Translate this page into:
Novel indan-1,3-dione derivatives: Design, green synthesis, effect against tomato damping-off disease caused by Fusarium oxysporum and in silico molecular docking study
⁎Corresponding author at: Chemistry Department, College of Science, Jouf University, P.O. Box: 2014, Sakaka, Saudi Arabia. nahasan@ju.edu.sa (Nadia A.A. Elkanzi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
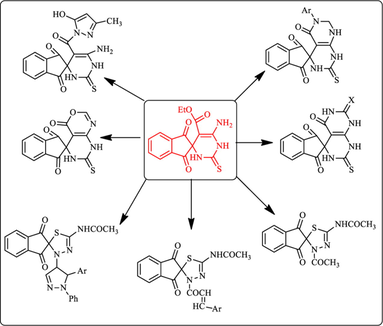
Abstract
An effective method for synthesizing series of twenty-two new compounds 1, 2a,b, 3, 4a,b, 5a-e, 7, 8, 9, 10, 12, 13a-d, 15a,b was performed starting from reaction of 1,2,3-indenetrione thiourea, and ethyl cyanoacetate under microwave irradiation and / or 2-(1,3-dioxo-1H-inden-2(3H)-ylidene) hydrazine carbothioamide with acetic anhydride. Chemical structure of the obtained products has been established by spectroscopic techniques including FTIR, 1H NMR, 13C NMR, DEPT-135, and mass spectroscopy. The designed new compounds have been successfully examined in-vitro for their antifungal activities. The relation between the structure of the synthesized compounds and their activity against tomato damping-off disease caused by Fusarium oxysporum fungi was studied and favourable results were obtained. The antifungal studies indicated that compounds 1, 7, 4a and 5a-d exerted the highest antifungal activities, while 3 and 4b recorded the lowest effect. The obtained results confirmed the possibility of the application of ethyl 6′-amino-1,3-dioxo-2′-thioxo-1,2′,3,3′-tetrahydro-1′H-spiro[indene-2,4′-pyrimidine]-5′-carboxylate 1 as a new effective regulator of the vegetative growth of tomatoes. The molecular docking analysis was performed within succinate dehydrogenase (SDH) as a target enzyme in order to rationalize the promising findings obtained for the active compounds 1, 2a,b, 5a-d, 7, 8, 9, 10, 12, and 13a.
Keywords
Pyrazole derivatives
Chalcones
Green synthesis
Antifungal activity
Molecular docking
1 Introduction
Pesticides are efficient means for protecting crops growing agricultural yields, within which fungicides exhibiting an essential role in modern agriculture (Kumar et al., 2019; (Shang et al., 2019). Due to the emergence of fungicide-resistance issues, it is essential to develop new antifungal agents with high efficiency and more selectivity. On the other hand, tomato (Solanum lycopersicum L.) is one of widely cultivated crops in different countries (Srivastava et al., 2010; (McGovern, 2015; (Kaiwart et al., 2020), Tomato are parasitized by different pathogenic bacteria, viruses, fungi and nematodes which are constraints to tomato farming such as tomato leaf curl disease, Fusarium wilt, bacterial wilt, Verticillium wilt and damping off (Al-ani et al., 2011; (Karthika et al., 2020; (Campos et al., 2021). Damping-off disease is one of the worst diseases of tomato, which can kill germinating seeds and young seedlings also (Lamichhane et al., 2017). Fusarium is soil borne pathogenic fungi, and the main prevalent, serious diseases of tomatoes that causes loss in tomato production in both field and greenhouse (Maurya et al., 2019). Several disease management strategies are available e.g. chemical control (Abo-Elyousr and Mohamed, 2009), using chloropicrin and methyl-bromide (McGovern et al., 1998) and inducing resistance in susceptible tomato plants via utilizing CuCl2 and MnSO4 (Mandal and Sinha, 1992). In 2020, symptoms of damping-off disease of tomato were shown in a field of Al-Jouf region, KSA. A diseased seedling was observed damping-off in which the fungus attacked the basal part of the stems and finally led to collapse of the plant on the soil surface. Many bare areas appeared in the diseased fields (see Fig. 1).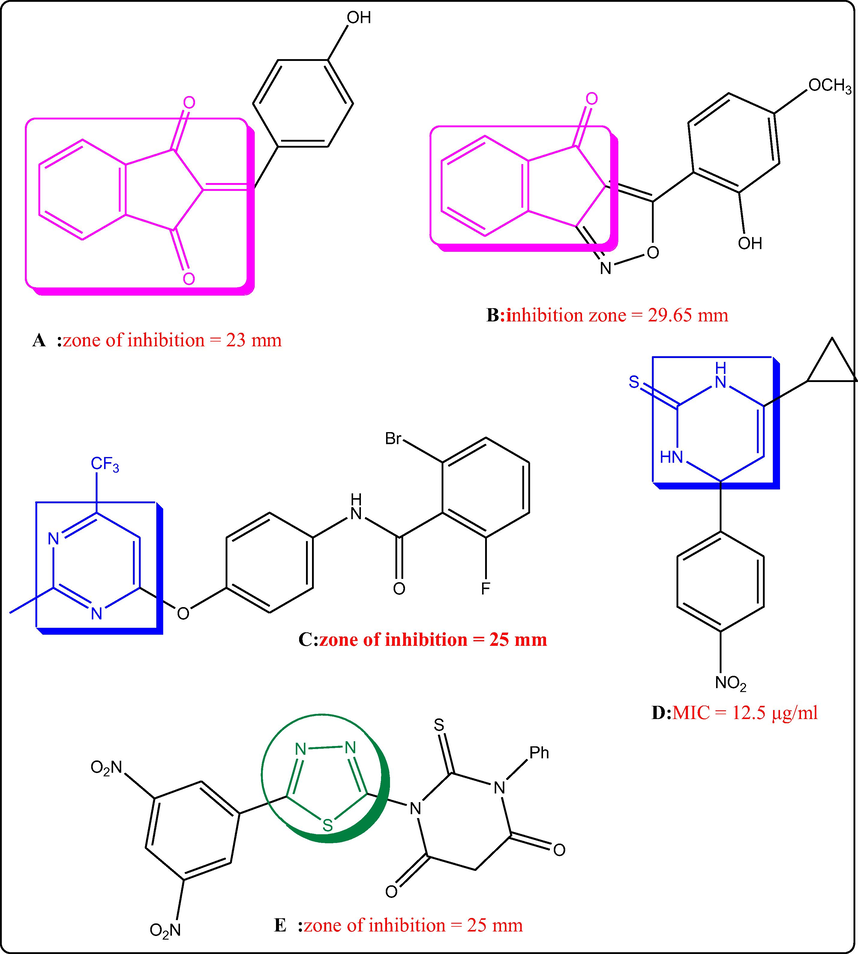
Examples of indane-1,3-diones (A, B), pyrimidines (C, D) and thiadiazole (E) with antifungal potential.
It was documented that heterocycles comprising indane-1,3-diones attracted considerable interest because of their diverse biological potentials as antiviral (Oliveira et al., 2018; (de Oliveira et al., 2019), α-glucosidase inhibitors (Mukhtar et al., 2021), Aβ1-40 fibrillogenesis inhibitor (Catto et al., 2010), antioxidant (Kouzi et al., 2019), anti-inflammatory (Giles et al., 2011; (A Muhammad et al., 2017), antiparkinson (Millan et al., 2004), urease inhibitor (Bano et al., 2018) and antifungal (Sarvesh and Nizamuddin, 2008; (Xu et al., 2009; (Mohil et al., 2014; (Meena et al., 2017). 4-Hydroxyphenylmethylene-indane-1,3-dione A was found to exert comparable antifungal effect towards A. niger (zone of inhibition = 23 mm) and C. albicans (zone of inhibition = 25 mm) to that recorded by griseofulvin (zone of inhibition = 26 and 24 mm, respectively) (Meena et al., 2017). Furthermore, the indeno derivative B revealed comparable zone of inhibition towards A. niger (inhibition zone = 29.65 mm) to that recorded by fluconazole (inhibition zone = 31.80 mm) at concentration 100 μg/mL (Patel et al., 2012). Heterocyclic compounds incorporating pyrimidine nucleus draw the attention of medicinal chemists owing to its versatile biological roles as anti-inflammatory (Abdelgawad et al., 2018; (Abdelgawad et al., 2019), anticancer (Al-Sanea et al., 2020; (Ghoneim et al., 2020), antidiabetic (Panahi et al., 2013), protein kinase inhibitor (B Bakr et al., 2017; (Abdellatif and Bakr, 2020), antimicrobial (Bakr et al., 2018; (Radwan et al., 2020; (Zhuang and Ma, 2020) and antifungal (Liu et al., 2005; (Maddila et al., 2016; (Sheikhi-Mohammareh et al., 2020). For example, pyrimidin-4-yl-oxy-phenylbenzamide derivative C recorded antifungal potential against Phomopsis Sp. with EC50 = 1.4 µg/mL which is more potent than pyrimethanil (EC50 = 32.1 µg/ml) (Wu et al., 2019). In addition, pyrimidine derivative D (MIC = 12.5 µg/ml) showed the same antifungal activity towards C. albicans as the standard drug Clotrimazole (MIC = 12.5 µg/ml) (Sharshira and Hamada, 2012). Moreover, thiadiazole incorporating heterocycles recorded antifungal potential (Keerthi Kumar et al., 2013; (Fadda et al., 2017). For instance, thiadiazole compound E (zone of inhibition = 25 mm) exhibited higher antifungal potency towards C. albicans than fluconazole (zone of inhibition = 22 mm) (El-Naggar et al., 2019). Prompted by all of these facts and in continuation of our work aiming at discovering novel antimicrobial agents (Bakr et al., 2018; (Ghoneim et al., 2018; (Elkanzi et al., 2019a; (Elkanzi et al., 2019b; (Bakr and Elkanzi, 2020; (Elkanzi and Bakr, 2020; (Elkanzi et al., 2020; (Ghoneim et al., 2020; (Hrichi et al., 2020), We report herein hybridizing the indane-1,3-dione scaffold with pyrimidine and/or thiadiazole heterocycles to get novel antifungal agents. To explore the antifungal potential of these newly prepared compounds, zones of inhibition of these compounds were estimated towards Fuserium oxysporum testing their effects on tomato seed germination. Furthermore, in silico molecular docking study had been conducted for the novel candidates within succinate dehydrogenase enzyme in an attempt to predict the binding mode of these novel compounds as antifungal agents. The objective of this study was to isolate and identifying causal organisms of tomato damping off disease based on the pathogens morphological and molecular characterization.
2 Experimental
2.1 Chemistry
All chemical reagents used for synthesis were purchased from Sigma Aldrich (Somatco Trading Co. Ltd., Sakaka, Aljouf, Saudi Arabia) and used without further purification. The reaction progress was controlled using thin layer chromatography (TLC) on silica gel pre-coated F254Merck plates (Darmstadt, Germany). The visualization of spots was performed with ultraviolet irradiation at 350–380 nm. Melting points (m.p.) of the synthesized compounds were determined on a Gallenkamp electrothermal melting point apparatus and are uncorrected. The elemental analyses (C, H, and N) of the synthesized compounds were realized on CE 440 Elemental Analyzer- Automatic Injector (Exeter Analytical, Inc., USA) at the Microanalytical Center of Cairo University. IR spectra were registered on Shimadzo infrared spectrophotometer (Chemistry department research laboratory, Jouf University) using potassium bromide disks. 1H, 13C NMR spectra were recorded on a Varian MercuryVXR-300 NMR spectrometer (Palo Alto, CA) at 400 and 125 MHz in dimethyl sulfoxide-d 6 (DMSO‑d6)and DEPT-135(ppm). The chemical shifts are given in ppm (δ) relative to the internal standard TMS., DEPT135 was used; Mass spectra were obtained at 70 eV on a shimadzu GCMS-QP 1000EX spectrometer. Microwave irradiations were carried out in a Kenstar OM9925E MW oven (2450 MHz, 800 W). Ultrasonic irradiation was performed in an ELO-150 ultrasonic cleaner with a frequency of 46 kHz and a nominal power of 200 W.
2.1.1 Synthesis of ethyl 6′-amino-1,3-dioxo-2′-thioxo-1,2′,3,3′-tetrahydro-1′H-spiro[indene-2,4′-pyrimidine]-5′-carboxylate (1)
-
Conventional method:
A mixture of thiourea (1.0 mmol), ethyl cyano acetate (1.0 mmol), 1, 2, 3-indenetrione (1.0 mmol) and potassium carbonate (1.0 mmol) in absolute ethanol (50.0 mL) was refluxed for 8 h. The reaction was monitored by thin-layer chromatography (TLC). After completion of the reaction, the reaction mixture was neutralized with concentrated hydrochloric acid, and the precipitate was filtered and recrystallized from ethanol.
-
b)
Microwave method:
A mixture of thiourea (1.0 mmol), ethyl cyanoacetate (1.0 mmol), 1, 2, 3-indenetrione (1.0 mmol) and potassium carbonate (a catalytic amount) was taken in about 30.0 mL of ethanol (one pot reaction). The reaction mixture was subjected to microwave for about 20 min. TLC monitored the completion of reaction, and the product was obtained in the form of potassium salt, which was dissolved in warm water and acidified by acetic acid to precipitate the product, and the obtained solid was recrystallized from ethanol.
Reddish brown; Yield: 96%; mp 290–292 °C; IR (Vmax, cm−1): 1489 (C⚌S), 1715 and 1772 (3C⚌O), 3100–3400 (2NH, NH2), 1H NMR (δ, DMSO‑d6): 1.31 (t, J = 7.2 Hz, 3H, CH3), 4.24 (q, J = 7.2 Hz, 2H, CH2), 7.01–8.02 (m, 6H, ArH, NH2), 12.76 (s, 2H, 2NH). 13CNMR (DMSO‑d6): 14.89 (CH3), 62.07 (CH2), 125.05, 126.04, 133.13, 141.12, 145.59 (Ar-C), 168.04 (C = S), 164.22, 165.08, 166.25 (3C = O) ppm; DEPT-135 δ (ppm) 21.99(CH3), 60.57(CH2), 123.21(CH), 123,46(CH),123.58 (CH), 123.65 (CH); Analysis calculated for C15H13N3O4S (331.35); C, 54.37; H, 3.95; N, 12.68; S, 9.68; Found; C, 54.41; H, 3.98; N, 12.72; S, 9.74. MS (m/z :): 331.35: [M+].
2.1.2 General procedure of compound 2a, b
A mixture of compound 1 (1.0 mmol), urea and / or thiourea (1.0 mmol), trimethylamine (few drops), and ethanol (20.0 mL) was subjected to microwave irradiation for about 20 min. After the reaction was completed (TLC), the mixture was concentrated and the obtained solid was collected by filtration and recrystallized from ethanol to provide 2a, b.
