Translate this page into:
Oxidative desulfurization catalyzed by magnetically recoverable CoFe2O4 nano-particles
⁎Corresponding author. xhinbj@126.com (Hang Xu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
In this study, the magnetic CoFe2O4 was fabricated and utilized as catalysts to activate peroxymonosulfate (PMS) for removal of dibenzothiophene (DBT) in model oil with the extraction-coupled catalytic combined with oxidation desulfurization system (ECODS). The prepared magnetic CoFe2O4 was characterized by scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), X-ray Photoelectron Spectroscopy (XPS), Vibrating Sample Magnetometer VSM etc. The results showed that the prepared magnetic CoFe2O4 with a large specific surface area and exhibited excellent magnetism, phase composition, crystallinity and uniform distribution of the elements. The sulfur removal of DBT in n-octane was 95 % in 60 min at 40 °C under the conditions: 6 mL of model oil (600 ppm), O/S = 3:1 and 125 mg of CoFe2O4 powder. The possible mechanism of desulfurization was proposed by GC–MS. In conclusion, CoFe2O4 magnetic nanoparticles function well in both desulfurization and catalysis for PMS.
Keywords
CoFe2O4 powders
Magnetic materials
Catalyst
Oxidation
Desulfurization
1 Introduction
In recent times, the escalating occurrence of air pollution has become a global concern. The emission of sulfur oxides (SOx gases) stands as the primary culprit behind respiratory problems, air pollution, acid rain, soil fertility degradation, and various other forms of environmental contamination (Daglar et al., 2022). Consequently, the removal of sulfur compounds such as sulfides, disulfides, and thiophene present in petroleum products has become an imperative, garnering substantial attention on an international scale (Haruna et al., 2022). In pursuit of controlling pollution issues and mitigating the impact of toxic gases, many nations have taken decisive steps by imposing restrictions and enacting stringent regulations regarding the allowable sulfur content in fossil fuels (Chen et al., 2022). In light of environmental and health considerations, the production of low-sulfur fuel plays a pivotal role in preserving a clean and sustainable environment.
To advance cutting-edge breakthroughs and enhance the efficiency of fuel production, all while maintaining cost-effectiveness and expediency, scientists and engineers have been actively involved in the development of diverse desulfurization technologies. These technologies aim to reduce the sulfur content in crude oil to comply with stringent regulations. As a result, a multitude of desulfurization methods have been proposed, including hydrodesulfurization (HDS) (Yue et al., 2022), biodesulfurization (BDS), adsorptive desulfurization (ADS) (Omar et al., 2022, Saeed et al., 2022a), extractive desulfurization (EDS) (Abro et al., 2022), oxidative desulfurization (ODS) (Cao et al., 2020, Jabar et al., 2022, Saeed et al., 2022b, Armandsefat et al., 2024), as well as processes based on alkylation, chlorinolysis, and supercritical water (Nasyrova et al., 2022).
Oxidative desulfurization (ODS), considered one of the most advanced and promising desulfurization methods, exhibits the capability to reduce refractory organosulfur compounds to less than 10 ppm under mild operating conditions and at a low cost. Utilizing molecular oxygen as the oxidant, Wei et al. (Jiang et al., 2022) have achieved high removal efficiency for stubborn sulfur compounds such as DBT, 4-methyldibenzothiophene (4-MDBT), and 4,6-DMDBT. They harnessed the combined strengths of robust single-atom catalysts derived from metal-support interactions. Ahmadian et al. (Ahmadian and Anbia 2022), on the other hand, explored the catalytic activity of mesoporous silica supports like SBA-15 and MOR-SBA-15 for the oxidative desulfurization of model fuel containing BT, dibenzothiophene (DBT), and 4,6-DMDBT. Mohammad Ali Rezvani reported on the synthesis and characterization of a new heterogeneous nanocatalyst comprised of the sandwich-type polyoxotungstate [(FeW9O34)2Fe4(H2O)2] -10 (Fe6W18O70) clusters and copper ferrite (CuFe2O4) nanoparticles (Rezvani and Imani 2021). Mohammad Ali Rezvani et al designed a series of organic–inorganic catalysts for the oxidative desulfurization and obtained ideal effects (Rezvani and Zonoz 2015, Rezvani et al., 2016, Rezvani et al., 2018, Rezvani and Fereyduni 2019, Rezvani et al., 2020a, Rezvani et al., 2020b, Rezvani et al., 2022a). Saeed et al reported that a Ni-WO3@g-C3N4 composite as an efficient and recoverable nanocatalyst for oxidative desulfurization of both and real oil. They obtained findings revealed that 97 for DBT, 89.5 for diesel and 91.2 % for kerosene (Jabar et al., 2022, Saeed et al., 2022).Typically, the ODS process comprises two stages: oxidation and sulfone generation, followed by separation. Notably, an extraction-coupled catalytic oxidative desulfurization (ECODS) approach has been adopted to enhance the conventional ODS process, achieving deep desulfurization at a relatively lower cost compared to other existing desulfurization technologies.
Traditionally, efficient catalytic oxidative reactions relied on the use of hydrogen peroxide (H2O2) (Harutyunyan et al., 2011, Rezvani et al., 2019, Rezvani et al., 2021, Rezvani and Imani 2021, Rezvani et al., 2022a, Rezvani et al., 2022b, Khalafi et al., 2023, Rezvani et al., 2023a) or aerobic oxidants like O2 or air (Jiang et al., 2019, Guo et al., 2022). However, these oxidants are prone to decomposition, resulting in reduced efficiency, and often come with drawbacks related to transportation, storage, and usage. In comparison, solid oxidants like peroxymonosulfate (PMS) (Li et al., 2021, Liu et al., 2021) have gained prominence for their stability and practical advantages. Recently, considerable attention has been drawn to sulfate radicals (SO − 4·) due to their numerous merits, including a high redox potential (2.5–3.1 V) and a prolonged half-life (30–40 ms). Significantly, SO − 4· can be readily generated from PMS through the activation of transition metal ions, such as Fe2+, Mn2+, Ni2+, and Co2+. Among these, Co2+ has emerged as the most efficient homogeneous catalyst for PMS activation (Tan et al., 2017). Consequently, sulfate radicals hold great promise for achieving outstanding performance in oxidative desulfurization (ODS).
For an oxidant to effectively function in an oxidative desulfurization (ODS) system, a catalyst is crucial to expedite the activation process of the oxidant. Nano-sized cobalt ferrite (CoFe2O4), a typical bimetallic spinel ferrite, is widely recognized as a highly effective catalyst in oxidation reactions. This recognition is due to its exceptional catalytic activity, stability, magnetic separation capabilities, low cost, abundant resources, electrical properties, magnetic anisotropy, and eco-friendliness (Zhao et al., 2017). Furthermore, the synergistic interaction between its iron (Fe) and cobalt (Co) components leads to a substantial enhancement in catalytic activity compared to the individual oxides. CoFe2O4 exhibits rich redox properties (both Co2+ and Fe3+ are active species), high electronic conductivity, superior catalytic activity, cost-effectiveness, and, most importantly, environmental friendliness (Zhu et al., 2022). These advantages position CoFe2O4 as a highly promising candidate for catalytic applications. Notably, recent research has extensively reported the activation of peroxymonosulfate (PMS) by CoFe2O4. Du et al. (Du et al., 2016) investigated CoFe2O4 and MnFe2O4 as heterogeneous catalysts for peroxymonosulfate (PMS) in the degradation of paracetamol (APAP) in water. The results demonstrated that both CoFe2O4 and MnFe2O4 magnetic nanoparticles activated PMS, representing promising technologies for addressing water pollution. Additionally, Peng et al. (Peng et al., 2022) successfully prepared a series of Fenton-like catalysts by loading CoFe2O4 nanoparticles onto the surface of MoS2 to activate PMS. Their findings revealed that these CoFe2O4@MoS2 catalysts, with varying mass ratios of CoFe2O4 and MoS2, exhibited superior catalytic performance in tetracycline (TC) degradation. To date, limited efforts have been dedicated to exploring the potential of CoFe2O4 nanoparticles as a heterogeneous catalyst for PMS activation in the removal of sulfur compounds from oil.
