Translate this page into:
Novel coumarin derivatives as potential tyrosinase inhibitors: Synthesis, binding analysis and biological evaluation
⁎Corresponding authors. xuetaoxu@wyu.edu.cn (Xuetao Xu), lpbai@must.edu.mo (Liping Bai), wyuchemlhg@126.com (Hongguang Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
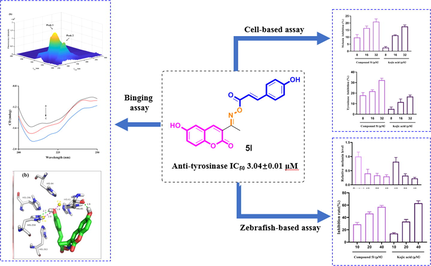
Abstract
A novel series of thirty coumarin derivatives (4a ∼ r, 5a ∼ l) constituted by coumarin and cinnamic acid/benzoic acid through oxime linkage were synthesized and evaluated for their potential anti-tyrosinase activity. Among them, compound 5l exhibited outstanding anti-tyrosinase activity with IC50 of 3.04 ± 0.01 μM compared to 14.13 ± 0.80 μM of kojic acid. Kinetic study revealed compound 5l to be a reversible and uncompetitive tyrosinase inhibitor. 3D fluorescence and CD spectra results showed treatment of compound 5l lead to the conformational changes of tyrosinase. Molecular docking revealed the binding between compound 5l and tyrosinase. Furthermore, compound 5l inhibited melanin content and cellular tyrosinase activity both in B16F10 cells and zebrafish model with no toxicity effect. Taken together, our findings suggested that compound 5l could be used as potential candidate to relieve tyrosinase-related hyperpigmentation.
Keywords
Tyrosinase
Melanogenesis
Coumarin
Cinnamic acid

1 Introduction
Melanin, produced by melanocytes, is widely distributed in humans, animals, and plants (Lin and Fisher, 2007). It is a vital biological pigment responsible for skin, hair, and eye color of humans (Moreiras et al., 2020). The production of melanin leads to the pigmentation that protects the skin against from ultraviolet related injuries (Pillaiyar et al., 2018). However, excess accumulation of melanin characterizes multiplex hyperpigmentary disorders, including melasma, freckles, and melanoma (Roberts et al., 2015). Melanogenesis is a complex process involving series of chemical and enzymatic reactions (Imokawa et al., 2015).
Tyrosinase as the only rate-limiting enzyme in melanogenesis, is crucial for melanogenesis in human skin cells (Zolghadri et al., 2019). Tyrosinase is responsible for the first two catalytic steps in biosynthetic process of melanin: hydroxylation reaction of tyrosine to L-dihydroxyphenylalanine (L-DOPA) and subsequent oxidation reaction of L-DOPA to dopaquinone (Parvez et al., 2007). Immediately, dopaquinone was catalytic converted to melanin by tyrosinase-related proteins (Sánchez-Ferrer et al., 1995). Thence, the regulation of tyrosinase activity is vital for the prevention of melanin synthesis (Yuan et al., 2020). To date, a huge number of natural and synthetic tyrosinase inhibitors have been developed (Li et al., 2021; Fu et al., 2021; Ashooriha et al., 2020). However, only a few inhibitors including arbutin and kojic acid are used as marketable therapeutic agents. But they still present the undesirable side-effects, including dermatitis, cytotoxicity, and skin cancer. These problems prompt us to discover safer and more active tyrosinase inhibitors.
Coumarin, an important core skeleton existing in natural products, presents various pharmacological activities, such as anti-oxidation, anti-inflammatory, anti-bacterial, and anti-cancer (Xu et al., 2020; Kostova, 2005; Musa and Cooperwood, 2008). Importantly, lots of coumarin derivatives presented potential inhibition on tyrosinase or/and melanin (Ashraf et al., 2015; Matos et al., 2015; Asthana et al., 2015). Liu et al. (Liu et al., 2012) reported the synthesis of coumarin esters with the most potent tyrosinase inhibition IC50 = 3.4 μM (Fig. 1). Matos et al. (Matos et al., 2011) developed halogenated phenylcoumarins as tyrosinase inhibitors (most potent tyrosinase inhibition IC50 = 0.25 μM) (Fig. 1). Pang et al. (Pang et al., 2017) synthesized novel isoxazole contained coumarin derivatives that showed better activity on melanin synthesis than positive control 8-methoxypsoralen (Fig. 1). On the other hand, cinnamic acid/benzoic acid, active ingredients widely found in natural plants, have been recognized as the privileged scaffolds in the drug research and development due to their broad-spectrum pharmacological activities (Jitareanu et al., 2013; Xu et al., 2005; Taofiq et al., 2017). Especially, cinnamic acid/benzoic acid, and their derivatives have been reported to exhibit anti-tyrosinase or/and anti-melanin ability effects (Kong et al., 2008; Sheng et al., 2018; Ullah et al., 2018). Romagnoli et al. (Romagnoli et al., 2022) reported arylpiperazine linked cinnamic acid derivatives presenting good inhibitory against tyrosinase activity and melanin synthesis (Fig. 1). Cinnamamide derivatives also showed excellent inhibitory activity toward tyrosinase and melanin (Fig. 1) (Ullah et al., 2019). Benzoic acid-thymol ester (Ashraf et al.) demonstrated good anti-tyrosinase activity (Ashraf et al., 2015). In addition, the oxime core prevalently exists in lots of anti-tyrosinase active compounds, including chalcone oximes (Radhakrishnan et al., 2016), and hydroxypyridinone oxime ether derivatives (Shao et al., 2018) (Fig. 1).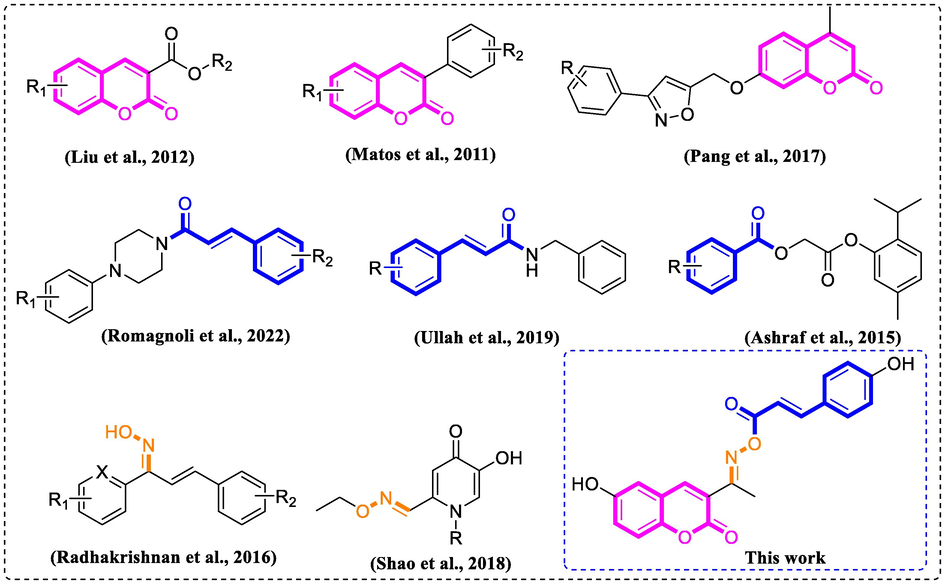
Rationale of the current study.
