Translate this page into:
Novel bimodal micro‐mesoporous Ni50Co50-LDH/UiO-66-NH2 nanocomposite for Tl(I) adsorption
⁎Corresponding author at: Ton Duc Thang University, Ho Chi Minh City, Viet Nam. azam.marjani@tdtu.edu.vn (Azam Marjani)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Ni50Co50-layered double hydroxide/UiO-66-NH2 metal–organic framework nanocomposite (Ni50Co50-LDH/UiO-66-NH2 NC) was synthesized through a facile ultrasonic-assisted hydrothermal method. UiO-66-NH2 MOF nanocrystals were in situ grown on the surface of ultrathin 2-dimensional functionalized Ni50Co50-LDH nanosheets. Using this method, a uniform nanocomposite architecture was obtained by uniformly distributing MOF nanocrystals on Ni50Co50-LDH. The synthesized LDH/MOF NC possesses essential properties of potential nanoadsorbent such as high surface area (907 m2 g−1), large pore volume (0.91 cm3 g−1), bimodal micro-mesoporous structure, and chemical functionality. Accordingly, Ni50Co50-LDH/UiO-66-NH2 NC was used as an adsorbent for the uptake of toxic thallium (I) from water. Isotherm, thermodynamic, and kinetic studies were conducted to gain a better insight into the adsorption mechanism (s) involved in the removal process. Langmuir and pseudo-first-order models present a better fit to the isotherm and kinetic data, respectively, and the maximum Langmuir adsorption capacity was found to be 601.3 mg g−1 after non-linear fitting analysis (pH=7.0, solution volume=30 mL, initial thallium (I) concentration=50 mg L–1, contact time=15 min, solution temperature=293 K). Thermodynamic parameters were estimated ( =+64.979 kJ mol−1, =+0.335 kJ mol−1 K−1, =–33.176 to –39.876 kJ mol−1) and it was found that the adsorption is endothermic and spontaneous with a physicochemical adsorption nature.
Keywords
Adsorbent
In situ growing
Isotherm studies
Metal-organic frameworks
Surface functionalization
Thermodynamics
1 Introduction
Highly toxic heavy metals such as thallium (Tl), chromium (Cr), cadmium (Cd), lead (Pb), mercury (Hg), arsenic (As), and manganese (Mn) cause significant health effects in both humans and animals. Among them, Tl with a relative atomic mass of 204.37 and a density of 11.83 g cm−1 is a highly toxic heavy metal and even more poisonous to mammals than many other heavy metals like Pb, Cd, Hg, Cu, and Zn. Accordingly Tl rates as an Environmental Protection Agency (EPA) priority pollutant with a maximum permissible concentration of 2 μg L–1 in drinking water (Peter and Viraraghavan, 2005). From the viewpoint of ecotoxicological and health importance, exposure to a low level of Tl(I) can cause diseases as well as various disorders, such as headache, psychic disturbance, alopecia, acute progressive paralysis, anorexia, temporary hair loss, and even cardiovascular effects, because it is easily absorbed through the skin and mucous membrane and accumulated in bones, renal medulla, and central nervous system (Cvjetko et al., 2010; Sangvanich et al., 2010; Dashti Khavidaki and Aghaie, 2013). Tl is a ubiquitous trace element, which has an average concentration of 0.013 mg L–1 in the oceanic crust, and 0.49 mg L–1 in the continental crust and has been used to manufacture scintillation counters for radioactivity quantitation, infrared spectrometers, ceramic semiconductor material, pigments (thallium chromate), imitation jewelry, catalyst, and low-temperature thermometers (Peter and Viraraghavan, 2005; Wan et al., 2014). Tl has been released into the environment (soil, water, and wastewater) by anthropogenic activities such as smelting and coal combustion, exploiting and machining mines, as well as other thriving industries, ranging from dozens of μg L–1 to several mg L–1 (Dashti Khavidaki and Aghaie, 2013; Twidwell and Williams-Beam, 2002). Consequently, removing Tl from aquatic environments and aqueous media, and controlling its contamination regarding humans and the environment is indispensable.
Current approaches to remove heavy metals pollutants from the aquatic environment include filtration, reverse osmosis, solvent extraction, chemical precipitation, electrochemical removal, etc. However, these techniques have disadvantages such as high energy consumption, incomplete removal, high-cost procedure, production of secondary pollution, process complexity, the need for specialized operators, sludge production, and the use of organic solvents in the process (Burakov et al., 2018; Carolin et al., 2017; Azimi et al., 2017). As a more general problem, these methods suffer from high selectivity for removing heavy metals. For these reasons, these methods are commonly used together during a remediation process. The adsorption method is another approach to removing heavy metals and other pollutants from water and wastewater and has attracted increasing attention in recent years (Pelalak et al., 2021; Marjani et al., 2020; Pelalak et al., 2021). The advantages of this method compared to the techniques mentioned above include designability, selectivity, lower costs, lack of sludge, lower energy consumption, and simplicity of the process (Burakov et al., 2018; Soltani et al., 2020; Soltani et al., 2020; Soltani et al., 2020; Yanyan et al., 226 (2018); Albadarin et al., 2017).
In recent years, nanomaterials including layered double hydroxides (LDHs) (Soltani et al., 2018; Zubair et al., 2017), metal–organic frameworks (MOFs) (Feng et al., 2018; Khan et al., 2013; Madden et al., 2020) covalent organic frameworks (COFs) (Li et al., 2018; Wang and Zhuang, 2019; Heydari et al., 2021); mesoporous silica and carbon materials (MSMs and MCMs) (Soltani et al., 2020; Soltani et al., 2019; Soltani et al., 2020; Gang et al., 2021); graphene and graphene oxides (Lim et al., 2018; Zhang et al., 2020), and polymer matrix nanocomposites (PMNCs) (Soltani et al., 2020; Zabihi et al., 2020; Zarei et al., 2019) have shown their potential as promising adsorbents. Among them, MOFs arouse worldwide interest during the past decade because of their remarkable properties including high surface area, regular porosity, designable structure, and dense aromatic linkers (Soltani et al., 2020; Zhang et al., 2019). Recently, among various kinds of MOFs, water-resistant functionalized MOFs such as UiO-66-NH2, UiO-66-(Zr)-(COOH)2, and NH2-MIL-68, have attracted more and more attention both because of the presence of functional groups (hydroxyl, amine, carboxyl, and thiol) in their structure and the ability to use them in the presence of moisture or water (Zhang et al., 2019; Wang et al., 2020; Yang et al., 2013; Soltani et al., 2021; Shi et al., 2020). LDHs are another group of nanomaterials that have received considerable interest among researchers, especially those who work on the environmental applications of LDHs (Soltani et al., 2018; Soltani et al., 2020; Yang et al., 2020; Yang et al., 2020). However, the most important disadvantages of LDHs and MOFs as adsorbents are low surface area and small particle size (nanometer-size particles), respectively (Soltani et al., 2021). The low surface area of an adsorbent restricts access to the adsorption sites and the small particle size of adsorbent particles makes it difficult to separate them from the aqueous medium (generally needs high‐speed centrifugation). Nevertheless, the simultaneous use of these two unique structures in the form of a composite can combine the properties and advantages of both materials and create a composite with hybrid features. Furthermore, by choosing the right strategy, it is possible to prepare a composite of LDH and MOF which solves the two aforementioned disadvantages, namely low surface area and small particle size of the adsorbent. Recently, we found that very few reports have been made on the synthesis and development of these hybrid LDH/MOF structures for practical applications especially adsorption and extraction. For instance, in 2017, Yang et al. (Yang et al., 2017) reported a method for in situ growth of Zeolite ZIF-8 MOF on Zn-Al LDH to prepare a ZIF-8/LDH nanocomposite for AsV removal from aqueous media. In 2018, Hu et al. (Hu et al., 2018) reported the preparation of ZIF-8/Zn-Al layered double oxides composite (LDO) using an in situ growth approach for photocatalytic degradation of an organic dye. In these works, Zn-Al LDH was fabricated by the co-precipitation of zinc and aluminum nitrate salts in the presence of urea. Although these LDO/MOF and LDH/MOF nanocomposites revealed acceptable performance for adsorption and degradation applications, the following drawbacks to these LDH/MOF nanocomposites are worth considering:
-
(1)
The surfaces of the Zn-Al LDH and Zn-Al LDO, as a scaffold, were not homogeneously covered by MOF nanoparticles. This might be due to the presence of impurities that influenced the growth of MOF nanoparticles on the rough surfaces of Zn/Al LDH material (Yang et al., 2017).