In this study, we synthesized magnetic CoFe2O4 nanoparticles using the hydrothermal method and employed them as catalysts to activate peroxymonosulfate (PMS) for the removal of sulfur-containing compounds, including dibenzothiophene (DBT), benzothiophene (BT), and 4,6-Dimethyldibenzothiophene (4,6-DMDBT) from n-octane. We conducted a comprehensive microstructural analysis of the CoFe2O4 catalyst using X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), transmission electron microscopy (TEM), scanning electron microscopy (SEM), Brunauer–Emmett–Teller (BET) surface area analysis, and vibrating sample magnetometry (VSM). Subsequently, we analyzed the products obtained after the desulfurization process using gas chromatography-mass spectrometry (GC–MS) and delved into the desulfurization mechanism.
2 Experimental
2.1 Materials
CoCl2·6H2O (>99.99 % purity, Aladdin), Fe(NO3)3·9H2O (>99.99 % purity, Aladdin), Ascorbic acid (AA, >99.99 % purity, Aladdin), Urea (CH4N2O, >99.99 % purity, Aladdin), Ethanol absolute (Kaitong), Dibenzothiophene (DBT, Aladdin), 1-Benzothiophene (BT, Aladdin), 4,6-Dimethyldibenzothiophene (4,6-DMDBT, Aladdin), Peroxymonosulfate (PMS, 2KHSO5·KHSO4·K2SO4, KHSO5 47 %, Meryer), Acetonitrile (C2H3N, DaMao), n-octane (DaMao) and deionized water (DI water, no lower than 18.3 MΩ·cm) were purchased from Sigma-Aldrich.
2.2 Preparation of CoFe2O4 powders
Cobalt salt (CoC12·6H2O) and ferric salt (Fe(NO3)3·9H2O), in a specific ratio, were dissolved in deionized water along with 0.5 g of ascorbic acid and urea (CH4N2O). This solution was then stirred with a magnetic force in a mixer for 20 min. Subsequently, the clear solution was transferred to a Teflon-lined autoclave. The hydrothermal process was carried out at various temperatures and durations. The resulting products were collected through magnetic separation, thoroughly washed with deionized water and ethanol, and finally, dried in a vacuum oven at 70 °C for 6 h. Ultimately, the dried precipitates underwent calcination in ambient air at specific temperatures and for specific durations to yield magnetic CoFe2O4 nanoparticles. The preparation process for CoFe2O4 powders is illustrated in Fig. 1.
Schematic illustration of CoFe2O4 through the Hydrothermal-Calcination process.
2.3 Desulfurization process
Dissolved thiophene sulfur in n-octane to prepare 300–1200 ppm DBT simulated oil. The model fuel oil is sealed and stored to prevent volatilization. Added a certain amount of CoFe2O4 and 1 mL acetonitrile to 6 mL prepared model oil, for 30 min to achieve adsorption and extraction equilibrium. After a specific amount of the catalyst and PMS (wt. 20 %) solution were added, the mixture was vigorously stirred, and the time was recorded as “0″. In the reaction process, the liquid in the upper oil phase was regularly extracted and diluted. The thiophene sulfur in the model fuel oil was detected by UV–visible spectrophotometer (DBT and 4,6-DMDBT at 312 nm; BT at 297 nm, the UV–visible spectra in Fig S1. The desulfurization rate in model oil is calculated by the following formula (1).
All other conditions are tested with the default values below when exploring the effect of one of these factors on desulfurization seen in Fig. S2. The hydrothermal reaction temperature is 160 ℃, the hydrothermal reaction time is 7 h, the calcination reaction temperature is 550 ℃, the calcination reaction time is 5 h, Co:Fe = 2:4, the catalyst dose is 125 mg, the temperature of desulfurization is 40 ℃, initial sulfur content is 600 ppm, O/S = 3:1, sulfur-containing compounds is DBT.
2.4 General information & characterization
The crystallographic information of the as-prepared samples was checked by the powered X-ray Diffractometer Technique (XRD) patterns obtained by Bruker D2 Phaser powder diffractometer having Cu Kα radiation at a step size of 2θ range of 10–80°. The particle size, crystallinity, and composition of the as-prepared samples were examined by Scanning Electron Microscope (SEM) using Gemini SEM 300, Transmission Electron Microscope (TEM) using FEI Tecnai G2 F20, Energy Dispersive X-ray spectroscopy (EDS) using SDD super-X instrument. X-ray Photoelectron Spectroscopy (XPS) measurements were performed by a photoelectron spectrometer with Al Kα source, a concentric hemispherical analyzer operating in a fixed-analyzer transmission mode, and a multichannel detector. Fourier transform infrared (FT-IR) spectra of the nanofibers were carried out by Shimadzu Trace-100 spectrometer in the region of 4000–400 cm−1 with the 4 cm−1 resolution and 32 times scanning. Magnetic hysteresis loops were obtained through the VSM system at room temperature in an applied magnetic field sweeping from −30,000 to +30,000 Oe. A Brunauer − Emmett − Teller (BET) method was employed to evaluate the surface area and pore size distribution by nitrogen gas absorption–desorption using a Micromeritics APSP 2460 system at 300 ℃.
3 Results and discussion
3.1 Characterizations of CoFe2O4
Fourier transform infrared spectroscopy (FT-IR) was conducted on CoFe2O4 within the range of 4000 to 400 cm−1, as shown in Fig. 2(a). Specifically, the peak observed at approximately 400 cm−1 corresponds to the stretching vibrations of Fe3+–O2− bonds in CoFe2O4, while the peak centered at 600 cm−1 indicates the stretching vibration of Co2+–O2− bonds within CoFe2O4 (Kumbhar et al., 2012). The peak at 1628 cm−1 corresponds to the bending vibration pattern of H2O molecules, and the broad peak at 3379 cm−1 represents the H-OH vibrations of adsorbed water molecules (Hosseini et al., 2018). The test results indicate the absence of significant impurity peaks, except for the water peak. This suggests that the Co-Fe precursors have undergone complete oxidation, resulting in the formation of pure CoFe2O4 nanoparticles.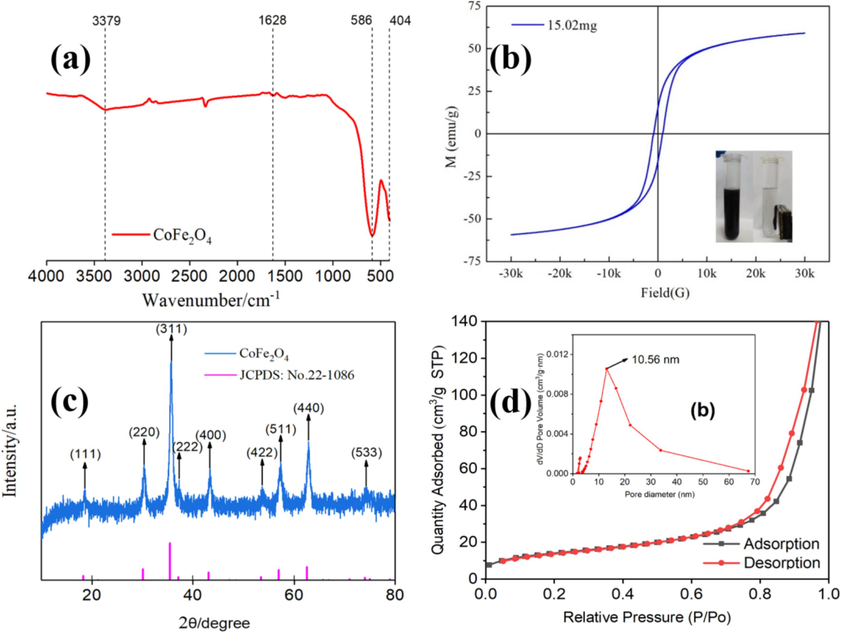
FT-IR (a); VSM (b); XRD (c); N2 adsorption/desorption isotherm and pore size distribution (inset) (d) of as-prepared CoFe2O4.
