Translate this page into:
Nucleophilicity and solvent effects on the kinetics of 4-(pyren-1-yl)thiazol-2-amine interaction with 4,6-dinitrobenzofuroxan
⁎Corresponding author at: Department of Chemistry, Faculty of Applied Science, Umm Al-Qura University, 21955 Makkah, Saudi Arabia. Tel./Fax: +966 530435760. saahmed@uqu.edu.sa (Saleh A. Ahmed) saleh_63@hotmail.com (Saleh A. Ahmed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
A multistep synthesis of novel pyrene-based thiazole moiety been has been realized following some synthetic challenges and complications. The chemical structure of the synthesized compound has been established on the basis of both spectroscopic and analytical tools. Its nucleophilic reactivity with 4,6-dinitrobenzofuroxan (DNBF) has been successfully studied in solution. A kinetic study of the covalent electrophile/nucleophile combination of dinitrobenzofuroxan (DNBF, electrophile) and 4-(pyren-1-yl)thiazol-2-amine (nucleophile) resulting in the formation of the corresponding σ-adduct in solution is reported. The rate constant (k1) of the second-order relating to the C—C bond forming step of this complexation process has been found to fit into the linear correlation log k = sN (N + E), thereby permitting the evaluation of the nucleophilicity parameter (N) of the 4-(pyren-1-yl)thiazol-2-amine. 4-(Pyren-1-yl)thiazol-2-amine has been subsequently ranked according to its reactivity profile on the general nucleophilicity scale developed recently by Mayr et al., leading to an interesting and direct comparison over a large domain of π-, σ-, and n-nucleophiles.
Keywords
Pyrene-based heterocycles
Nucleophilicity parameters
Dinitrobenzofuroxan
Kinetic study
1 Introduction
The chemical design of new fluorescent materials with tunable emissions is possible via the modification of chemical structures, thereby altering the transition-energy levels which may be monitored and characterized by physicochemical techniques (Wang et al., 2018; Liang et al., 2017; Lin et al., 2016; Wu et al., 2008). However, producing emission materials exhibiting desired properties to satisfy the requirements of high-performing devices remains an elusive task. Additionally, gaining an insight into the interdependence of the structure and reactivity of these targets still an attractive area of research. In the traditional organic synthesis methodologies, the dipolar push-pull chromophoric materials are ideal in fabricating tunable emission materials which can display a broad emission scope ranging from blue to red and to near-IR region (Merz et al., 2017; Kurata et al., 2017; Keller et al., 2017; Wang et al., 2015; Li et al., 2014).
Pyrene and its derivatives are well-known compounds related to polynuclear aromatic hydrocarbons (PAHs) which are characterized by the existence of four fused benzene rings forming a planar system. Pyrenes can undergo stacking by π–π interactions, with a tendency to form fluorescent excimers with a high exporter mobility in solution as well as in the solid state (Cabral et al., 2017; Wang et al., 2012a,b). Owing to these unique properties, pyrene chromophore is frequently used in sensors (Bobe et al., 2013) or fluorescent probes. Recently, pyrene-based probes have attracted substantial research interest (Bains et al., 2011) because of their exceptional spectroscopic properties and remarkably prolonged lifetimes of emission band structure (Khalifa et al., 2014) with emission exhibiting a reliable dependency on polarity of the solvent (Filichev et al., 2008). Pyrene containing chips can be associated to lipids (Machata et al., 2014), protein or DNA or RNA (Crawford et al., 2011) to construct probes designed for specific applications in biological findings. Furthermore, incorporating different substitutions on the pyrene moiety has been found to drastically change both, chemical properties, which provide a leverage to control the molecular construction, and subsequently the molecular packing. This is particularly useful in the potential applications of pyrene-based semiconductors (Wang et al., 2012a,b). Pyrene exhibits spectral features of emission which is highly sensitive to the micro-environment accessible in the standardized media (Duarte and Müllen, 2011; Solvas et al., 2014). Our earlier investigations established that pyrene-based compounds exhibit a significant effect on the emission with considerable red-shifts contingent upon the substitution position along the long axis or K-regions (Guerrini et al., 2009). Therefore, pyrene containing dipolar molecules are anticipated to exhibit desirable optical behaviors when functionalized along both long axis and K-regions (Boujday et al., 2009).
Due to the attracting properties of the pyrene-containing compounds outlined earlier, the molecular design and synthetic pathways of substituted pyrenes have become an important endeavor. As well, five membered heterocyclic systems-based chromophores also exhibit the desirable photochemical and electrochemical behaviors being targeted. Among the five-membered ring heterocyclic structures, the thiazole moiety is an important π-conjugated building block. Several commercial fluorescent dyes and brighteners bearing the thiazole unit have been produced worldwide (Pinto et al., 2001). Further, the thiazole motif is a useful heterocycle component used in drug design and research and has been incorporated in numerous notable drugs such as bleomycin anti-cancer (Tsukada et al., 2010; Cort et al., 2012). Furthermore, as an electron-rich aromatic group, the thiazole function may find useful application in photoelectric technique (Dessì et al., 2013). Compared to the preparation of other heterocyclic compounds, the synthesis of thiazoles is relatively simple. Hantzsch reaction of α-halocarbonyl compounds with thioureas provides an expedient method for the synthesis of thiazole derivatives (Hantzsch and Weber, 1887).
In the past decades, nitrobenzofuroxans, a class of electron-deficient heteroaromatics, have received a great deal of attention, as extremely strong neutral electrophiles that display high susceptibility towards substitution or covalent nucleophilic addition processes (Terrier, 1982, 1991; Buncel et al., 1984, 1987; Crampton et al., 1999, 2003; Boga and Forlani, 2001). Contrary to nitro-arene compounds represented by 1,3,5-trinitrobenzene (TNB), nitrobenzofuroxans are considered as traditional reference electrophiles, these new heteroaromatic compounds illustrated by 4,6-dinitrobenzofuroxan (DNBF) have been labelled as super-electrophiles that are capable of reacting readily with weak nucleophiles (Terrier et al., 1992, 1993a,b; Buncel et al., 1997; Crampton et al., 1999; Boga et al., 2005). This behavior is due to the facile carbon–carbon couplings of DNMF with π-excessive heterocycles (indoles, pyrroles, etc.) or benzenoid aromatics (e.g. alkoxybenzenes, polyhydroxybenzenes and anilines, etc.) affording σ-adducts, which exhibit high thermodynamic stability (Terrier et al., 1993a,b, 1998; Moutiers et al., 2001; Goumont et al., 2003; Asghar and Crampton, 2005).
In continuation of our earlier effort involving the preparation and photophysical properties of pyrene-based heterocycles (Hussein et al., 2017, 2018; El Guesmi et al., 2019), this work aims to shed more light on the synthesis of pyrene-based thiazole moiety and its nucleophilic reactivity with DNBF in solution. Moreover, the presented research describes a successful ranking of the nucleophilicity of 4-(pyren-1-yl)thiazol-2-amine within the N scale of Mayr. This finding will assist in assessing coupling reactions which can be envisioned for this type of aminothiazole derivatives.
