Paecilomyces variotii extract increases lifespan and protects against oxidative stress in Caenorhabditis elegans through SKN-1, but not DAF-16
⁎Corresponding author. qiaokang11-11@163.com (Kang Qiao)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
A natural extract from Paecilomyces variotii (P. variotii extract, PVE), an endophytic fungus, has been used widely to improve agricultural crop performance and control multiple plant pathogens. Most recent studies focused on its application as a plant growth promoter, while relatively few studies have been reported on the antioxidant potential in vivo and the underlying mechanism. The present study was designed to determine the antioxidant activities of PVE and its mechanisms using Caenorhabditis elegans. Results showed that, compared to the solvent control, PVE at 1.0, 10 and 100 ng/mL significantly extended the lifespan of C. elegans by 36.60%, 59.80% and 53.30%, respectively. PVE at 10 ng/mL consistently promoted nematodes growth, but all treatments did not influence nematode fecundity, locomotion behavior, and pharyngeal pumping. Furthermore, PVE at the three tested concentrations significantly reduced accumulation of reactive oxygen species (ROS), lipofuscin, lipid and malondialdehyde (MDA) content, meanwhile significantly promoted activities of superoxide dismutase (SOD), catalase (CAT) and glutathione S-transferase (GST) in the nematodes. Compared with the solvent control, PVE up-regulated gene expression of skn-1, mev-1, sod-3, and daf-2, but significantly down-regulated the expression of nhr-49 and daf-16. Further evidence revealed that PVE at the three concentrations significantly promoted nuclear localization of SKN-1, but not affected that of DAF-16, indicating the complex roles of DAF-16 and SKN-1 in stress resistance and longevity regulation. Overall, our results demonstrated that SKN-1 played a critical role in increasing lifespan of C. elegans and protecting the nematodes from oxidative stress, independent of DAF-16.
Keywords
Paecilomyces variotii extract
Caenorhabditis elegans
Oxidative stress
SKN-1
Insulin/IGF-1 signaling
1 Introduction
Fungal endophytes can improve agricultural crop performance and involve in plant defense systems against pathogens (Busby et al., 2016; Deng and Cao, 2017). There is an ever-increasing interest in finding new and environment-friendly bioactive compounds from endophytes for use in agriculture. Among the endophytic fungi, the ascomycete Paecilomyces variotii has drawn great attention for its application in improving plant health (Moreno-Gavíra et al., 2021). The potential of P. variotii as a biocontrol agent to control several phytopathogens has been extensively studied (Dai et al., 2020; Moreno-Gavíra et al., 2020). However, there are limitations in its commercial use such as lack of reliability and consistency that prevents its application on a large scale (Murphy et al., 2018).
Compared with endophytes, extracts from endophytes have greater potential in agriculture (Wang et al., 2021a,b). P. variotii extract (PVE), which is an extract from P. variotii SJ1, has been characterized as a plant growth promoter (Peng et al., 2020). A previous report indicated that PVE at 1.0–10 ng/mL promoted plant growth by inducing auxin accumulation and increased nitrogen absorption in Arabidopsis (Lu et al., 2019). PVE was also reported to improve the utilization efficiency of nitrogen or phosphorus in rice, corn and potato in the field, which subsequently increased crop yield (Cao et al., 2021; Chen et al., 2020; Wang et al., 2020). A latest study reported that guanine might be an active component of PVE (Wang et al., 2022). PVE is used extensively in the field to control a variety of pathogens and increase crop yield due to its high efficiency and broad spectrum. However, to date, no reports have been published on impacts of PVE on human health and the environment.
Caenorhabditis elegans is a powerful model organism to study ecotoxicology and biological effects of natural and synthetic compounds due to its easy culture conditions, short lifespan, and experimental flexibility (Kaletta and Hengartner, 2006). In addition, C. elegans and humans share homologous genes, which means that investigating the effects of xenobiotics on C. elegans could predict their impacts on humans (Herndon et al., 2002). Oxidative stress is an adverse effect caused by reactive oxygenated species (ROS) and is one of the main factors attributable to aging and various age-related diseases in human and C. elegans (Finkel and Holbrook, 2000). Growing evidence indicates that endophytic fungi improve tolerance of host plants to biotic and abiotic stresses through increased antioxidant activity (Hamilton et al. 2012). Zhang et al. (2015) reported that extracts from a marine-derived P. variotii EN-291 possessed the scavenging activity against 1,1-diphenyl-2-picryl-hydrazyl radicals, which was stronger than that of the positive control butylated hydroxytoluene. Recent studies on the antioxidant activity of PVE were primarily conducted in vitro, the antioxidant potency of PVE in vivo remains largely unknown (Lu et al., 2019; Peng et al., 2020).
In C. elegans, insulin/IGF-1-like signaling (IIS) is central to the growth and metabolisms, and transcription factor SKN-1 also enhances resistance to oxidative stress (Kenyon, 2005). In addition, SKN-1 can promote the activation of multiple genes such as gst-4 and gcs-1 in the nematodes (Hu et al., 2021; Song et al., 2020). DAF-16, a FoxO-family transcription factor, is the core gene involved in regulating stress resistance in this pathway, and regulates the expression of multiple genes such as ctl-2 and hsp-16.2 (Lee et al., 2003). It is well established that many natural compounds enhance stress resistance and extend longevity directly by IIS pathway and SKN-1 (Schaffitzel and Hertweck, 2006). PVE has a great antioxidant property in vitro, thus it is reasonable to hypothesize that PVE could increase oxidative stress resistance in vivo. To test this hypothesis, the antioxidant activities of PVE and its mechanisms using C. elegans were investigated. Lifespan, brood size, growth, locomotive behavior, and pharyngeal pumping of C. elegans were assessed to examine the effects of PVE. Several well-documented oxidative stress related parameters including intracellular ROS, lipid, lipofuscin, malondialdehyde (MDA) content, and related gene expression in the nematodes were also determined. Moreover, transgenic C. elegans strains were used to investigate the stress related genes involved in the antioxidant activities.