Magnetic measurements were performed to understand the magnetic behavior of CoFe2O4. We observed typical magnetic hysteresis loops of CoFe2O4 powder under a magnetic field of ±3000 Oe (Fig. 2(b)). The magnetic saturation (MS) value of the CoFe2O4 powder was determined to be 59 emu·g−1. VSM measurements also confirmed the typical magnetic behavior of CoFe2O4. The presence of magnetic properties indicates that CoFe2O4 can be efficiently and conveniently separated from the model oil using an external magnet.
As-synthesized CoFe2O4 was characterized using several spectroscopic and microscopic studies. The powder XRD of the as-synthesized CoFe2O4 powder showed the characteristic peaks of 2θ which are located at 18.29°, 30.08°, 35.43°, 37.06°, 43.06°, 53.42°, 56.94°, 62.53° and 73.97°. corresponded to (111), (220), (311), (222), (400), (422), (511), (440) and (533) plane of cubic CoFe2O4, respectively (Fig. 2(c))(Li et al., 2017). The XRD pattern is consistent with the JCPDS 22-1086 standard card. No distinct impurity peak was detected, indicating that the pure CoFe2O4 powders were obtained by hydrothermal method.
Subsequently, the porous nature of CoFe2O4 was determined through N2 adsorption studies. The N2 adsorption–desorption curves, as shown in Fig. 2(d), closely approach the X-axis in the low-pressure range (0–0.1) and the medium-pressure range (0.3–0.8), implying modest N2 adsorption forces on the material's surface. However, in the high-pressure range (0.8–1.0), the curves exhibit a significant increase in N2 self-adsorption capacity, trending more towards the y-axis. Additionally, the absorption–desorption curves display a typical hysteresis loop characteristic of type IV isotherms, according to the IUPAC classification. This hysteresis loop categorization aligns with H3 type features, underscoring the mesoporous structure of CoFe2O4 generated between the interstitial spaces of flake-like particles.
We employed various techniques to characterize the morphology of the CoFe2O4 film obtained. The scanning electron microscopy (SEM) characterization, as depicted in Fig. 3(a-b), reveals some degree of aggregation within the CoFe2O4 nanoparticles. This aggregation can be attributed to their magnetic properties and weak surface interactions between the primary particles, which are held together by van der Waals forces(Sagadevan et al., 2016). However, the surface morphology of the CoFe2O4 nanoparticles shows the presence of numerous pores. These pores can increase the specific surface area of the nanomaterials and enhance the number of catalytic active sites, ultimately resulting in increased catalytic activity. Transmission electron microscopy (TEM) characterization further confirms that the majority of the CoFe2O4 nanoparticles exhibit an approximately cubic shape, although there are also a few particles that appear elongated (Fig. 3(c-d)). The average particle size of the measured particles falls within the range of approximately 10 to 15 nm. Furthermore, high-resolution TEM (HRTEM) (Fig. 3(e)) reveals well-defined lattice spacing within the CoFe2O4, indicating that the prepared CoFe2O4 nanoparticles possess good crystallinity, with d-spacing values of 0.219 nm and 0.300 nm, corresponding to the (4 0 0) and (2 2 0) planes of CoFe2O4 (JCPDS 22-1086), respectively. The selected area electron diffraction (SAED) pattern in (Fig. 3(f)) displays distinct concentric circles, indicating that the CoFe2O4 sample is polycrystalline in nature. Moreover, the diffuse rings in the SAED pattern can be attributed to the lattice planes of (511), (400), (311), and (220) of CoFe2O4 (JCPDS 22-1086). The CoFe2O4 material, featuring a porous architecture and polycrystalline structure, is expected to exhibit robust catalytic activity. Notably, both SAED and XRD analyses reveal that the (311) lattice planes exhibit the most intense reflections. These results collectively affirmed the successful preparation of CoFe2O4 nanoparticles.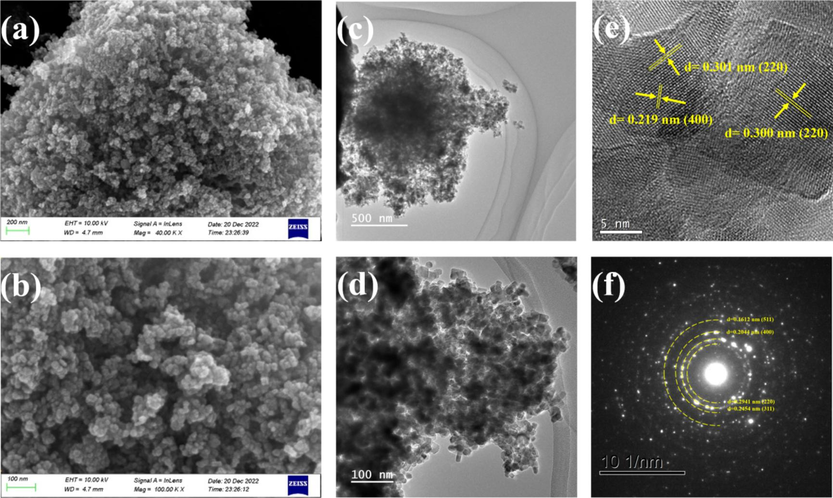
SEM at lower resolution (a); SEM at higher resolution (b); TEM at lower resolution (c); TEM at higher resolution(d); HR-TEM (e); SAED (f) of CoFe2O4.
Fig. 4 displays the EDS data for the CoFe2O4 samples. The EDS spectra reveals the presence of all elements corresponding to CoFe2O4, indicating the chemical purity of the samples. In Fig. 4(a), a table presents the weight and atomic percentages of the sample elements obtained from the EDS spectrum. The atomic ratio of Co:Fe:O, which is approximately 1.00:2.23:3.85, closely resembles the elemental atomic number ratio of Co:Fe:O, which is 1.00:2.00:4.00 in CoFe2O4. To gain insight into the distribution of various ions within the CoFe2O4 samples, we conducted elemental mapping of the materials in the selected sample region. The results, depicted in Fig. 4(b-f), show that all components of the sample are distributed relatively evenly throughout, without any noticeable segregation. This uniform distribution further supports the successful synthesis of CoFe2O4 nanoparticles.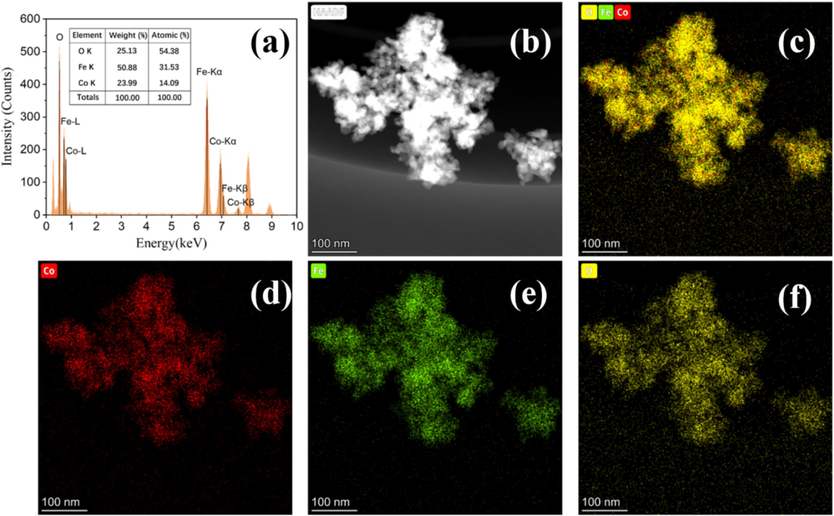
EDS (a); and EDS mapping of CoFe2O4 (Co, Fe, O) (b-f).
