Translate this page into:
Optimization and characterization of immobilized E. coli for engineered thermostable xylanase excretion and cell viability
⁎Corresponding author at : Siti Fatimah Zaharah Mohd Fuzi, Faculty of Applied Sciences and Technology, Universiti Tun Hussein Onn Malaysia, 84600 Pagoh, Muar, Johor, Malaysia. fatimahz@uthm.edu.my (Siti Fatimah Zaharah Mohd Fuzi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
There are many parameters that may have influenced the properties of cell during immobilization process. Particularly, the immobilization methods, carrier materials, and enzyme loading amount that have been proved to be important for immobilization process. The physiological responses of microorganisms are depending on the immobilization technique used. Typical alterations to the micro-environment of the immobilized cell involved the altered water activity, presence of ionic charges, cell confinement and modified surface tension. In this study, the graphene oxide was selected as a suitable carrier for immobilization process of recombinant E.coli and adsorption was chosen as an appropriate method to improve the production of engineered thermostable xylanase. High level production of thermostable xylanase by immobilized recombinant cell in the 5 L bioreactor was studied by using optimum research surface methodology (RSM) conditions was studied. The immobilization of E. coli onto nanoparticle matrix manages to improve the cell performance by improving the protein expression, reduced the occurrences of cell lysis as well as improved the plasmid stability of the host cell. Thus, immobilization contributes a physical support for both whole cells as well as enzymes to develop a better operative achievement system for industrialized fields and give rise to the biological advancement existing enzyme for instance xylanase.
Keywords
Engineered E. coli
Thermostable enzyme
Immobilization
Optimization
Graphene oxide
1 Introduction
Protein enzymes are important finding in the industry of bioprocess technology, which is produced by active cells that promote the transformation of chemical species in living systems (Zhang et al., 2013; Brena et al., 2013). The use of enzymes has become a necessary processing strategy with extensive applications in the nanotechnology, animal nutrition, pulp and paper industry, textile, organic synthesis, cellulose and detergent industries, medical, pharmaceutical, biofuels industry, fine chemicals, biomaterials, cosmetic and food industry (Jaqueline et al., 2016; Li et al., 2016).
There are numerous patents obtainable in commercially marketed systems and significant advancement in understanding the secretory production of foreign proteins by E. coli as well as the mechanism of the membrane transport. However, industrialized operation has been mounted with technical complications (Yoon et al., 2010). The primary impediments to using enzymes in the multiple practical processes were having low thermal stability, narrow pH range, along with the deficiency of enzymatic activeness after one cycle (Mohamad et al., 2016; Krajewska et al., 2014). To address these difficulties, researchers have tried a variety of strategies, including directed evolution (Damis et al., 2019), rational enzyme design (Hakimi et al., 2016), enzyme immobilization (Cui and Jia, 2015, Cui et al., 2016), and adjusting reaction conditions (Jamil et al., 2016). Among these approaches, enzyme immobilization has been hailed as a promising solution for overcoming these constraints. Immobilized enzymes have various advantages over soluble enzymes, including higher operational stability, increased enantioselectivity, reusability of the enzymes, and easier reactor operation and product separation (Cui et al., 2018, Ren et al., 2019, Feng et. al., 2021).
Whole-cell catalysts, in addition to free enzymes, can be immobilized. Immobilization is one of the most effective ways for improving the system performance of recombinant E. coli for protein excretion, as it offers various advantages over the traditional free cell fermentation procedure. For example, lowering the risk of contamination, reducing plasmid instability and cell lysis, improving the productiveness of recombinant cells, low sensitivity to pH and temperature when compared to free cells, reducing the fermentation time, accelerating the rate of substrate uptake, and resulting in estimated volatile component concentration for product (Zajkoska et al., 2013; Man et al., 2016). Adsorption-based cell immobilization is gaining popularity due to its potential to reduce or eliminate the cell-matrix mass transfer problem. As a result, by maintaining a continuous connection between the matrix and nutrients, immobilization using this approach benefits the entire cell system (Koo & Yamada., 2016).
The physiochemical factors, for instance; cell-matrix interaction, cultivation conditions such as a culture medium, pH, temperature, and inducer concentration and agitation rate influence the recombinant cell immobilization and cell growth (Shang et al., 2017). Immobilized cells are usually demonstrated with better performance towards temperature and pH conditions compared to free cells and can be reused or recovered for several cycles (Zhang et al., 2013; Xu et al., 2014). For example, one of the modified graphene oxide (GO) nanosheets with polyethylene glycol have been utilized as consistent aqueous carriers for insoluble drug delivery (Akhavan et al., 2015; Zhang et al., 2010; Adhikari et al., 2014). Additionally, owing to an exceptionally large particular surface region with the two operative sides have the sufficient oxygen for surface functionalities; it also can be utilized for both whole cell and enzymes immobilization (Akhavan et al., 2015).
Bioprocess development strategies generally start from shake flask cultivation modes to determine the optimum factors. However, these factors could not directly apply to the higher scale production by considering the incompatible conditions between large cultivation mode e.g bioreactor and small cultivation mode e.g shake flask (Li et al., 2015). Hence, it is crucial to perform the shake-flask cultivations by using the both free cell and immobilized cell culture, screening the cultural conditions using full-factorial design (FFD), optimization of the cultural conditions RSM and study of high-level xylanase excretion in stirred tank bioreactor (using RSM condition) to determine the cells performance (Abdul Manaf et. al., 2021).
Complex interactions among many physicochemical characteristics are addressed by statistical experimental designs such as FFD and RSM. RSM is a powerful statistical and mathematical tool that aids in the identification of effective factors, the study of interactions, the selection of optimum conditions, and the quantification of relationships between one or more measured responses and key input factors in a small number of experiments (Zhang et al., 2010, Cui and Zhang 2011, Cui et al., 2016).
Previously, the highest xylanase excretion was exhibited by the immobilized recombinant E. coli onto untreated GO (Nor Ashikin el al., 2017). The strong cell attachment on untreated graphene oxide led to the higher stability of the plasmid with lower cell lysis. Thus, it offered better performance to the strain and demonstrated higher efficiency on excretion of engineered thermostable xylanase.
