Translate this page into:
Optimization of the solvent system used to prepare cannabis inflorescence samples for cannabidiol and Δ9-tetrahydrocannabinol quantification using D-optimal design
⁎Corresponding author. chaowalit@rsu.ac.th (Chaowalit Monton)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The solvent most commonly used to prepare cannabis inflorescence samples is a mixture of methanol and chloroform (9:1), which poses a health hazard. This study aimed to develop a solvent system that maximizes the extraction of two major cannabinoids, cannabidiol (CBD) and Δ9-tetrahydrocannabinol (Δ9-THC), with superior performance to conventional solvents. The cannabinoid content was determined using high-performance liquid chromatography. The stability of these two cannabinoids in the optimal solvent system was also evaluated. The volume ratios of acetonitrile, methanol, and water were varied for extracting cannabinoids from two cannabis cultivars, Hang Kra Rog Phu Phan and Charlotte’s Angel, based on a D-optimal design. The solvent system developed in this study showed superior extraction efficiency to acetonitrile, as specified in the Thai Herbal Pharmacopoeia, and the methanol:chloroform mixture (9:1), as specified in the United Nations Office on Drugs and Crime manual. The optimal solvent system that provided the highest cannabinoid content consisted of acetonitrile, methanol, and water at a volume ratio of 0.511:0.289:0.200. This solvent system could extract 0.141 % ± 0.023 % of CBD and 3.255 % ± 0.511 % of Δ9-THC from the Hang Kra Rog Phu Phan cultivar, and 2.120 % ± 0.143 % of CBD and 3.813 % ± 0.219 % of Δ9-THC from the Charlotte’s Angel cultivar. The stability of cannabinoids in the optimal solvent system was comparable to that of the methanol:chloroform mixture (9:1) but superior to acetonitrile when stored at 4 °C and − 20 °C for 28 days. In summary, the optimal solvent system developed in this study is less toxic than existing systems and can be valuable for extracting cannabinoids from cannabis during inflorescence sample preparation for analyzing their content.
Keywords
Cannabinoids
High-performance liquid chromatography
Marijuana
Mixture design
Thai Stick
1 Introduction
Cannabis is a plant in the Cannabaceae family that has recently been used for medicinal purposes. Previous studies have identified over 750 compounds in cannabis plants, including cannabinoids, terpenoids, flavonoids, and sterols (Jin et al., 2020; Kanabus et al., 2021; Siracusa et al., 2023). More than 104 cannabinoids and 441 non-cannabinoids were reported in several parts of cannabis (ElSohly and Gul, 2014), with over 150 cannabinoids identified more recently (Lu et al., 2023). Cannabinoids and terpenes derived from cannabis show great potential in treating medical conditions such as cancer, Alzheimer’s disease, Parkinson’s disease, and neurological disorders such as depression, anxiety, insomnia, epilepsy, and seizures (Pattnaik et al., 2022). Cannabinoids are the prominent compounds found in cannabis plants used for medicinal purposes. The cannabinoids predominantly used for medicinal purposes are non-psychoactive cannabidiol (CBD) and psychoactive Δ9-tetrahydrocannabinol (Δ9-THC) (Freeman et al., 2019).
When chemically analyzing cannabis samples, it is essential to extract cannabinoids using an appropriate solvent. While various solvents are available, they vary in their ability to efficiently and selectively extract cannabinoids. Choosing the proper solvent for the specific analysis is paramount (United Nations Office on Drugs and Crime, 2022). Cannabinoids readily dissolve in a wide range of organic solvents, including methanol, petroleum ether, n-hexane, toluene, and chloroform, as well as solvent combinations such as methanol:chloroform (9:1, v/v), which are equally effective for their extraction (United Nations Office on Drugs and Crime, 2022). The stability of standard solutions depends on the selected solvents and storage conditions. Preparations of Δ9-THC in methanol or methanol:chloroform (9:1) solutions are favored over chloroform or petroleum ether since they demonstrate higher cannabinoid stability (Smith and Vaughan, 1977). Furthermore, Δ9-THC is more stable in ethanol than carbon tetrachloride and hexane (WHO Expert Committee on Drug Dependence, 2018). In chemical analysis, the primary objective of sample preparation is to achieve thorough extraction. Therefore, toxic solvents are occasionally used for this purpose.
Previous studies predominantly used a solvent system consisting of a 9:1 mixture of methanol and chloroform for sample preparation (Brighenti et al., 2017; De Backer et al., 2009; Gul et al., 2015; Hazekamp et al., 2004; Jin et al., 2020; Lehmann and Brenneisen, 1995; Mano-Sousa et al., 2023; McRae and Melanson, 2020; Mehmedic et al., 2010; Rovetto and Aieta, 2017; Smith and Vaughan, 1976; Stolker et al., 2004; Swift et al., 2013; Zivovinovic et al., 2018). This choice is attributed to its high capacity for extracting substantial quantities of cannabinoids such as CBD and Δ9-THC. Moreover, it demonstrates a better ability to preserve cannabinoids against degradation than other solvent systems (Smith and Vaughan, 1977; United Nations Office on Drugs and Crime, 2022). The United Nations Office on Drugs and Crime (UNODC) recommends this solvent system for preparing decarboxylated samples (United Nations Office on Drugs and Crime, 2022). Conversely, in Thailand, the Thai Herbal Pharmacopoeia (THP) recommends acetonitrile as a solvent for preparing cannabis samples (Bureau of Drug and Narcotic, 2021); however, it is less commonly used in previous studies than the widely favored methanol:chloroform mixture (9:1).
A solvent system that is less toxic but capable of efficiently extracting cannabinoids in high quantities and maintaining their stability is ideal. A Design of Experiment approach can achieve significant savings in terms of time, money, and resources. Additionally, it allows for identifying factor interactions and characterizing the response surface (Gibson, 2016; Steele, 2018). Moreover, it enables using a statistical model to predict the simultaneous impact of multiple factors (JMP Statistical Discovery LLC, 2023).
Therefore, this study aimed to identify a solvent system that optimizes the extraction of two major cannabinoids, CBD and Δ9-THC, surpassing conventional solvents. This study chose methanol and acetonitrile while avoiding carcinogenic chloroform. Additionally, based on the authors’ experience, a mixture of acetonitrile and water was found suitable for extracting cannabinoids. Therefore, methanol, acetonitrile, and water were selected for this study. Additionally, it evaluated the stability of these two cannabinoids in the optimal solvent system using high-performance liquid chromatography (HPLC). It used a D-optimal design—an algorithm that selects experimental runs that reduce the determinant of the variance–covariance matrix, effectively minimizing the volume of the joint confidence ellipsoid for the coefficients—to vary the volume ratios of acetonitrile, methanol, and water for extracting cannabinoids from two cannabis cultivars: Hang Kra Rog Phu Phan and Charlotte’s Angel. These two cultivars were selected based on their CBD and Δ9-THC contents: Hang Kra Rog Phu Phan has a high Δ9-THC content, and Charlotte’s Angel has a high CBD content. We anticipate this research will provide valuable guidance for extracting cannabinoids while preparing inflorescence samples to quantify their content.