2.1.2.1 2′-Thioxo-2′,3′-dihydro-1′H-spiro[indene-2,4′-pyrimido[4,5-d]pyrimidine]-1,3, 5′,7′(6′H,8′H)-tetraone (2a)
Red; Yield: 95%; mp 235–237 °C; IR (Vmax, cm−1), 1476 (C⚌S), 1675 (C⚌O), 3100–3400 (3NH); 1H NMR (δ, DMSO‑d6): 7.01–8.02 (m, 4H, ArH), 10.55 (s, 1H, NH), 11.38 (s, 1H, NH), 11.99 (s, 1H, NH), 12.27 (s, 2H, NH); 13CNMR (DMSO‑d6): 120.0, 128.25, 131.13, 141.12, 161.07 (Ar-C), 170.04 (C⚌S), 162.14, 163.28, 195.25 (3C⚌O) ppm:. Analysis calculated for C14H8N4O4S (328.30); C, 51.22; H, 2.46; N, 17.07; S, 9.77; Found; C, 51.26; H, 2.51; N, 17.13; S, 9.82. MS (m/z :): 328.30: [M+].
2.1.2.2 2′,7-dithioxo-2′,3′,7′,8′-tetrahydro-1′H-spiro[indene-2,4′-pyrimido[4,5-d] pyrimidine] −1,3,5′(6′H)-trione (2b)
Reddish brown ; yield: 97%; mp 260–262 °C; IR (Vmax, cm−1): 1491 (C⚌S), 1667 (C⚌O), 3100–3400 (4NH); 1H NMR (δ, DMSO‑d6): 7.22–7.91 (m, 4H, ArH), 9.79 (s, 1H, NH), 10.28 (s, 1H, NH), 11.81 (s, 1H, NH), 12.25 (s, 1H, NH). 13CNMR (DMSO‑d6): 120.55, 126.04, 133.13, 141.12, 161.07 (Ar-C), 165.36, 185.78 (C⚌O), 169.03, 170.04, (C⚌S) ppm; analysis calculated for C14H8N4O3S2 (344.37); C, 48.83; H, 2.34; N, 16.27; S, 18.62; Found; C, 48.86; H, 2.39; N, 16.31; S, 18.66. MS (m/z): 344.37: [M+].
2.1.3 7′-Thioxo-7′,8′-dihydrospiro[indene-2,5′-pyrimido[4,5-d][1,3]oxazine]-1,3,4′(6′H)-trione (3)
A mixture of compound (1) with equimolecular amount of formic acid was refluxed for 10 h. The solid product was collected and recrystallized from ethanol to provide (3).
Pale yellow ;Yield: 69%; m.p. 276–278 °C; IR (Vmax, cm−1): 1240 (C⚌S), 1654, 1707 (3C⚌O); 3100–3400 (2NH); 1H NMR (δ, DMSO‑d6):7.50–8.00 (m, 5H, ArH), 10.35 (s, 1H, NH), 11.67 (s, 1H, NH); 13CNMR (DMSO‑d6): 129.16, 130.79, 133.86, 134.05, 135.16, 137.28, 140.57, 141.71 (Ar-C), 163.05 (CH⚌N), 163.25, 196.58 (3C⚌O), 180.56 (C⚌S) ppm. Analysis calculated for C14H7N3O4S (313.29); C, 53.67; H, 2.25; N, 13.41; S, 10.23; Found; C, 53.69; H, 2.27; N, 13.46; S, 10.28. MS (m/z): 313.29 [M+].
2.1.4 Synthesis of Schiff base (4a, b)
Equimolar concentrations of compound 1 (1.0 mmol) and different aryl aldehydes (1.0 mmol) were stirred for 6–8 h at room temperature using ethanol (20.0 mL) and then 2–3 drops of glacial acetic acid were added to the mixture. The progress of the reaction was followed by TLC until the reaction was complete. It was cooled to 0 °C, the precipitate was filtered, washed with diethyl ether and the residue was recrystallized from ethanol to give the corresponding (4a, b).
2.1.4.1 Ethyl 6′-((4-hydroxy-2-nitrobenzylidene)amino)-1,3-dioxo-2′-thioxo-1,2′,3,3′-tetrahydro-1′H-spiro[indene-2,4′-pyrimidine]-5′-carboxylate (4a)
Orange; Yield: 76%; m.p. 231–232; IR (Vmax, cm−1): 1250 (C⚌S), 1685, 1712 (C⚌O), 3100–3400 (2NH); 1H NMR (δ, DMSO‑d6): 1.62 (t, 3H, CH3), 4.55 (q, 2H, CH2–), 6.86–8.20 (m, 7H, ArH), 8.61 (s, 1H, HC⚌N), 10.27 (s, 1H, NH), 11.28 (s, 1H, NH), 11.96 (s, 1H, OH); 13CNMR (DMSO‑d6): 10.9 (CH3), 57.82 (O—CH2—), 95.26 (O⚌C—C—C⚌O), 85.68, 162.82, 115.16, 122.79, 125.86, 133.05, 131.16, 143.57, 141.28, 158.71 (Ar-C), 163.05 (CH⚌N), 163.25, 196.58 (3C⚌O), 180.56 (C⚌S) ppm. Analysis calculated for C22H16N4O7S (480.45); C, 55.00; H, 3.36; N, 11.66; S, 6.67; Found; C, 55.07; H, 3.39; N, 11.67; S, 6.71. MS (m/z): 480.45 [M+].
2.1.4.2 Ethyl 6′-(4-hydroxybenzylideneamino)-1,3-dioxo-2′-thioxo-1,2′,3,3′-tetrahydro-1′H-spiro [indene-2, 4′-pyrimidine]-5′-carboxylate (4b)
Red; Yield: 82%; m.p. 215–218; IR (Vmax, cm−1): 1237 (C⚌S), 1712 (C⚌O), 2942 (Ar-H), 3100–3400 (2NH); 1H NMR (δ, DMSO‑d6):1.29 (t, J = 6.9 Hz, 3H, CH3), 4.55 (q, J = 6.9 Hz, 2H, O—CH2—), 7.01–8.02 (m, 9H, ArH), 10.48 (s, H, 2NH); 13CNMR (DMSO‑d6): 15.9 (CH3), 62.5 (O-CH2-), 95.26 (O⚌C—C—C⚌O), 85.68, 115.16, 122.79, 125.86, 131.16, 143.57, 141.28, 158.71, 162.82, (Ar-C), 163.05 (CH⚌N), 163.25, 196.58 (2C⚌O), 180.56 (C⚌S) ppm. Analysis calculated for C22H17N3O4S (419.45); Calcd, C, 63.00; H, 4.09; N, 10.02; S, 7.64; Found; C, 63.05; H, 4.12; N, 10.7; S, 7.69. MS (m/z): 419.45 [M+].
2.1.5 General procedure for synthesis of compounds (5a-e):
A mixture of compound 1 (1.0 mmol) and equivalent amount of p-substituted aniline (p-nitoaniline, p-methoxy aniline, p-methylaniline, aniline) and or 2-amino pyridine (1.0 mmol) and excess of formaldehyde (37%) in acetonitrile at room temperature was stirred for 4 h. The solid formed was collected and crystalized from ethanol to provide (5a-e) in good yield.
2.1.5.1 Synthesis of 6′-(4-nitrophenyl)-2′-thioxo-2′,3′,7′,8′-tetrahydro-1′H-spiro[indene-2,4′-pyrimido[4,5-d]pyrimidine]-1,3,5′(6′H)-trione (5a)
Yellow; Yield: 95%; m.p. 232–234 °C; IR (Vmax, cm−1): 1245 (C═S), 1710 and 1597 (3C⚌O), 3100–3400 (3NH); 1H NMR (δ, DMSO‑d6): 4.81 (s, 2H, CH2), 7.01–8.02 (m, 8H, ArH), 10.25 (s, 2H, 2NH), 10.56 (s, 1H, NH); 13CNMR (DMSO‑d6): 58.22 (CH2), 116.88, 117,58, 118.66, 119.86, 120.01, 123.27, 124.50, 125.45, 126.23, 141.71 (Ar-C), 153.93, 156.45, 160.89 (3C⚌O), 162.77 (C⚌S) ppm. Analysis calculated for C20H13N5O5S (435.41); C, 55.17; H, 3.01; N, 16.08; S, 7.36; Found; C, 55.21; H, 3.15; N, 16.13; S, 7.41. MS (m/z):435.41 [M+].
2.1.5.2 6′-(4-methoxyphenyl)-2′-thioxo-2′,3′,7′,8′-tetrahydro-1′H-spiro[indene-2,4′-pyrimido[4,5-d]pyrimidine]-1,3,5′(6′H)-trione (5b)
Brown; Yield: 76%; m.p. 261–262 °C; Yield: 76%; m.p. 261–262 °C; IR (Vmax, cm−1): 1461(C⚌S), 1672 (C⚌O), 3100–3400 (3NH); 1H NMR (δ, DMSO‑d6): 3.89 (s, 3H, CH3) 4.85 (s, 2H, CH2), 6.69–8.01 (m, 8H, ArH), 10.28 (s, 2H, 2NH), 11.17 (s, 1H, NH); 13CNMR (DMSO‑d6): 15.07 (CH3), 63.05 (CH2), 95.26 (O⚌C—C—C⚌O), 162.82, 116.16, 117.79, 118.86, 131.16, 133.05, 140.28, 141.57, 142.71 (Ar-C), 158.79, 160.13, 161.42 (C⚌O), 163.90 (C⚌S) ppm. Analysis calculated for C21H16N4O4S (420.44); C, 59.99; H, 3.84; N, 13.33; S, 7.63; Found; C, 60.03; H, 3.87; N, 13.37; S, 7.68. MS (m/z): 420.44 [M+].
2.1.5.3 2′-Thioxo-6′-(p-tolyl)-2′,3′,7′,8′-tetrahydro-1′H-spiro[indene-2,4′-pyrimido[4,5-d]pyrimidine]-1,3,5′(6′H)-trione (5c)
Green; Yield: 70%; m.p. 242-–244 °C; IR (Vmax, cm−1): 1354 (C⚌S), 1678 (C⚌O), 3100–3400 (3NH); 1H NMR (δ, DMSO‑d6): 2.81 (s, 3H, CH3), 4.75 (s, 2H, CH2), 6.86–8.69 (m, 8H, ArH), 11.25 (s, 2H, 2NH), 11.87 (s, 1H, NH); 13CNMR (DMSO‑d6): 21.25 (CH3), 63.05 (CH2), 95.26 (O⚌C—C—C⚌O), 85.68, 162.82, 117.16, 118.79, 119.86, 120.16, 129.75, 141.28, 143.57, 158.71 (Ar-C), 163.25, 196.58 (C⚌O), 180.56 (C⚌S) ppm. Analysis calculated for: C21H16N4O3S (404.44); C, 62.36; H, 3.99; N, 13.85; S, 7.93; Found; C, 62.39; H, 4.02; N, 13.86; S, 7.97. MS (m/z): 404.44 [M+].
2.1.5.4 6′-Phenyl-2′-thioxo-2′,3′,7′,8′-tetrahydro-1′H-spiro[indene-2,4′-pyrimido[4,5-d]pyrimidine]-1,3,5′(6′H)-trione (5d)
Red; Yield: 72%; m.p. 217-–219 °C; IR (Vmax, cm−1):1246(C⚌S), 1654, 1716 (3C⚌O), 3100–3400 (3NH); 1H NMR (δ, DMSO‑d6): 5.19 (s, 2H, CH2), 7.01–8.02 (m, 9H, ArH), 12.19 (s, 3H, 3NH); 13CNMR (DMSO‑d6): 62.70 (CH2), 112.17, 122.28, 125.22, 128.33, 130.46, 141.26, 143.57, 158.71 (Ar-C), 162.12, 185.01 (C⚌O), 158.65 (C⚌S) ppm; DEPT135 spectrum (ppm): 62.70 (CH2), 122.28, 123.35, 125.22, 128.33(Ar-); Analysis calculated for C21H16N4O3S (390.42); C, 61.53; H, 3.61; N, 14.35; S, 8.21; Found; C, 61.56; H, 3.63; N, 14.38; S, 8.26. MS (m/z): 390.42 [M+].
2.1.5.5 Synthesis of 6′-(pyridin-2-yl)-2′-thioxo-2′,3′,7′,8′-tetrahydro-1′H-spiro[indene-2,4′-pyrimido[4,5-d]pyrimidine]-1,3,5′(6′H)-trione (5e)
Reddish brown; Yield: 69%; m.p. 252–253 °C; IR (Vmax, cm−1): 1247(C⚌S),1654, 1720 (3C⚌O); 3100–3400 (3NH); 1H NMR (δ, DMSO‑d6): 5.28 (s, 2H, CH2), 7.01–8.02 (m, 8H, ArH), 10.26 (s, 2H, 2NH), 11.59 (s, 1H, NH); 13CNMR (DMSO‑d6): 56.32 (CH2), 95.26 (O⚌C—C—C⚌O), 85.68, 112.48, 122.79, 125.86, 131.16, 133.05, 137.38, 139.10, 142.06, 147.94, 158.71 (Ar-C), 163.25 (C⚌O), 196.58 (2C⚌O), 180.56 (C⚌S) ppm. Analysis calculated for C19H13N5O3S (391.40); C, 58.30; H, 3.35; N, 17.89; S, 8.19; Found; C, 58.36; H, 3.41; N, 17.93; S, 8.23. MS (m/z): 391.40 [M+].
2.1.6 Synthesis of 6′-amino-1,3-dioxo-2′-thioxo-1,2′,3,3′-tetrahydro-1′H-spiro[indene-2,4′-pyrimidine]-5′-carbohydrazide (7)
A mixture of compound 1 (1.0 mmol) and hydrazine hydrate (3.0 mL) in 30.0 mL EtOH was refluxed for 5 h and / or subjected to microwave for ten minutes. Then, the reaction mixture was concentrated under reduced pressure, and the residue washed with acidified cold H2O and then triturated with MeOH. The formed orange product was filtered off, washed well with MeOH, and recrystallized from ethanol solvent to afford the hydrazinyl derivatives 7.
Orang ;Yield: 90%; mp 248–250 °C; IR (Vmax, cm−1): 1484 (C⚌S), 1652 (C⚌O), 3100–3400 (3NH, 2NH2); 1H NMR (δ, DMSO‑d6): 6.30–8.45 (m, 8H, for ArH and 2NH2), 10.19 (s, 1H, NH), 10.33(s, 2H, 2NH); 13CNMR (DMSO‑d6): 125.05, 126.04, 133.13, 141.12, 145.59 (Ar-C), 168.04 (C⚌S), 165.08, 167.02 (C⚌O) ppm. Analysis calculated for: C13H11N5O3S (317.32); C, 49.21; H, 3.49; N, 22.07; S, 10.10; Found; C, 49.26; H, 3.53; N, 22.13; S, 10.15. MS (m/z): 317.32[M+].
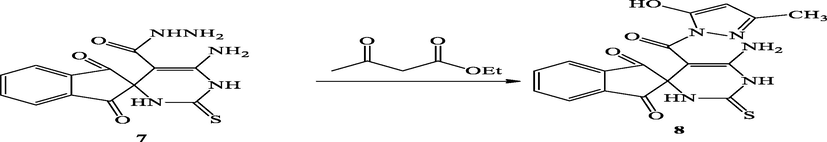
2.1.6.1 Synthesis of 6′-amino-5′-(5-hydroxy-3-methyl-1H-pyrazole-1-carbonyl)-2′-thioxo-2′,3′-dihydro-1′H-spiro[indene-2,4′-pyrimidine]-1,3-dione (8)
A mixture of compound (7) (1.0 mmol) and ethyl acetoacetate (1.0 mmol) in ethanol (20.0 mL) and three drops of glacial acetic acid the reaction mixture was subjected to microwave for about 10 min. The reaction mixture was allowed to cool at room temperature and then poured into crushed-ice. The separated solid was filtered off, dried and crystallized from EtOH/DMF (2:1) to give compound 8.