This study demonstrates the use of GO as a convenient immobilization matrix for recombinant E. coli to enhance the immobilization cell efficiency and excrete high xylanase expression using statistical optimization method and upscaling the production in 5 L bioreactor.
2 Material and methods
2.1 E. coli strains and plasmid selection
The recombinant E. coli strain carrying xylanase gene (xyng) from Aspergillus fumigatus af293 used for this research was constructed by Hakimi et al. (2016). In this study, xyng with thermostability characteristic at 70 °C was cloned into an expression host, E. coli BL21 (DE3) containing vector pET21a (+). This vector contains signal peptide M5 that can direct the expressed protein into extracellular space. E. coli BL21 (DE3) served as the host for heterologous expression while E. coli JM109 was utilized for storage purposes (Hakimi et al., 2016). The SDS-PAGE examination revealed E. coli excretion of xylanase into the extracellular space, according to the authors. The existence of a single protein band of 23 kDa on SDS-PAGE revealed that the recombinant protein was discovered in the extracellular space and was relatively pure, with the exception of a few additional bands identified by SDS-PAGE (Hakimi et al., 2016).
2.2 Chemical reagents
The chemicals and reagents used in this study were all analytical grades which were obtained from several different suppliers including Merck (Merck KGaA, Darmstadt, Germany), Sigma-Aldrich (Merck KGaA, Darmstadt, Germany), Fisher (Thermo Fisher Scientific Inc, US).
2.3 Cell immobilization
Immobilization of cells was performed by adding 10 mg of GO into 50 mL LB broth with 100 μg/mL ampicillin. The GO was cultivated with recombinant E. coli strain from bacterial glycerol stock at 37 °C, agitation rate 200 rpm. Then, the growth medium was taken out from the flask after the overnight cultivation period (12 h). The GO was then washed thoroughly with sterilized water to remove the non-immobilized E. coli. Meanwhile, the immobilized E. coli were transferred to 250-mL flasks consisted of 50 mL TB broth with 100 µg/mL ampicillin concentration for cultivation medium. The free cell suspension (2% v/v) was used as a control in the study and operated under the equal expression and growth conditions as immobilized cell.
2.4 Screening of the cultural conditions by using FFD
A 25 full factorial design was implemented to indicate the statistical significance of the concentration of IPTG, post induction time, post induction temperature, pH, inoculum size and agitation rate on the xylanase excretion and occurrence of cell lysis (indicated by release of β-galactosidase into the medium). A two-level factorial design was a statistically based technique that consists of concurrent adjustment of analysis factors by using only two levels which were low and high. The settings of ranges for variables were depending on the analysis of single factors as mentioned by Rahman et al. 2016, Che et al. 2016. The overall of 67 sets of experimentations were carried out in this research to determine the critical parameters affecting the xylanase excretion and β-galactosidase activity (Supplementary Data 1).
2.5 Optimization of the cultural conditions by RSM
The optimization was designed based on a central composite design (CCD) with six parameters as the independent variables and xylanase excretion production and β-galactosidase activity as the dependent variables with a total of 16 experiments that are shown in Table 1. The xylanase excretion and β-galactosidase activity were collected as the responses. The parameters investigated for this study were inducer concentration, mM (x1), post induction temperature, °C (x2), post induction time, h (x3), agitation rate, rpm (x4), pH (x5) and inoculum size, % (x6) with the xylanase excretion and β-galactosidase activity collected as the responses.
Run
Actual Value
Response
x1
x2
x3
x4
x5
x6
Xylanase excretion (U/mL)
β-galactosidase activity (U/mL)
1
0.00
30
4
150.0
7.00
15.0
0.0371
0.8644
2
0.26
30
4
150.0
7.00
15.0
0.1508
0.0343
3
0.26
30
1
150.0
7.00
15.0
0.0748
0.0261
4
0.26
30
7
150.0
7.00
15.0
0.0518
0.9514
5
0.26
30
4
150.0
7.00
15.0
0.0596
0.9407
6
0.26
30
4
150.0
7.00
32.0
0.0496
0.9722
7
0.67
30
4
150.0
7.00
15.0
0.0715
21.808
8
0.26
30
4
150.0
7.00
15.0
0.0569
0.7340
9
0.26
30
4
150.0
4.00
15.0
0.0969
0.0040
10
0.26
30
4
150.0
7.00
00.0
0.0628
0.7913
11
0.26
30
4
150.0
7.00
15.0
0.0684
0.0431
12
0.26
30
4
150.0
10.0
15.0
0.0356
1.2414
13
0.26
47
4
150.0
7.00
15.0
0.0817
0.6990
14
0.26
30
4
00.00
7.00
15.0
0.0659
0.0144
15
0.26
13
4
150.0
7.00
15.0
0.0521
0.7579
16
0.26
30
4
318.0
7.00
15.0
0.0627
0.0620
The interpretation of the experimental design results was performed by analysis of variance (ANOVA) in a random order. The significance of the quadratic model was resolved by P-value. A P-value ≤ 0.05 was considered to be significant.
2.6 Reusability of immobilized cells
The expression was conducted using 0.5 mM IPTG at 20 °C and 50 rpm until 20 h of post induction time. After that, the immobilized cells were separated from the expression medium (TB), washed with sterilized distilled water for the removal of the non-immobilized cells and reintroduce into the fresh medium. The process was continued for the next cycle. Subsequent batches were run at 24 h intervals. The parallel experiment was also carried out with the free cell, whereby the supernatant from the earlier cycle was utilized for the next cycle’s inoculation (Man et al. 2016 & Rahman, 2016).
2.7 Batch cultivation in stirred-tank bioreactor
The production of recombinant xylanase by E. coli were performed in 5 L stirred tank bioreactor (Infors HT Minifors 109,726 model, Bottmingen, Switzerland), with a working volume of 2 L. The production medium (TB) comprised of 48 g/L yeast extract, 40 mL glycerol (20% from the working volume), 24 g/L tryptone, 25.08 g/L K2HPO4 and 4.62 g/L KH2PO4. Prior to inoculation, the stirred tank bioreactor was autoclaved at 121 °C for 15 min. The 5% (v/v) of inoculum was added into the 2 L TB medium complemented with 0.4 g sterile graphene oxide and 100 µg/mL ampicillin. The cells were cultured at 200 rpm, 37 °C and pH 5.0 before expression until the 4.5 h of induction time using 0.5 mM IPTG concentration (Fuzi et al. 2011).