-
(2)
Due to the lack of strong chemical bonds between the two components, there is a possibility of separation of LDH and MOF nanoparticles caused by environmental factors or ultrasonic waves during the adsorption and desorption procedures.
-
(3)
The Zn-Al LDH and Zn-Al LDO particles used are only a few micrometers in size, which results in the production of adsorbents with very fine particles, which in turn complicate the process of separating the adsorbent from the aqueous media.
-
(4)
The lack of organic functional groups (–OH, –NH, –SH, –C=O) as active adsorption sites on the pores and surface of the MOF nanoparticles potentially leads to a decrease in adsorption performance of the LDO/MOF and LDH/MOF adsorbents in comparison with their functionalized-homologs.
In 2020, Soltani and colleagues (Soltani et al., 2020) reported the synthesis of an LDH/MOF composite with a hierarchical trimodal micro-meso-macro porosity as a superior adsorbent for simultaneous adsorption of Cr(VI) and orange II reactive dye, in which bimetallic zeolitic imidazole frameworks (BMZIF20) uniformly grow on the surface of Ni50Co50-LDH nanosheets. In order to establish strong chemical bonds between the LDH surface and MOF nanocrystals, the surface of the LDH was first modified with an appropriate silane coupling agent and then MOF nanocrystals were in situ grown on the LDH surface. Another important point in this work compared to the method used by the aforementioned works is the use of the Ni50Co50-LDH with extended 2D ultrathin nanosheets, which can play the role of an appropriate scaffold for growing MOF nanocrystals. Although the method reported by Soltani and colleagues solves the three above-mentioned shortcomings (drawbacks 1–3), the problem of functionality (problem 4) remains unresolved. In 2021, Soltani and co-workers (Soltani et al., 2021) reported the synthesis of an LDH/MOF composite via a facile and “green” synthesis approach in which UiO-66-(Zr)-(COOH)2 nanoparticles were in situ grown on the surface of carboxylic-functionalized Ni50Co50-LDH sheets in water as solvent under reflux condition. Although this reported LDH/MOF composite possesses abundant amine and carboxylic functional groups in its hierarchical structure and solved the problem of functionality, the LDH/MOF adsorbent does not possess a high surface area (surface area=41 m2 g−1).
Here, for the first time, in line with our previous research and with the aim of solving the problem of functionality, a novel Ni50Co50-layered double hydroxide/UiO-66-NH2 metal–organic framework nanocomposite (denoted as Ni50Co50-LDH/UiO-66-NH2 NC) was synthesized using in situ growth of amine-decorated MOF nanocrystals on the surface of functionalized-Ni50Co50-LDH under a mild hydrothermal condition. Due to the presence of abundant adsorption groups in the Ni50Co50-LDH/UiO-66-NH2 NC structure, this material was used as an adsorbent to remove Tl(I) cations from the aqueous solution and its adsorption performance was systematically investigated.
2 Experimental section
2.1 Materials
2-Aminoterephthalic acid (2-amino-1,4-benzenedicarboxylic acid, H2N-H2BDC, 99%), zirconium(IV) chloride (ZrCl4, ≥99.5%), cobalt(II) nitrate hexahydrate (Co(NO3)2⋅6H2O, ≥98%), nickel(II) nitrate hexahydrate (Ni(NO3)2⋅6H2O, ≥98.5%), and (3-chloropropyl)trimethoxysilane (CPTMS, ≥97%) were purchased from Sigma-Aldrich (Germany). Thallium(I) nitrate (TlNO3, ≥99.9%), urea (≥99.5%), ethylene glycol (EG, ≥99.5%), fuming hydrochloric acid (HCl, 37%), sodium hydroxide pellets (NaOH, ≥99%), acetone (≥99.8%), ethanol (absolute), N, N-dimethylformamide (DMF, ≥99.8%), and toluene (≥99.9%) were purchased from Merck (Germany). Ethanol (96% v/v) was purchased from Dr. Mojallali Chemical Laboratories (Tehran, Iran). Double-distilled water (DDW) was used for the synthesis of materials and Tl(I) adsorption experiments.
2.2 Synthesis of the samples
2.2.1 Synthesis of Ni50Co50-LDH ultrathin nanosheets
Ni50Co50-LDH was synthesized according to the large-scale preparation and environmentally-friendly synthetic method described by Soltani and co-workers (Soltani et al., 2018; Soltani et al., 2020). For a typical synthesis, 90 mL EG and 36 mL DDW were added into a 250 mL round-bottom flask containing 12.0 mmol (3.492 g) Co(NO3)2⋅6H2O and 6.0 mmol (1.745 g) Ni(NO3)2⋅6H2O (Co:Ni=2:1), and the mixture was magnetically stirred until the entire salts were dissolved. Once the temperature of the reaction mixture reached 363 K, 90 mmol (5.405 g) urea was added to the flask, and the mixture was magnetically stirred under reflux for 3 h at 363 K. After cooling the mixture to room temperature (RT), the green precipitates were filtered through a Whatman No. 42 filter paper by means of vacuum filtration and repeatedly rinsed with DDW and ethanol 96% to eliminate the remains of reactants and EG. The pale green Ni50Co50-LDH ultrathin nanosheets were obtained after oven-drying the precipitates at 333 K for 24 h.
2.2.2 Synthesis of Ni50Co50-LDH-Cl
Post-modification of Ni50Co50-LDH was carried out by adding a certain amount of CPTMS into a 250 mL round-bottom flask containing 100 mL dry toluene and 1.00 g synthesized Ni50Co50-LDH, followed by ultrasonication (15 min, RT), refluxing under stirring (N2 atmosphere, 383 K, 24 h), repeatedly washing (toluene and acetone), and drying (under vacuum at RT overnight and 343 K for another 12 h).
2.2.3 Synthesis of UiO-66-NH2
UiO-66-NH2 was prepared according to the method described by Lillerud and co-workers with a slight modification (Kandiah et al., 2010). In a volumetric flask, 9.6 mmol (1.739 g) H2N-H2BDC and 9.6 mmol ZrCl4 (2.237 g) were dissolved in 200 mL DMF at RT and the flask was transferred to a preheated electric oven at 353 K overnight and then held at 373 K for 1 day. After cooling the mixture to RT, the obtained solid was filtered through a Whatman No. 42 filter paper using vacuum filtration and rinsed several times with absolute ethanol for 2 days while heated at 333 K in a water bath. Finally, the obtained yellow powder was separated using vacuum filtration, transferred to a Schlenk flask, and oven-dried under vacuum at RT.
2.2.4 Synthesis of Ni50Co50-LDH/UiO-66-NH2 NC
2.25 g Ni50Co50-LDH-Cl was added into a volumetric flask containing 150 mL dry DMF and the mixture was ultrasonicated for 15 min at RT. Then, a mixture containing 9.6 mmol H2N-H2BDC and 9.6 mmol ZrCl4 in 100 mL dry DMF was added to the above mixture and stirred for 15 min at RT. Afterward, the volumetric flask was sealed and transferred in a preheated electric oven at 353 K overnight and then held at 373 K for a day. Finally, the reaction mixture was cooled to RT, filtered, washed with absolute ethanol, and dried as mentioned in the previous section.
After the synthesis of the aforementioned samples, the residual solvents (DMF, ethanol, toluene, and acetone) were distilled and purified according to the known procedures.
2.3 Characterization of the samples
Powder X-ray diffraction (XRD) measurements were performed on the samples at ambient atmosphere and 293 K on a Bruker D5000 (Siemens, Germany) diffractometer using monochromatic Cu Kα radiation (λ = 1.540 A°).
The molecular bond structure and chemical nature of the samples were studied using Fourier transform infrared (FTIR, Thermo Nicolet, Avatar 330 FTIR Spectrometer, USA) spectroscopy in the wavelength range of 4000 cm−1-400 cm−1 by making 60 scans at a resolution of 4 cm−1.
The microscopic observations and energy dispersive spectroscopy (EDS)-Mapping were performed by a Field Emission Scanning Electron Microscope (FESEM, MIRA3 TESCAN-XMU, Kohoutovice, Czech Republic).
N2 adsorption–desorption measurements at 77 K was carried out using a porosimetry analyzer (Belsorp-mini II, BEL Japan). The Langmuir, Brunauer-Emmett-Teller (BET), and t-plot equations were used to estimate the surface area of the samples. The pore diameter (D) of the samples was calculated using nonlocal density functional theory (NLDFT).
The concentrations of Tl(I) cations in aqueous media were measured using a flame atomic absorption spectrophotometer (FAAS - SHIMADZU AA 6800 model).