Molecular hybridization has been recognized as an important structural modification strategy to design new drugs, that combine two or more pharmacophoric moieties to yield a new hybrid molecule. As part of our ongoing efforts to develop safer and more active tyrosinase inhibitors, the hybrids of coumarin with cinnamic acid/benzoic acid through oxime linkage (Fig. 1) were designed and synthesized. Then the synthetic derivatives were assayed for their anti-tyrosinase activity, followed by the investigation of B16F10 cell-based and zebrafish-based experiments.
2 Results and discussion
2.1 Chemistry
All coumarin-cinnamic acid/benzoic acid derivatives (4a ∼ r, 5a ∼ l) were synthesized as synthetic route in Scheme 1. Substituted salicylaldehyde (1) reacted with ethyl acetaacetate to produce substituted acetyl coumarin (2), followed by the reaction with hydroxylamine hydrochloride to obtain substituted coumarin containing oxime (3). Finally, coumarin containing oxime underwent esterification with substituted cinnamic acid/benzoic acid chloride to yield target compounds. All synthetic derivatives (4a ∼ r, 5a ∼ l) were identified by 1H NMR, 13C NMR and HRMS.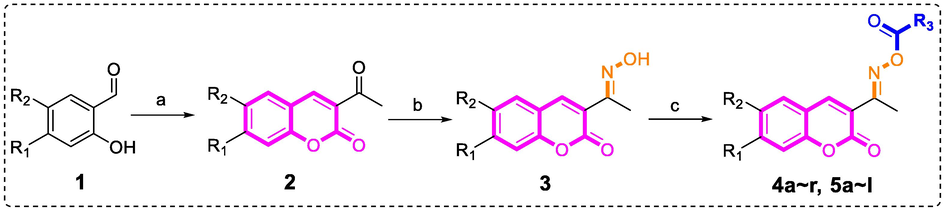
Synthesis of coumarin-cinnamic acid/benzoic acid derivatives 4a ∼ r and 5a ∼ l. Reagents and condition: (a) Ethyl acetaacetate, Piperidine, EtOH, 80 °C; (b) Hydroxylamine hydrochloride, Pyridine, EtOH, rt; (c) Substituted benzoic acid chloride/cinnamic acid chloride, triethylamine, DCM, rt.
2.2 Anti-tyrosinase activity and SAR analysis
For finding more active tyrosinase inhibitors, coumarin-cinnamic acid derivatives (4a ∼ i) and coumarin-benzoic acid derivatives (4j ∼ r) were firstly synthesized. Due to the solubilities of some coumarin derivatives at enzyme activity test system were not good enough, ∼ 64 μM. For better comparing the inhibition activity of coumarin derivatives and future structure–activity relationship analysis, we firstly tested the anti-tyrosinase activity of compounds at concentration of 64 μM using kojic acid as positive control. As depicted in Table 1, among 18 derivatives, compound 4i with 4-hydroxyl group in benzene ring of cinnamic acid exerted strongest tyrosinase inhibition (61.24 ± 0.17%) at concentration of 64 μM, while other derivatives showed weaker inhibition against tyrosinase activity (<50%). Interesting, compound 4i (IC50: 6.40 ± 0.39 μM) presented about ∼ 2.2 folds stronger tyrosinase inhibitory effect than kojic acid (14.13 ± 0.80 μM).
