Translate this page into:
Nutra-pharmaceutical potential and phytonutrients profiling of wild jujube fruits along with bioactivities studies
⁎Corresponding authors. mnavedahmad@yahoo.com (Naveed Ahmad), bosalvee@yahoo.com (Munawar Iqbal)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The present study reports the phenolic antioxidants and phytonutrients profiling in the wild jujube fruits which are naturally grown in Soon valley of Pakistan. Phenolic antioxidant components were recovered from shade-dried wild jujube fruits using various extracting solvents. Among all extracting solvents tested, aqueous ethanol recovered the maximum amount of extractable antioxidant compounds from the fruits of wild jujube. Crude concentrated extracts (CCEs) and phenolic rich fractions (PRFs) recovered from wild jujube fruits using aqueous ethanol contained higher concentration of total phenolics and flavonoids along with superior biological potential. ICP-OES analysis disclosed the occurrence of twenty-five minerals, where potassium (14.80 g/kg) and calcium (1.81 g/kg) were the dominant macro elements. The tested wild fruits juice was found to contain individual natural sugars including galactose (1.27 g/100 g dry weight), glucose (1.07 g/100 g dry weight), sucrose (0.70 g/100 g dry weight) and xylose (0.04 g/100 g dry weight); and gluconic acid (2.10 mg/100 g of dry matter) as dominant organic acid when analyzed on HPLC. The concentration of phenolic antioxidants and biological activities vary significantly (p < 0.05) among extracting systems used. A strong correlation was also recorded among total phenolic (TP), total flavonoids (TF) and biological attributes of tested wild fruits. The results of this study explored wild jujube fruits as a propitious source of natural phenolic components and valuable nutrients which advocate its potential use in the development of functional food and nutraceutical industry.
Keywords
Wild Jujube
Nutra-pharmaceutical
Phenolics
Biological attributes
ICP-OES
1 Introduction
Lipid oxidation is a severe problem in food industry due to its deleterious effects including off flavors and rancidity. The loss of nutritional and organoleptic value of various food stuffs has been attributed to its deleterious effects (Manian et al., 2008). In biological systems, the perceived toxicity of reactive oxygen species (ROS) and free radicals is associated to the incidence of various ailments including cardiovascular disorders, inflammations, certain cancers, aging and metabolic syndromes and aging (Anwar et al., 2013; Raghuveer, 2009). Plants have extensively been used as promising source of folk medicine, fuel and food. Due to occurrence of different class of phytochemicals including terpenoids, alkaloids, phytosterols and polyphenols, plants impart medicinal benefits. Phenolic components exhibit different medicinal properties including lower incidence of certain cancers, metabolic syndromes and inflammation ((Arabshahi-Delouee and Urooj, 2007; Hussnain Siddique et al., 2022; Kamarazaman et al., 2022; Malik et al., 2022).
Though the conventional food plants have been extensively used to regulate and maintain various bodily processes. However, to cope the challenges of growing concerns of public health and food insecurity with growing population, there is dire need for exploration of vast resources of under-utilized (wild and semi wild) food plants for potent bioactive components and valuable nutrients (Shahidi, 2009). The fruits of naturally grown plants have been documented as potential source of folk medicines and food in various civilizations of the world (Danbury et al., 2000).
Pakistan has huge reserves of potent medicinal flora which are available for bioprospecting. A variety of plant species, naturally distributed in Pakistan, are traditionally very popular to be consumed as folk medicine due to the presence of valuable bioactives and optimum quantity of nutrients (Huang and Wang, 2004). Among others, wildly grown fruit plants like Jujube are potential candidates to be investigated for the presence of high-value phytochemicals to develop nutraceuticals and functional foods with therapeutic properties.
Jujube (Ziziphus jujuba Mill), member of Rhamnaceae family, is grown in subtropical and tropical regions of America and Asia. Its fruits have extensive applications in different processed products including cakes, loaf, jams and jelly. Different parts (fruits, seeds, bark, leaves and roots) of jujube have been utilized in different folk medicine systems to provide protection against various ailments due to occurrence of considerable amount of different vitamins, individual sugars, valuable minerals, fatty acids and potent bioactives (Abdel-Zaher et al., 2005; Memon et al., 2012). Red date or jujuba fruit has been stated as ‘‘fruit of life’’ which is promising source of functional ingredients including flavonoids, phenolics, saponins and polysaccharides. These potent ingredients have been believed for different biological potential such as immune function regulation, lowering of blood triglyceride and anti-proliferation activity of cancer cell (Dahiru and Obidoa, 2008; Li et al., 2011).
Recently, scientists and food biotechnologist have focused different vegetables and fruits including jujube for the presence of phenolic components which have extensively contributed to establishing their antioxidant potential. Antioxidant and anti-inflammation potential of jujube have been ascribed to the occurrence of various phenolic acids including p-hydroxybenzoic, caffeic, ferulic and p-coumaric acids (Kamiloǧlu et al., 2009; Muchuweti et al., 2005). The seeds of jujube have been found very effective to improve the level of glucose in blood (Al-Reza et al., 2010). Due to high nutritional and therapeutic value of jujube fruits, it has commonly been consumed as folklore medicine to cure different ailments including palliative, antibechic, analeptic (Memon et al., 2012).
Due to the growing interest in medicinal properties of wild plants, exploration of composition of under-utilized wild fruits is promptly needed. The importance of phenolic bioactives as chemo-preventive agent together with the existing information gap invigorated us to characterize phytonutrients and phenolic antioxidant compounds in wild jujube fruit. This is a first report on analytical characterization of phytonutrients and phenolic antioxidant components in wild Jujube fruits native to Pakistan. Therefore, this research project (Fig. 1) was planned to evaluate various phenolic antioxidant compounds, high-value phytonutrients and biological activities in the fruits of wild Jujube from Soon valley of Punjab, Pakistan for exploring their potential to develop functional foods and nutraceuticals.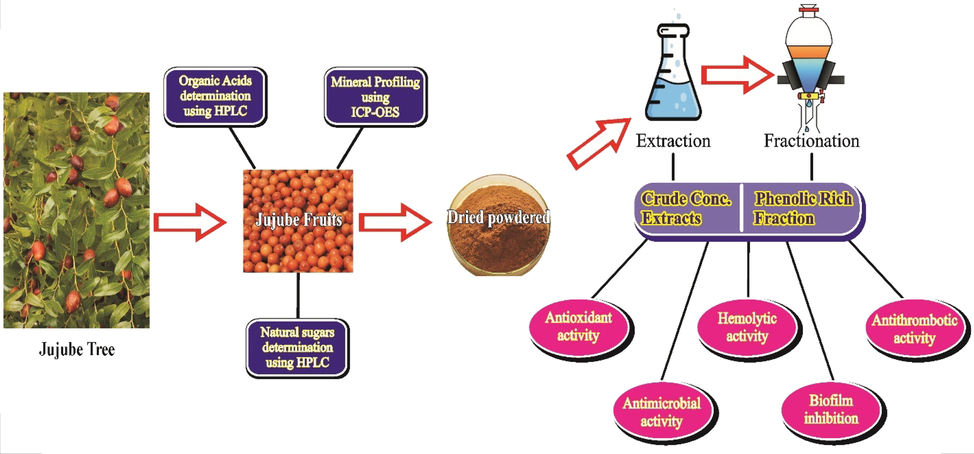
Schematic presentation of the present research work undertaken.
2 Material and methods
2.1 Extraction, fractionation and pretreatment of samples
The fruit samples were taken at full ripening stage from Jujube trees, grown naturally in Soon valley of Punjab, Pakistan (Specific Geographical Coordinates: 32o58/N 72o15/E). This valley has specific agroclimatic conditions where annual precipitation is 50 cm, minimum and maximum average temperatures are 1 °C and 36 °C, respectively and annual precipitation (50 cm). After authentication by a taxonomist, collected fruits samples were washed using distilled deionized water and shade-dried followed by storing at −20 °C in polythene bags till further analysis. These samples were pulverized using an electric grinder machine (Tector–Cemotec 1090 sample mill, Hognas, Sweden). These minced samples were soaked in different extraction solvents such as aqueous methanol (80: 20, methanol: water), absolute methanol (100 %), aqueous ethanol (80: 20, ethanol: water), absolute ethanol (100 %), aqueous acetone (80: 20, acetone: water) and absolute acetone (100 %) for five days.
These mixtures were filtered through filter paper (Whatman, 8µ) to separate insoluble residue followed the removal of excessive solvent using rotavapor (EYELA, Tokyo, Japan) to produce crude concentrated extracts (CCEs). These recovered CCEs were processed for fractionation to achieve phenolic rich fraction (PRFs) following a method (Maheshwari et al., 2011). The CCEs and PRFs were preserved at −20 °C freezer to be analyzed further.
2.2 Total flavonoid content (TFC) in CCEs/PRFs
TFC in the ripened fruits of wild jujube was quantified following a protocol stated by Ahmad et al. (Ahmad et al., 2011). Briefly, CCEs/PRFs (100 mg/mL) was mixed with NaNO2 (5 %; 0.3 mL) in distilled water (5.0 mL). After incubation, resulting solution was allowed to react with AlCl3 (10 %; 0.6 mL) and NaOH (1.0 M; 2 mL). The optical density (OD) resultant solution was taken at 510 nm. The concentration of TFC was estimated as catechin equivalents (CE) g/100 g dry matter using catechin calibration curve.