2 Materials and methods
2.1 Preparation of PVE
The P. variabilis SJ1 strain was isolated from wild sea buckthorn (Hippophae rhamnoides) and deposited in China General Microbiological Culture Collection Center (CGMCC) as P. variabilis CGMCCNO.10114. PVE was extracted from P. variabilis SJ1 strain according to Wang et al. (2021a,b). Briefly, the SJ1 hyphal culture by three-stage liquid fermentation was filtered with a 300-mesh filter cloth under pressures of 0.2 and 0.4 MPa, respectively, and the filtrate was mixed with the same volume of 95% alcohol. The mixture was sealed in a container for 14 days to allow extraction of PVE. Then the mixture was injected into a 500- liter tank at 25 °C with a stirring speed of 1000 g/min and a frequency of 20.0 kHz. Then, a filter press was used to remove residue of the mycelia. After drying under vacuum at 40 °C, PVE was obtained from the infiltrate. The average molecular weight of PVE was 758 Da as determined by liquid chromatography electrospray mass spectrometry (LC-ESI-MS) (Wang et al., 2021a,b). In PVE, the organic matter content was 81.5%, with the content of saccharide, protein, amino acid, nucleoside, and lipid of 33.33%, 19.24%, 28.96%, 7.4% and 3.75%, respectively (Wang et al., 2021a,b). PVE at 1.0 mg/mL was prepared in 20% alcohol as stock solution and stored at 4 °C prior to use.
2.2 C. elegans strains and culture conditions
The wild-type Bristol N2, the transgenic nematode strains and Escherichia coli OP50 were provided by the Caenorhabditis Genetics Center (University of Minnesota, USA). The transgenic nematode strains used in this work were: GR1307 [daf-16(mgDF50)], LD1 (ldIs7 [skn-1b/c::GFP + rol-6(su1006)]), EU1 [skn-1(zu67)], and TJ356 (zIs356[daf-16p::daf-16a/b::GFP + rol-6(su1006)]). All worms were cultured at 20 °C in nematode growth medium (NGM) plates with E. coli OP50 as food. C. elegans strains were synchronized according to Brenner (1974). After synchronization, the L4 C. elegans were prepared for further experiments.
2.3 Toxicity assay
PVE was dissolved in M9 buffer to obtain the final concentrations of 1.0, 10, 100, 1000 and 10000 ng/mL. M9 buffer only was served as the solvent control. Each individual well of a 24-well plate was filled with equal volumes (1.0 mL) of PVE at corresponding doses and C. elegans suspension containing 60 synchronized L4 worms at 20 °C. The survival of nematodes was recorded every day and data were expressed as the survival of nematodes after 72 h. The experiment was conducted in independent triplicates.
2.4 Bacterial growth assay
Bacterial growth assay was performed to exclude caloric restriction induced by PVE. Briefly, the final concentration of PVE at 100 ng/mL was added to freshly prepared E. coli OP50 solutions. M9 buffer only was included as the solvent control. E. coli OP50 was cultured for 6 h at 37 °C and the OD600 values were measured with a microtiter plate reader (Synergy H1, BioTek, USA). The experiment was conducted in independent triplicates.
2.5 Measurement of lifespan and brood size assays
Sixty L4 synchronized Bristol N2 worms were transferred to fresh NGM plates with or without PVE at 20 °C. 5-fluoro-2-deoxyuridine (Sigma-Aldrich) at 50 μM was used to inhibit progeny growth. Worms were transferred every other day to fresh extracts. The number of living nematodes was counted daily until the last nematode was dead. For the brood size assay, twenty synchronized L4 nematodes were individually transferred to new NGM plates pretreated with PVE at various concentrations or the solvent control and allowed to lay eggs. The number of eggs was counted daily until the worms stopped laying eggs. Both experiments were conducted in independent triplicates.
2.6 Growth, locomotive ability and pharyngeal pumping rate assays
Body length, width and area of wild-type C. elegans were measured following Liu et al. (2022a). Briefly, after treatment with PVE for 24 h, the worms were burned with an alcohol lamp for 10 s and immobilized. The body length and width of C. elegans was measured using an Olympus SZX10 stereoscope. Body area was the product of body length and width. Locomotion assay was performed according to Yu et al. (2021). After exposure, the nematodes were washed with M9 buffer and then placed onto new NGM plates. After 1 min recovery, the frequency of head thrashes and body bends in 30 s was recorded under a stereomicroscope. Pharyngeal pumping rate was measured following Huang et al. (2004). After treatment, worms were washed and transferred to fresh NGM plates without E. coli OP50 and the pharyngeal pumping rate within 30 s was counted after 2 min of exercise. These experiments were conducted in independent triplicates with 20 nematodes for each treatment.
2.7 Measurement of intracellular ROS, lipofuscin and lipid accumulation
The L4 stage wild-type N2, GR1307 [daf-16(mgDf50)] and EU1 [skn-1(zu67)] of nematodes were treated with PVE at 1.0, 10 and 100 ng/mL for 24 h. Then worms were washed three times using M9 buffer. Subsequently, the nematodes were transferred into a 2-mL centrifuge tube, followed by centrifugation at 4000 rpm for 2 min, and the supernatant was removed. Precipitated nematodes were used in the following assays. (1) ROS production: the nematodes were pre-incubated in 1 μM of CM-H2DCFDA (Beyotime Biotechnology, China) for 2 h at 20 °C. (2) Lipofuscin accumulation: the nematodes were pre-incubated in levamisole at 1 mM. (3) Lipid accumulation: the nematodes were treated by paraformaldehyde (4%) for 1 h. Then the nematodes were stained with Nile red solution (1 μg/mL for 2 h. The worms were washed with S-basal [5.85 g NaCl, 6 g KH2PO4, 1 g K2HPO4, and 1 mL cholesterol (5 mg/mL in ethanol) in 1 L H2O, and sterilized by autoclaving]. Fluorescence was detected with a fluorescence microscope (Olympus TH4-200, Japan). Fluorescence intensity was measured using ImageJ. These experiments were conducted in independent triplicates with 20 nematodes for each treatment.
2.8 Determination of MDA content and antioxidant enzyme activities
The content of MDA and activities of antioxidant enzyme assays were determined according to Hui et al. (2020). The nematodes were pretreated with PVE at different concentrations for 24 h at 20 °C. Worms (∼7000) were washed thrice with M9 buffer, and mixed with PBS (phosphate-buffered saline) buffer. The mixture was homogenized in an ice bath, centrifuged at 4000 rpm for 3 min at 4 °C. The supernatant was collected for further analyses. The MDA content and activities of superoxide dismutase (SOD), catalase (CAT) and glutathione S-transferase (GST) were measured using the manufacturer’s protocol (JianCheng, NanJing, China). These experiments were conducted in independent triplicates.
