Translate this page into:
Novel hydroxy-tagged bis-dihydrazothiazole derivatives: Synthesis, antimicrobial capacity and formation of some metal chelates with iron, cobalt and zinc ions
⁎Corresponding authors. rkassab@sci.cu.edu.eg (Refaie M. Kassab), zeinab.a.muhammad@gmail.com (Zeinab A. Muhammad)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Until today, microbial infections epitomize a serious universal health threat. Subsequently, developing a potential antimicrobial prototype remains an urgent, global necessity. Despite several reports discussing the biological utility of some bis-thiazoles, the use of bis-dihydrazothiazoles and their corresponding metal chelates as potent antimicrobials, is yet to be explored. The current report outlines an attempt to fill that void by representing a successful synthesis of a new class of bis-dihydrazothiazoles as well as three of their transition metal chelates and their use as potential antimicrobials. This report presents the design of some novel hydroxy-tagged bis-dihydrazothiazoles 6a-e and bis-dihydrazothiazolones 9a-e via the reaction of a bis-aldehyde thiosemicarbazone 3, as a common synthetic precursor, with two groups of hydrazonoyl halide derivatives 4a-e and 7a-e. The chelation affinity of these novel bis-dihydrazothiazoles to behave as neutral tridentate ligands coordinating to the metal ions; Fe(III), Co(II), or Zn(II) through azomethine-N, thiazole-S, and azo-N atoms to form metal complexes with a metal/ligand ratio of 2:1 was confirmed. In-vitro, antimicrobial screening of the novel hydroxy-tagged bis-dihydrazothiazole derivatives, as well as a selected group of their transition metal chelate complexes [M2L], was inspected, and the data were measured in comparison to those of ketoconazole and gentamycin as antimicrobial reference standards. Most bis-dihydrazothiazoles 6a-e and bis-dihydrazothiazolones 9a-e showed decent to excellent antimicrobial activities against the screened microbes, but the chloro-substituted bis dihydrazothiazole derivatives 6c and 9c showed exceptional inhibition of Bacillus subtilis (20 mm), Escherichia coli (25 mm), and Candida albicans (19 mm), and Salmonella typhimurium (19 mm), respectively. Additionally, amongst the three synthesized metal complexes [M2L], only the zinc complex, [Zn2L] showed remarkable inhibition of both Staphylococcus aureus (21 mm) and Salmonella typhimurium (19 mm) compared to the reference standard Gentamycin, and was more potent than the free ligand, 6a itself (no activity and 11 mm respectively). Antimicrobial inspection was assessed by the agar well diffusion assay method against all bacterial and fungal strains. Magnetic moment, diffused reflectance, FT-IR, 1H NMR, molar conductivity, thermogravimetric analysis (TGA), X-ray diffraction (XRD), and scanning electron microscope (SEM) were among the many techniques used to elucidate the structures of all novel hydroxy-tagged bis-heterocyclic ligands and their metal chelate complexes.
Keywords
Bis-aldehyde thiosemicarbazone
Hydrazonoyl halides
Transition metal complexes
Antibacterial, antifungal prototypes
1 Introduction
The thiazole ring has been one of the more interesting heterocycles amongst the synthetic and pharmaceutical communities (Potewar et al., 2007; Rajanarendar et al., 2012; Petrou et al., 2021). The wide spectrum of its pharmacological applications makes the thiazole ring an attractive pharmacophore nucleus. The thiazole nucleus presents the pharmacophoric center in various biologically active compounds (Potewar et al., 2007; Rajanarendar et al., 2012; Petrou et al., 2021; Abdelhamid et al., 2015; Dawood and Gomha, 2015; Gomha et al., 2015; Sayed et al., 2020; Abdel-Wahab et al., 2008; Verma and Saraf, 2008). Several thiazole derivatives are well-known biologically active scaffolds (Petrou et al., 2021; Verma and Saraf, 2008), which make them outstanding nominees for structural-based pharmacological screening (Oka et al., 2012; Watanabe and Uesugi, 2013).
Many thiazole derivatives span a wide range of pharmacological activities such as antifungal (Zhang et al., 2023); antimicrobial (Limban et al., 2008); antibacterial (Kassab et al., 2022); anti-inflammatory (Franklin et al., 2008; Kouatly et al., 2009); analgesic (Yücel et al., 2024); antihypertensive (Abdel-Wahab et al., 2008); anti-HIV agents (Israr et al., 2024); or anticancer (Rana et al., 2023). Additionally, the thiazole nucleus was incorporated into numerous clinically approved antimicrobial drugs (Fig. 1) (Niu et al., 2023).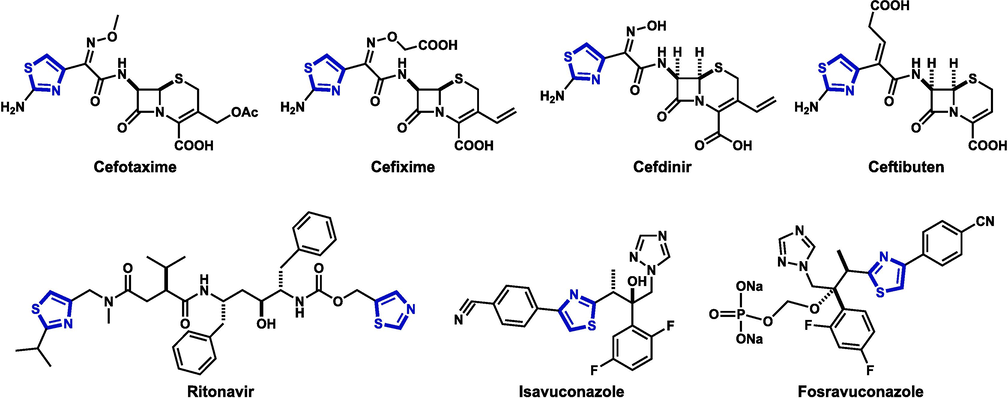
Some thiazole-containing clinically used antimicrobial drugs.
Similar to their parent thiazole analogs, numerous bis-thiazole derivatives were found to be biologically active as antimicrobial (Kassab et al., 2021; Borcea et al., 2021); antifungal (Clough et al., 2006); antiviral (Kassab et al., 2024); antibiotics (Cascioferro et al., 2020); or anticancer (Rana et al., 2023). Moreover, the ability of many organic molecules in general and bis-heterocycles, aka, bidentate and tridentate ligands in particular, to coordinate with metal ions forming metal complexes make them very attractive targets for various, including medicinal, applications (Karges et al., 2021). As a result, several recent reports have discussed the rich biological diversity of several transition metal ion complexes, especially with azo-containing ligands (Kyhoiesh and Hassan, 2024; Kyhoiesh and Al-Adilee, 2023; Kyhoiesh and Al-Adilee, 2022). However, up to the present moment, microbial infections represent a serious global health threat. Subsequently, developing new antimicrobial drug prototypes remains an urgent, universal necessity. Despite several reports discussing the biological functionality of some bis-thiazoles (Kassab et al., 2021; Borcea et al., 2021; Clough et al., 2006; Kassab et al., 2024); the use of bis-dihydrazothiazole and their corresponding transition metal chelates as potential antimicrobials, is yet to be investigated.
Considering these verdicts, and in extension of our everlasting conquest in the construction of heterocyclic (Kassab et al., 2023; Al-Hussain et al., 2024; Kassab et al., 2024) and bis-heterocyclic compounds (Kassab et al., 2016; Sanad et al., 2016; Mahmoud et al., 2019; Kassab et al., 2020; Kassab et al., 2022; Kassab et al., 2022; Kassab et al., 2023); we dedicated the current study to build some novel hydroxy-tagged bis-dihydrazothiazole derivatives with prominent biological capacity. In this account, the construction protocol of some hydroxy-tagged bis-dihydrazothiazoles and bis-dihydrazothiazolone derivatives was outlined using a hydroxy-tagged bis-thiosemicarbazone derivative 3 as a common synthetic scaffold. The hydroxyl group was incorporated to serve the purpose of future fine-tuning of the structural activity relationship (SAR) via several possible synthetic modifications (Ou et al., 2013).
Additionally, the preliminary coordination characteristics of these novel hydroxy-tagged bis-dihydrazothiazole derivatives were probed by studying their interactions with Fe(III), Cr(II), and Zn(II) transition metal ions. Furthermore, the antimicrobial behavior of these novel hydroxy-tagged bis-dihydrazothiazole derivatives as well a selected group of their metal chelates with some transition metals was examined via in-vitro screening. The antimicrobial activity data were measured in comparison to those of ketoconazole and gentamycin as antimicrobial standards.
2 Experimental
2.1 Chemicals and instruments
The chemicals, and reagents (analytical grade) used in the research were acquired from Float chemicals and reagents, BDH laboratory chemicals, and Sigma-Aldrich. They include 4-sbstituted anilines (99 %), 4-hydroxybenzaldehyde (99 %), epichlorohydrin (99 %), thiosemicarbazide (98 %), Co(OCOCH3)2·4H2O (99 %), Fe(NO3)3·9H2O (99 %), and Zn(OCOCH3)2·4H2O (99 %). Melting points of the ligands and complexes were determined by using a Gallen Kamp apparatus. The carbon, hydrogen, and nitrogen content of the solid complexes was determined using a CHNS-932 (LECO) Vario elemental analyzer. The Fourier transform infrared (FT-IR) spectra were recorded using a Perkin Elmer 1650 spectrophotometer with potassium bromide (KBr) pellets as the sample matrix. The range of analysis was 400–4000 cm−1. Molar magnetic susceptibility was measured on powdered samples using the Faraday method. Diamagnetic corrections were made using Pascal’s constant and Hg[Co(SCN)4] was used as a calibrant. Molar conductivities of 10-3 M solutions of the solid complexes in ethanol were measured using a Jenway 4010 conductivity meter. The 1H NMR spectra were recorded on a 300 MHz Varian-Oxford Mercury spectrometer at room temperature as solutions in DMSO‑d6, with tetramethyl silane as an internal standard. Chemical shifts are expressed as parts per million (ppm) to TMS. The mass spectra were recorded using a Hewlett-Packard MS-5988 GS-MS apparatus with the electron ionization method at 70 eV. 1 × 10-3 M solutions of the solid complexes in dimethylformamide (DMF) were used to measure the molar conductivities using a Jenway 4010 conductivity meter. Thermogravimetric (TG) and differential thermogravimetric (DTG) analysis of the solid complexes were carried out at ambient temperature and up to 800 °C using a Shimadzu TG-50H thermal analyzer. At the Cairo University, Faculty of Nanotechnology, X-ray diffractions (XRDs) were measured using a Bruker D8 Discover (Bruker AXS Inc., 35 KV, 30 mA) X-ray diffractometer with a step size of 0.02 and a speed scan of 0.016. The Cu Kα radiation (λ = 1.5406 Å) was used for the measurement for two hours, with 2θ ranging from 5 to 80. A Japanese Jol 2000 scanning electron microscope (SEM) was used to take pictures of all transition metal complexes. Under measuring conditions (size 500 X 500 nm, contact mode, speed 0.5 in./sec), the suspension was placed to a mica slide.