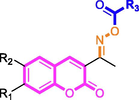
Compound
R1
R2
R3
Inhibition (%)a
IC50 (μM)
4a
H
H
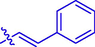
7.48 ± 0.12
–
4b
H
H
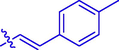
44.87 ± 0.17
–
4c
H
H
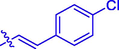
37.94 ± 0.35
–
4d
H
H
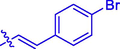
40.32 ± 0.10
–
4e
H
H
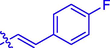
30.96 ± 0.14
–
4f
H
H
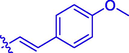
37.29 ± 0.28
–
4g
H
H
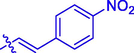
9.62 ± 0.32
–
4h
H
H
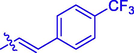
27.52 ± 0.30
–
4i
H
H

61.24 ± 0.17
6.40 ± 0.39
4j
H
H
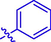
2.45 ± 0.32
–
4k
H
H
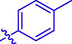
9.26 ± 0.21
–
4l
H
H
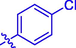
20.71 ± 0.12
–
4m
H
H
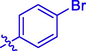
5.17 ± 0.24
–
4n
H
H
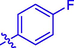
7.70 ± 0.14
–
4o
H
H
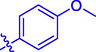
5.75 ± 0.10
–
4p
H
H
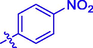
6.08 ± 0.10
–
4q
H
H
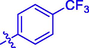
5.14 ± 0.20
–
4r
H
H
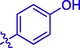
3.90 ± 0.10
–
5a
Cl
H
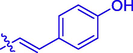
26.02 ± 0.30
–
5b
Br
H
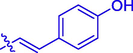
35.44 ± 0.21
–
5c
F
H
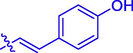
56.57 ± 0.23
22.91 ± 0.14
5d
CH3
H
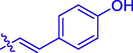
23.79 ± 0.23
–
5e
OCH3
H
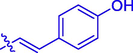
60.20 ± 0.23
23.86 ± 0.15
5f
OH
H
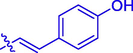
62.39 ± 0.17
6.04 ± 0.30
5 g
H
Cl
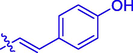
9.43 ± 0.20
–
5 h
H
Br
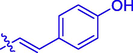
48.55 ± 0.17
44.12 ± 0.36
5i
H
F
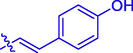
53.73 ± 0.30
17.00 ± 0.15
5j
H
CH3

58.27 ± 0.40
58.85 ± 0.19
5 k
H
OCH3
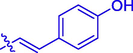
51.31 ± 0.20
52.32 ± 0.67
5l
H
OH
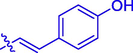
66.24 ± 0.20
3.04 ± 0.01
Kojic acid
14.13 ± 0.80
The main differences between derivatives 4a ∼ i and 4j ∼ r were the incorporation moiety: cinnamic acid and benzoic acid. Generally speaking, coumarin-cinnamic acid derivatives (4a ∼ i) were more active than coumarin-benzoic acid derivatives (4j ∼ r) with the same substitute group. This might due to the potential binding between the cinnamic acid of 4a ∼ i and tyrosinase. Furthermore, the substituents in cinnamic acid moiety also had effects on their anti-tyrosinase activity. Compared to compound 4a with no substituent in cinnamic acid, compounds 4b ∼ i showed stronger anti-tyrosinase activity, indicating that the substituent groups including CH3, Cl, Br, F, OCH3, NO2, CF3, and OH were benefit for their inhibitory activity.
Coumarin-cinnamic acid derivative 4i with strongest anti-tyrosinase activity was further structural modified by introduction of various substituents in the coumarin moiety, obtaining derivatives 5a ∼ l. Their anti-tyrosinase activity results revealed that the introduction of substituent group (Cl, Br, F, CH3, and OCH3) in the C-6 and C-7 position of coumarin caused a negative effect, however the introduction of hydroxyl would increase their inhibitory activity. Among them, compound 5l with OH at C-6 position of coumarin showed the best anti-tyrosinase activity (IC50: 3.04 ± 0.01 μM), ∼ 4.6 folds higher than kojic acid (14.13 ± 0.80 μM).
2.3 Inhibitory mechanism and kinetic type assay
Compound 5l with the strongest anti-tyrosinase activity was selected as representative molecule to investigate the inhibitory mechanism and kinetic type. The mechanism assay by the plots of ν versus [E] in the presence of compound 5l gave a family of straight lines passing the origin (Fig. 2a), revealing that compound 5l was a reversible inhibitor. The kinetic type was detected by Lineweaver-Burk plots of ν versus 1/[S] in the presence of compound 5l, that obtained a group of data lines intersecting at × axis (Fig. 2a). The results showed that compound 5l showed a noncompetitive inhibition. Moreover, thire slop increasesed with the increasing compound 5l concentration, and the inhibition constant (Ki) was calculated as 1.5 μM.![(a) The plots of ν versus [E] in the presence of compound 5l; (b) Lineweaver-Burk plots of ν versus 1/[S] in the presence of compound 5l.](/content/184/2023/16/6/img/10.1016_j.arabjc.2023.104724-fig4.png)
(a) The plots of ν versus [E] in the presence of compound 5l; (b) Lineweaver-Burk plots of ν versus 1/[S] in the presence of compound 5l.
2.4 3D fluorescence spectra and CD spectra assay
The 3D fluorescence spectra and CD spectra were operated to monitor the tyrosinase conformational changes interacting with compound 5l. The 3D fluorescence spectra of tyrosinase (Fig. 3a) appeared two typical spectra: Tyr with Trp residues at excitation of 280 nm and emission of 332 nm (Peal 1), and polypeptide backbone structure at excitation of 232 nm and emission of 335 nm (Peal 2). Treatment with compound 5l (3.33 μM) reduced the fluorescence intensity of Peak 1 by 17.87%, and Peak 2 by 28.19% (Fig. 3b), respectively, declaring that the binding of compound 5l with tyrosinase resulted in the changes of microenvironments and polypeptide backbone structure of tyrosinase.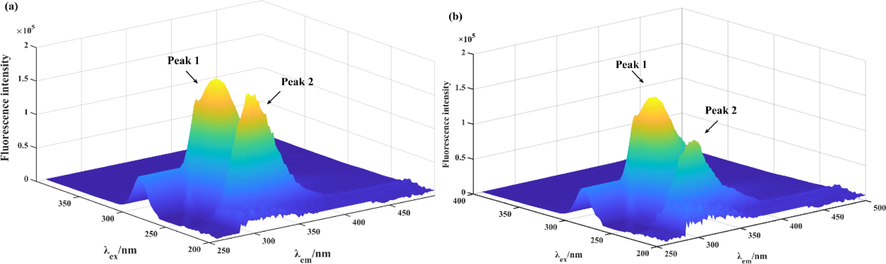
(a) The 3D fluorescence spectra of tyrosinase. (b) The 3D fluorescence spectra of tyrosinase with compound 5l.