2.3 Total phenolic content (TPC) in CCEs/PRFs
TPC in the ripened fruits of wild jujube were estimated using Folin–Ciocalteu reagent method stated by Ahmad et al. (Ahmad et al., 2011). Concisely, CCEs/PRFs (1 mg/mL) was diluted in deionized water (7.5 mL), mixed with sodium carbonate (20 % (w/v); 1.5 mL) and Folin-Ciocalteu reagent (0.5 mL) followed by heating at 40 °C for 20 min using water bath. The absorbance of final solution was taken at 755 nm using a spectrophotometer (Hitachi U-2001). TPC were determined as Gallic Acid Equivalents (GAE) g/100 g dry matter using gallic acid standard curve.
2.4 Free radical scavenging attributes of CCEs/PRFs
DPPH radical scavenging assay was conducted following a protocol Tepe etal. (Tepe et al., 2005) to evaluate the potential of CCEs/PRFs, recovered from ripened fruits of wildly grown jujube, to inhibit DPPH radical in terms of IC50 value. The methanolic solution of DPPH (0.004 %; 5 mL) was mixed with each concentration (0.10 to 5.0 mg/mL) by incubating in ambient conditions for 30 mins. OD of each solution was recorded at 517 nm (Eq. (1)).
Where, As and Ab are OD of sample and blank, respectively, while I is inhibition potential.
2.5 Reducing capacity of CCEs/PRFs
Reducing ability of CCEs/PRFs were estimated following a methodology stated by (Yen et al., 2000). Concisely, equal amount of potassium ferricyanide (1.0 %), sodium phosphate buffer (0.2 M, pH 6.6) and CCEs/PRFs (5–20 mg) were mixed and allowed to incubate at 50 °C for 20 min. Then trichloroacetic acid (10 %; 5 mL) was reacted with resultant solution to spin (980 g;10 min) using centrifuge machine (CHM-17; Kokusan Denki, Japan). The supernatant (2.5 mL) was recovered and then reacted with FeCl2 solution (0.1 %; 0.5 mL) and distilled water (2.5 mL). The OD of the final solution was recorded at 700 nm using spectrophotometer (Hitachi U-2001).
2.6 Inhibition of peroxidation
The potential of various CCEs/PRFs was measured by inhibiting peroxidation in linoleic acid system following a protocol stated by Ahmad et al. (Ahmad et al., 2011). Sample solution was prepared by mixing CCEs/PRFs (5 mg) with sodium phosphate buffer (0.2 M; pH = 7; 10.0 mL), ethanol (99.8 %; 10 mL) and linoleic acid (0.13 mL) followed by addition of distilled water (5 mL) and incubation at 40 °C. The method developed by Yen et al., (2000) was used to measure degree of oxidation. Concisely, ammonium thiocyanate (30 %; 0.2 mL), ethanol (75 %;10 mL), ferrous chloride (20 mM in 3.5 % HCl; 0.2 mL) and sample solution (0.2 mL) were mixed and its OD was taken at 500 nm (Eq. (2)).
Where, I (%) is a percentage inhibition, As and Ac are absorbance values of sample and control (treatment without CCE/PRF) at 350 h. Positive controls: ascorbic acid and butylated hydroxytoluene.
2.7 Antimicrobial capacity
Antimicrobial attribute of CCEs/PRFs was determined against a set of pathogenic microbial strains (Escerichia coli, Bacillus cereus, Fusarium oxysporum, Aspergilus flavus, Staphylococcus aureus and Aspergilus niger) following disc diffusion and micro dilution broth methods (NCCLS, 1999, 1997). Fungal and bacterial strains were grown at 29 ± 2 °C and 37 ± 2 °C on potato dextrose agar and nutrient agar (Oxoid), respectively.
Briefly, broth culture (100 µL) of bacterial cell (with 108 cfu/mL) and fungal strains (104 cfu/mL spores) were spread on their respective growth media. Filter paper discs (6 mm in diameter) were autoclaved and then placed on microbial cultured plates after soaking in CCEs/PRFs (100 mg/mL). Negative control (without sample) and positive control (rifamycin and fluconazole) were also run under same experimental conditions. Sample and control plates were processed for incubation at 29 °C for 48 h for fungal and 37 °C for 24 h for bacterial strains. The inhibition of tested microbial growth was appraised measuring the zone of inhibition (mm) against the control to express antimicrobial potential.
Minimal inhibitory concentration (MIC) was calculated by culturing bacterial and fungal strains in nutrient broth (NB) and sabouraud dextrose broth (SDB) augmented with Tween 80. Positive controls (tested microorganisms without extracts), sterility control (Tween 80 + test extract + NB) and growth control (Tween 80 + NB) were also run under similar experimental conditions. Rifamycin and fluconazole were employed as standard bactericidal and fungicidal compounds, respectively. After incubation, the lowest concentration of CCE/PRF without growth was recorded as MIC value.
2.8 Inhibition of biofilm formation
In this analysis, the potential of various CCEs/PRFs was assessed by inhibiting the formation of biofilm by different bacterial culture such as E.coli and S. aureus (Stepanović et al. 2000). Briefly, samples (2.5 and 5.0 μg) were dissolved in DMSO and mixed with nutrient broth (100 μL) in wells of microtiter plate followed inoculation with bacterial culture (20 μL with 109 CFU/mL). Negative control (growth medium and bacterial culture) and positive control (rifampicin and growth medium) were also run under similar experimental conditions. Microtiter plate containing sample and control treatment were allowed for incubation at 37 °C for 24 h. followed by washing with sterile phosphate buffer (pH: 7.2; 220 μL) to detach loosely attached bacterial cells. Then aqueous methanol (99 %, 220 μL) was added to each well for 15 min followed by emptied and dried. Each well of microtiter plate was rinsed with distilled water after staining with crystal violet (50 %, 220 μL) for 5 mins. After drying each well, bound stain was resolubilized in glacial acetic acid (33 %, 220 μL). OD each well was recorded by microplate reader (Biotek, USA) at 630 nm. Inhibition (%) of bacterial growth was estimated as shown in Eq. (3).
2.9 Haemolytic activity of CCEs/PRFs
A spectrophotometric technique was utilized to assess haemolytic potential of CCEs/PRFs (Yang et al., 2005). Briefly, equal volume of each concentration of plant extract (125, 250, 500 and 1000 µg/ml) was reacted with cell suspension by incubating (30 min) at 37 °C. The resulting mixture was allowed to spin at 1500 rpm. Consequently, free hemoglobin was estimated in recovered supernatant by processing at 540 nm using UV–vis spectrophotometer. Minimal (phosphate buffer saline) and maximal (distilled water) hemolytic controls were also processed under same experimental conditions and percentage hemolysis is estimated Eq. (4).
Where, At, An and Ac represented absorbance values of sample, control (saline control) and control (water control).
2.10 Antithrombotic activity
Antithrombotic activity of CCEs/PRFs was carried out following a method (Prasad et al. 2006). Briefly, blood sample (0.5 mL) was mixed with different concentrations of CCEs/PRFs in separate micro-centrifuge tubes. Positive control (streptokinase) and blank (normal saline only) were also run along with sample treatments. Reaction between blood sample and plant extract was noted. All reactions were processed in water bath (37 °C).
2.11 Quantification of minerals
The fully ripened fruits of wildly grown jujube were examined for minerals by inductively coupled plasma-optical emission spectroscopy (ICP-OES) (Link et al., 1998). Fruit samples were digested with concentrated HNO3 and HCl (1:3) in microwave oven for 9.5 min at 182 °C following USEPA method (3051A). The reaction mixture was cooled down, filtered, centrifuged and then diluted to required volume with water followed by analysis using ICP-OES. Preparation of samples and standards, dilution and washing were carried out using high-purity water (18 MΩ cm).
2.12 Analysis of organic acids and HPLC conditions
The organic acid profiling of wild jujube fruits were performed according to the protocol (Mahmood et al., 2012). Concisely, fresh wild olive fruits (10 g) were crushed with distilled water (20 mL) in a blender machine (Ika-Labortechnik). The resulting fruit juice was subjected to spin for 10 min at 3000 g. The suspended debris in supernatant were removed by filtering through filter paper (0.25 µm).
The recovered fruit juice was analyzed for organic acid using HPLC (Varian Pro star, USA) coupled with UV–vis detector and column (RP-C18, 250 × 2.0 mm × 1/4″). The flow rate of mobile phase (0.001 N H2SO4) was kept 1.0 mL/min. Organic acids were analyzed at 230 nm and their quantitative was done by calibration method of external standards (acetic, gluconic, succinic, oxalic, malic and citric acids). The processing of obtained chromatographic data was done using Varian Chem. Station.
2.13 Analysis of individual sugars and HPLC conditions
Quantitative analysis of sugars in wild jujube fruits was done following a protocol reported by Mahmood et al. (Mahmood et al., 2012). Concisely, fresh wild olive fruits (10 g) were crushed with distilled water (20 mL) in a blender machine (Ika-Labortechnik). The resulting fruit juice was subjected to spin for 10 min at 3000 g. The suspended debris in supernatant were removed by filtering through filter paper (0.25 µm).
The recovered fruit juice was analyzed for individual sugars using HPLC (Shimadzu, Japan) coupled with refractive index detector (RID-10A) and column (Lichrospher R-100). Acetonitrile was chosen as mobile phase whose flow rate was adjusted at 0.6 mL/min. Retention times of samples were compared with those of standards to detect and quantify natural sugars.
2.14 Statistical treatment
All experimental data was statistically analyzed using STATISTICA 5.5 (Stat SoftInc, Tulsa, Oklahoma, USA) software.
