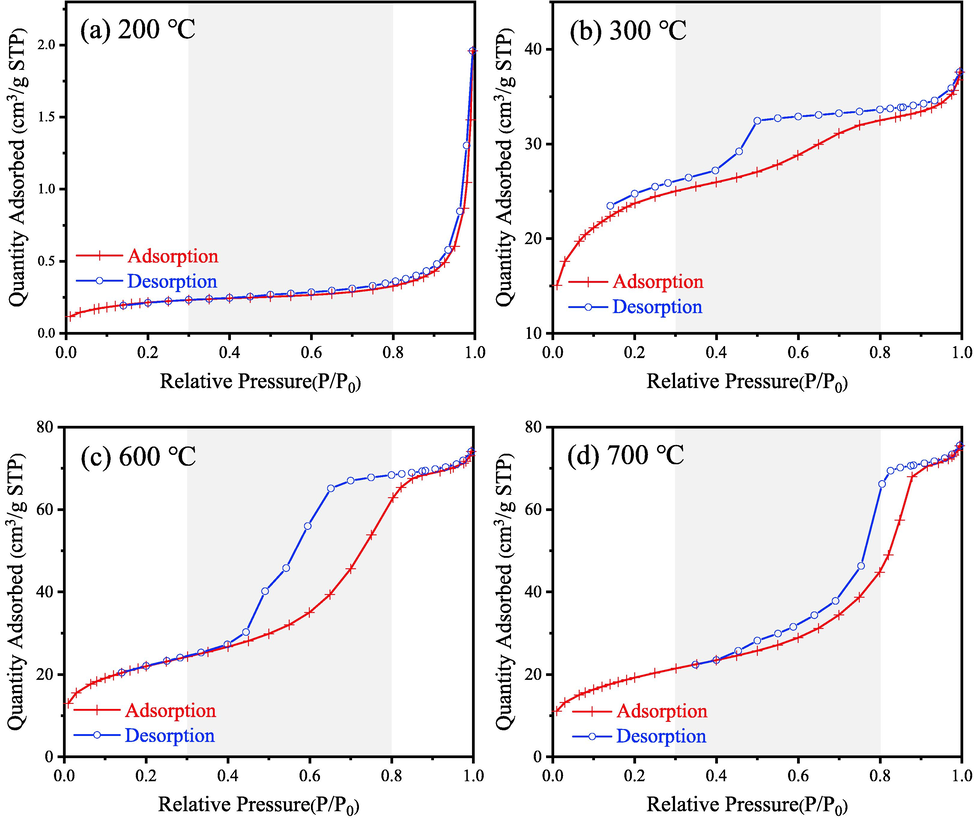
N2 adsorption–desorption isotherms of samples calcined at different temperatures: (a) 200 °C (b) 300 °C (c) 600 °C (d) 700 °C.
temperatures
BET specific surface area(m2g−1)
Pore volume
(cm3g−1)Pore diameter
(nm)
200
0.79
0.001
26.8
300
84
0.05
3.7
600
79
0.11
6.2
700
70
0.12
7.5
As shown in Fig. 18(a), sample M3-200 has the smallest amount of adsorption of N2, which is about 2 cm3/g, and the BET of M3-200 is only 0.79 m2/g. However, in Fig. 18(b-d), the amount of N2 adsorption and BET specific surface area of M3-300, M3-600 and M3-700 are evidently higher than M3-200. Besides, all the three isotherms (Fig. 18b-d) show the hysteresis loop when the P/P0 in the range of 0.3–0.8. Based on the IUPAC classification (Zhang et al., 2016a), this kind of isotherms could be classified as the type IV curve, suggesting the presence of mesopores in their structure. Moreover, the hysteresis loop obtained in M3-300 and M3-600 are evidently broader than M3-700 within the range of P/P0 = 0.3–0.8. It is well-known that the phenomenon of capillary condensation will occur in the middle-pressure sections (P/P0 = 0.3–0.8) when the sample has a mesoporous structure, and this is the root cause for the hysteresis of N2 desorption curve. Therefore, it can be concluded that higher quantity of mesoporous exist in M3-300 and M3-600 than M3-700. Besides, it can be seen in Fig. 18(d) that M3-700 has large amount of adsorption in the high-pressure sections (0.8–1.0), which is nearly 30 cm3/g. Essentially, the quantity of N2 adsorption belong to M3-600 shows a flatlining growth, which is only 11 cm3/g. This flatlining growth indicates that less large pores exist in the sample calcined at 600 °C. These results indicate that the sample M3-300 and M3-600 shows a mesoporous structure, which is favorite to the adsorption of CR solution.