2.9 Quantitative real-time PCR (qRT-PCR)
Total RNA was isolated using Trizol reagent, and the cDNA was produced by the Quantscript RT kit (Accurate Biology, China). The qRT-PCR was conducted to test the expression of antioxidant-related genes by using a QuantStudio™ 3 Real-Time PCR System (Thermo Fisher Scientific, Waltham, US). The 2-△△Ct method was used to quantify the relative gene expression, and act-1 was selected as the reference gene (Livak and Schmittgen, 2001). The genes and their PCR primers are listed in Table S1. The experiment was conducted in independent triplicates.
2.10 Intracellular localization of DAF-16 and SKN-1
Intracellular localization of DAF-16 and SKN-1 was analyzed with the transgenic C. elegans strains TJ356 and LD1, respectively. Synchronized mutant nematodes were cultured on NGM plates with PVE (1.0, 10 and 100 ng/mL) for 24 h at 20 °C. After exposure, mutant nematodes were fixed by sodium azide at 20 mM, and a fluorescence microscope was used to observe subcellular distribution. Intracellular location of DAF-16 and SKN-1 was classified into three categories: cytosolic, intermediate and nuclear. The experiment was conducted in independent triplicates with 30 nematodes for each treatment.
2.11 Statistical analysis
Data were expressed as the mean ± SEM and analyzed with SPSS (V16.0, IBM, Chicago, IL). Significant differences were measured using analysis of variance (ANOVA) with Student-Newman-Keuls test at p = 0.05.
3 Results and discussion
3.1 PVE promoted the lifespan in C. elegans and had no obvious effect on bacteria growth and nematodes fecundity
Before conducting other experiments, an in vivo toxicity study was performed. As depicted in Fig. S1, PVE at all tested concentrations were non-toxic to C. elegans. Then lifespan assays were performed and the results showed that PVE at the tested concentrations significantly (p < 0.05) prolonged the mean lifespan of C. elegans as compared to the solvent control (Fig. 1). The mean lifespan of worms treated with PVE at 1, 10, 100, 1000, and 10000 ng/mL was between 12.78 and 15.98 days, while that of the solvent control group was only 10.00 days (Table S2). The result was consistent with previous reports that many extracts had potent lifespan extension properties (Martel et al., 2020). Cuong et al. (2019) reported that lifespan was significantly extended by 35.84% after exposure to extract from Ganoderma lucidum (Basidiomycetes). In our study, exposure to PVE at 1.0, 10 and 100 ng/mL significantly (p < 0.001) extended lifespan by 36.60%, 59.80% and 53.30%, respectively, as compared to the solvent control group. Thus, the concentrations of 1.0, 10 and 100 ng/mL of PVE, which were most effective in improving longevity of C. elegans, were selected for further tests.
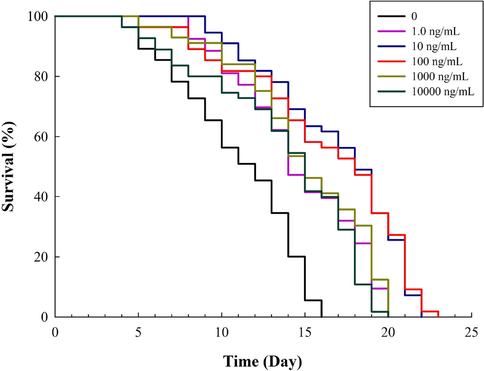
- Effect of Paecilomyces variotii extract (PVE) on the lifespan of C. elegans. L4 stage wild-type N2 nematodes were exposed to PVE at 0 (M9 buffer), 1.0, 10, 100, 1000 and 10000 ng/ml.
Previous studies have shown that PVE contained antioxidants, showing antimicrobial and anti-inflammation activities in vitro (Dai et al., 2020; Moreno-Gavíra et al., 2020). It is also suggested that proliferating bacteria adversely affected the nematodes by yielding harmful metabolite (Garigan et al., 2002). It is possible that PVE extended the lifespan via restricting bacterial growth. To determine the influence of PVE on the growth of E. coli OP50, the growth of bacteria in the control and PVE at 100 ng/mL group was assessed (Fig. S2). Results showed that PVE did not inhibit E. coli OP50 growth. Therefore, it is unlikely that PVE extended the lifespan of the nematodes via its antimicrobial effects or restricting bacterial growth.
In a previous study, it was observed that longevity was negatively correlated with fecundity (Arantes-Oliveira et al., 2003). To clarify the effect of PVE on brood size, the number of offspring was measured. Our result showed no significant differences were observed in brood size between PVE-treated and the solvent control groups (Fig. S3). This indicated that PVE had no effect on the reproduction of the nematodes.
3.2 PVE promoted growth but did not influence locomotion behavior and pharyngeal pumping in C. elegans
To determine whether PVE had side effects on the health of C. elegans, we evaluated its effects on growth, locomotion behavior, and pharyngeal pumping of the nematodes. Compared to the solvent control, PVE at 1.0 and 10 ng/mL significantly (p < 0.05) increased the body length of C. elegans (Fig. 2A). There were no significant differences between 100 ng/mL of PVE treatment and the solvent control (p = 0.158). The body width of the nematodes treated with PVE at 10 and 100 ng/mL was significantly (p < 0.001) increased compared to the solvent control (Fig. 2B). There were no significant differences between the nematodes exposed to 1.0 ng/mL of PVE and the solvent control (p = 0.402). Only PVE at 10 ng/mL significantly (p < 0.001) increased the body area of C. elegans compared to the solvent control (Fig. 2C). Body growth is an important indicator for assessing C. elegans health. Our results showed that PVE at 10 ng/mL consistently increased the growth of the worms. This agreed with previous studies, indicating that the worms had larger size after exposure to extracts from G. lucidum or Lentinus edodes (Liu et al., 2022b; Peng et al., 2021).