3 Results and discussion
3.1 Extraction yield
Due to difference in the polarity of individual plant phenolic compounds, pure solvents and their aqueous mixtures were used to extract potent bioactives from the wild jujube fruits. The yield of extraction (g/100 g) of crude concentrated extracts (CCEs) from the tested fruits with different solvent systems including aqueous ethanol (80:20, ethanol: water), absolute ethanol, aqueous methanol (80:20, methanol: water), absolute methanol, aqueous acetone (80:20, acetone: water) and absolute acetone was recorded (Fig. 2., S1). The yield of extractable bioactives, recovered from tested wild fruits was found to vary noticeably over the range of 6.95–55.63 g/100 g of dry matter. The maximum yield (55.63 %) of CCE was recovered using aqueous ethanol while the absolute acetone extracted the minimum yield (6.95 %) of CCE from tested wild fruits.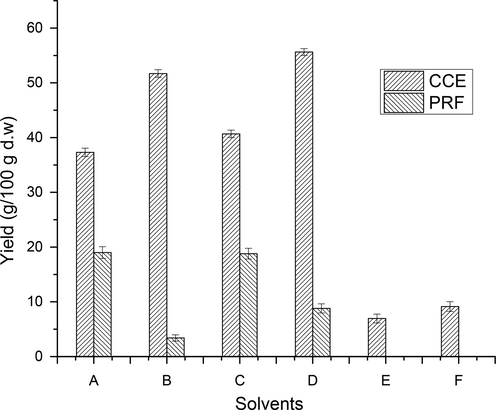
Extraction yield of CCEs/PRFs of wild jujube fruits (A = Absolute Methanol, B = Aqueous Methanol, C = Absolute Ethanol, D = Aqueous Ethanol, E = Absolute Acetone and F = Aqueous Acetone).
The CCEs of tested wild jujube fruits have some impurities including lipid molecules which are responsible to hinder the detection and eventually affect the true antioxidant potential of recovered extracts. To remove these types of interfering substances, CCEs were further purified/fractioned using sequential liquid–liquid fractionation. The yield of PRFs from CCEs, recovered using different extraction solvents from fruits of wild jujube, was calculated (Fig. 2, S1). The yield of PRFs from wild jujube fruits varied over the range of 3.40–19.00 g/100 g of crude extract. The highest PRF yield (19.00 %) was purified from absolute ethanol derived CCE while the minimum PRF yield (3.4 %) was produced from aqueous methanolic extract.
The higher yield of extracts from jujube fruits with aqueous ethanol supports its better efficiency to recover extractable antioxidant molecules. The yield of phenolic antioxidant molecules, recovered from tested wild fruits with various extracting solvent systems, were found to vary significantly (p < 0.05). The differences in the extraction yield with different solvents was also reported (Shabir et al., 2011; Sultana et al., 2009). According to different reports, aqueous ethanol and aqueous methanol have been proven to be efficient to recover phenolic bioactives from tested samples (Chen et al., 2001; Jiao and Zuo, 2009; Manzoor et al., 2013; Shabir et al., 2011; Zuo et al., 2002). In current analysis, aqueous ethanol can be a good selection for optimal extraction food-grade bioactives. Afroz et al., (2014) employed methanol as extracting solvent to recover crude extract (11.25 %) from the fruits of jujube cultivar, however, this extraction yield is lower than those of extracted with methanol in present analysis. The variation in yield of extract might be ascribed to nature of soil, amount of extractable components and agro-climatic conditions of experimental sites (Mahmood et al., 2012).
3.2 Total phenolics content (TPC) and total flavonoids content (TFC)
Due to the availability of various potent bioactives in plant phenolics, its utilization in food industry has continuously been increasing over the past decades (Wojdyło et al., 2007). Antioxidant potential of various vegetables and fruits was attributed to their phenolic and flavonoid molecules (Katalinic et al., 2006).
TPC and TFC in CCEs/PRFs of the wild jujube fruits with different extracting solvent systems are estimated (Figs. 3-4), S1). The amount of TPC and TF in CCEs of tested fruits was found to be in the range of 0.01–2.45 GAE (g/100 g) & 0.01–0.17 CE (g/100 g), respectively. The highest TPC (2.45 g/100 g of DW) and TF (0.17 CE g/100 g) was found in CCE extracted with aqueous ethanol while minimum amount of TPC (0.01 g/100 g of DW) and TFC (0.01 CE g/100 g) was found in CCE of absolute acetone. The amount of TP recovered from wild jujube fruits with various extraction solvents was found to vary significantly (p < 0.05).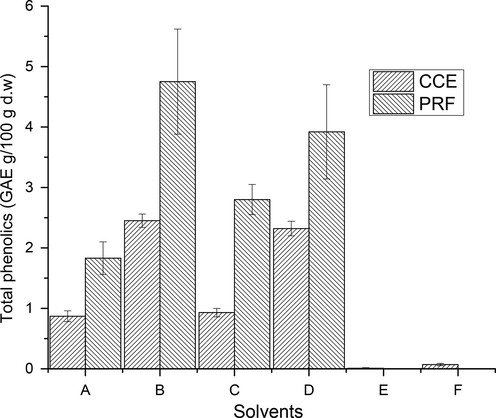
Total phenolic content in CCEs/PRFs of wild jujube fruits (Explanation as given in Fig. 2).
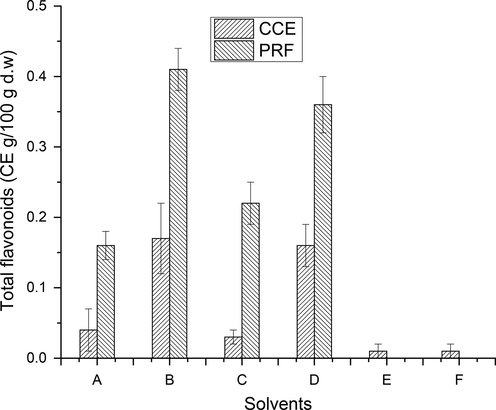
Total flavonoid content in CCEs/PRFs of wild jujube fruits (Explanation as given in Fig. 2).
TPC and TFC in wild jujube fruit PRFs was found to vary from 1.83 to 4.75 GAE (g/100 g) & 0.16–0.41 CE (g/100 g). The PRF, purified from aqueous ethanol CCE, contained highest TPC (4.75 GAE g/100 g) and TFC (0.41 CE g/100 g), respectively. The minimum amount of total phenolics (1.83 GAE) and total flavonoids (0.16 CE g/100 g) was recorded in PRF, purified from absolute methanol CCE. The amount of TP and TF in CCEs and PRFs of tested wild fruits varied significantly (p < 0.05) relative to extracting solvents. Though, the yield of PRFs was lower than that of CCEs, but their total phenolic and flavonoid content were approximately 3–4 times higher than those of CCEs. The data thus obtained in this study indicated a successful purification of phenolic compounds through sequential liquid–liquid extractions.
TPC and TFC in the fruits of wild jujube were determined rarely before. However, TPC in the present analysis was found to be higher than those of fruits from different jujube cultivars (Das, 2012; Gao et al., 2012, 2011; Kamiloǧlu et al., 2009; Li et al., 2005; Wang et al., 2019; Wu et al., 2012; Xue et al., 2009; Zhang et al., 2021; Zhao et al., 2014), however, lower than the findings of Gunduz and Saracoglu, (Gunduz and Saracoglu, 2014). TFC in tested wild jujube fruit was lower than those of different jujube fruit cultivars (Zhao et al., 2014). The TPC of tested wild fruits of jujube was higher than those of methanolic extract of wild orange (Lala et al., 2020), acidified methanolic extract of wild black berry (Radovanović et al., 2013), aqueous methanolic extract of raspberry fruit (Bobinaite et al., 2013). The amount of TF in tested fruit sample was superior to those reported by (Nakilcioğlu and Hışıl, 2013), but comparable to methanolic extract of wild orange (Lala et al., 2020). These differences in the amounts of TFC and TPC among various varieties of fruit from different origin may be attributed to stage of maturity, harvesting season, agro-climatic conditions and genetic makeup of the plants.
3.3 Antioxidant potential in terms of reducing power
The reducing capacity of CCEs/PRFs, recovered from wild jujube fruit, was assessed by measuring the reduction of ferric into ferrous with the help of reducing agents present in the CCEs/PRFs (Joshi et al., 2010). Antioxidant potential of CCEs/PRFs is directly linked to the amount of reducing agents in it (Zou et al., 2004). In this work, regular pattern of rise in reducing potential as a function of concentration of CCEs/PRFs was observed (Figs. 5-6), S2). The reducing potential of CCEs and PRFs, recovered from wild jujube fruit, were significantly (p < 0.05) differ from each other. Li et al. (Li et al., 2005) noticed similar trend of reducing potential in relation to the concentration extracts of different Chinese jujube cultivars.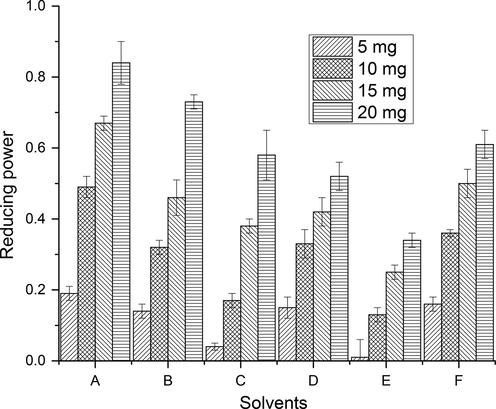
Reducing potential of CCEs of wild jujube fruits (Explanation as given in Fig. 2).