Fig. 19 illustrates the TEM images of M3-600. It is clearly seen that numerous mesopores distributed in the sample. Fig. 19 (b) show the pore diameter is about 6.56 nm, which is in good agreement with the result determined via Barrett-Joyner-Halenda (BJH) method shown in Table.4.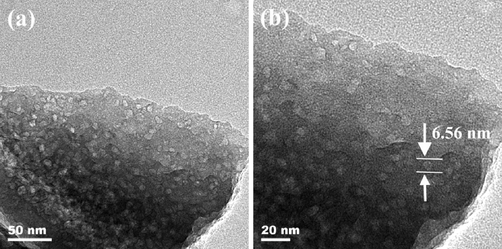
(a)(b) TEM images of sample M3-600.
Fig. 20 shows the SEM (a) and EDS mapping (b-e) of adsorbent M3-300. The images displayed in Fig. 20 demonstrated that the signal of C is appeared, verifying the existence of C in the adsorbent. Moreover, the C element distributes homogeneously, and matches with the dispersion of Mg, Al and O elements. These findings indicate that the in-situ carbon-containing composite can be prepared by the NHSG method, through forming a carbon-containing precursor. The element composition of M3-300 was studies by EDS as shown in Fig.S1, the atomic fractions of C is 13.496 %.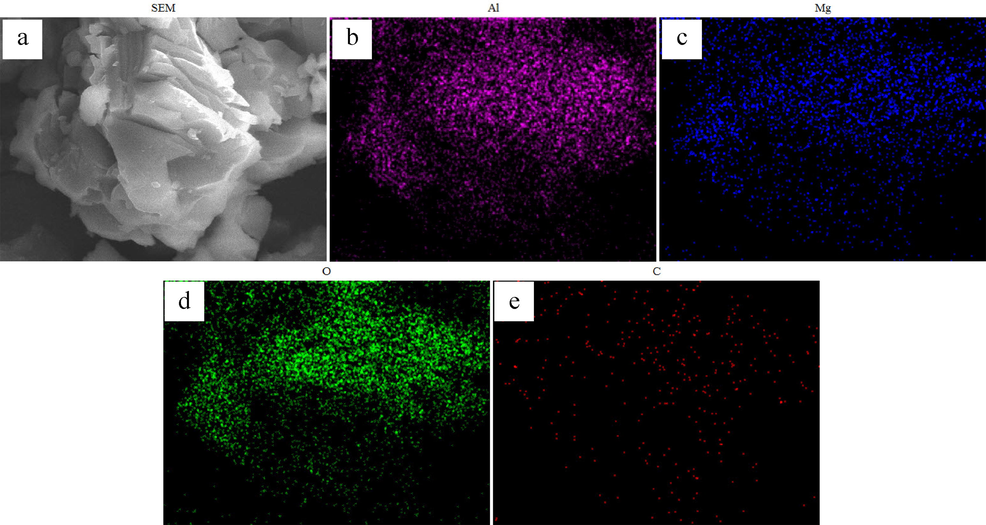
SEM image of (a) MgAl2O4/C adsorbent, EDS mapping of (b-e) Al, Mg, O and C.