2 Experimental
2.1 Materials and methods
All solvents used in this work were of analytical grade purchased from Sigma-Aldrich and were used without any further purification. Melting points were measured using Stuart melting point apparatus and are uncorrected. The IR spectra were recorded on Shimadzu IR-3600 FT-IR spectrometer with KBr as pallets. All 1H and 13C NMR spectra were acquired on a Bruker Avance 500 instrument (at 500 and 125 MHz for 1H and 13C, respectively) in deuterated chloroform solution CDCl3, using residual fully deuterated solvent signals as internal standards. The electronic absorption spectra and kinetic determinations were recorded with a conventional UV–Vis spectrophotometer (Shimadzu model 1800 PC), whose temperature of cell compartments were maintained around 20 ± 0.1 °C. All kinetic runs were performed no less than three times under conditions of pseudo-first-order with the 4,6-dinitrobenzofuroxan (5) concentration of ∼3 × 10−5 mol dm−3 and a concentration of 4-(pyren-1-yl)thiazol-2-amine (4) in the range 3 × 10−4–1 × 10−3 mol dm−3. In all of the experiments carried out, the rates data were found to be reproducible to 2–3%. Fluorescence spectra were performed using Horiba Spectrofluorometer (model FluoroMax4).
2.2 General procedure
2.2.1 Synthesis of 1-(pyren-1-yl)ethenone (2) (Weng et al., 2012)
In an ice-cold bath, a solution of pyrene (16.16 g, 80 mmol) in 100 mL CH2Cl2 was added dropwise over a period of 2 h to an ice solution containing a mixture of AlCl3 (11.8 g, 88 mmol), acetyl chloride (6.4 mL, 88 mmol) in CH2Cl2 (500 mL). The mixture was stirred at 0 °C for 3 h then kept at room temperature overnight. The mixture was poured slowly into deionized water (1.0 L) containing conc. HCl (150 mL) and stirred for 2 h. The formed complex was decomposed, and clear yellow solution was separated, the organic layer was collected, dried over anhydrous magnesium sulphate (MgSO4), then filtered off and concentrated under reduced pressure. The obtained residue was purified using silica gel column chromatography method with DCM as eluent to give the titled compound 2 (16.78 g, 86% yield).
Light-yellow crystals; mp 88–89 °C. IR (KBr): νmax = 3040 (CH arom.), 2890 (CH aliph.), 1666 (C⚌O), 1622 (C⚌N) cm−1. 1H NMR (500 MHz, CDCl3): δ 9.04 (1H, d, J = 8.5 Hz, pyrenyl-H), 8.22–8.21 (1H, m, pyrene-H), 8.14–8.12 (3H, m, pyrene-H), 8.02–7.96 (3H, m, pyrene H), 7.89–7.88 (1H, m, pyrene-H), 2.86 (3H, s, CH3); 13C NMR (125 MHz, CDCl3): δ 202.05 (C⚌O), 133.87 (C), 131.56 (C), 130.93 (C), 130.36 (C), 129.6 (CH), 129.50 (CH), 129.39 (CH), 127.12 (CH), 126.95 (CH), 126.27 (CH), 126.01 (CH), 124.94 (CH), 124.78 (C), 124.09 (C), 123.86 (CH), 30.43 (CH3) ppm.
2.2.2 Synthesis of 2-bromo-1-(pyren-1-yl)ethanone (3)
A slurry of cuprous bromide (17.22 g, 120 mmol) in 250 mL of EtOAc was heated to reflux. A solution of 1-(pyren-1-yl)ethenone 2 (14.64 g, 60 mmol) in 150 mL of chloroform was added over 45 min. The resulting solution was heated at reflux for 7 h and then filtered while still hot through a 1 in. thick pad of Celite. The filter cake was washed several times with ethyl acetate (EtOAc) and the combined filtrate was evaporated under reduced pressure to provide 14.68 g (76% yield) of 3 which was kept in the dark and was used in the next step without further purification.
Yellow needles, mp 128–130 °C. IR (KBr): νmax = 3038 (CH arom.), 2887 (CH aliph.), 1670 (C⚌O), 1628 (C⚌N) cm−1. 1H NMR (500 MHz, CDCl3): δ 9.19 (1H, d, J = 9.0 Hz, pyrene-H), 8.54 (1H, d, J = 9.0 Hz, pyrene-H), 8.48–8.44 (3H, m, pyrene-H), 8.39–8.33 (2H, m, pyrene-H), 8.29–8.24 (2H, m, pyrene-H), 4.96 (2H, s, CH2); 13C NMR (125 MHz, CDCl3): δ 189.83 (C⚌O), 129.86, 126.18, 125.83, 125.65, 125.57, 125.46, 123.52, 122.24, 122.05, 121.99, 121.86, 121.74, 120.24, 119.83, 119.24, 119.17, 29.55 (CH2) ppm. Anal. Calcd. for C18H11BrO (323.18): C, 66.89; H, 3.43; Br, 24.72. Found: C, 66.71; H, 3.40; Br, 24.50%.
2.2.3 Synthesis of 4-(pyren-1-yl)thiazol-2-amine (4)
In 500 mL round bottom flask fitted with reflux condenser, 2-bromo-1-(pyren-1-yl)ethanone 3 (9.66 g, 30 mmol) in 200 mL ethanol was refluxed until clear solution appear. To this hot solution, thiourea (2.66 g, 35 mmol) dissolved in 50 mL ethanol was added dropwise over 20 min. The reaction was reflux for 2 h then cooled to room temperature. The formed precipitate was washed with NaHCO3 solution until effervescence subsided and the product was collected by filtration and dried in vacuum oven for 48 h to get the target compound 4 in 91% yield (8.19 g).
Yellow crystals; mp = 186–187 °C. IR (KBr): νmax = 3260, 3170 (NH2), 3040 (CH arom.), 2890 (CH aliph.), 1622 (C⚌N) cm−1; 1H NMR (500 MHz, DMSO‑d6): δ 8.86 (1H, d, J = 9.5.0 Hz, pyrene-H), 8.28–8.25 (4H, m, pyrene-H), 8.18–8.16 (3H, m, pyrene-H), 8.07–8.04 (1H, m, pyrene-H), 7.28 (2H, s, NH2), 6.95 (1H, s, thiazole-H); 13C NMR (125 MHz, CDCl3): δ 168.71 (C⚌N), 150.70 (C), 131.44 (C), 130.89 (C), 130.69 (C), 128.22 (C), 127.82 (CH), 127.80 (CH), 127.73 (CH), 126.77 (CH), 126.18 (CH), 125.69 (CH), 125.40 (C), 125.28 (C), 124.49 (C), 106.32 (CH-thiazole) ppm. Anal. Calcd. for C19H12N2S (300.38): C, 75.97; H, 4.03; N, 9.33; S, 10.67. Found: C, 75.80; H, 3.88; N, 9.20; S, 10.51%.