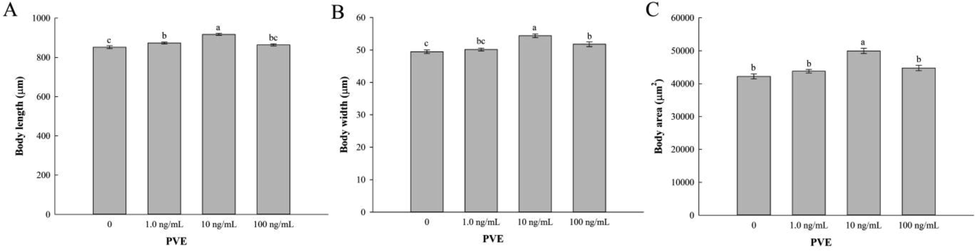
- Effect of Paecilomyces variotii extract (PVE) on growth of C. elegans. L4 stage wild-type N2 nematodes were exposed to PVE at 0 (M9 buffer), 1.0, 10 and 100 ng/ml for 24 h. (A) body length; (B) body width; (C) body area. The results were expressed as mean ± standard error of the mean. The statistical significance of difference was analyzed by one-way ANOVA with Student-Newman-Keuls test. Bars with no letters in common are significantly different (p < 0.05).
Pharyngeal pumping and locomotion behavior are regulated by complex neural networks and muscles, and motor abnormality reflects function defects in neurological or muscular (Zhen and Samuel, 2015). As shown in Fig. S4, no statistical differences were observed between PVE treatments and the solvent control in locomotion behaviors (head thrash and body bend) and pharyngeal pumping, indicating that PVE treatments had no effects on nematode motility and pharyngeal pumping. This disagrees with a previous study, which showed that Cordyceps bassiana spore (Ascomycetes) extract at 0.145 mg/mL significantly increased the frequency of head thrashes and body bends of the nematodes (Ye et al., 2021). Results from Tiwari et al. (2014) also indicated that no negative effects by an endophytic fungus of Curcuma amada extract were detected on the pharyngeal pumping of the nematodes.
3.3 PVE decreased oxidative stress in C. elegans
Extended lifespan is often accompanied by enhanced stress resistance (Song et al., 2020), so we detected the effect of PVE on oxidative stress and antioxidant enzyme activities. Intracellular ROS, lipofuscin and lipid accumulations are important biomarkers of oxidative stress and aging in the nematodes (Huang et al., 2004). Results from our present study showed that PVE at three tested concentrations significantly reduced the levels of ROS compared to the solvent control, with PVE at 10 ng/mL being the best (Fig. 3A and B). There were no differences in ROS levels between 1.0 and 100 ng/mL of PVE treatment groups (p = 0.660). PVE treatments also significantly reduced the lipofuscin content in the nematodes (p < 0.001) compared to the solvent control (Fig. 3C). A dose-response relationship was found between PVE dose and lipofuscin content. Moreover, compared with the solvent control, PVE at 100 ng/mL significantly (p < 0.001) inhibited the accumulation of lipid (Fig. 3D). PVE at lower doses (1.0 and 10 ng/mL) did not significantly (p = 0.074 and 0.122, respectively) affect the accumulation of lipid in the nematodes.
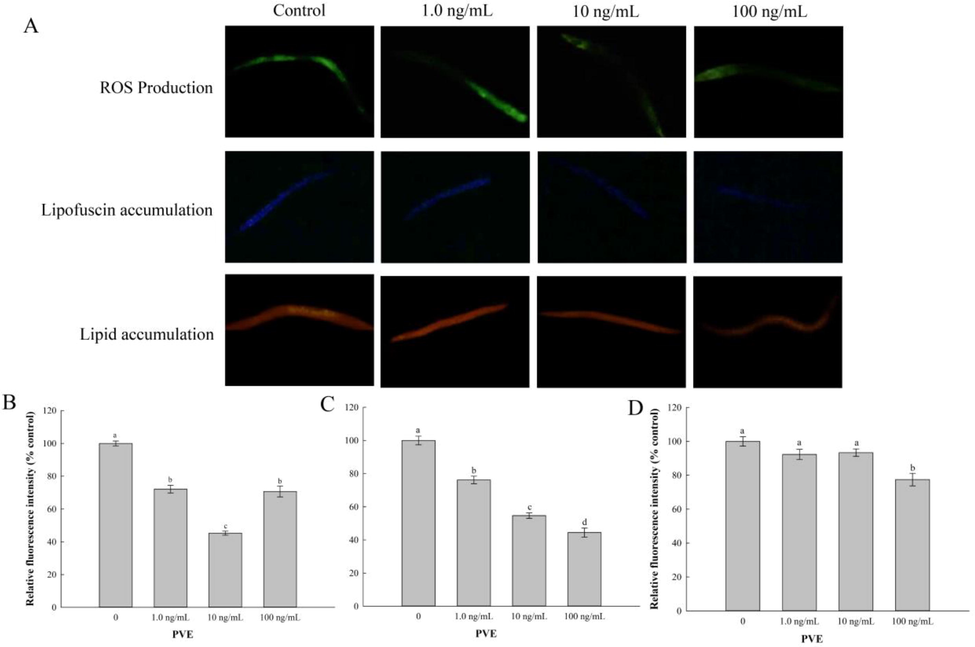
- Effect of Paecilomyces variotii extract (PVE) on oxidative stress in C. elegans. L4 stage wild-type N2 nematodes were exposed to PVE at 0 (M9 buffer), 1.0, 10 and 100 ng/ml for 24 h. The results were shown as the percentage relative to the solvent control. (A) Representative images showing accumulations of ROS, lipofuscin and lipid, respectively; (B) Comparison of ROS production; (C) Comparison of lipofuscin accumulation, and (D) Comparison of lipid accumulation. The results were expressed as mean ± standard error of the mean. The statistical significance of difference was analyzed by one-way ANOVA with Student-Newman-Keuls test. Bars with no letters in common are significantly different (p < 0.05).
Natural compounds have been well-documented to promote stress resistance in C. elegans (Benedetti et al., 2008). Oxidative stress can be induced by an excessive increase of ROS in C. elegans, and lipofuscin and lipid accumulations are promoted by oxidative stress (Brunk and Terman, 2002). In the present study, PVE decreased accumulation of ROS, lipofuscin and lipid. This indicated that PVE mitigated the damage of intracellular DNA, proteins and other biological macromolecules caused by excessive ROS, reduced lipid peroxidation and enhanced stress resistance, thus improving the survival of C. elegans. The results were agree with Lin et al. (2018) and Cuong et al. (2019), who reported that extract from Cordyceps sobolifera (Ascomycetes) and G. lucidum ameliorated oxidative stress in the nematodes. As for the accumulation of ROS, PVE at 10 ng/mL was lower than that of 100 ng/mL. Our results were in agreement with Azevedo et al. (2019), who reported that an aqueous leaf extract (ALE) from Uncaria tomentosa reduced intracellular accumulations of ROS in N2 worms. The accumulation of ROS in ALE at 40 μg/mL treatment was significantly lower than that of ALE at 80 μg/mL, which might be the reason that ALE at 40 μg/mL switched from an antioxidant to a pro-oxidant behavior. Further investigations are needed to validate if this is the reason for the results obtained in the present study.