2 Materials and methods
2.1 Materials
Female cannabis inflorescences were harvested from Nonthaburi and Sa Kaeo Provinces for the Hang Kra Rog Phu Phan and Charlotte’s Angel cultivars, respectively, in July 2023. The samples were identified by Ajan Nirun Vipunngeun, a plant taxonomist in the Department of Pharmacognosy, College of Pharmacy, Rangsit University. Voucher specimens were assigned the codes TMRC 065 and TMRC 066 and deposited at the Drug and Herbal Product Research and Development Center, College of Pharmacy, Rangsit University. CBD (purity = 99.38 %) and Δ9-THC (purity = 93.03 %) standards were obtained from the Department of Medical Sciences, Ministry of Public Health. Acetonitrile and methanol (HPLC grade) were obtained from Fisher Chemicals (Leicestershire, UK). Chloroform (AR grade) was obtained from VWR International S.A.S. (Briare, France). Water was produced by a Direct-Q 3UV water purifier (Merck KGaA, Darmstadt, Germany).
2.2 Preparation of cannabis samples
Female cannabis inflorescence samples from the two cultivars were placed in a hot air oven (Memmert UFB 400; Memmert GmbH, Schwabach, Germany) at 150 °C for 60 min to decarboxylate the cannabinoids from their acid to their neutral form. Peduncles were removed. Next, they were individually pulverized using a grinder and passed through a 40-mesh sieve. They were stored in a dry place and protected from light until used.
2.3 Sample preparation for CBD and Δ9-THC content analysis
Samples for the CBD and Δ9-THC content analysis were prepared by accurately weighing 10 mg of cannabis powder for the Hang Kra Rog Phu Phan cultivar and 5 mg for the Charlotte’s Angel cultivar and placing it into glass test tubes (n = 3). Next, 10 mL of the specific solvents at room temperature (Table 1) were added to each tube, which was then sealed with a cap. Then, the samples were prepared in three sets for extraction with different durations. The first set of samples was sonicated for 10 min, the second set for 20 min, and the third set for 30 min, using an ultrasonic bath (Elmasonic Easy 300H; Elma Schrnidbauer GmbH, Singen, Germany). After sonication, the samples were thoroughly mixed, filtered through a 0.45-µm nylon syringe filter, and transferred to amber vials. Finally, the samples were analyzed by HPLC (see Section 2.6). The CBD and Δ9-THC contents obtained with each solvent system were compared to those obtained with the methanol:chloroform mixture (9:1), as recommended by the UNODC, and acetonitrile, as recommended in the THP. Note: Runs 3 & 4, 2 & 11, 6 & 12, 9 & 14, and 10 & 16 were intentionally replicated.
Run
Factor
Response
Volume ratio
Hang Kra Rog Phu Phan
Charlotte’s Angel
Acetonitrile
Methanol
Water
CBD content (%)
Δ9-THC content (%)
CBD content (%)
Δ9-THC content (%)
1
0
0.9
0.1
0.117 ± 0.016
3.015 ± 0.095
1.932 ± 0.134
3.799 ± 0.274
2
0
1
0
0.115 ± 0.010
3.094 ± 0.134
2.000 ± 0.145
3.799 ± 0.369
3
0
0.8
0.2
0.124 ± 0.002
3.056 ± 0.159
2.020 ± 0.112
3.948 ± 0.198
4
0
0.8
0.2
0.130 ± 0.112
3.145 ± 0.077
2.072 ± 0.073
3.680 ± 0.159
5
0.225
0.725
0.05
0.126 ± 0.009
3.080 ± 0.144
1.855 ± 0.084
3.871 ± 0.349
6
1
0
0
0.111 ± 0.014
2.845 ± 0.114
1.863 ± 0.239
3.621 ± 0.367
7
0.9
0
0.1
0.123 ± 0.012
2.961 ± 0.079
1.870 ± 0.143
3.922 ± 0.196
8
0.725
0.225
0.05
0.125 ± 0.011
3.115 ± 0.221
2.031 ± 0.172
3.973 ± 0.128
9
0.8
0
0.2
0.126 ± 0.007
3.068 ± 0.134
1.935 ± 0.090
3.938 ± 0.149
10
0.5
0.5
0
0.112 ± 0.012
2.910 ± 0.214
2.162 ± 0.031
3.805 ± 0.096
11
0
1
0
0.117 ± 0.015
2.954 ± 0.141
2.036 ± 0.228
3.780 ± 0.018
12
1
0
0
0.111 ± 0.017
2.855 ± 0.020
2.052 ± 0.159
3.454 ± 0.409
13
0.45
0.45
0.1
0.130 ± 0.014
3.212 ± 0.228
1.915 ± 0.263
4.065 ± 0.214
14
0.8
0
0.2
0.130 ± 0.009
3.065 ± 0.028
2.065 ± 0.232
4.339 ± 0.225
15
0.225
0.625
0.15
0.125 ± 0.018
3.199 ± 0.154
2.151 ± 0.105
3.851 ± 0.109
16
0.5
0.5
0
0.123 ± 0.007
3.109 ± 0.160
1.994 ± 0.188
3.809 ± 0.172
2.4 Experimental design
This study used a D-optimal design, where the volume ratios of acetonitrile (A), methanol (B), and water (C) were adjusted within the following specified design constraints:
0 ≤ A ≤ 1.0
0 ≤ B ≤ 1.0
0 ≤ C ≤ 0.2
A + B + C = 1.0
The CBD and Δ9-THC contents in raw cannabis materials obtained with various solvent systems (Table 1) were analyzed using Design-Expert® (version 11; Stat-Ease Inc., Minneapolis, MN, USA). This analysis included the generation of contour plots, trace (Piepel) plots, and an analysis of variance. Two design spaces were established. The first examined the scenario where the model solvent system surpassed acetonitrile (recommended in the THP) or the methanol:chloroform mixture (9:1; recommended by the UNODC). The second examined the scenario where the model solvent system surpassed both acetonitrile and the methanol:chloroform mixture (9:1).
The optimal solvent system, identified based on the highest CBD and Δ9-THC contents according to the desirability function and within the design space, was chosen for verification. This optimal solvent system was used to prepare cannabis extract solutions according to the method described in Section 2.3, and their CBD and Δ9-THC contents were analyzed. The CBD and Δ9-THC contents obtained experimentally were compared to the predicted values and reported as a percent error using the following equation:
2.5 Stability test
The cannabis samples from the two cultivars were extracted using the optimal solvent system. Next, they were filtered into amber vials and stored at 4 °C or − 20 °C for up to 28 days. Then, their CBD and Δ9-THC contents were determined after 7, 14, 21, and 28 days. These results were compared to the stability of CBD and Δ9-THC in acetonitrile and the methanol:chloroform mixture (9:1).