Based on the MgAl2O4/C before and after adsorbing CR identified by FT-IR spectra, XPS spectra, zeta potential, TEM and N2 adsorption–desorption results, along with the adsorption kinetics and isotherms, a possible adsorption mechanism of MgAl2O4/C composite to CR is proposed and shown in Fig. 21.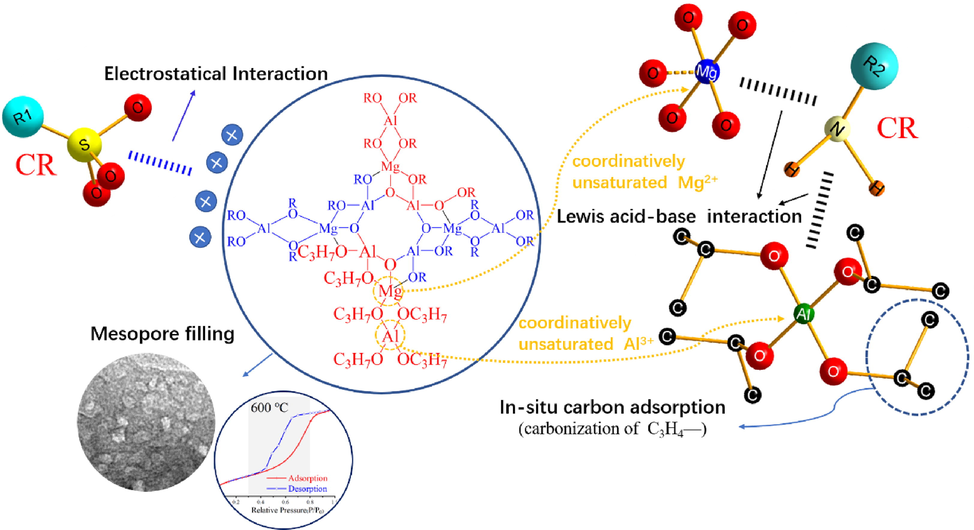
Schematic illustration of the CR adsorption process on to MgAl2O4/C.
According to the zeta potential analysis, electrostatic attraction involves in the adsorption process. The negatively charged sulfate group (-SO32-) on CR molecules are attracted by the unsaturated Mg2+ and Al3+ on the MgAl2O4/C composite surfaces. Furthermore, according to the pseudo-second-order kinetics evaluation, chemical adsorption dominates the adsorption process. It is confirmed that such chemical adsorption is Lewis acid-base interaction between unsaturated Mg2+/Al3+ on the MgAl2O4/C nanocomposite as the hard Lewis acid site and —NH2 on CR as the hard Lewis base site (Ansari et al., 2019). Meanwhile, the presence of in-situ carbon is benefit to improve the wetting process between organic pollutants and adsorbent, leading to the promoting of the adsorption. Besides, the adsorption process is also driven by the pore filling, this was confirmed by the mesoporous structure of as-prepared MgAl2O4/C nanocomposite observed from TEM and N2 adsorption–desorption analysis.
Overall, these mechanisms might take effect synergistically to endow the MgAl2O4/C composite with such a high adsorption capacity. Specifically, it is the uniformly dispersed microstructure, electrostatic attractions, mesoporous structure, homogenous mixing of unsaturated Mg, Al and C elements, and more exposure of Lewis acid sites (Guo et al., 2020) that have enabled such high adsorption properties.
4 Conclusions
The MgAl2O4/C composite adsorbent was prepared by the NHSG method. The MgAl2O4/C composite with pure MgAl2O4 phase and without impurity phase could be obtained only when using magnesium powder as magnesium source. The in-situ C came from the organic groups in Mg(Al(OiPr)4)2, which was formed in the xerogel reacted from the precursors of Mg powder, Al wire and isopropanol. The uniform-dispersed C was originated from residual organic groups through removal of C3H+, C3H2+, C3H3+, C3H5+, C3H6+, OH–, H2O+, CO2+ at 225 °C, 260 °C and 188 °C &301 °C &455 °C. The adsorption kinetics and isotherms studies for MgAl2O4/C composites followed the pseudo-second-order and Langmuir model. The MgAl2O4/C composite adsorbent prepared at 300 °C exhibited a maximum adsorption capacity for CR was up to 5690 mg/g. This was due to abundant coordinatively unsaturated Mg and Al irons in amorphous MgAl2O4, the electrostatic interactions between the anionic dye CR and MgAl2O4/C surface, in-situ formed C as well as pore filling.
Acknowledgments
This work was supported by the National Natural Science Foundation of China [grant numbers 52072162, 51962014,]; Jiangxi Provincial Natural Science Foundation, China, [grant numbers 20202ACBL214006, 20202BABL214013]; the Jingdezhen ceramics major special project, China, (2021ZDGG001); the Jingdezhen science and technology planning project, China, (2020GYZD013-09); the Science Foundation of Jiangxi Provincial Department of Education, China, (GJJ211315).
References
- Shape controlled synthesis of high surface area MgO microstructures for highly efficient congo red dye removal and peroxide sensor. J. Environ. Chem. Eng.. 2019;7:103347
- [CrossRef] [Google Scholar]
- NiO nanoparticle doped-PVA-MF polymer nanocomposites: preparation, Congo red dye adsorption and antibacterial activity. Arabian J. Chem.. 2020;13:5724-5739.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of nanocrystalline MgAl2O4 spinel via sucrose process. Mater. Res. Bull.. 2008;43:1188-1194.