2.4 Adsorption experiments
Batch adsorption tests for Tl(I) adsorption by Ni50Co50-LDH/UiO-66-NH2 NC were conducted in a thermostatically controlled shaking water bath (Precision Scientific Inc., Chicago, IL) using traditional bottle-point method in 100-mL narrow-mouth polypropylene (PP) bottles. TlNO3 was dissolved in DDW to form Tl(I) stock solution (1000 mg L–1). The working solutions were prepared daily from the stock solution with DDW. The effect of some important factors such as solution pH, adsorbent dose ( ), initial Tl(I) concentration ( ), contact time ( ), and temperature ( ) on the adsorption process were systematically monitored.
The adsorption capacity and removal percentage were calculated using equations (1–3) in Table 1. Different non-linear isotherm (Eqs. (4–6)) equations, thermodynamic equations (Eqs. (7–9)), and non-linear kinetic (Eqs. (10–12)) equations were used for predicting adsorption mechanism (s) and the probable interactions between Tl(I) cations and the adsorbent. Non-linear regression analysis (NLRA) was used in this work for fitting the isotherm and kinetic data by using the statistical analysis function in Origin Pro 8.6 software (Origin Lab Corporation, Northampton, USA).
Equations
Parameters (units)
: initial concentration (mg L–1)
: equilibrium concentration (mg L–1)
: adsorption capacity at any time
(mg g−1)
: equilibrium adsorption capacity (mg g−1)
: solution volume (mL)
: adsorbent dose (mg)
(mg g−1): calculated maximum adsorption capacity
(L mg−1): Langmuir isotherm constant
,
(dimensionless): separation factor
(mg g−1)(L g−1)n: Freundlich isotherm constant related to the adsorption capacity
(dimensionless): adsorption intensity
(L g−1): Redlich-Peterson isotherm constant
(L mg−1): Redlich-Peterson isotherm constant
(dimensionless): Redlich-Peterson isotherm binding constant,
(dimensionless): thermodynamic equilibrium constant
(1 mol L–1): the unitary standard concentration of the adsorbate
(g mol−1): molecular weight of the adsorption
(L mg−1): Langmuir isotherm constant
: (dimensionless) the coefficient of activity
(kJ mol−1 K−1): entropy change
(kJ mol−1): enthalpy change
(K): temperature
(kJ mol−1): Gibbs free energy
(min−1): the rate constant of PFO kinetic model
(mg g−1): calculated equilibrium adsorption capacity
(g mg−1 min−1): the rate constant of PSO kinetic mode
(mg g−1 min−1)=
: the initial adsorption rate
(min): time
(mg g−1 min−1): the initial adsorption rate
(g mg−1): a parameter related to the activation energy for chemisorption and extent of surface coverage
It is reported that the non-linear method is a more appropriate method than the linear method to estimate either the isotherm parameters or kinetic parameters because in the non-linear analysis technique the error distribution does not get altered as in the linear analysis method (Soltani et al., 2020; Soltani et al., 2020; Kumar and Sivanesan, 2006; Soltani et al., 2019).
The detailed experimental procedures for adsorption of Tl(I) by Ni50Co50-LDH/UiO-66-NH2 NC were illustrated in the following sections.
2.4.1 The effect of pH and adsorbent dose
The simultaneous influence of pH and adsorbent dose on Tl(I) adsorption by the adsorbent was investigated first. The pH of a 30 mL solution containing 30 mg L–1 of Tl(I) cations and a certain dose of the adsorbent ( =0.001, 0.002, and 0.005 g) was adjusted to a target set between 5.5 and 8.5 using 0.01 mol L–1 NaOH and HCl. After shaking for 120 min ( =293 K, shaking speed=180 rpm), samples were taken and the powder adsorbent was isolated from the solution by centrifugation, and then the Tl(I) concentration was measured immediately using FAAS.
2.4.2 The effect of initial Tl(I) concentration and temperature
For isotherm and thermodynamic studies, the initial concentration of Tl(I) cations and temperature values were varied from 0.5 to 250 mg L–1 and 293 to 313 K, respectively. The samples (pH=6.5, =30 mL, =0.002 g) were shaken for 120 min and then taken and centrifuged and the residual metal concentration in the supernatant was measured immediately afterward.
2.4.3 The effect of contact time
For kinetic studies, the solutions (pH=6.5, =30 mL, =0.002 g, =303 K) were shaken and samples were taken at specific times ( =1, 3, 5, 10, 15, 30, 60, 120 min) and measured immediately afterward.
3 Results and discussion
3.1 Synthesis of the samples
Ni50Co50-LDH/UiO-66-NH2 NC was synthesized by the in situ growth of UiO-66-NH2 MOF nanocrystals on the surface of ultrathin nanosheets of functionalized Ni50Co50-LDH. UiO-66-NH2 MOF was chosen because this MOF possesses free amine functional groups in its linker that can act as adsorption sites for the capture of heavy metals and, more importantly, this MOF shows good water and organic solvent resistance. Ni50Co50-LDH was chosen because it can be synthesized by a facile, large-scale, and environmentally friendly synthetic protocol in which no toxic organic solvents are used. Due to its extended 2D-ultrathin nanosheets, Ni50Co50-LDH can be a potential substrate to covalently anchor MOF nanocrystals. However, it is worth pointing out that before the in situ growth of MOF nanocrystals, in order to establish a strong chemical bond between LDH nanosheets and MOF nanocrystals, it is necessary to modify the surface of the LDH with a suitable silane coupling agent. Herein, we have functionalized Ni50Co50-LDH with CPTMS prior to the in situ growth of UiO-66-NH2 nanocrystals (Fig. 1). Methoxy groups of CPTMS molecules can easily react with surface hydroxyl groups of LDH, and a number of carboxylic groups of MOF linkers (H2N-H2BDC) can form chemical bonds with carbon attached to chlorine atoms in CPTMS to form ester bonds (Fig. 1). This synthetic approach, in addition to establishing a strong chemical bond between the composite components (LDH and MOF), also results in a uniform distribution of MOF nanocrystals throughout Ni50Co50-LDH nanosheets, and accordingly the formation of a homogeneous composite structure. Ni50Co50-LDH/UiO-66-NH2 NC decorated with abundant amine groups in its structure could be a potential adsorbent for capturing cationic heavy metals.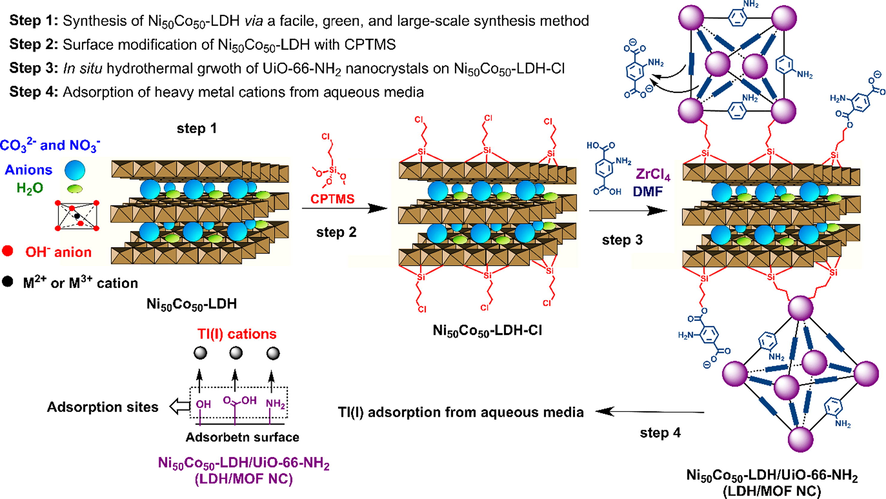
The overall synthesis procedure of the Ni50Co50-LDH/UiO-66-NH2 NC for the adsorption of Tl(I) from aqueous medium.