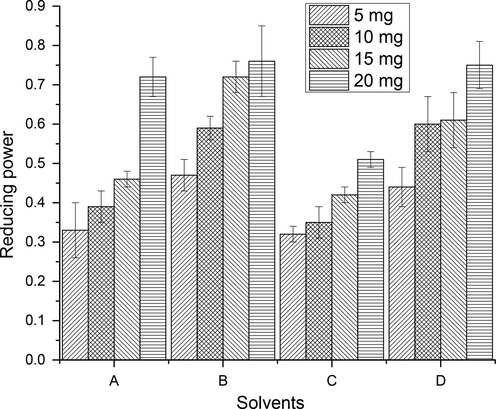
Reducing potential of PRFs of wild jujube fruits (Explanation as given in Fig. 2).
The occurrence of reducing agents in the extracts induces the reduction of Fe3+/ferricyanide complex into Fe2+. Color intensity of resultant complex depends on the reducing ability of bioactive components in extracts. So, the absorption of light was enhanced with higher color intensity of complex and consequently antioxidant capacity was improved (Zou et al., 2004).
3.4 Free radical scavenging potential
DPPH, a stable nitrogen-centered free radical, has absorption maxima in the range of 515–528 nm. By having proton from phenolic components, DPPH becomes yellow by losing its chromophore. With the increasing concentration of phenolic components in plant extracts, its antioxidant potential (DPPH radical scavenging ability) also enhanced (Larrauri et al., 1999). Various extracts (CCEs and PRFs), recovered from wild jujube fruits using different solvents exhibited an appreciable DPPH radical scavenging activity by exhibiting IC50 values over the range of 0.13–1.73 mg/mL and 0.09–0.37 mg/mL, respectively (Fig. 7, S2). Among all extracts, CCE/PRF recovered using aqueous ethanol showed highest free radical scavenging potential with corresponding IC50 value (0.13 mg/mL, 0.09 mg/mL). All the tested CCEs/PRFs showed lower antioxidant potential in comparison to positive control (BHT). DPPH radical scavenging ability of tested CCEs/PRFs of wild jujube fruits can be linked with phenolic compounds present in them (Siddhuraju et al., 2002).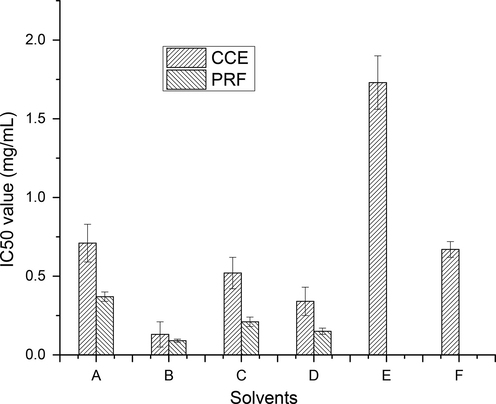
Free radical scavenging potential of CCEs/PRFs of wild jujube fruits (Explanation as given in Fig. 2).
DPPH radical scavenging ability of PRFs (IC50 value; 0.09–0.37 mg/mL) was noticed to be significantly (p < 0.05) lower than those of CCEs (IC50 value; 0.13–1.73 mg/mL). This increment in antioxidant potential in terms of DPPH radical scavenging activity may be ascribed to enhanced content of phenolic components in PRF after successful fractionation of CCEs.
There is no report available on DPPH radical scavenging activity of CCEs/PRFs, recovered from wild jujube fruits. The results of DPPH radical scavenging assay of CCEs/PRFs of wild jujube fruit was in line with those of reported by Afroz et al. (Afroz et al., 2014). IC50 value of present analysis was found to be higher than the findings of Plastina et al. (Plastina et al., 2012) and lower than the results of Das, (Das, 2012). Zhang et al. (Zhang et al., 2010) and Gao et al. (Gao et al., 2011) estimated DPPH radical scavenging capacity of different jujube fruit cultivar over the range of (28.56–51.30 mg AEAC/100 g DW) and (1.35–3.38 mmol trolox eq/100 g FW), respectively. DPPH radical scavenging potential of tested jujube fruit’s CCEs/PRFs was found to be higher than those of hydroxymethanolic extract of apple (Pires et al., 2018), aqueous-ethanolic extract of wild apricot (Qin et al., 2019).
3.5 Inhibition of peroxidation
Various CCEs/PRFs of wild jujube fruits were evaluated to assess the ability to inhibit the process of peroxidation in lipid molecules (Fig. 8., S2). Lipid molecule such as linoleic acid produces peroxides ions by oxidation. These peroxides were found to produce a complex by combing with SCN- whose concentration was estimated at 500 nm using double beam spectrophotometer. Wild jujube fruit’s CCEs/PRFs inhibited the lipid peroxidation in the range of 34.46–74.91 % and 74.02–81.91 %, respectively. The highest potential to inhibit the peroxidation was exhibited by CCE (74.91.65 %) and PRF (81.91 %) recovered using aqueous ethanol while the lowest was exhibited by CCE (34.46 %) and PRF (74.02 %) using absolute acetone and aqueous ethanol, respectively.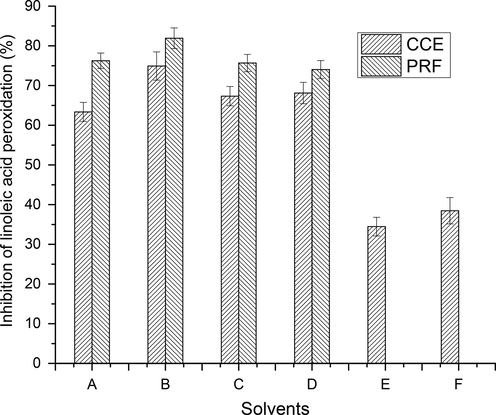
Inhibition of peroxidation by CCEs/PRFs of wild jujube fruits (Explanation as given in Fig. 2).
Among all extracts, CCE/PRF recovered with aqueous ethanol exhibited highest ability to retard the process of peroxidation in lipid molecules showing its superior antioxidant activity that is ascribed to higher concentration phenolic components in them. The ability of various CCEs/PRFs to inhibit the peroxidation in lipid molecules were found to be lower than that of BHT (92.01 %). Wild jujube fruit’s PRFs showed considerably higher potential to retard the process of oxidation in lipidic molecules in comparison to their corresponding CCEs, which revealed increment in the concentration of phenolic components due to the successful pre-concentration step. No reports are present on inhibition of lipid peroxidation by tested wild jujube fruit. Li et al. (2005) and Zhao et al. (2014) reported the potential of crude extract, recovered from jujube fruit cultivars, to inhibit lipid peroxidation in the range of 28.4–87.8 % and 73.3 %, respectively.
3.6 Antimicrobial activity of CCEs and PRFs
A variety of antimicrobial drugs have been isolated from different plant materials to cure different health disorders (Ahmad et al., 2011; Hussain et al., 2011, 2008). Antimicrobial potential of CCEs/PRFs, recovered from wild jujube fruits, was evaluated against different microbial strains (Table 1) by measuring the zone of inhibition and MIC. These MIC values provide information about the concentration of antimicrobial agents to retard the bacterial growth (Walsh et al., 2003). The letters (superscripts) with the given data (mean ± SD) in a column shows significant difference (p< 0.05) among the solvents.
SolventsZone of inhibition (mm)
MIC (µg/mL)
CCE
PRF
CCE
PRF
S. aureus
E.coli
B. cereus
S. aureus
E.coli
B. cereus
S. aureus
E.coli
B. cereus
S. aureus
E.coli
B. cereus
Absolute Methanol
10.0 ± 0.7de
12.0 ± 0.5c
8.0 ± 0.4d
11.0 ± 0.7d
14.0 ± 0.9c
9.0 ± 0.6d
225 ± 8b
236 ± 6b
241 ± 9ab
209 ± 8a
195 ± 6a
220 ± 8b
Aqueous Methanol
15.0 ± 0.8b
15.0 ± 0.7b
14.0 ± 0.5b
17.0 ± 0.8b
16.0 ± 0.6b
14.0 ± 0.8b
193 ± 7d
201 ± 8d
219 ± 9bc
165 ± 9c
169 ± 10b
184 ± 9c
Absolute Ethanol
11.0 ± 0.8d
13.0 ± 0.7bc
10.0 ± 0.7c
14.0 ± 0.7c
15.0 ± 0.8bc
12.0 ± 0.8c
219 ± 9bc
209 ± 9 cd
249 ± 9a
199 ± 8bc
188 ± 9a
244 ± 9a
Aqueous Ethanol
13.0 ± 0.6c
14.0 ± 0.5bc
11.0 ± 0.6c
14.0 ± 0.8c
15.0 ± 0.9bc
10.0 ± 0.7 cd
209 ± 6c
223 ± 8c
234 ± 8b
187 ± 9b
176 ± 7ab
235 ± 10a
Absolute Acetone
7.0 ± 0.2e
5.0 ± 0.2e
8.0 ± 0.5d
–
–
–
252 ± 11a
270 ± 11a
221 ± 6bc
–
–
–
Aqueous Acetone
8.0 ± 0.3e
9.0 ± 0.3d
10.0 ± 0.8c
–
–
–
242 ± 8ab
225 ± 10bc
216 ± 8c
–
–
–
Rifamycin
24a
26a
18a
24a
26a
18a
124e
96e
146e
124c
96d
146c
Staphylococcus aureus was the most sensitive bacterial strain with greater zone of inhibition ranging (7–15 mm and 11–17 mm) with lower crossponding MIC values (193–252 µg/mL and 165–209 µg/mL), respectively, when exposed to CCEs and PRFs of wild jujube fruit. Highest antimicrobial activity with lowest MIC value was recorded for CCE (193 µg/mL) and PRF (165 µg/mL), recovered with aqueous methanol, against Staphylococcus aureus. Lowest antimicrobial activity of wild jujube fruit’s extract, recovered with absolute acetone, was recorded with relatively smaller zone of inhibition (5–8 mm) and corresponding higher MIC values (221–252 µg/mL). Antimicrobial activity of CCEs and PRFs was significantly (p < 0.05) lower in comparison to positive control (rifamycin).