3 Results and discussion
3.1 Synthesis of 4-(pyren-1-yl)thiazol-2-amine
The synthesis of the target 4-(pyren-1-yl)thiazol-2-amine (4) was carried out in three steps as outlined in Scheme 1. A Friedel–Crafts acylation of pyrene (1) with acetyl chloride in the presence of anhydrous aluminum chloride in dried dichloromethane at 0 °C for 3 h, followed by stirring at ambient temperature overnight, afforded the corresponding 1-(pyren-1-yl)ethenone (2). The pure product was obtained in 86% yield as pale-yellow crystals after column chromatography on silica gel using DCM as eluent. In the subsequent step, a convenient and selective bromination of compound (2) was carried-out using cuprous bromide as brominating agent in refluxing CHCl3/CH3CO2C2H5 for 7 h to afford 2-bromo-1-(pyren-1-yl)ethenone (3). The pure product was obtained in 76% yield as yellow needles after filtration of the black residue and evaporation of the solvents under reduced pressure and was used in the next step without further purification. Finally, the target compound, 4-(pyren-1-yl)thiazol-2-amine (4) was obtained in 91% yield as yellow crystals by reacting compound 3 with thiourea (1.1 eq.) in refluxing ethanol for 2 h. The pure product 4 was obtained as yellow crystals after crystallization from ethanol. The synthesized compounds 2–4 were well characterized using both analytical and spectroscopic tools (see experimental section and supporting information).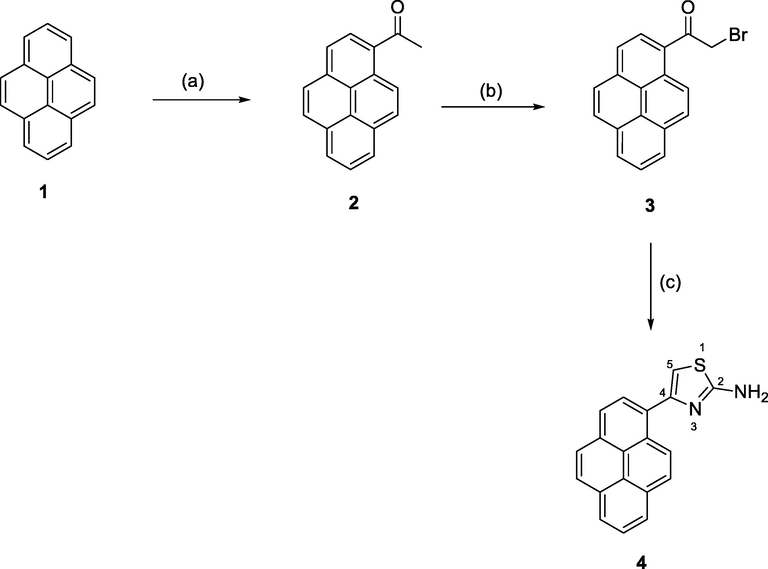
Synthesis of 4-(pyren-1-yl)thiazol-2-amine (4): (a) CH3COCl, AlCl3, DCM, 0 °C/3 h then rt/overnight; (b) Cu2Br2, CHCl3/CH3CO2C2H5/rt/reflux/7 h; (c) 1.1 eq. H2NCSNH2/EtOH, reflux/2 h.
3.2 Kinetic studies
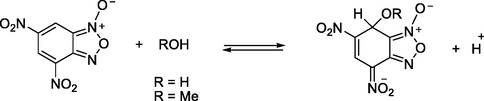
The kinetic study was performed under pseudo-first-order experimental conditions in which the concentration of 4-(pyren-1-yl)thiazol-2-amine (4) was used in excess over DNBF 5 substrate concentration. Experiments were performed by directly mixing a solution of 3 × 10−5 mol dm−3 of DNBF with a solution of thiazole with concentrations in the range of 3.0 × 10−4–1.0 × 10−3 mol dm−3. The reaction rates as described in scheme 2, were recorded in acetonitrile at 20 °C, resulting the formation of the σ-adducts by conservative spectrophotometry. The formation of σ-adduct 7 characterized by λmax = 482 nm was studied by mixing in acetonitrile a solutions of 4-(pyren-1-yl)thiazol-2-amine (4) with DNBF (5) solution (Scheme 2), where neither the electrophilic (DNBF (5); λmax = 418 nm), nor its nucleophilic partners have a notable absorption. Fig. 1 shows the UV–Vis spectra for the progressive formation of σ-adduct (7). Figs. S1–S8 (c.f. supporting information) show the oscilloscope traces presenting a process by only one relaxation relating to the formation of anionic C-adduct 7 at various concentrations of 4-(pyren-1-yl)thiazol-2-amine (4). By choosing the excitation wavelength at λex = 344 nm and acetonitrile as solvent, the characteristic data for the fluorescence of 4-(pyren-1-yl)thiazol-2-amine is λem = 438 nm (Fig. 2(a)) agrees with the known range for aminothiazoles derivatives (λem = 430–480 nm) (Murai et al., 2019). Notably, compared with literature data, a remarkable increase in fluorescence intensity was observed with the incorporation of the pyrene fragment (Fig. 2(a)).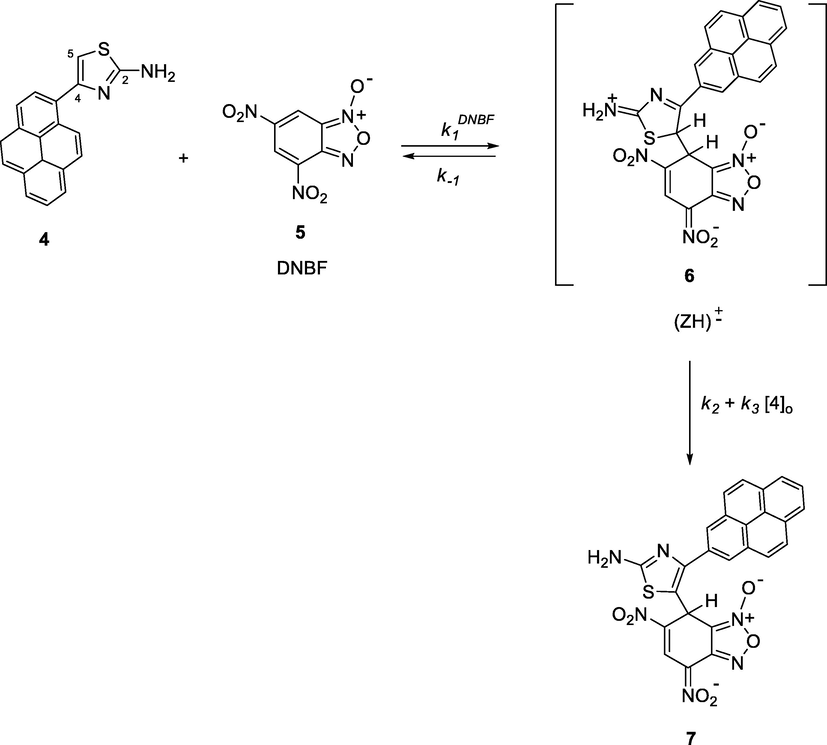
The interaction of 4-(pyren-1-yl)thiazol-2-amine with DNBF.
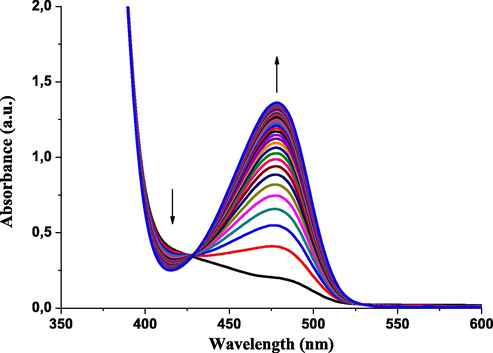
UV–vis absorption spectra and kinetic relaxation processes of the formation of adduct 7 (λmax = 482 nm) from the reaction of DNBF (λmax = 418 nm) with 4-(pyren-1-yl)thiazol-2-amine 4 (c = 1 × 10−3 mol dm−3) (cycle time = 40 × 100 s) in acetonitrile at T = 20 °C.