Light brown; Yield: 98%; m.p.: 190–192 °C; IR (Vmax, cm−1): 1271(C⚌S),1616 (C⚌O), 3100–3400 (2NH, NH2), 3590 (OH); 1H NMR (δ, DMSO‑d6:1.21 (s, 3H, CH3), 6.32 (s, 1H, pyrazole H), 7.50–8.00 (m, 4H, ArH), 10.27 (s, 2H, NH2), 11.12 (s, 2H, 2NH), 12.41 (s, 1H, OH); 13CNMR (DMSO‑d6): 15.02 (CH3), 92.88, 115.29, 120.04, 125.23, 129.67, 141.12, 153.02, 180.59 (Ar-C), 163.52, 163.89, 165.85 (3C⚌O), 177.36 ppm (C⚌S) ppm. Analysis calculated for C17H13N5O4S (383.38); C, 53.26; H, 3.42; N, 18.27; S, 8.36; Found; C, 53.31; H, 3.46; N, 18.30; S, 8.39. MS (m/z): 383.38 [M+].
2.1.6.2 Synthesis of 6′-amino-5′-(3-amino-5-hydroxy-1H-pyrazole-1-carbonyl)-2′-thioxo-2′,3′-dihydro-1′H-spiro[indene-2,4′-pyrimidine]-1,3-dione (9)
A mixture of compound (7) (1.0 mmol) and ethyl cyanoacetate (1.0 mmol) in ethanol (20.0 mL) and three drops of glacial acetic acid was subjected to microwave for about 10 min. After cooling, the reaction mixture was poured into crushed-ice, and the Separated solid was filtered off, dried well and crystallized from acetic acid to afford compound 9.
Orange; Yield 96%; m.p.: 225–227 °C; IR (Vmax, cm−1): 1245 (C⚌S), 1624 (C⚌O), 3100–3400 (2NH, 2NH2), 3596(OH), 1H NMR (δ, DMSO‑d6): 6.11 (s, 1H, pyrazole H), 6.52 (s, 2H, NH2), 7.50–8.00 (m, 4H, ArH), 10.27 (s, 2H, NH2), 11.12 (s, 2H, 2NH), 12.53 (s, 1H, OH); 13CNMR (DMSO‑d6): 118.88, 119.29, 120.04, 125.23, 129.67, 133.13, 137.12, 138.02, 142.59 (Ar-C), 163.52, 163.89, 165.85 (3C⚌O); 177.36 (C⚌S) ppm. Analysis calculated for C16H12N6O4S (384.37); C, 50.00; H, 3.15; N, 21.86; S, 8.34; Found; C, 50.14; H, 3.20; N, 21.89; S, 8.39. MS (m/z): 384.37 [M+].
2.1.6.3 Synthesis of 6′-amino-5′-(3,5-dihydroxy-1H-pyrazole-1-carbonyl)-2′-thioxo-2′,3′-dihydro-1′H-spiro[indene-2,4′-pyrimidine]-1,3-dione (10)
A mixture of (7) (1.0 mmol) and diethyl malonate (1.0 mmol) in ethanol (20.0 mL) and three drops of glacial acetic acid was subjected to microwave for about 10 min. After cooling, the reaction mixture was poured into crushed-ice and the separated solid was filtered off, dried well and crystallized from EtOH/ DMF (2:1) to give compound 10.
Brown; Yield: 99%; m.p. 206–208 °C; IR (Vmax, cm−1): 1489 (C⚌S), 1716, (C⚌O), 3100–3400 (2NH, NH2), 3596(2OH); 1H NMR (δ, DMSO‑d6): 6.00 (s,1H, pyrazole proton), 7.01–8.02 (m, 4H, ArH), 10.29 (s, 2H, NH2), 11.98 (s, 2H, 2NH), 12.16 (s, 2H, 2OH); 13CNMR (DMSO‑d6): 120.04, 123.13, 126.12, 128.11, 34.84, 135.79, 141.12, 145.02 (Ar-C), 168.04 (C⚌S), 167.78, 171.56, (2C⚌O) ppm. Analysis calculated for: C16H11N5O5S (385.35); C, 49.87; H, 2.88; N, 18.17; S, 8.32; Found; C, 49.89; H, 2.91; N, 18.22; S, 8.36;MS (m/z): 385.35 [M+].
2.1.7 Synthesis of compound (11)
Compound (11) was prepared according to the reported method Reddy,1986.
Green method for preparation of (11)
A Mixture of 1, 2, 3-indenetrione (1.0 mmol) and thiosemicarbazide (1.0 mmol) in water was stirred at room temperature (23 °C) using ultrasonic irradiation for 7 min to provide the corresponding (11) in 99% yield.
2.1.7.1 N-(3′-acetyl-1,3-dioxo-1,3-dihydro-3′H-spiro[indene-2,2′-[1,3,4]thiadiazol]-5′-yl)acetamide (12)
A mixture of thiosemicarbazone derivative (11, 1.0 mmol) and acetic anhydride was subjected to microwave irradiation for 10 min at 50 °C to provide the corresponding (12)
Reddish brown; Yield: 99%, m.p. 220–222 °C; IR (Vmax, cm−1): 1665, 1716 (4C⚌O), 2970 (Ar-H), 3074 (NH), 1H NMR (δ, DMSO‑d6): 1.33 (s, 3H, CH3), 2.0 (s, 3H, CH3), 7.01–8.02 (m, 4H, ArH), 10.58 (s, 1H, NH); 13CNMR (DMSO‑d6):120.01, 139.15, 140.03, 141.06, 142.21 (Ar-C), 161.03, 183.42, (2C⚌O), 184.27 (C⚌O) ppm. Analysis calculated for: C14H11N3O4S (317.32); C, 52.99; H, 3.49; N, 13.24;; S, 10.10; Found; C, 53.03; H, 3.53; N, 13.26; S, 10.14; MS (m/z): 317.32 [M+].
2.1.8 General synthesis of compounds (13a-d)
A mixture of compound 12 (1.0 mmol), the appropriate aromatic aldehyde (1.0 mmol), NaOH 40% and solvent were taken in a beaker and sonicated for 15–30 min in the water bath of an ultrasonic cleaner bath. The progress of the reaction was monitored by TLC. The reaction mixture was then cooled in ice-water bath. The formed precipitate was filtered, washed with cool water, dried and recrystallized from alcohol to afford chalcones (13a-d).
2.1.8.1 N-(1,3-dioxo-3′-(3-phenylacryloyl)-1,3-dihydro-3′H-spiro[indene-2,2′-[1,3,4] thiadiazol]-5′-yl)acetamide (13a)
Pale Yellow ;Yield :96%; m.p. 240–242 °C; IR (Vmax, cm−1): 1600 (C⚌C), 1683 (C⚌O), 2981 (Ar-H), 3070 (NH); 1H NMR (δ, DMSO‑d6): 1.29 (s, 3H, CH3), 7.05–7.61 (m, 4H, ArH), 7.65 (d, J = 15.63 Hz, 1H, H) 7.65–7.72 (m, 3H, ArH), 7.76 (d, J = 15.61 Hz, 1H, H), 8.02 (d, J = 9.11 Hz, 2H, ArH); 10.23 (s, 1H, NH); 13CNMR (DMSO‑d6): 126.23, 127.14, 128.15, 129.27, 130.12, 131.06, 133.02, 134.14 (Ar-C), 165.13 (C⚌O) ppm. Analysis calculated for C21H15N3O4S (405.43); C, 62.21; H, 3.73; N, 10.36; S, 7.91; Found; C, 62.25; H, 3.79; N, 10.39; S, 7.95; MS (m/z): 405.43 [M+].
2.1.8.2 N-(3′-(3-(4-hydroxy-2-nitrophenyl)acryloyl)-1,3-dioxo-1,3-dihydro-3′H-spiro [indene −2,2′-[1,3,4] thiadiazol]-5′-yl)acetamide (13b)
Yellow; Yield: 93%, m.p.252–254 °C; IR (Vmax, cm−1): 1600 (C⚌C), 1683, 1716 (C⚌O), 2972 (Ar-H), 3100–3400 (NH); 1H NMR (δ, DMSO‑d6): 2.31 (s, 3H, CH3), 7.06–7.12 (m, 4H, ArH), 7.64 (d, J = 15.57 Hz, 1H, H), 7.75 (d, J = 15. 63 Hz, 1H, H), 8.02–8.16 (m, 3H, ArH), 10.21 (s, 1H, NH), 12.13(s, 1H, OH); 13CNMR (DMSO‑d6): 126.25, 127.17, 128.18, 129.29, 130.15, 131.12, 133.11, 134.18 (Ar-C), 165.26, (C⚌O) ppm, Analysis calculated for C21H14N4O7S (466.42); C, 54.08; H, 3.03; N, 12.01;; S, 6.87; Found; C, 54.14; H, 3.16; N, 12.07; S, 6.89; MS (m/z): 466.42 [M+].
2.1.8.3 N-(3′-(3-(4-(dimethylamino)phenyl)acryloyl)-1,3-dioxo-1,3-dihydro-3′H-spiro [indene-2,2′-[1,3,4]thiadiazol]-5′-yl)acetamide (13c)
Orang ;Yield :95%; m.p.236–238 °C; IR (Vmax, cm−1): 1616 (C⚌C), 1639 (C⚌O), 3100–3400 (NH); 1H NMR (δ, DMSO‑d6): 1.22 (s, 3H, CH3), 2.32 (s, 6H, 2CH3), 7.05–7.38 (m, 4H, ArH), 7.63 (d, J = 15.53 Hz, 1H, H), 7.76 (d, J = 15. 62 Hz, 1H, H), 7.81–8.02 (d, J = 9. 14 Hz, 2H, ArH), 12.25 (s, 1H, NH); 13CNMR (DMSO‑d6):39.05 (CH3), 40.03 (CH3), 111.03, 125.25, 126.23, 129.27, 135.14, 136.16, 138.22, 139.24, 141.03 (Ar-C), 163.13, 164.23, 190.26, 195.08 (C⚌O) ppm. Analysis calculated for C23H20N4O4S (448.49); C, 61.59; H, 4.49; N, 12.49; S, 7.15; Found; C, 61.63; H, 4.52; N, 12.55; S, 7.19. MS (m/z): 448.49 [M+].
2.1.8.4 N-(3′-(3-(2-hydroxy-4-methoxyphenyl)acryloyl)-1,3-dioxo-1,3-dihydro-3′H-spiro [indene-2,2′-[1,3,4]thiadiazol]-5′-yl)acetamide (13d)
Yellowish; Yield: 94%; m.p. 263–265 °C; IR (Vmax, cm−1): 1618 (C⚌C), 1638 (C⚌O), 3100–3400 (NH), 3551 (OH). 1H NMR (δ, DMSO‑d6): 1.03 (s, 3H, CH3), 3.74 (s, 3H, OCH3), 7.05–7.26 (m, 4H, ArH), 7.63 (d, J = 15.53 Hz, 1H, H), 7.76 (d, J = 15. 62 Hz, 1H, H), 7.80–8.02 (m, 2H, ArH), 12.25 (s, 1H, NH). 13CNMR (DMSO‑d6): 25.67, 39.05 (2CH3), 111.03, 125.25, 126.23, 129.27, 135.14, 136.16, 138.22, 139.24, 141.03 (Ar-C), 163.13, 164.23, 190.26, 195.08 (C⚌O) ppm. Analysis calculated for: C22H17N3O6S (451.45); C, 58.53; H, 3.80; N, 9.31; S, 7.10; Found; C, 58.56; H, 3.85; N, 9.33; S, 7.16. MS (m/z): 451.45 [M+].
2.1.9 Synthesis of pyrazoles (15a, b)
A mixture of chalcones (13a, b) (1.0 mmol) and phenyl hydrazine (1.0 mmol) in ethanol was heated under reflux for 6 h and / or subjected to microwave for 10 min. The solvent was evaporated and the solid crystalized by diethyl ether to provide the corresponding pyrazoles (15a, b) in good yield.
2.1.9.1 N-(3′-(1,5-diphenyl-4,5-dihydro-1H-pyrazol-4-yl)-1,3-dioxo-1,3-dihydro-3′H-spiro [indene-2,2′-[1,3,4]thiadiazol]-5′-yl)acetamide (15a)
Brown ;Yield :88%; m.p: 270–272 °C; IR (Vmax, cm−1):, 1697, 1714 (C⚌O), 3100–3400 (NH); 1H NMR (δ, DMSO‑d6): 1.38(s,3H,CH3),3.68(s,1H,CH2),4.24(s,1H,CH), 7.01–8.02 (m,15H,Ar-H,NH); 13CNMR (DMSO‑d6): 18.67 (CH3), 55.66, 60.57 (CH,CH2), 100.05, 121.12, 122.14, 125.03, 128.25, 129.21, 131.05 (Ar-C), 155.21, 165.03 (3C⚌O) ppm. Analysis calculated for C27H21N5O3S (495.55); C, 65.44; H, 4.27; N, 14.13; S, 6.47; Found; C, 65.45; H, 4.31; N, 14.15; S, 6.51. MS (m/z): 495.55 [M+].
2.1.9.2 N-(3′-(5-(5-hydroxy-2-nitrophenyl)-1-phenyl-4,5-dihydro-1H-pyrazol-4-yl)-1,3-dioxo-1,3-dihydro-3′H-spiro[indene-2,2′-[1,3,4]thiadiazol]-5′-yl)acetamide (15b)
Reddish-brown; Yield: 86%; m.p: 285–287 °C; IR (Vmax, cm−1): 1699, 1714 (C⚌O), 3100–3400 (NH). 1H NMR (δ, DMSO‑d6): 2.41(s,3H,CH3),3.68(s,1H,CH),4.92(s,1H,CH), 7.01–8.02(m,13H,Ar-H,NH), 12.35 (s,1H,OH); 13CNMR (DMSO‑d6): 21.64(CH3), 56,67(CH2), 66.89(CH), 96.89, 121.51, 128,08, 128.88,128.27, 130.15,130.84,132.70, 136.06, 146.36, 147,32(Ar-C), 155.66,161.65(3CO)ppm,Analysis calculated for C27H20N6O6S (556.55); C, 58.27; H, 3.62; N, 15.10; S, 5.76; Found; C, 58.29; H, 3.66; N, 15.14; S, 5.79 . MS (m/z): 556.55 [M+].
2.2 Antifungal activity
2.2.1 Collection of samples
Tomato plants showing the damping-off disease symptoms were collected from tomato fields of Al-Jouf region. The infected plants were taken in plastic bags, transported to the laboratory for isolating the fungal causative agent of disease.
2.2.2 Isolation of fungi
basal portions of infected roots and stem were washed from the adhered soil and sterilized with NaClO2 (2%) solution for 3 min, after that these parts were washed with sterile distilled H20 for 30 min. By using Sterile forceps transferred pieces of 0.5 cm from each end of the roots to plates containing sterile Potato Dextrose Agar (PDA) medium with Rose Bengal (0.001 gm). Incubation was done at 27 °C ± 2 for 7 days Ofunne, 1999.
2.2.3 Identification of fungi:
isolated fungi were identified based on microscopic and macroscopic characteristics Rahjoo, 2008. Pathogenic fungi were additionally identified using molecular criteria according to sequence analysis of ITS1-5.8S rRNA–ITS2 region (Animal health research institute, Dokki, Giza, Egypt).