After the expression process, the culture was conducted at 20 °C, 100 rpm and pH 5.0 until 24 h of post induction time for each set, before the samples were collected. The supernatant of immobilized cell after centrifugation at 13 000 rpm for 5 min was used for analysis. During the experiment, the sterile air was supplied to the bioreactor with 1 vessel volumes per min (VVM) of volumetric flow rate per min to ensure that enough oxygen will be supplied to the cells. The pH was regulated with 2 M HCl and 2 M NaOH to maintain the neutral pH during the process (Fuzi et al. 2011). The pH was determined by using sterilizable electrode (Mettler, Toledo) while the temperature was detected by the traceable electrode (Cole-Parmer, USA).
2.8 Analysis procedures
Several analyses were done in each experiment to determine the xylanase activity, β-galactosidase activity, plasmid stability, cell density, extracellular enzyme size and the surface morphology of immobilized cell.
2.8.1 Xylanase activity
Xylanase excretion was evaluated by incubating appropriately diluted enzyme in sodium acetate buffer (pH 5.0) and temperature of 50 °C for 10 min by using a substrate solution of 1% (w/v) beechwood xylan (Merck). Reducing sugars were assayed by adding 500 μL of DNS (2-hydroxy-3, 5 dinitrosalicylic acid) reagent, boiling for 5 min, followed by cooling and reading the absorbance at 540 nm using UV–Vis spectrophotometer. One unit of xylanase excretion was described as the amount of enzyme releasing 1 μmol of reducing sugar (xylose equivalent) per min under the experimental condition (Hakimi et al. 2016).
2.8.2 Beta-galactosidase activity (cell lysis)
Cell lysis was measured by determining the quantity of β-galactosidase in the extracellular medium using 0- nitrophenyl-β-D-galactopyranosid (ONPG). A total of 1 mL of substrate buffer consisting of 4 mg/mL of ONPG in 0.1 M phosphate buffer (pH 7.4) was added with 0.1 mL of sample before it was incubated in 37 °C water bath for 10 min. The absorbance at 420 nm was recorded after the reaction was ended by adding 0.5 mL of 1 M sodium carbonate. One unit of xylanase excretion was described as the quantity of enzyme that forms 10-8 mol of ONPG per min under the investigation condition (Hakimi et al. 2016).
2.8.3 Plasmid stability
The plasmid stability was determined from the proportion of the cell counts on the LB agar ampicillin plate and non-ampicillin LB agar plate. The immobilized E. coli were collected in 2 mL micro-centrifuge tube consisting of 1 mL of sterilized distilled water. After that, the samples were vortex vigorously for 2 min. These methods were made to detach the portion of cells from GO. Both immobilized and free cell samples were diluted by using 10 fold serial dilutions to achieve cell counts in the range of 30–300. All colony counts were verified from the average of minimum three repeated experiments (Man et al. 2016).
2.8.4 Cell density
The dry cells weight of immobilized cell was measured by subtracting the mass of recovered GO 95% from the initial GO (9.5 mg). The amount of recovered GO was obtained from the average value of 3 batch experiments in the early stage of immobilization process. The difference in the weight of initial and recovered GO showed the number of cells attached to the matrix (Rahman, 2016). The dry cell weight of immobilized and free cell was calculated based on the Equation (3.1) and Equation (3.2) respectively.
Where, X is the cell density, W0 is the filter paper weight, W1 is the filter paper weight, dry cell and the dry GO, and V is the volume of sample, 9.5 mg is the standardized initial mass of recovered GO.
2.9 Scanning electron microscope (SEM)
Scanning electron microscopy (SEM) was performed to observe the structure formed when the cell was immobilized with the carbon nanomaterial. The samples were prepared by fixation in 3% glutaraldehyde (24 h) and in 1% osmium tetroxide (3 h), followed by dehydration with ethanol (30, 50, 70, 80, 90, 95 and 100%, each for 10 min) and drying by lyophilization, and were then covered with gold. (Abdul Manaf et al. 2021). The samples were observed under a high-resolution electron microscope, FEI Versa 3D Dual Beam (Czech Republic).
2.10 Xylan reaction process
The xylan reaction process was determined by pre-incubating the enzyme at the temperature 70 °C, using 50 mM sodium acetate buffer and pH 7 in the present of xylan substrate for 30 min before standard xylanase activity assay was carried out (Hakimi et al., 2016).
2.11 Liquid chromatography mass spectrometry (LCMS)
The production of xylooligosaccharides was determined using a Liquid Chromatography Mass Spectrometry (LCMS), equipped with a Waters ACQUITY UPLC BEH Amide column (100 mm × 2.1 mm × 1.7 µm). The mobile phase used for solution A was LCMS Grade Water and 0.1% Ammonium Hydroxide. While for solution B was LCMS Grade Acetonitrile and 0.1% Ammonium Hydroxide. Before measurements, all samples were centrifuged down and filtered through 0.22 µm syringe filter. The extracted samples were used for sugar content detection.
3 Results and Discussion
3.1 Screening of cultural conditions of immobilized E. coli on xylanase excretion and cell viability using design of experiment (full factorial design)
In this study, the two-level of full factorial design (FFD) was implemented to determine the significant variables of cultivation conditions for cell viability and xylanase excretion of immobilized E. coli. The significant of post induction temperature, IPTG concentration, pH, post induction time, agitation rate and inoculum size were analyzed by 26 full factorial design. The results and the experimental designs were tabulated in Supplementary Data 2. The P-value indicates that the post induction temperature (x2) was the significant variable that affected the xylanase excretion, Supplementary Data 3. Meanwhile, the pH condition (x5) was the significant variable that affected the incidence of cell lysis (Supplementary Data 4).
The model precision is denoted based on the correlation coefficient (R) and coefficient (R2) are shown in Supplementary Data 5. All terms despite of their significant were comprised in the regression equations (Equation (4.1), 4.2) that were achieved from ANOVA method.
Final equation in terms of coded factors:
Therefore, the most significant parameters with P-value which is less than 0.05 were then further optimized by means of Central Composite Design (CCD) as these factors manage to influence both of xylanase excretion and β-galactosidase activity.