- [CrossRef] [Google Scholar]
- Removal of Congo red dyes from aqueous solutions by porous γ-alumina nanoshells. Chemosphere. 2022;286:131769
- [CrossRef] [Google Scholar]
- Synthesis of mesoporous nanocrystalline MgAl2O4 spinel via surfactant assisted precipitation route. Powder Technol.. 2010;198:275-278.
- [CrossRef] [Google Scholar]
- CuO sputtered flexible polyaniline@graphene thin films: a recyclable photocatalyst with enhanced electrical properties. Compos. B Eng.. 2019;175:107092
- [CrossRef] [Google Scholar]
- Amine-functionalized and hydroxyl-functionalized magnesium ferrite nanoparticles for congo red adsorption. ACS Appl. Nano Mater.. 2019;2:5329-5341.
- [CrossRef] [Google Scholar]
- Selective high adsorption capacity for Congo red dye of a new 3D supramolecular complex and its magnetic hybrid, Inorganic Chemistry. Frontiers. 2018;5:694-704.
- [CrossRef] [Google Scholar]
- Synthesis of nanoscale zeolitic imidazolate framework-8 (ZIF-8) using reverse micro-emulsion for Congo red adsorption. Sep. Purif. Technol.. 2021;260:118062
- [CrossRef] [Google Scholar]
- Evidence for the instability of surface oxygen at the Zn(0001)-O-Cu interface from core-level and X-ray induced Auger spectroscopies. Top. Catal.. 1996;3:91.
- [CrossRef] [Google Scholar]
- Preparation of monolithic binary oxide gels by a nonhydrolytic sol-gel process. Chem. Mater.. 1992;4:961-963.
- [CrossRef] [Google Scholar]
- Electrospun adsorptive nanofibrous membranes from ion exchange polymers to snare textile dyes from wastewater. Adv. Mater. Technol.. 2021;6:2000955.
- [CrossRef] [Google Scholar]
- Direct fabrication of hierarchically processed pineapple peel hydrogels for efficient Congo red adsorption. Carbohydr. Polym.. 2020;230:115599
- [CrossRef] [Google Scholar]
- Template-free synthesis of nanoparticle-built MgO and Zn-doped MgO hollow microspheres with superior performance for Congo red adsorption from water. Dalton Trans.. 2018;47:17421-17431.
- [CrossRef] [Google Scholar]
- Synthesis of MgAl2O4 spinel by thermal plasma and its synergetic structural study. J. Alloys Compd.. 2017;726:1186-1194.
- [CrossRef] [Google Scholar]
- Mesoporous mixed oxide catalysts via non-hydrolytic sol-gel: a review. Appl. Catal. A. 2013;451:192-206.
- [CrossRef] [Google Scholar]
- Functionalization of walnut shell by grafting amine groups to enhance the adsorption of Congo red from water in batch and fixed-bed column modes. J. Environ. Chem. Eng.. 2021;9:106301
- [CrossRef] [Google Scholar]
- Mechanically induced phase transformation of γ-Al2O3 into α-Al2O3. Access to structurally disordered γ-Al2O3 with a controllable amount of pentacoordinated Al sites. The Journal of Physical Chemistry C. 2011;115:22770-22780.
- [CrossRef] [Google Scholar]
- An FT-IR and flow reactor study of the conversion of propane on γ-Al2O3 in oxygen-containing atmosphere. Appl. Catal. A. 2000;190:157-167.
- [CrossRef] [Google Scholar]
- Novel facile nonaqueous precipitation in-situ synthesis of mullite whisker skeleton porous materials. Ceram. Int.. 2018;44:22904-22910.
- [CrossRef] [Google Scholar]
- A review on magnesium aluminate (MgAl2O4) spinel: synthesis, processing and applications. Int. Mater. Rev.. 2013;58:63-112.
- [CrossRef] [Google Scholar]
- Microwave assisted solid state reaction synthesis of MgAl2O4 spinel powders. J. Eur. Ceram. Soc.. 2004;24:201-207.