The metallic state and surface composition of the synthesized spinel CoFe2O4 was investigated using X-ray photoelectron spectroscopy (XPS). The XPS spectrum of the synthesized nanoparticles in Fig. 5(a) confirms the presence of Co, Fe, and O elements. In Fig. 5(b), the XPS spectra of Co 2p for both CoFe2O4 and Co3O4 show peaks at 779.6 eV and 779.8, while peaks at 781.9 eV and 782.1 eV correspond to Co3+ [23]. These results indicate the coexistence of Co3+ and Co2+ in the samples. It's worth noting that only Co2+ (from CoC12·6H2O) was employed in the sample preparation, and the presence of Co3+ may be due to the precursor oxidizing Co2+ during the calcination process. In Co3O4, the peak area of Co3+ and Co2+ was 48.8 % and 51.2 %, respectively, while in CoFe2O4, it was 46.9 % and 53.1 %, respectively. Besides, the XPS spectra of Fe 2p for both CoFe2O4 and Fe2O3 show peaks at 779.6 eV and 779.8 eV; while peaks at 781.9 eV and 782.1 eV correspond to Fe 3+. These results indicate the coexistence of Fe 3+ and Fe 2+ in the samples. In Fe2O3, the peak area of Fe3+ and Fe2+ was 63.8 % and 36.2 %, respectively, while in CoFe2O4, it was 28.8 % and 71.2 %, respectively. This suggests that in CoFe2O4, Co and Fe atoms interact to varying degrees, leading to electron rearrangements and a certain degree of electronic synergistic effect between the metals. The O 1 s spectra (Fig. 5(d)) can be deconvoluted into three oxygen contributions peaks. Hydroxyl oxygen (531.4 eV) and adsorbed oxygen (533.2 eV) in CoFe2O4, Co3O4, and Fe2O3 have similar binding energies, around 533.2 eV and 531.4 eV, respectively. However, the binding energies of lattice oxygen in CoFe2O4, Co3O4, and Fe2O3 materials show slight variations, with values of 529.8 eV, 530.0 eV, and 529.5 eV, respectively. This suggests that lattice oxygen plays a significant role in the synthesis of CoFe2O4. The coexistence of Co and Fe results in a shift in the electron binding energy on the O 1 s orbital in CoFe2O4, indicating an interaction between Co and Fe. The content of Co2+ and Fe2+ in prepared CoFe2O4 is higher than that in Co3O4 and Fe3O4 monometallic oxides. This higher content enhances the reducibility of the material, making it more catalytically active toward oxidants. Consequently, compared with Co3O4 and Fe2O3, CoFe2O4 is expected to exhibit superior catalytic performance in future catalytic oxidation desulfurization applications.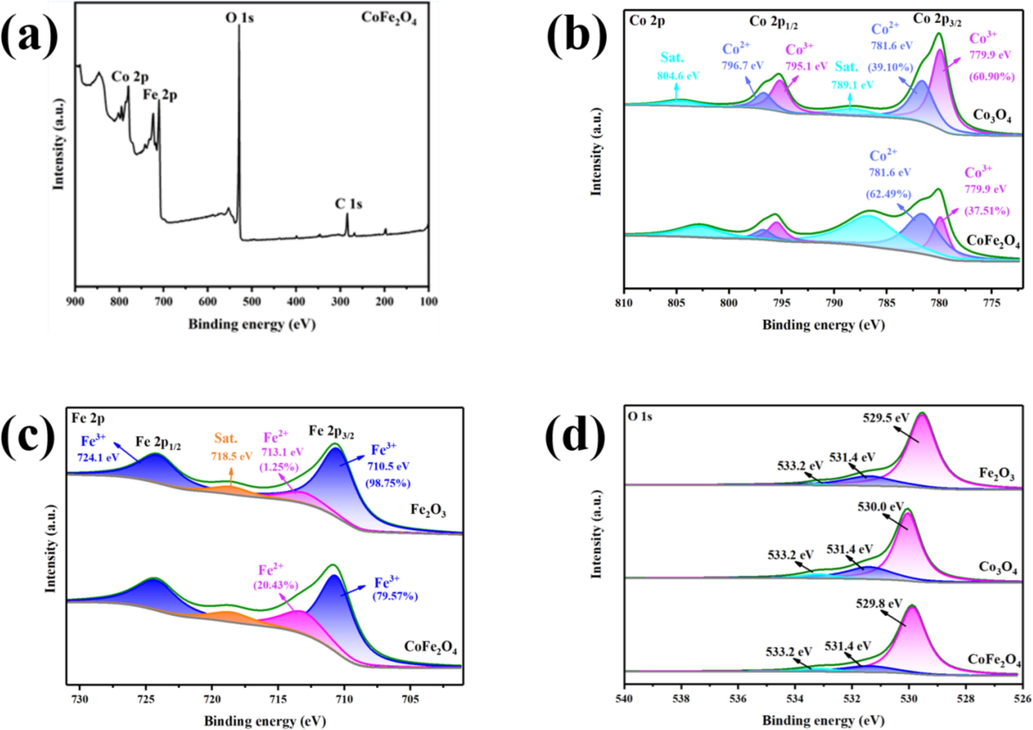
XPS survey spectra of the CoFe2O4 (a); The XPS spectra for Co 2p of both CoFe2O4 and Co3O4 (b); The XPS spectra for Fe 2p of both CoFe2O4 and Fe2O3 (c); The XPS spectra for O 1s of both CoFe2O4, Co3O4, and Fe2O3 (d).
3.2 Optimization of desulfurization conditions
Acetonitrile, characterized by its low surface tension, facilitates efficient mass transfer at the biphasic solvent interface. Additionally, it exhibits superior extraction properties for dibenzothiophene, making it an ideal choice as a solvent, resulting in higher desulfurization rates. The CoFe2O4 catalyst enhances the oxidative desulfurization (ODS) efficiency, and the resultant DBTO2 can be efficiently extracted from model oil using acetonitrile, further enhancing the overall desulfurization efficiency(Muhammad et al., 2018). Therefore, the use of acetonitrile as an extractant is pivotal in investigating the catalytic performance of CoFe2O4 in Extractive Catalytic Oxidative Desulfurization (ECODS) experiments.