2.6 HPLC condition
Cannabinoid contents were determined using a validated HPLC method, as previously described (Monton et al., 2024; Monton et al., 2022; Monton et al., 2023; Yangsud et al., 2021). The cannabinoids were quantified using an Agilent 1260 Infinity HPLC instrument (Agilent Technologies Inc., Santa Clara, CA, USA). The quantification process involved an isocratic system with an ultrapure water and methanol mixture (17:83, v/v) and a flow rate of 0.4 mL/min. The analysis used an ACE 3 C18-PFP column (150 × 3.0 mm, internal diameter, 3 μm; Advanced Chromatography Technologies Ltd., Aberdeen, UK) coupled to a guard SecurityGuard Cartridge C18 column (4.0 × 3.0 mm, internal diameter; Phenomenex Inc., Torrance, CA, USA), maintained at 25 °C. The injection volume was set at 5 μL, and signals were detected with a photodiode array detector at a wavelength of 222 nm.
2.7 Statistical analysis
The data were analyzed using SPSS Statistics (version 22.0; IBM Corp., Armonk, NY, USA). Data were compared among groups using a one-way analysis of variance followed by a post-hoc Fisher’s least significant difference test. A p-value of < 0.05 was considered statistically significant.
3 Results and discussion
3.1 Effect of sonication time on CBD and Δ9-THC contents
This study used sonication to extract CBD and Δ9-THC from two cannabis cultivars. It examined the CBD and Δ9-THC contents of cannabis inflorescence samples from the Hang Kra Rog Phu Phan and Charlotte’s Angel cultivars prepared using different solvent systems and sonication times of 10, 20, and 30 min (Fig. 1). It also compared the CBD and Δ9-THC contents between the methanol:chloroform mixture (9:1) and acetonitrile (Runs 6 and 12). Generally, sonication time did not significantly affect the CBD and Δ9-THC contents extracted with the different solvent systems, except for condition 5 for CBD and condition 16 for Δ9-THC from the Charlotte’s Angel cultivar. Specifically, these exceptions revealed that a sonication time of 30 min resulted in significantly higher CBD and Δ9-THC contents than sonication times of 10 and 20 min. With the methanol:chloroform mixture (9:1), a sonication time of 30 min resulted in significantly higher Δ9-THC content than a sonication time of 10 min.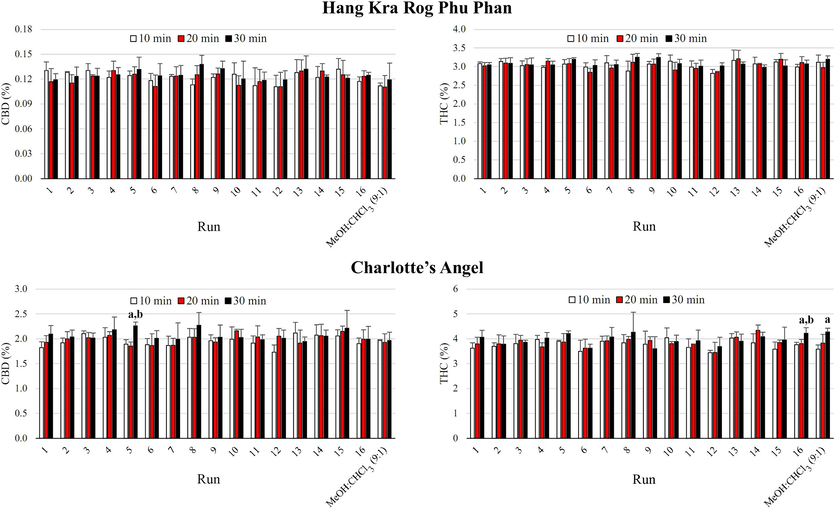
The CBD and Δ9-THC contents of cannabis inflorescence samples from the Hang Kra Rog Phu Phan and Charlotte’s Angel cultivars prepared with different solvent systems and sonication times (10, 20, and 30 min). The CBD and Δ9-THC contents of samples prepared using the methanol:chloroform mixture (9:1; MeOH:CHCl3) and acetonitrile were also compared (Runs 6 and 12). In the bar graph, “a” and “b” indicate significant differences (p < 0.05) compared to sonication times of 10 and 20 min, respectively.
In studies using a methanol:chloroform mixture (9:1) for cannabis sample preparation, sonication times varied between 10 and 30 min, with specific values of 10 min (Hazekamp et al., 2004; Smith and Vaughan, 1976), 15 min (Gul et al., 2015; Lehmann and Brenneisen, 1995; Mano-Sousa et al., 2023; Zivovinovic et al., 2018), 30 min (Swift et al., 2013), and 10–30 min (Jin et al., 2020). The UNODC recommends a sonication time of 15 min with the methanol:chloroform mixture (9:1), while the THP recommends a sonication time of 20 min with acetonitrile. As shown in Fig. 1, varying the sonication time between 10 and 30 min did not significantly affect the CBD and Δ9-THC contents. Therefore, a sonication time of 20 min was chosen for the optimization step to ensure an adequate sonication time for both solvents.
While the naïve Charlotte’s Angel cultivar has a high CBD content, its raw materials used in this study contained more Δ9-THC than CBD. This discrepancy can be attributed to the fact that cannabis is recognized as a wind-pollinated plant and is particularly susceptible to cross-breeding. Even under controlled conditions, maintaining genetic purity becomes challenging when relying on sexual reproduction (Duggan, 2021). Furthermore, genetic characteristics, plant development stage, and environmental factors such as climate and elevation in the cultivation area have been observed to influence the chemical constituents in cannabis plants (Tipparat et al., 2012).
3.2 Optimal solvent system for preparing samples for CBD and Δ9-THC analyses
Contour plots of the CBD and Δ9-THC contents in cannabis inflorescence samples from the Hang Kra Rog Phu Phan and Charlotte’s Angel cultivars prepared using different solvent systems are shown in Fig. 2. Trace (Piepel) plots illustrating how the solvent ratio affects the CBD and Δ9-THC contents of cannabis inflorescence samples are shown in Fig. 3. For the Hang Kra Rog Phu Phan cultivar, the contour plots revealed a similar pattern between CBD and Δ9-THC contents. They gradually increased as the acetonitrile and methanol ratios increased from low to medium and then rapidly decreased as the ratios increased from medium to high. In contrast, they increased continually with the water ratio.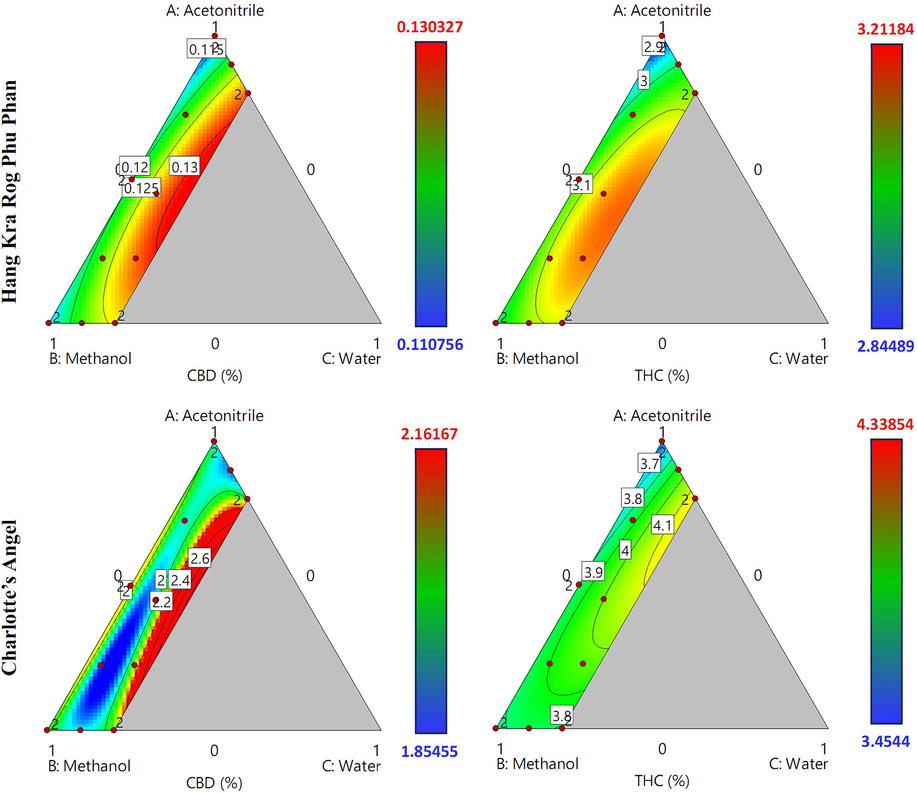
Contour plots of the CBD and Δ9-THC contents of cannabis inflorescence samples from the Hang Kra Rog Phu Phan and Charlotte’s Angel cultivars using different solvent systems.