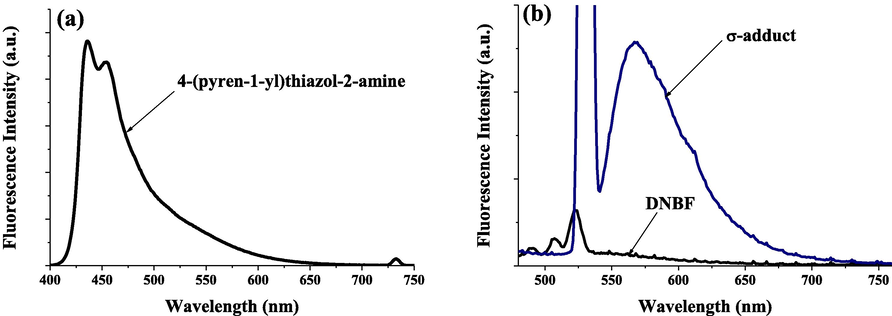
Normalized emission spectra of 1.0 × 10−5 M solution of (a) 4-(pyren-1-yl)thiazol-2-amine; (b) DNBF and σ-adduct, in acetonitrile.
In the case of 4,6-dinitrobenzofuroxan (DNBF), no fluorescent property was detected (Fig. 2(b)) probably due to the presence of two electron-withdrawing NO2 groups which significantly decrease its fluorescent property. The reaction of 4-(pyren-1-yl)thiazol-2-amine with 4,6-dinitrobenzofuroxane gave a stable Meisenheimer σ-complex which exhibited pronounced fluorescent properties with λem = 568 nm (Fig. 2(b)). These fluorescence and paramagnetic properties of 4-(pyren-1-yl)thiazol-2-amine may lead to applications as a molecular probe for biological processes.
The reaction of 4-(pyren-1-yl)thiazol-2-amine (4) with DNBF (5) obeyed first-order kinetics. Thus, from the equation ln(A∞ − At) = −kobsd t + ln(A∞ − Ao), pseudo-first order rate constants (kobsd) have been determined, where Ao represents the initial absorbance while At represent the absorbance at time t (s) and A∞ stands for absorbance at infinite dilution. The use of integration method by plotting ln(A∞ - At) vs. time allowed us to collect kinetic data relating to the coupling reaction. Linear plots were obtained for DNBF (5) with constant concentration and varying concentrations of 4-(pyren-1-yl)thiazol-2-amine (4). These straight lines plots indicate that the reaction is first order (Figs. S14–S21 (c.f. supporting information)), which was defined through the first-order rate constant integral equation. The pseudo-first order rate constants kobsd values are summarized in Table 1.
[aminothiazole] × 104 (mol dm−3)
3.0
4.0
5.0
6.0
7.0
8.0
9.0
10.0
kobsd × 104 (s−1)
4.30
6.64
7.45
8.19
9.67
11.91
12.90
14.71
On the basis of Scheme 2, the conventional expression corresponding to the observed rate constant for the formation of 7 can be expressed by assuming that low concentration of the zwitterion ZH± as follows:
Thus, excellent straight line with negligible ko (≈ 0) intercepts in present system was obtained according the above equation when plotting kobsd vs the aminothiazole 4 concentration (Nu). Furthermore, no detection of third-order term or higher-order were observed, and therefore no difficulty was found in the determination of pseudo-first order rate constants kobsd or in the linear plot of Eq. (1). From the slope of the plot kobsd vs. [2-aminothiazole (4)] (Fig. 3), the second-order rate constants k1 was readily derived (k1 = 1.42 dm3 mol−1 s−1). Therefore, this leads us to believe that the electrophilic attack at the reactive nucleophilic center according to this process is largely rate-limiting, as depicted in Scheme 2 for 4-(pyren-1-yl)thiazol-2-amine system. In other words, the proton removal step from the zwitterion ZH± can be considered as rapid in acetonitrile solution, allowing for the identification of the composite rate constant k to the rate constant k1 for the carbon-carbon coupling step (Taylor, 1990; Jackson and Lynch, 1987). The present situation is in good agreement with that observed for the same type of reactions for the σ-complexation reactions of 4,6-dinitrobenzofuroxan with indole nucleophiles (Lakhdar et al., 2006; Terrier et al., 2005) which is predominant in a great number of heteroaromatic/aromatic electrophilic substitution reactions in which the formation of intermediates of Wheland-Meisenheimer type (here ZH±) is rate determining step, e.g., the azo couplings of pyrrole and indole derivatives by p-nitrobenzenediazonium cations (Taylor, 1990; Jackson and Lynch, 1987; Challis and Rzepa, 1975).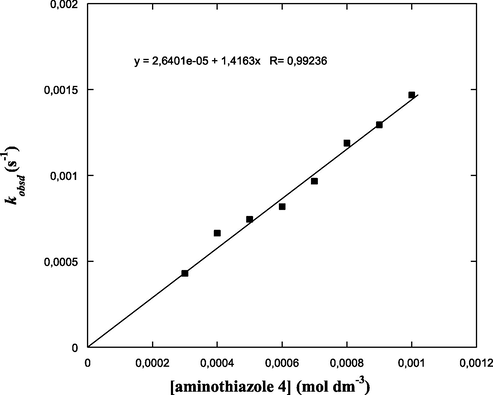
Effect of the concentration of 4-(pyren-1-yl)thiazol-2-amine (4) on kobsd of the formation of adduct 7 in acetonitrile at T = 20 °C.
3.3 Determination of the estimated nucleophilicity parameters N of 4-(pyren-1-yl)thiazol-2-amine
A quantitative assessment of the intrinsic nucleophilicity of 4-(pyren-1-yl)thiazol-2-amine is feasible by referring to the general electrophilicity/nucleophilicity approach developed by Mayr and co-workers (Mayr et al., 2004, 2005, 1992; Phan et al., 2006; Minegishi et al., 2004; Lucius et al., 2002; Herrlich et al., 2001; Gotta and Mayr, 1998). By using a large series of diarylcarbenium ions as reference sets for electrophiles and various π-excessive systems as nucleophiles, indeed, these authors have demonstrated that it is possible to characterize the reactivities of a wide variety of nucleophile–electrophile combinations by a simple equation of only three parameters as shown in Eq. (2). It is clear from this equation that the estimated E parameter reflects the reactivity strength of the electrophile, while N expresses the strength of the nucleophile and s is a nucleophile-specific parameter which depicts the sensitivity of the rate constant of coupling reaction upon variation of the electrophile partner.