3.2 Characterization of the samples
The powder XRD patterns obtained for the samples are shown in Fig. 2 and demonstrate that the synthesized samples are indeed crystalline. Ni50Co50-LDH revealed characteristic X-ray diffraction peaks centered at 11.7, 21.7, 34.2, and 60.7, and Ni50Co50-LDH-Cl revealed peaks centered at 10.5, 20.5, 34.4, and 60.5 which are related to 2θ=(0 0 3), (0 0 6), (0 1 2), and (1 1 0) planes of nickel cobalt carbonate hydroxide hydrate (JCPDS 33–0429) which are in close agreement with previously reported results (Soltani et al., 2018; Soltani et al., 2020; Li et al., 2016). These XRD results clearly exhibited that both Ni50Co50-LDH and Ni50Co50-LDH-Cl possess a low degree of crystallinity and surface modification has no significant effect on the degree of crystallinity. For the UiO-66-NH2 sample, it can be observed that the XRD peaks are consistent with those previously reported (Kandiah et al., 2010; Decoste et al., 2013; Cavka et al., 2008). The two featured intensive peaks at 2θ=7.3° and 8.55° are attributed to the (1 1 1) and the (2 0 0) crystal planes, respectively. Also, less prominent peaks were observed at 26°, 30.8°, 43.8°, 50.5°, and 57.5°. The XRD pattern of Ni50Co50-LDH/UiO-66-NH2 NC exhibited two featured diffraction peaks at 2θ=7.4° and 8.6°, which could be related to (1 1 1) and (0 0 2) planes of UiO-66-NH2, demonstrating that MOF nanocrystals are grown on the Ni50Co50-LDH-Cl sheets.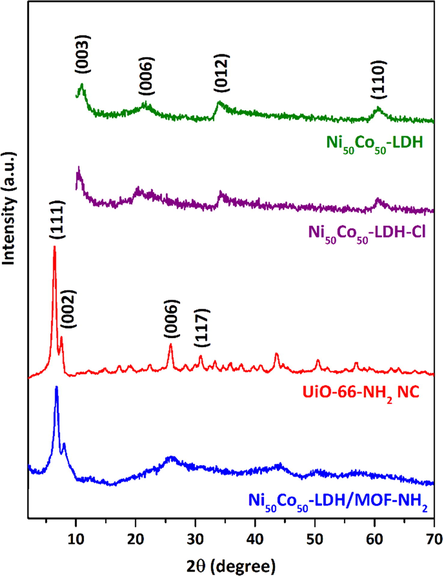
Powder XRD patterns of the samples.
FTIR spectra of the prepared specimens are illustrated in Fig. 3. Both Ni50Co50-LDH and Ni50Co50-LDH-Cl samples have similar FTIR bands at around 3443, 1628, 1382, and 632 cm−1 which are attributed to the O-H stretching vibration, bending mode of water, the vibration of interlayer anions (
and
), and metal-oxide vibrations, respectively (Soltani et al., 2018; Soltani et al., 2020). After surface modification of Ni50Co50-LDH with CPTMS new absorption bands appeared at 1450, 720, and 698 cm−1 which are due to C–H bending mode, Si–C stretching vibration, and C–Cl stretching vibration, respectively. The appearance of these absorption bands, along with a significant reduction in the intensity of the absorption band at 3443 cm−1 is indicative of the successful grafting of CPTMS on the surface of Ni50Co50-LDH. For UiO-66-NH2, the absorption bands at 1402 and 1650 cm−1 is attributed to the C-N stretching mode and N–H scissoring vibration in aromatic amines. The absorption bands at 1584 and 1710 cm−1 are attributed to the symmetric and asymmetric modes of Zr-bound carboxylate. Also, the absorption bands at 3455 and 3384 cm−1 represent, respectively, the symmetric and asymmetric N-H stretching vibration. After the in situ growth of MOF nanocrystals on the LDH surface, characteristic absorption bands related to both UiO-66-NH2 and modified Ni50Co50-LDH are observed in the FTIR spectrum of Ni50Co50-LDH/UiO-66-NH2 NC, indicating the simultaneous presence of both structures in the nanocomposite.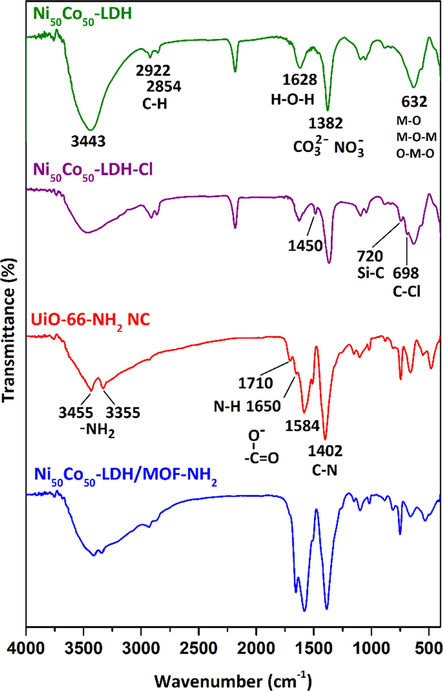
FTIR spectra of the samples.
FESEM images of the samples are shown in Fig. 4. It can be seen that polygonal nanocrystals of UiO-66-NH2 possess a uniform size distribution (Fig. 4a-c). Ultrathin nanosheets of Ni50Co50-LDH can be clearly observed in Fig. 4d-f. After surface modification of Ni50Co50-LDH with CPTMS, no significant difference was observed, indicating that surface modification has no significant effect on the surface morphology of the LDH nanosheets (Fig. 4g-i). Fig. 4j-l reveal the simultaneous presence of both structural components: UiO-66-NH2 MOF crystals and Ni50Co50-LDH sheets are clearly visible in the structure of the Ni50Co50-LDH/UiO-66-NH2 NC, indicating the successful synthesis of Ni50Co50-LDH/UiO-66-NH2 NC by the in situ growing method. Interestingly, compared to previous works (Yang et al., 2017; Hu et al., 2018), the simultaneous presence of both MOF crystals (with well-dispersed nanoparticles) and LDH sheets in the nanocomposite structure is well visible, which is probably due to 1) the chemical surface modification of LDH, 2) synthesis method, and 3) the selection of suitable MOF and LDH for the nanocomposite formation.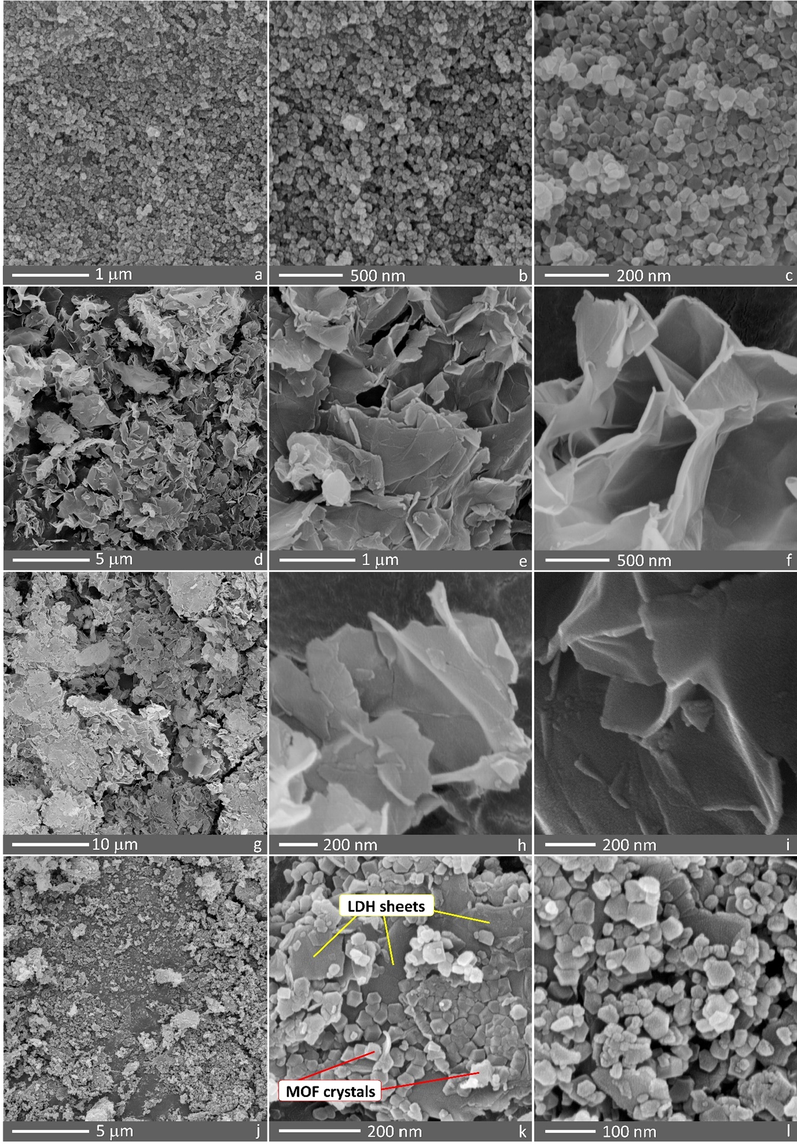
FESEM images of (a-c) UiO-66-NH2, (d-f) Ni50Co50-LDH, (g-i) Ni50Co50-LDH-Cl, and (j-l) Ni50Co50-LDH/UiO-66-NH2 NC.