The catalyst dosage significantly influences the oxidation of DBT. We evaluated the impact of CoFe2O4 powder dosage on sulfur removal, as shown in Fig. 6(a). Desulfurization efficiencies increased as the CoFe2O4 powder dosage ranged from 50 mg to 125 mg within a 20 min timeframe. This result suggests that higher catalyst loadings generate more active sites for the activation of PMS. A remarkable sulfur removal efficienc was up to 95 % was achieved within just 60 min when a catalyst loading of 125 mg was used. However, desulfurization rates declined when the catalyst amount exceeded 125 mg. The mesoporous structure of CoFe2O4 facilitated substrate diffusion, with its relatively large channels proving more suitable for adsorption and desorption of large sulfur-containing compounds compared to narrower materials. Furthermore, this outcome underscores the exceptional desulfurization performance of the CoFe2O4 and PMS combined system.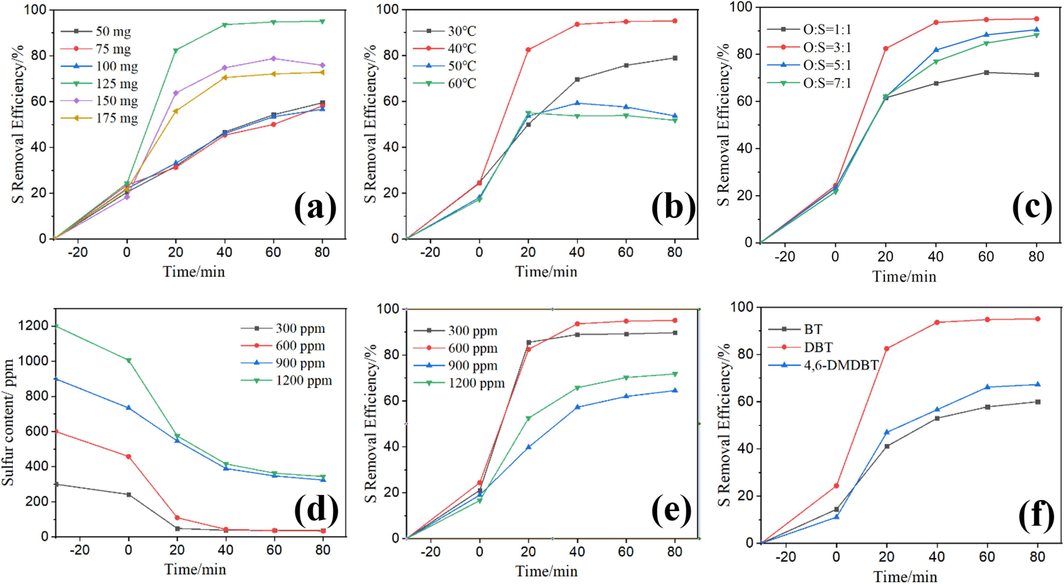
The S removal efficiency of different factors on oxidative desulfurization: the effect of catalyst dosage (a), the effect of reaction temperature (b), the effect of O/S molar ratio (c), the effect of initial sulfur content (d-e), the effect of substrates (f).
Fig. 6(b) illustrates the desulfurization rates at different desulfurization temperatures. During the extraction stage, DBT diffused from the oil phase into the extraction phase. Following the addition of PMS to the system, the desulfurization rate improved as temperatures increased. Sulfur removal efficiencies notably increased between 30 °C and 40 °C within 80 min. However, desulfurization rates decreased at temperatures of 50 °C and 60 °C, as excessively high temperatures are unfavorable for catalytic oxidation(Li et al., 2009). Additionally, PMS is unstable and readily decomposes at 65 °C(Kermani et al., 2018). Taking into consideration both heat source utilization efficiency and catalytic performance, catalytic ODS is most effective at 40 °C.
Achieving an appropriate oxygen-to-sulfur ratio (O/S), using the correct amount of PMS (20 wt%), is crucial for an efficient ODS process. If the O/S ratio is too low, the system's ability to oxidize DBT in the model oil will be compromised(Zhang et al., 2012). Conversely, excessive PMS is detrimental to the catalytic oxidation ability of cobalt ions. Therefore, determining the right PMS dosage is crucial. As depicted in Fig. 6(c), the desulfurization rate increased from 71.49 % to 95.10 % as the O/S ratio increased from 1:1 to 3:1. However, when the O/S ratios further increased from 3:1 to 7:1, the desulfurization rate decreased noticeably. This observation can be attributed to the PMS content exceeding the optimal value, as the strong acidity of hydrogen persulfate is unfavorable for the catalytic oxidation of cobalt ions. Furthermore, hydrogen persulfate also acts as a scavenger of sulfate radicals(Madhavan et al., 2008), which is detrimental to the reaction. Consequently, the optimal O/S ratio for the desulfurization system is found to be 3:1.
In Fig. 6(d), the effect of the initial DBT concentration on the desulfurization process is shown. Increasing the initial sulfur content from 300 to 1200 ppm resulted in decreased removal efficiencies, dropping from 20.05 % to 16.60 % in the initial extraction phase. However, in the second extraction-oxidation catalytic desulfurization (EOCDS) phase, sulfur removal efficiencies significantly increased within the first 20 min for all initial sulfur content concentrations. CoFe2O4 catalyzes PMS to produce a large number of HSO5− ions due to its excellent catalytic activity, leading to a rapid and significant improvement in desulfurization efficiency. At a constant initial CoFe2O4 dosage and PMS dosage, sulfur removal efficiency decreased as the initial sulfur content increased (Fig. 6(e)).
Desulfurization tests were conducted on BT, DBT, and 4,6-DMDBT under identical conditions to investigate the effects of CoFe2O4 nanoparticles on various sulfur-containing compounds. The results revealed that the catalytic oxidation reaction of sulfur-containing compounds had a more pronounced removal effect on DBT compared to 4,6-DMDBT and BT, as depicted in Fig. 6(f). This variation in reactivity can be attributed to the electron density of the sulfur atom in each compound, with BT having the lowest electron density (5.696), which is lower than that of DBT (5.758) and 4,6-DMDBT (5.760) (Shujiro Otsuki et al., 2000). Consequently, BT exhibited lower reactivity. While the electron concentrations of the sulfur atoms in DBT and 4,6-DMDBT are similar, the presence of two methyl groups in 4,6-DMDBT may introduce steric hindrance, reducing the interaction between the CoFe2O4 nanoparticle catalyst and the oxide. As a result, the removal of 4,6-DMDBT is more challenging than that of DBT.
Table 1 presents a comparison with other cobalt-containing or iron-containing catalysts, highlighting the advantages of the CoFe2O4 catalyst. It exhibits the shortest reaction time, requires the lowest temperature, and uses the least amount of oxidant while achieving the same level of efficiency as other catalysts.
Catalyst
Oxidant
Dosage (g/L)
Concentration (ppm)
O/S Ratio
Temperature (℃)
Time (min)
S Removal Efficiency (%)
Ref.