Based on Eq. (2), N and E scales, covering a reactivity range of about 40 (−8.80 ≤ N ≤ 30.82) and 35 (−24.69 ≤ E ≤ 8.02) orders of magnitude, respectively, have been constructed and thus used successfully to predict the feasibility and rate of many electrophile-nucleophile interactions (Mayr et al., 2002, 2001, 1998, 1994; Ofial et al., 2003; Lemek and Mayr, 2003; Minegishi and Mayr, 2003; Bug and Mayr, 2003; Schindele et al., 2002; Würthwein et al., 2002). It is important to note that among the large variety of nucleophiles studied by Mayr, a number of selected enamines have been classified on the N scale with the finding that the corresponding nucleophile-specific s parameter does not vary much with the change of enamine structure (0.79 < s < 1.03) (Kempf et al., 2003). Accordingly, approximating the N values for 4-(pyren-1-yl)thiazol-2-amine (4) is possible through Eq. (2) by combination of the average value of s = 0.90 for the nucleophile with the E value determined for the strong electrophile DNBF (E = −5.22) (Terrier et al., 2005), in which the log k values in Mayr’s equation (Eq. (2)) refers to our kinetic (k1DNBF) measurements in acetonitrile. The N parameter of our nucleophile 4 (N = 5.39) is collected in Table 2 along with the values of aminothiazole analogue, such as 2-aminothiazole, 2-aminobenzothiazole, 2-amino-4-methylthiazole and 2-amino-4,5-dimethylthiazole.
R1 = H
R2 = HR1 = H
R2 = CH3
R1 = CH3
R2 = CH3
N
5.56
6.80
5.39
6.42
4.17
The quantitative ranking of the nucleophile 4-(pyren-1-yl)thiazol-2-amine (4) on the nucleophilicity scale (Fig. 4) enabled comparison of its reactivity as a nucleophile with that of related amino-compounds previously classified by Mayr and coworkers in the N scale. Thus, the experimental N value of 4 shows that the addition of one pyrene group on 2-aminothiazole (N = 5.56) resulting in 4-(pyren-1-yl)thiazol-2-amine (4) (N = 5.39). decreasing its nucleophilicity strength by approx. 0.2 order of magnitude. On the other hand, the experimental N values of 4-(pyren-1-yl)thiazol-2-amine (4) (N = 5.39) and 2-amino-4-methylthiazole (N = 6.80) show that the substitution of one methyl group by a pyrene group to give 2 decrease its nucleophilicity strength by approx. 1.5 order of magnitude.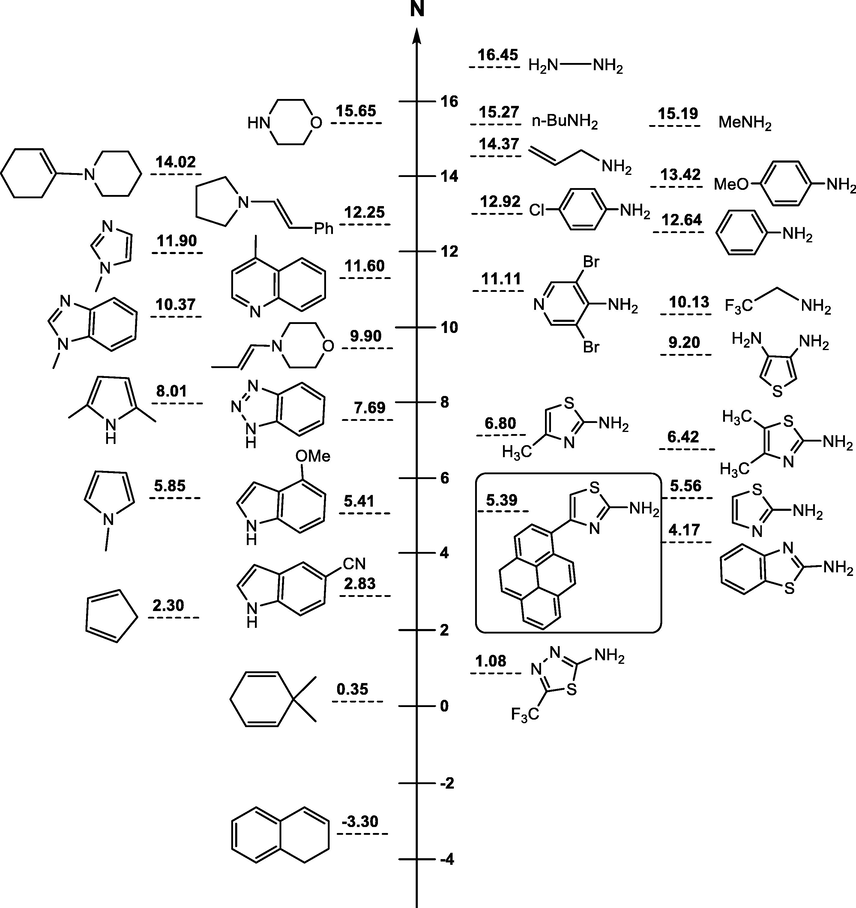
Ranking of 4-(pyren-1-yl)thiazol-2-amine on the Nucleophilicity scale (N), as developed by Mayr et al.
The nucleophilicity of 4 (N = 5.39) compares well with that of the 4-methoxylindole (N = 5.41) and found to approach that of N-methylpyrrole (N = 5.85) but show lower reactivity than that of other primary amines such as 4-amino-3,5-dibromo-pyridine, aniline and its derivatives or aliphatic amines such as butylamine and hydrazine, as shown in Fig. 4.