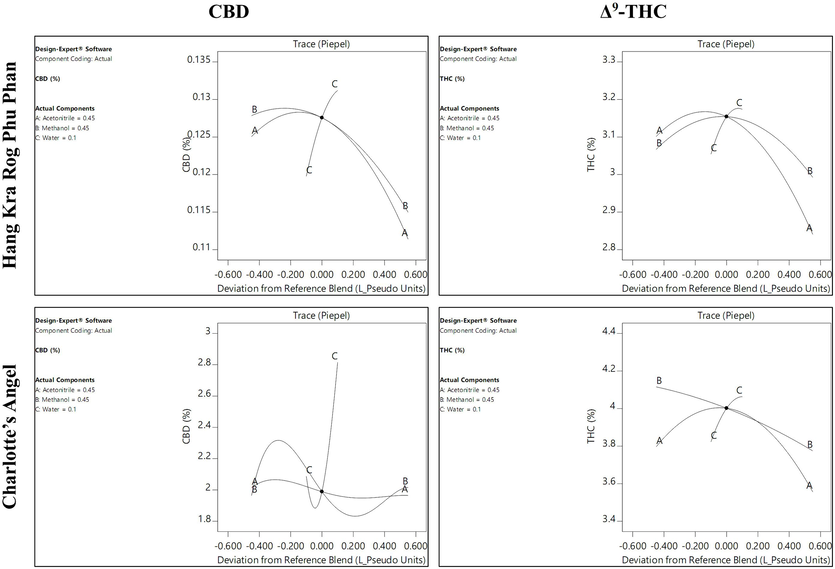
Trace (Piepel) plots illustrating how the solvent ratio affects the CBD and Δ9-THC contents of cannabis inflorescence samples from the Hang Kra Rog Phu Phan and Charlotte’s Angel cultivars. The volume ratio of acetonitrile, methanol, and water was set at 0.45, 0.45, and 0.1, respectively.
For the Charlotte’s Angel cultivar, the CBD content increased and then decreased as the acetonitrile and methanol ratios increased from low to medium. However, the CBD content showed different patterns as the acetonitrile and methanol ratios increased from medium to high. Specifically, the CBD content decreased and then increased as the methanol ratio increased from medium to high but remained almost constant as the acetonitrile ratio increased from medium to high. In contrast, the Δ9-THC content increased gradually as the acetonitrile ratio increased from low to medium but decreased as the acetonitrile ratio increased from medium to high. The Δ9-THC content decreased continuously as the methanol ratio increased from low to high. Regarding the water ratio, CBD content initially decreased and then increased as the water ratio increased from low to high, while the Δ9-THC content increased gradually as the water ratio increased from low to high.
The analyses of variance of the CBD and Δ9-THC contents in the Hang Kra Rog Phu Phan and Charlotte’s Angel cultivars are shown in Table S1 (Supplementary Material). The CBD and Δ9-THC contents of the Hang Kra Rog Phu Phan cultivar and the Δ9-THC content of the Charlotte’s Angel cultivar were found to fit quadratic models. However, the CBD content of the Charlotte’s Angel cultivar instead fits a special quartic model. The models for the CBD and Δ9-THC contents of the Hang Kra Rog Phu Phan and Δ9-THC content of Charlotte’s Angel cultivars were statistically significant, and there was no significant lack of fit, indicating that the desired model was achieved. However, it should be noted that the model for the CBD content of the Charlotte’s Angel cultivar did not reach statistical significance. The linear mixture model was significant for the CBD and Δ9-THC contents of the Hang Kra Rog Phu Phan cultivar and the Δ9-THC content of the Charlotte’s Angel cultivar. Furthermore, the interaction between the acetonitrile and methanol ratios was significant for the CBD and Δ9-THC contents of the Hang Kra Rog Phu Phan cultivar. Notably, the interaction between the ratios of the solvents, especially acetonitrile and methanol, enhanced the extraction efficiency of both CBD and Δ9-THC.