2.1.1 Synthesis of bis-thiosemicarbazone derivative 3
Bis-thiosemicarbazone derivative 3 was prepared using a modified reported procedure by our research group (Kassab et al., 2021; Kassab et al., 2022). To an ethanolic (30 ml) mixture of hydroxy-bis-aldehyde 1 (3.00 g, 0.01 mol) containing a few drops of conc. HCl, thiosemicarbazide (4.46 g, 0.01 mol) was added and the mixture was refluxed for 3 h. The formed ppt upon cooling was collected and recrystallized from a mixture of ethanol/dioxane 3:1 (v/v) as yellow crystals,72 % yield; mp > 300 °C; IR: 3471, 3350 (NH2), 3185 (NH), 1510 (C=N) cm−1; 1H NMR: δ 2.50 (br, 1H, OH), 4.1–4.3 (m, 5H, OCH2, OCH), 6.90–8.00 (m, 12H, ArH, NH2), 8.15 (s, 2H, CH=N), 11.25 (s, 2H, NH); MS m/z (%): 446 (M+, 57), 450 (69), 432 (57), 380 (44), 346 (53), 232 (47), 218 (38), 195 (82), 135 (1 0 0), 121 (80), 99 (58). Anal. Calcd for C19H22N6O3S2 (446.54 g/mol): C, 51.11; H, 4.97; N, 18.82. Found: C, 51.0; H, 4.79; N, 18.75.
2.2 General procedure for the synthesis of bis-dihydrazothiazole derivatives 6a-e, 9a-e
To a stirred solution of thiosemicarbazone derivative 3 (1.1 g, 2.5 mmol) and tow equivalent of hydrazonoyl chloride derivative 4a-e, 7a-e (5 mmol) in dioxane (30 mL), was added triethylamine (0.7 mL) and the mixture was refluxed for 5 h. The precipitated triethylamine hydrochloride was filtered off, and the solvent of the filtrate was subjected to evaporation under reduced pressure. The residue was triturated with methanol. The formed solid was collected by filtration, washed with water, dried, and recrystallized from dioxane to afford the corresponding bis-thiazole derivatives 6a-e, 9a-e respectively.
2.2.1 1,3-Bis(4-((2-(4-methyl-5-(phenyldiazenyl)thiazol-2-yl)hydrazono)methyl)phenoxy)propan-2-ol (6a)
Following the general procedure, thiosemicarbazone derivative 3 (1.1 g, 2.5 mmol) and hydrazonoyl chloride derivative 4a (0.98 g, 5 mmol) gave compound 6a as red solid, 74 % yield; mp 240–241 °C; IR: v 3430 (2NH), 3030, 2930 (C–H) cm−1; 1H- NMR δ 2.43 (s, 6H, 2CH3), 4.16–4.21 (m, 5H, OCH2, OCH), 5.53 (s, 1H, OH), 6.98–8.00 (m, 18H, Ar-H), 8.56 (s, 2H, 2CH=N), 10.50 (s, 2H, 2NH) ppm; MS m/z (%): 730 (M+, 57), 711 (46), 692 (30), 613 (28), 585 (45), 565 (45), 459 (69), 421 (57), 381 (44), 357 (53), 235 (43), 218 (38), 197 (44), 137 (1 0 0), 123 (80), 99 (58). Anal. Calcd for C37H34N10O3S2 (730.87 g/mol): C, 60.81; H, 4.69; N, 19.16. Found: C, 60.53; H, 4.40; N, 18.85.
2.2.2 1,3-Bis(4-((2-(4-methyl-5-(p-tolyldiazenyl)thiazol-2-yl)hydrazono)methyl)phenoxy)propan-2-ol (6b)
Following the general procedure, thiosemicarbazone derivative 3 (1.1 g, 2.5 mmol) and hydrazonoyl chloride derivative 4b (1.05 g, 5 mmol) gave compound 6b as red solid, 71 % yield; mp 204–205 °C; IR: v 3425 (2NH), 3014, 2920 (C–H) cm−1; 1H NMR: δ 2.24 (s, 6H, 2CH3), 2.49 (s, 6H, 2CH3), 4.11–4.21 (m, 5H, OCH2, OCH), 5.50 (s, 1H, OH), 7.09–7.81 (m, 16H, Ar-H), 8.54 (s, 2H, 2CH=N), 10.54 (s, 2H, 2NH) ppm; 13C NMR: δ 21.1, 24.3 (4CH3), 26.3 (2CH), 52.3 (2CH2), 115.1, 115.6, 124.4, 126.7, 128.2, 128.4, 128.5 129.5, 130.9, 136.5, 140.4, 152.7, 160.3 (Ar-H and C=N) ppm; MS m/z (%): 759 (M+, 26), 755 (35), 725 (43), 691 (22), 619 (30), 568 (25), 526 (1 0 0), 506 (38), 483 (35), 391 (26), 353 (36), 266 (52), 249 (60), 92 (42), 62 (43). Anal. Calcd. for C39H38N10O3S2 (758.92 g/mol): C, 61.72; H, 5.05; N, 18.46. Found: C, 61.50; H, 4.78; N, 18.28.
2.2.3 1,3-Bis(4-((2-(5-((4-chlorophenyl)diazenyl)-4-methylthiazol-2-yl)hydrazono)methyl) phenoxy)propan-2-ol (6c)
Following the general procedure, thiosemicarbazone derivative 3 (1.1 g, 2.5 mmol) and hydrazonoyl chloride derivative 4c (1.15 g, 5 mmol) gave compound 6c as red solid, 75 % yield; mp 199–200 °C; IR: v 3402 (2NH), 3098, 2931 (C–H) cm−1; 1H NMR: δ 2.43 (s, 6H, 2CH3), 4.16–4.21 (m, 5H, OCH2, OCH), 5.54 (s, 1H, OH), 6.98–8.00 (m, 16H, Ar-H), 8.55 (s, 2H, 2CH=N), 10.35 (s, 2H, 2NH) ppm; MS m/z (%): 799 (M+, 9), 777 (35), 760 (16), 670 (17), 622 (23), 588 (16), 550 (24), 535 (26), 480 (45), 449 (61), 426 (47), 383 (38), 301 (68), 299 (96), 254 (86), 223 (66), 163 (43), 150 (1 0 0), 120 (98), 93 (47). Anal. Calcd. for C37H32Cl2N10O3S2 (799.75 g/mol): C, 55.57; H, 4.03; N, 17.51. Found: C, 55.35; H, 3.75; N, 17.25.
2.2.4 1,3-Bis(4-((2-(5-((4-bromophenyl)diazenyl)-4-methylthiazol-2-yl)hydrazono)methyl) phenoxy)propan-2-ol (6d)
Following the general procedure, thiosemicarbazone derivative 3 (1.1 g, 2.5 mmol) and hydrazonoyl chloride derivative 4d (1.37 g, 5 mmol) gave compound 6d as red solid, 76 % yield; mp 224–225 °C; IR: v 3433 (2NH), 3065, 2929 (CH) cm−1; 1H- NMR: δ 2.43 (s, 6H, 2CH3), 4.17–4.21 (m, 5H, OCH2, OCH), 5.40 (s, 1H, OH), 7.10–7.82 (m, 16H, Ar-H), 8.55 (s, 2H, 2CH=N), 10.25 (s, 2H, 2NH) MS m/z (%): 888 (M+, 21), 870 (33), 841 (44), 824 (94), 774 (42), 761 (38), 702 (50), 667 (67), 632 (63), 606 (51), 542 (42), 501 (48), 447 (41), 405 (57), 366 (74), 301 (45), 280 (91), 201 (1 0 0), 151 (95). Anal. Calcd. for C37H32Br2N10O3S2 (888.66 g/mol): C, 50.01; H, 3.63; N, 15.76. Found: C, 50.45; H, 3.45; N, 15.55.
2.2.5 1,3-Bis(4-((2-(5-((4-fluorophenyl)diazenyl)-4-methylthiazol-2-yl)hydrazono)methyl) phenoxy)propan-2-ol (6e)
Following the general procedure, thiosemicarbazone derivative 3 (1.1 g, 2.5 mmol) and hydrazonoyl chloride derivative 4e (1.07 g, 5 mmol) gave compound 6e as red solid, 78 % yield; mp 209–210 °C; IR: v 3448 (2NH), 2988, 2935 (CH) cm−1; 1H NMR: δ 2.48 (s, 6H, 2CH3), 4.16–4.21 (m, 5H, OCH2, OCH), 5.62 (s, 1H, OH), 7.09–7.82 (m, 16H, Ar-H), 8.55 (s, 2H, 2CH=N), 10.50 (s, 2H, 2NH) ppm; 13C NMR: δ 25.8 (2CH3), 43.2, 63.2, 67.5 (2CH2, CH-OH), 113.5, 115.3, 119.2, 127.8, 129.1, 131.6, 133.5, 134.6, 144.4, 146.5, 158.3 (Ar-H and C=N) ppm; MS m/z (%): 766 (M+, 15), 742 (12), 710 (15), 667 (21), 657 (24), 610 (11), 568 (16), 566 (20), 524 (13), 466 (28), 386 (10), 285 (21), 238 (53), 201 (1 0 0), 135 (34), 94 (22). Anal. Calcd. for C37H32F2N10O3S2 (766.85 g/mol): C, 57.95; H, 4.21; N, 18.27. Found: C, 58.20; H, 3.95; N, 18.15.