The CD spectra of tyrosinase with compound 5l were also monitored at wavelength of 190 ∼ 250 nm and shown in Fig. 4. The CD spectra had two negative bands at 210 and 222 nm, those were the typical features of α-helixes due to n → π* and π → π* electron transfer of amide. Treatment of compound 5l caused the visible reduce of negative bands intensity, revealing that binding of compound 5l with tyrosinase generated the conformation change. The secondary structure contents of tyrosinase in the presence of compound 5l were calculated and listed in Table 2. Treatment of compound 5l (molar ratios: 2:1) caused increase in the content of the α-helix (from 7.3 to 9.6%), and β-turn (from 19.8 to 20.6%), while decrease in β-Sheet (from 38.7 to 29.9%) and random coil (from 36.1 to 35.5%).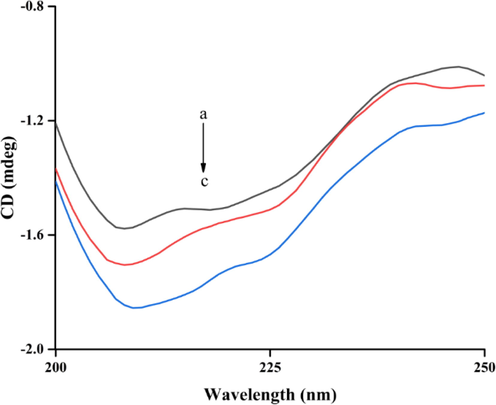
The CD spectra of tyrosinase and compound 5l complex. The molar ratios of tyrosinase to 5l were 1: 0, 1: 1, and 1: 2 for curves a-c, respectively.
[Tyrosinase]: [Compound 5l]
α-Helix
(%)β-Sheet
(%)β-Turn
(%)Rndm Coil
(%)
1:0
7.3
38.7
19.8
36.1
1:1
7.8
36.5
20
35.9
1:2
9.6
29.9
20.6
35.5
2.5 Molecular docking
To obtain more binding mode of compound 5l within the active site of tyrosinase, molecular docking was performed using SYBYL software. As illustrated in Fig. 5(a), compound 5l was well stabilized into the active site of tyrosinase with a “U shaped” conformation, and 4-hydroxy cinnamic acid moiety nested inside. The detailed interaction results (Fig. 5(b)) showed that 6-hydroxy in coumarin formed a hydrogen bond with His85 (bond length: 1.9 Å), 4-hydroxy in cinnamic acid also formed a hydrogen bond with His259 (bond length: 2.7 Å). Moreover, 4-hydroxy in cinnamic acid was oriented toward two Cu ions at distances of 1.9 and 2.8 Å, respectively. All interactions helped compound 5l to anchor into the active site of tyrosinase.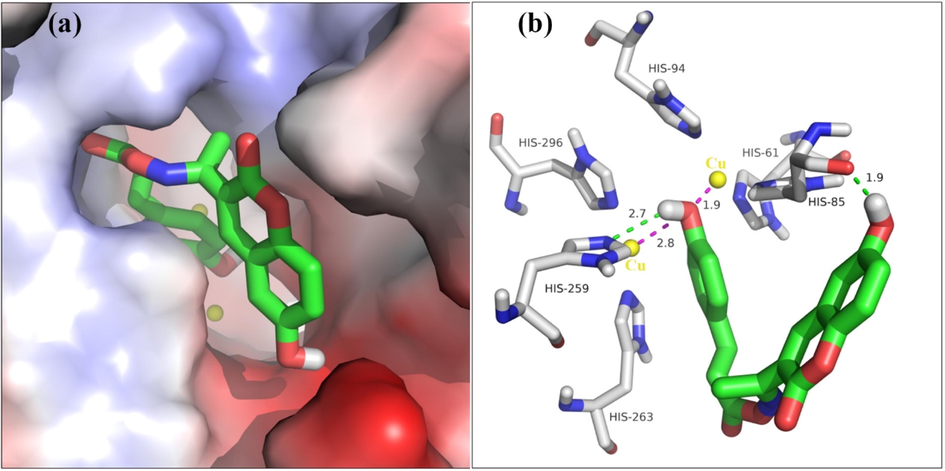
(a) Compound 5l is positioned into the active pocket. (b) Detailed interaction of compound 5l with tyrosinase.
2.6 Melanogenesis and tyrosinase activity assay in B16F10 cells
The cytotoxicity of representative compound 5l on B16F10 cells was firstly assayed after treatment of compound 5l for 48 h (Fig. 6). The results verified that compound 5l had no toxicity effect on B16F10 cells up to concentration 32 μM, while compound 5l showed obvious cytotoxicity at 64 μM. On the other hand, kojic acid (8–64 μM) showed no cytotoxicity. Thererfore, the effects of compound 5l on melanogenesis and tyrosinase activity in vitro were performed at concentrations (8–32 μM) with no cytotoxicity on B16F10 cells.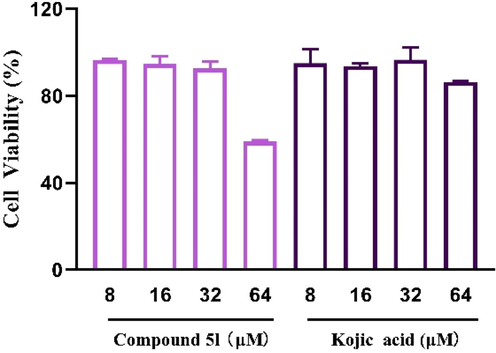
The cytotoxicity assay of compound 5l and kojic acid in B16F10 cells.
The inhibitory effects of compound 5l on melanogenesis were ascertained by measuring the melanin content of B16F10 cells that were treated with compound 5l (8–32 μM) for 48 h. As can be seen in Fig. 7, compound 5l (8–32 μM) presented effectively melanin inhibition in a dose-dependent manner, showing (9.69 ± 1.69)%, (16.41 ± 1.15)%, and (20.96 ± 1.62)%, respectively. Moreover, compound 5l existed stronger melanin inhibitory activity than kojic acid at same tested concentrations.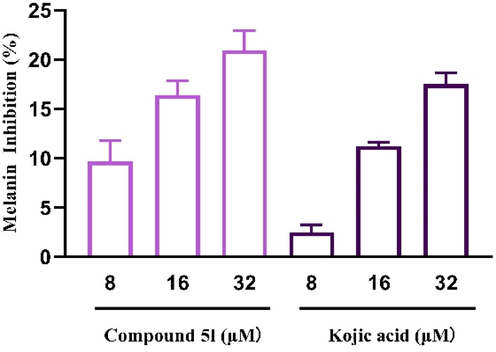
The melanin inhibition of compound 5l and kojic acid in B16F10 cells.