Fig. 5 shows the EDS mapping images of Ni50Co50-LDH/UiO-66-NH2 NC. These images demonstrated that structural elements of C, N, O, Co, Ni, Si, and Zr were uniformly distributed on the composite structure. Also, the EDS spectrum obtained from the Ni50Co50-LDH/UiO-66-NH2 NC shows the peaks corresponding to all the aforementioned elements.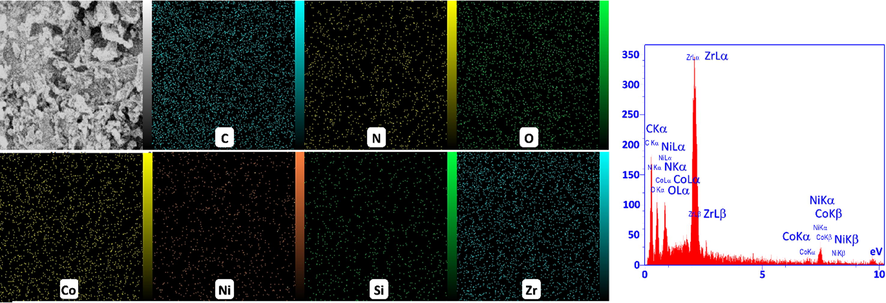
Elemental mapping images for C, N, O, Co, Ni, Si, and Zr and the EDS spectrum of the Ni50Co50-LDH/UiO-66-NH2 NC.
The N2 adsorption–desorption isotherm profiles of the samples are revealed in Fig. 6 and their textural properties are given in Table 2. Ni50Co50-LDH and Ni50Co50-LDH-Cl show a characteristic type III isotherm with an H1 hysteresis loop, indicating the existence of mesopores and macropores, which is due to gaps and large space between the adjacent interlayers of overlapping ultrathin nanosheets (Soltani et al., 2020; Li et al., 2016). After surface modification, the pore volume and surface area decreased slightly which is a common phenomenon during the surface modification with silane coupling agents via the post-modification method. Type I shape for UiO-66-NH2 indicates that MOF nanocrystals possess microporous features with a pore diameter (D) of 1.2 nm. The shape of the isotherm for Ni50Co50-LDH/UiO-66-NH2 NC is the same as that of UiO-66-NH2 (Type I), except that the hysteresis loop in the UiO-66-NH2 sample is wider, indicating the presence of larger pores (mesopores). The origin of these mesopores located in the composite structure is the existence of the LDH component in the composite structure. Compared to UiO-66-NH2, a decrease in surface area and pore volume was observed for Ni50Co50-LDH/UiO-66-NH2 NC which was predictable. The surface area of Ni50Co50-LDH, Ni50Co50-LDH-Cl, UiO-66-NH2, Ni50Co50-LDH/UiO-66-NH2 NC were found to be 48, 31, 1223, and 907 m2 g−1 according to the BET equation. The microporous surface area calculated using the t-plot method was 32, 20, 1218, and 554 m2 g−1 for Ni50Co50-LDH, Ni50Co50-LDH-Cl, UiO-66-NH2, Ni50Co50-LDH/UiO-66-NH2 NC, respectively. The large surface area and a high degree of porosity with a bimodal micro/mesoporosity, as well as amine-decorated pores, make Ni50Co50-LDH/UiO-66-NH2 material a potential adsorbent for removal of cationic heavy metals.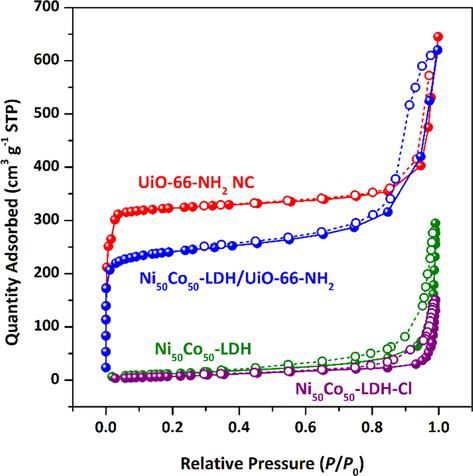
N2 adsorption–desorption isotherms of the samples at 77 K.
Surface area (m2 g−1)
Pore volume (
, cm3 g−1)
Samples
BET
Langmuir
t-plot
(nm)
Ni50-Co50 LDH
48
45
32
0.459
0.221
3.2
Ni50-Co50 LDH-Cl
31
29
20
0.232
0.108
2.4
UiO-66-NH2
1223
1250
1218
0.456
0.444
1.2
Ni50-Co50 LDH/UiO-66-NH2
907
916
554
0.909
0.111
2.1
3.3 Adsorption studies
3.3.1 The effect of pH and adsorbent dose
Solution pH is an important parameter in adsorption because it affects the surface charge of adsorbents and the chemistry of metal solutions (Glocheux et al., 2014). As shown in Fig. 7, the removal percentage of Tl(I) rises with increasing pH until it reaches a constant value in the range between 6.5 and 7.5 and then starts to decrease gradually from 7.5 to 8.5. This is a common adsorption behavior in many metal adsorption processes by adsorbents possessing functional groups like hydroxyl, amines, thiols, and ketones, known as the increasing-maximum-decreasing (IMD) pattern. According to the IMD model proposed by Soltani and co-workers (Soltani et al., 2020; Soltani et al., 2021; Soltani et al., 2019), in an acidic medium, the adsorption quantity of target adsorbate is low because of the competition between metal cations and H3O+ cations for interaction with surface functional groups of adsorbents. By enhancing the pH of solution and reducing competition, the adsorption is increased to reach its highest level. As shown in Fig. 7, with an increase in the adsorbent dose from 0.001 g to 0.002 g the removal percentage of Tl(I) cations increased considerably, but with an increase to 0.005 g, there was no significant increase in its removal percentage. For an adsorbent dose of 0.002 g and the pH range of 6.5–7.5, the removal percentage obtained was ≥ 98%. Accordingly, pH 7.0 and an adsorbent dose of 0.002 g were selected as optimal conditions for further adsorption investigations.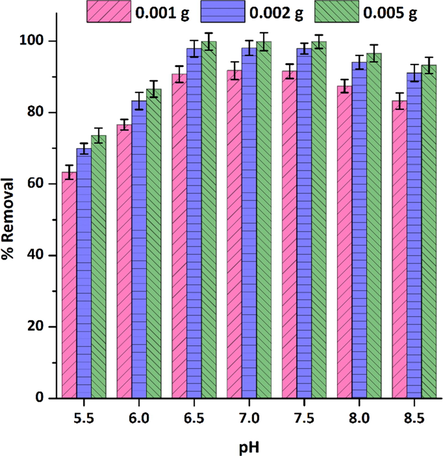
The effect of pH and adsorbent dose on Tl(I) adsorption by Ni50Co50-LDH/UiO-66-NH2 NC (pH=5.5–8.5,
=30 mg L–1,
=30 mL,
=0.001–0.005 g,
=293 K, shaking speed=180 rpm).