CoFe2O4
PMS
20
600
3
40
60
95
Current study
[Co3(oba)3(O) (Py)0.5]n·4DMF·Py
TBHP
4
500
3
60
480
75.2
(Guo et al., 2022)
Fe–Ni–Mo/Al2O3
H2O2
40
800
15.8
60
150
99
(Muhammad et al., 2018)
CoPcTcCl8
O2
20
800
−
20
180
94.42
(Gao et al., 2019)
Co3O4/Al2O3
Air
−
1000
−
200
180
81
(Nawaf et al., 2015)
H3PW12O40/nano-Fe3O4/SiO2
H2O2
32
500
20
60
180
91.40
(Liu et al., 2017)
PTA@MIL-100(Fe)
H2O2
15
500
4
70
120
92
(Wang et al., 2017b)
PMo11Cd@MnFe2O4,
H2O2/CH3COOH
2
500
−
35
60
96
(Rezvani et al., 2020c)
(Gly)3PMo12O40@MnFe2O4
H2O2/CH3COOH
2
500
35
60
95
(Rezvani et al., 2022a)
((n-C4H9)4N)4H[PW11FeO39]@NiO)
CH3COOH/H2O2
2
500
−
60
120
95
(Rezvani et al., 2023b)
t-B.PWFe/NiO
CH3COOH/H2O2
2
500
−
35
60
97
(Rezvani and Aghmasheh 2021)
[C16H33N(CH3)3]7H3SiV3W9O40-TiO2
H2O2
20
200
−
30–80
60
98..4
(Rezvani and Zonoz 2015)
CoFe2O4
H2O2
5
272.8
20
25
8
99.82
(Rezvani et al., 2023c)
CoFe2O4
H2O2
5
272.8
20
25
8
(Zhang et al., 2021)
3.3 Recycling of CoFe2O4 powder
After the ODS tests, the CoFe2O4 catalyst was separated from the solution using a magnet due to its strong magnetic properties. It was subsequently cleaned, dried, and subjected to a cycle of testing. Remarkably, the CoFe2O4 catalyst maintained a consistent sulfur removal efficiency of approximately 90 % even after three usage cycles, as illustrated in Fig. 7(a). However, following the fourth cycle of desulfurization testing, the desulfurization rate dropped to 82 %, indicating a decrease in the catalyst's activity by approximately 13 %. Fig. 7(b) shows the XRD spectra of CoFe2O4 before and after the recycling tests, revealing no significant differences. This suggests that the decline in the catalyst's reuse performance was not due to damage to its crystalline structure. The diminished performance may be attributed to several factors: (1) Contamination of the catalyst's active sites; (2) A minor leaching of metal ions from the catalyst during the catalytic process, recovery, and washing stage.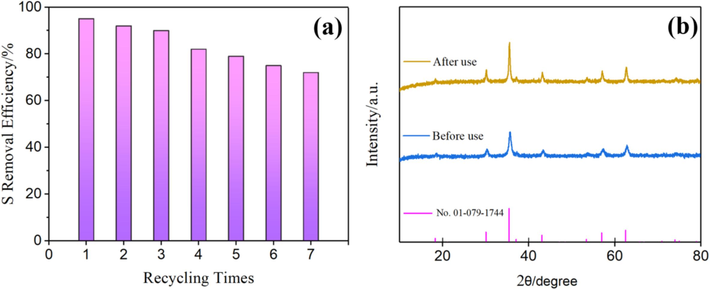
Recycle test of cofe2O4 (a); XRD of CoFe2O4 before and after use (b).
3.4 Desulfurization mechanism
Therefore, possible desulfurization mechanism was constructed as seen in Fig. 8. According to similarity-intermiscibility theory (Wang et al., 2017a), nonpolar DBT is readily soluble in nonpolar octane. Part of the DBT dissolved into the acetonitrile phase when acetonitrile was added to the simulated oil, which is actually an extraction process. Added PMS to the reaction system, DBT was oxidized to polar DBTO/DBTO2 and extracted by acetonitrile and separated from oil phase. DBTO/DBTO2 can be detected by gas chromatography-mass spectrometry (GC–MS). In acetonitrile phase, the possible specific oxidation reaction mechanism as follow.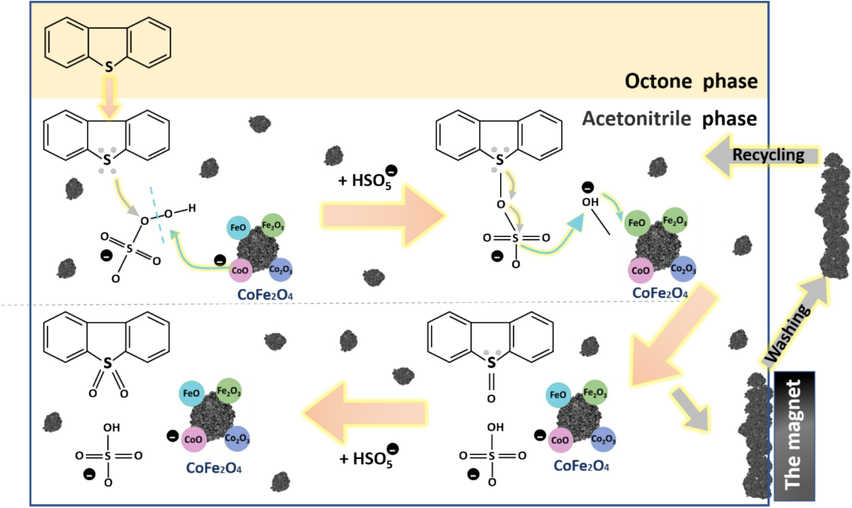
Catalytic oxidative desulfurization of CoFe2O4 possible desulfurization mechanism.
In acetonitrile phase, the CoO can catalyze the cleavage of the peroxide bridge of HSO5− to produce sulfate radicals (SO4−) and hydroxyl radicals. Meanwhile, CoO can give one of its electrons to the hydroxyl radical and form an unstable Co–O covalent bond. The sulfate radical can attack lone pair electrons of sulfur atom in DBT molecule. The electron cloud density of sulfur is relatively high, which is conducive to the formation of covalent double bonds with an oxygen atom. Therefore, DBT can be oxidized to form DBTO. The unstable Co–O covalent bond can obtain an electron from the S–O bond in HSO5− to form a stable CoO. Briefly, the HSO5− can release the active oxygen to form S⚌O bond under CoFe2O4 as a catalyst. DBTO2 can also be produced in a similar mechanism.
After the reaction, the interaction of CoFe2O4 with DBTO2 is reduced because the electron-bonding ability of S atoms in DBTO2 is severely decreased. Therefore, the oxidation product (DBTO2) can be desorbed and released from the surface of the catalyst. In addition, the DBTO2 can thoroughly dissolve in acetonitrile because the polarity of its S⚌O bonds is considerably stronger than the polarity of the S–C bonds in DBT using electronegativity analysis(Yuan et al., 2022).
Gas chromatography-mass spectrometry (GC–MS) was employed to identify and assess the compounds present in the acetonitrile phase at various time intervals(Armandsefat et al., 2024), as depicted in Fig. 9. At the point of extraction equilibrium (0 min), the DBT peak was clearly discernible in the acetonitrile phase, indicating that oxidative desulfurization (ODS) had not yet commenced. After the addition of PMS at 1 min, the intensity of the DBT peak slightly diminished. Importantly, small peaks corresponding to DBTO and DBTO2 emerged, signifying the initiation of ODS and the continuous conversion of DBT into DBTO and DBTO2. As the oxidation reaction progressed, the distinctive DBT peak in the simulated oil gradually decreased, while the characteristic peak intensity of DBTO initially increased and then declined, and the characteristic peak intensity of DBTO2 continued to rise. This observation implies that the remaining DBT in the simulated oil was being continuously oxidized. After 20 min of reaction time, the distinctive DBT peak was nearly completely oxidized. The insets in Fig. 9 display the Mass Spectra of DBT, DBTO, and DBTO2, respectively. These experimental results elucidate that DBT undergoes initial oxidation to the intermediate transition product DBTO, followed by further oxidation of DBTO to yield the final product DBTO2 during the catalytic oxidation process of DBT.“.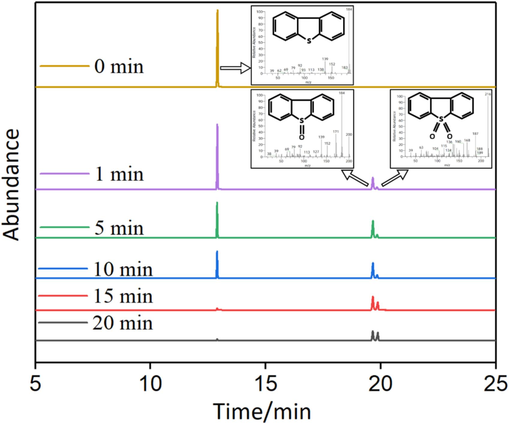
GC–MS diagrams of DBT and its oxidation products the acetonitrile phase at different oxidation reaction times.