The cellular tyrosinase inhibitory effects of compound 5l were also determined in B16F10 cells and the results were listed in Fig. 8. Compound 5l (8–32 μM) exerted markedly inhibitory activity on cellular tyrosinase in a dose-dependent manner, with inhibition rate of (17.94 ± 2.06)%, (21.77 ± 1.17)%, and (32.30 ± 1.47)%, respectively, which were higher than those of kojic acid, respectively. Those results established that compound 5l suppressed melanogenesis by the inhibition of tyrosinase.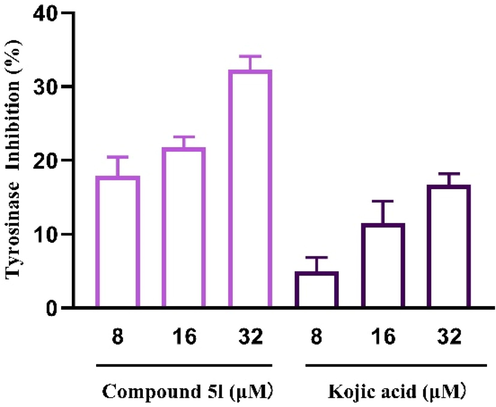
The cellular tyrosinase inhibitory of compound 5l and kojic acid in B16F10 cells.
2.7 Melanogenesis and tyrosinase activity assay in zebrafish
To address whether the depigment effects of compound 5l also occurs in vivo, we employed zebrafish modle that had been believed as the typical in vivo model for the evaluation of tyrosinase activity and melanin. The acute toxicity of compound 5l was firstly evaluated. Fish embryos were exposed to compound 5l for 48 h to observe the embryo survival. The survival rate of compound 5l and kojic acid ranged from 85% to 95% (Fig. 9), revealing that compound 5l and kojic acid did not show acute toxicity at the tested concentrations (10–160 μM).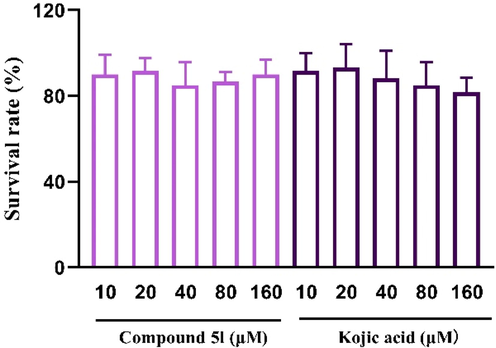
The acute toxicity assay of compound 5l and kojic acid in zebrafish.
Then, the effects of compound 5l (10–40 μM) on melanogenesis in zebrafish were assayed by analyzing the grayscale value of the photo of embryo (Fig. 10). The results indicated that fish embryos in control group presented obvious pigmentation, especially in the eyes, yolk sac and notum. However, treatment with compound 5l (10–40 μM) could effectively reduce the pigmentation in a dose-dependent manner to (30 ± 5)%, while kojic acid showed (23 ± 3)% at 40 μM. Additionally, compound 5l (10–20 μM) exhibited better depigmenting effects, relative to kojic acid.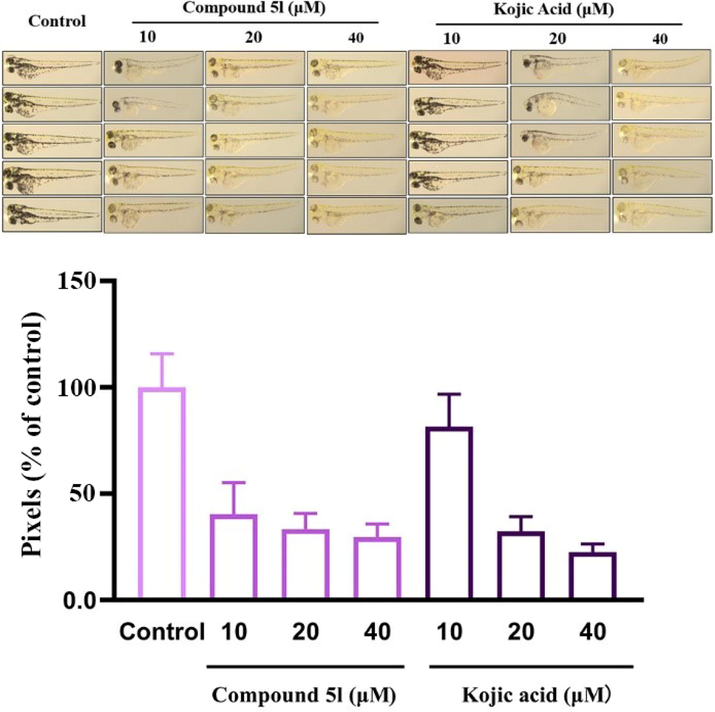
The melanin inhibition of compound 5l and kojic acid in zebrafish.
The effects of compound 5l (10–40 μM) on tyrosinase activity in zebrafish were finally detected and the results were shown in Fig. 11. Compound 5l effectively inhibited tyrosinase activity in zebrafish in a dose-dependent manner to (57.03 ± 2.19)% at 40 μM, which was slightly lower than that of kojic acid (63.09 ± 3.82)% at 40 μM. Additionally, compound 5l (10–20 μM) exhibited better anti-tyrosinase activity than kojic acid at the same concentrations. This linear reduction of melanogenesis and tyrosinase activity in zebrafish embryos matched that obtained in the B16 cell, revealing the positive in vitro-in vivo correlation.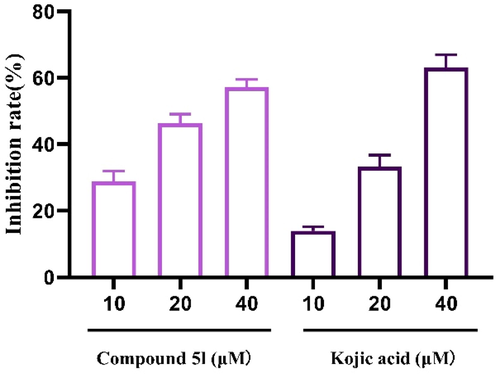
The tyrosinase inhibitory of compound 5l and kojic acid in zebrafish.