3.4 Solvent effect on the DNBF behavior on reaction with 4-(pyren-1-yl)thiazol-2-amine
The broad-range reactivity of DNBF offers renders it suitable to explore SEAr reaction in various organic solvents. The UV–visible absorption spectra of 4,6-dinitrobenzofuroxan with 4-(pyren-1-yl)thiazol-2-amine (Fig. 5a′–f′) were recorded using polar protic solvents such as methanol, ethanol, propanol, butanol and polar aprotic solvent like dimethylformamide (DMF) and tetrahydrofuran (THF), respectively. As shown in Fig. 4a, the UV–visible absorption spectra at 460 nm reveal that the formation of the adduct at this wavelength results most likely from the attack of a molecule of methanol on the parent DNBF substrate. The result obtained is in good agreement with the literature (Terrier, 1982, 1991; Buncel et al., 1984, 1987; Crampton et al., 1999, 2003; Boga and Forlani, 2001). Indeed, Terrier and co-workers have focused particularly on the reactivity of 4,6-dinitrobenzofuroxan which exhibit high electrophilicity in various organic processes, including also the addition to carbon with very weak nucleophilic character, proceeding even under mild conditions providing quantitatively stable carbon bonded σ-adducts (Terrier, 1982, 1991; Buncel et al., 1984, 1987; Crampton et al., 1999, 2003; Boga and Forlani, 2001). More importantly, 4,6-dinitrobenzofuroxan (DNBF) undergoes facile addition of one molecule of solvent (water or methanol) according to Eq. (3), resulting in hydroxide or methoxide adducts with rate coefficient determined as kH2O = 0.035 s−1 and kMeOH = 0.030 s−1 (Crampton et al., 1999; 2003, Terrier et al., 1984; Strauss et al., 1983; Buncel et al., 1987; Manderville and Buncel, 1993). On the other hand, (Fig. 5a′) corresponds to the band of absorption resulting from the addition of excess of 4-(pyren-1-yl)thiazol-2-amine to DNBF in methanol. The same band of absorption is observed in Fig. 5a at 460 nm corresponding to the formation of methoxide adduct, this observation can be interpreted according the fact that our super-electrophile DNBF in solution is in presence of two species with nucleophilic characters, the methanol (N = 7.54) and 4-(pyren-1-yl)thiazol-2-amine (N = 5.39) in which the methanol is more nucleophilic than 4-(pyren-1-yl)thiazol-2-amine by two logarithmic unit (ΔN = 2). Fig. S10 in the supplementary information section supports this interpretation since the change in concentration of 4-(pyren-1-yl)thiazol-2-amine (4) has no effect on the kinetics of the reaction. In view of the known reactivity of 4,6-dinitrobenzofuroxan toward a number of compounds having weak nucleophilic character, including water and methanol, the ease of reaction of ethanol (Fig. 5b) and propanol (Fig. 5c) with this compound is perhaps not unexpected. Interestingly, despite the DNBF interaction with the mentioned solvents, this reaction proceeds slower than that with methanol, which leads us to conclude that these solvents acts as nucleophiles, which are weaker than methanol but stronger than 4-(pyren-1-yl)thiazol-2-amine (4). This is supported by Fig. 5b′ and Fig. 5c′ which reveal a UV–Visible spectrum illustrating the conversion of ethoxide adduct formed at 462 nm into σ-adduct 7 formed at 482 nm, and the conversion of propoxide adduct formed at 464 nm into σ-adduct 7 formed at 484 nm, respectively. It appears that two competitive processes have been identified in this case with the initial addition of solvent molecule to give ethoxide and propoxide adducts and a subsequent and slow conversion of these species into the σ-adduct 7.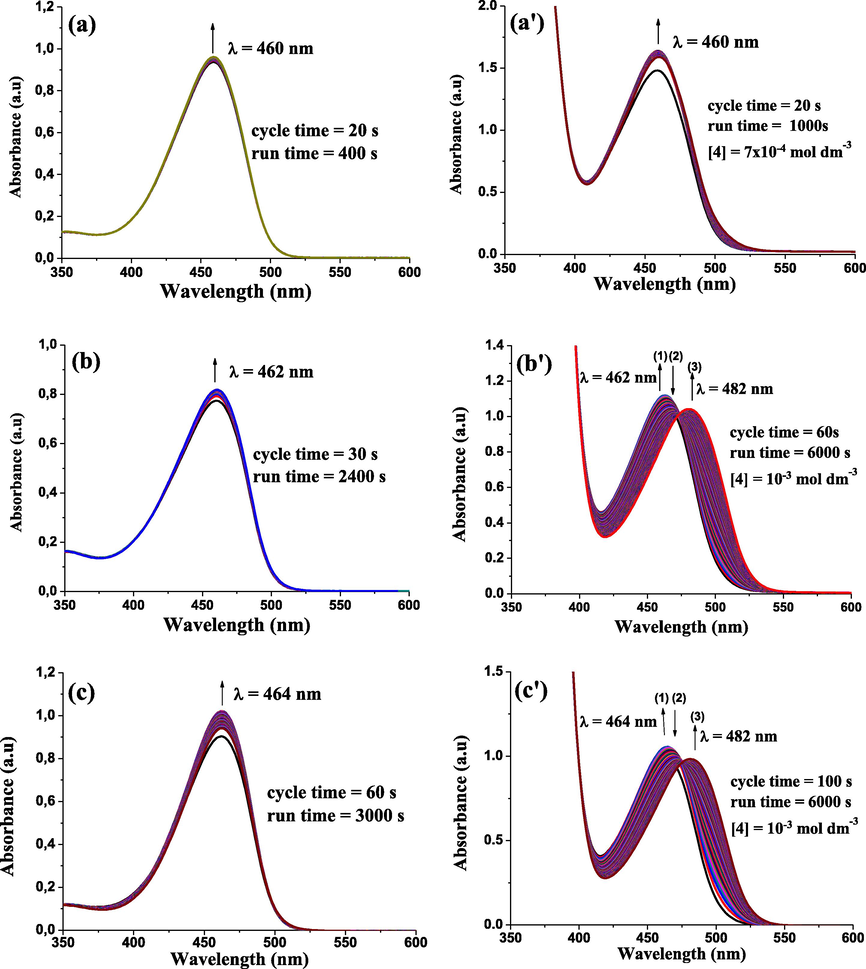
UV–Vis absorption spectra illustrating the behavior of 4,6-dinitrobenzofuroxan 5: (1) in solvent: (a) methanol; (b) ethanol; (c) propanol; (d) butanol; (e) DMF; (f) THF, (2) in presence of 4-(pyren-1-yl)thiazol-2-amine (4) in (a′) methanol; (b′) ethanol; (c′) propanol; (d′) butanol; (e′) DMF; (f′) THF, at T = 20 °C.
UV–Vis absorption spectra illustrating the behavior of 4,6-dinitrobenzofuroxan 5: (1) in solvent: (a) methanol; (b) ethanol; (c) propanol; (d) butanol; (e) DMF; (f) THF, (2) in presence of 4-(pyren-1-yl)thiazol-2-amine (4) in (a′) methanol; (b′) ethanol; (c′) propanol; (d′) butanol; (e′) DMF; (f′) THF, at T = 20 °C.
Fig. 4d and f corresponding to butanol and THF, respectively and show that these species have a unique role in solution as a solvent, so the addition of 4-(pyren-1-yl)thiazol-2-amine (4) is followed only by the processes of formation of σ-adduct 7 at 483 nm and 479 nm, respectively, as showed in Fig. 5d′ and f′. In the case of DMF, the behavior of DNBF is different from the above, indeed, Fig. 5e shows formation of an absorption band at 472 nm, illustrating the reaction of DNBF with DMF, whereas Fig. 5e′ only shows the process of formation of the σ-adduct 7 without the DMF intervening in reaction. This can be explained by the fact that 4-(pyren-1-yl)thiazol-2-amine (4) has a stronger nucleophilic reactivity than DMF which prevents it from participating in the reaction. Clearly, the exceptional electrophilic reactivity of 4,6-dinitrobenzofuroxan (DNBF) provides an entry to the superelectrophilic dimension in σ-complexation process, permitting considerable extension to the utility of this reaction in synthesis.
4 Conclusion
The kinetic study of 4-(pyren-1-yl)thiazol-2-amine and dinitrobenzofuroxan (DNBF) reveals that the nucleophilic reactivity of the 4-(pyren-1-yl)thiazol-2-amine (4) is found to undergo perfectly the linear-free energy relationship developed by Mayr et al. (Eq. (2)). With a N value in the order of 5.39, 4-(pyren-1-yl)thiazol-2-amine is well located in the domain of the enamines compounds studied recently by Mayr. Moreover, consistent with the considerably great C-basicity, our aminothiazole 4 displays N value in the range of 5.0–6.0, comparable to those of 4-methoxylindole (N = 5.41) and N-methylpyrrole (N = 5.85). This finding is noteworthy since it allows us to extend the applicability of Mayr’s equation to σ-complexation processes employing neutral electrophiles thus broadening markedly the field of coupling reactions which can be predicted with 4,6-dinitrobenzofuroxans.