2.2.4 Effect of tested chemical compounds on the mycelial growth of Fusarium oxysporium:
Under sterile conditions, petri dish (9 cm diameter) was supplemented with sterilized PDA medium. A circular hole of 10 mm, 0.5 mm depth was made (using special instrument). Two hundred µl of each tested chemical compound (100 mg) which dissolved in 1.0 mL of DMSO was added into each well. Before adding the tested chemical solution, each Petri dish was inoculated with two discs of the tested fungus. Negative control was done using DMSO (100 µL) in well without chemicals, and positive control was done using Metalaxyl (100 µL). Incubation was performed at 27 °C for 6 days in the dark.
2.2.5 Determination of MIC
Diameter of inhibition zone (mm) around the well in different concentration (10, 15, 25, 50 and 100, mg/mL) of synthetic compound was determined for measuring the antifungal activity. All tested compound were measured in triplicate and expressed as the average ± standard deviation of the measurements. Controls were done using DMSO without chemicals.
Inhibition percent (%) of fungal growth was calculated using the following formula Hmouni et al., 1996: Where, C = Diameter of colony growth of the Fusarium oxysporum in control.
F = Diameter of colony growth of the Fusarium oxysporum in treated.
2.2.6 Microscopic analysis of the effect of tested chemical compounds on the mycelium growth
The microscopic images were taken at the faculty of Agriculture, Cairo University, using a SEM at 1000 and 3000 x. Samples were taken from mycelium treatment with tested chemical compounds at 25 mg/mL, then the mycelium was placed on a glass slide and using a metal coating equipment of samples (EDWARDS E306A), a copper bath was applied to the samples for 20 min for subsequent visualization. Finally, the general morphological and structural characteristics of the F. oxysporium mycelium were evaluated.
2.2.7 Testing the effect of chemical compounds on tomato seeds
2.2.7.1 Agar plates
One hundred ml of water agar (2%) with 10.0 mL of different chemical compounds (25.0 mg/mL) were poured each in conical flasks (250.0 mL) and then sterilized by autoclave. Tomato seeds were sterilized by 5% sodium hypochlorite solution for 5 min and then seeds were washed with sterilized distilled water for 30 min. Seeds were germinated for 2 days at 25 °C to select viable ones. Six Tomato seeds were planted in each flask with three discs of F. oxysporum. All flasks were incubated in a growth chamber at 25 °C with 12 h photoperiod. Experiments were performed with three replications were imposed on tomato seedling to record.
2.2.8 Statistical analysis
The mean of analysis/determination results was formed by the statistical analysis by using ANOVA software. Statistical significance was defined as P < 0.05.
2.3 Docking study
In this study, we utilized MOE software Version 2008, Chemical Computing Group Inc, Montreal, Quebec, Canada) to do this study (Bakr et al., 2021). Succinate dehydrogenase enzyme (SDH) with the cocrystallized ligand was downloaded from protein data bank (PDB: 2FBW). All the novel compounds were docked within the SDH enzyme. Binding energy scores and hydrogen bonds are listed within Table 6.
3 Results and discussion
3.1 Chemistry
1, 2, 3-indenetrione, which contains three carbonyl groups, is a highly active electrophile. Initially, we tried to synthesize the spiro compound 1 via a cascade three-component protocol. The reaction was carried out by successive addition of reactants: 1, 2, 3-indenetrione, thiourea, and ethyl cyanoacetate in the existence of a catalytic portion of K2CO3 in a flask with ethanol at refluxed temperature (Scheme 1). TLC analysis showed that the reaction was achieved in 5 h to afford the target spiro compound 1 in 85% yield. To obtain the optimal reaction conditions, we investigated another energy source such as irradiation with microwave, which afforded a better yield (96%) in comparison to conventional conditions. To our delight, the Knoevenagel condensation product (Kundu and Pramanik, 2014) between 1,2,3-indenetrione and ethyl cyanoacetate did not observe in this cascade reaction and only a single product was detected spiro compound1. The molecular structure of the spiro compound 1 was interpreted from its mass spectrometric analysis, FT-IR, 1H NMR, and 13C NMR. The FT-IR spectrum of spiro compound 1 demonstrated wide absorption bands at 3100–3400 cm−1 attributed to the stretching frequencies of (2NH, NH2). Besides, the absorption bands at 1772, 1715, and 1489 cm−1 are assigned to C⚌O and C⚌S groups. 1H NMR spectrum of spiro compound 1 exhibited a singlet signal that related to NH group at δ = 12.76 ppm, one triplet signal is detected at δ = 1.31 ppm for the methyl protons (CH3), and one quartet at δ = 4.24 ppm for the methylene protons (CH2). Also, the multiples at δ = 7.01–8.02 ppm were attributed to the aromatic protons and NH2 proton. The existence of distinguished signals in the 13C NMR spectrum of spiro compound1 is compatible with the suggested structure. Moreover DEPT-135 δ (ppm) spectrum appeared negative signal in the opposite direction due to the CH2 group at 60.57 ppm. The mass spectra agreement with the molecular structure of spiro compound 1. The Suggested mechanism support the formation of spiro compound 1 was found in Scheme 2.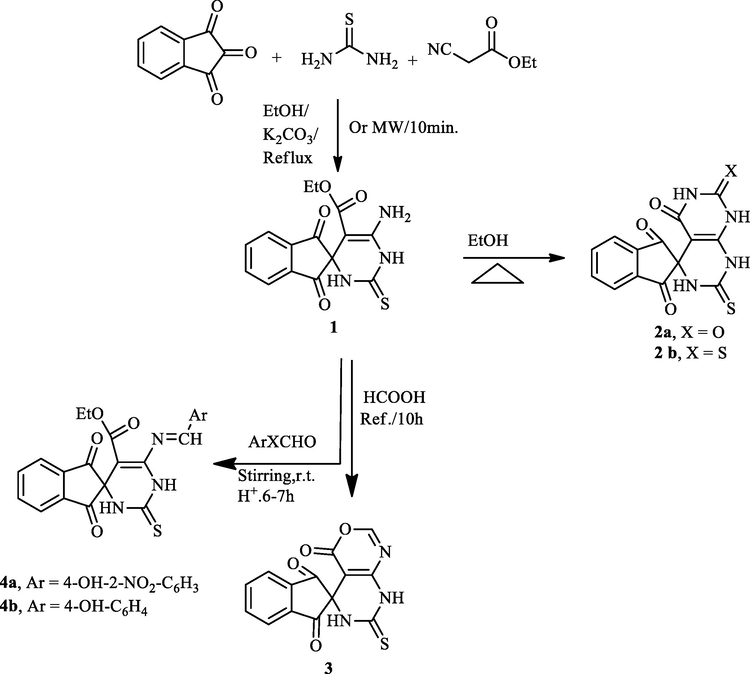
Synthesis of indene-2,4′-pyrimidine-2-thione derivatives 1, 2a,b, 3, and 4a,b.
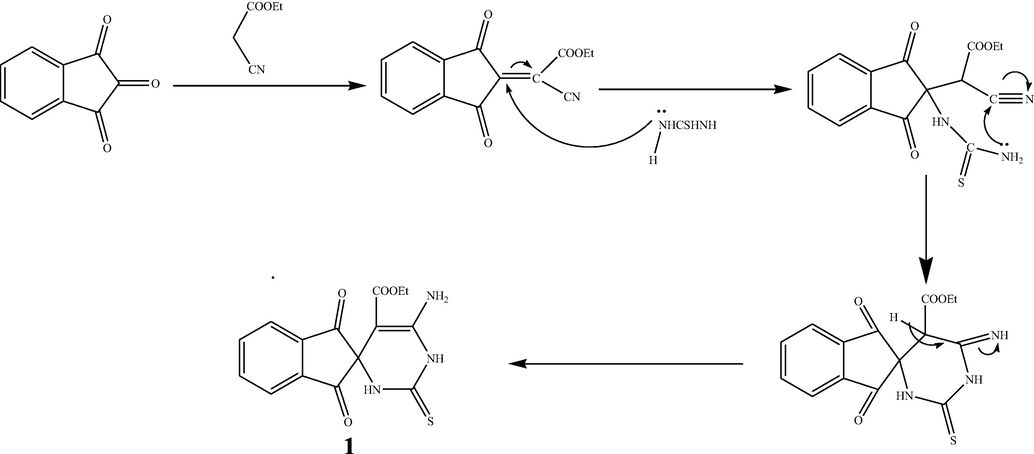
A proposed mechanism for compound 1.
Treatment of the obtained ethyl spiro[indene-2,4′-pyrimidine]-5′-carboxylate 1 with urea and/or thiourea furnished the corresponding spiro[indene-2,4′-pyrimido[4,5-d]pyrimidine] derivatives 2a,b in quantitative yields (Scheme 1). The structure of pyrimidine derivatives 2a, b was established by IR, 13C NMR, and mass spectra. The 7′-thioxo-7′,8′-dihydrospiro[indene-2,5′-pyrimido[4,5-d][1,3]oxazine]-1,3,4′(6′H)-trione 3 was constructed via the reaction of spiro compound 1 with formic acid. While the reaction of spiro compound 1 with two aromatic aldehydes (4-hydroxy-2-nitrobenzaldhyde and 4-hydroxybenzaldhyde) provided the appropriate Schiff bases 4a, b in excellent yields (Scheme 1). FTIR, H-NMR, and mass spectra were utilized to establish the structure of obtained compounds 3 and 4a,b. The IR spectrum of compound 3 indicated the existence of bands at 1654, 1707 and 1240 due to C⚌O and C⚌S, respectively. The 13C NMR spectrum of compounds 3 revealed signals at 163.05, 163.25, 196.5 and 180.56 ppm for CH⚌N, C⚌O, and C⚌S groups, respectively. The mass spectrum indicated that the compound 3 has a molecule of water due to the presence of a peak at m/z 313 (see Scheme 3).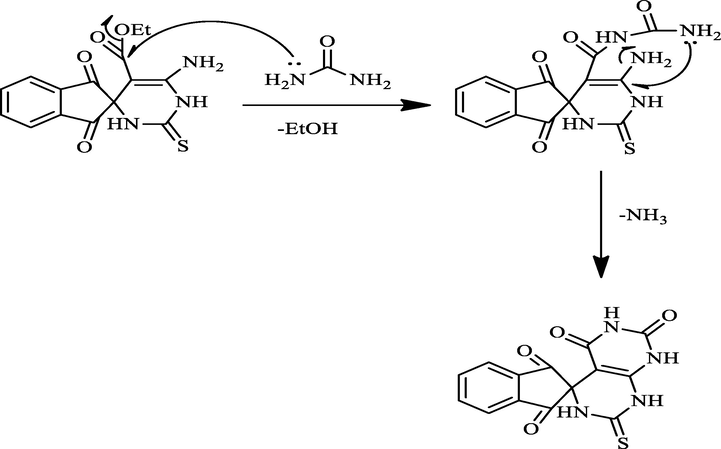
A plausible mechanism for formation of compound 2a.
Treating the spiro compound (1) with one mole equivalent of p-substituted anilines and or 2- aminopyridine in excess of formaldehyde (37%) in acetonitrile at ambient temperature could proceed to give the spiro[indene-2,4′-pyrimido[4,5-d]pyrimidine] (5a-d) or the other isomeric product spiro[indene-2,6′-pyrimido[2,1-b][1,3,5]thiadiazine] 6a-d (Scheme 4). The TLC analysis revealed that the reaction produced only one product 5a-d in high yield, and the other derivatives were undetectable (Scheme 4). The IR spectrum of the product 5a as example is devoid of the C⚌O (ester) absorption peak, instead of showing the C⚌O (amide) peak at 1710 cm−1. Moreover, the 1H NMR spectrum of the compound 5a was distinguished by the presence of one signal at δ = 4.81 ppm, attributed to the methylene group (NCH2N) besides the other protons at the anticipated chemical shifts. Also, the mass spectrum of the isolated compound 5a revealed the required molecular ion peak.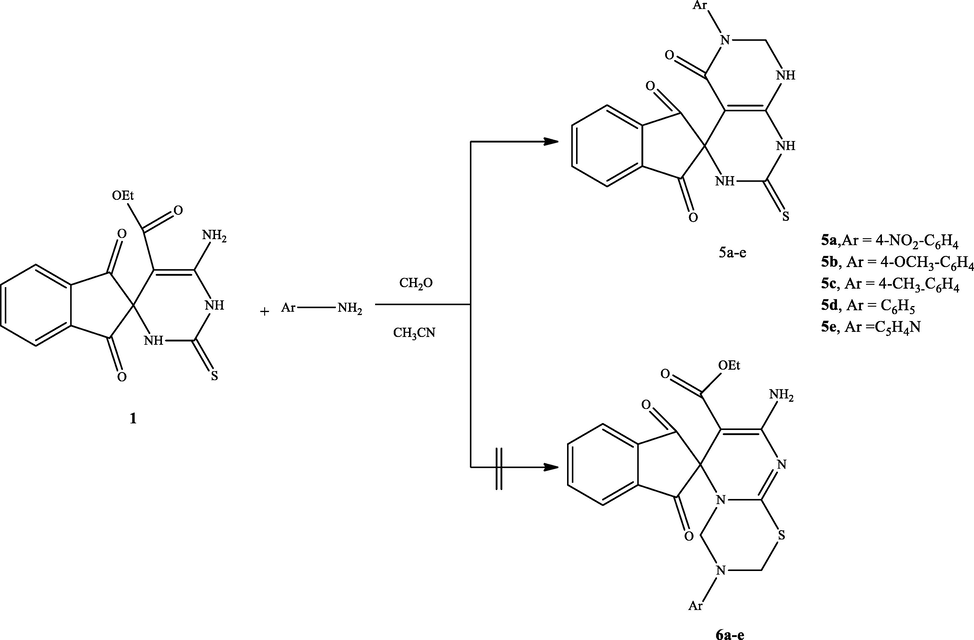
Synthesis of derivative 5a-d.
On the other hand, the hydrazone derivative bearing pyrimidine-2-thiol core 7 was successfully synthesized utilizing the microwave irradiation technique. Treatment of spiro compound 1 with hydrazine hydrate in ethanol produced the corresponding hydrazone 7 in an excellent yield (Scheme 5). IR spectra of hydrazone 7 revealed the existence of bands in the frequency of 1608, 3100–3400 cm−1 corresponding to (C⚌O), (NH, NH2), respectively, 1HNMR spectra indicate the absence of triplet and quartet peak for ethyl ester group, and its elemental analyses were aligned with the calculated values.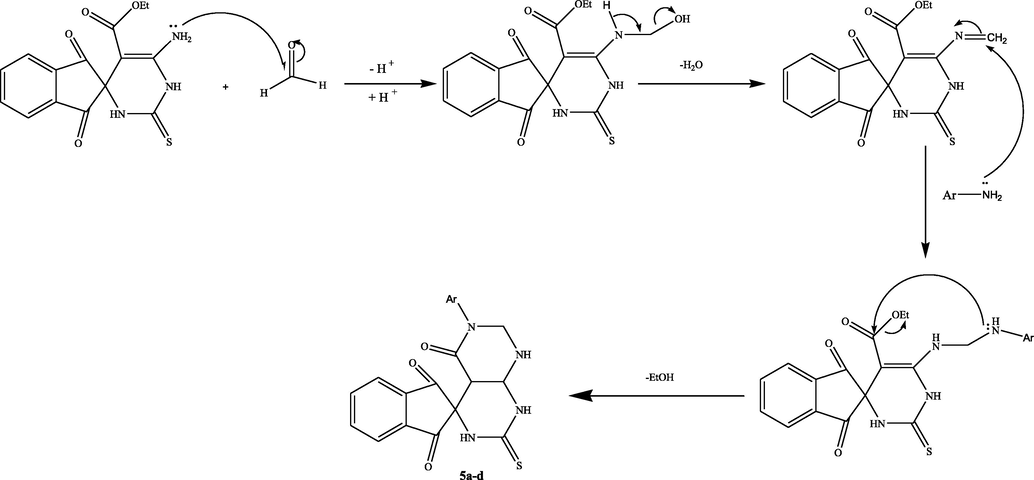
A suggested mechanism for formation of compounds 5a-d.