Aspergilus niger was the most sensitive fungal strain against wild jujube fruit’s CCEs/PRFs exhibiting greater inhibition zone (5–16 mm and 13–19 mm) with corresponding lower MIC values (191–289 µg/mL and 160–207 µg/mL) (Table 2), respectively. Fusarium oxysporum was the least sensitive fungal strain against CCEs and PRFs with relatively larger inhibition zones (5–13 mm; 9–13 mm) with their corresponding higher MIC values (209–301 µg/mL; 129–251 µg/mL). The higher antimicrobial potential of CCE and PRF, recovered using aqueous ethanol, is ascribed to the higher concentration of phenolic components in them. Plant phenolic bioactives have a variety of biological attributes including antimicrobial, antioxidant, astringent, anti-inflammation and astringent activity (Anwar et al., 2015; Jacob Vaya and Saeed Mahmood, 2006; Rubnov et al., 2001). The letters (superscripts) with the given data (mean ± SD) in a column shows significant difference (p< 0.05) among the solvents.
SolventsZone of inhibition (mm)
MIC(µg/mL)
CCE
PRF
CCE
PRF
A. niger
A. flavus
F. oxysporum
A. niger
A. flavus
F. oxysporum
A. niger
A. flavus
F. oxysporum
A. niger
A. flavus
F. oxysporum
Absolute Methanol
12.0 ± 0.5bc
10.0 ± 0.6c
9.0 ± 0.6d
14.0 ± 0.8c
11.0 ± 0.7c
9.0 ± 0.4c
208 ± 7 cd
231 ± 7bc
265 ± 9b
199 ± 9ab
224 ± 6b
237 ± 8b
Aqueous Methanol
16.0 ± 0.9b
15.0 ± 0.8b
13.0 ± 0.8b
19.0 ± 1.2b
16.0 ± 0.4b
13.0 ± 0.8b
191 ± 9d
183 ± 9d
209 ± 8d
160 ± 6b
198 ± 5c
205 ± 6c
Absolute Ethanol
12.0 ± 1.0bc
14.0 ± 1.1b
10.0 ± 0.7 cd
14.0 ± 0.9c
16.0 ± 0.5b
10.0 ± 0.4bc
251 ± 8b
210 ± 10c
276 ± 10b
190 ± 7ab
181 ± 7d
129 ± 5d
Aqueous Ethanol
13.0 ± 0.8c
9.0 ± 0.9 cd
11.0 ± 0.5c
13.0 ± 0.6c
10.0 ± 0.9c
10.0 ± 0.7bc
219 ± 10c
245 ± 10b
237 ± 9c
207 ± 9a
240 ± 9a
251 ± 9a
Absolute Acetone
5.0 ± 0.6e
6.0 ± 0.5e
5.0 ± 0.3e
–
–
–
289 ± 9a
281 ± 7a
301 ± 12a
–
–
–
Aqueous Acetone
9.0 ± 0.2d
8.0 ± 0.4d
8.0 ± 0.5de
–
–
–
251 ± 7b
262 ± 9ab
271 ± 10b
–
–
–
Fluconazole
23a
24a
20a
23a
24a
20a
134e
126f
160e
134d
126d
160c
Indian jujube was evaluated for antimicrobial traits against a set of pathogenic microbial strains including E.coli, B. cereus, S. aureus, and A. niger with zone of inhibition as 14.33, 13, 15.33 and 7.67 mm, respectively (Das, 2012). Daneshmand et al. (Daneshmand et al., 2013) appraised Iranian jujube for their antimicrobial potential of against a panel of bacterial strains including S. aureus, E. coli and B. cereus with MIC value as 0.65, 2.26 and 1.5 mg/mL, respectively, which is found to be lower than that of results of present analysis. Similarly, antimicrobial activity of Bangladeshi jujube fruits was estimated against E. coli and S. aureus by measuring the zone of inhibition as 17.50 and 16.83 mm, respectively (Afroz et al., 2014), which is higher than those of our present analysis.
The CCEs/PRFs of wild jujube fruit was found to exhibit superior antimicrobial potential against E.coli, S. aureus and B. cereus in comparison to: methanolic extract of wild orange against staphylococcus sp. (Lala et al., 2020); aqueous ethanolic extract of wild apricot against S. aureus, E.coli and B. cereus (Qin et al., 2019); acidified methanolic extract of wild black berry against E.coli. (Radovanović et al., 2013).
3.7 Biofilm inhibition by CCEs and PRFs
Biofilm formation offers shielding effect to bacterial cells against different antibiotics (Parsek and Singh, 2003). This predominant mode of growth exhibited low susceptibility various kind of inhibitors which is a matter of concern (Limsong et al., 2004). As an alternatives, different plant-based phyto-medicines were isolated and used in traditional medicine systems against microbial infections (Prabu et al., 2006).
Plants extracts (CCEs and PRFs) of wild jujube fruits were evaluated for their potential to control biofilm formation caused by Staphylococcus aureus and Escherichia coli. Biofilm inhibition potential of CCEs and PRFs of wild jujube fruits was found to be in the range of 31.62–75.23 % and 58.09–79.72 %, respectively (Table 3). Among all extracts tested, aqueous-methanolic CCE/PRF have shown highest potential (73.49–75.23 % and 76.95–79.72 %) to inhibit biofilm formation while the minimum biofilm inhibition potential was displayed by absolute acetone CCE (31.62–46.56 %) and PRF extracted from absolute ethanolic extract (58.09–70.08 %). This superior biofilm inhibition potential of hydroxymethanolic CCE/PRF is linked to the availability of higher concentration of phenolic components. Therefore, PRFs showed considerably better potential to inhibit biofilm formation compared to those of corresponding CCEs due successful pre-concentration of phenolic molecules. Biofilm inhibition capacity of tested CCEs/PRFs were found to be lower than that of standard drug, Rifamycin (87.91–89.43 %). The letters (superscripts) with the given data (mean ± SD) in a column shows significant difference (p< 0.05) among the solvents.
SolventsBiofilm inhibition (%)
Hemolytic activity (%)
Thrombolytic activity (%)
CCEs
PRFs
CCEs
PRFs
CCEs
PRFs
S.aureus
E.coli
S.aureus
E.coli
Absolute Methanol
67.51 ± 1.40c
65.24 ± 1.56c
71.37 ± 2.02d
68.83 ± 1.90c
2.76 ± 0.12b
2.67 ± 0.07a
37.13 ± 1.92bc
43.31 ± 1.74d
Aqueous Methanol
73.49 ± 1.35b
75.23 ± 2.17b
79.72 ± 1.75b
76.95 ± 1.68b
2.65 ± 0.08c
2.41 ± 0.05b
48.72 ± 1.90a
62.87 ± 1.29a
Absolute Ethanol
64.07 ± 1.62d
40.74 ± 1.43d
70.08 ± 1.85d
58.09 ± 1.57d
2.15 ± 0.09d
2.27 ± 0.04c
41.39 ± 1.73ab
50.65 ± 1.18c
Aqueous Ethanol
70.51 ± 2.19bc
61.76 ± 2.20bc
75.16 ± 1.69c
69.90 ± 1.52c
2.31 ± 0.11d
2.15 ± 0.05d
43.02 ± 1.21b
57.44 ± 1.78b
Absolute Acetone
46.56 ± 0.97f
31.62 ± 1.21e
–
–
3.58 ± 0.13a
–
37.02 ± 0.87bc
–
Aqueous Acetone
59.73 ± 1.72e
43.13 ± 2.07d
–
–
2.82 ± 0.12b
–
39.65 ± 0.72c
–
Control (Rifamycin)
87.91 ± 4.12a
89.43 ± 3.94a
87.91 ± 4.12a
89.43 ± 3.94a
–
–
–
–
PBS
–
–
–
–
1.54
–
Triton X-100
–
–
–
–
100
–
Water
–
–
–
–
–
3.84
Streptokinase
–
–
–
–
–
92.34
This is the first report in which potential of wild jujube fruit’s CCEs/PRFs was evaluated to inhibit biofilm formation.
3.8 Thrombolytic activity of CCEs and PRFs
Synthetic drugs (urokinase and streptokinase) have been employed to cure thrombosis but their use have few serious health complications (Collen, 1990). Therefore, several herbs have been reported to express promising thrombolytic potential (Mclaughlin et al., 1998). Plant-based components (flavonoids, polyphenols and hydrotyrosols) affect platelet function and thrombogenicity (Maheshwari et al., 2011; Singh et al., 2008).
Thrombolytic potential of CCEs and PRFs, recovered from the fruits of wild jujube, was found to be varied over the range of 37.02–48.72 % and 43.31–62.87 %, respectively (Table 3). The maximum thrombolytic capacity was exhibited by hydoxymethanolic CCE (48.72 %) and PRF (71.42 %) while the lowest for absolute acetone extract (37.02 %) and absolute methanolic PRF (43.31 %). Aqueous methanolic CCE and PRF showed superior thrombolytic capacity due to occurrence of higher concentration of phenolic contents in it. As expected, PRFs were found to contain better thrombolytic potential than those of their crossponding CCEs due to pre-concentration/fractionation of phenolic components. All tested CCEs/PRFs exhibited lower thrombolytic potential in comparison to streptokinase (87.91–89.43 %). Although no evaluation of CCEs/PRFs of wild jujube fruits for thrombolytic profiling on human was not reported yet, Therefore, we report first evaluation of wild jujube’s CCEs/PRFs for thrombolytic effect.