2.2.6 2,2′-(((((2-Hydroxypropane-1,3-diyl)bis(oxy))bis(4,1-phenylene))bis(methanylylidene))bis (hydrazin-1-yl-2-ylidene))bis(5-(2-phenylhydrazono)thiazol-4(5H)-one) (9a)
Following the general procedure, thiosemicarbazone derivative 3 (1.1 g, 2.5 mmol) and hydrazonoyl chloride derivative 7a (1.13 g, 5 mmol) gave compound 9a as yellow solid, 71 % yield; mp 158–159 °C; IR: v 3390, 3280 (4NH), 3073, 3015, 2975 (CH), 1720 (2C=O) cm−1; 1H NMR: δ 4.12–4.40 (m, 5H, OCH2, OCH), 5.48 (s, 1H, OH), 6.86–7.91 (m, 18H, Ar-H), 8.38 (s, 2H, 2CH=N), 10.78 (s, 2H, 2NH), 11.21 (s, 2H, 2NH) ppm; MS m/z (%): 734 (M+, 24), 709 (34), 682 (55), 634 (30), 595 (33), 430 (44), 319 (39), 309 (1 0 0), 236 (46), 200 (30), 159 (38), 151 (46), 131 (34), 59 (74), 54 (50). Anal. Calcd. for C35H30N10O5S2 (734.81 g/mol): C, 57.21; H, 4.12; N, 19.06. Found: C, 57.00; H, 3.94; N, 18.88.
2.2.7 2,2′-(((((2-Hydroxypropane-1,3-diyl)bis(oxy))bis(4,1-phenylene))bis(methanylylidene))bis (hydrazin-1-yl-2-ylidene))bis(5-(2-(p-tolyl)hydrazono)thiazol-4(5H)-one) (9b)
Following the general procedure, thiosemicarbazone derivative 3 (1.1 g, 2.5 mmol) and hydrazonoyl chloride derivative 7b (1.2 g, 5 mmol) gave compound 9b as yellow solid, 73 % yield; mp 168–169 °C; IR: v 3441, 3275 (4NH), 3073, 2931 (CH), 1728 (2C=O) cm−1; 1H NMR: δ 1.90 (s, 6H, 2CH3), 4.13–4.34 (m, 5H, OCH2, OCH), 5.47 (s, 1H, OH), 6.87–7.99 (m, 16H, Ar-H), 8.45 (s, 2H, 2CH=N), 10.49 (s, 2H, 2NH), 11.29 (s, 2H, 2NH) ppm; 13C NMR: δ 14.8 (2CH3), 28.9 (CH-OH), 32.1 (2CH2), 65.6, 89.4, 113.5, 116.6, 118.5, 121.0, 123.7, 125.5, 128.2, 129.9, 130.8 (Ar-H and C=N), 158.0 (2C=O) ppm; MS m/z (%): 762 (M+, 15), 758 (24), 662 (19), 560 (28), 516 (71), 506 (74), 481 (38), 448 (50), 433 (81), 414 (86), 377 (86), 343 (1 0 0), 109 (53), 53 (72), 43 (68). Anal. Calcd. for C37H34N10O5S2 (762.86 g/mol): C, 58.26; H, 4.49; N, 18.36. Found: C, 58.55; H, 4.24; N, 18.14.
2.2.8 2,2′-(((((2-Hydroxypropane-1,3-diyl)bis(oxy))bis(4,1-phenylene))bis(methanylylidene))bis (hydrazin-1-yl-2-ylidene))bis(5-(2-(4-chlorophenyl)hydrazono)thiazol-4(5H)-one) (9c)
Following the general procedure, thiosemicarbazone derivative 3 (1.1 g, 2.5 mmol) and hydrazonoyl chloride derivative 7c (1.3 g, 5 mmol) gave compound 9c as yellow solid, 74 % yield; mp 165–166 °C; IR: v 3430, 3250 (4NH), 3150, 2968 (CH), 1729 (C=O) cm−1; 1H NMR: δ 4.09–4.32 (m, 5H, OCH2, OCH), 5.42 (s, 1H, OH), 6.87–7.89 (m, 16H, Ar-H), 8.45 (s, 2H, 2CH=N), 10.49 (s, 2H, 2NH), 11.28 (s, 2H, 2NH) ppm; 13C NMR: δ 28.9 (CH-OH), 32.9 (2CH2), 61.0, 115.5, 116.6, 120.8, 127.5, 129.3, 133.3, 138.0, 142.1, 143.1, 152.0 (Ar-H and C=N), 162.6 (2C=O) ppm; MS m/z (%): 803 (M+, 6), 798 (10), 760 (10), 686 (33), 550 (42), 504 (31), 463 (38), 431 (81), 368 (79), 354 (34), 323 (34), 255 (52), 236 (1 0 0), 139 (32), 114 (38), 96 (41), 59 (40). Anal. Calcd. for C35H28Cl2N10O5S2 (803.69 g/mol): C, 52.31; H, 3.51; N, 17.43. Found: C, 52.55; H, 3.30; N, 17.16.
2.2.9 2,2′-(((((2-hydroxypropane-1,3-diyl)bis(oxy))bis(4,1-phenylene))bis(methanylylidene))bis (hydrazin-1-yl-2-ylidene))bis(5-(2-(4-bromophenyl)hydrazono)thiazol-4(5H)-one) (9d)
Following the general procedure, thiosemicarbazone derivative 3 (1.1 g, 2.5 mmol) and hydrazonoyl chloride derivative 7d (1.5 g, 5 mmol) gave compound 9d as yellow solid, 70 % yield; mp 160–162 °C; IR (KBr):v 3441, 3200 (4NH), 2978, 2947 (C–H), 1726 (C=O) cm−1; 1H NMR: δ 4.14–4.19 (m, 5H, OCH2, OCH), 5.33 (s, 1H, OH), 7.08–7.88 (m, 16H, Ar-H), 8.45 (s, 2H, 2CH=N), 10.49 (s, 2H, 2NH), 11.22 (s, 2H, 2NH) ppm; MS m/z (%): 892 (M+, 60), 737 (35), 707 (25), 650 (29), 551 (48), 513 (30), 474 (61), 388 (31), 341 (35), 268 (30), 214 (32), 199 (23), 75 (88), 57 (1 0 0). Anal. Calcd for C35H28N12O9S2 (892.60 g/mol): C, 47.10; H, 3.16; N, 15.69. Found: C, 47.32; H, 3.35; N, 15.51.
2.2.10 2,2′-(((((2-Hydroxypropane-1,3-diyl)bis(oxy))bis(4,1-phenylene))bis(methanylylidene))bis (hydrazin-1-yl-2-ylidene))bis(5-(2-(4-fluorophenyl)hydrazono)thiazol-4(5H)-one) (9e)
Following the general procedure, thiosemicarbazone derivative 3 (1.1 g, 2.5 mmol) and hydrazonoyl chloride derivative 7e (1.22 g, 5 mmol) gave compound 9e as yellow solid, 73 % yield; mp 172–173 °C; IR (KBr): v 3415, 31,522 (4NH), 3154, 2970 (CH), 1728 (2C=O) cm−1; 1H NMR: δ 4.06–4.39 (m, 5H, OCH2, OCH), 5.46 (s, 1H, OH), 6.87–7.89 (m, 16H, Ar-H), 8.61 (s, 2H, 2CH=N), 11.22 (s, 2H, 2NH) ppm; 13C NMR: δ 27.5 (CH-OH), 30.0 (2CH2), 65.3, 112.7, 114.1, 116.7, 120.8, 128.6, 129.9, 131.8, 132.3, 139.5, 143.5 (Ar-H and C=N), 171.3 (2C=O) ppm; MS m/z (%): 770 (M+, 12), 710 (10), 665 (20), 640 (90), 585 (24), 501 (57), 467 (16), 320 (26), 255 (38), 160 (43), 148 (81), 117 (1 0 0), 90 (68). Anal. Calcd. For C35H28F4N10O5S2 (770.79 g/mol): C, 54.54; H, 3.66; N, 18.17. Found: C, 54.76; H, 3.52; N, 18.00.
2.3 General procedure for the synthesis of bis-dihydrazothiazole metal chelates M2L, M=Fe(III), Co(II), and Zn(II); L=bis-dihydrazothiazole derivative 6a
A DMF/ethanol 3:1 (v/v) solution of the synthesized bis-dihydrazothiazole ligand 6a (400 mg, 0.548 mmol) was mixed dropwise with an ethanolic salt solution of ferric nitrate nonahydrate (430 mg, 1.096 mmol), cobalt acetate tetrahydrate (273 mg, 1.096 mmol), or zinc acetate tetrahydrate (241 mg, 1.096 mmol). The mixture was refluxed for 4 h. The solid formed upon cooling was filtered off, washed with ethanol, and vacuum dried.