3.4 PVE reduced MDA content and enhanced antioxidant enzyme activities in C. elegans
MDA affects the activity of antioxidant enzymes and aggravates the damage to membranes in organisms to accelerate aging process (Lapointe and Hekimi, 2010). Results from our study exhibited that PVE at 1.0, 10, 100 ng/mL significantly (p < 0.05) reduced the level of MDA compared to the solvent control group, with PVE at 10 ng/mL being the best (Fig. 4A). Antioxidant enzymes mitigate biological oxidative damage, and reduce the production of excessive ROS, playing a key role in the defense system (Beckman and Ames, 1998). All PVE treatments significantly increased activities of SOD, CAT and GST in the nematodes, with PVE at 10 ng/mL being the best (Fig. 4B-D). SOD and CAT had a same trend that the highest activity was observed in PVE at 10 ng/mL treatment, followed by PVE at 100 and 1.0 ng/mL with PVE at 1.0 mg/mL being the least. For GST activity, there was no statistically significant difference between PVE at 1.0 and 100 ng/mL (p = 0.165).
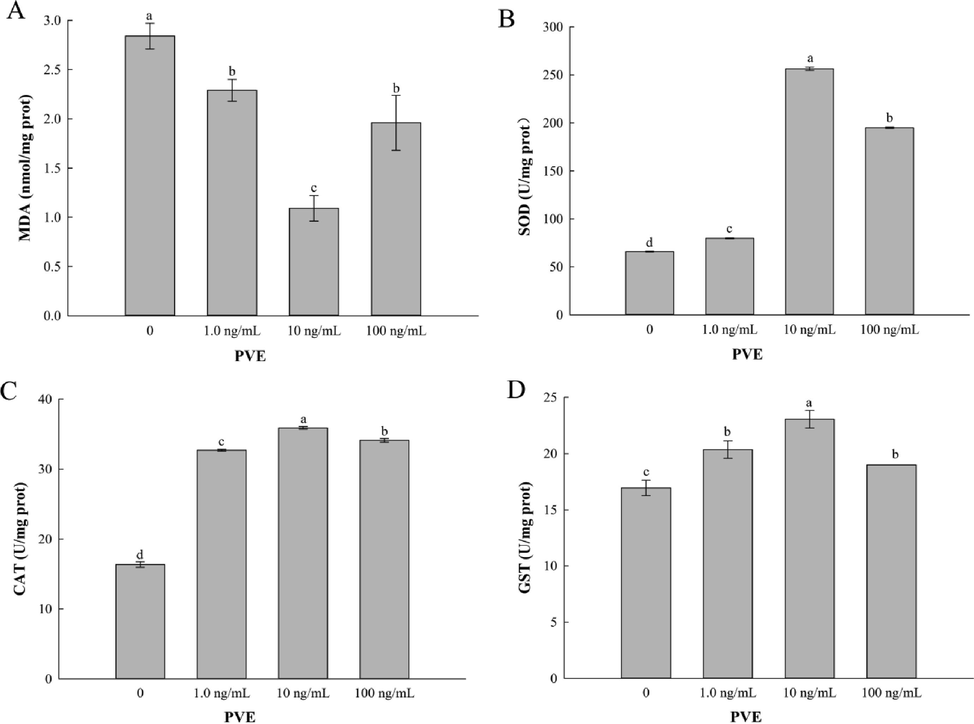
- Effect of Paecilomyces variotii extract (PVE) on MDA content and antioxidant enzyme activities in C. elegans. L4 stage wild-type N2 nematodes were exposed to PVE at 0 (M9 buffer), 1.0, 10 and 100 ng/ml for 24 h. (A) MDA content; (B) SOD activity; (C) CAT activity; (D) GST activity. The results were expressed as mean ± standard error of the mean. The statistical significance of difference was analyzed by one-way ANOVA with Student-Newman-Keuls test. Bars with no letters in common are significantly different (p < 0.05).
MDA is the end product of lipid peroxidation, which is considered to be a key indicator to evaluate oxidative damage (Li et al., 2016). SOD, CAT, and GST are antioxidant enzymes that scavenge oxygen free radicals in organisms. For instance, Hu et al. (2021) found that flavonoids from Folium Artemisia Argyi reduced MDA level and improved the antioxidant defense system. The results agreed with ours that PVE treatment enhanced the oxidative stress resistance and improved the antioxidant defense system.
3.5 PVE affected gene expression related to oxidative stress
Our results showed that PVE reduced ROS content and increased lifespan of the nematodes. To explore the pathways involved, the expression of several genes related to oxidative stress in the nematodes was investigated. In the wild-type N2, PVE exposure increased mRNA level of skn-1, mev-1, sod-3, and daf-2, but significantly down-regulated gene expression of nhr-49 and daf-16 (Fig. 5), suggesting that these genes played important roles in mediating oxidative stress in the nematodes. Our results agreed with previous reports, in which extracts from rhodiola and raspberry substantially increased gene expressions of daf-16, sod-3, gst-4, daf-2 and skn-1 (Jiang et al., 2021; Song et al., 2020). Contrary to their results, our results showed that PVE treatment significantly down-regulated gene expression of daf-16. Deng et al. (2020) found that activation of SKN-1 caused strong inhibition of DAF-16 reporters, indicating that SKN-1 may be a negative regulator of DAF-16. This discrepancy could be explained by the complex roles of DAF-16 and SKN-1 in stress resistance and longevity regulation (Deng et al., 2020; Tullet et al., 2017). Havermann et al. (2016) reported that baicalein modulated stress-resistance and lifespan in C. elegans through SKN-1, but not DAF-16. Therefore, whether there is a tradeoff between daf-16 expression and lifespan of C. elegans will need further investigation.