3 Conclusion
In order to develop potent tyrosinase inhibitors, we designed and synthesized hybrids (4a ∼ r, 5a ∼ l) coumarin and cinnamic acid/benzoic acid constituted by coumarin and cinnamic acid/benzoic acid through oxime linkage. Among them, compound 5l exhibited outstanding anti-tyrosinase activity with IC50 of 3.04 ± 0.01 μM compared to 14.13 ± 0.80 μM of kojic acid. Kinetic study revealed compound 5l to be a reversible and uncompetitive tyrosinase inhibitor. 3D fluorescence and CD spectra results showed treatment of compound 5l lead to the conformational changes of tyrosinase. Molecular docking revealed the binding between compound 5l and tyrosinase. Furthermore, compound 5l inhibited melanin content and cellular tyrosinase activity both in B16F10 cells and zebrafish model with no toxicity effect. Taken together, our findings suggested that compound 5l could be used as potential candidate to relieve tyrosinase-related hyperpigmentation. All studies highlight the importance of coumarin derivatives as promising anti-melanin agents and provided a method for developing this inhibitors.
4 Experimental
4.1 Materials and methods
Tyrosinase from mushroom (EC 1.14.18.1) and 3,4-Dihydroxyphenylalanine (L-DOPA) were purchased from Sigma-Aldrich. All other reagents and solvents were commercial available. 1H NMR and 13C NMR spectra were recorded in CDCl3 on 500 MHz instruments. High-resolution mass spectral analysis (HRMS) data were measured on the Apex II by means of the ESI technique.
4.2 Synthesis of derivatives 4a ∼ r and 5a ∼ l
Substituted salicylaldehyde (1) (1.0 mmol) and ethyl acetoacetate (1.1 mmol) were added into 10 mL of ethanol containing piperidine (0.02 mmol), then reflowed at 80 °C until the reaction completed. Ice-cold water was added to quench the reaction, followed by filtration and recrystallization to obtain substituted acetyl coumarin (2). To the solution of 2 (1.0 mmol) in 10 mL of ethanol, hydroxylamine hydrochloride (3.0 mmol) and pyridine (0.04 mmol) were added and reacted at room temperature. The product was washed with ethanol to obtain substituted acetyl coumarin containing oxime (3). To the solution of 3 (1.0 mmol) in 3 mL of DCM, triethylamine (1.1 mmol) and substituted benzoic acid chlorides/cinnamic acid chlorides (1.0 mmol) were added, respectively, and reacted at room temperature. Then the reaction was quenched, extracted, washed, and dried, followed by column chromatography purification to produce derivatives 4a ∼ r and 5a ∼ l. All NMR, HRMS, yield, and m.p. data were summarized in supplementary material.
4.3 Tyrosinase activity assay
The synthetic derivatives were assayed for anti-tyrosinase activity using mushroom tyrosinase and L-DOPA (He et al., 2021; Song et al., 2017). All derivatives were dissolved in DMSO and diluted to various concentrations. 10 μL of mushroom tyrosinase (1333.4 U/mL) and 10 μL of test compound were added into 130 μL of phosphate buffer (50 M, pH 6.8), respectively, followed by the incubation for 10 min at room temperature. Then 50 μL of L-DOPA solution (2 mM) was added and the absorbance change was determined at 475 nm. The inhibition percentage was calculated respect to blank group and the 50% inhibitory concentration (IC50) was calculated by interpolation of the dose–response curves. Kojic acid was used as the positive standard. Each experiment was done four times in parallel.
The enzyme inhibitory kinetics of compound 5l were determined by the plots of enzymatic reaction vs enzyme concentration in the presence of compound 5l (Hu et al., 2016), and the substrate inhibitory kinetics were obtained by the Lineweaver-Burk plot of enzymatic reaction vs substrate concentration in the presence of compound 5l (Zhao et al., 2019). The final L-DOPA concentrations were 0.5, 1, 0.67, and 2, respectively.
4.4 3D fluorescence spectra assay
10 μL of mushroom tyrosinase (Final concentration: 168 μM) and 10 μL of compound 5l (Final concentration: 3.33 μM) were added into 2.98 mL of phosphate buffer (50 M, pH 6.8) and incubated for 5 min (Song et al., 2020). The 3D fluorescence spectra of this mixture were measured at excitation and emission wavelengths of 200–600 nm.
4.5 CD spectroscopy
10 μL of mushroom tyrosinase (Final concentration: 33 μM) and 10 μL of compound 5l were added into 180 μL of phosphate buffer (50 M, pH 6.8) and incubated for 5 min (Song et al., 2021). The CD spectrum of this mixture was recorded. The proportion of secondary conformation of protein was analyzed using CDNN.
4.6 Molecular docking
Molecular docking was performed by SYBYL software using the crystal structure of tyrosinase from Agaricus bisporus (PDBID: 2Y9X) obtained from the RCSB Protein Data Bank (Peng et al., 2021). The tyrosinase protein was prepared by the remove of ligand and water, followed by the addition of hydrogen atoms and charge, repair of end residues, and the generation of active pocket. Compound 5l was prepared by addition of charge with Gasteiger-Hückle and energy minimization. Then, the docking between Compound 5l and tyrosinase was operated in the default format, and the results were visualized by Pymol software.
4.7 Cell culture
B16F10 cells (ATCC, Manassas, VA, USA) were cultured in DMEM supplemented with 10% FBS and 1% penicillin/streptomycin at 37 °C in a humidified atmosphere with 5% CO2.
4.8 Cytotoxicity assay
The cells viability after exposure to compound was determined by MTT method as described previously (Lee et al., 2019). B16F10 cells were seeded in a 96 well plate (5 × 103 cells/well) for 24 h and treated with compound 25 (10–40 μM) for another 24 h. Then cells were labelled with MTT solution (0.5 mg/mL) for 4 h. The violet formazan precipitates were dissolved in 100 μL DMSO, then the absorbance was measured at 570 nm. Kojic acid was used as a positive control.