3.9 Haemolytic activity of CCEs and PRFs
Erythrocytes are used in drug delivery due to its morphological and physiological properties (Hamidi and Tajerzadeh, 2003). Membrane of these erythrocyte have been documented to be damaged by oxidative stress to cause hemolysis (Ko et al., 1997). The hemolytic activity of CCEs and PRFs of wild jujube fruits was recorded in the range of 2.15–3.58 %, 2.15–2.67 %, respectively (Table 3). The maximum (3.58 %) and minimum (2.15 %) hemolytic activity was recorded for CCE recovered with absolute acetone and absolute ethanol while among PRFs, derived from absolute methanol and aqueous ethanol exhibited highest (2.67 %) and lowest (2.15 %) hemolytic activity, respectively.
The decrease (1–2.50 times) in hemolysis activity of PRF (2.15–2.67 %) compared to those of CCEs (2.15–3.58 %) is due to higher amounts of phenolics in them which provide better shielding effects to erythrocytes against hemolysis. This variation in hemolytic activity of tested plant matrices might be attributed to availability of varying concentration of phenolic contents that were extracted with different extracting solvents. Hemolytic potential of wild jujube fruit’s CCEs/PRFs was found to be higher in comparison to PBS (1.54 %).
3.10 Estimation of organic acids and individual sugars
Individual sugars and organic acids were reported as main contributors in the taste development and organoleptic properties of different vegetables and fruits (Slavin and Lloyd Beate, 2012; Zhang et al., 2021). Metabolic activity has been attributed to organic acids which are obtained when different compounds are formed and degraded (Cunha et al., 2001).
Wild jujube fruits were processed for estimation of individual sugars including sucrose, galactose, xylose and glucose (Table 4). The most dominant individual sugar was galactose (1.27 %) followed by glucose (1.07 %), sucrose (0.70 %) and xylose (0.04 %) were also detected in tested wild fruit. The results of present analysis advocate the consumption of wild jujube fruit due to occurrence of natural sugars in it. Gluconic acid was detected as dominant organic acid at a concentration of 2.10 mg/100 g DW while small amount of malic acid (0.05 mg/100 g DW) was also present in in tested wild jujube fruits (Tables 4). Different amount of organic acids are available in various vegetables and fruits due to varietal differences and environmental conditions (Poyrazoğlu et al., 2002). The given data (mean ± SD) are obtained by analyzing samples in triplicates. ND: not determined.
Sugar content (mg/100 g DW)
Organic acid (mg/100 g of dry matter)
Glucose
Sucrose
Galactose
Xylose
Total Sugar
Succinic acid
Gluconic acid
Malic acid
Oxalic acid
Citric acid
Acetic acid
100.07 ± 0.140
70.00 ± 0.09
127.00 ± 0.27
4.00 ± 0.01
308
ND
2.10 ± 0.08
0.05 ± 0.02
ND
ND
ND
Gunduz and Saracoglu, (Gunduz and Saracoglu, 2014) have reported the determination of organic acids and individual sugars in Turkish Jujube using HPLC coupled with UV–vis and refractive index (RI) detector, respectively. Ascorbic, malic, tartaric and citric acids were quantified as main organic acids over the concentration range of 0.06–0.10, 0.09–0.17, 0.01–0.04 and 0.19–0.31 g/100 g, while fructose, glucose and sucrose were detected in the range of 4.0–5.2, 6.2–8.1 and 2.2–11.6 g/100 g, respectively. Muchuweti et al., (2005) utilized paper chromatography to determine qualitatively citric, malonic and malic acids in Zimbabwean jujube fruits cultivars. In another study, Gao et al., (2012) reported the occurrence of organic acids (malic (294–740 mg/100 g), citric (39.4–196.6 mg/100 g) and succinic acid (13.8–177.9 mg/100 g) and individual sugars [sucrose (557.3–2801 mg/100 FW) and glucose (1156–2667.8 mg/100 FW)] and with small amount of other sugar molecules in Chinese jujube using HPLC equipped with UV–visible detector and refractive index detector, respectively.
3.11 Minerals profiling
Wild jujube fruits were analyzed using ICP-OES for quantification of minerals present in it. Potassium, calcium, magnesium and phosphorus were detected at a concentration of 14801.10, 1812.37, 811.79 and 630.53 mg/kg as dominant minerals while considerable amount of other minerals were also found in wild jujube fruits (Tables 5). To maintain a normal blood pressure, optimum concentration of sodium and potassium are required by the body (NRC, 1989). The tested wild fruits are promising source of essential minerals to carry out different physiological functions in human body. The given data (mean ± SD) are obtained by analyzing samples in triplicates. ND: Not Detected.
Elements
Concentration (mg/kg)
Elements
Concentration (mg/kg)
Al
325.25 ± 15
Se
4.31 ± 0.04
Ca
1812.37 ± 54
Cu
6.59 ± 0.09
Ba
13.36 ± 0.09
Cr
4.30 ± 0.04
Mg
811.79 ± 45
Na
176.90 ± 4.89
La
0.59 ± 0.03
Fe
444.19 ± 9.65
Mn
20.30 ± 2.12
B
21.70 ± 1.20
Ni
1.24 ± 0.05
Be
ND
Sr
46.22 ± 6.69
Co
0.37 ± 0.01
Zn
5.05 ± 1.02
P
630.53 ± 18.25
K
14801.10 ± 145
Sb
0.35 ± 0.05
Ti
14.77 ± 1.1
Si
109.33 ± 8.40
Cd
1.13 ± 0.05
V
ND
Pb
0.61 ± 0.02
Mineral compositional analysis of Indian Jujube fruit cultivars revealed the presence of calcium (256.2 mg/100 g), sodium (181.6 mg/100 g) and magnesium (156.4 mg/100 g) and potassium (1502.3 mg/100 g) as major macro-elements with small amount of other micro-elements (Valvi, S.R. and Rathod, 2011). San et al. (San et al., 2009) analyzed Turkish jujube fruit cultivars to reveal potassium (314.67–420 mg/100 g DW), nitrogen (170–506 mg/100 g DW) and calcium (79.33–121.33 mg/100 g DW) as dominant minerals with trace amount of other elements in it. Similarly, Indian jujube fruits were found to contain phosphorus (26.8 mg/100 g), calcium (25.6 mg/100 g) and iron (0.76–1.8 mg/100 g) (Pareek, 2013).
3.12 Correlation analysis among TP, TF and biological activities
We have conducted a correlation analysis using Pearson Correlation Method with significance level at P = 0.001 (Table 6, 7). The results of correlation analysis revealed TP and TF have significant positive relationship with inhibition potential, antimicrobial, biofilm and thrombolytic activities (P = 0.866, 0.586–0.920, 0.309–0.837 and 0.838), (P = 0.732, 0.470–0.851, 0.292–0.742 and 0.838) while there was also a significant negative relationship with IC50 value and haemolytic activity (P = -0.768, 0.682), (P = -0.583, −0.427), respectively. The occurrence of eight phenolic and flavonoid components in the fruits of wild jujube has already been reported by our research group (Ahmad et al., 2016), therefore these potent biological attributes of wild jujube fruits are attributed to presence of TP and TF components in it.
TP
IC50 Value
Inhibition Potential
Antibacterial activity
Antifungal activity
Biofilm inhibition
Hemolytic activity
Thrombolytic activity
S.aureus
E.coli
B. cereus
A. niger
A. flavus
F. oxysporum
S. aureus
E. coli
TP
1
IC50 Value
-0.768**
1
Inhibition Potential
0.866**
-0.817**
1
Antibacterial (S.aureus)
0.920**
-0.809**
0.907**
1
Antibacterial (E.coli)
0.839**
-0.916**
0.932**
0.891**
1
Antibacterial (B. cereus)
0.753**
-0.747**
0.627**
0.749**
0.673**
1
Antifungal (A. niger)
0.846**
-0.913**
0.914**
0.924**
0.945**
0.758**
1
Antifungal (A. flavus)
0.586*
-0.718**
0.799**
0.685**
0.775**
0.639**
0.763**
1
Antifungal (F. oxysporum)
0.854**
-0.905**
0.866**
0.824**
0.896**
0.834**
0.911**
0.757**
1
Biofilm inhibition (S. aureus)
0.837**
-0.955**
0.910**
0.872**
0.956**
0.709**
0.956**
0.714**
0.915**
1
Biofilm inhibition (E.coli)
0.309
-0.177
0.170
0.108
0.124
0.204
0.066
-0.165
0.255
0.195
1
Hemolytic activity
-0.583*
0.843**
-0.770**
-0.622**
-0.830**
-0.432
-0.728**
-0.648**
-0.719**
-0.795**
-0.212
1
Thrombolytic activity
0.838**
-0.760**
0.683**
0.803**
0.717**
0.899**
0.775**
0.707**
0.830**
0.699**
0.127
-0.485*
1
TF
IC50 Value
Inhibition Potential
Antibacterial activity
Antifungal activity
Biofilm inhibition
Hemolytic activity
Thrombolytic activity
S.aureus
E.coli
B. cereus
A. niger
A. flavus
F. oxysporum
S. aureus
E.coli
TF
1
IC50 Value
-0.682**
1
Inhibition Potential
0.732**
-0.817**
1
Antibacterial (S.aureus)
0.851**
-0.809**
0.907**
1
Antibacterial (E.coli)
0.744**
-0.916**
0.932**
0.891**
1
Antibacterial (B. cereus)
0.755**
-0.747**
0.627**
0.749**
0.673**
1
Antifungal (A. niger)
0.752**
-0.913**
0.914**
0.924**
0.945**
0.758**
1
Antifungal (A. flavus)
0.470*
-0.718**
0.799**
0.685**
0.775**
0.639**
0.763**
1
Antifungal (F. oxysporum)
0.780**
-0.905**
0.866**
0.824**
0.896**
0.834**
0.911**
0.757**
1
Biofilm inhibition (S. aureus)
0.742**
-0.955**
0.910**
0.872**
0.956**
0.709**
0.956**
0.714**
0.915**
1
Biofilm inhibition (E.coli)
0.292
-0.177
0.170
0.108
0.124
0.204
0.066
-0.165
0.255
0.195
1
Hemolytic activity
-0.427
0.843**
-0.770**
-0.622**
-0.830**
-0.432
-0.728**
-0.648**
-0.719**
-0.795**
-0.212
1
Thrombolytic activity
0.838**
-0.760**
0.683**
0.803**
0.717**
0.899**
0.775**
0.707**
0.830**
0.699**
0.127
-0.485*
1
An increasing attention in the pharmacological effects of the wild fruits prompts the requirement to explore them for presence of high-value phytonutrients and potent bioactives. This is the first report on characterization of valuable phytonutrients and phenolic bioactives in wild jujube fruits native to Soon valley of Pakistan. This study contributed significantly to fill the existing scientific gap of information by authenticating the occurrence of valuable phytonutrients and potent phenolic antioxidant components in tested fruits with remarkable biological potential which were rarely determined before. These wild jujube fruits could be a potential candidate to be used in formulation of nutra-pharamaceuticals with wide range of health benefits.