3.3.2 The isotherm and thermodynamic studies (effect of initial concentration and temperature)
The simultaneous influence of initial Tl(I) concentration and solution temperature on the adsorption process were studied. As shown in Fig. 8a, for all three temperatures (293, 303, and 313 K), with increasing Tl(I) concentration the adsorption capacity goes up sharply until it is almost fixed at a point (
) and the adsorption reaches the equilibrium state. In order to gain a better understanding of the equilibrium adsorption isotherms, three non-linear isotherm models, namely Langmuir, Freundlich, and Redlich-Peterson (R-P) (Eqs. (4–6) in Table 1), were used to fit experimental adsorption equilibrium data. These adsorption isotherm curves, after non-linear fitting, are shown in Fig. 8a for three temperatures, and the corresponding parameters and R2 values (adjusted R2) are listed in Table 3. The adsorption isotherms of Tl(I) at all three temperatures present a good Langmuir fit (R2 ∼ 0.94–0.98) in comparison with Freundlich (R2 ∼ 0.88), implying that the active sites (adsorption functional groups) were distributed homogenously on the Ni50Co50-LDH/UiO-66-NH2 NC, and that monolayer adsorption occurred on a limited number of identical and equivalent localized sites (Soltani et al., 2020). The calculated maximum adsorption capacity of Tl(I) reached 601.3 mg g−1, surpassing the largest reported adsorption capacities in Table 4. Also, the closeness of the calculated maximum adsorption capacities (
= 601.3, 626.5, and 650.7 mg g−1 at 293, 303, and 313 K, respectively) with their experimental values (
= 600.0, 626.3, 649.5 mg g−1 at 293, 303, and 313 K, respectively) indicates a very good agreement of the Langmuir isotherm model with experimental data. LDH/MOF NC: Ni50Co50-LDH/UiO-66-NH2 nanocomposite; TMS-SMSs: thiol-modified mesoporous silica submicrospheres; HMO: amorphous hydrous manganese dioxide; FC-Cu-EDA-SAMMS: copper(II) ferrocyanide on mesoporous silica.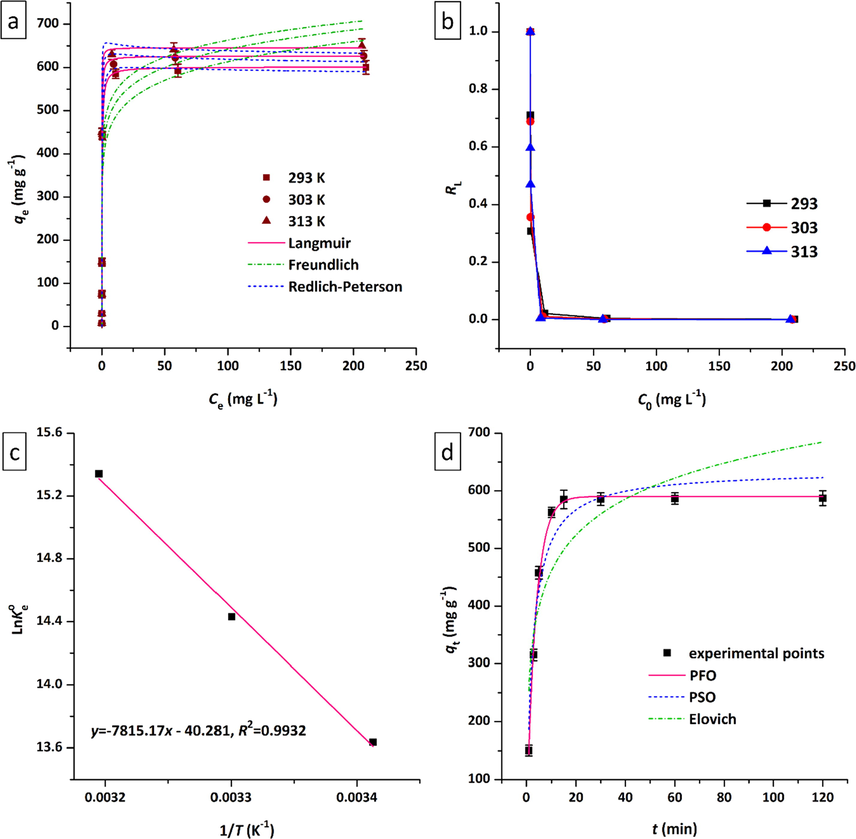
Diagrams related to (a) the effect of initial concentration of Tl(I) on the adsorption capacity and isotherm curves, (b)
values, and (c) corresponding thermodynamic curve for adsorption of Tl(I) on Ni50Co50-LDH/UiO-66-NH2 NC (pH=7.0,
=30 mL,
=0.002 g,
=120 min, shaking speed=180 rpm). (d) The effect of time on the adsorption of Tl(I) by Ni50Co50-LDH/UiO-66-NH2 NC and corresponding kinetic curves (pH=7.0,
=50 mg L–1,
=30 mL,
=0.002 g,
=293 K, shaking speed=180 rpm).
Values
Isotherms
Parameters
293 K
303 K
313 K
(mg g−1)
600.0
626.3
649.5
Langmuir
(mg g−1)
601.3
626.5
650.7
(L mg−1)
4.087
9.051
22.512
0.9817
0.9775
0.9401
Freundlich
(mg g−1)(L g−1)n
377.3
409.6
440.7
(-)
9.483
10.247
11.259
0.8854
0.8862
0.8775
R-P
(L g−1)
2287.5
5271.2
13658.0
(L mg−1)
3.626
8.006
20.195
(-)
0.998
0.992
0.989
0.9788
0.9740
0.9289
Conditions
Adsorbents
Groups
Year
(mg g−1)
pH
(min/h)
(℃)
Ref
LDH/MOF NC
Soltani et al.
2020
601.3
6.5
20
20
This work
TMS-SMSs
Soltani et al.
2019
452.8
6.0
50 min
25
(Soltani et al., 2019)
titanium peroxide
Zhang et al.
2018
412.0
7.0
4 h
25
(Zhang et al., 2018)
MnO2@ pyrite cinder
Li et al.
2018
290.7
12.0
30 min
25
(Li et al., 2018)
Fe-Mn binary oxides
Li et al.
2017
236.4
10.0
24 h
35
(Li et al., 2017)
HMO
Wan et al.
2014
352.9
5.0
40 min
15
(Wan et al., 2014)
Eucalyptus leaves
Dashti et al.
2013
80.7
8.5
30 min
25
(Dashti Khavidaki and Aghaie, 2013)
FC-Cu-EDA-SAMMS
Sangvanich et al.
2010
28.3
7.8
NR
RT
(Sangvanich et al., 2010)
nano-Al2O3
Zhang et al.
2008
6.3
4.5
10 min
40
(Zhang et al., 2008)
R-P isotherm is a three-parameter model that combines features of both Freundlich and Langmuir isotherms in one equation (Redlich and Peterson, 1959). It can be applied to determine whether the adsorption isotherm follows Langmuir or Freundlich's characteristics. In this equation (Eq. (6)), when =1 it becomes the Langmuir equation and when =0 it approaches the Freundlich equation ( and ). As shown in Table 3, the values of parameter for adsorption of Tl(I) on the adsorbent at all three temperatures were very close to unity, implying that the Langmuir model showed a better fit than the Freundlich.
According to Hall et al. (Hall et al., 1966), an essential characteristic of the Langmuir equation could be illustrated in terms of separation factor ( , Table 1) which is a unitless equilibrium factor and proposes the adsorption nature and possibility of the adsorption phenomenon: >1, unfavorable; =1, linear; 0< <1, favorable; =0, irreversible. As shown in Fig. 8b, for the adsorption of Tl(I) on the Ni50Co50-LDH/UiO-66-NH2 NC, the values obtained are between zero and one, demonstrating a favorable adsorption process.
The influence of solution temperature on the adsorption of Tl(I) cations by Ni50Co50-LDH/UiO-66-NH2 NC was studied. As shown in Fig. 8a, the adsorption capacity increases with increasing temperature. For example, with increasing temperature from 293 K to 303 K and 313 K, the adsorption capacity reaches 626.3 and 649.5 mg g−1, respectively (Table 3). In the Langmuir equation, constant indicates the affinity between an adsorbent and a target adsorbate. The higher the value of this parameter, the greater the affinity to adsorb the target species. As the solution temperature rises, the value of parameter and accordingly the adsorption affinity of Ni50Co50-LDH/UiO-66-NH2 NC toward Tl(I) cations continually increases. This trend is in close agreement with the changes in the uptake capacities of Tl(I) with temperature, as can be seen in Fig. 8a and Table 3. In order to gain more insight into the temperature-dependent behavior of Tl(I) adsorption on the adsorbent and assess the feasibility of the adsorption procedure thermodynamic studies were conducted. For this purpose, important thermodynamic parameters such as , , and were obtained from the equations 7–9 given in Table 1.
It is reported that the right method for estimating
values in the adsorption procedure is to determine the best fitted-nonlinear isotherm (generally the Langmuir isotherm) at several temperatures (Lima et al., 273 (2019)). Furthermore, it is assumed that the concentration of target adsorbate in an aqueous medium is very low to presume that the activity coefficient (
) is unitary. So, in these circumstances, for each Langmuir isotherm at a certain temperature (Fig. 8a), a value of
is determined, resulting in a value of
(Eq. (7)), which can be used to estimate the value of
and
(Eq. (8)), and, consequently,
values (Eq. (9)). The estimated values of
,
, and
are given in Table 5 and the corresponding thermodynamic curve is shown in Fig. 8c.