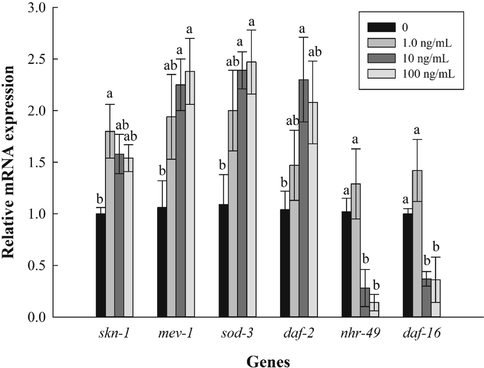
- Effect of Paecilomyces variotii extract (PVE) on expression of genes related to oxidative stress. L4 stage wild-type N2 nematodes were exposed to PVE at 0 (M9 buffer), 1.0, 10 and 100 ng/ml for 24 h. The results were expressed as mean ± standard error of the mean. The statistical significance of difference was analyzed by one-way ANOVA with Student-Newman-Keuls test. Bars with no letters in common are significantly different (p < 0.05).
3.6 PVE induced nuclear translocation of SKN-1::GFP, but not affect DAF-16::GFP
SKN-1 and DAF-16 are important transcription factors that modulate stress response and longevity of C. elegans by natural compounds (Baumeister et al., 2006). In this study, PVE at three concentrations significantly promoted the nuclear localization of SKN-1 (Fig. 6A). Compared with the solvent control, the nuclear localization was significantly (p < 0.05) enhanced by 31.67%, 45.00% and 41.67%, respectively, in the nematodes treated with PVE at 1.0, 10 and 100 ng/mL (Fig. 6B). In contrast to SKN-1, PVE treatments did not affect nuclear translocation of DAF-16 (Fig. 6C). Our results suggested that SKN-1 was the key transcription factor that modulated oxidative stress and lifespan in C. elegans induced by PVE. Most studies indicated that SKN-1 and DAF-16 were activated simultaneously to increase stress resistance and extend lifespan in the nematodes (Deng et al., 2020; Jiang et al., 2021; Song et al., 2020). Contrary to results mentioned above, our results showed that PVE induced nuclear translocation of SKN-1, but not affect DAF-16. Tullet et al. (2017) suggested SKN-1 promoted longevity by a mechanism other than protection against oxidative damage. Thus, a balance between DAF-16 and SKN-1 may appear to promote health in C. elegans with reduced IIS (Park et al., 2021).
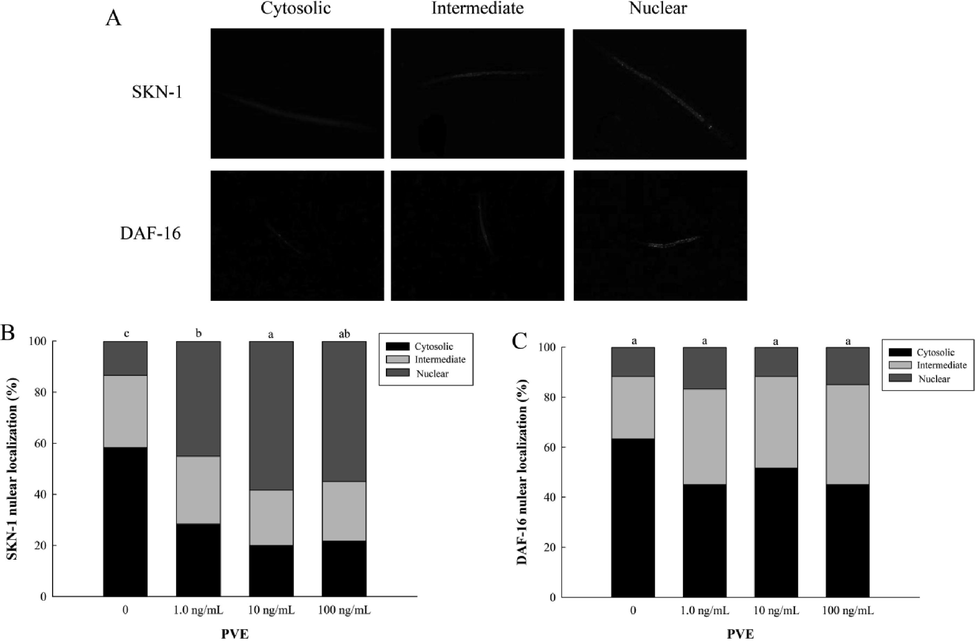
- Effect of Paecilomyces variotii extract (PVE) on intracellular localization of SKN-1 and DAF-16 in C. elegans. L4 stage wild-type N2 nematodes were exposed to PVE at 0 (M9 buffer), 1.0, 10 and 100 ng/ml for 24 h. (A) The cytosolic localization of SKN-1::GFP and DAF-16::GFP; (B) Intracellular localization of SKN-1; (C) Intracellular localization of DAF-16. The results were expressed as mean ± standard error of the mean. The statistical significance of difference was analyzed by one-way ANOVA with Student-Newman-Keuls test. Bars with no letters in common are significantly different (p < 0.05).
3.7 PVE promoted the expression of skn-1 downstream genes, but not affected that of daf-16
To further confirm the role of SKN-1, the expression levels of its downstream genes (gcs-1 and gst-4) were determined. Our results showed that PVE at 10 ng/mL significantly influenced the gene expression of gst-4 and gcs-1 in wild-type C. elegans (Fig. S5). However, these changes disappeared in the skn-1 (zu67) mutant nematodes. Similarly, we detected the effect of PVE on daf-16 downstream genes, including ctl-2 and hsp-16.2. The results showed that PVE at three concentrations significantly (p < 0.05) down-regulated the expression of hsp-16.2 in N2 and GR1307 worms, while, the expression of ctl-2 had no significant differences for all of the PVE treatments and the solvent control (Fig. S6).
3.8 Effects of PVE on the ROS levels of daf-16 and skn-1 mutant strains
Mutant strains lacking daf-16 (GR1307) and skn-1 (EU1) genes were used to quantify the intracellular ROS levels. In GR1307 worms, the ROS levels were significantly lower in all of the PVE treatments compared with the solvent control (Fig. 7C and D). On the other hand, in the EU1 worms, PVE at 1.0 ng/mL showed a prooxidant activity, with the ROS accumulation increasing by 23.40% in comparison with the solvent control (p < 0.001; Fig. 7A and B). No significant differences were observed between PVE treatments at 10 and 100 ng/mL and the solvent control. This result correlates with a study by Tullet et al. (2017) showing that SKN-1 was constitutively active in increasing lifespan independent of DAF-16.