4.9 Melanin assay in B16F10 cells
The effect of compound 5l on melanin content was studied in Murine B16F10 melanoma cells using the standard method with slight modification (Ullah et al., 2019). B16F10 cells were seeded in a 6 well plate (105 cells/well) for 24 h, and then treated with compound 5l (8 ∼ 32 μM) for another 48 h. Harvested cells were added 200 μL NaOH (1 M, containing 10% DMSO), heated at 100 °C for 1 h, and then the absorbance at 405 nm was detected. Kojic acid (8 ∼ 32 μM) was used as a positive control.
4.10 Tyrosinase activity in B16F10 cells
The cellular tyrosinase inhibition of compound 5l was performed as previously described with slight modification (Tang et al., 2021). B16F10 cells were seeded in a 6 well plate (105 cells/well) for 24 h, and then treated with compound 5l (8–32 μM) for 48 h. Harvested cells were washed with ice-cold PBS two times, added 300 μL Triton X-100 (1%, 50 mM PBS), and frozen at −80 °C for 1 h. Then cellular extracts were thawed and centrifuged (12000 rpm, 4 °C) for 30 min to obtain the supernatant, followed by the protein concentration determination using BCAKit. To 80 μL protein solution (equal amount of protein), 20 μL of L-DOPA solution (10 mM) was added, and then the absorbance change was determined at 475 nm. Kojic acid (8–32 μM) was used as a positive control.
4.11 Zebrafish maintenance
Sexual maturity, health, and no deformity Wild-type (AB) zebrafish were cultured at 28 °C under a 14/10‐hr light/dark cycle with the pH around 7.4.
4.12 Fish embryo acute toxicity test
For the in vivo toxicity test (Ramlan et al., 2017); 6 ∼ 8 hpf embryos were seeded in 24 well plate with M3 culture medium (1 embryo/well), treated with test compound (0 ∼ 160 μM) and Kojic acid (0 ∼ 160 μM), respectively, then observed the survival at 72 hpf. Kojic acid (0 ∼ 160 μM) was used as a positive control. Each condition had 12 embryos and was repeated 5 times.
4.13 Melanin determination in zebrafish
Phenotype-based melanogenesis inhibition of compound 5l in zebrafish was determined following reported method (Abbas et al., 2017). Embryos were seeded in 6 well plate with M3 culture medium (5 embryo/well) at 24 hpf, treated with test compound (0 ∼ 40 μM) and Kojic acid (0 ∼ 40 μM), respectively. Then the photo of embryo was taken to determine the melanin using image J software. The embryo photo was prepared with built-in program of image J software, including convering picture to grayscale, framing embryo photo, closing resulting curve, reading grayscale. The grayscale of sample embryo photo was expressed as relative melanin level of the control (100%).
4.14 Tyrosinase activity in zebrafish
Tyrosinase activity was measured according to the method by Chen et al. (Chen et al., 2019). The treated embryos was were lysed in 150 μL sodium deoxycholate solution (5.0 mg/mL), and centrifuged (10000 rpm, 4 °C) for 5 min to obtain the supernatant. 100 μL supernatant was mixed with 100 μL L-DOPA solution (5 mg/mL), followed by the absorbance change record at 475 nm.
4.15 Statistical analysis
All data were presented as mean ± SD. One-way ANOVA was performed to evaluate the difference between groups. P < 0.05 was considered significant.
Acknowledgements
This work was financially supported by the Fundamental and Applied Basic Research Fund of Guangdong Province (No. 2022A1515011657), Department of Education of Guangdong Province (Nos. 2019KZDXM035, 2021KTSCX135, 2021KCXTD044), Jiangmen Science and Technology Plan Project (2021030103150006664), Joint research fund for Wuyi university and Hong Kong and Macao (2021WGALH08), the Science and Technology Development Fund, Macau SAR (0043/2020/AGJ) and the Department of Science and Technology of Guangdong Province for the support of Guangdong-Hong Kong-Macao Joint Laboratory of Respiratory Infectious Disease.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Development of highly potent melanogenesis inhibitor by in vitro, in vivo and computational studies. Drug Des. Dev. Ther.. 2017;11:2029.
- [Google Scholar]
- Kojic acidenatural product conjugates as mushroom tyrosinase inhibitors. Eur. J. Med. Chem.. 2020;201:112480
- [Google Scholar]
- Kinetic and in silico studies of novel hydroxy-based thymol analogues as inhibitors of mushroom tyrosinase. Eur. J. Med. Chem.. 2015;98:203-211.
- [Google Scholar]
- Design, synthesis and bioevaluation of novel umbelliferone analogues as potential mushroom tyrosinase inhibitors. J. Enzyme Inhib. Med. Chem.. 2015;30:874-883.
- [Google Scholar]
- Structure-activity relationship study of hydroxycoumarins and mushroom tyrosinase. J. Agric. Food Chem.. 2015;63:7236-7244.
- [Google Scholar]
- Anti-melanogenesis of novel kojic acid derivatives in B16F10 cells and zebrafish. Int. J. Biol. Macromol.. 2019;123:723-731.
- [Google Scholar]
- Design, synthesis and biological evaluation of tyrosinase-targeting PROTACs. Eur. J. Med. Chem.. 2021;226:113850
- [Google Scholar]
- Design, synthesis, molecular modeling, and biological evaluation of novel kojic acid derivatives containing bioactive heterocycle moiety as inhibitors of tyrosinase and antibrowning agents. Food Chem.. 2021;362:130241
- [Google Scholar]
- 4-Hydroxy cinnamic acid as mushroom preservation: anti-tyrosinaseactivity kinetics and application. Int. J. Biol. Macromol.. 2016;86:489-495.
- [Google Scholar]
- Biological mechanisms underlying the ultraviolet radiation-induced formation of skin wrinkling and sagging II: overexpression of neprilysin plays an essential role. Int. J. Mol. Sci.. 2015;16:7776-7795.