4 Conclusions
Wild fruits are promising source of phytonutrients and potent bioactives with multiple medicinal properties. Appraisal of natural individual sugars, organic acids, valuable minerals, TPC, TFC and biological properties (antioxidant, antimicrobial, hemolytic, biofilm inhibition and thrombolytic capacity) of CCEs/PRFs of wild jujube fruits were carried out using different spectro-analytical techniques. Potassium, calcium, magnesium and phosphorus were detected as dominant elements while gluconic acid was found as main organic acid in tested fruit. Aqueous-ethanolic and aqueous methanolic CCEs/PRFs exhibited superior TPC, TFC and higher antioxidant, and biological potential which were rarely determined before. Strong correlation among TP, TF and biological attributes was also recorded revealing the facts that biological potential of tested wild fruits was attributed to the concentration of bioactive components. Generally, the results of this project advocate wild jujube fruits are a rich source of phenolic antioxidants and valuable nutrients, and hence can be explored as potent ingredients in developing functional foods and pharma-nutraceuticals.
Acknowledgements
Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R158) Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University, Saudi Arabia for funding this work through the Research Groups Program under Grant No R.G.P.2: 187/43.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antidiabetic activity and toxicity of Zizyphus spina-christi leaves. J. Ethnopharmacol.. 2005;101:129-138.
- [CrossRef] [Google Scholar]
- Potential antioxidant and antibacterial properties of a popular jujube fruit: Apple kul (Zizyphus mauritiana) J. Food Biochem.. 2014;38:592-601.
- [CrossRef] [Google Scholar]
- Antioxidant and antimicrobial attributes of different solvent extracts from leaves and flowers of akk [Calotropis procera (Ait.) Ait. F.)] J. Med. Plant Res.. 2011;5:4879-4887.
- [Google Scholar]
- Characterization of free and conjugated phenolic compounds in fruits of selected wild plants. Food Chem.. 2016;190:80-89.
- [CrossRef] [Google Scholar]
- Anti-inflammatory activity of seed essential oil from Zizyphus jujuba. Food Chem. Toxicol.. 2010;48:639-643.
- [CrossRef] [Google Scholar]
- Enzyme-aided cold pressing of flaxseed (Linum usitatissimum L.): Enhancement in yield, quality and phenolics of the oil. Grasas y Aceites. 2013;64:463-471.
- [CrossRef] [Google Scholar]
- Antioxidant and antimicrobial attributes of different solvent extracts from leaves of four species of mulberry. Int. J. Pharmacol.. 2015;11:757-765.
- [CrossRef] [Google Scholar]
- Antioxidant properties of various solvent extracts of mulberry (Morus indica L.) leaves. Food Chem.. 2007;102:1233-1240.
- [CrossRef] [Google Scholar]
- Phytochemical composition, antioxidant and antimicrobial properties of raspberry fruit, pulp, and marc extracts. CYTA - J. Food. 2013;11:334-342.
- [CrossRef] [Google Scholar]
- Separation and determination of flavonoids and other phenolic compounds in cranberry juice by high-performance liquid chromatography. J. Chromatogr. A. 2001;913:387-395.
- [CrossRef] [Google Scholar]
- Coronary thrombolysis: Streptokinase or recombinant tissue-type plasminogen activator? Ann. Intern. Med.. 1990;112:529-538.
- [CrossRef] [Google Scholar]
- Cunha, S.C., Ferreira, I.M.P.L.V.O., Fernandes, J.O., Faria, M.A., Beatriz, M., Oliveira, P.P., Ferreira, M.A., 2001. Determination of lactic, acetic, succinic, and citric acids in table olives by HPLC/UV. J. Liq. Chromatogr. Relat. Technol. 24, 1029–1038. https://doi.org/10.1081/JLC-100103429.
- Evaluation of the antioxidant effects of Ziziphus mauritiana lam. leaf extracts against chronic ethanol-induced hepatotoxicity in rat liver. African J. Tradit. Complement. Altern. Med.. 2008;5:39-45.
- [CrossRef] [Google Scholar]
- Self-selection of the analgesic drug carprofen by lame broiler chickens. Vet. Rec.. 2000;146:307-311.
- [CrossRef] [Google Scholar]
- Crude extract from Ziziphus Jujuba fruits, a weapon against pediatric infectious disease. Iran. J. Pediatr. Hematol. Oncol.. 2013;3:216-221.
- [Google Scholar]
- Antimicrobial and antioxidant activities of green and ripe fruits of Averrhoa carambola Linn. and Zizyphus mauritiana Lam. Asian J. Pharm. Clin. Res.. 2012;5:102-105.
- [Google Scholar]
- Physico-chemical properties and antioxidant capacity of different jujube (Ziziphus jujuba Mill.) cultivars grown in loess plateau of China. Sci. Hortic. (Amsterdam). 2011;130:67-72.
- [CrossRef] [Google Scholar]
- Textural characteristic, antioxidant activity, sugar, organic acid, and phenolic profiles of 10 promising Jujube (Ziziphus jujuba Mill.) selections. J. Food Sci.. 2012;77
- [CrossRef] [Google Scholar]
- Changes in chemical composition, total phenolic content and antioxidant activities of jujube (Ziziphus jujuba Mill.) fruits at different maturation stages. Acta Sci. Pol. Hortorum Cultus. 2014;13
- [Google Scholar]
- Carrier erythrocytes: an overview. Drug Deliv. J. Deliv. Target. Ther. Agents. 2003;10:9-20.
- [CrossRef] [Google Scholar]
- Antioxidant capacity and lipophilic content of seaweeds collected from the Qingdao coastline. J. Agric. Food Chem.. 2004;52:4993-4997.
- [CrossRef] [Google Scholar]
- Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem.. 2008;108:986-995.
- [CrossRef] [Google Scholar]
- Antioxidant attributes of four Lamiaceae essential oils. Pakistan J. Bot.. 2011;43:1315-1321.
- [Google Scholar]
- Integration of in silico and in vitro approaches to evaluate antioxidant and anticancer properties of Tribulus terrestris extracts. Arab. J. Chem.. 2022;15:103984
- [CrossRef] [Google Scholar]
- Ultrasonic extraction and HPLC determination of anthraquinones, aloe-emodine, emodine, rheine, chrysophanol and physcione, in roots of Polygoni multiflori. Phytochem. Anal.. 2009;20:272-278.
- [CrossRef] [Google Scholar]
- Antioxidant and antibacterial activities of the leaf essential oils of Himalayan Lauraceae species. Food Chem. Toxicol.. 2010;48:37-40.
- [CrossRef] [Google Scholar]
- In vitro wound healing evaluation, antioxidant and chemical profiling of Baeckea frutescens leaves ethanolic extract. Arab. J. Chem.. 2022;15:103871
- [CrossRef] [Google Scholar]
- Total phenolics and antioxidant activity of jujube (Zizyphus jujube Mill.) genotypes selected from Turkey. African J. Biotechnol.. 2009;8:303-307.
- [Google Scholar]
- Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem.. 2006;94:550-557.
- [CrossRef] [Google Scholar]
- Protection of oxidative hemolysis by demethyldiisoeugenol in normal and β-thalassemic red blood cells. Free Radic. Biol. Med.. 1997;22:215-222.
- [CrossRef] [Google Scholar]
- Potent bioactive methanolic extract of wild orange (Citrus macroptera Mont.) shows antioxidative, anti-inflammatory, and antimicrobial properties in in vitro, in vivo, and in silico studies. Bull. Natl. Res. Cent.. 2020;44
- [CrossRef] [Google Scholar]
- Free radical scavenging capacity in the aging of selected red spanish wines. J. Agric. Food Chem.. 1999;47:1603-1606.
- [CrossRef] [Google Scholar]
- Comparison of antioxidant capacities of extracts from five cultivars of Chinese jujube. Process Biochem.. 2005;40:3607-3613.
- [CrossRef] [Google Scholar]
- Antioxidant activities of polysaccharides from the fruiting bodies of Zizyphus Jujuba cv. Jinsixiaozao. Carbohydr. Polym.. 2011;84:390-394.
- [CrossRef] [Google Scholar]
- Inhibitory effect of some herbal extracts on adherence of Streptococcus mutans. J. Ethnopharmacol.. 2004;92:281-289.