- [CrossRef] [Google Scholar]
- Construction of hydroxyethyl cellulose/silica/graphitic carbon nitride solid foam for adsorption and photocatalytic degradation of dyes. Arabian J. Chem.. 2022;15:104105
- [CrossRef] [Google Scholar]
- Facile preparation of MgO/graphene oxide nanocomposite for efficient removal of aqueous Congo red: adsorption performance and interaction mechanism. Res. Chem. Intermed.. 2021;47:945-971.
- [CrossRef] [Google Scholar]
- Green and facile synthesis of cobalt-based metal–organic frameworks for the efficient removal of Congo red from aqueous solution. J. Colloid Interface Sci.. 2020;578:500-509.
- [CrossRef] [Google Scholar]
- Sol–gel preparation of hierarchically porous magnesium aluminate (MgAl2O4) spinel monoliths for dye adsorption. J. Sol-Gel Sci. Technol.. 2018;88:114-128.
- [CrossRef] [Google Scholar]
- Ultra-high adsorption capacity of MgO/SiO2 composites with rough surfaces for Congo red removal from water. J. Colloid Interface Sci.. 2018;510:111-117.
- [CrossRef] [Google Scholar]
- Quartzite an efficient adsorbent for the removal of anionic and cationic dyes from aqueous solutions. Arabian J. Chem.. 2020;13:4731-4740.
- [CrossRef] [Google Scholar]
- Treatment of organic pollutants by homogeneous and heterogeneous Fenton reaction processes. Environ. Chem. Lett.. 2018;16:947-967.
- [CrossRef] [Google Scholar]
- Novel facile nonhydrolytic sol-gel synthesis of MgAl2O4 nanocrystal from bimetallic alkoxides. J. Sol-Gel Sci. Technol.. 2021;100:555-561.
- [CrossRef] [Google Scholar]
- Facile preparation of Fe2O3 Al2O3 composite with excellent adsorption properties towards Congo red. Ceram. Int.. 2021;47:13884-13894.
- [CrossRef] [Google Scholar]
- Removal of Congo red and Brilliant green dyes from aqueous solution using flower shaped ZnO nanoparticles, Journal of Environmental. Chem. Eng.. 2017;5:5420-5428.
- [CrossRef] [Google Scholar]
- Soybean peroxidase-catalyzed degradation of a sulfonated dye and its azo-cleavage product. journal of chemical technology and biotechnology. 2021;96(2):423-430.
- [CrossRef] [Google Scholar]
- Template-free method for the synthesis of high-pore-volume γ-Al2O3 nanofibers in a membrane dispersion microreactor. Nanotechnology. 2021;32:185601
- [CrossRef] [Google Scholar]
- Synthesis and characterisation of MgAl2O4 spinel nanopowders via nonhydrolytic sol-gel route. J. Ceram. Soc. Jpn.. 2017;125:100-104.
- [CrossRef] [Google Scholar]
- A simple polyacrylamide gel route for the synthesis of MgAl2O4 nanoparticles with different metal sources as an efficient adsorbent: Neural network algorithm simulation, equilibrium, kinetics and thermodynamic studies. Sep. Purif. Technol.. 2022;281:119855
- [CrossRef] [Google Scholar]
- Effects of chelation reactions between metal alkoxide and acetylacetone on the preparation of MgAl2O4 powders by sol-gel process. Adv. Powder Technol.. 2013;24:436-440.
- [CrossRef] [Google Scholar]
- Adsorption of congo red on mesoporous activated carbon prepared by CO2 physical activation. Chin. J. Chem. Eng.. 2020;28:1069-1076.
- [Google Scholar]
- Lewis acid and base sites at the surface of microcrystalline TiO2 anatase: relationships between surface morphology and chemical behaviour. Appl. Catal. A. 2000;200:275-285.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of MgAl2O4 spinel precursor sol prepared by inorganic salts. Ceram. Int.. 2021;47:4813-4819.
- [CrossRef] [Google Scholar]
- Flower-like Mg/Fe-layered double oxide nanospheres with ultrahigh adsorption efficiency for anionic organic dyes. Colloids Surf., A. 2021;618:126446
- [CrossRef] [Google Scholar]
- Construction of hierarchical MgAl2O4 spinel as catalytic supports. Mater. Lett.. 2015;159:204-206.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of nanocrystalline MgAl2O4 spinel via modified sol-gel method. J. Alloys Compd.. 2015;645:535-540.