Reaction of hydrazone 7 and ethyl acetoacetate giving the pyrazole derivative 8 in moderate yield (78%, 8 h) under thermal conditions (Scheme 6). In an attempt to improve the reaction rate and yield, we used microwave irradiation as a sustainable energy source. Consequently, by conducting the aforementioned model reaction under microwave irradiation, both reaction rate and yield were highly improved; the desired product was obtained in 98% within 10 min. The optimistic results of the aforementioned model reaction (Scheme 6) prompted us to study the behaviors of the other active methylene derivatives (Scheme 6). Interestingly, the reaction of hydrazone 7 with ethyl cyanoacetate and / or diethylmalonate afforded the corresponding pyrazole derivatives 9, 10 under microwave conditions in excellent yields (96 and 99%, respectively). The structure of compounds 8, 9 and 10 was established by utilizing NMR, IR, and MS spectra. The IR spectrum of derivative 8 displayed a characteristic band at 1616 cm−1, which was assigned to the carbonyl (C⚌O) stretching frequencies. The 1H NMR spectrum of 8 exhibited one singlet signal at 6.32 ppm which was assigned to the pyrazole-4-proton. These results were supported further by the MS spectrum, which displayed the molecular ion peak at m/z 383 in agreement with the molar mass of the proposed structure.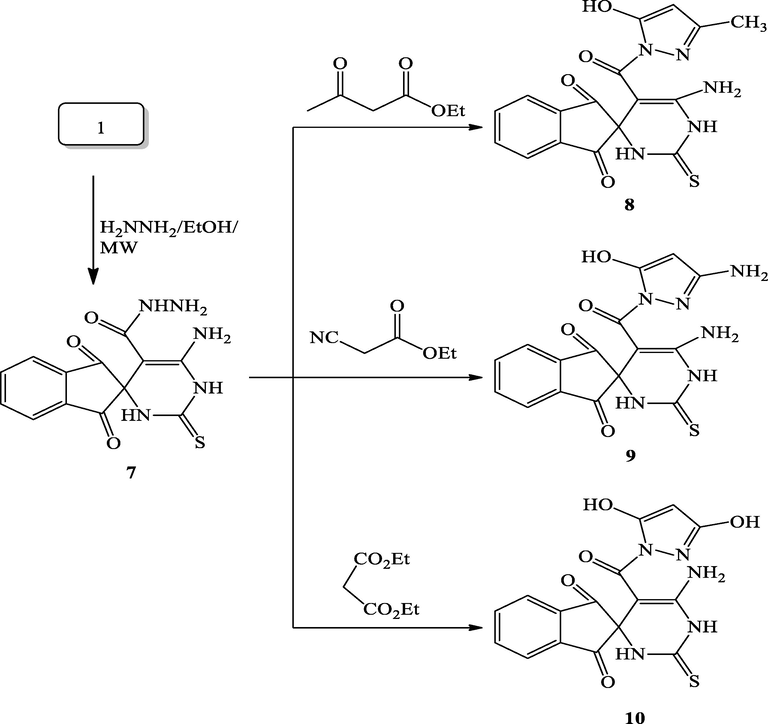
Synthesis of pyrazole derivatives 8–10.
From an industrial standpoint; we carried out a gram-scale assembly of pyrazole derivatives 8 under the optimal condition. Thus, irradiation of 7, and ethyl acetoacetate in ethanol (Scheme 5) for 10 min (TLC) afforded the desired product 8 in super yield (98%). As the result, the current protocol could be employed as a distinguished protocol for the manufacturing design of such pyrazole derivatives. Further, to assess the aforesaid protocol (Scheme 6) on the “greenness” scale, several green parameters including, yield economy, process mass intensity, reaction mass efficiency, E-factor, atom economy, and carbon efficiency (CE), and have been investigated (Tobiszewski et al., 2015). The elevated value of ecological-harmonious criteria, such as Yield Economy (9.80), Carbon efficiency (89.47%), Atom Economy (85.68%), and Reaction Mass Efficiency (83.96%) besides the low values of Process Mass Intensity (1.19) and E-factor (0.02) make the investigated microwave-assisted protocol ideal sustainable green process for construction of pyrazole vs. the conventional (thermal) process when comparable reaction chemistries are applied (Table 1).
Method
Yield (%)
YE (%)
CE (%)
RME (%)
AE (%)
PMI
E-Factor
Thermal
78
0.43
89.47
66.83
85.68
1.49
0.28
MW
98
9.80
89.47
83.96
85.68
1.19
0.02
The synthesis of N-(3′-acetyl-1,3-dioxo-1,3-dihydro-3′H-spiro[indene-2,2′-[1,3,4] thiadiazol]-5′-yl)acetamide 12 from thiosemicarbazone derivative 11 has been obtained by mixing equimolar amounts of thiosemicarbazide and 1,2,3-indenetrione 1, in ethanol/water in the existence of hydrochloric acid as a catalyst in a water bath for 30 min (Scheme 7) following the reported method (Reddy et al., 1985). This compound 11 could also be obtained by the sonication of ninhydrin and thiosemicarbazide in water at room temperature (23 °C). This clean and efficient modified protocol allowed the formation of 11 in 99% within 7 min in pure form. Besides, by applying the ultrasonic irradiation technique as a sustainable energy source, the drawbacks of the reported methods such as harsh reaction conditions, longer reaction time, use of volatile carcinogenic organic solvents, and low yield were avoided. By using an excess amount of acetic anhydride (solvent and reactant), the obtained thiosemicarbazone derivative 11 underwent a cyclization reaction and subsequent acetylation under microwave irradiation into the unreported spiro[indene-2,2′-[1,3,4]thiadiazol]-5′-yl)acetamide derivative 12 in excellent yield (99%) and short reaction time (10 min), (Scheme 7). Interestingly, on performing the aforesaid reaction under ultrasonic irradiation conditions, derivative 12 was obtained in 99% within 10 min at 50 °C (see Scheme 8).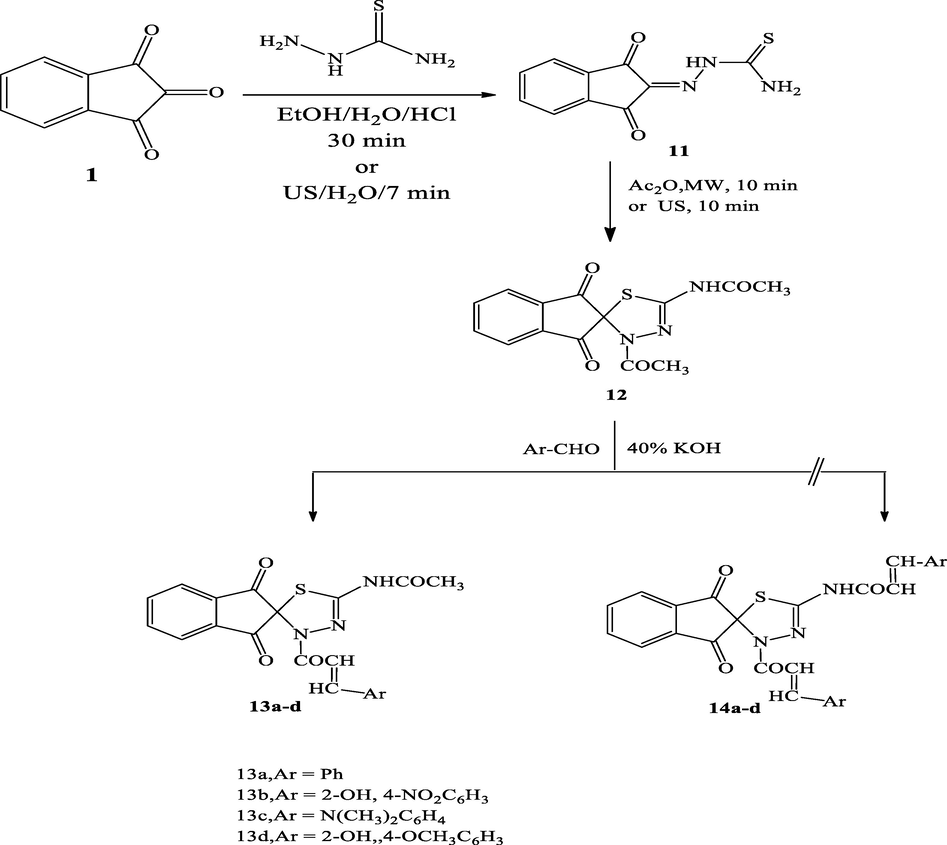
Synthesis of compound (12) and chalcones 13a-d.
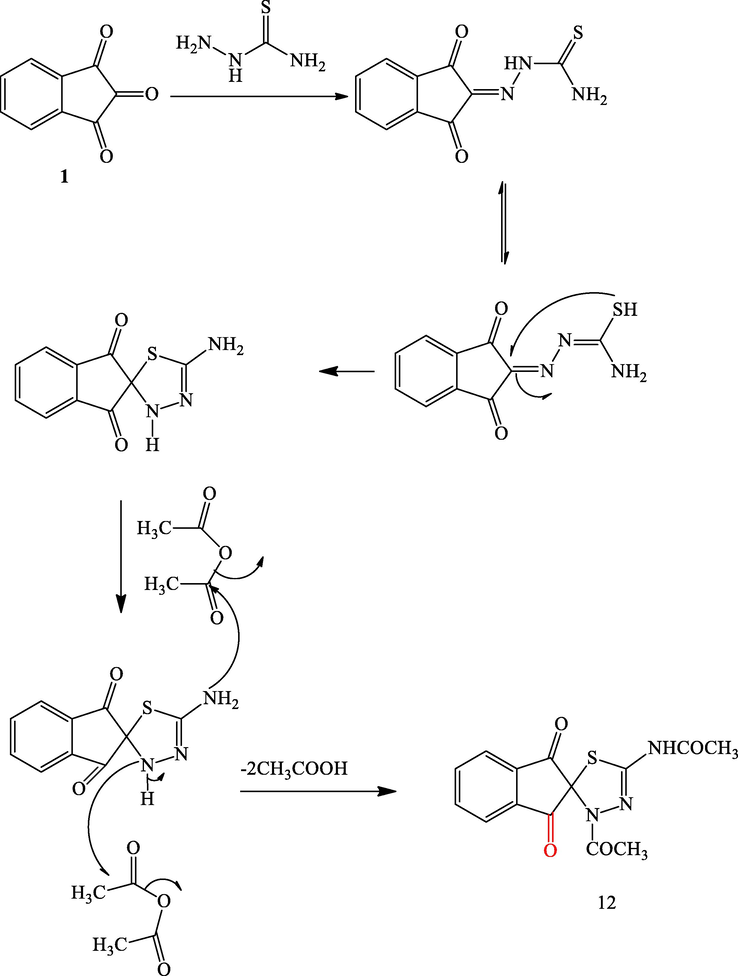
A plausible Mechanism for formation of compound 12.
The IR spectrum of derivative 12 displayed mainly a sharp strong absorption band around 1606 cm−1 which corresponds to the ninhydrin carbonyl groups with the disappearance of the amino (NH2) and thiocarbonyl (C⚌S) bands and appearance of the medium absorption band at about 1716 cm−1 attributed to the stretching of the amide carbonyl groups. The 1H NMR spectral analysis of the obtained compound 12, two characteristic singlet signals at δ = 1.33 and 2.0 ppm assigned to the two methyl groups. In addition, only one singlet at 10.58 ppm is attributed to the NH group, this is strong evidence to prove the cyclization of thiosemicarbazone. In view of the varied biological and pharmacological applications, we synthesized two unreported chalcone derivatives 13a-d using Claisen Schmidt condensation reaction. This reaction was carried out by the reaction of equimolar of the appropriate benzaldehyde with the acetyl derivative 12 in 40% NaOH solutions to produce the corresponding chalcones 13a-d in excellent yields (Scheme 6) while the chalcone 14a-d is not formed under the current reaction condition. The obtained products were characterized by using spectroscopic techniques such as IR and 13C NMR spectra.
On the other hand, we synthesized series chaconne derivatives 13a, b. via reaction between benzaldehyde and the acetyl derivative 12 and were chosen as a template protocol (Scheme 7). Some variables for instance solvents, temperature, catalysts, and energy sources were investigated aiming to obtain the optimal reaction conditions. At first, the mixture of model reactants was refluxed in ethanol (20.0 mL) without adding a catalyst. Even after refluxing the reaction solution over a significant period, the generation of the predicted chalcone (13a) was not recognized under those same conditions (Table 2, entry 1). After charging the model reaction with a 40% NaOH solution as a promoter, a considerable yield (60%) of the intended product 13a was achieved in 3 h (Table 2, entry 2). In repeating the same reaction but by using 40% KOH solution as catalyst instead of NaOH, the reaction rate slightly increased (Table 2, entry 3). The conversion of acetyl derivative 12 to the target chalcone 13a was enhanced to 80% by sonicating the template reactants at room temperature (23 °C) for 15 min (Table 2, entry 4). The performance of the reaction was drastically reduced in solvents such as dioxane and DMF, generating the necessary product 13a in lower yields however after 1 h of sonication (Table 2, entries 5 and 6). Water outperformed the other solvents evaluated for the present catalytic reaction (Table 2, entry 7). Diverse operational temperatures were utilized to enhance the efficiency of the current ultrasound-assisted protocol. The catalyst (KOH) successfully converted the modeled materials to the intended product 13a, 94%) in 10 min by elevating the temperature to 35 °C (Table 2, entry 8). It might be because the produced bubbles, which comprised catalyst and reactants, burst with significant energy to enhance both reaction yield and rate (Andraos, 2006). Surprisingly, increasing the temperature to 40 °C resulted in the required product 13a being produced in perfect yield (99%) in just 8 min (Table 2, entry 9). Such finding is based on the fact that when the temperature of the reaction mixture increased, the viscosity and surface tension of the reaction mixture decreased, promoting the formation and collapse of bubbles (Protti et al., 2007). Surprisingly, at temperatures exceeding 50 °C, both reaction yield and rate are moderately reduced (Table 2, entries 10 and 11). At higher temperatures, additional bubbles were formed, which act as a barrier to the waves being successfully transferred across the reaction solution, affecting the conversion marginally (Protti et al., 2007). The time influence also was investigated, and the best duration was found to be 7 min.