4 Conclusion
Under favourable reaction conditions, involving 6 mL of simulated oil with a dibenzothiophene concentration of 600 ppm, 1 mL of acetonitrile, 125 mg of CoFe2O4 nanoparticles (hydrothermal reaction temperature of 160 °C, hydrothermal reaction time of 5 h, calcination temperature of 550 °C, calcination time of 7 h, cobalt-iron molar ratio of 2:4) at a desulfurization system temperature of 40 °C and an O/S ratio of 3, the S removal efficiency exceeded 95 % after 60 min of reaction time. It is noteworthy that compared to other cobalt or iron-containing catalysts reported in the literature, CoFe2O4 exhibited shorter ODS reaction times and required lower temperatures and oxidant amounts to achieve the same level of desulfurization efficiency. The GC–MS analysis of the products in the acetonitrile phase unveiled the mechanism by which DBT is first oxidized to dibenzothiophene sulfoxide (DBTO) as a transitional intermediate, ultimately culminating in the formation of dibenzothiophene sulfone (DBTO2). This insightful mechanism showcases the tremendous potential of CoFe2O4 catalysts for application in oxidative desulfurization processes, owing to their high activity and recyclability.
CRediT authorship contribution statement
Fengmin Wu: Writing – review & editing, Project administration, Investigation, Funding acquisition, Conceptualization. Qinlin Yuan: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. Jinlong Wang: Investigation, Data curation. Xiaowei Wang: Writing – review & editing, Investigation. Jie Luo: Writing – review & editing, Methodology, Funding acquisition. Yafei Guo: Writing – review & editing. Hang Xu: Writing – review & editing, Funding acquisition. Xuefeng Wei: Writing – review & editing.
Acknowledgments
This work is supported by the Nature science Foundation of Henan Province, China (No: 202300410155), National Nature Science Foundation of China, China (No: 21576073), PhD research startup foundation of Henan University of Science and Technology, China (No: 13480069), Programs for Science and Technology Development of Henan Province, China (No. 222102320065), Huaiyin Institute of Technology Mine salt resources deep utilization technology of national and local joint engineering research Center open fund, China (No. SF202410).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Extractive desulfurization of fuel oils using deep eutectic solvents – a comprehensive review. J. Environ. Chem. Eng.. 2022;10:107369
- [CrossRef] [Google Scholar]
- Highly efficient oxidative desulfurization catalyzed by copper-based materials using hydrogen peroxide as oxidant. Fuel. 2022;324:0016-2361.
- [CrossRef] [Google Scholar]
- Efficient and promising oxidative desulfurization of fuel using Fenton like deep eutectic solvent. Sci. Rep.. 2024;14
- [CrossRef] [Google Scholar]
- Highly efficient oxidative desulfurization of dibenzothiophene using Ni modified MoO3 catalyst. Appl. Catal. A. 2020;589:117308
- [CrossRef] [Google Scholar]
- Removal characteristics of particulate matters and hazardous trace elements in a 660 MW ultra-low emission coal-fired power plant. Fuel. 2022;311:122535
- [CrossRef] [Google Scholar]
- Metal-organic framework-based materials for the abatement of air pollution and decontamination of wastewater. Chemosphere. 2022;303:0045-6535.
- [CrossRef] [Google Scholar]
- Magnetic CoFe2O4 nanoparticles supported on titanate nanotubes (CoFe2O4/TNTs) as a novel heterogeneous catalyst for peroxymonosulfate activation and degradation of organic pollutants. J. Hazard. Mater.. 2016;308:58-66.
- [CrossRef] [Google Scholar]
- Oxidative desulfurization of dibenzothiophene by central metal ions of chlorophthalocyanines-tetracarboxyl complexes. Inorg. Chim. Acta. 2019;485:58-63.
- [CrossRef] [Google Scholar]
- Self-templated fabrication of CoMoO4-Co3O4 hollow nanocages for efficient aerobic oxidative desulfurization. Appl. Surf. Sci.. 2022;579:152251
- [CrossRef] [Google Scholar]
- Sulfur removal technologies from fuel oil for safe and sustainable environment. Fuel. 2022;329:125370
- [CrossRef] [Google Scholar]
- H5 PMo10V2O40 as a green, reusable, and highly efficient catalyst for the oxidation of dithiols in intermolecular reactions using permanganate as an oxidizing reagent. Synth. React. Inorg., Met.-Org., Nano-Met. Chem.. 2011;41:94-99.
- [CrossRef] [Google Scholar]
- Photocatalytic desulfurization of dibenzothiophene by NiCo2O4 nanospinel obtained by an oxidative precipitation process modeling and optimization. J. Sulfur Chem.. 2018;39:119-129.
- [CrossRef] [Google Scholar]
- Development of graphitic carbon nitride supported novel nanocomposites for green and efficient oxidative desulfurization of fuel oil. Nanomater. Nanotechnol.. 2022;12
- [CrossRef] [Google Scholar]
- Boric acid-based ternary deep eutectic solvent for extraction and oxidative desulfurization of diesel fuel. Green Chem.. 2019;21:3074-3080.
- [CrossRef] [Google Scholar]
- Enhanced oxygen activation achieved by robust single chromium atom-derived catalysts in aerobic oxidative desulfurization. ACS Catal.. 2022;12:8623-8631.
- [CrossRef] [Google Scholar]
- Deep oxidative desulfurization of dibenzothiophene with Mo-132 nanoballs supported on activated carbon as an efficient catalyst at room temperature. New J. Chem.. 2018;42:12188-12197.
- [CrossRef] [Google Scholar]
- Facile synthesis of new hybrid nanocomposite sandwich-type polyoxometalate@lead (II) oxide@polyvinyl alcohol as an efficient and reusable amphiphilic nanocatalyst for ODS of real fuel. Adv. Powder Technol.. 2023;34
- [CrossRef] [Google Scholar]
- Chemical synthesis of spinel cobalt ferrite (CoFe2O4) nano-flakes for supercapacitor application. Appl. Surf. Sci.. 2012;259:39-43.
- [CrossRef] [Google Scholar]
- Correlation of active sites to generated reactive species and degradation routes of organics in peroxymonosulfate activation by co-loaded carbon. Environ. Sci. Tech.. 2021;55:16163-16174.
- [CrossRef] [Google Scholar]
- Surfactant-assisted solvothermal synthesis of NiCo2O4 as an anode for lithium-ion batteries. RSC Adv.. 2017;7:36909-36916.
- [CrossRef] [Google Scholar]
- Deep oxidative desulfurization of fuels in redox ionic liquids based on iron chloride. Green Chem.. 2009;11:810-815.
- [CrossRef] [Google Scholar]
- Oxidative desulfurization of fuel oil catalyzed by magnetically recoverable nano-Fe3O4/SiO2 supported heteropoly compounds. J. Clean. Prod.. 2017;168:1048-1058.
- [CrossRef] [Google Scholar]
- Direct and efficient reduction of perfluorooctanoic acid using bimetallic catalyst supported on carbon. J. Hazard. Mater.. 2021;412:125224
- [CrossRef] [Google Scholar]
- Kinetic studies on visible light-assisted degradation of acid red 88 in presence of metal-ion coupled oxone reagent. Appl. Catal. B. 2008;83:8-14.
- [CrossRef] [Google Scholar]
- Oxidative desulfurization of dibenzothiophene over Fe promoted Co-Mo/Al2O3 and Ni-Mo/Al2O3 catalysts using hydrogen peroxide and formic acid as oxidants. Chin. J. Chem. Eng.. 2018;26:593-600.
- [CrossRef] [Google Scholar]
- The effect of supercritical water on conversion of resins, asphaltenes and kerogens in rocks of different lithofacies of Domanic deposits of Tatarstan. Fuel. 2022;329:125429
- [CrossRef] [Google Scholar]
- Improvement of fuel quality by oxidative desulfurization: design of synthetic catalyst for the process. Fuel Process. Technol.. 2015;138:337-343.