3.2 Optimization of cultural conditions on engineered thermostable xylanase excretion and cell viability of immobilized E. coli using response surface methodology (central composite design)
In this study, the optimization using FFD conjunction with CCD model has been developed. The results gain from the total of 67 experimental design runs from FFD were augmented to another 16 experimental design runs of RSM using central composite design method (Table 1).
Analysis of Variance (ANOVA) is substantial to identify the significance and adequacy of the quadratic models in Supplementary Data 6 & 7. The significant models were designated by the P-value which is below 0.05. The model terms x4, x52and x2x3 were significant parameters that affected on xylanase excretion. While, the model terms x3, x5, x22, x62 and x4x5 were significant parameters that affected on cell lysis as showed by the amount of β-galactosidase released into the cultivation media. The regression equivalent Equations (4.5) and (4.6) were achieved from the Analysis of Variance (ANOVA) and all parameters were comprised in the equivalent equations below:
Final equation in terms of coded factors:
Table 2 shows the actual production of xylanase was 0.4821 U/mL which representing 99.47% of the predicted value (0.4847 U/mL). This amount is close enough to the predicted value than that in free cell (0.1264 U/mL). While, for the cell lysis, the actual production of β-galactosidase was 0.0299 U/mL, that was slightly higher than the predicted one (0.0237 U/mL), which is still close with the predicted value. Hence, this model was measured as successfully validated. From the analysis, the response surface plots shows that the optimum predicted cultural conditions for the optimum xylanase production were found to be: IPTG 0.5 Mm, 20 °C, induction time 4.5 h, 50 rpm, pH 7 and inoculum size 5%.
Before optimization
After optimization
Cultural condition:
Inducer concentration (mM)
0.1
0.5
Post induction temperature (°C)
30
20
Post induction time (h)
2
4.5
Agitation rate (rpm)
200
50
pH
5
7
Inoculum size (%)
5
5
Response:
xylanase excretion (U/mL)
Predicted
0.4847
Actual
0.0603
0.4821
Free cell (using optimized conditions)
0.1264
β-galactosidase activity (U/mL)
Predicted
0.0237
Actual
0.1487
0.0299
Free cell (using optimized conditions)
0.1273
3.3 Reusability of immobilized recombinant E. coli
The free cells and immobilized cells were reused in seven sequential fermentation cycles of 24 h cultivation each. Under the optimal cultural conditions that were established before, the immobilized E. coli demonstrated a higher and stable xylanase excretion until the 4th reused cycles before the activity was continuously declined until the end of 7th cycles, Fig. 1(a). Conversely, the free cell displayed lower stability from the 2nd cycle until the end of 7th cycles.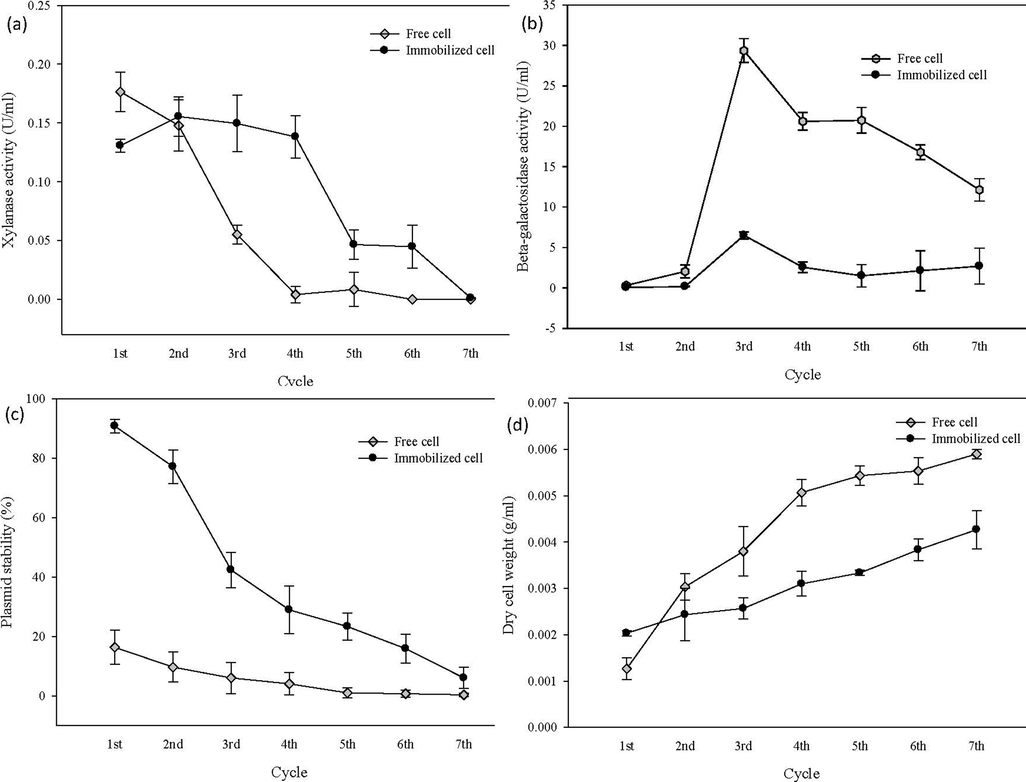
(a) Xylanase excretion between immobilized cell (onto non-treated graphene oxide) and free cell by repeated fermentation (b) β-galactosidase activity of immobilized cell (onto non-treated graphene oxide) and free cell by repeated fermentation (c) Plasmid stability of immobilized cell (onto non-treated graphene oxide) and free cell by repeated fermentation (d) Dry cell weight of immobilized cell (onto non-treated graphene oxide) and free cell by repeated fermentation.