Entry
Method
Catalyst
Solvent
Temp. (oC)
Time (min)
Yield (%)
Silent
---
EtOH
100
180
0
Silent
NaOH (40%)
EtOH
100
180
60
Silent
KOH (40%)
EtOH
100
180
71
US
KOH (40%)
EtOH
rt(23)
15
80
US
KOH (40%)
Dioxane
rt(23)
60
65
US
KOH (40%)
DMF
rt(23)
60
62
US
KOH (40%)
H2O
rt(23)
15
90
US
KOH (40%)
H2O
35
10
94
US
KOH (40%)
H2O
40
8
99
US
KOH (40%)
H2O
50
7
96
US
KOH (40%)
H2O
60
7
96
US
KOH (40%)
H2O
40
7
99
To assess the feasibility of the current approach, the model reaction was carried out on a gram scale using the optimum conditions, yielding the required product 13a in outstanding yield (98%, Scheme 7). Besides, diverse green measures such as Process Mass Intensity (PMI), Carbon Efficiency (CE), Reaction Mass Efficiency (RME), Atom Economy (AE), and E-factor (EF) were also studied (Scheme 7) to investigate the present method in order of waste and carbon efficiency (Sheldon et al., 2007). The increased ecological suitability criteria, such as lower PMI (1.05) and EF (0.05) values, along with higher values of YE (14.14%), CE (100%), AE (95.74%), and RME (95.01%), verified the established protocol's eco-friendly strategy.
The optimal conditions were then employed for the assembly of series of chalcones using several aldehydes. Different benzaldehydes worked successfully in the reaction and produced high yields of the chalcones. Despite this, aromatic aldehydes with electron-donating groups 13c, d yielded marginally less than those with electron-withdrawing groups 13b. This observation may be owing to o-substituted benzaldehydes 13b; d gave the required chalcone in slightly reduced yields, than the p-substituted derivative 13c, probably due to steric factors. The chalcone derivatives were recrystallized from a proper solvent and characterized by using spectroscopic techniques such as FT-IR and NMR. The FT-IR spectrum of compound 13a displayed bands at 1600, 1683, 2981, 3070 cm−1 corresponding to (C⚌C), C⚌O, Ar-H and, NH, respectively. The 1H NMR spectrum of derivative 13a afforded signals at 1.29 and 10.23 ppm for CH3, and NH, respectively. Treating the enones 13a,b with phenylhydrazine which might be furnished either 4,5-dihydro-1H-pyrazoles 15a,b or dehydrated products 16a,b. Interestingly, the cyclocondensation reaction of chalcone derivatives 13a,b with phenylhydrazine under refluxing conditions in ethanol afforded the unreported 4,5-dihydro-1H-pyrazoles 15a,b as the major products (TLC) without the detection of dehydrated derivatives 16a,b (Scheme 9). This observation might be explained based on the fact that the formation of 4,5-dihydro-1H-pyrazoles 15a,b, were likely due to the electronic conjugation between the nitrogen atom (N1) lone pair of the pyrazoline-ring and the π-system of the phenyl motif that making the aromatization of the pyrazole ring quite complicated (Buriol et al., 2010). The structures of 15a, b were confirmed by IR, 13C NMR analysis.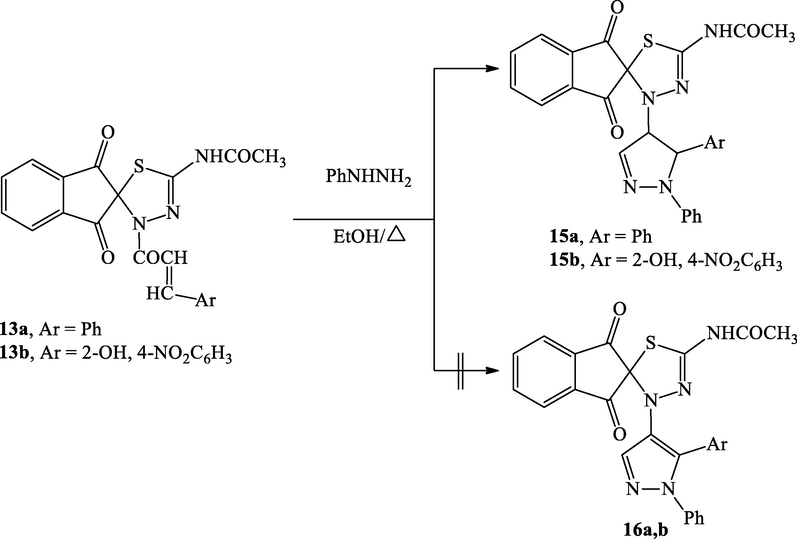
Synthesis of pyrazole derivatives (15a, b).
3.2 Antifungal potential
3.2.1 Antifungal activity and structure–activity relationship (SAR)
The newly synthesized compounds 1, 2a,b, 3, 4a,b, 5a-e, 7, 8, 9, 10, 12, 13a-d, and 15a,b were assayed in-vitro for their antifungal activity towards Fusarium oxysporum, Metalaxyl was used as a reference to evaluate the potency of the tested compounds under the same conditions Fig. S3. The mean inhibition zone diameters (MIZ, mm/mg sample) (n = 3) were determined as a parameter of the antifungal activity, the antifungal results are illustrated in Fig.S3, recorded that all the novel compounds exhibited antifungal potential against Fusarium oxysporum fungi. The compounds 1 (MIZ = 80 mm), 7 (MIZ = 80 mm), and 4a (MIZ = 75 mm) exhibited the highest antifungal effect and revealed a comparable potential to that shown by Metalaxyl. The structure–activity relationship (SAR) of the newly synthesized compounds 1, 7, and 4a suggested that the withdrawing effect of the substituent groups in the synthesized compounds induced an increase in their antifungal activity compared to the others. For instance, the presence of carbonyl group as substituent attached directly to pyrimidine ring in compounds 1, 7 and 4a as electron-withdrawing groups showed a significant improvement against Fusarium oxysporum fungi. Compound 5a (MIZ = 64 mm) showed moderate effect due to the presence of the nito group replacement of the hydrogen atom present at the phenyl group. The results showed that the presence of electron-donating groups such as methyl, amino group, hydroxyl group at the pyrazole ring in compounds 8 (MIZ = 45 mm), 9 (MIZ = 50 mm) and 10 (MIZ = 38 mm) decreased their antifungal activities towards Fusarium oxysporum fungi.
3.2.2 Antifungal studies
On the other hand, we studied the efficacy of new synthetic chemical compounds against tomato damping-off disease caused by Fusarium oxysporum. The results indicated that the combination of six chemical compounds in the culture medium showed that the compound 1 has the strongest effect (86.5%), followed by 7, 4a and 5a-d which have a percentage of (81.2%), (78.8%) and (76.4%) respectively. In addition, results showed that 3 and 4b of a lowest effect on the inhibition of Fusarium oxysporum. These chemical compounds showed an effective antifungal activity against Fusarium oxysporum .The chemical compound 12 showed lowest antifungal effect on Fusarium oxysporum (45%).While, chemical compound of 5a, 5b, 5c, 13a, and 13b, have no any antifungal effect on Fusarium oxysporum. These results indicated that F. oxysporum was probably more sensitive to the chemical compounds 1, 7, 4a and 5a than chemical compound 12. Moustafa and Elkanzi, 2021 evaluated the effect of Pyrazolo pyrimidine against P. aphanidermatum, which isolated from olive feeder roots. in addition study the ability of pyrazolo pyrimidine to decline the production of zoo-spores and oospores of the fungus. their results observed that the synthetic chemical compound of pyrazolo pyrimidine inhibited mycelial growth and production of zoo- and oospores) of P. aphanidermatum, The highest antifungal activity observed in pyrazolo pyrimidine is probably due to the presence of methyl group.so in this study the minimum inhibitory concentrations (MIC) was carried out to compare the effect of six chemical compound against F. oxysporum. Our in vitro tests showed that the treatments with 8, 9, 10, 2a, 2b, 3, 4b, and 12 have no effects on the mycelial growth of F. oxysporum exposed to 10, 15, 25, and 50 mg/ml concentrations. Only chemical compounds of 1 and 7 were effective at low concentrations (10 mg/ml) 42 ± 0.24 and 42 ± 0.24, respectively as compared to chemical compound 4a and 5a 38 ± 0.24 and 20 ± 0.1, respectively. However, higher concentrations (100 mg/ml) of chemical compound 1 and 7 reduced mycelial growth of F. oxysporum (80 ± 0.14, and 80 ± 0.14), respectively. In the comparison of the inhibitory effect of the various chemical compounds (9, 8, 10, 4b, 4a, 3, 4b and 12) higher concentrations with 100 mg/ml reduced mycelial growth to 50 ± 0.14, 45 ± 0.1, 38 ± 0.3, 38 ± 0.2, 38 ± 0.12, 25 ± 0.2, 25 ± 0.2 and 42 ± 0.12, respectively (Table 3). The results of the present study demonstrated that MIC (25 µg/ml) of chemical compound 1 and 7 reduced mycelial growths in vitro; the percentage of reduction varied between 42 and 42% as compared to the control. Numerous formulated chemical compounds were shown to effectively reduce the pre damping off disease caused by of F. oxysporum and increase symptomless plant stand in controlled trials. The suppression of F. oxysporum development in the agar bottles corresponds with the ability of these chemical compounds to reduce growth of Fusarium in agar bottles (Table 4 & fig. 8). The inhibitory effect of chemical compound 1 on fungal hyphal morphology was clearly detected by using Scanning electron microscopic. SEM images from the zone of interaction in culture showed the loss of structural integrity of the mycelium. A variety of aberrant features such as hyphal perforation, lysis, fragmentation and degradation of mycelia were observed in chemical compounds 1, 7, 4a and 5a. Higher concentrations (100 µg/ml) of chemical compound showing abnormal characters in F. oxysporum (fig 7).
New synthetic chemical compound
Average of Diameter of inhibition zone / mm
Mycelial growth inhibition (%)
Metalaxyl (control)
85 ± 0.2
96.50
DMSO (control)
0
0
1
80 ± 0.21
94.1
4a
75 ± 0.1
88.2
7
80 ± 0.13
94.1
5a
64 ± 0.20
75.3
9
50 ± 0.56
58.8
8
45 ± 0.13
52.9
10
38 ± 0.21
44.7
4b
38 ± 0.10
44.7
2a
38 ± 0.13
44.7
3
25 ± 0.22
29.4
4b
25 ± 0.5
29.4
5b
0
0
5c
0
0
5d
0
0
5e
0
0
12
42 ± 0.14
49.4
13a
0
0
13b
0
0
Chemical compounds
Average of diameter of inhibition zone (100 µg/mL)
Average of diameter of inhibition zone (50 µg/mL)
Average of diameter of inhibition zone (25 µg/mL)
Average of diameter of inhibition zone (15 µg/mL)
Average of diameter of inhibition zone (10 µg/mL)
DMSO (control)
0
0
0
0
0
Metalaxyl (control)
85 ± 0.14
60 ± 0.20
45 ± 0.16
45 ± 0.1
45 ± 0.18
1
80 ± 0.14
57 ± 0.14
42 ± 0.09
42 ± 0.18
42 ± 0.24
4a
75 ± 0.14
50 ± 0.14
38 ± 0.09
38 ± 0.18
38 ± 0.24
7
80 ± 0.14
57 ± 0.14
42 ± 0.09
42 ± 0.18
42 ± 0.24
5a
64 ± 0.01
33 ± 0.4
20 ± 0.12
18 ± 0.16
20 ± 0.1
9
50 ± 0.14
20 ± 0.18
10 ± 0.20
5 ± 0.1
5 ± 0.16
8
45 ± 0.1
18 ± 0.12
10 ± 0.22
10 ± 0.14
5 ± 0.2
10
38 ± 0.3
10 ± 0.1
7 ± 0.12
7 ± 0.12
7 ± 0.12
2b
38 ± 0.2
12 ± 0.1
5 ± 0.24
5 ± 0.3
5 ± 0.2
2a
38 ± 0.12
12 ± 0.23
5 ± 0.18
5 ± 0.1
5 ± 0.12
3
25 ± 0.2
10 ± 0.12
5 ± 0.12
5 ± 0.11
5 ± 0.18
4b
25 ± 0.2
10 ± 0.2
5 ± 0.18
5 ± 0.1
5 ± 0.2
12
42 ± 0.12
17 ± 0.18
8 ± 0.2
8 ± 0.2
8 ± 0.18
Several studies have reported that chemical compound applications can suppress diseases caused by several pathogens (Boumaaza et al., 2015). The chemicals documented as generally regarded as safe (GRAS) by the United States Food and Drug Administration (FDA) are considered as non-toxic since wide historical use did not cause any health hazards. Salts such as sodium benzoate, sodium methylparaben, sodium bicarbonate and potassium sorbate are considered as GRAS and their antifungal activity can be applied as part of post-harvest. However, GRAS compounds are generallnot considered as BCAs. In spite of they generally influence fungal cell membrane permeability and fungal nutrient transport but are nontoxic to humans and can be utilized as part of organic farming (Ons et al., 2020). The combined utilized of GRAS antagonists and fungicides, the addition of sodium bicarbonate to imazalil importantly improved its activity against both fungicide-sensitive and fungicide-resistant isolates of fungi on harvested lemons (Smilanick et al., 2005). Kanetis et al. (Moustafa and Elkanzi, 2021) mentioned that the efficiency of fungicides such as azoxystrobin, fludioxonil or pyrimethanil is significantly increased when mixed with GRAS sanitizers such as sodium bicarbonate when treating citrus green mold.
3.2.3 Isolation and molecular identification of damping-off fungi from infected tomato plants
F. oxysporum was one of the important pathogenic fungi present in soil observed adhering to tomato roots grown in the field studied. Identification of F. oxysporum was done based on morphological criteria and molecular identification according to (Rahjoo et al., 2008) (Fig. 2). The sequence of the pathogenic fungi (N. JF 505) was closely related to F. oxysporum (Genbank accession number, MW830121) with 100% similarity (Fig. 3). (
https://www.ncbi.nlm.nih.gov/nuccore/MW830121.1?report=GenBank
).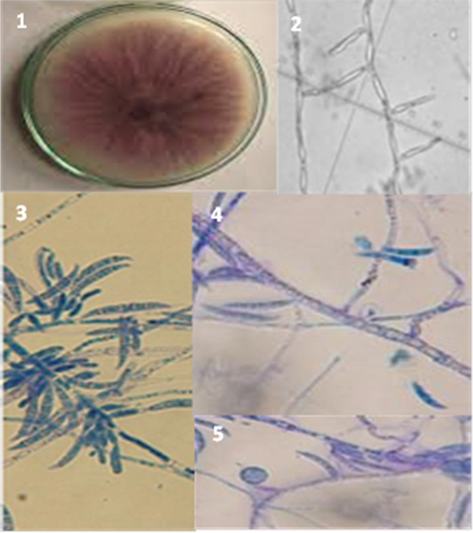
Cultural and morphological structures of Fusarium oxysporum. (1) Colony aspect on PDA medium. (2) Monophialides. (3) Macroconidia. (4) Macroconidia. (5) Chlamydospores.

Sequence analysis of the ITS1-5.8S rRNA–ITS2 region of F. oxysporum.
3.2.4 The effect of tested chemical compounds on the mycelial growth of Fusarium oxysporum
Measuring of the inhibition zones around each tested synthetic chemical compound against F. oxysporum indicated that some of these chemical compounds showed inhibition zones around F. oxysporum growth on PDA medium at 27 °C in the dark. The compounds 1 and 7 displayed the strongest effect (80.5%), followed by, 4a and 5a were (75%) and (64 %) respectively. In addition, results showed that 3 and 4b having the lowest effect on the inhibition of pathogenic isolated fungus. Other chemical compounds showed inhibition zones between 40 and 25 mm (Table 3, Fig. S1).