Acknowledgement
The authors are highly indebted to the Deanship of the Scientific Research (DSR), Umm Al-Qura University for the financial support of this work through the project number 18-SCI-1-01-0009. Also, S. A. Ahmed is highly indebted to Alexander von Humboldt Foundation (AvH) for fellwopship award and Prof. Dr. Karola Rück-Braun at Technical University Berlin (TU-Berlin), Germany for her valuable discussions.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
References
- Rate-limiting proton-transfer in the σ-adduct forming reactions of 1,3,5-trinitrobenzene and 4-nitrobenzofuroxan with substituted anilines in dimethyl sulfoxide. Org. Biomol. Chem.. 2005;3:3971-3978.
- [Google Scholar]
- Pyrene: a probe to study protein conformation and conformational changes. Molecules. 2011;16:7909-7935.
- [Google Scholar]
- Detection of trace amounts of water in organic solvent by 8-hydroxypyrene-1,3,6-trisulfonic acid trisodium salt. Aust. J. Chem.. 2013;67:615-619.
- [Google Scholar]
- Evidence for carbon-carbon Meisenheimer-Wheland complexes between superelectrophilic and supernucleophilic carbon reagents. Angew. Chem. Int. Ed.. 2005;44:3285-3289.
- [Google Scholar]
- Formation and stability of zwitterionic complexes between nitrobenzofuroxans and amines. J. Chem. Soc., Perkin Trans.. 2001;2:1408-1413.
- [Google Scholar]
- Surface IR applied to rapid and direct immunosensing of environmental pollutants. Talanta. 2009;78:165-170.
- [Google Scholar]
- Nucleophilic reactivities of carbanions in water: the unique behavior of the malodinitrile anion. J. Am. Chem. Soc.. 2003;125:12980-12986.
- [Google Scholar]
- In Electron-Deficient Aromatic- and Heteroaromatic-Base Interactions. Amsterdam: Elsevier; 1984.
- Ambident reactivity of aryloxide ions towards the super-electrophile, 4,6-dinitrobenzofuroxan. Kinetics, thermodynamics and stereoelectronic factors on regioselectivity. J. Chem. Soc., Perkin Trans.. 1997;2:1019-1026.
- [Google Scholar]
- Ambident nucleophilic reactivity in.sigma.-complex formations. 6. Reactivity-selectivity relationships in reactions of ambident nucleophiles with the superelectrophiles 4,6-dinibrobenzofuroxan and 4,6-dinitro-2-(2,4,6-trinitrophenyl) benzotriazole 1-oxide. J. Org. Chem.. 1987;52:488-495.
- [Google Scholar]
- Synthesis and structure of 2-substituted pyrene-derived scaffolds. Tetrahedron Lett.. 2017;58:4547-4550.
- [Google Scholar]
- The mechanism of diazo-coupling to indoles and the effect of steric hindrance on the rate-limiting step. J. Chem. Soc., Perkin Trans.. 1975;2:1209-1217.
- [Google Scholar]
- Effects of N-acetyl-L-cysteine on bleomycin induced oxidative stress in malignant testicular germ cell tumors. Biochimie. 2012;94:2734-2739.
- [Google Scholar]
- σ-Adduct formation and oxidative substitution in the reactions of 4-nitrobenzofurazan and some derivatives with hydroxide ions in water. Org. Biomol. Chem.. 2003;1:3438-3443.
- [Google Scholar]
- Electrophilic aromatic substitution in substituted anilines; kinetics of the reaction with 4,6-dinitrobenzofuroxan. Can. J. Chem.. 1999;77:639-646.
- [Google Scholar]
- Experimental and theoretical studies of the photophysical properties of 2- and 2,7-functionalized pyrene derivatives. J. Am. Chem. Soc.. 2011;133:13349-13362.
- [Google Scholar]
- Organic chromophores based on a fused bis-thiazole core and their application in dye-sensitized solar cells. Eur. J. Org. Chem. 2013:1916-1928.
- [Google Scholar]
- MCM-SO3H catalyzed synthesis of environment-sensitive fluorophores incorporating pyrene moiety: optimization, fluorescence emission and theoretical studies. J. Photochem. Photobiol. A. 2019;371:306-314.
- [Google Scholar]
- 1-, 2-, and 4-Ethynylpyrenes in the structure of twisted intercalating nucleic acids: structure, thermal stability, and fluorescence relationship. Chem. Eur. J.. 2008;14:9968-9980.
- [Google Scholar]
- Kinetics of the Friedel-Crafts alkylations of heterocyclic arenes: comparison of the nucleophilic reactivities of aromatic and nonaromatic π-systems. J. Org. Chem.. 1998;63:9769-9775.
- [Google Scholar]
- σ-Complex formation and oxidative nucleophilic aromatic substitution in 4-nitro-2,1,3-benzoxadiazoles. Org. Biomol. Chem.. 2003;1:2192-2199.
- [Google Scholar]
- Nanosensors based on viologen functionalized silver nanoparticles: few molecules surface-enhanced Raman spectroscopy detection of polycyclic aromatic hydrocarbons in interparticle hot spots. Anal. Chem.. 2009;81:1418-1425.
- [Google Scholar]
- Ueber Verbindungen des Thiazols (Pyridins der Thiophenreihe) Ber. Dtsch. Chem. Ges.. 1887;20:3118-3132.
- [Google Scholar]
- Nucleophilic reactivities of tributylstannyl-substituted furans and thiophenes. Org. Lett.. 2001;3:1633-1635.
- [Google Scholar]
- A convenient regioselective synthesis of spirooxindolinopyrrolizidines incorporating the pyrene moiety through a [3 + 2]-cycloaddition reaction. Heterocycl. Commun.. 2017;23:379-384.
- [Google Scholar]
- Facile access to regio- and stereoselective synthesis of highly functionalized spiro[indoline-3,2′-pyrrolidines] incorporating a pyrene moiety: experimental, photophysical and theoretical approach. RSC Adv.. 2018;8:24116-24127.
- [Google Scholar]
- Electrophilic substitution in indoles. Part 14. Azo-coupling of indoles with p-nitrobenzenediazonium fluoroborate. J. Chem. Soc., Perkin Trans.. 1987;2:1483-1488.
- [Google Scholar]
- Optical effect of varying acceptors in pyrene donor–acceptor–donor chromophores. Eur. J. Org. Chem. 2017:3980-3985.
- [Google Scholar]
- Structure-nucleophilicity relationships for enamines. Chem. Eur. J.. 2003;9:2209-2218.
- [Google Scholar]
- Synthesis, characterization and pharmacological investigations of some novel heterocyclic derivatives incorporating pyrene and sugar moieties. Res. Chem. Intermed.. 2014;40:1565-1574.
- [Google Scholar]
- Diarylamino- and diarylboryl-substituted donor-acceptor pyrene derivatives: influence of substitution pattern on their photophysical properties. J. Org. Chem.. 2017;82:5111-5121.
- [Google Scholar]
- Electrophilicity parameters for benzylidenemalononitriles. J. Org. Chem.. 2003;68:6880-6886.
- [Google Scholar]
- A 2,7-pyrene-based dye for solar cell application. New. J. Chem.. 2014;38:4404-4408.
- [Google Scholar]
- Colloidal metal oxide nanocrystals as charge transporting layers for solution-processed light-emitting diodes and solar cells. Chem. Soc. Rev.. 2017;46:1730-1759.