Fig. 4 presents the design spaces visualizing the solvent systems used to prepare cannabis inflorescence samples from the Hang Kra Rog Phu Phan and Charlotte’s Angel cultivars, which were superior to acetonitrile (recommended in the THP) and the methanol:chloroform mixture (9:1; recommended by the UNODC). Regarding the Hang Kra Rog Phu Phan cultivar, all acetonitrile/methanol/water systems examined in the design space provided superior CBD and Δ9-THC contents compared to acetonitrile alone (recommended in the THP) and the methanol:chloroform mixture (9:1; recommended by the UNODC). However, acetonitrile alone resulted in inferior CBD and Δ9-THC contents compared to the methanol:chloroform mixture (9:1). These findings suggest that the various solvent systems developed in this study are superior to those recommended in the THP and by the UNODC. Furthermore, the solvent system recommended in the THP (acetonitrile alone) exhibited lower extraction efficiency than the solvent system recommended by the UNODC (9:1 methanol:chloroform mixture). While the Charlotte’s Angel cultivar had a smaller design space than the Hang Kra Rog Phu Phan cultivar, the solvent systems developed in this study demonstrated higher extraction efficiency than those recommended in the THP and by the UNODC.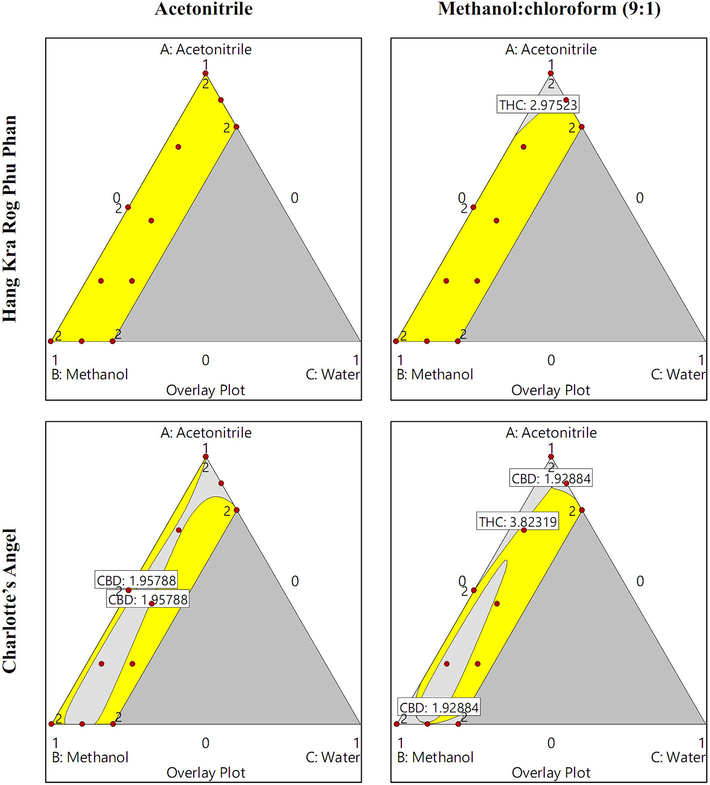
Design spaces illustrating the solvent systems used to prepare cannabis inflorescence samples from the Hang Kra Rog Phu Phan and Charlotte’s Angel cultivars. The yellow area represents the CBD and Δ9-THC contents, which were superior to those obtained using acetonitrile (recommended in the THP) or the methanol:chloroform mixture (9:1; recommended by the UNODC).
The primary consideration when selecting a solvent is the polarity of the target compound. Other critical factors include molecular affinity, mass transfer, cosolvents, environmental safety, human toxicity, and financial feasibility (Azmir et al., 2013). This study optimized a solvent system combining acetonitrile, methanol, and water as an alternative to the toxic methanol-chloroform mixture. Surprisingly, non-polar cannabinoids dissolved in this solvent system even with 20 % water content. Adding water modifies solvent properties, including melting and boiling points, with dielectric constant and polarity being crucial (Lazarjani et al., 2021; Moreno-Sanz et al., 2020; Tzimas et al., 2023), which may explain the high extraction efficiency observed. The solvent ratios enhanced the extraction efficiency of both CBD and Δ9-THC.
Solid-liquid extraction follows the “like dissolves like” principle. CBD is more polar than Δ9-THC (Monton et al., 2024; Yangsud et al., 2021). The dielectric constant values of acetonitrile, methanol, and chloroform are 37.5 (20 °C), 32.63 (25 °C), and 4.81 (20 °C), respectively (Maryott and Smith, 1951). Therefore, acetonitrile is more polar than the methanol:chloroform mixture (9:1) and can extract more CBD. In contrast, the methanol-chloroform mixture extracts more Δ9-THC. However, acetonitrile alone is less efficient for neutral cannabinoids than the methanol-chloroform mixture, as these non-polar compounds dissolve better in non-polar solvents. Therefore, the methanol:chloroform mixture (9:1) is more suitable for extracting neutral cannabinoids.
The two cannabis cultivars had differing contour plot patterns and design spaces, which can be attributed to the well-documented phytochemical diversity commonly observed in medicinal plants. Consequently, the quantitative variation of a specific compound typically differs across cannabis plant materials from different geographical locations (Dhami and Mishra, 2015). Additionally, genetic traits, plant developmental stages, and environmental factors such as climate and elevation in the cultivation area have been noted to influence the chemical constituents of cannabis plants (Tipparat et al., 2012). Therefore, the choice of analytical methods and sample preparation for chemical analysis should be carefully considered when working with various cannabis cultivars.
The above results obtained for both cannabis cultivars, we observed that the solvent system mentioned in the THP is effective in extracting a high content of CBD, whereas the solvent system recommended by the UNODC is proficient at extracting a high content of Δ9-THC. Furthermore, a design space was constructed based on the criterion that the mixed solvent should simultaneously surpass acetonitrile and the methanol:chloroform mixture (9:1). Fig. 5 illustrates the design space representing the solvent systems used for the sample preparation of cannabis inflorescence samples from the Hang Kra Rog Phu Phan and Charlotte’s Angel cultivars. These solvent systems were superior to both acetonitrile (recommended in the THP) and the methanol:chloroform mixture (9:1; recommended by the UNODC). Moreover, in the verification step, the optimal solvent system was identified based on the highest CBD and Δ9-THC contents according to the desirability function within the design space, which consisted of acetonitrile, methanol, and water at a volume ratio of 0.511:0.289:0.200, with a desirability value of 0.894. We emphasize that adding water to the solvent system used to prepare cannabis samples offers several advantages, such as being safe for workers, environmentally friendly, and cost-effective. Furthermore, the acetonitrile/methanol/water solvent system is less toxic than the methanol-chloroform mixture.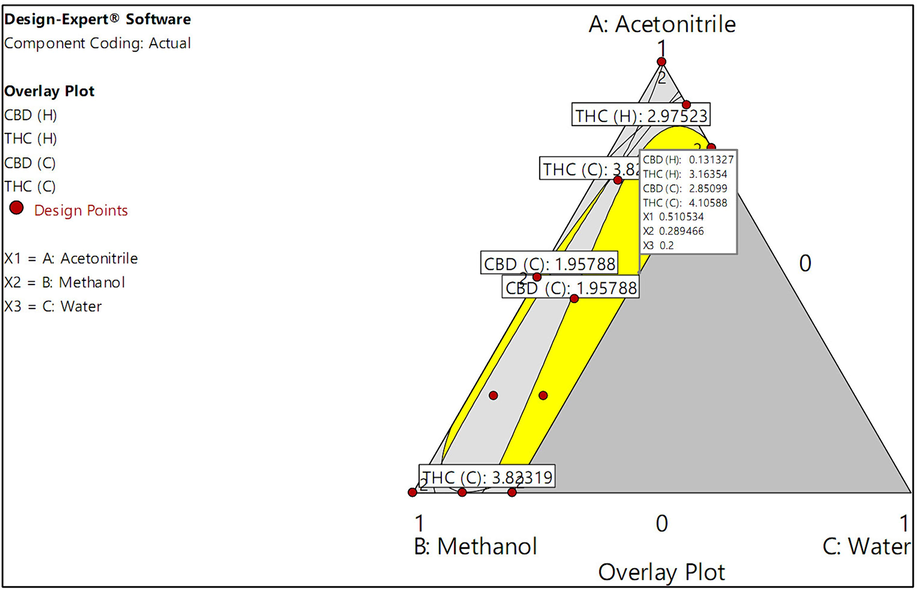
Design spaces illustrating the solvent systems used to prepare cannabis inflorescence samples from the Hang Kra Rog Phu Phan and Charlotte’s Angel cultivars. The yellow area represents the CBD and Δ9-THC contents, which were simultaneously superior to those obtained using acetonitrile (recommended in the THP) and the methanol:chloroform mixture (9:1; recommended by the UNODC). The flag indicates the optimal solvent system (acetonitrile, methanol, and water at a volume ratio of 0.511:0.289:0.200) and its predicted values for use in the verification step.