The β-galactosidase activities of immobilized and free cells were compared under repeated fermentation. In case of free cells, it showed the increased of cell lysis on the 3rd cycle; 29.3467 U/mL before it dropped to 20.966 U/mL and declined slowly until the end of 7 reused cycles, Fig. 1(b). Conversely, the immobilized cells display better resistant for cell lysis from the 1st cycle until the end of 7th cycles. The lower plasmid stability demonstrated by free cells may cause by the lower energy level reserved by the free cells as a result of high motion in the cultivation medium, Fig. 1(c). Hence, it proved that, the plasmid stability was improved by immobilization system compared with free cell cultivation. This data was supported by several studies conducted by Duarte et al. (2013) and Xu et al. (2014), the immobilization system generally showed better stability towards the physiochemical factor changes than the free system which consequently correlated with higher substrate conversion, higher production rate as well as can be recovered and reused multiple times. In Fig. 1(d), the dry cell weight was lower for immobilized cells compare to free cell. Adsorption-based cell immobilization can influence cell development in both positive and negative ways. Study conducted by Joanna et al. (2016) point out that the potential causes for the reduced growth rate perceived in immobilized cells is due to the nutrient and oxygen gradients formation within the matrices, which lead to the restriction of mass transfer as the immobilized cells focus on the exterior surface of the matrices, whereas the interior parts remain free deprived of nutrients. On the other hand, immobilized cells correlated with higher constant cell growth due to the protection by matrix that increase the tolerance to inhibitors or the change of environmental condition and toxic compounds (Gungormusler et al., 2015; Joanna et al., 2016).
During this study, non-immobilized cells were removed and the immobilized cells were reintroduced into a fresh expression medium, which encouraged the formation of new cells. The outcomes can be observed by the consistent rise of dry cell weight over the 7th cycles. Similarly, Hutchinson et al., 2020 found that the number of microbial cells on the surface of corncobs rose during cycled fermentation in the investigation of reusability of immobilized cells for successive balsamic-styled vinegar fermentations.
3.4 Xylanase excretion of free cells cultivation, FFD cultivation, RSM cultivation and bioreactor.
Fig. 2 shows the highest xylanase activity during the 6th h for bioreactor and RSM cultivation which demonstrates that the consistency in enzyme productivity from small scale to the larger scale before drastically drop at 8th h and continuously declined slowly to the end of the cultivation. According to Radwan et al. (2011) when undesired components were produced in parallel with a desired product, the xylanase activity will decline and leads to the reduction of enzyme production. Nonetheless, from the data shown in Fig. 2 it proved that, this research could be potentially brought to a mass scale production as the productivity of the reactor scale could be maintained from the prior scale (flask cultivation) as the enzyme productivity from the reactor scale that applied the optimized RSM condition was 6% higher (0.506 U/mL) than RSM flask cultivation (0.483 U/mL).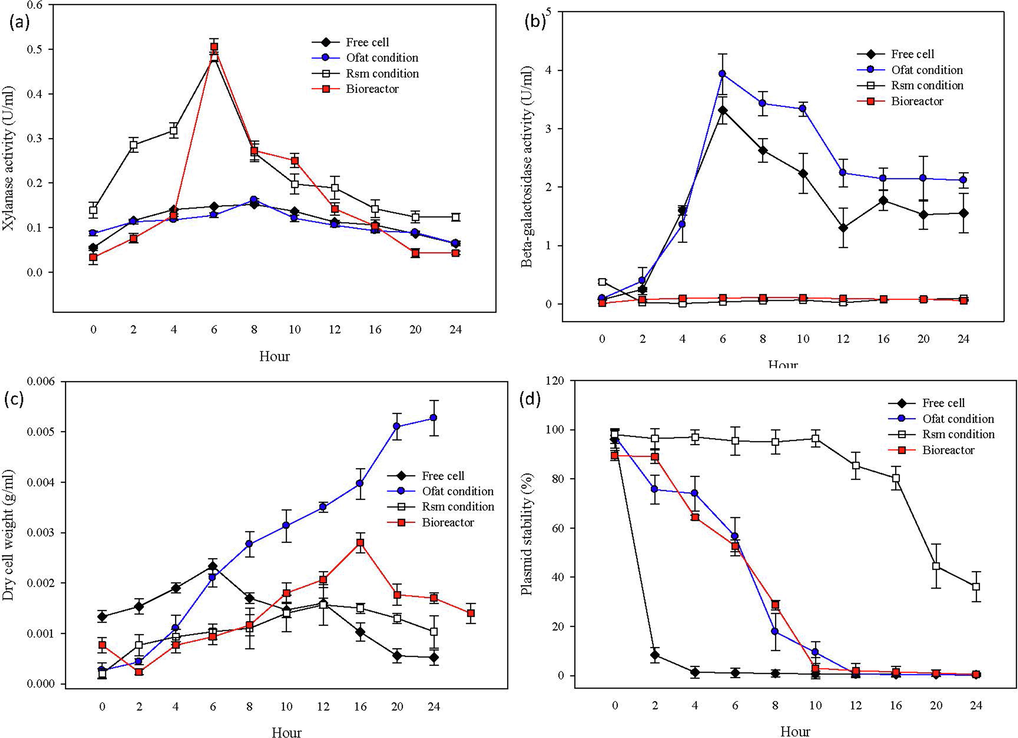
(a) Xylanase excretion of free cell, immobilized cell using FFD condition, immobilized cell using RSM condition and bioreactor (b) β-galactosidase activity of free cell, immobilized cell using FFD condition, immobilized cell using RSM condition and bioreactor (c) Dry cell weight of free cell, immobilized cell using FFD condition, immobilized cell using RSM condition and bioreactor (d) Xylanase excretion of free cell, immobilized cell using FFD condition, immobilized cell using RSM condition and bioreactor.
Bioreactor cultivation showed resistant in cell lysis through the low β-galactosidase activity occurred during the cultivation process due to the imobilization system that made the strong cell attachment to the surface of matrix. Hence, cell resistant to the higher shear stress and mechanical force that comes from shaking condition during the cultivation process (Ramírez-Nuñez et al., 2011).
In order to observe the growth phase of E. coli, the dry cell weight method can be used to determine the increase in cell mass and number of the cultivation cells. Thus, from Fig. 2(c) the dry cell weight of FFD cultivation was continuously raised until the end of the cultivation, while for bioreactor cultivation, the maximum exponential phase at 16 h then slowly declined until the end of 24 h cultivation. During bioreactor cultivation, as the maximum exponential phase at 16 h then slowly declined until the end of 24 h cultivation. Thus, as demonstrated from the Fig. 2(c) the first phase or starter phase observed during the cultivation process of bioreactor from 0 h to 4 h was the lag phase in which the growth rate was basically zero. This was happened when the inoculum was placed into the fresh medium, the growth phase after the first h was called as the lag phase. The lag phase was defined as the transition to the exponential phase after the initial population has doubled (Yates & Smotzer, 2007). The lag phase usually lasts from min to several hours.