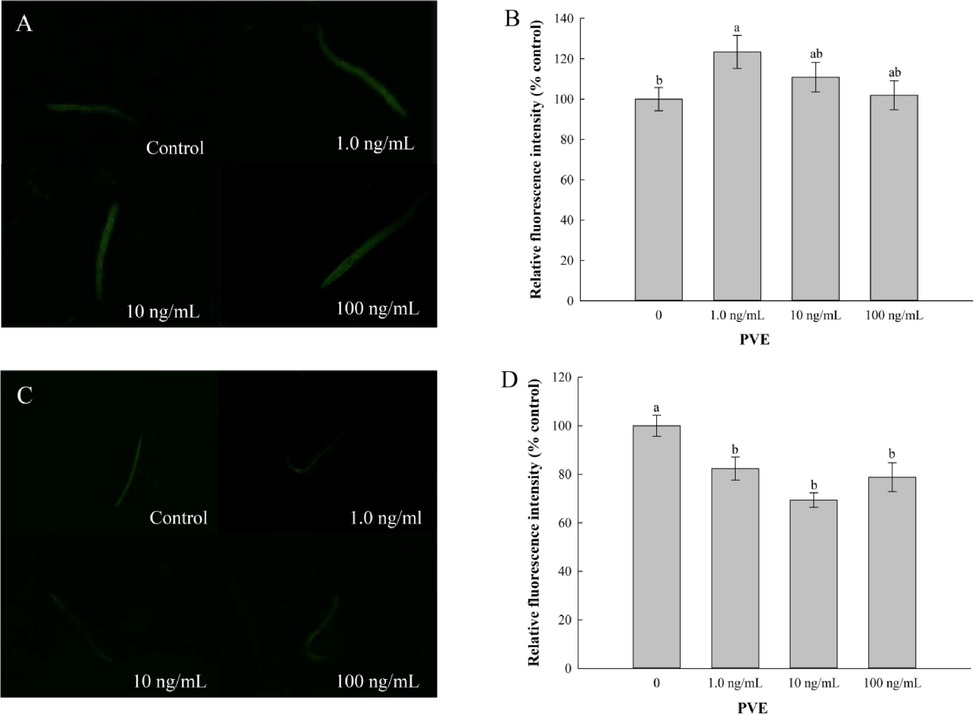
- Effect of Paecilomyces variotii extract (PVE) on reactive oxygen species (ROS) production of C. elegans. L4 stage mutant nematodes were exposed to PVE at 0 (M9 buffer), 1.0, 10 and 100 ng/ml for 24 h. (A) Representative images showing ROS production in EU1 (skn-1(zu67)); (B) Comparison of fluorescence intensity in EU1 (skn-1(zu67)); (C) Representative images showing ROS production in GR1307 (daf-16(mgDF50)); (D) Comparison of fluorescence intensity in GR1307 (daf-16(mgDF50)). The results were expressed as mean ± standard error of the mean. The statistical significance of difference was analyzed by one-way ANOVA with Student-Newman-Keuls test. Bars with no letters in common are significantly different (p < 0.05).
4 Conclusions
This is the first report that Paecilomyces variotii extract (PVE) extended lifespan and increased oxidative stress resistance in C. elegans. The results showed that PVE reduced accumulations of intracellular ROS, lipofuscin, lipid and MDA content. In addition, PVE enhanced activities of SOD, CAT and GST. The expression of antioxidant-related genes showed that PVE increased the gene expression of skn-1, mev-1, sod-3, and daf-2, but significantly down-regulated gene expression of nhr-49 and daf-16 in worms, thus improving the oxidative stress resistance of nematodes and alleviating oxidative damage. Moreover, nuclear localization assays revealed that PVE enhanced the migration of SKN-1 not DAF-16 into the nucleus. Further studies on C. elegans mutant strains also indicated that enhanced stress resistance of PVE was attributed to SKN-1. In conclusion, our results revealed that PVE could increase lifespan and protect C. elegans against oxidative stress through SKN-1. Results from this study demonstrated the antioxidant activities of PVE providing a basis for further research on PVE against oxidative stress.
Author contribution statement
KQ and XJ conceived and designed research. YW, HL, WS, GF and YL conducted experiments and analyzed data. All authors contributed to discussion of the results. KQ and SZ supervised the research, reviewed and revised the manuscript. YW and KQ wrote the paper. All authors approved its submission.
Acknowledgements
This work was supported by Shandong Provincial Natural Science Foundation (ZR2021MC065), Shandong Province Modern Agricultural Technology System Peanut Innovation Team, China (SDAIT-04-08), the Major Science and Technology Innovation Project of Shandong Province (2019JZZY020608), and National Natural Science Foundation of China (31601661).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antioxidant activity of an aqueous leaf extract from Uncaria tomentosa and its major alkaloids mitraphylline and isomitraphylline in Caenorhabditis elegans. Molecules. 2019;24:3299.
- [Google Scholar]
- Endocrine signaling in Caenorhabditis elegans controls stress response and longevity. J. Endocrinol.. 2006;190
- [Google Scholar]
- Compounds that confer thermal stress resistance and extended lifespan. Exp. Gerontol.. 2008;43:882-891.
- [Google Scholar]
- Lipofuscin: mechanisms of age-related accumulation and influence on cell function. Free Radic. Biol. Med.. 2002;33:611-619.
- [Google Scholar]
- Plant endophytic fungus extract ZNC improved potato immunity, yield, and quality. Front. Plant Sci.. 2021;12:707256
- [Google Scholar]
- Maize yield and root morphological characteristics affected by controlled release diammonium phosphate and Paecilomyces variotii extracts. Field Crops Res.. 2020;255:107862
- [Google Scholar]
- The anti-oxidation and anti-aging effects of Ganoderma lucidum in Caenorhabditis elegans. Exp. Gerontol.. 2019;117:99-105.
- [Google Scholar]
- Secondary metabolites and their bioactivities produced by Paecilomyces. Molecules. 2020;25:5077.
- [Google Scholar]
- SKN-1 is a negative regulator of DAF-16 and somatic stress resistance in Caenorhabditis elegans. G3-Genes Genom. Genetics. 2020;10:1707-1712.