(kJ mol−1)
(kJ mol−1)
(kJ mol−1 K−1)
293
303
313
64.979
0.335
–33.176
−36.526
−39.876
According to the literature, in an adsorption process, the magnitude of and can suggest the kind of interactions between the adsorbent and adsorbate: –20< <0 kJ mol−1, physical adsorption; –400< <–80 kJ mol−1, chemical adsorption; 4< <10 kJ mol−1, van der Waals forces; 2< <29 kJ mol−1, dipole–dipole interactions; 2< <40 kJ mol−1, hydrogen bonding forces; ≈40 kJ mol−1, exchange of dentate; >60 kJ mol−1, chemical bonding forces (Soltani et al., 2020; Zhou et al., 2014). The obtained and values for adsorption of Tl(I) cations on the adsorbent are + 64.979 kJ mol−1 and 0.335 kJ mol−1 K−1, respectively, indicating that the adsorption process is endothermic with an increase in randomness at the solid/liquid interface with rising temperature. Furthermore, became more negative with an increase in temperature, indicating a more efficient Tl(I) adsorption onto Ni50Co50-LDH/UiO-66-NH2 NC at a higher temperature. The values of and demonstrate that the adsorption of Tl(I) on the Ni50Co50-LDH/UiO-66-NH2 NC is more chemical than physical.
3.3.3 The kinetic studies (effect of contact time)
The effect of contact time on the adsorption of Tl(I) onto Ni50Co50-LDH/UiO-66-NH2 NC was studied. As shown in Fig. 8d, with increasing time, the amount of adsorption capacity increases continuously with a steep slope until it reaches a constant value in about 15 min. This observation reveals that the adsorption rate of Tl(I) by the adsorbent is high, which can be attributed to the presence of abundant active adsorption sites (amine groups and aromatic rings) in the adsorbent structure as well as the high surface area of the adsorbent.
In order to gain better insight into the kinetics of the adsorption, non-linear forms of three kinetic models, namely pseudo-first-order (PFO), pseudo-second-order (PSO), and Elovich, were used and fitted to the experimental data. Table 1 lists these kinetic equations, and the corresponding kinetic parameter values after non-linear fitting are tabulated in Table 6.
Kinetics
Parameters
Values
(mg g−1)
587.5
PFO
(mg g−1)
590.2
(min−1)
0.2798
0.9955
PSO
(mg g−1)
635.4
(g mg−1 min−1)
6.54 × 10–4
(mg g−1 min−1)
264.0
0.9420
Elovich
(mg g−1 min−1)
1501.9
(g mg−1)
0.0111
0.7218
The kinetic results are closely fitted by the PFO kinetic equation, with a high R2 value (R2=0.9955). In addition, compared with the PSO model, the calculated equilibrium adsorption capacity ( ) estimated from the PFO model ( =590.2) is much closer to the experimental equilibrium adsorption capacity ( =587.5).
4 Conclusion
Ni50Co50-LDH/UiO-66-NH2 NC was synthesized through a facile ultrasonic-assisted hydrothermal method. UiO-66-NH2 MOF nanocrystals were in situ grown on the surface of 2D-ultrathin functionalized Ni50Co50-LDH nanosheets. Using this method, a uniform nanocomposite architecture was obtained by uniformly distributing MOF nanocrystals on the Ni50Co50-LDH. XRD, FTIR, FESEM/EDS/Mapping, and N2 adsorption–desorption analyses were performed to characterize the physicochemical properties of the synthesized samples. The Ni50Co50-LDH/UiO-66-NH2 NC has remarkable features of potential nanoadsorbent, including chemical functionality, high surface area (907 m2 g−1), large pore volume (0.91 cm3 g−1), and bimodal micro-mesoporous architecture. Therefore, Ni50Co50-LDH/UiO-66-NH2 NC was used as an adsorbent for the removal of toxic Tl(I) from water. Different important parameters affecting the adsorption process were systematically studied and isotherm, thermodynamic, and kinetic investigations were conducted to gain a better understanding of the adsorption behavior and plausible mechanisms involved in the adsorption process. After non-linear fitting analysis, it was found that the Langmuir isotherm presents a better fit to the equilibrium data, and the maximum Langmuir adsorption capacity was found to be 601.3 mg g−1 (conditions: pH=7.0, adsorbent dose=0.002 g, solution volume=30 mL, time=15 min, temperature=293 K, shaking speed=180 rpm) surpassing the best previously reported adsorption capacities for Tl(I) by various adsorbents. Thermodynamic parameters were estimated ( =+64.979 kJ mol−1, =+0.335 kJ mol−1 K−1, =–33.176 to –39.876 kJ mol−1) and it was found that the adsorption of Tl(I) by the Ni50Co50-LDH/UiO-66-NH2 NC is endothermic and spontaneous and the adsorption nature is more chemical than physical.
CRediT authorship contribution statement
Roozbeh Soltani: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Writing - original draft, Writing - review & editing, Project administration. Rasool Pelalak: Software, Validation. Mahboubeh Pishnamazi: Software, Validation, Investigation. Azam Marjani: Resources, Supervision. Shaheen M. Sarkar: Validation, Investigation, Writing - review & editing. Ahmad B. Albadarin: Investigation, Writing - review & editing. Saeed Shirazian: Resources, Validation, Investigation, Supervision, Funding acquisition.
Acknowledgments
Saeed Shirazian acknowledges the supports by the Government of the Russian Federation (Act 211, contract 02.A03.21.0011) and by the Ministry of Science and Higher Education of Russia (grant FENU- 2020-0019).
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
References
- Thallium: A review of public health and environmental concerns. Environ. Int.. 2005;31:493-501.
- [CrossRef] [Google Scholar]
- Selective capture of cesium and thallium from natural waters and simulated wastes with copper ferrocyanide functionalized mesoporous silica. J. Hazard. Mater.. 2010;182:225-231.
- [CrossRef] [Google Scholar]
- Adsorption of Thallium(I) Ions Using Eucalyptus Leaves Powder. Clean - Soil, Air, Water.. 2013;41:673-679.
- [CrossRef] [Google Scholar]
- Selective capture of thallium(I) ion from aqueous solutions by amorphous hydrous manganese dioxide. Chem. Eng. J.. 2014;239:200-206.
- [CrossRef] [Google Scholar]
- Potential technologies for removing thallium from mine and process wastewater: an annotation of the literature. Eur. J. Miner. Process. Environ. Prot.. 2002;2:1-10.
- [Google Scholar]
- Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf.. 2018;148:702-712.
- [CrossRef] [Google Scholar]
- Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. J. Environ. Chem. Eng.. 2017;5:2782-2799.
- [CrossRef] [Google Scholar]
- Removal of Heavy Metals from Industrial Wastewaters: A Review. ChemBioEng Rev.. 2017;4:37-59.
- [CrossRef] [Google Scholar]
- Molecular dynamics simulation of novel diamino-functionalized hollow mesosilica spheres for adsorption of dyes from synthetic wastewater. J. Mol. Liq.. 2021;322 114812
- [CrossRef] [Google Scholar]
- Functionalized pollen-like mesoporous silica for Cr(VI) removal. Microporous Mesoporous Mater.. 2020;310 110531
- [CrossRef] [Google Scholar]
- Synthesis, molecular dynamics simulation and adsorption study of different pollutants on functionalized mesosilica. Sci. Rep.. 2021;11:1-13.
- [CrossRef] [Google Scholar]
- Mesostructured Hollow Siliceous Spheres for Adsorption of Dyes. Chem. Eng. Technol.. 2020;43:392-402.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of novel N-methylimidazolium-functionalized KCC-1: A highly efficient anion exchanger of hexavalent chromium. Chemosphere. 2020;239 124735
- [CrossRef] [Google Scholar]
- Hierarchical multi-shell hollow micro–meso–macroporous silica for Cr(VI) adsorption. Sci. Rep.. 2020;10:1-12.
- [CrossRef] [Google Scholar]
- Removal of acetaminophen from synthetic wastewater in a fixed-bed column adsorption using low-cost coconut shell waste pretreated with NaOH, HNO3, ozone, and/or chitosan. J. Environ. Manage.. 2018;226:365-376.
- [CrossRef] [Google Scholar]
- Single, simultaneous and consecutive biosorption of Cr(VI) and Orange II onto chemically modified masau stones. J. Environ. Manage.. 2017;204:365-374.
- [CrossRef] [Google Scholar]
- Environmentally-friendly and ultrasonic-assisted preparation of two-dimensional ultrathin Ni/Co-NO3 layered double hydroxide nanosheet for micro solid-phase extraction of phenolic acids from fruit juices. Ultrason. Sonochem.. 2018;40:395-401.
- [CrossRef] [Google Scholar]
- Recent progress in layered double hydroxides (LDH)-containing hybrids as adsorbents for water remediation. Appl. Clay Sci.. 2017;143:279-292.