- [CrossRef] [Google Scholar]
- Successive bacterial desulfurization and regeneration of liquid fuel over Ni-doped carbon beads using a single Enterococcus faecium strain isolated from an industrial wastewater. Fuel. 2022;309:106685
- [CrossRef] [Google Scholar]
- Mechanistic investigation of rapid catalytic degradation of tetracycline using CoFe2O4@MoS2 by activation of peroxymonosulfate. Sep. Purif. Technol.. 2022;287
- [CrossRef] [Google Scholar]
- Synthesis of t-B.PWFe/NiO nanocomposite as an efficient and heterogeneous green nanocatalyst for catalytic oxidative-extractive desulfurization of gasoline. Environ. Prog. Sustain. Energy. 2021;40
- [CrossRef] [Google Scholar]
- Extractive-oxidative desulfurization of real and model gasoline using (gly)3H SiW12O40 ⊂CoFe2O4 as a recoverable and efficient nanocatalyst. Energy Fuel 2023
- [CrossRef] [Google Scholar]
- Highly oxidative desulfurization of thiophenic model fuels and real gasoline by Keggin-type heteropolyanion immobilized on polyaniline and chitosan as an efficient organic-inorganic nanohybrid catalyst. J. Appl. Polym. Sci.. 2023;140
- [CrossRef] [Google Scholar]
- Synthesis of Organic-inorganic hybrid nanocomposite polyoxometalate/metal oxide/CS polymer (PMnW11@TiO2@CS): nanocatalyst for oxidative desulfurization of real fuel. ChemistrySelect. 2019;4:11467-11474.
- [CrossRef] [Google Scholar]
- Synthesis of new nanocomposite based on ceramic and heteropolymolybdate using leaf extract of <i>Aloe vera</i> as a high-performance nanocatalyst to desulfurization of real fuel. Appl. Organomet. Chem.. 2021;35
- [CrossRef] [Google Scholar]
- Highly efficient catalytic oxidative desulfurization of gasoline using PMnW11@PANI@CS as a new inorganic-organic hybrid nanocatalyst. Energy Fuel. 2022;36:7722-7732.
- [CrossRef] [Google Scholar]
- Deep oxidative desulfurization of gas oil by iron(III)-substituted polyoxometalate immobilized on nickel(II) oxide, ((n-C4H9) 4N)4HPW11FeO39@NiO, as an efficient nanocatalyst. Sci. Rep.. 2023;13
- [CrossRef] [Google Scholar]
- Ultra-deep oxidative desulfurization of real fuels by sandwich-type polyoxometalate immobilized on copper ferrite nanoparticles, Fe6W18O70 ⊂ CuFe2O4, as an efficient heterogeneous nanocatalyst. J. Environ. Chem. Eng.. 2021;9
- [CrossRef] [Google Scholar]
- An organic-inorganic hybrid based on an Anderson-type polyoxometalate immobilized on PVA as a reusable and efficient nanocatalyst for oxidative desulphurization of gasoline. RSC Adv.. 2016;6:53069-53079.
- [CrossRef] [Google Scholar]
- Green and efficient organic-inorganic hybrid nanocatalyst for oxidative desulfurization of gasoline. Appl. Organomet. Chem.. 2018;32
- [CrossRef] [Google Scholar]
- Synthesis and characterization of new inorganic-organic hybrid nanocomposite PMo11Cu@MgCu2O4@CS as an efficient heterogeneous nanocatalyst for ODS of real fuel. ChemistrySelect. 2019;4:6370-6376.
- [CrossRef] [Google Scholar]
- Synthesis of new nanocomposite based on nanoceramic andmonosubstituted polyoxometalate, PMo11Cd@MnFe2O4, with superior catalytic activity for oxidative desulfurization of real fuel. Appl. Organomet. Chem.. 2020;34
- [CrossRef] [Google Scholar]
- Synthesis and characterization of new nano organic-inorganic hybrid (TBA) 4PW11Fe@TiO2@PVA as a promising phase-transfer catalyst for oxidative desulfurization of real fuel. Polyhedron. 2020;177
- [CrossRef] [Google Scholar]
- Catalytic oxidative desulphurization of gasoline using amphiphilic polyoxometalate@polymer nanocomposite as an efficient, reusable, and green organic-inorganic hybrid catalyst. Environ. Technol.. 2020;41:1219-1231.
- [CrossRef] [Google Scholar]
- Synthesis of (Gly) 3PMo12<O40@MnFe2O4 organic/inorganic hybrid nanocomposite as an efficient and magnetically recoverable catalyst for oxidative desulfurization of liquid fuels. Int. J. Energy Res.. 2022;46:2617-2632.
- [CrossRef] [Google Scholar]
- An organic-inorganic hybrid compound constructed by polytungsto-vanadosilicate and hexadecyltrimethyl ammonium as an efficient catalyst for demercaptanization of crude oil. J. Ind. Eng. Chem.. 2015;22:83-91.
- [CrossRef] [Google Scholar]
- Facile synthesis of a novel Ni-WO3@g-C3N4 nanocomposite for efficient oxidative desulfurization of both model and real fuel. ACS Omega. 2022;7:15809-15820.
- [CrossRef] [Google Scholar]
- Synthesis, characterization and application of organoclays for adsorptive desulfurization of fuel oil. Sci. Rep.. 2022;12
- [CrossRef] [Google Scholar]
- Sagadevan, S., J. Podder, I. Das, 2016. Synthesis and Characterization of Cobalt Ferrite (CoFe2O4) Nanoparticles Prepared by Hydrothermal Method.
- Oxidative desulfurization of light gas oil and vacuum gas oil by oxidation and solvent extraction. Energy Fuel. 2000;14:1232-1239.
- [CrossRef] [Google Scholar]
- Efficient degradation of paracetamol with nanoscaled magnetic CoFe2O4 and MnFe2O4 as a heterogeneous catalyst of peroxymonosulfate. Sep. Purif. Technol.. 2017;175:47-57.
- [CrossRef] [Google Scholar]
- Boosting oxidative desulfurization of model and real gasoline over phosphotungstic acid encapsulated in metal-organic frameworks: the window size matters. ChemCatChem. 2017;9:971-979.
- [CrossRef] [Google Scholar]
- MoO3 subnanoclusters on ultrasmall mesoporous silica nanoparticles: an efficient catalyst for oxidative desulfurization. RSC Adv.. 2017;7:44827-44833.
- [CrossRef] [Google Scholar]
- Preparation of magnetic urchin-like NiCo2O4 powders by hydrothermal synthesis for catalytic oxidative desulfurization. RSC Adv.. 2022;12:32659-32666.
- [CrossRef] [Google Scholar]
- Cobalt promoted molybdenum carbide supported on gamma-alumina as an efficient catalyst for hydrodesulfurization of dibenzothiophene. J. Clean. Prod.. 2022;371:0959-6526.
- [CrossRef] [Google Scholar]
- Extraction-oxidation desulfurization by pyridinium-based task-specific ionic liquids. Fuel. 2012;102:580-584.
- [CrossRef] [Google Scholar]
- A coupled catalyst composed of CoFe2O4 magnetic nanoparticle and [HMIM]Br-FeCl3 to intensify the oxidative desulfurization of FCC diesel. Green Chem. Eng.. 2021;2:9.
- [Google Scholar]
- Spinels: controlled preparation, oxygen reduction/evolution reaction application, and beyond. Chem. Rev.. 2017;117:10121-10211.
- [CrossRef] [Google Scholar]
- Magnetic Co/Fe nanocomposites derived from ferric sludge as an efficient peroxymonosulfate catalyst for ciprofloxacin degradation. Chem. Eng. J.. 2022;432:134180
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
The UV–visible spectra of BT, DBT and 4,6-DMDBT, the optimization of CoFe2O4 preparation conditions and blank experiments. Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2024.106076.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