2.3.1 [Fe2(L)(NO3)6]
1,3-Bis(4-((2-(4-methyl-5-(phenyldiazenyl)thiazol-2-yl)hydrazono)methyl)phenoxy)propan-2-ol bis(ferric(III) trinitrate). Yield 84 %; mp > 300 °C; Brown solid. Anal. Calc. for C37H34N16O21S2Fe2 (%): C, 36.55; H, 2.80; N, 18.44; S, 5.27; Fe, 9.22. Actual (%): C, 36.51; H, 2.78; N, 18.27; S, 4.98; Fe, 8.93. Λm (Ω-1mol-1cm2) = 16; FT-IR (ν, cm−1): thiazole-N ring (C=N) 1680 s, azomethine (C=N) 1636sh, N=N azo 1470 m, thiazole-S ring (C-S) 1050 s, 741 s, (M−O) 510w, (M−N) 470w, (M−S) 430w. µeff (BM): 5.66.
2.3.2 [Co2(L)(H2O)4(AcO)2].2AcO·2H2O
1,3-Bis(4-((2-(4-methyl-5-(phenyldiazenyl)thiazol-2-yl)hydrazono)methyl)phenoxy)propan-2-ol bis(cobalt(II) acetate-diaquo)diacetatedihydrate. Yield 90 %; mp > 300 °C; Brown solid. Anal. Calc. for C37H58N10O17S2Co2 (%): C, 45.27; H, 4.86; N, 11.74; S, 5.37; Co, 9.89. Actual (%): C, 45.28; H, 4.80; N, 11.58; S, 5.18; Co, 9.73. Λm (Ω-1mol-1cm2) = 96; FT-IR (ν, cm−1): thiazole-N ring (C=N) 1659sh, azomethine (C=N) disappeared, N=N azo 1450 s, coordinated water stretching 875w, 850 s, thiazole-S ring (C-S) 1026 m, 750 s, (M−O) 575 s, (M−N) 500 s, (M−S) 450w. µeff (BM): 4.61.
2.3.3 [Zn2(L)(H2O)4(AcO)2].2AcO·2H2O
1,3-Bis(4-((2-(4-methyl-5-(phenyldiazenyl)thiazol-2-yl)hydrazono)methyl)phenoxy)propan-2-ol bis(zinc(II) acetate-diaquo)diacetatedihydrate. Yield 85 %; mp > 300 °C; Brown solid. Anal. Calc. for C37H58N10O17S2Zn2 (%): C, 44.82; H, 4.81; N, 11.62; S, 5.31; Zn, 10.79. Actual (%): C, 44.68; H, 4.80; N, 11.48; S, 5.11; Cd, 10.58. Λm (Ω-1mol-1cm2) = 99. FT-IR (ν, cm−1): thiazole-N ring (C=N) 1650 s, azomethine (C=N) 1630sh, N=N azo 1475 s, coordinated water stretching 880w, 825 s, thiazole-S ring (C-S) 1026 m, 756 m, (M−O) 575w, (M−N) 480 s, (M−S) 430w. µeff (BM): diamagnetic. 1H- NMR δ 2.49 (s, 6H, 2CH3), 4.14–4.40 (m, 5H, OCH2, OCH), 5.55 (s, 1H, OH), 6.99–7.94 (m, 18H, Ar-H), 8.61 (s, 2H, 2CH=N), 9.84 (s, 2H, 2NH) ppm.
2.4 Method of antimicrobial screening
The Agar well diffusion method was used to evaluate the antimicrobial activity of the newly synthesized bis-dihydrazothiazole derivatives 6a-e and 9a-e along with a few metal complexes of 6a as a free ligand and a representative example of these new bis-heterocyclic compounds. The surface of the agar plate is inoculated by spreading a volume of the microbial inoculum over the entire agar surface. Then, using a sterile cork-borer or a tip, a 6 mm diameter hole is punched aseptically, and a specific volume (50 µl) of the antimicrobial agent or extract solution at the desired concentration is introduced into the well. The agar plates are then incubated under suitable conditions depending on the test microorganism. The antimicrobial agent diffuses into the agar medium and inhibits the growth of the test microbial strain (Magaldi et al., 2004).DMSO was used as negative control, while ketoconazole and gentamycin were used positive controls.
3 Results and discussion
3.1 Synthesis of Hydroxy-Tagged Bis-Dihydrazothiazole derivatives
To initiate our synthetic approach, the parent bis-thiosemicarbazone derivative 3 was obtained by condensing two equivalents of thiosemicarbazide (2) with one equivalent of hydroxy-bearing bis-aldehyde 1 in boiling ethanol, in the presence of a catalytic amount of HCl, for two hours as shown in scheme 1. Bis-aldehyde 1 was prepared analogously to the method reported by Bin et al. (Zhao et al., 1996), by heating two equivalents of salicylaldehyde with one equivalent of epichlorohydrin in an aqueous solution of NaOH (Scheme 1).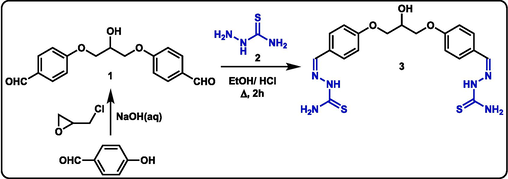
Synthesis of bis-thiosemicarbazone 3.
To create a novel set of bis-thiazoles, the chemical reactivity of the new hydroxy-bearing bis-thiosemicarbazone 3 towards a variety of hydrazonoyl chlorides 4 was investigated. As a result, the reaction of bis-thiosemicarbazone 3 with hydrazonoyl chlorides 4a-g in boiling dioxane with catalytic triethylamine yielded the desired bis-thiazoles 6a-g, presumably through intermediate 5a-g, as outlined in Scheme 2. Several spectral techniques were used to confirm the structures of all novel bis-thiazole derivatives. The IR spectrum of compound 6a, for example, showed a typical –NH absorption at v 3430 cm−1. Furthermore, the 1H NMR spectrum of 6a revealed four typical singlets for the CH3, CH-OH, CH=N, and NH groups at 2.43, 5.53, 8.56, and 10.50 ppm, respectively. The distinctive multiplet for the two CH2 groups, as well as the C–H multiplet, may be seen at 4.16–4.21 ppm.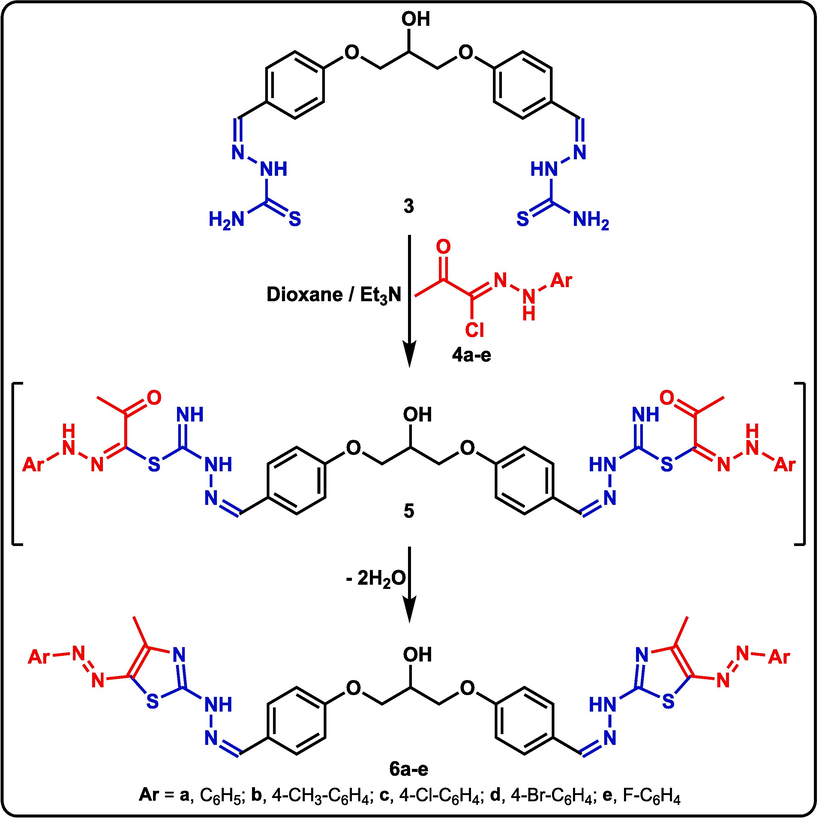
Synthesis of bis-thiazole derivatives 6a-e.
Under analogous reaction conditions, boiling dioxane and catalytic triethylamine, the parent hydroxy-tagged bis-thiosemicarbazone derivative 3 interacted with N-aryl-2-oxopropanehydrazonoyl halides 7a-e (the ester equivalents of compounds 4a-e) to create the desired bis-thiazole derivatives 9a-e, presumably through intermediates 8a-g as shown in scheme 3. The formation of the new bis-heterocycles 9a-e was supported by extensive spectral investigations. The IR spectrum of derivative 9b, for example, revealed the characteristic –NH absorption at 3441 and 3275 cm−1, which is characteristic of the two –NH groups. The isolated bis-thiazole derivatives 9a-e were structurally confirmed using their 1H NMR spectra, which had four singlets for the CH-OH, CH=N, and 2NH protons near 5.47, 8.45, 10.49, and 11.29 ppm, respectively.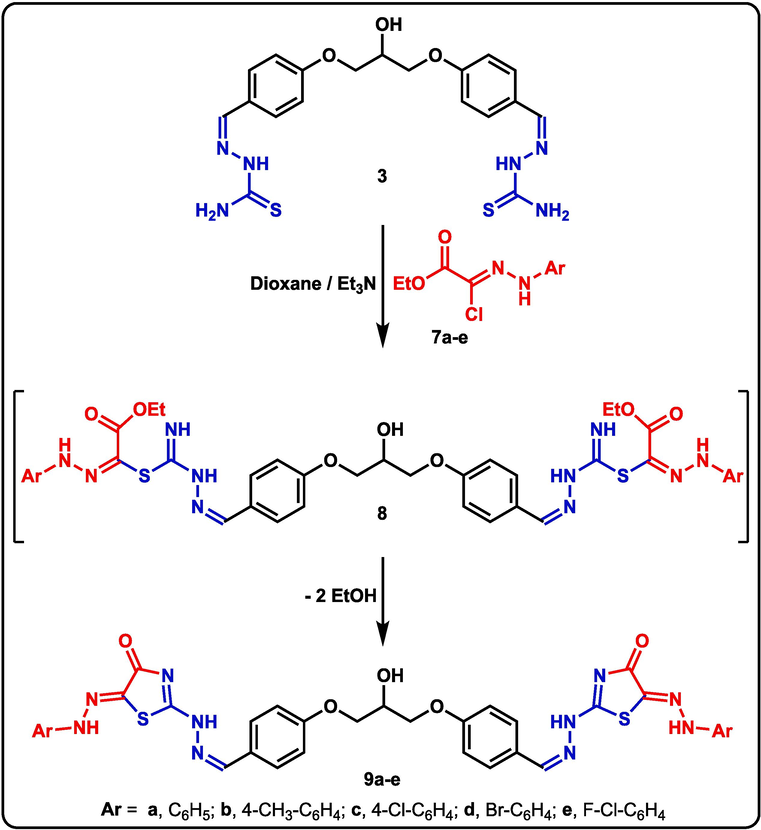
Synthesis of bis-thiazole derivatives 9a-e.