- [CrossRef] [Google Scholar]
- Highly porous, hierarchical peanut-like Ecandrewsite binary metal oxide nanostructures for the high-efficiency detoxification of organic dyes from wastewater. Ceram. Int.. 2022;48:1057-1067.
- [CrossRef] [Google Scholar]
- Temperature-dependent 4-, 5- and 6-fold coordination of aluminum in MOCVD-grown amorphous alumina films: a very high field 27Al-NMR study. J. Phys. Chem. C. 2013;117:21965-21971.
- [CrossRef] [Google Scholar]
- Mg–Al-layered double hydroxide (LDH) modified diatoms for highly efficient removal of Congo red from aqueous solution. Appl. Sci.. 2020;10:2285.
- [CrossRef] [Google Scholar]
- Model studies of metalloenzymes involving metal ions as Lewis acid catalysts. Acc. Chem. Res.. 1992;25:273-279.
- [CrossRef] [Google Scholar]
- Magnetic mesoporous γ-Al2O3/ZnFe2O4 micro-bowls realizing enhanced adsorption, separation and recycle performance towards waste water. Microporous Mesoporous Mater.. 2018;270:120-126.
- [CrossRef] [Google Scholar]
- Correlation of sol-gel processing parameters with microstructure and properties of a ceramic product. Mater. Charact.. 2003;50:325-337.
- [CrossRef] [Google Scholar]
- Synthesis of porous MgAl2O4 spinel and its superior performance for organic dye adsorption. RSC Adv.. 2015;5:5123-5130.
- [CrossRef] [Google Scholar]
- The Chemistry of Metal Alkoxides. Kluwer academic publishers; 2002.
- Catalytic degradation of organic dyes using biosynthesized silver nanoparticles. Micron. 2014;56:54-62.
- [CrossRef] [Google Scholar]
- Low-temperature hydrothermal synthesis of α-Fe/Fe3O4 nanocomposite for fast Congo red removal. Dalton Trans.. 2013;42:2572-2579.
- [CrossRef] [Google Scholar]
- Rational design, synthesis, adsorption principles and applications of metal oxide adsorbents: a review. Nanoscale. 2020;12:4790-4815.
- [CrossRef] [Google Scholar]
- Preparation, characterisation, and growth mechanism of mesoporous petal-like MgAl2O4 spinel. Ceram. Int.. 2022;48:3351-3361.
- [CrossRef] [Google Scholar]
- Selective adsorption of Congo red and Cu(II) from complex wastewater by core-shell structured magnetic carbon@zeolitic imidazolate frameworks-8 nanocomposites. Sep. Purif. Technol.. 2021;277:119053
- [Google Scholar]
- Template-free synthesis of MgO mesoporous nanofibers with superior adsorption for fluoride and Congo red. Ceram. Int.. 2018;44:9454-9462.
- [CrossRef] [Google Scholar]
- A solid-state chemical method for synthesizing MgO nanoparticles with superior adsorption properties. RSC Adv. 2019;9:2011-2017.
- [CrossRef] [Google Scholar]
- Ordered crystalline mesoporous γ-alumina fabricated by vacuum-promoted self-assembly and alkaline hydrothermal method. Mater. Lett.. 2016;163:122-125.
- [CrossRef] [Google Scholar]
- Mesoporous zinc aluminate (ZnAl2O4) nanocrystal: synthesis, structural characterization and catalytic performance towards phenol hydroxylation. Microporous Mesoporous Mater.. 2016;226:278-283.
- [CrossRef] [Google Scholar]
- Nonhydrolytic sol-gel in-situ synthesis of novel recoverable amorphous Fe2TiO5/C hollow spheres as visible-light driven photocatalysts. Mater. Des.. 2020;194
- [CrossRef] [Google Scholar]
- Solution Combustion Synthesis of Porous MgO Nanostructures for Efficient Removal of Congo Red. J. Nanosci. Nanotechnol.. 2020;20:810-818.
- [CrossRef] [Google Scholar]
- Hierarchical porous Al2O3@ZnO core-shell microfibres with excellent adsorption affinity for Congo red molecule. Appl. Surf. Sci.. 2019;473:251-260.
- [CrossRef] [Google Scholar]
- N, N-dimethylformamide assisted facile hydrothermal synthesis of boehmite microspheres for highly effective removal of Congo red from water. J. Colloid Interface Sci.. 2021;583:128-138.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104393.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