On the other hand, Fig. 2(d) is in contrast to the data in Fig. 2(c), which shows an increase in the dry cell weight for each time point during the FFD cultivation. However, as illustrated in Fig. 2(d), the plasmid was unstable and displayed a decline pattern at each time point. Accordingly, Chen et al. (2009) pointed out that the cells are exposed to the metabolic burden that occasioned in the development of plasmid loss cells at the time of cell division which occurred at the doubling time of the cell mass. The occurrences of plasmid loss in recombinant E. coli cultivations are perceived once the mass of cells become doubled after induction process, which correlated to the cell fission timing. Thus, it is necessary to control the doubling time of cells after induction to regulate the occurrence of plasmid free cells during the cell fission process (Soares et al., 2019). Interestingly, the immobilization technique was able to restrict the growth rate by slowing the doubling time due to the immobilized cells' limited mobility, which resulted in fewer cell divisions and, as a result, reduced plasmid partitioning. When cells divide infrequently, less energy is diverted toward replication, which lowers the metabolic load of plasmid maintenance on the cells.
Furthermore, the kinetic parameter values of free and immobilized recombinant E. coli were obtained for a better knowledge of the fermentation process and a higher production rate (Table 3). *The chosen time point was based on the highest kinetic values.
No.
Kinetic parameter valuesConditions
Free cell
FFD
RSM
Bioreactor scale-up
1.
Maximal recombinant xylanase excretion (U/mL)
0.152
0.162
0.482
0.506
2.
Maximum biomass produced, Xmax (g/L)
2.341 a
5.276b
1.578c
2.832c
3.
Productivity (U/mL/h)
0.019
0.020
0.080
0.084
4.
Time point (h)*
8
8
6
6
3.5 Characterization of immobilized E. coli
3.5.1 SEM characterization
Free cells and immobilized E. coli had dimensions of 435 nm and 772 nm, respectively, and lengths of 1.7–2.8 µm, as shown in Fig. 3(b) and Fig. 3(d). While the average size distribution or pore diameter of GO was in the range of nanometer scale, 5–100 nm as shown in Fig. 3(a). Hence, there was no possibility for the microbial cell to make an entrance inside the GO (Zhang et al., 2009; Rostro et al., 2016). It exhibited that the immobilization process takes placed by adsorption method onto the GO’s exterior surface Fig. 3(c). Meanwhile, the process occurs involved the physicochemical bonds or electrostatic interactions (van der Waals forces) among the charge support and microorganisms (Zajkoska et al., 2013; Gungormusler et al., 2015).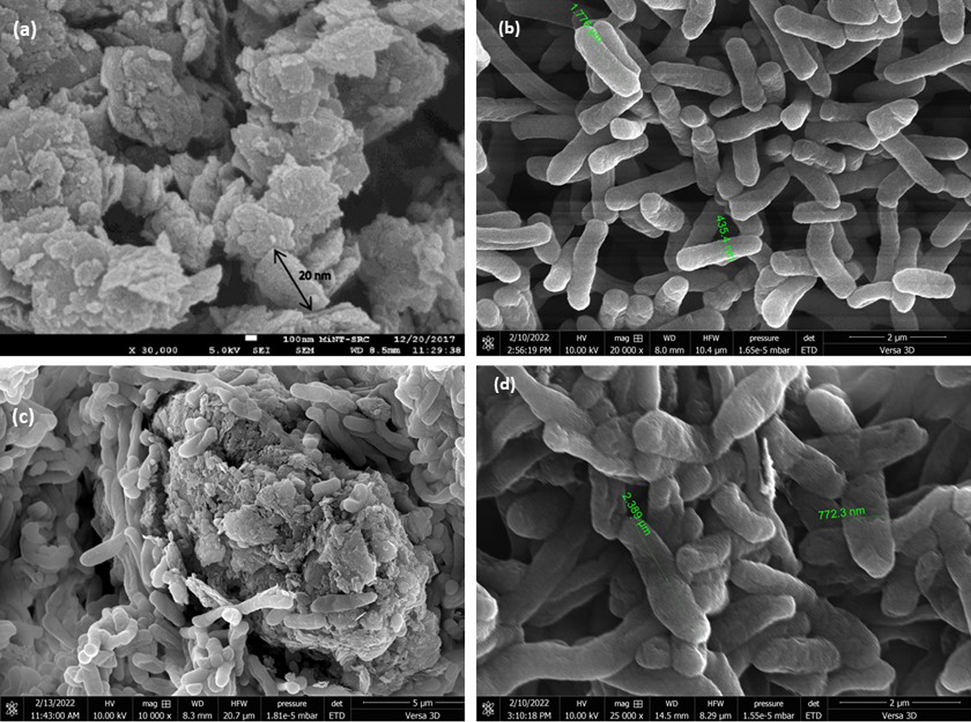
(a) SEM image of graphene oxide at 30 k × magnification (b) SEM image of free suspension recombinant Escherichia coli cells at 20 k × magnification (c) SEM image of immobilized recombinant Escherichia coli cells at 10 k × magnification (d) SEM image of immobilized recombinant Escherichia coli cells at 25 k × magnification.
The SEM results revealed that biofilm development is the primary cause of the most substantial alterations in microbial metabolism observed in cells immobilized by adsorption. Biofilm development is responsible for the most substantial alterations in microbial metabolism found in cells immobilized by adsorption as previously mentioned by Zur et al., 2016. Biofilm is a complex surface-attached or associated with the interfaces of microbial communities that forms in response to environmental circumstances like nutrition and oxygen availability. Bacteria produce high molecular weight biopolymers that enable cell-to-cell and cell-surface/interface adhesion during biofilm growth, which are referred to as extracellular polymeric subsatances (EPS), exopolysaccharide, exopolymer, microbial flocculants, biopolymers). EPS is a component of microbial aggregates that binds cells in a three-dimensional structure together. As shown in Fig. 3(b) and Fig. 3(d), the size of recombinant E. coli cells differs significantly between free and immobilized cells, which could be due to EPS generation. Biofilm formation depends on the EPS production, and the transport of recombinant protein to the cell exterior took its share of the translocation capacity of the cell, as mentioned by Soares et al., 2019. Additionally, the authors discuss the SWOT analysis of biofilm cultures for the production of recombinant proteins.