3.2 Synthesis and characterization of bis-dihydrazothiazole metal chelates
3.2.1 Synthesis of bis-dihydrazothiazole metal chelates M2L, M=Fe(III), Co(II), and Zn(II); L=bis-dihydrazothiazole derivative 6a
The chelating potential of the novel hydroxy-tagged bis-dihydrazothiazole derivative 6a was studied as an example of all the synthesized bis thiazole ligand as they all have the same coordination sites. Iron(III), cobalt(II), and zinc(II) metal ions were used to synthesize the corresponding metal chelates as an example of trivalent (Fe(III)), paramagnetic divalent (Co(II)) and diamagnetic divalent (Zn(II)) and they considered as biological important elements. Drawing from earlier research on the synthesis and characterization of several transition metal complexes of newly created molecules (Abd El Salam et al., 2023; Moustafa et al., 2022); metal complexes with a metal/ligand ratio of 2:1 were believed to be formed as depicted from the elemental analyses and other data (Table 1). The proposed complex structure was established based on a simple spatial arrangement that allows coordination of the chelating bis-dihydrazothiazole derivative 6a and the corresponding metal ions (Iron(III), cobalt(II), and zinc(II)) with the least steric hindrance.
Elemental Analyses
Complex
Color(% yield)
M.P (°C)
% Found (Calcd.)
µeff(B.M)
Λm Ω-1mol-1cm2
C
H
N
S
M
[Fe2(L)(NO3)6]
Brown(84)
>300
36.51 (36.55)
2.78 (2.80)
18.27(18.44)
4.98 (5.27)
9.93 (9.22)
5.66
16
[Co2(L)(OAc)2(H2O)4] (AcO)2·2H2O
Brown(90)
>300
45.28(45.27)
4.80 (4.86)
11.58(11.74)
5.18 (5.37)
9.73(9.89)
4.61
96
[Zn2(L)(OAc)2(H2O)4] (AcO)2·2H2O
Brown(85)
>300
44.68 (44.82)
4.80 (4.81)
11.48(11.62)
5.11 (5.31)
10.58 (10.79)
Diam
99
IR
ν(C=N)thiazole
ν(C=N)azomethine
ν(N=N)azo
ν(C-S)
ν(M−O)
ν(M−N)
ν(M−S)
[Fe2(L)(NO3)6]
1680 s
1636sh
1470 m
1050 m, 74as
510w
470 s
430 s
[Co2(L)(OAc)2(H2O)4] (AcO)2·2H2O
1659sh
disappeared
1450 s
1026 m, 750 s
575 s
500 s
450w
[Zn2(L)(OAc)2(H2O)4] (AcO)2·2H2O
1650 s
1630sh
1475 s
1026 m, 756 m
575w
480 s
430w
TG
TG range °C
DTGmax, °C
Mass loss%F (%Calcd)
Total mass loss
Assignment
Residue
[Fe2(L)(NO3)6]
30–200
62
37.96 (38.11)
87.18(86.76)
Loss of 6NO3 and C6H5N
Fe2O3
200–800
309, 550
49.22 (48.65)
Loss of C31H29N9S2
[Co2(L)(OAc)2(H2O)4] (AcO)2·2H2O
30–250
180
33.97 (33.78)
86.71 (87.35)
Loss of 4H2O, 2AcO and C10H11NO2Loss of remaining ligand
2 CoO
250–800
467
52.74 (53.57)
[Zn2(L)(OAc)2(H2O)4] (AcO)2·2H2O
30–110
67
7.40 (7.89)
86.04 (86.49)
Loss of 2H2O and C2H3O2
2 ZnO
110–800
287, 545
78.64 (78.60)
Loss of C43H51N10O11S2
Unlike the yellowish-orange color of their parent bis-dihydrazothiazole derivative 6a, dark, brown-colored metal chelates were formed. The color of the obtained complexes was attributed to the d → d transitions and the high effect of charge transfer which is a clear confirmation for the formation of the desired chelate complexes. Characterization of the synthesized chelate complexes was supported with elemental analyses, spectroscopic techniques, molar conductivity, magnetic moment measurements, as well as thermogravimetric analysis (TG). Based on elemental analyses data, all complexes conform to the general composition M2L, where M=Fe(III), Co(II), or Zn(II); L=bis-dihydrazothiazole ligand 6a (Table 1). The synthesized chelates were isolated in a powder form. The synthesized complexes were sufficiently soluble in DMF and dimethyl sulfoxide (DMSO) for molar conductivity measurements and insoluble in water, ethanol, chloroform or methanol. These complexes were found to have molar conductivity values of 16, 96, and 99 Ω-1mol-1cm2 for Fe(III), Co(II), and Zn(II) ions respectively (Table 1). These molar conductivity values reflect the electrolytic nature of the Co(II) and Zn(II) complexes and the non-electrolytic nature of the Fe(III) complex. Additionally, it confirmed the complexes' proposed formula, which was indicated by the elemental studies. The peaks in the 1H NMR spectra of the Zn(II) complex corresponded to the aromatic, NH, and OH protons, and they were exactly in the same positions as those in the spectrum of the bis-dihydrazothiazole derivative 6a. Attempts to isolate single crystals for the formed complexes were unsuccessful (see experimental section).
The diffused reflectance spectrum of Co(II) complex contained three bands at 10,290 (4T1g (F)→4T2g(F)(υ1)), 16,559 (4T1g(F)→4A2g(F)(υ2)), and 19,889 cm−1 (4T1g(F)→4T2g(P) (υ3)) transitions. The magnetic value was found to be 4.61 BM supporting octahedral geometry (Table 1) (Moustafa et al., 2022; Kyhoiesh et al., 2021). Fe(III) complex diffused reflectance spectrum exhibited three d-d bands at 11,780 (6A1g(S)→4T1g(G) (υ1)), 16,353 (6A2g(S)→4T2g(G) (υ2)), and 25,350 cm−1 (6A1g(S)→4A1g(G) (υ3)), transitions indicating an octahedral geometry around the Fe(III) ion, which is also supported by its magnetic moment value of 5.66 BM (Table 1) (Moustafa et al., 2022; Kyhoiesh et al., 2021). However, the diamagnetic nature of the Zn(II) complex suggested an octahedral geometry (Fig. 2). The geometry of the complexes supported the elemental analyses and hence correlate with the suggested formula.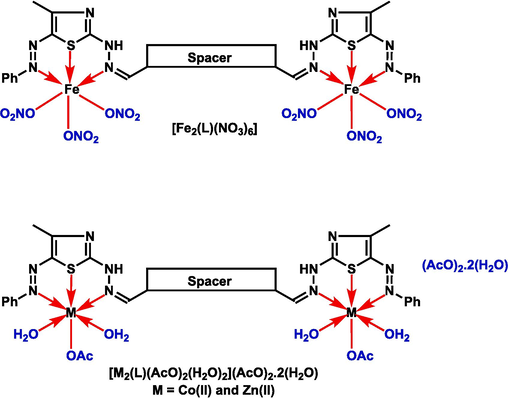
Geometrical structures of the metal chelate (Fe(III), Co(II), or Zn(II)) with bis-dihydrazothiazole ligand (L).
3.2.2 Thermal analysis study
For Fe(III), Co(II), and Zn(II)–L complexes, thermogravimetric (TG) and differential thermogravimetric (DTG) studies were performed under ambient conditions (Table 1 and Supplementary Fig. 1). The plausible complexes formula as well as correlations between the various decomposition processes of the complexes and the related weight losses are thoroughly discussed in this section.
Complex [Fe2(L)(NO3)6] underwent thermal decomposition over three successive steps (Supplementary Fig. 1a). The first step estimated mass loss of 37.96 % (calculated mass loss = 38.11 %) over a 30–200 °C temperature range could be attributed to the loss of 6NO3 and C6H5N fragments. The DTG curve had a peak at 62 °C (the maximum peak temperature). The second and third steps had an estimated mass loss of 49.22 % (calculated mass loss = 48.65 %) over a temperature range of 200–800 °C could be attributed to the loss of C31H29N9S2 fragment, leaving behind a Fe2O3 residue. The DTG curve had two peaks at 309 and 550 °C (the maximum peak temperature). Overall, thermal decomposition indicated a total estimated mass loss of 87.18 % (calculated mass loss = 86.76 %).
The thermal decomposition of complex [Co2(L)(H2O)4(AcO)2].2AcO·2H2O (Supplementary Fig. 1b) underwent thermal decomposition over two main degradation steps. The first step estimated mass loss of 33.97 % (calculated mass loss = 33.78 %) over a 30–250 °C temperature range could be attributed to the loss of hydrated and coordinated water molecules, two acetates, and C10H11NO2 fragments. The DTG curve had a maximum peak temperature of 180 °C. The second step estimated mass loss of 52.74 % (calculated mass loss = 53.57 %) over a temperature range of 250–800 °C could be attributed to the loss of the remaining portion of the ligand molecule, leaving behind a 2CoO residue. The DTG curve had an exothermic peak at 467 °C (the maximum peak temperature). Overall, thermal decomposition indicated a total estimated mass loss of 86.63 % (calculated mass loss = 87.35 %).