- [CrossRef] [Google Scholar]
- Development and validation of the new EPA microwave-assisted leach method 3051A. Environ. Sci. Technol.. 1998;32:3628-3632.
- [CrossRef] [Google Scholar]
- Antioxidant and hepatoprotective activities of phenolic rich fraction of Seabuckthorn (Hippophae rhamnoides L.) leaves. Food Chem. Toxicol.. 2011;49:2422-2428.
- [CrossRef] [Google Scholar]
- Effect of maturity on phenolics (Phenolic acids and flavonoids) profile of strawberry cultivars and mulberry species from Pakistan. Int. J. Mol. Sci.. 2012;13:4591-4607.
- [CrossRef] [Google Scholar]
- Malik, M.N., Haq, I. ul, Fatima, H., Ahmad, M., Naz, I., Mirza, B., Kanwal, N., 2022. Bioprospecting Dodonaea viscosa Jacq.; a traditional medicinal plant for antioxidant, cytotoxic, antidiabetic and antimicrobial potential. Arab. J. Chem. 15, 103688. https://doi.org/10.1016/j.arabjc.2022.103688.
- The antioxidant activity and free radical scavenging potential of two different solvent extracts of Camellia sinensis (L.) O. Kuntz, Ficus bengalensis L. and Ficus racemosa L. Food Chem.. 2008;107:1000-1007.
- [CrossRef] [Google Scholar]
- Variation of phenolics and antioxidant activity between peel and pulp parts of pear (Pyrus communis L.) fruit. Pakistan J. Bot.. 2013;45:1521-1525.
- [Google Scholar]
- The use of biological assays to evaluate botanicals. Ther. Innov. Regul. Sci.. 1998;32:513-524.
- [CrossRef] [Google Scholar]
- Phenolic compounds and seed oil composition of Ziziphus mauritiana L. fruit. Polish J. Food Nutr. Sci.. 2012;62:15-21.
- [CrossRef] [Google Scholar]
- Sugars, organic acid and phenolic compounds of Ziziphus mauritiana fruit. Eur. Food Res. Technol.. 2005;221:570-574.
- [CrossRef] [Google Scholar]
- Research on the Phenolic Compounds in Sarilop (Ficus Carica L.) Fig Variety (in English) Gida. 2013;38:267-274.
- [CrossRef] [Google Scholar]
- NCCLS, 1997. National Committee for Clinical Laboratory Standards (NCCLS). Approved Standard M2 A6, 1997, 5th edn . NCCLS: Wayne, PA.
- NCCLS, 1999. National Committee for Clinical Laboratory Standards (NCCLS). M100-S9., 1999, NCCLS: Wayne, PA.
- NRC, 1989. National Research Council. Recommended dietary allowances., 1989, (10th ed .). Washington: National Academy Press. 1989.
- Consumer acceptability of chocolate chip cookies using applesauce as a fat (butter) substitute. Emirates J. Food Agric.. 2013;25:463-470.
- [CrossRef] [Google Scholar]
- Bacterial biofilms: an emerging link to disease pathogenesis. Annu. Rev. Microbiol.. 2003;57:677-701.
- [CrossRef] [Google Scholar]
- Antioxidant and antimicrobial properties of dried Portuguese apple variety (Malus domestica Borkh. cv Bravo de Esmolfe) Food Chem.. 2018;240:701-706.
- [CrossRef] [Google Scholar]
- Identification of bioactive constituents of Ziziphus jujube fruit extracts exerting antiproliferative and apoptotic effects in human breast cancer cells. J. Ethnopharmacol.. 2012;140:325-332.
- [CrossRef] [Google Scholar]
- Organic acids and phenolic compounds in pomegranates (Punica granatum L.) grown in Turkey. J. Food Compos. Anal.. 2002;15:567-575.
- [CrossRef] [Google Scholar]
- Guaijaverin - a plant flavonoid as potential antiplaque agent against Streptococcus mutans. J. Appl. Microbiol.. 2006;101:487-495.
- [CrossRef] [Google Scholar]
- Development of an in vitro model to study clot lysis activity of thrombolytic drugs. Thromb. J.. 2006;4:9-12.
- [CrossRef] [Google Scholar]
- Phenolic composition, antioxidant and antibacterial properties, and in vitro anti-HepG2 cell activities of wild apricot (Armeniaca Sibirica L. Lam)kernel skins. Food Chem. Toxicol.. 2019;129:354-364.
- [CrossRef] [Google Scholar]
- Antioxidant and antimicrobial activity of polyphenol extracts from wild berry fruits grown in Southeast Serbia. Trop. J. Pharm. Res.. 2013;12:813-819.
- [CrossRef] [Google Scholar]
- Raghuveer, C., 2009. Review Article Consumption of Functional Food and Our Health Concerns 5, 76–83
- Suppressors of cancer cell proliferation from fig (Ficus carica) resin: Isolation and structure elucidation. J. Nat. Prod.. 2001;64:993-996.
- [CrossRef] [Google Scholar]
- Mineral composition of leaves and fruits of some promising jujube (zizyphus jujuba miller) genotypes. Asian J. Chem.. 2009;21:2898-2902.
- [Google Scholar]
- Antioxidant and antimicrobial attributes and phenolics of different solvent extracts from leaves, flowers and bark of gold mohar [Delonix regia (Bojer ex Hook.) Raf.] Molecules. 2011;16:7302-7319.
- [CrossRef] [Google Scholar]
- Nutraceuticals and functional foods: Whole versus processed foods. Trends Food Sci. Technol.. 2009;20:376-387.
- [CrossRef] [Google Scholar]
- Studies on the antioxidant activity of Indian Laburnum (Cassia fistula L.): a preliminary assessment of crude extracts from stem bark, leaves, flowers and fruit pulp. Food Chem.. 2002;79:61-67.
- [CrossRef] [Google Scholar]
- The effects of polyphenols in olive leaves on platelet function. Nutr. Metab. Cardiovasc. Dis.. 2008;18:127-132.
- [CrossRef] [Google Scholar]
- Slavin, J.U. of M., Lloyd Beate, P.N.G.R., 2012. Health Benefits Of Cassava-Karrapendalam. www.yadtek.com › Heal. › Diet Nutr. 3, 506–516. https://doi.org/10.3945/an.112.002154.506.
- A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods. 2000;40:175-179.
- [CrossRef] [Google Scholar]
- Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules. 2009;14:2167-2180.
- [CrossRef] [Google Scholar]
- Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae) Food Chem.. 2005;90:333-340.
- [CrossRef] [Google Scholar]
- Mineral composition of some wild edible fruits mineral composition of some wild edible fruits from. Biol., J. Appl. Tech., Phar.. 2011;2:392-396.
- [Google Scholar]
- Jacob Vaya, Saeed Mahmood, 2006. Flavonoid content in leaf extracts of the fig(Ficus carica L.),carob(Ceratonia siliquaL.)andpistachio(Pistacia lentiscusL.) 28, 169–175.
- Activity and mechanisms of action of selected biocidal agents on Gram-positive and -negative bacteria. J. Appl. Microbiol.. 2003;94:240-247.
- [CrossRef] [Google Scholar]
- Green synthesis of nanoparticles for the remediation of contaminated waters and soils: Constituents, synthesizing methods, and influencing factors. J. Clean. Prod.. 2019;226:540-549.
- [CrossRef] [Google Scholar]
- Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem.. 2007;105:940-949.
- [CrossRef] [Google Scholar]
- Effect of ripening stage on physicochemical properties and antioxidant profiles of a promising table fruit “pear-jujube” (Zizyphus jujuba Mill.) Sci. Hortic. (Amsterdam). 2012;148:177-184.
- [CrossRef] [Google Scholar]
- Antioxidant activity and total phenolic contents in peel and pulp of chinese jujube (ziziphus jujuba mill) fruits. J. Food Biochem.. 2009;33:613-629.
- [CrossRef] [Google Scholar]
- Haemolytic activities and adjuvant effect of Astragalus membranaceus saponins (AMS) on the immune responses to ovalbumin in mice. Vaccine. 2005;23:5196-5203.
- [CrossRef] [Google Scholar]
- Antioxidant activity of anthraquinones and anthrone. Food Chem.. 2000;70:437-441.
- [CrossRef] [Google Scholar]
- Systematic evaluation of antioxidant capacities of the ethanolic extract of different tissues of jujube (Ziziphus jujuba Mill.) from China. Food Chem. Toxicol.. 2010;48:1461-1465.
- [CrossRef] [Google Scholar]
- Zhang, X., Wei, X., Ali, M.M., Rizwan, H.M., Li, B., Li, H., Jia, K., Yang, X., Ma, S., Li, S., Chen, F., 2021. Changes in the content of organic acids and expression analysis of citric acid accumulation-related genes during fruit development of yellow (Passiflora edulis f. flavicarpa) and purple (passiflora edulis f. edulis) passion fruits. Int. J. Mol. Sci. 22. https://doi.org/10.3390/ijms22115765
- Apoptosis inducing effects of kuding tea polyphenols in human buccal squamous cell carcinoma cell line BcaCD885. Nutrients. 2014;6:3084-3100.
- [CrossRef] [Google Scholar]
- Antioxidant activity of a flavonoid-rich extract of Hypericum perforatum L. in vitro. J. Agric. Food Chem.. 2004;52:5032-5039.
- [CrossRef] [Google Scholar]
- Simultaneous determination of catechins, caffeine and gallic acids in green, oolong, black and pu-erh teas using HPLC with a photodiode array detector. Talanta. 2002;57:307-316.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104240.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