Overall, the choice of suitable matrix altered the morphology, physiology, metabolic reaction, and biochemical content of the microbial cell in this investigation. According to Zhang et al., 2013, the material should be biologically compatible for efficient cell-matrix interaction, non-hazardous, and readily available for the system's long-term sustainability. The high xylanase excretion reported in GO immobilized cells may be due to the ability of these cells to more easily incorporate nutrients in culture medium compared to free cells by obtaining from the adsorbed nutrient at the liquid–solid border (Man et al., 2015). Furthermore, GO has a high hydrophilicity, which allows it to penetrate hydrogen (H2) and oxygen (O2), allowing it to dissolve in culture media or other hydrogen bonding polar solvents. It is able to open the path for cell attachment to the matrix by having this feature (Ammar et al., 2016).
3.5.2 Liquid chromatography mass spectrometry (LCMS) analysis of xylooligosaccharides production
The process involved in the production of xylooligosaccharides from xylan substrate was degradation of xylan into monomers by random cleavage of β-1,4-glycosidic bonds of the xylan backbone (Zhao et al., 2015). However, the dinitrosalicylic acid (DNS) assay technique analyses only reducing sugars, specifically xylose, which explains why the analysis revealed minimal activity. While LCMS is used to determine the specific sugar hydrolyzed by xylanase from beechwood xylan. As a result, it could be higher in terms of xylanase activity, resulting in larger sugar molecules. The xylanase production after 8 min run time demonstrated that the products of beechwood xylan was hydrolyzed by xylanase enzyme at 1.86 min retention time to produce xylopentaose sugar which was exactly at the same retention time of standard chromatogram for xylopentaose sugar as shown in the Fig. 4.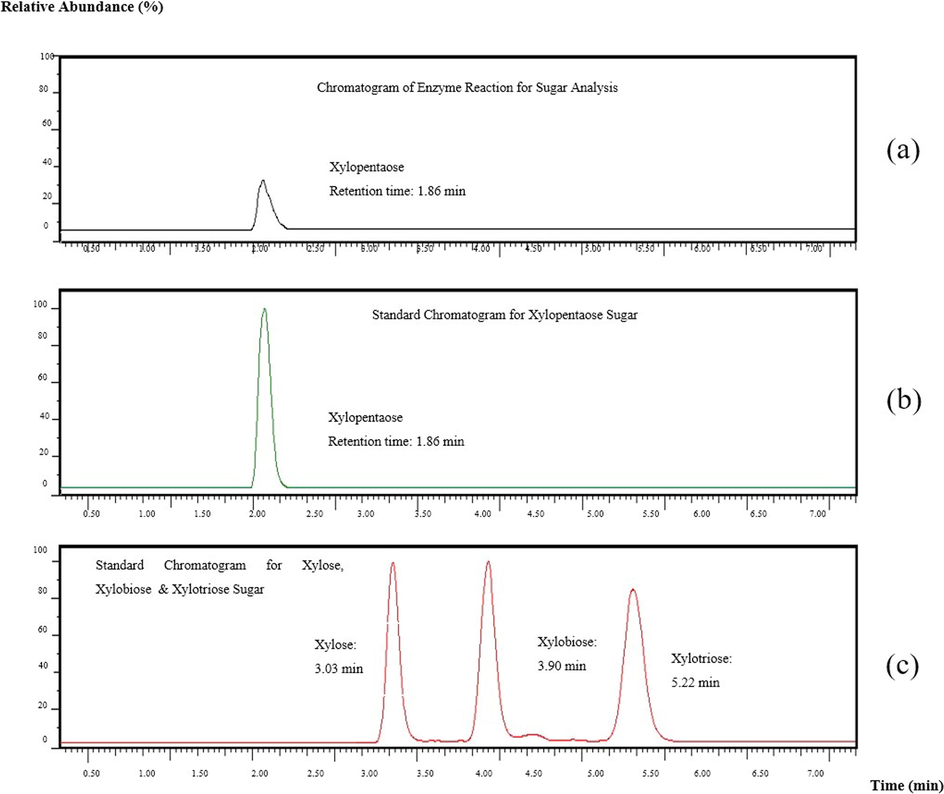
LCMS analysis for the quantification of xylooligosaccharides production using the waters ACQUITY UPLC BEH Amide column (100 mm × 2.1 mm × 1.7 µm).
This result was consistent with another two repetitions of chromatogram analysis using the same reaction sample. Therefore, the results indicated that the xylooligosaccharides product of beechwood xylan was found to be hydrolyzed by an endoxylanase to produce xylopentaose sugar by splitting the xylooligosaccharides intramolecular glycosidic
4 Conclusion
In this study, the cells immobilization onto GO exhibited certain efficient as well as practical preferences. For instance, more resilient to surrounding conditions due to the high plasmid stability than cell suspension cultures. In addition, it has better cell stability, high mechanical strength and manage to preserve the cells from shear damage. This boosts the rate of xylanase synthesis by reducing the possibility of cell lysis and plasmid instability. Apart from that, it was discovered that thermostable endoxylanase-producing E. coli hydrolyzed the xylooligosaccharides to produce high value xylopentaose sugar, instead of xylose. From the first cycle to the end of seven cycles of reusability cycle analysis, immobilized cells exhibit increased resistance to cell lysis. Moreover, the lower plasmid stability demonstrated by free cells may be due to the free cells' lower energy level reserved as a result of high motion in the cultivation medium.
Acknowledgement
We are grateful to the Ministry of Higher Education Malaysia for the funding from the research grant Research Acculturation Grant Scheme (RAGS) RAGS/1/2014/SG05/UTHM//1 as well as Universiti Tun Hussein Onn Malaysia (UTHM) GPPS Vot U488. Communication of this research is made possible through monetary assistance by Universiti Tun Hussein Onn Malaysia and the UTHM Publisher’s Office via Publication Fund E15216.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Carbon nanomaterial properties help to enhance xylanase production from recombinant Kluyveromyces lactis through a cell immobilization method. Appl. Microbiol. Biotechnol.. 2021;105:8531-8544.