The thermal decomposition of complex [Zn2(L)(H2O)4(AcO)2].2AcO·2H2O (Supplementary Fig. 1c) underwent thermal decomposition over three main degradation steps. The first step estimated mass loss of 7.40 % (calculated mass loss = 7.89 %) over a temperature range of 30–110 °C could be attributed to the liberation of two hydrate water molecules and C2H3O2 fragments. The DTG curve had a peak at 67 °C (the maximum peak temperature). The second and third steps estimated mass loss of 78.64 % (calculated mass loss = 78.60 %) over a temperature range of 110–800 °C could be attributed to the decomposition of the remaining portion of the ligand molecule (C43H51N10O11S2), leaving behind a 2ZnO residue. The DTG curve had two peaks at 287 and 545 °C (the maximum peak temperature). The overall thermal decomposition indicated a total estimated mass loss of 86.04 % (calculated mass loss = 86.49 %). The acquired results showed good correlations with the other data and elemental data.
3.2.3 IR spectral study
Examining the FT-IR spectra of the free bis-dihydrazothiazole ligand 6a and its corresponding metal chelates M2L; M=Fe(III), Co(II), and Zn(II); L=bis-dihydrazothiazole derivative 6a, more insights were revealed about the molecular structure of the formed chelate complex M2L and the coordination mechanism of the bis-dihydrazothiazole ligand (L) around the metal ion (M) (Table 1 and Supplementary Fig. 2a-c). First, the free bis-dihydrazothiazole ligand 6a and metal complexes showed the characteristic IR absorption bands which correspond to the –OH and –NH groups at typical positions. The thiazole C=N group appeared at 1674 cm−1 in the bis-dihydrazothiazole ligand which shifted to lower ranges upon formation of the metal complexes at 1650–1680 cm−1. The shift in the IR absorption band of the thiazole group for the metal complexes could be attributed to the skeletal bending of the bis-dihydrazothiazole ligand upon coordination with metal ions (Magaldi et al., 2004; Abd El Salam et al., 2023). Secondly, the wavenumbers of the azomethine (C=N) and the azo group (N=N) in the bis-dihydrazothiazole FT-IR spectrum revealed two peaks at 1620 and 1470 cm−1, respectively, which shifted to 1630 cm−1 (for the Fe(III) and Zn(II) complexes and completely vanished for the Co(II) complex) and 1450–1475 cm−1 (Magaldi et al., 2004; Abd El Salam et al., 2023). These changes indicated the formation of bonding coordination between both the azo- group and the azomethine nitrogen in the bis-dihydrazothiazole ligand and the metal ions (Magaldi et al., 2004; Abd El Salam et al., 2023). Thirdly, the υ(C-S) band, which is present in the free bis-dihydrazothiazole ligand at 1050 and 741 cm−1, was observed as two discrete bands in the FT-IR spectra of all metal complexes at 1018–1026 and 750–756 cm−1 (Kyhoiesh and Hassan, 2024; Kyhoiesh and Al-Adilee, 2023; Kyhoiesh and Al-Adilee, 2022). This provided additional proof that thiazole S and the metal ions were coordinated. Additionally, and as a result of this coordination process, three additional bands that corresponded to υ(M−O), υ(M−N), and υ(M−S) were observed in the FT-IR spectra of all metal complexes at 510–575 cm−1, 470–500 cm−1, and 430–450 cm−1, respectively. (Kyhoiesh and Hassan, 2024; Kyhoiesh and Al-Adilee, 2023; Kyhoiesh and Al-Adilee, 2022).
On account of the bis-dihydrazothiazole ligand coordination to metal ions via the N, O, and S donor atoms of the characteristic bands, C-S, C=N, and N=N, respectively, and based on our previously reported researches (Magaldi et al., 2004; Abd El Salam et al., 2023); it was concluded that the bis-dihydrazothiazole ligand behaves as a neutral tridentate ligand. (Kyhoiesh and Hassan, 2024; Kyhoiesh and Al-Adilee, 2023; Kyhoiesh and Al-Adilee, 2022).
Each metal ion is surrounded by a single ligand molecule as revealed by the CHN analyses and is coordinated by three donor atoms as evidenced by the IR spectral data. A detailed summary of all metal complexes FT-IR spectra was provided in Table 1. Based on these findings, it is clear why the structure depicted in Fig. 2 is the most plausible conformation of the newly synthesized metal complexes.
3.2.4 Surface morphology
3.2.4.1 XRD study
Using X-ray diffraction patterns, the phase and crystallinity of the ligand and its complexes have been examined. The PXRD pattern of the synthesized bis-dihydrazothiazole ligand 6a and its complexes (Supplementary Fig. 3a–d) was displayed. The large intensity peak implies that the synthesized bis-dihydrazothiazole ligand 6a and its complexes are crystallinity. Through comparing the bis-dihydrazothiazole ligand 6a and complex patterns, it was discovered that the most characteristic ligand peaks appeared in all synthesized complexes in addition to apparent characteristic peaks for the iron, cobalt, and zinc species, which further confirms the complexes' synthesis and the incorporation of the bis-dihydrazothiazole ligand 6a structure into them (Orooji et al., 2020; Ghanbari and Salavati-Niasari, 2021; Karami et al., 2021).
3.2.4.2 SEM study
Material morphologies were examined using a scanning electron microscope (SEM). The free bis-dihydrazothiazole ligand 6a and its complexes were subjected to SEM analysis as shown in Supplementary Figure 4a-d, which showed a compact, crystalline homogenous material with a porous structure. The atoms were arranged in a well-defined pattern with a smooth surface and a well-defined grain-like morphology (Orooji et al., 2020; Ghanbari and Salavati-Niasari, 2021; Karami et al., 2021).
4 Antimicrobial activity screening of the novel bis-dihydrazothiazole derivatives and three of their metal chelates with transition metal ions; Fe+3, Co+2, and Zn+2
The antibacterial capacity of the novel hydroxy-tagged bis-dihydrazothiazole derivatives along with the three synthesized metal complexes were evaluated using the agar well diffusion method. Six microbial species were screened; two Gram-negative bacterial strains, Salmonella typhimurium (ATCC 14028) and Escherichia coli (ATCC 25955); two Gram-positive bacterial strains, Bacillus subtilis (NRRL B-543) and Staphylococcus aureus (ATCC:13565); and two fungal species, Aspergillus fumigatus and Candida albicans (ATCC:10231).
Dimethyl sulfoxide (DMSO) was used as a negative control, and ketoconazole and gentamycin were used as positive standards for bacterial and fungal species, respectively. The collected data were summarized in Table 1 and graphically represented in Fig. 3.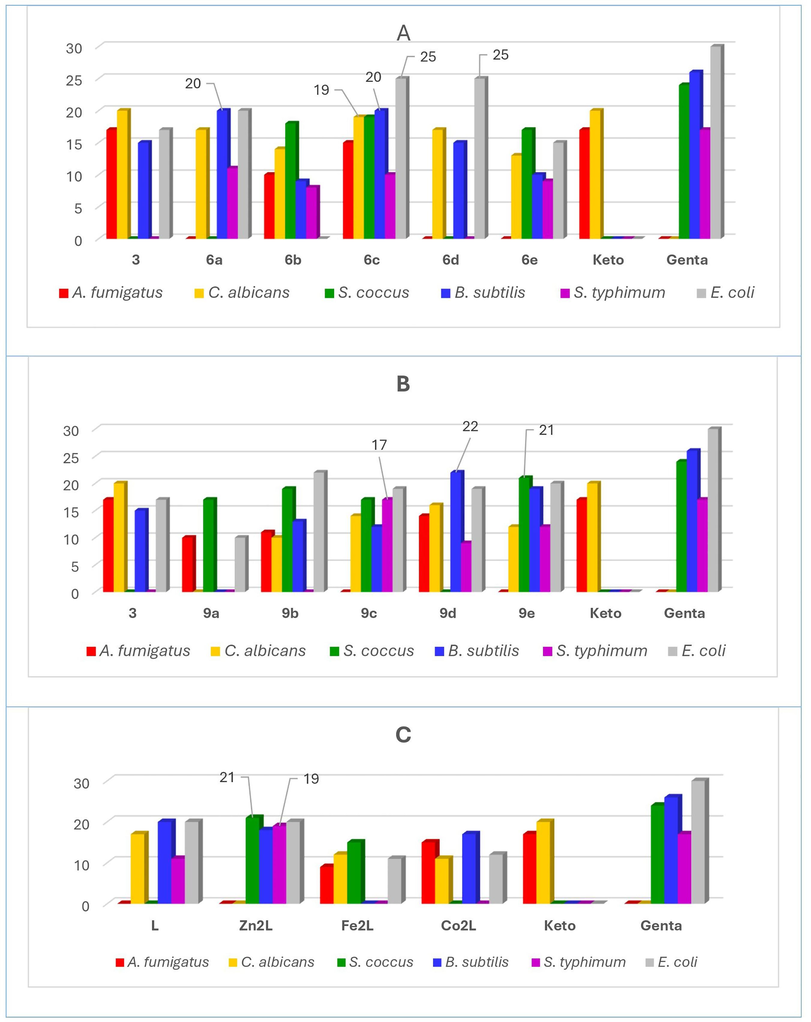
Antibacterial activity of the synthesized compounds represented by the diameter of zone of inhibition (mm), A) Series 6a-e, B) Series 9a-e, and C) Metal complexes M2L, M=Fe, Co, Zn; L=Ligand, 6a; Keto = ketoconazole, Genta = gentamycin, well diameter: 6.0 mm, sample volume: 50 µL, sample concentration: 10 μg/ml.