The minimum inhibitory concentrations (MIC) were determined and are given in Table 4; figure (S2, S3). The most active compound was 1, which presents against F. oxysporum, has value 25 µg/mL.
3.2.4.1 Micrograph of Fusarium oxysporum grown in tested chemical compound concentrations
The scanning electron microscopy analysis revealed that tested chemical compound (1) 25 µg/mL induced marked effects on the morphology and density of mycelium of F. oxysporum. Fig. S4 shows a greater morphological change as deformation of the mycelium perforation, lysis of cell and destruction of both mycelium and conidia of F. oxysporum; when compared to the control. While the Effect of adding tested chemical compounds on pre-emergence damping-off by F. oxysporum- In agar bottles indicate that Controlling damping-off of tomato seeds caused by F. oxysporum, by different new chemical compounds add to 2% water agar medium were showed in (Tables 5, fig S5, S6). All tested chemical compounds reduced the pre damping off disease caused by F. oxysporum, but in different ranges. The effective tested chemical compounds were 1 which improved seeds emergence when subjected to the tested pathogenic fungi and reduced the disease to 94%; chemical compounds 4a and 5a reduced the disease to 88%. The least chemical compounds were 3 and 4b which reduced the disease to only 61%. *Standard error for 3 replicates.
Treatment
Percentage of germination (PG)
Percent disease incidence (PDI)
Control : seeds without pathogenic fungi
100%
0%
Control : seeds with pathogenic fungi only
13%
86%
Seeds with Pathogenic fungi + 1
94%
7%
Seeds with Pathogenic fungi + 4a
88%
13%
Seeds with Pathogenic fungi + 7
83%
17%
Seeds with Pathogenic fungi + 5a
88%
12%
Seeds with Pathogenic fungi + 9
77.8%
25%
Seeds with Pathogenic fungi + 8
77.8%
24%
Seeds with Pathogenic fungi + 10
66%
35%
Seeds with Pathogenic fungi + 2b
66%
35%
Seeds with Pathogenic fungi + 2a
66%
35%
Seeds with Pathogenic fungi + 3
61%
42%
Seeds with Pathogenic fungi + 4b
61%
45%
Seeds with Pathogenic fungi + 12
72.2%
28%
3.3 Docking study
Succinate dehydrogenase (EC 1.3.5.1) is involved in Krebs cycle and respiration chain via catalysing the oxidation of succinate to fumarate in the mitochondrial matrix (Pollard et al., 2003; (Yu et al., 2020). SDH is considered as a promising target for agrochemical discovery due to its essential role in life processes (Guan et al., 2014; (Jeschke, 2018).
Molecular docking analysis was performed within succinate dehydrogenase (SDH) as a target enzyme in order to rationalize the promising findings obtained for the active compounds 1, 2a,b, 5a, 5b-d, 7, 8, 9, 10, 12, 13a using MOE, 2008, Chemical Computing Group Inc. The crystal structure of SDH with the cocrystallized ligand was downloaded from Protein Data Bank (code: 2FBW). The cocrystallized ligand (malate like intermediate) was redocked within SDH with root mean standard deviation (RMSD) = 1.5707. The main binding interactions resulted in hydrogen bondings with Ala411, Arg408, His364, Arg297, His253, Glu266, Thr265 and Gly62 (Fig. 4).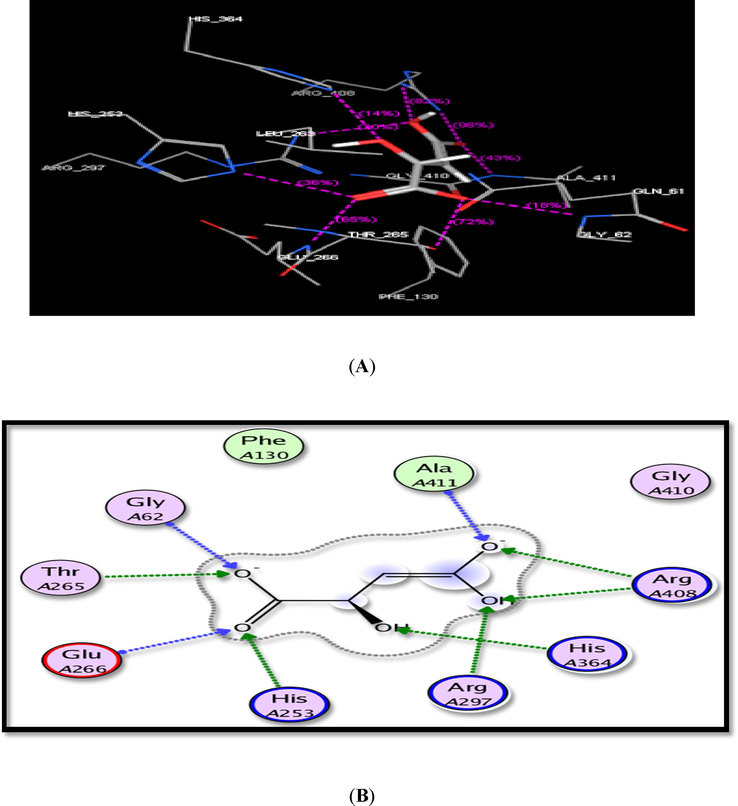
The suggested binding of the cocrystallized ligand within SDH active site. A) 3D, B) 2D binding interaction.
The formed hydrogen bonds and binding energy scores obtained from docking study are recorded in Table 6. From the docking results, compound (1) displayed the highest binding energy (-16.34 kcal/mol) with His56, Glu397, Ser413, Arg408 and His364 through binding with ester C⚌O, 2NH of pyrimidine ring and indene C⚌O (Fig. 5).
Compound
Affinity Kcal/mol
No. of hydrogen bonds
Distance (Å) from the main residue
Functional group
1
−16.34
5
His56
2.97
Ester C⚌O
Glu397
2.27
NH
Ser413
3.07
NH
Arg408
2.99
Indene C⚌O
His364
3.01
Indene C⚌O
7
−15.99
6
His364
2.98
Hydrazide C⚌O
Gln61
2.75
Indene C⚌O
Arg297
2.56
Hydrazide C⚌O
Arg408
3.05
Hydrazide C⚌O
Ala411
3.11
NH2
Arg408
3.22
NH2
2b
−12.65
3
Arg408
2.98
Pyrimidine C⚌O
Arg297
2.51
Pyrimidine C⚌O
His364
3.17
Pyrimidine C⚌O
2a
−12.34
3
Ser413
2.98
Pyrimidine C⚌O
His364
3.11
Pyrimidine C⚌O
Arg408
2.92
Pyrimidine C⚌O
8
−14.19
5
His56
3.24
OH
Thr224
3.05
OH
His56
2.98
NH2
Arg408
2.72
C⚌O
His364
3.11
C⚌O
9
−15.09
5
His56
2.59
Pyrimidine NH2
Thr224
2.84
Pyrazole NH2
Ala59
2.98
OH
His364
3.04
Indene C⚌O
Arg408
3.15
Indene C⚌O
10
−11.87
4
Arg408
2.35
Amide C⚌O
Arg408
2.94
Indene C⚌O
Ser413
2.80
NH2
His364
2.77
Indene C⚌O
5a
−15.78
3
Arg408
2.98
C⚌O
Arg297
2.78
C⚌O
His364
3.09
C⚌O
5b
−12.59
2
Gly62
Thr2653.09
2.93OCH3
OCH3
3
−11.45
3
Gln61
3.22
Oxazine C⚌O
‘His364
Arg4082.98
2.83Indene C⚌O
Indene C⚌O
12
−14.84
4
His364
2.85
Indene C⚌O
Arg408
2.74
Acetyl C⚌O
Ala411
2.94
Acetyl C⚌O
Ser413
3.06
Amide C⚌O
Cocrystallized Ligand
−15.40
9
Gly62
2.58
OH
Thr265
2.56
OH
Glu266
2.99
C⚌O
His253
2.93
C⚌O
Arg297
3.06
OH
His364
3.18
OH
Arg408
2.98
OH
Arg408
2.63
C⚌O
Ala411
2.63
OH
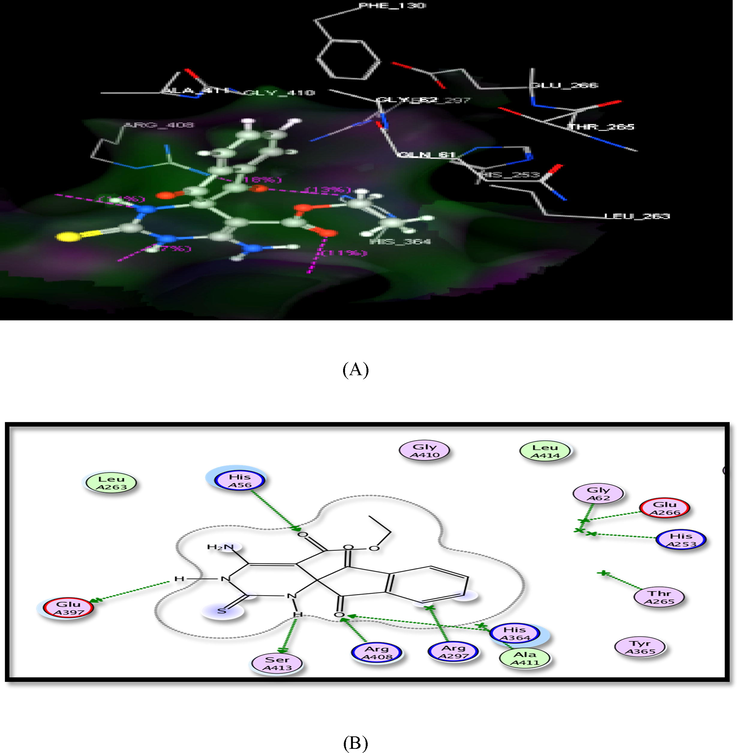
The suggested binding of compound 1 within SDH active site. A) 3D, B) 2D binding interaction.
Furthermore, compound (7) exhibited good fitting with SDH binding site with binding energy score = -14.99 kcal/mol. In addition, this candidate (7) revealed six H-bonds as followings (Fig. S7):
-
His364 with hydrazide C⚌O
-
Gln61 with indene C⚌O
-
Arg297 with hydrazide C⚌O
-
Arg408 with hydrazide C⚌O
-
Ala411 with NH2
-
Arg408 with NH2
Compound 5a recorded three H bonds with Arg408, Arg297 and His364 through binding with indene C⚌O moiety, in addition to the formation of arene cation interaction between Arg297 and the phenyl moiety (Fig. S8). The target compound 2b formed three H-bonds with Arg297, Arg408 and His364 through interaction with pyrimidine C⚌O. This candidate 2b displayed also two arene cation interactions with Arg408 and His364 with binding energy score = -12.65 Kcal/mol (Fig. S9). Furthermore, compound 2a displayed arene cation interaction with phenyl ring. Also, it showed three H-bonds as follows: i) His364 with C⚌O, ii) Arg408 with C⚌O and iii) Ser413 with C⚌O (Fig. S10). In addition, the target compound 8 showed five H-bonds with His56, Thr224, Arg408 and His364 amino acids with binding energy score equals to −15.29 Kcal/mol (Fig. S11). Moreover, derivative 9 showed good fitting inside SDH enzyme displaying five hydrogen bonds as follows: i) His56 with pyrimidine NH2, ii) Ala59 with OH, iii) Thr224 with pyrazole NH2, iv) His364 with indene C⚌O, and vi) Arg408 with indene C⚌O. in addition, the benzene ring of indene formed arene cation interaction with Arg408 (Fig. S12). The candidate 10 recoded binding energy score = -11.39 kcal/mol and the pyrazole moiety formed hydrophobic interaction with His364. Also, this candidate 10 demonstrated four H-bonds with Arg408, Ser413, and His364 through binding with carbonyl moieties and amino group (Fig.S13). On the other hand, the candidate 5b revealed only two H-bonds with Gly62 and Thr265 via binding with methoxy group and exhibited also arene cation interaction with the phenyl moiety (Fig. S14). On the other hand, the target compound 3 displayed three H-bond with Arg408, His364 and Gln61 through carbonyl moieties with binding energy = -11.45 kcal/mol (Fig. S15). Finally, the thiadiazole candidate 12 displayed good fitting inside SDH active site with binding energy score = -14.84 kcal/mol. Furthermore this compound showed four H-bonds with His364, Ala411, Arg408, and Ser413 in addition to formation arene cation interaction between Arg297 and the benzene moiety (Fig. S16).
In conclusion, all the docked target compounds displayed good binding within succinate dehydrogenase enzyme with docking scores = -16.34- −11.45 kcal/mol comparing with the cocrystallized ligand which recorded binding energy score = -15.40 kcal/mol). Furthermore, all the compounds showed 2 to 6 hydrogen bonds within the binding site. Moreover, compounds 1 and 7 (the most active candidates) revealed the best docking scores (-16.34 and −15.99 kcal/mol, respectively) and recorded 5 and / or 6 hydrogen bonds within the active site. In addition, compound 3 (the least active candidate) demonstrated the least docking score = -11.45 kcal/mol and formed 3 hydrogen bonds. These outcomes were in agreement with that recorded by antifungal studies.
4 Conclusion
Sustainable and simple preparation of spiro [indene-2,4′-pyrimidine]-5′-carboxylate 1 was achieved by microwave irradiation of ninhydrin, thiourea, and ethyl cyanoacetate. The target compound 1 had been characterized by FT-IR and NMR, and Mass spectra. An excellent reaction yield (96%) was obtained in the presence of potassium carbonate and irradiation with microwave for 20 min. Also, pyrazole derivatives (8, 9, and 10) were prepared in excellent yields (98, 96, and 99%, respectively) via microwave irradiation of derivative 7 with ethyl acetoacetate, ethyl cyanoacetate, and diethyl malonate. The green synthesis of the spiroacetamide derivative (12) via acetylation of thiosemicarbazone derivative 11 under microwave irradiation provided 12 in an excellent yield (99%). Besides, chalcone 13a-d was prepared in excellent yield via ultrasonic irradiation of 12 with aromatic aldehydes. The reaction of compounds 13a-b with phenylhydrazine provided 15a-b. The molecular docking study of the synthesized compound indicated the active compounds are 1, 2a, 2b, 5a, 5b-d, 7, 8, 9, 10, 12, and 13a. Ethyl 6′-amino-1,3-dioxo-2′-thioxo-1,2′,3,3′,5′,6′-hexahydro-1′H-spiro[indene-2,4′-pyrimidine]-5′-carboxylate 1 is considered as new effective regulators of vegetative growth of tomato depending on their evaluation against tomato damping-off disease caused by Fusarium oxysporum.
Acknowledgements
The financial support from Deanship of graduate studies at Jouf University for funding and supporting this research, through the initiative of DGS, Graduate students research support (GSR) at Jouf university, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Anti-inflammatory, analgesic and anti-ulcerogenic activities of novel bis-thiadiazoles, bis-thiazoles and bis-formazanes. Med. Chem.. 2017;13:226-238.