The optimal solvent system was used to prepare cannabis extract solutions to verify the accuracy of the predictions made by the Design-Expert® software. The verification data for cannabis inflorescence samples prepared using the optimal solvent system are presented in Table 2, comparing the experimental and predicted values and their respective percent errors. The percent error was < 10 % in all cases except the CBD content of the Charlotte’s Angel cultivar. The high percent error observed for the CBD content of the Charlotte’s Angel cultivar can be attributed to its nonsignificant model (Table S1, Supplementary Material), indicating that it could not be accurately predicted.
Cultivar
Compound
Predicted value (%)
Experimental value (%, n = 3)
Error (%)
Hang Kra Rog Phu Phan
CBD
0.131
0.141 ± 0.023
7.09
Δ9-THC
3.164
3.255 ± 0.511
2.80
Charlotte’s Angel
CBD
2.852
2.120 ± 0.143
−34.53
Δ9-THC
4.106
3.813 ± 0.219
−7.68
The HPLC chromatograms of the CBD and Δ9-THC standards and the cannabis inflorescence samples from the Hang Kra Rog Phu Phan and Charlotte’s Angel cultivars prepared using the optimal solvent system, acetonitrile (recommended in the THP), and the methanol:chloroform mixture (9:1; recommended by the UNODC) are shown in Fig. S1 (Supplementary Material). The HPLC chromatogram profiles of the samples extracted using the different solvent systems appear similar for the peak eluted between 3 and 20 min. However, they show notable differences in retention times from 1 to 3 min. Notably, these peaks were not identified within the scope of this study. Further investigation and analysis could help uncover the composition and nature of these unidentified peaks, potentially enhancing our understanding of the extraction process and the chemical profiles of the studied samples.
The CBD and Δ9-THC contents of the Hang Kra Rog Phu Phan and Charlotte’s Angel cultivars were extracted using the optimal solvent system (acetonitrile, methanol, and water at a volume ratio of 0.511:0.289:0.200) and compared to acetonitrile (recommended in the THP) and the methanol:chloroform mixture (9:1; recommended by the UNODC; Table 3). It can be concluded that samples prepared using the optimal solvent system were comparable to those prepared using the solvents recommended in the THP (acetonitrile) and by UNODC (9:1 methanol:chloroform mixture). However, the optimal solvent system was significantly superior to the other two solvents in CBD content from the Hang Kra Rog Phu Phan cultivar. The different superscript letters indicate significant differences (p < 0.05) when compared within the same row.
Cultivar
Compound
Solvent systems (n = 3)
Optimal solvent
Acetonitrile (THP)
Methanol:chloroform (9:1; UNODC)
Hang Kra Rog Phu Phan
CBD (%)
0.141 ± 0.023a
0.111 ± 0.014b
0.110 ± 0.014b
Δ9-THC (%)
3.255 ± 0.511a
2.850 ± 0.073a
2.975 ± 0.146a
Charlotte’s Angel
CBD (%)
2.120 ± 0.143a
1.958 ± 0.209a
1.929 ± 0.174a
Δ9-THC (%)
3.813 ± 0.219a
3.538 ± 0.360a
3.823 ± 0.359a
3.3 Stability of CBD and Δ9-THC in the optimal solvent system
Fig. 6 shows the stability of CBD and Δ9-THC from the Hang Kra Rog Phu Phan and Charlotte’s Angel cultivars in the optimal solvent system during storage at 4 °C and − 20 °C for 28 days, compared to acetonitrile (recommended in the THP) and the methanol:chloroform mixture (9:1; recommended by the UNODC). The CBD content remained stable across the measured time points compared to the initial time point for both cultivars (Fig. 6a and 6c). In contrast, while the Δ9-THC content of Hang Kra Rog Phu Phan cultivar samples remained stable in the optimal solvent system, it decreased significantly in acetonitrile and the methanol:chloroform mixture (9:1; Fig. 6b). Similarly, the Δ9-THC content of the Charlotte’s Angel cultivar samples remained stable in the optimal solvent system and acetonitrile, but decreased significantly in the methanol:chloroform mixture (9:1; Fig. 6d).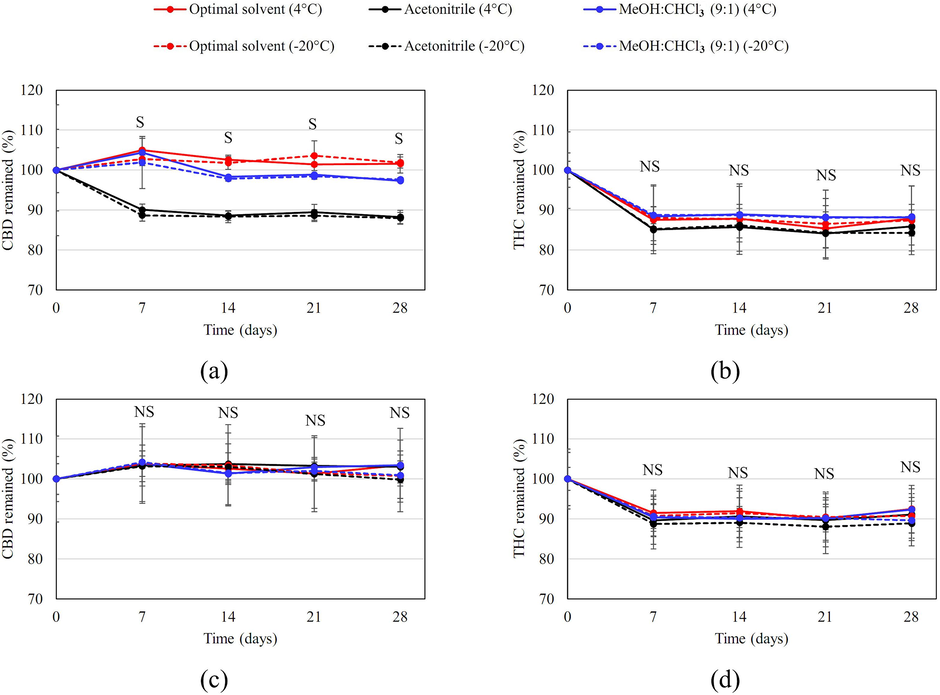
The stability of (a) CBD and (b) Δ9-THC from the Hang Kra Rog Phu Phan cultivar and (c) CBD and (d) Δ9-THC from the Charlotte’s Angel cultivar in the optimal solvent system (acetonitrile, methanol, and water at a volume ratio of 0.511:0.289:0.200), acetonitrile (recommended in the THP), and the methanol:chloroform mixture (9:1; recommended by the UNODC) during storage at 4 °C or − 20 °C for 28 days. “S” and “NS” denote significant and nonsignificant differences compared to the other systems at the same time point, respectively.