- [CrossRef] [Google Scholar]
- Water-stable metal-organic frameworks for aqueous removal of heavy metals and radionuclides: A review. Chemosphere. 2018;209:783-800.
- [CrossRef] [Google Scholar]
- Adsorptive removal of hazardous materials using metal-organic frameworks (MOFs): A review. J. Hazard. Mater.. 2013;244–245:444-456.
- [CrossRef] [Google Scholar]
- Metal-Organic Material Polymer Coatings for Enhanced Gas Sorption Performance and Hydrolytic Stability under Humid Conditions. ACS Appl. Mater. Interfaces. 2020;12:33759-33764.
- [CrossRef] [Google Scholar]
- Recent advances in facile synthesis and applications of covalent organic framework materials as superior adsorbents in sample pretreatment. TrAC - Trends Anal. Chem.. 2018;108:154-166.
- [CrossRef] [Google Scholar]
- Covalent organic frameworks (COFs) for environmental applications. Coord. Chem. Rev.. 2019;400 213046
- [CrossRef] [Google Scholar]
- Covalent triazine-based framework-grafted functionalized fibrous silica sphere as a solid-phase microextraction coating for simultaneous determination of fenthion and chlorpyrifos by ion mobility spectrometry. Microchim. Acta. 2021;188:1-11.
- [CrossRef] [Google Scholar]
- Preparation of COOH-KCC-1/polyamide 6 composite by in situ ring-opening polymerization: synthesis, characterization, and Cd(II) adsorption study. J. Environ. Chem. Eng. 2020 104683
- [CrossRef] [Google Scholar]
- Novel diamino-functionalized fibrous silica submicro-spheres with a bimodal-micro-mesoporous network: Ultrasonic-assisted fabrication, characterization, and their application for superior uptake of Congo red. J. Mol. Liq.. 2019;294 111617
- [CrossRef] [Google Scholar]
- Meso-architectured siliceous hollow quasi-capsule. J. Colloid Interface Sci.. 2020;570:390-401.
- [CrossRef] [Google Scholar]
- A review of adsorptive remediation of environmental pollutants from aqueous phase by ordered mesoporous carbon. Chem. Eng. J.. 2021;403 126286
- [CrossRef] [Google Scholar]
- Inamuddin, Recent trends in the synthesis of graphene and graphene oxide based nanomaterials for removal of heavy metals—A review. J. Ind. Eng. Chem.. 2018;66:29-44.
- [CrossRef] [Google Scholar]
- Removal of heavy metals in aquatic environment by graphene oxide composites: a review. Environ. Sci. Pollut. Res.. 2020;27:190-209.
- [CrossRef] [Google Scholar]
- In situ polymerized FDU-12/PMMA and FDU-12/polyamide 6 nanocomposites for Cd(II) adsorption. Chem. Eng. Technol. 2020
- [CrossRef] [Google Scholar]
- Novel and green nanocomposite-based adsorbents from functionalised mesoporous KCC-1 and chitosan-oleic acid for adsorption of Pb(II) Eur. Polym. J.. 2019;119:400-409.
- [CrossRef] [Google Scholar]
- A hierarchical LDH/MOF nanocomposite: Single, simultaneous and consecutive adsorption of a reactive dye and Cr(vi) Dalt. Trans.. 2020;49:5323-5335.
- [CrossRef] [Google Scholar]
- The preparation of defective UiO-66 metal organic framework using MOF-5 as structural modifier with high sorption capacity for gaseous toluene. J. Environ. Chem. Eng.. 2019;7 103405
- [CrossRef] [Google Scholar]
- Construction of ternary Ag/AgCl/NH2-UiO-66 hybridized heterojunction for effective photocatalytic hexavalent chromium reduction. J. Colloid Interface Sci.. 2019;555:342-351.
- [CrossRef] [Google Scholar]
- Bimetallic Fe/In metal-organic frameworks boosting charge transfer for enhancing pollutant degradation in wastewater. Appl. Surf. Sci.. 2020;528 147053
- [CrossRef] [Google Scholar]
- A water stable metal-organic framework with optimal features for CO2 capture. Angew. Chemie - Int. Ed.. 2013;52:10316-10320.
- [CrossRef] [Google Scholar]
- A novel and facile green synthesis method to prepare LDH/MOF nanocomposite for removal of Cd (II) and Pb (II) Sci. Rep.. 2021;11:1-15.
- [CrossRef] [Google Scholar]
- Effective toluene adsorption over defective UiO-66-NH2: An experimental and computational exploration. J. Mol. Liq.. 2020;316 113812
- [CrossRef] [Google Scholar]
- In-situ fabrication of a spherical-shaped Zn-Al hydrotalcite with BiOCl and study on its enhanced photocatalytic mechanism for perfluorooctanoic acid removal performed with a response surface methodology. J. Hazard. Mater.. 2020;399 123070
- [CrossRef] [Google Scholar]
- Highly effective adsorption removal of perfluorooctanoic acid (PFOA) from aqueous solution using calcined layer-like Mg-Al hydrotalcites nanosheets. Environ. Sci. Pollut. Res.. 2020;27:13396-13408.
- [CrossRef] [Google Scholar]
- In-situ growth of ZIF-8 on layered double hydroxide: Effect of Zn/Al molar ratios on their structural, morphological and adsorption properties. J. Colloid Interface Sci.. 2017;505:206-212.
- [CrossRef] [Google Scholar]
- In-situ fabrication of ZIF-8 decorated layered double oxides for adsorption and photocatalytic degradation of methylene blue. Microporous Mesoporous Mater.. 2018;271:68-72.
- [CrossRef] [Google Scholar]
- Synthesis and stability of tagged UiO-66 Zr-MOFs. Chem. Mater.. 2010;22:6632-6640.
- [CrossRef] [Google Scholar]
- Selection of optimum sorption kinetics: Comparison of linear and non-linear method. J. Hazard. Mater.. 2006;134:277-279.
- [CrossRef] [Google Scholar]
- Facile one-pot synthesis of thiol-functionalized mesoporous silica submicrospheres for Tl(I) adsorption: Isotherm, kinetic and thermodynamic studies. J. Hazard. Mater.. 2019;371:146-155.
- [CrossRef] [Google Scholar]
- Large scale synthesis of NiCo layered double hydroxides for superior asymmetric electrochemical capacitor. Sci. Rep.. 2016;6:1-9.
- [CrossRef] [Google Scholar]
- Stability and degradation mechanisms of metal-organic frameworks containing the Zr6O4(OH)4 secondary building unit. J. Mater. Chem. A. 2013;1:5642-5650.
- [CrossRef] [Google Scholar]
- A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc.. 2008;130:13850-13851.
- [CrossRef] [Google Scholar]
- Adsorption study using optimised 3D organised mesoporous silica coated with Fe and Al oxides for specific As(III) and As(V) removal from contaminated synthetic groundwater. Microporous Mesoporous Mater.. 2014;198:101-114.
- [CrossRef] [Google Scholar]
- Shell-in-shell monodispersed triamine-functionalized SiO2 hollow microspheres with micro-mesostructured shells for highly efficient removal of heavy metals from aqueous solutions. J. Environ. Chem. Eng.. 2019;7 102832
- [CrossRef] [Google Scholar]
- Pore- and solid-diffusion kinetics in fixed-bed adsorption under constant-pattern conditions. Ind. Eng. Chem. Fundam.. 1966;5:212-223.
- [CrossRef] [Google Scholar]
- Superior adsorption of thallium(I) on titanium peroxide: Performance and mechanism. Chem. Eng. J.. 2018;331:471-479.
- [CrossRef] [Google Scholar]
- Efficient removal of thallium(I) from wastewater using flower-like manganese dioxide coated magnetic pyrite cinder. Chem. Eng. J.. 2018;353:867-877.
- [CrossRef] [Google Scholar]
- Removal of thallium from aqueous solutions using Fe-Mn binary oxides. J. Hazard. Mater.. 2017;338:296-305.
- [CrossRef] [Google Scholar]
- Studies on the capability and behavior of adsorption of thallium on nano-Al2O3. J. Hazard. Mater.. 2008;157:352-357.
- [CrossRef] [Google Scholar]
- A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van’t Hoof equation for calculation of thermodynamic parameters of adsorption. J. Mol. Liq.. 2019;273:425-434.
- [CrossRef] [Google Scholar]
- Removal of crystal violet by a novel cellulose-based adsorbent: Comparison with native cellulose. Ind. Eng. Chem. Res.. 2014;53:5498-5506.
- [CrossRef] [Google Scholar]