From the data presented in Table 2 and Graph 3A for the hydroxy-tagged bis-dihydrazothiazole derivatives 6a-e series, the following observations could be made. Whereas compound 6c showed excellent inhibition of B. subtilis, E. coli, and C. albicans, it showed only good inhibition of S. coccus and A. fumigatus. While compounds 6a and 6d showed excellent inhibition of B. subtilis and E. coli, respectively, both compounds showed only good inhibition of C. albicans. Negative control is DMSO. Positive control for fungal species (ketoconazole, 100 μg/disc) and positive control for both Gram-negative bacteria and Gram-positive bacteria (gentamycin, 4 μg/disc), *NA: No activity.
Diameter of zone of inhibition (mm) at 10 μm/mL
Sample
A. fumigatus
C. albicans
S. aureus
B. subtilis
S. typhimurium
E. coli
3
38.11
44.84
NA
33.63
NA
38.11
6a
NA
23.28
NA
27.39
15.06
27.39
6b
13.19
18.46
23.74
11.87
10.55
NA
6c
18.77
23.77
23.77
25.03
12.51
31.28
6d
NA
19.14
NA
16.89
NA
28.15
6e
NA
16.97
22.19
13.05
11.74
19.58
9a
13.62
NA
23.16
NA
NA
13.62
9b
14.43
13.12
24.93
17.06
NA
28.87
9c
NA
17.43
21.17
14.94
21.17
23.66
9d
15.69
17.93
NA
24.66
10.08
21.30
9e
NA
15.58
27.27
24.67
15.58
25.97
[Zn2L(AcO)2(H2O)4]2AcO·2H2O
NA
NA
18.98
16.27
17.17
18.08
[Fe2L(NO3)6]
7.77
10.36
12.95
NA
NA
9.49
[Co2L(AcO)2(H2O)4]2AcO·2H2O]
14.47
10.61
NA
16.40
NA
11.58
Ketoconazole
32.01
37.66
−
−
−
−
Gentamycin
−
−
50.31
54.50
35.63
62.89
DMSO
0.0
0.0
0.0
0.0
0.0
0.0
From the data presented in Table 2 and graph 3B for the hydroxy-tagged bis-dihydrazothiazolone derivatives 9a-e series, the following observations could be made. Compound 9c showed excellent inhibition of S. typhimurium. However, it showed only good inhibition of S. coccus. While compound 9d showed excellent inhibition of B. subtilis, it showed only good inhibition of both A. fumigatus and C. albicans. Whereas compound 9e showed excellent inhibition of S. coccus, it showed only good inhibition of both B. subtilis and E. coli. This high antimicrobial potency of bis-dihydrazothiazole derivatives 6c and 9c could be attributed to an optimum charge/radius ratio of the chlorine atom.
Finally, and from the data presented in Table 2 and Graph 3. C for the metal chelates [M2L] series, the following observations could be made. Complex [Zn2L] showed great inhibition of both S. coccus and S. typhimurium compared to the reference standard gentamycin, and was more potent than the free ligand, 6a itself, nonetheless. While complexes [Fe2L] and [Co2L] showed only good inhibition against S. coccus and A. fumigatus compared to the reference standards gentamycin and ketoconazole, respectively, both complexes were more potent than the free ligand, nevertheless. Whereas complex [Co2L] showed only good inhibition of A. fumigatus compared to the reference standard ketoconazole, it was still more potent than the free ligand itself, nonetheless. The higher activity of the metal complexes, [M2L] against six microbial species, as compared to the free ligand, could be attributed to the chelation hypothesis effect. Chelation increases the lipophilic character of the complex metal atom and helps its crossover through the microbial lipid membrane enhancing, significantly, the complex overall antimicrobial activity (Sharma et al., 2022).
It is worth mentioning that according to Mahmoud and Abass (Mahmood and Pharmaceutical, 2020); the azo-Schiff base ligand exhibited antibacterial action against Staphylococcus aureus and Escherichia coli pathogens, while its Co(II) complex remained inert. This is not the case with the results presented in this article.
5 Conclusions
The synthesis of various hydroxy-tagged bis-dihydrazothiazole derivatives 6a-e and bis-dihydrazothiazolones derivatives 9a-e was successfully established using a bis-thiosemicarbazone derivative 3 as a common synthetic scaffold. The chelation affinity of the novel bis-dihydrazothiazole derivatives was explored by coordinating with the metal ions; Fe(III), Co(II), or Zn(II) using 6a as a representative bis-dihydrazothiazole ligand. It was elucidated that bis-dihydrazothiazole 6a behaves as a neutral tridentate ligand through coordination of the azomethine-N, thiazole-S, and azo-N atoms forming octahedral metal complexes with a metal/ligand ratio of 2:1. Both Co(II) and Zn(II) complexes were electrolytes. However, Fe(III) complex was nonelectrolyte. All complexes displayed two to three breakdown phases within the temperature range of 30–800 co., as inferred from the TG data, they showed two to three decomposition steps within the temperature range 30–800 °C as concluded from TG results. The XRD investigation showed that the bis-dihydrazothiazole derivative 6a and its metal chelates were crystalline in nature. The antimicrobial capacities of the novel hydroxy-tagged bis-dihydrazothiazole derivatives 6a-e and 9a-e, as well as some of their metal chelates with (Fe(III), Co(II), or Zn(II) were conducted against six different microbes using ketoconazole and gentamycin as antimicrobial reference standards utilizing the agar well diffusion method. Most compounds showed decent to excellent antimicrobial activities against the screened microbes. However, the chloro-substituted bis-dihydrazothiazole derivatives 6c and 9c showed exceptional reactivities. Compound 6c showed excellent inhibition of B. subtilis (20 mm), E. coli (25 mm), and C. albicans (17 mm), while compounds 9c showed excellent inhibition of S. typhimurium (17 mm) and E. coli (19 mm), respectively. This high antimicrobial potency of bis-dihydrazothiazole derivatives 6c and 9c could be endorsed by an optimum charge/radius ratio of the chlorine atom. On the other hand, amongst the three synthesized metal complexes, only the [Zn(II) complex showed remarkable inhibition of both S. aureus (21 mm), S. typhimurium (20 mm), and E. coli (20 mm) compared to the reference standard gentamycin.
It was significantly more potent than the free ligand, 6a itself. This improved reactivity could be attributed to increased lipophilicity of the complex metal atom which, sequentially, helps its crossover through the microbial lipid membrane enhancing, significantly, the complex overall antimicrobial activity compared to the free ligand itself. On another note, both Fe(III) and Co(II) complexes showed decent antifungal activity against A. fumigatus, 9 and 15 mm, respectively.
Further functionalization of the free –OH group and extensive investigation of the biological spectrum of the new hydroxy-tagged bis-dihydrazothiazole derivative, and its metal complexes are currently underway.
CRediT authorship contribution statement
Refaie M. Kassab: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Sami A. Al-Hussain: Writing – review & editing, Project administration, Funding acquisition. Magdi E.A. Zaki: Writing – review & editing, Supervision, Funding acquisition, Conceptualization. Gehad G. Mohamed: Writing – original draft, Resources, Formal analysis, Data curation. Zeinab A. Muhammad: Writing – review & editing, Writing – original draft, Software, Resources, Project administration, Methodology, Formal analysis, Data curation.
Acknowledgment
This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (grand number IMSIU-RP23080).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Isophthaloylbis (Azanediyl) Dipeptide Ligand and Its Complexes: Structural Study, Spectroscopic, Molecular Orbital, Molecular Docking, and Biological Activity Properties. Polycycl. Aromat. Compd. [Internet]. 2023;43(6):4866-4888. Available from:
- [Google Scholar]
- Synthesis and antimicrobial evaluation of some novel thiazole, 1,3,4-thiadiazole and pyrido[2,3-D][1,2,4]- TRIAZOLO[4,3-A]pyrimidine derivatives incorporating pyrazole moiety. Heterocycles.. 2015;91(11):2126-2142.
- [Google Scholar]
- Synthesis and reactions of thiosemicarbazides, triazoles, and Schiff bases as antihypertensive α-blocking agents. Monatshefte Fur Chemie.. 2008;139(9):1083-1090.
- [Google Scholar]
- The anti-breast cancer activity of indeno[1,2-b]pyridin-5-one and their hydrazonal precursors endowed with anti-CDK-2 enzyme activity. J. Mol. Struct.. 2024;1295:136692
- [Google Scholar]
- An overview of the synthesis and antimicrobial, antiprotozoal, and antitumor activity of thiazole and bisthiazole derivatives. Molecules [internet]. 2021;26(3):624. Available from
- [Google Scholar]
- Thiazoles, Their Benzofused Systems, and Thiazolidinone Derivatives: Versatile and Promising Tools to Combat Antibiotic Resistance. J. Med. Chem. [Internet]. 2020;63(15):7923-7956. Available from:
- [Google Scholar]
- Total synthesis of myxothiazols, novel bis-thiazole β-methoxyacrylate- based anti-fungal compounds from myxobacteria. Org. Biomol. Chem. [Internet]. 2006;4(15):2906-2911. Available from:
- [Google Scholar]
- Synthesis and Anti-cancer Activity of 1,3,4-Thiadiazole and 1,3-Thiazole Derivatives Having 1,3,4-Oxadiazole Moiety. J. Heterocycl. Chem.. 2015;52(5):1400-1405.
- [Google Scholar]
- 2-Amino-5-thiazolyl motif: A novel scaffold for designing anti-inflammatory agents of diverse structures. Eur. J. Med. Chem.. 2008;43(1):129-134.
- [Google Scholar]
- Copper iodide decorated graphitic carbon nitride sheets with enhanced visible-light response for photocatalytic organic pollutant removal and antibacterial activities. Ecotoxicol. Environ. Saf.. 2021;208:111712
- [Google Scholar]
- Synthesis and anti-hypertensive ?-blocking activity evaluation of thiazole derivatives bearing pyrazole moiety. Heterocycles.. 2015;91(9):1763-1773.