- [Google Scholar]
- Evaluation of phytotoxic and mutagenic effects of some cinnamic acid derivatives using the Triticum test. Turk. J. Biol.. 2013;37:748-756.
- [Google Scholar]
- Inhibitory effects of cinnamic acid on melanin biosynthesis in skin. Biol. Pharm. Bull.. 2008;31:946-954.
- [Google Scholar]
- Synthetic and natural coumarins as cytotoxic agents. Curr. Med. Chem. Anti Cancer Agents. 2005;5:29-46.
- [Google Scholar]
- Inhibitory effects of N-(acryloyl)benzamide derivatives on tyrosinase and melanogenesis. Bioorg. Med. Chem.. 2019;27:3929-3937.
- [Google Scholar]
- Recent advances in the design and discovery of synthetic tyrosinase inhibitors. Eur. J. Med. Chem.. 2021;224:113744
- [Google Scholar]
- Biological evaluation of coumarin derivatives as mushroom tyrosinase inhibitors. Food Chem.. 2012;135:2872-2878.
- [Google Scholar]
- New halogenated phenylcoumarins as tyrosinase inhibitors. Bioor. Med. Chem. Lett.. 2011;21:3342-3345.
- [Google Scholar]
- Design and discovery of tyrosinase inhibitors based on a coumarin scaffold. RSC Adv.. 2015;5:94227.
- [Google Scholar]
- The exocyst is required for melanin exocytosis from melanocytes and transfer to keratinocytes. Pigm. Cell. Melanoma. R.. 2020;33:366-371.
- [Google Scholar]
- A review of coumarin derivatives in pharmacotherapy of breast cancer. Curr. Med. Chem.. 2008;15:2664-2679.
- [Google Scholar]
- Synthesis and in vitro biological evaluation of novel coumarin derivatives containing isoxazole moieties on melanin synthesis in B16 cells and inhibition on bacteria. Bioor. Med. Chem. Lett.. 2017;27:2674-2677.
- [Google Scholar]
- Naturally occurring tyrosinase inhibitors: mechanism and applications in skin health, cosmetics and agriculture industries. Phytother Res.. 2007;21:805-816.
- [Google Scholar]
- Synthesis, antioxidant and anti-tyrosinase activity of 1,2,4-triazole hydrazones as antibrowning agents. Food Chem.. 2021;341:128265
- [Google Scholar]
- Evaluation of novel chalcone oximes as inhibitors of tyrosinase and melanin formation in B16 cells. Arch. Pharm. Chem. Life Sci.. 2016;349:20-29.
- [Google Scholar]
- Time dependent effect of chronic embryonic exposure to ethanol on zebrafish: Morphology, biochemical and anxiety alterations. Behav. Brain Res.. 2017;332:40-49.
- [Google Scholar]
- The pigment in alkaptonuria relationship to melanin and other coloured substances: a review of metabolism, composition and chemical analysis. JIMD Rep.. 2015;24:51-66.
- [Google Scholar]
- Cinnamic acid derivatives linked to arylpiperazines as novel potent inhibitors of tyrosinase activity and melanin synthesis. Eur. J. Med. Chem.. 2022;231:114147
- [Google Scholar]
- Tyrosinase: a comprehensive review of its mechanism. Biochim. Biophys. Acta. 1995;1247:1-11.
- [Google Scholar]
- Novel hydroxypyridinone derivatives containing an oxime ether moiety: synthesis, inhibition on mushroom tyrosinase and application in antibrowning of fresh-cut apples. Food Chem.. 2018;242:174-181.
- [Google Scholar]
- Design, synthesis and evaluation of cinnamic acid ester derivatives as mushroom tyrosinase inhibitors. Medchemcomm. 2018;9:853-861.
- [Google Scholar]
- Inhibitory mechanism of epicatechin gallate on tyrosinase: inhibitory interaction, conformational change and computational simulation. Food Funct.. 2020;11:4892-4902.
- [Google Scholar]
- Comparing the inhibitory abilities of epigallocatechin-3-gallate and gallocatechin gallate against tyrosinase and their combined effects with kojic acid. Food Chem.. 2021;349:129172
- [Google Scholar]
- Study on the design, synthesis and structure-activity relationships of new thiosemicarbazone compounds as tyrosinase inhibitors. Eur. J. Med. Chem.. 2017;139:815-825.
- [Google Scholar]
- Design, synthesis of Cinnamyl-paeonol derivatives with 1, 3-Dioxypropyl as link arm and screening of tyrosinase inhibition activity in vitro. Bioorg. Chem.. 2021;106:104512
- [Google Scholar]
- Hydroxycinnamic acids and their derivatives: cosmeceutical significance, challenges and future perspectives, a review. Molecules. 2017;22:281-304.
- [Google Scholar]
- Design, synthesis and anti-melanogenic effect of cinnamamide derivatives. Bioorg. Med. Chem.. 2018;26:5672-5681.
- [Google Scholar]
- Synthesis of cinnamic amide derivatives and their anti-melanogenic effect in alpha-MSH-stimulated B16F10 melanoma cells. Eur. J. Med. Chem.. 2019;161:78-92.
- [Google Scholar]
- Tyrosinase inhibition and anti-melanin generation effect of cinnamamide analogue. Bioorg. Chem.. 2019;87:43-55.
- [Google Scholar]
- Synthesis and biological evaluation of coumarin derivatives as α-glucosidase inhibitors. Eur. J. Med. Chem.. 2020;189:112013
- [Google Scholar]
- Anticancer activity of sodium caffeate and its mechanism. Acta Pharmacol. Sin.. 2005;26:1248-1252.
- [Google Scholar]
- Tyrosinase inhibitors as potential antibacterial agents. Eur. J. Med. Chem.. 2020;187:111892
- [Google Scholar]
- Synthesis and anti-tyrosinase mechanism of the substituted vanillyl cinnamate analogues. Bioorg. Chem.. 2019;93:103316
- [Google Scholar]
- A comprehensive review on tyrosinase inhibitors, J. Enzym. Inhib. Med. Chem.. 2019;34:279-309.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.104724.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1