A comparison among the different solvents and temperatures at the same time point revealed no significant differences in CBD content in Charlotte’s Angel cultivar samples (Fig. 6c) and in Δ9-THC content in both Hang Kra Rog Phu Phan (Fig. 6b) and Charlotte’s Angel (Fig. 6d) cultivar samples. However, the CBD content of the Hang Kra Rog Phu Phan cultivar samples was significantly lower in acetonitrile samples than in the optimal solvent system and the methanol:chloroform mixture (9:1) samples on day 7. Additionally, between days 14 and 28, the CBD content was significantly higher in the optimal solvent system samples than in both the acetonitrile and methanol:chloroform mixture (9:1) samples (Fig. 6a).
Previous studies have preferentially used methanol or methanol:chloroform (9:1) over chloroform or petroleum ether to prepare Δ9-THC samples. These solvents provide greater cannabinoid stability (Smith and Vaughan, 1977). Additionally, it has been observed that Δ9-THC is more stable in ethanol than carbon tetrachloride and hexane (WHO Expert Committee on Drug Dependence, 2018). The stability of CBD and Δ9-THC in methanol, chloroform, and the methanol:chloroform mixture (9:1) was compared. Both cannabinoids remained stable in the three solvent systems when stored at − 18 °C, 4 °C, and 20 °C in the dark. In contrast, CBD and Δ9-THC both degraded when stored at 20 °C in daylight (Smith and Vaughan, 1977).
Based on our stability data, it can be concluded that the optimal solvent system was superior to acetonitrile (recommended in the THP) and the methanol:chloroform mixture (9:1; recommended by the UNODC) in preserving CBD and Δ9-THC. Furthermore, the CBD and Δ9-THC content was unaffected when samples were stored at 4 °C or − 20 °C for 28 days.
In the Globally Harmonized System, chloroform is classified as acutely toxic and a health hazard due to its carcinogenicity, reproductive toxicity, and specific target organ toxicity (repeated exposure) (National Center for Biotechnology Information, 2023b). Methanol is classified as flammable, acutely toxic, and a health hazard due to specific target organ toxicity (single exposure) (National Center for Biotechnology Information, 2023a). Acetonitrile is classified as flammable and an irritant (National Center for Biotechnology Information, 2023c). Conversely, water is the safest solvent. Reducing chloroform use by substituting it with less toxic solvents such as acetonitrile, methanol, and water can protect the health of analysts.
The optimal solvent system developed in this study, comprising acetonitrile, methanol, and water at a specific volume ratio, provided similar cannabinoid stability without using chloroform, a recognized carcinogenic solvent. This solvent combination ensured the preservation and robustness of cannabinoid compounds during analysis while addressing the critical health and safety considerations associated with chloroform exposure. By reducing chloroform use in analytical procedures, researchers can uphold stringent scientific standards and prioritize the well-being of laboratory personnel and environmental sustainability.
4 Conclusions
This study highlighted the importance of solvent selection when preparing samples for chemical analysis, particularly when extracting key compounds from cannabis inflorescence samples. The most commonly recommended solvents, the methanol:chloroform mixture (9:1) recommended by the UNODC and acetonitrile recommended in the THP, have been integral in this field. This study had two primary objectives: to identify a solvent system that maximized the extraction efficiency of two major cannabinoids (CBD and Δ9-THC), surpassing the performance of traditional solvents, and to evaluate their stability in the optimal solvent system. A systematic exploration of various volume ratios of acetonitrile, methanol, and water in the extraction process, conducted using a D-optimal design, provided significant findings. Our results unequivocally demonstrated that adding water to the acetonitrile and methanol solution substantially enhanced cannabinoid extraction. In addition, the solvent system developed in this study exhibited superior extraction efficiency compared to acetonitrile (recommended in the THP) and the methanol:chloroform mixture (9:1; recommended by the UNODC). The optimal solvent system comprised acetonitrile, methanol, and water at a volume ratio of 0.511:0.289:0.200. It could extract 0.141 % ± 0.023 % of CBD and 3.255 % ± 0.511 % of Δ9-THC from the Hang Kra Rog Phu Phan cultivar, and 2.120 % ± 0.143 % of CBD and 3.813 % ± 0.219 % of Δ9-THC from the Charlotte’s Angel cultivar. Notably, their stability in the optimal solvent system exceeded that in acetonitrile and the methanol:chloroform mixture (9:1), even after storage at 4 °C or − 20 °C for 28 days.
In summary, the optimal solvent system developed in this comprehensive study can be a valuable guide for improving cannabinoid extraction when preparing cannabis inflorescence samples for cannabinoid quantification. These findings contribute significantly to advancing analytical techniques in the cannabis research field and offer practical insights for its researchers and professionals.
Declaration of generative AI in scientific writing
During the preparation of this work the authors used ChatGPT 3.5 and Gemini in order to proofread and correct grammatical errors during the manuscript writing process. After using these tools, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
CRediT authorship contribution statement
Chaowalit Monton: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Visualization, Writing – original draft, Writing – review & editing. Warittiya Chanduaykit: Formal analysis, Investigation, Methodology, Writing – original draft. Tisana Mongkhonvanich: Formal analysis, Investigation, Methodology, Writing – original draft. Suphannisa Srikaenchan: Formal analysis, Investigation, Methodology, Writing – original draft. Jirapornchai Suksaeree: Formal analysis, Investigation, Methodology, Writing – original draft. Laksana Charoenchai: Methodology, Resources, Writing – original draft. Thanapat Songsak: Methodology, Resources, Writing – original draft.
Acknowledgement
This work was supported by the College of Pharmacy, Rangsit University, research funding for senior project of undergraduate pharmacy students (the academic year 2023).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Techniques for extraction of bioactive compounds from plant materials: A review. J. Food Eng.. 2013;117:426-436.
- [CrossRef] [Google Scholar]
- Development of a new extraction technique and HPLC method for the analysis of non-psychoactive cannabinoids in fibre-type Cannabis sativa L. (hemp) J. Pharm. Biomed. Anal.. 2017;143:228-236.
- [CrossRef] [Google Scholar]
- Bureau of Drug and Narcotic, 2021. Thai Herbal Pharmacopoeia 2021. https://bdn.go.th/thp/ebook/qQWcZ3tlpR9gC3q0GT5gMJq0qT5co3uw. Accessed 19 August 2023.
- Innovative development and validation of an HPLC/DAD method for the qualitative and quantitative determination of major cannabinoids in cannabis plant material. J. Chromatogr. B. 2009;877:4115-4124.
- [CrossRef] [Google Scholar]
- Phytochemical variation: How to resolve the quality controversies of herbal medicinal products? J. Herb. Med.. 2015;5:118-127.
- [CrossRef] [Google Scholar]
- The chemistry of cannabis and cannabinoids. Aust. J. Chem.. 2021;74:369-387.
- [CrossRef] [Google Scholar]
- Constituents of Cannabis sativa. In: Pertwee R., ed. Handbook of Cannabis. Oxford University Press; 2014. p. :1-22.
- [Google Scholar]
- Medicinal use of cannabis based products and cannabinoids. BMJ. 2019;365:l1141
- [CrossRef] [Google Scholar]
- Pharmaceutical preformulation and formulation: A practical guide from candidate drug selection to commercial dosage form (2 ed.). New York: Informa Healthcare; 2016.