- [Google Scholar]
- Synthesis, anti-HIV and cytotoxicity evaluation of chiral 2,5-disubstituted 1,3,4-Thiadiazole derivatives bearing the sulfonamide scaffold. Zeitschrift Fur Naturforsch. - Sect. B J. Chem. Sci. [internet]. 2024;79(2–3):89-97. Available from:
- [Google Scholar]
- Facile fabrication of Tl4HgI6nanostructures as novel antibacterial and antibiofilm agents and photocatalysts in the degradation of organic pollutants. Inorg. Chem. Front. [Internet]. 2021;8(10):2442-2460. Available from:
- [Google Scholar]
- Metal complexes for therapeutic applications. Trends Chem. [Internet].. 2021;3(7):523-534. Available from:
- [Google Scholar]
- 1, ω-Bis(formylphenoxy)alkane: versatile precursors for novel bis-dihydropyridine derivatives. Monatshefte Fur Chemie.. 2016;147(7):1227-1232.
- [Google Scholar]
- Kassab RM, Gomha SM, Al-Hussain SA, et al. Synthesis and In-silico Simulation of Some New Bis-thiazole Derivatives and Their Preliminary Antimicrobial Profile: Investigation of Hydrazonoyl Chloride Addition to Hydroxy-Functionalized Bis-carbazones. Arab. J. Chem. [Internet]. 14(11), 6 (2021). Available from: https://www.sciencedirect.com/science/article/pii/S1878535221004111.
- Kassab RM, A Zaki ME, Abo Dena AS, Al-Hussain SA, Abdel-Aziz MM, Muhammad ZA. Novel Set of Highly Substituted Bis-pyridines: Synthesis, Molecular Docking and Drug-Resistant Antibacterial Profile. Future Med. Chem. [Internet]. 14(24), 1881–1897 (2022). Available from: https://www.future-science.com/doi/10.4155/fmc-2022-0196.
- Kassab RM, Zaki MEA, Al-Hussain SA, Abdelmonsef AH, Muhammad ZA. Two Novel Regioisomeric Series of Bis-pyrazolines: Synthesis, In Silico Study, DFT Calculations, and Comparative Antibacterial Potency Profile against Drug-Resistant Bacteria; MSSA, MRSA, and VRSA. ACS Omega [Internet]. (2023). Available from: https://pubs.acs.org/doi/full/10.1021/acsomega.3c06348.
- Biological Profile, and Molecular Docking of Some New Bis- Imidazole Fused Templates and Investigation of their Cytotoxic Potential as Anti-tubercular and/or Anticancer Prototypes. Med. Chem. (los. Angeles).. 2020;17(8):875-886.
- [Google Scholar]
- Tackling Microbial Resistance with Isatin-Decorated Thiazole Derivatives: Design, Synthesis, and in vitro Evaluation of Antimicrobial and Antibiofilm Activity. Drug Des. Devel. Ther. [Internet]. 2022;16:2817-2832. Available from:
- [Google Scholar]
- Synthesis and Antimicrobial Activities of Some New Bis(Schiff Bases) and Their Triazole-Based Lariat Macrocycles. Polycycl. Aromat. Compd.. 2022;42(5):2751-2766.
- [Google Scholar]
- Indeno[1,2-b]pyridin-5-one derivatives containing azo groups and their hydrazonal precursors: Synthesis, antimicrobial profile, DNA gyrase binding affinity, and molecular docking. J. Heterocycl. Chem. [Internet] 2023 Available from:
- [Google Scholar]
- Novel xylenyl-spaced bis-thiazoles/thiazines: synthesis, biological profile as herpes simplex virus type 1 inhibitors and in silico simulations. Future Med. Chem. [Internet]. 2024;16(1):27-41. Available from:
- [Google Scholar]
- kassab RM, Ibrahim MH, Rushdi A, et al. Comprehensive study for synthesis, antiviral activity, docking and ADME study for the new fluorinated hydrazonal and indeno[1,2-b]pyridine derivatives. J. Mol. Struct. [Internet]. 1305, 137752 (2024). Available from: https://linkinghub.elsevier.com/retrieve/pii/S0022286024002758.
- Adamantane derivatives of thiazolyl-N-substituted amide, as possible non-steroidal anti-inflammatory agents. Eur. J. Med. Chem.. 2009;44(3):1198-1204.
- [Google Scholar]
- Pt(IV) and Au(III) complexes with tridentate-benzothiazole based ligand: synthesis, characterization, biological applications (antibacterial, antifungal, antioxidant, anticancer and molecular docking) and DFT calculation. Inorganica Chim. Acta.. 2023;555:121598
- [Google Scholar]
- Synthesis, spectral characterization, lethal dose (LD50) and acute toxicity studies of 1,4-Bis(imidazolylazo)benzene (BIAB) Heliyon.. 2021;7(9)
- [Google Scholar]
- Kyhoiesh HAK, Al-Adilee KJ. Synthesis, spectral characterization and biological activities of Ag(I), Pt(IV) and Au(III) complexes with novel azo dye ligand (N, N, O) derived from 2-amino-6-methoxy benzothiazole. Chem. Pap. [Internet]. 76(5), 2777–2810 (2022). Available from: https://link.springer.com/article/10.1007/s11696-022-02072-9.
- Synthesis, Characterization, in silico DFT, Molecular docking, ADMET Profiling Studies and Toxicity Predictions of Ag(I) Complex Derived from 4-Aminoacetophenone. ChemistrySelect [Internet]. 2024;9(4):e202304429. Available from
- [Google Scholar]
- Antimicrobial activity of some new thioureides derived from 2-(4-chlorophenoxymethyl)benzoic acid. Molecules.. 2008;13(3):567-580.
- [Google Scholar]
- Well diffusion for antifungal susceptibility testing. Int. J. Infect. Dis.. 2004;8(1):39-45.
- [Google Scholar]
- Mahmood R, Pharmaceutical HA-IJ of, 2020 undefined. Synthesis, Characterization and Biological Activities of Azo and Azomethine Ligand with Some Transition Metal Complexes. Res. Mahmood, H AbassInternational J. Pharm. Res. 2020•researchgate.net [Internet]. 12(2), 258–263 (2020). Available from: https://www.researchgate.net/profile/Rasha-Mahmood/publication/348906855_Synthesis_Characterization_and_Biological_Activities_of_Azo_and_Azomethine_Ligand_with_Some_Transition_Metal_Complexes/links/60155a9945851517ef273a28/Synthesis-Characterization-and-Biological-Activities-of-Azo-and-Azomethine-Ligand-with-Some-Transition-Metal-Complexes.pdf.
- Synthesis and characterization of some novel bis-thiazoles. J. Heterocycl. Chem. [internet].. 2019;56(11):3157-3163. Available from:
- [Google Scholar]
- Structural characterization, spectroscopic studies, and molecular docking studies on metal complexes of new hexadentate cyclic peptide ligand. Appl. Organomet. Chem. [Internet]. 2022;36(2):e6515. Available from:
- [Google Scholar]
- Application and synthesis of thiazole ring in clinically approved drugs. Eur. J. Med. Chem.. 2023;250:115172
- [Google Scholar]
- Discovery and optimization of a series of 2-aminothiazole-oxazoles as potent phosphoinositide 3-kinase γ inhibitors. Bioorganic Med. Chem. Lett.. 2012;22(24):7534-7538.
- [Google Scholar]
- Facile fabrication of silver iodide/graphitic carbon nitride nanocomposites by notable photo-catalytic performance through sunlight and antimicrobial activity. J. Hazard. Mater.. 2020;389:122079
- [Google Scholar]
- Synthesis and characterization of macrocyclic compounds with a hydroxyl functional group. Chinese Chem. Lett.. 2013;24(10):869-872.
- [Google Scholar]
- Petrou A, Fesatidou M, Geronikaki A. Thiazole ring—a biologically active scaffold. Molecules [Internet]. 26(11), 3166 (2021). Available from: https://www.mdpi.com/1420-3049/26/11/3166/htm.
- Efficient synthesis of 2,4-disubstituted thiazoles using ionic liquid under ambient conditions: a practical approach towards the synthesis of Fanetizole. Tetrahedron.. 2007;63(45):11066-11069.
- [Google Scholar]
- Synthesis of novel isoxazolyl bis-thiazolo[3,2-a]pyrimidines. Chinese Chem. Lett.. 2012;23(8):899-902.
- [Google Scholar]
- A comprehensive review on thiazole based conjugates as anti-cancer agents. J. Mol. Struct.. 2023;1292:136194
- [Google Scholar]
- Microwave assisted multi-component synthesis of novel bis(1,4-dihydropyridines) based arenes or heteroarenes. Heterocycles.. 2016;92(5):910-924.
- [Google Scholar]
- One-pot synthesis of novel thiazoles as potential anti-cancer agents. Drug Des. Devel. Ther.. 2020;14:1363-1375.
- [Google Scholar]
- Antimicrobial Agents Based on Metal Complexes: Present Situation and Future Prospects. Int. J. Biomater.. 2022;2022
- [Google Scholar]
- 4-Thiazolidinone - A biologically active scaffold. Eur. J. Med. Chem.. 2008;43(5):897-905.
- [Google Scholar]
- Small-molecule inhibitors of SREBP activation-potential for new treatment of metabolic disorders. Medchemcomm.. 2013;4(11):1422-1433.
- [Google Scholar]
- Design and synthesis of novel dithiazole carboxylic acid Derivatives: In vivo and in silico investigation of their Anti-Inflammatory and analgesic effects. Bioorg. Chem.. 2024;144:107120
- [Google Scholar]
- Chalcone derivatives containing thiazole fragment: Synthesis and antifungal activity. J. Saudi Chem. Soc.. 2023;27(6):101773
- [Google Scholar]
- Studies on the syntheses of hydroxy-bearing benzo-azacrown ethers and their complexing behaviour. Polyhedron.. 1996;15(7):1197-1202.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2024.105933.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