- [Google Scholar]
- Fungal endophytes and their interactions with plants in phytoremediation: A review. Chemosphere. 2017;168:1100-1106.
- [Google Scholar]
- Genetic analysis of tissue aging in Caenorhabditis elegans: a role for heat-shock factor and bacterial proliferation. Genetics. 2002;161:1101-1112.
- [Google Scholar]
- Endophytic mediation of reactive oxygen species and antioxidant activity in plants: a review. Fungal Divers.. 2012;54:1-10.
- [Google Scholar]
- Baicalein modulates stress-resistance and life span in C. elegans via SKN-1 but not DAF-16. Fitoterapia. 2016;113:123-127.
- [Google Scholar]
- Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;419:808-814.
- [Google Scholar]
- Measurements of age-related changes of physiological processes that predict lifespan of Caenorhabditis elegans. P. Natl. Acad. Sci. USA. 2004;101:8084-8089.
- [Google Scholar]
- Anti-aging effects on Caenorhabditis elegans of a polysaccharide, O-acetyl glucomannan, from roots of Lilium davidii var. unicolor Cotton. Int. J. Biol. Macromol.. 2020;155:846-852.
- [Google Scholar]
- Antioxidant capacity of flavonoids from Folium Artemisiae Argyi and the molecular mechanism in Caenorhabditis elegans. J. Ethnopharmacol.. 2021;279:114398
- [Google Scholar]
- Rhodiola extract promotes longevity and stress resistance of Caenorhabditis elegans via DAF-16 and SKN-1. Food Funct.. 2021;12:4471-4483.
- [Google Scholar]
- Finding function in novel targets: C. elegans as a model organism. Nat. Rev. Drug Discov.. 2006;5:387-399.
- [Google Scholar]
- DAF-16 target genes that control C. elegans life-span and metabolism. Science. 2003;300:644-647.
- [Google Scholar]
- Astragalus polysaccharide suppresses 6-hydroxydopamine-induced neurotoxicity in Caenorhabditis elegans. Oxid. Med. Cell. Longev.. 2016;2016:4856761.
- [Google Scholar]
- Antioxidant activity of water extract from fermented mycelia of Cordyceps sobolifera (Ascomycetes) in Caenorhabditis elegans. Int. J. Med. Mushromms. 2018;20:61-70.
- [Google Scholar]
- Lentinan extends lifespan and increases oxidative stress resistance through DAF-16 and SKN-1 pathways in Caenorhabditis elegans. Int. J. Biol. Macromol.. 2022;202:286-295.
- [Google Scholar]
- Oxidative stress, intestinal damage, and cell apoptosis: Toxicity induced by fluopyram in Caenorhabditis elegans. Chemosphere. 2022;286:131830
- [Google Scholar]
- Analysis of relative gene expression data using real-time quantitative PCR and the 2- ΔΔCT method. Methods. 2001;25:402-408.
- [Google Scholar]
- Paecilomyces variotii extracts (ZNC) enhance plant immunity and promote plant growth. Plant Soil. 2019;441:383-397.
- [Google Scholar]
- Plant and fungal products that extend lifespan in Caenorhabditis elegans. Micro. Cell. 2020;7:255-269.
- [Google Scholar]
- Biocontrol effects of Paecilomyces variotii against fungal plant diseases. J. Fungi. 2021;7:415.
- [Google Scholar]
- Paecilomyces and its importance in the biological control of agricultural pests and diseases. Plants. 2020;9:1746.
- [CrossRef] [Google Scholar]
- From concept to commerce: developing a successful fungal endophyte inoculant for agricultural crops. J. Fungi. 2018;4:24.
- [Google Scholar]
- A PTEN variant uncouples longevity from impaired fitness in Caenorhabditis elegans with reduced insulin/IGF-1 signaling. Nat. Commun.. 2021;12:1-15.
- [Google Scholar]
- Ultrahigh-activity immune inducer from Endophytic Fungi induces tobacco resistance to virus by SA pathway and RNA silencing. BMC Plant Biol.. 2020;20:1-14.
- [Google Scholar]
- Ganoderma lucidum stimulates autophagy-dependent longevity pathways in Caenorhabditis elegans and human cells. Aging. 2021;13:13474-13495.
- [Google Scholar]
- Raspberry extract ameliorates oxidative stress in Caenorhabditis elegans via the SKN-1/Nrf2 pathway. J. Funct. Foods. 2020;70:103977
- [Google Scholar]
- Isolation, structure determination, and antiaging effects of 2, 3-pentanediol from endophytic fungus of Curcuma amada and docking studies. Protoplasma. 2014;251:1089-1098.
- [Google Scholar]
- The SKN-1/Nrf2 transcription factor can protect against oxidative stress and increase lifespan in C. elegans by distinct mechanisms. Aging Cell. 2017;16:1191-1194.
- [Google Scholar]
- A novel guanine elicitor stimulates immunity in Arabidopsis and rice by ethylene and jasmonic acid signaling pathways. Front. Plant Sci.. 2022;13:841228
- [Google Scholar]
- A technical system for the large-scale application of metabolites from Paecilomyces variotii SJ1 in agriculture. Front. Bioeng. Biotechnol.. 2021;9:671879
- [Google Scholar]
- Paecilomyces variotii extracts and controlled-release urea synergistically increased nitrogen use efficiency and rice yield. ACS Omega. 2020;22:13303-13311.
- [Google Scholar]
- The potential antioxidant ability of hydroxytyrosol on Caenorhabditis elegans against oxidative damage via the insulin signaling pathway. Arab. J. Chem.. 2021;14:103149
- [Google Scholar]
- Optimization of in vitro culture conditions for production of Cordyceps bassiana spores (Ascomycetes) and the effect of spore extracts on the health of Caenorhabditis elegans. Int. J. Med. Mushromms. 2021;23:45-55.
- [Google Scholar]
- Long-term toxicity of lindane through oxidative stress and cell apoptosis in Caenorhabditis elegans. Environ. Pollut.. 2021;272:116036
- [Google Scholar]
- New butenolide derivatives from the marine-derived fungus Paecilomyces variotii with DPPH radical scavenging activity. Phytochem. Lett.. 2015;11:85-88.
- [Google Scholar]
- C. elegans locomotion: small circuits, complex functions. Curr. Opin. Neurobiol.. 2015;33:117-126.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104073.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