- Determination of 11 cannabinoids in biomass and extracts of different varieties of cannabis using high-performance liquid chromatography. J. AOAC Int.. 2015;98:1523-1528.
- [CrossRef] [Google Scholar]
- Quantitative analysis of cannabinoids from Cannabis sativa using 1H-NMR. Chem. Pharm. Bull.. 2004;52:718-721.
- [CrossRef] [Google Scholar]
- Secondary metabolites profiled in cannabis inflorescences, leaves, stem barks, and roots for medicinal purposes. Sci. Rep.. 2020;10:3309.
- [CrossRef] [Google Scholar]
- JMP Statistical Discovery LLC, 2023. Design of experiments. https://www.jmp.com/en_ph/statistics-knowledge-portal/what-is-design-of-experiments.html. Accessed 24 September 2023.
- Cannabinoids-characteristics and potential for use in food production. Molecules. 2021;26
- [CrossRef] [Google Scholar]
- Processing and extraction methods of medicinal cannabis: a narrative review. J. Cannabis Res.. 2021;3:32.
- [CrossRef] [Google Scholar]
- High performance liquid chromatographic profiling of cannabis products. J. Liq. Chromatogr.. 1995;18:689-700.
- [CrossRef] [Google Scholar]
- Green method for recovery of cannabinoids from Cannabis sativa flowers: pH-controlled aqueous leaching. Sep. Purif. Technol.. 2023;326:124754
- [CrossRef] [Google Scholar]
- Validation of analytical method of cannabinoids: Novel approach using turbo-extraction. Talanta. 2023;254:124108
- [CrossRef] [Google Scholar]
- Maryott, A.A., Smith, E.R., 1951. Table of dielectric constants of pure liquids. https://nvlpubs.nist.gov/nistpubs/Legacy/circ/nbscircular514.pdf. Accessed 29 September 2023.
- Quantitative determination and validation of 17 cannabinoids in cannabis and hemp using liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem.. 2020;412:7381-7393.
- [CrossRef] [Google Scholar]
- Potency trends of Δ9-THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. J. Forensic Sci.. 2010;55:1209-1217.
- [CrossRef] [Google Scholar]
- Optimization of supercritical carbon dioxide fluid extraction of seized cannabis and self-emulsifying drug delivery system for enhancing the dissolution of cannabis extract. J. Supercrit. Fluids.. 2022;179:105423
- [CrossRef] [Google Scholar]
- Monton, C., Chankana, N., Duangjit, S., Suksaeree, J., Naksuriya, O., Charoenchai, L., Songsak, T., 2024. Fabrication and optimization of directly compressible self-emulsifying tablets containing cannabis extract obtained from supercritical carbon dioxide extraction. Appl. Sci. Eng. Progress 17, 6973. 10.14416/j.asep.2023.08.004.
- Cannabidiol, Δ9-tetrahydrocannabinol, and cannabinol contents of Cannabis sativa L. inflorescences claimed to be Hang Kra Rog Phu Phan cultivar cultivated outdoors in various locations of Thailand. Phytochem. Lett.. 2023;57:126-132.
- [CrossRef] [Google Scholar]
- Biological activity of Cannabis sativa L. extracts critically depends on solvent polarity and decarboxylation. Separations. 2020;7:56.
- [CrossRef] [Google Scholar]
- National Center for Biotechnology Information, 2023a. PubChem Compound LCSS for CID 887, Methanol. https://pubchem.ncbi.nlm.nih.gov/compound/Methanol#datasheet=LCSS. Accessed 24 September 2023.
- National Center for Biotechnology Information, 2023b. PubChem Compound LCSS for CID 6212, Chloroform. https://pubchem.ncbi.nlm.nih.gov/compound/Chloroform#datasheet=LCSS. Accessed 24 September 2023.
- National Center for Biotechnology Information, 2023c. PubChem Compound LCSS for CID 6342, Acetonitrile. https://pubchem.ncbi.nlm.nih.gov/compound/Acetonitrile#datasheet=LCSS. Accessed 24 September 2023.
- Cannabis: Chemistry, extraction and therapeutic applications. Chemosphere. 2022;289:133012
- [CrossRef] [Google Scholar]
- Supercritical carbon dioxide extraction of cannabinoids from Cannabis sativa L. J. Supercrit. Fluids.. 2017;129:16-27.
- [CrossRef] [Google Scholar]
- Siracusa, L., Ruberto, G., Cristino, L., 2023. Recent research on Cannabis sativa L.: Phytochemistry, new matrices, cultivation techniques, and recent updates on its brain-related effects (2018-2023). Molecules 28. 10.3390/molecules28083387.
- High-pressure liquid chromatography of cannabis quantitative analysis of acidic and neutral cannabinoids. J. Chromatogr.. 1976;129:347-354.
- [CrossRef] [Google Scholar]
- The decomposition of acidic and neutral cannabinoids in organic solvents. J. Pharm. Pharmacol.. 1977;29:286-290.
- [CrossRef] [Google Scholar]
- Quality by Design (QbD) and the development and manufacture of drug substance. In: Schlindwein W.S., Gibson M., eds. Pharmaceutical Quality by Design: A Practical Approach. New Jersey: John Wiley & Sons Ltd.; 2018. p. :61-95.
- [Google Scholar]
- Determination of cannabinoids in cannabis products using liquid chromatography-ion trap mass spectrometry. J. Chromatogr. A. 2004;1058:143-151.
- [CrossRef] [Google Scholar]
- Analysis of cannabis seizures in NSW, Australia: cannabis potency and cannabinoid profile. PLoS One. 2013;8:e70052.
- [Google Scholar]
- Characteristics of cannabinoids composition of Cannabis plants grown in Northern Thailand and its forensic application. Forensic Sci. Int.. 2012;215:164-170.
- [CrossRef] [Google Scholar]
- Extraction solvent selection for Cannabis sativa L. by efficient exploration of cannabinoid selectivity and phytochemical diversity. Phytochem. Anal. 2023
- [CrossRef] [Google Scholar]
- United Nations Office on Drugs and Crime, 2022. Recommended methods for the identification and analysis of cannabis and cannabis products. https://www.unodc.org/documents/scientific/Recommended_methods_for_the_Identification_and_Analysis_of_Cannabis_and_Cannabis_products.pdf. Accessed 19 August 2023.
- WHO Expert Committee on Drug Dependence, 2018. Delta-9-tetrahydrocannabinol. https://cdn.who.int/media/docs/default-source/controlled-substances/thcv1.pdf. Accessed 24 September 2023.
- Stability of cannabidiol, Δ9-tetrahydrocannabinol, and cannabinol under stress conditions. Adv. Tradit. Med.. 2021;21:475-484.
- [CrossRef] [Google Scholar]
- Determination of cannabinoids in Cannabis sativa L. samples for recreational, medical, and forensic purposes by reversed-phase liquid chromatography-ultraviolet detection. J. Anal Sci. Technol.. 2018;9:27.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2024.105847.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