- [Google Scholar]
- High-performance electron acceptor with thienyl side chains for organic photovoltaics. J. Am. Chem. Soc.. 2016;138:4955-4961.
- [Google Scholar]
- Kinetic studies of carbocation-carbanion combinations: key to a general concept of polar organic reactivity. Angew. Chem. Int. Ed. Engl.. 2002;41:92-95.
- [Google Scholar]
- Regioregular electrochromic polymers based on thienyl derivatives of fluorescent pyrene monomers: optical properties, spectroelectrochemistry and quantum chemical study. Electrochim. Acta. 2014;122:57-65.
- [Google Scholar]
- Regioselectivity and stereoelectronic factors in the reactions of aryloxide nucleophiles with 4-nitrobenzofuroxan. J. Chem. Soc., Perkin Trans.. 1993;2:1887-1894.
- [Google Scholar]
- Rate constants for the attack of carbenium ions at arenes—a link between the chemistry of aliphatic and aromatic π systems. Angew. Chem., Int. Ed. Engl.. 1992;31:1613-1615.
- [Google Scholar]
- Reference scales for the characterization of cationic electrophiles and neutral nucleophiles. J. Am. Chem. Soc.. 2001;123:9500-9512.
- [Google Scholar]
- Determination of the electrophilic reactivites of 1,1,3-triarylallyl cations. J. Chem. Soc., Perkin Trans.. 2002;2:1435-1440.
- [Google Scholar]
- Mayr, H., Ofial, A.R., 2004. In carbocation chemistry. Olah, G.A., Prakash, G.K.S. (Eds.). Wiley: Hoboken. Chapter 13, pp. 331–358.
- Kinetics of electrophile-nucleophile combinations: a general approach to polar organic reactivity. Pure Appl. Chem.. 2005;77:1807-1821.
- [Google Scholar]
- Scales of nucleophilicity and electrophilicity: a system for ordering polar organic and organometallic reactions. Angew. Chem., Int. Ed. Engl.. 1994;33:938-957.
- [Google Scholar]
- Reactivites and selectivities of free and metal-coordinated carbocations. Pure Appl. Chem.. 1998;70:1993-2000.
- [Google Scholar]
- Pyrene molecular orbital shuffle—controlling excited state and redox properties by changing the nature of the frontier orbitals. Chem. Eur. J.. 2017;23:13164-13180.
- [Google Scholar]
- How constant are Ritchie's “Constant selectivity relationships”? – a general reactivity scale for n-, π-, and σ-nucleophiles. J. Am. Chem. Soc.. 2003;125:286-295.
- [Google Scholar]
- Electrochemical oxidation of σ-complex-type intermediates in aromatic nucleophilic substitutions. Chem. Eur. J.. 2001;7:1712-1719.
- [Google Scholar]
- 2-(2-Hydroxy-phenyl)-5-aminothiazoles: synthesis and properties involving dual emissions. Asian. J. Org. Chem.. 2019;8:1102-1106.
- [Google Scholar]
- Role of electron transfer processes in reactions of diarylcarbenium ions and related quinone methides with nucleophiles. J. Am. Chem. Soc.. 2003;125:10906-10912.
- [Google Scholar]
- Towards a general scale of nucleophilicity? Angew. Chem., Int. Ed.. 2006;45:3869-3874.
- [Google Scholar]
- Photophysical properties of 2,5-diphenyl-thiazolo[5,4-d]thiazole. J. Photochem. Photobiol. A: Chem.. 2001;143:119-127.
- [Google Scholar]
- Relationships between carbocation stabilities and electrophilic reactivity parameters, E: Quantum mechanical studies of Benzhydryl cation structures and stabilities. J. Am. Chem. Soc.. 2002;124:11208-11214.
- [Google Scholar]
- Synthesis of substituted pyrenes by indirect methods. Org. Biomol. Chem.. 2014;12:212-232.
- [Google Scholar]
- Ambident aniline reactivity in Meisenheimer complex formation. J. Am. Chem. Soc.. 1983;105:2473-2474.
- [Google Scholar]
- In Electrophilic Aromatic Substitutions. New York: Wiley; 1990.
- Rate and equilibrium studies in Jackson-Meisenheimer complexes. Chem. Rev.. 1982;82:77.
- [Google Scholar]
- Terrier, F., 1991. In Nucleophilic Aromatic Displacement. Feuer, H. (Ed.). VCH, New York.
- Methanol attack on highly electrophilic 4,6-dinitrobenzofurazan and 4,6-dinitrobenzofuroxan derivatives. A kinetic study. J. Org. Chem.. 1984;49:4176-4181.
- [Google Scholar]
- 4,6-Dinitrobenzofuroxan: a stronger electrophile than the p-nitrobenzenediazonium cation and proton. J. Am. Chem. Soc.. 1992;114:1740-1742.
- [Google Scholar]
- Ranking the reactivity of superelectrophilic heteroaromatics on the electrophilicity scale. J. Org. Chem.. 2005;70:6242-6253.
- [Google Scholar]
- Electrophilic heteroaromatic substitutions: reactions of 5-X-substituted indoles with 4,6-dinitrobenzofuroxan. J. Chem. Soc., Perkin Trans.. 1993;2:1665-1672.
- [Google Scholar]
- Electrophilic aromatic substitutions: reactions of hydroxy- and methoxy-substituted benzenes with 4,6-dinitrobenzofuroxan: kinetics and mechanism. J. Phys. Org. Chem.. 1998;11:707-714.
- [Google Scholar]
- Evidence for a strong enaminic character of 3,4-diaminothiophene: a fast carbon-carbon coupling with 4,6-dinitrobenzofuroxan. J. Org. Chem.. 1993;58:4696-4702.
- [Google Scholar]
- Structure-based drug design of tricyclic 8H-indeno[1,2-d][1,3]thiazoles as potent FBPase inhibitors. Bioorg. Med. Chem. Lett.. 2010;20:1004-1007.
- [Google Scholar]
- Pyrene-based color-tunable dipolar molecules: synthesis, characterization and optical properties. Dyes Pigments. 2018;153:125-131.
- [Google Scholar]
- Photophysical and self-assembly properties of asymmetrical multi-aralkyl and arylaldehyde substituted pyrene derivatives. Tetrahedron. 2012;68:6338-6342.
- [Google Scholar]
- Combining an ionic transition metal complex with a conjugated polymer for wide-range voltage-controlled light-emission color. ACS Appl. Mater. Interfaces. 2015;7:2784-2789.
- [Google Scholar]
- Novel pyrene derivatives: synthesis, properties and highly efficient non-doped deep-blue electroluminescent device. Dyes Pigm.. 2012;92:732-736.
- [Google Scholar]
- A new colorimetric and fluorescent ratiometric sensor for Hg2+ based on 4-pyren-1-yl-pyrimidine. Tetrahedron. 2012;68:3129-3134.
- [Google Scholar]
- The photophysical properties of dipyrenylbenzenes and their application as exceedingly efficient blue emitters for electroluminescent devices. Adv. Funct. Mater.. 2008;18:67-75.
- [Google Scholar]
- Rate-equilibrium relationships in hydride transfer reactions: the role of intrinsic barriers. J. Am. Chem. Soc.. 2002;124:4084-4092.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2019.12.016.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