- [Google Scholar]
- Immobilization of carbon nanotubes on functionalized graphene film grown by chemical vapor deposition and characterization of the hybrid material. Sci. Technol. Adv. Mater.. 2014;17(1):541-553.
- [Google Scholar]
- Wrapping bacteria by graphene nanosheets for isolation from environment, reactivation by sonication, and inactivation by near - infrared irradiation. J. Phys. Chem. B. 2015:1-17.
- [Google Scholar]
- Influence of graphene oxide on mechanical, morphological, barrier, and electrical properties of polymer membranes. Arab. J. Chem.. 2016;9(2):274-286.
- [Google Scholar]
- Immobilization of enzymes: a literature survey. Methods Mol. Biol.. 2013;1051:15-31.
- [Google Scholar]
- A fermentation strategy for production of recombinant protein subjected to plasmid instability. Korean J. Chem. Eng.. 2009;25(5):1110-1114.
- [Google Scholar]
- Comparison of culture methods on exopolysaccharide production in the submerged culture of Cordyceps militaris and process optimization. Lett Appl Microbiol.. 2011;52(2):123-128.
- [Google Scholar]
- Optimization protocols and improved strategies of cross-linked enzyme aggregates technology: current development and future challenges. Crit. Rev. Biotechnol.. 2015;35(1):15-28.
- [Google Scholar]
- Surfactant-activated lipase hybrid nanoflowers with enhanced enzymatic performance. Sci. Rep.. 2016;6:27928.
- [Google Scholar]
- Shielding effects of Fe3+-tannic acid nanocoatings for immobilized enzyme on magnetic Fe3O4@silica core shell nanosphere. Chem. Eng. J.. 2018;343:629-637.
- [Google Scholar]
- Protein engineering of GH11 xylanase from Aspergillus fumigatus RT-1 for catalytic efficiency improvement on kenaf biomass hydrolysis. Enzyme Microb. Technol.. 2019;131:109383
- [Google Scholar]
- Effect of immobilized cells in calcium alginate beads in alcoholic fermentation. AMB Express.. 2013;3(1):31.
- [Google Scholar]
- Three-dimensional ordered magnetic macroporous metal-organic frameworks for enzyme immobilization. J. Colloid Interface Sci.. 2021;590:436-445.
- [Google Scholar]
- Cell immobilization for microbial production of 1,3-propanediol. Biotechnol. 2015:1-13.
- [Google Scholar]
- Thermostability enhancement of xylanase Aspergillus fumigatus RT-1. J. Mol. Catal. B: Enzymatic.. 2016;134(A):154-163.
- [Google Scholar]
- Reusability of Immobilized Cells for Subsequent Balsamic-Styled Vinegar Fermentations. Ferment.. 2020;6:103.
- [Google Scholar]
- Optimization of enzymatic hydrolysis condition and functional properties of eel (Monopterus sp.) protein using response surface methodology (RSM) Int. Food Res. J.. 2016;23:1-9.
- [Google Scholar]
- Industrial and biotechnological applications of ligninolytic enzymes of the basidiomycota : A review. Electron J. Biotechnol.. 2016;13(6):1-13.
- [Google Scholar]
- Metabolic responses of bacterial cells to metabolic responses of bacterial cells. Molecules.. 2016;21(958):1-15.
- [Google Scholar]
- Dynamic cell-matrix interactions modulate microbial biofilm and tissue 3D microenvironments. Curr. Opin. Cell. Biol.. 2016;42:102-112.
- [Google Scholar]
- Technology prospecting on enzymes: application, marketing and engineering. Comput. Struct. Biotechnol. J.. 2016;2(3):1-14.
- [Google Scholar]
- Effects of culture conditions of immobilized recombinant Escherichia coli on cyclodextrin glucanotransferase (CGTase) excretion and cell stability. Process Bioch. J.. 2016;51(4):474-483.
- [Google Scholar]
- An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip.. 2016;29(2):205-220.
- [Google Scholar]
- Comparison of Thermostable Xylanase Production by Escherichia coli Immobilised onto Different Nanoparticles Chem. Eng. Trans.. 2017;56:1825-1830.
- [Google Scholar]
- Optimization of a fed-batch fermentation process for production of bleomycin by Streptomyces mobaraensis ATCC 15003. Afr. J. Biotechnol.. 2011;10(9):1690-1695.
- [Google Scholar]
- Xylitol production of recombinant Escherichia coli immobilized on multi walled carbon nanotubes. Universiti Teknologi Malaysia; 2016. Master thesis
- Effect of pH and salt gradient on the autolysis of Lactococcus lactis strains. Braz. J. Microbiol.. 2011;52:1495-1499.
- [Google Scholar]
- Recent progress in multienzymes co-immobilization and multienzyme system applications. Chem. Eng. J.. 2019;373:1254-1278.
- [Google Scholar]
- Nanobiocatalysis: nanostructured materials: a mini review. Biocatal.. 2016;2(1):1-24.
- [Google Scholar]
- Enhancement of thermoalkaliphilic xylanase production by Pichia pastoris through novel fed-batch strategy in high cell-density fermentation. BMC Biotechnol.. 2017;17(55):1-10.
- [Google Scholar]
- Application of iron magnetic nanoparticles in protein immobilization. Molecules.. 2014;19(8):11465-11486.
- [Google Scholar]
- The lag phase and initial decline of microbial growth curves. J. Theor. Biol.. 2007;244:511-517.
- [Google Scholar]
- Secretory production of recombinant proteins in Escherichia coli. Recent Pat. Biotechnol.. 2010;4(1):23-29.
- [Google Scholar]
- Biocatalysis with immobilized Escherichia coli. Appl. Microbiol. Biotechnol.. 2013;97:1441-1455.
- [Google Scholar]
- Graphene oxide as a matrix for enzyme immobilization. Langmuir.. 2010;26(9):6083-6085.
- [Google Scholar]
- Enhancement of chitosanase production by cell immobilization of Gongronella sp. JG. Braz. J. Microbiol.. 2013;44(1):189-195.
- [Google Scholar]
- Expression of the Thermobifida fusca xylanase xyn11a in Pichia pastoris and its characterization. BMC Biotechnol.. 2015;15(18):1-12.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.103803.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