- [Google Scholar]
- New pyrimidine-benzoxazole/benzimidazole hybrids: Synthesis, antioxidant, cytotoxic activity, in vitro cyclooxygenase and phospholipase A2-V inhibition. Bioorg. Chem.. 2019;92:103218.
- [Google Scholar]
- Novel pyrimidine-pyridine hybrids: synthesis, cyclooxygenase inhibition, anti-inflammatory activity and ulcerogenic liability. Bioorg. Chem.. 2018;77:339-348.
- [Google Scholar]
- Pyrimidine and fused pyrimidine derivatives as promising protein kinase inhibitors for cancer treatment. Med. Chem. Res. 2020:1-19.
- [Google Scholar]
- Note biological control of Fusarium wilt in tomato by plant growth-promoting yeasts and rhizobacteria. Plant Pathol. J.. 2009;25:199-204.
- [Google Scholar]
- Tomato yellow leaf curl virus (TYLCV), identification, virus vector relationship, strains characterization and a suggestion for its control with plant extracts in Iraq. Afr. J. Agric. Res.. 2011;6:5149-5155.
- [Google Scholar]
- Design, Synthesis, and In Vitro Cytotoxic Activity of Certain 2-[3-Phenyl-4-(pyrimidin-4-yl)-1 H-pyrazol1-yl] acetamide Derivatives. Russ. J. Org. Chem.. 2020;56:514-520.
- [Google Scholar]
- On using tree analysis to quantify the material, input energy, and cost throughput efficiencies of simple and complex synthesis plans and networks: Towards a blueprint for quantitative total synthesis and green chemistry. Org. Process Res. Dev.. 2006;10:212-240.
- [Google Scholar]
- B Bakr, R., BM Mehany, A., RA Abdellatif, K., 2017. Synthesis, EGFR Inhibition and Anti-cancer Activity of New 3, 6-dimethyl-1-phenyl-4-(substituted-methoxy) pyrazolo [3, 4-d] pyrimidine Derivatives. Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Cancer Agents) 17, 1389–1400.
- Thiochromene candidates: design, synthesis, antimicrobial potential and in silico docking study. J. Iranian Chem. Soc. 2021:1-11.
- [Google Scholar]
- Preparation of some novel thiazolidinones, imidazolinones, and azetidinone bearing pyridine and pyrimidine moieties with antimicrobial activity. J. Heterocycl. Chem.. 2020;57:2977-2989.
- [Google Scholar]
- Synthesis, MOLECULAR DOCKING STUDIES AND IN VITRO ANTIMICROBIAL EVALUATION OF NOVEL PYRIMIDO [1, 2-A] QUINOXALINE AND TRIAZINO [4, 3-a] Quinoxaline derivatives. Heterocycles: Int. J. Rev. Commun. Heterocyclic Chem.. 2018;96:1941-1957.
- [Google Scholar]
- Benzylidine indane-1, 3-diones: As novel urease inhibitors; synthesis, in vitro, and in silico studies. Bioorg. Chem.. 2018;81:658-671.
- [Google Scholar]
- Boumaaza, B., Benkhelifa, M., Belkhoudja, M., 2015. Effects of two salts compounds on mycelial growth, sporulation, and spore germination of six isolates of Botrytis cinerea in the western north of Algeria. Int. J. Microbiol., 2015.
- Pyrazole synthesis under microwave irradiation and solvent-free conditions. J. Braz. Chem. Soc.. 2010;21:1037-1044.
- [Google Scholar]
- Campos, M.D., Félix, M.d.R., Patanita, M., Materatski, P., Varanda, C., 2021. High throughput sequencing unravels tomato-pathogen interactions towards a sustainable plant breeding. Horticult. Res., 8.
- Design, synthesis and biological evaluation of indane-2-arylhydrazinylmethylene-1, 3-diones and indol-2-aryldiazenylmethylene-3-ones as β-amyloid aggregation inhibitors. Eur. J. Med. Chem.. 2010;45:1359-1366.
- [Google Scholar]
- de Oliveira, A.S., Gazolla, P.A., Oliveira, A.F.C.d.S., Pereira, W.L., de S. Viol, L.C., Maia, A.F.d.S., Santos, E.G., da Silva, Í.E., Mendes, T.A.d.O., da Silva, A.M., 2019. Discovery of novel West Nile Virus protease inhibitor based on isobenzonafuranone and triazolic derivatives of eugenol and indan-1, 3-dione scaffolds. PloS one 14:e0223017.
- El-Naggar, M., Sallam, H.A., Shaban, S.S., Abdel-Wahab, S.S., E Amr, A.E.-G., Azab, M.E., Nossier, E.S., Al-Omar, M.A., 2019. Design, synthesis, and molecular docking study of novel heterocycles incorporating 1, 3, 4-thiadiazole moiety as potential antimicrobial and anticancer agents. Molecules 24, 1066.
- Microwave Assisted, Antimicrobial Activity and Molecular Modeling of Some Synthesized Newly Pyrimidine Derivatives Using 1, 4-diazabicyclo [2.2. 2] octane as a Catalyst. Lett. Drug Des. Discovery. 2020;17:1538-1551.
- [Google Scholar]
- Design, Synthesis, Molecular Modeling Study, and Antimicrobial Activity of Some Novel Pyrano [2, 3-b] pyridine and Pyrrolo [2, 3-b] pyrano [2.3-d] pyridine Derivatives. J. Heterocycl. Chem.. 2019;56:406-416.
- [Google Scholar]
- Synthesis, in vitro evaluation and molecular docking of new pyrazole derivatives bearing 1, 5, 10, 10a-tetrahydrobenzo [g] quinoline-3-carbonitrile moiety as potent antibacterial agents. J. Iranian Chem. Soc. 2020:1-15.
- [Google Scholar]
- Design, efficient synthesis and antimicrobial evaluation of some novel Pyrano [2, 3-b][1, 8] Naphthyridine and Pyrrolo [2, 3-f][1, 8] naphth-yridine derivatives. Pharma Chem.. 2019;11:6-13.
- [Google Scholar]
- Synthesis and insecticidal assessment of some innovative heterocycles incorporating a thiadiazole moiety against the cotton leafworm, Spodoptera littoralis. RSC Adv.. 2017;7:39773-39785.
- [Google Scholar]
- Synthesis and studies molecular docking of some new thioxobenzo [g] pteridine derivatives and 1, 4-dihydroquinoxaline derivatives with glycosidic moiety. J. Taibah Univ. Sci.. 2018;12:774-782.
- [Google Scholar]
- Design, Synthesis, Molecular Docking of Novel Substituted Pyrimidinone Derivatives as Anticancer Agents. Polycyclic Aromatic Compd. 2020:1-17.
- [Google Scholar]
- Heterocyclic Indane-1, 3-Dione Derivatives as Topical Anti-Inflammatory Agents. J. Pharmaceut. Sci. Res.. 2011;3:1253.
- [Google Scholar]
- Application of the intermediate derivatization approach in agrochemical discovery. Chem. Rev.. 2014;114:7079-7107.
- [Google Scholar]
- Novel β-lactams and thiazolidinone derivatives from 1, 4-dihydroquinoxaline schiff’s base: synthesis, antimicrobial activity and molecular docking studies. Chem. J. Moldova. 2020;15:86-94.
- [Google Scholar]
- Résistance de Botrytis cinerea aux benzimidazoles et aux dicarboximides dans les cultures abritées de tomate en Tunisie. EPPO Bull.. 1996;26(3–4):697-705.
- [Google Scholar]
- Evaluation of tomato (Solanum lycopersicum L.) Hybrids for quality parameter in Allahabad agroclimatic condition. J. Pharmacogn. Phytochem.. 2020;9:2089-2091.
- [Google Scholar]
- Exploring the efficacy of antagonistic rhizobacteria as native biocontrol agents against tomato plant diseases. 3 Biotech. 2020;10:1-17.
- [Google Scholar]
- Keerthi Kumar, C.T., Keshavayya, J., Rajesh, T.N., Peethambar, S.K., Shoukat Ali, A.R., 2013. Synthesis, characterization, and biological activity of 5-phenyl-1, 3, 4-thiadiazole-2-amine incorporated azo dye derivatives. Organ. Chem. Int., 2013.
- 2-Arylidene-1-indandiones as Pleiotropic Agents with Antioxidant and Inhibitory Enzymes Activities. Molecules. 2019;24:4411.
- [Google Scholar]
- Nano-based smart pesticide formulations: Emerging opportunities for agriculture. J. Control. Release. 2019;294:131-153.
- [Google Scholar]
- Synthesis of different isoindolone embedded heterocycles with phenolic subunits from a common intermediate, 3-(2′-hydroxyaroyl)-2, 3-dihydroisoindol-1-ones. Tetrahedron Lett.. 2014;55:4466-4474.
- [Google Scholar]
- Integrated management of damping-off diseases. A review. Agron. Sustain. Develop.. 2017;37:10.
- [Google Scholar]
- Genome-wide expression profiling of the response to azole, polyene, echinocandin, and pyrimidine antifungal agents in Candida albicans. Antimicrob. Agents Chemother.. 2005;49:2226-2236.
- [Google Scholar]
- Synthesis, antibacterial and antifungal activity of novel benzothiazole pyrimidine derivatives. Arabian J. Chem.. 2016;9:681-687.
- [Google Scholar]
- An alternative approach for the chemical control of Fusarium wilt of tomato. Indian Phytopathol.. 1992;45:194-198.
- [Google Scholar]
- Management tactics for fusarium wilt of tomato caused by Fusarium oxysporum f. sp. lycopersici (Sacc.): A review. Management. 2019;4:1-7.
- [Google Scholar]
- Management of tomato diseases caused by Fusarium oxysporum. Crop Prot.. 2015;73:78-92.
- [Google Scholar]
- Evaluation of application methods of metam sodium for management of Fusarium crown and root rot in tomato in southwest Florida. Plant Dis.. 1998;82:919-923.
- [Google Scholar]
- One pot three component synthesis of spiro [indolo-3, 10′-indeno [1, 2-b] quinolin]-2, 4, 11′-triones as a new class of antifungal and antimicrobial agents. Chin. Chem. Lett.. 2017;28:136-142.
- [Google Scholar]
- The serotonin1A receptor partial agonist S15535 [4-(benzodioxan-5-yl) 1-(indan-2-yl) piperazine] enhances cholinergic transmission and cognitive function in rodents: a combined neurochemical and behavioral analysis. J. Pharmacol. Exp. Ther.. 2004;311:190-203.
- [Google Scholar]
- Synthesis and Antimicrobial Activity of Some 1, 3-Disubstituted Indeno [1, 2-c] pyrazoles. J. Heterocycl. Chem.. 2014;51:203-211.
- [Google Scholar]
- Effect of the Newly Synthesized pyrazole, and pyrazolo pyrimidine derivatives on Pythium aphanidermatum (Edson) Fitzp. Egypt. J. Chem.. 2021;64:8-9.
- [Google Scholar]
- Indane-1, 3-diones: As Potential and Selective α-glucosidase Inhibitors, their Synthesis, in vitro and in silico Studies. Med. Chem.. 2021;17:887-902.
- [Google Scholar]
- Oliveira, A.F.C.d.S., de Souza, A.P.M., de Oliveira, A.S., da Silva, M.L., de Oliveira, F.M., Santos, E.G., da Silva, Í.E.P., Ferreira, R.S., Villela, F.S., Martins, F.T., 2018. Zirconium catalyzed synthesis of 2-arylidene Indan-1, 3-diones and evaluation of their inhibitory activity against NS2B-NS3 WNV protease. Eur. J. Med. Chem., 149, 98–109.
- Combining biocontrol agents with chemical fungicides for integrated plant fungal disease control. Microorganisms. 2020;8:1930.
- [Google Scholar]
- J. I. Ofunne, “Bacteriological examination of clinical specimens”, Achugo Publ. Ama JK Recreat. Park. Owerri, Niger., 1999.
- Synthesis of new pyrimidine-fused derivatives as potent and selective antidiabetic α-glucosidase inhibitors. Carbohydr. Res.. 2013;380:81-91.
- [Google Scholar]
- Synthesis, pharmacological evaluation and molecular docking studies of indanone derivatives. Med. Chem. Res.. 2012;21:4403-4411.
- [Google Scholar]
- The TCA cycle and tumorigenesis: the examples of fumarate hydratase and succinate dehydrogenase. Ann. Med.. 2003;35:634-635.
- [Google Scholar]
- Photochemistry in synthesis: Where, when, and why. Pure Appl. Chem.. 2007;79:1929-1938.
- [Google Scholar]
- Synthesis, molecular docking and antimicrobial activity of new fused pyrimidine and pyridine derivatives. Bioorg. Chem.. 2020;96:103516
- [Google Scholar]
- Morphological and molecular identification of Fusarium isolated from maize ears in Iran. J. Plant Pathol. 2008:463-468.
- [Google Scholar]
- Sensitive and selective spectrophotometric method for the determination of trace amounts of osmium with 1, 2, 3-indanetrione monothiosemicarbazone. Microchim. Acta. 1985;86:319-330.
- [Google Scholar]
- Synthesis and Antimicrobial Study of Novel 1-Aryl-2-oxo-indano [3, 2-d] pyrido/pyrimido [1, 2-b] pyrimidines. Archiv der Pharmazie: Int. J. Pharmaceut. Med. Chem.. 2008;341:418-423.
- [Google Scholar]
- Applications of nanotechnology in plant growth and crop protection: a review. Molecules. 2019;24:2558.
- [Google Scholar]
- Synthesis, antibacterial and antifungal activities of some pyrazole-1-carbothioamides and pyrimidine-2 (1H)-thiones. Am. J. Org. Chem.. 2012;2:26-31.
- [Google Scholar]
- Synthesis of Various Derivatives of [1, 3] Selenazolo [4, 5-d] pyrimidine and Exploitation of These Heterocyclic Systems as Antibacterial, Antifungal, and Anticancer Agents. ChemistrySelect. 2020;5:10060-10066.
- [Google Scholar]
- Green chemistry and catalysis. John Wiley & Sons; 2007.
- Influence of pH and NaHCO3 on effectiveness of imazalil to inhibit germination of Penicillium digitatum and to control postharvest green mold on citrus fruit. Plant Dis.. 2005;89:640-648.
- [Google Scholar]
- Evaluation of arbuscular mycorrhizal fungus, fluorescent Pseudomonas and Trichoderma harzianum formulation against Fusarium oxysporum f. sp. lycopersici for the management of tomato wilt. Biol. Control. 2010;53:24-31.
- [Google Scholar]
- Green chemistry metrics with special reference to green analytical chemistry. Molecules. 2015;20:10928-10946.
- [Google Scholar]
- Novel pyrimidine derivatives containing an amide moiety: design, synthesis, and antifungal activity. Chem. Pap.. 2019;73:719-729.
- [Google Scholar]
- Comparative research of chemical constituents, antifungal and antitumor properties of ether extracts of Panax ginseng and its endophytic fungus. Phytomedicine. 2009;16:609-616.
- [Google Scholar]
- Design, Synthesis, and Evaluation of the Antifungal Activity of Novel Pyrazole-Thiazole Carboxamides as Succinate Dehydrogenase Inhibitors. J. Agric. Food. Chem.. 2020;68:7093-7102.
- [Google Scholar]
- Recent Development of Pyrimidine-Containing Antimicrobial Agents. ChemMedChem. 2020;15:1875-1886.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.103731.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







